Published online Dec 14, 2023. doi: 10.3748/wjg.v29.i46.6022
Peer-review started: October 11, 2023
First decision: November 6, 2023
Revised: November 6, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 14, 2023
Processing time: 62 Days and 10.6 Hours
Patients with inflammatory bowel diseases (IBDs) require repeated endoscopic evaluations over time by colonoscopy to weigh disease activity but also for different and additional indications (e.g., evaluation of postoperative recurrence, colorectal cancer surveillance). Colonoscopy, however, requires adequate bowel preparation to be of quality. The latter is achieved as long as the patient takes a certain amount of product to have a number of bowel movements suitable to clean the colon and allow optimal visualization of the mucosa during endoscopy. However, significant guidelines recommend preparations for patients with IBD not excelling in palatability. This recommendation originates from the fact that most of the studies conducted on bowel preparations in patients with IBD have been done with isosmolar preparations based on polyethylene glycol (PEG), for which, therefore, more safety data exist. As a result, the low-volume non-PEG preparations (e.g., magnesium citrate plus picosulphate, oral sulphate solutions) have been set aside for the whole range of warnings to be heeded because of their hyperosmolarity. New studies, however, are emerging, leaning in overall for a paradigm shift in this matter. Indeed, such non-PEG preparations seem to show a particularly encouraging and engaging safety profile when considering their broad potential for tolerability and patient preference. Indeed, such evidence is insufficient to indicate such preparations in all patients with IBD but may pave the way for those with remission or well-controlled disease. This article summa
Core Tip: Preparations based on polyethylene glycol (PEG) are most recommended for patients with inflammatory bowel disease (IBD) undergoing colonoscopy. However, these solutions are not always palatable because they often require the intake of large volumes of solution, making it difficult for the patient to complete the entire preparation. This leads to a reduction in the quality of the endoscopic examination. Low-volume non-PEG-based, although excluded in the major guidelines for patients with IBD, are emerging as potentially safe in this setting, especially in remission or mild disease conditions.
- Citation: Pellegrino R, Gravina AG. Emerging space for non-polyethene-glycol bowel preparations in inflammatory bowel disease-related colonoscopy: Veering toward better adherence and palatability. World J Gastroenterol 2023; 29(46): 6022-6027
- URL: https://www.wjgnet.com/1007-9327/full/v29/i46/6022.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i46.6022
Quality colonoscopy in the inflammatory bowel disease (IBD) patient has adequate bowel preparation as an essential prerequisite. Colonoscopy is, in fact, an imperative examination of IBD and encompasses a long list of indications, among which initial diagnosis, patient follow-up to weigh response to therapy, and colorectal cancer surveillance are recognized[1-4]. Bowel preparation requires the patient to take an oral formulation of varying constitution and volume to obtain a functional number of bowel movements to determine the cleanliness of rectal, colonic and ileal segments that can be explored by colonoscopy[5]. However, new retrograde modalities are emerging (i.e. colonic lavage)[6]. The pharmacodynamics of bowel preparations require patient compliance so that the patient ingests the entire formulation and follows any complementary indications (i.e. a low-fiber diet).
As a result, it tends to follow that patients’ preferred preparations are those with lower volume, good taste (palatable), suitable dose dilution over time (e.g., split dose), and good safety profile such that they do not experience symptoms while taking the product[7].
However, the widely recommended preparations for patients with IBD are low (< 3 L) or high volume (at least 3 L) polyethylene glycol (PEG)-based preparations[5]. This choice, while not axiomatically pursuing the goal of palatability, is necessary because most studies in IBD are available for this type of preparation and because safety studies have shown a significantly better safety profile for PEG solutions than for non-PEG-based solutions (e.g., magnesium citrate plus picosulphate, oral sulfate solutions)[8].
This paradigm, which has been going on for several years, has the potential to change the view of the new studies that are increasingly emerging on non-PEG-based and low-volume solutions.
Non-PEG preparations ordinarily have osmotic power, which partly affects their safety profile compared to isosmolar PEG solutions, especially for electrolyte disturbances[5,9] and mucosal damage[5]. PEG preparations are based on the principle that they, being nonabsorbable and isosmolar fluids, result in minimal electrolyte and fluid absorption or secretion by the gastrointestinal wall. Therefore, to achieve a purgative effect, they must generally be used in large volumes[10]. The opposite principle applies to non-PEG preparations in that their cleansing effect is based precisely on osmotic potency rather than on accumulating nonabsorbable fluids[10]. Despite this, in a general sense, low-volume non-PEG preparations have several potential advantages for the patient employing them for colonoscopy.
First and foremost, these preparations do not have the disadvantage of requiring large volumes of preparation associated with lower adherence and patient preference or gastrointestinal overload symptoms (e.g., nausea, vomiting, abdominal pain) that often prevent completion of the preparation[11]. Additionally, PEG solutions are often poorly palatable due to their salty taste[12]. The more excellent tolerability of non-PEG low-volume solutions compared with PEG-based preparations has also been demonstrated in a large meta-analysis of more than 20 randomized placebo-controlled trials in which, in terms of efficacy, these were also found to be non-inferior to PEG solutions[13].
However, several studies are already available for non-PEG low-volume preparations in IBD (Table 1). These studies have produced compelling results in both clinical trial settings and real-world ones on such bowel preparation efficacy/effectiveness rates and safety.
| Ref. | Bowel preparation | IBD sample size | IBD type, n | Sample age | IBD duration | Disease activity trend | Split regimen as Y/N or % | Clear diet as Y/N or % |
| Briot et al[14], 2019 | MPS, NaP, PicoS | 12 (MPS), 26 (NaP), 80 (PicoS) | CD: 8 (MPS), 14 (NaP), 57 (PicoS); UC: 4 (MPS), 11 (NaP), 21 (PicoS); Undetermined IBD: 1 (NaP), 2 (PicoS) | Median: 42.9 (MPS), 41 (NaP), 40.8 (PicoS) | 13.9 yr (MPS), 12.9 yr (NaP), 10.6 yr (PicoS) | Remission, mild | 58.3% (MPS), 84.6% (NaP), 55.6% (PicoS) | 100% (MPS), 100% (NaP), 95.1% (PicoS) |
| Mohsen et al[17], 2021 | PicoS + magnesium citrate + PEG | 61 | N/A | Mean: 39.7 | N/A | Unknown | Y | Y |
| Rueda García et al[18], 2023 | PicoS | 31 | CD: 18, UC: 12: Undetermined IBD: 1 | Mean: 51.7 | N/A | Mild | Y | Y |
| Kim et al[19], 2022 | OST | 55 | CD: 18, UC: 37 | Mean: 44.4 | 93.2 mo | Remission, mild | Y | Y |
| Lee et al[20], 2023 | OSS | 92 | UC: 92 | Mean: 47.9 | 7 yr | Remission, mild | Y | Y |
For example, the prospective CLEAN study in 2019 provided some results about sodium, magnesium and potassium sulphate (trisulphate solutions), sodium phosphate and sodium picosulphate preparations in 119 IBD patients[14]. In all the above regimens, patients had followed a split regimen and a low-fiber diet before colonoscopy in more than half of the cases. In only 18 cases, however, the indication was a flare-up of IBD, and, as a result, most of the patients had a Mayo endoscopic subscore[15] of less than 2 (i.e. endoscopically inactive IBD). Picosulphate preparation showed, in this study, a higher mean Boston bowel preparation scale[16] than 2 or 4 L PEG solutions (i.e. increased cleaning capacity). The safety profile, within the limit of low numbers, was acceptable in comparison with PEG preparations. Regarding tolerability, however, sodium picosulfate showed substantially more excellent palatability as well as less nausea, vomiting, and bloating.
Mohsen et al[17] also later weighed the efficacy and safety of an osmotically active solution of sodium picosulphate and magnesium citrate (with PEG added) in 56 patients with IBD. These determined less abdominal pain than the PEG-ascorbate comparison, albeit with a greater serum increase in magnesium and no significant differences in efficacy and safety between the groups. This study, however, did not operate a particular stratification by colonoscopy indication type and IBD activity. Another trial (i.e. EII-PREP) showed promising results regarding sodium picosulphate but, unfortunately, in only 31 IBD patients[18].
In a subsequent trial targeting patients with inactive IBD, Kim et al[19] reported a lower bubble score rate in the novel oral sulphate table preparations and higher palatability (including a willingness to reuse the preparation for subsequent colonoscopy) than the PEG-ascorbate comparison. The problem of bubbles is another technical aspect to consider when discussing mucosal visibility, so much so that the use of simethicone for this purpose is bleached out by European guidelines and also found beneficial for IBD[5,8].
The safety and efficacy profile was comparable. Lee et al[20] also reported a similar efficacy and safety profile (even considering changes in serum electrolytes) between novel oral sulphate solution and PEG-ascorbate in 92 patients with inactive ulcerative colitis.
Studies evaluating non-PEG preparations, specifically in the population with IBD vs the counterpart PEG preparations, have shown some homogeneity in reporting good palatability, tolerability, and safety results.
However, there are some drawbacks to consider. Such studies are generally conducted in low sample size settings. This phenomenon poses the problem of requiring more and more evidence from studies with larger samples and, therefore, greater statistical power and generalizability. The absence of recommendations for non-PEG preparations by major guidelines also limits the use of these preparations in clinical practice and thus counteracts the conduct of real-world studies weighing the real-life effectiveness of these preparations by adding new data[5].
In addition, in the available studies, the different disease activity IBD subgroups are not well represented, sometimes unreported and not always weighted by scores widely recognized as usable in IBD (e.g., partial and total Mayo scores, Crohn's disease index of severity, Harvey-Bradshaw index[15]).
Moreover, most available studies enrolled patients with mild or no endoscopic activity. This severely limits interpretation in cases of moderate and moderate-severe endoscopic activity. Indeed, the problem does not arise for severe acute activity since, in those cases, at most, rectoscopy is enough to perform endoscopic evaluation and exclude over-infections (e.g., cytomegalovirus).
One of the reasons for European guidelines to deny the indication of non-PEG solutions in IBD is that they have been described to be associated with a risk of mucosal damage about ten times higher than PEG solutions[5].
However, this recommendation, although seemingly supportable, encapsulates in the definition of IBD the broad spectrum of different degrees of endoscopic activity. Therefore, a conditional stratification of recommendations that considers a balance of the efficacy/safety profile for individual indications for colonoscopy should probably be advocated. In other words, one cannot treat an indication for assessment of disease activity (perhaps in a patient with moderate-severe clinical and biochemical activity) and an oncologic surveillance colonoscopy performed in a long-term remission setting in the same way. In the former case, safety weighs more than efficacy since the primary purpose of the endoscopist is to consider disease activity to select treatment, and the mucosal status is already objectively compromised variably. In the second case, where the disease is already well controlled, and the patient has a colon much closer to that of the healthy population, it is undoubtedly efficacy a parameter to observe (especially in patients without safety concerns) as a colonoscopy with a lower quality of bowel preparation may significantly reduce the adenoma detection rate[21]. This evaluation becomes even more relevant compared to high-volume centers performing high-quality chromoendoscopy for surveillance colonoscopy in IBD patients.
A large proportion of patients with IBD receive the diagnosis at a young age and are largely destined for repeated endoscopic evaluations over time. The most recent treat-to-target strategies[22] place endoscopic outcomes among the pillars for eventual IBD treatment discontinuation or switch. For such patients, therefore, under conditions where there are no particular safety red flags, palatable bowel preparations should be reserved, and research should move in this direction. The current evidence, even better if evaluated meta-analytically, could potentially argue for a modification of the recommendations in future guideline updates, probably by including those with IBD in stable remission, or at most in already known mild endoscopic activity, in the pool of patients who can take advantage of low-volume non-PEG preparations. These evaluations are tentative and need to be weighed by ad hoc meta-analyses that consider the efficacy-safety ratio of non-PEG low-volume solutions against the current standard of care to assess whether the formulation of recommendations (even with low evidence level) is possible.
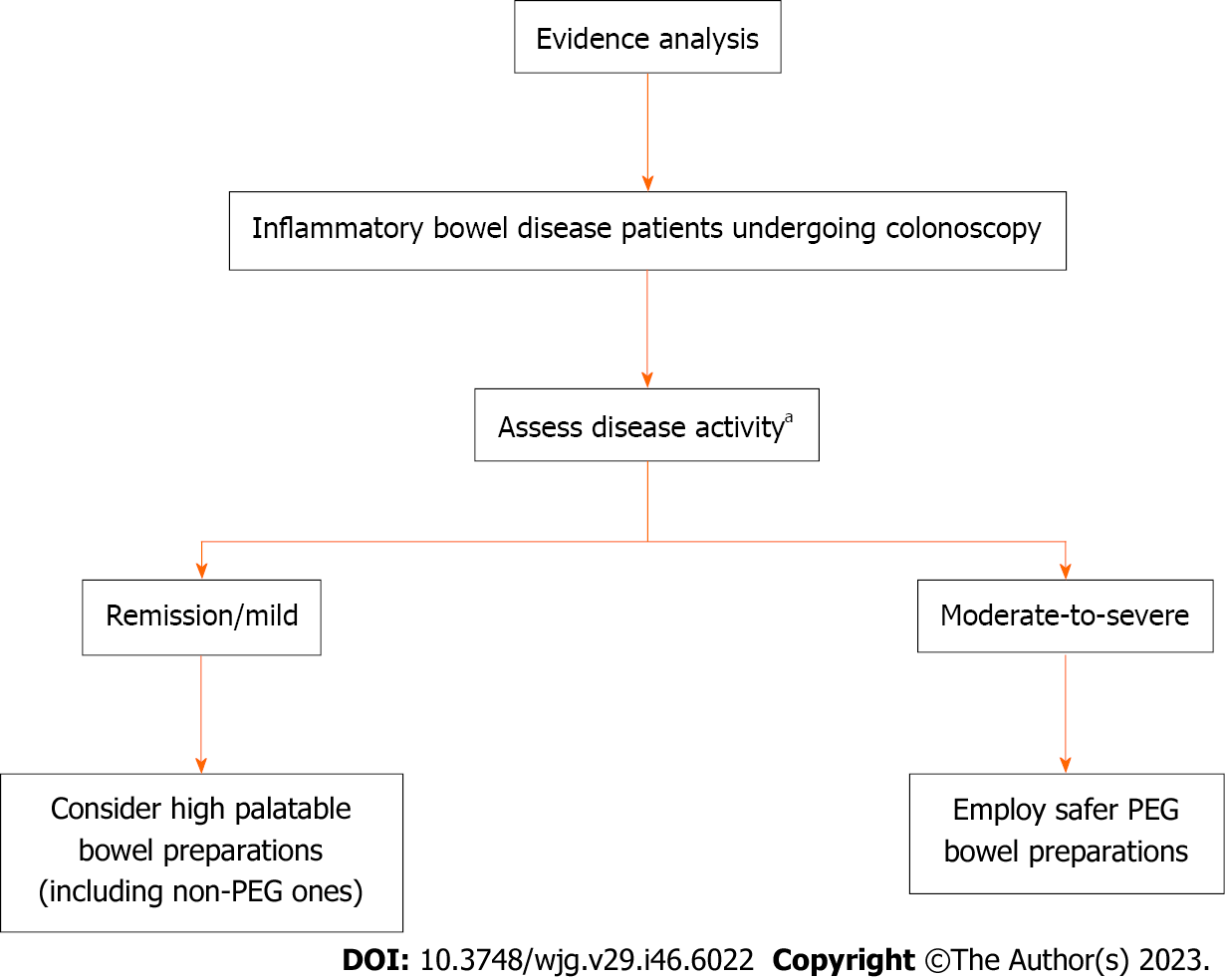
New evidence has emerged regarding using non-PEG-based bowel preparations for colonoscopy in patients with IBD. Although limited by initial data, these preparations appear to possess sustainable and potentially safe use in patients with IBD in remission or with mild activity with no particular safety concerns of adverse events or post-colonoscopy flare-ups. However, net of this, major guidelines, including the European ones, indicate and recommend only PEG solutions for patients with IBD, encompassing all patients with IBD in this indication without making stratifications based on disease activity. Future guidelines for bowel preparation should weigh whether the currently available studies allow such stratification (Figure 1) by opening the possibility for patients with well-controlled IBD to the use of more palatable preparations for better patient compliance, greater validity of colonoscopy with adequate bowel preparation, and ultimately for greater patient comfort.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: United European Gastroenterology
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Tu J, China; Wang YD, China; Hasan Ozen, Turkey S-Editor: Lin C L-Editor: Filipodia P-Editor: Lin C
| 1. | Kucharzik T, Ellul P, Greuter T, Rahier JF, Verstockt B, Abreu C, Albuquerque A, Allocca M, Esteve M, Farraye FA, Gordon H, Karmiris K, Kopylov U, Kirchgesner J, MacMahon E, Magro F, Maaser C, de Ridder L, Taxonera C, Toruner M, Tremblay L, Scharl M, Viget N, Zabana Y, Vavricka S. ECCO Guidelines on the Prevention, Diagnosis, and Management of Infections in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15:879-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 265] [Article Influence: 66.3] [Reference Citation Analysis (32)] |
| 2. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1162] [Article Influence: 193.7] [Reference Citation Analysis (0)] |
| 3. | Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 300] [Article Influence: 50.0] [Reference Citation Analysis (1)] |
| 4. | Gordon H, Biancone L, Fiorino G, Katsanos KH, Kopylov U, Al Sulais E, Axelrad JE, Balendran K, Burisch J, de Ridder L, Derikx L, Ellul P, Greuter T, Iacucci M, Di Jiang C, Kapizioni C, Karmiris K, Kirchgesner J, Laharie D, Lobatón T, Molnár T, Noor NM, Rao R, Saibeni S, Scharl M, Vavricka SR, Raine T. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2023;17:827-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 152] [Reference Citation Analysis (0)] |
| 5. | Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Fuccio L, Awadie H, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Vanella G, Mangas-Sanjuan C, Frazzoni L, Van Hooft JE, Dumonceau JM. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:775-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (4)] |
| 6. | Gajera A, South C, Cronley KM, Ziebert JJ, Wrigh CH, Levitan O, Burleson DB, Johnson DA. High-Volume Colonic Lavage Is a Safe and Preferred Colonoscopy Preparation for Patients With Inflammatory Bowel Disease. Crohns Colitis 360. 2022;4:otac024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Millien VO, Mansour NM. Bowel Preparation for Colonoscopy in 2020: A Look at the Past, Present, and Future. Curr Gastroenterol Rep. 2020;22:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (2)] |
| 8. | Gravina AG, Pellegrino R, Romeo M, Palladino G, Cipullo M, Iadanza G, Olivieri S, Zagaria G, De Gennaro N, Santonastaso A, Romano M, Federico A. Quality of bowel preparation in patients with inflammatory bowel disease undergoing colonoscopy: What factors to consider? World J Gastrointest Endosc. 2023;15:133-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 9. | Reumkens A, van der Zander Q, Winkens B, Bogie R, Bakker CM, Sanduleanu S, Masclee AAM. Electrolyte disturbances after bowel preparation for colonoscopy: Systematic review and meta-analysis. Dig Endosc. 2022;34:913-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Lichtenstein G. Bowel preparations for colonoscopy: a review. Am J Health Syst Pharm. 2009;66:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Rutherford CC, Calderwood AH. Update on Bowel Preparation for Colonoscopy. Curr Treat Options Gastroenterol. 2018;16:165-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Parra-Blanco A, Ruiz A, Alvarez-Lobos M, Amorós A, Gana JC, Ibáñez P, Ono A, Fujii T. Achieving the best bowel preparation for colonoscopy. World J Gastroenterol. 2014;20:17709-17726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (10)] |
| 13. | Jin Z, Lu Y, Zhou Y, Gong B. Systematic review and meta-analysis: sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur J Clin Pharmacol. 2016;72:523-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Briot C, Faure P, Parmentier AL, Nachury M, Trang C, Viennot S, Altwegg R, Bulois P, Thomassin L, Serrero M, Ah-Soune P, Gilletta C, Plastaras L, Simon M, Dray X, Caillo L, Del Tedesco E, Abitbol V, Zallot C, Degand T, Rossi V, Bonnaud G, Colin D, Morel B, Winkfield B, Danset JB, Filippi J, Amiot A, Attar A, Levy J, Peyrin-Biroulet L, Vuitton L; CLEAN Study Group. Efficacy, Tolerability, and Safety of Low-Volume Bowel Preparations for Patients with Inflammatory Bowel Diseases: The French Multicentre CLEAN Study. J Crohns Colitis. 2019;13:1121-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kishi M, Hirai F, Takatsu N, Hisabe T, Takada Y, Beppu T, Takeuchi K, Naganuma M, Ohtsuka K, Watanabe K, Matsumoto T, Esaki M, Koganei K, Sugita A, Hata K, Futami K, Ajioka Y, Tanabe H, Iwashita A, Shimizu H, Arai K, Suzuki Y, Hisamatsu T. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J Gastroenterol. 2022;57:246-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 930] [Cited by in RCA: 924] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 17. | Mohsen W, Williams AJ, Wark G, Sechi A, Koo JH, Xuan W, Bassan M, Ng W, Connor S. Prospective single-blinded single-center randomized controlled trial of Prep Kit-C and Moviprep: Does underlying inflammatory bowel disease impact tolerability and efficacy? World J Gastroenterol. 2021;27:1090-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Rueda García JL, Suárez Ferrer C, Martín-Arranz E, García-Ramírez L, Sánchez-Azofra M, Poza Cordón J, Noci J, Vergés T, Blanco San Miguel P, Martín-Arranz MD; IdiPAZ Study Group for Immune-Mediated Gastrointestinal Diseases. Randomized clinical trial evaluating three low-volume preparations for colonoscopy in outpatients with Inflammatory Bowel Disease: the EII-PREP trial. Scand J Gastroenterol. 2023;58:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 19. | Kim KO, Kim EY, Lee YJ, Lee HS, Kim ES, Chung YJ, Jang BI, Kim SK, Yang CH. Efficacy, safety and tolerability of oral sulphate tablet for bowel preparation in patients with inflammatory bowel disease: A multicentre randomized controlled study. J Crohns Colitis. 2022;16:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Lee JM, Lee KM, Kang HS, Koo JS, Lee HS, Jeong SH, Kim JH, Kim DB. Oral Sulfate Solution Is as Effective as Polyethylene Glycol with Ascorbic Acid in a Split Method for Bowel Preparation in Patients with Inactive Ulcerative Colitis: A Randomized, Multicenter, and Single-Blind Clinical Trial. Gut Liver. 2023;17:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Moein HR, Pervez E, Faidhalla S, Habbal H, Khan H, Wadehra A, Khalid M, Kakos D, Naylor P, Mohamad B. Role of Bowel Preparation in Adenoma Detection Rate and Follow-up Recommendations in African American Dominant Patient Population. Cureus. 2021;13:e16065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1637] [Article Influence: 409.3] [Reference Citation Analysis (1)] |