Published online Oct 7, 2023. doi: 10.3748/wjg.v29.i37.5339
Peer-review started: June 25, 2023
First decision: July 23, 2023
Revised: July 26, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 7, 2023
Processing time: 92 Days and 6.3 Hours
Nonalcoholic fatty liver disease (NAFLD) is chronic, with its progression leading to liver fibrosis and end-stage cirrhosis. Although NAFLD is increasingly common, no treatment guideline has been established. Many mechanistic studies and drug trials have been conducted for new drug development to treat NAFLD. An up-to-date overview on the knowledge structure of NAFLD through bibliometrics, focusing on research hotspots, is necessary to reveal the rational and timely directions of development in this field.
To research the latest literature and determine the current trends in treatment for NAFLD.
Publications related to treatment for NAFLD were searched on the Web of Science Core Collection database, from 2010 to 2023. VOSviewers, CiteSpace, and R package “bibliometrix” were used to conduct this bibliometric analysis. The key information was extracted, and the results of the cluster analysis were based on network data for generating and investigating maps for country, institution, journal, and author. Historiography analysis, bursts and cluster analysis, co-occurrence analysis, and trend topic revealed the knowledge structure and research hotspots in this field. GraphPad Prism 9.5.1.733 and Microsoft Office Excel 2019 were used for data analysis and visualization.
In total, 10829 articles from 120 countries (led by China and the United States) and 8785 institutions were included. The number of publications related to treatment for NAFLD increased annually. While China produced the most publications, the United States was the most cited country, and the United Kingdom collaborated the most from an international standpoint. The University of California-San Diego, Shanghai Jiao Tong University, and Shanghai University of Traditional Chinese Medicine produced the most publications of all the research institutions. The International Journal of Molecular Sciences was the most frequent journal out of the 1523 total journals, and Hepatology was the most cited and co-cited journal. Sanyal AJ was the most cited author, the most co-cited author was Younossi ZM, and the most influential author was Loomba R. The most studied topics included the epidemiology and mechanism of NAFLD, the development of accurate diagnosis, the precise management of patients with NAFLD, and the associated metabolic comorbidities. The major cluster topics were “emerging drug,” “glucagon-like peptide-1 receptor agonist,” “metabolic dysfunction-associated fatty liver disease,” “gut microbiota,” and “glucose metabolism.”
The bibliometric study identified recent research frontiers and hot directions, which can provide a valuable reference for scholars researching treatments for NAFLD.
Core Tip: A total of 10829 articles published between 2010-2023 were identified through a bibliometric analysis to explore the trends and hotspots of treatment for nonalcoholic fatty liver disease (NAFLD). Replacing NAFLD/nonalcoholic steatohepatitis with metabolic dysfunction-associated fatty liver disease has been shown to greatly promote transformation of the treatment strategy of the disease. Research on gut microbiomes and traditional medicine will continue to be a short-term research hotspot. Obeticholic acid (phase 3 clinical validation) and semaglutide (under study) are likely to become the first approved drugs for NAFLD treatment.
- Citation: Dai JJ, Zhang YF, Zhang ZH. Global trends and hotspots of treatment for nonalcoholic fatty liver disease: A bibliometric and visualization analysis (2010-2023). World J Gastroenterol 2023; 29(37): 5339-5360
- URL: https://www.wjgnet.com/1007-9327/full/v29/i37/5339.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i37.5339
Nonalcoholic fatty liver disease (NAFLD) was first proposed by Ludwig et al[1] in 1980 to describe fatty liver disease without a history of alcoholism. NAFLD is further divided histologically into nonalcoholic fatty liver and nonalcoholic steatohepatitis (NASH)[2]. NASH can progress to fibrosis, eventually leading to cirrhosis and cirrhosis-related complications such as end-stage liver disease and hepatocellular carcinoma (HCC)[3]. In the United States, NASH cirrhosis is the leading indicator and fastest-growing cause of candidates waiting for liver transplantation (LT)[4] and is expected to become the prevalent indication for LT by 2030[5]. Currently, NAFLD is an epidemic among chronic diseases, threatening the health of nearly 1.7 billion people worldwide and placing a huge burden on individuals, families, and healthcare systems[6].
The clinical burden of NAFLD extends beyond liver-related morbidity and mortality as there is growing evidence suggesting its association with various metabolic comorbidities, including obesity, type 2 diabetes, cardiovascular disease, chronic kidney disease, hypertension, and hypercholesterolemia[7]. The global epidemics of obesity, diabetes, and metabolic syndrome (MetS) have led to an increasing prevalence of NAFLD[8]. The presence of multiple metabolic comorbidities increases the risk of NAFLD and the risk of progressive liver disease. The primary liver pathology in NAFLD affects liver structure and function, increasing the risk of morbidity and mortality associated with cirrhosis, end-stage liver disease, and HCC, with cardiovascular disease accounting for the most deaths in patients with NAFLD[9]. Therefore, Eslam et al[10], representing the International Consensus Group in 2020, recommended renaming NAFLD/NASH to metabolic dysfunction-associated fatty liver disease (MAFLD) to more accurately reflect its pathogenesis and accelerate the transition to novel therapeutics.
The most effective strategy for addressing chronic disease is to reduce the disease burden through prevention. However, this goal has not been achieved for NAFLD. Lifestyle changes focused on weight loss remain the cornerstone of NASH treatment, with a 10% weight loss able to resolve steatohepatitis or improve fibrosis and portal vein inflammation[11]. Nonetheless, achieving significant weight loss in clinical practice is challenging, as its effects are slow and can easily be reversed. Studies have shown that only 30% of patients can lose more than 5% of their weight through lifestyle changes after 13 mo[12].
New drug research and development for NAFLD treatment has been slow. Currently, clinical phase 2b and phase 3 studies have achieved certain efficacy. It is anticipated that new drugs will be available in the near future. However, no drugs have been approved yet, and the efficacy of the various compounds under development is not deemed satisfactory. The response rates for the investigational drugs in current studies range from 20%-40%, with no significant difference from the placebo response rate of 10%-20%[10].
Bibliometric analysis is a statistical method of literature analysis that examines the publication status of studies in a specific field from both quantitative and qualitative perspectives. It includes various factors such as authorship, country of origin, affiliated institutions, journal publications, and keywords[13]. Bibliometric tools such as VOSviewer, the R package “bibliometrix”[14], and CiteSpace[15] were used to visualize the results of this literature analysis, and these tools are gradually being used more frequently in the medical field. The application of bibliometrics to analyze research in the treatment of NAFLD/MAFLD is exceedingly helpful for identifying hotspots and research trends in this field of study, and in identifying those countries, institutions, and researchers that contribute the most. The advantages of this research methodology also include the possibility of analyzing and summarizing the evolution of research directions and content for a given institution or individual, as well as of quantitatively analyzing the collaboration between them. However, there are not many bibliometric statistical analyses in the field of liver disease, with few articles related to NAFLD[16-20]. In analyzing research that focuses on the possible causes of classical liver diseases, the research hotspots are mainly viral hepatitis and alcoholic liver disease. In recent years, with the overall decline in the prevalence of viral hepatitis and the annual increase in the incidence of NAFLD, there has been a growing interest in the latter. Indeed, the collective research and resultant advancement in knowledge of the disease from the proposal of "NAFLD" in 1980[1], to the proposed renaming of the disease to "MAFLD" in 2020[10], to the recent publication of the “multi-society Delphi consensus statement on new fatty liver disease nomenclature”[21] is serving as a signal to the end of the diagnosis and research into NAFLD and the entry into a brand new era of the more accurately termed “metabolic dysfunction-associated steatotic liver disease (MASLD)". Therefore, at the time of this new naming of the disease, it is necessary to analyze and summarize the research results for the treatment of this disease through the method of bibliometric statistical analysis, so as to accurately analyze the research hotspots and the trend of future development through a more objective approach and global perspective.
As mentioned earlier, NAFLD is widespread and harmful, and new treatment strategies are urgently needed to halt the progression of hepatic steatosis and fibrosis[22]. Since the renaming of NAFLD to MAFLD in 2020, a major shift has been observed in the direction of its treatment. With the publication of research results in the past 2 years, it is crucial to conduct a statistical analysis of relevant literature, identify key contributors and the current research status in this field, pinpoint current research areas of focus, and outline future research trends and development prospects.
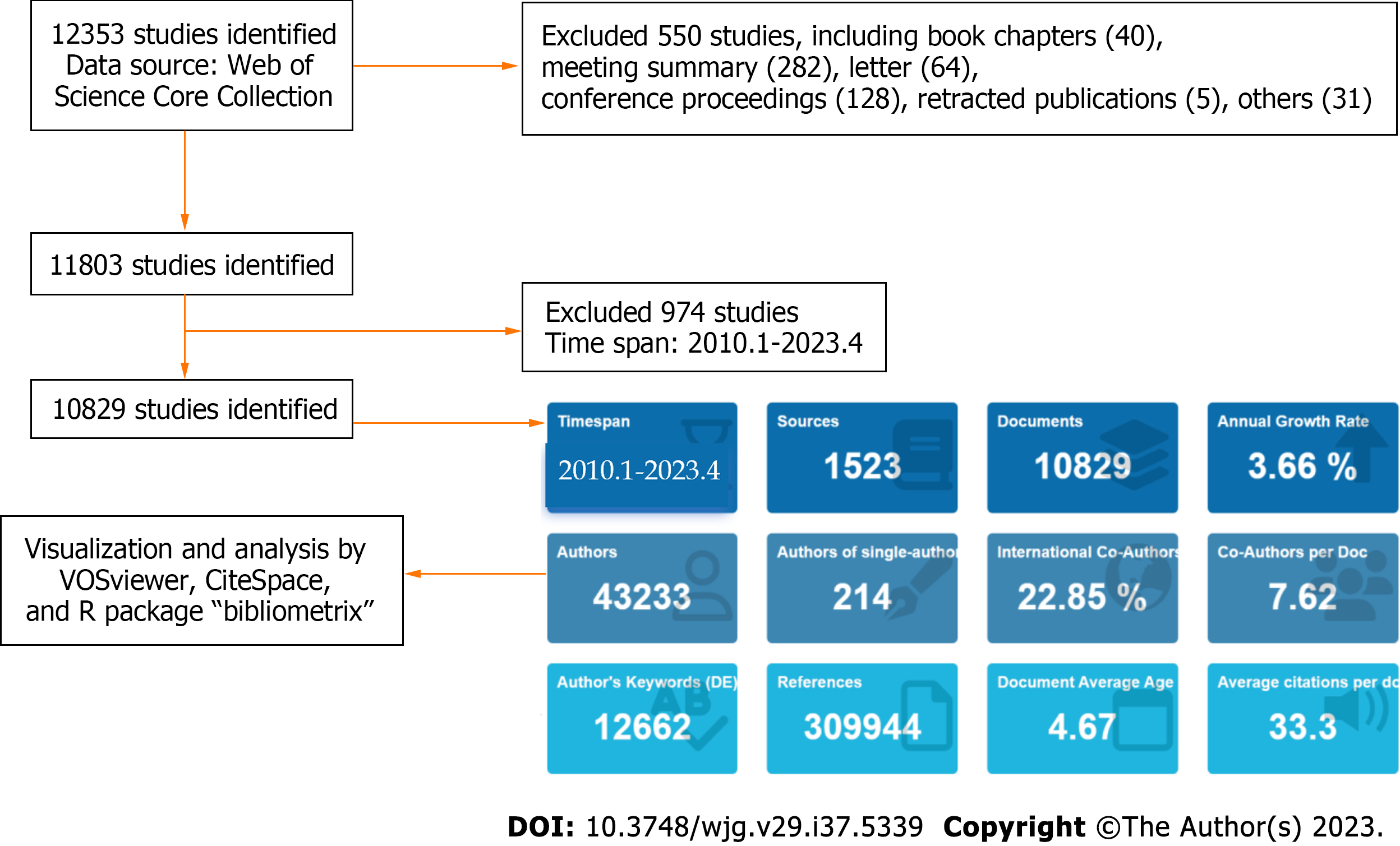
We conducted a literature search on the Web of Science Core Collection database (https://www.webofscience.com/wos/woscc/basic-search) on April 10, 2023 to collect all literature data related to therapy for NAFLD. We completed the search and retrieval of data within 1 d in order to reduce the bias caused by frequent database updates. The search formula was set to [TS = (nonalcoholic fatty liver) or (non-alcoholic fatty liver disease) or (NAFLD) or (MAFLD) or (metabolic dysfunction-associated fatty liver disease) or (metabolic associated fatty liver disease)] and TS = [(therapy) or (treatment)] and LA = (English). The type of documents was set to “articles” and “review” (editorials, proceeding papers, abstracts, and book chapters articles were excluded) with a timespan ranging from January 1, 2010 to April 12, 2023 (Figure 1).
VOSviewer (version 1.6.19) is a bibliometric analysis software released in 2010 by van Eck and Waltman[23]. VOSviewer can extract the key information and show the results of cluster analysis based on network data for generating and investigating maps[24,25]. Country and institution analysis, journal and co-cited journal analysis, author and co-cited author analysis, and author keywords co-occurrence analysis were performed by the VOSviewer software[26]. Of note, reference co-citation cluster and keyword co-occurrence analysis have the ability to recognize the knowledge structure and research hotspots in a field[27].
The R package “bibliometrix”[28] (version 3.2.1) (https://www.bibliometrix.org) was applied for a thematic evolution analysis and to construct a global distribution network of publications of therapy for NAFLD. CiteSpace (version 6.2.R2 Advanced) is the most popular and recognized bibliometric visualization tool[29] developed by Synnestvedt et al[15]. In our study, the CiteSpace map primarily completed the dual-map overlay of journals’ analysis and the timeline cluster analysis of keywords. GraphPad Prism 9.5.1.733 and Microsoft Office Excel 2019 were used for data analysis and visualization.
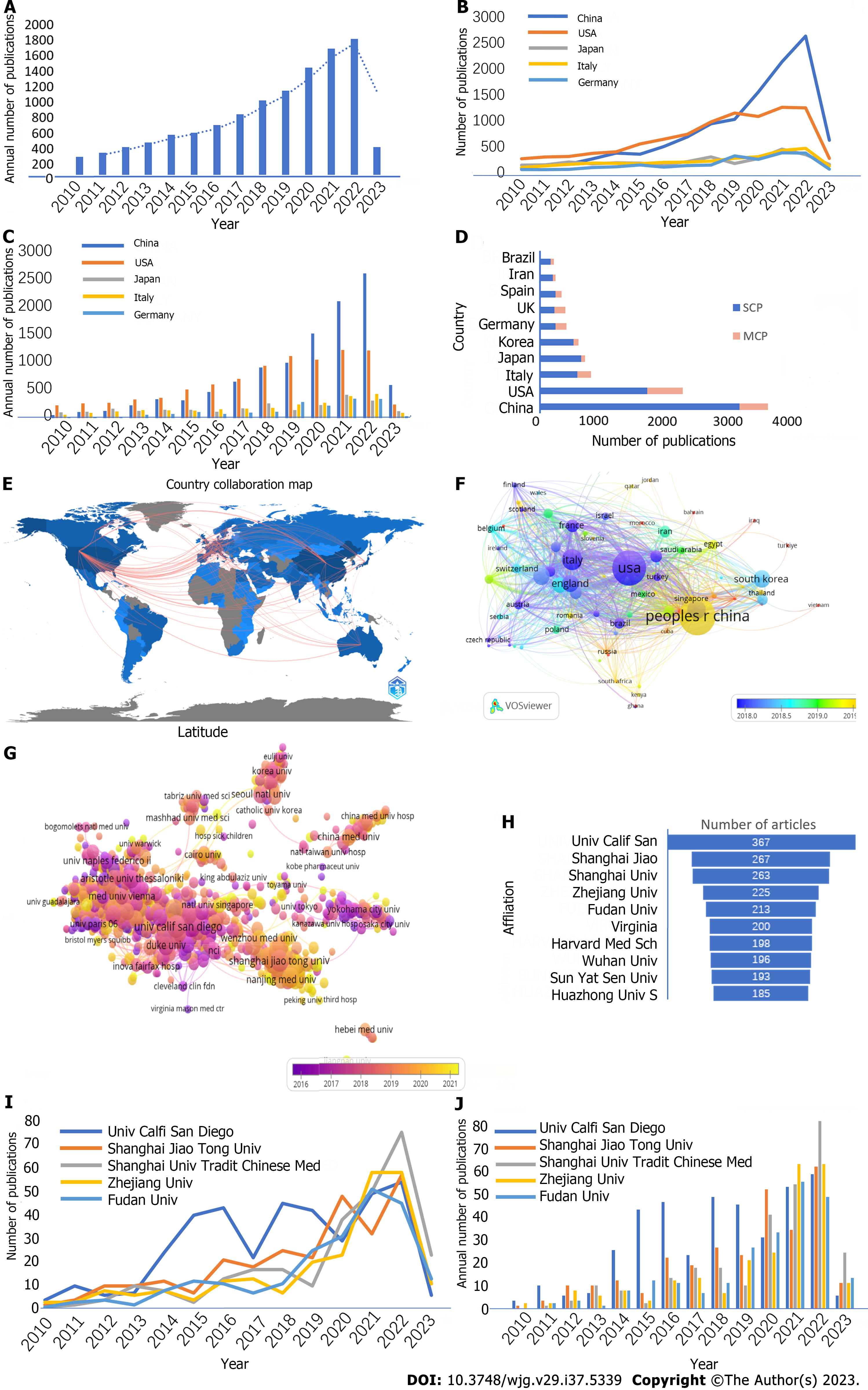
According to our search strategy, there were a total of 10829 studies on therapy or treatment for NAFLD since 2010, including 7826 articles and 3003 reviews. The annual number of publication increased year over year, and it showed explosive growth from 2019 to 2022. Although only a quarter of 2023 was available, there were more than 352 articles, suggesting a continuation of the upward trend (Figure 2A).
These publications came from 120 countries and 8785 institutions. More than half of the publications came from China and the United States (54.9%). The United States had the most publications until 2020 when publications from China increased massively (Figure 2B and C). The top 10 countries were distributed in Europe, Asia, and North America (Figure 2D).
China had the highest single-country publications (SCP) ratio of all countries (total of 3160 papers; SCP: 2763; SCP ratio: 87.4%). The SCP ratio in the United States was 75.0%, with 1978 papers. Of note, there was an abundance of active cooperation between different countries and regions (Figure 2E). Germany and the United Kingdom placed the greatest emphasis on international cooperation; their multiple country publication ratios were 42.1% and 43.3%, respectively (Figure 2D).
Subsequently, we filtered and visualized 76 countries based on the minimum number of five publications and constructed a collaborative network based on the number and relationship of publications in each country. In 2018, the United States was the most important and biggest research center in the world, which was transferred to China in 2020 (Figure 2F).
Then, we selected 1173 institutions based on the minimum number of five publications for visualization and constructed a collaborative network based on the number and relationship of publications of each institution (Figure 2G). The top 10 institutions were located in two countries; seven of the institutions were located in China (Figure 2H). The three institutions that published the most relevant papers were University of California-San Diego, Shanghai Jiao Tong University, and Shanghai University of Traditional Chinese Medicine. University of California-San Diego was the leader in publication output and maintained a high level of publications in recent years (Figure 2I and J). In the past 3 years, Chinese institutions have increasingly published NAFLD research. In particular, Shanghai Jiao Tong University ranked second and Shanghai University of Traditional Chinese Medicine ranked third, publishing frequently since 2020 and showing expansive growth in 2022.
Publications related to therapy for NAFLD were published in 1523 journals. The International Journal of Molecular Sciences published the most papers (n = 281, 2.6%). The top three journals with the most citations were Hepatology (n = 21451), Journal of Hepatology (n = 20843), and the World Journal of Gastroenterology (n = 12454). Among the top 20 journals of total link strength, the journal with the highest impact factor (IF) was Nature Reviews Gastroenterology and Hepatology (IF = 73.082).
Among the top 20 co-cited journals, five journals were cited more than 10000 times. Hepatology (co-citation = 49058) was the most cited journal, followed by the Journal of Hepatology (co-citation = 26782), Gastroenterology (co-citation = 21448), Journal of Biological Chemistry (co-citation = 12220), and PLOS ONE (co-citation = 12083). In addition, the IF of The Lancet was the highest (IF = 202.731) followed by the New England Journal of Medicine (IF = 176.079).
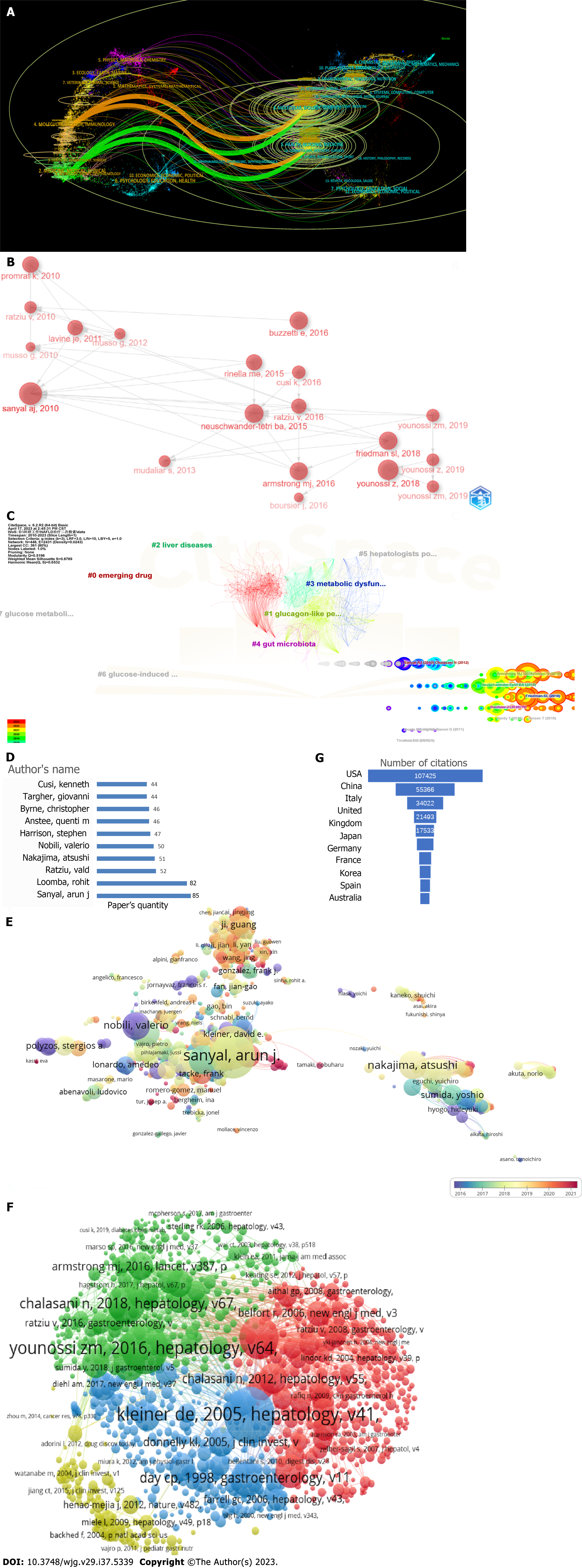
The dual-map overlay of journals can show the citation relationships between citing journals on the left and the clusters of co-cited journals on the right through the colored paths[29]. As shown in Figure 3A, the orange path is the main citation path, which represents the studies published in molecular/biology/immunology journals primarily cited by literature in molecular/biology/gene and health nursing/medicine journals. The green path is the main document flow path, which represents the research published in molecular/biology/gene and health nursing/medicine journals primarily cited by studies published in medicine/medical clinical journals.
Through historiography analysis by R package “bibliometrix,” we observed that the publications with the highest importance in chronological order in the dataset were Sanyal et al[30], Neuschwander-Tetri et al[31], Rinella[32], and Younossi et al[33] (Figure 3B and Table 1). Table 1 clearly showed that the vast majority of important studies (local cited documents and global cited documents were both high) were randomized controlled trials[30,31,34-36]. In addition, two important reviews in 2018 were “mechanisms of NAFLD development and therapeutic strategies”[37] and “global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention”[33].
| Ref. | Year | Title | LCS | GCS |
| Musso et al[93] | 2010 | A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease | 213 | 410 |
| Sanyal et al[30] | 2010 | Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis | 986 | 2063 |
| Promrat et al[35] | 2010 | Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis | 426 | 832 |
| Ratziu et al[22] | 2010 | A position statement on NAFLD/NASH based on the EASL 2009 special conference | 245 | 731 |
| Lavine et al[94] | 2011 | Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the toninc randomized controlled trial | 363 | 711 |
| Musso et al[105] | 2012 | Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomised trials | 224 | 435 |
| Mudaliar et al[96] | 2013 | Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease | 234 | 631 |
| Neuschwander-Tetri et al[31] | 2015 | Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial | 586 | 1416 |
| Rinella et al[32] | 2015 | Nonalcoholic fatty liver disease: A systematic review | 403 | 1443 |
| Buzzetti et al[97] | 2016 | The multiple-hit pathogenesis of NAFLD | 538 | 1386 |
| Boursier et al[98] | 2016 | The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota | 213 | 717 |
| Cusi et al[106] | 2016 | Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial | 277 | 525 |
| Ratziu et al[107] | 2016 | Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and-δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening | 350 | 642 |
| Armstrong et al[36] | 2016 | Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study | 529 | 1006 |
| Friedman et al[37] | 2018 | Mechanisms of NAFLD development and therapeutic strategies | 550 | 1526 |
| Younossi et al[33] | 2018 | Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention | 739 | 2379 |
| Younossi[56] | 2019 | Non-alcoholic fatty liver disease-a global public health perspective | 243 | 906 |
| Younossi et al[6] | 2019 | Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis | 254 | 808 |
| Younossi et al[34] | 2019 | Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial | 263 | 541 |
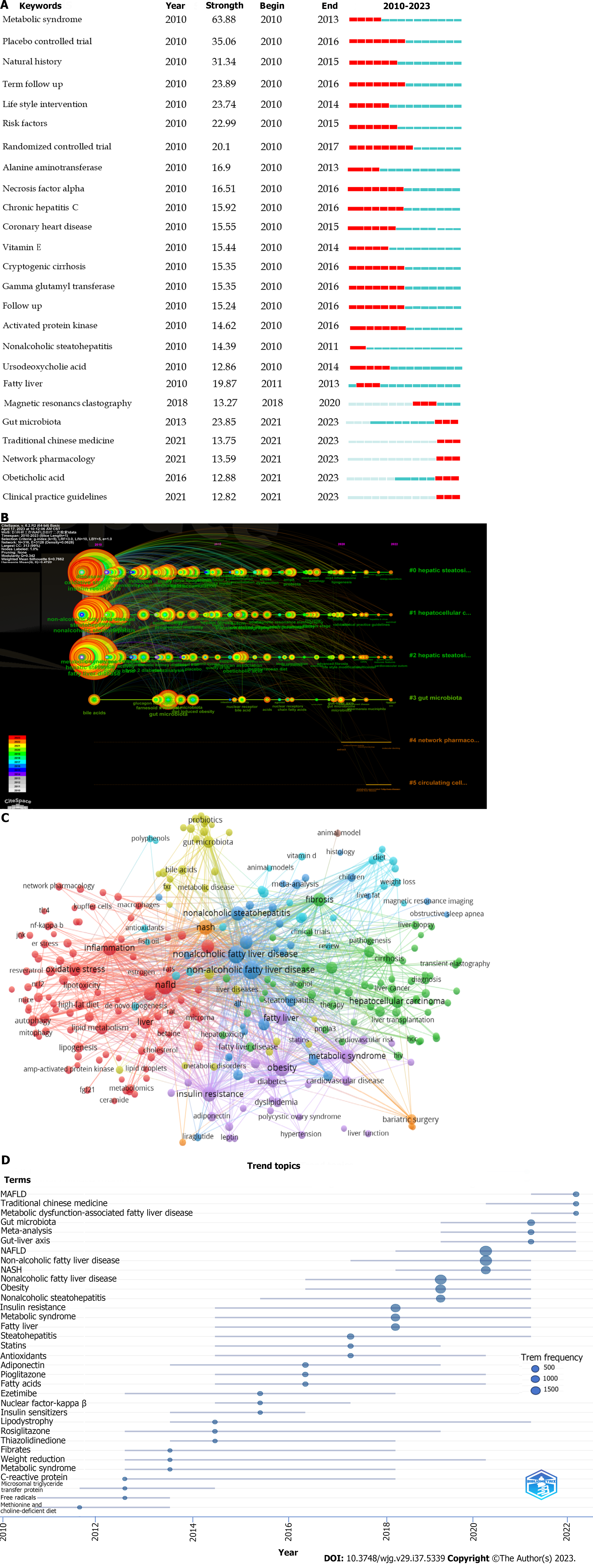
Reference with citation bursts refers to those references that are frequently cited by scholars in a certain field over a period of time. In our study, the details of 25 references with strong citation bursts by R package “bibliometrix” were listed in Table 2. As shown in Table 2, every bar represents a year, and the red bar represents strong citation burstiness. Citation bursts for references appeared as early as 2010 and as late as 2023. The burst strength of these 25 references ranged from 49.79 to 161.62, and endurance strength was from 2-5 years.
| Rank | Ref. | Year | Primary research content | Strength | Beginning | Ending |
| 1 | Sanyal et al[30] | 2010 | Vitamin E showed improvement in nonalcoholic steatohepatitis in adults without diabetes | 161.62 | 2010 | 2015 |
| 2 | Chalasani et al[3] | 2018 | The diagnosis and management of nonalcoholic fatty liver disease practice guideline | 150.14 | 2013 | 2017 |
| 3 | Neuschwander-Tetri et al[31] | 2015 | Obeticholic acid improved the histological features of nonalcoholic steatohepatitis | 102.84 | 2016 | 2020 |
| 4 | Vernon et al[111] | 2011 | Systematic review: The epidemiology and natural history of NAFDL and NASH in adults | 97.78 | 2012 | 2016 |
| 5 | Chalasani et al[2] | 2012 | A practice guideline of the diagnosis and management of NAFLD | 87.88 | 2013 | 2017 |
| 6 | Williams et al[116] | 2011 | Prevalence of NAFLD was higher than estimated, especially in hispanics and diabetes patients | 82.64 | 2012 | 2016 |
| 7 | Promrat et al[35] | 2010 | Weight reduction achieved through lifestyle intervention led to improvements in liver histology in NASH | 80.62 | 2011 | 2015 |
| 8 | Vilar-Gomez et al[11] | 2015 | NASH resolution/fibrosis regression occurred in patients with ≥ 10% weight loss induced by lifestyle changes | 77.63 | 2016 | 2020 |
| 9 | Lavine et al[94] | 2011 | Neither vitamin E nor metformin was effective in reducing ALT in patients with pediatric NAFLD | 76.39 | 2012 | 2016 |
| 10 | Rinella et al[32] | 2015 | A systematic review of NAFLD | 73.68 | 2016 | 2020 |
| 11 | Cohen et al[101] | 2011 | NAFLD is strongly associated with obesity and insulin resistance | 72.23 | 2012 | 2016 |
| 12 | Angulo et al[110] | 2015 | Fibrosis stage was associated with long-term overall mortality, liver transplantation, and liver-related events | 71.71 | 2016 | 2020 |
| 13 | Ratziu et al[22] | 2010 | Collaboration between hepatologists and specialists in the endocrine, nutritional, and cardiology fields should be encouraged to optimize clinical management | 62.53 | 2011 | 2015 |
| 14 | Targher et al[7] | 2010 | NAFLD was associated with an increased risk of cardiovascular disease | 58.16 | 2011 | 2015 |
| 15 | Byrne et al[5] | 2015 | NAFLD is a multisystem disease, affecting extrahepatic organs and regulatory pathways | 57.94 | 2016 | 2020 |
| 16 | Ekstedt et al[108] | 2015 | Fibrosis stage predicted both overall and disease-specific mortality | 57.38 | 2016 | 2020 |
| 17 | Wong et al[109] | 2015 | NASH has been predicted to become the leading indication for liver transplantation | 54.86 | 2016 | 2020 |
| 18 | Anstee et al[112] | 2013 | NAFLD is associated with liver-related morbidity and mortality and an increased risk of developing cardiovascular disease and T2DM | 50.86 | 2014 | 2018 |
| 19 | Younossi et al[53] | 2016 | As the global epidemic of obesity fuels metabolic conditions, the clinical and economic burden of NAFLD will become enormous | 125.36 | 2018 | 2021 |
| 20 | Loomba et al[114] | 2013 | The epidemiology and demographic characteristics of NAFLD vary worldwide | 63.19 | 2015 | 2018 |
| 21 | Tilg et al[115] | 2010 | Many parallel hits derived from the gut and/or the adipose tissue may promote liver inflammation. endoplasmic reticulum stress and related signaling networks, (adipo) cytokines, and innate immunity are emerging as central pathways that regulate key features of NASH | 58.39 | 2012 | 2015 |
| 22 | Buzzetti et al[97] | 2016 | The multiple-hit pathogenesis of NAFLD | 49.79 | 2018 | 2021 |
| 23 | Eslam et al[102] | 2020 | A diagnosis of MAFLD requires positive criteria | 75.68 | 2021 | 2023 |
| 24 | Eslam et al[10] | 2020 | Renaming NAFLD to MAFLD was proposed to accelerate the translational path to new treatments | 68.10 | 2021 | 2023 |
| 25 | Newsome et al[103] | 2021 | Treatment with semaglutide resulted in a higher percentage of NASH resolution than placebo | 55.16 | 2021 | 2023 |
A total of eight major clusters (Q = 0.519; S = 0.8789; Q/S = 0.6532) were generated from the co-citation networks of references after cluster analysis by CiteSpace (Figure 3C). The cluster nomenclature may reflect the study hotspots and frontiers in treatment of NAFLD. The largest cluster was emerging drug, followed by glucagon-like peptide-1 (GLP-1) receptor agonist, liver diseases, MAFLD, gut microbiota, hepatologists points, glucose-induced glucagon-like peptide, and glucose metabolism.
A total of 51987 authors participated in research on the therapy for NAFLD. Among the top 10 authors, 5 (who were from the United States, the United Kingdom, Italy, and France) each published more than 50 papers (Figure 3D). We built a collaborative network based on authors whose number of published papers was ≥ 5 (Figure 3E). Sanyal et al[30], Loomba et al[38], and Ratziu et al[22] had the largest nodes due to publishing the most related publications. In addition, we observed close collaboration among multiple authors.
Sanyal et al[30] was considered one of the most distinguished scholars because of his prolific research output (Figure 3D) and the highest number of citations (Figure 3E). His research on fatty liver encompassed a multidisciplinary interactive approach, covering areas such as apoptosis, cancer research, obesity, and microRNA. He hypothesized that NASH was associated with altered liver microRNA expression and alterations in the human gut microbiome were associated with cirrhosis and its complications[39]. He has committed to the field of effective therapeutic development for NASH and has provided summaries of new drugs under development or key ongoing research[40]. These include highly anticipated treatments such as farnesoid X receptor agonists, peroxisome proliferator-activated receptor agonists, GLP-1 receptor agonists, C-C chemokine receptor type 2 and 5 inhibitors, caspase inhibitors, lysyl oxidase-like 2 inhibitors, galectin-3 inhibitors, and others.
According to the H-index, which is a measure used to assess the scientific productivity and impact of researchers, the most influential scientists in this field of NAFLD are Loomba et al[38], Sanyal et al[30]. Loomba et al[38] has focused on various aspects of NAFLD, including aging, epidemiology, genetic and environmental susceptibility, natural history, and the treatment of NASH. His research has also explored the efficacy of metformin[40], ezetimibe[41], obeticholic acid (OCA)[31], sitagliptin[42], cenicriviroc[43], selonsert[44], polyethylene glycol[45], and semaglutide[46] in the treatment of NAFLD.
Among the 163234 co-cited authors, five authors were co-cited more than 2000 times. The most co-cited author was Younossi et al[33] (n = 4007) followed by Chalasani et al[3] (n = 2363) and Sanyal et al[30] (n = 2088). Younossi et al[33] concluded that lifestyle modifications to achieve weight loss remains a first-line intervention for patients with NAFLD[47]. He also found that a dosage of 25 mg of OCA significantly improved fibrosis and NASH disease activity scores in patients with NASH[34,48]. Chalasani et al[3] suggested that bariatric surgery may be a viable option for patients with cirrhosis and extreme obesity[49]. Furthermore, Chalasani et al[3]was involved in research on the treatment of NASH using saroglitazar[50], belapectin[51], and OCA[34,48].
There are 309771 co-cited references on research on the therapy for NAFLD over the past 12 years. In the top 10 co-cited references, all references were co-cited more than 550 times. We selected references with co-citation ≥ 20 for the construction of the co-citation network map (Figure 3F). Four articles occupied an important position and became the center of the co-citation network diagram. They were Kleiner et al[52], Younossi et al[33], Sanyal et al[30], and Chalasani et al[3]. The most cited country is the United States (Figure 3G).
Keywords with citation bursts refers to those keywords or topics that are frequently discussed in a certain field over a period of time. In our study, 25 keywords with strong citation bursts were identified by R package “bibliometrix” (Figure 4A). Judging from the top 25 keywords with the strongest citation bursts, the whole period can be divided into two: Period I (2010-2017) and period II (2019-2023). Keywords in period I primarily included MetS, insulin resistance, lifestyle intervention, and focus on pathogenesis such as lipid peroxidation. Subsequently, keywords in period II primarily included traditional Chinese medicine, network pharmacology, lifestyle modification, autophagy, and gut microbiota. Cluster analysis of the keyword timeline graph built by CiteSpace showed the evolution of high-frequency keywords (Figure 4B).
Through the co-occurrence analysis of author keywords (n = 12665), we could quickly capture research hotspots in a certain field. Table 3 showed the top 20 high-frequency keywords excluding the name of the disease (such as NAFLD, MAFLD, NASH, etc) for publications regarding therapy for NAFLD. Among these keywords, obesity, insulin resistance, inflammation, and MetS appeared more than 500 times, which represented the main research direction of therapy for NAFLD. We filtered keywords with the number of occurrences ≥ 20 and performed cluster analysis through VOSviewer (Figure 4C). We obtained five clusters representing five research directions. The keywords in red clusters consisted of inflammation, oxidative stress, lipid metabolism, high-fat diet, lipogenesis, macrophages, etc. The keywords in yellow clusters consisted of gut microbiota, probiotics, bile acids, etc. The keywords in blue clusters consisted of vitamin E, weight loss, diet, etc. The keywords in purple clusters consisted of obesity, MetS, insulin resistance, metformin, adiponectin, diabetes, liraglutide, etc. The keywords in green clusters consisted of HCC, fibrosis, cirrhosis, live transplantation, immunotherapy, etc.
| Rank | Keyword | Occurrence |
| 1 | Obesity | 898 |
| 2 | Insulin resistance | 684 |
| 3 | Inflammation | 670 |
| 4 | Metabolic syndrome | 547 |
| 5 | Oxidative stress | 444 |
| 6 | Steatosis | 418 |
| 7 | Fibrosis | 414 |
| 8 | Hepatocellular carcinoma | 306 |
| 9 | Lipid metabolism | 304 |
| 10 | Gut microbiota | 260 |
| 11 | Diabetes | 258 |
| 12 | Cirrhosis | 241 |
| 13 | Treatment | 177 |
| 14 | Autophagy | 165 |
| 15 | High-fat diet | 141 |
| 16 | Bariatric surgery | 124 |
| 17 | Cardiovascular disease | 123 |
| 18 | Metformin | 121 |
| 19 | Apoptosis | 118 |
| 20 | Lipogenesis | 118 |
The trend topic analysis of the keywords showed that from 2010 to 2016, the research focused on diet, lifestyle intervention, vitamin E, etc. Since 2017, researchers have begun to focus on MetS and actively explore the precise diagnosis and effective treatment for NAFLD. Since the suggestion of renaming NAFLD to MAFLD in 2020, the main keywords were insulin resistance, obesity, inflammation, microbiome, OCA, etc. It is worth noting that gut microbiota, gut-liver axis, and traditional Chinese medicine are the latest trending topics (Figure 4D).
Since 2010, the annual publications regarding NAFLD treatment have increased, particularly in the past 5 years. The number of related studies has also grown rapidly, indicating that research on NAFLD treatment is in an explosive period, and China and the United States are the leading countries studying the treatment of NAFLD. The United States has been the largest center for publications on the treatment of NAFLD worldwide. However, 2020 was a turning point when the number of publications from China exploded. The cumulative number of publications from China surpassed those from the United States in 2022. China accounts for 70% of the top 10 research institutions regarding publication volume, and the remaining institutions are in the United States. University of California San Diego has been a leader in the NAFLD field; however, research institutions in China have developed rapidly in recent years.
China is developing rapidly in the NAFLD field, surpassing the United States in the number of related publications. There are several reasons for this, which we analyzed below.
The first reason is that the incidence of NAFLD is increasing in China. The global prevalence of NAFLD is 25.2%[53] and 29.2% in China. This has increased rapidly (2008-2010: 25.4%; 2015-2018: 32.3%)[54]. Over the past 20 years, the economic development, urbanization, and dietary patterns in China have changed (e.g., increased consumption of animal-derived foods, refined grains, and highly processed, high-sugar, and high-fat foods)[55]. This has led to sedentary lifestyles and overnutrition, undergirding obesity and the NAFLD epidemic[56].
The second reason is that the burden of NAFLD in China will continue to increase. With the development of the global economy, the global prevalence of NASH will increase by 15%-56%, and the highest prevalence of NAFLD is expected to occur in China[6]. The Chinese population is large, and the burden of the disease increases exponentially[57,58]. In a NAFLD disease burden modeling analysis, it was estimated that by 2030, the NASH population in China is expected to increase by 48% compared to that in 2016 (48.26 million), with an 86% increase in HCC (14090)[59].
The third reason is that MetS is a significant public health problem in China[60]. MetS is the strongest risk factor for NAFLD and NASH. The association between NAFLD and MetS may be bidirectional, particularly for diabetes and systemic hypertension, while traditional Chinese culinary culture (high in fat, salt, and fiber) adversely affects these diseases. Given the two-way causal relationship between NAFLD and type 2 diabetes mellitus, a rapid increase in diabetes and obesity rates directly leads to an increase in the prevalence of NAFLD[61].
The fourth reason is that the prevalence of hepatitis B in China has decreased. China has the highest prevalence of hepatitis B. However, government control measures, effective hepatitis B virus vaccination, and standardized anti-hepatitis B virus treatment have had a positive impact on decreasing the prevalence. Hepatitis B in China has been largely controlled, making chronic liver disease caused by NAFLD increasingly prominent.
The fifth reason is that the government attached importance to scientific research and improved the level of scientific research. In 2010, China spent less than half the amount on research and development as the United States (208280000 USD vs 444709000 USD) (https://data.oecd.org/rd/gross-domestic-spending-on-r-d.htm). Recently, due to economic development and robust government support, China has become the world’s second-largest industry research and development center, with its total research and development expenditure reaching 80% of that of the United States’ research and development expenditure (631845000 USD) in 2019 (https://www.oecd.org/sti/msti-highlights-march-2021.pdf). Over the years, basic and clinical research on liver disease in China has been listed as a priority investment area by the National Natural Science Foundation of China (NSFC). From 2010 to 2022, the total number of research projects related to fatty liver funded by the NSFC was 7515, with a total funding of 535000000 USD. The number of projects and funding provided by the NSFC has increased annually. During the same period the National Institutes of Health in the United States funded 3304 projects (1267000000 USD) in the field of fatty liver disease. Although the investment gap between China and the United States is still significant, the number of NSFC-funded projects has surpassed that of the United States.
The final reason is the emphasis on traditional medicine. The NSFC has funded 960 projects related to traditional Chinese medicine and integrated traditional Chinese medicine and western medicine, while there are few funded projects related to traditional medicine in the United States. This also reflects the advantages and characteristics of the fatty liver research field in China. For example, Shanghai University of Traditional Chinese Medicine systematically and comprehensively analyzes the multitarget mechanism of action of traditional Chinese medicine compounds[62-64] and explores the metabolite-target-disease network of traditional Chinese medicine[65-67] in the treatment of NAFLD. The concept of multitarget therapy with traditional Chinese medicine compounds is consistent with the multiple pathogeneses of NAFLD. It is challenging for a single drug to address the diversity of the pathogenesis of NAFLD. Therefore, traditional Chinese medicine and compound multitarget active ingredients may usher in a new era in the treatment of NAFLD.
Although the number of publications in China has grown rapidly in recent years, the citation volume is still relatively low. Possible causes are: (1) Insufficient international cooperation in China. China’s SCP ratio was as high as 87.4%, the highest among all countries; (2) the international discourse of China in this field needs to be improved. The top experts in this field are all American scholars who have decades of experience in the field. Their research directions are focused and deep, which allows the publication of their research in top journals. Conversely, researchers in China lack international academic authority and have few articles published in top journals; and (3) the quality and innovation of publications needs to be improved. Research in China is primarily basic, and there is a lack of multicenter randomized controlled trials and large-sample prospective cohort studies with high clinical evidence levels.
Although Chinese scientists are not ranked high regarding personal influence, they are ranked second only to the United States in the ranking of cited countries, which indicates that the overall scientific research strength in China cannot be underestimated. The top 30 cited Chinese studies focused on epidemiology[59,61,68,69], basic research, particularly pathogenesis[70-76], the search for therapeutic targets[77-89], in vitro tests[76,82,86], animal experiments[87-89], and review articles[81,83]. At present, most data on drug treatment originate from foreign research, and there is a lack of research on the Chinese population. This is an important direction that needs to be addressed in future research on NAFLD treatments in China.
Most studies on NAFLD treatment have been published in the International Journal of Molecular Sciences (IF = 6.208, second quartile), indicating that it is currently the most popular journal in this field of research. Among the top 20 journals, Nature Reviews Gastroenterology and Hepatology (IF = 73.082) had the highest IF. For co-cited journals, we found that most were high-impact top quartile journals. These high-quality international journals provide research support for the treatment of NAFLD. Among them, Hepatology and Journal of Hepatology are the most popular co-cited journals, indicating the high quality of the journal in the field of NAFLD research. Additionally, the research results in molecular/biology/immunology journals mainly flow to molecular/biology/genetic and health care/medical journals, which also indicates that the development of therapeutics is inseparable from basic research fields such as molecular biology, genetics, and immunity. The articles in the field of NAFLD treatment research have been cited by medical/clinical journals, which illustrates the clinical application value of treatment research.
Co-cited references are regarded as the basis of research in a certain field, and the development and evolutionary dynamics of a discipline can be explored by studying co-cited networks[90,91]. We selected the 10 most co-cited articles to determine the research basis for the treatment of NAFLD. Among the 309771 cited publications, the design and validation of the histology scoring system for NAFLD published by Kleiner et al[52] in 2005 was the most cited study. The top 10 citations covered five main topics: diagnosis; epidemiology[33,53]; pathogenesis; therapeutic drug development[30,31]; and review of NAFLD[92]. Epidemiology is the exploration of disease prevalence and incidence[33], global burden trend prediction and prevention[53], and the overall understanding of the disease. The standardization of diagnostic methods[3,52] is a prerequisite for disease management and guiding treatment. The exploration of pathogenesis[37,92] is conducive to the development of new drugs and individualized and precise treatment. Vitamin E, pioglitazone[30], and OCA[31] are the most promising NAFLD therapeutics in development. These main topics also reflect the evolution of NAFLD drug research directions.
Historiography analysis is commonly described as “the history of history” and seeks to explain origins and evolution providing a clearer understanding of the future. Results from the first randomized controlled trial in 2010 of adult NASH patients using lifestyle modification as an active therapeutic intervention suggested that lifestyle changes focused on diet, exercise, and behavioral changes could successfully improve overall histological activity, degree of steatosis, and liver chemistry of NASH[35]. Weight loss, physical activity, reduction of a sedentary lifestyle, and dietary changes should be treated as the first-line treatment of NAFLD/NASH and assessed after 6 mo. If this is not effective, then additional treatment options, such as medication, may be considered[30].
Simultaneously, it was hypothesized that oxidative stress dealt a second “blow” to the liver, and there was a strong correlation between the severity of NASH and the degree of oxidative stress. However, the results from antioxidant treatment are inconsistent in the treatment of NAFLD[93]. Only vitamin E, through inhibiting fatty acid oxidation, is superior to placebo in the treatment of NAFLD[30]. NAFLD/NASH is closely related to the global diabetes and obesity epidemics. Some hypoglycemic drugs, such as metformin, can improve aminotransferase levels, and pioglitazone can improve steatohepatitis (recommended NASH regimen: pioglitazone or vitamin E combined with high-dose ursodeoxycholic acid)[30]. However, in 2011, it was found that neither vitamin E nor metformin was superior to placebo for the primary outcome of a sustained reduction in alanine aminotransferase levels in children with NAFLD[94]. A 2012 meta-analysis noted that vitamin E improved histological indices after 2 years of use, but increased insulin resistance and plasma triacylglycerol were observed. Long-term use may increase the risk of prostate cancer, and there is a lack of efficacy in reducing liver fibrosis[37]. In patients who did not respond to lifestyle interventions, pioglitazone improved histological disease activity, slowed fibrosis progression, and broadly improved the cardiometabolic endpoints[95]. However, the use of thiazolidinediones has been limited by adverse effects, such as weight gain, fluid retention, increased risk of fractures (particularly in older women), and bladder cancer.
OCA is a semi-synthetic derivative of human cholic acid, which is a natural agonist of farnesoid X receptors. Farnesoid X receptors are nuclear hormone receptors that regulate glycolipid metabolism, can block the conversion of cholesterol to bile acids, increase serum cholesterol concentration, and promote the reverse transport of cholesterol from tissues. A phase 2 trial in 2013[96] and a multicenter randomized controlled trial in 2015 confirmed that OCA was well tolerated in the treatment of NAFLD, increased insulin sensitivity and weight loss, and reduced markers of liver inflammation and fibrosis[31]. An interim analysis of a phase 3 clinical trial in 2019 concluded that 25 mg/day of OCA resulted in significant histological improvements in NASH[34] and unfortunately in itching and a moderate increase in low-density lipoprotein cholesterol.
The 2016 “two-hit” hypothesis became obsolete, and the “multiple blows” hypothesis more accurately explained the pathogenesis of NAFLD, which includes insulin resistance, hormones secreted by adipose tissue, nutritional factors, gut microbiota, and genetic and epigenetic factors[97]. In 2016, Buzzetti et al[97] evaluated the association between intestinal dysbiosis and liver fibrosis in human NAFLD and found that animal diets favor the accumulation of branched-chain fatty acids of Bacteroides, thereby promoting insulin resistance and increasing the risk of NASH. The diet of agricultural societies is rich in fiber, starch, and plant polysaccharides, which promotes the abundance of Prevobacterium, and its abundance decreases with an increase in liver lesions. A gut microbiota analysis provided a theoretical basis for NAFLD patients to regulate diet structure and intestinal microbial preparations[98].
In 2018, probiotics containing endotoxin antibodies and bovine colostrum were evaluated for their efficacy in the treatment of NASH by enhancing brown adipose tissue to burn glucose and ameliorate obesity and glucose abnormalities[37]. Insulin resistance has long been considered an important component of the pathogenesis of NAFLD and worsens as the disease progresses. GLP-1 analogs reduce hepatic steatosis, liver enzyme concentrations, and insulin resistance by inducing insulin secretion and reducing glucagon secretion in a glucose-dependent manner. Armstrong et al[36] reported the effects of GLP-1 analogs on liver histology in patients with NASH in a randomized, placebo-controlled trial. Liraglutide was well tolerated in this study, improving several key components of MetS, including weight and glycemic control, and improving NASH histology.
The pathogenesis of NAFLD involves multiple drivers, and no more than 40% of patients in clinical trials have shown benefits from monotherapy, which is likely insufficient to prompt regulatory approval of monotherapy for long-term treatment of NAFLD. Current research favors combination therapies including drug combinations, single-agent searches for multitarget effects[99], and comorbidities in patients with NAFLD. We hypothesize that NAFLD has progressed to become the most common cause of chronic liver disease worldwide[57]. The global burden of NAFLD and NASH is growing rapidly. Future research should focus on accurate non-invasive measurement of biomarkers and clarification of pathogenic pathways, which will facilitate the development and effective evaluation of the efficacy of new drugs. Simultaneously, we continue to actively explore effective treatments, including the development of effective treatments for patients with NASH and prevention methods for individuals at high risk of progression[56].
Citation bursts represent emerging topics in a particular field of study[100]. Based on the main research content[5,30,97,100,101] of the strongly cited burst references (Table 2), we found that renaming NAFLD to MAFLD[10,102] and the development of semaglutide for NAFLD treatment[103] may represent the main hotspots of current NAFLD treatment research. Both of these topics were cited in 2021. As shown in Table 2, 16 of the 25 citations were reviewed, and high-quality reviews reflected understanding of the disease at a certain time. With the exploration of the pathogenesis of NAFLD, the naming and diagnosis of the disease have been gradually updated and standardized[104], and its treatment strategy has gradually evolved from vitamin E, weight loss, and lifestyle interventions[105] for the management of metabolism-related diseases[106,107].
In addition to citation bursts, keywords can also quickly capture the distribution and evolution of hot topics in our research field. The top keywords from the last 2 years included gut microbiota, traditional Chinese medicine, network pharmacology, OCA, and clinical practice guideline. In keyword trend topic analysis, it was evident that MAFLD, traditional Chinese medicine, gut-liver axis, and gut microbiota were the most frequently discussed keywords. Interestingly, insulin sensitizers such as pioglitazone for the treatment of fatty liver were popular in 2012, reached their peak from 2014 to 2016, and gradually faded in 2020.
Due to the complex pathogenesis of NAFLD, it is challenging to treat[108-110]. Effective treatment requires precise localization based on the patients’ phenotype[111] and genetic background[10]. The study of treatment strategies[112,113], epidemiological knowledge[114], pathogenesis[115], and precise diagnosiss[116] (including non-invasive diagnostic techniques) may all be integrated into the consideration of heterogeneity in disease treatment. The current mainstream view is to replace the diagnosis of NAFLD/NASH with MAFLD. Hence, this name change has greatly promoted the transformation of the treatment strategy of the disease. The previous treatment plan mainly included two directions: (1) Correction of insulin resistance and reduction of fat mass with a focus on lifestyle changes for weight loss, including physical activity, diet, insulin sensitizers, and anti-obesity surgery; and (2) prevention/reversal of lipotoxicity-induced hepatocellular damage by inhibiting lipid peroxidation and oxidative stress or by using anti-inflammatory, anti-apoptotic, or other hepatoprotective agents.
Future therapeutic research on NAFLD/MAFLD should focus on comprehensive therapies tailored to individual patients, considering the above two therapeutic directions, and targeting multitarget and multiaction mechanisms[29]. Research on gut microbiomes and traditional medicine will continue to be a short-term research hotspot. With the official name change to MASLD[117], more emphasis was given to the management of associated comorbid, particularly metabolic abnormalities. OCA, which has entered phase 3 clinical validation, and semaglutide, which is currently under study, are likely to become the first approved drugs for the treatment of NAFLD. A clinical trial called Semaglutide treatment in the real-world for fibrosis due to NAFLD in obesity and type 2 diabetes mellitus (SAMARA) to evaluate the efficacy of semaglutide in improving liver scarring associated with NAFLD is currently ongoing in the US. Once SAMARA is completed, a larger multi-centred global trial may follow.
Advantages and shortcomings: This study had several advantages. First, we systematically analyzed the research on NAFLD/MAFLD treatment through bibliometrics, which provided comprehensive guidance for scholars who are interested in related research. Second, we used three bibliometric tools simultaneously for the survey, hence our data analysis process was objective. Third, bibliometric analysis provided a more complete insight into hot topics and frontiers than traditional reviews.
This study also had some shortcomings. First, the data for this study was extracted from the Web of Science Core Collection database only. Other databases were not searched, and some relevant studies may have been missed. This may increase the risk of bias in the selection of the literature. Second, we filtered studies published in English, which means that non-English language papers were underestimated. In addition, the 2023 publication data was not fully included, resulting in insufficient statistics for 2023. The research directions of NAFLD/MAFLD therapeutic research, cluster analysis, and trend topic analysis may not be comprehensive and may not cover marginal and emerging topics. Last but not least, bibliometric analysis is an analytical method of bibliometrics that focuses on the analysis of measures such as the number of articles issued, cited and quoted, with particular consideration of research countries, institutions and individuals, but pays less attention to the content of the articles themselves; as such, it may not be able to detect and temporally count research for a new type of topic, especially for the beginning stage of a certain research field that may become a hot spot in the future.
The treatment of NAFLD has important research value and application prospects, as indicated by the rapidly increasing number of documents. The study of treatments and therapies for NAFLD, particularly NASH, is highly valued worldwide. The leading countries publishing NAFLD research are the United States and China. NAFLD research in China is developing rapidly, with their number of published articles quickly surpassing the United States in recent years. However, it is worth noting that most of the top scientists in the field are from the United States. Conducting an in-depth study of the pathogenesis can foster the development and research of new drugs, while accurate diagnosis, specifically through non-invasive diagnostic technology, contributes to better patient management and efficacy evaluation. The multiple-hit pathogenesis of NAFLD and the renaming of NAFLD to MAFLD require enhanced multidisciplinary and multicenter cooperation. Mechanism studies provide guidance for therapeutic strategies, while clinical application and treatment research represent the transformation of basic research.
Nonalcoholic fatty liver disease (NAFLD) is a chronic disease that threatens the lives of numerous people globally. However, there are limited bibliometric statistical analyses on NAFLD. In this study, we conducted a bibliometric analysis to examine previous research on NAFLD, aiming to identify key contributors and assess the current research status in the field of liver health. Moreover, we identified prospects for future research trends and development.
The progression of NAFLD leads to liver fibrosis and end-stage cirrhosis. However, no treatment has been established. Many mechanistic studies and drug trials have been undertaken for the development of new drugs for NAFLD treatment. In particular, NAFLD was renamed metabolic dysfunction-associated fatty liver disease (MAFLD), and there was a major shift in treatment strategy. It is necessary to understand the knowledge structure of NAFLD through bibliometrics, focusing on research hotspots in order to explore the direction of development in this field.
The treatment of NAFLD has important research value and application prospects. It is anticipated that drugs may become available in the near future. However, no drugs are currently approved. Clinical phase 2b and phase 3 studies have achieved certain efficacy. While trends and hotspots can be clearly studied through bibliometric analysis, clues can reveal possible future therapeutic strategies for scholars in the field.
Bibliometric analysis was applied to provide a comprehensive understanding of the knowledge structure of a research field, and visualization analysis was used to visualize the results. Historiography analysis, bursts and cluster analysis, co-occurrence analysis, and trend topic analysis were also utilized to reveal the knowledge structure and research hotspots in this field.
The bibliometric study identified recent research frontiers and hotspot directions, which will provide a valuable reference for scholars researching treatments for NAFLD. The leading countries publishing NAFLD research are the United States and China. The NAFLD research field in China has developed rapidly in the past 3 years.
Research on the treatment and therapeutics of NAFLD, especially nonalcoholic steatohepatitis, is highly valued by the global academic community. It is likely that obeticholic acid, which has entered phase 3 clinical validation, and semaglutide, which is currently under study, will become the first approved drugs for the treatment of NAFLD.
The multiple-hit pathogenesis of NAFLD and the renaming of NAFLD to MAFLD requires enhanced multidisciplinary and multicenter cooperation. More clinical trials are needed to verify the safety and efficacy of drugs and to discover new ones.
We would like to thank Hao Xing from Hefei Third Hospital and Xiaodan Hong, MD from the Second Hospital of Anhui Medical University for their comments on drafting the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gutierrez-Castrellon P, Mexico; Zamani M, Iran S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 2. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1357] [Article Influence: 104.4] [Reference Citation Analysis (4)] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 4. | Stepanova M, Kabbara K, Mohess D, Verma M, Roche-Green A, AlQahtani S, Ong J, Burra P, Younossi ZM. Nonalcoholic steatohepatitis is the most common indication for liver transplantation among the elderly: Data from the United States Scientific Registry of Transplant Recipients. Hepatol Commun. 2022;6:1506-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 6. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1317] [Article Influence: 219.5] [Reference Citation Analysis (0)] |
| 7. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1487] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 8. | Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3909] [Cited by in RCA: 3859] [Article Influence: 296.8] [Reference Citation Analysis (0)] |
| 9. | Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM. Mortality Risk Detected by Atherosclerotic Cardiovascular Disease Score in Patients With Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2019;3:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2211] [Article Influence: 442.2] [Reference Citation Analysis (1)] |
| 11. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1627] [Article Influence: 162.7] [Reference Citation Analysis (1)] |
| 12. | Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: Facts and figures. JHEP Rep. 2019;1:468-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 168] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 13. | Ke L, Lu C, Shen R, Lu T, Ma B, Hua Y. Knowledge Mapping of Drug-Induced Liver Injury: A Scientometric Investigation (2010-2019). Front Pharmacol. 2020;11:842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 14. | Arruda H, Silva ER, Lessa M, Proença D Jr, Bartholo R. VOSviewer and Bibliometrix. J Med Libr Assoc. 2022;110:392-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 177] [Reference Citation Analysis (0)] |
| 15. | Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724-728. [PubMed] |
| 16. | Zhang TS, Qin HL, Wang T, Li HT, Li H, Xia SH, Xiang XH. Global publication trends and research hotspots of nonalcoholic fatty liver disease: a bibliometric analysis and systematic review. Springerplus. 2015;4:776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Zhang TS, Qin HL, Wang T, Li HT, Li H, Xia SH, Xiang XH. Bibliometric analysis of top 100 cited articles in nonalcoholic fatty liver disease research. World J Hepatol. 2016;8:1478-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Trifan A, Stanciu C, Jurcău M, Zenovia S, Frunzuc G, Timofte D. Nonalcoholic steatohepatitis: A scientometric analysis of publications during 1980-2018. Medicine (Baltimore). 2019;98:e18221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Li Z, Cao S, Zhao S, Kang N. A bibliometric analysis and visualization of nonalcoholic fatty liver disease from 2012 to 2021. Clin Exp Med. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Yang S, Yu D, Liu J, Qiao Y, Gu S, Yang R, Chai X, Wang W. Global publication trends and research hotspots of the gut-liver axis in NAFLD: A bibliometric analysis. Front Endocrinol (Lausanne). 2023;14:1121540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 21. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne C, Narro GEC, Chowdhury A, Cortez-Pinto H, Cryer D, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot B, Korenjak M, Kowdley K, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell E, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN (senior); NAFLD Nomenclature consensus group. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2023;101133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 322.0] [Reference Citation Analysis (0)] |
| 22. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010; 53: 372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 789] [Article Influence: 52.6] [Reference Citation Analysis (1)] |
| 23. | van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4505] [Cited by in RCA: 5153] [Article Influence: 322.1] [Reference Citation Analysis (1)] |
| 24. | Yeung AWK, Mozos I. The Innovative and Sustainable Use of Dental Panoramic Radiographs for the Detection of Osteoporosis. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Zhang XL, Zheng Y, Xia ML, Wu YN, Liu XJ, Xie SK, Wu YF, Wang M. Knowledge Domain and Emerging Trends in Vinegar Research: A Bibliometric Review of the Literature from WoSCC. Foods. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 26. | Wu H, Cheng K, Guo Q, Yang W, Tong L, Wang Y, Sun Z. Mapping Knowledge Structure and Themes Trends of Osteoporosis in Rheumatoid Arthritis: A Bibliometric Analysis. Front Med (Lausanne). 2021;8:787228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Zhang Q, Ma J, Yu Y. Mapping research trends of insulin resistance in polycystic ovary syndrome from 2017 to 2021: A bibliometric analysis. Front Endocrinol (Lausanne). 2022;13:963213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Aria M, Cuccurullo C. bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. Journal of Informetrics. 2018;11:959-975. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1736] [Cited by in RCA: 2277] [Article Influence: 284.6] [Reference Citation Analysis (1)] |
| 29. | Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1:5303-5310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1314] [Article Influence: 62.6] [Reference Citation Analysis (1)] |
| 30. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2471] [Article Influence: 164.7] [Reference Citation Analysis (2)] |
| 31. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E; NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1799] [Article Influence: 179.9] [Reference Citation Analysis (3)] |
| 32. | Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1508] [Cited by in RCA: 1758] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 33. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3798] [Article Influence: 542.6] [Reference Citation Analysis (2)] |
| 34. | Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 929] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 35. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 973] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 36. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1470] [Article Influence: 163.3] [Reference Citation Analysis (1)] |
| 37. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2920] [Article Influence: 417.1] [Reference Citation Analysis (1)] |
| 38. | Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, Hoofnagle JH. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 39. | Albhaisi SAM, Bajaj JS, Sanyal AJ. Role of gut microbiota in liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;318:G84-G98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 40. | Albhaisi SAM, Sanyal AJ. New drugs for NASH. Liver Int. 2021;41 Suppl 1:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, Hooker J, Kono Y, Bhatt A, Hernandez L, Nguyen P, Noureddin M, Haufe W, Hooker C, Yin M, Ehman R, Lin GY, Valasek MA, Brenner DA, Richards L; San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015;61:1239-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 42. | Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 43. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 534] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 44. | Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR; GS-US-384-1497 Investigators. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2018;67:549-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 45. | Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, Lawitz EJ, Halegoua-DeMarzio D, Kundu S, Noviello S, Luo Y, Christian R. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019; 392: 2705-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 46. | Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, Lawitz E, Ratziu V, Sanyal AJ, Schattenberg JM, Newsome PN; NN9931-4492 investigators. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 246] [Article Influence: 123.0] [Reference Citation Analysis (0)] |
| 47. | Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 332] [Article Influence: 83.0] [Reference Citation Analysis (2)] |
| 48. | Ratziu V, Sanyal AJ, Loomba R, Rinella M, Harrison S, Anstee QM, Goodman Z, Bedossa P, MacConell L, Shringarpure R, Shah A, Younossi Z. REGENERATE: Design of a pivotal, randomised, phase 3 study evaluating the safety and efficacy of obeticholic acid in patients with fibrosis due to nonalcoholic steatohepatitis. Contemp Clin Trials. 2019;84:105803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 49. | Vuppalanchi R, McCabe ME 4th, Tandra SR, Parcha SP, Ghafoor A, Schuh L, Inman MM, Selzer DJ, Stefanidis D, Chalasani N. Safety and Efficacy of Bariatric Surgery in Cirrhosis Patients With Extreme Obesity. Ann Surg. 2022;275:e174-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Gawrieh S, Noureddin M, Loo N, Mohseni R, Awasty V, Cusi K, Kowdley KV, Lai M, Schiff E, Parmar D, Patel P, Chalasani N. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology. 2021;74:1809-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 51. | Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, Noureddin M, Pyko M, Shiffman M, Sanyal A, Allgood A, Shlevin H, Horton R, Zomer E, Irish W, Goodman Z, Harrison SA, Traber PG; Belapectin (GR-MD-02) Study Investigators. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients With Nonalcoholic Steatohepatitis With Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334-1345.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 52. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8239] [Article Influence: 412.0] [Reference Citation Analysis (5)] |
| 53. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7541] [Article Influence: 837.9] [Reference Citation Analysis (0)] |
| 54. | Lee HW, Wong VW. Changing NAFLD Epidemiology in China. Hepatology. 2019;70:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 893] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 56. | Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70:531-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1458] [Article Influence: 243.0] [Reference Citation Analysis (1)] |
| 57. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1710] [Article Influence: 244.3] [Reference Citation Analysis (0)] |
| 58. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 944] [Article Influence: 85.8] [Reference Citation Analysis (4)] |
| 59. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1309] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 60. | Du LJ, He ZY, Gu X, Hu X, Zhang XX, Yang LJ, Li J, Pan LY, Li YQ, Yang B, Gu XJ. Inverse Association of Fruit and Vegetable Consumption with Nonalcoholic Fatty Liver Disease in Chinese Patients with Type 2 Diabetes Mellitus. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 61. | Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (1)] |
| 62. | Zhang Q, Zhang L, Liu K, Shang H, Ruan J, Yu Z, Meng S, Liang F, Wang T, Zhang H, Peng W, Wang Y, Chen J, Xiao T, Wang B. A Network Pharmacology Study on the Active Components and Targets of the Radix Ginseng and Radix Bupleuri Herb Pair for Treating Nonalcoholic Fatty Liver Disease. Evid Based Complement Alternat Med. 2022;2022:1638740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Liu H, Xu J, Li H, Zhang L, Xu P. Network pharmacology-based investigation to explore the effect and mechanism of Erchen decoction against the nonalcoholic fatty liver disease. Anat Rec (Hoboken). 2021;304:2605-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Jia W, Wang K, Zhang S, Lu W, Du A, Li J, Ji L, Xu H. Integrating network pharmacology and in vivo experimental validation to reveal the alleviation of mailuoning oral liquid on non-alcoholic fatty liver disease. Phytomedicine. 2022;104:154326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 65. | Zhao L, Zhang H, Li N, Chen J, Xu H, Wang Y, Liang Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 374] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 66. | Zhou W, Zhu Z, Xiao X, Li C, Zhang L, Dang Y, Ge G, Ji G, Zhu M, Xu H. Jiangzhi Granule attenuates non-alcoholic steatohepatitis by suppressing TNF/NFκB signaling pathway-a study based on network pharmacology. Biomed Pharmacother. 2021;143:112181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Mao FF, Gao SS, Huang YJ, Zhou N, Feng JK, Liu ZH, Zhang YQ, Yuan LY, Wei G, Cheng SQ. Network pharmacology-based analysis of Resinacein S against non-alcoholic fatty liver disease by modulating lipid metabolism. Front Nutr. 2023;10:1076569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 68. | Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, Yang H, Liu C, Kam LY, Tan XXE, Chien N, Trinh S, Henry L, Stave CD, Hosaka T, Cheung RC, Nguyen MH. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 568] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 69. | Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 454] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 70. | Liu YZ, Wang YX, Jiang CL. Inflammation: The Common Pathway of Stress-Related Diseases. Front Hum Neurosci. 2017;11:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 480] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 71. | Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Blüher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20:1436-1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 72. | Li R, Xin T, Li D, Wang C, Zhu H, Zhou H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: The role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 73. | Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, Zhang Z, Zhang D, Fan D, Nie Y, Shao F, Wu K, Liang J. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 74. | Yang L, Roh YS, Song J, Zhang B, Liu C, Loomba R, Seki E. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology. 2014;59:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 75. | Li L, Chen L, Hu L, Liu Y, Sun HY, Tang J, Hou YJ, Chang YX, Tu QQ, Feng GS, Shen F, Wu MC, Wang HY. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 76. | Cao J, Dai DL, Yao L, Yu HH, Ning B, Zhang Q, Chen J, Cheng WH, Shen W, Yang ZX. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364:115-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 77. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 540] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 78. | Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512-10522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 358] [Cited by in RCA: 453] [Article Influence: 50.3] [Reference Citation Analysis (4)] |
| 79. | Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 280] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 80. | Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121:4477-4490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 81. | Xu Y, Wang N, Tan HY, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front Microbiol. 2020;11:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 82. | Dai J, Liang K, Zhao S, Jia W, Liu Y, Wu H, Lv J, Cao C, Chen T, Zhuang S, Hou X, Zhou S, Zhang X, Chen XW, Huang Y, Xiao RP, Wang YL, Luo T, Xiao J, Wang C. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115:E5896-E5905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 83. | Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, Wang BM. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol. 2015;21:102-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 161] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (2)] |
| 84. | Wang PX, Ji YX, Zhang XJ, Zhao LP, Yan ZZ, Zhang P, Shen LJ, Yang X, Fang J, Tian S, Zhu XY, Gong J, Zhang X, Wei QF, Wang Y, Li J, Wan L, Xie Q, She ZG, Wang Z, Huang Z, Li H. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat Med. 2017;23:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 85. | Li S, Liao X, Meng F, Wang Y, Sun Z, Guo F, Li X, Meng M, Li Y, Sun C. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One. 2014;9:e86724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Zhao XJ, Yu HW, Yang YZ, Wu WY, Chen TY, Jia KK, Kang LL, Jiao RQ, Kong LD. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018;18:124-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 87. | Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML, Tipoe GL. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 88. | Zhang P, Wang PX, Zhao LP, Zhang X, Ji YX, Zhang XJ, Fang C, Lu YX, Yang X, Gao MM, Zhang Y, Tian S, Zhu XY, Gong J, Ma XL, Li F, Wang Z, Huang Z, She ZG, Li H. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med. 2018;24:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 89. | Liu D, Wong CC, Fu L, Chen H, Zhao L, Li C, Zhou Y, Zhang Y, Xu W, Yang Y, Wu B, Cheng G, Lai PB, Wong N, Sung JJY, Yu J. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 90. | Barlow P, McKee M, Basu S, Stuckler D. The health impact of trade and investment agreements: a quantitative systematic review and network co-citation analysis. Global Health. 2017;13:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 91. | Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. Journal of the Association for Information Science and Technology. 2006;57:359-377. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2057] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 92. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3719] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 93. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 94. | Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Ünalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR; Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 849] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 95. | Bellanti F, Lo Buglio A, Dobrakowski M, Kasperczyk A, Kasperczyk S, Aich P, Singh SP, Serviddio G, Vendemiale G. Impact of sodium glucose cotransporter-2 inhibitors on liver steatosis/fibrosis/inflammation and redox balance in non-alcoholic fatty liver disease. World J Gastroenterol. 2022;28:3243-3257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 96. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-82.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 738] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 97. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2119] [Article Influence: 235.4] [Reference Citation Analysis (1)] |
| 98. | Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 1051] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 99. | Promrat K, Longato L, Wands JR, de la Monte SM. Weight loss amelioration of non-alcoholic steatohepatitis linked to shifts in hepatic ceramide expression and serum ceramide levels. Hepatol Res. 2011;41:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 100. | Amjad T, Shahid N, Daud A, Khatoon A. Citation burst prediction in a bibliometric network. Scientometrics. 2022;127:2773-2790. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 101. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1660] [Article Influence: 118.6] [Reference Citation Analysis (2)] |
| 102. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2836] [Article Influence: 567.2] [Reference Citation Analysis (1)] |
| 103. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1216] [Article Influence: 304.0] [Reference Citation Analysis (0)] |
| 104. | Eslam M, Sanyal AJ, George J. Toward More Accurate Nomenclature for Fatty Liver Diseases. Gastroenterology. 2019;157:590-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 105. | Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 106. | Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med. 2016;165:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 738] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 107. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 108. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1703] [Article Influence: 170.3] [Reference Citation Analysis (1)] |
| 109. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 110. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015; 149: 389-97.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2231] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 111. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2293] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 112. | Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013; 10: 330-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1318] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 113. | Anstee QM, Day CP. A lipid to treat non-alcoholic fatty liver disease - the dawn of 'lipo-rehabilitation'? J Hepatol. 2012;56:987-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 114. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1330] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 115. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 116. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 117. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne C, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer D, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot B, Korenjak M, Kowdley K, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell E, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, WaiSun Wong V, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;20:S0168-8278(23)00418-X. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1306] [Article Influence: 653.0] [Reference Citation Analysis (1)] |