Published online Oct 28, 2023. doi: 10.3748/wjg.v29.i40.5582
Peer-review started: August 7, 2023
First decision: August 28, 2023
Revised: August 31, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: October 28, 2023
Processing time: 81 Days and 6.4 Hours
Programmed death 1 (PD-1) and CD4+CD25+FoxP3+ expression in peripheral blood T-cells has been previously reported in various types of cancer. However, the specific variation tendency during surgery and chemotherapy, as well as their relationship in gastric cancer patients, still remain unclear. Understanding this aspect may provide some novel insights for future studies on tumor recurrence and tumor immune escape, and also serve as a reference for determining the optimal timing and dose of clinical anti-PD-1 antibodies.
To observe and analyze the expression characteristics of peripheral lymphocyte PD-1 and FoxP3+ regulatory T cells (FoxP3+ Tregs) before and after surgery or chemotherapy in gastric cancer patients.
Twenty-nine stomach cancer patients undergoing chemotherapy after a D2 gastrectomy provided 10 mL peripheral blood samples at each phase of the perioperative period and during chemotherapy. This study also included 29 age-matched healthy donors as a control group. PD-1 expression was detected on lymphocytes, including CD4+CD8+CD45RO+, CD4+CD45RO+, and CD8+CD45RO+ lymphocytes as well as regulatory T cells.
We observed a significant increase of PD-1 expression on immune subsets and a larger number of FoxP3+ Tregs in gastric cancer patients (P < 0.05). Following D2 gastrectomy, peripheral lymphocytes PD-1 expression and the number of FoxP3+ Tregs notably decrease (P < 0.05). However, during postoperative chemotherapy, we only observed a decrease in PD-1 expression on lymphocytes in the CD8+CD45RO+ and CD8+CD45RO+ populations. Additionally, linear correlation analysis indicated a positive correlation between PD-1 expression and the number of CD4+CD45RO+FoxP3high activated Tregs (aTregs) on the total peripheral lymphocytes (r = 0.5622, P < 0.0001).
The observed alterations in PD-1 expression and the activation of regulatory T cells during gastric cancer treatment may offer novel insights for future investigations into tumor immune evasion and the clinical application of anti-PD-1 antibodies in gastric cancer.
Core Tip: In short, this paper shows that programmed death 1 (PD-1) expression on immune subsets and the number of FoxP3+ Treg were higher in peripheral blood of patients with gastric cancer than healthy donors. PD-1 expression and the number of FoxP3+ Treg decrease notably after D2 gastrectomy. PD-1 expression declines on lymphocytes, CD8+, CD45RO+ and CD8+CD45RO+ populations during postoperative chemotherapy. PD-1 expression correlates with the number of CD4+CD45RO+FoxP3 high activated Treg in peripheral lymphocytes. This paper is particularly timely, as the studies of PD-1 expression on immune subsets in peripheral blood are of expanding interest. As well as providing some novel insight for future studies of tumor recurrence and tumor immune escape, our results might also be a reference to determining the timing and dose of clinical anti-PD-1 antibodies, and we anticipate that this study will be widely cited.
- Citation: Li H, Cao GM, Gu GL, Li SY, Yan Y, Fu Z, Du XH. Expression characteristics of peripheral lymphocyte programmed death 1 and FoxP3+ Tregs in gastric cancer during surgery and chemotherapy. World J Gastroenterol 2023; 29(40): 5582-5592
- URL: https://www.wjgnet.com/1007-9327/full/v29/i40/5582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i40.5582
Global cancer statistics have revealed that there were 1089103 newly diagnosed gastric cancer cases in 2020, resulting in a significant number of newly diagnosed gastric cancer cases and deaths worldwide[1]. Specifically, the National Cancer Centre of China estimated that 679000 new gastric cancer cases and 498000 gastric cancer-related deaths would occur in China in 2015[2]. Further compounding this issue is the fact that almost 70% of newly diagnosed cases are advanced gastric cancer, with the overall five-year survival rate being less than 30%[3].
With the advances of Chimeric antigen receptor T-cells, genetically engineered T cells, cytotoxic T-lymphocyte-associated antigen-4, and the programmed death-1/programmed death-ligand (PD-1/PD-L) pathway, tumor immunotherapy has rapidly emerged as a field of advanced research[4].
Multiple clinical trials have confirmed the efficacy and safety of PD-1 monoclonal antibody[5,6], and PD-1 monoclonal antibody was already approved for advanced gastric cancer in the United States and Japan. However, a recently randomized and open Phase III trial, Keymat-61[7], showed no significant improvement on objective response rate or progression-free survival (PFS) from paclitaxel. Meanwhile, in the randomized III trials, JAVELIN Gastric 300, reported by Bang et al[8], Avelumab in the treatment of advanced gastric/gastroesophageal joint cancer patients did not improve OS and PFS compared with the third-line treatment.
A growing body of evidence suggests that Tregs might be involved in the treatment of PD-1/PD-L1 blockade and PD-1/PD-L1 axis could influence Treg differentiation and function. However, the complex relationship between PD-1/PD-L1 pathway and Tregs is yet to be fully elucidated[9,10].
Although research into peripheral PD-1 expression in patients with gastric cancer has been reported previously, few studies have sequentially investigated such expression from the time of surgery to the completion of postoperative chemotherapy[11]. Furthermore, studies investigating the relationship between peripheral PD-1 expression and FoxP3+ Tregs in gastric cancer patients are largely lacking.
In this study, we detected PD-1 expression on peripheral blood T cell subsets and the population of FoxP3+ Tregs in gastric cancer from surgery to the completion of postoperative chemotherapy to analyse how these indexes change in expectation of being helpful to decide when and how the anti-PD-1 antibodies should be applied and deeper under-standing on the mechanism of tumor immune escape in gastric cancer in the future.
Patients with a histologically confirmed diagnosis of gastric adenocarcinoma following a D2 gastrectomy and treated with postoperative adjuvant chemotherapy, as well as age-matched healthy donors were eligible for this prospective observational study. Other inclusion criteria consisted of: (1) A healthy physical examination of the donors performed within the past three months; and (2) the age of patients and donors within the range of 18-75 years old. The exclusion criteria consisted of: (1) Patients diagnosed with other types of cancer within five years; (2) patients that received preoperative adjuvant chemotherapy, radiotherapy, or immunotherapy; (3) patients and donors who were diagnosed with chronic hepatitis, human immunodeficiency virus, syphilis, or any other acute infectious disease; (4) patients and donors who suffered from rheumaimmune systemic diseases (e.g., systemic lupus erythematosus or hyperthyroidism); (5) patients with any other severe disease that might render them incapable of completing the entire course of chemotherapy; and (6) patients suffered gastric cancer recurrence or failed to finish the entire course of chemotherapy.
At the beginning of this study, 33 patients (alongside 33 age-matched donors) were enrolled from February 2020 to February 2021 in the Chinese People’s Liberation Army General Hospital (PLAGH). However, one patient experienced tumor recurrence and three who failed to complete the entire cycles were excluded. A total of 29 patients (15 men and 14 women; mean age: 59.72 years) and 29 age-matched donors (17 men and 12 women; mean age: 59.62 years) were ultimately included in this study.
This study received approval from the ethics commission of the General Hospital of PLA, and all patients and donors provided signed informed consent.
The treatment strategy for the enrolled patients consisted of MDT and a D2 gastrectomy performed by a chief physician at the General Hospital of PLA. The patients were treated with the following doses of chemotherapy and affiliated schedules: Oral capecitabine (1000 mg/m2 twice daily on days 1-14 of each cycle) plus intravenous oxaliplatin (130 mg/m2 on day 1 of each cycle) for eight three-week cycles.
A sample volume of 10 mL peripheral blood was obtained from the patients into ananticoagulation tube on the day before surgery, the first and fourth cycles of chemotherapy and the day after chemotherapy. All blood samples were analysed within 6 h. Each sample was divided into two tubes and the detection of PD-1 expression and CD4+CD25+
The detection of PD-1 expression involved staining the cells from the whole blood with fluorescently labelled antibodies, including anti-CD4-PerCP-Cy5.5 (clone RPA-4), anti-CD8-FITC (clone RPA-T8), anti-CD45RO-PE (clone UCHL-1) and anti-CD279 (PD-1)-APC (clone MIH4), from BD Biosciences (Franklin Lakes, NJ, United States) for the detection of PD-1 expression.
The detection of CD4+CD25+FoxP3+ regulatory T cells was performed by staining PBMCs with anti-CD3-APC (clone SP34-2), anti-CD4-Percp (clone RPA-T4), anti-CD25-FITC (clone M-A251) andanti-CD45RO-PE (clone UCHL-1) from BD Biosciences (Franklin Lakes, NJ, United States) first, followed by anti-FoxP3-PE (clone 236A/E7, BD Biosciences) was added after permeabilizing the cell and nuclear membranes.
Data in this paper is represented as the mean ± SD. Comparisons of the differences in the continuous variables between the patients and donors were made using a Student’s t-test or Wilcoxon rank sum test. A Chi-square test was performed for the categorical data. A paired-t test was adopted to compare the measurement data before and after the operation. Changes in the measurement data during chemotherapy were evaluated by an analysis of variance repeated-measures function. A Pearson correlation analysis was applied to dispose of the relativity between PD-1 expression and a population of FoxP3+ Tregs. P values were based on two-tailed tests, with a value of P < 0.05 considered to be statistically significant.
Statistical analysis revealed no significant difference in age (59.72 ± 15.33 vs 59.62 ± 15.64, P = 0.9798), gender (15/14 vs 17/12, P = 0.7918), and body mass index (23.90 ± 5.00 vs 25.10 ± 3.18, P = 0.2824) between the patients and healthy donors. We also assessed the daily life activity of both patients and donors using the Karnofsky score; all patients scored above 60 (indicating occasional care required for most needs), which is a score above the threshold at which patients with advanced gastric cancer are advised to transition from systemic therapy to supportive therapy according to the NCCN Guidelines. The descriptive statistics for CEA levels, TNM stages, and tumor differentiation are detailed in Table 1.
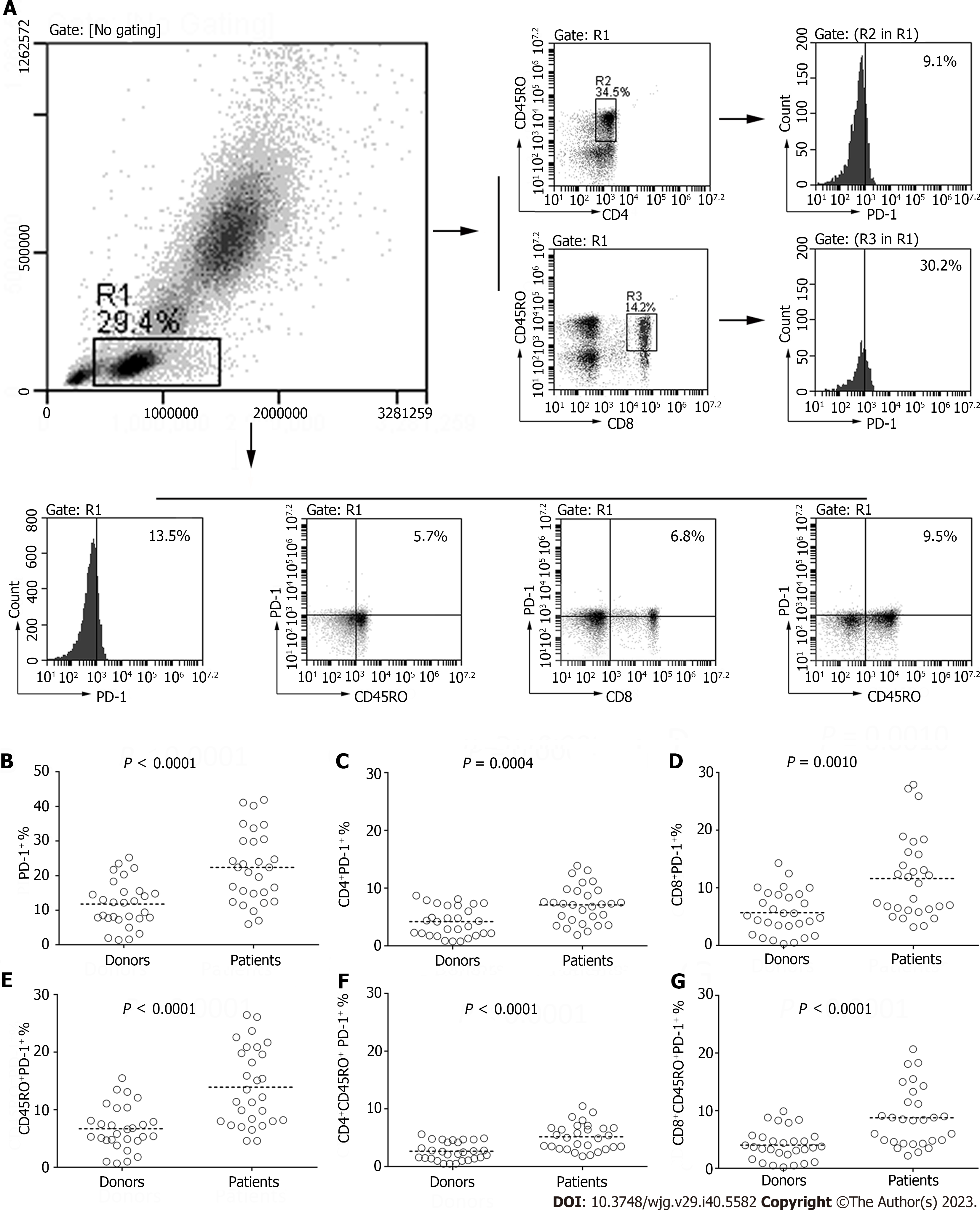
We performed an analysis of PD-1 expression on fresh peripheral blood sample subsets using flow cytometry (Figure 1A). The PD-1 expression on lymphocytes obtained from patients was significantly higher than those derived from donors (22.37% ± 10.35% vs 11.77% ± 6.67%, P < 0.0001; Figure 1B-G). Similarly, PD-1 expression on the CD4+ and CD8+ lymphocytes was notably higher in the patient group compared to the donor group (7.10% ± 3.27% vs 4.18% ± 2.53%, P = 0.0004; 11.63% ± 7.06% vs 5.71% ± 3.74%, P = 0.001; Figure 1B-G). Further, we assessed the PD-1 expression in lymphocytes through CD45RO phenotyping, a crucial marker utilized to distinguish memory T cells from naive T cells. This analysis revealed a significant difference between the patients and donors (13.94% ± 6.75% vs 6.71% ± 3.86%, P < 0.0001; Figure 1B-G). Moreover, PD-1 expression on CD4+CD45RO+ and CD8+CD45RO+ lymphocytes was also markedly higher in the gastric cancer patients (5.16% ± 2.31% vs 2.67% ± 1.57%, P < 0.0001; 8.79% ± 5.15% vs 4.05% ± 2.67%, P < 0.0001; Figure 1B-G).
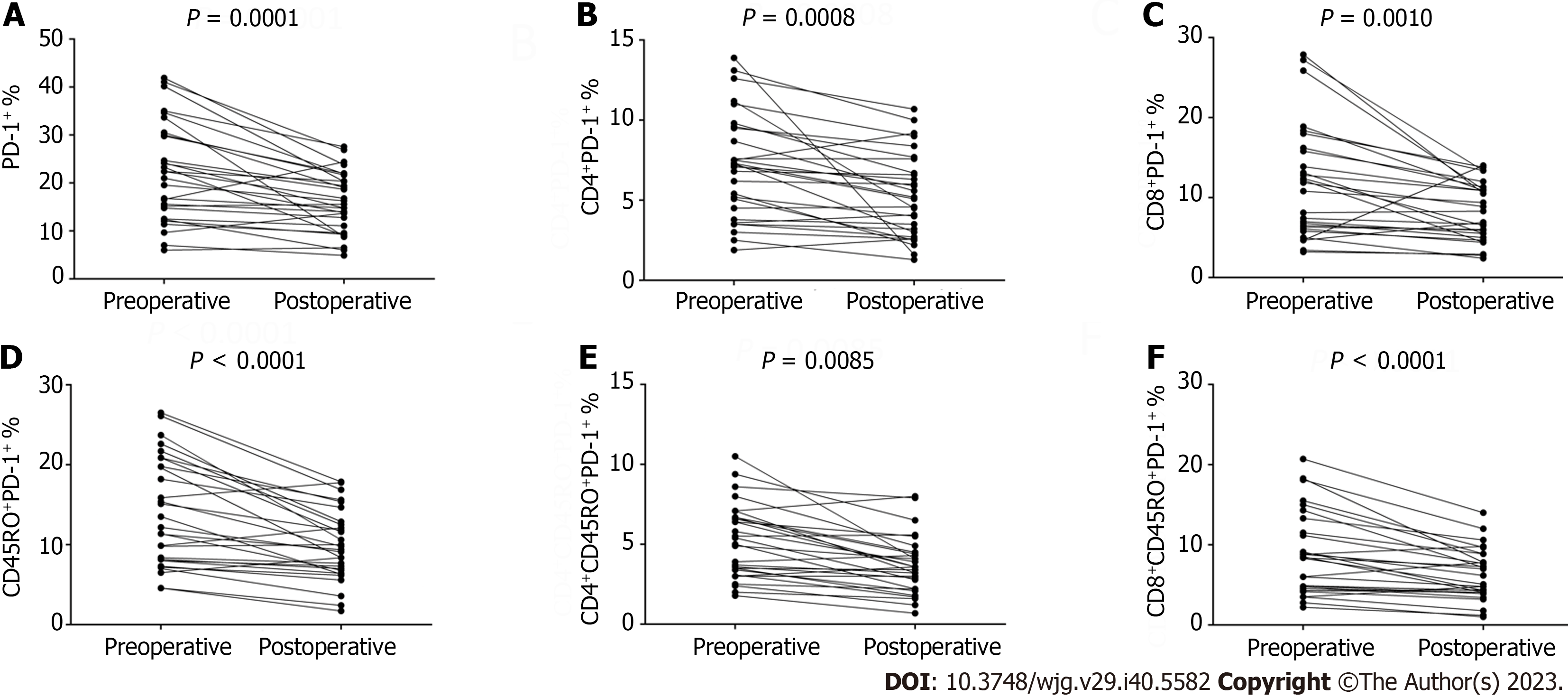
In this part, we detected the expression of PD-1 on the lymphocytes from blood samples drawn from postoperative patients. Blood samples were drawn on average of 27.24 d ± 5.06 d (range: 19-35 d) following surgery. A paired-t test was applied to compare the frequency of PD-1 on lymphocytes between the preoperative and operative blood samples. Strikingly, we found that surgery might be able to reduce the level of PD-1 expression on T cells. There was a significant decline in the PD-1 expression on lymphocytes from postoperative peripheral blood (22.37% ± 10.35% vs 16.00% ± 6.29%, P = 0.0001; Figure 2). A significant decrease was also observed for the level of PD-1 expression on CD4+ and CD8+ lymphocytes (7.10% ± 3.27% vs 5.26% ± 2.62%, P = 0.0008; 11.63% ± 7.06% vs 8.05% ± 3.60%, P = 0.001; Figure 2). The frequency of PD-1 expression on CD4+CD45RO+ and CD8+CD45RO+ lymphocytes from postoperative patients was also significantly lower than that derived from preoperative patients (5.16% ± 2.31% vs 3.67% ± 1.80%, P = 0.0085; 8.79% ± 5.15% vs 6.19% ± 3.20%, P < 0.0001; Figure 2). Similarly, the PD-1 expression on CD45RO+ lymphocytes was also significantly lower (13.94% ± 6.75% vs 9.86% ± 4.41%, P < 0.0001; Figure 2). Since the increase of PD-1 expression is dependent on antigen stimulation, one explanation for the observed results might be the lack of stimulation from tumor antigens following surgery.
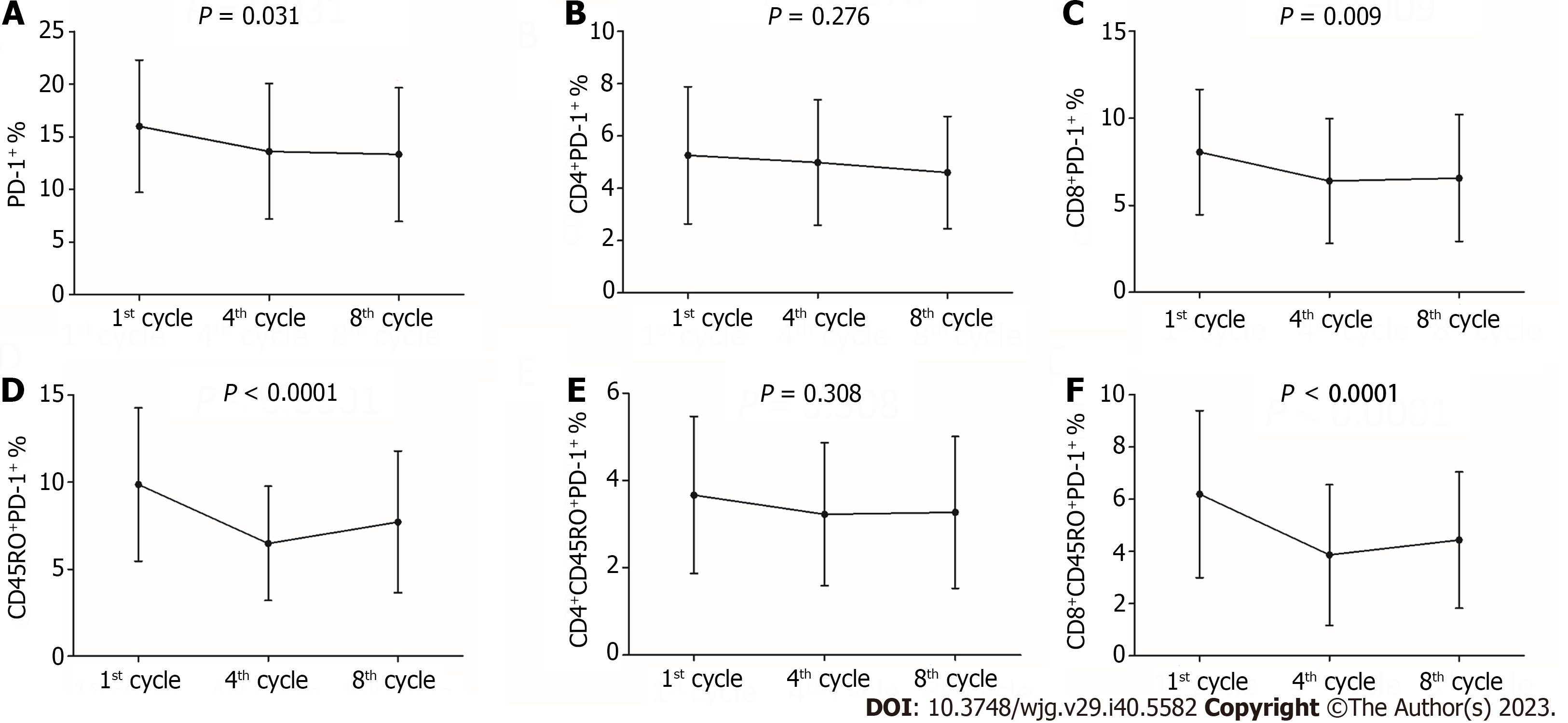
A total of 29 patients in the trial accepted an eight three-week cycles course of chemotherapy with oxaliplatin and capecitabine following a D2 gastrectomy. We drew peripheral blood samples on the day before the first cycle of chemotherapy (an average of 27.24 d ± 5.06 d after surgery), fourth cycle of chemotherapy (an average of 97.90 d ± 6.64 d after surgery) and the day after the eighth cycle of chemotherapy (an average of 171.14 d ± 8.73 d after surgery). There was a statistically significant decrease in PD-1 expression on the total lymphocytes (16.00% ± 6.29%, 13.62% ± 6.43% vs 13.33% ± 6.35%, P = 0.031; Figure 3) and CD8+T cells (8.05% ± 3.60%, 6.39% ± 3.59% vs 6.56% ± 3.64%, P = 0.009; Figure 3). A notably significant decline was also observed for the PD-1 expression on CD45RO+ lymphocytes (9.86% ± 4.41%, 6.48% ± 3.28% vs 7.71% ± 4.07%, P < 0.0001; Figure 3) and CD8+CD45RO+ lymphocytes (6.19% ± 3.20%, 3.86% ± 2.69% vs 4.44% ± 2.61%, P < 0.0001; Figure 3). However, the difference in PD-1 expression on CD4+ lymphocytes (5.26% ± 2.62%, 4.98% ± 2.40% vs 4.60% ± 2.15%, P = 0.276; Figure 3) and CD4+CD45RO+ lymphocytes (3.67% ± 1.80%, 3.23% ± 1.64% vs 3.27% ± 1.74%, P = 0.308; Figure 3) was not statistically significant.
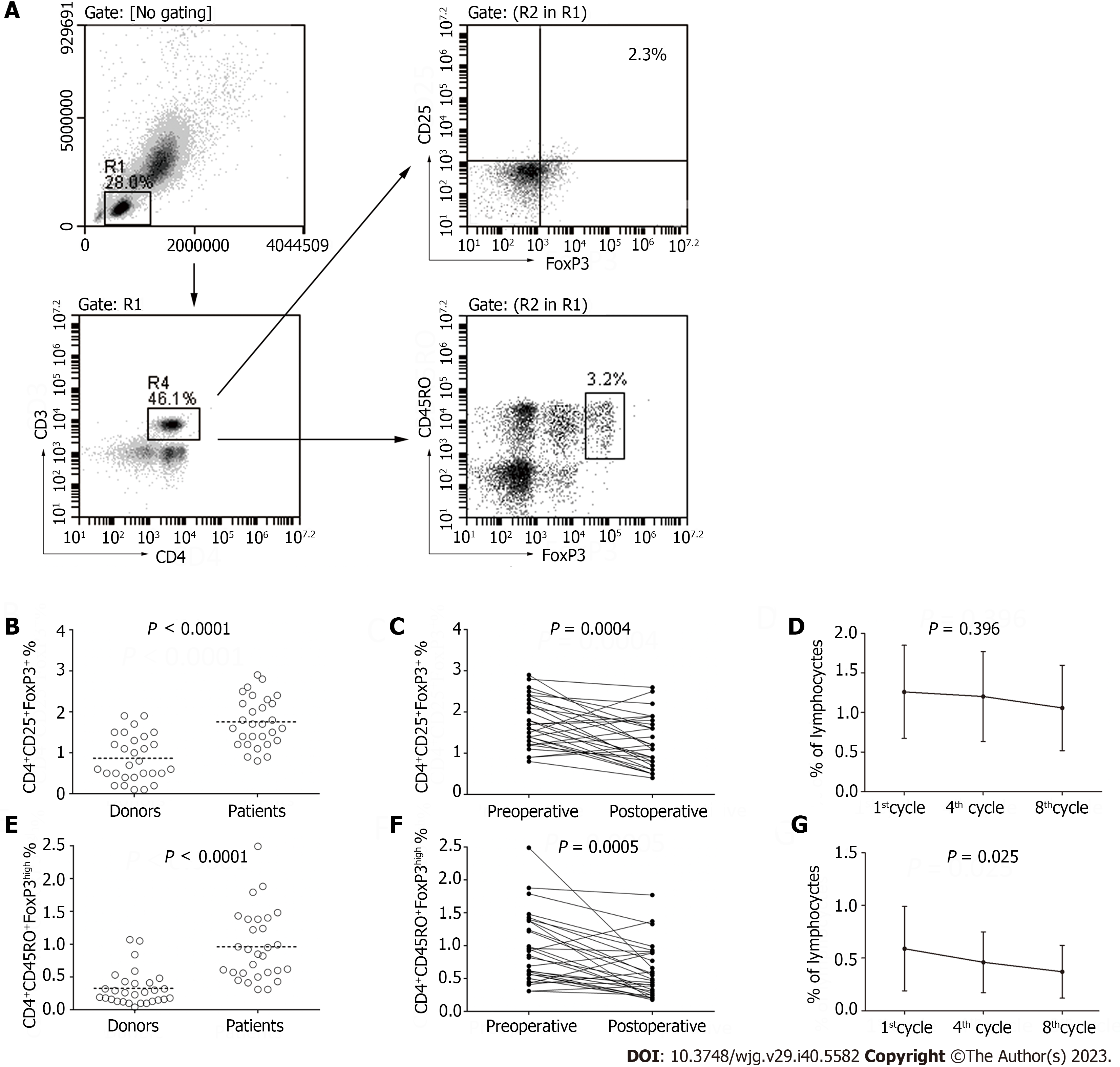
While analyzing the PD-1 expression on lymphocytes, we also detected the frequency of CD4+CD25+FoxP3+ T cells and CD4+CD45RO+FoxP3high T cellsin the lymphocyte population (Figure 4A). The frequency of FoxP3+T cells and CD4+CD45RO+FoxP3high T cellswas higher in the patients than in the healthy donors (1.76% ± 0.59% vs 0.87% ± 0.56%, P < 0.0001; 0.92% ± 0.45% vs 0.33% ± 0.27%, P < 0.0001; Figure 4B-G) and declined after the D2 gastrectomy was performed (1.76% ± 0.59% vs 1.26% ± 0.62%, P = 0.0004; 0.92% ± 0.45% vs 0.59% ± 0.40%, P = 0.0005; Figure 4B-G). Statistical difference was also observed in the frequency of CD4+CD45RO+FoxP3high T cells in the peripheral lymphocytes during chemotherapy (0.59% ± 0.40%, 0.46% ± 0.29% vs0.37% ± 0.25%, P = 0.025; Figure 4B-G).
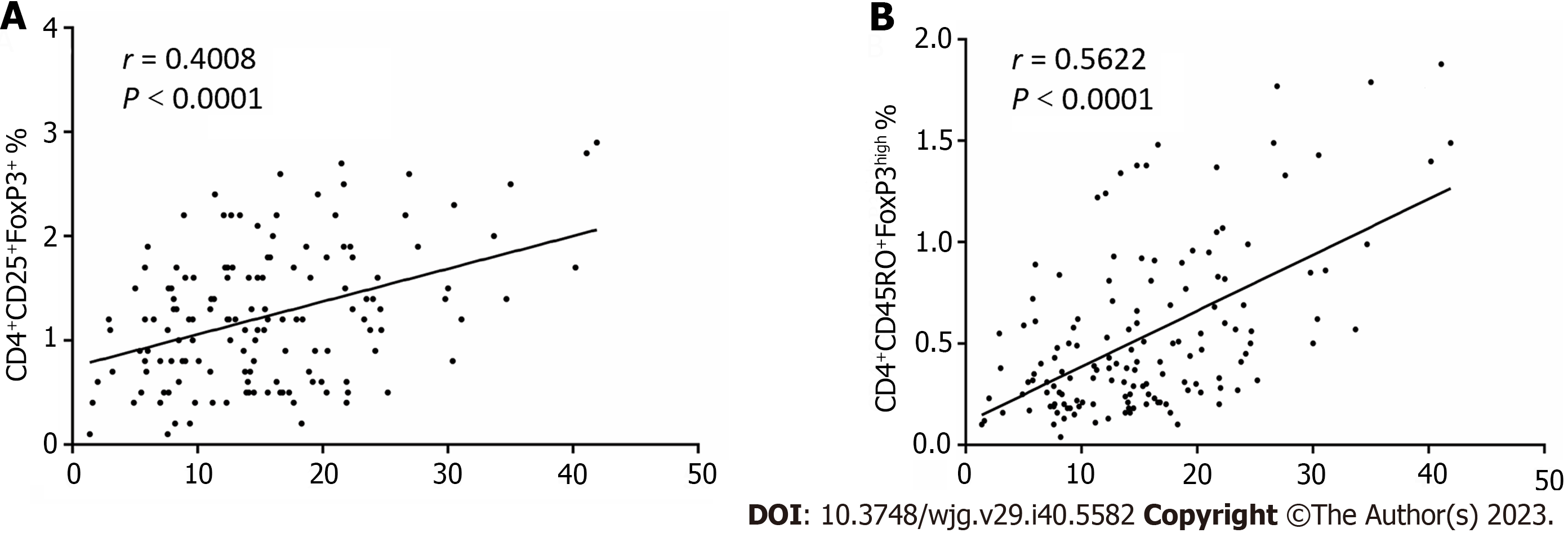
Previous research has suggested that PD-1+ T cells and Tregs arecorrelative in the tumor tissues and tumor-involved lymph nodes in both breast and papillary thyroid cancer[12,13]. However, no clear correlations between the frequency of PD-1 expression and the number of CD4+CD25+FoxP3+ T cellsobserved in the peripheral blood of preoperative patients (r = 0.4008, P < 0.0001; Figure 5) but significant correlation between CD4+CD45RO+FoxP3high T cells and PD-1 expression (r = 0.5622, P < 0.0001; Figure 5) were observed in our study.
Studies have shown that the PD-1/PD-L1 pathway plays a critical role in promoting T cell exhaustion[14]. In many types of tumor tissues, overexpression of PD-L1 has been found, with a similar pattern of PD-1 overexpression observed on tumor-infiltrating lymphocytes[15,16].
Supported by T-helper cells, CD8+ T cells can be activated, leading to the destruction of tumor cells through the perforin/granzyme and Fas/Fas ligand (FasL) apoptosis pathways. However, tumor cells have been found to inhibit the function of CD4+ and CD8+ T cells by boosting PD-1 expression[17,18]. This scenario has been observed in the peripheral blood of various cancer types, such as non-small cell lung cancers, actinic cheilitis, oral squamous cell carcinoma, and head and neck cancer[19-22].
Our analysis unveiled a notable increase in PD-1 expression on CD45RO+ lymphocytes in gastric cancer. CD45RO is a surface antigen employed to differentiate memory T cells from naive T cells. When antigens are reintroduced, CD4+CD45RO+ T cells are seen to respond rapidly and robustly, migrating to the antigen source and aiding B cells in antibody production[23,24].
Several studies have suggested that CD8+CD45RO+ T cells serve as a more effective independent prognostic factor for metastatic colorectal cancer in Cox regression multivariate analysis compared to traditional markers like CEA and LDH[25-27]. Given this evidence, it seems likely that the upregulation of PD-1 on CD45RO+ T cells is a consequence of tumor cell stimulation, thereby facilitating tumor immune escape from memory T cells.
PD-1/PD-L1 expression can be induced or maintained by many cytokines, such as type I IFn and IFNγ. Research has shown that tumor-associated plasmacytoid DCs produce large amounts of type I IFn[28], which can in turn induce PD-1/PD-L1 expression[29]. The decline in these cytokines following tumor resection might be the underlying cause of the observed reduction in PD-1 expression.
Research conducted by Maeda et al[30] found that the populations of CD4+, CD8+ and NK cells in the peripheral blood of patients with metastatic colorectal cancer remained stable following FOLFOX administration, yet the number of regulatory cells exhibited a significant decline. A similar drop in the Tregs population was observed in patients treated with paclitaxel-based chemotherapy[31]. Data from our present study reveal a trend of significant decline or reduction in PD-1 expression and population of FoxP3+ Tregs in patients undergoing surgery and chemotherapy.
We observed that the population of regulatory T cells was higher in patients compared to donors, which is consistent with prior findings in prostate, lung, pancreatic, and breast cancer studies[32,33]. The increased population of Tregs in tumor-bearing patients could potentially arise from the secretion of TGF-β, IL-10, and H-ferritin, which have been known to induce CD4+CD25− T cells to transition into CD4+CD25+ T cells and upregulate the expression of FoxP3[34-36].
Interestingly, this mechanism might also be responsible for the observed decrease in the number of peripheral Tregs following tumor tissue removal. Moreover, several studies have indicated that drugs like cyclophosphamide, fludarabine, and paclitaxel could down-regulate the quantity and function of Tregs in cancer patients[37,38].
These findings provide a theoretical foundation for the treatment of tumors with PD-1/PDL-1 blockers in combination with chemotherapy drugs, offering a potential strategy to optimize cancer immunotherapy.
In this study, CD4+CD45RO+FoxP3+ appears to have a stronger correlation to PD-1 expression than Tregs in peripheral blood. Previous detection of FoxP3 at both the mRNA and protein levels has shown that human CD25highCD4+ T cells indeed express FoxP3[39,40], indicating that the population of CD4+CD45RO+FoxP3+ cells can serve as a reflection of CD25highCD45RA-FoxP3high activated Treg cells (aTreg cells).
Moreover, the CD4+FoxP3+T cells in peripheral blood comprise three subpopulations, distinguished by differing levels of FoxP3 and cell surface molecules CD45RA and CD25. Notably, only CD25highCD45RA-FoxP3high cells (aTregs) are terminally differentiated and exhibit high suppressive capacities[41].
The exploration of PD-1 expression impacts on immune subsets and the abundance of FoxP3+ Tregs in peripheral blood could provide invaluable insights for future research on the PD-1/PDL-1 axis, tumor recurrence, and tumor immune escape. Additionally, these findings may also serve as potential biomarkers for studies involving the timing and dosing of clinical anti-PD-1 antibodies.
Programmed death 1 (PD-1) and CD4+CD25+FoxP3+ expression in peripheral blood T-cells have been identified in multiple cancer types, but their variation during surgery and chemotherapy in gastric cancer remains elusive. Understanding this could illuminate tumor recurrence mechanisms and guide optimal anti-PD-1 antibody treatment strategies.
Despite known PD-1 and CD4+CD25+FoxP3+ expression in various cancers, the specific changes during surgery and chemotherapy, and their relationship in gastric cancer, remain undefined. This study seeks to shed light on these variations, potentially offering insights into tumor recurrence, immune evasion, and the clinical application of anti-PD-1 antibodies in gastric cancer.
The study aims to observe and analyze the expression characteristics of peripheral lymphocyte PD-1 and FoxP3+ regulatory T cells (FoxP3+Tregs) in gastric cancer patients, both prior to and following surgery or chemotherapy, to better understand their roles and implications in gastric cancer treatment.
In this study, 29 gastric cancer patients, post-D2 gastrectomy and undergoing chemotherapy, provided 10 mL peripheral blood samples during various perioperative phases. PD-1 expression was analyzed on specific lymphocyte subsets, with an additional 29 age-matched healthy donors serving as a control group.
The study found a significant elevation in PD-1 expression and FoxP3+ Tregs in gastric cancer patients, which decreased notably post-D2 gastrectomy. A positive correlation was identified between PD-1 expression and the number of activated FoxP3high Tregs in peripheral lymphocytes, especially during postoperative chemotherapy.
Alterations in PD-1 expression and regulatory T cell activation during gastric cancer treatment could provide valuable insights for understanding tumor immune evasion. These findings may also influence the clinical application of anti-PD-1 antibodies in gastric cancer therapy.
The changes observed in PD-1 expression and regulatory T cell activation during gastric cancer treatments pave the way for deeper exploration into tumor immune evasion mechanisms. These findings could also shape the future clinical application and optimization of anti-PD-1 antibodies in treating gastric cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Colorectal Anal Surgery Professional Committee; Chinese Research Hospital Society; Member of Colorectal Surgery Group; Surgery Society of Chinese Medical Association; Standing member of General Surgery Committee of the PLA.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho JY, South Korea; Toshimori A, Japan S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Cunningham SC, Schulick RD. Palliative management of gastric cancer. Surg Oncol. 2007;16:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 5. | Bang YJ, Muro K, Fuchs CS, Golan T, Geva R, Hara H, Jalal SI, Borg C, Doi T, Wainberg ZA, Wang JD, Koshiji M, Dalal RP, Chung HC. Keynote-059 cohort2: safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-Fu) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clini Onco. 2017;35 Suppl 15:4012-4012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1715] [Article Influence: 214.4] [Reference Citation Analysis (0)] |
| 7. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 999] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 8. | Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 414] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 9. | Cai J, Wang D, Zhang G, Guo X. The Role Of PD-1/PD-L1 Axis In Treg Development And Function: Implications For Cancer Immunotherapy. Onco Targets Ther. 2019;12:8437-8445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 10. | Woods DM, Ramakrishnan R, Laino AS, Berglund A, Walton K, Betts BC, Weber JS. Decreased Suppression and Increased Phosphorylated STAT3 in Regulatory T Cells are Associated with Benefit from Adjuvant PD-1 Blockade in Resected Metastatic Melanoma. Clin Cancer Res. 2018;24:6236-6247. [PubMed] |
| 11. | Saito H, Kuroda H, Matsunaga T, Osaki T, Ikeguchi M. Increased PD-1 expression on CD4+ and CD8+ T cells is involved in immune evasion in gastric cancer. J Surg Oncol. 2013;107:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC Jr, Klopper JP, Haugen BR. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E934-E943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1578] [Cited by in RCA: 1576] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 15. | Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1283] [Article Influence: 75.5] [Reference Citation Analysis (1)] |
| 16. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3543] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 17. | Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1375] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 18. | Wu X, Zhang H, Xing Q, Cui J, Li J, Li Y, Tan Y, Wang S. PD-1(+) CD8(+) T cells are exhausted in tumours and functional in draining lymph nodes of colorectal cancer patients. Br J Cancer. 2014;111:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 19. | Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Malaspina TS, Gasparoto TH, Costa MR, de Melo EF Jr, Ikoma MR, Damante JH, Cavassani KA, Garlet GP, da Silva JS, Campanelli AP. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Baruah P, Lee M, Odutoye T, Williamson P, Hyde N, Kaski JC, Dumitriu IE. Decreased levels of alternative co-stimulatory receptors OX40 and 4-1BB characterise T cells from head and neck cancer patients. Immunobiology. 2012;217:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Waki K, Yamada T, Yoshiyama K, Terazaki Y, Sakamoto S, Matsueda S, Komatsu N, Sugawara S, Takamori S, Itoh K, Yamada A. PD-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci. 2014;105:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Clement LT, Vink PE, Bradley GE. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990;145:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 24. | Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 342] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 25. | Formica V, Massara MC, Portarena I, Fiaschetti V, Grenga I, Del Vecchio Blanco G, Sileri P, Tosetto L, Skoulidis F, Pallone F, Roselli M. Role of CA19.9 in predicting bevacizumab efficacy for metastatic colorectal cancer patients. Cancer Biomark. 2009;5:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Ferroni P, Palmirotta R, Spila A, Martini F, Formica V, Portarena I, Del Monte G, Buonomo O, Roselli M, Guadagni F. Prognostic value of carcinoembryonic antigen and vascular endothelial growth factor tumor tissue content in colorectal cancer. Oncology. 2006;71:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Formica V, Cereda V, di Bari MG, Grenga I, Tesauro M, Raffaele P, Ferroni P, Guadagni F, Roselli M. Peripheral CD45RO, PD-1, and TLR4 expression in metastatic colorectal cancer patients treated with bevacizumab, fluorouracil, and irinotecan (FOLFIRI-B). Med Oncol. 2013;30:743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Gupta S, Maheshwari A, Parab P. Reply to V.G. Gupta et al and W. Zou et al. J Clin Oncol. 2018;36:2813-2814. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1006] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 30. | Ma C, Dong X. Colorectal cancer-derived Foxp3(+) IL-17(+) T cells suppress tumour-specific CD8+ T cells. Scand J Immunol. 2011;74:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Zhang L, Dermawan K, Jin M, Liu R, Zheng H, Xu L, Zhang Y, Cai Y, Chu Y, Xiong S. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Loskog A, Ninalga C, Paul-Wetterberg G, de la Torre M, Malmström PU, Tötterman TH. Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J Urol. 2007;177:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Lu L, Ma J, Li Z, Lan Q, Chen M, Liu Y, Xia Z, Wang J, Han Y, Shi W, Quesniaux V, Ryffel B, Brand D, Li B, Liu Z, Zheng SG. All-trans retinoic acid promotes TGF-β-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS One. 2011;6:e24590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Gray CP, Arosio P, Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood. 2002;99:3326-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 945] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 38. | Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, Knolle PA, Thomas RK, von Bergwelt-Baildon M, Debey S, Hallek M, Schultze JL. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 39. | Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, Banham AH. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 40. | Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 41. | Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1812] [Article Influence: 113.3] [Reference Citation Analysis (0)] |