Published online Oct 7, 2023. doi: 10.3748/wjg.v29.i37.5313
Peer-review started: June 15, 2023
First decision: August 26, 2023
Revised: September 8, 2023
Accepted: September 14, 2023
Article in press: September 14, 2023
Published online: October 7, 2023
Processing time: 102 Days and 2.1 Hours
Colorectal cancer (CRC) has become the second most deadly malignancy in the world, and the exploration of screening markers and precise therapeutic targets is urgent. Our previous research identified leukocyte immunoglobulin-like receptor B2 (LILRB2) protein as a characteristic protein of CRC, but the association between LILRB2 expression and clinicopathological features, the internal mechanism related to CRC progression, and screening diagnostic efficacy are not clear. Therefore, we hypothesized that LILRB2 is significantly highly expressed in CRC tissues, correlated with advanced stage and a poor prognosis, and could be used as a therapeutic target and potential screening biomarker for CRC.
To explore whether LILRB2 can be used as a potential therapeutic target and noninvasive screening biomarker for CRC.
Patients who underwent radical surgery for CRC at China-Japan Friendship Hospital between February 2021 and October 2022 were included. Cancer and paracancerous tissues were collected to verify LILRB2 expression, and the association between LILRB2 expression and clinicopathological features was analysed. Serum was collected from CRC patients, adenoma patients and healthy controls during the same period to assess the diagnostic value of LILRB2 as a noninvasive screening biomarker, and its diagnostic value was further compared with that of the traditional markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9).
A total of 58 CRC patients were included, and LILRB2 protein was significantly overexpressed in cancer tissues compared with paracancerous tissues (P < 0.001). Angiopoietin-like protein 2 (ANGPTL2) protein, as the ligand of LILRB2, was synergistically overexpressed in CRC tissues (P < 0.001), and overexpression of LILRB2 and ANGPTL2 protein was significantly correlated with poor to moderate differentiation, vascular involvement, lymph node metastasis, distant metastasis, advanced tumor-node-metastasis stage and a poor prognosis (P < 0.05), which suggested that LILRB2 and ANGPTL2 are closely associated with CRC progression. In addition, serum LILRB2 concentrations increased stepwise in healthy individuals, adenoma patients and CRC patients with statistically significant differences. The sensitivity of serum LILRB2 for the diagnosis of CRC was 89.74%, the specificity was 88.89%, the area under the curve was 0.95, and the diagnostic efficacy was better than that of conventional CEA and CA19-9.
LILRB2 protein can be used as a potential novel therapeutic target and noninvasive screening biomarker for CRC, which is beneficial for early screening and precise treatment.
Core Tip: Based on prior proteomic research rather than simple data mining, this study innovatively proposed and validated that leukocyte immunoglobulin-like receptor B2 (LILRB2) and its ligand, the angiopoietin-like protein 2 protein, are significantly overexpressed in colorectal cancer (CRC) and closely associated with tumour progression and a poor prognosis. In addition, this study is the first to propose that serum LILRB2 concentration can be used as a novel screening biomarker with a sensitivity of 89.74%, a specificity of 88.89% and an accuracy rate of 89.63% for the diagnosis of CRC. The sensitivity and accuracy were significantly higher than those of carcinoembryonic antigen and carbohydrate antigen 19-9. Therefore, LILRB2 could be a promising therapeutic target and noninvasive screening biomarker for CRC.
- Citation: Wang QQ, Zhou L, Qin G, Tan C, Zhou YC, Yao SK. Leukocyte immunoglobulin-like receptor B2 overexpression as a promising therapeutic target and noninvasive screening biomarker for colorectal cancer. World J Gastroenterol 2023; 29(37): 5313-5326
- URL: https://www.wjgnet.com/1007-9327/full/v29/i37/5313.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i37.5313
The latest cancer statistics show that more than 1.9 million new cases of colorectal cancer (CRC) and 935000 deaths occurred in 2020, accounting for approximately one-tenth of all new cancer cases and deaths. CRC has become the third ranked malignancy worldwide in terms of incidence and the second ranked in terms of mortality, second only to lung cancer[1-3]. The 5-year overall survival (OS) rate for early-stage CRC can be as high as 80%-90%, while the prognosis for advanced-stage patients is poor, with a 5-year survival rate of less than 20%[4,5]. Although targeted therapy or immunotherapy has improved the OS of CRC patients, limited treatment options, moderate response rates and drug resistance remain major challenges for CRC treatment. Therefore, the exploration of novel therapeutic targets and noninvasive screening biomarkers is essential to improve the outcomes of CRC patients.
Proteomics has been widely used in cancer research[6,7]. In preliminary research, we screened leukocyte immunoglobulin-like receptor B2 (LILRB2) protein as a characteristic protein of CRC[8]. LILRB2 is a transmembrane glycoprotein with a structure similar to immune checkpoint protein programmed cell death protein-1 (PD-1)/PD-ligand 1; it has four extracellular immunoglobulin-like structural domains and intracellular tyrosine-based immune receptor inhibitory motifs (ITIMs); it is widely expressed on the surface of dendritic cells, macrophages, and other myeloid cells and can inhibit immune cell function and participate in important pathological processes such as tumour immune microenvironment (TME), immune escape, and promotion of tumour progression[9]. Angiopoietin-like proteins (ANGPTLs) are a family of seven secreted glycoproteins that play an important role in angiogenesis, the inflammatory response, and tumour progression[10]. In recent years, it has been revealed that LILRB2 is a receptor for ANGPTLs, with the strongest affinity for ANGPTL2, ending the status of ANGPTLs as “orphan ligands”[11]. The interaction of the LILRB2 receptor with the ANGPTL2 ligand has been reported to promote the progression of tumours such as acute leukaemia and non-small cell lung cancer (NSCLC)[10,12,13]. However, such interactions have not been reported in CRC.
Therefore, this study hypothesized that LILRB2 could be a potential novel therapeutic target and screening biomarker for CRC, but validation of LILRB2 and ANGPTL2 protein expression and interaction in CRC tissues is first needed to further explore the feasibility of LILRB2 protein as a noninvasive screening biomarker.
Patients who underwent radical surgery for CRC at China-Japan Friendship Hospital between February 2021 and October 2022 were recruited. Inclusion criteria were as follows: patients diagnosed with CRC by the department of pathology who intended to undergo radical resection. The exclusion criteria were as follows: (1) Patients with familial adenomatous polyposis, hereditary nonpolyposis CRC (lynch syndrome), synchronous multiple tumours, inflammatory bowel disease-related CRC; (2) CRC with neuroendocrine manifestations; (3) Patients who received radiotherapy, targeted therapy, immunotherapy, etc., within 6 mo prior to radical surgery; and (4) Patients who lacked informed consent or patients with missing information.
Colorectal adenoma patients and healthy controls were recruited at the Endoscopy Center of China-Japan Friendship Hospital during the same period. Inclusion criteria for adenoma patients were as follows: Patients with pathologically confirmed colorectal adenoma who were intended to undergo elective endoscopic mucosal resection. The exclusion criteria were as follows: (1) Severe atypical hyperplasia, high-grade intraepithelial neoplasia or carcinoma in situ; (2) Familial adenomatous polyposis or inflammatory bowel disease-associated colorectal polyps; and (3) Refusal to sign the informed consent form. Patients with no abnormalities according to colonoscopy were included in the healthy control group. The study was approved by the ethics committee of China-Japan Friendship Hospital and conducted in accordance with the Declaration of Helsinki.
Cancer and paracancerous tissues (> 5 cm from the cancer margin) were collected from CRC patients after surgical resection, and the samples were rinsed with ice-cold saline, soaked in 10% formalin solution with a volume ratio of 1:7, fixed for 24 h, rinsed under running water, dehydrated and waxed at 55 °C and embedded in paraffin. The paraffin blocks were stored at room temperature and subsequently used for immunohistochemical staining.
Fresh whole blood samples were collected from CRC patients before (within 7 d) and after (within 24 h) surgery, adenoma patients before surgery (within 7 d) and healthy controls and centrifuged at 2-8 °C and 3000 r/min for 15 min at room temperature. The supernatant serum was collected into 2 mL freezing tubes, transferred to a -80 °C refrigerator in liquid nitrogen for freezing and storage, and subsequently used for enzyme-linked immunosorbent assay (ELISA), and the samples avoided repeated thawing before the examination.
In the electronic medical record system, applications were made to view the medical records of the enrolled patients and collect clinical information (age, sex), tumour markers [carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9)], histopathological features [tumour location, size, pathological type, degree of differentiation, vascular involvement, nerve involvement, lymph node metastasis, and tumor-node-metastasis (TNM) stage] and molecular pathology (KRAS, NRAS, PIK3CA, BRAF, MS). The normal reference ranges were CEA < 5 ng/mL and CA19-9 ≤ 30 U/mL. TNM staging was based on the American Joint Committee on Cancer 8th edition TNM staging system.
Paraffin blocks were cut into 4-μm-thick sections, and the sections were sequentially placed in dewaxing solution I for 15 min, dewaxing solution II for 15 min, dewaxing solution III for 15 min, anhydrous ethanol I for 5 min, anhydrous ethanol II for 5 min, 85% alcohol for 5 min, and 75% alcohol for 5 min and then washed with distilled water. The slides were placed in a repair box filled with EDTA antigen repair buffer (pH 9.0) in a microwave oven for antigen repair, and after natural cooling, the slides were placed in phosphate buffered saline (PBS) (pH = 7.4) on a decolorization shaker and washed 3 times for 5 min each. Sections were placed in 3% hydrogen peroxide solution, incubated for 25 min at room temperature and protected from light, and the slides were placed in PBS (pH = 7.4) on a decolorizing shaker and washed three times for 5 min each time to block endogenous peroxidase. The tissue slides were covered uniformly with 3% BSA in a dropwise manner and then blocked at room temperature for 30 min; the slide was gently shaked to remove the blocking solution, and a primary antibody (anti-LILRB2, LSBio, Cat No. LS-B9762-50, 1:150; anti-ANGPTL2, Proteintech, Cat No. 12316-1-AP, 1:200) in a certain ratio of PBS was added dropwise onto the section. Sections were incubated flat in a wet box overnight at 4 °C. After washing and shaking the slides dry, HRP-labelled goat anti-rabbit secondary antibody (Servicebio, GB23303, 1:200) was added dropwise in the circle to cover the tissue and incubated at room temperature for 50 min. After washing and shaking the slides dry, freshly prepared DAB colour development solution was added dropwise, the colour development time was controlled under the microscope, and the positive colour was brownish yellow. Haematoxylin was used to restain the nuclei for approximately 3 min, and the sections were washed with tap water. Finally, microscopic examination was performed, and images were acquired for analysis. Image-Pro Plus image analysis software was used to quantify the immunohistochemical images; five fields of view were randomly selected after magnification of each section at 200 × to assess the integrated optical density and area, and the average optical density value was calculated.
The Gene Expression Profiling Interactive Analysis (GEPIA) platform can analyse differentially expressed genes or mRNAs between normal and cancer tissues online for survival and correlation analysis in a variety of tumours. In this study, we used GEPIA V2.0 (http://gepia2.cancer-pku.cn/) for survival analysis and correlation analysis of CRC at the mRNA level as a complement to proteomics and immunohistochemistry (IHC) at the protein level.
The assay was performed according to the instructions of the Human LILRB2 ELISA Kit (Abcam, Cat No. ab269551). Prepare blank wells and wells with multiply diluted standards, add 100 μL of properly diluted serum samples to be tested in the reaction wells, seal the plate with sealing film and incubate at 37 °C for 1-2 h. Discard the liquid, add 300 μL of washing solution to each well, soak for 1-2 min, pat dry on absorbent paper and repeat 3-5 times. Then, 100 μL of diluted biotinylated antibody working solution was added to each well, the plate was sealed with sealing film and incubated at 37 °C for 1 h. The liquid was discarded, 300 μL of washing solution was added to each well, and the washing was repeated. Then, 100 μL of diluted enzyme conjugate working solution was added to each well, the plate was sealed with sealing film and incubated for 30 min at 37 °C, and the washing process was repeated. TMB substrate solution (100 μL) was added to each well and incubated for 10-30 min at 37 °C, protected from light, until a clear colour gradient appeared in the wells of the standards diluted in multiples. The reaction was terminated by adding 100 μL of 2 M sulfuric acid to each well, and the colour changed from blue to yellow. The OD value of each well, including wells with enzyme standards and blank wells, was measured at 450 nm. The blank control wells were adjusted to zero. A standard curve was made according to the concentration and OD value of the standards, and then the sample concentration was calculated according to the equation of the standard curve, and the result was multiplied by the dilution factor for the sample.
SPSS 25.0 was used for statistical analysis. Quantitative data are expressed as the mean ± SD, and an independent sample t test was used for comparisons between two groups. If the quantitative data did not obey a normal distribution or the variance was not uniform, the median (quartiles) was used, the Mann-Whitney U test was used for two-group comparisons, the Kruskal-Wallis H test was used for multigroup comparisons, and the Bonferroni method was required to correct the significance level for multiple two-group comparisons. Categorical data were expressed as percentages, and two or more groups were compared using the χ2 test or Fisher’s exact test. Quantitative data correlation analysis was performed using Pearson correlation or Spearman correlation analysis (nonnormally distributed). OS curves and progression-free survival (PFS) curves were constructed using the Kaplan-Meier method and analysed using the log rank test. P < 0.05 was considered to indicate a significant difference.
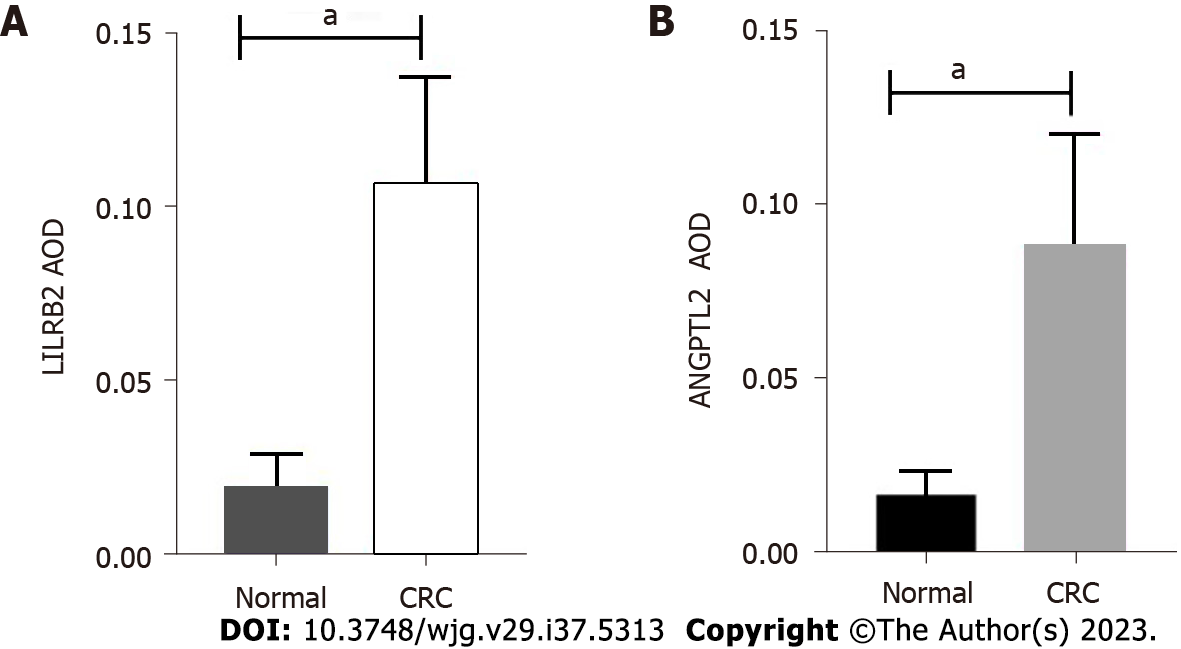
From February 2021 to October 2022, a total of 58 CRC patients met the criteria, and 58 pairs of tissues were successfully collected. Figure 1 demonstrates the actual expression of LILRB2 and ANGPTL2 proteins in primary CRC tissues and paracancerous tissues. Figure 1A shows that LILRB2 protein expression significantly higher in CRC tissues than in paracancerous tissues (P < 0.001), and ANGPTL2 protein was significantly overexpressed in CRC tissues compared with paracancerous tissues (P < 0.001) (Figure 1B).
The associations between LILRB2 and ANGPTL2 protein expression and clinicopathological characteristics of CRC are shown in Table 1. The results showed that the overexpression of LILRB2 protein and ANGPTL2 protein was significantly correlated with poor to moderate tumour differentiation, vascular involvement, lymph node metastasis and advanced TNM stage. In addition, high LILRB2 protein expression was significantly correlated with nerve involvement (P = 0.019), confirming that LILRB2 and ANGPTL2 protein are closely associated with the progression of CRC.
| LILRB2 | P value | ANGPTL2 | P value | |||
| Low (n = 19) | High (n = 39) | Low (n = 30) | High (n = 28) | |||
| Clinical features | ||||||
| Age (yr) | 0.670 | 0.986 | ||||
| ≤ 50 | 4 (21.1%) | 5 (12.8%) | 14 (46.7%) | 13 (46.4%) | ||
| > 50 | 15 (78.9%) | 34 (87.2%) | 16 (53.3%) | 15 (53.6%) | ||
| Sex | 1.000 | 0.473 | ||||
| Male | 10 (52.6%) | 21 (53.8%) | 24 (80.0%) | 25 (89.3%) | ||
| Female | 9 (47.4%) | 18 (46.2%) | 6 (20.0%) | 3 (10.7%) | ||
| Histopathological features | ||||||
| Location | 0.737 | 0.065 | ||||
| Right-side colon | 2 (10.5%) | 7 (17.9%) | 2 (6.60%) | 7 (25.0%) | ||
| Left-side colon | 6 (31.6%) | 9 (23.1%) | 11 (36.7%) | 4 (14.3%) | ||
| Rectum | 11 (57.9%) | 23 (59.0%) | 17 (56.7%) | 17 (60.7%) | ||
| Size (cm) | 0.224 | 0.436 | ||||
| > 4.9 | 7 (36.8%) | 21 (53.8%) | 13 (43.3%) | 15 (53.6%) | ||
| ≤ 4.9 | 12 (63.2%) | 18 (46.2%) | 17 (56.7%) | 13 (46.4%) | ||
| Pathological type | 0.287 | 0.589 | ||||
| Ulcer | 12 (63.2%) | 29 (74.4%) | 23 (76.7%) | 18 (64.3%) | ||
| Bulge | 6 (31.6%) | 5 (12.8%) | 5 (16.7%) | 6 (21.4%) | ||
| Mix | 1 (5.20%) | 5 (12.8%) | 2 (6.60%) | 4 (14.3%) | ||
| Differentiation | 0.009 | 0.013 | ||||
| Poor | 0 (0.00%) | 10 (25.6%) | 1 (3.40%) | 9 (32.1%) | ||
| Moderate | 15 (78.9%) | 27 (69.2%) | 25 (83.3%) | 17 (60.7%) | ||
| High | 4 (21.1%) | 2 (5.20%) | 4 (13.3%) | 2 (7.20%) | ||
| Vascular involvement | 0.001 | 0.016 | ||||
| Yes | 1 (5.30%) | 19 (48.7%) | 6 (20.0%) | 14 (50.0%) | ||
| No | 18 (94.7%) | 20 (51.3%) | 24 (80.0%) | 14 (50.0%) | ||
| Nerve involvement | 0.019 | 0.754 | ||||
| Yes | 7 (36.8%) | 27 (69.2%) | 17 (56.7%) | 17 (60.7%) | ||
| No | 12 (63.2%) | 12 (30.8%) | 13 (43.3%) | 11 (39.3%) | ||
| Lymph node metastasis | < 0.001 | 0.008 | ||||
| Yes | 2 (10.5%) | 29 (74.4%) | 11 (36.7%) | 20 (71.4%) | ||
| No | 17 (89.5%) | 10 (25.6%) | 19 (63.3%) | 8 (28.6%) | ||
| Molecular pathology | ||||||
| KRAS | 0.186 | 0.249 | ||||
| + | 6 (31.6%) | 19 (48.7%) | 11 (36.7%) | 14 (50.0%) | ||
| - | 13 (68.4%) | 20 (51.3%) | 19 (63.3%) | 14 (50.0%) | ||
| NRAS | 0.594 | 0.613 | ||||
| + | 2 (10.5%) | 2 (5.10%) | 3 (10.0%) | 1 (3.60%) | ||
| - | 17 (89.5%) | 37 (94.9%) | 27 (90.0%) | 27 (96.4%) | ||
| PIK3CA | 1.000 | 1.000 | ||||
| + | 0 (0.0%) | 1 (2.60%) | 1 (3.30%) | 0 (0.0%) | ||
| - | 19 (100%) | 38 (97.4%) | 29 (96.7%) | 28 (100%) | ||
| BRAF | 0.548 | 1.000 | ||||
| + | 0 (0.0%) | 2 (5.10%) | 1 (3.30%) | 1 (3.60%) | ||
| - | 19 (100%) | 37 (94.9%) | 29 (96.7%) | 27 (96.4%) | ||
| MS | 1.000 | 1.000 | ||||
| MSS | 18 (94.7%) | 37 (94.9%) | 28 (93.3%) | 27 (96.4%) | ||
| MSI-H | 1 (5.30%) | 2 (5.10%) | 2 (6.70%) | 1 (3.60%) | ||
| Clinical stage | ||||||
| pT | 0.001 | 0.006 | ||||
| T1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| T2 | 7 (36.8%) | 1 (2.60%) | 7 (23.3%) | 1 (3.60%) | ||
| T3 | 11 (57.9%) | 27 (69.2%) | 21 (70.0%) | 17 (60.7%) | ||
| T4 | 1 (5.30%) | 11 (28.2%) | 2 (6.70%) | 10 (35.7%) | ||
| pN | < 0.001 | 0.001 | ||||
| N0 | 19 (100%) | 11 (28.2%) | 22 (73.3%) | 8 (28.6%) | ||
| N1 | 0 (0.0%) | 15 (38.5%) | 6 (20.0%) | 9 (32.1%) | ||
| N2 | 0 (0.0%) | 13 (33.3%) | 2 (6.70%) | 11 (39.3%) | ||
| pM | 0.044 | 0.002 | ||||
| M0 | 19 (100%) | 31 (79.5%) | 30 (100%) | 20 (71.4%) | ||
| M1 | 0 (0.0%) | 8 (20.5%) | 0 (0.0%) | 8 (28.6%) | ||
| pTNM stage | < 0.001 | < 0.001 | ||||
| I-II | 18 (94.7%) | 10 (25.6%) | 22 (73.3%) | 6 (21.4%) | ||
| III-IV | 1 (5.30%) | 29 (74.4%) | 8 (26.7%) | 22 (78.6%) | ||
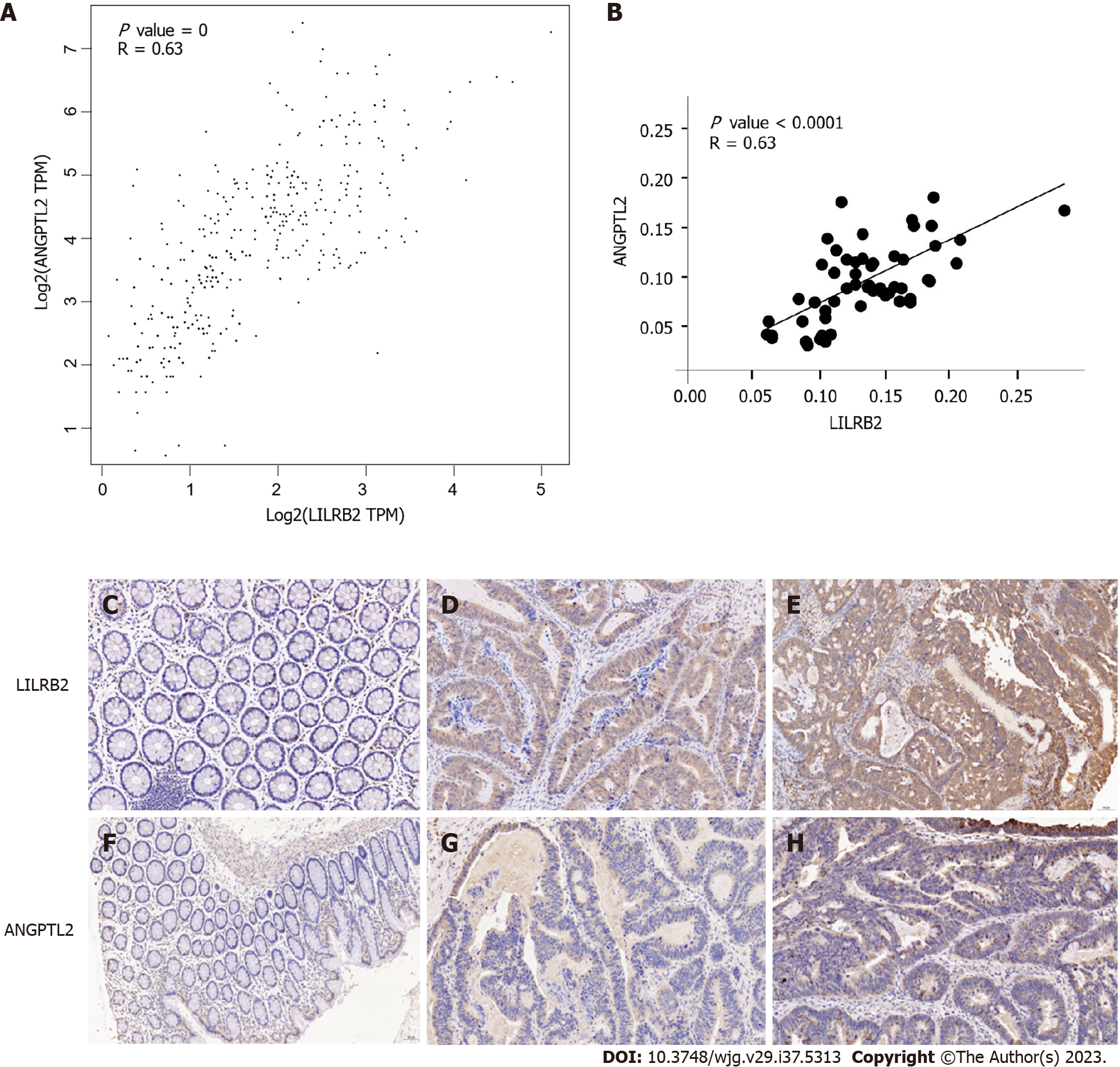
Figure 2 demonstrates the correlation between LILRB2 and ANGPTL2 expression at the mRNA and protein levels in primary CRC tissues. The correlation between LILRB2 and ANGPTL2 expression at the mRNA level was first analysed using the GEPIA database, and the results showed that LILRB2 mRNA expression was significantly and positively correlated with ANGPTL2 mRNA expression in CRC tissues (r = 0.63, P = 0) (Figure 2A); similarly, LILRB2 protein expression was significantly and positively correlated with ANGPTL2 protein expression (r = 0.63, P < 0.0001) (Figure 2B).
Observation of IHC images showed that LILRB2 protein was expressed in the cell membrane, cytoplasm or both in CRC cells, and inflammatory cells also showed brown staining/positive expression, while ANGPTL2 protein was mainly expressed in the cytoplasm of CRC cells. LILRB2 protein was highly expressed in 67.2% of CRC tissues, and ANGPTL2 protein was highly expressed in 48.3% of CRC tissues. However, in the paracancerous tissues, LILRB2 protein and ANGPTL2 protein expression was significantly lower or not detected (Figures 2C-H).
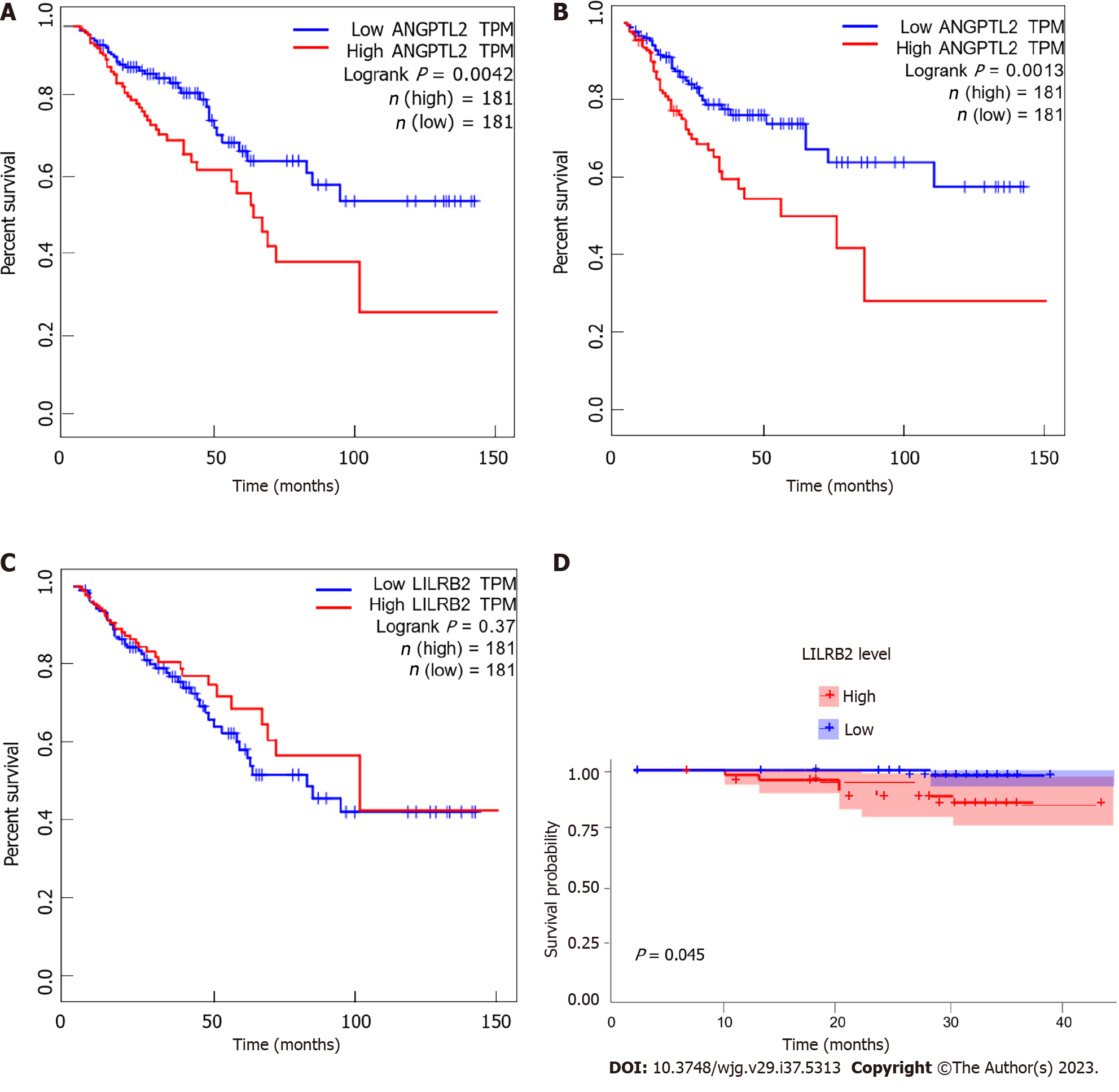
ANGPTL2 mRNA was significantly negatively correlated with the OS (P = 0.0042) and PFS (P = 0.0013) of CRC patients, while no association was found at the protein level (Figures 3A and B). In contrast, LILRB2 mRNA expression was not associated with the OS or PFS of CRC patients (P > 0.05) (Figure 3C), while LILRB2 protein overexpression was significantly associated with reduced OS (P = 0.045) (Figure 3D), suggesting a poor prognosis in CRC patients and indicating the procancer role of LILRB2 protein in CRC progression.
From February 2021 to October 2022, 313 serum samples were collected, including 117 preoperative and 85 postoperative serum samples from CRC patients, 93 serum samples from adenoma patients and 18 serum samples from healthy controls.
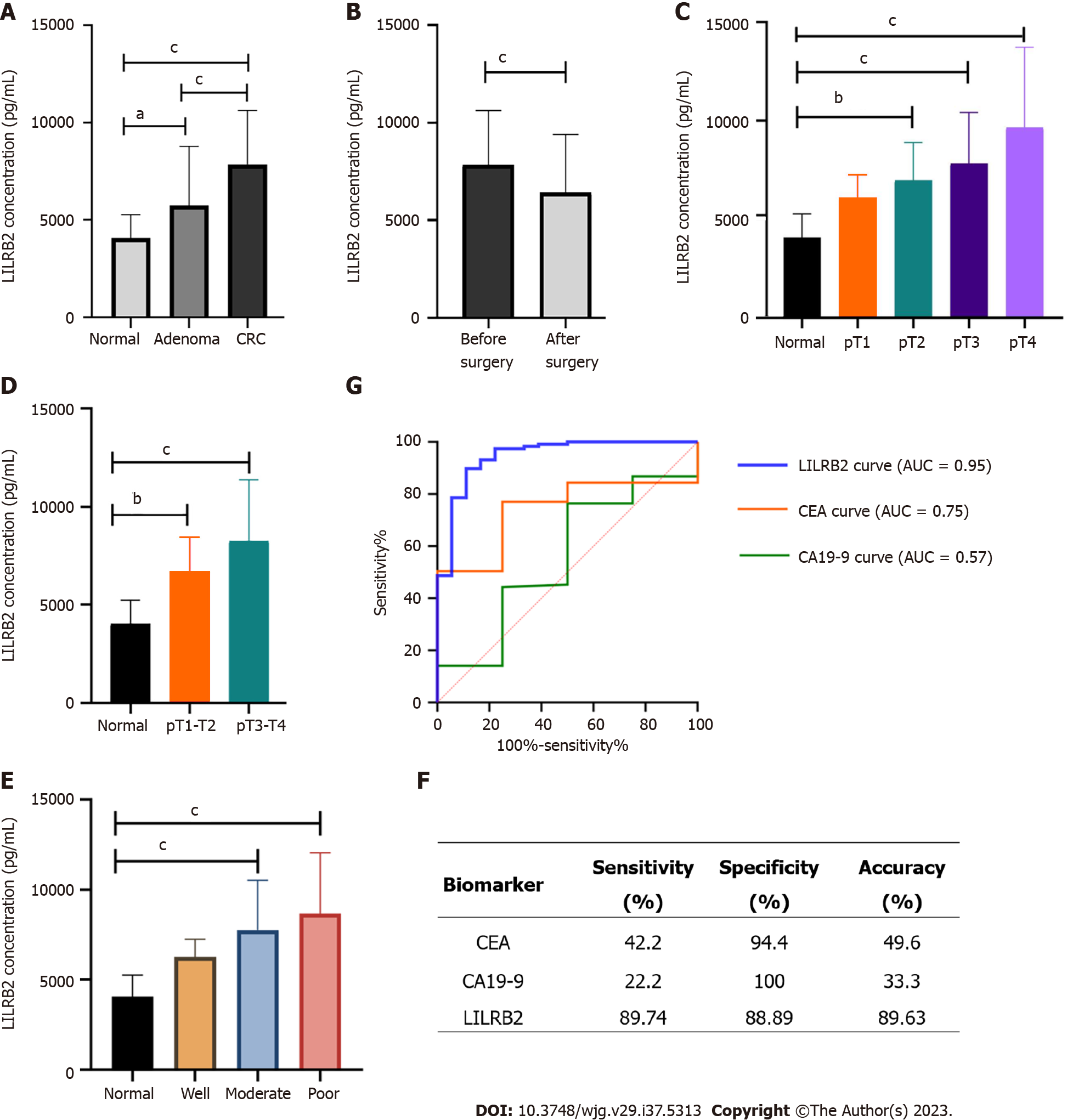
Comparison of the differences in serum LILRB2 concentrations among CRC patients, adenoma patients and healthy controls showed that serum LILRB2 concentrations were not identical in the three groups, and the differences were statistically significant (H = 75.25, P < 0.001). The Bonferroni method was used to correct for significance levels, and a two-by-two comparison showed that serum LILRB2 concentrations in patients with CRC (7074.69 pg/mL vs 3931.33 pg/mL, adjusted P < 0.001) and in adenoma patients (4855.88 pg/mL vs 3931.33 pg/mL, adjusted P = 0.036) were significantly higher than those in healthy controls (Figure 4A). The serum LILRB2 concentration in CRC was again significantly higher than that in the adenoma group (7074.69 pg/mL vs 4855.88 pg/mL, adjusted P < 0.001). When compared before and after CRC radical surgery, postoperative serum LILRB2 concentrations were significantly lower than preoperative concentrations (5665.30 pg/mL vs 7074.69 pg/mL, adjusted P < 0.001) (Figure 4B), and this result was consistent with the analysis of 50 paired serum samples (5431.44 pg/mL vs 6579.17 pg/mL, P = 0.002).
Further stratified analysis of CRC patients showed significant differences in serum LILRB2 concentrations between tumours with different infiltration depths (H = 42.13, P < 0.001). Compared with those in healthy controls, serum LILRB2 concentrations at pT2 (adjusted P = 0.004), pT3 (adjusted P < 0.001) and pT4 (adjusted P < 0.001) were significantly higher (Figure 4C). LILRB2 concentrations were significantly higher in pT1-T2 stage (adjusted P = 0.002) and pT3-T4 stage (adjusted P < 0.001) patients than in healthy controls (Figure 4D). There was also a significant difference between samples with different degrees of differentiation (H = 38.525, P < 0.001), with significantly higher serum LILRB2 concentrations in both the poor differentiation (adjusted P < 0.001) and moderate differentiation groups (adjusted P < 0.001) than in the healthy controls (Figure 4E).
The recommended cut-off value of serum LILRB2 concentration for screening CRC was 5256 pg/mL, with a sensitivity of 89.74% (105/117), a specificity of 88.89% (16/18), an accuracy rate of 89.63% (121/135), an area under the curve (AUC) of 0.95 (0.89, 1.00) and P < 0.0001 (Figure 4F). The diagnostic efficacy of LILRB2 was superior to that of the traditional serum markers CEA and CA19-9 (Figure 4G).
The high morbidity and mortality of CRC has created a more urgent clinical need for novel therapeutic targets and screening biomarkers. Preliminary high-throughput proteomics research identified LILRB2 as a signature protein for CRC. In this study, IHC was used to confirm that LILRB2 protein was significantly highly expressed in CRC and that increased LILRB2 protein expression was significantly associated with clinicopathological features such as poor to moderate degree of differentiation, lymph node metastasis, advanced TNM stage and poor prognosis. ANGPTL2, as the ligand for LILRB2, was significantly highly expressed in CRC tissues and synergistically overexpressed with LILRB2 at both the mRNA and protein levels, suggesting that their overexpression and interaction are associated with CRC progression. In addition, serum LILRB2 concentrations increased in healthy controls, adenoma patients and CRC patients sequentially with statistically significant differences; the sensitivity for the diagnosis of CRC was 89.74%, the specificity was 88.89%, and the AUC value was 0.95, and these results were superior to those of traditional CEA and CA19-9 in terms of diagnostic efficacy. Therefore, we believe that LILRB2 protein can be used as a potential therapeutic target and noninvasive screening biomarker for CRC; the biomarker could facilitate early screening and precise treatment and improve the prognosis of CRC patients.
At present, there are relatively few basic experimental or clinical studies on the relevance of LILRB2 to CRC. One study explored the expression of LILRB2 in human normal colorectal mucosal cells and various CRC cell lines, and the results showed that LILRB2 was significantly highly expressed in CRC cell lines, and the malignant biological behaviour of cancer cells was inhibited after blocking LILRB2, suggesting that LILRB2 is involved in and regulates the proliferation, migration and invasion of CRC cells and promotes cancer development[14,15]. The results of this cellular assay complement and validate the results of our clinical study. In addition to the basal cell assay, another study analysed clinical samples from CRC patients, but unlike our study using IHC, the researchers prepared cell suspensions from frozen CRC tissues and analysed the immune checkpoint protein LILRB2 by flow cytometry; the results showed that LILRB2 expression was associated with distant lymph node metastasis, advanced stage, and shorter survival and that LILRB2 overexpression may be an independent prognostic risk factor for CRC patients[15]. This study used flow cytometry analysis, and the study technique is innovative, but because flow cytometry requires fresher of tissue samples, prolonged cryopreservation may affect the accuracy of LILRB2 expression detection by flow cytometry. Our used traditional immunohistochemical staining, and the findings with the two study methods corroborated each other. Ultimately, the findings further supported that LILRB2 promotes colorectal carcinogenesis and development.
Since the discovery of the LILRB2 receptor, ANGPTLs have lost their designation as “orphan ligands”; the LILRB2 receptor interacts with the highest affinity with ANGPTL2 to support the development of a variety of malignancies, such as CRC, acute myeloid leukaemia (AML)[12] and NSCLC[13]. The present study confirmed the synergistic expression of LILRB2 and ANGPTL2 in CRC: There was a significant positive correlation between their expression at both the mRNA and protein levels and a significant association with clinicopathological parameters, suggesting that LILRB2 receptor and ANGPTL2 ligand interactions are closely associated with the development of CRC. A study analysing more than 9000 human leukaemia samples revealed that LILRB2 mRNA levels could be elevated several-fold in human AML cells. In a mouse leukaemia model, defects in the mouse immunoglobulin-like receptor (PirB), a homologue of human LILRB2, led to increased differentiation of leukaemic stem cells and significant downregulation of the expression of many pro-oncogenes, suggesting that PirB promotes leukaemia development[10]. Another study further indicated that the LILRB2 receptor accelerated leukaemia development by binding to overexpressed ANGPTL2, and the binding of both induced activation of tyrosine phosphatases SHP-1/SHP-2 and calmodulin-dependent protein kinase downstream of iTIMs, stimulating leukaemia development, and suggested that targeting ANGPTL2 ligands could serve as a certain type of AML as a potential disruption strategy[12,16]. LILRB2 is significantly highly expressed in NSCLC tissues and correlates with a poor prognosis[17]. There have been corresponding advances in the study of the mechanisms by which LILRB2 promotes NSCLC development and immunotherapy tolerance, which are expected to bring new therapeutic opportunities to NSCLC patients. On the one hand, tumour cell-derived ANGPTL2 overexpression, by acting on LILRB2 receptors, promotes increased tumour angiogenesis, epithelial mesenchymal transition, reduced intercellular adhesion, and enhanced cell motility, thus conferring high invasive and metastatic potential to tumour cells[18]. On the other hand, in NSCLC cells, activation of epidermal growth factor receptor-protein kinase B and extracellular signal-related kinases 1 and 2 signalling induces LILRB2 production, and LILRB2 overexpression reprograms the TME, recruits tumour-associated macrophages of the M2-like phenotype, inhibits dendritic cell maturation and antigen presentation, and promotes the progression of NSCLC[13]. In addition, LILRB2 expression was also significantly increased in hepatocellular carcinoma and breast cancer, but the underlying mechanisms by which LILRB2 regulates the progression of hepatocellular carcinoma and breast cancer need to be further investigated[19,20].
In the last three years, several LILRB-targeted therapies have entered clinical trials, but most are in phase I and phase II clinical trials. JTX-8064 is a specific antibody targeting LILRB2 that exerts antitumour effects by blocking the binding of the receptor to MHC-I class molecules, ANGPTLs and other relevant ligands in the TME, and its combination therapy with the PD-1 inhibitor pablizumab has also been conducted in clinical trials[21]. IO-108 is a novel inhibitory antibody that specifically binds LILRB2 with high affinity and blocks the binding of the receptor to ligands in the TME while reprogramming immunosuppressed myeloid cells to exhibit a proinflammatory phenotype, thereby enhancing the antitumour effects of both intrinsic and adaptive immunity[22]. In addition to LILRB2, the targeting of other LILRB family members has been a hot topic of research in recent years. For example, BND-22, a new class of humanized immunoglobulin G4 monoclonal antibodies targeting the LILRB1 receptor, can be targeted for the treatment of solid tumours; this blocks immunosuppression caused by the binding of the LILRB1 receptor and HLA-G ligand, which in turn activates T-cell-mediated innate and adaptive immunity and acts as a tumour killer[23,24]. IO-202 is a monoclonal antibody targeting the inhibition of LILRB4 for the indications of AML and chronic granulocytic leukaemia and has been granted orphan drug status by the United States Food and Drug Administration for the treatment of AML[25]. In terms of the latest progress of several clinical trials, the indications are broad, and no drugs targeting LILRBs have had breakthrough therapeutic effects in tumours; the present study provides a theoretical basis for assessing LILRB2 expression in CRC to guide immune checkpoint blockade therapy to achieve early screening and precision medicine.
Based on this study, we proposed for the first time that serum LILRB2 concentration could be used as a noninvasive screening biomarker for CRC with better diagnostic efficacy than traditional serum tumour markers to facilitate early diagnosis and treatment of CRC[26,27]. We analysed the source of serum LILRB2, since LILRB2 molecules are expressed on the surface of myeloid cells and tumour cells, and we hypothesized that serum LILRB2 may originate from circulating exfoliated cells of CRC, inflammatory cells or immune cells associated with CRC. Postoperative serum LILRB2 concentrations were significantly lower than preoperative serum concentrations in CRC, indicating a strong association between serum LILRB2 source and CRC; to further verify the relationship, a comparison of paired serum from 50 patients before and after surgery was performed and revealed that serum LILRB2 concentrations were significantly lower after surgery than before surgery, confirming that serum LILRB2 level is associated with tumour burden in CRC. It was hypothesized that serum LILRB2 might originate from circulating tumour cells shed in the blood and that the decreased LILRB2 concentration is associated with a reduced CRC cell load in the blood after surgery. Another source is hypothesized to be CRC-associated immune cells because tumorigenesis is closely related to the chronic inflammatory state in the body and during tumour development, chemotaxis and infiltration of myeloid cells, including neutrophils, dendritic cells, and tumour-associated macrophages, which are common cell types with LILRB2 expression[28,29]. There are no relevant studies on the source of serum LILRB2 globally, this study can only make reasonable speculations on its source. However, considering the half-life of protein, the time is not long enough to detect the serum LILRB2 concentration only 24 h after operation, and this is also not long enough for the immune cells to disappear gradually from circulation. Therefore, follow-up after the surgical removal, including measurements of the serum LILRB2 concentration, circulating tumor cell load and immune cell infiltration, is necessary. It is more meaningful that the serum LILRB2 decreases after 2 wk or longer of follow-up and increases when the tumor progresses or recurs.
There are advantages but also limitations to this study. The advantages are as follows: First, this study is based on the results of preliminary high-throughput proteomics screening and further protein expression validation and correlation analysis, with a solid prior experimental foundation, rather than through mere database mining. Second, this study confirms that LILRB2 can be a novel therapeutic target for CRC with high potential for clinical translation, providing new therapeutic opportunities for CRC. Third, this study is the first to suggest that ELISA of serum LILRB2 concentration can be used as a noninvasive screening method for CRC, with better diagnostic efficacy than traditional serum tumour markers. The shortcomings of this study are that further testing and analysis of circulating tumour cell load and immune cell infiltration in the blood of CRC patients are needed in the future to clarify the source of serum LILRB2. Furthermore, LILRB2 levels could be confounded by the presence of other tumors concurrently, such as NSCLC, hepatocellular and breast cancer, which needs to be excluded. In addition, although the results of this study corroborate those of previous basic studies, the single center study type and small sample size are still limitations of this study, and more efforts are needed in the future to eventually achieve the goal of translation to clinical treatment.
In conclusion, LILRB2 protein is significantly highly expressed in CRC tissues, and high LILRB2 protein expression is significantly correlated with tumour progression and a poor prognosis. The LILRB2 receptor is synergistically expressed with the ANGPTL2 ligand, and their binding and interaction are closely related to CRC development. Serum LILRB2 concentration showed higher diagnostic efficacy than traditional markers, highlight its potential as an innovative screening marker for CRC. Therefore, LILRB2 protein is a potential novel therapeutic target and noninvasive screening biomarker, which could be beneficial for early screening and precise treatment and is important for the development of new therapeutic strategies for CRC.
The incidence and mortality of colorectal cancer (CRC) have been rising continuously. CRC has become the second leading cause of cancer-related death worldwide, and the 5-year survival rate of patients with advanced CRC is below 20%.
Early screening and precise targeted therapy are very important to improve the prognosis of CRC patients. Therefore, identifying novel therapeutic targets and early screening biomarkers is urgent for clinical practice.
To explore whether leukocyte immunoglobulin-like receptor B2 (LILRB2) can be used as a potential therapeutic target and noninvasive screening biomarker for CRC.
On the basis of previous proteomics, immunohistochemical staining was used to verify the expression of LILRB2 protein and its ligand angiopoietin-like protein 2 (ANGPTL2) protein in CRC cancer tissues and paired paracarcinoma tissues, and to explore the association between their expression and the clinicopathological features. Enzyme-linked immunosorbent assay was used to detect serum LILRB2 concentration and explore the diagnostic efficacy for CRC.
LILRB2 protein is significantly overexpressed in CRC cancer tissues and is closely associated with peritumoral infiltration, distant metastasis and poor prognosis. LILRB2 may bind to the ligand ANGPTL2 protein, and the synergistic expression and interaction of the two proteins promotes CRC progression and metastasis. Serum LILRB2 concentration has high sensitivity, specificity and accuracy in screening CRC, which is better than traditional carcinoembryonic antigen and carbohydrate antigen 19-9.
Therefore, LILRB2 protein can be used as a potential novel therapeutic target and noninvasive screening biomarker.
Novel LILRB2 protein is beneficial for early screening and precise treatment, which provides new opportunities to improve the prognosis of CRC patients.
We thank all medical staff and patients who agreed to participate in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Herold Z, Hungary; Vega KJ, United States S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15299] [Article Influence: 3059.8] [Reference Citation Analysis (4)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64436] [Article Influence: 16109.0] [Reference Citation Analysis (176)] |
| 3. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1548] [Reference Citation Analysis (3)] |
| 4. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1425] [Article Influence: 356.3] [Reference Citation Analysis (0)] |
| 5. | Shen C, Tannenbaum D, Horn R, Rogers J, Eng C, Zhou S, Johnson B, Kopetz S, Morris V, Overman M, Parseghian C, Chang GJ, Lopez-Olivo MA, Kanwal R, Ellis LM, Dasari A. Overall Survival in Phase 3 Clinical Trials and the Surveillance, Epidemiology, and End Results Database in Patients With Metastatic Colorectal Cancer, 1986-2016: A Systematic Review. JAMA Netw Open. 2022;5:e2213588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Mani DR, Krug K, Zhang B, Satpathy S, Clauser KR, Ding L, Ellis M, Gillette MA, Carr SA. Cancer proteogenomics: current impact and future prospects. Nat Rev Cancer. 2022;22:298-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 7. | Li C, Sun YD, Yu GY, Cui JR, Lou Z, Zhang H, Huang Y, Bai CG, Deng LL, Liu P, Zheng K, Wang YH, Wang QQ, Li QR, Wu QQ, Liu Q, Shyr Y, Li YX, Chen LN, Wu JR, Zhang W, Zeng R. Integrated Omics of Metastatic Colorectal Cancer. Cancer Cell. 2020;38:734-747.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 8. | Wang Q, Zhou Y, Zhou G, Qin G, Tan C, Yin T, Zhao D, Yao S. Age-stratified proteomic characteristics and identification of promising precise clinical treatment targets of colorectal cancer. J Proteomics. 2023;277:104863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 9. | Chen HM, van der Touw W, Wang YS, Kang K, Mai S, Zhang J, Alsina-Beauchamp D, Duty JA, Mungamuri SK, Zhang B, Moran T, Flavell R, Aaronson S, Hu HM, Arase H, Ramanathan S, Flores R, Pan PY, Chen SH. Blocking immunoinhibitory receptor LILRB2 reprograms tumor-associated myeloid cells and promotes antitumor immunity. J Clin Invest. 2018;128:5647-5662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 10. | Zheng J, Umikawa M, Cui C, Li J, Chen X, Zhang C, Huynh H, Kang X, Silvany R, Wan X, Ye J, Cantó AP, Chen SH, Wang HY, Ward ES, Zhang CC. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485:656-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Deng M, Lu Z, Zheng J, Wan X, Chen X, Hirayasu K, Sun H, Lam Y, Chen L, Wang Q, Song C, Huang N, Gao GF, Jiang Y, Arase H, Zhang CC. A motif in LILRB2 critical for Angptl2 binding and activation. Blood. 2014;124:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Kang X, Cui C, Wang C, Wu G, Chen H, Lu Z, Chen X, Wang L, Huang J, Geng H, Zhao M, Chen Z, Müschen M, Wang HY, Zhang CC. CAMKs support development of acute myeloid leukemia. J Hematol Oncol. 2018;11:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Chen X, Gao A, Zhang F, Yang Z, Wang S, Fang Y, Li J, Wang J, Shi W, Wang L, Zheng Y, Sun Y. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation. Theranostics. 2021;11:3392-3416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 14. | Cai Z, Wang L, Han Y, Gao W, Wei X, Gong R, Zhu M, Sun Y, Yu S. Immunoglobulinlike transcript 4 and human leukocyte antigenG interaction promotes the progression of human colorectal cancer. Int J Oncol. 2019;54:1943-1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Chen QY, Chen YX, Han QY, Zhang JG, Zhou WJ, Zhang X, Ye YH, Yan WH, Lin A. Prognostic Significance of Immune Checkpoints HLA-G/ILT-2/4 and PD-L1 in Colorectal Cancer. Front Immunol. 2021;12:679090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Huang D, Sun G, Hao X, He X, Zheng Z, Chen C, Yu Z, Xie L, Ma S, Liu L, Zhou BO, Cheng H, Zheng J, Cheng T. ANGPTL2-containing small extracellular vesicles from vascular endothelial cells accelerate leukemia progression. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Zhang P, Guo X, Li J, Yu S, Wang L, Jiang G, Yang D, Wei Z, Zhang N, Liu J, Sun Y. Immunoglobulin-like transcript 4 promotes tumor progression and metastasis and up-regulates VEGF-C expression via ERK signaling pathway in non-small cell lung cancer. Oncotarget. 2015;6:13550-13563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Endo M, Nakano M, Kadomatsu T, Fukuhara S, Kuroda H, Mikami S, Hato T, Aoi J, Horiguchi H, Miyata K, Odagiri H, Masuda T, Harada M, Horio H, Hishima T, Nomori H, Ito T, Yamamoto Y, Minami T, Okada S, Takahashi T, Mochizuki N, Iwase H, Oike Y. Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 2012;72:1784-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Li X, Wei X, Xu H, Sha Z, Gao A, Sun Y, Li J, Xu L. Expression of leukocyte immunoglobulin-like receptor B2 in hepatocellular carcinoma and its clinical significance. J Cancer Res Ther. 2018;14:1655-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Liu J, Wang L, Gao W, Li L, Cui X, Yang H, Lin W, Dang Q, Zhang N, Sun Y. Inhibitory receptor immunoglobulin-like transcript 4 was highly expressed in primary ductal and lobular breast cancer and significantly correlated with IL-10. Diagn Pathol. 2014;9:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Umiker B, Hashambhoy-Ramsay Y, Smith J, Rahman T, Mueller A, Davidson R, Meyer C, Patankar G, Alam MM, Jaffe S, Krukenberg K, Goodman A, Spaulding V, Priess M, Dhaneshwar A, Wong M, Diiorio A, O'Malley K, McGrath L, Willer M, Pepper L, Gostissa M, Kis-Toth K, Wiederschain D, Cohen H, Shaffer DR. Inhibition of LILRB2 by a Novel Blocking Antibody Designed to Reprogram Immunosuppressive Macrophages to Drive T-Cell Activation in Tumors. Mol Cancer Ther. 2023;22:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 22. | Taylor MH, Patel MR, Powderly JD, Woodard P, Chung L, Tian H, Hong X, Hong K, Valencia D, Huang T, Schebye XM, Liao C, Naing A. Abstract CT040: A first-in-human phase 1 trial of IO-108, an antagonist antibody targeting LILRB2 (ILT4), as monotherapy and in combination with pembrolizumab in adult patients with advanced relapsed or refractory solid tumors: Dose escalation study. Cancer Res. 2023;83:CT040. [DOI] [Full Text] |
| 23. | Mandel I, Haves Ziv D, Goldshtein I, Peretz T, Alishekevitz D, Fridman Dror A, Hakim M, Hashmueli S, Friedman I, Sapir Y, Greco R, Qu H, Nestle F, Wiederschain D, Pao L, Sharma S, Ben Moshe T. BND-22, a first-in-class humanized ILT2-blocking antibody, promotes antitumor immunity and tumor regression. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 24. | Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, George BM, Markovic M, Ring NG, Tsai JM, McKenna KM, Ho PY, Cheng RZ, Chen JY, Barkal LJ, Ring AM, Weissman IL, Maute RL. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 426] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 25. | Anami Y, Deng M, Gui X, Yamaguchi A, Yamazaki CM, Zhang N, Zhang CC, An Z, Tsuchikama K. LILRB4-targeting Antibody-Drug Conjugates for the Treatment of Acute Myeloid Leukemia. Mol Cancer Ther. 2020;19:2330-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep. 2018;8:2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 27. | Chen F, Dai X, Zhou CC, Li KX, Zhang YJ, Lou XY, Zhu YM, Sun YL, Peng BX, Cui W. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut. 2022;71:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 173] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 28. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11271] [Article Influence: 490.0] [Reference Citation Analysis (2)] |
| 29. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1623] [Article Influence: 162.3] [Reference Citation Analysis (0)] |