Published online Nov 28, 2023. doi: 10.3748/wjg.v29.i44.5907
Peer-review started: September 2, 2023
First decision: October 16, 2023
Revised: October 29, 2023
Accepted: November 14, 2023
Article in press: November 14, 2023
Published online: November 28, 2023
Processing time: 86 Days and 2.3 Hours
The efficacy and safety profile of tenofovir amibufenamide (TMF) in chronic hepatitis B (CHB) patients is not well-established.
To compare the efficacy and safety of TMF and tenofovir alafenamide (TAF) over a 48-wk period in patients with CHB.
A total of 215 subjects meeting the inclusion criteria were enrolled and divided into two groups: TMF group (n = 106) and the TAF group (n = 109). The study included a comparison of virological response (VR): Undetectable hepatitis B virus DNA levels, alanine transaminase (ALT) normalization rates, renal function parameters, and blood lipid profiles.
At 24 and 48 wk, VR rates for the TMF group were 53.57% and 78.57%, respectively, compared with 48.31% and 78.65% for the TAF group (P > 0.05). The VR rates were also similar in both groups among patients with low-level viremia, both hepatitis B e antigen (HBeAg)-positive and HBeAg-negative subgroups. The TMF cohort showed ALT normalization and renal safety profiles similar to the TAF group. There was a notable increase in total cholesterol levels in the TAF group (P = 0.045), which was not observed in the TMF group (P > 0.05). In patients with liver cirrhosis, both groups exhibited comparable VR and ALT normalization rates and renal safety profiles. However, the fibrosis 4 score at 48 wk showed a significant reduction in the TAF group as compared to the TMF group within the liver cirrhosis subgroup.
Our study found TMF is as effective as TAF in treating CHB and has a comparable safety profile. However, TAF may be associated with worsening lipid profiles.
Core Tip: This is a retrospective study to compare the efficacy and safety of tenofovir amibufenamide (TMF) and tenofovir alafenamide (TAF) for 48 wk in patients with chronic hepatitis B (CHB). Our study found that TMF is as effective as TAF in treating CHB and has comparable safety profiles. In addition, TAF may cause deterioration of lipid profiles. These results suggest that TMF may be a viable alternative to TAF for CHB treatment.
- Citation: Peng WT, Jiang C, Yang FL, Zhou NQ, Chen KY, Liu JQ, Peng SF, Fu L. Tenofovir amibufenamide vs tenofovir alafenamide for treating chronic hepatitis B: A real-world study. World J Gastroenterol 2023; 29(44): 5907-5918
- URL: https://www.wjgnet.com/1007-9327/full/v29/i44/5907.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i44.5907
Hepatitis B virus (HBV) infection represents a significant economic and health burden worldwide. As of 2019, over 1.5 million preventable new infections continue to occur annually, and there are approximately 296 million people living with chronic HBV infection, resulting in over 820,000 deaths annually due to liver cirrhosis and hepatocellular carcinoma (HCC)[1]. Achieving complete suppression of HBV in a safe and effective manner is crucial for preventing HBV-related adverse health events[2]. Consequently, efforts in this regard have primarily focused on antiviral treatment over the past decades. Current international guidelines recommend as first-line treatments newer antiviral agents with a high genetic barrier to HBV mutation, such as entecavir, tenofovir (TFV) disoproxil fumarate (TDF) and tenofovir alafenamide (TAF)[3-5]. Previous studies have shown that these drugs are safe and effective in treating chronic hepatitis B (CHB). However, long-term use of TDF leads to high levels of circulating TFV, resulting in kidney and bone toxicity, particularly in aging populations[6]. TAF, a TFV prodrug, is converted into the active form of TFV diphosphate in vivo, similar to TDF. At a dose of ≤ 25 mg, TAF reduces the total body exposure of TFV by more than 90%[7]. The correlativity study demonstrated that TAF, with its low concentration of TFV in the circulation, reduces the drug load on the kidneys and bones, thereby improving their safety[8].
Currently, tenofovir amibufenamide (TMF) is recommended as the fourth nucleoside analog for first-line treatment of CHB in mainland China[9]. TMF, another prodrug of TFV, is produced by ProTide technology and features an additional methyl group compared to TAF. This extra methyl group may enhance TMF's stability in peripheral blood and facilitate intracellular conversion[10]. In vitro studies have shown that TMF has a lower EC50 in HepG2.2.15 cells than TAF and TDF[11]. In randomized clinical trials and prospective clinical studies with treatment durations of 48 and 96 wk, TMF was found to be similarly effective in viral suppression to TDF, but with significantly less bone and renal toxicity[12,13]. TMF was approved in June 2021 and was included in the 2021 China National Reimbursement Drug List for CHB treatment.
Due to the recent introduction of TMF in the Chinese market and the limited real-world research data for the Chinese population, there is currently a knowledge gap regarding the drug’s safety and efficacy. Therefore, we conducted this clinical study to assess the safety and effectiveness of TMF in treating patients with CHB in China.
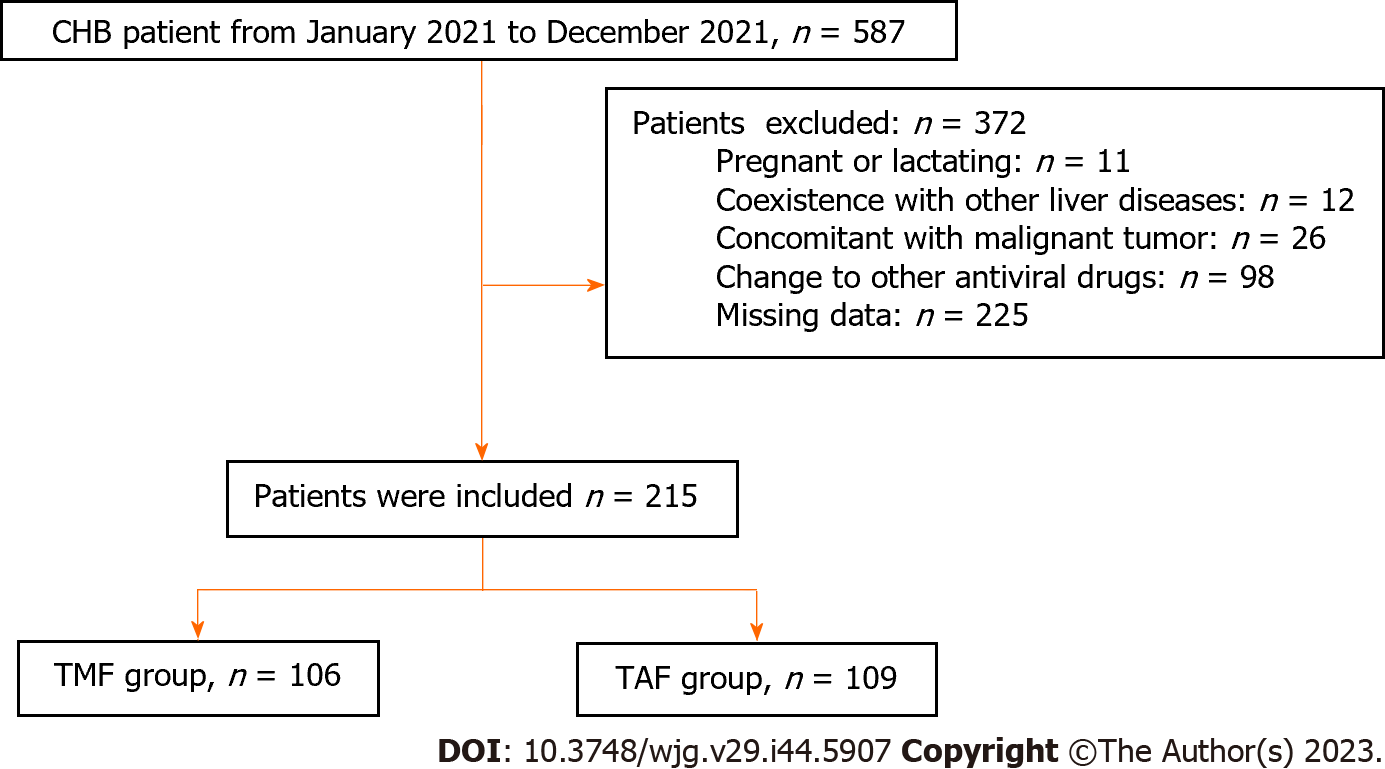
In this retrospective study, we enrolled a total of 587 patients aged 18 and above who had been HBsAg positive for more than 6 mo. These patients were treated at Xiangya Hospital of Central South University between July 2021 and April 2022. Patients were excluded if they met any of the following criteria: (1) Concomitant with other liver diseases, such as alcoholic liver disease, nonalcoholic fatty liver disease, autoimmune liver disease, drug-induced liver injury, hepatolenticular degeneration, or other viral infections [hepatitis A, C, and E virus or human immunodeficiency virus (HIV)]; (2) pregnant or lactating women; (3) concomitant with malignant tumors or other serious diseases affecting survival time; (4) added or changed to other antiviral drugs during treatment; and (5) patients with missing data. Of the enrolled patients, 215 were included in the final analysis and were divided into two groups based on their drug selection: The TMF group and the TAF group.
The study protocol was approved by the Medical Ethics Committee of Xiangya Hospital Central South University (approval No. 202303047).
During the study period, all patients received anti-HBV treatment with 25 mg of TMF (Hansoh Pharmaceuticals Co., Ltd, Jiangsu, China) or 25 mg of TAF (Gilead Sciences, Inc.) once daily immediately after diagnosis of CHB. Additionally, liver protection drugs were used according to the needs of the disease as prescribed by clinicians. Clinical results and related indicators were collected for each participant during the 48-week follow-up period. These parameters were recorded at baseline, approximately at week 24, and again at week 48.
The efficacy endpoint at week 48 was defined as the proportion of patients achieving a virological response (VR), which is characterized by a reduction in serum HBV DNA levels to less than 10 IU/mL, as measured by the real-time polymerase chain reaction method. Additionally, a pre-specified safety outcome included the percentage change in renal function markers and lipid profiles at weeks 24 and 48 in comparison with the baseline values.
Clinical and laboratory data were collected during hospitalization, including clinical characteristics, routine blood test results [including white blood cells (WBC) and platelets (PLT)], liver function tests [including albumin, globulin, total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST)], renal function tests [including serum creatinine (Cr), blood urea nitrogen (BUN), and estimated glomerular filtration rate (eGFR)], HBV DNA quantification, serological biomarkers, blood lipids [including triglycerides, total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL)], serum phosphorus, alpha-fetoprotein (AFP), and liver stiffness measurement (LSM). The model for fibrosis 4 (FIB-4) score was calculated using the following formula[14]: FIB-4 = age [(year) × AST (U/L)] /[(PLT (10(9)/L) × [ALT (U/L) (1/2)]. In our study, ultrasound examinations were employed to diagnose liver cirrhosis in patients. Low-level viremia (LLV) was characterized as either persistent or intermittent detection of HBV DNA at levels below 2000 IU/mL, with a detection threshold of 10 IU/mL, following 48 wk of antiviral therapy.
The sample size for this study was calculated using G Power version 3.1.9.2 (Heinrich-Heine-Universität Düsseldorf). We predetermined the effect size f to range between 0.1 (small) and 0.4 (large), with a type I error rate (alpha) of 0.05 and a power of 0.8, considering two independent groups: TMF and TAF. Employing a one-way ANOVA model, the estimated sample size necessary varied from 84 for a large effect size to 788 for a small effect size. We ultimately recruited 215 participants for the study.
Statistical analyses were performed using SPSS for Windows, version 25.0. Continuous variables were reported as mean ± SD or median (interquartile range), while categorical variables were reported as percentages. The Student t-test and rank sum test were used to compare continuous variables, while the chi-squared test was used for categorical variables. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant.
A total of 587 patients with CHB were identified at our hospital between April 2022 and December 2021, of which 372 patients were excluded for various reasons (Figure 1). The final study population consisted of 215 patients, with 106 patients receiving TMF treatment and 109 patients receiving TAF treatment. The mean age of the study population was 40.57 ± 10.54 years, with 145 (67.74%) male patients. The mean LSM using FibroScan was 10.43 ± 3.99, and 42 (19.53%) of patients were diagnosed with cirrhosis. As shown in Table 1, there were no significant differences in baseline characteristics between the two treatment groups, including age, gender proportion, underlying disease, serum Cr, eGFR, BUN, albumin, globulin, AST, ALT, TBIL, DBIL, AFP, blood phosphorus, WBC, PLT, LSM, and FIB-4 score (all P > 0.05). These findings suggest that the two treatment groups were comparable.
| Variable | TMF group 25 mg (n = 106) | TAF group 25 mg (n = 109) | P value |
| Male | 71 (66.98) | 74 (67.89) | 0.887 |
| Age (yr) | 40.96 ± 11.25 | 40.19 ± 9.84 | 0.707 |
| Routine blood test | |||
| WBC (× 109/L) | 5.59 ± 1.64 | 5.59 ± 1.49 | 0.792 |
| PLT (× 109/L) | 193.97 ± 67.44 | 181.98 ± 68.63 | 0.218 |
| Liver function | |||
| Albumin (g/L) | 45.57 ± 3.64 | 45.46 ± 3.59 | 0.746 |
| Globulin (g/L) | 29.32 ± 3.66 | 29.49 ± 3.70 | 0.617 |
| TBIL (μmol/L) | 4.55 (3.70, 5.90) | 4.75 (2.20, 18.78) | 0.070 |
| DBIL (μmol/L) | 2.50 (1.50, 3.90) | 5.85 (4.10, 8.33) | 0.152 |
| ALT (U/L) | 28.30 (20.10, 46.70) | 32.40 (22.35, 49.60) | 0.203 |
| AST (U/L) | 30.50 (24.90, 39.00) | 30.00 (24.83, 40.80) | 0.740 |
| Kidney function | |||
| BUN (mmol/L) | 4.75 (3.99, 6.11) | 4.88 (4.22, 5.70) | 0.856 |
| Creatinine (μmol/L) | 79.50 (66.05, 91.00) | 83.30 (73.00, 93.90) | 0.177 |
| eGFR (ml/min/1.73 m2) | 90.58 (79.84, 103.80) | 97.19 (87.335, 106.38) | 0.180 |
| Viral load | |||
| HBV DNA < 10 IU/mL | 34 (32.08) | 44 (40.37) | 0.206 |
| HBeAg positive | 39 (36.79) | 46 (42.20) | 0.417 |
| Blood lipid | |||
| Triglycerides (mmol/L) | 1.57 ± 0.82 | 1.65 ± 1.19 | 0.719 |
| Total cholesterol (mg/dl) | 4.83 ± 1.09 | 4.30 ± 1.54 | 0.173 |
| HDL | 1.18 ± 0.21 | 1.10 ± 0.14 | 0.341 |
| LDL | 3.19 ± 0.91 | 3.20 ± 0.94 | 0.877 |
| Phosphorus (mmol/L) | 1.64 ± 3.84 | 1.05 ± 0.44 | 0.958 |
| AFP (ng/mL) | 5.19 ± 8.90 | 5.24 ± 7.89 | 0.167 |
| LSM (Kpa) | 10.17 ± 4.41 | 10.94 ± 3.37 | 0.108 |
| FIB-4 score | 1.15 (0.75, 1.77) | 1.27 (0.87, 2.03) | 0.552 |
| Underlying diseases | |||
| Diabetes | 5 (4.72) | 6 (5.50) | 0.793 |
| Cirrhosis | 23 (21.70) | 19 (17.43) | 0.430 |
| Decompensated cirrhosis | 4 (3.77) | 4 (3.67) | 0.968 |
| Hepatocellular carcinoma | 2 (1.89) | 3 (2.75) | 0.674 |
| NAFLD | 26 (24.53) | 28 (25.69) | 0.845 |
| Treatment naïve | 63 (59.43) | 61 (55.96) | 0.607 |
During the 48-wk follow-up period, no significant drug-related adverse reactions were observed with either oral antiviral drug.
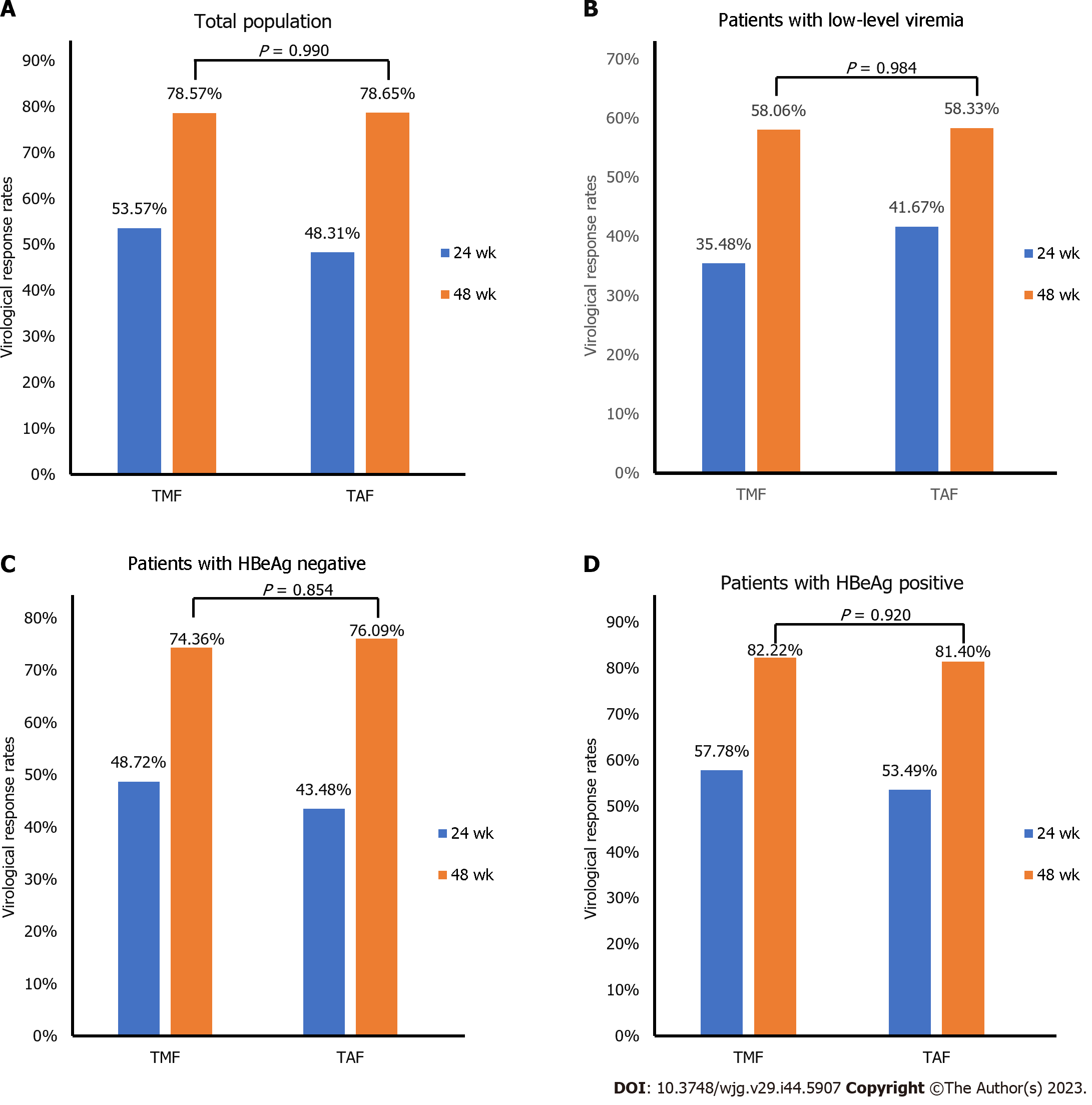
At week 48, the rate of undetectable HBV DNA (HBV DNA < 10 IU/mL) was slightly higher in the TMF treatment group (78.57%), compared to the TAF group (78.65%), although the difference was not statistically significant (Figure 2A). Similarly, the VR rates were similar in both treatment groups for patients with LLV (P > 0.05) (Figure 2B). Among the hepatitis B e antigen (HBeAg)-positive population, 74.36% of patients receiving TMF and 76.09% receiving TAF achieved HBV DNA less than 10 IU/mL (Figure 2C). In the HBeAg-negative population, 82.22% and 81.40% of patients in the TMF and TAF groups, respectively, achieved HBV DNA less than 10 IU/mL (Figure 2D).
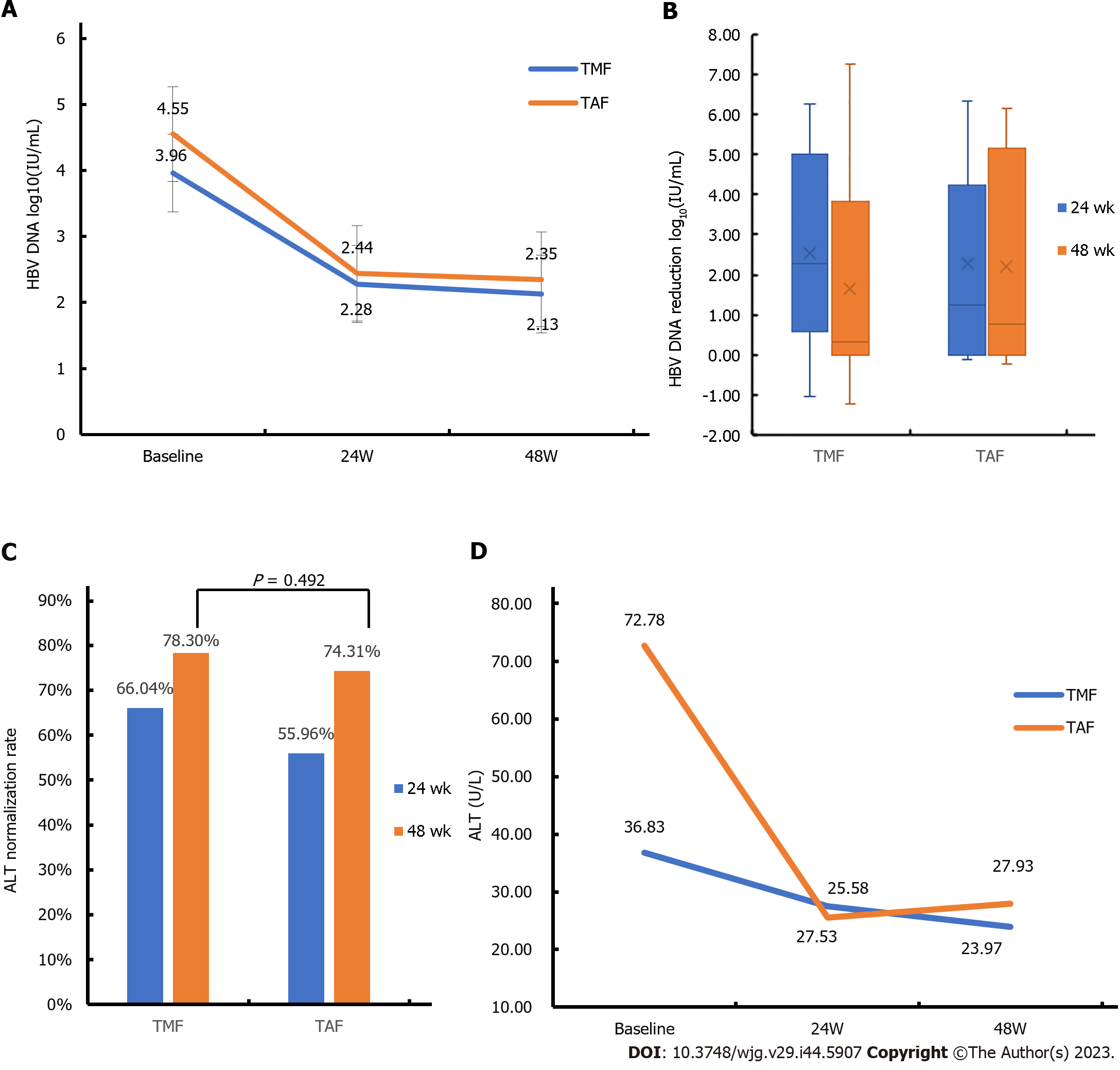
The carrying capacity of HBV DNA decreased from 3.96 ± 2.18 to 2.13 ± 0.84 Log10 (IU/mL) in the TMF group and from 4.55 ± 2.31 to 2.35 ± 1.33 Log10 (IU/mL) in the TAF group (Figure 3A). While the TMF group showed a similar VR within 48 wk compared with the TAF group, there was no statistical difference between the two groups (Figure 3B).
The ALT normalization rate in the TMF group was 66.04% and 78.30% at 24 and 48 wk, respectively. In the TAF group, the ALT normalization rate was 55.96% and 74.31% at 24 and 48 wk, respectively. Although the ALT normalization rate in the TAF group showed a higher trend compared to the TMF group from baseline to 48 wk, this difference did not reach statistical significance (Figure 3C). As shown in Figure 3D, both TMF and TAF groups had similar trends in ALT changes during the 48-wk period.
After 48 wk of treatment, Cr levels in the TMF group decreased from 79.50 (66.05, 91.00) μmol/L to 72.00 (63.00, 83.00) μmol/L, and that in the TAF group decreased from 83.30 (73.00, 93.90) μmol/L to 78.10 (61.00, 90.70) μmol/L. Meanwhile, eGFR in both groups increased slightly. However, there was no significant difference in the changes of Cr and eGFR between the two groups within 48 wk (Table 2).
| TMF group (n = 106) | TAF group (n = 109) | P value | |
| Creatinine (μmol/L) | |||
| Before treatment | 79.50 (66.05, 91.00) | 83.30 (73.00, 93.90) | 0.856 |
| After 48 wk | 72.00 (63.00, 83.00) | 78.10 (61.00, 90.70) | 0.194 |
| Reduction | 4.00 (-19.65, 19.50) | 3.37 (-7.96, 26.13) | 0.728 |
| P (baseline vs. 48 wk) | 0.053 | 0.105 | |
| eGFR (mL/min/1.73 m2) | |||
| Before treatment | 90.58 (79.84, 103.80) | 97.19 (87.35, 106.38) | 0.180 |
| After 48 wk | 106.37 (94.58, 113.15) | 105.17 (88.15,129.56) | 0.617 |
| Reduction | -2.22 (-9.72, 16.75) | -4.17 (-227.89, 7.67) | 0.093 |
| P (baseline vs. 48 wk) | 0.301 | 0.108 |
In this study, plasma lipids consisted primarily included triglycerides, TC, LDL, and HDL. There was no significant change observed in the triglycerides, HDL, and LDL levels at 48 wk. Specifically, in the TMF group, the TC levels demonstrated a mean change of -0.23 ± 0.71 mg/dL at the 48-wk mark (P = 0.822) (Table 3). Conversely, in the TAF group, TC values exhibited a continuous rise from 4.30 ± 1.54 mg/dL at baseline to 5.2 ± 0.99 mg/dL at week 48 (P = 0.045) (Table 3).
| TMF group (n = 106) | TAF group (n = 109) | P value | |
| Triglycerides (mmol/L) | |||
| Before treatment | 1.57 ± 0.82 | 1.65 ± 1.19 | 0.719 |
| After 48 wk | 2.16 ± 1.34 | 1.81 ± 0.87 | 0.931 |
| Reduction | -0.64 ± 1.02 | 0.19 ± 0.31 | 0.103 |
| P (baseline vs. 48 wk) | 0.099 | 0.359 | |
| Total cholesterol (mg/dl) | |||
| Before treatment | 4.83 ± 1.09 | 4.30 ± 1.54 | 0.173 |
| After 48 wk | 4.82 ± 1.52 | 5.20 ± 0.99 | 0.581 |
| Reduction | -0.23 ± 0.95 | -1.02 ± 1.18 | 0.182 |
| P (baseline vs. 48 wk) | 0.822 | 0.045 | |
| HDL (mmol/L) | |||
| Before treatment | 1.18 ± 0.21 | 1.10 ± 0.14 | 0.341 |
| After 48 wk | 1.43 ± 0.74 | 1.23 ± 0.31 | 0.977 |
| Reduction | -0.23 ± 0.71 | -0.09 ± 0.16 | 0.672 |
| P (baseline vs. 48 wk) | 0.430 | 0.225 | |
| LDL (mmol/L) | |||
| Before treatment | 3.19 ± 0.91 | 3.20 ± 0.94 | 0.877 |
| After 48 wk | 3.15 ± 1.18 | 3.40 ± 0.71 | 0.428 |
| Reduction | 0.10 ± 0.94 | -0.04 ± 0.9 | 0.791 |
| P (baseline vs. 48 wk) | 0.807 | 0.332 |
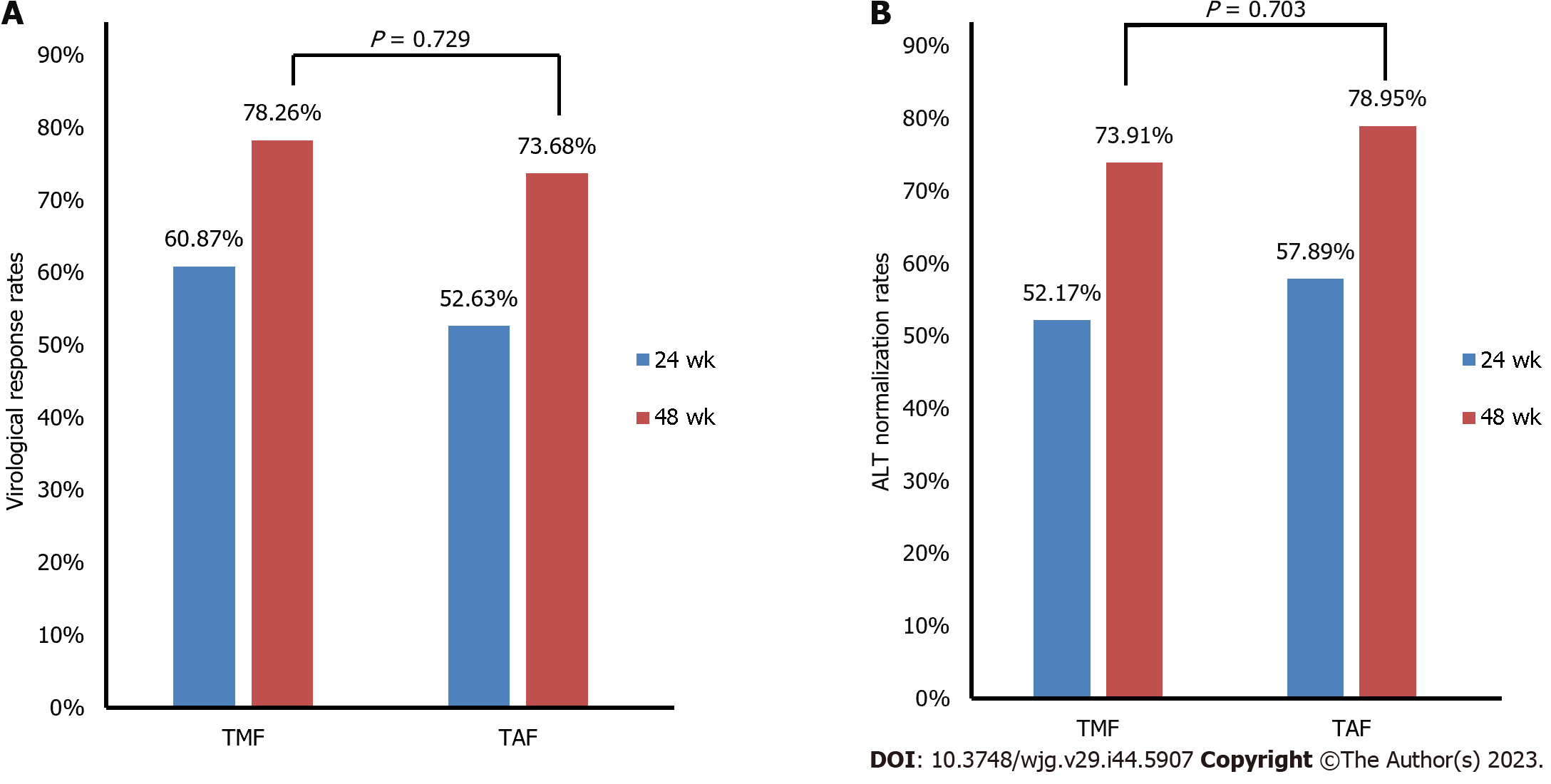
In our study, 23 patients in the TMF group and 19 patients in the TAF group had liver cirrhosis. The complete VR rates after 48 wk of treatment were 78.26% in the TMF group and 73.68% in the TAF group, with no significant difference between the two groups (Figure 4A). The normalization rate of ALT was similar in the two groups after 48 wk of treatment (Figure 4B). Renal safety profiles for TMF were similar to those observed for TAF at the 48-wk mark (Supplementary Table 1).
The regression of liver fibrosis was evaluated using FIB-4 scores and LSM in this study (Supplementary Table 2). Liver stiffness was measured using FibroScan. The LSM of cirrhotic patients in the TAF group decreased from baseline to the 48th week (P > 0.05). For the TMF cohort, both LSM and FIB-4 scores demonstrated a marginal increase after 48 wk of treatment; however, these differences did not reach statistical significance. In patients with liver cirrhosis, there was no significant difference between the two groups in the reduction of LSM from baseline level at the 48th week. However, the decrease in FIB-4 in patients with TAF was significantly greater than in patients with TMF [0.29 (0.06, 0.77) vs. -0.43 (1.16, -0.08); P = 0.001].
TFV ester prodrugs, a class of nucleotide analogs (NAs), are the first-line clinical anti-HBV drugs with potent antiviral efficacy, low resistance rates, and high safety. Various types of ester prodrugs of TFV have been designed in recent decades to improve its antiviral activity and reduce its adverse reactions[15,16].
TMF, the third commercially available TFV ester prodrug, was developed by modifying TAF through the addition of a single methyl group. It received approval from China’s National Medical Products Administration for the treatment of HBV infection in 2021. Clinical trials have demonstrated that TMF possesses superior plasma stability compared to TDF and exhibits a similar potency in inhibiting HBV, even when administered at a mere 1/30 of TDF’s dosage[17]. Another study, however, revealed that both TMF and TAF displayed enhanced anti-HBV activity and corrective effects on liver biochemical metabolism disturbances relative to TDF in vitro and in vivo, with TMF exhibiting marginally superior performance to TAF[11]. Given the relatively recent introduction of TMF to the Chinese market and the scarcity of real-world research data for the Chinese population, limited information exists regarding its safety and efficacy.
Consequently, we conducted a real-world investigation to assess the safety and effectiveness of TMF in treating patients with CHB in Southern China. Our findings indicated that, apart from a few mild side effects, TMF did not induce any serious reactions, thus establishing its safety for the treatment of CHB.
In our present study, we observed that the antiviral effectiveness of the TMF and TAF treatment groups was comparable across various patient subpopulations, including the general population, those with LLV, and HBeAg-positive and HBeAg-negative individuals. Throughout the 48-wk TMF treatment duration, no instances of virological breakthrough were encountered. In the 48th week, prior research demonstrated the sustained non-inferiority of VR rates between TMF and TDF treatments, regardless of HBeAg status[13]. Another study corroborated that TAF maintained its efficacy in inhibiting HBV replication relative to TDF, with no emergence of virologic resistance[18]. These findings align with our results, substantiating the equivalent antiviral potency of TMF and TAF in patients with CHB following 48 wk of therapy.
The significance of achieving on-treatment ALT normalization in CHB patients has been emphasized in recent literature. A large-scale observational study revealed that patients who attained normal on-treatment ALT in the first 48 wk of antiviral treatment exhibited a reduced risk of hepatic events[19]. Liu et al[13] reported a notably higher ALT normalization rate for TMF-treated patients compared to those receiving TDF. Concurrently, Agarwal et al[18] observed a significantly greater ALT normalization rate among CHB patients treated with TAF relative to TDF recipients. In contrast, our study established that, at week 48, the rate of ALT normalization in the TMF group was comparable to that in the TAF group. These findings, taken together with the virological inhibition rate and biochemical response, confirm the equivalent efficacy of TMF and TAF in the treatment of CHB patients over a 48-wk period.
Prior research has demonstrated the nephrotoxic and osteotoxic effects of TFV[20], emphasizing the need to consider nephrotoxicity when developing TFV prodrugs. Renal impairment associated with TDF primarily arises from proximal tubulopathy[21], with the ensuing tubular dysfunction evidenced by increased serum Cr and reduced serum phosphate levels. The superior renal safety profile of TAF, compared to TDF, is attributable to the primary elimination of TAF through fecal excretion, with less than 1% excreted renally[22].
Since all NAs are eliminated via the kidneys, it is crucial for clinicians to monitor for progression of renal dysfunction[23]. The ability of TAF to reduce the risk of renal damage renders it a favorable option for CHB patients who have potential or associated risk factors for renal damage. Studies have confirmed that TAF can continuously enhance renal function and maintain bone safety in patients with CHB[7,24]. Our findings indicate that the renal safety profile of the TMF group is comparable to that of the TAF group, suggesting that TMF could emerge as a novel therapeutic option for CHB patients, particularly those with an elevated risk of renal damage.
TDF and TAF are both efficacious nucleoside analogs, with TAF being preferred over TDF due to its lower incidence of renal and bone toxicities. However, there is evidence indicating a worsening of the lipid profile following the transition from TDF- to TAF-containing antiretroviral regimens in patients with HIV, as documented in clinical trials and observational studies[25,26]. Given the association between dyslipidemia, cardiovascular disease, and metabolic/non-alcoholic fatty liver disease-which may elevate the risk of HCC-it becomes imperative to determine whether TAF monotherapy alone adversely affects lipid profiles in CHB patients. This study compared lipid profile alterations in a cohort of CHB patients managed with either TMF or TAF over a 48-wk observation period. The findings indicated a significant increase in serum TC levels in the TAF group (4.3 ± 1.54 vs. 5.2 ± 0.99, mg/dL, P < 0.05) compared to the TMF cohort (4.83 ± 1.09 vs. 4.82 ± 1.52 mg/dL, P > 0.05). Therefore, this study suggests that TAF might contribute to the worsening of lipid profiles, whereas TMF appears to have a negligible impact on serum lipids. These conclusions are in contrast with the findings presented by Li et al[27] Despite these insights, the underlying mechanism by which TFV affects serum lipids remains to be elucidated. Further research is essential to fully understand this aspect. Nevertheless, physicians should monitor lipid levels vigilantly in patients at the higher end of the normal range when prescribing TAF.
Chronic HBV infection constitutes the primary cause of liver cirrhosis in China and may progress to decompensated liver cirrhosis and primary liver cancer, severely impacting the quality of life of patients. An increasing body of evidence indicates that sustained and effective antiviral therapy can reverse liver fibrosis and cirrhosis[4,28]. Therefore, our study evaluated the efficacy and safety of treatment in patients with cirrhosis.
In cirrhotic patients, the FIB-4 score reduction observed in the TAF cohort was significantly more pronounced than that in the TMF cohort. This could be partly attributed to the marginally higher ALT normalization rate associated with TAF treatment and the limited sample size of both groups. In contrast, no significant difference was discerned in the LSM values between the TMF and TAF groups. However, implications of these findings are not entirely clear, as it remains uncertain if the changes reflect true fibrosis regression or merely a biochemical variation. The observed decline in FIB-4 scores is noteworthy, warranting further research to ascertain if such biochemical alterations correspond to actual histological improvements. Where appropriate, liver tissue biopsies should be considered for conclusive evidence.
Our study is not without limitations. Firstly, the follow-up period of 48 wk may be insufficient to fully capture the antiviral effect, and a more extended timeframe would provide a clearer representation. Secondly, serum Cr and eGFR were employed as markers of renal function in this study, but incorporating indicators reflecting renal tubular function could bolster the study’s reliability based on established clinical pharmacological research. Thirdly, the applicability of our findings is restricted, as TMF is not available worldwide. Fourthly, our study did not include data on bone health, such as that obtained via DEXA scans, and relied on serum Cr as a surrogate marker for renal function. Lastly, as a single-center retrospective study, future multi-center investigations with larger cohort and longer follow-up durations for CHB patients are essential to corroborate our findings.
In summary, our results indicate that TMF demonstrates comparable efficacy to TAF in terms of VR, ALT normalization rate, and renal safety among CHB patients in China. Nevertheless, TMF has an advantage over TAF in patients with hyperlipidemia. Additionally, TMF exhibits effectiveness and safety in cirrhotic patients. Collectively, these results suggest that TMF presents a viable therapeutic alternative for patients with CHB.
Hepatitis B virus (HBV) infection may lead to cirrhosis and hepatocellular carcinoma, and the exploration of optimal antiviral drugs can improve patient prognosis.
Tenofovir amibufenamide (TMF) is a new antiviral drug with limited research on its safety and efficacy. Our research may provide new evidence for the treatment of patients with HBV infection.
To compare the efficacy and safety of TMF and tenofovir alafenamide (TAF) for 48 wk in patients with chronic hepatitis B (CHB). The primary outcome was the proportion of virological responses (VR) at 48 wk. Additional outcomes included the changes of renal function and lipid characteristic markers at weeks 24 and 48 compared to baseline.
In this retrospective study, we enrolled a total of 587 patients who had been HBsAg positive for more than 6 mo. Of the enrolled patients, 215 were included in the final analysis and were divided into two groups based on their drug selection: The TMF group and the TAF group.
The VR rates of the TMF group and TAF group were comparable at 24 and 48 wk of treatment (P > 0.05). In patients with low-level viremia, hepatitis B e antigen (HBeAg) positive, and HBeAg negative, their VR rates are also similar. The alanine transaminase (ALT) normalization rate and renal safety of TMF are also comparable to those of TAF. However, total cholesterol levels increased in the TAF group (P = 0.045). In patients with liver cirrhosis, the renal safety, VR, and ALT normalization rate were comparable between the TMF group and the TAF group.
TMF is as effective as TAF in treating CHB and has considerable safety. Moreover, TMF may have more advantages in lipid profile compared to TAF.
The design and research of new nucleotide analogs should continue in the hope of achieving clinical cure of hepatitis B infection as soon as possible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martinez-Camacho A, United States; Mucenic M, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Hepatitis B Fact Sheet. World Health Organization. 2021. Available from: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b. |
| 2. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J; Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3798] [Article Influence: 474.8] [Reference Citation Analysis (1)] |
| 4. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1958] [Article Influence: 217.6] [Reference Citation Analysis (0)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2840] [Article Influence: 405.7] [Reference Citation Analysis (0)] |
| 6. | Jung CY, Kim HW, Ahn SH, Kim SU, Kim BS. Tenofovir is Associated With Higher Risk of Kidney Function Decline Than Entecavir in Patients With Chronic Hepatitis B. Clin Gastroenterol Hepatol. 2022;20:956-958.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, Hui AJ, Janssen HL, Chowdhury A, Tsang TY, Mehta R, Gane E, Flaherty JF, Massetto B, Gaggar A, Kitrinos KM, Lin L, Subramanian GM, McHutchison JG, Lim YS, Acharya SK, Agarwal K; GS-US-320-0110 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 8. | Murakami E, Wang T, Park Y, Hao J, Lepist EI, Babusis D, Ray AS. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59:3563-3569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Chinese Society of Hepatology; Chinese Medical Association. [Expert opinion on expanding anti-HBV treatment for chronic hepatitis B]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Mehellou Y, Rattan HS, Balzarini J. The ProTide Prodrug Technology: From the Concept to the Clinic. J Med Chem. 2018;61:2211-2226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 11. | Hong X, Cai Z, Zhou F, Jin X, Wang G, Ouyang B, Zhang J. Improved pharmacokinetics of tenofovir ester prodrugs strengthened the inhibition of HBV replication and the rebalance of hepatocellular metabolism in preclinical models. Front Pharmacol. 2022;13:932934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 12. | Liu Z, Jin Q, Zhang Y, Gong G, Wu G, Yao L, Wen X, Gao Z, Huang Y, Yang D, Chen E, Mao Q, Lin S, Shang J, Gong H, Zhong L, Yin H, Wang F, Hu P, Xiao L, Li C, Wu Q, Sun C, Niu J, Hou J; TMF Study Group. Randomised clinical trial: 48 weeks of treatment with tenofovir amibufenamide versus tenofovir disoproxil fumarate for patients with chronic hepatitis B. Aliment Pharmacol Ther. 2021;54:1134-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Jin Q, Zhang Y, Gong G, Wu G, Yao L, Wen X, Gao Z, Huang Y, Yang D, Chen E, Mao Q, Lin S, Shang J, Gong H, Zhong L, Yin H, Wang F, Hu P, Wu Q, Pan C, Jia W, Li C, Sun C, Niu J, Hou J; TMF Study Group. 96-Week Treatment of Tenofovir Amibufenamide and Tenofovir Disoproxil Fumarate in Chronic Hepatitis B Patients. J Clin Transl Hepatol. 2023;11:649-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3561] [Article Influence: 187.4] [Reference Citation Analysis (0)] |
| 15. | Beadle JR, Aldern KA, Zhang XQ, Valiaeva N, Hostetler KY, Schooley RT. Octadecyloxyethyl benzyl tenofovir: A novel tenofovir diester provides sustained intracellular levels of tenofovir diphosphate. Antiviral Res. 2019;171:104614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Wang A, Wu S, Tao Z, Li X, Lv K, Ma C, Li Y, Li L, Liu M. Design, Synthesis, and Anti-HBV Activity of New Bis(l-amino acid) Ester Tenofovir Prodrugs. ACS Med Chem Lett. 2019;10:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Zhang H, Hu Y, Wu M, Liu J, Zhu X, Li X, Chen H, Li C, Liu C, Niu J, Ding Y. Randomised clinical trial: safety, efficacy and pharmacokinetics of HS-10234 versus tenofovir for the treatment of chronic hepatitis B infection. Aliment Pharmacol Ther. 2021;53:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Agarwal K, Brunetto M, Seto WK, Lim YS, Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, Sharma M, Janssen HLA, Pan CQ, Çelen MK, Furusyo N, Shalimar D, Yoon KT, Trinh H, Flaherty JF, Gaggar A, Lau AH, Cathcart AL, Lin L, Bhardwaj N, Suri V, Mani Subramanian G, Gane EJ, Buti M, Chan HLY; GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. J Hepatol. 2018;68:672-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 19. | Wong GL, Chan HL, Tse YK, Yip TC, Lam KL, Lui GC, Wong VW. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Wong GL, Seto WK, Wong VW, Yuen MF, Chan HL. Review article: long-term safety of oral anti-viral treatment for chronic hepatitis B. Aliment Pharmacol Ther. 2018;47:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Samuels R, Bayerri CR, Sayer JA, Price DA, Payne BAI. Tenofovir disoproxil fumarate-associated renal tubular dysfunction: noninvasive assessment of mitochondrial injury. AIDS. 2017;31:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Gilead Sciences. Prescribing information for VEMLIDY (tenofovir alafenamide). Available from: https://www.gilead.com/-/media/files/pdfs/medicines/liver-disease/vemlidy/vemlidy_pi.pdf. |
| 23. | Lo AO, Wong GL. Current developments in nucleoside/nucleotide analogues for hepatitis B. Expert Rev Gastroenterol Hepatol. 2014;8:607-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL, Acharya SK, Flaherty JF, Massetto B, Cathcart AL, Kim K, Gaggar A, Subramanian GM, McHutchison JG, Pan CQ, Brunetto M, Izumi N, Marcellin P; GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 25. | Kauppinen KJ, Kivelä P, Sutinen J. Switching from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide Significantly Worsens the Lipid Profile in a Real-World Setting. AIDS Patient Care STDS. 2019;33:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Hagins D, Orkin C, Daar ES, Mills A, Brinson C, DeJesus E, Post FA, Morales-Ramirez J, Thompson M, Osiyemi O, Rashbaum B, Stellbrink HJ, Martorell C, Liu H, Liu YP, Porter D, Collins SE, SenGupta D, Das M. Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Med. 2018;19:724-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Li L, Zhou J, Li Y, Wang F, Zhang D, Wang M, Tao Y, Chen E. Effectiveness and safety of tenofovir amibufenamide and its comparison with tenofovir alafenamide in patients with chronic hepatitis B: results from a retrospective real-world study. Front Pharmacol. 2023;14:1165990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 720] [Cited by in RCA: 749] [Article Influence: 68.1] [Reference Citation Analysis (0)] |