Published online Oct 14, 2023. doi: 10.3748/wjg.v29.i38.5428
Peer-review started: June 7, 2023
First decision: July 7, 2023
Revised: July 21, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: October 14, 2023
Processing time: 126 Days and 15.6 Hours
Treatment of infantile-onset inflammatory bowel disease (IO-IBD) is often challenging due to its aggressive disease course and failure of standard therapies with a need for biologics. Secondary loss of response is frequently caused by the production of anti-drug antibodies, a well-known problem in IBD patients on biologic treatment. We present a case of IO-IBD treated with therapeutic drug monitoring (TDM)-guided high-dose anti-tumor necrosis factor therapy, in which dose escalation monitoring was used as a strategy to overcome anti-drug antibo
A 5-mo-old boy presented with a history of persistent hematochezia from the 10th d of life, as well as relapsing perianal abscess and growth failure. Hypoalbuminemia, anemia, and elevated inflammatory markers were also present. Endo
TDM-guided high-dose ADA treatment as a monotherapy overcame ATA production. This strategy could be a good alternative to combination therapy, especially in very young patients.
Core Tip: Infantile-onset inflammatory bowel disease (IBD) frequently has a more severe course and a greater resistance to standard therapy than IBD in older children. Anti-tumor necrosis factor agents often lead to the production of anti-drug antibodies, resulting in loss of clinical response and disease progression. For this reason, the early detection of anti-drug antibodies is important, which may be possible with therapeutic drug monitoring. To date, commonly used strategies to overcome anti-drug antibodies are switching drugs or adding an immunomodulator, but a better option may be dose escalation.
- Citation: Ancona S, Signa S, Longo C, Cangemi G, Carfora R, Drago E, La Rosa A, Crocco M, Chiaro A, Gandullia P, Arrigo S. Dose escalation of adalimumab as a strategy to overcome anti-drug antibodies: A case report of infantile-onset inflammatory bowel disease. World J Gastroenterol 2023; 29(38): 5428-5434
- URL: https://www.wjgnet.com/1007-9327/full/v29/i38/5428.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i38.5428
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract, and its incidence and prevalence in the pediatric population are rising in most countries[1]. Patients with IBD can be categorized by age at diagnosis: IBD cases diagnosed before 6 years of age are classified as very early onset IBD (VEO-IBD), whereas those with onset by 2 years of age are classified as infantile onset IBD (IO-IBD)[2]. Children with VEO-IBD and IO-IBD tend to have a more severe disease course[3,4] and higher rates of treatment resistance to standard therapy, including biologics[5-8]. Therefore, treatment of IO-IBD is challenging and frequently requires an aggressive approach[9].
Anti-tumor necrosis factor (TNF) agents are highly effective drugs for the treatment of pediatric IBD; however, some patients do not respond to induction therapy (primary non-responders) and some initial responders later experience loss of response (LOR; secondary non-responders). Primary and secondary non-responses are often the result of low trough concentration or high levels of anti-drug antibodies[10]. The use of therapeutic drug monitoring (TDM) has modified the biologic therapeutic approach in pediatric IBD by allowing the measurement of drug and anti-drug antibody serum concentrations[11,12]. Trough and antibody levels can guide appropriate dosing and interval schedules, allowing the development of an individualized treatment plan and leading to higher remission rates[13]. TDM has consequently resulted in higher treatment intensification rates, making it useful for guiding high-dose therapy in IO-IBD. A recent case series by Assa et al[14] suggested that an accelerated high-dose anti-TNF induction protocol could help recapture the response in children with IO-IBD who experienced an initial non-response or secondary LOR with infliximab (IFX).
Herein, we present a patient with IO-IBD who experienced secondary LOR due to anti-drug antibodies, and was successfully treated with TDM-guided high-dose anti-TNF therapy.
A 5-mo-old boy presented with a history of persistent hematochezia from the 10th d of life, as well as relapsing perianal abscess and growth failure.
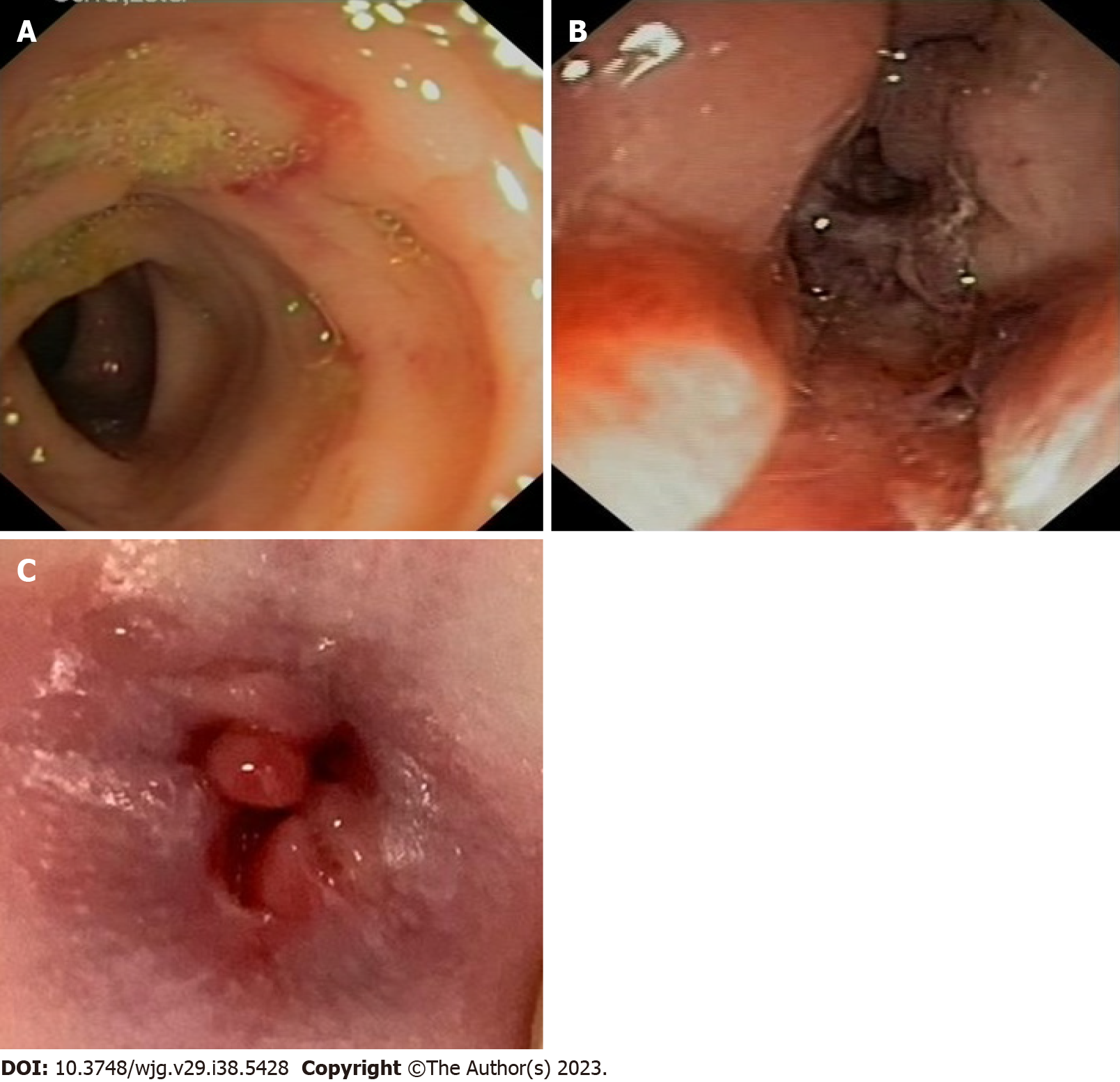
The ileocolonoscopy revealed skip lesions with deep colic ulcerations, especially in the descending colon, and inflammatory anal sub-stenosis with deep fissures and tags (Figure 1). Histological findings demonstrated patchy severe chronic active colitis, characterized by crypt distortion and abscesses, loss of glands, and basal plasma cell expansion, all of which strongly suggested IBD. Pelvic magnetic resonance imaging was performed, and no additional perianal lesions were discovered.
Blood tests revealed microcytic anemia (hemoglobin 7.4 g/dL, normal range: 11-13 g/dL; mean corpuscular volume 67.5 fL, normal range: 77-101 fL) and hypoalbuminemia (2740 mg/dL). Inflammatory markers were moderately increased [C-reactive protein (CRP) 3.61 mg/dL]. Total immunoglobulin levels and immunological screening results were normal. Stool cultures and the Clostridium difficile test were negative.
On physical examination, the infant was pale and mildly hyporeactive, with adequate hydration. A perianal fistula, without drainage, and an anal fissure were found. No abdominal tenderness or mass was found. An auxological evaluation demonstrated growth failure. The rest of the examination was unremarkable.
His parents denied a family history of autoimmune or gastrointestinal diseases.
At the age of 6 wk, he presented for the first time to the Emergency Department of our hospital with complaint of a perianal abscess and a history of persistent hematochezia from the 10th d of life. He was also unresponsive to a cow’s milk protein-free diet. His growth was regular, and his psychomotor development was normal. Blood tests showed elevated inflammatory markers (CRP: 2.38 mg/dL, normal range: < 0.46 mg/dL) and mild hypoalbuminemia (2970 mg/dL, normal range: 3800-5400 mg/dL). Stool cultures were negative. A rectosigmoidoscopy was performed and showed macroscopic signs of unspecific proctocolitis, without hypereosinophilia at the histological exam. Intravenous antibiotic therapy led to transient resolution of hematochezia and improvement of the perianal abscess.
Two months later, the patient was readmitted to the hospital due to the recurrence of perianal abscess and bloody stools, so a second rectosigmoidoscopy was performed, which showed evidence of a macroscopic micronodular proctosigmoiditis. No specific histological alterations were found and blood tests were normal. The patient was discharged with topical steroid therapy.
In the last several weeks before hospital admission, his clinical condition worsened: he developed diarrhea, characterized by more than eight completely unformed bloody stools, and had painful defecation.
Taking into consideration the endoscopic assessment and perianal disease, a diagnosis of IO-IBD Crohn-like was made. Targeted IO-IBD next-generation sequencing panel was negative, and whole exome sequencing results are pending; an eventual pathogenic mutation for a monogenic IBD could explain the severity of the disease course in a very young child and may suggest a more targeted treatment.
The patient was initially treated with oral steroids (prednisone 1.5 mg/kg/d) with a clinical response and fistulotomy. After the perianal abscess healed, adalimumab (ADA) was administered [20 mg (3.3 mg/kg) for the first two doses, and then 10 mg (1.6 mg/kg) every 2 wk] with concomitant gradual steroid tapering. Of note, ADA has currently been approved for the treatment of moderate-to-severe Crohn’s disease in children from six years of age; in particular, for patients < 40 kg, the drug label recommends 80 mg at the first dose (week 0), 40 mg at week 2 and 20 mg every two weeks (from week 4 onwards). IFX was avoided due to extremely difficult venous access. Clinical and biochemical steroid-free remission was achieved with good trough levels.
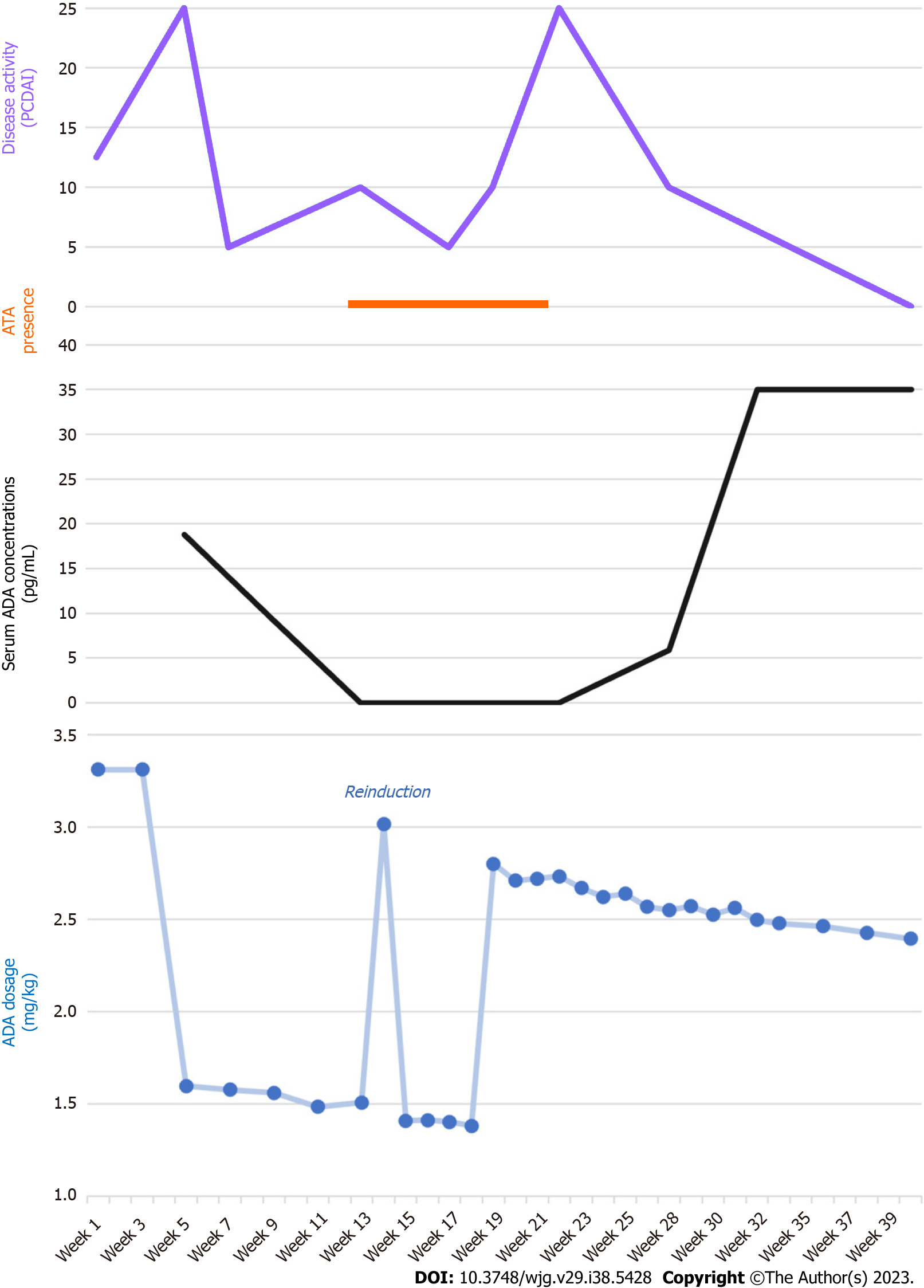
After 3 mo, antibodies to ADA (ATA) were found with undetectable trough levels: therefore, we decided to optimize the therapy schedule, first administering 10 mg weekly and subsequently up to 20 mg weekly (2.8 mg/kg/dose). After 2 mo of high-dose treatment, the ATA disappeared, with concomitant high trough levels and stable clinical and biochemical remission of the disease. In Figure 2, ADA dosage and trough levels are shown, which correlated with ATA and disease activity, as assessed with the Pediatric Crohn’s Disease Activity Index.
Four months after the ATA disappearance, the child is still in clinical and biochemical remission. No adverse events have been reported, and the high dose of ADA has been well tolerated.
We describe a case of successful TDM-guided high-dose ADA treatment of a patient with IO-IBD. He experienced secondary LOR due to ATA production, which was overcome with dose intensification of ADA in monotherapy, reaching high trough levels.
Treatment of pediatric IBD with anti-TNF agents can result in immunogenicity and the formation of anti-drug antibodies[15], which are associated with loss of clinical response and worsening disease. Similar to adults[16,17], studies in the pediatric population have demonstrated that the combination of anti-TNF with an immunomodulator, such as azathioprine or methotrexate, lowers the risk of antibody formation and associated secondary LOR[18-20]. In line with these findings, the 2020 ECCO-ESPGHAN guidelines recommend combination therapy in patients with pediatric Crohn’s disease, starting with IFX, and prudentially suggest a concomitant immunomodulator when starting ADA in patients previously sensitized to IFX or in high-risk patients when used as a primary anti-TNF agent[10]. However, long-term treatment with immunomodulators, especially thiopurines, is controversial because of the risk of malignancy[21,22].
To date, the management of patients who develop antibodies to IFX or ADA is often empiric. In our case, we administered accelerated high-dose ADA treatment, which overcame ATA production, resulting in stable clinical and biochemical remission after a period of transient LOR. This strategy of dose optimization has been previously suggested in adults[23]. Regarding the pediatric IBD literature, Cohen et al[24] showed suppression of antibodies in pediatric IBD patients with lower antibody levels (< 10 U/mL)[24].
Although a stable clinical remission has been achieved for almost a year, a recurrence of ATA could happen again in the future. Indeed, it is important to continue a strict trough levels and antibodies monitoring, in order to adjust ADA dosage and prevent ATA production. Other possible therapeutic approaches in the case of a recurrence of LOR, could be a combination with an immunomodulator, such as azathioprine or methotrexate, or a switch to another off-label biologic drug, like ustekinumab or vedolizumab. IFX has been previously avoided for the extremely difficult venous access, but a more stable venous device could be placed for making an attempt with IFX.
We describe the first case of successful TDM-guided high-dose ADA treatment of a patient with IO-IBD. We overcame ATA production and subsequent transient LOR with a combination of interval shortening and dose escalation of ADA in monotherapy, reaching high trough levels. This strategy may be a good alternative to combination therapy, particularly in IO-IBD where an underlying primary immunodeficiency needs to be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan; Knudsen T, Denmark; Lee WS, Malaysia S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | Kuenzig ME, Fung SG, Marderfeld L, Mak JWY, Kaplan GG, Ng SC, Wilson DC, Cameron F, Henderson P, Kotze PG, Bhatti J, Fang V, Gerber S, Guay E, Kotteduwa Jayawarden S, Kadota L, Maldonado D F, Osei JA, Sandarage R, Stanton A, Wan M; InsightScope Pediatric IBD Epidemiology Group, Benchimol EI. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology. 2022;162:1147-1159.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 335] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 2. | Arai K. Very Early-Onset Inflammatory Bowel Disease: A Challenging Field for Pediatric Gastroenterologists. Pediatr Gastroenterol Hepatol Nutr. 2020;23:411-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Kelsen JR, Sullivan KE, Rabizadeh S, Singh N, Snapper S, Elkadri A, Grossman AB. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper on the Evaluation and Management for Patients With Very Early-onset Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2020;70:389-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Kelsen JR, Conrad MA, Dawany N, Patel T, Shraim R, Merz A, Maurer K, Sullivan KE, Devoto M. The Unique Disease Course of Children with Very Early onset-Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Ouahed J, Spencer E, Kotlarz D, Shouval DS, Kowalik M, Peng K, Field M, Grushkin-Lerner L, Pai SY, Bousvaros A, Cho J, Argmann C, Schadt E, Mcgovern DPB, Mokry M, Nieuwenhuis E, Clevers H, Powrie F, Uhlig H, Klein C, Muise A, Dubinsky M, Snapper SB. Very Early Onset Inflammatory Bowel Disease: A Clinical Approach With a Focus on the Role of Genetics and Underlying Immune Deficiencies. Inflamm Bowel Dis. 2020;26:820-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 6. | Kelsen JR, Grossman AB, Pauly-Hubbard H, Gupta K, Baldassano RN, Mamula P. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr. 2014;59:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Kerur B, Fiedler K, Stahl M, Hyams J, Stephens M, Lu Y, Pfefferkorn M, Alkhouri R, Strople J, Kelsen J, Siebold L, Goyal A, Rosh JR, LeLeiko N, Van Limbergen J, Guerrerio AL, Maltz RM, Karam L, Crowley E, Griffiths AM, Heyman MB, Deneau M, Benkov K, Noe J, Moulton D, Pappa H, Galanko J, Snapper S, Muise AM, Kappelman MD, Benchimol EI. Utilization of Antitumor Necrosis Factor Biologics in Very Early Onset Inflammatory Bowel Disease: A Multicenter Retrospective Cohort Study From North America. J Pediatr Gastroenterol Nutr. 2022;75:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Shim JO. Recent Advance in Very Early Onset Inflammatory Bowel Disease. Pediatr Gastroenterol Hepatol Nutr. 2019;22:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Kammermeier J, Dziubak R, Pescarin M, Drury S, Godwin H, Reeve K, Chadokufa S, Huggett B, Sider S, James C, Acton N, Cernat E, Gasparetto M, Noble-Jamieson G, Kiparissi F, Elawad M, Beales PL, Sebire NJ, Gilmour K, Uhlig HH, Bacchelli C, Shah N. Phenotypic and Genotypic Characterisation of Inflammatory Bowel Disease Presenting Before the Age of 2 years. J Crohns Colitis. 2017;11:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, Gasparetto M, Gerasimidis K, Griffiths A, Henderson P, Koletzko S, Kolho KL, Levine A, van Limbergen J, Martin de Carpi FJ, Navas-López VM, Oliva S, de Ridder L, Russell RK, Shouval D, Spinelli A, Turner D, Wilson D, Wine E, Ruemmele FM. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 11. | Kapoor A, Crowley E. Advances in Therapeutic Drug Monitoring in Biologic Therapies for Pediatric Inflammatory Bowel Disease. Front Pediatr. 2021;9:661536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | van Hoeve K, Hoffman I, Vermeire S. Therapeutic drug monitoring of anti-TNF therapy in children with inflammatory bowel disease. Expert Opin Drug Saf. 2018;17:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Conrad MA, Kelsen JR. The Treatment of Pediatric Inflammatory Bowel Disease with Biologic Therapies. Curr Gastroenterol Rep. 2020;22:36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 14. | Assa A, Dorfman L, Shouval DS, Shamir R, Cohen S. Therapeutic Drug Monitoring-guided High-dose Infliximab for Infantile-onset Inflammatory Bowel Disease: A Case Series. J Pediatr Gastroenterol Nutr. 2020;71:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Aardoom MA, Veereman G, de Ridder L. A Review on the Use of Anti-TNF in Children and Adolescents with Inflammatory Bowel Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2376] [Article Influence: 158.4] [Reference Citation Analysis (1)] |
| 17. | Cosnes J, Sokol H, Bourrier A, Nion-Larmurier I, Wisniewski A, Landman C, Marteau P, Beaugerie L, Perez K, Seksik P. Adalimumab or infliximab as monotherapy, or in combination with an immunomodulator, in the treatment of Crohn's disease. Aliment Pharmacol Ther. 2016;44:1102-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Kansen HM, van Rheenen PF, Houwen RHJ, Tjon A Ten W, Damen GM, Kindermann A, Escher JC, Wolters VM; Kids with Crohnʼs, Colitis (KiCC) Working Group for Collaborative Paediatric IBD Research in the Netherlands. Less Anti-infliximab Antibody Formation in Paediatric Crohn Patients on Concomitant Immunomodulators. J Pediatr Gastroenterol Nutr. 2017;65:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Chi LY, Zitomersky NL, Liu E, Tollefson S, Bender-Stern J, Naik S, Snapper S, Bousvaros A. The Impact of Combination Therapy on Infliximab Levels and Antibodies in Children and Young Adults With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, Rick J, Mack D, Bousvaros A, Rosh J, Grossman A, Saeed S, Kay M, Boyle B, Oliva-Hemker M, Keljo D, Pfefferkorn M, Faubion W, Kappelman MD, Sudel B, Markowitz J, Hyams JS. Concomitant Use of Immunomodulators Affects the Durability of Infliximab Therapy in Children With Crohn's Disease. Clin Gastroenterol Hepatol. 2015;13:1748-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Hyams JS, Dubinsky MC, Baldassano RN, Colletti RB, Cucchiara S, Escher J, Faubion W, Fell J, Gold BD, Griffiths A, Koletzko S, Kugathasan S, Markowitz J, Ruemmele FM, Veereman G, Winter H, Masel N, Shin CR, Tang KL, Thayu M. Infliximab Is Not Associated With Increased Risk of Malignancy or Hemophagocytic Lymphohistiocytosis in Pediatric Patients With Inflammatory Bowel Disease. Gastroenterology. 2017;152:1901-1914.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 22. | Biancone L, Onali S, Petruzziello C, Calabrese E, Pallone F. Cancer and immunomodulators in inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:674-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 23. | Dreesen E, Van Stappen T, Ballet V, Peeters M, Compernolle G, Tops S, Van Steen K, Van Assche G, Ferrante M, Vermeire S, Gils A. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn's disease who lose clinical response. Aliment Pharmacol Ther. 2018;47:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Cohen RZ, Schoen BT, Kugathasan S, Sauer CG. Management of Anti-drug Antibodies to Biologic Medications in Children With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2019;69:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |