Published online Nov 7, 2023. doi: 10.3748/wjg.v29.i41.5657
Peer-review started: July 10, 2023
First decision: September 1, 2023
Revised: September 14, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: November 7, 2023
Processing time: 120 Days and 3.2 Hours
Functional constipation (FC) and constipation-predominant irritable bowel syndrome (IBS-C) represent a spectrum of constipation disorders. However, the majority of previous clinical investigations have focused on Western populations, with limited data originating from China.
To determine and compare the colorectal motility and psychiatric features of FC and IBS-C in an Eastern Chinese population.
Consecutive chronic constipation patients referred to our motility clinic from December 2019 to February 2023 were enrolled. FC and IBS-C diagnoses were established using ROME IV criteria, and patients underwent high-resolution anorectal manometry (ARM) and a colonic transmit test using the Sitz marker study. Constipation-related symptoms were obtained through questionnaires. Anxiety and depression were assessed by the Hamilton anxiety rating scale and the Hamilton Depression Rating Scale-21. The clinical characteristics and colorectal motility patterns of FC and IBS-C patients were compared.
No significant differences in sex, age or abdominal discomfort symptoms were observed between IBS-C and FC patients (all P > 0.05). The proportion of IBS-C patients with delayed colonic transit was higher than that of patients with FC (36.63% vs 15.91%, P < 0.05), while rectosigmoid accumulation of radiopaque markers was more common in the FC group than in the IBS-C group (50% vs 26.73%, P < 0.05). Diverse proportions of these dyssynergic patterns were noted within both the FC and IBS-C groups by ARM. IBS-C patients were found to have a higher prevalence of depression than FC patients (66.30% vs 42.42%, P < 0.05). The scores for feelings of guilt, suicide, psychomotor agitation, diurnal variation, obsessive/compulsive disorder, hopelessness, self-abasedment and gastrointestinal symptoms were significantly higher in IBS-C patients than that in FC patients (P < 0.05). For IBS-C
Our findings highlight both overlapping and distinctive patterns of colon transit, dyssynergic patterns, anorectal sensation, psychological distress, and associations of psychiatric and colorectal motility characteristics in FC and IBS-C patients in an Eastern Chinese population, providing valuable insights into the pathophysiological underpinnings of these disorders.
Core Tip: Functional constipation (FC) and constipation-predominant irritable bowel syndrome (IBS-C) are the two primary subtypes of constipation. Previous clinical studies that attempted to illuminate distinctive physiological mechanisms between FC and IBS-C patients were predominantly from Western countries, with limited data originating from China. Our study has revealed distinctive elements of FC and IBS-C across multifaceted parameters, namely colonic transmit time, psychological distress, and dyssynergic patterns, and the relationship among these parameters. These findings extend our comprehension of the intricate pathophysiological mechanisms underlying FC and IBS-C. These findings could provide guidance for constipation patients to choose appropriate colorectal tests.
- Citation: Lv CL, Song GQ, Liu J, Wang W, Huang YZ, Wang B, Tian JS, Yin MQ, Yu Y. Colorectal motility patterns and psychiatric traits in functional constipation and constipation-predominant irritable bowel syndrome: A study from China. World J Gastroenterol 2023; 29(41): 5657-5667
- URL: https://www.wjgnet.com/1007-9327/full/v29/i41/5657.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i41.5657
Chronic constipation (CC) represents a prevalent health challenge globally, impacting approximately 4% to 10% of the population in China[1,2]. The process of fecal evacuation is governed by a complex interplay of brain-gut axis interactions, intestinal peristalsis, and the function of the pelvic floor muscles and anal sphincter. The multifaceted pathogenesis of CC involves a dynamic interplay between biological and psychosocial factors. Two principal contributors to the pathophysiology of CC are colonic sensorimotor disturbances and pelvic floor dysfunction[3,4]. A multitude of diagnostic tools, including high-resolution anorectal manometry (HR-ARM), the colonic transmit test (CTT), barium and magnetic resonance defecography, and the balloon expulsion test (BET) can provide valuable insights into the underlying mechanisms of CC. In a clinical setting, the assessment of colorectal motility and psychiatric evaluations are often essential when patients with CC do not respond to conventional laxative treatments[5].
Functional constipation (FC) and constipation-predominant irritable bowel syndrome (IBS-C) are the two primary subtypes of CC. As per the Rome IV criteria, an IBS-C diagnosis requires the presence of abdominal pain or discomfort, a criterion that is not required for FC. The Rome IV guidelines also suggest that IBS-C and FC are not distinct disorders but rather exist on a continuum of constipation disorders. Despite overlaps in symptoms, pathophysiological mechanisms and treatment responses, distinctions between IBS-C and FC exist[6]. A thorough understanding of these physiological mechanisms could enhance our ability to differentiate between IBS-C and FC more effectively than relying solely on symptoms. While several clinical studies, predominantly from Western countries, have attempted to illuminate this issue[7-9], data from Chinese populations remain scarce.
In China, the first choice of treatment for patients with treatment-resistant constipation is often ARM, the only minimally invasive tool available to measure anorectal pressures. Due to its affordability, ease of execution, and ready availability, the radiopaque marker technique is widely utilized to assess colonic transit[10]. Therefore, in our study, we selected these two modalities to examine the physiological mechanisms of CC. However, controversy persists regarding the correlation between these two tests, and it remains unclear which test provides more meaningful data for IBS-C or FC[7-11]. Thus, our study aimed to compare the psychiatric (depression and anxiety) and colorectal motility (colonic transit and anorectal motility and sensation) characteristics between FC and IBS-C patients in an Eastern Chinese population. We also sought to investigate the correlations between psychiatric and colorectal motility characteristics in both FC and IBS-C patients.
For this study, we recruited patients diagnosed with FC and IBS-C from the Anhui Provincial Hospital’s motility clinic between December 2019 and February 2023. The diagnoses of FC and IBS-C were made according to the Rome IV criteria[6]. The exclusion criteria included pregnancy or lactation; diabetes; thyroid dysfunction; and cardiovascular, hepatic or renal disease. All patients underwent standardized HR-ARM and CTT. The study received approval from the Ethics Committee of Anhui Provincial Hospital.
HR-ARM was conducted using a water-perfusion HR-ARM device (GAP-08A, Maida Instruments, Ningbo, China) according to the London consensus protocol[12]. Patients remained in the left lateral decubitus position during simulated evacuation. Four patterns of dyssynergia were classified according to the Rao classification, along with a normal and an unclassified pattern. Type I dyssynergia showed an adequate increase in rectal pressure (≥ 45 mmHg) accompanied by a paradoxical simultaneous increase in anal pressure; type II dyssynergia showed an inadequate increase in rectal pressure of (< 45 mmHg) (poor propulsive force) accompanied by a paradoxical simultaneous increase in anal pressure; type III dyssynergia showed an adequate increase in rectal pressure (≥ 40 mmHg) accompanied by failure of reduction in anal pressure (≤ 20% baseline pressure); and type IV dyssynergia showed an inadequate increase in rectal pressure of (< 45 mmHg) (poor propulsive force) accompanied by failure of reduction in anal pressure (≤ 20% baseline pressure). The normal pattern showed an adequate increase in rectal pressure (≥ 45 mmHg) accompanied by a simultaneous reduction in anal pressure. The unclassified pattern showed anorectal pressure changes not consistent with any patterns mentioned above[13]. The rectal sensory test was subsequently performed, recording sensory thresholds based on balloon volumes at first constant sensation, desire to defecate, maximum tolerance, and sustained urgency[10].
Colonic transit time was assessed using radiopaque marker techniques (Sitzmarks; Konsyl Pharmaceuticals, TX, United States). Medications that might affect gastrointestinal transmission were discontinued for 1 wk before and during the CTT study. Patients were instructed to adhere to their regular diet and avoid laxatives throughout the study. The patients ingested a single capsule containing 24 radiopaque markers on day 1, and an abdominal X-ray was obtained 48 h later. The X-ray analysis determined the number and distribution of the markers as per the protocol described by Metcalf et al[14]. Spinal processes and imaginary lines from the fifth lumbar vertebra to the pelvic outlets served as landmarks by which the right colon (RC), left colon (LC) and rectosigmoid (RS) colon were defined. Patients were classified as positive for evidence of normal transit constipation (NTC) when less than 10% of the markers were visible throughout the colon at 48 h. Slow transit constipation (STC) was defined as retention of more than 50% of the markers in the RC and LC on imaging. RS accumulation of radiopaque markers (RSARM), defined as retention of more than 50% of the markers in the RS region, suggested the possibility of functional defecation disorders[10,14,15].
All patients were asked to complete the clinical symptoms questionnaires capturing data such as age, sex, stool frequency, Bristol stool form scale score, abdominal pain, abdominal bloating, relationship between abdominal discomfort and defecation, straining during a bowel movement, feeling of incomplete emptying, sensation that stool cannot be passed and feeling of defecation urgency. The Hamilton anxiety rating scale (HAMA) and the Hamilton Depression Rating Scale (HAMD)-21 were used to evaluate patients’ mental health, with higher scores signifying more severe anxiety and depression. A score ranging from 7 to 13 was indicative of possible anxiety, a score ranging from 14 to 20 was indicative of anxiety, and a score ≥ 21 was indicative of severe anxiety. A score ranging from 8 to 19 was indicative of mild depression, a score ranging from 20 to 34 was indicative of moderate depression, and a score ≥ 35 was indicative of severe depression[16,17].
Baseline demographic, clinical, ARM, and CTT variables were compared between IBS-C patients and FC patients. The proportions of each variable were compared using the chi-square test, while means and medians were evaluated using the Student’s t test and the Wilcoxon rank-sum test, respectively. The association between variables was determined using the Pearson correlation coefficient. All data were analyzed using SPSS 19.0. P < 0.05 was considered statistically significant.
Our study comprised 230 patients with CC, of whom 149 were diagnosed with IBS-C and 81 with FC. Females represented a larger portion of both groups (82.39%) compared to males (17.61%). No statistically significant differences were observed in terms of sex, age, or abdominal discomfort symptoms between the IBS-C and FC cohorts (Table 1; P > 0.05).
| Variable | IBS-C | FC | P value |
| Age (yr) | 42.32 ± 14.41 | 43.95 ± 18.04 | > 0.05 |
| Sex | > 0.05 | ||
| Male | 17 | 8 | |
| Female | 84 | 33 | |
| Stool frequency | > 0.05 | ||
| > 6 d | 23 | 6 | |
| < 6 d | 72 | 27 | |
| Feeling of incomplete emptying | > 0.05 | ||
| Yes | 76 | 26 | |
| No | 18 | 13 | |
| Sensation that stool cannot be passed | > 0.05 | ||
| Yes | 54 | 16 | |
| No | 16 | 11 | |
| Feeling of defecation urgency | > 0.05 | ||
| Yes | 39 | 7 | |
| No | 56 | 24 | |
| Straining during a bowel movement | > 0.05 | ||
| Yes | 91 | 28 | |
| No | 5 | 5 |
IBS-C patients demonstrated a higher prevalence of delayed colonic transit than FC patients (36.63% vs 15.91%, P < 0.05). Conversely, RSARM was more common among FC patients (50% vs 26.73%, P < 0.05). No significant differences were observed in the proportions of patients with a normal colon transit time between the two groups (36.63% vs 34.09%, P > 0.05) (Table 2).
| Variable | IBS-C | FC | P value |
| ARM | |||
| Dyssynergic patterns | > 0.05 | ||
| I | 19 | 12 | |
| II | 18 | 8 | |
| III | 5 | 1 | |
| IV | 14 | 10 | |
| Normal | 6 | 1 | |
| Unclassified | 7 | 1 | |
| Anorectal sensation thresholds | |||
| First constant sensation | > 0.05 | ||
| Low | 2 | 1 | |
| High | 38 | 14 | |
| Normal | 25 | 10 | |
| Desire to defecate | > 0.05 | ||
| Low | 30 | 10 | |
| High | 4 | 1 | |
| Normal | 22 | 10 | |
| Sustained urgency | > 0.05 | ||
| Low | 30 | 12 | |
| High | 4 | 1 | |
| Normal | 29 | 14 | |
| CTT | < 0.05 | ||
| STC | 37 | 7 | |
| RS accumulation | 27 | 22 | |
| Normal | 37 | 15 | |
| Psychiatric characteristics | |||
| Scores | > 0.05 | ||
| HAMA | 11.71 ± 9.48 | 12.00 ± 9.15 | |
| HAMD | 11.51 ± 8.61 | 9.41 ± 0.33 | |
| Anxiety | > 0.05 | ||
| No | 34 | 13 | |
| Possible | 24 | 7 | |
| Yes | 35 | 13 | |
| Depression | < 0.05 | ||
| No | 31 | 19 | |
| Possible | 46 | 9 | |
| Yes | 15 | 5 |
There were no significant differences in the prevalence of dyssynergic patterns (I-IV) observed on HR-ARM between the FC (81%) and IBS-C (85.2%) groups. Moreover, diverse proportions of these dyssynergic patterns were noted within both groups. In FC patients, the type I pattern was most prevalent (41.94%), while the type II pattern was most commonly observed in IBS-C patients (34.55%). The type III pattern was found to be the least common in both groups (3.23% in FC and 10.91% in IBS-C). Although a marginal increase in the prevalence of type II and type IV patterns was noticed in FC patients compared to IBS-C patients, the difference was not statistically significant (56.3% vs 55.9%, P > 0.05).
In the assessment of sensory thresholds, a majority of both IBS-C (58.46%) and FC (56%) patients exhibited high thresholds for the first constant sensation. Low thresholds for the desire to defecate were more commonly observed in IBS-C patients (53.57%) and in approximately half of the FC patients (47.62%). Slightly higher proportions of high thresholds for the first constant sensation, low thresholds for the desire to defecate, and anxiety or potential anxiety (63.44% vs 60.61%) were observed in IBS-C patients compared to FC patients, although these differences lacked statistical significance (P > 0.05) (Table 3).
| Variable | IBS-C | FC | ||||||
| Anxiety | Without anxiety | Depression | Without depression | Anxiety | Without anxiety | Depression | Without depression | |
| STC | 21 | 16 | 25 | 12 | 4 | 1 | 3 | 2 |
| NTC | 24 | 6 | 21 | 9 | 8 | 3 | 5 | 6 |
| First sensation | ||||||||
| High | 26 | 8 | 21 | 11 | 10 | 1 | 5 | 6 |
| Normal | 14 | 9 | 11 | 12 | 4 | 5 | 4 | 5 |
| Desire toa | ||||||||
| Low | 19 | 11 | 13 | 16 | 6 | 4 | 6 | 4 |
| Normal | 16 | 6 | 15 | 6 | 8 | 2 | 4 | 6 |
| High | 4 | 0 | 3 | 1 | 1 | 0 | 0 | 1 |
| Urgency | ||||||||
| Low | 15 | 12 | 14 | 12 | 4 | 4 | 5 | 3 |
| Normal | 21 | 5 | 15 | 10 | 10 | 2 | 5 | 7 |
| High | 3 | 0 | 2 | 1 | 1 | 0 | 0 | 1 |
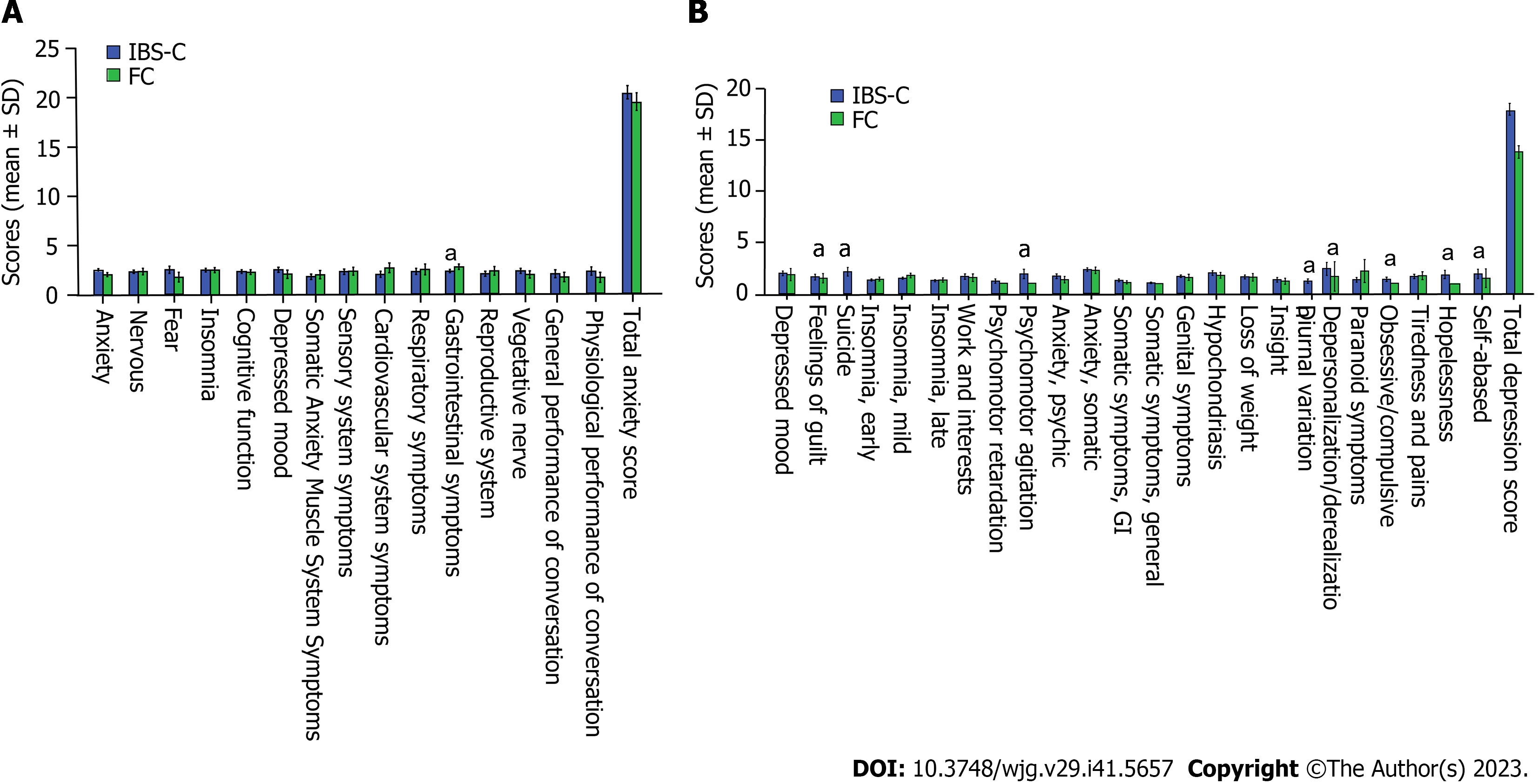
A significantly higher prevalence of depression was observed among IBS-C patients compared to their FC counterparts (66.30% vs 42.42%, P < 0.05). Although the incidence of anxiety was slightly higher among IBS-C patients, this difference was not statistically significant (63.44% vs 60.61%, P > 0.05). The mean scores for HAMA and HAMD were similar for both groups (Table 1; P > 0.05). However, IBS-C patients reported significantly higher scores for specific symptoms, such as feelings of guilt, suicidal ideation, psychomotor agitation, diurnal variation, obsessive-compulsive tendencies, hopelessness, self-abasement, and gastrointestinal symptoms (Figure 1; P < 0.05).
In both the IBS-C and FC patient cohorts, a significant percentage of individuals with NTC or STC demonstrated symptoms of anxiety or depression. However, the incidence of depression was comparable among patients with NTC and STC (70% vs 67.57% for IBS-C, 45.45% vs 60% for FC, P > 0.05). Interestingly, in the IBS-C group (but not the FC group), patients with NTC exhibited a notably higher prevalence of anxiety than those with STC (80% vs 56.76%, χ2 = 5.438, P < 0.05).
In the IBS-C group, again not observed in the FC group, weak correlations were identified between the degrees of anxiety and the thresholds for first constant sensation, desire to defecate, and sustained urgency (r = 0.414, r = 0.404, and r = 0.418, respectively, P < 0.05). Furthermore, IBS-C patients suffering from depression exhibited a lower prevalence of low thresholds for the desire to defecate than those without depression (69.6% vs 41.9%, χ2 = 4.054, P < 0.05). No substantial correlation was observed between depression and other anorectal sensations (P > 0.05) (Table 3 and 4).
| Thresholds of first sensation | Thresholds of desire to defecate | Thresholds of urgency | |||||||||||||
| High | Normal | r | P value | Low | Normal | High | r | P value | Low | Normal | High | r | P value | ||
| IBS-C | Anxiety degree | 0.414 | < 0.05 | 0.404 | < 0.05 | 0.407 | < 0.05 | ||||||||
| Depression degrees | 0.803 | > 0.05 | 0.019 | > 0.05 | -0.018 | > 0.05 | |||||||||
| FC | Anxiety degree | 0.241 | > 0.05 | 0.111 | > 0.05 | 0.242 | > 0.05 | ||||||||
| Depression degrees | -0.098 | > 0.05 | -0.166 | > 0.05 | -0.233 | > 0.05 | |||||||||
The probability of normal patterns in ARM was 9.68% for patients who exhibited RSARM during CTT tests. Conversely, a 16.67% probability of RSARM was found in patients devoid of type I-IV patterns. RSARM was prominent in 77.78% of FC patients with a type IV pattern, while only 16.67% of IBS-C patients with a type I pattern exhibited RSARM. Notably, all patients with a type III pattern demonstrated RSARM in both the IBS-C and FC cohorts.
We observed high proportions of dyssynergic patterns in both IBS-C and FC patients. Interestingly, these patterns also occurred in a substantial proportion of asymptomatic individuals, potentially due to the unnatural posture adopted during simulated defecation[18]. A previous study by Grossi et al[19] suggested that the type IV pattern could be helpful for differentiating FC patients from healthy volunteers, with rectal pressure measurements proving more indicative than anal pressure measurements. Furthermore, a study conducted in India highlighted a higher prevalence of insufficient rectal force in FC patients than in those with IBS-C[9]. Consistent with these findings, our study detected a marginal increase in the incidence of insufficient rectal force and type IV dyssynergia among FC patients compared with their IBS-C counterparts, although these differences were not statistically significant. The potential for abnormal rectal pressure shifts during a push maneuver to serve as an effective discriminator between IBS-C or FC and a healthy state within the Chinese population requires further investigation.
Beyond the evaluation of anorectal pressure, ARM also offers the opportunity to gather additional physiological data relating to anorectal sensation[20]. Abnormal visceral sensitivity has been linked with intestinal dysfunction. It is common for constipation patients to exhibit rectal hyposensitivity, whereas rectal hypersensitivity often accompanies IBS[3,4]. In our study, we observed a substantial proportion of both IBS-C and FC patients displaying high thresholds for initial sensation, potentially contributing to fecal retention. This occurrence was slightly more prevalent in IBS-C patients than in FC patients; however, the difference between the two patient cohorts was not statistically significant.
Psychiatric conditions have been suggested to influence constipation[5]. In our study, we observed a higher incidence of depression among IBS-C patients than among FC patients, a finding consistent with prior studies conducted in China[21]. Moreover, our results showed a positive correlation between anxiety levels and anorectal sensory thresholds in IBS-C patients, suggesting that elevated anxiety may decrease rectal sensitivity, potentially leading to constipation in this patient group.
Impaired peristaltic motility within the intestine is another potential factor contributing to CC. Utilizing radiopaque markers, we evaluated colonic transit in our study population. Our results showed comparable proportions of NTC in IBS-C and FC patients and an increased prevalence of STC among IBS-C patients. Previous studies reported varied findings regarding the prevalence of STC and NTC in IBS-C and FC patients. For example, research by Lam et al[22] indicated that STC was more common in FC patients than in IBS-C patients, a finding corroborated by Patcharatrakul and Gonlachanvit[23]. Conversely, Shekhar et al[24] did not observe a significant difference in STC prevalence between the two patient groups. The discrepancies in these findings may be attributable to variations in diet, ethnicity, or methodology across studies. Psychological stress may also affect colonic motor activity[25]. We further explored the relationship between CTT and psychological stress. Our data showed that IBS-C patients with NTC were more likely to experience anxiety compared to those with STC. However, we found no significant correlations between psychological stress and colonic motility in FC patients. This difference indicates that emotional factors may have varying effects on colonic motility between these two patient groups.
Dyssynergic defecation (DD) is a condition characterized by a patient’s inability to effectively coordinate abdominal and pelvic floor muscles to eliminate stool, commonly seen in functional defecatory disorders. According to the ROME IV classification, DD can be diagnosed in both FC and IBS-C patients. Given the high diagnostic sensitivity of ARM for DD, a normal ARM result can be quite effective in excluding DD[26]. Radiopaque transit studies not only assess colonic transit but can also indicate outlet obstruction based on marker accumulation in the RS region. RSARM has been proven to distinguish DD from STC and NTC, with specificity ranging between 81.2% and 88.2%[27-29]. In our study, most CC patients with normal ARM showed no RS marker accumulation during CTT, with two IBS-C patients being exceptions. We hypothesize that these two patients might have structural anorectal abnormalities causing difficulty in eliminating the radiopaque markers. Alternatively, overlapping colon segments might have made marker separation challenging, potentially leading to classification errors. The relationship between DD and IBS-C and FC is currently a subject of debate. Our study indicated a higher prevalence of RSARM and elevated anal resting pressure in FC patients than in IBS-C patients. High resting pressures associated with anal dyssynergia have been proposed as a useful diagnostic tool to distinguish DD patients from healthy individuals[19,30]. Hence, our findings suggest that DD might be more prevalent among Chinese FC patients. Furthermore, we observed that nearly half of the FC patients with RSARM exhibited type IV dyssynergia, nearly double the prevalence of IBS-C patients, implying distinct pathogeneses of DD in FC and IBS-C patients.
Recognizing DD is crucial because it is a substantial indicator that patients may benefit from biofeedback therapy[13]. The Rome IV criteria define DD according to CC symptoms and at least two abnormal anorectal tests, such as BET, ARM, or defecography. However, no single method is sufficient to diagnose DD[20,31-33]. It is important to note that many institutions may not have access to these diagnostic tools. In our study, we found that most FC patients with type IV dyssynergia and IBS-C patients with type III dyssynergia displayed RSARM. Given that RSARM indicates a possibility of DD, we hypothesize that FC patients with type IV dyssynergia and IBS-C patients with type III dyssynergia are more likely to have DD. Consequently, further BET or defecography might not be necessary for these patients. However, the exact mechanisms underlying the absence of RSARM in most IBS-C patients with type I or II dyssynergia remain elusive. Although RSARM shows a fair correlation with DD, the absence of markers in the RS region does not conclusively rule out DD due to high false-negative rates[31]. Therefore, type I or II dyssynergia cannot rule out the need for CTT in CC patients. On the other hand, it might not be necessary for FC patients with type IV dyssynergia and IBS-C patients with type III dyssynergia to undergo CTT. Although RSARM can somewhat differentiate STC from DD, it cannot distinguish NTC from DD[29,34]. Therefore, further BET or defecography may still be necessary in all aforementioned scenarios.
Our study has several limitations. The clinical features of hospital-based constipation patients are somewhat different from those of community-based patients[35]. As all patients were from one tertiary hospital, our results may represent more severe cases of constipation, and these psychosocial profiles and colorectal motility patterns may not be generalizable to a broader population. Future studies adding community-based patients will be more informative. Deeper and wider psychosocial studies should also be performed in the future. Additionally, our sample size was relatively small, which may have limited our ability to detect significant differences.
In conclusion, our study revealed elements that can distinguish between FC and IBS-C across multifaceted parameters, namely colonic transmit time, psychological distress, the association between colonic transit and psychiatric conditions, variations in dyssynergic patterns, and the relationship between RSARM and these patterns. Clinical manifestations, manometric evidence of dyssynergia, and elevated thresholds for first sensation, although valuable, have limited discriminatory power in distinguishing FC from IBS-C. These findings extend our comprehension of the intricate patho
The comparation of colorectal motility, psychiatric features, and the association of colorectal motility patterns and psychiatric traits between functional constipation (FC) and constipation-predominant irritable bowel syndrome (IBS-C) groups, especially in the Chinese population has not been fully studied.
Controversy persists regarding the correlation between high-resolution anorectal manometry (HR-ARM) and the colonic transmit test (CTT), and it remains unclear which test provides more meaningful data for IBS-C or FC.
We aimed to compare the psychiatric and colorectal motility characteristics between FC and IBS-C patients in an Eastern Chinese population. We also sought to investigate the correlations between psychiatric and colorectal motility characteristics in both FC and IBS-C patients.
Colorectal motility patterns were obtained by HR-ARM and CTT. Anxiety and depression were assessed by the Hamilton anxiety rating scale (HAMA) and the Hamilton Depression Rating Scale (HAMD)-21.
Our study indicated a higher prevalence of rectosigmoid accumulation of radiopaque markers (RSARM) and elevated anal resting pressure in FC patients compared to IBS-C patients. Furthermore, we observed that nearly half of the FC patients with RSARM exhibited type IV dyssynergia, a prevalence nearly double that of IBS-C patients. Our data also showed that IBS-C patients with normal transit time were more likely to experience anxiety compared to those with slow transit time. However, we found no significant correlations between psychological stress and colonic motility in FC patients. FC patients with type IV dyssynergia and IBS-C patients with type III dyssynergia are more likely to have dyssynergic defecation. Type I or II dyssynergia cannot rule out the need for CTT in chronic constipation patients, while it might not be necessary for FC patients with type IV dyssynergia and IBS-C patients with type III dyssynergia to undergo CTT, but further balloon expulsion test or defecography might still be necessary.
The associations of psychological stress and colonic motility in our study are discrepant from results of Western studies, indicating that emotional factors may have varying effects on colonic motility between these two patient groups. The associations we found between CTT results and dyssynergia patterns by ARM could provide guidance for different constipation groups to choose appropriate colorectal tests.
We compared not only colorectal motility and psychiatric features, but also the correlations between psychiatric and colorectal motility characteristics in FC and IBS-C patients. What we found could provide guidance for constipation patients to choose appropriate colorectal tests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chakrabarti S, India; Kumar A, India; Setiawati Y, Indonesia S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Chen YX
| 1. | Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. [A community population-based epidemiologic study of chronic constipation in Guandong provinece]. Chin J Dig. 2004;24:488-491. |
| 2. | Guo XF, Ke MY, Pan GZ, Han SM, Fang XC, Lu SC, Guo HP. [Cluster, stratified, randomized epidemiological survey and related factor analysis of adult chronic constipation in Beijing]. Chin J Dig. 2002;22:637-638. [DOI] [Full Text] |
| 3. | Wald A, Bharucha AE, Limketkai B, Malcolm A, Remes-Troche JM, Whitehead WE, Zutshi M. ACG Clinical Guidelines: Management of Benign Anorectal Disorders. Am J Gastroenterol. 2021;116:1987-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Bharucha AE, Lacy BE. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology. 2020;158:1232-1249.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (1)] |
| 5. | Person H, Keefer L. Psychological comorbidity in gastrointestinal diseases: Update on the brain-gut-microbiome axis. Prog Neuropsychopharmacol Biol Psychiatry. 2021;107:110209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 6. | Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150:1257-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 1032] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 7. | Whitehead WE, Palsson OS, Simrén M. Biomarkers to distinguish functional constipation from irritable bowel syndrome with constipation. Neurogastroenterol Motil. 2016;28:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Siah KT, Wong RK, Whitehead WE. Chronic Constipation and Constipation-Predominant IBS: Separate and Distinct Disorders or a Spectrum of Disease? Gastroenterol Hepatol (N Y). 2016;12:171-178. [PubMed] |
| 9. | Goyal O, Bansal M, Sood A. Clinical and anorectal manometry profile of patients with functional constipation and constipation-predominant irritable bowel syndrome. Indian J Gastroenterol. 2019;38:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Gastrointestinal Dynamics Group; Gastroenterology Branch of Chinese Medical Association; Functional Gastrointestinal Disease Collaborative Group. [Expert consensus of Chinese Chronic Constipation]. Chin J Dig. 2019;39:577-598. [DOI] [Full Text] |
| 11. | Gwee KA, Ghoshal UC, Chen M. Irritable bowel syndrome in Asia: Pathogenesis, natural history, epidemiology, and management. J Gastroenterol Hepatol. 2018;33:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Carrington EV, Scott SM, Bharucha A, Mion F, Remes-Troche JM, Malcolm A, Heinrich H, Fox M, Rao SS; International Anorectal Physiology Working Group and the International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: Advances in the evaluation of anorectal function. Nat Rev Gastroenterol Hepatol. 2018;15:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Rao SS. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin North Am. 2008;37:569-586, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 14. | Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 641] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Zhou LV, Ke MY. Gastrointestinal dynamics: basic and clinical. 1th ed. Beijing: Sci Pub, 1999: 429-437. |
| 16. | Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6169] [Cited by in RCA: 6770] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 17. | Carrozzino D, Patierno C, Fava GA, Guidi J. The Hamilton Rating Scales for Depression: A Critical Review of Clinimetric Properties of Different Versions. Psychother Psychosom. 2020;89:133-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 148] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 18. | Oblizajek NR, Gandhi S, Sharma M, Chakraborty S, Muthyala A, Prichard D, Feuerhak K, Bharucha AE. Anorectal pressures measured with high-resolution manometry in healthy people-Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol Motil. 2019;31:e13597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Grossi U, Carrington EV, Bharucha AE, Horrocks EJ, Scott SM, Knowles CH. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016;65:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 20. | Bharucha AE, Basilisco G, Malcolm A, Lee TH, Hoy MB, Scott SM, Rao SSC. Review of the indications, methods, and clinical utility of anorectal manometry and the rectal balloon expulsion test. Neurogastroenterol Motil. 2022;34:e14335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Zhang Q, Zhang QX, Zuo XY, Xiao AH, Tan XP. Comparison of psychological characteristics between patients with irritable bowel syndrome with constipation and those with functional constipation. Shijie Huaren Xiaohua Zazhi. 2014;22:5615-5622. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Lam C, Chaddock G, Marciani L, Costigan C, Paul J, Cox E, Hoad C, Menys A, Pritchard S, Garsed K, Taylor S, Atkinson D, Gowland P, Spiller R. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol Motil. 2016;28:861-870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Patcharatrakul T, Gonlachanvit S. Outcome of biofeedback therapy in dyssynergic defecation patients with and without irritable bowel syndrome. J Clin Gastroenterol. 2011;45:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Shekhar C, Monaghan PJ, Morris J, Issa B, Whorwell PJ, Keevil B, Houghton LA. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within a spectrum of sensitization, regulated by serotonin. Gastroenterology. 2013;145:749-57; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Heitmann PT, Vollebregt PF, Knowles CH, Lunniss PJ, Dinning PG, Scott SM. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat Rev Gastroenterol Hepatol. 2021;18:751-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 26. | Ortengren AR, Ramkissoon RA, Chey WD, Baker JR, Staller K, Iturrino J, Shah ED. Anorectal manometry to diagnose dyssynergic defecation: Systematic review and meta-analysis of diagnostic test accuracy. Neurogastroenterol Motil. 2021;33:e14137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Abe T, Kunimoto M, Hachiro Y, Ohara K, Inagaki M, Murakami M. Rectosigmoid Localization of Radiopaque Markers for Identifying Defecation Disorders in Patients With Chronic Constipation: A Retrospective Cohort Study. J Neurogastroenterol Motil. 2021;27:419-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Nullens S, Nelsen T, Camilleri M, Burton D, Eckert D, Iturrino J, Vazquez-Roque M, Zinsmeister AR. Regional colon transit in patients with dys-synergic defaecation or slow transit in patients with constipation. Gut. 2012;61:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Lee YJ. Is There a Role for Radiopaque Markers in Identifying Defecation Disorders? J Neurogastroenterol Motil. 2021;27:312-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Ratuapli SK, Bharucha AE, Noelting J, Harvey DM, Zinsmeister AR. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology. 2013;144:314-322.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 31. | Blackett JW, Gautam M, Mishra R, Oblizajek NR, Kathavarayan Ramu S, Bailey KR, Bharucha AE. Comparison of Anorectal Manometry, Rectal Balloon Expulsion Test, and Defecography for Diagnosing Defecatory Disorders. Gastroenterology. 2022;163:1582-1592.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Staller K, Barshop K, Ananthakrishnan AN, Kuo B. Rectosigmoid Localization of Radiopaque Markers Does Not Correlate with Prolonged Balloon Expulsion in Chronic Constipation: Results from a Multicenter Cohort. Am J Gastroenterol. 2015;110:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Heinrich H, Fox M. One and Done: Is Measurement of the Rectoanal Pressure Gradient Enough to Diagnose Defecatory Disorders and Guide the Management of Constipation? Gastroenterology. 2022;163:1488-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 34. | Tanner S, Chaudhry A, Goraya N, Badlani R, Jehangir A, Shahsavari D, Malik Z, Parkman HP. Prevalence and Clinical Characteristics of Dyssynergic Defecation and Slow Transit Constipation in Patients with Chronic Constipation. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Staudacher HM, Mikocka-Walus A, Ford AC. Common mental disorders in irritable bowel syndrome: pathophysiology, management, and considerations for future randomised controlled trials. Lancet Gastroenterol Hepatol. 2021;6:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |