Published online Nov 14, 2023. doi: 10.3748/wjg.v29.i42.5699
Peer-review started: June 16, 2023
First decision: August 31, 2023
Revised: September 13, 2023
Accepted: November 8, 2023
Article in press: November 8, 2023
Published online: November 14, 2023
Processing time: 149 Days and 23.5 Hours
Esophageal cancer (EC) has a high incidence and mortality rate and is emerging as one of the most common health problems globally. Owing to the lack of sensitive detection methods, uncontrollable rapid metastasis, and pervasive treatment resistance, EC is often diagnosed in advanced stages and is susceptible to local recurrence. Exosomes are important components of intercellular communication and the exosome-mediated crosstalk between the cancer and surrounding cells within the tumor microenvironment plays a crucial role in the metastasis, progression, and therapeutic resistance of EC. Considering the critical role of exosomes in tumor pathogenesis, this review focused on elucidating the impact of exosomes on EC metastasis and therapeutic resistance. Here, we summarized the relevant signaling pathways involved in these processes. In addition, we discussed the potential clinical applications of exosomes for the early diagnosis, prognosis, and treatment of EC.
Core Tip: Esophageal cancer (EC) is a highly malignant type of cancer, and its early diagnosis and effective treatment are lacking. We highlighted the following: (1) Exosomes are involved in altering the number or function of cells in the tumor microenvironment of EC; (2) all steps of EC metastasis and various therapeutic resistance are closely related to exosomes; and (3) exosomes can serve as reliable biomarkers and effective treatment tools for EC. Finally, we presented the urgent challenges and described the future research directions for exosome application in the diagnosis and treatment of EC.
- Citation: Ning XY, Ma JH, He W, Ma JT. Role of exosomes in metastasis and therapeutic resistance in esophageal cancer. World J Gastroenterol 2023; 29(42): 5699-5715
- URL: https://www.wjgnet.com/1007-9327/full/v29/i42/5699.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i42.5699
Esophageal cancer (EC) is a common malignancy of the digestive system, ranking seventh in incidence and sixth in mortality worldwide. According to the GLOBOCAN Project, approximately 604000 new cases of EC were reported, and more than 544000 patients died worldwide in 2020[1]. EC has two main histological subtypes, esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). ESCC is the primary subtype of EC found in East and Central Asia, whereas EAC is prevalent in Western Europe and North America. Early EC is difficult to diagnose because of its insidious onset, as most patients are diagnosed at stage 4 or an advanced stage. Surgery and systematic drug therapy play an important role in preventing further spread of cancer cells. A distant metastasis or therapeutic resistance also occurs in approximately half of patients after treatment, and the 5-year survival rate for patients is less than 25%. Therefore, rapid metastasis and therapeutic resistance have emerged as two major issues that need to be addressed in EC.
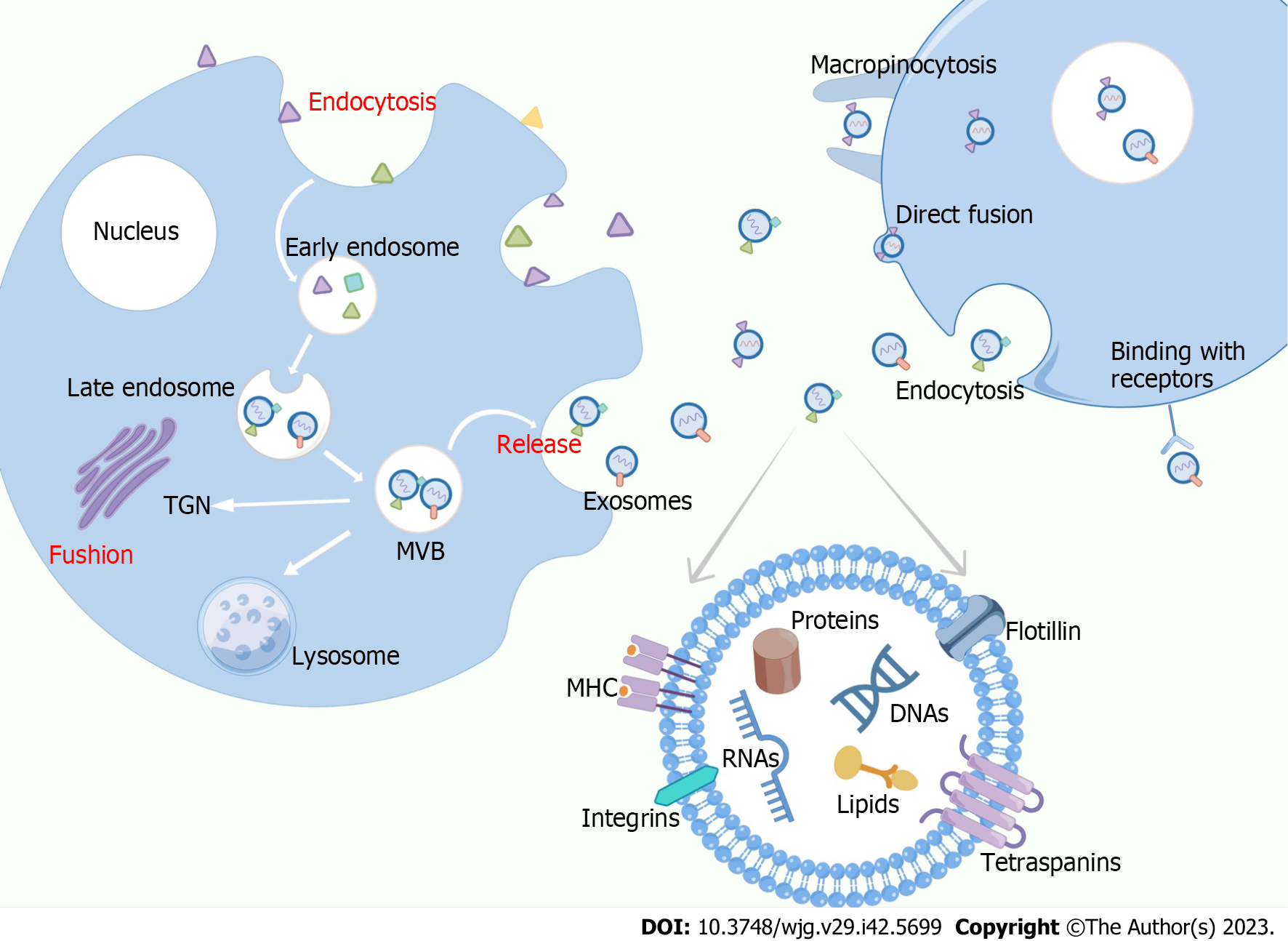
Extracellular vesicles (EVs) were first described by Chargaff and West as “procoagulant platelet-derived particles” in 1946[2]. Subsequently, Wolf distinguished minute particulate materials (platelet dust) from intact platelets[3]. With in-depth investigation, the biology of EVs has gradually been revealed and their functions have been explored[4-7]. Research on EVs has increased markedly over the past decade, with exosomes being the most widely discussed topic[8]. According to the canonical classification scheme, EVs can be divided into exosomes (< 150 nm in diameter) and microvesicles (≤ 1000 nm in diameter) based on their size and biogenesis route[9]. With a diameter of 30-150 nm, exosomes carry abundant cargo, including selected RNAs [miRNAs, regulatory mRNAs, and long noncoding RNAs (lncRNAs)], specific DNA sequences, proteins, lipids, glycoconjugates, and metabolites[10,11]. Exosomes secreted by all cell types, such as fibroblasts, tumor cells, and various immune cells, can be found in blood and other biological fluids, including urine[12], saliva[13], cerebral spinal fluid[14], breast milk[15] amniotic fluid[16], and gastric acid[17]. Multiple studies have revealed that exosomes play an important role in processes related to cancer progression, including carcinoma cell proliferation[18], apoptosis[19], invasion[20], epithelial-mesenchymal transition (EMT)[21], angiogenesis[22], immunosuppression[23], and tumor implantation[24] (Table 1).
| Molecules in exosomes | Biological process | Targets | Ref. |
| lncRNA ZFAS1 | Regulate proliferation, invasion, migration, and apoptosis | MicroRNA-124, STAT3 | [2] |
| miR-103a-2-5p | Promote proliferation and migration of ESCC cells | - | [3] |
| miR-93-5p | Promote the proliferation of esophageal cancer cells | PTEN, p21, CCND1 | [4] |
| miR-200a | Promote the proliferation, migration, and invasion of esophageal cells and inhibits apoptosis | KEAP1, NRF2 | [5] |
| miR-19b-3p | Inhibit apoptosis, promote cell migration and invasion | PTEN | [6] |
| LINC01410 | Promote metastasis and epithelial-mesenchymal transition | miR-122-5p, PKM2 | [7] |
| lncRNA FAM225A | Accelerate progression and angiogenesis | miR-206, NETO2, FOXP1 | [8] |
| miRNA-21-5p | Promote angiogenesis and malignant progression | - | [9] |
| miR-154-5p | Attenuate progression and angiogenesis | KIF14 | [10] |
| miR-301a-3p | Promote angiogenesis | PTEN | [11] |
| uc.189 | Promote proliferation and lymph angiogenesis of human lymphatic endothelial cells and thus facilitate lymph node metastasis | EPHA2 | [12] |
The aim of this review was to provide a brief introduction to the underlying mechanisms through which exosomes affect oncogenesis, metastasis, and resistance to different therapies in EC. Here, we summarized the applications of exosomes as diagnostic and/or prognostic biomarkers and therapeutic tools to enhance the treatment efficacy of EC.
Exosomes are phospholipid bilayer-encapsulated nanosized vesicles formed via the endocytic pathway[25]. There are multiple ways in which exosomes are transported from donors to recipient cells (Figure 1). Depending on the cell source, physiological conditions, and sorting mechanisms, exosomal contents can be divided into two main categories: those related to the exosome formation mechanism and those related to exosome functions[26]. The cargos of exosomes play important roles in cancer metastasis, modulating stromal reactions, inducing angiogenesis, immune response, and other biological processes through paracrine, autocrine, and endocrine effects. The fact that exosomes have substantial effects on the abovementioned processes explains their essential role in cell-to-tissue communication as well as intercellular communication, which robustly alters the function of cells and promotes the development of cancer and drug resistance[27,28]. Extensive research has proposed that proteins in exosomes not only participate in the biogenesis of exosomes, but also mediate signal transduction and immunogenic regulators, including the transferrin receptor, epidermal growth factor receptor (EGFR), major histocompatibility complex-I (MHC-I), and MHC-II[29]. Protein studies have revealed that exosomes have their own conserved components that may serve as biomarkers for other exosomes[30]. The main RNAs carried by exosomes, including miRNAs, mRNA, rRNAs, tRNA, lncRNAs, and circular RNAs, have been revealed to play a crucial role in tumorigenesis by modulating gene expression and regulating inter-organ communication during disease progression[31-34]. Another type of nucleic acid, DNA, is an exosomal cargo. Jeppesen et al[35] observed the presence of a large fraction of cell-free DNA in plasma exosomes during the qualitative analysis of plasma exosomes. Lipids are abundant in exosomes, and their expression level and status vary with exosome type, suggesting that lipids are important tools that can be used to improve cancer diagnosis. Studies on prostate cancer have revealed that urinary exosomes in patients with cancer contain lipids, such as phosphatidylserine, lactosylceramide, and cholesterol, which are notably different from those in healthy controls[36].
As exosomes are more representative of their originating cells, are more abundant, and have higher biological stability in body fluids than other cellular factors, developing accurate methods for exosome isolation is critical[37]. Current separation methods for exosomes have limitations such as low reproducibility, low accuracy, complicated extraction procedures, low exosome yield, extensive duration, and high cost. The small size and intrinsic heterogeneity of exosomes limit their applications[38]. Although ultracentrifugation is usually applied as the standard method and is the most commonly used protocol for exosome isolation, it requires expensive machinery and considerable time. Nonetheless, purity requirements are met with this technology, albeit at the expense of recovery.
Ultrafiltration operates based on the specific sizes and molecular weights of membranes, obtaining exosomes in less time and with lower equipment and reagent costs; however, its purity is lower than that of ultracentrifugation[39-41]. Size-exclusion chromatography has proven to outperform other technologies in the following areas: purity, efficiency, integrity, and retention of major characteristics of exosomes[42,43]. However, size-exclusion chromatography involves high equipment costs and additional methods for exosome extraction[44]. As mentioned above, each method for isolating exosomes has its advantages and disadvantages, and the choice must be made according to the specific situation. Further efforts are warranted to create a high-purity, high-utilization, and low-cost method of exosome isolation that can be widely used in the future.
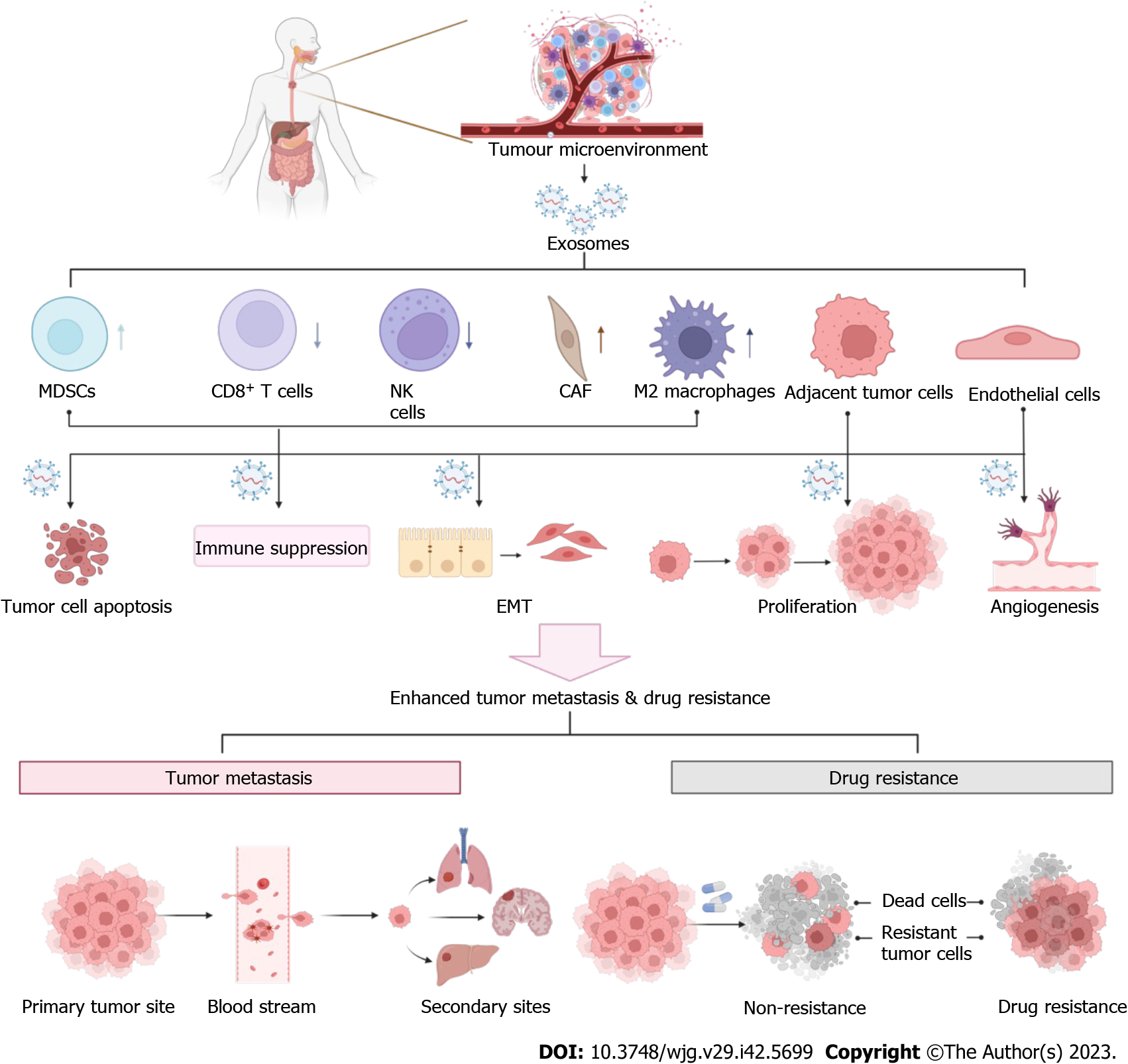
The tumor microenvironment (TME), comprising malignant cells, peripheral blood vasculature, noncancer host cells, and noncellular components, plays a central role in tumor invasion and metastasis[45,46]. According to the “seed and soil” theory, metastasized tumor cells invade tissue and modulate it to create a suitable “soil” called the TME, which is amenable to the ectopic growth and survival of metastasized cancer cells[47]. In addition to the therapeutic resistance caused by tumor cell changes, emerging evidence has revealed the role of TME in therapeutic resistance[48]. Nontumor cell components in the TME can also drive tumor cell resistance by secreting cytokines, chemokines, growth factors, and exosomes[49,50]. Extracellular matrix (ECM) modeling can influence drug delivery, promote immune escape, and mediate signal transduction, consequently diminishing the therapeutic effects of antitumor agents[51]. The acidic and hypoxic status of the TME can lead to resistance to anticancer treatments[52]. We focused on how cells in the TME influence EC development and the role of exosomes in this process (Figure 2).
Endothelial cells play a vital role in angiogenesis, thereby initiating tumors and facilitating distant metastasis. EC angiogenesis involves growth factors, cytokines, and ECM, among which vascular endothelial growth factor acts as a key factor[53,54]. Studies on EC have demonstrated that the expression of vascular endothelial growth factor is negatively correlated with the degree of tissue differentiation and positively correlated with the degree of cell malignancy and angiogenic ability[55]. Exosomes derived from ESCC cells cultured under hypoxic conditions promote angiogenesis by altering the phenotype and transcriptome of endothelial cells[56]. Exosomes derived from ESCC cells cultured under hypoxia have been reported to promote angiogenesis by altering the phenotype and transcriptome of endothelial cells[57]. Therefore, exosomes may participate in angiogenesis by altering the levels of angiogenesis-related factors in the EC.
T cells, which can generally be divided into CD4+, CD8+, helper, and regulatory T cells (Tregs), play both pro- and anti-tumorigenic roles in the TME[58]. Fibroblast growth factor 2 produced by tumor fibroblasts can impair the activation of CD8+ T cells in EC, increasing the growth and metastasis of cancer cells in vitro and in vivo by regulating the expression of recombinant sprouty homologue 1[59]. Irradiated esophageal carcinoma-infiltrating T-cell derived exosomes can directly upregulate β-catenin, nuclear factor-κB (NF-κB), and snail in cancer cells, which is related to EC development, indicating a protumorigenic role of T-cell derived exosomes in EC[60].
Under the influence of exosomes, fibroblasts involved in tissue remodeling and repair transform into cancer-associated fibroblasts (CAFs)[61]. CAFs can provide suitable conditions for metastatic tumor cells and are thus an important determinant of EC. A study involving the analysis of clinical samples and establishment of an orthotopic metastasis model of EC indicated that CAFs could promote lymph node metastasis[62]. CAFs accelerate the progression and metastasis of tumors by secreting different tumor-promoting factors and regulating communication between tumor cells and microenvironment components[63]. CAFs can promote the growth and migration of ESCC cells by generating exosomes containing sonic hedgehog factors; inhibition of the Hedgehog signaling pathway can partly neutralize this phenomenon, suggesting a new strategy for ESCC treatment[64]. In 2022, Shi et al[65] designed in vitro experiments that revealed that CAF-derived exosomes facilitate the metastasis and EMT of ESCC cells via the LINC01410/miR-122-5p/PKM2 axis. CAFs enhance tumor cell evasion during immune surveillance by inducing M2 polarization of macrophages and apoptosis of T and NK cells[66]. Additionally, Zhang et al[67] observed a role of CAFs in establishing resistance of ESCC cells to chemotherapeutic drugs, which they accomplished via exosome-mediated FOXO1/TGF1 signaling. In addition to being associated with the formation of CAFs, exosomes play an indispensable role in immune dysfunction in patients with EC, which may lead to disease progression and poor response to therapy.
Owing to the high variability of tumor-associated macrophages (TAMs), different stimuli in the TME, including exosomes and cytokines secreted by tumor cells, can drive the tilt differentiation of TAMs towards M1 or M2 macrophages[68]. The transfer of miR-21-5p from EC109 or EC9706 cells to M0 macrophages via exosomes resulted in M2 macrophage polarization and EMT in EC cells[69]. Shou et al[70] verified that another EC-derived exosomal cargo, miR-301a-3p, could also facilitate angiogenesis by inducing macrophage polarization The transfer of hsa-circ-0048117 from hypoxic ESCC cells to macrophages via exosomes results in M2 macrophage polarization[71]. EC-derived exosomes can alter the function of macrophages; similarly, exosomes secreted by macrophages can also affect EC progression. Emerging evidence indicates that M2 macrophages are associated with the migration, invasion, poor prognosis of ESCC, and the subsequent induction of EMT[72]. By transmitting lncRNA AFAP1-AS1 and microRNA-26a, exosomes derived from M2 macrophages promoted the migration, invasion, and lung metastasis of EC cells. Mechanistically, M2 macrophage-derived exosomes increase EC metastasis by transferring lncRNA AFAP1-AS1 to upregulate ATF2 and downregulate miR-26a, revealing that targeting M2 macrophages and the lncRNA AFAP1AS1/miR-26a/ATF2 signaling axis may be a useful therapeutic strategy for EC[73].
Myeloid-derived suppressor cells (MDSCs) can suppress various types of immune responses and participate in the formation of the premetastatic niche and are immature myeloid cells closely linked to tumor development and prognosis[74,75]. Chen et al[76] found that the level of MDSCs, which cause immune escape and promote metastasis by eliciting T cell dysfunction, is considerably related to ESCC formation. Notably, MDSC differentiation has been reported to aggravate immunosuppression in EC through transcriptomic alterations induced by the lncRNA Lnc-17Rik[77]. Based on the alterations in cells in the TME, the use of exosomes to intervene in this process to regulate TME is worth studying.
High metastatic rate is a major issue in EC, resulting in high mortality and poor prognosis. Tumor metastasis is a complex and multistep process that includes the growth of primary tumors, neovascularization, interactions between tumor and stromal cells, EMT, intravasation of tumor cells, extravasation of surviving tumor cells, and colonization and growth at secondary sites[78], which are affected by a number of signaling pathways. Existing studies on EC have revealed that exosomes derived from tumor and nontumor cells participate in various steps of tumor metastasis by mediating intercellular communication (Figure 3).
EMT, which involves epithelial cells losing their apical polarity, gaining anteroposterior polarity, and losing cell adhesion to transform into mesenchymal cells, is a key driver of tumor metastasis[79]. Primary tumors undergo EMT through the regulation of various factors, including EMT transcription factors, paired related homeobox protein 1 (Prrx1), cancer stem cells, N-cadherin, vimentin, E-cadherin, occludin, cytokeratin, and claudin[80]. Through the transmission of these elements, exosomes can cause epithelial cells to undergo morphological and functional changes and acquire invasive properties that facilitate EMT.
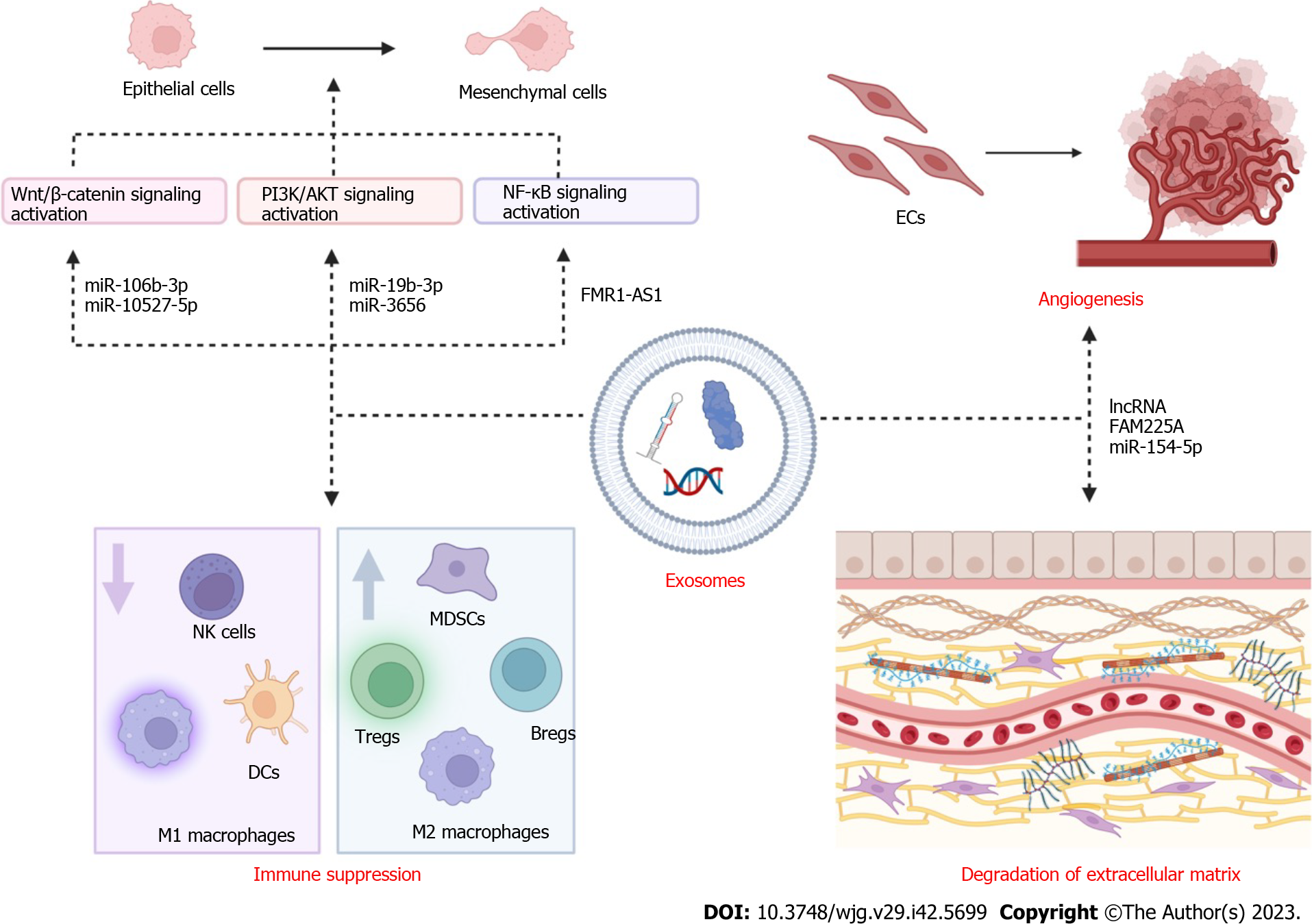
We summarized the relevant literature on exosomes and EMT process, focusing on changes in signaling pathways. Qiao et al[81] compared 50 paired ESCC tumor tissues and matched normal adjacent tissues and found that the expression of EMT-related proteins was notably upregulated in ESCC cells in the presence of miR-106b-3p. Then, the researchers performed gene and protein expression analyses, and the results revealed that Wnt, GSK3, and β-catenin related to Wnt/β-catenin pathway were increased in the miR-106b-3p mimic group compared with the control group. In ESCC, miR-106b-3p may be transported by exosomes to induce these effects. By transmitting miR-10527-5p, exosomes derived from human ESCC cell lines notably downregulated N-cadherin and vimentin (mesenchymal markers) and upregulated the epithelial marker E-cadherin by activating the Wnt/β-catenin pathway[82]. Additionally, one study emphasized the function of CAF-derived exosomal miR-3656 in the TME, which could serve as a biomarker for EC diagnosis. MiR-3656 in CAF-derived exosomes promotes the proliferation, migration, and invasion of EC cells by triggering the ACAP2/phosphatidylinositol 3-kinase (PI3K/AKT) signaling pathway[83].
Exosomes extracted from T cells after radiotherapy significantly promote the EMT of EC cells by increasing the expression of β-catenin, NF-κB, and snail, as revealed using western blot analysis[60]. Li et al[84] demonstrated that FMR1-AS1 was increased in ESCC tissues, and a comparison of lncRNA levels between 179 pairs of ESCC tissues and their adjacent normal tissues showed that the upregulation of FMR1-AS1 was associated with poor prognosis in female patients. According to further experiments and analyses, ESCC-derived exosomal FMR1-AS1 maintains the interconversion state of cancer stem cells by activating the TLR7-NF-κB signaling pathway and upregulating c-Myc levels in target cells. All these studies demonstrate that when studying the relationship between exosomes and EC, we can focus on changes in the molecules of the relevant signaling pathways, which opens up possibilities for diagnosis and subsequent treatment.
The ECM is composed of collagen, glycoprotein, proteoglycans, and glucosamine and is a constantly changing part of the TME, supporting the survival and activity of cells, mediating the intercellular signal transduction system, and maintaining homeostasis. ECM remodeling depends on the functions of MMPs, adamalysins, meprins, growth factors secreted by tumor cells, monocytes, CAFs, polymorphonuclear cells, and leukocytes[85,86]. During tumor metastasis, collagen decomposes and destroys the basement membrane[79]. CAFs can generate exosomes that transmit miR-451 to the esophageal TME, mediating changes in the ECM and degradation of basement membrane collagen. As miR-451 is overexpressed in the serum of patients with EC compared with healthy individuals, it may serve as a potential diagnostic biomarker for EC[80].
The importance of exosomes in EC angiogenesis has been demonstrated in three reports. Zhang et al[87] showed that the exosome-encapsulated lncRNA FAM225A was highly expressed in ESCC tissues and cell lines and could sponge miR-206, which targets NETO2 and FOXP1 to accelerate angiogenesis. Exosomal cation-dependent mannose-6-phosphate receptors from SRGN-overexpressing ESCC cells facilitate neovascularization[88]. Tumor cell-derived exosomes can negatively regulate ESCC progression and angiogenesis by transmitting miR-154-5p, which downregulates kinesin family member 14[89].
Immune cells constitute an essential part of the immune system and play diverse protumor and antitumor roles in the genesis and metastasis of tumors. Tumor-derived exosomes can mediate the expansion of Tregs, MDSCs, and regulatory B cells (Bregs); inhibit the function of NK cells; and control the differentiation of myeloid progenitors, lymphoid progenitors, and dendritic cells (DCs) by delivering contents obtained from parent tumor cells to recipient cells[90]. Yuan et al[91] observed that exosomes produced from EC stem cells could transfer the nutrient sensor O-GlcNAc transferase to neighboring CD8+ T cells, silencing antitumor immune responses by increasing PD-1 expression in CD8+ T cells. These studies will bring new promise for immunotherapy of EC.
The steps underlying the formation of a premetastatic niche in target organs, which is a fundamental process required for tumor metastasis, include the arrival of tumor cell-secreted components, infiltration of bone marrow-derived cells, and alteration of the matrix microenvironment[92]. In addition to the vascular metastatic niche, the formation of a lymphatic metastatic niche in the local lymph nodes that induces immunosuppression, reduces proliferation, and enhances apoptosis is vital for metastasis in patients with ESCC[93]. Available evidence suggests that exosomes play an indispensable role in mediating signals between the primary tumor and target organs to construct a premetastatic niche in breast[94], pancreatic[95], and ovarian cancers[96]. As mentioned above, it is reasonable to hypothesize that exosomes participate in the generation of a premetastatic niche in ESCC.
Various types of treatment, including surgery, endoscopy, chemotherapy, radiotherapy, and immunotherapy, can be employed for EC; they can be used as monotherapy or in combination and should be selected carefully according to the cancer stage[96]. However, the use of multitarget drugs and the presence of cellular heterogeneity and immunoediting have led to the emergence of widespread treatment resistance, which greatly affects the survival rate of patients with EC[97,98]. Although detailed mechanisms of tumor therapy resistance have rarely been explored, exosomes, as tools for cell-to-cell communication, are speculated to play a vital role in the transmission of therapy resistance. In addition to the role of exosomes in tumor angiogenesis and immunosuppression, a large number of studies have reported that exosomes can mediate changes in signal transduction, drug efflux, and drug sequestration, leading to the development of tumor treatment resistance[99]. Therapeutic resistance remains the main factor that limits the application and efficacy of therapeutic measures for EC. In the following sections, we explain the role of exosomes in therapeutic resistance using specific examples.
When receiving chemotherapy, patients with EC can develop multidrug resistance via several mechanisms, including increase in drug efflux, attenuation of drug influx, alteration of cell apoptosis machinery, changes in molecular drug targets, and enhancement of drug metabolism, ultimately leading to treatment failure[98,100-102]. Exosomes derived from drug-resistant cells can induce sensitive tumor cells to acquire antitumor characteristics by transmitting proteins and nucleic acid cargo to regulate oncogene expression[61]. Additionally, exosomes can lead to drug resistance by directly or indirectly regulating immune cells in the EC microenvironment[103,104]. Owing to the complex and unknown mechanisms of chemotherapy resistance, it is difficult to find an effective solution.
Recently, CAF-derived exosomes have been reported to participate in EC resistance by establishing a physical barrier that inhibits the delivery of drugs and rays to tumor cells[105]. A previous study demonstrated that CAFs can generate monocytic myeloid-derived suppressor cells by activating signal transducer and activator of transcription 3 (STAT3) by secreting interleukin-6 and exosomal miR-21. Monocytic myeloid-derived suppressor cells not only dampen the function and promote apoptosis of some immune cells but also strengthen the cisplatin resistance of EC cells[106]. In 2020, a series of experiments conducted by Tong et al[59] suggested that ESCC cell-derived exosomal lncRNA POU3F3 affects the proliferation of tumor cells and cisplatin resistance of ESCC by activating normal fibroblasts.
Considering the importance of signaling pathways in mediating the development of drug resistance and their potential in treating EC, we focused on signaling pathways related to the exosome-based modulation of chemotherapy resistance. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, which includes more than 50 cytokines and growth factors, plays an important role in immune fitness, inflammation, and cell apoptosis; the pathway begins with the activation of JAK, which leads to the phosphorylation of STAT[107,108]. In 2020, a study reported 189 overexpressed and 304 downregulated miRNAs in exosomes secreted by cisplatin-resistant EC cells. Analysis of the correlation between small RNA-seq and RNA-seq revealed that exosomes derived from drug-resistant cells decreased the inhibitory effects of cisplatin on the cell cycle in sensitive cells and thus increased their cisplatin resistance in a manner that was potentially related to JAK/STAT signaling[109]. Exosomal circ_0000337 overexpression in cisplatin-resistant EC tissues and cells promoted cisplatin resistance by regulating the miR-377-3p/JAK2 axis[110]. JAK2 is a crucial component of the JAK2/STAT3 signaling pathway and its activation has been reported to mediate resistance to chemotherapy and radiotherapy[111]. Exosomal miR-21 was significantly overexpressed in EC cells with low sensitivity to cisplatin[112]. Further experiments indicated that exosomal miR-21 can inhibit the expression of PTEN, ultimately activating STAT3 signaling[106]. In summary, exosomes mediate chemotherapy resistance, especially cisplatin resistance, in EC through the JAK/STAT signaling pathway. However, whether JAK/STAT signaling participates in other forms of therapy resistance remains unknown. A recent study demonstrated that exosomal PD-L1 Leads to paclitaxel resistance in EC cells by targeting the STAT3/miR-21/PTEN/Akt axis[113]. CAF-derived exosomes induce cell proliferation, suppress cell apoptosis, and decrease tumor chemosensitivity to cisplatin in ESCC, and Cui et al[114] reported that RIG-I/IFN-β signaling plays a crucial role in these processes. Exploring additional signaling pathways that play an essential role in EC drug resistance and focusing on the relationship between exosomes and these pathways may help develop strategies to overcome the widespread problem of chemotherapy resistance[115].
Radiation can directly or indirectly kill cells within a limited range and restrict tumor growth by damaging DNA or generating free radicals and ions. However, tumors tend to exhibit radiation resistance via EMT, immunosuppression, repair of DNA damage, abnormal expression of noncoding RNAs, activation of autophagy and initiation of related signaling pathways[116]. The importance of exosomes in radiotherapy resistance in EC has been demonstrated in two reports. In vitro assays and xenograft models showed that hypoxic cell-derived exosomes transfer miR-340-5p to normoxic cells, thereby inducing the proliferation of radiotherapy-resistant normoxic ESCC cells by influencing the KLF10/UVRAG axis. The level of plasma exosomal miR-340-5p is upregulated and is associated with the in-field recurrence-free survival of patients with ESCC, suggesting that plasma exosomal miR-340-5p can serve as a biomarker for prognostic evaluation[117]. Additionally, NORAD is associated with local recurrence in patients with ESCC after radiotherapy, and in vitro experiments further confirmed that cell-to-cell transfer of exosome-encapsulated miR-199a-5p improved the radiosensitivity of radioresistant cells[118]. Further, miR-26b-5p contained in EVs derived from dying tumor cells after irradiation promotes cancer cell metastasis by potentiating the deposition of premetastatic niche components and the expansion and activation of MDSCs in the TME[119]. After radiotherapy, EC cells may acquire radiation resistance by secreting specific exosomes, and it is crucial to identify effective interference targets to improve the efficacy of radiotherapy and reduce the recurrence rate.
Immunotherapy exerts antitumor effects by strengthening the body's immune response and disrupting immune tolerance by employing tumor cells, nucleic acids, cytokines, antibodies, and/or immune cells[120]. Recently, based on the role of immune cells in the TME during EC development, immune checkpoint blockade, chimeric antigen receptor T-cell therapy, and tumor vaccines have been used for EC treatment. Despite significant breakthroughs in these immunotherapies, primary resistance lowers the overall response rate of immunotherapy and acquired resistance leads to a gradual decrease in immunotherapy efficacy in EC treatment[121]. Sensitive EC cells can acquire resistance via the downregulation of tumor antigens to escape the attack of T cells, the acquisition of immune escape ability via mutation, interferon-γ (IFN-γ) signaling, and neoantigen consumption[122]. Programmed death 1 (PD-1), which can interact with programmed death-ligand 1 (PD-L1), is a critical immune checkpoint that helps tumor cells escape T cell attack by disguising malignant cells as normal cells and promoting T cell depletion. PD-1/PD-L1 blockade strategies have been developed to overcome this problem by decreasing T cell suppression and activation[123]. However, changes in PD-L1 expression, decreased presentation of tumor neoantigens, and immune suppression may be responsible for the failure of anti-PD-1/PD-L1 immunotherapy. Exosomes derived from M2 TAMs regulate PD-1/PD-L1 expression and mediate PD-1/PD-L1 immunosuppression[124]. Tumor cell-derived exosomal PD-L1 can be used to supplement PD-1 on the cell surface, thereby antagonizing the effects of PD-1/PD-L1-blocking antibodies[125]. Exosomes may participate in immunotherapy resistance by disseminating immune checkpoint molecules, inhibitory molecules, cytokines, and growth factors, resulting in a poor clinical response[126]. In addition to PD-1/PD-L1, the relationship between exosomes and other immunotherapy approaches for EC remains to be explored. Identifying a reliable and universal relationship may help address immunotherapy resistance, although this remains a severe challenge.
As some exosomes can be found in the early stages of EC and change specifically as malignancy progresses, it is reasonable to use exosomes for the early diagnosis of ESCC to prevent EC progression, which will improve the cure rate of EC[127,128]. Compared with RNAs in plasma, exosomal RNAs have higher applicability and specificity in diagnosing EC for the following reasons: exosomes are stable in the body fluids of patients and can be used as an effective transport tool for RNAs; the level of exosomes correlates with that of donor cells; and exosomes can protect RNAs from degradation[127,129]. In addition, studies on EAC have reported that serum is a better sample type to test exosome content than plasma because it has fewer interfering components, and miRNAs of non-vesicle origins exist in plasma[130].
Recent studies have demonstrated that exosome-encapsulated miR-21 is prominently upregulated in patients with ESCC. In addition, the level of exosomal miR-21 was related to the clinicopathological features, clinical stage, and prognosis of patients with ESCC. In summary, exosomal miR-21 may serve as a clinical biomarker for human EC[131]. In a multicenter prospective study in 2022, researchers found that the newly discovered “small RNA identified in exosome from saliva of patients with ESCC” could be combined with tRNA-GlyGCC-5 to diagnose and determine the prognosis of EC. These two types of small RNAs can be found in saliva-derived exosomes, indicating the possible application of saliva-derived exosomal small RNAs as accurate and noninvasive predictive markers of EC[132]. Searching for ideal diagnostic molecules to be applied in combination may greatly improve diagnostic accuracy and reduce the false-positive rate. As the levels of circulating exosomal miRNAs differ between patients with Barrett's esophagus (BE) who develop EAC and those who do not and between patients with EAC and those with BE, they may predict and distinguish the prognosis of BE/EAC and distinguish them[27,133]. Previous reports suggest that the expression level of miR-93-5p is notably upregulated in patients with ESCC compared to healthy controls[134]. CircFNDC3B encapsulated in exosomes participates in the migration and invasion of EC cells by sponging miR-490-5p and regulating thioredoxin reductase 1 expression and is abundant in the tissues and cells of EC samples[135].
Currently, the diagnosis of EC mainly depends on medical imaging and endoscopy; laboratory examinations are insufficient and inaccurate. Owing to the disadvantages of imaging and endoscopy, such as being time consuming, expensive, and invasive, laboratory examinations should be the focus of research to identify strategies for screening and diagnosing EC. The application of exosome-based liquid biopsy for the early diagnosis of cancer has pioneering significance; however, this strategy cannot be widely applied because of its high false-positive rate and high cost[136]. Consequently, finding accurate and readily available contents of exosomes as a new strategy for diagnosing EC is complicated but crucial and urgent (Table 2).
| Clinical application | Biofluids | Exosomes or exosomal cargos | Expression | Ref. |
| Diagnosis | Plasma | miR-106a, miR-18a, miR-20b, miR-486-5p, miR-584 | High | [155] |
| Serum | lncRNA UCA1, POU3F3, ESCCAL-1 and PEG10 | High | [156] | |
| Plasma | lncRNAs NR_039819, NR_036133, NR_003353, ENST00000442416.1, and ENST00000416100.1 | High | [157] | |
| Plasma | miR-223-3p | Low | [122] | |
| Prognosis | Serum | miR-182 | High | [158] |
| Serum | miR-766-3p | High | [159] | |
| Serum | hsa_circ_0026611 | High | [160] | |
| Serum | lncRNA-POU3F3 | High | [156] | |
| Plasma | hsa_circ_0001946 | Low | [161] |
Only a limited number of patients can receive direct esophagectomy, the most effective treatment, whereas others need to choose an alternative comprehensive therapy to prolong survival if they have middle- or advanced-stage disease[137,138]. Although esophagectomy is the primary method of EC treatment, it can lead to a loss of appetite, dysphagia, reflux, and other complications, thereby seriously damaging the quality of life of patients[139]. Furthermore, patients treated with esophagectomy are susceptible to local recurrence owing to the presence of cells resistant to radiotherapy, immunotherapy, or chemotherapy. Therefore, there is an urgent need to develop novel therapeutic strategies for EC. Exosomes can serve as delivery tools by selectively transporting miRNAs, mRNAs, lncRNAs, and proteins to target cells, thereby providing new avenues for EC treatment[140]. Although no direct evidence has shown that exosomal miRNAs can alter target cell functions in BE/EAC, several studies have provided evidence supporting the application of exosomal miRNAs as therapeutic modalities to inhibit the progression from BE to EAC[141,142]. Exosome-derived miR-154-5p attenuates the invasion of EC cells and inhibits their angiogenic capability in vitro, curbing the malignant progression of ESCC[89]. Whether specific miRNAs regulate radiation resistance or can be used as tumor radiosensitizers in ESCC remains unclear. Luo et al[143] observed that some miRNAs, such as miR-339-5p, selectively secreted by exosomes, can promote sensitivity to radiation therapy by downregulating the cell division cycle 25 A (Cdc25A), and high levels of miR-339-5p in tumor tissues and serum indicated good prognosis, providing a theoretical basis for developing new tumor radiosensitizers based on the use of exosomal miRNAs.
In addition, exosomes derived from immune cells can exert antitumor effects by displaying specific surface antigens that initiate the human immune system, thus helping kill tumor cells[144]. Evidence suggests that MHC molecules and CD86 expressed on the surface membranes of exosomes derived from DCs are associated with T cell activation[145]. The activation of T cells, including CD4+ and CD8+ T cells, is vital for tumor immunity. Exosomes derived from tumor antigen-stimulated DCs carrying anti-CD3 and anti-EGFR antibodies promote the interaction of T cells with cancer cells[146]. We believe that exosomes can achieve similar regulatory effects in EC, suggesting new approaches for EC treatment.
Synthetic drugs must pass through the cell membrane and enter target cells to function[147,148]. Concomitantly, exosomes are better for delivering synthetic drugs than other materials, including liposomes, polymers, and dendrimers, as they are more biocompatible, biodegradable, less toxic, better at penetrating the blood-brain barrier, better at traveling deep into tissues, and have better target specificity for recipient cells[149,150]. In addition, exosomes cause minimal acute immune reactions, more accurately identify tumors, and are not targeted by early attack by circulating immune cells, ultimately prolonging the half-life of drugs and reducing drug resistance[148,151]. Similar to exosome isolation, there are multiple techniques for loading drugs into exosomes, including sonication, transfection, incubation, transgenesis, pH gradient loading, extrusion, and hypotonic dialysis[148].
When considering the application of exosomes as drug delivery systems, it is necessary to focus on engineered exosomes. Engineered exosomes are exosomes with particular molecules attached to their surface or loaded with specific molecules for delivery, which enables the exosomes to target cells or tissues and enhances the local enrichment of the delivered substance. In addition, the engineered exosomes considerably increased the output and shortened the time of exosome production. Engineered exosomes of different origins have different lipid and surface protein compositions that may affect their functions. For example, tumor cell-derived exosomes may mediate immune responses, immune cell-derived exosomes may induce immune evasion, and exosomes isolated from fruits, milk, or plants are usually safe, inexpensive, and scalable[152]. Therefore, it is important to select a suitable source of exosomes based on their applications and available conditions. Existing evidence shows that engineered M1 macrophage-derived exosomes can inhibit tumor growth and transform M2-type TAMs into M1-like macrophages by targeting IL4 receptors on the surfaces of M2 macrophages[153]. In summary, we can expect engineered exosomes to have great potential for use in EC treatment.
Based on the above data, the components of exosomes affect the progression of EC through some key signaling pathways, and we propose that we can not only use exosomes as vessels for the delivery of signaling pathway inhibitors or activators but also change the content of key substances in exosomes to affect downstream signaling. Additionally, exosomes can act as carriers to deliver critical components for gene therapy and cancer vaccines. Exogenous exosomes also hold tremendous potential for optimizing the therapeutic outcomes of the surgical treatment of EC. Notably, there are challenges that urgently need to be overcome for the successful application of exosomes in EC treatment. These may be addressed by developing strategies to enable the controlled or continuous release of therapeutic drugs, increasing the ability of exosomes to carry therapeutic agents, determining what cells should be used to produce exosomes, and determining how exosomes can escape cell and/or enzyme[154]. More efforts are needed to address these problems in order to apply exosomes in clinical therapy.
Valuable biomarkers with high sensitivity and availability for the diagnosis and prognosis of EC, as well as effective therapeutic strategies for EC to confine the disease to a manageable stage and improve disease-free survival in patients, need to be urgently identified. Increasing evidence has shown that exosomes are robustly involved in the metastasis and treatment resistance of EC by facilitating carcinoma cell proliferation, EMT, angiogenesis, and immunosuppression. Factors in the TME, especially cells, have an important impact on tumor growth and development. Furthermore, exosomes are differentially expressed and widely present in various biofluids, indicating that they can be used as novel biomarkers for the diagnosis and prognosis of EC and delivery of antitumor drugs. However, there remain several challenges to overcome for the clinical application of exosomes. Exosomes with considerable specificity and high sensitivity for discriminating patients from healthy individuals and EC from other cancers are urgently needed. New biomarkers should not only be easy and inexpensive to detect but should also show changes at an early stage of EC. Based on the findings of previous studies, we concluded that specific signaling pathways are associated with the exosome regulation of metastasis and drug resistance in EC. To identify suitable and precise targets for EC treatment, it is worth exploring more relevant signaling pathways. Overall, there is still a long way to go before exosomes can be used for clinical diagnosis, treatment, and prognosis of EC.
We also thank Figdraw.com vs Biorender.com for figure painting.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Christodoulidis G, Greece; Falasca M, Australia; Jeyaraman M, India S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64700] [Article Influence: 16175.0] [Reference Citation Analysis (177)] |
| 2. | Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189-197. [PubMed] |
| 3. | Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1220] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 4. | Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 2629] [Article Influence: 438.2] [Reference Citation Analysis (0)] |
| 5. | Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell Communication by Extracellular Vesicles: Focus on Microglia. Neuroscience. 2019;405:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 270] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 6. | Tarasov VV, Svistunov AA, Chubarev VN, Dostdar SA, Sokolov AV, Brzecka A, Sukocheva O, Neganova ME, Klochkov SG, Somasundaram SG, Kirkland CE, Aliev G. Extracellular vesicles in cancer nanomedicine. Semin Cancer Biol. 2021;69:212-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Maas SLN, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27:172-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1083] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 8. | Zhao Z, Yang S, Zhou A, Li X, Fang R, Zhang S, Zhao G, Li P. Small Extracellular Vesicles in the Development, Diagnosis, and Possible Therapeutic Application of Esophageal Squamous Cell Carcinoma. Front Oncol. 2021;11:732702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1613] [Cited by in RCA: 1748] [Article Influence: 249.7] [Reference Citation Analysis (0)] |
| 10. | Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1478] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 11. | Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 12. | Street JM, Koritzinsky EH, Glispie DM, Star RA, Yuen PS. Urine Exosomes: An Emerging Trove of Biomarkers. Adv Clin Chem. 2017;78:103-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, Fang X, Zhang X. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 489] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 14. | Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, Zhou C, Liang F, Shi S, Wang S, Qin W, Wang Q, Li F, Li Y, Shen L, Wei Y, Jia J. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15:1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 15. | Liu T, Zhang Q, Zhang J, Li C, Miao YR, Lei Q, Li Q, Guo AY. EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019;47:D89-D93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 16. | Gebara N, Scheel J, Skovronova R, Grange C, Marozio L, Gupta S, Giorgione V, Caicci F, Benedetto C, Khalil A, Bussolati B. Single extracellular vesicle analysis in human amniotic fluid shows evidence of phenotype alterations in preeclampsia. J Extracell Vesicles. 2022;11:e12217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | He Q, Liu L, Wei J, Jiang J, Rong Z, Chen X, Zhao J, Jiang K. Roles and action mechanisms of bile acid-induced gastric intestinal metaplasia: a review. Cell Death Discov. 2022;8:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Chen B, Sang Y, Song X, Zhang D, Wang L, Zhao W, Liang Y, Zhang N, Yang Q. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932-3947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 19. | Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 652] [Article Influence: 93.1] [Reference Citation Analysis (0)] |
| 20. | Jiang TY, Shi YY, Cui XW, Pan YF, Lin YK, Feng XF, Ding ZW, Yang C, Tan YX, Dong LW, Wang HY. PTEN Deficiency Facilitates Exosome Secretion and Metastasis in Cholangiocarcinoma by Impairing TFEB-mediated Lysosome Biogenesis. Gastroenterology. 2023;164:424-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 21. | Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 180] [Reference Citation Analysis (0)] |
| 22. | Hu N, Cai Z, Jiang X, Wang C, Tang T, Xu T, Chen H, Li X, Du X, Cui W. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 118] [Reference Citation Analysis (0)] |
| 23. | Hu Z, Chen G, Zhao Y, Gao H, Li L, Yin Y, Jiang J, Wang L, Mang Y, Gao Y, Zhang S, Ran J. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer. 2023;22:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 189] [Reference Citation Analysis (0)] |
| 24. | Zhang Q, Deng T, Zhang H, Zuo D, Zhu Q, Bai M, Liu R, Ning T, Zhang L, Yu Z, Ba Y. Adipocyte-Derived Exosomal MTTP Suppresses Ferroptosis and Promotes Chemoresistance in Colorectal Cancer. Adv Sci (Weinh). 2022;9:e2203357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 117] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 25. | Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, Zhou QA. Exosomes─Nature's Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano. 2022;16:17802-17846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 280] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 26. | Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 792] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 27. | Lv J, Zhao HP, Dai K, Cheng Y, Zhang J, Guo L. Circulating exosomal miRNAs as potential biomarkers for Barrett's esophagus and esophageal adenocarcinoma. World J Gastroenterol. 2020;26:2889-2901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Li K, Chen Y, Li A, Tan C, Liu X. Exosomes play roles in sequential processes of tumor metastasis. Int J Cancer. 2019;144:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 29. | Wang X, Huang J, Chen W, Li G, Li Z, Lei J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp Mol Med. 2022;54:1390-1400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 73] [Reference Citation Analysis (0)] |
| 30. | He J, Ren W, Wang W, Han W, Jiang L, Zhang D, Guo M. Exosomal targeting and its potential clinical application. Drug Deliv Transl Res. 2022;12:2385-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 31. | Chang W, Wang J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 32. | Lakshmi S, Hughes TA, Priya S. Exosomes and exosomal RNAs in breast cancer: A status update. Eur J Cancer. 2021;144:252-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 33. | Cheng J, Meng J, Zhu L, Peng Y. Exosomal noncoding RNAs in Glioma: biological functions and potential clinical applications. Mol Cancer. 2020;19:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 34. | O'Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 1193] [Article Influence: 238.6] [Reference Citation Analysis (0)] |
| 35. | Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of Exosome Composition. Cell. 2019;177:428-445.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 2071] [Article Influence: 345.2] [Reference Citation Analysis (1)] |
| 36. | Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K, Llorente A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017;70:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 37. | Lai JJ, Chau ZL, Chen SY, Hill JJ, Korpany KV, Liang NW, Lin LH, Lin YH, Liu JK, Liu YC, Lunde R, Shen WT. Exosome Processing and Characterization Approaches for Research and Technology Development. Adv Sci (Weinh). 2022;9:e2103222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 275] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 38. | Wang X, Xia J, Yang L, Dai J, He L. Recent progress in exosome research: isolation, characterization and clinical applications. Cancer Gene Ther. 2023;30:1051-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 39. | He L, Zhu D, Wang J, Wu X. A highly efficient method for isolating urinary exosomes. Int J Mol Med. 2019;43:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Gao H, Zhong S, Dangayach R, Chen Y. Understanding and Designing a High-Performance Ultrafiltration Membrane Using Machine Learning. Environ Sci Technol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, Du L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. 2021;9:811971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 302] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 42. | Sidhom K, Obi PO, Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 456] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 43. | Monguió-Tortajada M, Gálvez-Montón C, Bayes-Genis A, Roura S, Borràs FE. Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci. 2019;76:2369-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 44. | Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-3707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 707] [Cited by in RCA: 668] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 45. | Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 777] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 46. | Novoa Díaz MB, Martín MJ, Gentili C. Tumor microenvironment involvement in colorectal cancer progression via Wnt/β-catenin pathway: Providing understanding of the complex mechanisms of chemoresistance. World J Gastroenterol. 2022;28:3027-3046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Fokas E, Engenhart-Cabillic R, Daniilidis K, Rose F, An HX. Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev. 2007;26:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Wu P, Gao W, Su M, Nice EC, Zhang W, Lin J, Xie N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front Cell Dev Biol. 2021;9:641469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 49. | Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, Huang D, Zhao J, Yang L, Liao D, Su F, Li M, Liu Q, Song E. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172:841-856.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 879] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 50. | Nwabo Kamdje AH, Seke Etet PF, Kipanyula MJ, Vecchio L, Tagne Simo R, Njamnshi AK, Lukong KE, Mimche PN. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front Endocrinol (Lausanne). 2022;13:927390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu X, Zhang Z, Yang S, Xiao M. Extracellular matrix remodeling in tumor progression and immune escape: from mechanisms to treatments. Mol Cancer. 2023;22:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 52. | Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021;18:751-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 53. | De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 1306] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 54. | Arii S, Mori A, Uchida S, Fujimoto K, Shimada Y, Imamura M. Implication of vascular endothelial growth factor in the development and metastasis of human cancers. Hum Cell. 1999;12:25-30. [PubMed] |
| 55. | Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1083] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 56. | He G, Peng X, Wei S, Yang S, Li X, Huang M, Tang S, Jin H, Liu J, Zhang S, Zheng H, Fan Q, Yang L, Li H. Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications. Mol Cancer. 2022;21:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 57. | Mao Y, Wang Y, Dong L, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X, Li X, Fu Z. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 58. | Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 59. | Tong Y, Yang L, Yu C, Zhu W, Zhou X, Xiong Y, Wang W, Ji F, He D, Cao X. Tumor-Secreted Exosomal lncRNA POU3F3 Promotes Cisplatin Resistance in ESCC by Inducing Fibroblast Differentiation into CAFs. Mol Ther Oncolytics. 2020;18:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 60. | Min H, Sun X, Yang X, Zhu H, Liu J, Wang Y, Chen G. Exosomes Derived from Irradiated Esophageal Carcinoma-Infiltrating T Cells Promote Metastasis by Inducing the Epithelial-Mesenchymal Transition in Esophageal Cancer Cells. Pathol Oncol Res. 2018;24:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist Updat. 2019;45:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 62. | Kashima H, Noma K, Ohara T, Kato T, Katsura Y, Komoto S, Sato H, Katsube R, Ninomiya T, Tazawa H, Shirakawa Y, Fujiwara T. Cancer-associated fibroblasts (CAFs) promote the lymph node metastasis of esophageal squamous cell carcinoma. Int J Cancer. 2019;144:828-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 63. | Zarin B, Rafiee L, Daneshpajouhnejad P, Haghjooy Javanmard S. A review on the role of CAFs and CAF-derived exosomes in progression and metastasis of digestive system cancers. Tumour Biol. 2021;43:141-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Zhao G, Li H, Guo Q, Zhou A, Wang X, Li P, Zhang S. Exosomal Sonic Hedgehog derived from cancer-associated fibroblasts promotes proliferation and migration of esophageal squamous cell carcinoma. Cancer Med. 2020;9:2500-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 65. | Shi Z, Jiang T, Cao B, Sun X, Liu J. CAF-derived exosomes deliver LINC01410 to promote epithelial-mesenchymal transition of esophageal squamous cell carcinoma. Exp Cell Res. 2022;412:113033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Qiu L, Yue J, Ding L, Yin Z, Zhang K, Zhang H. Cancer-associated fibroblasts: An emerging target against esophageal squamous cell carcinoma. Cancer Lett. 2022;546:215860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 67. | Zhang H, Xie C, Yue J, Jiang Z, Zhou R, Xie R, Wang Y, Wu S. Cancer-associated fibroblasts mediated chemoresistance by a FOXO1/TGFβ1 signaling loop in esophageal squamous cell carcinoma. Mol Carcinog. 2017;56:1150-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 68. | Yuan X, Li Y, Zhang AZ, Jiang CH, Li FP, Xie YF, Li JF, Liang WH, Zhang HJ, Liu CX, Pang LJ, Shen XH, Li F, Hu JM. Tumor-associated macrophage polarization promotes the progression of esophageal carcinoma. Aging (Albany NY). 2020;13:2049-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Song J, Yang P, Li X, Zhu X, Liu M, Duan X, Liu R. Esophageal Cancer-Derived Extracellular Vesicle miR-21-5p Contributes to EMT of ESCC Cells by Disorganizing Macrophage Polarization. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 70. | Shou Y, Wang X, Chen C, Liang Y, Yang C, Xiao Q, Li H, Wang S, Shu J, Tian X, Chen K. Exosomal miR-301a-3p from esophageal squamous cell carcinoma cells promotes angiogenesis by inducing M2 polarization of macrophages via the PTEN/PI3K/AKT signaling pathway. Cancer Cell Int. 2022;22:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 71. | Lu Q, Wang X, Zhu J, Fei X, Chen H, Li C. Hypoxic Tumor-Derived Exosomal Circ0048117 Facilitates M2 Macrophage Polarization Acting as miR-140 Sponge in Esophageal Squamous Cell Carcinoma. Onco Targets Ther. 2020;13:11883-11897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 72. | Zhou J, Zheng S, Liu T, Liu Q, Chen Y, Ma R, Tan D, Lu X. Infiltrated M2 tumour-associated macrophages in the stroma promote metastasis and poor survival in oesophageal squamous cell carcinoma. Histol Histopathol. 2019;34:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Mi X, Xu R, Hong S, Xu T, Zhang W, Liu M. M2 Macrophage-Derived Exosomal lncRNA AFAP1-AS1 and MicroRNA-26a Affect Cell Migration and Metastasis in Esophageal Cancer. Mol Ther Nucleic Acids. 2020;22:779-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 74. | Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 991] [Article Influence: 247.8] [Reference Citation Analysis (0)] |
| 75. | Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 76. | Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH, Chen WC, Lee KD. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget. 2014;5:8716-8728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 77. | Wen J, Xuan B, Gao Y, Liu Y, Wang L, He L, Meng X, Zhou T, Tao Y, Guo K, Wang Y. Lnc-17Rik promotes the immunosuppressive function of Myeloid-Derived suppressive cells in esophageal cancer. Cell Immunol. 2023;385:104676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Colozza G, Koo BK. Wnt/β-catenin signaling: Structure, assembly and endocytosis of the signalosome. Dev Growth Differ. 2021;63:199-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 79. | Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;35:2871-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 338] [Cited by in RCA: 411] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 80. | Khazaei S, Nouraee N, Moradi A, Mowla SJ. A novel signaling role for miR-451 in esophageal tumor microenvironment and its contribution to tumor progression. Clin Transl Oncol. 2017;19:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Qiao G, Dai C, He Y, Shi J, Xu C. Effects of miR106b3p on cell proliferation and epithelialmesenchymal transition, and targeting of ZNRF3 in esophageal squamous cell carcinoma. Int J Mol Med. 2019;43:1817-1829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Xiao Z, Feng X, Zhou Y, Li P, Luo J, Zhang W, Zhou J, Zhao J, Wang D, Wang Y, Tian Z, Zhao X. Exosomal miR-10527-5p Inhibits Migration, Invasion, Lymphangiogenesis and Lymphatic Metastasis by Affecting Wnt/β-Catenin Signaling via Rab10 in Esophageal Squamous Cell Carcinoma. Int J Nanomedicine. 2023;18:95-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 83. | Jin Y, Meng Q, Zhang B, Xie C, Chen X, Tian B, Wang J, Shih TC, Zhang Y, Cao J, Yang Y, Chen S, Guan X, Hong A. Cancer-associated fibroblasts-derived exosomal miR-3656 promotes the development and progression of esophageal squamous cell carcinoma via the ACAP2/PI3K-AKT signaling pathway. Int J Biol Sci. 2021;17:3689-3701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 84. | Li W, Zhang L, Guo B, Deng J, Wu S, Li F, Wang Y, Lu J, Zhou Y. Exosomal FMR1-AS1 facilitates maintaining cancer stem-like cell dynamic equilibrium via TLR7/NFκB/c-Myc signaling in female esophageal carcinoma. Mol Cancer. 2019;18:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 85. | Brassart-Pasco S, Brézillon S, Brassart B, Ramont L, Oudart JB, Monboisse JC. Tumor Microenvironment: Extracellular Matrix Alterations Influence Tumor Progression. Front Oncol. 2020;10:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 86. | Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2221] [Cited by in RCA: 3036] [Article Influence: 303.6] [Reference Citation Analysis (0)] |
| 87. | Zhang C, Luo Y, Cao J, Wang X, Miao Z, Shao G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020;9:8600-8611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 88. | Yan D, Cui D, Zhu Y, Chan CKW, Choi CHJ, Liu T, Lee NPY, Law S, Tsao SW, Ma S, Cheung ALM. M6PR- and EphB4-Rich Exosomes Secreted by Serglycin-Overexpressing Esophageal Cancer Cells Promote Cancer Progression. Int J Biol Sci. 2023;19:625-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 89. | Shou Y, Wang X, Liang Y, Liu X, Chen K. Exosomes-derived miR-154-5p attenuates esophageal squamous cell carcinoma progression and angiogenesis by targeting kinesin family member 14. Bioengineered. 2022;13:4610-4620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 90. | Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. 2016;126:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 446] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 91. | Yuan Y, Wang L, Ge D, Tan L, Cao B, Fan H, Xue L. Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8(+) T cells. Cancer Lett. 2021;500:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 92. | Han P, Cao P, Hu S, Kong K, Deng Y, Zhao B, Li F. Esophageal Microenvironment: From Precursor Microenvironment to Premetastatic Niche. Cancer Manag Res. 2020;12:5857-5879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 93. | Otto B, Koenig AM, Tolstonog GV, Jeschke A, Klaetschke K, Vashist YK, Wicklein D, Wagener C, Izbicki JR, Streichert T. Molecular changes in pre-metastatic lymph nodes of esophageal cancer patients. PLoS One. 2014;9:e102552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Yuan X, Qian N, Ling S, Li Y, Sun W, Li J, Du R, Zhong G, Liu C, Yu G, Cao D, Liu Z, Wang Y, Qi Z, Yao Y, Wang F, Liu J, Hao S, Jin X, Zhao Y, Xue J, Zhao D, Gao X, Liang S, Song J, Yu S. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11:1429-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 95. | Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1554] [Cited by in RCA: 2061] [Article Influence: 206.1] [Reference Citation Analysis (0)] |
| 96. | Feng W, Dean DC, Hornicek FJ, Shi H, Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol Cancer. 2019;18:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 97. | Sharma U, Barwal TS, Acharya V, Singh K, Rana MK, Singh SK, Prakash H, Bishayee A, Jain A. Long Non-Coding RNAs as Strategic Molecules to Augment the Radiation Therapy in Esophageal Squamous Cell Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Yang W, Ma J, Zhou W, Zhou X, Cao B, Zhang H, Zhao Q, Fan D, Hong L. Molecular mechanisms and clinical implications of miRNAs in drug resistance of esophageal cancer. Expert Rev Gastroenterol Hepatol. 2017;11:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1034] [Article Influence: 172.3] [Reference Citation Analysis (0)] |
| 100. | Liu L, Ju Y, Wang J, Zhou R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol Res Pract. 2017;213:1242-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 101. | Qiang F, Guangguo R, Yongtao H, Dandan D, Hong Y. Multidrug resistance in primary tumors and metastases in patients with esophageal squamous cell carcinoma. Pathol Oncol Res. 2013;19:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Eichelmann AK, Matuszcak C, Lindner K, Haier J, Hussey DJ, Hummel R. Complex role of miR-130a-3p and miR-148a-3p balance on drug resistance and tumor biology in esophageal squamous cell carcinoma. Sci Rep. 2018;8:17553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Baba Y, Nomoto D, Okadome K, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Baba H. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020;111:3132-3141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 104. | Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front Immunol. 2021;12:656364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 105. | Li C, Teixeira AF, Zhu HJ, Ten Dijke P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer. 2021;20:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 106. | Zhao Q, Huang L, Qin G, Qiao Y, Ren F, Shen C, Wang S, Liu S, Lian J, Wang D, Yu W, Zhang Y. Cancer-associated fibroblasts induce monocytic myeloid-derived suppressor cell generation via IL-6/exosomal miR-21-activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma. Cancer Lett. 2021;518:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 107. | Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 1351] [Article Influence: 337.8] [Reference Citation Analysis (0)] |
| 108. | Darnell JE Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4322] [Cited by in RCA: 4587] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 109. | Shi S, Huang X, Ma X, Zhu X, Zhang Q. Research of the mechanism on miRNA193 in exosomes promotes cisplatin resistance in esophageal cancer cells. PLoS One. 2020;15:e0225290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Zang R, Qiu X, Song Y, Wang Y. Exosomes Mediated Transfer of Circ_0000337 Contributes to Cisplatin (CDDP) Resistance of Esophageal Cancer by Regulating JAK2 via miR-377-3p. Front Cell Dev Biol. 2021;9:673237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 111. | Mengie Ayele T, Tilahun Muche Z, Behaile Teklemariam A, Bogale Kassie A, Chekol Abebe E. Role of JAK2/STAT3 Signaling Pathway in the Tumorigenesis, Chemotherapy Resistance, and Treatment of Solid Tumors: A Systemic Review. J Inflamm Res. 2022;15:1349-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 112. | Yang YC, Liu GJ, Yuan DF, Li CQ, Xue M, Chen LJ. Influence of exosome-derived miR-21on chemotherapy resistance of esophageal cancer. Eur Rev Med Pharmacol Sci. 2019;23:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 113. | Wang H, Qi Y, Lan Z, Liu Q, Xu J, Zhu M, Yang T, Shi R, Gao S, Liang G. Exosomal PD-L1 confers chemoresistance and promotes tumorigenic properties in esophageal cancer cells via upregulating STAT3/miR-21. Gene Ther. 2023;30:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (1)] |
| 114. | Cui Y, Zhang S, Hu X, Gao F. Tumor-associated fibroblasts derived exosomes induce the proliferation and cisplatin resistance in esophageal squamous cell carcinoma cells through RIG-I/IFN-β signaling. Bioengineered. 2022;13:12462-12474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 115. | Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE, Liang J, Zhang Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 116. | Huang RX, Zhou PK. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 685] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 117. | Chen F, Xu B, Li J, Yang X, Gu J, Yao X, Sun X. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. 2021;40:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 118. | Sun Y, Wang J, Ma Y, Li J, Sun X, Zhao X, Shi X, Hu Y, Qu F, Zhang X. Radiation induces NORAD expression to promote ESCC radiotherapy resistance via EEPD1/ATR/Chk1 signalling and by inhibiting pri-miR-199a1 processing and the exosomal transfer of miR-199a-5p. J Exp Clin Cancer Res. 2021;40:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 119. | Yin X, Tian M, Zhang J, Tang W, Feng L, Li Z, Zheng C, Liu C, Yan L, Yu X, Li B. MiR-26b-5p in small extracellular vesicles derived from dying tumor cells after irradiation enhances the metastasis promoting microenvironment in esophageal squamous cell carcinoma. Cancer Lett. 2022;541:215746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 120. | Helmy KY, Patel SA, Nahas GR, Rameshwar P. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4:1307-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 121. | Li R, Huang B, Tian H, Sun Z. Immune evasion in esophageal squamous cell cancer: From the perspective of tumor microenvironment. Front Oncol. 2022;12:1096717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 122. | Fang P, Zhou J, Liang Z, Yang Y, Luan S, Xiao X, Li X, Zhang H, Shang Q, Zeng X, Yuan Y. Immunotherapy resistance in esophageal cancer: Possible mechanisms and clinical implications. Front Immunol. 2022;13:975986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 123. | Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15:1111-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 124. | Pu Y, Ji Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front Immunol. 2022;13:874589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 125. | Yin Z, Yu M, Ma T, Zhang C, Huang S, Karimzadeh MR, Momtazi-Borojeni AA, Chen S. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: a key role of exosomal PD-L1. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 126. | Cheng C, Zhuge L, Xiao X, Luan S, Yuan Y. Overcoming resistance to PD-1/PD-L1 inhibitors in esophageal cancer. Front Oncol. 2022;12:955163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 127. | Zhao G, Zhou A, Li X, Zhu S, Wang Y, Zhang S, Li P. The Significance of Exosomal RNAs in the Development, Diagnosis, and Treatment of Gastric Cancer. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 128. | Lorenc T, Klimczyk K, Michalczewska I, Słomka M, Kubiak-Tomaszewska G, Olejarz W. Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 129. | Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 306] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 130. | Chiam K, Mayne GC, Wang T, Watson DI, Irvine TS, Bright T, Smith LT, Ball IA, Bowen JM, Keefe DM, Thompson SK, Hussey DJ. Serum outperforms plasma in small extracellular vesicle microRNA biomarker studies of adenocarcinoma of the esophagus. World J Gastroenterol. 2020;26:2570-2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 131. | Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M, Baba H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 132. | Li K, Lin Y, Luo Y, Xiong X, Wang L, Durante K, Li J, Zhou F, Guo Y, Chen S, Chen Y, Zhang D, Yeung SJ, Zhang H. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: a multicenter prospective study. Mol Cancer. 2022;21:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 133. | Xu J, Yin Z, Yang L, Wu F, Fan J, Huang Q, Jin Y, Yang G. Evidence that dysplasia related microRNAs in Barrett's esophagus target PD-L1 expression and contribute to the development of esophageal adenocarcinoma. Aging (Albany NY). 2020;12:17062-17078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Liu MX, Liao J, Xie M, Gao ZK, Wang XH, Zhang Y, Shang MH, Yin LH, Pu YP, Liu R. miR-93-5p Transferred by Exosomes Promotes the Proliferation of Esophageal Cancer Cells via Intercellular Communication by Targeting PTEN. Biomed Environ Sci. 2018;31:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 135. | Tang B, Zhang Q, Liu K, Huang Y. Exosomal circRNA FNDC3B promotes the progression of esophageal squamous cell carcinoma by sponging miR-490-5p and regulating thioredoxin reductase 1 expression. Bioengineered. 2022;13:13829-13848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 136. | Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 557] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 137. | Kakeji Y, Oshikiri T, Takiguchi G, Kanaji S, Matsuda T, Nakamura T, Suzuki S. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus. 2021;18:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 138. | Abdelhakeem A, Blum Murphy M. Adjuvant Therapies for Esophageal Cancer. Thorac Surg Clin. 2022;32:457-465. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 139. | Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, Imamura Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 140. | Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 798] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 141. | van Baal JW, Verbeek RE, Bus P, Fassan M, Souza RF, Rugge M, ten Kate FJ, Vleggaar FP, Siersema PD. microRNA-145 in Barrett's oesophagus: regulating BMP4 signalling via GATA6. Gut. 2013;62:664-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 142. | Craig MP, Rajakaruna S, Paliy O, Sajjad M, Madhavan S, Reddy N, Zhang J, Bottomley M, Agrawal S, Kadakia MP. Differential MicroRNA Signatures in the Pathogenesis of Barrett's Esophagus. Clin Transl Gastroenterol. 2020;11:e00125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 143. | Luo A, Zhou X, Shi X, Zhao Y, Men Y, Chang X, Chen H, Ding F, Li Y, Su D, Xiao Z, Hui Z, Liu Z. Exosome-derived miR-339-5p mediates radiosensitivity by targeting Cdc25A in locally advanced esophageal squamous cell carcinoma. Oncogene. 2019;38:4990-5006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 144. | Zhao Y, Liu T, Zhou M. Immune-Cell-Derived Exosomes for Cancer Therapy. Mol Pharm. 2022;19:3042-3056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 145. | Pitt JM, André F, Amigorena S, Soria JC, Eggermont A, Kroemer G, Zitvogel L. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 474] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 146. | Fan M, Liu H, Yan H, Che R, Jin Y, Yang X, Zhou X, Yang H, Ge K, Liang XJ, Zhang J, Li Z. A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy. Biomaterials. 2022;282:121424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 147. | Arafiles JVV, Futaki S. Chemical passports to cross biological borders. Nat Chem. 2021;13:517-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Zhao X, Wu D, Ma X, Wang J, Hou W, Zhang W. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020;128:110237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 149. | Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 909] [Article Influence: 227.3] [Reference Citation Analysis (0)] |
| 150. | Chinnappan M, Srivastava A, Amreddy N, Razaq M, Pareek V, Ahmed R, Mehta M, Peterson JE, Munshi A, Ramesh R. Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. 2020;486:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 151. | Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J, Fang Y, Xia Y, Cheng G, He X, Zheng SY. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018;78:798-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 152. | Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 856] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 153. | Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 302] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 154. | Wang J, Chen D, Ho EA. Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 2021;329:894-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 155. | Zhou X, Wen W, Zhu J, Huang Z, Zhang L, Zhang H, Qi LW, Shan X, Wang T, Cheng W, Zhu D, Yin Y, Chen Y, Zhu W, Shu Y, Liu P. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8:34468-34480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 156. | Yan S, Du L, Jiang X, Duan W, Li J, Xie Y, Zhan Y, Zhang S, Wang L, Li S, Wang C. Evaluation of Serum Exosomal lncRNAs as Diagnostic and Prognostic Biomarkers for Esophageal Squamous Cell Carcinoma. Cancer Manag Res. 2020;12:9753-9763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 157. | Jiao Z, Yu A, Rong W, He X, Zen K, Shi M, Wang T. Five-lncRNA signature in plasma exosomes serves as diagnostic biomarker for esophageal squamous cell carcinoma. Aging (Albany NY). 2020;12:15002-15010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 158. | Qiu ML, Li X, Lin JB, Luo RG, Liu B, Feng Z. Serum exosomal miR-182 upregulation predicts unfavorable prognosis of esophageal squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2020;24:5412-5418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 159. | Liu S, Lin Z, Zheng Z, Rao W, Lin Y, Chen H, Xie Q, Chen Y, Hu Z. Serum exosomal microRNA-766-3p expression is associated with poor prognosis of esophageal squamous cell carcinoma. Cancer Sci. 2020;111:3881-3892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 160. | Liu S, Lin Z, Rao W, Zheng J, Xie Q, Lin Y, Lin X, Chen H, Chen Y, Hu Z. Upregulated expression of serum exosomal hsa_circ_0026611 is associated with lymph node metastasis and poor prognosis of esophageal squamous cell carcinoma. J Cancer. 2021;12:918-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 161. | Fan L, Cao Q, Liu J, Zhang J, Li B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer. 2019;18:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |