Published online Sep 28, 2023. doi: 10.3748/wjg.v29.i36.5198
Peer-review started: April 25, 2023
First decision: July 9, 2023
Revised: July 23, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 28, 2023
Processing time: 148 Days and 9.7 Hours
Despite advances in cross-sectional imaging and endoscopic technology, bile duct strictures remain a challenging clinical entity. It is crucial to make an early determination of benign or malignant nature of biliary strictures. Early diagnosis not only helps with further management but also minimizes mortality and morbidity associated with delayed diagnosis. Conventional imaging and endoscopic techniques, particularly endoscopic retrograde cholangiopancreatography (ERCP) and tissue sampling techniques play a key in establishing a diagnosis. Indeterminate biliary strictures (IDBSs) have no definite mass on imaging or absolute histopathological diagnosis and often warrant utilization of multiple diagnostics to ascertain an etiology. In this review, we discuss possible etiologies, clinical presentation, diagnosis, and management of IDBSs. Based on available data and expert opinion, we depict an evidence based diagnostic algorithm for management of IDBSs. Areas of focus include use of traditional tissue sampling techniques such as ERCP with brush cytology, intraductal biopsies, fluorescence in situ hybridization and flow cytometry. We also describe the role of endoscopic ultrasound (EUS)-guided fine needle aspiration and biopsies, cholangioscopy, confocal laser endomicroscopy, and intraductal EUS in management of IDBSs.
Core Tip: Despite advances in imaging and endoscopy, bile duct strictures remain challenging. Timely detection of malignant strictures is crucial. Conventional techniques play a crucial role in diagnosis, but indeterminate biliary strictures (IDBSs) require multiple diagnostic tools. This review discusses etiology, diagnosis, and workup of IDBSs, presenting an evidence-based algorithm focusing on traditional tissue sampling techniques and innovative technologies. The importance of future research is emphasized.
- Citation: Yadlapati S, Mulki R, Sánchez-Luna SA, Ahmed AM, Kyanam Kabir Baig KR, Peter S. Clinical approach to indeterminate biliary strictures: Clinical presentation, diagnosis, and workup. World J Gastroenterol 2023; 29(36): 5198-5210
- URL: https://www.wjgnet.com/1007-9327/full/v29/i36/5198.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i36.5198
Bile duct strictures are commonly encountered in clinical practice and pose a diagnostic challenge to endoscopists. The diagnostic difficulty lies in differentiating malignant strictures from benign strictures. Failure to diagnose malignant strictures or subjecting patients with benign strictures to unwarranted surgery can result in increased morbidity and mortality. Biliary strictures are characterized as indeterminate when no overt mass or differentiating characteristics are seen on cross-sectional imaging or after performing endoscopic retrograde cholangiopancreatography (ERCP) with standard diagnostic tools[1]. At present, up to 20% of biliary strictures are indeterminate. These strictures include both benign and malignant conditions. Biliary strictures may result from intrinsic biliary pathology as well as from extrinsic compression or infiltration from benign or malignant lesions.
Based on previous observational studies, up to one-third of biliary strictures are benign, and two-thirds are malignant in nature[2]. Inflammatory strictures, particularly those related to primary sclerosing cholangitis (PSC), often mimic malignant strictures. Close to 25% of suspected neoplastic strictures are ultimately found to be benign after surgical intervention[2]. Treatment of benign strictures typically involves medical and endoscopic therapy, with only a few patients requiring surgical intervention. Alternatively, malignant strictures often require surgical resection or palliative biliary drainage.
Establishing histological diagnosis is key in determining further management of biliary strictures. ERCP with traditional diagnostics such as brush cytology and forceps biopsies have limited sensitivity in establishing a definitive diagnosis[3]. In some cases, it may be necessary to use additional diagnostic tools such as endoscopic ultrasound (EUS)-guided sampling or cholangioscopy to improve diagnostic yield. Our review aims to explore potential causes of indeterminate biliary strictures (IDBSs) and provide guidance on how to use diagnostic testing and technologies to confirm a diagnosis and plan further management. Based on the available data and expert opinion, we depict an evidence based diagnostic algorithm for management of IDBSs.
Clinical presentation in these patients is often variable. Strictures may be found incidentally on cross sectional imaging in asymptomatic patients undergoing testing for other reasons or in those who are symptomatic with abdominal pain or jaundice. Clinical presentation can range from lack of symptoms to fulminant cholangitis or sepsis. A detailed history can help determine underlying etiology to some extent. Painless jaundice and rapid weight loss raise suspicion of underlying malignancy, whereas a more progressive course is seen in those with benign strictures.
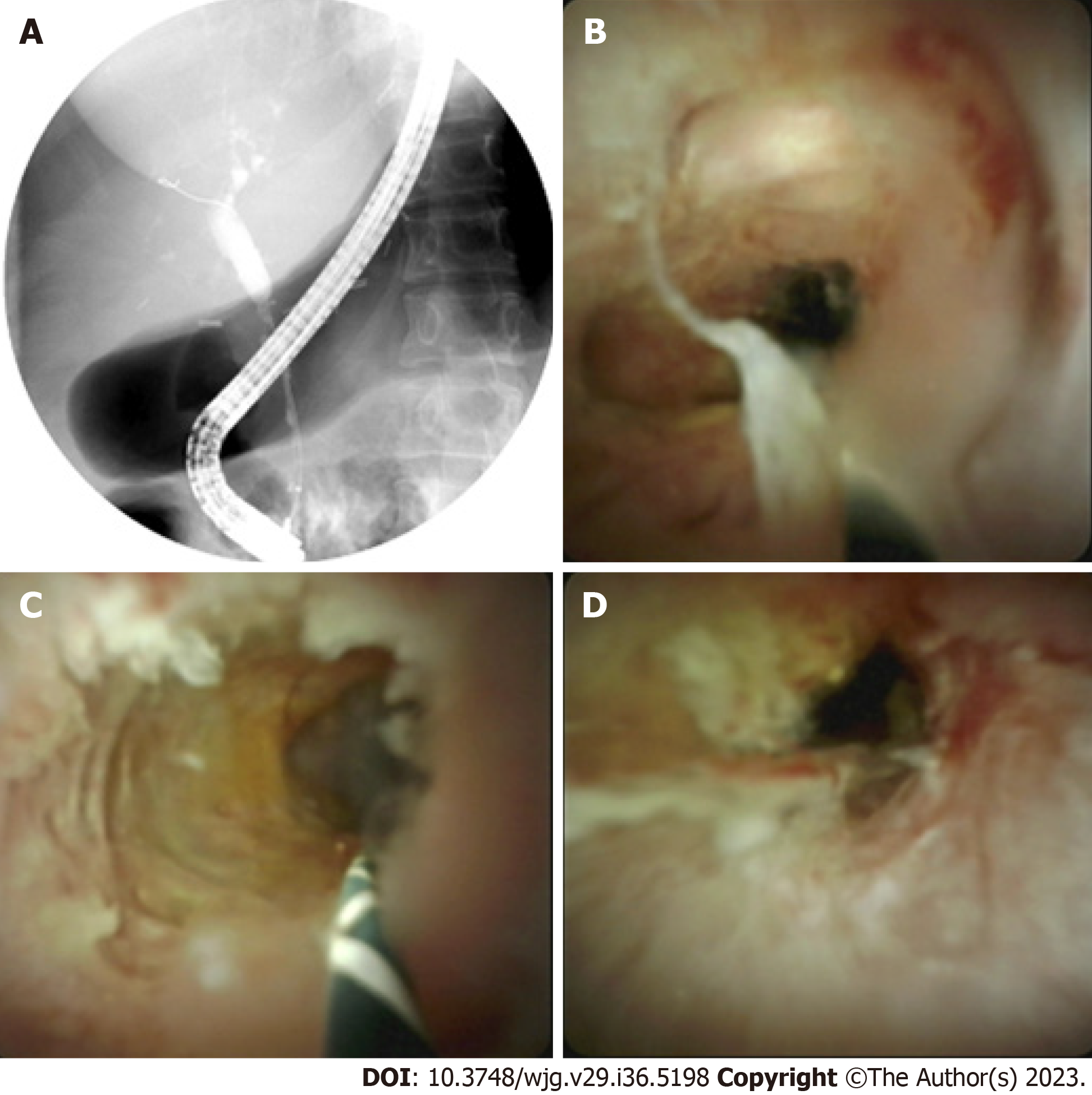
Biliary strictures can have benign or malignant origins and may arise from either intraductal pathology or external compression on the biliary tract (Table 1). The most frequent causes of benign biliary strictures include postoperative strictures and those associated with chronic pancreatitis. Postoperative strictures can develop after cholecystectomy, bile duct surgery, or liver transplant (Figure 1). These strictures may be caused by several factors: ischemic injury, mechanical injury, anastomotic leakage, and postoperative infections causing inflammation, underlying autoimmune disease, or malignancy. Chronic inflammation and fibrosis of the pancreatic ducts (PDs) in chronic pancreatitis can cause scarring and narrowing of the adjacent bile ducts, leading to the development of biliary strictures. In some cases, chronic inflammation may result in extrinsic compression of bile ducts due to adhesions and fibrosis.
| Benign | Malignant |
| Postoperative or iatrogenic strictures: After cholecystectomy, liver transplantation or surgical procedures involving pancreas. Local cancer treatment including radiation therapy, chemoembolization, and microwave ablation | Pancreatic adenocarcinoma |
| Chronic pancreatitis | Cholangiocarcinoma |
| Primary sclerosing cholangitis | Gallbladder malignancy |
| Trauma | Hepatocellular carcinoma |
| Infection: Recurrent pyogenic cholangitis, AIDS cholangiopathy | Ampullary carcinoma |
| Autoimmune: IgG4 related sclerosing cholangitis, sarcoidosis | Metastatic tumors or perihilar malignant nodes (colon cancer, breast cancer, renal cell cancer, squamous cell carcinoma) |
| Vascular: Ischemia, portal hypertensive biliopathy | Less common: Lymphomas or neuroendocrine tumors |
| Primary biliary cirrhosis | |
| Rarely COVID-19 cholangiopathy, gallstones, inflammatory bowel disease or medications |
Other causes of benign strictures include systemic or inflammatory diseases such as acquired immunodeficiency syndrome, PSC or IgG4 cholangiopathy. PSC is an inflammatory disorder characterized by focal biliary strictures and dilations involving both intra- and extrahepatic bile ducts. Close to 13% of patients with PSC eventually develop cholangiocarcinoma (CCA). Biliary strictures in PSC can be managed with endoscopic therapy. Oncological treatment is warranted in those with CCA and this highlights the importance of endoscopic tissue acquisition for management of biliary stricture[3]. IgG4 disease is a fibroinflammatory condition characterized by the infiltration of IgG4 plasma cells into the bile duct wall and pancreas, leading to the development of sclerosing cholangitis and autoimmune pancreatitis.
Malignant biliary strictures are commonly associated with pancreatic cancer, CCA, gallbladder cancer, and metastatic cancer. Cross-sectional imaging can detect a mass lesion involving surrounding structures, such as the pancreas, liver, gallbladder, or regional lymph nodes. In some cases of malignant biliary obstruction, a stricture may be visible without an obvious mass in the early stages of the disease. Although rare, malignant biliary strictures can also be caused by biliary lymphoma, sarcoma, or intraductal papillary neoplasm of the bile duct. Biliary strictures in the setting of malignancy occur due to tumor growth and invasion into bile ducts or surrounding structures, inflammation and scarring, or metastases.
Initial testing often involves a combination of laboratory markers and cross-sectional imaging. This is followed by endoscopic procedures for tissue sampling. Although many tests are available to determine the nature of biliary strictures, no single test is considered absolute. A combination of tests may need to be utilized to evaluate indeterminate strictures. Serum markers play a limited role in detection and diagnosis of CCA and pancreatic adenocarcinoma. Carbohydrate antigen (CA) 19-9 is commonly used but has variable sensitivity and specificity. It is worth noting that CA 19-9 can also be elevated in certain benign conditions that lead to cholestasis, such as biliary tract inflammation, infection, or diabetes. Carcinoembryonic antigen was previously used for colorectal cancer diagnosis but has limited performance in CCA. Elevated IgG4 levels are associated with IgG4-related sclerosing cholangitis and can aid in diagnosis when used in conjunction with other tests. Other potential tumor markers, such as interleukin-6, transthyretin, mitochondrial-mediated apoptotic pathway-7, mucin-5AC, and miRNA-16 and 21, require further validation through larger studies to determine their clinical utility.
Transabdominal US is typically performed early in work up of biliary obstruction. US is highly sensitive for detecting biliary dilation however has limited ability to identify biliary strictures. Therefore, follow up cross sectional imaging is generally required. Transabdominal US is readily available in all institutions, cost-effective, easy to perform, and lacks exposure to radiation, which makes it an optimal screening test.
Computed tomography (CT) has a higher sensitivity for detecting biliary strictures and pancreaticobiliary malignancies. The sensitivity of CT in detecting malignant strictures is 40%–77%[4-6]. With the advent of enhanced detectors and contrast agents, the performance of CT scans has dramatically improved. In initial stages, CCA can present as a biliary stricture without an obvious mass. CT findings in such cases include a hypoenhancing biliary lesion during the arterial phase and hyperenhancement during the delayed phase. Several studies have concluded that multidetector CT is nearly 100% sensitive in detecting hilar malignancies, but it has low specificity (60%–80%) for identifying malignant versus biliary strictures.
Magnetic resonance imaging/magnetic resonance cholangiopancreatography (MRI/MRCP) can provide high-quality cholangiograms, making them an ideal choice for evaluating biliary strictures. The cholangiogram performed during MRCP is similar in diagnostic quality to that performed at the time of ERCP. MRCP can help determine the location and extent of a biliary stricture. A large meta-analysis looking at the performance found a sensitivity of 91% and specificity of 98% in determining the presence of biliary obstruction and ~98% combined sensitivity and specificity in the ability to detect the location of a biliary stricture. The sensitivity and specificity in the ability to delineate benign from malignant strictures were 88% and 95%, respectively[7]. Diffusion-weighted imaging allows for an improvement in the ability to determine the nature of biliary strictures. Compared to ERCP, this test eliminates several risks, including pancreatitis and cholangitis. However, it is not readily available, expensive, and does not allow for tissue sampling.
With the increasing availability of MRI, the diagnostic utility of ERCP in detecting strictures has significantly decreased. Nonetheless, ERCP continues to serve as a valuable diagnostic tool for tissue acquisition to determine the nature of these strictures. Cholangiogram performed after successful biliary cannulation is used to evaluate location, anatomy, and extent of strictures. Physical characteristics of a biliary stricture, although indicative, are often inadequate to definitively differentiate between malignant and benign etiologies. Double duct sign, dilation of common bile duct and PD, is more likely to be seen in malignancy. Malignant strictures are often long and irregular with abrupt cut off “shouldering” resulting in upstream biliary dilation (Figure 2A). However, benign biliary strictures often appear smoother[2]. Brush cytology and intraductal biopsies during ERCP are routinely performed to establish histological diagnosis. A recent meta-analysis by Navaneethan et al[8] showed that brush cytology and biopsies during ERCP had limited sensitivity of 45% and 48%, respectively, in establishing diagnosis of strictures. Combining both diagnostic modalities only increased sensitivity to 59% [95% confidence interval (CI): 54%–65%], however, specificity for confirming malignancy was close to 100% (95%CI: 90%–100%)[8]. In this regard, patients may need multiple ERCPs with brushings and biopsies to establish a conclusive diagnosis.
Several techniques have been proposed to enhance tissue acquisition during ERCP, such as the use of novel brushes, biliary stricture dilation followed by biopsies and brushing, and repetitive brushings. Pörner et al[9] have suggested that balloon dilation of the stricture before forceps biopsies can improve sensitivity from 40% to > 70% without increasing the complication rate. In another study by Sugimoto et al[10] post brushing biliary lavage fluid was obtained by injecting small amount of saline into bile duct. This increased cumulative sensitivity by > 24%. Submucosal tumor growth and presence of extrinsic malignancy with biliary involvement are difficult to detect with standard biliary brushings. Inadequate brushings or biopsy specimens are primary reason for nondiagnostic samples. At present wire-guided endobiliary forceps, free-hand biopsies, and endoscopic scrapers are available to obtain tissue. Free-hand biopsies are performed by using forceps to grasp the stricture and obtain a tissue sample, while wire-guided endobiliary biopsies use a wire guide to navigate to the stricture and obtain a tissue sample. There is no clear consensus on whether free-hand biopsies have a greater yield than wire-guided endobiliary biopsies. Some studies have suggested that free-hand biopsies may have a higher yield, while others have found no significant difference in yield between the two methods. The choice between the two techniques may depend on individual factors such as the location and characteristics of the stricture, as well as the expertise and experience of the endoscopist. Endoscopic devices with scraping loops have recently been proposed for sampling of biliary strictures. This type of device can be considered in institutions where cholangioscopy is unavailable. A recent study compared one endoscopic scraper device to cholangioscopy guided tissue acquisition. Sufficient samples were obtained in 87.5% of cases with endoscopic scraping loops compared to 90% with cholangioscopy forceps. This study described 83% sensitivity and 86% specificity with endoscopic scrapers compared to 90% sensitivity and specificity with samples obtained during cholangioscopy[11]. Endoscopic sphincterotomy was less frequently needed when sampling was done with endoscopic scrapers[11].
Fluorescence in situ hybridization (FISH) is a tissue-based diagnostic tool that uses fluorescent-labeled DNA probes to detect chromosomal abnormalities in cells. It has been found that about 80% of pancreaticobiliary malignancies exhibit abnormalities such as aneuploidy or polysomy of chromosomes[12]. FISH probes typically target chromosomes 3, 7, 9 and 17. FISH has shown a sensitivity of 89% and specificity of 97% for detecting malignancy, especially when combined with other techniques like biopsies and cytology[13,14]. However, false-positive results can occur in chronic inflammatory conditions. Flow cytometry has lower sensitivity (42%) and moderate specificity (77%) for detecting neoplastic cells in cytology samples[15]. Bile aspirate analysis for mutations and proteomic profiling may aid in distinguishing CCA from benign strictures, but further validation is required for routine use.
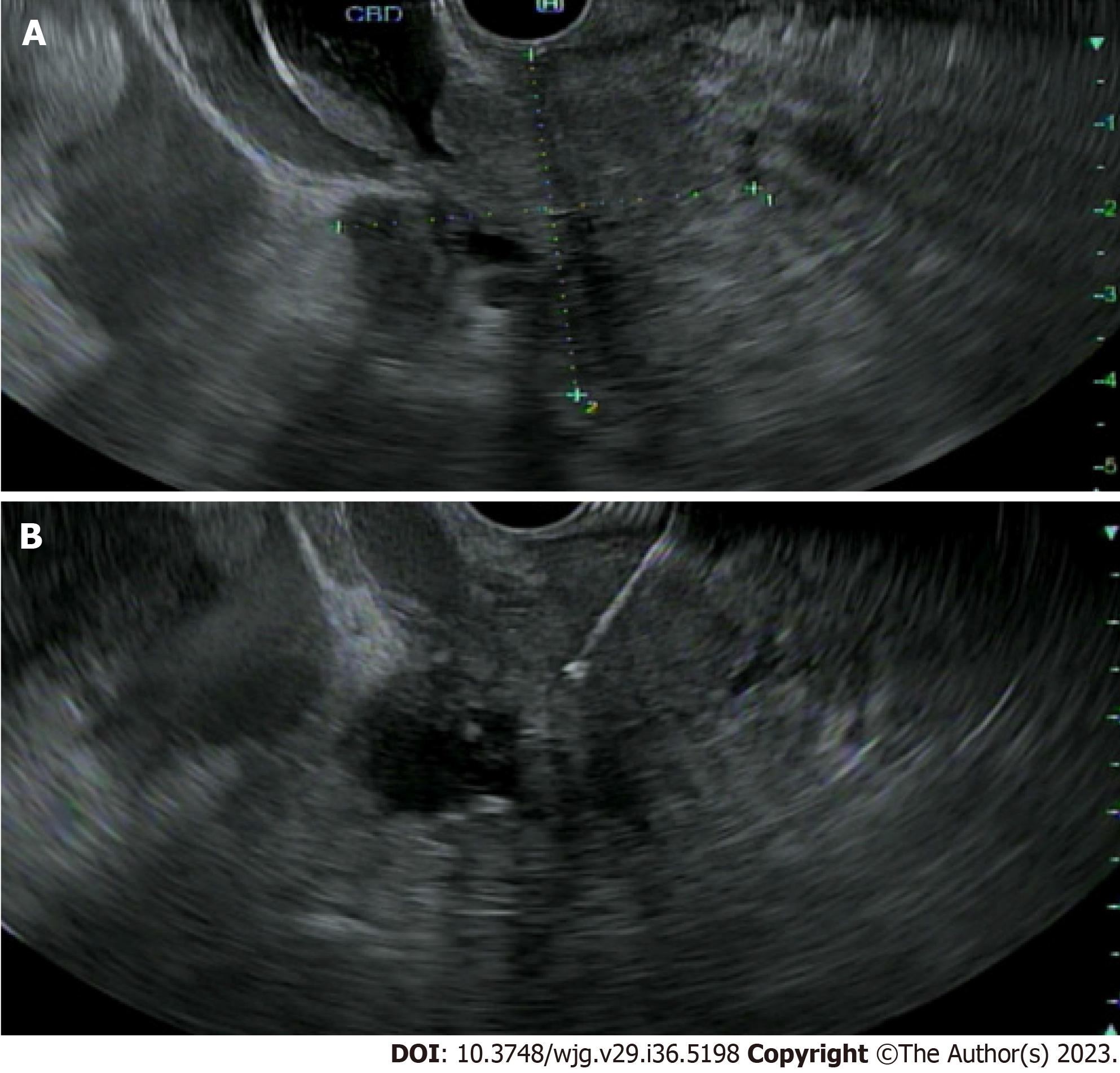
EUS can play a pivotal role in evaluation of IDBSs. EUS allows for high-resolution imaging of the pancreaticobiliary system and tissue acquisition by fine-needle aspiration and biopsy (FNA/FNB) at the same time (Figure 3). This has been proven to be safe and is considered a primary modality of tissue acquisition from biliary lesions. An overall sensitivity ranging from 47% to 87% has been reported in several studies looking at role of EUS-FNA in work up of hilar strictures[16-18]. EUS has some benefits over ERCP in determining etiology of extrahepatic biliary strictures. In a single center study, EUS-FNA was found to be superior to ERCP with brush cytology and transpapillary biopsies with a sensitivity of 93.8% versus 60% and accuracy of 94% versus 62% (P = 0.034)[19]. However, when ERCP and EUS were combined during a single session, the two procedures reached a sensitivity and accuracy of > 97%[19]. Zaheer et al[20] noted diagnostic accuracy of both procedures was > 90% for both benign and malignant strictures. Similarly, in a multicenter retrospective study of 263 patients with suspected malignant biliary strictures, combining EUS/ERCP had diagnostic sensitivity and accuracy of 85.8% and 87.1% respectively[21]. This approach has better outcomes for distal bile duct strictures and those > 15 mm[19]. No significant difference in combination procedure versus EUS-FNA alone was noted in patients with large biliary or pancreatic mass of ≥ 4 cm. One advantage of performing both procedures during same session is being able to better triage the need for ERCP in patients with biliary obstruction.
Lee et al[16] concluded that sonographic features could provide clues in determining etiology of indeterminate strictures. The presence of pancreatic mass or irregular bile duct wall is suggestive of malignancy. Bile duct wall thickness of ≥ 3 mm has a sensitivity and specificity close to 79%. EUS-FNA may have low sensitivity but is highly specific for diagnosis of malignancy. One drawback of EUS-FNA in diagnosis of CCA is concern for potential seeding along the needle tract. A small-scale study by Heimbach et al[22] revealed peritoneal metastasis was noted in 83% of patients with positive transperitoneal biopsies for CCA. Metastasis was not found in those with negative biopsies and only 8% of patients who did not undergo FNA had peritoneal metastasis. In contrast, no difference in overall survival was noted among patients who did or did not undergo EUS-FNA. Seeding after EUS-FNA of pancreatic adenocarcinoma is rare with an estimated incidence ~2%[23]. No large-scale studies are available to investigate EUS-FNA related peritoneal carcinomatosis in cases of CCA. Patients being considered for liver transplant should ideally not undergo EUS-FNA.
A high frequency (12–30 MHz) US probe is typically placed into extrahepatic bile duct during ERCP to perform intraductal US (IDUS). This allows for real time imaging of bile ducts and surrounding structures. Features suggestive of malignancy include hypoechoic mass or evidence of infiltration to adjacent structures, interruption of three-layer architecture of a bile duct, eccentric wall thickening, heterogenous or irregular margins, malignant appearing perihilar or periductal lymph nodes or periportal vascular invasion. A cohort study of 397 patients undergoing IDUS concluded that IDUS could differentiate benign from malignant biliary strictures with a sensitivity of 93%, specificity of 90%, and accuracy of ~91%[24]. Other studies have concluded ERCP with IDUS significantly improved diagnostic accuracy in identifying malignant strictures compared to ERCP alone. In addition, IDUS was also found to be superior in sensitivity (91% vs 76%), specificity (80% vs 75%), accuracy (89% vs 76%) and cancer staging (78% vs 54%) compared to EUS alone[25]. Limitations of IDUS include inability to obtain tissue to confirm histopathological diagnosis. Adverse events associated with IDUS include pancreatitis, bleeding, cholangitis, and perforation of the bile duct. It is also challenging to evaluate proximal biliary tree as advancing IDUS probe may be technically difficult.
Cholangioscopy was first introduced in 1976 and has undergone significant technological advancement since its inception[26]. Cholangioscopy allows for direct visualization and targeted biopsies of bile ducts. This technique failed to gain popularity a few decades ago due to poor reliability and need for two operators to manipulate the mother–baby endoscopies. Traditional peroral cholangioscopy requires insertion of a dedicated video-cholangioscope through an accessory channel of the duodenoscope and requires two skilled endoscopists to operate the system. However, with development of single operator cholangioscopy (SOC) systems, cholangioscopy has gained renewed interest. At present there are two SOC system: duodenoscope-assisted SOC called Spyglass and peroral direct cholangioscopy using an ultrathin gastroscope.
Chen et al[27] concluded that sensitivity and specificity of SOC in determining etiology of biliary strictures was 78% and 82% respectively, compared to 51% and 54% with ERCP alone. A meta-analysis conducted by Navaneethan et al[28] concluded that pooled sensitivity and specificity for detection of CCA using cholangioscopy was 60% and 98% respectively. Another meta-analysis performed by Korrapati et al[29] compared diagnostic accuracy of SOC based on visual images to histology and reported overall accuracy of 89% for making a phenotypic diagnosis and 79% for making a histopathological diagnosis. Similar results of higher diagnostic yield with visual impression compared to visually targeted biopsies was seen in multiple other studies[30,31]. This may be a result of sampling error at time of biopsy. Cholangioscopy has also been found useful to differentiate PSC related strictures from those of IgG-4 disease[32].
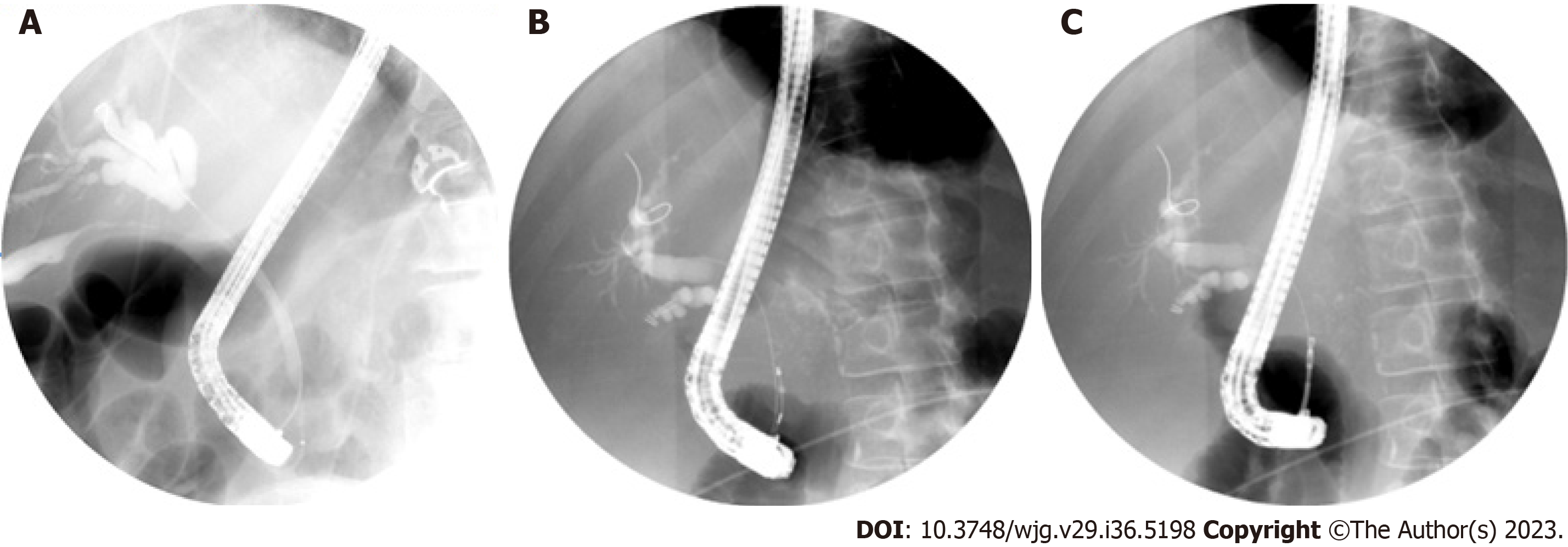
Cholangioscopy-guided biopsies improve diagnosis of IDBSs in patients with negative ERCP brushings and biopsies (Figure 2B and 2C). In two meta-analyses performed by Navaneethan et al[28] and Wen et al[33] pooled sensitivity and specificity of cholangioscopy-guided biopsies were 60% and 98%, and 74% and 98% respectively. Mean number of biopsies performed in these studies were 2–5. A recent prospective trial looking at rapid on-site evaluation (ROSE) of the biopsy specimen in these patients did not significantly alter diagnostic accuracy[34]. Two prior cost analysis studies have shown that cholangioscopy was more cost efficient for establishing CCA diagnosis compared to conventional ERCP[35,36]. One study showed a reduction in number of procedures by over 30% and cost by 5% when cholangioscopy was utilized[36].
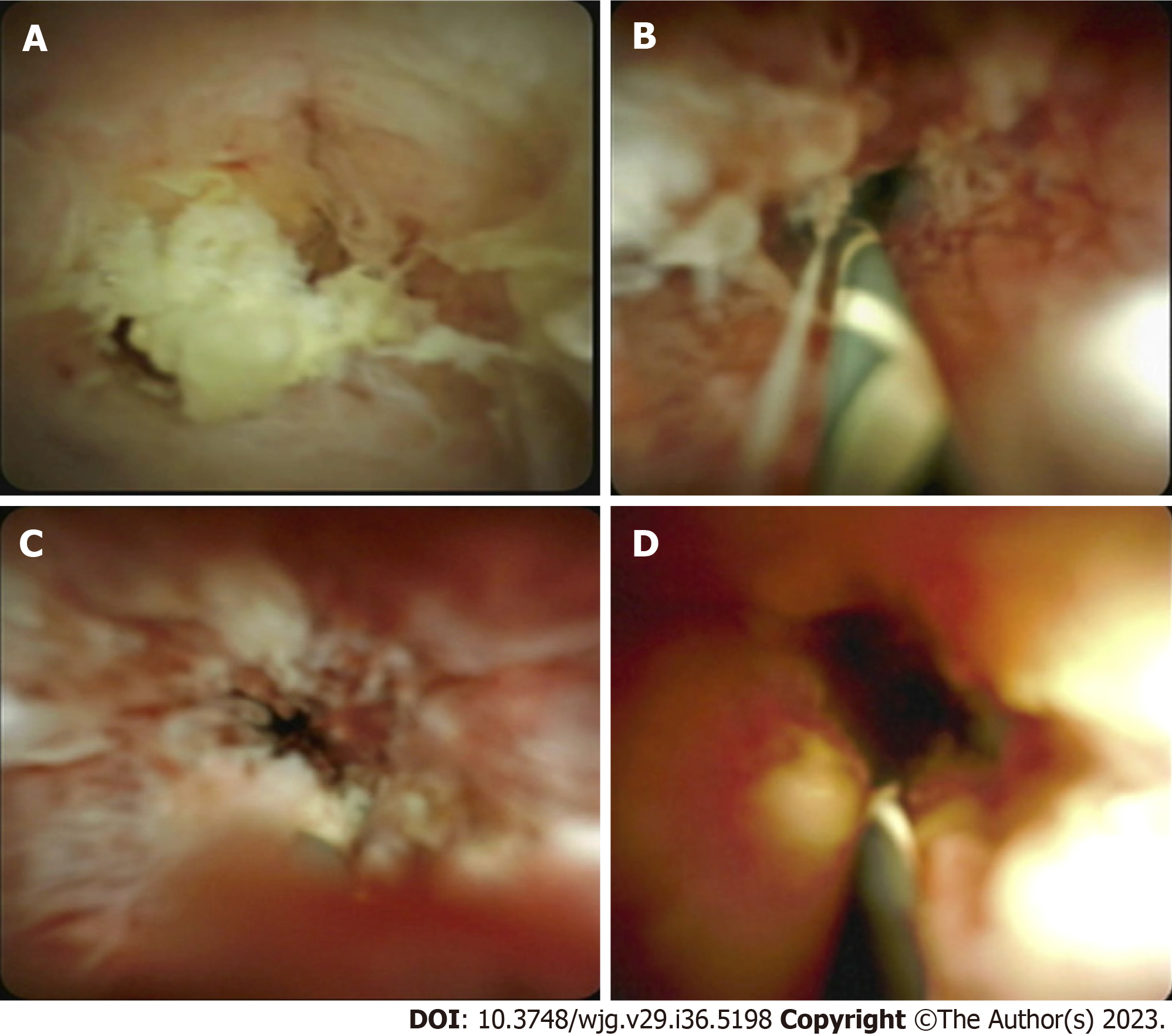
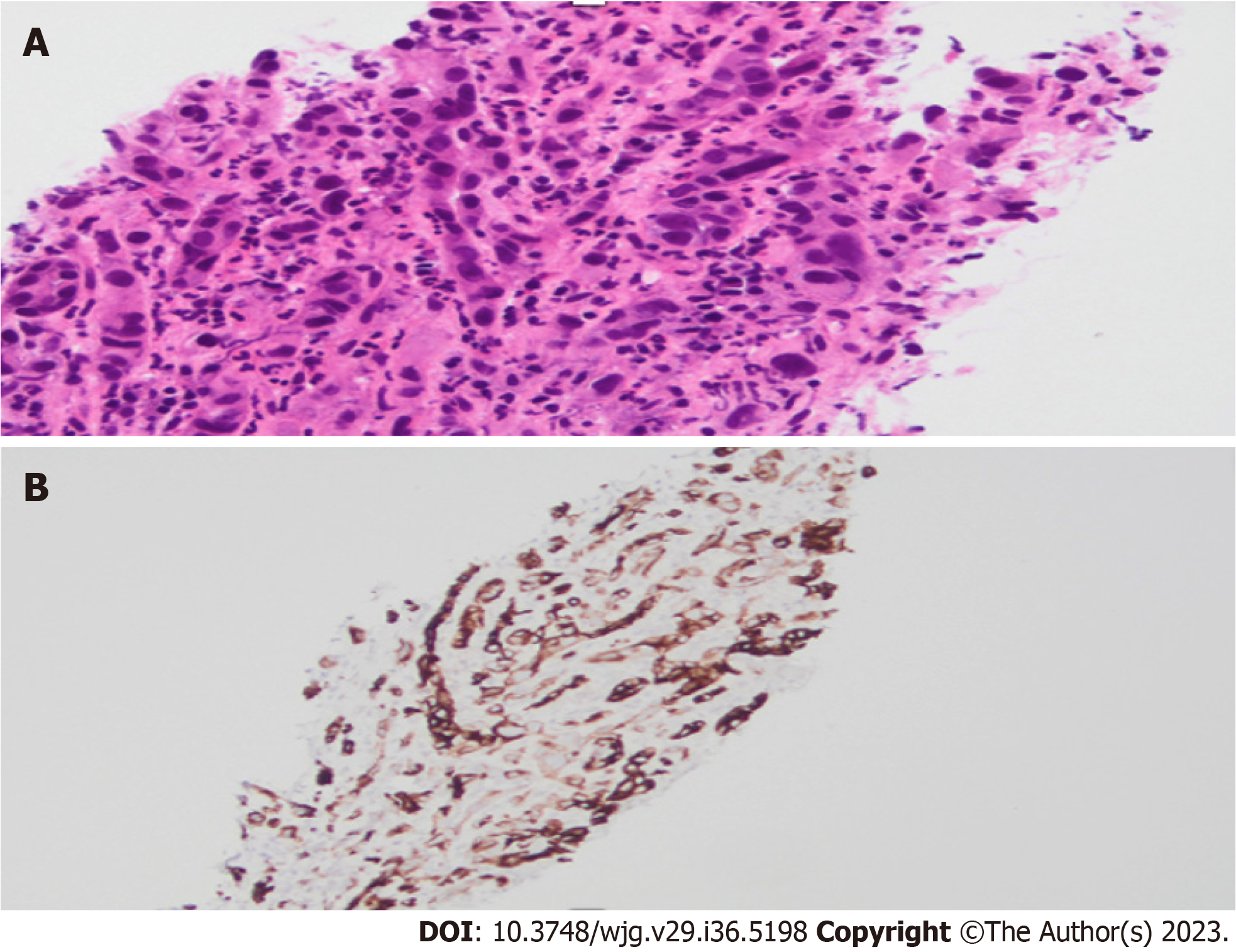
Interobserver variability is often noted when it comes to performing a visual diagnosis during cholangioscopy. This variability exists due to a lack of consensus on what constitutes a malignant stricture. The Mendoza criteria are a set of guidelines used to describe high-risk features during cholangioscopy, and these features have been found to be statistically associated with presence of malignancy. Based on this criteria, high risk features include presence of neovascularization or dilated tortuous vessels, irregular or nodular biliary mucosa, tumors or masses, irregular surface with ulcerated, infiltrative, or friable appearance (Figure 4). Biopsies obtained from malignant strictures often reveal malignant cells arranged in inflamed desmoplastic stroma; moreover, immunostaining for CK7 on the biopsy specimen exhibits positive results in tumor cells with appropriate control (Figure 5). Diagnostic accuracy using this method has been found to be close to 77%[37].
Limitations to the use of the Spyglass Visualization System include cost, specialized training requirements for endoscopists and technical staff, and associated risks. An extended biliary sphincterotomy is required to allow for cholangioscopy equipment to advance into biliary tree and this increases risk of cholangitis, pancreatitis, and bleeding. Sethi et al[38] reported an increase in overall complication rate to 7% with use of cholangioscopy compared with 2.9% with ERCP alone. Rate of cholangitis was significantly higher in patients undergoing cholangioscopy in this study. Similar data were also reported by Korrapati et al[29]. Risk of infection after cholangioscopy is attributed to bacterial accumulation in setting of biliary obstruction and disruption of microbial flora with introduction of cholangioscopy. For this reason, prophylactic antibiotics are usually given prior to the procedure. Cholangioscopy should therefore be used in conjunction to ERCP when conventional techniques fail to provide a diagnosis despite high suspicion for malignancy.
Confocal laser endomicroscopy (CLE) is an imaging technique that allows for real-time optical biopsies during ERCP. A contrast agent is typically injected intravenously, and the biliary tissue is imaged with a CLE probe. This technique results in specific patterns that correlate with histology and help differentiate between malignant and normal biliary mucosa. Miami classification is used to differentiate probe-based CLE visual findings as benign or malignant strictures. Presence of thick dark bands (> 40 m), thick white bands (> 20 m), dark clumps, epithelial strictures, villous glands, and contrast leakage can differentiate nature of strictures[39]. Paris classification is a newer classification which includes additional features like vascular congestion, dark glandular patterns, interglandular spaces and thickened reticular structures[40]. A multicenter study found that CLE provided higher diagnostic accuracy (90% vs 73%) for identifying malignant biliary strictures than conventional ERCP[41].
Use of CLE in clinical practice is limited by several factors. One limitation is that CLE quires specialized equipment and trained personnel to operate it. Cost of the equipment can be prohibitive for some healthcare facilities. Another limitation is the need for specialized training for endoscopists to ensure that the procedure is performed correctly and safely.
During chromoendoscopy different stains are applied to surface of biliary mucosa at time of cholangioscopy. Methylene blue can be used to delineate malignant lesions and ischemic strictures. High-risk lesions concerning for malignancy such as increased vascularity and infiltrative or irregular mucosal surface can be better examined using narrow-band imaging. Initial studies looking at use of autofluorescence during cholangioscopy have been less promising given lack of specificity and high false-positive rates seen with this method of testing. This technique is not widely use.
Optical coherence tomography (OCT) is an imaging technique that allows acquisition of high-resolution images of the intra- and extrahepatic bile ducts. OCT is performed by advancing a probe through accessory channel of a duodenoscope. Disturbed bile duct layers and nonreflective areas, indicating presence of tumor vessels are indicative of malignant strictures. A second-generation OCT system using volumetric laser endomicroscopy was recently introduced. Tyberg et al[42] reported findings of epithelial thickening, hyper-reflective surfaces with shadowing and haziness of inner mucosal layers in patients with CCA. A pilot study noted at least one such finding in 79% of study population[43]. However, large, randomized trials are necessary to confirm clinical utility of adding this technique to ERCP and conventional tissue acquisition techniques.
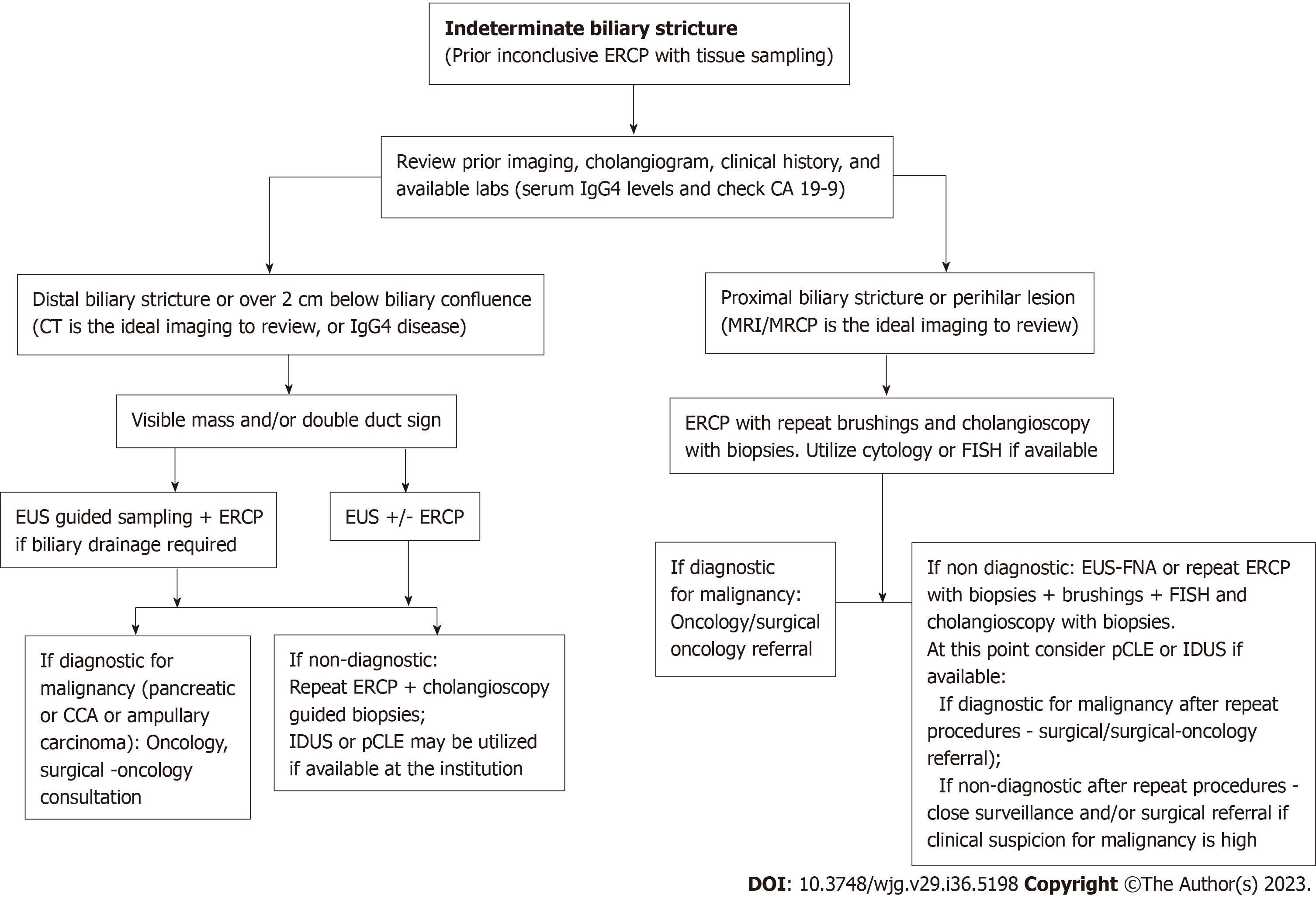
Management of biliary strictures can be complex and requires a personalized approach that considers the specific clinical presentation and the diagnostic techniques available at the institution (Table 2). Biliary strictures can have a variable presentation and can be caused by a variety of underlying conditions, which can make it difficult to have a standardized approach to their management. Drawing on the data available, we propose an approach to address IDBSs (Figure 6). Additionally, it can be challenging to rule out neoplastic biliary strictures prior to surgery, despite the evolution of endoscopic techniques for diagnosis and treatment. It is important for healthcare providers to carefully evaluate each patient and consider the complete clinical picture to develop an appropriate plan of care.
| Tissue acquisition technique | Advantages | Disadvantages | Comments |
| ERCP-guided techniques | |||
| Intraductal biopsy | Direct sampling of larger tissue samples | Risk of bleeding, perforation, pancreatitis | Used in conjunction to other techniques to maximize yield |
| Brush cytology | Less invasive. Can be used in cases of advanced liver or pancreatic disease | Smaller tissues sample. Low diagnostic yield | Used in conjunction to other techniques to maximize yield |
| FISH | Can detect genetic abnormalities associated with malignancy | Requires specialized equipment for analysis. Limited sensitivity and specificity | Used in conjunction with biopsies or brushings to improve diagnostic yield |
| Flow cytometry | Can detect abnormal cellular DNA associated with malignancy | Requires specialized equipment for analysis. Limited sensitivity and specificity | Used in conjunction with other techniques |
| Cholangioscopy with biopsy | Direct visualization of stricture and targeted biopsies | Requires specialized equipment and expertise. Associated with higher risk of complications such as cholangitis, pancreatitis, and bleeding. More expensive than conventional ERCP | Can perform interventions under direct visualization |
| Intraductal ultrasound | High-resolution imaging of the biliary tract and surrounding structures, can detect structural abnormalities associated with malignancy | Invasive, requires specialized equipment and expertise | Not available in every institution |
| Risks associated with ERCP - bleeding, perforation, pancreatitis, infection | |||
| EUS guided sampling | |||
| EUS with FNA | High diagnostic yield, ability to sample adjacent lymph nodes or lesions | Invasive, risk of bleeding and infection, requires expertise | Can be used as a complementary technique to ERCP or alone. Should be used with ROSE if available and if malignancy suspected |
| EUS with FNB | High diagnostic yield, ability to sample lymph nodes, larger tissue | Invasive, risk of bleeding and infection, requires expertise | Can be used as a complementary technique to ERCP or alone |
| EUS/ERCP combination | Ability to obtain tissue samples and perform therapeutic interventions in the same encounter | Invasive, requires specialized equipment and expertise | Used in complex cases and in cases where biliary drainage is needed |
| Confocal laser endomicroscopy | Real-time in vivo imaging of biliary tissue at the cellular level | Limited availability and expertise, expensive | Can be used to improve diagnostic accuracy and guide targeted biopsies |
| Optical coherence tomography | High-resolution imaging of biliary tissue, can detect structural abnormalities associated with malignancy | Limited availability and expertise, expensive | Can be useful in cases where other techniques have failed or when precise localization of the stricture is necessary |
During the initial evaluation of biliary strictures, it is important to obtain a detailed history and physical examination to assess the patient’s risk factors and determine the type of biliary stricture. Basic laboratory tests, particularly liver function tests, should be performed. In cases with clinical suspicion of certain conditions such as PSC, IgG4 or human immunodeficiency virus (HIV), additional testing for tumor markers, HIV antibodies, and IgG4 levels may be necessary. Imaging studies, such as US and CT with IV contrast, can be useful in the evaluation of biliary strictures. If intra- and extrahepatic biliary dilation is visualized on US, this is more suggestive of a distal bile duct stricture, which may be caused by an ampullary or pancreatic mass. CT with intravenous contrast is the preferred imaging modality in such cases. If intrahepatic biliary dilation is seen on US, this is more suggestive of a proximal biliary stricture, for which MRI/MRCP is the preferred imaging modality.
In instances where extrahepatic biliary strictures are suspected to be a result of a pancreatic mass, EUS-guided fine-needle sampling is the preferred method to assess for presence of malignancy. EUS-guided FNB or EUS with FNA plus ROSE is recommended over EUS with FNA without ROSE. EUS can help identify minute lesions, in about 40% of cases that may not be readily visible on CT. Single session EUS and ERCP may be warranted in cases where biliary decompression is warranted in addition to establishing diagnosis. Cholangioscopy-guided biopsies or IDUS may be needed if conventional ERCP is unable to determine nature of stricture. Maintaining stable positioning of cholangioscope is key to performing targeted biopsies and this can often be challenging if there is a distal bile duct stricture. Endoscopists may use techniques such as balloon dilation to widen the stricture and improve access to the bile ducts. In cases of proximal or hilar lesions, ERCP is often the primary diagnostic and therapeutic procedure. In the assessment of hilar strictures, multimodal sampling during ERCP is preferred over relying solely on brush cytology. EUS may be warranted if ERCP and traditional sampling techniques, cytology and FISH are inconclusive. Since the location of proximal biliary strictures tends to be more intra-hepatic and away from duodenal wall, it may be challenging to adequately visualize using EUS and obtain biopsies. In cases that are being considered for liver transplant, caution should be exercised before perming biopsies of biliary or hilar lesions. Lymph nodes may be biopsied safely with low concern for seeding.
Lee et al[16] conducted a study of 181 patients with suspected malignant biliary strictures. Accuracy of cholangioscopy in diagnosis of malignancy in proximal strictures was 93.6% whereas accuracy of EUS-FNA or biopsy in diagnosis malignant biliary strictures was 96.3%. Currently there are no randomized control trials comparing cholangioscopy guided biopsies and EUS-FNA in cases of IDBSs where conventional ERCP based tissue sampling is inconclusive. However, based on available data and expert opinions this could be considered a diagnostic approach.
Despite advancement in diagnostic testing, nonsurgical evaluation of biliary strictures is often challenging. Management of IDBSs often involves a multidisciplinary approach and multiple diagnostic modalities. The entire clinical picture should be considered to come up with a management strategy. Goal of management should be early establishment of diagnosis in cases of malignant strictures and avoidance of unnecessary surgical exploration in cases of benign biliary strictures. A single test may be inadequate to diagnose IDBSs. However, a combination of validated serum or bile-based markers, imaging and endoscopic testing will improve diagnostic yield.
We thank Leona Council, MD. Department of Pathology, University of Alabama at Birmingham.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khalil MTASH, Egypt; Kim HJ, South Korea; Wang LM, China S-Editor: Wang JJ L-Editor: Kerr C P-Editor: Wang JJ
| 1. | Oleas R, Alcívar-Vasquez J, Robles-Medranda C. New technologies for indeterminate biliary strictures. Transl Gastroenterol Hepatol. 2022;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 2. | Novikov A, Kowalski TE, Loren DE. Practical Management of Indeterminate Biliary Strictures. Gastrointest Endosc Clin N Am. 2019;29:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Sun B, Moon JH, Cai Q, Rerknimitr R, Ma S, Lakhtakia S, Ryozawa S, Kutsumi H, Yasuda I, Shiomi H, Li X, Li W, Zhang X, Itoi T, Wang HP, Qian D, Wong Lau JY, Yang Z, Ji M, Hu B; Asia-Pacific ERCP Club. Review article: Asia-Pacific consensus recommendations on endoscopic tissue acquisition for biliary strictures. Aliment Pharmacol Ther. 2018;48:138-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998;171:651-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Heinzow HS, Kammerer S, Rammes C, Wessling J, Domagk D, Meister T. Comparative analysis of ERCP, IDUS, EUS and CT in predicting malignant bile duct strictures. World J Gastroenterol. 2014;20:10495-10503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Rösch T, Meining A, Frühmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Romagnuolo J, Bardou M, Rahme E, Joseph L, Reinhold C, Barkun AN. Magnetic resonance cholangiopancreatography: a meta-analysis of test performance in suspected biliary disease. Ann Intern Med. 2003;139:547-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 264] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015;81:168-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 9. | Pörner D, Kaczmarek DJ, Heling D, Hausen A, Mohr R, Hüneburg R, Matthaei H, Glowka TR, Manekeller S, Fischer HP, Toma M, Nattermann J, Strassburg CP, Gonzalez-Carmona MA, Weismüller TJ. Transpapillary tissue sampling of biliary strictures: balloon dilatation prior to forceps biopsy improves sensitivity and accuracy. Sci Rep. 2020;10:17423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Sugimoto S, Matsubayashi H, Kimura H, Sasaki K, Nagata K, Ohno S, Uesaka K, Mori K, Imai K, Hotta K, Takizawa K, Kakushima N, Tanaka M, Kawata N, Ono H. Diagnosis of bile duct cancer by bile cytology: usefulness of post-brushing biliary lavage fluid. Endosc Int Open. 2015;3:E323-E328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Kato M, Onoyama T, Takeda Y, Kawata S, Kurumi H, Koda H, Yamashita T, Hamamoto W, Sakamoto Y, Matsumoto K, Isomoto H. Peroral Cholangioscopy-Guided Forceps Biopsy and Endoscopic Scraper for the Diagnosis of Indeterminate Extrahepatic Biliary Stricture. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Levy MJ, Baron TH, Clayton AC, Enders FB, Gostout CJ, Halling KC, Kipp BR, Petersen BT, Roberts LR, Rumalla A, Sebo TJ, Topazian MD, Wiersema MJ, Gores GJ. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol. 2008;103:1263-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Gonda TA, Glick MP, Sethi A, Poneros JM, Palmas W, Iqbal S, Gonzalez S, Nandula SV, Emond JC, Brown RS, Murty VV, Stevens PD. Polysomy and p16 deletion by fluorescence in situ hybridization in the diagnosis of indeterminate biliary strictures. Gastrointest Endosc. 2012;75:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Nanda A, Brown JM, Berger SH, Lewis MM, Barr Fritcher EG, Gores GJ, Keilin SA, Woods KE, Cai Q, Willingham FF. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therap Adv Gastroenterol. 2015;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Ryan ME, Baldauf MC. Comparison of flow cytometry for DNA content and brush cytology for detection of malignancy in pancreaticobiliary strictures. Gastrointest Endosc. 1994;40:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U, Werner M. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Moura DTH, de Moura EGH, Matuguma SE, Dos Santos ME, Moura ETH, Baracat FI, Artifon E, Cheng S, Bernardo WM, Chacon D, Tanigawa R, Jukemura J. EUS-FNA versus ERCP for tissue diagnosis of suspect malignant biliary strictures: a prospective comparative study. Endosc Int Open. 2018;6:E769-E777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 20. | Zaheer A, Anwar MM, Donohoe C, O'Keeffe S, Mushtaq H, Kelleher B, Clarke E, Kirca M, McKiernan S, Mahmud N, Keeling N, MacMathuna P, O'Toole D. The diagnostic accuracy of endoscopic ultrasound in suspected biliary obstruction and its impact on endoscopic retrograde cholangiopancreatography burden in real clinical practice: a consecutive analysis. Eur J Gastroenterol Hepatol. 2013;25:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 21. | Jo JH, Cho CM, Jun JH, Chung MJ, Kim TH, Seo DW, Kim J, Park DH; Research Group for Endoscopic Ultrasonography in KSGE. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: A multicenter experience. J Gastroenterol Hepatol. 2019;34:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford). 2011;13:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 23. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Meister T, Heinzow HS, Woestmeyer C, Lenz P, Menzel J, Kucharzik T, Domschke W, Domagk D. Intraductal ultrasound substantiates diagnostics of bile duct strictures of uncertain etiology. World J Gastroenterol. 2013;19:874-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Menzel J, Poremba C, Dietl KH, Domschke W. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastroenterol. 2000;35:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Turowski F, Hügle U, Dormann A, Bechtler M, Jakobs R, Gottschalk U, Nötzel E, Hartmann D, Lorenz A, Kolligs F, Veltzke-Schlieker W, Adler A, Becker O, Wiedenmann B, Bürgel N, Tröger H, Schumann M, Daum S, Siegmund B, Bojarski C. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS™: results of a multicenter retrospective cohort study. Surg Endosc. 2018;32:3981-3988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Chen YK, Parsi MA, Binmoeller KF, Hawes RH, Pleskow DK, Slivka A, Haluszka O, Petersen BT, Sherman S, Devière J, Meisner S, Stevens PD, Costamagna G, Ponchon T, Peetermans JA, Neuhaus H. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 2011;74:805-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc. 2015;82:608-14.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Korrapati P, Ciolino J, Wani S, Shah J, Watson R, Muthusamy VR, Klapman J, Komanduri S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endosc Int Open. 2016;4:E263-E275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Nishikawa T, Tsuyuguchi T, Sakai Y, Sugiyama H, Miyazaki M, Yokosuka O. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: a prospective study. Gastrointest Endosc. 2013;77:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S, Sekaran A, Rao GV. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc. 2011;74:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Itoi T, Kamisawa T, Igarashi Y, Kawakami H, Yasuda I, Itokawa F, Kishimoto Y, Kuwatani M, Doi S, Hara S, Moriyasu F, Baron TH. The role of peroral video cholangioscopy in patients with IgG4-related sclerosing cholangitis. J Gastroenterol. 2013;48:504-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Wen LJ, Chen JH, Xu HJ, Yu Q, Liu K. Efficacy and Safety of Digital Single-Operator Cholangioscopy in the Diagnosis of Indeterminate Biliary Strictures by Targeted Biopsies: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Guvendir I, Zemheri IE, Ozdil K. Impact of rapid on-site evaluation on diagnostic accuracy of EUS-guided fine-needle aspiration of solid pancreatic lesions: experience from a single center. BMC Gastroenterol. 2022;22:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 35. | Njei B, McCarty TR, Varadarajulu S, Navaneethan U. Cost utility of ERCP-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastrointest Endosc. 2017;85:773-781.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Deprez PH, Garces Duran R, Moreels T, Furneri G, Demma F, Verbeke L, Van der Merwe SW, Laleman W. The economic impact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy. 2018;50:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 37. | Kahaleh M, Gaidhane M, Shahid HM, Tyberg A, Sarkar A, Ardengh JC, Kedia P, Andalib I, Gress F, Sethi A, Gan SI, Suresh S, Makar M, Bareket R, Slivka A, Widmer JL, Jamidar PA, Alkhiari R, Oleas R, Kim D, Robles-Medranda CA, Raijman I. Digital single-operator cholangioscopy interobserver study using a new classification: the Mendoza Classification (with video). Gastrointest Endosc. 2022;95:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 38. | Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, Khan AH, Shah RJ. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. 2011;73:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Meining A, Shah RJ, Slivka A, Pleskow D, Chuttani R, Stevens PD, Becker V, Chen YK. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy. 2012;44:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | Caillol F, Filoche B, Gaidhane M, Kahaleh M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris Classification. Dig Dis Sci. 2013;58:1784-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Meining A, Chen YK, Pleskow D, Stevens P, Shah RJ, Chuttani R, Michalek J, Slivka A. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc. 2011;74:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Tyberg A, Xu MM, Gaidhane M, Kahaleh M. Second generation optical coherence tomography: Preliminary experience in pancreatic and biliary strictures. Dig Liver Dis. 2018;50:1214-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Arvanitakis M, Hookey L, Tessier G, Demetter P, Nagy N, Stellke A, De Maertelaer V, Devière J, Le Moine O. Intraductal optical coherence tomography during endoscopic retrograde cholangiopancreatography for investigation of biliary strictures. Endoscopy. 2009;41:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |