Published online Dec 14, 2023. doi: 10.3748/wjg.v29.i46.6028
Peer-review started: September 13, 2023
First decision: October 17, 2023
Revised: November 8, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 14, 2023
Processing time: 90 Days and 17.9 Hours
Frailty and sarcopenia are frequently observed in patients with end-stage liver disease. Frailty is a complex condition that arises from deteriorations across various physiological systems, including the musculoskeletal, cardiovascular, and immune systems, resulting in a reduced ability of the body to withstand stressors. This condition is associated with declined resilience and increased vulnerability to negative outcomes, including disability, hospitalization, and mortality. In cirrhotic patients, frailty is influenced by multiple factors, such as hyperammonemia, hormonal imbalance, malnutrition, ascites, hepatic encephalopathy, and alcohol intake. Assessing frailty is crucial in predicting morbidity and mortality in cirrhotic patients. It can aid in making critical decisions regarding patients’ eligibility for critical care and transplantation. This, in turn, can guide the development of an individualized treatment plan for each patient with cirrhosis, with a focus on prioritizing exercise, proper nutrition, and appropriate treatment of hepatic complications as the primary lines of treatment. In this review, we aim to explore the topic of frailty in liver diseases, with a particular emphasis on pathophysiology, clinical assessment, and discuss strategies for preventing frailty through effective treatment of hepatic complications. Further
Core Tip: Frailty is a common condition in patients with cirrhosis, and it is associated with increased morbidity and mortality. This review provides a comprehensive overview of the etiology, pathophysiology, assessment, and management of frailty in cirrhosis. It places particular emphasis on the management of frailty during complications, while also delving into the future of managing this condition.
- Citation: Elsheikh M, El Sabagh A, Mohamed IB, Bhongade M, Hassan MM, Jalal PK. Frailty in end-stage liver disease: Understanding pathophysiology, tools for assessment, and strategies for management. World J Gastroenterol 2023; 29(46): 6028-6048
- URL: https://www.wjgnet.com/1007-9327/full/v29/i46/6028.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i46.6028
Frailty is a complex and ever-evolving syndrome that arises from a combination of deteriorating events across various physiological systems. This results in a reduction in the body’s physical ability to withstand stressors, leading to decreased resilience and increased susceptibility to adverse outcomes such as disability, hospitalization, and mortality[1-3]. Half of the patients diagnosed with cirrhosis of any cause exhibit frailty[4]. This condition has been shown to have a significant impact on the health outcomes of individuals with liver diseases, leading to higher rates of morbidity and mortality[5]. Despite its importance, there is still a lack of knowledge in the field of frailty in liver cirrhosis and an absence of universal assessment and treatment protocols, especially in the presence of decompensation with ascites and encephalopathy. This review offers an analysis of the pathophysiological mechanisms that give rise to the clinical manifestations of frailty, various assessment tools employed to evaluate frailty in patients with cirrhosis, and highlights the management options available for frail cirrhotic patients including future therapeutic options for this condition.
Although frailty, sarcopenia, malnutrition, and cachexia are distinct terms in literature, they are deeply interconnected[6]. All four terminologies can be used to express patients with muscle loss and measures targeting management of any of these conditions can improve the others[7]. Here is a breakdown of each terminology (Table 1).
| Definition | |
| Frailty | A condition where patients undergo a reduction in their physical abilities and become more vulnerable to health-related challenges, leading to negative health consequences. It is a multifaceted concept that involves different aspects such as physical, psychological, social, and environmental factors[8] |
| Malnourishment | An imbalance in the consumption of nutrients, whether it be a deficiency or an excess, can have detrimental effects on the body’s tissues and overall physical form[9] |
| Cachexia | A metabolic syndrome that is complex and linked to an underlying illness. It is distinguished by the reduction of muscle mass, with or without a decrease in fat mass[10] |
| Sarcopenia | A debilitating syndrome that is marked by a gradual and widespread decline in both skeletal muscle mass and strength[11] |
| Dynapenia | The pre-sarcopenia stage, in which only muscle strength is reduced[12] |
| Primary sarcopenia | The loss of anatomical skeletal muscle mass in the aging population[13] |
| Secondary sarcopenia | The loss of skeletal muscle mass in various chronic diseases[14] |
| Compound sarcopenia | The combination of primary (i.e., age-related) and secondary (i.e., disease-related) sarcopenia. It occurs in older patients with chronic diseases[15] |
| Sarcopenic obesity | A state of decreased muscle mass in the setting of increased fat mass. The muscle wasting can be obscured by increased muscle mass, making specialized testing and management necessary[16] |
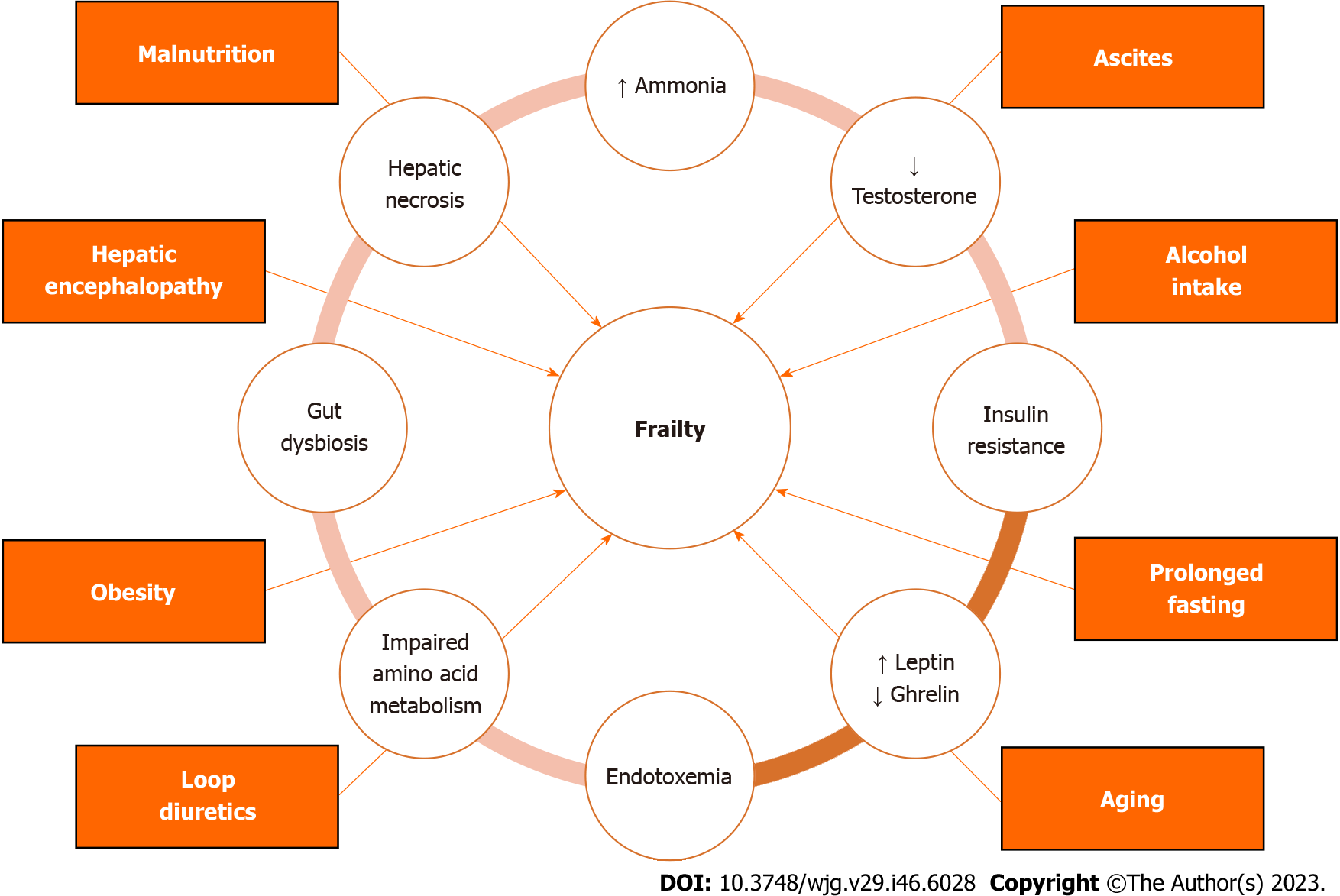
The precise reason for frailty in liver disease is not known, but it is thought to result from the failure of several organ systems, including the neuromuscular, endocrine, immune, and skeletal muscle systems[17] (Figure 1). In this section, we outline a brief overview of different factors that have been cited in literature as potential causes of frailty in cirrhosis[17,18].
Hyperammonemia is a major factor that induces sarcopenia and frailty in patients with liver cirrhosis[19-21]. There are multiple mechanisms through which hyperammonemia can contribute to frailty in individuals with cirrhosis. These mechanisms include upregulation of myostatin expression inhibiting skeletal muscle mass, mitochondrial dysfunction, heightened production of reactive oxygen species that impede protein synthesis, and enhanced proteolysis[22,23].
Testosterone plays a crucial role in activating the Akt/mTOR pathway, which is responsible for the development and maintenance of muscle tissue[24]. Individuals with cirrhosis often experience low levels of circulating testosterone. This can be attributed to the detrimental effects of cirrhosis on the hypothalamic-pituitary-gonadal axis[25]. Additionally, an increased activity of the hepatic aromatase enzyme converts testosterone into estrogen, further contributing to the decline in testosterone levels and subsequent loss of muscle mass[26]. Moreover, research has shown that androgens suppress the expression of myostatin, a key inhibitor of muscle mass[27,28]. This explains the elevated levels of myostatin in cirrhosis patients which contributes to their muscle mass loss[29].
Insulin resistance is a common occurrence in individuals with cirrhosis, as supported by the literature[30]. Insulin resistance can lead to muscle loss through several mechanisms. Insulin has anabolic effects on muscle tissue. But, when there is insulin resistance, it reduces its ability to stimulate protein synthesis, resulting in muscle mass loss[31]. Insulin resistance can also impair mitochondrial function, leading to decreased energy availability and further promoting muscle loss[32]. Finally, individuals with insulin resistance may be less physically active, which can cause muscle atrophy and a decline in muscle function[33]. All these factors contribute to the development of muscle mass loss and frailty in patients with cirrhosis.
Leptin and ghrelin are two hormones that play a crucial role in regulating energy balance, appetite, and body weight[34]. Leptin, which is produced mainly by adipose tissue, functions by suppressing hunger and promoting satiety[35]. In individuals with cirrhosis, there is typically an elevation in serum leptin levels, which can contribute to frailty by decreasing appetite, altering energy balance, and causing muscle wasting[36]. Conversely, ghrelin, which stimulates hunger, gastric motility, and gastric acid secretion, is found to be reduced in liver cirrhosis compared to healthy individuals[37,38]. These changes can affect satiety, digestive functions, and muscle mass, which predispose individuals with cirrhosis to frailty.
Liver diseases trigger proinflammatory mediators release due to hepatic necrosis and endotoxemia. In advanced liver disease, the death of liver cells triggers the recruitment of immune cells such as macrophages and neutrophils[39]. This leads to an increase in the production of substances promoting inflammation, such as cytokines, resulting in inflammation both locally and systemically[40]. Additionally, patients with cirrhosis have elevated levels of proinflammatory mediators due to endotoxemia[41]. This occurs when bacterial endotoxins circulate in the bloodstream due to impaired gut barrier function[42], portosystemic shunts[43], compromised Kupffer cell function[44], and altered gut microbiota[45]. The presence of endotoxemia triggers the release of tumor necrosis factor-alpha, interleukin-6, and reactive oxygen species which promote systemic inflammation, cause oxidative stress, and impair mitochondrial function[43,46]. The presence of these proinflammatory mediators can give rise to various frailty components, including muscle wasting, loss of appetite, fatigue, energy deficiency, and impaired immune function[18].
The human gut is a complex ecosystem that harbors a diverse array of microbial species, which engage in multifaceted interactions with our body[47]. One of the key benefits provided by the gut microbiota is their remarkable ability to ferment indigestible substances, such as dietary fibers, yielding short-chain fatty acids (SCFAs) like acetate, propionate, and butyrate. Moreover, the gut microbiota plays a pivotal role in generating a wide range of metabolites, including secondary bile acids and indols[48]. These metabolites, along with bacterial components, can circulate throughout the human body, exerting their influence on organs beyond the gut itself[49]. In the context of cirrhosis, there is a notable prevalence of gut dysbiosis, which involves an imbalance in the gut microbiota. This imbalance is marked by a decrease in the diversity of bacterial species and an overall reduction in the number of bacteria present in the gut[50,51].
In cirrhotic patients, dysbiosis plays a significant role in frailty development through various mechanisms[52-55]. Firstly, the presence of a high proportion of harmful bacteria in the gut can lead to systemic inflammation as bacterial lipopolysaccharides translocate through the compromised gut barriers[56,57]. This inflammation can cause muscle wasting and energy deficiency, further contributing to frailty. Secondly, individuals with cirrhosis often have a lower abundance of beneficial gut bacteria, which reduces their capacity to contribute to metabolism. This reduction leads to a decrease in the production of SCFAs, which are not only essential as an energy source but also serve as metabolic mediators in skeletal muscles[58-60]. The decrease in SCFAs may contribute to the observed skeletal muscle loss in cirrhotic patients and further exacerbate the development of frailty[61]. Lastly, small intestinal bacterial overgrowth (SIBO) is common in cirrhosis, and the increased population of gut microbes can lead to heightened competition with the human body for nutrients[62]. This competition can worsen the loss of muscle mass and frailty, as evidenced by the link between SIBO and malnutrition in cirrhosis[63].
Amino acid metabolism is a crucial process that plays a vital role in maintaining energy balance, promoting muscle growth, and regulating critical processes such as inflammation and insulin sensitivity[64,65]. When this process is disrupted, it can lead to the development of frailty and degenerative diseases[66]. Individuals with liver cirrhosis experience imbalances in body metabolism, resulting in higher levels of fatty acid oxidation and gluconeogenesis. This leads to an imbalance in amino acid metabolism, causing changes in amino acid levels in the blood[67,68]. As a consequence, there are low levels of branched-chain amino acids (BCAAs) in the blood, which are essential for muscle tissue. BCAAs are crucial for protein synthesis in skeletal muscles and serve as a preferred source of energy. Therefore, low levels of BCAAs in the blood can lead to muscle degradation and impaired muscle strength[69]. Moreover, patients with end-stage liver disease (ESLD) have impaired ammonia detoxification in the liver, leading to high levels of ammonia in the blood. To compensate for this, the body breaks down BCAAs stored in skeletal muscles to help eliminate ammonia[70]. This constant consumption of BCAAs further contributes to low levels of BCAAs in the blood, leading to sarcopenia and frailty. Fortunately, supplementing with BCAAs has shown significant benefits for individuals with liver cirrhosis and frailty. In a recent 16-wk randomized controlled trial, BCAA supplementation was found to improve the liver frailty index (LFI), serum albumin levels, and quality of life in frail compensated cirrhotic patients[69]. This finding is consistent with previous studies that have used BCAAs as a therapy for sarcopenia in liver cirrhosis[71,72].
Cirrhosis often leads to malnutrition, which is associated with negative consequences[73]. Malnutrition in cirrhosis has various contributing factors, including inadequate oral intake, early satiety caused by ascites, imbalance in ghrelin and leptin levels, maldigestion, malabsorption, gut microbial dysbiosis, and encephalopathy[73,74]. Frailty is strongly associated with malnutrition. Research has shown that malnourished patients have a 3.381 times higher risk of developing frailty compared to well-nourished patients[75].
In cirrhotic patients, ascites can cause several symptoms such as loss of appetite, difficulty moving, reduced stomach capacity, and poor digestion[76] (Table 2). These symptoms can ultimately result in malnutrition and frailty. However, therapeutic paracentesis has been shown to effectively counteract these effects and provide several benefits for cirrhotic patients, including improving appetite, decreasing feelings of fullness, enhancing caloric intake, and improving exercise tolerance[77]. Large-volume paracentesis can increase fasting gastric volumes, which leads to better tolerance of nutrient ingestion and improved caloric intake[78]. This could potentially be explained by the procedure providing more space for the stomach to relax within the peritoneal cavity with albumin infusion helping relieve gastric wall edema[78].
| Predisposing factor | Pathophysiology | Morbidity and mortality | Recommendations |
| Ascites | Loss of appetite; Difficult ambulation; Reduced stomach capacity; Poor digestion | Odds of frailty were higher in ascitic than non-ascitic patients [adjusted odd ratio 1.56, 95% confidence interval (CI): 1.15-2.14][129]. Ascitic patients identified as frail had a 29% waitlist mortality rate, higher than the 17% rate for non-frail patients[129] | Large volume paracentesis with iv albumin; Salt intake not < 5 g NaCl/d to preserve food palatability |
| Hepatic encephalopathy (HE) | Decreased voluntary oral intake; Decreased capacity for ambulance and exercise | Odds of frailty were higher in HE than in non-HE patients (odd ratio 2.45, 95%CI: 1.80-3.33)[129]. Waitlist mortality was higher for HE patients identified as frail (30%) than non-frail (20%)[129] | Enteral nutrition with precautions to avoid aspiration and hyperglycemia; Parenteral nutrition if indicated; Avoid unnecessary protein restriction |
| Alcohol intake | Decreased oral intake; Gastrointestinal upset; Vitamin and mineral deficiency; Increased resting energy expenditure; Alcohol direct toxic muscular and neurologic effects | Frail alcoholic liver disease patients had a significantly higher risk of death or liver transplantation compared to non-frail patients (P < 0.001)[130] | Alcohol abstinence; Healthy diet with approximately 30 kcal/kg to 40 kcal/kg per day; Small and frequent meals; Enteral feeding in severe disease |
| Sarcopenic obesity | Challenging to diagnose; Physical disability due to decreased muscle size and high muscle fat | MASLD cirrhotic patients have an increased risk of worsening frailty over time and higher waitlist mortality than non-MASLD patients[131] | Structured exercise program to help preserve muscle mass; If caloric restriction is necessary, maintain adequate protein intake (1.2-1.5 g/kg/d) |
| Prolonged fasting | Accelerated catabolic state with Increased muscle breakdown | Limit fasting period to a maximum of 12 h; Daily calorie intake should be divided into 4-6 meals; Late evening snacks | |
| Loop diuretics | May worsen muscle mass loss | Loop diuretics inversely correlated with skeletal muscle mass in cirrhotic patients | Regular frailty assessments are recommended for patients who have been on prolonged courses of loop diuretics, particularly when the dosage exceeds 20 mg/d; Spironolactone may be a preferable option for long-term use due to its promising efficacy in treating sarcopenia |
| Aging | Combined muscle loss due to aging and hepatic illness (compound sarcopenia) | Elderly sarcopenic patients with cirrhosis have longer hospital stays, higher hospitalization costs, and increased risk of in-hospital mortality[15] | Frequent frailty assessment and management in elderly patients with cirrhosis |
Salt restriction is currently a crucial component of ascites treatment at all stages, as it helps prevent sodium overload and promotes the mobilization of fluid retention[79,80]. Previous studies have indicated that a tight restriction of sodium intake (< 5 g salt daily) can result in a reduced need for diuretics, faster resolution of ascites, and shorter hospital stays[81-83]. However, strict sodium restriction may not always be the optimal approach for managing ascites in cirrhotic patients. A study by Gauthier et al[81] provides an example of this. The researchers conducted a trial on patients who were receiving diuretic treatment. They compared the resolution of ascites in a group with strict salt restriction (only 1230 mg salt daily) to a group with no sodium restriction[81]. Surprisingly, despite the rapid resolution of ascites in the restricted salt group at the beginning of the study, there was no significant difference between the two groups after 90 d[81].
While strict sodium restriction has demonstrated short-term benefits, it may increase the risk of sarcopenia and frailty in cirrhotic patients due to the unpleasant taste of food and the additional effort required for food acquisition and preparation, ultimately resulting in a loss of appetite[81,84-86]. This can lead to malnutrition and an increased risk of developing ascites. Two randomized controlled trials have provided strong evidence that adhering to a strict low-salt diet increases the risk of malnutrition and can worsen ascites in cirrhotic patients[87,88]. In a study conducted by Gu et al[87], the disappearance of ascites in cirrhotic patients was compared between a group with restricted salt intake and a group with unrestricted salt intake. The results demonstrated that cirrhotic patients in the unrestricted salt group (8.8 g NaCl/d) consumed more calories than those in the restricted salt group (4.2 g NaCl/d), leading to higher albumin levels with better nutritional status and rapidly resolving ascites[87]. These findings suggest that strict salt restriction may not always be the optimal approach for managing ascites in cirrhotic patients. Instead, individualized dietary recommendations are necessary to ensure adequate patient nutrition and prevent complications[84]. For this reason, leading hepatology scientific societies recommend a daily salt intake of no less than 5 g NaCl/d (equivalent to 2 g Na/d) for cirrhotic patients with ascites[79,89-92]. To meet the recommended salt intake, it is highly recommended to avoid consuming canned foods or pre-packaged meals. Instead, prioritize the consumption of fresh, home-cooked dishes that are rich in fruits, vegetables, and dairy products[93]. Furthermore, to enhance the flavor of meals it is advised to substitute salt with a variety of herbs and spices[84].
Patients with hepatic encephalopathy are at a high risk of malnutrition and frailty due to a decrease in voluntary oral intake[94]. This can result in longer hospital stays, worsening of their condition, and increased susceptibility to infections[95]. When dealing with such cases, enteral nutrition is advised, but it is important to take precautions to avoid the risk of aspiration and hyperglycemia[7]. Parenteral nutrition, on the other hand, should only be used for patients who are unable to tolerate enteral feeding or cannot meet their caloric and nutrient needs orally[7].
It is important to note that protein intake should not be restricted in patients with encephalopathy[7]. While there used to be a debate about whether protein supplementation could worsen hepatic encephalopathy, recent research has shown that protein does not cause encephalopathy and may even improve cognitive function in the long term[96,97]. On the contrary, restricting protein intake could exacerbate frailty[7]. Therefore, to avoid the risk of worsening frailty, it is generally recommended that patients consume protein from a diverse range of sources, including vegetables and dairy products[98]. The American Association for the Study of Liver Diseases (AASLD) suggests that adults with cirrhosis should consume 1.2-1.5 g of protein per kilogram of ideal body weight daily[7,97]. However, for critically ill cirrhotic patients, the recommended protein intake is slightly higher, ranging from 1.2-2.0 g/kg of ideal body weight daily[7]. For children with cirrhosis, a protein intake of up to 4 g/kg of body weight per day is both safe and successful in improving anthropometric measurements without the development of hepatic encephalopathy[99].
Alcohol consumption has a significant and complex impact on nutritional status, affecting both nutrient intake and absorption. Initially, alcohol itself contributes to approximately 50% of daily caloric intake, leading to a decreased intake of other essential nutrients[100]. Moreover, alcohol-induced anorexia, nausea, vomiting, and gastritis exacerbate the situation by further reducing oral intake of nutrients. Alcohol also interferes directly with nutrient absorption by damaging the mucosal lining of the stomach and small intestine[101]. This damage impairs the absorption of important nutrients such as protein, vitamins A, B1, B12, folic acid, and zinc, leading to deficiencies of these micronutrients and vitamins in individuals with alcohol-associated liver disease (ALD)[98,100]. Furthermore, chronic alcohol consumption has been found to increase resting energy expenditure, which over time can lead to significant muscle wasting and frailty[102]. These factors cause malnutrition and frailty in alcohol-related cirrhosis, which has a detrimental impact on survival rates and quality of life, with an increased risk of various complications. These include variceal bleeding, development of ascites and hepatic encephalopathy, susceptibility to infections, longer hospital stays, and occurrence of hepatorenal syndrome[103].
Given the multifaceted impact of alcohol on liver diseases and nutritional status, lifelong abstinence is recommended[104]. Additionally, it is crucial to address nutritional deficiencies and promote a healthy diet in individuals with ALD[105]. This can help to mitigate the negative effects of alcohol on nutrient intake and absorption, as well as prevent muscle wasting and frailty. To prevent these issues, current guidelines recommend approximately 30 kcal/kg to 40 kcal/kg per day for these patients, with an emphasis on small, frequent meals as some patients may not be able to tolerate large meals three times a day[106]. In severe cases of ALD, supplementation in the form of enteral nutrition should be considered to ensure adequate nutrition and prevent complications associated with malnutrition[107].
Sarcopenic obesity is a condition where there is a simultaneous loss of skeletal muscle and gain of adipose tissue, which is a common finding in patients with cirrhosis[108]. This condition is characterized by a decrease in muscle size and an increase in muscular fat, known as myosteatosis. It can be challenging to diagnose, particularly in patients with metabolic dysfunction-associated steatotic liver disease (MASLD), as the presence of morbid obesity can obscure the signs of sarcopenia, making it difficult to detect[109]. Sarcopenic obesity has detrimental effects on the morbidity and mortality of patients with liver cirrhosis[108]. It poses a greater risk of physical impairment and disability than either sarcopenia or myosteatosis alone, and it is considered a negative prognostic marker for the progression of liver cirrhosis and outcomes of liver transplantation[110]. Additionally, both sarcopenia and myosteatosis are associated with a higher risk of long-term mortality in cirrhosis[108].To manage sarcopenia and MASLD, increasing physical activity and following a healthy diet can be helpful[111].
Currently, there are no specific exercise recommendations available for cirrhotic patients with sarcopenic obesity[112]. However, several studies have indicated that exercise can have positive effects on these patients[113-115]. These studies have shown that exercise can lead to a reduction in body weight and fat mass, as well as an improvement in skeletal muscle mass and physical capacity in patients with cirrhosis. These positive outcomes are particularly advantageous for patients with sarcopenic obesity. In a randomized controlled trial conducted by Román et al[114], functional capacity, body composition, and the risk of falls were measured in patients with cirrhosis before and after a moderate exercise program. The results of the study demonstrated that the exercise group experienced significant improvements in functional capacity, increased muscle mass, decreased body fat, and a reduced risk of falls, while no changes were observed in the control group[114]. Studies have also shown that elderly men who engage in moderate-to-vigorous exercise for at least thirty minutes a day have a lower risk of developing sarcopenic obesity[116]. Therefore, it can be concluded that a moderate exercise program can be beneficial for patients with cirrhosis, especially those with sarcopenic obesity. Additionally, while cirrhotic patients with sarcopenic obesity have increased body mass index (BMI) and body fat, it is important to be cautious when recommending weight loss to affected patients, in order not to exacerbate frailty[16]. If caloric restriction is deemed necessary, it is imperative to closely monitor the body composition, muscle strength, and physical activity levels of these individuals using appropriate assessment tools[117]. Moreover, it is recommended to implement a comprehensive approach that includes ensuring adequate protein intake (1.2-1.5 g/kg/d) and applying a structured exercise program to help preserve muscle mass and promote overall health[7].
Individuals with cirrhosis experience a more rapid breakdown of their body tissues due to starvation than healthy individuals[118]. After an overnight fast, the type of energy sources utilized by cirrhotic patients is comparable to that of healthy individuals who have been fasting for 2-3 d[119]. This implies that cirrhotic patients who undergo medical procedures, such as gastrointestinal endoscopy, with prolonged fasting are at risk of developing a severe catabolic state, which can lead to the breakdown of their body tissues[118]. Therefore, it is recommended that patients with liver cirrhosis limit their fasting period to a maximum of 12 h[120,121]. It is also advised that their daily calorie intake should be divided into 4-6 meals, which can include snacks at night[122,123]. This dietary approach can help maintain stable blood sugar levels and prevent the breakdown of body tissues due to prolonged fasting[118].
The evidence supporting the notion that loop diuretics may worsen sarcopenia and frailty is mounting. For instance, a study discovered that bumetanide and furosemide administration had an adverse effect on myogenic differentiation and exercise-induced muscle hypertrophy[124]. Additionally, loop diuretics have been associated with a decrease in thigh and arm circumference among heart failure patients, regardless of the severity of their disease[125]. Furthermore, a retrospective study involving 266 cirrhotic patients found that high doses of loop diuretics were associated with rapid muscle mass loss and poor survival rates, independent of liver disease severity[126]. This study found that therapeutic dosages of loop diuretics were inversely correlated with skeletal muscle mass in cirrhotic patients, as demonstrated by both simple (r = -0.27, P < 0.0001) and multiple regression analyses (t = -3.07, P = 0.002)[126]. Patients receiving more than 20 mg of loop diuretics per day had lower overall survival rates compared to those receiving < 20 mg (median, 66 mo vs 97 mo; P = 0.002), and higher doses of loop diuretics were independently associated with mortality among cirrhotic patients [hazard ratio, 1.86; 95% confidence interval (CI): 1.03-3.24; P = 0.039][126]. Compared to loop diuretics, spironolactone has been suggested to have potential benefits in preventing muscle mass loss, enhancing muscle blood flow, and boosting contractile power[127]. Given these findings, future research needs to investigate the impact of different types of diuretics on muscle health, particularly in patients who are on long-term or high-dose loop diuretic therapy, with regular assessments of frailty.
The loss of muscle mass due to aging, known as primary sarcopenia, and the loss of muscle mass due to chronic illness, known as secondary sarcopenia, combine to form a health condition called compound sarcopenia[15]. This condition can have a significant impact on the clinical outcomes of older adults with chronic diseases[128]. In patients with hospitalized cirrhotic patients, compound sarcopenia has been associated with increased length and cost of hospital stay with detrimental effects on patient survival[15]. As a result, it is crucial to regularly assess and provide aggressive treatment for elderly patients with sarcopenia.
Frailty is a condition that is associated with reduced cognitive abilities, increased risk of falls, and lower quality of life[132-134]. Depression is a common occurrence in patients with ESLD and is closely linked to frailty, rather than the severity of liver disease[135]. A prospective cohort study was conducted on 542 patients with ESLD who were referred for liver transplantation to investigate the relationship between frailty, depression, and the severity of liver disease[135]. The study found a significant association between frailty and depression, with an odds ratio of 2.78, P < 0.001. However, no significant association was found between the Model for End-Stage Liver Disease (MELD) score and depression[135]. This highlights the importance of addressing frailty as a potential risk factor for depression in patients with ESLD.
In another study, the relationship between frailty and disability was examined in cirrhotic individuals receiving outpatient care. Disability was evaluated through the measurement of individuals’ capacity to carry out essential activities of daily living (ADLs), such as feeding and bathing, as well as more complex tasks known as instrumental ADLs (IADLs), which encompass activities like shopping and managing finances. The study found a strong link between frailty and disability in patients with cirrhosis[136]. The LFI was used to measure frailty, and each point increase in the LFI was associated with a higher likelihood of experiencing difficulty with ADLs and IADLs[136]. The odds of experiencing current difficulty with at least one ADL and IADL were 3.3-fold and 4.6-fold higher, respectively, for each point increase in the LFI[136]. In participants who initially did not have any baseline disability, the study revealed that for every point increase in the LFI, the odds of having trouble with at least one ADL and IADL after 6 mo were 2.6 times and 1.7 times higher, respectively[136]. These findings suggest that even a slight increase in frailty can significantly impact the ability to perform essential tasks and responsibilities, thereby affecting both patients and their caregivers’ quality of life.
Frailty is a distinctive risk factor that is independently associated with a range of cirrhosis-related complications, such as ascites, encephalopathy, hepatorenal syndrome, and sepsis, which often necessitate hospitalization[129,137,138]. Moreover, frailty has been linked to an increased risk of acquiring nosocomial infections[139]. Patients with significant frailty may require prolonged ICU and hospital stays, and they are more prone to respiratory complications and sepsis[140]. In a prospective study involving 373 pre-transplant patients, researchers found that gait speed, a measure of frailty, played a significant and influential role in the risk of hospitalization for various complications related to cirrhosis[138]. The study found that for every 0.1 m/s decrease in gait speed, there was a 22% increase in the number of hospital days (P < 0.001), indicating a robust correlation between frailty and the need for hospital care[138].
Frailty has been identified as a significant risk factor for non-home discharge among hospitalized patients, which can lead to increased healthcare and financial burden[139]. The results of a prospective study conducted on 211 cirrhotic patients from three Liver transplantation centers revealed that frailty was strongly associated with discharge to physical rehabilitation, a skilled nursing facility, or hospice, rather than being discharged to the patient’s home[139]. The study’s odds ratio indicated that for every one-point increase in the LFI, the likelihood of non-home discharge increased by 1.81 times, (95%CI: 1.14-2.86)[139].
Frailty is an established factor that significantly increases the likelihood of severe complications and mortality among liver cirrhotic patients, both before and after liver transplantation[141]. In a comprehensive study conducted across nine transplant centers in the United States, researchers assessed frailty in pretransplant patients on the waitlist and found a significant correlation between the presence of frailty, as measured by the LFI, and an increased risk of mortality. Specifically, patients with frailty had an adjusted risk of death that was nearly twice as high as those without frailty (sub-hazard ratio 1.82, 95%CI: 1.31-2.52)[129]. Furthermore, a systematic review examining the impact of frailty on post-transplant mortality revealed that frailty had a negative effect on post-transplant outcomes. The review suggested that severe frailty was associated with a two-fold reduction in early survival and a 50% reduction in late survival[140].
The early and prompt assessment of frailty in cirrhotic patients is crucial for healthcare providers to enhance comprehensive care for ESLD patients[142]. Frailty has been observed to be a valuable predictor of outcomes both before and following therapeutic interventions. For instance, frailty has been associated with post-transjugular intrahepatic portosystemic shunt (TIPS) and post-liver transplant morbidity and mortality[143,144]. Additionally, early identification of frailty is also crucial as it is possible to reverse frailty to some extent, and identifying it early on enables healthcare providers to intervene more effectively to enhance the health outcomes of patients with frailty[145]. Therefore, all patients with this ailment need to undergo a frailty assessment to aid in making critical decisions regarding their life and death, including determining their eligibility for critical care and transplantation and prioritizing prehabilitation services such as nutrition, physiotherapy, and psychotherapy[146].
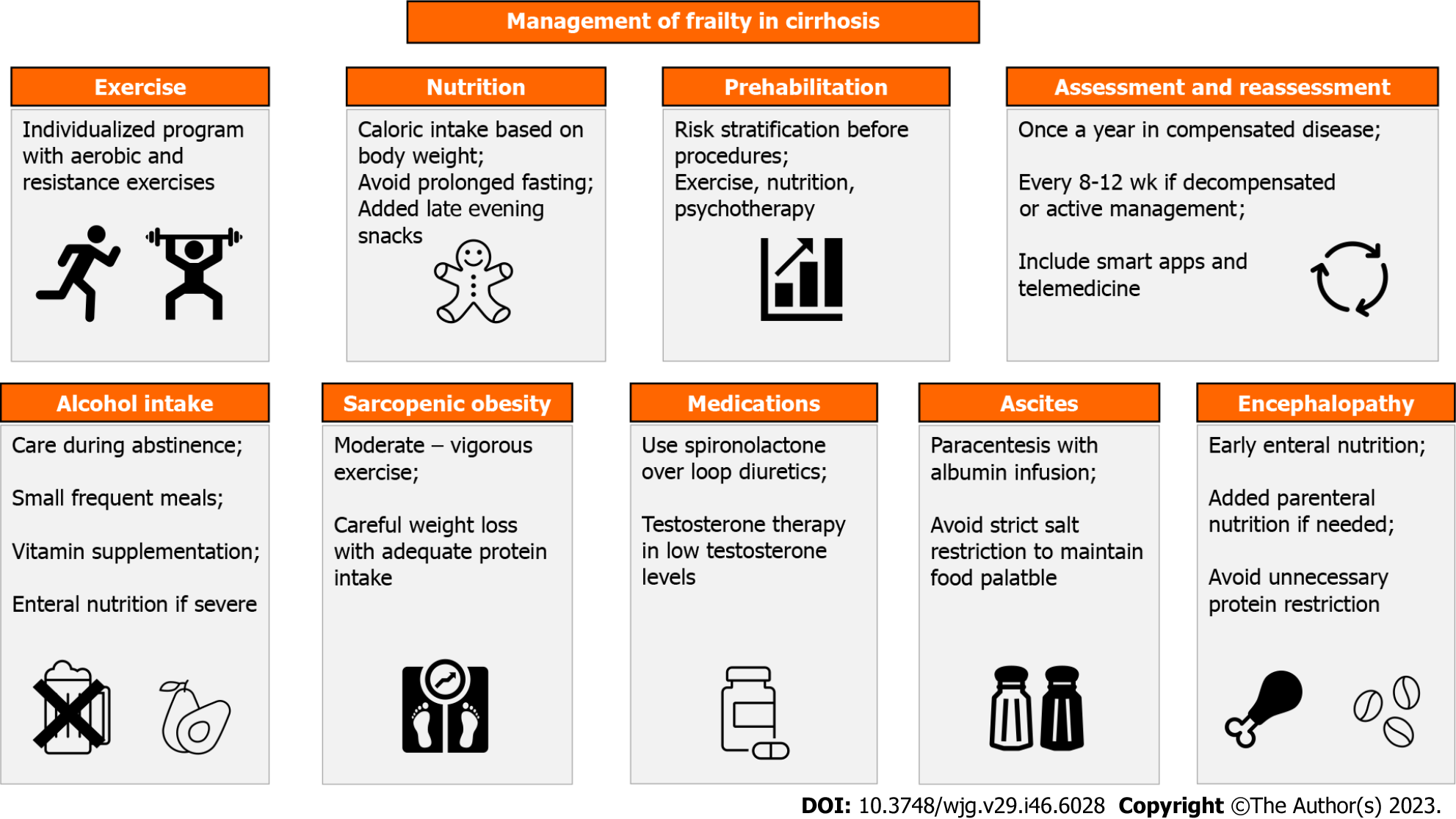
Frailty reassessment is also crucial to monitor the response to treatment of cirrhotic patients who have been diagnosed with frailty. Patients with well-compensated liver cirrhosis should be reassessed at least once a year, while those with decompensated cirrhosis or those receiving active management for these conditions should be reassessed more frequently, every 8 wk to 12 wk[7]. This will help healthcare providers to identify any changes in the patient’s condition and adjust their treatment plan accordingly, which can improve the patient’s quality of life and overall health outcomes[7].
Assessing frailty in cirrhotic patients requires the use of various assessment tools, each with its own methodology, time requirements, and limitations[146]. It is important to note that clinical interpretation of these tools can also vary among cirrhotic patients. Therefore, healthcare providers should choose the most appropriate assessment tool for each patient and interpret the results accurately. In the following paragraphs, we will summarize the three most used assessment tools for evaluating frailty in cirrhotic patients, including their strengths and limitations.
The fried frailty index (FFI) is a commonly used assessment tool for frailty that encompasses both subjective and objective components. It states self-reported fatigue, weight loss, and limited physical activity, along with objective measurements of walking speed and grip strength[8]. It is a quick assessment that can be completed in less than 10 min. The use of FFI has been linked to predicting morbidity and mortality in patients with liver cirrhosis. Higher FFI scores have been associated with elevated MELD scores, reduced albumin levels, ascites, and hepatic encephalopathy[147]. Moreover, the degree of physical weakness observed in individuals on the liver transplant waiting list, as determined by the FFI, is a noteworthy indicator of total hospitalized days per year, regardless of the severity of their liver disease[148]. Additionally, a one-unit increase in FFI leads to a 50% increase in mortality rates among those on the waiting list[149]. Despite these benefits, the accuracy of the FFI may be questionable when assessing frailty in decompensated cirrhosis. In a cohort of 685 pre-transplant patients, the FFI was found to have no association with survival among hepatic encephalopathy patients[147]. This could be attributed to the challenges that HE patients face in reporting subjective FFI components and the suboptimal performance of objective components such as grip strength and walking speed[147].
The clinical frailty scale (CFS) is a 1-min consistent method utilized to evaluate frailty in patients[150]. It is a comprehensive subjective clinical assessment of frailty that is user-friendly and has demonstrated its ability to anticipate mortality or the requirement for institutionalized care[151]. The scale rates individuals’ level of comorbidity, function, and dependence on others for daily activities, ranging from 1 (very fit) to 9 (terminally ill)[151]. Frail patients (CFS > 4) were associated with increased rates of unplanned hospitalization or death among outpatient cirrhotic patients[150]. Additionally, CFS has linked frailty to acute kidney injury and hepatorenal syndrome in patients with hospitalized liver cirrhotic patients[137]. However, the CFS provides only a brief overview of frailty and is not detailed enough to track changes in frailty resulting from therapeutic interventions[146].
The LFI is a valuable tool for assessing frailty in hepatic patients, as it combines three performance-based evaluations: grip strength of the hand, the duration taken to perform five chair stands, and the duration of maintaining three different balance positions[152]. This quick test (3-5 min) is specifically designed to assess frailty in hepatic patients, making it a reliable and efficient tool for healthcare professionals[152]. A higher LFI score indicates a greater degree of frailty, and an LFI cut-off of > 4.62 has been identified as the most effective in distinguishing between cirrhotic patients who are at high risk for rehospitalization within 30 d and those who are not[153]. This makes the LFI a crucial tool for identifying patients who require additional care and support to prevent rehospitalization. Moreover, healthcare professionals can use the LFI to assess a patient’s frailty before transplantation, identifying those who are at high risk for waitlist mortality and prolonged hospital stay after transplantation[154]. Incorporating LFI into the subjective clinical evaluation has been demonstrated to correctly reclassify the survival status of 34% of waitlist patients[154].
In addition to assessing frailty in hospitalized patients, the LFI is also employed to diagnose frailty in out-patient individuals with cirrhosis, making it a versatile tool that can be used in a variety of healthcare settings[155]. Compared to the Karnofsky Performance Status scale, which only measures one aspect of frailty, the LFI is a more comprehensive and accurate tool for assessing the risk of mortality as it captures multiple components of frailty[156].
In the management of frailty in liver cirrhosis, a continuous process is involved, which includes multiple interventions and regular reassessment of the patient to monitor their response and guide the next steps. In this section, we will provide a summary of the interventions for treating frailty in cirrhosis, as depicted in Figure 2.
Recent studies have shown that a sedentary lifestyle is linked to lower survival rates among decompensated cirrhotic patients. In fact, low moderate-vigorous activity has been associated with increased mortality among liver transplant wait-listed patients[157]. Conversely, exercise interventions have the potential to safely improve exercise capacity, peak oxygen consumption, muscle mass and function, and quality of life, while decreasing the hepatic venous pressure gradient in individuals with cirrhosis[158,159]. This can even reverse the process of frailty in cirrhotic patients.
A study was conducted to evaluate the feasibility and benefits of a 6-wk exercise program for cirrhotic patients who were waiting for a liver transplant[160]. The program involved supervised exercise on a stationary bike three times a week. The results of the study showed that the exercise program was both feasible and beneficial for these patients[160]. Specifically, 56% of the patients (9 out of 16) were able to complete the full 6-wk program, and the exercise group showed a significant improvement in oxygen consumption compared to the control group[160].
Although exercise has potential benefits for cirrhotic patients, incorporating it into their routine can be challenging due to the risk of complications. This is especially true for decompensated patients, for whom there have been few studies on the safety and effects of exercise[161]. Cirrhotic patients, especially those who are decompensated, face numerous challenges in performing exercises, such as muscle wasting, fatigue, fluid retention, risks of falls, bleeding tendency, and portal hypertension[162,163]. To fully benefit from exercise, it is crucial to customize the exercise program to the individual patient’s needs and risk of complications[7]. For instance, patients experiencing muscle mass loss may benefit from incorporating resistance training into their program, while those with a history of falls should exercise with caution when engaging in aerobic activities[7]. Moreover, while regular exercise has been found to decrease chronic portal hypertension, it is important to note that in acute situations, exercise can lead to an increase in portal pressure. Therefore, it is crucial to apply appropriate primary or secondary prophylaxis for variceal rupture to mitigate any potential risks[162].
To enhance the physical frailty and quality of life of both compensated and decompensated cirrhotic patients, it is generally recommended to incorporate a combination of aerobic and resistance exercises into their routine for at least 12 wk[161]. However, to prevent possible complications and maximize the benefits of exercise, an individualized exercise and nutrition plan should be created based on the patient’s degree of frailty. This approach involves categorizing patients into one of three groups: absent/mild, moderate, or severe frailty, and creating an individualized plan for each patient accordingly[164]. For patients with severe frailty, an inpatient rehabilitation program is recommended as they are likely to derive the greatest benefit from it[164]. This approach ensures close monitoring of the patient’s progress and helps in avoiding complications. Patients with moderate frailty are advised to participate in home-based exercises, with a focus on enhancing ADLs[164]. For patients with mild or no frailty, it is recommended that they engage in moderate-intensity exercise for a minimum of 150 min/wk[164]. These individuals should gradually work on improving their physical capacity and strength. Regular reassessment of all patients is necessary to modify their exercise programs based on their current state[164]. By following these guidelines, cirrhotic patients can safely and effectively improve their physical health and quality of life.
Studies have shown that interventions aimed at improving nutrition, such as providing nutritional education, protein-energy supplementation, and oral nutritional support, can effectively enhance frailty[165]. The Practice Guidance by the AASLD recommends developing a personalized nutrition plan for all individuals with cirrhosis, considering their current nutritional status[7]. To tailor the nutritional plan for cirrhotic patients, AASLD suggests considering their weight and BMI. For individuals with a BMI between 30-40 kg/m2, the recommended energy intake is 25-35 kcal/kg/d, while for those with a BMI ≥ 40 kg/m2, the recommended energy intake is 20-25 kcal/kg/d[7]. Moreover, AASLD recommends a safe protein intake of 1.2-1.5 g/kg/d for adults with cirrhosis, which should come from a diverse range of sources[7]. A recent randomized controlled trial showed that intensive nutrition therapy administered at home for six months can enhance frailty and sarcopenia in patients with decompensated cirrhosis[166]. The study found that the intervention group showed a greater improvement in the LFI compared to the control group (0.8 vs 0.4; P < 0.001)[166]. AASLD highlights the importance of identifying and overcoming obstacles to proper nutrition for patients with cirrhosis who are frail[7]. This may involve loosening restrictions on salt intake in cases of diet unpalatability, encouragement of enteral feeding in encephalopathy, prevention of prolonged fasting hours, and providing late evening snacks[7].
Prehabilitation, also known as preoperative habilitation, involves a range of interventions that are implemented before a medical procedure or treatment to mitigate or prevent any adverse effects that may arise[167]. In the context of liver transplantation, prehabilitation aims to identify high-risk patients as early as possible and enhance their physical capacity before surgery[168]. This is achieved through various interventions, including exercise, nutrition, and psychological stress management[169]. According to a recent systematic review of eight studies, prehabilitation could potentially boost the aerobic capacity of patients who are awaiting orthotopic liver transplantation and is deemed a safe and feasible approach[170]. The review also revealed notable improvements in several metrics, such as VO2 peak, 6-min walking distance, hand grip strength, LFI, and quality of life[170]. Despite the evidence supporting the role of prehabilitation before liver transplantation, there is limited research on the benefits of prehabilitation for frail cirrhotic patients after liver transplantation. While a single study has demonstrated that an exercise training program can lead to post-transplant short-term benefits, such as reduced 90-d readmission rates and shorter hospital stays[171], further research is needed to confirm the potential long-term benefits of prehabilitation after liver transplantation.
Although pharmacological interventions are not commonly used in frailty treatment, there is evidence to suggest that testosterone supplementation may be a safe and effective option for enhancing muscle mass and strength in males with cirrhosis and low testosterone levels. A 54-wk randomized controlled trial conducted by Sinclair et al[172] concluded that testosterone supplementation can safely enhance muscle mass and strength in this population. However, there is limited information regarding the effect of testosterone therapy in treating frailty after liver transplantation. A single retrospective study suggested that short testosterone therapy may be useful in treating frailty after liver transplantation[173]. In this study, administering a single dose of testosterone replacement therapy along with regular exercise has been associated with patient and graft survival rates of 93.8% and 87.5% at one and five years, respectively[173]. Large randomized controlled trials are necessary to validate the safety and potential benefits of administering testosterone as a treatment for frailty following liver transplantation.
Frailty assessment tools are now available online, making it easier to assess frailty in the geriatric population. These web-based tools are user-friendly and can be accessed through internet-connected devices[174]. In addition, smartphone applications have shown great potential in evaluating and quantifying physical activity and patient mobility, which are crucial indicators of frailty. For instance, a recent app was used to prehabilitate liver transplant candidates, and it was found that the app’s training level matched that of a physical therapist in 89% of cases[175]. The app also motivated patients through videos and gamification features, leading to a 35% increase in physical activity performance among participants[175]. However, these tools require further advancements to fully comprehend the current patient frailty status, especially in cirrhotic patients who require special clinical evaluation. Validation studies are also needed to ensure that these smart tools can be integrated into clinical decision-making for patients.
A novel tool called the tele-liver frailty index (TeLefI) has been proposed for frailty assessment and follow-up in cirrhotic patients[176]. It utilizes telemedicine to virtually measure frailty in liver transplant candidates with cirrhosis. Wang et al[176] introduced this tool by comparing frailty assessment using in-person LFI and then by TelefI assessment tool[176]. The telemedicine-based TeLefI tool has been statistically validated in predicting LFI > 4.4, indicating that it can effectively identify patients who require more frequent follow-up or in-person assessment[176]. This tool has the potential to revolutionize frailty assessment and follow-up in cirrhotic patients, especially those who live in remote or underserved areas. This has the potential to not only decrease healthcare expenses but also enhance patient outcomes. While this tool has shown promising results, more research is necessary to fully understand the benefits and limitations of this novel approach to frailty assessment.
Targeting pathways that contribute to the progression of frailty shows promise as a potential treatment approach[17]. In fact, there are several medications that could potentially be used to treat frailty in cirrhosis. Here are some examples (Table 3).
| Suggested medication | Target of action | Side effects | Dose used in clinical trials | Clinical trial results |
| Metformin | Insulin resistance; Proinflammatory cytokines | Gastrointestinal upset; Non responders[209] | - | - |
| Rifaximin | Gut dysbiosis | - | 1200 mg daily for 24 wk | No significant changes in skeletal muscle index[186] |
| Myostatin antagonists | Hyperammonemia; Muscle mass inhibition | Spontaneous bone fractures[210]; Vascular (nasal and gum bleeding, telangiectasia)[211] | - | - |
| L-Carnitine | Hyperammonemia; Proinflammatory cytokines (antioxidant) | Gastrointestinal upset | (1000 mg/d) + exercise for 6 mo[201] | No significant changes in muscle mass, leg, and handgrip strength[201] |
| L-ornithine L-aspartate | Hyperammonemia | Gastrointestinal upset | 6 g three times daily for 2 wk[207] | No significant increase in prealbumin level after use[207] |
| Testosterone therapy | Testosterone deficiency | Cardiovascular diseases; Prostate cancer; Erythrocytosis[212] | Intramuscular injection of testosterone undecanoate 1000 mg at 0, 6, 18, 30, 42 wk[172] | The intervention group had increased muscle and bone mass with lower fat mass[172] |
Metformin: In general, the research on the effectiveness of metformin in treating frailty is inconclusive. Although some studies indicate that metformin may not be useful in decreasing the occurrence of frailty[177,178], other studies suggest that it could be advantageous in managing age-related illnesses and ailments including frailty[179-181]. The reason for this protective effect may be attributed to the reduction of insulin resistance, which is involved in the pathophysiology of frailty[182]. Additionally, the correlation discovered between metformin usage and lower levels of proinflammatory cytokines, regardless of blood glucose levels, may also contribute to the mechanism[183]. However, further investigation is necessary to gain a complete understanding of metformin’s potential in treating frailty in cirrhosis.
Rifaximin: Rifaximin is an antibiotic that boasts a highly favorable safety profile. Its primary function is to specifically target and eradicate harmful bacteria in the intestinal tract[184]. Due to its low absorption rate, rifaximin is considered an excellent choice for patients who require a safe and effective treatment option[185]. One of the key benefits of rifaximin is its ability to modify the composition of the gut microbiota, resulting in a notable decrease in harmful bacterial taxa. This, in turn, helps to prevent hyperammonemia, bacterial endotoxemia, and translocation, which have been identified as contributing factors to muscle loss in cirrhosis patients[62]. In a recent uncontrolled study, the long-term use of rifaximin in cirrhosis patients had a positive impact on their nutritional status[186]. Furthermore, the study demonstrated that rifaximin enabled these patients to maintain a consistent amount of muscle mass[186]. These findings shed light on the potential of rifaximin as an intervention for addressing malnutrition and potentially mitigating muscle loss in individuals with cirrhosis. However, further research is needed, specifically randomized controlled trials, to establish the efficacy of rifaximin in treating frailty in liver cirrhosis. Additionally, probiotics and fecal microbial transplantation are being explored as potential therapies for frailty induced by gut dysbiosis in liver cirrhosis[62]. However, their effectiveness in human subjects is still under investigation, and there is a lack of comprehensive studies in this area.
Myostatin antagonists: Myostatin is a type of signaling molecule that falls under the transforming growth factor-β superfamily[187]. It has a crucial function in skeletal muscle metabolism, and it works as an inhibitor of muscle mass, leading to a decrease in muscle size[188]. Higher levels of myostatin are linked to poorer survival rates in cirrhosis patients, and increased serum myostatin levels are associated with decreased muscle mass[29]. Studies have demonstrated that blocking myostatin can result in muscle hypertrophy and the reversal of muscle atrophy in both young and old mice[189-191], making it a potential solution to prevent muscle wasting in liver cirrhosis patients[192]. There exist various therapeutic interventions that can counteract the impact of myostatin, such as monoclonal antibodies, myostatin propeptide, and follistatin[190,193-196]. Additional studies are needed to ascertain the efficiency and safety of myostatin antagonists in this specific context.
Carnitine: L-carnitine, an endogenous compound with antioxidant properties, has been shown to promote muscle growth by increasing muscle blood flow[197]. Carnitine also has an essential role in fatty acid metabolism, and its deficiency leads to increased hepatic steatosis, hyperammonemia, cardiac and skeletal muscle disease[198]. Despite its potential benefits, there is limited information available on the effectiveness of L-carnitine in treating frail cirrhotic patients. Two retrospective studies have suggested that taking L-carnitine supplements may help prevent the loss of skeletal muscle mass in individuals with liver cirrhosis[199,200]. On the contrary, a clinical trial involving the administration of a daily dose of 1000 mg of L-carnitine for over six months to liver cirrhosis patients did not result in significant changes in muscle mass, handgrip, and leg strength[201]. Further research is necessary to evaluate the potential benefits of L-carnitine in the treatment of frailty in cirrhosis.
L-ornithine L-aspartate: L-ornithine L-aspartate (LOLA) has been proven to be an effective treatment for hepatic encephalopathy as it helps to reduce ammonia levels[202]. It achieves this by stimulating the production of urea in the liver and promoting muscle glutamine synthesis[203,204]. Administering LOLA to mice with non-alcoholic steatohepatitis has been shown to enhance muscle protein function[205]. By decreasing ammonia levels and improving muscle function in mice, LOLA has been suggested as a potential treatment for sarcopenia and frailty in individuals with cirrhosis[206]. However, a randomized controlled trial administering LOLA of 6 g three times daily for 2 wk to a group of 17 liver cirrhotic patients did not produce a significant increase in prealbumin levels[207]. Therefore, future research is necessary to validate the beneficial role of LOLA in treating frailty in patients with decompensated cirrhosis.
Hormonal therapy: Sinclair et al[172] conducted a randomized controlled trial that lasted for 12 mo, which showed notable enhancements in muscle mass, bone mineral mass, and a decrease in total fat mass among cirrhotic patients who received testosterone therapy. Nevertheless, further research is required to ascertain the safety and effectiveness of this therapy, particularly due to potential adverse effects such as an increased risk of hepatocellular carcinoma, cardiovascular diseases, and elevated hematocrit levels[208]. Additionally, the efficacy and safety of testosterone therapy after liver transplantation require further investigation.
There is an ongoing debate surrounding the use of TIPS as a potential therapy for sarcopenia and frailty in liver cirrhosis patients. While several studies have shown promising results in terms of improving muscle mass and nutritional status, as well as reducing the risk of hepatic encephalopathy and mortality in sarcopenic patients[213-215], other studies have raised concerns about the use of TIPS as a therapy for sarcopenia and frailty, particularly in severe cases. These studies have demonstrated that these conditions are associated with a higher risk of post-TIPS complications, such as non-home discharge, prolonged hospital stay, hepatic encephalopathy, and even mortality[143,216,217].
Moreover, while all studies conducted have focused on sarcopenic assessment after TIPS, only one novel study has included the effect of TIPS on frailty parameters[218]. In this study, 12 cirrhosis patients showed improvement in skeletal muscle mass six months after TIPS compared to their baseline measurements before the procedure[218]. However, there was no improvement in LFI, handgrip strength, or physical performance measurements[218]. This study adds to the concerns about using TIPS as a potential therapy for cirrhotic frail patients. To address these concerns and obtain more conclusive evidence, we strongly recommend that future studies on this topic prioritize larger sample sizes and control groups.
Frailty is a complex medical condition that arises from multiple predisposing factors in patients with cirrhosis. It has a significant impact on the morbidity and mortality of cirrhotic patients and can even predict the outcomes of therapeutic interventions. Therefore, it is crucial to properly assess, reassess, and intervene in cirrhotic patients to prevent, treat, or even reverse this hazardous process. Looking ahead, future research should explore the possibility of utilizing telemedicine and smart apps for frailty assessment to make clinical decisions for patient treatment and follow-up. This could potentially improve patient outcomes and reduce healthcare costs. Furthermore, future research must investigate the safety and long-term potential benefits of therapeutic strategies, including TIPS, prehabilitation, and medications for managing frailty in cirrhotic patients, especially after liver transplantation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jiang W, China; Li Z, China S-Editor: Gao CC L-Editor: A P-Editor: Yuan YY
| 1. | Doody P, Lord JM, Greig CA, Whittaker AC. Frailty: Pathophysiology, Theoretical and Operational Definition(s), Impact, Prevalence, Management and Prevention, in an Increasingly Economically Developed and Ageing World. Gerontology. 2023;69:927-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 306] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 3. | Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1488] [Cited by in RCA: 1316] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 4. | Karakousis ND, Chrysavgis L, Chatzigeorgiou A, Papatheodoridis G, Cholongitas E. Frailty in metabolic syndrome, focusing on nonalcoholic fatty liver disease. Ann Gastroenterol. 2022;35:234-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Siramolpiwat S, Kiattikunrat K, Soontararatpong R, Pornthisarn B, Vilaichone RK, Chonprasertsuk S, Bhanthumkomol P, Nunanun P, Issariyakulkarn N. Frailty as tested by the Liver Frailty Index is associated with decompensation and unplanned hospitalization in patients with compensated cirrhosis. Scand J Gastroenterol. 2021;56:1210-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Jeejeebhoy KN. Malnutrition, fatigue, frailty, vulnerability, sarcopenia and cachexia: overlap of clinical features. Curr Opin Clin Nutr Metab Care. 2012;15:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 397] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 8. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13384] [Cited by in RCA: 15967] [Article Influence: 665.3] [Reference Citation Analysis (1)] |
| 9. | Lochs H, Allison SP, Meier R, Pirlich M, Kondrup J, Schneider S, van den Berghe G, Pichard C. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, definitions and general topics. Clin Nutr. 2006;25:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr. 2008;27:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1652] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 11. | Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Yoh K, Nishikawa H, Enomoto H, Iwata Y, Ikeda N, Aizawa N, Nishimura T, Iijima H, Nishiguchi S. Grip Strength: A Useful Marker for Composite Hepatic Events in Patients with Chronic Liver Diseases. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Rahman R, Wilson BP, Paul TV, Yadav B, Kango Gopal G, Viggeswarpu S. Prevalence and factors contributing to primary sarcopenia in relatively healthy older Indians attending the outpatient department in a tertiary care hospital: A cross-sectional study. Aging Med (Milton). 2021;4:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E, Baracos VE, Crawford JA, Doehner W, Heymsfield SB, Jatoi A, Kalantar-Zadeh K, Lainscak M, Landi F, Laviano A, Mancuso M, Muscaritoli M, Prado CM, Strasser F, von Haehling S, Coats AJS, Anker SD. Sarcopenia: A Time for Action. An SCWD Position Paper. J Cachexia Sarcopenia Muscle. 2019;10:956-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 490] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 15. | Welch N, Attaway A, Bellar A, Alkhafaji H, Vural A, Dasarathy S. Compound Sarcopenia in Hospitalized Patients with Cirrhosis Worsens Outcomes with Increasing Age. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38:1706-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Warner Ii ER, Satapathy SK. Sarcopenia in the Cirrhotic Patient: Current Knowledge and Future Directions. J Clin Exp Hepatol. 2023;13:162-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 18. | Tandon P, Montano-Loza AJ, Lai JC, Dasarathy S, Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75 Suppl 1:S147-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 235] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 19. | Redman JS, Kaspar M, Puri P. Implications of pre-transplant sarcopenia and frailty in patients with non-alcoholic steatohepatitis and alcoholic liver disease. Transl Gastroenterol Hepatol. 2022;7:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Yang YJ, Kim DJ. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Chen HW, Dunn MA. Muscle at Risk: The Multiple Impacts of Ammonia on Sarcopenia and Frailty in Cirrhosis. Clin Transl Gastroenterol. 2016;7:e170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Kumar R, Prakash SS, Priyadarshi RN, Anand U. Sarcopenia in Chronic Liver Disease: A Metabolic Perspective. J Clin Transl Hepatol. 2022;10:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, McDonald C, Almasan A, Hazen SL, Naga Prasad SV, Dasarathy S. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983-E993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | White JP, Gao S, Puppa MJ, Sato S, Welle SL, Carson JA. Testosterone regulation of Akt/mTORC1/FoxO3a signaling in skeletal muscle. Mol Cell Endocrinol. 2013;365:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Deng N, Mallepally N, Peng FB, Kanji A, Marcelli M, Hernaez R. Serum testosterone levels and testosterone supplementation in cirrhosis: A systematic review. Liver Int. 2021;41:2358-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Johnson PJ. Sex hormones and the liver. Clin Sci (Lond). 1984;66:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Mendler L, Baka Z, Kovács-Simon A, Dux L. Androgens negatively regulate myostatin expression in an androgen-dependent skeletal muscle. Biochem Biophys Res Commun. 2007;361:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Ma K, Mallidis C, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Characterization of 5'-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab. 2001;281:E1128-E1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Dasarathy S. Myostatin and beyond in cirrhosis: all roads lead to sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8:864-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 30. | Goswami A, Bhargava N, Dadhich S, Kulamarva G. Insulin resistance in euglycemic cirrhosis. Ann Gastroenterol. 2014;27:237-243. [PubMed] |
| 31. | Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 32. | Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 761] [Article Influence: 69.2] [Reference Citation Analysis (1)] |
| 33. | Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Pathophysiology of Physical Inactivity-Dependent Insulin Resistance: A Theoretical Mechanistic Review Emphasizing Clinical Evidence. J Diabetes Res. 2021;2021:7796727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 869] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 35. | Al-Hussaniy HA, Alburghaif AH, Naji MA. Leptin hormone and its effectiveness in reproduction, metabolism, immunity, diabetes, hopes and ambitions. J Med Life. 2021;14:600-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Rachakonda V, Borhani AA, Dunn MA, Andrzejewski M, Martin K, Behari J. Serum Leptin Is a Biomarker of Malnutrition in Decompensated Cirrhosis. PLoS One. 2016;11:e0159142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Elaghori A, Salem PES, Azzam E, Abu Elfotoh N. GHRELIN LEVEL IN PATIENTS WITH LIVER CIRRHOSIS. Acta Endocrinol (Buchar). 2019;-5:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Diz-Lois MT, Garcia-Buela J, Suarez F, Sangiao-Alvarellos S, Vidal O, Cordido F. Altered fasting and postprandial plasma ghrelin levels in patients with liver failure are normalized after liver transplantation. Eur J Endocrinol. 2010;163:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765-783.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 575] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 40. | Dirchwolf M, Ruf AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J Hepatol. 2015;7:1974-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 41. | Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:77-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 43. | Sawant P, Vashishtha C, Nasa M. Management of cardiopulmonary complications of cirrhosis. Int J Hepatol. 2011;2011:280569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 45. | Acharya C, Bajaj JS. Altered Microbiome in Patients With Cirrhosis and Complications. Clin Gastroenterol Hepatol. 2019;17:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 46. | Lee FY, Lu RH, Tsai YT, Lin HC, Hou MC, Li CP, Liao TM, Lin LF, Wang SS, Lee SD. Plasma interleukin-6 Levels in patients with cirrhosis. Relationship to endotoxemia, tumor necrosis factor-alpha, and hyperdynamic circulation. Scand J Gastroenterol. 1996;31:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Altveş S, Yildiz HK, Vural HC. Interaction of the microbiota with the human body in health and diseases. Biosci Microbiota Food Health. 2020;39:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 237] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 48. | Su X, Gao Y, Yang R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 173] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 49. | Ohtani N, Kamiya T, Kawada N. Recent updates on the role of the gut-liver axis in the pathogenesis of NAFLD/NASH, HCC, and beyond. Hepatol Commun. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 50. | Woodhouse CA, Patel VC, Singanayagam A, Shawcross DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. 2018;47:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 51. | Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 348] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 52. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Gut-liver axis in cirrhosis: Are hemodynamic changes a missing link? World J Clin Cases. 2021;9:9320-9332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (3)] |
| 53. | Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen J, Yang B, Ou Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 54. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 55. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 838] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 56. | Casati M, Ferri E, Azzolino D, Cesari M, Arosio B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp Gerontol. 2019;124:110639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol. 2017;23:8355-8366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Jin M, Kalainy S, Baskota N, Chiang D, Deehan EC, McDougall C, Tandon P, Martínez I, Cervera C, Walter J, Abraldes JG. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019;39:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 59. | Shimizu Y. Gut microbiota in common elderly diseases affecting activities of daily living. World J Gastroenterol. 2018;24:4750-4758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Bloemen JG, Olde Damink SW, Venema K, Buurman WA, Jalan R, Dejong CH. Short chain fatty acids exchange: Is the cirrhotic, dysfunctional liver still able to clear them? Clin Nutr. 2010;29:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, Van Remmen H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14:957-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 62. | Maslennikov R, Alieva A, Poluektova E, Zharikov Y, Suslov A, Letyagina Y, Vasileva E, Levshina A, Kozlov E, Ivashkin V. Sarcopenia in cirrhosis: Prospects for therapy targeted to gut microbiota. World J Gastroenterol. 2023;29:4236-4251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 63. | Yao J, Chang L, Yuan L, Duan Z. Nutrition status and small intestinal bacterial overgrowth in patients with virus-related cirrhosis. Asia Pac J Clin Nutr. 2016;25:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 64. | Le Couteur DG, Solon-Biet SM, Cogger VC, Ribeiro R, de Cabo R, Raubenheimer D, Cooney GJ, Simpson SJ. Branched chain amino acids, aging and age-related health. Ageing Res Rev. 2020;64:101198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 65. | Kelly B, Pearce EL. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020;32:154-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 66. | Calvani R, Picca A, Rodriguez-Mañas L, Tosato M, Coelho-Júnior HJ, Biancolillo A, Laosa O, Gervasoni J, Primiano A, Santucci L, Giampaoli O, Bourdel-Marchasson I, Regueme SC, Sinclair AJ, Urbani A, Landi F, Gambassi G, Marini F, Marzetti E. Amino Acid Profiles in Older Adults with Frailty: Secondary Analysis from MetaboFrail and BIOSPHERE Studies. Metabolites. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 67. | Kinny-Köster B, Bartels M, Becker S, Scholz M, Thiery J, Ceglarek U, Kaiser T. Plasma Amino Acid Concentrations Predict Mortality in Patients with End-Stage Liver Disease. PLoS One. 2016;11:e0159205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Plauth M, Schütz T. Branched-chain amino acids in liver disease: new aspects of long known phenomena. Curr Opin Clin Nutr Metab Care. 2011;14:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Siramolpiwat S, Limthanetkul N, Pornthisarn B, Vilaichone RK, Chonprasertsuk S, Bhanthumkomol P, Nunanan P, Issariyakulkarn N. Branched-chain amino acids supplementation improves liver frailty index in frail compensated cirrhotic patients: a randomized controlled trial. BMC Gastroenterol. 2023;23:154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 70. | Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition. 2015;31:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 71. | Hernández-Conde M, Llop E, Gómez-Pimpollo L, Fernández Carrillo C, Rodríguez L, Van Den Brule E, Perelló C, López-Gómez M, Abad J, Martínez-Porras JL, Fernández-Puga N, Ferre C, Trapero M, Fraga E, Calleja JL. Adding Branched-Chain Amino Acids to an Enhanced Standard-of-Care Treatment Improves Muscle Mass of Cirrhotic Patients With Sarcopenia: A Placebo-Controlled Trial. Am J Gastroenterol. 2021;116:2241-2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 72. | Uojima H, Sakurai S, Hidaka H, Kinbara T, Sung JH, Ichita C, Tokoro S, Masuda S, Sasaki A, Koizumi K, Egashira H, Kako M, Kobayashi S. Effect of branched-chain amino acid supplements on muscle strength and muscle mass in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1402-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Traub J, Reiss L, Aliwa B, Stadlbauer V. Malnutrition in Patients with Liver Cirrhosis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (1)] |
| 74. | Verslype C, Cassiman D. Cirrhosis and malnutrition: assessment and management. Acta Gastroenterol Belg. 2010;73:510-513. [PubMed] |
| 75. | Liang H, Li X, Lin X, Ju Y, Leng J. The correlation between nutrition and frailty and the receiver operating characteristic curve of different nutritional indexes for frailty. BMC Geriatr. 2021;21:619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 76. | Moore CM, Van Thiel DH. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5:251-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Makhlouf NA, Mahran ZG, Sadek SH, Magdy DM, Makhlouf HA. Six-minute walk test before and after large-volume paracentesis in cirrhotic patients with refractory ascites: A pilot study. Arab J Gastroenterol. 2019;20:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Aqel BA, Scolapio JS, Dickson RC, Burton DD, Bouras EP. Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2005;3:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 80. | Pedersen JS, Bendtsen F, Møller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 81. | Gauthier A, Levy VG, Quinton A, Michel H, Rueff B, Descos L, Durbec JP, Fermanian J, Lancrenon S. Salt or no salt in the treatment of cirrhotic ascites: a randomised study. Gut. 1986;27:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Reynolds TB, Lieberman FL, Goodman AR. Advantages of treatment of ascites without sodium restriction and without complete removal of excess fluid. Gut. 1978;19:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Eisenmenger WJ, Ahrens EH. The effect of rigid sodium restriction in patients with cirrhosis of the liver and ascites. J Lab Clin Med. 1949;34:1029-1038. [PubMed] |
| 84. | Haberl J, Zollner G, Fickert P, Stadlbauer V. To salt or not to salt?-That is the question in cirrhosis. Liver Int. 2018;38:1148-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Morando F, Rosi S, Gola E, Nardi M, Piano S, Fasolato S, Stanco M, Cavallin M, Romano A, Sticca A, Caregaro L, Gatta A, Angeli P. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 86. | Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, Garcia-Tsao G, Lee SS. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30:937-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 87. | Gu XB, Yang XJ, Zhu HY, Xu BY. Effect of a diet with unrestricted sodium on ascites in patients with hepatic cirrhosis. Gut Liver. 2012;6:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Sorrentino P, Castaldo G, Tarantino L, Bracigliano A, Perrella A, Perrella O, Fiorentino F, Vecchione R, D' Angelo S. Preservation of nutritional-status in patients with refractory ascites due to hepatic cirrhosis who are undergoing repeated paracentesis. J Gastroenterol Hepatol. 2012;27:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Singh V, De A, Mehtani R, Angeli P, Maiwall R, Satapathy S, Singal AK, Saraya A, Sharma BC, Eapen CE, Rao PN, Shukla A, Shalimar, Choudhary NS, Alcantara-Payawal D, Arora V, Aithal G, Kulkarni A, Roy A, Shrestha A, Mamun Al Mahtab, Niriella MA, Siam TS, Zhang CQ, Huei LG, Yu ML, Roberts SK, Peng CY, Chen T, George J, Wong V, Yilmaz Y, Treeprasertsuk S, Kurniawan J, Kim SU, Younossi ZM, Sarin SK. Asia-Pacific association for study of liver guidelines on management of ascites in liver disease. Hepatol Int. 2023;17:792-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 90. | Aithal GP, Palaniyappan N, China L, Härmälä S, Macken L, Ryan JM, Wilkes EA, Moore K, Leithead JA, Hayes PC, O'Brien AJ, Verma S. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 91. | Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K, Kawaguchi T, Kurosaki M, Sakaida I, Shimizu M, Taniai M, Terai S, Nishikawa H, Hiasa Y, Hidaka H, Miwa H, Chayama K, Enomoto N, Shimosegawa T, Takehara T, Koike K. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J Gastroenterol. 2021;56:593-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 92. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 675] [Article Influence: 112.5] [Reference Citation Analysis (2)] |
| 93. | Pavić E, Martinis I, Orec I, Vrdoljak I. Use and importance of salt in hospital nutrition. Acta Med Croatica. 2010;64:133-142. [PubMed] |
| 94. | Blei AT, Córdoba J; Practice Parameters Committee of the American College of Gastroenterology. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 426] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 95. | Chadalavada R, Sappati Biyyani RS, Maxwell J, Mullen K. Nutrition in hepatic encephalopathy. Nutr Clin Pract. 2010;25:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Maharshi S, Sharma BC, Sachdeva S, Srivastava S, Sharma P. Efficacy of Nutritional Therapy for Patients With Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol. 2016;14:454-460.e3; quiz e33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 97. | Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 98. | Shah ND, Barritt AS 4th. Nutrition as Therapy in Liver Disease. Clin Ther. 2022;44:682-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 99. | Charlton CP, Buchanan E, Holden CE, Preece MA, Green A, Booth IW, Tarlow MJ. Intensive enteral feeding in advanced cirrhosis: reversal of malnutrition without precipitation of hepatic encephalopathy. Arch Dis Child. 1992;67:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res Health. 2003;27:220-231. [PubMed] |
| 101. | Roggin GM, Iber FL, Kater RM, Tabon F. Malabsorption in the chronic alcoholic. Johns Hopkins Med J. 1969;125:321-330. [PubMed] |
| 102. | Jhangiani SS, Agarwal N, Holmes R, Cayten CG, Pitchumoni CS. Energy expenditure in chronic alcoholics with and without liver disease. Am J Clin Nutr. 1986;44:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Singal AK, Charlton MR. Nutrition in alcoholic liver disease. Clin Liver Dis. 2012;16:805-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Ayares G, Idalsoaga F, Díaz LA, Arnold J, Arab JP. Current Medical Treatment for Alcohol-Associated Liver Disease. J Clin Exp Hepatol. 2022;12:1333-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 105. | Kamran U, Towey J, Khanna A, Chauhan A, Rajoriya N, Holt A. Nutrition in alcohol-related liver disease: Physiopathology and management. World J Gastroenterol. 2020;26:2916-2930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (5)] |
| 106. | Mitchell MC, Friedman LS, McClain CJ. Medical Management of Severe Alcoholic Hepatitis: Expert Review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 107. | Stickel F, Hoehn B, Schuppan D, Seitz HK. Review article: Nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. 2003;18:357-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, Esfandiari N, Ma M, Baracos VE. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7:126-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 109. | El Sherif O, Dhaliwal A, Newsome PN, Armstrong MJ. Sarcopenia in nonalcoholic fatty liver disease: new challenges for clinical practice. Expert Rev Gastroenterol Hepatol. 2020;14:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 110. | Schiavo L, Busetto L, Cesaretti M, Zelber-Sagi S, Deutsch L, Iannelli A. Nutritional issues in patients with obesity and cirrhosis. World J Gastroenterol. 2018;24:3330-3346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 111. | Wijarnpreecha K, Panjawatanan P, Aby E, Ahmed A, Kim D. Nonalcoholic fatty liver disease in the over-60s: Impact of sarcopenia and obesity. Maturitas. 2019;124:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 112. | Nishikawa H, Fukunishi S, Asai A, Nishiguchi S, Higuchi K. Sarcopenia and Frailty in Liver Cirrhosis. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Kruger C, McNeely ML, Bailey RJ, Yavari M, Abraldes JG, Carbonneau M, Newnham K, DenHeyer V, Ma M, Thompson R, Paterson I, Haykowsky MJ, Tandon P. Home Exercise Training Improves Exercise Capacity in Cirrhosis Patients: Role of Exercise Adherence. Sci Rep. 2018;8:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 114. | Román E, García-Galcerán C, Torrades T, Herrera S, Marín A, Doñate M, Alvarado-Tapias E, Malouf J, Nácher L, Serra-Grima R, Guarner C, Cordoba J, Soriano G. Effects of an Exercise Programme on Functional Capacity, Body Composition and Risk of Falls in Patients with Cirrhosis: A Randomized Clinical Trial. PLoS One. 2016;11:e0151652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 115. | Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, Tandon P. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:1920-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 116. | Aggio DA, Sartini C, Papacosta O, Lennon LT, Ash S, Whincup PH, Wannamethee SG, Jefferis BJ. Cross-sectional associations of objectively measured physical activity and sedentary time with sarcopenia and sarcopenic obesity in older men. Prev Med. 2016;91:264-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 117. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4955] [Article Influence: 707.9] [Reference Citation Analysis (9)] |
| 118. | Nakaya Y, Harada N, Kakui S, Okada K, Takahashi A, Inoi J, Ito S. Severe catabolic state after prolonged fasting in cirrhotic patients: effect of oral branched-chain amino-acid-enriched nutrient mixture. J Gastroenterol. 2002;37:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 119. | Owen OE, Trapp VE, Reichard GA Jr, Mozzoli MA, Moctezuma J, Paul P, Skutches CL, Boden G. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72:1821-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 234] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Plauth M, Cabré E, Campillo B, Kondrup J, Marchesini G, Schütz T, Shenkin A, Wendon J; ESPEN. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 121. | ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA-138SA. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 367] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 122. | Hanai T, Shiraki M, Imai K, Suetsugu A, Takai K, Shimizu M. Late Evening Snack with Branched-Chain Amino Acids Supplementation Improves Survival in Patients with Cirrhosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 123. | Yao J, Han W, Ren X, Yuan L, Xu J, Duan Z. Improvement of energy substrate metabolism by late evening snack supplementation in patients with liver cirrhosis: a meta-analysis. Ther Clin Risk Manag. 2019;15:659-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 124. | Mandai S, Furukawa S, Kodaka M, Hata Y, Mori T, Nomura N, Ando F, Mori Y, Takahashi D, Yoshizaki Y, Kasagi Y, Arai Y, Sasaki E, Yoshida S, Furuichi Y, Fujii NL, Sohara E, Rai T, Uchida S. Loop diuretics affect skeletal myoblast differentiation and exercise-induced muscle hypertrophy. Sci Rep. 2017;7:46369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 125. | Nakano I, Tsuda M, Kinugawa S, Fukushima A, Kakutani N, Takada S, Yokota T. Loop diuretic use is associated with skeletal muscle wasting in patients with heart failure. J Cardiol. 2020;76:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 126. | Hanai T, Shiraki M, Miwa T, Watanabe S, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M. Effect of loop diuretics on skeletal muscle depletion in patients with liver cirrhosis. Hepatol Res. 2019;49:82-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 127. | Burton LA, McMurdo ME, Struthers AD. Mineralocorticoid antagonism: a novel way to treat sarcopenia and physical impairment in older people? Clin Endocrinol (Oxf). 2011;75:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Attaway A, Bellar A, Dieye F, Wajda D, Welch N, Dasarathy S. Clinical impact of compound sarcopenia in hospitalized older adult patients with heart failure. J Am Geriatr Soc. 2021;69:1815-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 129. | Lai JC, Rahimi RS, Verna EC, Kappus MR, Dunn MA, McAdams-DeMarco M, Haugen CE, Volk ML, Duarte-Rojo A, Ganger DR, O'Leary JG, Dodge JL, Ladner D, Segev DL. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology. 2019;156:1675-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 130. | Skladany L, Molcan P, Vnencakova J, Vrbova P, Kukla M, Laffers L, Koller T. Frailty in Nonalcoholic Fatty Liver Cirrhosis: A Comparison with Alcoholic Cirrhosis, Risk Patterns, and Impact on Prognosis. Can J Gastroenterol Hepatol. 2021;2021:5576531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 131. | Lai JC, Dodge JL, Kappus MR, Dunn MA, Volk ML, Duarte-Rojo A, Ganger DR, Rahimi RS, McCulloch CE, Haugen CE, McAdams-DeMarco M, Ladner DP, Segev DL, Verna EC; Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study. Changes in frailty are associated with waitlist mortality in patients with cirrhosis. J Hepatol. 2020;73:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 132. | Berry K, Duarte-Rojo A, Grab JD, Dunn MA, Boyarsky BJ, Verna EC, Kappus MR, Volk ML, McAdams-DeMarco M, Segev DL, Ganger DR, Ladner DP, Shui A, Tincopa MA, Rahimi RS, Lai JC; from the Multi-Center Functional Assessment in Liver Transplantation (FrAILT) Study. Cognitive Impairment and Physical Frailty in Patients With Cirrhosis. Hepatol Commun. 2022;6:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 133. | Deng LX, Bischoff KE, Kent DS, O'Riordan DL, Pantilat SZ, Lai JC. Frailty is strongly associated with self-reported symptom burden among patients with cirrhosis. Eur J Gastroenterol Hepatol. 2021;33:e395-e400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 134. | Román E, Parramón M, Flavià M, Gely C, Poca M, Gallego A, Santesmases R, Hernández E, Nieto JC, Urgell E, Alvarado-Tapias E, Vidal S, Ferrero-Gregori A, Vargas V, Guarner C, Soriano G. Frailty in outpatients with cirrhosis: A prospective observational study. Liver Int. 2021;41:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Cron DC, Friedman JF, Winder GS, Thelen AE, Derck JE, Fakhoury JW, Gerebics AD, Englesbe MJ, Sonnenday CJ. Depression and Frailty in Patients With End-Stage Liver Disease Referred for Transplant Evaluation. Am J Transplant. 2016;16:1805-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 136. | Lai JC, Dodge JL, McCulloch CE, Covinsky KE, Singer JP. Frailty and the Burden of Concurrent and Incident Disability in Patients With Cirrhosis: A Prospective Cohort Study. Hepatol Commun. 2020;4:126-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 137. | Schleicher EM, Kremer WM, Kalampoka V, Gairing SJ, Kaps L, Schattenberg JM, Galle PR, Wörns MA, Nagel M, Weinmann-Menke J, Labenz C. Frailty as Tested by the Clinical Frailty Scale Is a Risk Factor for Hepatorenal Syndrome in Patients With Liver Cirrhosis. Clin Transl Gastroenterol. 2022;13:e00512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 138. | Dunn MA, Josbeno DA, Tevar AD, Rachakonda V, Ganesh SR, Schmotzer AR, Kallenborn EA, Behari J, Landsittel DP, DiMartini AF, Delitto A. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am J Gastroenterol. 2016;111:1768-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 139. | Serper M, Tao SY, Kent DS, Garren P, Burdzy AE, Lai JC, Gougol A, Bloomer PM, Reddy KR, Dunn MA, Duarte-Rojo A. Inpatient Frailty Assessment Is Feasible and Predicts Nonhome Discharge and Mortality in Decompensated Cirrhosis. Liver Transpl. 2021;27:1711-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Ferreira AP, Machado MV. Impact of pretransplant frailty and sarcopenia on the post-transplant prognosis of patients with liver cirrhosis: a systematic review. Eur J Gastroenterol Hepatol. 2021;33:e883-e897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Wang S, Whitlock R, Xu C, Taneja S, Singh S, Abraldes JG, Burak KW, Bailey RJ, Lai JC, Tandon P. Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology. 2022;75:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 142. | Pradhan F, Narang N, Fallon M. Tale of the Frail: Understanding Frailty in Cirrhosis. South Med J. 2021;114:186-191. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 143. | Sohal A, Chaudhry H, Kohli I, Arora K, Patel J, Dhillon N, Singh I, Dukovic D, Roytman M. Frailty as a risk-stratification tool in patients undergoing transjugular intrahepatic portosystemic shunt (TIPS). J Frailty Sarcopenia Falls. 2023;8:83-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 144. | Chapman B, Goh SK, Parker F, Romero S, Sinclair M, Gow P, Ma R, Angus P, Jones R, Luke J, Muralidharan V, Testro A. Malnutrition and low muscle strength are independent predictors of clinical outcomes and healthcare costs after liver transplant. Clin Nutr ESPEN. 2022;48:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Montgomery E, Macdonald PS, Newton PJ, Chang S, Wilhelm K, Jha SR, Malouf M. Reversibility of Frailty after Lung Transplantation. J Transplant. 2020;2020:3239495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 146. | Williams FR, Milliken D, Lai JC, Armstrong MJ. Assessment of the Frail Patient With End-Stage Liver Disease: A Practical Overview of Sarcopenia, Physical Function, and Disability. Hepatol Commun. 2021;5:923-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 147. | Tapper EB, Konerman M, Murphy S, Sonnenday CJ. Hepatic encephalopathy impacts the predictive value of the Fried Frailty Index. Am J Transplant. 2018;18:2566-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 148. | Sinclair M, Poltavskiy E, Dodge JL, Lai JC. Frailty is independently associated with increased hospitalisation days in patients on the liver transplant waitlist. World J Gastroenterol. 2017;23:899-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 149. | Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 385] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 150. | Tandon P, Tangri N, Thomas L, Zenith L, Shaikh T, Carbonneau M, Ma M, Bailey RJ, Jayakumar S, Burak KW, Abraldes JG, Brisebois A, Ferguson T, Majumdar SR. A Rapid Bedside Screen to Predict Unplanned Hospitalization and Death in Outpatients With Cirrhosis: A Prospective Evaluation of the Clinical Frailty Scale. Am J Gastroenterol. 2016;111:1759-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 151. | Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4103] [Cited by in RCA: 5827] [Article Influence: 291.4] [Reference Citation Analysis (0)] |
| 152. | Lai JC, Covinsky KE, Dodge JL, Boscardin WJ, Segev DL, Roberts JP, Feng S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66:564-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 373] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 153. | Kaps L, Lukac L, Michel M, Kremer WM, Hilscher M, Gairing SJ, Galle PR, Schattenberg JM, Wörns MA, Nagel M, Labenz C. Liver Frailty Index for Prediction of Short-Term Rehospitalization in Patients with Liver Cirrhosis. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 154. | Lai JC, Covinsky KE, McCulloch CE, Feng S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Am J Gastroenterol. 2018;113:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 155. | Siyu L, Yuan Y, Ran A, Minyan L. Frailty as tested by the Liver Frailty Index in out-patient patients with cirrhosis in China: a cross-sectional study. Eur J Gastroenterol Hepatol. 2023;35:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 156. | Xu CQ, Yao F, Mohamad Y, Wong R, Kent D, Seetharaman S, Srisengfa Y, Lai JC. Evaluating the Associations Between the Liver Frailty Index and Karnofsky Performance Status With Waitlist Mortality. Transplant Direct. 2021;7:e651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 157. | Dunn MA, Josbeno DA, Schmotzer AR, Tevar AD, DiMartini AF, Landsittel DP, Delitto A. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl. 2016;22:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 158. | Farrugia MA, Le Garf S, Chierici A, Piche T, Gual P, Iannelli A, Anty R. Therapeutic Physical Exercise Programs in the Context of NASH Cirrhosis and Liver Transplantation: A Systematic Review. Metabolites. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 159. | Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (1)] |
| 160. | Morkane CM, Kearney O, Bruce DA, Melikian CN, Martin DS. An Outpatient Hospital-based Exercise Training Program for Patients With Cirrhotic Liver Disease Awaiting Transplantation: A Feasibility Trial. Transplantation. 2020;104:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 161. | Williams FR, Berzigotti A, Lord JM, Lai JC, Armstrong MJ. Review article: impact of exercise on physical frailty in patients with chronic liver disease. Aliment Pharmacol Ther. 2019;50:988-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 162. | West J, Gow PJ, Testro A, Chapman B, Sinclair M. Exercise physiology in cirrhosis and the potential benefits of exercise interventions: A review. J Gastroenterol Hepatol. 2021;36:2687-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 163. | Yildirim M. Falls in Patients With Liver Cirrhosis. Gastroenterol Nurs. 2017;40:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 164. | Lai JC, Sonnenday CJ, Tapper EB, Duarte-Rojo A, Dunn MA, Bernal W, Carey EJ, Dasarathy S, Kamath BM, Kappus MR, Montano-Loza AJ, Nagai S, Tandon P. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019;19:1896-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 165. | Ni Lochlainn M, Cox NJ, Wilson T, Hayhoe RPG, Ramsay SE, Granic A, Isanejad M, Roberts HC, Wilson D, Welch C, Hurst C, Atkins JL, Mendonça N, Horner K, Tuttiett ER, Morgan Y, Heslop P, Williams EA, Steves CJ, Greig C, Draper J, Corish CA, Welch A, Witham MD, Sayer AA, Robinson S. Nutrition and Frailty: Opportunities for Prevention and Treatment. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 166. | Meena BL, Taneja S, Tandon P, Sahni N, Soundararajan R, Gorsi U, De A, Verma N, Premkumar M, Duseja A, Dhiman RK, Singh V. Home-based intensive nutrition therapy improves frailty and sarcopenia in patients with decompensated cirrhosis: A randomized clinical trial. J Gastroenterol Hepatol. 2023;38:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 167. | Santa Mina D, van Rooijen SJ, Minnella EM, Alibhai SMH, Brahmbhatt P, Dalton SO, Gillis C, Grocott MPW, Howell D, Randall IM, Sabiston CM, Silver JK, Slooter G, West M, Jack S, Carli F. Multiphasic Prehabilitation Across the Cancer Continuum: A Narrative Review and Conceptual Framework. Front Oncol. 2020;10:598425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 168. | Volk ML, Sonnenday C. Patient-centered liver transplantation. Clin Liver Dis (Hoboken). 2016;8:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 169. | Gurlit S, Gogol M. Prehabilitation is better than cure. Curr Opin Anaesthesiol. 2019;32:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 170. | Jetten WD, Hogenbirk RNM, Van Meeteren NLU, Cuperus FJC, Klaase JM, De Jong R. Physical Effects, Safety and Feasibility of Prehabilitation in Patients Awaiting Orthotopic Liver Transplantation, a Systematic Review. Transpl Int. 2022;35:10330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 171. | Al-Judaibi B, Alqalami I, Sey M, Qumosani K, Howes N, Sinclair L, Chandok N, Eddin AH, Hernandez-Alejandro R, Marotta P, Teriaky A. Exercise Training for Liver Transplant Candidates. Transplant Proc. 2019;51:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 172. | Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol. 2016;65:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 173. | Jain A, Haussner D, Hranjec T, Butt F, Stine JG, Ankola A, Al Yousif H, Dicristina R, Krok KL, Arenas J. Review of Sarcopenia and Testosterone Deficiency With Chronic Liver Disease and Postoperative Liver Transplant Utility of Short-Term Testosterone Replacement Therapy. Exp Clin Transplant. 2022;20:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 174. | Chang R, Low H, McDonald A, Park G, Song X. Web-based software applications for frailty assessment in older adults: a scoping review of current status with insights into future development. BMC Geriatr. 2021;21:723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 175. | Duarte-Rojo A, Bloomer PM, Rogers RJ, Hassan MA, Dunn MA, Tevar AD, Vivis SL, Bataller R, Hughes CB, Ferrando AA, Jakicic JM, Kim WR. Introducing EL-FIT (Exercise and Liver FITness): A Smartphone App to Prehabilitate and Monitor Liver Transplant Candidates. Liver Transpl. 2021;27:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 176. | Wang M, Shui AM, Barry F, Verna E, Kent D, Yao F, Seetharaman S, Berry K, Grubbs RK, George G, Huang CY, Duarte-Rojo A, Lai JC. The tele-liver frailty index (TeLeFI): development of a novel frailty tool in patients with cirrhosis via telemedicine. Am J Transplant. 2023;23:966-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 177. | Hazuda HP, Pan Q, Florez H, Luchsinger JA, Crandall JP, Venditti EM, Golden SH, Kriska AM, Bray GA. Association of Intensive Lifestyle and Metformin Interventions With Frailty in the Diabetes Prevention Program Outcomes Study. J Gerontol A Biol Sci Med Sci. 2021;76:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 178. | Soukas AA, Hao H, Wu L. Metformin as Anti-Aging Therapy: Is It for Everyone? Trends Endocrinol Metab. 2019;30:745-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 179. | Liu P, Pan Y, Song Y, Zhou Y, Zhang W, Li X, Li J, Li Y, Ma L. Association of metformin exposure with low risks of frailty and adverse outcomes in patients with diabetes. Eur J Med Res. 2023;28:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 180. | Baskaran D, Aparicio-Ugarriza R, Ferri-Guerra J, Milyani R, Florez H, Ruiz JG. Is There an Association Between Metformin Exposure and Frailty? Gerontol Geriatr Med. 2020;6:2333721420924956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 181. | Piskovatska V, Stefanyshyn N, Storey KB, Vaiserman AM, Lushchak O. Metformin as a geroprotector: experimental and clinical evidence. Biogerontology. 2019;20:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 182. | Pérez-Tasigchana RF, León-Muñoz LM, Lopez-Garcia E, Gutierrez-Fisac JL, Laclaustra M, Rodríguez-Artalejo F, Guallar-Castillón P. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing. 2017;46:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 183. | Perez A, Jacks R, Arora V, Spanheimer R. Effects of pioglitazone and metformin fixed-dose combination therapy on cardiovascular risk markers of inflammation and lipid profile compared with pioglitazone and metformin monotherapy in patients with type 2 diabetes. J Clin Hypertens (Greenwich). 2010;12:973-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 184. | Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 185. | Shayto RH, Abou Mrad R, Sharara AI. Use of rifaximin in gastrointestinal and liver diseases. World J Gastroenterol. 2016;22:6638-6651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 186. | Ishikawa T, Endo S, Imai M, Azumi M, Nozawa Y, Sano T, Iwanaga A, Honma T, Yoshida T. Changes in the Body Composition and Nutritional Status after Long-term Rifaximin Therapy for Hyperammonemia in Japanese Patients with Hepatic Encephalopathy. Intern Med. 2020;59:2465-2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 187. | Lee SJ. Myostatin: A Skeletal Muscle Chalone. Annu Rev Physiol. 2023;85:269-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 188. | Abati E, Manini A, Comi GP, Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79:374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 189. | Jang J, Park S, Kim Y, Jung J, Lee J, Chang Y, Lee SP, Park BC, Wolfe RR, Choi CS, Kim IY. Myostatin Inhibition-Induced Increase in Muscle Mass and Strength Was Amplified by Resistance Exercise Training, and Dietary Essential Amino Acids Improved Muscle Quality in Mice. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 190. | Latres E, Pangilinan J, Miloscio L, Bauerlein R, Na E, Potocky TB, Huang Y, Eckersdorff M, Rafique A, Mastaitis J, Lin C, Murphy AJ, Yancopoulos GD, Gromada J, Stitt T. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skelet Muscle. 2015;5:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 191. | Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 377] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 192. | Kim Y. Emerging Treatment Options for Sarcopenia in Chronic Liver Disease. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 193. | Suh J, Lee YS. Myostatin Inhibitors: Panacea or Predicament for Musculoskeletal Disorders? J Bone Metab. 2020;27:151-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 194. | Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39:283-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 195. | Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 196. | Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 627] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 197. | Fielding R, Riede L, Lugo JP, Bellamine A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 198. | Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012;7:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 199. | Hiramatsu A, Aikata H, Uchikawa S, Ohya K, Kodama K, Nishida Y, Daijo K, Osawa M, Teraoka Y, Honda F, Inagaki Y, Morio K, Morio R, Fujino H, Nakahara T, Murakami E, Yamauchi M, Kawaoka T, Miki D, Tsuge M, Imamura M, Tanaka J, Chayama K. Levocarnitine Use Is Associated With Improvement in Sarcopenia in Patients With Liver Cirrhosis. Hepatol Commun. 2019;3:348-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 200. | Ohara M, Ogawa K, Suda G, Kimura M, Maehara O, Shimazaki T, Suzuki K, Nakamura A, Umemura M, Izumi T, Kawagishi N, Nakai M, Sho T, Natsuizaka M, Morikawa K, Ohnishi S, Sakamoto N. L-Carnitine Suppresses Loss of Skeletal Muscle Mass in Patients With Liver Cirrhosis. Hepatol Commun. 2018;2:906-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 201. | Hiraoka A, Kiguchi D, Ninomiya T, Hirooka M, Abe M, Matsuura B, Hiasa Y, Michitaka K. Can L-carnitine supplementation and exercise improve muscle complications in patients with liver cirrhosis who receive branched-chain amino acid supplementation? Eur J Gastroenterol Hepatol. 2019;31:878-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 202. | Butterworth RF, Kircheis G, Hilger N, McPhail MJW. Efficacy of l-Ornithine l-Aspartate for the Treatment of Hepatic Encephalopathy and Hyperammonemia in Cirrhosis: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Exp Hepatol. 2018;8:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 203. | Kumar A, Davuluri G, Silva RNE, Engelen MPKJ, Ten Have GAM, Prayson R, Deutz NEP, Dasarathy S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology. 2017;65:2045-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 204. | Rose C, Michalak A, Pannunzio P, Therrien G, Quack G, Kircheis G, Butterworth RF. L-ornithine-L-aspartate in experimental portal-systemic encephalopathy: therapeutic efficacy and mechanism of action. Metab Brain Dis. 1998;13:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 205. | Pichon C, Nachit M, Gillard J, Vande Velde G, Lanthier N, Leclercq IA. Impact of L-ornithine L-aspartate on non-alcoholic steatohepatitis-associated hyperammonemia and muscle alterations. Front Nutr. 2022;9:1051157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 206. | Butterworth RF. L-Ornithine L-Aspartate for the Treatment of Sarcopenia in Chronic Liver Disease: The Taming of a Vicious Cycle. Can J Gastroenterol Hepatol. 2019;2019:8182195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 207. | Ndraha S, Hasan I, Simadibrata M. The effect of L-ornithine L-aspartate and branch chain amino acids on encephalopathy and nutritional status in liver cirrhosis with malnutrition. Acta Med Indones. 2011;43:18-22. [PubMed] |
| 208. | Shin MJ, Jeon YK, Kim IJ. Testosterone and Sarcopenia. World J Mens Health. 2018;36:192-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 209. | Florez JC. The pharmacogenetics of metformin. Diabetologia. 2017;60:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 210. | Suh J, Kim NK, Lee SH, Eom JH, Lee Y, Park JC, Woo KM, Baek JH, Kim JE, Ryoo HM, Lee SJ, Lee YS. GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone. Proc Natl Acad Sci U S A. 2020;117:4910-4920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 211. | Campbell C, McMillan HJ, Mah JK, Tarnopolsky M, Selby K, McClure T, Wilson DM, Sherman ML, Escolar D, Attie KM. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 212. | Grech A, Breck J, Heidelbaugh J. Adverse effects of testosterone replacement therapy: an update on the evidence and controversy. Ther Adv Drug Saf. 2014;5:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 213. | Gazda J, Di Cola S, Lapenna L, Khan S, Merli M. The Impact of Transjugular Intrahepatic Portosystemic Shunt on Nutrition in Liver Cirrhosis Patients: A Systematic Review. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 214. | Wu CH, Ho MC, Kao JH, Ho CM, Su TH, Hsu SJ, Huang HY, Lin CY, Liang PC. Effects of transjugular intrahepatic portosystemic shunt on abdominal muscle mass in patients with decompensated cirrhosis. J Formos Med Assoc. 2023;122:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 215. | Liu J, Yang C, Yao J, Bai Y, Li T, Wang Y, Shi Q, Wu X, Ma J, Zhou C, Huang S, Xiong B. Improvement of sarcopenia is beneficial for prognosis in cirrhotic patients after TIPS placement. Dig Liver Dis. 2023;55:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 216. | Yin L, Chu SL, Lv WF, Zhou CZ, Liu KC, Zhu YJ, Zhang WY, Wang CX, Zhang YH, Lu D, Cheng DL. Contributory roles of sarcopenia and myosteatosis in development of overt hepatic encephalopathy and mortality after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2023;29:2875-2887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (2)] |
| 217. | Li T, Liu J, Zhao J, Bai Y, Huang S, Yang C, Wang Y, Zhou C, Wang C, Ju S, Chen Y, Yao W, Xiong B. Sarcopenia Defined by Psoas Muscle Thickness Predicts Mortality After Transjugular Intrahepatic Portosystemic Shunt. Dig Dis Sci. 2023;68:1641-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 218. | Hey P, Chapman B, Wong D, Gow P, Testro A, Terbah R, Sinclair M. Transjugular intrahepatic portosystemic shunt insertion improves muscle mass but not muscle function or frailty measures. Eur J Gastroenterol Hepatol. 2023;35:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |