Published online Oct 21, 2023. doi: 10.3748/wjg.v29.i39.5471
Peer-review started: July 14, 2023
First decision: September 6, 2023
Revised: September 11, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: October 21, 2023
Processing time: 91 Days and 13.4 Hours
The small intestine is known to play a crucial role in the development and remission of diabetes mellitus (DM). However, the exact mechanism by which mid-small intestinal bypass improves glucose metabolism in diabetic rats is not fully understood.
To elucidate the mechanisms by which mid-small intestinal bypass improves glucose metabolism.
Streptozotocin (STZ) was used to induce DM in Sprague-Dawley (SD) rats at a dose of 60 mg/kg. The rats were then randomly divided into two groups: The mid-small intestine bypass (MSIB) group and the sham group (underwent switch laparotomy). Following a 6-wk recovery period post-surgery, the rats underwent various assessments, including metabolic parameter testing, analysis of liver glycogen levels, measurement of key gluconeogenic enzyme activity, characterization of the gut microbiota composition, evaluation of hormone levels, determination of bile acid concentrations, and assessment of the expression of the intestinal receptors Takeda G protein-coupled receptor 5 and farnesoid X receptor.
The MSIB group of rats demonstrated improved glucose metabolism and lipid metabolism, along with increased hepatic glycogen content. Furthermore, there was a decrease in the expression of the key gluconeogenic enzymes phosphoenolpyruvate carboxykinase 1 and glucose-6-phosphatase. Importantly, the MSIB group exhibited a substantial increase in the abundances of intestinal Lactobacillus, Clostridium symbiosum, Ruminococcus gnavus, and Bilophila. Moreover, higher levels of secondary bile acids, such as intestinal lithocholic acid, were observed in this group. Remarkably, the changes in the gut microbiota showed a significant correlation with the expression of key gluconeogenic enzymes and glucagon-like peptide 1 (GLP-1) at 6 wk postoperatively, highlighting their potential role in glucose regulation. These findings highlight the beneficial effects of mid-small intestine bypass on glucose metabolism and the associated modulation of the gut microbiota.
The findings of this study demonstrate that the introduction of postoperative intestinal Clostridium symbiosum in the mid-small intestine contributes to the enhancement of glucose metabolism in nonobese diabetic rats. This improvement is attributed to the increased inhibition of hepatic gluconeogenesis mediated by GLP-1, resulting in a favorable modulation of glucose homeostasis.
Core Tip: Intestinal function plays a pivotal role in the onset, progression, and alleviation of diabetes. However, research on surgical procedures and functions involving the mid-small intestine is limited. The precise mechanisms by which the mid-small intestine improves glucose metabolism in diabetic rats remain largely unclear. This study explores the effects of mid-small intestine bypass surgery on diabetic rats. Post-surgery, there was an increase in the abundance of Clostridium symbiosum in the rat gut, which contributed to improved glucose metabolism through the inhibition of hepatic gluconeogenesis mediated by glucagon-like peptide 1. These findings provide a theoretical basis for non-surgical interventions in the treatment of metabolic disorders associated with diabetes.
- Citation: Luo X, Tao F, Tan C, Xu CY, Zheng ZH, Pang Q, He XA, Cao JQ, Duan JY. Enhanced glucose homeostasis via Clostridium symbiosum-mediated glucagon-like peptide 1 inhibition of hepatic gluconeogenesis in mid-intestinal bypass surgery. World J Gastroenterol 2023; 29(39): 5471-5482
- URL: https://www.wjgnet.com/1007-9327/full/v29/i39/5471.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i39.5471
Diabetes and obesity have become global health concerns, and their strong association has been widely recognized[1,2]. Bariatric surgery has gained considerable attention as a therapeutic approach for obesity and diabetes improvement[3]. The small intestine, a pivotal organ in dietary metabolism, plays a crucial role in the development of diabetes[4]. Each segment of the small intestine exhibits distinct functions[5], including the “ileal brake” mechanism at the distal ileum[6,7]. Additionally, the gut microbiota composition varies among different segments of the small intestine. While gastric bypass, a commonly employed bariatric surgery, involves bypassing a portion of the upper small intestine[8], less research has focused on the procedure and function of the middle small intestine. To investigate the role of the middle small intestine in glucose metabolism, we utilized a STZ-induced nonobese diabetic SD rat model[9] and conducted middle small intestinal bypass surgery to assess the changes in metabolic function and related factors postoperatively. This study aimed to elucidate the metabolic function and underlying mechanisms of the mid-segment small intestine, with the ultimate goal of providing a theoretical foundation for nonsurgical interventions targeting glucose metabolism disorders.
In this study, 8-wk-old male SD rats were used to investigate the metabolic improvement effect of mid-small intestine bypass (MSIB). The rats were obtained from Shanghai Slaughter Laboratory Animal Co., Ltd. and were fed a normal diet. After a one-week acclimatization period, a diabetic rat model was established by intraperitoneal administration of STZ at a dose of 60 mg/kg, divided into two doses administered on the same day. Following successful model establishment, the rats were randomly assigned to undergo either MSIB or sham surgery (sham). The rats were maintained under identical environmental conditions for 6 wk until euthanasia. Glucose tolerance [oral glucose tolerance test (OGTT)] and insulin sensitivity [insulin tolerance test (ITT)] were assessed at 2 and 6 wk postoperatively. Venous blood, liver tissue, ileal tissue, and fecal samples were collected for analysis at 6 wk postoperatively.
The rats were individually housed in well-ventilated cages with ad libitum access to water and food. Weekly measurements of body weight and food intake were recorded. All animal experiments adhered to the relevant ethical guidelines for animal research and were approved by the Animal Ethics Committee of Nanchang University. Standard animal care and laboratory protocols were followed in accordance with the ARRIVE guidelines.
The rats were fasted for approximately 14 h and underwent surgery after anesthesia (isoflurane, 2% for maintenance and 4% for induction). The abdomen was prepared, and a 4-cm median incision was made to access the abdominal cavity.
In the MSIB group, the point close to the ileocecal flap was used as the reference point. Approximately 60% of the small bowel was bypassed, starting 20 cm proximal to this point and extending 20 cm distal to the flexural ligament. Intestinal continuity was restored through a lateral anastomosis between the distal and proximal small bowel segments. The lumen of the bypassed segment was occluded by silk ligation at the site of the lateral anastomosis. For the sham group, the bowel was gently manipulated upon entering the abdominal cavity. The abdominal cavity was closed with 3-0 silk sutures. The duration of the surgery was approximately 45 min. Subsequently, the rats were subcutaneously injected with 10 mL of sterile saline for fluid resuscitation and placed individually in cages to recover from anesthesia.
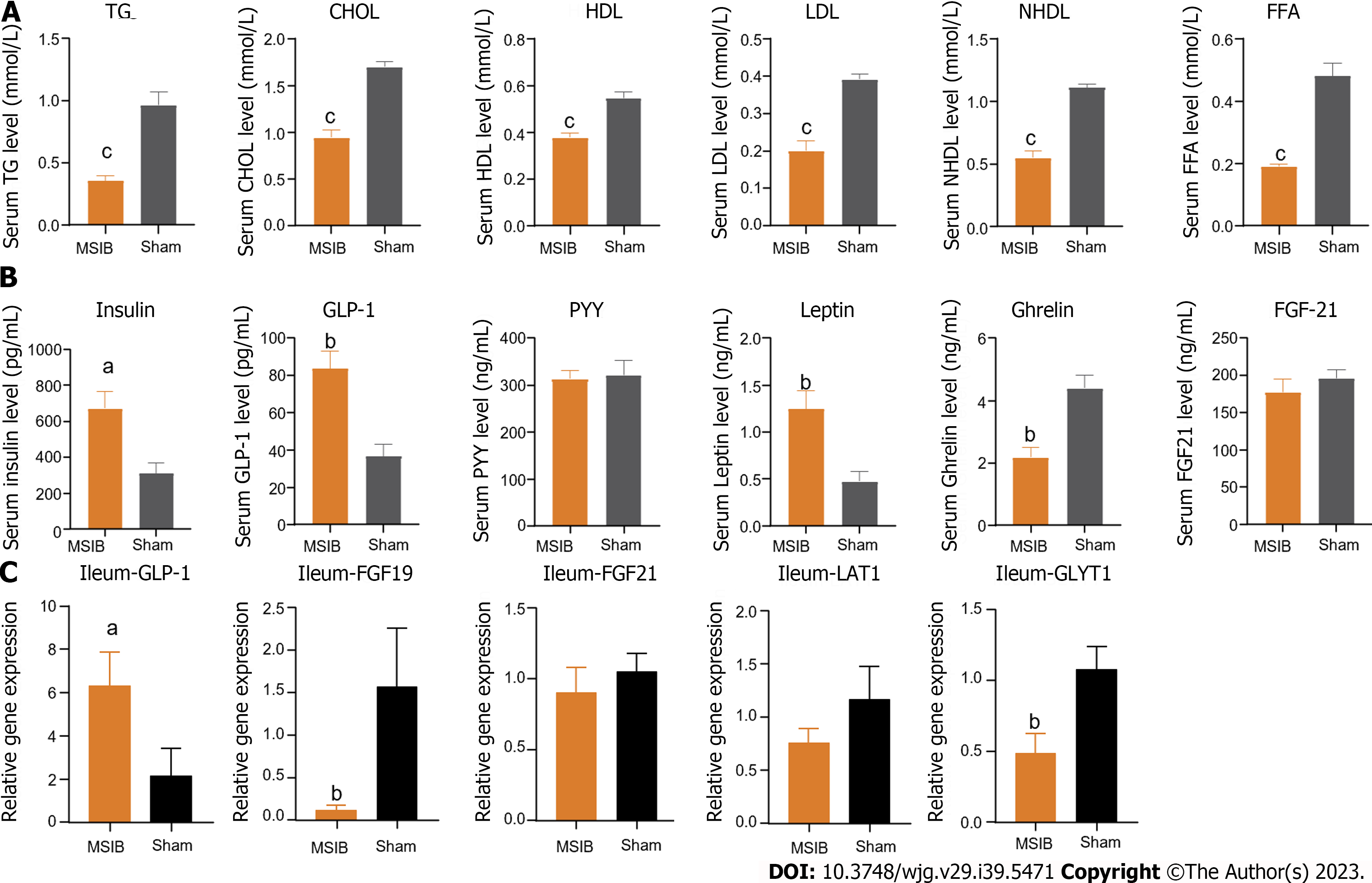
Blood was collected from the rat tail vein, and the blood glucose level was measured using an electronic glucometer (Accu-Chek Performa, Roche Diagnostics, Switzerland). The blood was then centrifuged at 3000 rpm for 15 min at 4 °C, and the resulting sera were immediately transferred to new tubes and stored at -80 °C until assay analysis was performed. Serum concentrations of triglycerides (TG), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein, non-HDL, and free fatty acids were determined using a fully automated biochemical analyzer. The analysis and testing procedures were conducted by the Biochemistry Laboratory at the Second Affiliated Hospital of Nanchang University. Insulin concentrations in serum were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Millipore, Billerica, MA, United States). Additionally, serum concentrations of glucagon-like peptide-1 (GLP-1), peptide tyrosine tyrosine, leptin, ghrelin, and fibroblast growth factor 21 (FGF21) were determined using ELISA kits (Uscn Life Sciences, Wuhan, China).
The OGTT and ITT were conducted at 2 and 6 wk postoperatively, respectively. The rats were fasted for 14 h prior to the tests. The OGTT was performed at 8 am by measuring the initial blood glucose level from the tail vein. Subsequently, a gavage of 20% glucose solution (1 g/kg) was administered to the rats with STZ-induced diabetes mellitus (STZ-DM). Blood glucose measurements were taken at 0, 15, 30, 60, 90, and 120 min after glucose administration, and the area under the curve (AUC) of the glucose tolerance test was calculated. Furthermore, six hours after fasting, the rats underwent ITT. After measuring the initial blood glucose level, 0.5 IU/kg human insulin (Wanbang Biopharmaceuticals, Jiangsu, China) was injected intraperitoneally, and blood glucose levels were measured at 0, 15, 30, 60, 90, and 180 min. Insulin sensitivity was assessed by calculating the ratio of blood glucose to basal blood glucose at each time point, and the AUC of the ITT was calculated.
After 6 wk of surgery, each rat was placed in an individual collection box, and fecal samples were collected within 48 h. The collected samples were immediately frozen in a -80 °C freezer. Bile acid extraction was performed using methanol. For each sample, methanol (5 mL/g) was added, and the mixture was shaken and incubated at room temperature for a specified period. Subsequently, the mixture was centrifuged at 1200 rpm for 2 min at room temperature, and the supernatant was carefully transferred into a new centrifuge tube. High-performance liquid chromatography (HPLC) was employed for bile acid determination. The filtered supernatant was injected into the HPLC apparatus and passed through the separation column. Gradient elution was performed using an appropriate mobile phase, and the absorbance of bile acids was detected by an ultraviolet detector. The concentration of bile acids in rat feces was determined by referencing a known concentration of bile acids in the standard curve.
RNA extraction from the collected liver and ileal samples was performed using the TRIzol Plus RNA Purification Kit (Thermo Fisher) following the manufacturer’s instructions. The extracted RNA samples were assessed for quantity and quality using a nanophotometer (NanoDrop-2000, Thermo Fisher Scientific, Massachusetts, United States). Reverse transcription reactions were carried out using the Reverse Transcription Kit (XYZ Company, Country) to convert RNA into cDNA, utilizing SuperScript™ III First-Strand Synthesis SuperMix (Thermo Fisher). The reverse transcription reaction mixture consisted of RNA template, reverse transcriptase, random primers, and reverse transcription buffer. The reaction conditions involved incubation at 37 °C for 30 min, followed by heat inactivation at 95 °C for 5 min. To amplify the target genes, preamplification reactions were performed using the Preamplification Kit (XYZ Company, Country). The preamplification reaction mixture included cDNA template, target gene-specific primer mixture, and preamplification buffer. The reaction conditions consisted of initial denaturation at 95 °C for 2 min, followed by 14 cycles of denaturation (95 °C, 15 s), annealing (60 °C, 1 min), and extension (72 °C, 30 s), with a final extension at 72 °C for 5 min. Real-time fluorescent quantitative polymerase chain reaction (PCR) analysis was performed using an ABI Prism 7700 sequence detector system (Applied Biosystems, Foster City, CA, United States). The PCR mixture consisted of the preamplified product, primer mix, fluorescent probe, and PCR Master Mix. The concentrations of primers and probes were adjusted based on optimization experiments. The reaction conditions included initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation (95 °C, 15 s) and annealing (60 °C, 1 min).
Microbial DNA was extracted from frozen fecal samples using the MagPure Fecal DNA KF Kit B (Magen, China). The extraction process was performed following the manufacturer’s instructions, and the concentration and purity of the DNA were measured using a nanophotometer with the Qubit dsDNA BR Analysis Kit (Invitrogen, United States). The V3-V4 region of the 16S rRNA gene was selected as the target for amplification. The PCR amplification reaction consisted of 10 ng of template DNA, 2.5 μM of each primer, and KAPA HiFi HotStart ReadyMix PCR Master Mix (Kapa Biosystems, United States). The PCR amplification conditions were as follows: Predenaturation at 95 °C for 3 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 30 s, and a final extension step at 72 °C for 5 min.
The PCR products were verified by gel electrophoresis and then purified using Ampure XP beads (Beckman Coulter, United States). The purified products underwent secondary PCR amplification using Illumina aptamers and sample-specific index sequences. The secondary PCR products were again validated by gel electrophoresis and purified using Ampure XP beads.
Sequencing was performed on the PacBio Sequel platform (UW Genetics, Shenzhen, China), and the obtained sequences were assigned to operational taxonomic units (OTUs) based on 97% sequence similarity. Clean tags were clustered into OTUs using USEARCH software (v7.0.1090), and species classification of the OTUs was completed. The representative sequences of the OTUs were compared to the Greengene_2013_5_99 database using RDP classifier (v2.2) software for species annotation, with a confidence threshold set to 0.5[10]. Diversity indices of the gut flora were calculated, and the beta diversity index of the gut microbiota was visualized using principal coordinate analysis, with the Shannon index representing the alpha diversity index. The differences in OTU abundance between groups were compared, and significantly different flora were identified using the linear discriminant analysis effect sizes (LEfSe) method, available on the Galaxy (harvard.edu) website.
Graphical data are presented as the mean ± SEM. All analyses were performed using R software version 4.1.3 and GraphPad Prism version 8.4 (GraphPad Software, San Diego, CA, United States), with a significance level set at 0.05. The AUC was calculated using trapezoidal integration. Differences between the two groups were analyzed using a t test. Changes in body weight, food intake, fasting blood glucose, OGTT, and ITT over time were analyzed using a two-way analysis of variance (ANOVA). Student’s t test was used for pairwise comparisons between the groups. Statistical significance was defined as follows: aP < 0.05, bP < 0.01, and cP < 0.001.
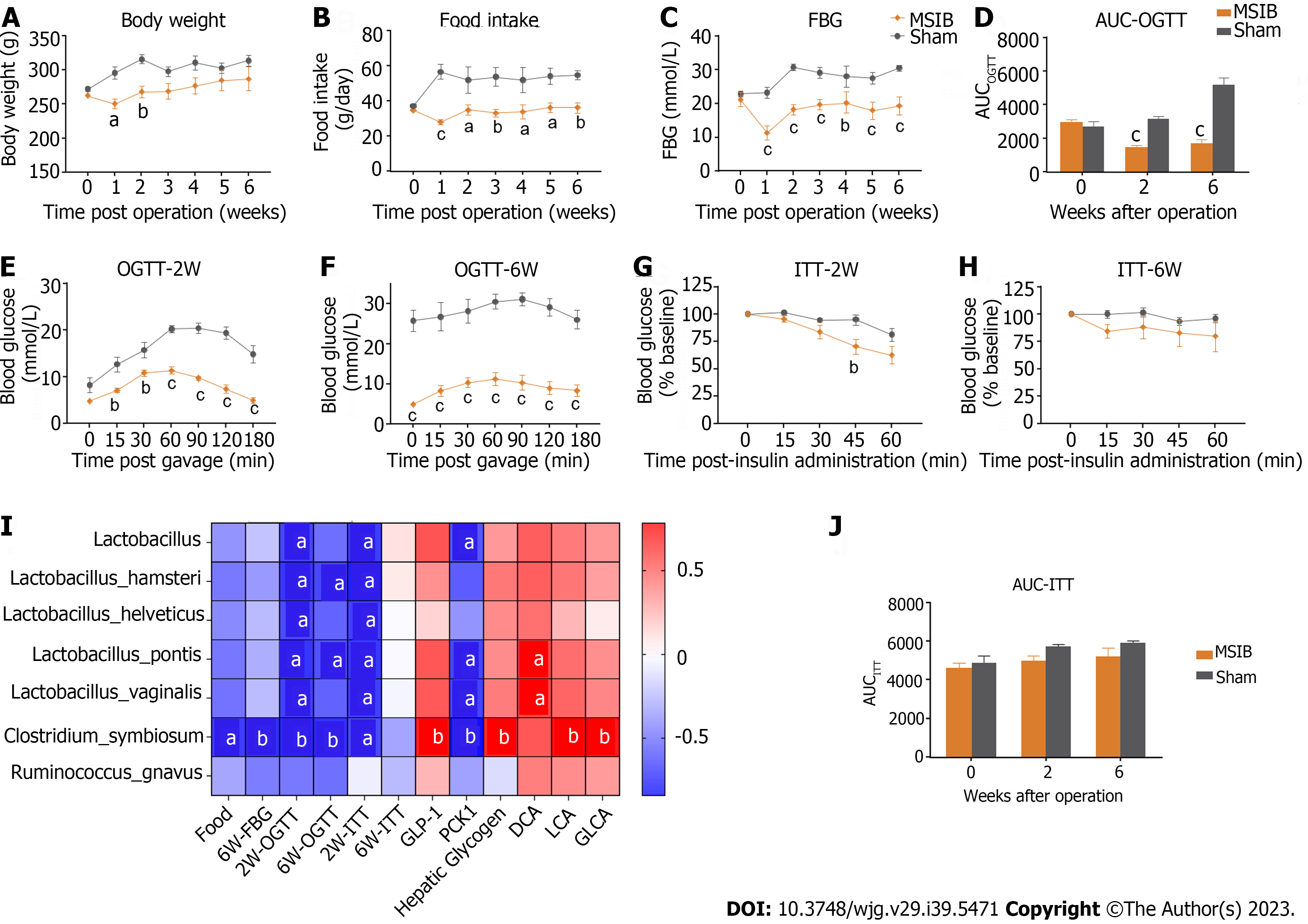
Significant metabolic improvements were observed after MSIB (Figures 1 and 2). At 2 wk after surgery, the body weight of rats in the MSIB group decreased compared to that of rats in the sham group (Figure 1A). Additionally, mean blood glucose levels were significantly lower in the MSIB group than in the sham group after surgery (Figure 1C). The OGTT results showed a significant decrease in both the OGTT values and the area under the OGTT curve (OGTT-AUC) in the MSIB group compared to the sham group (Figure 1D-F). Moreover, the MSIB group exhibited a significant reduction in food intake compared to the sham group (Figure 1B). However, there was no significant improvement in insulin sensitivity (ITT) in the MSIB group compared to the sham group, except for the 45-min mark in the ITT test two weeks after surgery and the 60-min mark in the ITT test six weeks after surgery (Figures 1G, H and J). In conclusion, these findings suggest that MSIB has a significant positive effect on glucose metabolism in diabetic rats.
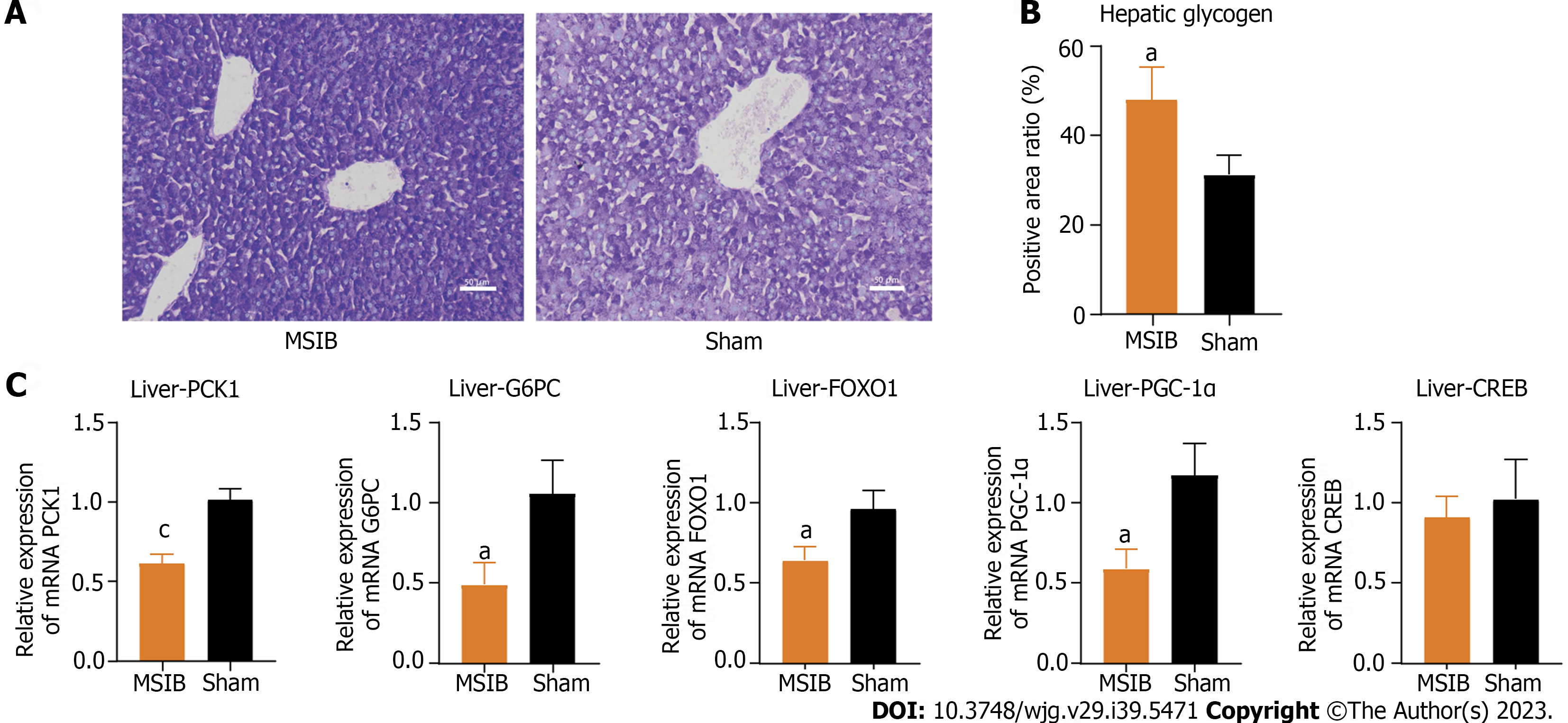
After MSIB, there was an observed increase in hepatic glycogen stores, a reduction in gluconeogenic key enzymes, and a decrease in the expression of transcription factors (Figure 3C). These changes were significantly correlated with glucose metabolism. Examination of liver tissue sections at 6 wk postoperatively revealed a higher hepatic glycogen content in the MSIB group than in the sham group (Figures 3A and B). Furthermore, the MSIB group showed reduced expression of phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (G6PC), which are key enzymes involved in hepatic gluconeogenesis. Additionally, there was a decrease in the expression of Forkhead box O1 (FOXO1) and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), which are transcription factors that regulate gluconeogenesis (Figure 3C).
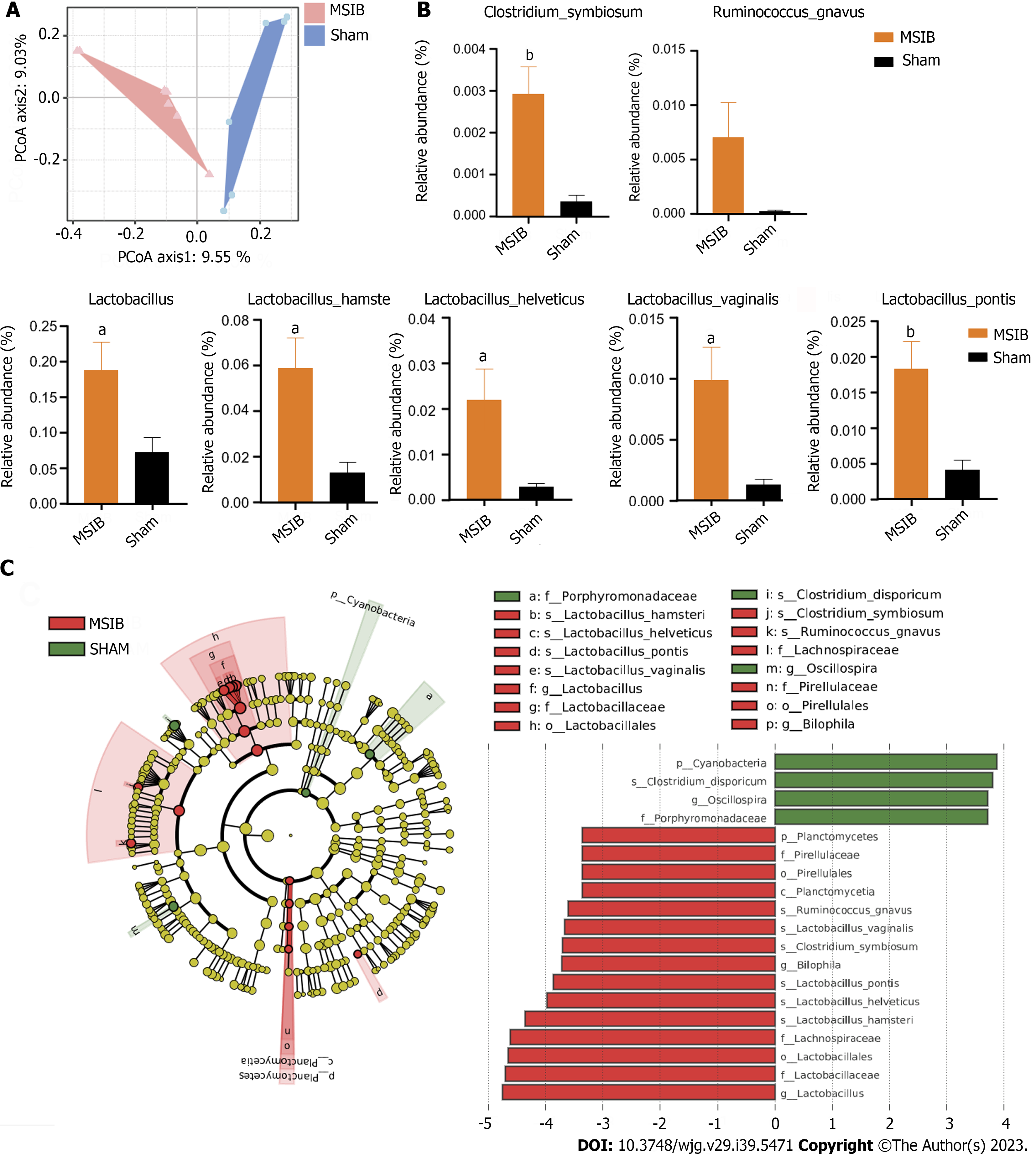
The gut microbiome of rats in the MSIB group exhibited notable alterations compared to that of the sham group following surgery, resulting in discernible differences in beta diversity between the two groups (Figure 4A). Specifically, there was a significant increase in the abundances of several Lactobacillus spp. at the species level, including Lactobacillus hamsteri, Lactobacillus helveticus, Lactobacillus pontis, and Lactobacillus vaginalis. Additionally, there was a significant increase in the abundances of Clostridium symbiosum, Bilophila and Ruminococcus gnavus in the gut microbiome of rats in the MSIB group (Figures 4B and C). Notably, these upregulated microbial species were correlated with changes in key enzymes involved in gluconeogenesis and bile acid metabolism.
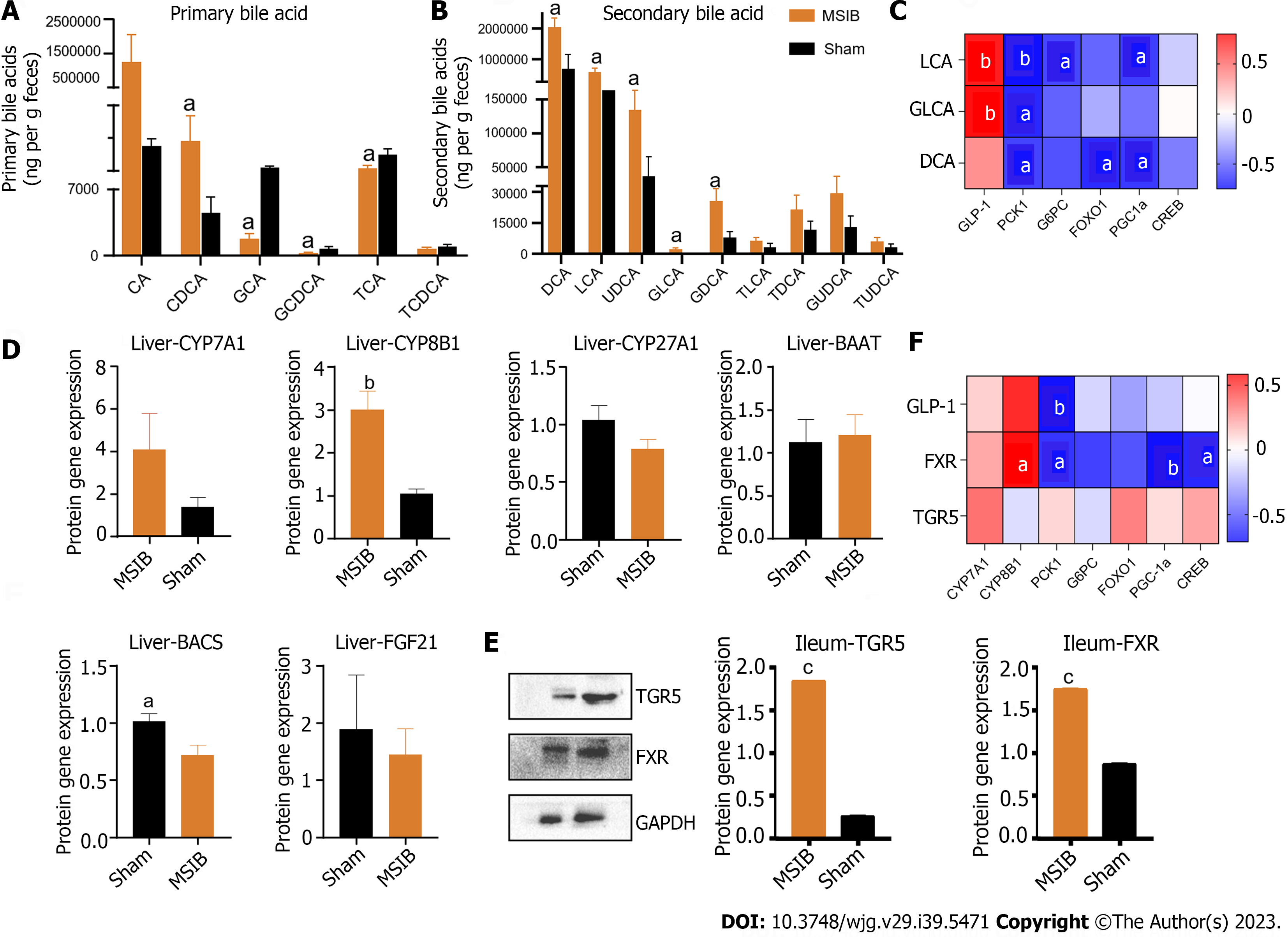
After surgery, the MSIB group exhibited increased expression of farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5) (Figure 5E), as well as elevated levels of fecal bile acids and secondary bile acids (Figure 5B). Metabolomics analysis revealed a significant increase in fecal bile acids in the MSIB group compared to the sham group (Figure 5). Specifically, there was an increase in secondary bile acids, including chenodeoxycholic acid, deoxycholic acid (DCA), glyco-deoxycholic acid, lithocholic acid (LCA), glycolithocholic acid (GLCA), and ursodeoxycholic acid. Correlation analysis further demonstrated a significant positive correlation between the levels of the intestinal secondary bile acids LCA and GLCA and the abundance of Clostridium_symbiosum in the intestines (Figure 5C). Moreover, LCA showed a significant positive correlation with intestinal GLP-1 expression and a significant negative correlation with the key hepatic gluconeogenic enzyme PCK1 (Figures 5C and F).
The decrease in serum glucose levels after MSIB is associated with the inhibition of hepatic gluconeogenesis. At 6 wk post-MSIB, diabetic rats in the MSIB group exhibited a significant decrease in blood glucose levels compared to those in the sham group, as well as a significant difference in the OGTT-AUC. These findings confirmed that a 60% small intestinal resection could still improve glucose metabolism, although the effect on reducing body weight was not significant. To explore the underlying mechanism of improved glucose metabolism after MSIB, we assessed the liver tissue glycogen content of rats at 6 wk post-surgery. The results revealed a significantly higher hepatic glycogen content in the MSIB group than in the sham group, indicating that the improved glucose metabolism after MSIB is associated with enhanced serum glucose conversion. Previous studies have reported increased hepatic glucose uptake and decreased endogenous glucose production following metabolic surgery, which aligns with our findings of elevated hepatic glycogen levels[11]. Moreover, bariatric surgery has been shown to inhibit hepatic glucose production[12], further supporting our observation. Analysis of liver tissue also demonstrated significantly reduced expression of the key gluconeogenic enzymes PCK1 and G6PC in the MSIB group compared to the sham group, along with decreased expression of the transcription factors FOXO1 and PGC-1α, which regulate gluconeogenesis. Correlation analysis revealed a significant positive correlation between PCK1, G6PC, and glucose levels. Based on these findings, we conclude that the decrease in serum glucose levels after MSIB is attributed to the inhibition of gluconeogenesis.
The gut microbiome of rats underwent significant changes after MSIB, as revealed by LEfSe analysis. Specifically, there was a significant increase in the abundance of Lactobacillus spp., including specific species such as Lactobacillus hamsteri, Lactobacillus helveticus, Lactobacillus pontis, Lactobacillus vaginalis, and Ruminococcus gnavus. Additionally, the abundances of Bilophila and Clostridium symbiosum, which are known to metabolize bile acids, also increased significantly. Our experiments demonstrated an increase in the fecal levels of various secondary bile acids in the MSIB group compared to the sham group. Correlation analysis revealed a significant positive correlation between the levels of LCA and GLCA and the abundance of intestinal Clostridium symbiosum, suggesting the involvement of the intestinal flora in metabolic regulation through the catabolic production of secondary bile acids.
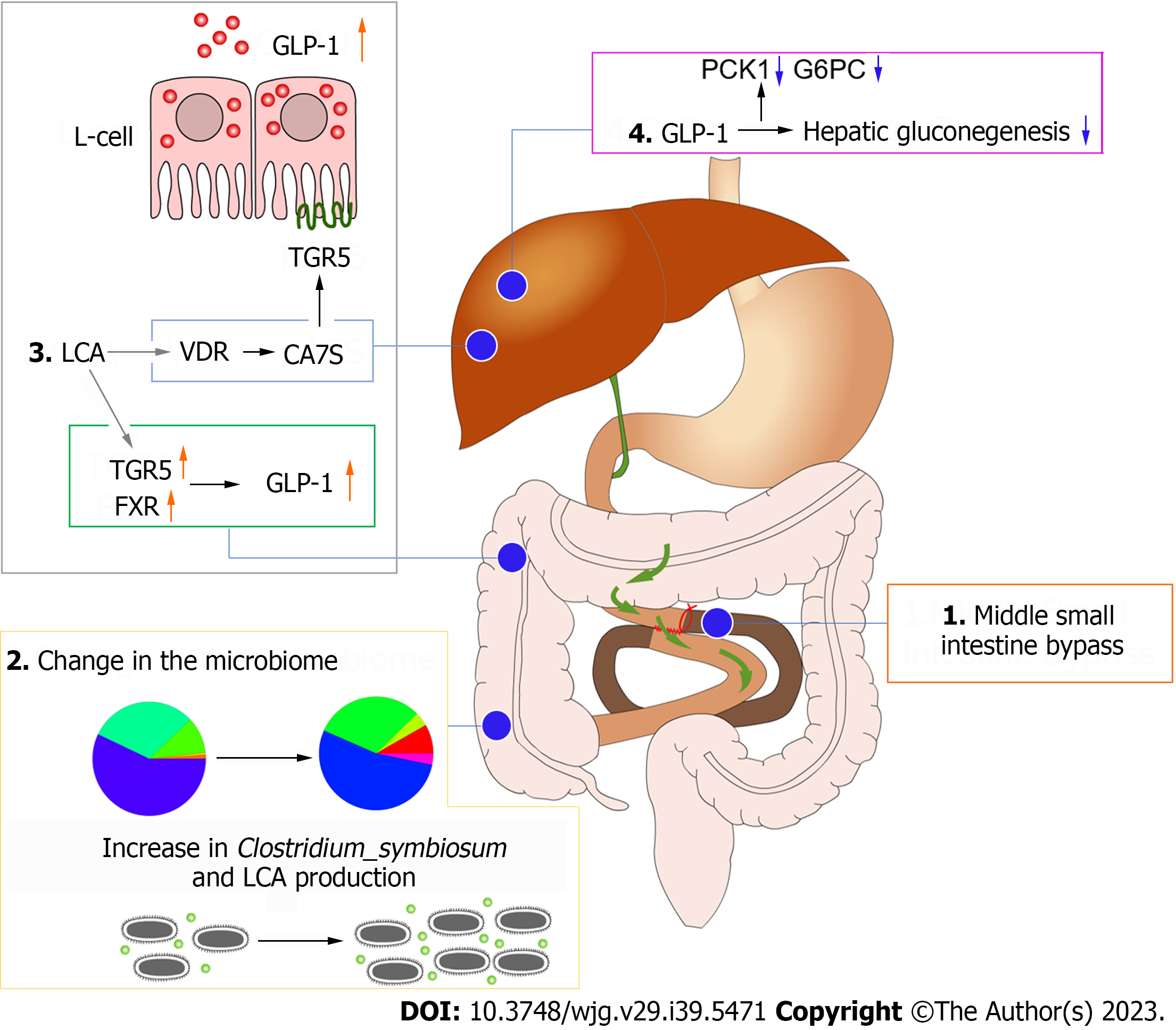
Remarkably, the expression levels of cytochrome P450 family 8 subfamily B member 1, an enzyme involved in bile acid synthesis, were significantly increased in the experimental group, suggesting that the elevated bile acid levels after MSIB may be attributed to augmented classical bile acid pathway synthesis. Bile acids play a crucial role in metabolic regulation as important signaling molecules. Previous studies by Steenackers et al[8] demonstrated that LCA can bind to hepatic vitamin D receptors, thereby promoting GLP-1 secretion from intestinal L cells (Figure 6). In our experiments, we also observed an increase in intestinal LCA levels and GLP-1 transcription in rats following MSIB surgery.
Moreover, bile acids can modulate metabolic homeostasis by activating the nuclear receptor FXR and the membrane receptor TGR5[13-15]. TGR5 receptor activation is generally believed to confer metabolic benefits, while the precise role of FXR in metabolic regulation remains to be fully elucidated. Pathak et al[16] demonstrated that the dual agonist INT-767 induces GLP-1 secretion from cells, which aligns with our observations. We found increased transcript levels of TGR5 and FXR in the MSIB group of rats after surgery, along with elevated GLP-1 transcript levels. GLP-1, as a gastrointestinal hormone, reduces blood glucose levels and improves insulin resistance. Importantly, we observed a positive correlation between insulin sensitivity and GLP-1 in the experimental group of rats. Based on these findings, we propose that secondary bile acids promote GLP-1 secretion through the activation of TGR5 and FXR, thus benefiting glucose metabolism in rats after MSIB. Additionally, the gut microbiota plays a crucial role as an important mediator of this process.
There was a significant negative correlation between GLP-1 levels and the transcript levels of the key enzymes PCK1 and G6PC after MSIB. We observed increased glycogen stores in liver tissue and a significant decrease in the expression of the gluconeogenic enzymes PCK1 and G6PC in the MSIB group compared to the sham group after surgery. Additionally, the transcription factors FOXO1 and CREB, which regulate gluconeogenesis, were also found to be inhibited. These findings suggest that the inhibition of gluconeogenesis played a significant role in the reduction in blood glucose levels in rats after MSIB.
Furthermore, correlation analysis revealed a significant negative correlation between PCK1 expression and GLP-1 levels, indicating that GLP-1 may be involved in the inhibition of gluconeogenesis. However, further experimental evidence is required to confirm this relationship. Previous studies by Lee et al[17] demonstrated that GLP-1 gene therapy reduces hepatic gluconeogenesis. Other scholars have suggested that GLP-1 may inhibit hepatic gluconeogenesis through FGF21[18] and regulate hepatic glucose production through neural circuits[19]. Additionally, intraperitoneal injection of GLP-1 has been shown to inhibit gluconeogenesis and related enzyme activity in obese mice[20]. These studies support the notion that GLP-1 can influence hepatic gluconeogenesis to improve blood glucose levels, which aligns with our observation of the decreased expression of key gluconeogenic enzymes. Therefore, we conclude that the increased levels of GLP-1 were responsible for the inhibition of postoperative gluconeogenesis in the MSIB group of rats.
In summary, our findings suggest that hepatic gluconeogenesis is inhibited by the decrease in serum glucose levels in rats after MSIB. Increased GLP-1 secretion, attributed to the elevated abundance of intestinal Clostridium symbiosum, serves as a potential pathway mediating these effects.
The study has some limitations, including a small sample size and a relatively short observation period. To further investigate the relationship between the intestinal Clostridium symbiosum abundance, bile acids, and GLP-1, future studies should consider conducting microbiota transplantation experiments and cell-based assays. Additionally, the cellular pathways connecting GLP-1 and gluconeogenesis still require further investigation.
Postoperative intestinal bypass of the midsection small intestine in nonobese diabetic rats improves glucose metabolism by increasing GLP-1 levels and inhibiting hepatic gluconeogenesis through the increased abundance of intestinal Clostridium symbiosum.
The exact mechanism by which mid-small intestinal bypass (MSIB) improves glucose metabolism in diabetic rats is not fully understood.
To explore the role of the mid-small intestine in the onset and progression of diabetes.
The aim of this study was to elucidate the mechanisms by which MSIB improves glucose metabolism.
Streptozotocin was used to induce diabetes mellitus in Sprague-Dawley rats at a dose of 60 mg/kg. The rats were then randomly divided into two groups: The MSIB group and the sham group (underwent switch laparotomy). Following a 6-wk recovery period post-surgery, the rats underwent various assessments, including metabolic parameter testing, analysis of liver glycogen levels, measurement of key gluconeogenic enzyme activity, characterization of the gut microbiota composition, evaluation of hormone levels, determination of bile acid concentrations, and assessment of the expression of the intestinal receptors Takeda G protein-coupled receptor 5 and farnesoid X receptor.
The MSIB group of rats exhibited improved glucose and lipid metabolism, increased hepatic glycogen content, and decreased expression of key gluconeogenic enzymes (phosphoenolpyruvate carboxykinase 1 and glucose-6-phosphatase). Notably, this group showed a substantial rise in specific intestinal bacteria, including Lactobacillus, Clostridium symbiosum, Ruminococcus gnavus, and Bilophila. Additionally, elevated levels of secondary bile acids, such as lithocholic acid, were observed. Importantly, changes in the gut microbiota were significantly correlated with the expression of gluconeogenic enzymes and glucagon-like peptide 1 (GLP-1) at 6 wk post-surgery, suggesting their potential involvement in regulating glucose. These findings underscore the beneficial impact of mid-small intestine bypass on glucose metabolism and its modulation of the gut microbiota.
This study shows that postoperative introduction of intestinal Clostridium symbiosum in the mid-small intestine improves glucose metabolism in non-obese diabetic rats. This enhancement is linked to increased inhibition of hepatic gluconeogenesis mediated by GLP-1, leading to a positive impact on glucose regulation.
This study explores partial mechanisms of the interaction between gut microbiota and host metabolism, providing a theoretical foundation for non-surgical interventions in diabetes-related metabolic disorders.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mata-Torres G, Mexico; Thongon N, Thailand S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4666] [Article Influence: 583.3] [Reference Citation Analysis (2)] |
| 2. | Molina-Montes E, Coscia C, Gómez-Rubio P, Fernández A, Boenink R, Rava M, Márquez M, Molero X, Löhr M, Sharp L, Michalski CW, Farré A, Perea J, O'Rorke M, Greenhalf W, Iglesias M, Tardón A, Gress TM, Barberá VM, Crnogorac-Jurcevic T, Muñoz-Bellvís L, Dominguez-Muñoz JE, Renz H, Balcells J, Costello E, Ilzarbe L, Kleeff J, Kong B, Mora J, O'Driscoll D, Poves I, Scarpa A, Yu J, Hidalgo M, Lawlor RT, Ye W, Carrato A, Real FX, Malats N; PanGenEU Study Investigators. Deciphering the complex interplay between pancreatic cancer, diabetes mellitus subtypes and obesity/BMI through causal inference and mediation analyses. Gut. 2021;70:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Fink J, Seifert G, Blüher M, Fichtner-Feigl S, Marjanovic G. Obesity Surgery. Dtsch Arztebl Int. 2022;119:70-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE, Baskin DG, Havel PJ. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437-2446, 2446.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Zhang X, Young RL, Bound M, Hu S, Jones KL, Horowitz M, Rayner CK, Wu T. Comparative Effects of Proximal and Distal Small Intestinal Glucose Exposure on Glycemia, Incretin Hormone Secretion, and the Incretin Effect in Health and Type 2 Diabetes. Diabetes Care. 2019;42:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Soper NJ, Chapman NJ, Kelly KA, Brown ML, Phillips SF, Go VL. The 'ileal brake' after ileal pouch-anal anastomosis. Gastroenterology. 1990;98:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Jarvie BC, Knight ZA. Breaking down a gut-to-brain circuit that prevents malabsorption. Cell. 2022;185:2393-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Steenackers N, Vanuytsel T, Augustijns P, Tack J, Mertens A, Lannoo M, Van der Schueren B, Matthys C. Adaptations in gastrointestinal physiology after sleeve gastrectomy and Roux-en-Y gastric bypass. Lancet Gastroenterol Hepatol. 2021;6:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 592] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10:57-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3022] [Cited by in RCA: 2672] [Article Influence: 222.7] [Reference Citation Analysis (0)] |
| 11. | Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, Borra R, Parkkola R, Mari A, Lehtimäki T, Pham T, Laine J, Kärjä V, Pihlajamäki J, Nelimarkka L, Nuutila P. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol. 2014;60:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Ben-Haroush Schyr R, Al-Kurd A, Moalem B, Permyakova A, Israeli H, Bardugo A, Arad Y, Hefetz L, Bergel M, Haran A, Azar S, Magenheim I, Tam J, Grinbaum R, Ben-Zvi D. Sleeve Gastrectomy Suppresses Hepatic Glucose Production and Increases Hepatic Insulin Clearance Independent of Weight Loss. Diabetes. 2021;70:2289-2298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1683] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 14. | Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 347] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 15. | Yan Y, Niu Z, Sun C, Li P, Shen S, Liu S, Wu Y, Yun C, Jiao T, Jia S, Li Y, Fang ZZ, Zhao L, Wang J, Xie C, Jiang C, Feng X, Hu C, Jiang J, Ying H. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat Commun. 2022;13:6408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 16. | Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, Chiang JYL. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292:11055-11069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 17. | Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, Yoon JW, Jun HS. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Liu J, Yang K, Yang J, Xiao W, Le Y, Yu F, Gu L, Lang S, Tian Q, Jin T, Wei R, Hong T. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine. 2019;41:73-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Yang M, Wang J, Wu S, Yuan L, Zhao X, Liu C, Xie J, Jia Y, Lai Y, Zhao AZ, Boden G, Li L, Yang G. Duodenal GLP-1 signaling regulates hepatic glucose production through a PKC-δ-dependent neurocircuitry. Cell Death Dis. 2017;8:e2609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Ip W, Shao W, Chiang YT, Jin T. GLP-1-derived nonapeptide GLP-1(28-36)amide represses hepatic gluconeogenic gene expression and improves pyruvate tolerance in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2013;305:E1348-E1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |