Published online Nov 28, 2023. doi: 10.3748/wjg.v29.i44.5872
Peer-review started: October 8, 2023
First decision: November 1, 2023
Revised: November 6, 2023
Accepted: November 14, 2023
Article in press: November 14, 2023
Published online: November 28, 2023
Processing time: 50 Days and 14.2 Hours
Anxiety is common in patients with inflammatory bowel disease (IBD), including those with ulcerative colitis (UC) and Crohn’s disease (CD); however, the causal relationship between IBD and anxiety remains unknown.
To investigate the causal relationship between IBD and anxiety by using bidirectional Mendelian randomization analysis.
Single nucleotide polymorphisms retrieved from genome-wide association studies (GWAS) of the European population were identified as genetic instrument variants. GWAS statistics for individuals with UC (6968 patients and 20464 controls; adults) and CD (5956 patients and 14927 controls; adults) were obtained from the International IBD Genetics Consortium. GWAS statistics for individuals with anxiety were obtained from the Psychiatric Genomics Consortium (2565 patients and 14745 controls; adults) and FinnGen project (20992 patients and 197800 controls; adults), respectively. Inverse-variance weighted was applied to assess the causal relationship, and the results were strengthened by heterogeneity, pleiotropy and leave-one-out analyses.
Genetic susceptibility to UC was associated with an increased risk of anxiety [odds ratio: 1.071 (95% confidence interval: 1.009-1.135), P = 0.023], while genetic susceptibility to CD was not associated with anxiety. Genetic susceptibility to anxiety was not associated with UC or CD. No heterogeneity or pleiotropy was observed, and the leave-one-out analysis excluded the potential influence of a particular variant.
This study revealed that genetic susceptibility to UC was significantly associated with anxiety and highlighted the importance of early screening for anxiety in patients with UC.
Core Tip: Our study provides evidence that genetic susceptibility to ulcerative colitis (UC) is associated with an increased risk of anxiety [odds ratio: 1.071 (95% confidence interval: 1.009, 1.135), P = 0.023], while genetic susceptibility to Crohn’s disease (CD) is not associated with an increased risk of anxiety. No causal effects of anxiety on UC and CD were observed in this study. In conclusion, our study demonstrates the causal effect of UC on anxiety. These findings may be helpful to increase physicians’ awareness of the need to recognize anxiety in UC patients and influence the management of anxiety in clinical practice.
- Citation: He Y, Chen CL, He J, Liu SD. Causal associations between inflammatory bowel disease and anxiety: A bidirectional Mendelian randomization study. World J Gastroenterol 2023; 29(44): 5872-5881
- URL: https://www.wjgnet.com/1007-9327/full/v29/i44/5872.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i44.5872
Inflammatory bowel disease (IBD), mainly composed of ulcerative colitis (UC) and Crohn’s disease (CD), is a debilitating chronic inflammatory disease with varying degrees of severity[1,2]. UC and CD influence not only the gastrointestinal tract but also other systems[3]. Emerging evidence has reported the role of the gut-brain axis in the interactions between gastrointestinal diseases and neuropsychiatric disorders[4,5]. Anxiety is a common comorbidity in patients with IBD (prevalence varies from 19.1% to 35.1%) compared with the general population (3.4%)[4,6]. The association between IBD and anxiety, or vice versa, has received considerable attention due to the putative pathophysiological mechanisms regulated by the gut-brain axis.
Some observational studies have investigated the temporal relationship between IBD and anxiety and have suggested that the relationship between IBD and anxiety may be bidirectional[7]. Patients with IBD might be at higher risk for anxiety than control individuals. Specifically, newly diagnosed patients with IBD had a rising prevalence (incidence rate ratio: 1.39) of anxiety when compared with matched control individuals during 10 years of follow-up[7]. However, some observational studies have shown that patients with anxiety are prone to suffering from IBD[8,9]. A cohort study suggested a higher prevalence of IBD in patients with newly diagnosed anxiety compared with control individuals during 6.7 years of follow-up[9]. Collectively, the existing findings from observational studies demonstrated the bidirectional relationships between IBD and anxiety, which is partly influenced by residual confounders. Therefore, more evidence is needed to clarify the causal relationships between IBD and anxiety. A recent Mendelian randomization (MR) study inferred the causal relationships between IBD and depression and demonstrated a causal effect of depression on IBD but no causal effect of IBD on depression[10]. Depression and anxiety are common co-occurrence in IBD, while the causality between IBD and anxiety has not been investigated.
MR is a genetic approach to estimate causality between the exposure and the outcome by using genetic instrument variants (IVs) identified through genome-wide association studies (GWAS), usually using single-nucleotide polymorphisms (SNPs) as IVs. Since genetic makeup is assigned at conception and is unlikely to be affected by disease later in life, unidirectional causality can be deduced by MR analysis. Potential confounders that could affect the outcomes were eliminated from the analysis, effectively forming naturally blinded randomized controlled trials[11]. Therefore, this study aimed to evaluate the causal associations between IBD and anxiety by performing bidirectional two-sample MR analysis.
The International IBD Genetics Consortium (IIBDGC), a large-scale consortium consisting of hundreds of researchers from more than 20 countries worldwide, collects GWAS data from over 75000 patients with IBD. The IIBDGC is an authoritative organization aimed at identifying genetic risk factors for IBD. GWAS summary statistics for UC and CD were obtained from the IIBDGC[12], which contains adult individuals of European descent with UC (6968 patients, 20464 control individuals) and CD (5956 patients, 14927 control individuals) (Supplementary Table 1). UC and CD were diagnosed by physicians based on comprehensive evidence of clinical symptoms, endoscopic findings, and histopathological and imaging results.
GWAS summary statistics for anxiety were obtained from two separate databases (Supplementary Table 1): (1): The Psychiatric Genomics Consortium (PGC) (https://pgc.unc.edu/) from Otowa et al[13]; and (2) the FinnGen project (https://www.finngen.fi/en). The PGC[14], the largest international psychiatric consortium consisting of more than 800 investigators from 38 countries, is dedicated to finding the genetic variants of psychiatric disorders. The FinnGen project is an academic-industrial collaboration aimed at deciphering genotype-phenotype relationships from more than 500000 Finnish participants. The participants in the GWAS databases for anxiety were adults of European descent. The number of patients and control individuals for anxiety were 2565/14745 in PGC and 20992/197800 in FinnGen, respectively.
We used published GWAS statistics and did not collect initial data. Patient informed consent and ethics approval were not needed for this study, as these materials had already been obtained in each of the preliminary studies.
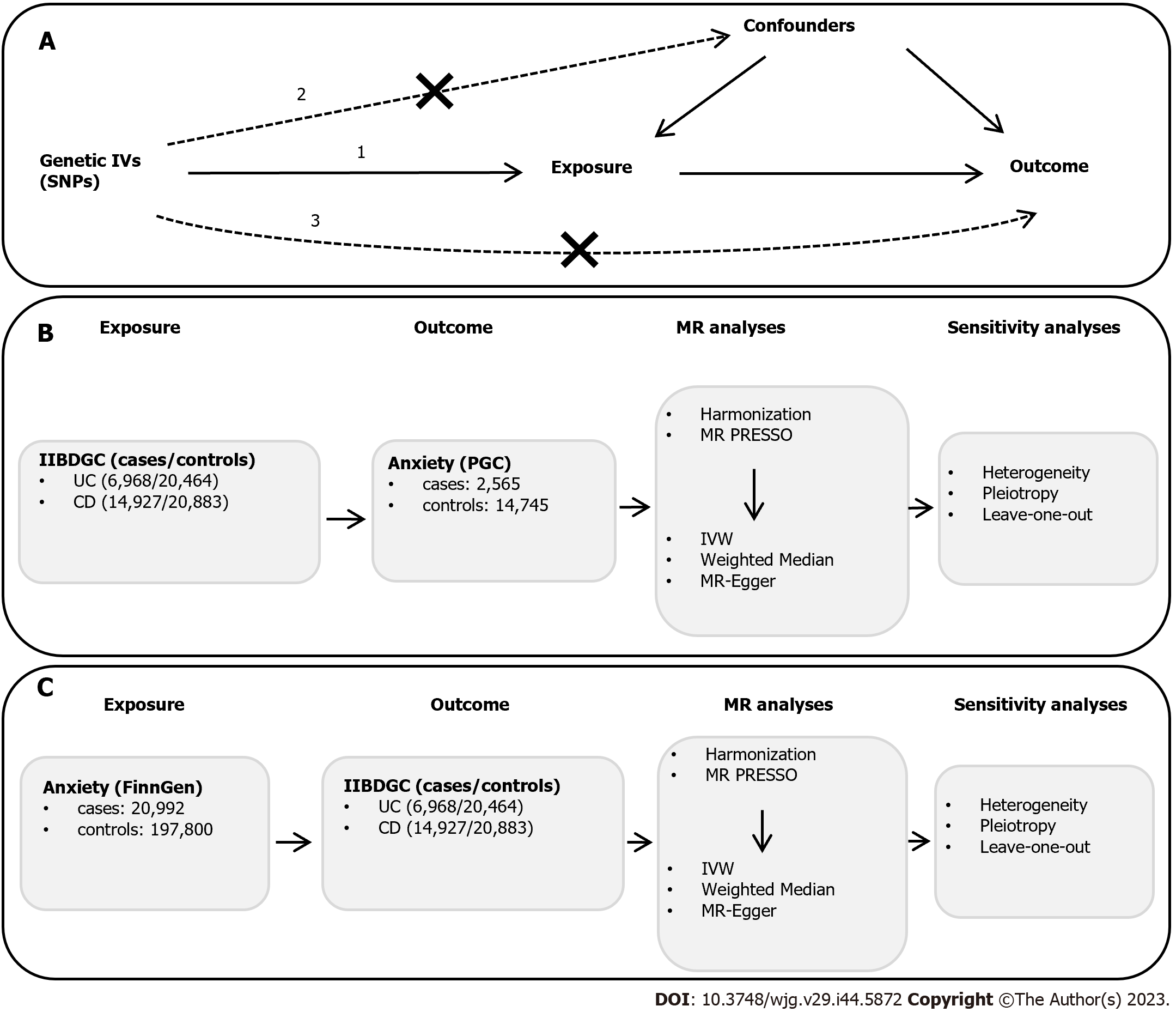
To select eligible SNPs as IVs from the GWAS statistics, a series of quality control steps were applied. The three following assumptions must be satisfied[11]: (1) Correlation assumption: The IV is strongly correlated with the exposure; (2) independence assumption: The IV does not influence the outcome through the confounding factors; and (3) exclusion assumption: the IV does not directly influence the outcome, but only influences the outcome via indirect exposure (Figure 1).
To satisfy the correlation assumption, the following criteria were set for identifying instrumental SNPs: (1): Genome-wide strongly significant (F > 10, P < 5 × 10-8) association with the exposure. For a single variant, the F statistic, which should be over 10 to avoid weak instrument bias, was calculated by the following equation[15]: F = [β/se]2, where β means estimated effect size and se means standard error of β; and (2) independent SNPs are selected by linkage disequilibrium (LD) clumping (r2 < 0.001, window size = 1 Mb). To satisfy the independence and exclusion assumption, we checked each SNP associated with the exposure at PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/) and eliminated SNPs significantly related to the potential confounders and the outcome. The potential confounders that may influence anxiety include smoking, body mass index, neuropsychiatric disease, drinking, and hypertension[16,17]. The potential confounders that may influence UC or CD include smoking, body mass index, and intestinal malabsorption[18,19]. The subsequent harmonization process was used to unify the effect direction and effect allele, ensure SNPs with a minor allele frequency (> 0.01), and remove the palindromic and incompatible SNPs.
For MR estimation from UC or CD to anxiety, the selection criteria of SNPs associated with UC or CD satisfy the abovementioned three assumptions. For MR estimation from anxiety to UC or CD, no eligible SNPs associated with anxiety could be obtained from GWAS statistics in PGC after LD clumping, so we used GWAS statistics from the FinnGen project to extract anxiety-related SNPs. Because anxiety-related SNPs could not be obtained by the statistical P value of < 5 × 10-8, we used a suggested P value of < 5 × 10-6 to extract SNPs, which had been applied in a previous study to decipher bidirectional relationships between prescription opioid use and anxiety risk by MR analysis[20].
The MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) test was used to detect the outlier SNPs in MR analysis[21]. The MR-PRESSO test consists of three main parts: (1) Detecting horizontal pleiotropy; (2) correcting horizontal pleiotropy by removing the outlier; and (3) testing significant distortion in the causal estimates before and after removing the outlier. The MR-PRESSO test requires that more than 50% of the SNPs are efficient IVs with balanced pleiotropy. The number of distributions was set to 3000 in the MR-PRESSO test.
Three different methods [inverse-variance weighted (IVW), MR Egger, and weighted median] were used in the MR analysis[15]. IVW, the most efficient causal estimation method allowing balanced pleiotropy in MR analysis, was used as the main method[22]. The assessments by the IVW method are efficient, consistent and close to the true effect when the sample size of IVs is large enough and the pleiotropy of IVs is not significant[23]. If no significant heterogeneity (IVW-derived Cochran Q statistic P ≥ 0.05) was detected, the fixed-effects IVW model was adopted; otherwise, the random-effects IVW model was applied. Since unbalanced pleiotropy may result in bias to the causal estimates by IVW, supplementary MR and sensitivity analyses are usually needed to verify the robustness of causal estimation in MR analysis[24]. MR Egger and weighted median were applied as supplementary MR methods to verify the causal estimates obtained by the IVW method[15,25]. Although they have less statistical power [wider confidence intervals (CIs)], they can provide more robust and reliable causal estimations across a wider range of scenarios. All statistical analyses were conducted using R (version 4.2.1), the Two-Sample MR package (version 0.5.6), and the MR-PRESSO package (version 1). P < 0.05 was considered indicative of statistical significance.
The MR Egger method was applied to assess the potential horizontal pleiotropy of SNPs. If the intercept P value was < 0.05, there was significant pleiotropy. Heterogeneity was tested by the IVW-derived Cochran Q statistic. A Cochran Q statistic P value of < 0.05 was considered to indicate the presence of significant heterogeneity. Scatter plots were used to visualize the results from MR analysis to show efficiency and reliability. The leave-one-out analysis aimed to verify that a single SNP does not affect the results by eliminating a single SNP one by one and performing MR analysis on the remaining SNPs.
First, the abovementioned three assumptions were used to identify the genetic IVs (SNPs) of the exposure. Second, after the harmonization process, MR-PRESSO analysis was applied to detect and remove the outlier SNPs. Third, after the outlier SNPs were removed, MR analysis was performed, and subsequently, sensitivity analyses were conducted to determine whether pleiotropy existed and which IVW model was adopted according to heterogeneity. A schematic overview of the procedure is detailed in Figure 1.
Data for 34652 individuals of European descent who participated in the GWAS cohorts were obtained from seven CD and eight UC collections with combined genome-wide SNP data. After LD clumping and harmonization processes were completed, the MR-PRESSO test did not detect any CD- or UC-related outlier SNPs. Finally, 46-51 confounder-independent CD-related SNPs and 34-36 UC-related SNPs were identified to evaluate the causal effects on anxiety (Table 1). Twenty-one anxiety-related SNPs from the FinnGen database were obtained to evaluate the causal effects on UC and CD (Table 1). The single-variant F statistic was calculated, and the F statistic values were all over 10, indicating that these selected SNPs were strongly associated with the exposures. Independent UC-related SNPs are listed in Supplementary Table 2, CD-related SNPs are listed in Supplementary Table 3, and anxiety-related SNPs are listed in Supplementary Tables 4 and 5.
| Exposure | Outcome | SNPs (n) | IVW | Weighted median | MR Egger | |||||
| OR | P value | Cochran’s Q | OR [95% CI] | P value | OR [95%CI] | P value | Intercept P value | |||
| UC | Anxiety | 34 | 1.071 (1.009-1.135) | 0.023 | 0.709 | 1.064 (0.981-1.154) | 0.131 | 1.138 (0.945-1.371) | 0.182 | 0.501 |
| CD | Anxiety | 46 | 1.005 (0.960-1.052) | 0.825 | 0.713 | 1.027 (0.960-1.100) | 0.435 | 1.023 (0.914-1.145) | 0.689 | 0.734 |
| Anxiety | UC | 21 | 0.920 (0.779-1.086) | 0.325 | 0.858 | 0.820 (0.654-1.027) | 0.085 | 0.807 (0.594-1.096) | 0.187 | 0.333 |
| Anxiety | CD | 21 | 1.012 (0.851-1.204) | 0.892 | 0.808 | 0.998 (0.771-1.291) | 0.989 | 1.016 (0.744-1.388) | 0.919 | 0.974 |
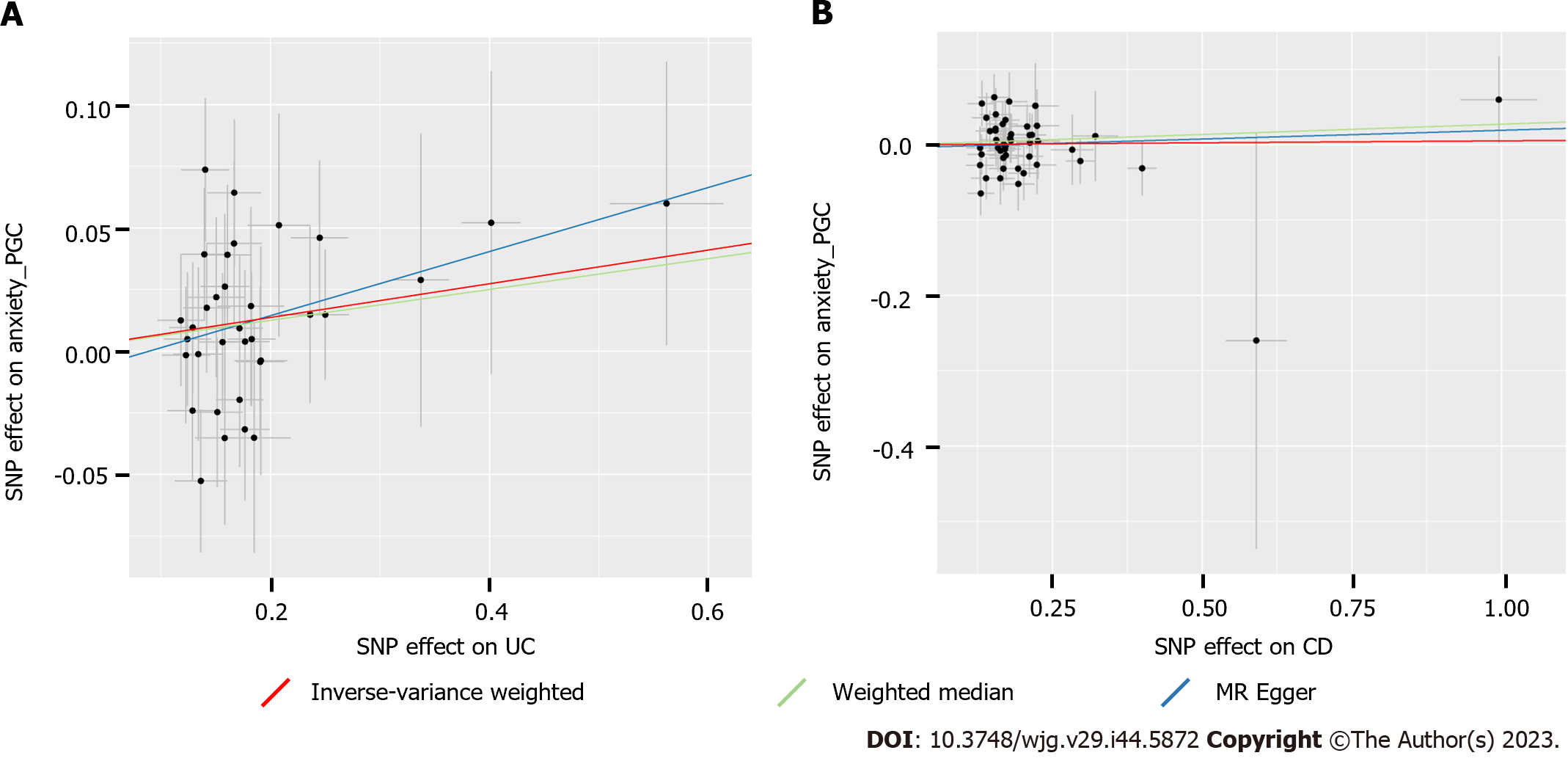
We used IVW as the primary method to assess the causal estimates. We only observed a causal effect of UC on anxiety [odds ratio (OR): 1.071, 95%CI: 1.009-1.135, P = 0.0226] (Table 1). However, CD had no causal effect on anxiety. Additionally, MR Egger and weighted median methods verified the reliability of the causality estimated by IVW. Even though the MR Egger-derived P value and weighted median-derived P value were over 0.05, the ORs from MR Egger (OR = 1.138; 95%CI: 0.945-1.371) and weighted median (OR = 1.064; 95%CI: 0.981-1.154) methods were in the same direction as IVW estimation from UC to anxiety, indicating the robustness of causality (Table 1). Moreover, the supplementary MR analysis also did not provide evidence for the causal effect of CD on anxiety.
Heterogeneity was tested by the IVW-derived Cochran’s Q statistic. The results showed that no statistically significant heterogeneity (all P > 0.05) was observed for the causal effects of UC and CD on anxiety (Table 1). Thus, we used the fixed-effects IVW model in the MR analysis. In addition, the effects of individual UC- or CD-related SNPs on anxiety are presented in scatter plots (Figure 2). The horizontal pleiotropic effect was performed by the MR Egger method to determine whether genetic IVs associated with UC or CD could affect anxiety through other potential pathways. Significant horizontal pleiotropy was not observed in our MR analysis (Table 1), which verified the robustness and credibility of the IVW-derived causal estimates. After one-by-one removal of each individual SNP, the following causal estimates of the remaining SNPs on anxiety were tested by leave-one-out analysis, which was consistent with the results of MR analysis and demonstrated that the causal effects were unlikely to be caused by any individual SNP (Supplementary Figure 1). Overall, our MR analysis showed that genetic susceptibility to UC, but not CD, was associated with an increased risk of anxiety.
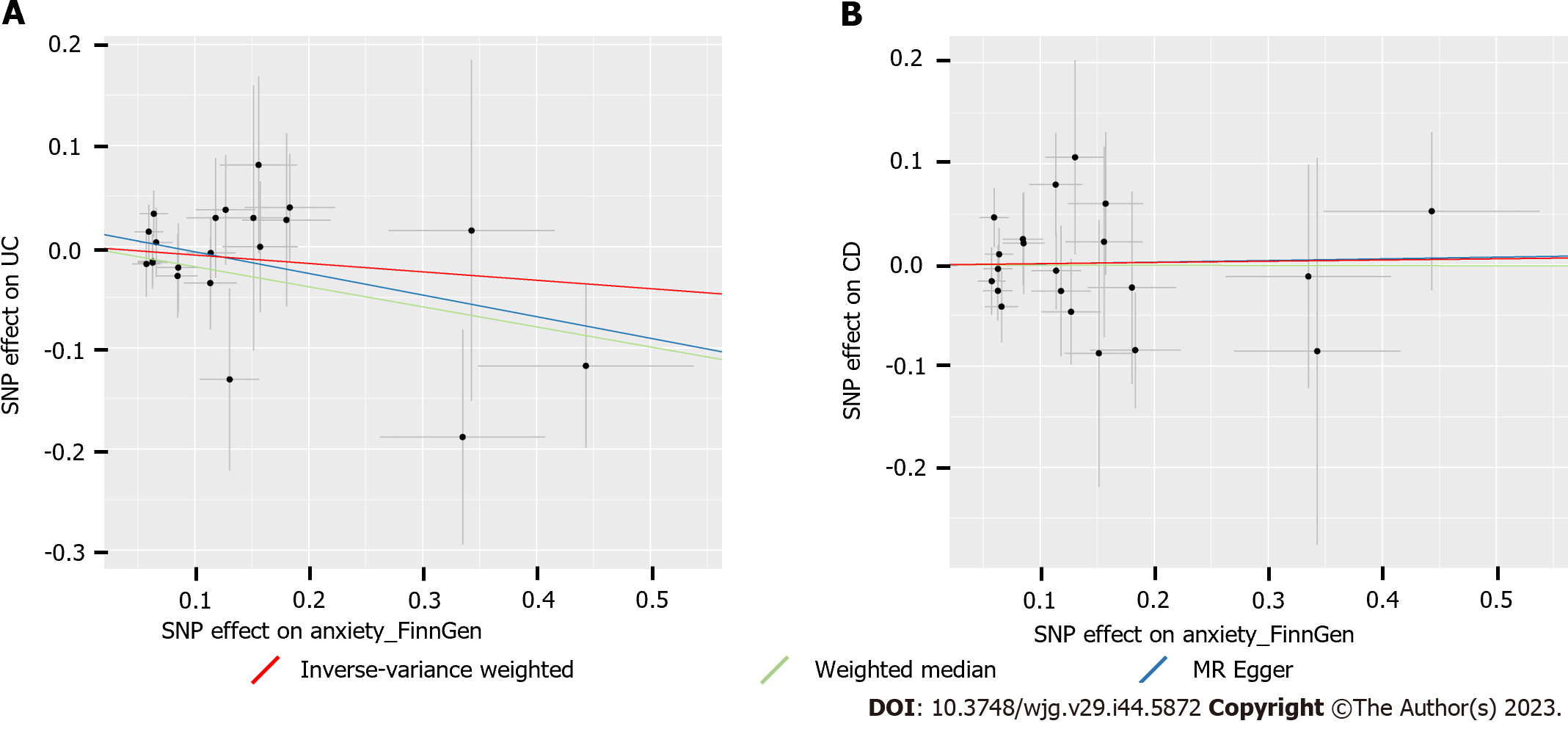
Both IVW and the supplementary methods showed no causal effects (all P > 0.05) of anxiety on UC or CD (Table 1). In addition, no heterogeneity or horizontal pleiotropy (all P > 0.05) was observed in the sensitivity analyses (Table 1), which indicated that these MR estimations were reliable and robust (Figure 3). The leave-one-out analysis also suggested that the MR analysis was reliable (Supplementary Figure 2). Taken together, our MR analysis suggested that genetic susceptibility to anxiety was not associated with UC or CD.
In this study, we estimated the bidirectional causal relationships between IBD and anxiety by MR analysis. Our results suggested that genetic susceptibility to UC, but not CD, was associated with anxiety; however, genetic susceptibility to anxiety was not associated with UC or CD.
Previous observational studies showed that IBD was a risk factor for anxiety. A recent meta-analysis showed that the pooled prevalence of anxiety in IBD patients was 12% (95%CI, 8%-18%)[26]. Another population-based cohort study in the United Kingdom demonstrated that young IBD patients had a significantly higher incidence and risk of anxiety (adjusted hazard ratio, 1.25; 95%CI, 1.06-1.48)[27]. In addition, two large nationwide cohort studies showed that anxiety was more commonly seen in both patients with adult-onset IBD and those with childhood-onset IBD[28,29]. However, the abovementioned results could not be used to clarify the causality and directionality of the relationship between IBD and anxiety. Our study is the first to estimate the causal associations between IBD and anxiety using MR analysis and provides evidence that UC has a causal effect on anxiety.
Some studies observed that CD patients had a higher OR value of anxiety symptoms than UC patients[30-32]. However, in our study, genetic susceptibility to UC but not CD was associated with an increased risk of anxiety. This result may be explained as follows: (1) UC and CD are two different diseases of the gut, with UC mainly restricted to the colonic mucosa, whereas CD involves the immune response of the entire gastrointestinal tract. Differences in the distribution of the enteric nervous system in the gastrointestinal tract may affect the function of different brain regions via the gut-brain axis[33,34]; and (2) discrepancies in gut microbiota and immune cell populations between UC and CD may have different influences on the brain through the microbiota-gut-brain axis[35-37]. These results reminded us that many factors other than genetic predisposition to IBD can also increase the risk of developing anxiety during the progression of IBD.
The biological route from UC or CD to anxiety has not yet been fully clarified. Increased evidence indicates that the gut-brain axis regulated by inflammation can affect neuronal development and subsequent behavioral phenotypes[5,38,39]. Circulating leukocytes and cytokines can reach the brain by crossing the blood-brain barrier, even leading to neuropsychiatric disorders[40]. For example, induced colitis in mice can result in increased levels of circulating cytokines, which influence certain brain regions, especially the hippocampus[41,42]. The hippocampus is related to memory and emotions, and damage to the hippocampus is closely associated with anxiety and depression[18,43-45]. Additionally, the gut microbiota plays a critical role in the interconnections between the gut and the brain[46,47]. The key routes or mediators between the gut microbiota and the brain are the enteric vagus nervous system, tryptophan metabolites, and microbial products[38,48]. Therefore, dysregulated inflammation and gut microbiota in patients with UC may lead to the progression of neuropsychiatric conditions, such as anxiety.
There are two main strengths in this study. First, this is the first study to assess the causal associations between IBD and anxiety using rigorous MR analysis. Second, the large-scale GWAS summary statistics for UC, CD and anxiety were all obtained from individuals of European descent, which would avoid the bias caused by a sample of individuals with different ethnicities. Meanwhile, some limitations should be considered. First, patients with CD or UC came from different medical units, and discrepancies in diagnostic approaches, data collection, and data processing may generate bias. Second, stratification analyses, especially those for sex, diverse severity, age, and drug use, are not viable due to using summary statistics for MR analysis. Third, all GWAS statistics included in the MR analysis came from European individuals; thus, the results of this study might not be generalizable to other ethnic groups.
Although this study investigated the causal relationship between IBD and anxiety, the precise biological mechanisms by which UC affects the development of anxiety remain unclear, such as whether and how the gut-brain axis plays a role. Hence, more basic and clinical studies regarding the identification of key regulators and pathways are needed to further uncover the biological mechanisms.
This study revealed that genetic susceptibility to UC was significantly associated with anxiety and highlighted the importance of early screening for anxiety in patients with UC, which may be helpful to strengthen physicians’ awareness of recognizing anxiety.
Inflammatory bowel disease (IBD), mainly consisted of Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disease. Depression and anxiety are common co-occurrence in IBD. A recent Mendelian randomization (MR) study has inferred the causal effect of depression on IBD, while the causality between IBD and anxiety has not been investigated.
Previous observational studies showed that IBD patients had a significantly higher incidence and risk of anxiety. Despite the existing findings demonstrated the bidirectional relationship between IBD and anxiety, the causal association between them remain unclear. This study seeks to find out causal association between IBD and anxiety from the genetic perspective by using MR analysis, potentially offering new insights into the pathogenesis and clinical significance of anxiety in IBD.
The study aims to investigate the causal relationship between IBD and anxiety by performing bidirectional MR analysis, to better understand the gene susceptibility of anxiety in IBD.
Single nucleotide polymorphisms retrieved from genome-wide association studies (GWAS) were identified as instrument variants. GWAS statistics for UC and CD were obtained from the International IBD Genetics Consortium. GWAS statistics for anxiety were obtained from the Psychiatric Genomics Consortium and FinnGen project. Inverse-variance weighted was applied to assess the causal relationship, and the results were strengthened by sensitivity analyses.
This study found that the genetic susceptibility to UC was associated with the increased risk of anxiety [odds ratio: 1.071 (95% confidence interval: 1.009, 1.135), P = 0.023]; while genetic susceptibility to CD was not associated with anxiety. However, genetic susceptibility to anxiety was not associated with UC or CD. No heterogeneity and pleiotropy were found and leave-one-out analysis excluded the potential influence of a particular variant.
This study identified that the genetic susceptibility to UC was significantly associated with anxiety, and provided the insight that early screening for the trait of anxiety is important for patients with UC.
Although this study investigated the causal relationship between IBD and anxiety, the precise biological mechanisms by which UC affects the development of anxiety remain unclear, such as whether and how the gut-brain axis plays a role in this process. Hence, more basic and clinical studies are needed for the identification of key regulators and pathways to further uncover the biological mechanisms.
We want to acknowledge the participants and investigators of the International Inflammatory Bowel Disease Genetics Consortium, Psychiatric Genomics Consortium, FinnGen study, and IEU Open genome-wide association studies project.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT, Brazil; Serban ED, Romania S-Editor: QuXL L-Editor: A P-Editor: Chen YX
| 1. | Baumgart DC, Le Berre C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N Engl J Med. 2021;385:1302-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 2. | Wehkamp J, Götz M, Herrlinger K, Steurer W, Stange EF. Inflammatory Bowel Disease. Dtsch Arztebl Int. 2016;113:72-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 4. | Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 281] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 5. | Fairbrass KM, Lovatt J, Barberio B, Yuan Y, Gracie DJ, Ford AC. Bidirectional brain-gut axis effects influence mood and prognosis in IBD: a systematic review and meta-analysis. Gut. 2022;71:1773-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 126] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 6. | Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 398] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 7. | Bernstein CN, Hitchon CA, Walld R, Bolton JM, Sareen J, Walker JR, Graff LA, Patten SB, Singer A, Lix LM, El-Gabalawy R, Katz A, Fisk JD, Marrie RA; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 8. | Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, Patten SB, Singer A, Lix LM, Hitchon CA, El-Gabalawy R, Katz A, Fisk JD, Bernstein CN; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. 2019;28:333-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 9. | Frolkis AD, Vallerand IA, Shaheen AA, Lowerison MW, Swain MG, Barnabe C, Patten SB, Kaplan GG. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68:1606-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 10. | Luo J, Xu Z, Noordam R, van Heemst D, Li-Gao R. Depression and Inflammatory Bowel Disease: A Bidirectional Two-sample Mendelian Randomization Study. J Crohns Colitis. 2022;16:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 11. | Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 2189] [Article Influence: 273.6] [Reference Citation Analysis (0)] |
| 12. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1867] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 13. | Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, Bigdeli T, Aggen SH, Adkins D, Wolen A, Fanous A, Keller MC, Castelao E, Kutalik Z, Van der Auwera S, Homuth G, Nauck M, Teumer A, Milaneschi Y, Hottenga JJ, Direk N, Hofman A, Uitterlinden A, Mulder CL, Henders AK, Medland SE, Gordon S, Heath AC, Madden PA, Pergadia ML, van der Most PJ, Nolte IM, van Oort FV, Hartman CA, Oldehinkel AJ, Preisig M, Grabe HJ, Middeldorp CM, Penninx BW, Boomsma D, Martin NG, Montgomery G, Maher BS, van den Oord EJ, Wray NR, Tiemeier H, Hettema JM. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. 2016;21:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 341] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 14. | Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, Cichon S, Edenberg HJ, Faraone SV, Gelernter J, Mathews CA, Nievergelt CM, Smoller JW, O'Donovan MC; Psychiatric Genomics Consortium. Psychiatric Genomics: An Update and an Agenda. Am J Psychiatry. 2018;175:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 15. | Thompson S G, Burgess S. Mendelian randomization: methods for using genetic variants in causal estimation. 1st ed. Chapman and Hall/CRC. 2015; 224. [DOI] [Full Text] |
| 16. | Baurecht H, Welker C, Baumeister SE, Weidnger S, Meisinger C, Leitzmann MF, Emmert H. Relationship between atopic dermatitis, depression and anxiety: a two-sample Mendelian randomization study. Br J Dermatol. 2021;185:781-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Freuer D, Linseisen J, Meisinger C. Association Between Inflammatory Bowel Disease and Both Psoriasis and Psoriatic Arthritis: A Bidirectional 2-Sample Mendelian Randomization Study. JAMA Dermatol. 2022;158:1262-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 18. | He J, Luo X, Xin H, Lai Q, Zhou Y, Bai Y. The Effects of Fatty Acids on Inflammatory Bowel Disease: A Two-Sample Mendelian Randomization Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Chen X, Kong J, Pan J, Huang K, Zhou W, Diao X, Cai J, Zheng J, Yang X, Xie W, Yu H, Li J, Pei L, Dong W, Qin H, Huang J, Lin T. Kidney damage causally affects the brain cortical structure: A Mendelian randomization study. EBioMedicine. 2021;72:103592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 20. | Rosoff DB, Smith GD, Lohoff FW. Prescription Opioid Use and Risk for Major Depressive Disorder and Anxiety and Stress-Related Disorders: A Multivariable Mendelian Randomization Analysis. JAMA Psychiatry. 2021;78:151-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 21. | Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 5399] [Article Influence: 771.3] [Reference Citation Analysis (0)] |
| 22. | Borges MC, Haycock PC, Zheng J, Hemani G, Holmes MV, Davey Smith G, Hingorani AD, Lawlor DA. Role of circulating polyunsaturated fatty acids on cardiovascular diseases risk: analysis using Mendelian randomization and fatty acid genetic association data from over 114,000 UK Biobank participants. BMC Med. 2022;20:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 23. | Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40:304-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4015] [Cited by in RCA: 5757] [Article Influence: 639.7] [Reference Citation Analysis (0)] |
| 24. | Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, Hartwig FP, Kutalik Z, Holmes MV, Minelli C, Morrison JV, Pan W, Relton CL, Theodoratou E. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019;4:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 458] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 25. | Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32:377-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 2905] [Article Influence: 363.1] [Reference Citation Analysis (0)] |
| 26. | Arp L, Jansson S, Wewer V, Burisch J. Psychiatric Disorders in Adult and Paediatric Patients With Inflammatory Bowel Diseases - A Systematic Review and Meta-Analysis. J Crohns Colitis. 2022;16:1933-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 27. | Cooney R, Tang D, Barrett K, Russell RK. Children and Young Adults With Inflammatory Bowel Disease Have an Increased Incidence and Risk of Developing Mental Health Conditions: A UK Population-Based Cohort Study. Inflamm Bowel Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 28. | Butwicka A, Olén O, Larsson H, Halfvarson J, Almqvist C, Lichtenstein P, Serlachius E, Frisén L, Ludvigsson JF. Association of Childhood-Onset Inflammatory Bowel Disease With Risk of Psychiatric Disorders and Suicide Attempt. JAMA Pediatr. 2019;173:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (2)] |
| 29. | Ludvigsson JF, Olén O, Larsson H, Halfvarson J, Almqvist C, Lichtenstein P, Butwicka A. Association Between Inflammatory Bowel Disease and Psychiatric Morbidity and Suicide: A Swedish Nationwide Population-Based Cohort Study With Sibling Comparisons. J Crohns Colitis. 2021;15:1824-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 30. | Keefer L. What can we do to tackle anxiety and depression in patients with inflammatory bowel disease? Lancet Gastroenterol Hepatol. 2021;6:337-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Hu S, Chen Y, Wang C. Depression and Anxiety Disorders in Patients With Inflammatory Bowel Disease. Front Psychiatry. 2021;12:714057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res. 2016;87:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 33. | Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17:338-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 34. | Niesler B, Kuerten S, Demir IE, Schäfer KH. Disorders of the enteric nervous system - a holistic view. Nat Rev Gastroenterol Hepatol. 2021;18:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 35. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 2791] [Article Influence: 465.2] [Reference Citation Analysis (2)] |
| 36. | Mayorga L, Serrano-Gómez G, Xie Z, Borruel N, Manichanh C. Intercontinental Gut Microbiome Variances in IBD. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Mitsialis V, Wall S, Liu P, Ordovas-Montanes J, Parmet T, Vukovic M, Spencer D, Field M, McCourt C, Toothaker J, Bousvaros A; Boston Children’s Hospital Inflammatory Bowel Disease Center; Brigham and Women’s Hospital Crohn’s and Colitis Center, Shalek AK, Kean L, Horwitz B, Goldsmith J, Tseng G, Snapper SB, Konnikova L. Single-Cell Analyses of Colon and Blood Reveal Distinct Immune Cell Signatures of Ulcerative Colitis and Crohn's Disease. Gastroenterology. 2020;159:591-608.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 38. | Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 420] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 39. | Gracie DJ, Hamlin PJ, Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. 2019;4:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 40. | D'Mello C, Swain MG. Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. Curr Top Behav Neurosci. 2017;31:73-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 41. | Haj-Mirzaian A, Amiri S, Amini-Khoei H, Hosseini MJ, Haj-Mirzaian A, Momeny M, Rahimi-Balaei M, Dehpour AR. Anxiety- and Depressive-Like Behaviors are Associated with Altered Hippocampal Energy and Inflammatory Status in a Mouse Model of Crohn's Disease. Neuroscience. 2017;366:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Vitali R, Prioreschi C, Lorenzo Rebenaque L, Colantoni E, Giovannini D, Frusciante S, Diretto G, Marco-Jiménez F, Mancuso M, Casciati A, Pazzaglia S. Gut-Brain Axis: Insights from Hippocampal Neurogenesis and Brain Tumor Development in a Mouse Model of Experimental Colitis Induced by Dextran Sodium Sulfate. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 43. | Ghasemi M, Navidhamidi M, Rezaei F, Azizikia A, Mehranfard N. Anxiety and hippocampal neuronal activity: Relationship and potential mechanisms. Cogn Affect Behav Neurosci. 2022;22:431-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 44. | Tang M, Huang H, Li S, Zhou M, Liu Z, Huang R, Liao W, Xie P, Zhou J. Hippocampal proteomic changes of susceptibility and resilience to depression or anxiety in a rat model of chronic mild stress. Transl Psychiatry. 2019;9:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 45. | Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 46. | Margolis KG, Cryan JF, Mayer EA. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology. 2021;160:1486-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 531] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 47. | Quigley EMM. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr Neurol Neurosci Rep. 2017;17:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 517] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 48. | Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A, Poleszak E, Fichna J, Wlaź P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021;172:105840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 403] [Article Influence: 100.8] [Reference Citation Analysis (0)] |