Published online Nov 21, 2023. doi: 10.3748/wjg.v29.i43.5865
Peer-review started: August 1, 2023
First decision: September 28, 2023
Revised: October 15, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: November 21, 2023
Processing time: 102 Days and 16.5 Hours
Patients with autoimmune conditions receiving immunosuppressants are at risk of non-Hodgkin lymphomas (NHL). Vedolizumab (anti-α4β7-integrin antibody), a treatment-of-choice for Crohn’s disease (CD), reduces inflammatory lymphocyte trafficking into the intestinal mucosa. This effect is believed to be confined to the colon.
We report the case of a CD patient on vedolizumab for five years who developed pediatric-type follicular lymphoma. Work-up prior to therapy revealed a reduction in circulating T-lymphocytes and their suppressed response to mitogens. Rituximab, cyclophosphamide, vincristine, and prednisone chemo-immunotherapy resulted in durable lymphoma remission, and vedolizumab treatment was continued. While the patient’s T-lymphocyte population and immunoglobulin production recovered, the T-lymphocyte mitogen response remained suppressed.
This patient’s NHL may be linked to receiving anti-α4β7 therapy. Further research could be beneficial to determine if proactive surveillance for NHL and other systemic diseases is indicated in patients on vedolizumab.
Core Tip: The literature is inconclusive on the association between anti-α4β7-integrin therapy and oncogenesis. This case report highlights a young adult on chronic vedolizumab, a monoclonal antibody targeting α4β7-integrin, who develops pediatric-type follicular lymphoma. The patient recovered with rituximab, cyclophosphamide, vincristine, prednisone immunotherapy, but T-lymphocyte mitogen response remained suppressed.
- Citation: Yerigeri K, Buhtoiarov I. Pediatric-type follicular lymphoma in a Crohn’s disease patient receiving anti-α4β7-integrin therapy: A case report. World J Gastroenterol 2023; 29(43): 5865-5871
- URL: https://www.wjgnet.com/1007-9327/full/v29/i43/5865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i43.5865
Follicular lymphoma (FL) is one of the most common non-Hodgkin lymphomas (NHL) worldwide[1]. Pediatric-type FL (PTFL) was first identified as a distinct clinicopathological condition in the 4th edition of the World Health Organization classification of lymphoid neoplasms[2]. PTFL lacks the t(14;18) translocation characteristic of adult-type FL that leads to overexpression of the B-cell leukemia/lymphoma 2 (BCL2) oncogene. Instead, the most commonly affected gene in PTFL is MAP2K1 on chromosome 15 encoding the serine/threonine kinase MEK1[3]. MEK1 plays a role in the RAS-MAPK signaling pathway and directs cell growth, differentiation, and apoptosis. PTFL commonly presents with localized disease (stage I or II) involving lymph nodes of the head and neck region. Hilar and mediastinal lymph nodes may also be involved. The adenopathy is typically painless and without mass effect on adjacent anatomical structures[4]. The disease course of PTFL is indolent and cure rate is high. Standard-of-care treatment approaches are surgical resection versus rituximab, cyclophosphamide, vincristine, and prednisone-like regimens for patients with advanced or unresectable conditions[5].
Patients with autoimmune conditions such as inflammatory bowel disease (IBD) and those treated with systemic immunosuppressants are at risk of developing lymphoid malignancies[6,7]. The incidence of such comorbidities remains low, and reports are limited to adult patient cohorts. Crohn’s disease (CD) is a chronic IBD with increasing incidence in the United States over the past several decades. The pathogenesis of Crohn’s is mediated by auto-reactive T-cells migrating into intestinal tissue, perpetuating inflammation and tissue necrosis. Targeted therapies have been developed to block inflammatory cell activation and migration in an organ-specific manner[8,9]. Vedolizumab is an anti-α4β7 integrin monoclonal antibody that inhibits interaction of T cells, monocytes, and dendritic cells with the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expressed on vascular endothelium. Paradoxically, ligation of α4β7 results in downregulation of genes controlling expression of innate immune receptors, chemokines, and their cognate receptors. Previous clinical studies suggest that vedolizumab does not have a systemic immunosuppressive effect and does not increase risk for malignancy[10,11].
We present a case of a 27-year-old male with CD who was diagnosed with PTFL five years into treatment with vedolizumab. Surprisingly, at the time of diagnosis, the patient had reduced numbers and suppressed function of circulating T cells, potentially contributing to a permissive immune environment for PTFL development. This case provides a useful addition to the existing knowledge of PTFL putative risk factors and vedolizumab systemic effects on the immune system.
A 27-year-old male presented with a painless mass in his right mandibular fossa.
Review of systems was negative. The patient denied constitutional B symptoms (fever, night sweats, weight loss), signs of gastrointestinal distress (e.g., pain, nausea, vomiting, diarrhea), and oral pain or dysphagia.
Past medical history was pertinent for CD on long-term vedolizumab therapy.
All other personal and family medical history was noncontributory.
Physical exam appreciated submandibular and upper cervical lymphadenopathy; there were no other concerning findings. Abdomen was soft, non-tender, non-distended, and without palpable masses; bowel sounds were present.
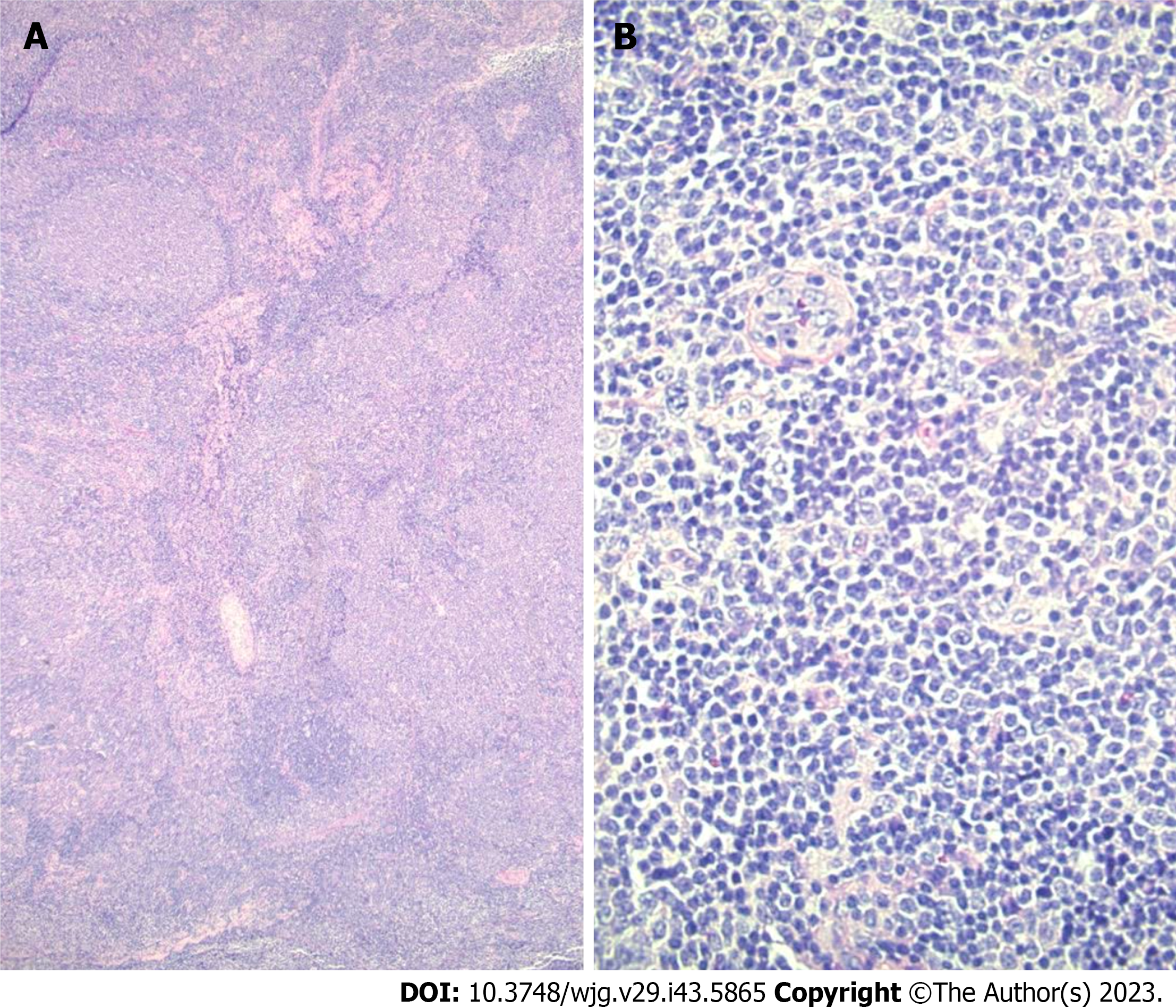
Core-needle biopsy of the parotid mass demonstrated neoplastic infiltrate with a follicular pattern. The follicles did not have polarized germinal centers and immunophenotype. The neoplastic cells were intermediate-sized with scant cytoplasm and irregular nuclei. The neoplastic cells expressed CD10, CD20, BCL6, PAX5, and CD23; they did not express CD3, CD5, CD21, cyclin D1, BCL2, MUM1, or BCL2. Ki-67 stain was positive in approximately 70% of cells (Figure 1). Molecular studies identified a MAP2K1p.g128D mutation.
Computed tomography confirmed the mass within the parotid gland encasing the right-sided facial nerve.
The above pathomorphological and molecular features were consistent with FL, pediatric-type.
Pre-therapy baseline immunologic testing revealed reduced absolute and relative levels of circulating CD3+CD4+ and CD3+CD8+ T-cells (Table 1). In vitro mitogen stimulation with phytohemagglutinin (PHA) and concanavalin A (ConA) also revealed suppressed proliferative response (Table 2). Of interest, while the peripheral blood B-cell population had a polyclonal pattern, the T-lymphocyte pool demonstrated expansion of two distinct T cell receptor (TCR)-rearranged monoclonal populations (Table 1), a phenomenon characteristic for autoimmune conditions such as IBD[12,13]. The patient was also positive for Epstein-Barr virus (EBV) viral capsid antigen (VCA) immunoglobulin (Ig)G [antibody index (AI) 7.1]; negative for EBV VCA IgM (0.2 AI); positive for EBV early antigen Ab (1.5 AI); and positive for EBV nuclear antigen Ab (7.8 AI), indicating chronic EBV viremia/reactivation. The parotid mass tissue was not stained for EBV, and EBV DNA quantification in blood was not performed.
| Component | Ref range & units | Testing time point | ||
| Pre-Tx | EOTx | 12 mo off Tx | ||
| CD3+ T cell | 60%-89% | 43 | 72 | 52 |
| CD3+ T cell | 958-2388 cells/uL | 473 | 792 | 869 |
| CD3+CD4+ T cell | 34%-61% | 27 | 39 | 32 |
| CD3+CD4+ T cell | 533-1674 cells/uL | 304 | 425 | 526 |
| CD3+CD8+ T cell | 10%-41% | 14 | 28 | 18 |
| CD3+CD8+ T cell | 175-958 cells/uL | 151 | 303 | 293 |
| CD19+ B cell | 5%-22% | 20 | 0 | 23 |
| CD19+ B cell | 75-660 cells/uL | 224 | 0 | 385 |
| NK cell | 5%-25% | 37 | 27 | 24 |
| NK cell | 102-565 cells/uL | 406 | 297 | 394 |
| CD4/CD8 ratio | 1.10-3.25 | 2.02 | 1.40 | 1.79 |
| B cell clonality | ||||
| IGH FR1 | Nonclonal | Nonclonal | ||
| IGH FR2 | Nonclonal | Nonclonal | ||
| IGH FR3 | Nonclonal | Nonclonal | ||
| IGH DH1-6-J | Nonclonal | Nonclonal | ||
| IGK V-J | Nonclonal | Nonclonal | ||
| IGK V-Kde | Nonclonal | Nonclonal | ||
| TCR clonality | ||||
| TCRB A (V-J) | Nonclonal | Nonclonal | ||
| TCRB B (V-J) | Nonclonal | Nonclonal | ||
| TCRB C (V-J) | Clonal peak (180 bp) | Clonal peak (180 bp) | ||
| TCRG D | Clonal peak (189 bp) | Nonclonal | ||
| Component | Reference range & units | Testing time point | |
| Pre-Tx | 12 mo off Tx | ||
| Mitogen control | > 50 CPM | 434 | 2297 |
| Phytohemagglutinin | ≥ 188800 CPM | 173643 | 125008 |
| Pokeweed mitogen | > 68549 CPM | 77631 | 76811 |
| Concanavalin A | > 81283 CPM | 49198 | 92755 |
The parotid gland was not amenable to surgical resection or local radiation therapy; therefore, 6 cycles of rituximab, cyclophosphamide, vincristine, and prednisone chemo-immunotherapy were administered concurrently with monthly vedolizumab. Complete remission for the lymphoma was successfully achieved with good control of CD symptoms (no recurrence or flares).
Repeat assessment at 12 mo off-therapy revealed ongoing complete remission of both PTFL and CD. Of note, one of the TCR-rearranged T-lymphocyte clones became undetectable (Table 1). Surprisingly, functional suppression of peripheral blood T-cells persisted. It remains unclear whether recurrent immune suppression at this clinical stage may only be attributed to continued vedolizumab therapy.
Lymphoproliferative disorders (including NHL) have long been recognized as complications for autoimmune inflammatory conditions[14]. Latent infection with EBV (as in this patient) or human herpesvirus 8 may also contribute to cancer genesis[15]. Vedolizumab, the monoclonal antibody against α4β7-integrin, uniquely inhibits interaction of T cells, monocytes, and dendritic cells with the vascular endothelium to reduce local inflammation for disease control[10]. While serious adverse events are reported in 41% of Crohn’s patients, benign and malignant neoplasms are noted in only 6.8% of treated patients at an incidence rate of 20.8 per 1000 person-years[16]. Several other studies suggested that treatment with vedolizumab is rather safe and does not carry an excessive burden of new or recurrent malignancies[17].
However, recent insights into the mechanisms of vedolizumab’s biologic activity suggest it may extend beyond inhibition of the α4β7-integrin interaction with MAdCAM-1. It appears that several important genes regulating immune effectors and mechanisms of their cross-talk via chemokines and cytokines [e.g., CXC chemokine ligand (CXCL)9, CXCL10, FCGR3B, interleukin (IL)23A, IL17, interferon-γ] were down-regulated in IBD patients who achieved clinical remission with vedolizumab[18,19]. Additional findings suggest that the interaction of α4β7-integrin with MAdCAM-1 may serve as an alternative co-stimulatory pathway for T cells, triggering expression of genes encoding multiple cytokines (e.g., IL-2, IL-3, IL-4, IL-8, IL-13, IL-17A, IL-17F, IL-22) in a similar fashion to CD28-mediated signaling. This potentially implies that inhibition of α4β7-integrin-MAdCAM-1 interaction results in down-regulation of T-cell function, although the argument requires further research[20].
Our patient was found to have decreased numbers of both CD3+CD4+ and CD3+CD8+ T-cells as well as a diminished response to ConA and PHA at the time of NHL diagnosis, i.e., while receiving ongoing vedolizumab therapy. At the end of lymphoma chemo-immunotherapy and at 12 mo post-therapy, peripheral blood lymphocyte subset quantification demonstrated improvement of the absolute CD3+CD4+ and CD3+CD8+ T-cell counts; however, the proliferative response to ConA remained suppressed. At this phase of the clinical course, reduced response to the in vitro mitogen stimulation may be attributable to vedolizumab, although the suppressive effect of an auto-inflammatory state cannot be ruled out. Further research is necessary to discern if vedolizumab has responsible mechanisms. Regardless, the patient was able to recover his CD19+ B-cell population, which was completely depleted by rituximab at the end of treatment, as well as maintain immunoglobulin production (Table 3). Surprisingly, one of the two clonally expanded populations of T-cells were not detectable following therapy completion, suggesting eradication by the anti-lymphoma chemotherapy.
| Component | Ref range & units | Testing time point | ||
| Pre-Tx | EOTx | 12 mo off Tx | ||
| IgA | 68-408 mg/dL | 250 | 177 | 170 |
| IgM | 35-263 mg/dL | 55 | 30 | 31 |
| IgG | 768-1632 mg/dL | 1376 | 921 | 927 |
| IgG Subclass 1 | 240-1118 mg/dL | 763 | - | 503 |
| IgG Subclass 2 | 124-549 mg/dL | 371 | - | 335 |
| IgG Subclass 3 | 21-134 mg/dL | 162 (H) | - | 64 |
| IgG Subclass 4 | 1-123 mg/dL | 64 | - | 40 |
PTFL is a rare type of B-cell NHL. The clinical course is indolent despite a high proliferative index and blastoid histopathological features. Due to its rarity, predisposing factors have not been clearly postulated. The role of underlying immune suppression in PTFL pathogenesis has not been established. Nodal PTFL is believed to arise from B-lymphocytes in follicle germinal centers. Immune system workup and cytogenetic analysis in PTFL patients may help reveal an etiological correlation between the malignancy and immunomodulatory processes. Attention must also be paid to possible systemic consequences of vedolizumab therapy hitherto unreported in the literature.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma V, India; Zhu L, China S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, Patmore R, Jack A, Roman E. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 308] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019-5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1444] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 3. | Louissaint A Jr, Schafernak KT, Geyer JT, Kovach AE, Ghandi M, Gratzinger D, Roth CG, Paxton CN, Kim S, Namgyal C, Morin R, Morgan EA, Neuberg DS, South ST, Harris MH, Hasserjian RP, Hochberg EP, Garraway LA, Harris NL, Weinstock DM. Pediatric-type nodal follicular lymphoma: a biologically distinct lymphoma with frequent MAPK pathway mutations. Blood. 2016;128:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Martin AR, Weisenburger DD, Chan WC, Ruby EI, Anderson JR, Vose JM, Bierman PJ, Bast MA, Daley DT, Armitage JO. Prognostic value of cellular proliferation and histologic grade in follicular lymphoma. Blood. 1995;85:3671-3678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Attarbaschi A, Abla O, Arias Padilla L, Beishuizen A, Burke GAA, Brugières L, Bruneau J, Burkhardt B, d'Amore ESG, Klapper W, Kontny U, Pillon M, Taj M, Turner SD, Uyttebroeck A, Woessmann W, Mellgren K. Rare non-Hodgkin lymphoma of childhood and adolescence: A consensus diagnostic and therapeutic approach to pediatric-type follicular lymphoma, marginal zone lymphoma, and nonanaplastic peripheral T-cell lymphoma. Pediatr Blood Cancer. 2020;67:e28416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Aithal GP, Mansfield JC. Review article: the risk of lymphoma associated with inflammatory bowel disease and immunosuppressive treatment. Aliment Pharmacol Ther. 2001;15:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Farrell RJ, Ang Y, Kileen P, O'Briain DS, Kelleher D, Keeling PW, Weir DG. Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Marsal J, Agace WW. Targeting T-cell migration in inflammatory bowel disease. J Intern Med. 2012;272:411-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Al-Bawardy B, Shivashankar R, Proctor DD. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front Pharmacol. 2021;12:651415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 10. | Luzentales-Simpson M, Pang YCF, Zhang A, Sousa JA, Sly LM. Vedolizumab: Potential Mechanisms of Action for Reducing Pathological Inflammation in Inflammatory Bowel Diseases. Front Cell Dev Biol. 2021;9:612830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 11. | Loftus EV Jr, Feagan BG, Panaccione R, Colombel JF, Sandborn WJ, Sands BE, Danese S, D'Haens G, Rubin DT, Shafran I, Parfionovas A, Rogers R, Lirio RA, Vermeire S. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1353-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 12. | Werner L, Nunberg MY, Rechavi E, Lev A, Braun T, Haberman Y, Lahad A, Shteyer E, Schvimer M, Somech R, Weiss B, Lee YN, Shouval DS. Altered T cell receptor beta repertoire patterns in pediatric ulcerative colitis. Clin Exp Immunol. 2019;196:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Hodges E, Krishna MT, Pickard C, Smith JL. Diagnostic role of tests for T cell receptor (TCR) genes. J Clin Pathol. 2003;56:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wang SS, Vajdic CM, Linet MS, Slager SL, Voutsinas J, Nieters A, de Sanjose S, Cozen W, Alarcón GS, Martinez-Maza O, Brown EE, Bracci PM, Lightfoot T, Turner J, Hjalgrim H, Spinelli JJ, Zheng T, Morton LM, Birmann BM, Flowers CR, Paltiel O, Becker N, Holly EA, Kane E, Weisenburger D, Maynadie M, Cocco P, Foretova L, Staines A, Davis S, Severson R, Cerhan JR, Breen EC, Lan Q, Brooks-Wilson A, De Roos AJ, Smith MT, Roman E, Boffetta P, Kricker A, Zhang Y, Skibola C, Chanock SJ, Rothman N, Benavente Y, Hartge P, Smedby KE. Associations of non-Hodgkin Lymphoma (NHL) risk with autoimmune conditions according to putative NHL loci. Am J Epidemiol. 2015;181:406-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Natkunam Y, Gratzinger D, Chadburn A, Goodlad JR, Chan JKC, Said J, Jaffe ES, de Jong D. Immunodeficiency-associated lymphoproliferative disorders: time for reappraisal? Blood. 2018;132:1871-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D'Haens G, Panaccione R, Loftus EV Jr, Sankoh S, Fox I, Parikh A, Milch C, Abhyankar B, Feagan BG. The safety of vedolizumab for ulcerative colitis and Crohn's disease. Gut. 2017;66:839-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 609] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 17. | Hong SJ, Zenger C, Pecoriello J, Pang A, Vallely M, Hudesman DP, Chang S, Axelrad JE. Ustekinumab and Vedolizumab Are Not Associated With Subsequent Cancer in IBD Patients with Prior Malignancy. Inflamm Bowel Dis. 2022;28:1826-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Zeissig S, Rosati E, Dowds CM, Aden K, Bethge J, Schulte B, Pan WH, Mishra N, Zuhayra M, Marx M, Paulsen M, Strigli A, Conrad C, Schuldt D, Sinha A, Ebsen H, Kornell SC, Nikolaus S, Arlt A, Kabelitz D, Ellrichmann M, Lützen U, Rosenstiel PC, Franke A, Schreiber S. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut. 2019;68:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 19. | Veny M, Garrido-Trigo A, Corraliza AM, Masamunt MC, Bassolas-Molina H, Esteller M, Arroyes M, Tristán E, Fernández-Clotet A, Ordás I, Ricart E, Esteve M, Panés J, Salas A. Dissecting Common and Unique Effects of Anti-α4β7 and Anti-Tumor Necrosis Factor Treatment in Ulcerative Colitis. J Crohns Colitis. 2021;15:441-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | DeBerg HA, Konecny AJ, Shows DM, Lord JD. MAdCAM-1 Costimulates T Cells through Integrin α(4)β(7) to Cause Gene Expression Events Resembling Costimulation through CD28. Immunohorizons. 2022;6:211-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |