Published online Dec 7, 2023. doi: 10.3748/wjg.v29.i45.5988
Peer-review started: September 6, 2023
First decision: September 23, 2023
Revised: September 26, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: December 7, 2023
Processing time: 85 Days and 22.7 Hours
Traditional Chinese medicine has used the drug Pien Tze Huang (PTH), a classic prescription, to treat autoimmune hepatitis (AIH). However, the precise mode of action is still unknown.
To investigate the mechanism of PTH in an AIH mouse model by determining the changes in gut microbiota structure and memory regulatory T (mTreg) cells functional levels.
Following induction of the AIH mouse model induced by Concanavalin A (Con A), prophylactic administration of PTH was given for 10 d. The levels of mTreg cells were measured by flow cytometry, and intestinal microbiota was analyzed by 16S rRNA analysis, while western blotting was used to identify activation of the toll-like receptor (TLR)2, TLR4/nuclear factor-κB (NF-κB), and CXCL16/CXCR6 signaling pathways.
In the liver of mice with AIH, PTH relieved the pathological damage and reduced the numbers of T helper type 17 cells and interferon-γ, tumor necrosis factor-alpha, interleukin (IL)-1β, IL-2, IL-6, and IL-21 expression. Simultaneously, PTH stimulated the abundance of helpful bacteria, promoted activation of the TLR2 signal, which may enhance Treg/mTreg cells quantity to produce IL-10, and suppressed activation of the TLR4/NF-κB and CXCL16/CXCR6 signaling pathways.
PTH regulates intestinal microbiota balance and restores mTreg cells to alleviate experimental AIH, which is closely related to the TLR/CXCL16/CXCR6/NF-κB signaling pathway.
Core Tip: Intestinal microbiota disorder plays an important role in the pathogenesis of autoimmune hepatitis (AIH), in which one of the main characteristics is the hypofunction of memory regulatory T (mTreg) cells to maintain immune tolerance. The interaction between Treg cells and intestinal microbiota may provide a feasible therapeutic strategy for AIH. As a well-known traditional Chinese medicine, Pien Tze Huang (PTH) can effectively treat AIH in the clinic. However, its mechanism is still unclear. In the present study, PTH regulated intestinal microbiota balance and restored mTreg cells to alleviate experimental AIH, which was closely related to the toll-like receptor/CXCL16/CXCR6/nuclear factor-κB signaling pathway.
- Citation: Zeng X, Liu MH, Xiong Y, Zheng LX, Guo KE, Zhao HM, Yin YT, Liu DY, Zhou BG. Pien Tze Huang alleviates Concanavalin A-induced autoimmune hepatitis by regulating intestinal microbiota and memory regulatory T cells. World J Gastroenterol 2023; 29(45): 5988-6016
- URL: https://www.wjgnet.com/1007-9327/full/v29/i45/5988.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i45.5988
Autoimmune hepatitis (AIH) is an interfacial hepatitis characterized by lymphocyte infiltration and the production of autoantibodies; however, its etiology is unknown. Some studies have suggested that AIH is associated with abnormal intestinal permeability[1]. In the case of increased intestinal permeability or intestinal leakage, abnormal intestinal microbiota can enter the submucosa to activate immune cells and produce inflammatory factors or metabolites, which can invade the liver through the portal vein and cause liver injury. Among the many mechanisms that the immune system uses to recognize microbiota are the toll-like receptors (TLRs), which directly promote regulatory T (Treg) cell proliferation and interleukin (IL)-10 production, thereby preventing imiquimod’s ability to induce psoriasis-like symptoms on the skin[2]. Moreover, in the peripheral blood of patients with AIH, some studies have discovered a reduced frequency of Treg cells, essential agents which maintain immune homeostasis, indicating that a deficiency in Treg cell quantity and abnormal Treg cell function may play a vital role in the pathogenesis of AIH[3].
The treatment for AIH is usually high doses of a steroid, which can suppress the immune system. Recent studies have suggested using intestinal microbiota as a potential strategy to treat AIH[4,5]. In their study it was shown that compound probiotics may regulate intestinal microbiota and intestinal permeability, reduce the level of T helper type 1 (Th1) and Th17 cells, improve the level of Treg cells, suppress activation of the TLR4/nuclear factor-κB (NF-κB) pathway, and promote the emergence of AIH[6]. Therefore, for patients with AIH, the interaction between Treg cells and intestinal microbiota may provide a feasible therapeutic strategy. However, the activation degree of effector Treg cells is closely related to memory Treg (mTreg) cells[7], suggesting that mTreg cells could quickly transform into effector Treg cells, playing a crucial part in preserving immunological tolerance.
As a well-known traditional Chinese medicine, Pien Tze Huang (PTH) contains Moschus, Bovis calculus, Snake bile, Notoginseng radix et rhizoma, and other ingredients[8,9] and is regularly used to treat liver conditions, including AIH[10-14]. PTH controls the NF-κB signaling pathway and the NLRP3 inflammasome to reduce IL-1, IL-6, and IL-17 production in blood and to reduce joint inflammation in mice with collagen-induced arthritis[10]. However, the definite mechanism of PTH is not fully understood. PTH has a physiological basis for controlling Treg cells and intestinal microbiota. Bile acid, the active component of Bovis calculus and Snake bile, and notoginsenoside R1, is a potent saponin that is extracted from Panax notoginseng. The immune system’s signals to the microbiota may be mediated by bile acids[15]. Song et al[16] reported that dietary adjustment and flora transplantation could improve a significant population of colonic Foxp3+ Treg cells with highly-expressed RORγ, and optimize the constituents of the gut bile acids pool. Moreover, Gong et al[17] found that notoginsenoside R1 modulates NF-κB and MAPK signaling to exert anti-inflammatory effects to reduce carbon tetrachloride-induced liver fibrosis. Based on the above evidence, we hypothesized that PTH could treat intestinal microbiota imbalance and a lack of mTreg cells. Thus, this research aimed to determine the mechanism of PTH in the AIH mouse model by observing the changes in intestinal microbiota structure and mTreg functional levels.
Forty male specific pathogen-free C57BL/6J mice (aged 8-9 wk; 20-22 g) were bought from GemPharmatech Co., Ltd. (Nanjing, China) (Animal Certificate Number: SCXK (Su)2018-0008). The mice were housed at a temperature of 22 ± 1 ºC, relative humidity of 50% ± 10%, and a light/dark cycle of 12/12 h. All animal experiments (including euthanasia of mice) were performed in accordance with the Institutional Animal Care and Use Committee’s guidelines (Nanchang, China). The experimental protocols (Permit Number: JZLLSC20210090) were approved by The Jiangxi University of Chinese Medicine’s Animal Care and Use Committee.
Concanavalin A was bought from Sigma-Aldrich Trading Co., Ltd. (CA, United States, No. C2010-250MG). Dexa
In order to determine the optimal dose of PTH in treating AIH, an animal experiment was carried out on the treatment of AIH with high, middle, and low doses of PTH.
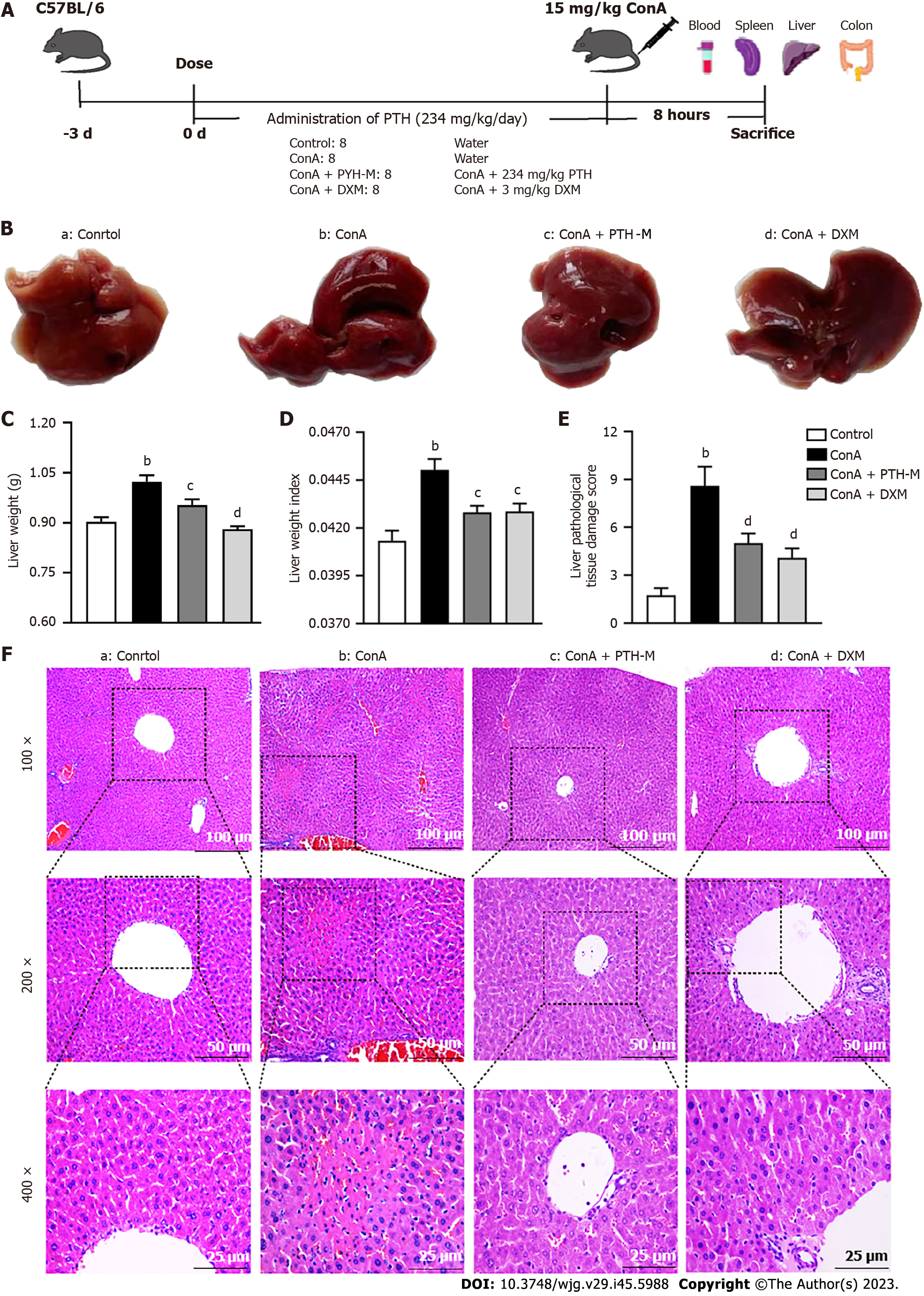
According to earlier research[20,21], the mice were randomly allocated into 6 groups (10 mice in each group): Control group (control), Concanavalin A (Con A) group (model), Con A + low-dose PTH group (PTH-L), Con A + middle-dose PTH group (PTH-M), Con A + high-dose PTH group (PTH-H), and Con A + DXM group (DXM). Mice in the Con A + PTH-L, Con A + PTH-M, and Con A + PTH-H groups received PTH (117 mg/kg, 234 mg/kg, and 468 mg/kg) every day for 10 d by gavage, respectively, while mice in the normal group and Con A group received the same amount of normal drinking water. The DXM group was intraperitoneally injected with DXM (3 mg/kg) every day. On the 10th d, the AIH mouse model was established, three hours after the last administration of PTH, the mice in the normal control group were injected with 0.2 mL of 0.9% sodium chloride solution through the tail vein, and mice in the other 5 groups were injected with 15 mg/kg Con A through the tail vein. At 8 h after Con A injection, fresh blood was collected from the mice after deep anesthesia with 2% sodium pentobarbital (20 mg/kg i.p.). The abdominal cavity was then quickly opened to collect mouse liver, spleen and colon tissues.
Mice in the Con A + PTH-M group received PTH (234 mg/kg) every day for 10 days by gavage, while mice in the control group and Con A group received the same amount of normal drinking water. The DXM group was intraperitoneally injected with DXM (3 mg/kg) every day. On the 10th day, the AIH mouse model was established as originally stated; three hours after the last administration of PTH, 0.2 mL of a 0.9% sodium chloride solution was injected into the tail vein of the mice in the normal control group, and the mice in the other three groups received an intravenous injection of 15 mg/kg Con A into the tail vein. At 8 h after Con A injection, fresh blood was collected from the mice after deep anesthesia with 2% sodium pentobarbital (20 mg/kg i.p.). The abdominal cavity was swiftly opened to collect mouse colon, spleen, and liver tissues.
Mouse peripheral blood was centrifuged at 1123 g for 15 min to extract the serum. An automated chemistry analyzer was used to measure the concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin (ALB), globulin (GLB), total protein (TP), indirect bilirubin (IBIL), triglyceride (TG), and high-density lipoprotein (HDL) IBIL in mice (AU480, Beckman Coulter, Pasadena, CA, United States).
After one week of fixing in 4% buffered paraformaldehyde, the sample was dehydrated in a 50% to 100% alcohol gradient, made completely transparent in dimethyl benzene, and embedded in paraffin. Following deparaffinization and rehydration, the samples were cut into sections 4 μm thick and stained with hematoxylin-eosin (Solarbio, Beijing, China). Thereafter, the slices were randomly and double-blindly observed, and scored by pathologists using a biomicroscope (Lecia, Wetzlar, Germany). Using the Ishak scoring system[22] shown in Supplementary Table 2, liver damage was graded with regard to portal and intralobular inflammation, degeneration, and necrosis. The degree of pathology was graded from 0 (no pathology) to 6 (severe pathology).
Immunofluorescence of intestinal mucosae was carried out to evaluate occludin expression and distribution. The tissues were quickly deparaffinized, rehydrated, and cleaned in 1% phosphate buffered saline. The samples were then blocked with 5% bovine serum albumin, and subsequently incubated with occludin (1:100, Abcam, MA, United States) at 4 °C overnight. After three washes, at room temperature, the samples were incubated with anti-FITC IgG secondary antibody for two hours. Finally, the slides were then counterstained with DAPI for 5 min. Images were captured using a fluorescent microscope (Leika, Wetzlar, Germany).
Mouse liver tissues were removed and cut into pieces in lysis buffer at the proportion of 1:10, homogenized in ice water using an electric homogenizer, incubated at 4 °C for 30 min, and the supernatants were then removed by centrifugation at 15493 g for 15 min. The expression levels of IL-4 (No. MM-0165M1), IL-17A (No. MM-0170M1), interferon (IFN)-γ (No. MM-0182M1), IL-1β (No. MM-0040M1), IL-2 (No. MM-0701M1), IL-6 (No. MM-0163M1), IL-10 (No. MM-0176M1, MM-0176M1), IL-21 (No. MM-0688M1) and tumor necrosis factor-alpha (TNF-α) (No. MM-0132M1) were detected by a commercial enzyme-linked immunosorbent assay (ELISA) kit (Jiangsu Meimian Industry Co., Ltd., Jiangsu, China). According to the instructions the absorbance was measured at 450 nm by a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, United States).
Mouse spleen was ground in a sterile culture dish and filtered through nylon mesh to prepare a single cell suspension. RPMI 1640 tissue medium was used to suspend the splenocytes, 10% heat-inactivated fetal bovine serum, 50 units per milliliter of penicillin, and 50 nanograms per milliliter of streptomycin were added, and cultivated for three hours at 37 °C with Cell Stimulation Cocktail after erythrocyte lysis (BD Biosciences, Franklin Lakes, NJ, United States). Extracellular antigens were stained for 20 min at 4 °C in RPMI 1640 medium following FcγR blockage with anti-CD16/32 antibodies (BD Biosciences, Franklin Lakes, NJ, United States). Foxp3/Transcription Factor Buffer Set (BD Biosciences, Franklin Lakes, NJ, United States) was used to fix and permeabilize the cells before staining for intracellular cytokines. CellQuest (BD Biosciences, CA, United States), and a BD FACSCalibur flow cytometer were used to collect the samples, and FlowJo software (BD Biosciences) was used to analyze the data. Cell populations were identified by staining with APC-H7 rat anti-mouse CD4 (1:200, BD Biosciences, NJ, United States), PE rat anti-mouse IL-17A (1:100, BD Biosciences, NJ, United States) FITC-CD25 (1:100, BD Biosciences, NJ, United States), BV421 rat anti-mouse Foxp3 (1:100, BD Biosciences, NJ, United States), APC rat anti-mouse programmed cell death ligand 1 (PD-L1)+(1:100, BD Biosciences, NJ, United States), Alexa Fluor 647 rat anti-mouse CCR7 (1:100, BD Biosciences, NJ, United States), and BV510 rat anti-mouse CD45RA (1:100, BD Biosciences, NJ, United States).
According to the instructions above, the liver tissue supernatant utilized for western blotting was produced (see the ELISA section). The traditional BCA protein determination method was used to measure the protein concentration (Cwbiotech, Beijing, China). Sodium-dodecyl sulfate gel electrophoresis was used to separate the same amount of protein by 10%-15% before being transferred to polyvinylidene fluoride membranes. After being sealed with 5% skimmed milk, the membranes were incubated with primary antibodies at 4 °C overnight. The antibodies were anti-GAPDH (1:10000, Proteintech, Wuhan, China), Tubulin (1:1000, Abclonal, Wuhan, China), TLR2 (1:1000, Abcam, MA, United States), TLR4 (1:1000, Abcam, MA, United States), CXCR6 (1:1000, Boster, Wuhan, China), NF-κB p65 (1:1000, Cell Signaling Technology, Boston, United States), CXCL16 (1:1000, Bioss, Beijing, China),and Occludin (1:1000, Abcam, MA, United States). The membranes were then rinsed with Tris buffered saline and Tween (TBST) and incubated at 37 °C for 1 h with the secondary antibody (1:10000, Proteintech, Wuhan, China). The tagged protein bands were scanned using the ChemiDoc XRS+Gel Imaging System (Hercules, CA, United States). after a second TBST wash.
According to the directions on the DNA extraction kit (Omega Bio-tek, Norcross, GA, United States), the DNA of the microbial community was collected. DNA concentration and purity were assessed using a NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, United States), and the quality of the DNA extracted was determined using 1% agarose gel electrophoresis. The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with primer pairs 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, United States). The 16S rRNA gene was amplified using PCR in the following manner: A first denaturation was performed at 95 °C for three minutes, followed by 27 cycles of denaturation at 95 °C for thirty seconds, annealing at 55 °C for thirty seconds, and extension at 72 °C for forty-five seconds, followed by a third extension at 72 °C for ten minutes, before finishing at 4 °C. Purified PCR products were quantified after removal from 2% agarose gels using a Quantus TM Fluorometer (Promega, United States) after purification using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States).
Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) used an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, United States) to sequence the purified amplicons in a paired-end manner using equimolar pooling. The NCBI Sequence Read Archive database received the raw reads (Accession Number: SRP392420). In addition, I-sanger was used to conduct co-occurrence network analysis, correlation analysis, and microbiological difference analysis (Majorbio Bio-Pharm Technology Co., Ltd.; www.i-sanger.com).
Data were expressed as the mean ± SEM. The Student t-test or one-way analysis of variance (ANOVA), followed by the Wilcoxon rank-sum test and Kruskal-Wallis’s test were used for multiple comparisons. P < 0.05 or P < 0.01 was considered significantly different. GraphPad Prism 8.0 and SPSS 21.0 were used for statistical analysis.
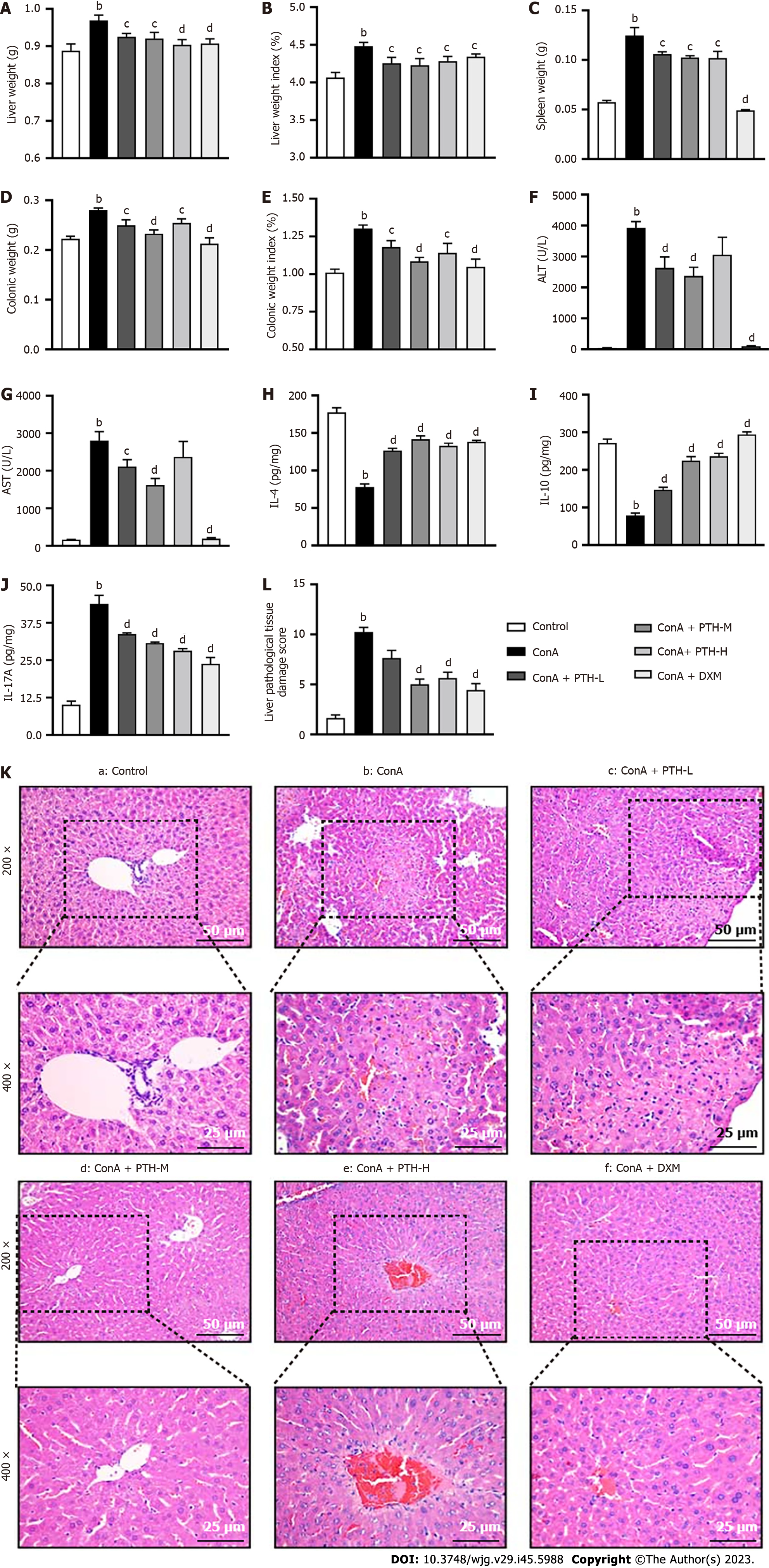
Con A-induced AIH is a confirmable model for studying human AIH, as it simulates many aspects of this disease, including liver damage, abnormal elevation of serum transaminase level and excessive generation of pro-inflammatory cytokines[23]. The liver weight (Figure 1A) and liver weight index (Figure 1B), spleen weight (Figure 1C), colonic weight (Figure 1D), and colonic weight index (Figure 1E), serum ALT (Figure 1F) and AST (Figure 1G), and the levels of IL-17A (Figure 1J) were raised in the Con A group compared with mice not treated with Con A (P < 0.01), while following PTH-L, PTH-M, PTH-H or DXM treatment, these results were the opposite (P < 0.05 or P < 0.01). The expression of IL-4 (Figure 1H) and IL-10 (Figure 1I) in Con A-induced AIH mice without treatment was lower than in control mice and AIH mice treated with PTH-L, PTH-M, PTH-H or DXM (P < 0.01). Compared with the normal control group, mice in the Con A group showed interfacial hepatitis, death of a large number of liver cells, structural disorder of liver lobules, increased infiltration of inflammatory cells, focal (or spotted) lytic necrosis or apoptosis (Figure 1K), and higher Ishak scores (Figure 1L) (P < 0.01). After 10 d of PTH treatment, compared with mice in the Con A group, mice in the PTH-L, PTH-M, PTH-H groups showed less interfacial hepatitis and mild focal (or spotted) lytic necrosis or apoptosis, more complete liver lobular structure, reduced inflammatory cell infiltration (Figure 1K), and lower Ishak scores (Figure 1L) (P < 0.01); thus, relieving AIH. However, pathological damage of the liver and the expression level of inflammatory factors were further reduced and liver function was further improved following the middle dose of PTH compared with the high dose of PTH and the low dose of PTH in AIH mice. The middle dose of PTH was the best dose to relieve AIH, and this dose was selected for the follow-up study.
Compared with the control group, the livers in the Con A group were dark red and swollen. Compared with the Con A group, the livers in the Con A + PTH-M and Con A + DXM groups were bright red with smooth surfaces (Figure 2B). Moreover, the liver weight (Figure 2C) and liver weight index (Figure 2D) were increased in the Con A group compared with mice not treated with Con A (P < 0.01), while PTH or DXM treatment decreased the increase in liver weight (Figure 2C) and liver weight index (Figure 2D) (P < 0.05). Compared with the normal control group, mice in the Con A group showed interfacial hepatitis, death of a large number of liver cells, structural disorder of liver lobules, increased infiltration of inflammatory cells, focal (or spotted) lytic necrosis or apoptosis (Figure 2F), and higher Ishak scores (Figure 2E) (P < 0.01). After 10 d of PTH treatment, compared with mice in the Con A group, mice in the Con A + PTH-M group and Con A + DXM groups showed less interfacial hepatitis and mild focal (or spotted) lytic necrosis or apoptosis, more complete liver lobular structure, reduced inflammatory cell infiltration (Figure 2F), and lower Ishak scores (Figure 2E) (P < 0.01). These findings demonstrate that PTH efficaciously relieves the pathological liver injury in Con A-induced AIH mice.
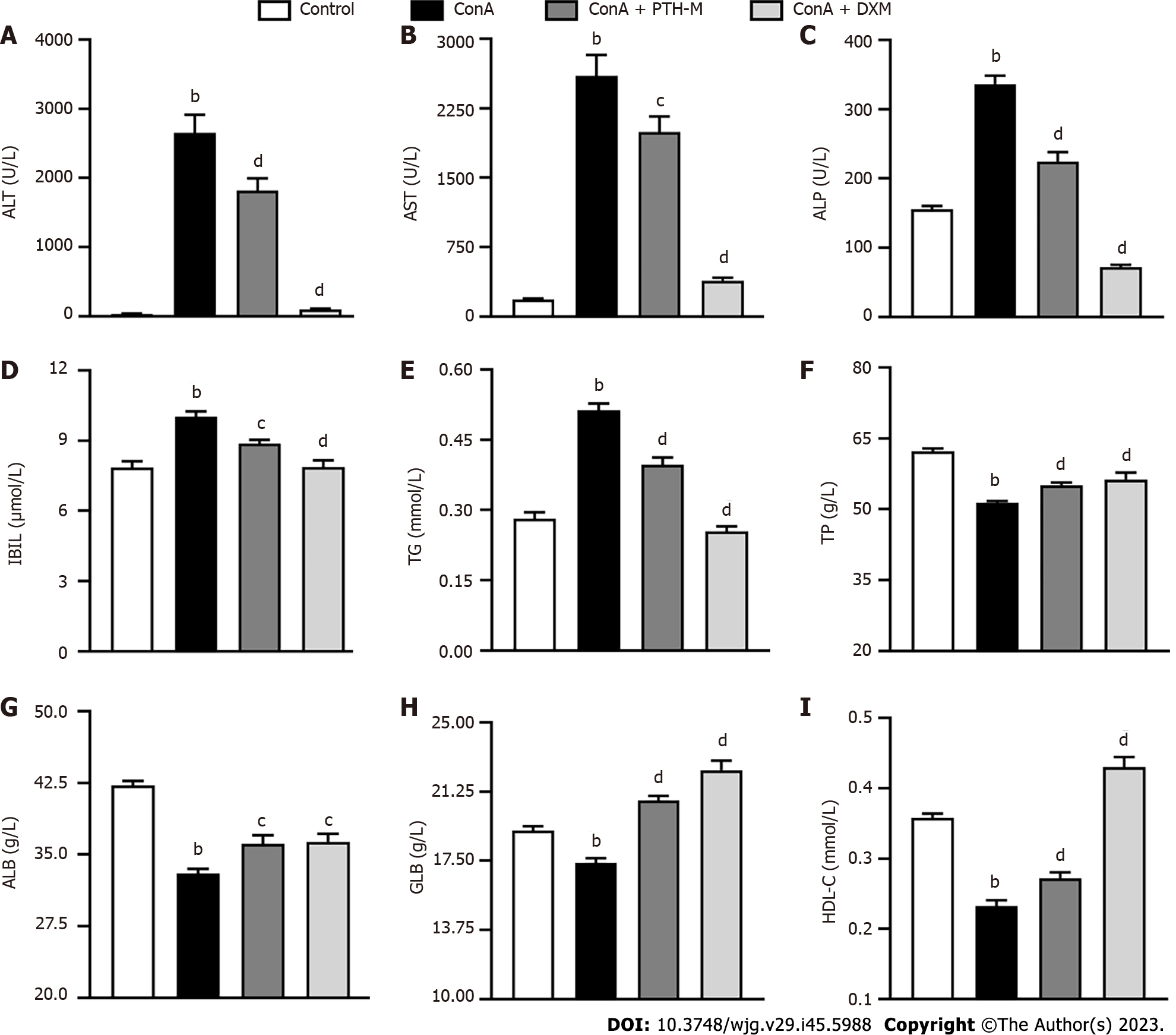
A biochemical liver test is an important method to judge whether there is liver damage, evaluate the severity of liver illness, and judge the treatment effect and prognosis[24-26]. The levels of serum enzymes, including ALT (Figure 3A), AST (Figure 3B), ALP (Figure 3C), IBIL (Figure 3D), and TG (Figure 3E) were raised in mice who received Con A compared with control mice (P < 0.01), and mice with Con A-induced AIH treated with PTH and DXM. In addition, decreased levels of TP (Figure 3F), ALB (Figure 3G), GLB (Figure 3H), and HDL (Figure 3I) were detected in serum from mice with Con A-induced AIH treatment (P < 0.01), and these levels were increased after treatment with PTH and DXM (P < 0.05 or P < 0.01). The above results show that PTH can improve liver function in mice with Con A-induced AIH.
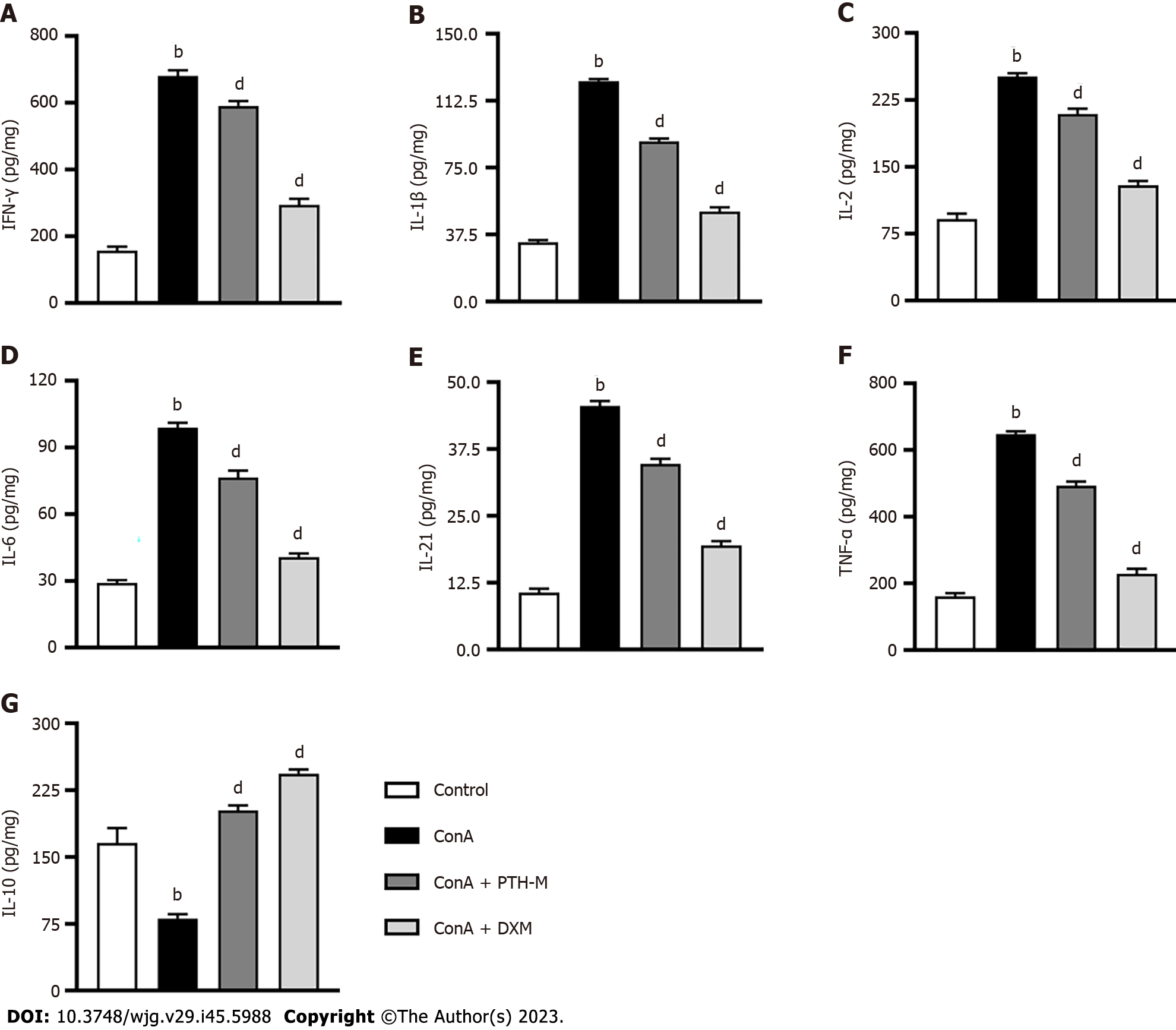
An essential aspect of immunological damage in AIH is abnormal expression of inflammatory cytokines, such as IFN-γ, IL-1β, IL-2, IL-6, IL-21, TNF-α, and IL-10. Th17, Treg, and mTreg cells thereafter undergo transformation and migration into the liver. The secretion of IFN-γ (Figure 4A), IL-1β (Figure 4B), IL-2 (Figure 4C), IL-6 (Figure 4D), IL-21 (Figure 4E), TNF-α (Figure 4F) in liver tissues from AIH mice in the Con A group was higher than that in the control, Con A + PTH-M and Con A + DXM groups (P < 0.01). In contrast, the expression of IL-10 (Figure 4G) in Con A-induced AIH mice without treatment was lower than in control mice and AIH mice treated with PTH or DXM (P < 0.01). According to these findings, PTH modulates the equilibrium of pro- and anti-inflammatory cytokines in AIH mice.
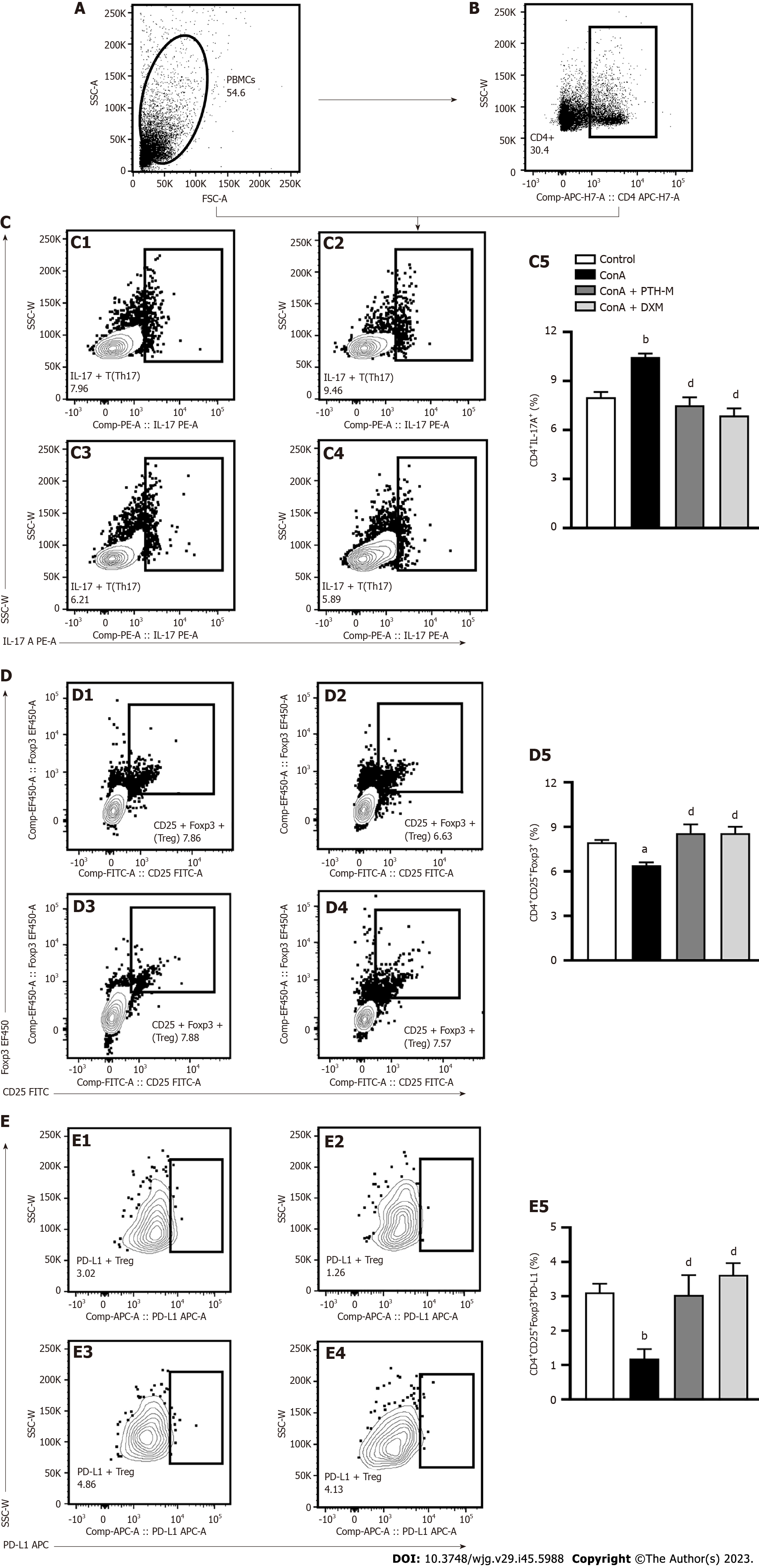
Production of the pro-inflammatory cytokine IL-17A is a characteristic of Th17 cells, the phenotype of Treg cells is CD4+CD25+Foxp3+, and they play a significant role in autoimmune disease such as AIH and so on[27,28]. Programmed death-1 (PD-1) is a member of the CD28/B7 superfamily of costimulatory molecules that plays an inhibitory effect in the periphery. PD-L1 (also known as B7-H1) and PD-L2 (also known as B7-DC) are ligands for PD-1[29]. In the present study, the mononuclear cells in peripheral blood and CD4+ lymphocytes were measured by flow cytometry (Figures 5A and B). The percentage of CD4+IL-17A+ (Th17) cells (Figure 5C) in the peripheral blood of AIH mice was markedly raised (P < 0.01), while the levels of CD4+IL-17A+ (Th17) cells (Figure 5C) were considerably lower in PTH-treated AIH mice than in untreated AIH mice (P < 0.01). However, the percentage of CD4+CD25+Foxp3+ Treg cells (Figure 5D) and CD4+CD25+Foxp3+PD-L1+ cells (Figure 5E) in the peripheral blood of mice with Con A-induced AIH were significantly decreased (P < 0.05 or P < 0.01), while PTH treatment significantly increased the percentages of CD4+CD25+Foxp3+ Treg cells (Figure 5D) and CD4+CD25+Foxp3+PD-L1+ cells (Figure 5E) in AIH mice (P < 0.01). The above results demonstrate that PTH controls the balance of Th17 cells and Treg cells in mice with Con A-induced AIH.
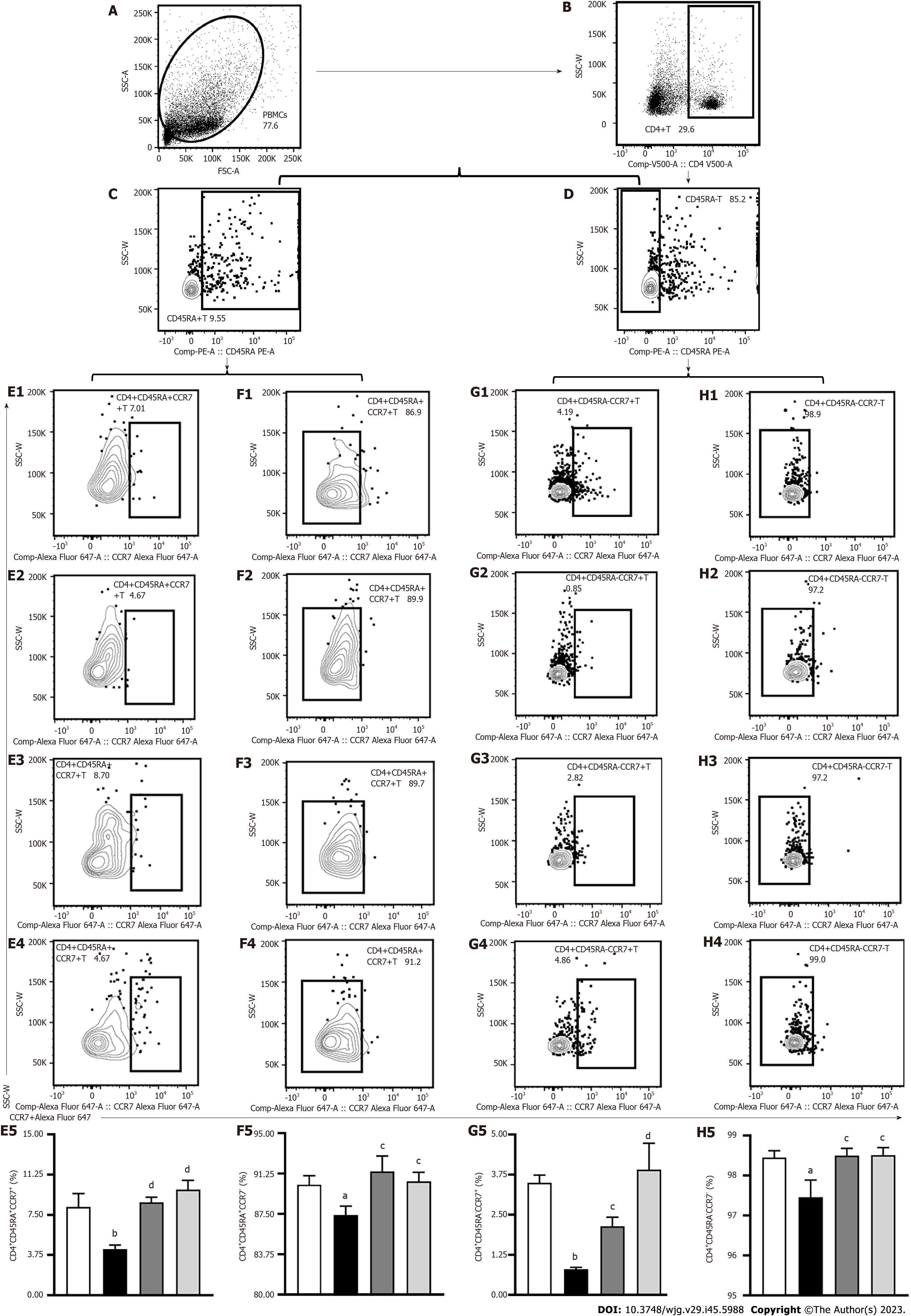
Central memory T (TCM) cells, whose phenotype is CD4+CD45RA-CCR7+, mainly exist in lymphoid organs. Following antigen stimulation, TCM cells can rapidly transform into effector T (TEM) cells. The TEM phenotype is CD4+CD45RA-CCR7- and these cells exist in the periphery for a long time to produce IFN-γ, IL-17, and other inflammatory factors. This study used lymph node homing receptors CD45RA-, CD45RA+, and chemokine CC receptor (CCR)7 (Figures 6A-D) to recognize and count memory T cells. The levels of CD4+CD45RA+CCR7+ (Figure 6E), CD4+CD45RA+CCR7- (Figure 6F), CD4+CD45RA-CCR7+ (TCM) (Figure 6G), and CD4+CD45RA-CCR7- (TEM) (Figure 6H) cells were markedly decreased in untreated AIH mice in contrast to control mice (P < 0.05 or P < 0.01). After PTH treatment, the levels of CD4+CD45RA+CCR7+ (Figure 6E), CD4+CD45RA+CCR7- (Figure 6F), CD4+CD45RA-CCR7+ (TCM) (Figure 6G) and CD4+CD45RA-CCR7- (TEM) (Figure 6H) cells were significantly increased (P < 0.05 or P < 0.01), which suggests that PTH controlled the quantity of TCM cells and TEM cells in AIH mice.
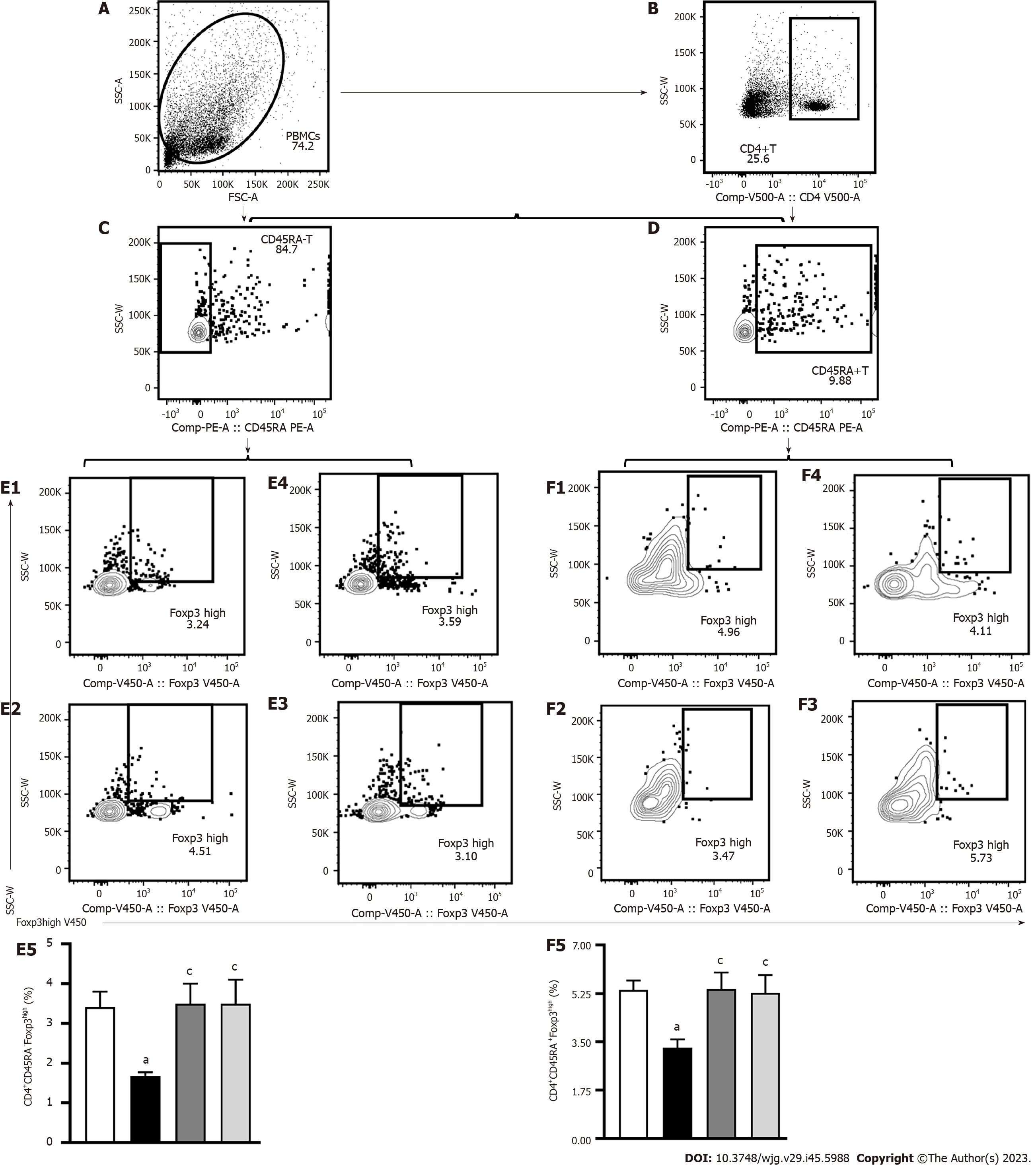
CD45RA+Foxp3low cells are static mTreg cells, which become CD45RA-Foxp3high cells after activation. This study used lymph node homing receptors CD45RA- and CD45RA+ (Figures 7A-D) to recognize and count memory Treg cells. In contrast to control mice, the number of CD4+CD45RA-Foxp3high mTreg (Figure 7E) and CD4+CD45RA+Foxp3high (Figure 7F) cells were significantly decreased in untreated AIH mice (P < 0.05), which implies that this is one of the primary characteristics in mice with Con A-induced AIH. After PTH treatment, the numbers of CD4+CD45RA-Foxp3high mTreg (Figure 7E), and CD4+CD45RA+Foxp3high (Figure 7F) significantly increased (P < 0.05). These results show that PTH improves mTreg cells level in AIH mice.
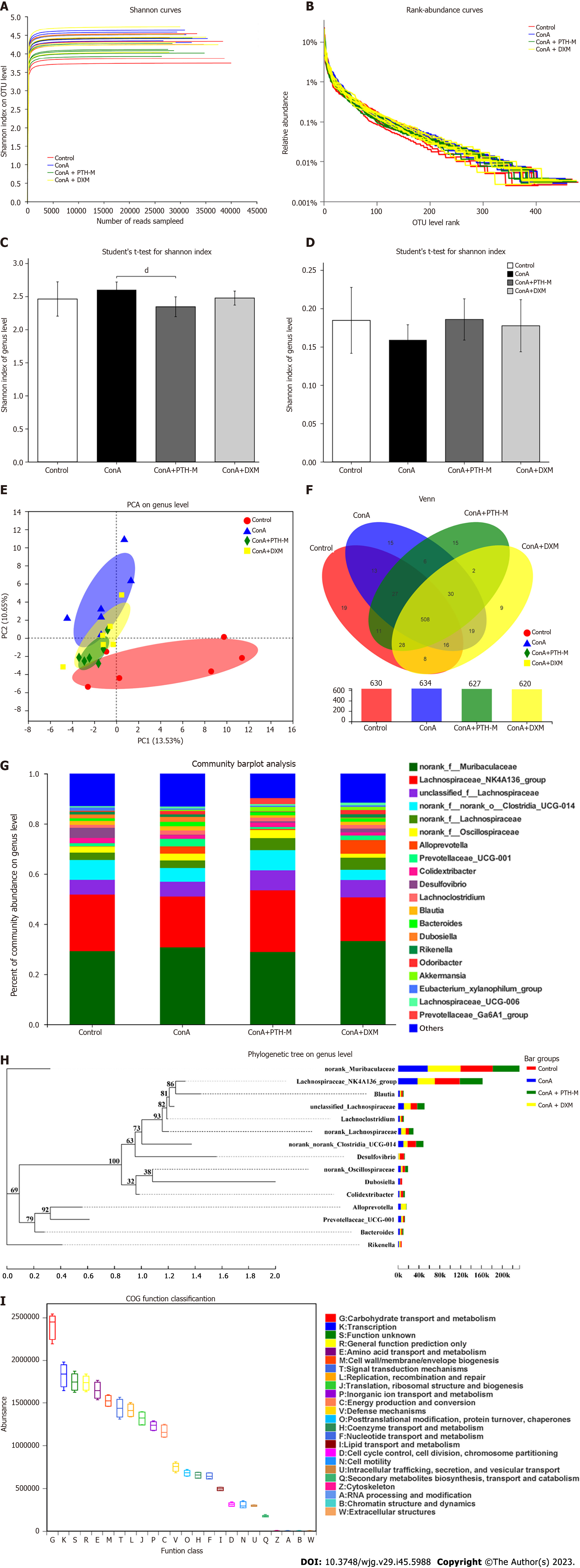
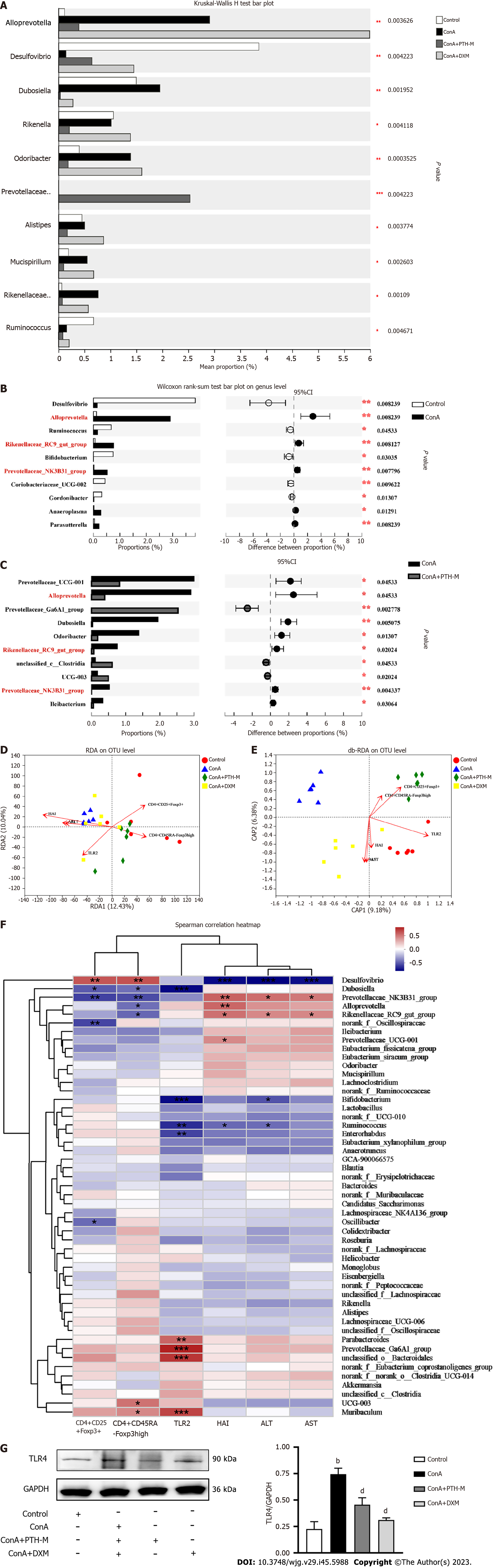
Increasing evidence has suggested that disordered intestinal microbiota is a significant characteristic in mice with Con A-induced AIH[30]. The operational taxonomic unit (OTU) level (Figure 8A) rarefaction curve of the Shannon index at the OTU level levelled off when the sequencing data reached saturation, covering the vast majority of species in the gut microbiome community of AIH mice. The abundance grade curve at the OTU level (Figure 8B) showed the species richness and evenness of species diversity in the sample. When the abscissa position of the extension end point of the sample curve is more backward, this implies that the species richness of the sample is higher, the curve is smoother, and the species distribution of the sample is uniform. The abundance and diversity of the microbial population were expressed by single sample diversity (Alpha diversity) analysis, including the microbial richness indices Shannon and Simpson (Figures 8C-D). Figure 8C shows a substantial variation in the Shannon index at the OTU level between the Con A group and Con A + PTH-M group. Although there was no substantial variation in the Shannon index among the control, Con A, and Con A + DXM groups, the Shannon index at the OTU level was higher in the Con A group than in the control mice and Con A + DXM groups. Interestingly, the result of the Simpson index (Figure 8D), which also represented species richness, showed the opposite trend. The Simpson index at the OTU level was lower in the Con A group than in the control, Con A + PTH-M, and Con A + DXM groups.
Subsequently, in order to compare the diversity of the intestinal microbiota among these four groups, the diversity analyses were used, including principal co-ordinates analysis (PCoA) (Figure 8E). The PCoA analyses proved that the distribution of species in the Con A and Con A + PTH-M groups was distinct from the control group, the Con A + PTH-M group and the control groups were separated from one another by a smaller distance than the Con A and control groups (Figure 8E). Venn diagram analysis at the OTU level revealed additional variations in the intestinal microbiota composition across the four groups (630, 634, 627, and 620 OTUs were seen in the control, Con A, Con A + PTH-M, and Con A + DXM groups, respectively) (Figure 8F).
The percent of community abundance at the genus level (Figure 8G) was used to analyze and compare the species abundance of each group of samples and observe their biological composition and structure. Lachnospiraceae-NK4A136, norank-f-norank-o-Clostridia-UCG-014, and Desulfovibrio were lower in the Con A group than in the control and Con A + PTH-M groups. Alloprevotella, Prevotellaceae-UCG-001, Dubosiella, and Odoribacter were superior in the Con A group than in the control and Con A + PTH-M groups.
Figure 8H shows the phylogenetic tree at the genus level. Each branch in the evolutionary tree represented a species. According to the higher taxonomic rank that the species belong to, the branches are colored differently. The bar graph on the right displays the percentage of reads of species in various groups, and the lengths of the branches represent the evolutionary distance between the two species, or the level of species difference. Picrust was used to predict the function of the 16S amplicon sequencing results (Figure 8I). The functions of enrichment mainly include the transport and metabolism of hydrates; transcription; amino acid transport and metabolism; signal transduction mechanism; copy, recombine, repair; translation, ribosome structure, and biogenesis; inorganic ion transport and metabolism; energy production and transformation.
To identify the species with different abundances in various groups (samples) of microbial communities, hypothesis tests were conducted, and the significance of the observed differences were assessed, and rigorous statistical methods of the Kruskal-Wallis H test (Figure 9A) and Wilcoxon rank-sum test (Figures 9B and C) were used. As shown in Figure 9A, Dubosiella and Rikenellaceae-RC9-gut-group were higher in the Con A group than in the control, Con A + PTH-M, and Con A + DXM groups, while Desulfovibrio were lower. As shown in Figures 9B and C, Alloprevotella, Rikenellaceae-RC9-gut-group and Prevotellaceae-NK3B31-group were superior in the Con A group than in the control and Con A + PTH-M groups. The above results show that PTH can improve Con A-induced intestinal microbial composition in AIH mice.
Based on the actual effects of PTH on Treg/mTreg and intestinal microbiota in AIH mice, the correlation between AST, ALT, HAI, TLR4, CD4+CD25+Foxp3+ Treg cells, CD4+CD45RA-Foxp3high mTreg cells, and intestinal microbiota was further investigated using a Spearman correlation heat map, redundancy analysis (RDA), and distance-based RDA (db-RDA). In addition, environmental factors such as AST, ALT, HAI, TLR4, CD4+CD25+Foxp3+ Treg, and CD4+CD45RA-Foxp3high mTreg cells were used to examine their relationship with the intestinal microbiota. On the basis of RDA analysis (Figure 9D), the abundance of AST, ALT, HAI, and TLR4 were accordant with the Con A group’s intestinal microbiota, while the abundance of CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells were accordant with the gut microbiota of mice in the control and Con A + PTH-M groups. On the basis of db-RDA analysis (Figure 9E), the abundance of AST, ALT, HAI and TLR4 were compatible with the gut microbiota of the Con A group, while the abundance of CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells were similar to the gut microbiota of mice in the Con A + PTH-M group. According to the Spearman correlation heat map at the genus level (Figure 9F), Desulfovibrio was negatively correlated with HAI, AST, ALT, TLR4 and positively connected with CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells; Prevotellaceae-NK3B31-group was positively connected with HAI, AST, ALT, TLR4, and negatively connected with CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells; Rikenellaceae-RC9-gut-group was positively connected with HAI, AST, ALT, and negatively connected with CD4+CD45RA-Foxp3high mTreg cells; Dubosiella was negatively connected with CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells.
According to Western blot analysis, AIH mice had higher levels of TLR4 protein expression (Figure 9G) than the control group (P < 0.01), and the Con A + PTH-M group had lower levels of TLR4 protein expression (Figure 9G) than AIH mice (P < 0.01) (Figure 9G). To sum up, PIH regulates gut microbiota-associated important environmental factors in AIH mice.
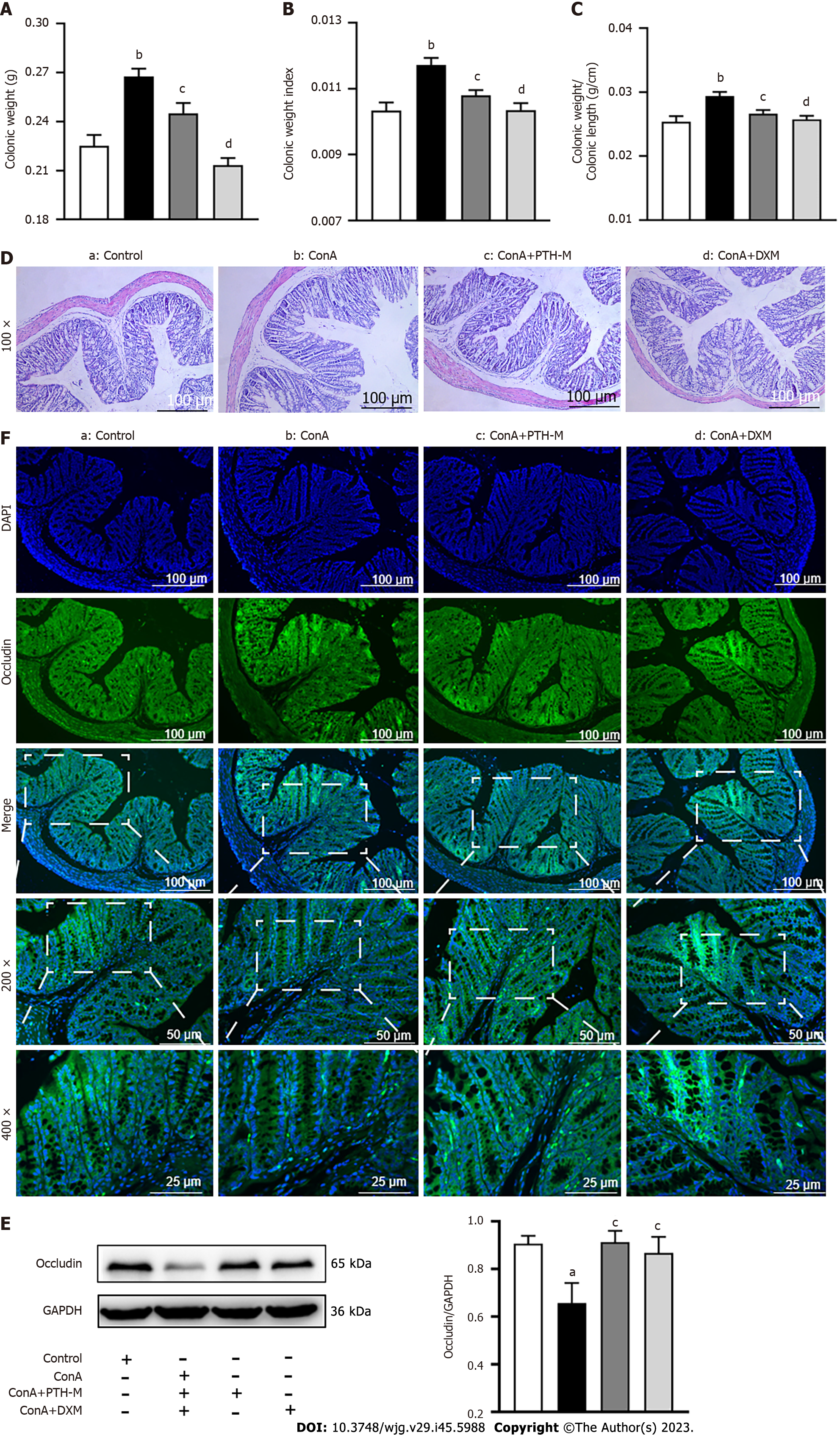
In order to further study whether intestinal microflora and/or other pathogens (or immunocomplexes) can enter the liver through the injured intestinal microbiota barrier and whether PTH can treat AIH by protecting the intestinal microbiota barrier and preventing bacterial translocation, the intestinal microbiota permeability test was performed. In the present study, the colonic weight (Figure 10A), colonic weight index (Figure 10B), and colonic weight/colonic length (Figure 10C) of AIH mice in the Con A group were higher than in the control group (P < 0.01), while colonic weight (Figure 10A), colonic weight index (Figure 10B), and colonic weight/colonic length (Figure 10C) of AIH mice in the Con A + PTH-M group were lower than in the Con A group (P < 0.05). Also, colonic inflammation was more pronounced in the colonic mucosa and submucosa of AIH model mice than in the control group (Figure 10D). Compared with the untreated Con A group, histological colonic damage was improved following 10-d of PTH and DXM therapy, with fewer inflammatory cells in the colonic mucosa and submucosa (Figure 10D).
Occludin content in tight junction proteins may be an indicator of the intestinal barrier’s permeability. The expression of occludin was reduced in the colon tissues of mice in the Con A group than in the control group (Figures 10E and F), while the expression of occludin was elevated in the colon tissues of mice in the Con A + PTH-M group than in the Con A group (Figures 10E and F). The above data suggest that PTH has a protective effect on the intestinal barrier.
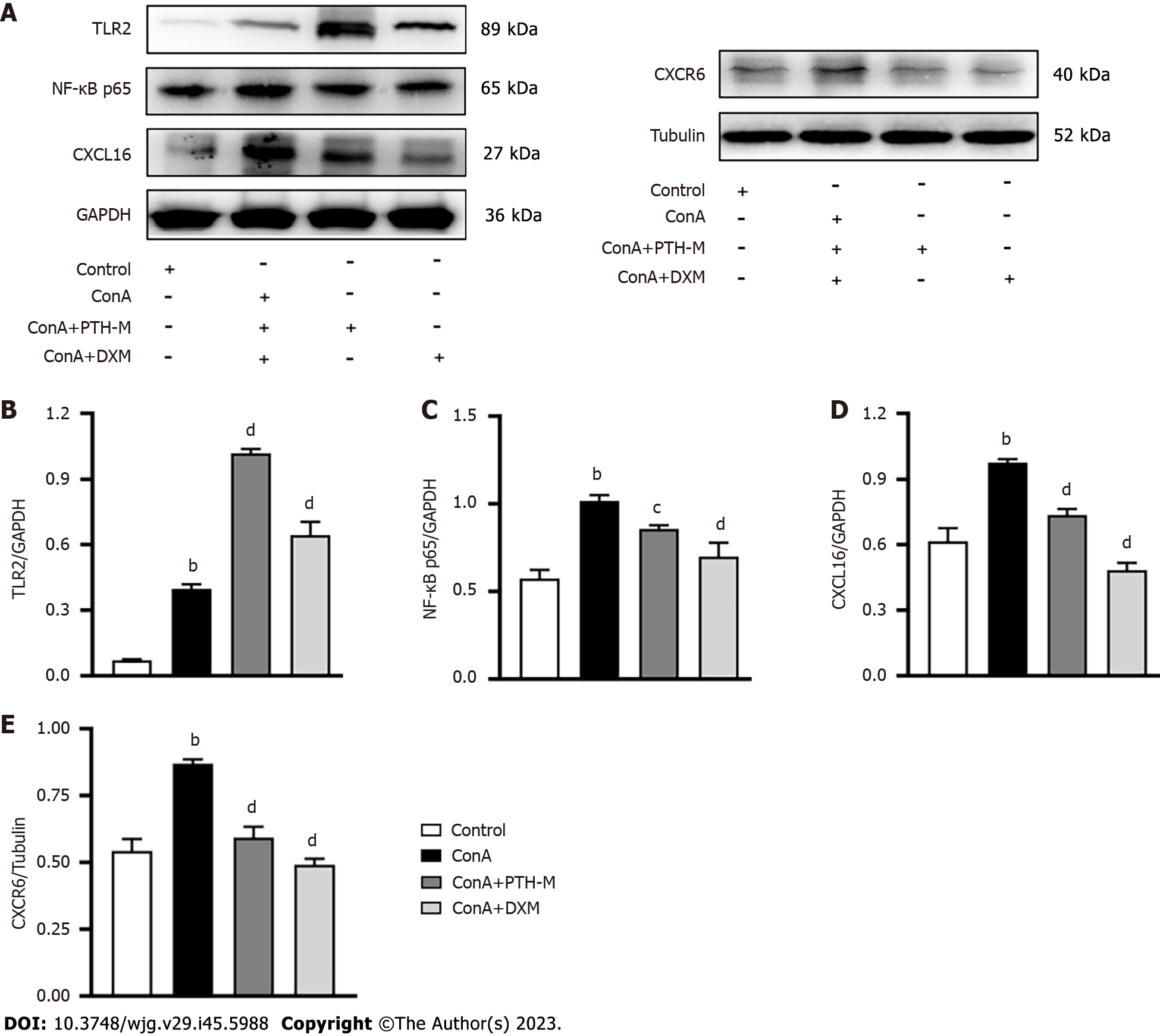
Emerging evidence has identified that TLRs are interrelated with autoimmune diseases by recognizing the change in gut microbiota and/or other pathogens (or immunocomplexes). Figures 9D-G show that in the treatment groups, there was a sizable association between the distribution of sample microflora and mTreg cells. The small intestine’s epithelial cells are stimulated by Lactiplantibacillus plantarum 22A-3 to secrete transforming growth factor-beta, and retinoic acid is likely produced through TLR2, resulting in an increase in CD103+ DCs and the Foxp3+ Treg population[31], and the activation of TLR4 by lipopolysaccharide (LPS) leads to the induction of pro-inflammatory Th17 cells and reduces the immunosuppressive function of Treg cells[3,32]. Differentiation of Th17 and Treg cells is adjusted by NF-κB[33,34]. CXCL16 promotes the migration of immune cells to secondary lymphoid organs and inflammatory sites, and CXCL16 is also an effective direct activator of NF-κB. The findings of western blot analysis revealed that the proteins TLR4 (Figure 9G), NF-κB p65 (Figure 11C), CXCL16 (Figure 11D), and CXCR6 (Figure 11E) in the liver were highly expressed in AIH model mice vs the control group (P < 0.01), while the expression of TLR4 (Figure 9G), NF-κB p65 (Figure 11C), CXCL16 (Figure 11D), and CXCR6 (Figure 11E) proteins were markedly decreased in mice in the Con A + PTH-M group than in mice in the Con A group (P < 0.01). However, the protein TLR2 (Figure 11B) in the liver was decreased in AIH model mice compared with the control group (P < 0.01). After PTH treatment, the protein TLR2 (Figure 11B) in the liver was lower in AIH model mice than in the Con A group (P < 0.01). Hence, these data suggest that PTH regulates Treg cell expression by inhibiting the NF-kB pathway and promoting TLR2 expression.
PTH has anti-inflammatory, neuroprotective, and immunoregulatory properties and is used in traditional Chinese medicine[10,35], which can reduce the serum homocysteine level and inhibit endoplasmic reticulum stress and the PERK/eIF2α pathway to reduce the severity of alcohol and high-fat diet in rats[36]. PTH can also inhibit apoptosis of intestinal crypt cells induced by 5-fluorouracil by inhibiting the ratio of Bax/Bcl-2, which can effectively reduce 5-fluorouracil-induced intestinal mucositis[37]. The above results suggest that PTH has the potential to treat immune liver injury and sustain the integrity of the gut mucosal barrier.
In order to further investigate the mechanism of PTH in the treatment of AIH, we analyzed the expression of various cytokines in AIH mice, and found that PTH treatment downregulated the expression of pro-inflammatory cytokines, such as IFN-γ, IL-1β, IL-2, IL-6, IL-21, and TNF-α, and upregulated the expression of anti-inflammatory factors, such as IL-10 in mice with Con A-induced HIA. IFN-γ[38]. TNF-α[39], IL-1β[40], IL-2[41], IL-21[42], and IL-6[43] promote autoimmune hepatic damage, whereas IL-10[44] protects the liver from damage. Thus, these data suggest that PTH could control the proportion of pro- and anti-inflammatory factors to improve Con A-induced AIH.
Previous research demonstrated a link between the poor prognosis of AIH and the low expression and functioning of Treg cells in the liver’s inflammatory microenvironment[45-47]. It is known that mTreg cells can control memory effector reactions to induce differentiation and proliferation of Treg cells, and subsequently reduce collateral damage to tissues[48,49]. Treg cells have a significant role in adjusting the diversity of intestinal microbiota. They are also key cells that can inhibit intestinal inflammation, inhibit the abnormally activated immune response, and maintain intestinal immune tolerance[50]. When exposed to a foreign antigen again, TCM cells, which are long-lived T cells with the capacity for self-renewal and proliferation, can quickly develop into effector cells (TEM)[51]. Moreover, in mice lacking Foxp3 enhancer CNS1, Firmicutes were less abundant in the gut relative to the control group[52]. Additionally, Zhou et al[53] found that Foxp3+ expression could be increased by PD-1/PD-L1+ to improve its inhibitory effect. In the present research, the expression of CD4+CD25+Foxp3+PD-L1+, CD4+CD25+Foxp3+ Treg, CD4+CD45RA-Foxp3high, TCM, and TEM cells were lower in the Con A group than in control mice, while the expression of CD4+IL-17A+ Th17 cells was higher. These findings imply that the decreased PD-L1 expression in CD4+CD25+Foxp3+ Treg cells downregulates the number of CD4+CD25+Foxp3+ Treg cells. The decreased immune memory function leads to reduced differentiation of Treg cells and more activation of Th17 cell differentiation. The total number of Treg cells was insufficient to preserve immunological tolerance and stop pathogenic elements-such as heteroantigen and pathogenic bacteria-from continuing to have a milder immunosuppressive effect on CD4+CD25+Foxp3+ Treg cells. Following PTH treatment for 10 d, the number of CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells was raised to maintain immune tolerance. In order to activate the immunosuppressive function and limit the severity of excessive immunological damage, PTH additionally enhanced the expression of PD-L1 in CD4+CD25+Foxp3+ Treg cells and decreased the number of CD4+IL17A+ Th17 cells. Also, PTH effectively reduced Con A-induced AIH in mice. These findings imply that the equilibrium between Th17, mTreg, and Treg cells may be controlled by PTH to provide its therapeutic impact in mice with Con A-induced AIH.
It is widely recognized that an imbalance in intestinal microbiota is a key trait and pathogenic component of AIH. Intestinal bacteria and/or other pathogens (or immunocomplexes) can enter the liver through the damaged gut mucosal barrier. PTH has a protective role in the liver of mice with Con A-induced AIH by encouraging the growth of particular helpful bacteria and repressing the growth of some potentially harmful bacteria. In this study, lower abundances of Alloprevotella, Prevotellaceae-UCG-001, Dubosiella, and Odoribacter, and higher abundances of Lachnospiraceae-NK4A136, norank-f-norank-o-Clostridia-UCG-014, and Desulfovibrio were seen in the PTH group. Moreover, psyllium elicits a broader microbial shift, increasing the abundance of Parasutterella and Akkermansia, and decreasing Enterorhabdus and Odoribacter[54]. Chen et al[55] found that chondroitin sulfate can ameliorate diet-induced insulin resistance, improve non-alcoholic fatty liver disease via the AKT pathway, and modulate gut microbiota composition, increasing the abundance of Desulfovibrio and the levels of hydrogen sulfide (H2S). Lachnospiraceae_NK4A136, Parabacteroides, and Clostridium likely strengthened the availability of Bifidobacterium pseudocatenulatum LI09 against D-GalN-induced rat liver injury[56]. In this study, species differences and correlation analysis of intestinal microbiota showed that PTH could reduce the abundance of Bacteroides-caecimuris and uncultured-bacterium-g-Prevotellaceae-NK3B31-group and increase the abundance of unclassified-g-norank-f-Muribaculaceae. Simultaneously, correlation analysis showed that Desulfovibrio was negatively related to HAI, AST, ALT, TLR4, and positively related to CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells; Prevotellaceae-NK3B31-group was positively related to HAI, AST, ALT, TLR4, and negatively related to CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells; Rikenellaceae-RC9-gut-group was positively related to HAI, AST, ALT, and negatively related to CD4+CD45RA-Foxp3high mTreg cells; Dubosiella was negatively related to CD4+CD25+Foxp3+ Treg and CD4+CD45RA-Foxp3high mTreg cells.
The destruction of intestinal mucosa was the main reason for induced bacterial translocation. Tight junction proteins have a prominent role in maintaining the intestinal barrier. Consequently, it is particularly important to reverse the deterioration of the intestinal barrier, bacterial translocation, and/or other pathogens (or immunocomplexes) to the liver. The present results showed that Con A increases intestinal permeability in mice by decreasing occludin protein expression; intestinal microbiota and/or other pathogens (or immunocomplexes) arrive at the liver through the portal vein to induce AIH. In order to treat mice with Con A-induced AIH, PTH controlled the interreaction between the intestinal microbiota and the number of mTreg cells.
Correlation analysis of environmental factors showed that Treg/mTreg cells were positively correlated with intestinal microbiota, and TLR4 was negatively correlated with intestinal microbiota. Treg/mTreg cells could affect the intestinal microbiota, which in turn could stimulate pattern recognition receptors on the surface of immune cells or metabolites of flora to affect Treg cells. The immune system can sense the intestinal microbiota through the TLRs mechanism[57]. Treg cells mainly express TLR1, TLR2, TLR4, TLR5, and TLR8. It has been demonstrated that the stimulation of TLR2 by the intestinal microbiota or polysaccharide A from Bacteroides fragilis plays a defensive role in the gut, lowering inflammation by raising IL-10 production by dendritic and B cells and encouraging Treg proliferation[58]. Moreover, the activation of TLR4 by LPS causes the emergence of pro-inflammatory Th17 cells and reduces the immunosuppressive function of Treg cells[3]. Lai et al[59] found that indirubin downregulates LPS-induced inflammation by blocking TLR4 and NF-κB and MAPK activation, which is used to treat mastitis. Studies have also suggested that CXCL16/CXCR6 are elevated in some autoimmune diseases. Treg cells could be inhibited by CXCL16[60]; LPS was discovered to enhance the transcription of CXCL16 via the NF-κB signaling pathway, thus promoting the pathogenesis of acute lung injury[61]. In the current study, TLR4, NF-κB p65, CXCR6, and CXCL16 proteins in the liver were markedly expressed in AIH mice, and the protein TLR2 in the liver showed low expression. However, after PTH treatment, the expression of TLR4, NF-κB p65, CXCR6, and CXCL16 was significantly reduced, and TLR2 protein was significantly increased. However, it is still unclear which precise PTH constituents collectively control the interaction between the gut microbiota, TLR/CXCL16/CXCR6/NF-κB signal pathway, and mTreg cells, and which specific bacterium and their metabolites would be significantly enhanced or have a significant role in PTH treatment of AIH mice.
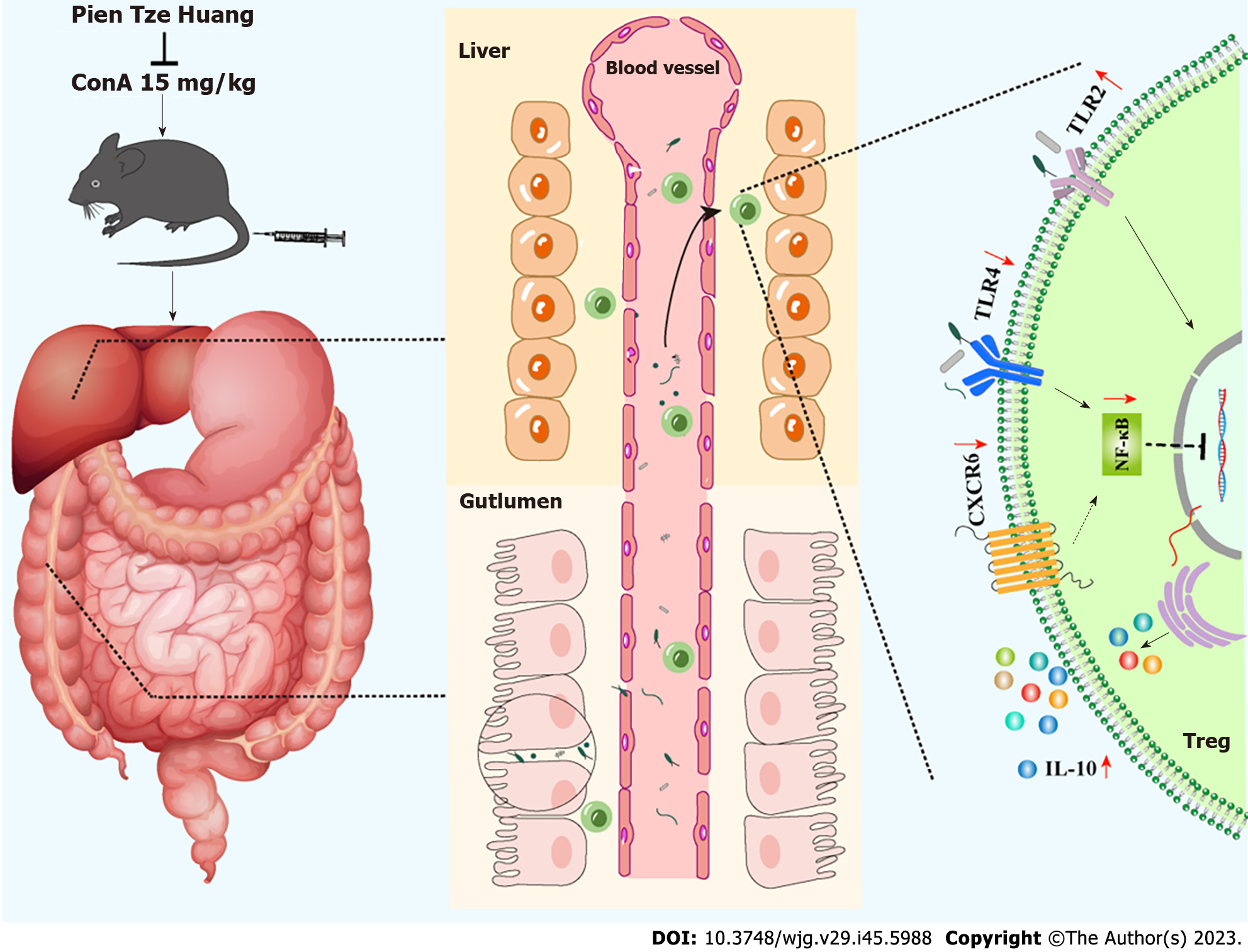
In conclusion, these findings suggest that PTH controls interactions between mTreg cells and intestinal microbiota to reduce experimental AIH. The effect of PTH was similar to that of DXM; yet, in the PTH group, there was obvious immunoregulation. PTH may enhance the colon barrier effect and the variety of intestinal microbiota, in order to prevent the movement of potentially hazardous intestinal substances to the liver, suppression of the TLR4/NF-κB, CXCL16/CXCR6 pathway, and activation of the TLR2 pathway could increase the expression of mTreg cells to secrete more Treg cells, thus limiting the expression of Th17 cells, reducing the creation of inflammatory substances, and achieving the purpose of treating AIH. These results suggest that PTH may balance intestinal microbiota and interfere with mTreg cells to regulate differentiated Treg cells, which is a natural alternative therapy for AIH (as shown in Figure 12).
Although our studies hoped to determine the mechanism of PTH treatment in mice with AIH, as a compound preparation with multiple and complex components, the definite effective ingredients of PTH in the treatment of AIH are ambiguous. Although specific bacteria had interactions with mTreg cells, these interactions were not defined. Crosstalk between the gut microbiota, mTreg cells, the TLR/CXCL16/CXCR6/NF-κB signaling pathway and PTH requires further investigation in future studies. It is very fortunate that PTH regulates intestinal microbiota balance and restores mTreg cells to alleviate experimental AIH, which is closely related to the TLR/CXCL16/CXCR6/NF-κB signaling pathway. These results will have directive significance and help to determine more immunomodulatory effects of PTH, to widen the scope of the clinical application of PTH treatment in AIH, and to develop new drugs related to PTH. In the future, we will plan to separate and co-cultured mTreg cells with PTH, or culture medium from PTH and the specific bacteria using a transwell microfluidic control chip, and to compare the effect of the inhibitor or agonist of the TLR/CXCL16/CXCR6/NF-κB signaling pathway on mTreg cells, and ultimately discover the definite action target and effective ingredients of PTH in the treatment of AIH.
Autoimmune hepatitis (AIH) is a common and frequently-occurring interfacial hepatitis, and its etiology remains unknown. The interaction between regulatory T (Treg) cells and intestinal microbiota may provide a feasible therapeutic strategy for AIH.
As a well-known traditional Chinese medicine, Pien Tze Huang (PTH) is regularly used to treat liver conditions, including AIH. PTH has a physiological basis for controlling Treg cells and intestinal microbiota. However, it is unknown whether PTH can regulate intestinal microbiota and memory Treg (mTreg) cells to treat AIH.
To explore the mechanism of PTH treated AIH by determining the changes in the structure of intestinal microbiota and the level of mTreg cells.
Following establishment of the mouse model of Concanavalin A (Con A)-induced AIH, prophylactic administration of PTH was given for 10 d. The levels of mTreg cells were measured by flow cytometry, and intestinal microbiota was analyzed by 16S rDNA analysis, while western blotting was used to identify the activation of the Toll-like receptor (TLR)2, TLR4/nuclear factor-κB (NF-κB), and CXCL16/CXCR6 signaling pathways.
In the present study, after PTH administration in mice with AIH, the expression of pro-inflammatory factors [such as tumor necrosis factor-alpha, interferon-γ, interleukin (IL)-1β, IL-2, IL-6, and IL-21] and Th17 cells was decreased, followed by improved pathological liver injury. In addition, the number of Treg/mTreg cells and IL-10 level were markedly increased, and the diversity of intestinal microbiota was regulated. Furthermore, activation of the TLR4/NF-κB, CXCL16/CXCR6 signaling pathway was suppressed in hepatic tissue.
PTH effectively relieved pathological liver injury in mice with Con A-induced AIH, which was realized by improving intestinal microbiota balance and mTreg levels, and inhibiting the TLR/CXCL16/CXCR6/NF-κB signaling pathway.
This research indicated that the mechanism of PTH treated mice with AIH was closely related to the regulation of intestinal microbiota balance and improving mTreg cell levels. These results provide scientific evidence for the application of PTH in AIH treatment and for the development of new drugs for AIH.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Velikova TV, Bulgaria; Zhong Y, China; Sipos F, Hungary S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Yu HG
| 1. | Wei Y, Li Y, Yan L, Sun C, Miao Q, Wang Q, Xiao X, Lian M, Li B, Chen Y, Zhang J, Huang B, Cao Q, Fan Z, Chen X, Fang JY, Gershwin ME, Tang R, Ma X. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 2. | Nakao M, Sugaya M, Fujita H, Miyagaki T, Morimura S, Shibata S, Asano Y, Sato S. TLR2 Deficiency Exacerbates Imiquimod-Induced Psoriasis-Like Skin Inflammation through Decrease in Regulatory T Cells and Impaired IL-10 Production. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Niño DF, Prindle T Jr, Ozolek JA, Hackam DJ. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016;126:495-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Ma L, Zhang L, Zhuang Y, Ding Y, Chen J. Lactobacillus improves the effects of prednisone on autoimmune hepatitis via gut microbiota-mediated follicular helper T cells. Cell Commun Signal. 2022;20:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Zhang H, Liu M, Liu X, Zhong W, Li Y, Ran Y, Guo L, Chen X, Zhao J, Wang B, Zhou L. Bifidobacterium animalis ssp. Lactis 420 Mitigates Autoimmune Hepatitis Through Regulating Intestinal Barrier and Liver Immune Cells. Front Immunol. 2020;11:569104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Liu Q, Tian H, Kang Y, Tian Y, Li L, Kang X, Yang H, Wang Y, Tian J, Zhang F, Tong M, Cai H, Fan W. Probiotics alleviate autoimmune hepatitis in mice through modulation of gut microbiota and intestinal permeability. J Nutr Biochem. 2021;98:108863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 7. | Petrarca C, Lanuti P, Petrosino MI, Di Pillo S, Mistrello G, Compalati E, Otzuki T, Marchisio M, Pierdomenico L, Paganelli R, Di Gioacchino M. Peripheral effector memory regulatory T cells are incremented and functionally enhanced in successful mite monomeric allergoid sublingual immunotherapy. Allergy. 2021;76:2208-2211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Liu C, Chen Z, Wu SLY, Chow TCH, Cheng RSY, Lee JTC, Yew DT. Comparative Review of Effects of Pien Tze Huang and AnGong NiuHuang Pill and their Potential on Treatment of Central Nervous System Diseases. Mini Rev Med Chem. 2022;22:2350-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Chen Z. Pien Tze Huang (PZH) as a Multifunction Medicinal Agent in Traditional Chinese Medicine (TCM): a review on cellular, molecular and physiological mechanisms. Cancer Cell Int. 2021;21:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Deng Y, Luo H, Shu J, Shu H, Lu C, Zhao N, Geng Y, He X, Lu A. Pien Tze Huang alleviate the joint inflammation in collagen-induced arthritis mice. Chin Med. 2020;15:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Lian B, Cai L, Zhang Z, Lin F, Li Z, Zhang XK, Jiang F. The anti-inflammatory effect of Pien Tze Huang in non-alcoholic fatty liver disease. Biomed Pharmacother. 2022;151:113076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Zhao J, Hu H, Wan Y, Zhang Y, Zheng L, Hong Z. Pien Tze Huang Gan Bao ameliorates carbon tetrachloride-induced hepatic injury, oxidative stress and inflammation in rats. Exp Ther Med. 2017;13:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Zheng H, Wang X, Zhang Y, Chen L, Hua L, Xu W. Pien-Tze-Huang ameliorates hepatic fibrosis via suppressing NF-κB pathway and promoting HSC apoptosis. J Ethnopharmacol. 2019;244:111856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Zhu J, Zhang D, Wang T, Chen Z, Chen L, Wu H, Huai C, Sun J, Zhang N, Wei M, Hong F, Qin S. Target identification of hepatic fibrosis using Pien Tze Huang based on mRNA and lncRNA. Sci Rep. 2021;11:16980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Liston A, Whyte CE. Bile acids mediate signaling between microbiome and the immune system. Immunol Cell Biol. 2020;98:349-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Song X, Sun X, Oh SF, Wu M, Zhang Y, Zheng W, Geva-Zatorsky N, Jupp R, Mathis D, Benoist C, Kasper DL. Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature. 2020;577:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 696] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 17. | Gong X, Shan L, Cao S, Li K, Wu Y, Zhang Q. Notoginsenoside R1, An Active Compound from Panax notoginseng, Inhibits Hepatic Stellate Cell Activation and Liver Fibrosis via MAPK Signaling Pathway. Am J Chin Med. 2022;50:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Li W, Jiang Z, Li H, Tu P, Song Q, Yu J, Song Y. [Chemome profiling of Pien-Tze-Huang by online pressurized liquid extraction-ultra-high performance liquid chromatography-ion trap-time-of-flight mass spectrometry]. Se Pu. 2021;39:478-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 19. | Xu W, Zhang Y, Zhou C, Tai Y, Zhang X, Liu J, Sha M, Huang M, Zhu Y, Peng J, Lu JJ. Simultaneous quantification six active compounds in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study of Pien-Tze-Huang. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1061-1062:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Ling Q, Hu X, Jiang R, Liu H, Qiu H, Jiang X, Zubreri A, Zhu H, Wan J, Liu Y. CQMUH-011 mitigates autoimmune hepatitis via inhibiting the function of T lymphocytes. Drug Dev Res. 2021;82:1111-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Wang T, Men R, Hu M, Fan X, Yang X, Huang X, Ye T, Yang L. Protective effects of Punica granatum (pomegranate) peel extract on concanavalin A-induced autoimmune hepatitis in mice. Biomed Pharmacother. 2018;100:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3782] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 23. | Hao J, Sun W, Xu H. Pathogenesis of Concanavalin A induced autoimmune hepatitis in mice. Int Immunopharmacol. 2022;102:108411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Floreani A, Restrepo-Jiménez P, Secchi MF, De Martin S, Leung PSC, Krawitt E, Bowlus CL, Gershwin ME, Anaya JM. Etiopathogenesis of autoimmune hepatitis. J Autoimmun. 2018;95:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 25. | Schmeltzer PA, Russo MW. Clinical narrative: autoimmune hepatitis. Am J Gastroenterol. 2018;113:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Mieli-Vergani G, Zen Y, Vergani D. Reassessement of the histological features of autoimmune hepatitis. Liver Int. 2022;42:954-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Liang M, Liwen Z, Yun Z, Yanbo D, Jianping C. The Imbalance between Foxp3(+)Tregs and Th1/Th17/Th22 Cells in Patients with Newly Diagnosed Autoimmune Hepatitis. J Immunol Res. 2018;2018:3753081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Huang C, Shen Y, Shen M, Fan X, Men R, Ye T, Yang L. Glucose Metabolism Reprogramming of Regulatory T Cells in Concanavalin A-Induced Hepatitis. Front Pharmacol. 2021;12:726128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Mataki N, Kikuchi K, Kawai T, Higashiyama M, Okada Y, Kurihara C, Hokari R, Kawaguchi A, Nagao S, Kondo T, Itoh K, Miyakawa H, Miura S. Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases. Am J Gastroenterol. 2007;102:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Brahim I, Brahim I, Hazime R, Admou B. [Autoimmune hepatitis: Immunological diagnosis]. Presse Med. 2017;46:1008-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Lamubol J, Ohto N, Kuwahara H, Mizuno M. Lactiplantibacillus plantarum 22A-3-induced TGF-β1 secretion from intestinal epithelial cells stimulated CD103(+) DC and Foxp3(+) Treg differentiation and amelioration of colitis in mice. Food Funct. 2021;12:8044-8055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Zhou M, Zhang Y, Tang R, Liu H, Du M, Gao Z, Ji Z, Fang H. HMGB1/TLR4 Signaling Affects Regulatory T Cells in Acute Lung Injury. J Inflamm Res. 2021;14:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Bian J, Wang T, Sun J, He X, Wu Z, Zhang S, Chi H, Fan T, Wang S, Shi W, Ruan Q. Targeting NF-κB c-Rel in regulatory T cells to treat corneal transplantation rejection. Am J Transplant. 2021;21:3858-3870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Tong L, Hao H, Zhang Z, Lv Y, Liang X, Liu Q, Liu T, Gong P, Zhang L, Cao F, Pastorin G, Lee CN, Chen X, Wang JW, Yi H. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics. 2021;11:8570-8586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 171] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 35. | Qiu X, Luo H, Liu X, Guo Q, Zheng K, Fan D, Shen J, Lu C, He X, Zhang G, Lu A. Therapeutic Potential of Pien Tze Huang on Experimental Autoimmune Encephalomyelitis Rat. J Immunol Res. 2018;2018:2952471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Chen Z, Deng L, Yu J, Wang K, Zhang X, Ji G, Li F. Pien Tze Huang ameliorates liver injury by inhibiting the PERK/eIF2α signaling pathway in alcohol and high-fat diet rats. Acta Histochem. 2018;120:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Fu C, Chu J, Shen A, Liu L, Chen H, Lin J, Sferra TJ, Chen Y, Peng J. Pien Tze Huang alleviates 5-fluorouracil-induced intestinal mucositis in CT-26 tumor-bearing mice. Exp Ther Med. 2017;14:2291-2297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Zheng C, Yin S, Yang Y, Yu Y, Xie X. CD24 aggravates acute liver injury in autoimmune hepatitis by promoting IFN-γ production by CD4(+) T cells. Cell Mol Immunol. 2018;15:260-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | El-Kashef DH, Abdelrahman RS. Montelukast ameliorates Concanavalin A-induced autoimmune hepatitis in mice via inhibiting TNF-α/JNK signaling pathway. Toxicol Appl Pharmacol. 2020;393:114931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, Zai W, Wang Y, Chen M, Meng G, Ju D. NOD-Like Receptor Protein 3 Inflammasome-Dependent IL-1β Accelerated ConA-Induced Hepatitis. Front Immunol. 2018;9:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | Mohammed MM, Okasha AMM, Naiem AHA, Mohamed RF, Abdelwahab SF, Mohamed HA. Cyclosporine Ameliorates Silica-Induced Autoimmune Hepatitis in Rat Model by Altering the Expression of Toll-Like Receptor-4, Interleukin-2, and Tumor Necrosis Factor-α. Curr Mol Med. 2023;23:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 42. | Ma L, Zhang LW, Zhuang Y, Ding YB, Chen JP. Exploration the significance of Tfh and related molecules on C57BL/6 mice model of experimental autoimmune hepatitis. J Microbiol Immunol Infect. 2021;54:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Jiang R, Tang J, Zhang X, He Y, Yu Z, Chen S, Xia J, Lin J, Ou Q. CCN1 Promotes Inflammation by Inducing IL-6 Production via α6β1/PI3K/Akt/NF-κB Pathway in Autoimmune Hepatitis. Front Immunol. 2022;13:810671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Liberal R, Grant CR, Yuksel M, Graham J, Kalbasi A, Ma Y, Heneghan MA, Mieli-Vergani G, Vergani D, Longhi MS. Regulatory T-cell conditioning endows activated effector T cells with suppressor function in autoimmune hepatitis/autoimmune sclerosing cholangitis. Hepatology. 2017;66:1570-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Richardson N, Wootton GE, Bozward AG, Oo YH. Challenges and opportunities in achieving effective regulatory T cell therapy in autoimmune liver disease. Semin Immunopathol. 2022;44:461-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | McEachern E, Carroll AM, Fribourg M, Schiano TD, Hartzell S, Bin S, Cravedi P, Levitsky J. Erythropoietin administration expands regulatory T cells in patients with autoimmune hepatitis. J Autoimmun. 2021;119:102629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Longhi MS, Mieli-Vergani G, Vergani D. Regulatory T cells in autoimmune hepatitis: an updated overview. J Autoimmun. 2021;119:102619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 48. | Rosenblum MD, Way SS, Abbas AK. Regulatory T cell memory. Nat Rev Immunol. 2016;16:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 49. | van der Veeken J, Gonzalez AJ, Cho H, Arvey A, Hemmers S, Leslie CS, Rudensky AY. Memory of Inflammation in Regulatory T Cells. Cell. 2016;166:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 50. | Kehrmann J, Effenberg L, Wilk C, Schoemer D, Ngo Thi Phuong N, Adamczyk A, Pastille E, Scholtysik R, Klein-Hitpass L, Klopfleisch R, Westendorf AM, Buer J. Depletion of Foxp3(+) regulatory T cells is accompanied by an increase in the relative abundance of Firmicutes in the murine gut microbiome. Immunology. 2020;159:344-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Pathakumari B, Devasundaram S, Raja A. Altered expression of antigen-specific memory and regulatory T-cell subsets differentiate latent and active tuberculosis. Immunology. 2018;153:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 666] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 53. | Zhou BG, Liu FC, Zhao HM, Zhang XY, Wang HY, Liu DY. Regulatory effect of Zuojin Pill on correlation with gut microbiota and Treg cells in DSS-induced colitis. J Ethnopharmacol. 2020;262:113211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Pontifex MG, Mushtaq A, Le Gall G, Rodriguez-Ramiro I, Blokker BA, Hoogteijling MEM, Ricci M, Pellizzon M, Vauzour D, Müller M. Differential Influence of Soluble Dietary Fibres on Intestinal and Hepatic Carbohydrate Response. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Chen L, Gao Y, Zhao Y, Yang G, Wang C, Zhao Z, Li S. Chondroitin sulfate stimulates the secretion of H(2)S by Desulfovibrio to improve insulin sensitivity in NAFLD mice. Int J Biol Macromol. 2022;213:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 56. | Zha H, Si G, Wang C, Zhang H, Li L. Multiple Intestinal Bacteria Associated with the Better Protective Effect of Bifidobacterium pseudocatenulatum LI09 against Rat Liver Injury. Biomed Res Int. 2022;2022:8647483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Lu H, Liu P, Zhang X, Bao T, Wang T, Guo L, Li Y, Dong X, Li X, Dong Y, Sha L, He L, Wang H. Inulin and Lycium barbarum polysaccharides ameliorate diabetes by enhancing gut barrier via modulating gut microbiota and activating gut mucosal TLR2+ intraepithelial γδ T cells in rats. J Funct Foods. 2021;79:104407. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, Bulik-Sullivan E, Carroll IM, Hansen JJ, Chen L, Wilson JE, Fisher NC, Ting JP, Nochi T, Wahl A, Garcia JV, Karp CL, Sartor RB. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 2019;129:3702-3716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 59. | Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu XF, Zhou QG, Chen YY, Guo AZ, Hu CM. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation. 2017;40:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 351] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 60. | Xing YN, Zhang JY, Xu HM. The roles of serum CXCL16 in circulating Tregs and gastrointestinal stromal tumor cells. Onco Targets Ther. 2016;9:3939-3949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Tu GW, Ju MJ, Zheng YJ, Hao GW, Ma GG, Hou JY, Zhang XP, Luo Z, Lu LM. CXCL16/CXCR6 is involved in LPS-induced acute lung injury via P38 signalling. J Cell Mol Med. 2019;23:5380-5389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |