Published online Sep 21, 2023. doi: 10.3748/wjg.v29.i35.5138
Peer-review started: June 28, 2023
First decision: July 23, 2023
Revised: August 6, 2023
Accepted: August 28, 2023
Article in press: August 28, 2023
Published online: September 21, 2023
Processing time: 78 Days and 2 Hours
Biliary microlithiasis/sludge is detected in approximately 30% of patients with idiopathic acute pancreatitis (IAP). As recurrent biliary pancreatitis can be pre
To develop a machine learning (ML) based decision tool for the use of endosonography (EUS) in pancreatitis patients to detect sludge and microlithiasis.
We retrospectively used routinely recorded clinical and laboratory parameters of 218 consecutive patients with confirmed AP admitted to our tertiary care hospital between 2015 and 2020. Patients who did not receive EUS as part of the diagnostic work-up and whose pancreatitis episode could be adequately explained by other causes than biliary sludge and microlithiasis were excluded. We trained super
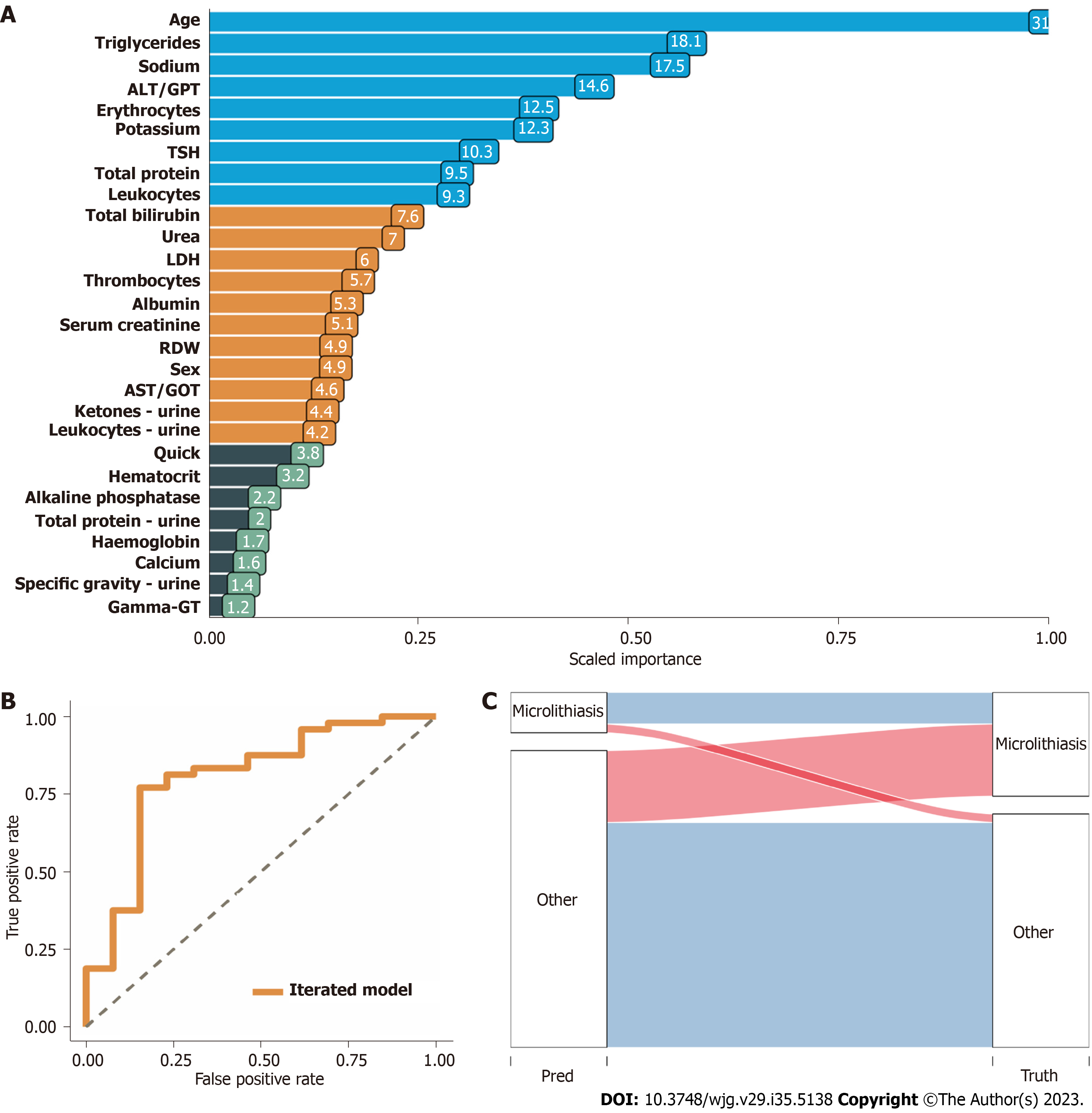
Twenty-eight categorized patients’ variables recorded at admission were identified to compute the predictor model with an accuracy of 0.84 [95% confidence interval (CI): 0.791-0.9185], positive predictive value of 0.84, and negative predictive value of 0.80 in the identification cohort (218 patients). In the validation cohort, the robustness of the prediction model was confirmed with an accuracy of 0.76 (95%CI: 0.673-0.8347), positive predictive value of 0.76, and negative predictive value of 0.78 (117 patients).
We present a robust and validated ML-based predictor model consisting of routinely recorded parameters at admission that can predict biliary sludge and microlithiasis as the cause of AP.
Core Tip: Occult biliary lithiasis represents the largest monocausally treatable aetiology group within idiopathic acute pancreatitis cases. The identification of this subgroup protects patients from pancreatitis recurrences and over- or underdiagnosis. Based on 28 easy-to-collect and widely available patient variables, a machine learning-based prediction score can be used to predict the presence or absence of biliary sludge or microlithiasis in the context of pancreatitis hospitalisation. We provide a web-based prediction tool to select patients for endosonography to investigate microlithiasis or sludge as the cause of pan
- Citation: Sirtl S, Żorniak M, Hohmann E, Beyer G, Dibos M, Wandel A, Phillip V, Ammer-Herrmenau C, Neesse A, Schulz C, Schirra J, Mayerle J, Mahajan UM. Machine learning-based decision tool for selecting patients with idiopathic acute pancreatitis for endosonography to exclude a biliary aetiology. World J Gastroenterol 2023; 29(35): 5138-5153
- URL: https://www.wjgnet.com/1007-9327/full/v29/i35/5138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i35.5138
Pancreatitis is a high incidence disease and the underlying cause for the highest number of patients admitted to hospital admission of all benign gastrointestinal-disorders[1]. In approximately 25% of patients with acute pancreatitis (AP), aetiology cannot be established during the first episode of pancreatitis[2,3]. If the aetiology of AP cannot be identified by history, laboratory chemistry, and imaging, it is classified as “idiopathic” [idiopathic AP (IAP)]. Unclassified or idiopathic pancreatitis represents the third largest group of pancreatitis and is therefore of great importance from both a medical and a socioeconomic point of view requiring thorough workup[3,4]. All efforts should be made to elucidate a treatable aetiology to prevent further episodes of AP. A recent meta-analysis has shown that biliary aetiology is the most common cause of idiopathic pancreatitis with a prevalence of 30%[5]. Specifically, in light of morbidity and mortality of AP, it is crucial to differentiate the potentially treatable aetiology of AP triggered by biliary sludge and microlithiasis from idiopathic or other causes of AP. Unfortunately, due to a lack of unifying definition of biliary sludge and microlithiasis, it is currently impossible to assess the risk of sludge and/or microlithiasis as the cause of AP. In the absence of clear evi
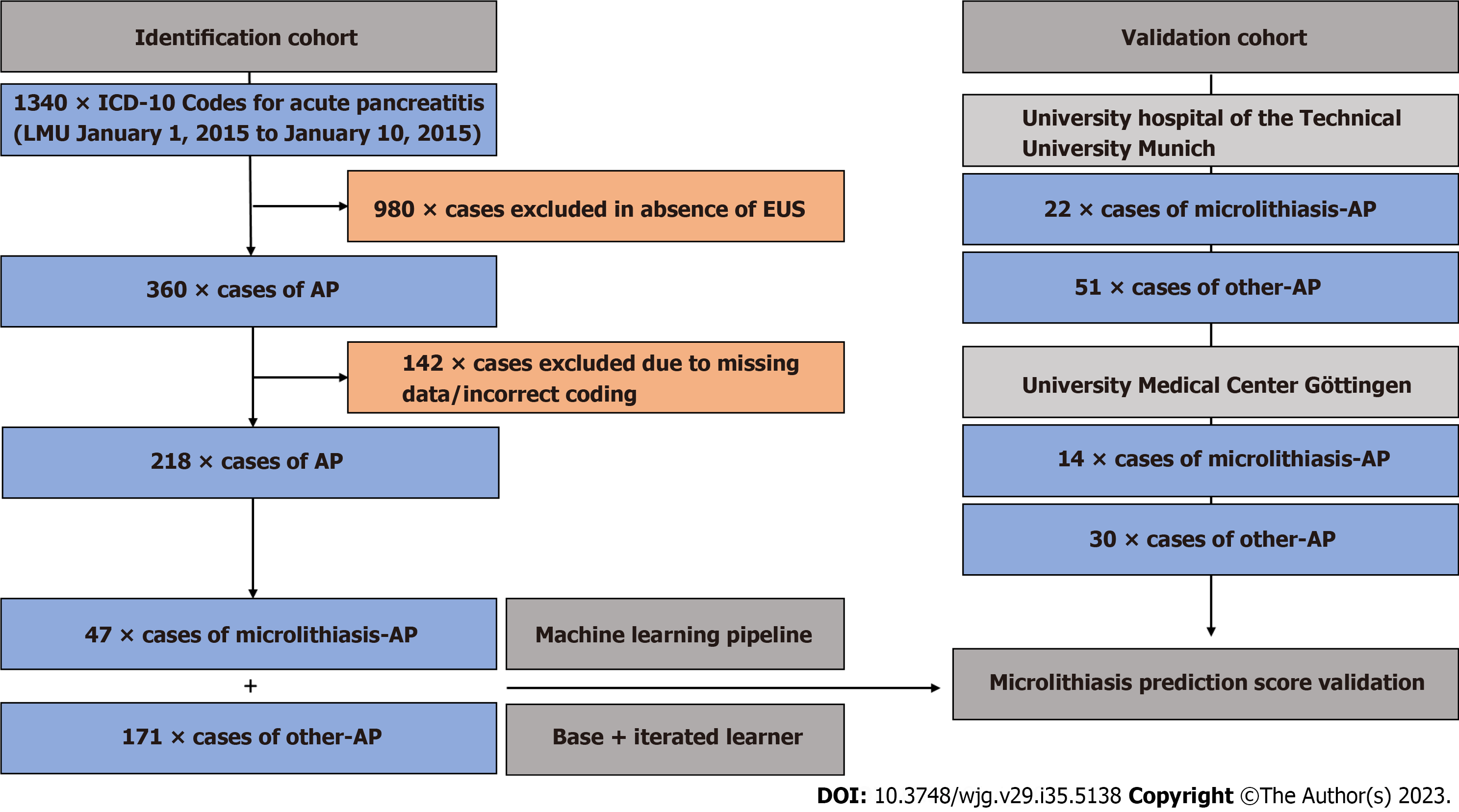
We retrospectively studied 1340 confirmed and hospitalized patient cases of AP treated at LMU University Hospital Munich (tertiary care hospital) between January 1, 2015 and October 1, 2020 (ICD-10 codes used: K85.00-K85.91). Patient cohorts with identical inclusion criteria from the University Hospital of the Technical University Munich and the University Medical Center Goettingen served as the validation cohort. The study was conducted in accordance with the updated STARD guideline of 2015[8].
Only patients meeting the diagnostic criteria for AP as set in the APA/IAP guidelines and adapted in the German S3-Guideline were enrolled in the analysis[9,10]. The first classifier used was whether patients received an EUS during their initial hospital stay, reducing the number of patients for further analysis to 360. The endosonographies were each per
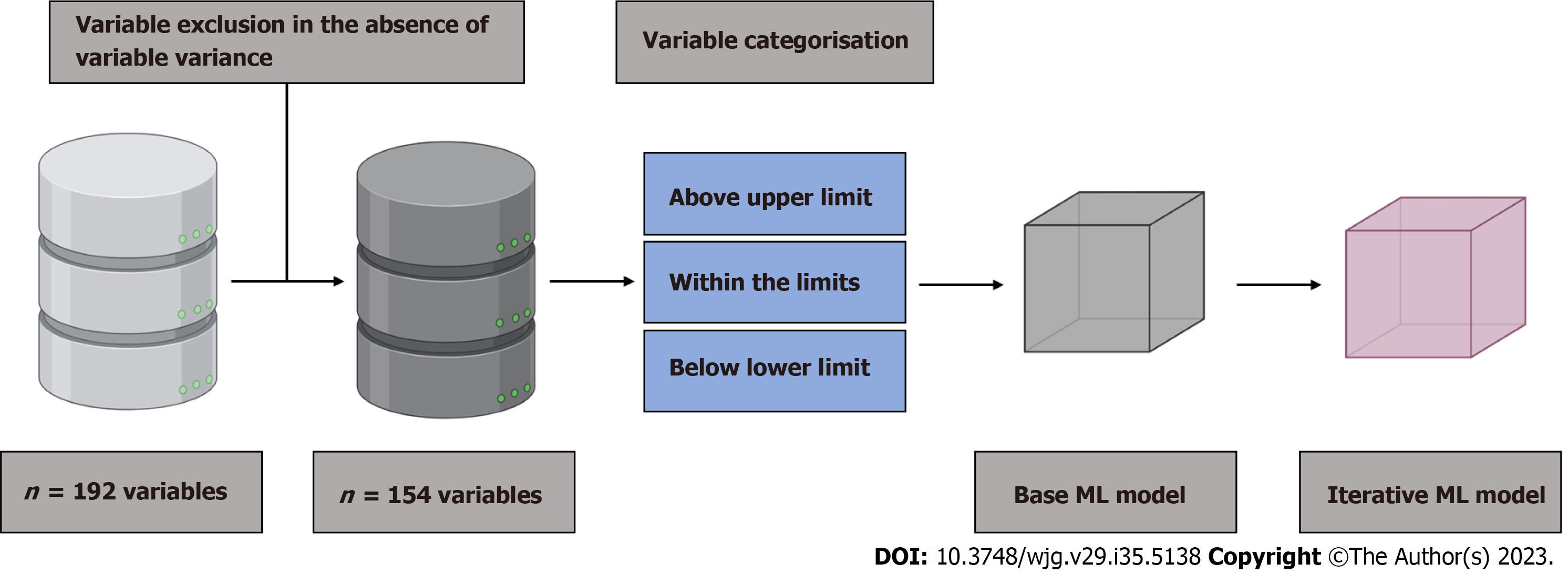
All aspects of data reporting, predictive modeling, and validation reporting were performed in accordance with the TRIPOD guidelines[11]. A diagnostic reference standard for laboratory or imaging-based prediction of biliary sludge or microlithiasis in the context of AP has not yet been published. To derive the ML-based predictor model (index test), the following steps were performed (Figure 2): (1) Baseline variables (n = 192) were filtered leaving out variables with zero and near zero variance; (2) All numeric variables were classified into within limit, above upper limit, and below lower limit, based on clinical reference limits. All categorised variables were retained; (3) The training cohort was divided into a training (80%) and a test set (20%). Endpoint balancing was achieved by stratifying the classes by inducing the sampling rate of patients with microlithiasis and reducing the sampling rate of patients with other-AP. ML was performed based on all filtered baseline variables and data from the training set, resulting in a predictor based on all variables (base pre
All predictor models were constructed using the H2O.ai platform (https://www.h2o.ai) selecting (with h2o.automl) the best suitable ML method in the training set. The parameters of each method were optimized by employing an internal ten-fold cross-validation on the training set. The optimal method was then applied to the test set to assess the final performance. In each loop, the best performing predictor model was identified from all predicted outcomes obtained using the performance measure logloss. Variables with a higher proportion of missing data (> 25% missing data) were also not excluded per se in order to base the final model on the broadest possible number of routinely available variables in the early phase of AP. The iterative predictive model obtained was externally validated in an independent retros
All data processing, modeling, and assessment of performances were done using R [version 4.0.4 (2021-02-15, “Lost Library Book”)] and visualized in R-studio (version 1.3.9.59). No unique algorithm was developed for this study. All data R scripts or functions are available online at the following link: https://github.com/mayerlelab/microlithiasisPredict. P values of < 0.05 were considered statistically significant if appropriate for the tests used.
Between January 1, 2015 and October 1, 2020, 218 patients with AP received an EUS during their initial admission with AP at LMU University Hospital meeting the study inclusion criteria (Figure 1). In 47 of 218 pancreatitis patients, no causal pancreatitis aetiology other than endosonographically detected biliary microconcrements/sludge was found during the respective inpatient stay. Among 171 out of 218 pancreatitis patients with EUS, 52.6% (90/171) were classified as ‘idio
| Variable | Microlithiasis (n = 47) | Other (n = 171) | Total (n = 218) | P value |
| Age (yr) | 0.122 | |||
| mean ± SD | 59.1 ± 18.8 | 54.6 ± 17.1 | 55.6 ± 17.6 | |
| Range | 30-92 | 24-90 | 24-92 | |
| Sex | 0.474 | |||
| Female | 16/47 (34%) | 68/171 (39.8%) | 84/218 (38.5%) | |
| Male | 31/47 (66%) | 103/171 (60.2%) | 134/218 (61.5%) | |
| Albumin | 0.706 | |||
| N-Miss | 32/47 (68%) | 90/171 (52.6%) | 122/218 (55.9%) | |
| LLN | 2/47 (4.2%) | 14/171 (8.1%) | 16/218 (7.3%) | |
| WL | 13/47 (27.6%) | 67/171 (39.1%) | 80/218 (36.6%) | |
| Alkaline phosphatase | 0.667 | |||
| N-Miss | 1/47 (2.1%) | 5/171 (2.9%) | 6/218 (2.7%) | |
| LLN | 0/47 (0%) | 1/171 (0.5%) | 1/218 (0.4%) | |
| ULN | 22/47 (46.8%) | 69/171 (40.3%) | 91/218 (41.7%) | |
| WL | 24/47 (51.0%) | 96/171 (56.1%) | 120/218 (55.0%) | |
| Total bilirubin | 0.110 | |||
| N-Miss | 0/47 (0%) | 2/171 (1.1%) | 2/218 (0.9%) | |
| ULN | 23/47 (48.9%) | 61/171 (35.6%) | 84/218 (38.5%) | |
| WL | 24/47 (51.0%) | 108/171 (63.1%) | 132/218 (60.5%) | |
| Calcium | 0.033 | |||
| N-Miss | 20/47 (42.5%) | 60/171 (35.0%) | 80/218 (36.6%) | |
| LLN | 6/47 (12.7%) | 7/171 (4.0%) | 13/218 (5.9%) | |
| ULN | 0/47 (0%) | 2/171 (1.1.%) | 2/218 (0.9%) | |
| WL | 21/47 (44.6%) | 102/171 (59.6%) | 123/218 (56.4%) | |
| Creatine kinase | 0.073 | |||
| N-Miss | 29/47 (61.7%) | 107/171 (62.5%) | 136/218 (62.3%) | |
| ULN | 0/47 (0%) | 10/171 (5.8%) | 10/218 (4.5%) | |
| WL | 18/47 (38.2%) | 54/171 (31.5%) | 72/218 (33%) | |
| CRP | 0.391 | |||
| ULN | 37/47 (78.7%) | 124/171 (72.5%) | 161/218 (73.9%) | |
| WL | 10/47 (21.3%) | 47/171 (27.5%) | 57/218 (26.1%) | |
| Total protein | 0.743 | |||
| N-Miss | 30/47 (63.8%) | 104/171 (60.8%) | 134/218 (61.4%) | |
| LLN | 0/47 (0%) | 1/171 (0.5%) | 1/218 (0.4%) | |
| ULN | 3/47 (6.3%) | 16/171 (9.3%) | 19/218 (8.7%) | |
| WL | 14/47 (29.7%) | 50/171 (29.2%) | 64/218 (29.3%) | |
| Erythrocytes | 0.880 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 14/47 (29.7%) | 53/171 (30.9%) | 67/218 (30.7%) | |
| ULN | 3/47 (6.3%) | 8/171 (4.6%) | 11/218 (5.0%) | |
| WL | 29/47 (61.7%) | 110/171 (64.3%) | 139/218 (63.7%) | |
| Gamma-GT | 0.108 | |||
| N-Miss | 0/47 (0%) | 1/171 (0.5%) | 1/218 (0.4%) | |
| ULN | 37/47 (78.7%) | 113/171 (66%) | 150/218 (68.8%) | |
| WL | 10/47 (21.3%) | 57/171 (33.3%) | 67/218 (30.7%) | |
| AST/GOT | 0.444 | |||
| N-Miss | 17/47 (36.1%) | 51/171 (29.8%) | 68/218 (31.1%) | |
| ULN | 21/47 (44.6%) | 75/171 (43.8%) | 96/218 (44.0%) | |
| WL | 9/47 (19.1%) | 45/171 (26.3%) | 54/218 (24.7%) | |
| ALT/GPT | 0.016 | |||
| ULN | 34/47 (72.3%) | 90/171 (52.6%) | 124/218 (56.9%) | |
| WL | 13/47 (27.7%) | 81/171 (47.4%) | 94/218 (43.1%) | |
| Urea | 0.429 | |||
| N-Miss | 31/47 (65.9%) | 80/171 (46.7%) | 111/218 (50.9%) | |
| LLN | 0/47 (0%) | 7/171 (4%) | 7/218 (3.2%) | |
| ULN | 3/47 (6.3%) | 11/171 (6.4%) | 14/218 (6.4%) | |
| WL | 13/47 (27.6%) | 73/171 (42.6%) | 86/218 (39.4%) | |
| Hematocrit | 0.304 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 0/47 (0%) | 2/171 (1.1%) | 2/218 (0.9%) | |
| ULN | 41/47 (87.2%) | 160/171 (93.5%) | 201/218 (92.2%) | |
| WL | 5/47 (10.6%) | 9/171 (5.2%) | 14/218 (6.4%) | |
| Haemoglobin | 0.574 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 12/47 (25.5%) | 45/171 (26.3%) | 57/218 (26.1%) | |
| ULN | 0/47 (0%) | 4/171 (2.3%) | 4/218 (1.8%) | |
| WL | 34/47 (72.3%) | 122/171 (71.3%) | 156/218 (71.5%) | |
| INR | 0.443 | |||
| N-Miss | 3/47 (6.3%) | 9/171 (1.1%) | 12/218 (5.5%) | |
| ULN | 8/47 (17.0%) | 22/171 (12.8%) | 30/218 (13.7%) | |
| WL | 36/47 (76.5%) | 140/171 (81.8%) | 176/218 (80.7%) | |
| Potassium | 0.270 | |||
| N-Miss | 9/47 (19.1%) | 2/171 (1.1%) | 11/218 (5%) | |
| LLN | 1/47 (2.1%) | 7/171 (4%) | 8/218 (3.6%) | |
| ULN | 0/47 (0%) | 10/171 (5.8%) | 10/218 (4.5%) | |
| WL | 37/47 (78.7%) | 152/171 (88.8%) | 189/218 (86.6%) | |
| Serum creatinine | 0.738 | |||
| N-Miss | 6/47 (12.7%) | 0/171 (0%) | 6/218 (2.7%) | |
| LLN | 1/47 (2.1%) | 5/171 (2.9%) | 6/218 (2.7%) | |
| ULN | 5/47 (10.6%) | 29/171 (16.9%) | 34/218 (15.5%) | |
| WL | 35/47 (74.4%) | 137/171 (80.1%) | 172/218 (78.8%) | |
| LDH | 0.020 | |||
| N-Miss | 7/47 (14.8%) | 19/171 (11.1%) | 26/218 (11.9%) | |
| ULN | 30/47 (63.8%) | 83/171 (48.5%) | 112/218 (51.8%) | |
| WL | 10/47 (21.2%) | 69/171 (40.3%) | 79/218 (36.2%) | |
| Leukocytes | 0.347 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 1/47 (2.1%) | 3/171 (1.7%) | 4/218 (1.8%) | |
| ULN | 16/47 (34%) | 80/171 (46.7%) | 96/218 (44%) | |
| WL | 29/47 (61.7%) | 88/171 (51.5%) | 117/218 (53.6%) | |
| Lipase | 0.653 | |||
| N-Miss | 0/47 (0%) | 1/171 (0.5%) | 1/218 (0.4%) | |
| LLN | 0/47 (0%) | 3/171 (1.7%) | 3/218 (1.3%) | |
| ULN | 44/47 (93.6%) | 157/171 (91.8%) | 201/218 (92.2%) | |
| WL | 3/47 (6.4%) | 10/171 (5.8%) | 13/218 (5.9%) | |
| MCH | 0.498 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 3/47 (6.3%) | 20/171 (11.6%) | 23/218 (10.5%) | |
| ULN | 4/47 (8.5%) | 10/171 (5.8%) | 14/218 (6.4%) | |
| WL | 39/47 (82.9%) | 141/171 (82.4%) | 180/218 (82.5%) | |
| MCHC | 0.108 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 0/47 (0%) | 13/171 (7.6%) | 13/218 (5.9%) | |
| ULN | 1/47 (2.1%) | 8/171 (4.6%) | 9/218 (4.1%) | |
| WL | 45/47 (95.7%) | 150/171 (87.7%) | 195/218 (89.4%) | |
| Triglycerides | 0.004 | |||
| N-Miss | 27/47 (57.4%) | 110/171 (64.3%) | 137/218 (62.8%) | |
| ULN | 1/47 (2.1%) | 24/171 (14%) | 25/218 (11.4%) | |
| WL | 19/47 (40.4%) | 37/171 (21.6%) | 56/218 (25.6%) | |
| RDW | 0.329 | |||
| N-Miss | 5/47 (10.6%) | 36/171 (21%) | 41/218 (18.8%) | |
| LLN | 1/47 (2.1%) | 11/171 (6.3%) | 12/218 (5.5%) | |
| ULN | 5/47 (10.6%) | 21/171 (12.2%) | 26/218 (11.9%) | |
| WL | 36/47 (76.5%) | 103/171 (60.2%) | 139/218 (63.7%) | |
| MCV | 0.893 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 4/47 (8.5%) | 13/171 (7.6%) | 17/218 (7.7%) | |
| ULN | 4/47 (8.5%) | 12/171 (7.0%) | 16/218 (7.3%) | |
| WL | 38/47 (80.8%) | 146/171 (85.3%) | 184/218 (84.4%) | |
| Sodium | 0.020 | |||
| N-Miss | 8/47 (17.0%) | 1/171 (0.5%) | 9/218 (4.1%) | |
| LLN | 1/47 (2.1%) | 29/171 (16.9%) | 30/218 (13.7%) | |
| WL | 38/47 (80.8%) | 141/171 (82.4%) | 179/218 (82.1%) | |
| Quick’s value | 0.479 | |||
| N-Miss | 3/47 (6.3%) | 7/171 (4%) | 10/218 (4.5%) | |
| LLN | 8/47 (17%) | 23/171 (13.4%) | 31/218 (14.2%) | |
| ULN | 20/47 (42.5%) | 65/171 (38%) | 85/218 (38.9%) | |
| WL | 16/47 (34%) | 76/171 (44.4%) | 92/218 (42.2%) | |
| Thrombocytes | 0.434 | |||
| N-Miss | 1/47 (2.1%) | 0/171 (0%) | 1/218 (0.4%) | |
| LLN | 8/47 (17%) | 22/171 (12.8%) | 30/218 (13.7%) | |
| ULN | 4/47 (8.5%) | 26/171 (15.2%) | 30/218 (13.7%) | |
| WL | 34/47 (72.3%) | 123/171 (71.9%) | 157/218 (72%) | |
| TSH | 0.567 | |||
| N-Miss | 27/47 (57.4%) | 118/171 (69%) | 145/218 (66.5%) | |
| LLN | 2/47 (4.2%) | 4/171 (2.3%) | 6/218 (2.7%) | |
| ULN | 4/47 (8.5%) | 6/171 (3.5%) | 10/218 (4.5%) | |
| WL | 14/47 (29.7%) | 43/171 (25.1%) | 57/218 (26.1%) | |
| Bilirubin-urine | 0.027 | |||
| N-Miss | 26/47 (55.3%) | 102/171 (59.6%) | 128/218 (58.7%) | |
| Normal | 8/47 (17%) | 45/171 (26.3%) | 53/218 (24.3%) | |
| Abnormal | 13/47 (27.6%) | 24/171 (14.0%) | 37/218 (16.9%) | |
| Total protein-urine | 0.231 | |||
| N-Miss | 26/47 (55.3%) | 102/171 (59.6%) | 128/218 (58.7%) | |
| Normal | 10/47 (21.3%) | 43/171 (25.1%) | 53/218 (24.3%) | |
| Abnormal | 11/47 (23.4%) | 26/171 (15.2%) | 37/218 (16.9%) | |
| Ketones-urine | 0.020 | |||
| N-Miss | 26/47 (55.3%) | 106/171 (61.9%) | 132/218 (60.5%) | |
| Normal | 21/47 (44.6%) | 51/171 (29.8%) | 72/218 (33%) | |
| Abnormal | 0/47 (0%) | 14/171 (8.1%) | 14/218 (6.4%) | |
| Leukocytes-urine | 0.162 | |||
| N-Miss | 26/47 (55.3%) | 102/171 (59.6%) | 128/218 (58.7%) | |
| Normal | 7/47 (14.8%) | 35/171 (20.4%) | 42/218 (19.2%) | |
| Abnormal | 14/47 (29.7%) | 34/171 (19.8%) | 48/218 (22%) | |
| Specific gravity-urine | 0.918 | |||
| N-Miss | 29/47 (61.7%) | 113/171 (66%) | 142/218 (65.1%) | |
| mean ± SD | 1018.33 ± 5.941 | 1018.103 ± 8.777 | 1018.158 ± 8.159 | |
| Range | 1005.000-1025.000 | 1005.000-1030.000 | 1005.000-1030.000 |
| Accuracy | Sensitivity | Specificity | PPV | NPV |
| ID: 0.8361; 95%CI: 0.7191-0.9185 | ID: 0.9792 | ID: 0.3077 | ID: 0.8393 | ID: 0.800 |
| VD: 0.7607; 95%CI: 0.673-0.8347 | VD: 0.9630 | VD: 0.3056 | VD: 0.7573 | VD: 0.7857 |
Data from two large-volume university pancreas centers were used for score validation. In total, a validation cohort of 36 patients with microlithiasis and 81 non-microlithiasis AP patients were retrieved from the clinical database at the University Hospital of the Technical University Munich (22 × microlithiasis-AP, 51 × other-AP) as well as the University Hospital Göttingen (14 × microlithiasis-AP, 30 × other-AP; Figure 1 and Table 3). In the Technical University Munich cohort, the group of other-AP patients was mainly alcohol-related [31/51 (60.8%)], while in the Göttingen cohort biliary macrolithiasis was held responsible for the majority of AP patients [16/33 (53.3%)]. Idiopathic aetiology was named as the second most frequent aetiology group in both external cohorts with about 30% each [Technical University Munich 17/51 (33.3%), Göttingen 10/33 (33.3%)] (Supplementary Table 1). Microlithiasis patients in the validation cohort were on average 60.1 (SD 18.4) years old, while patients from the other-AP cohort were 55.3 (SD 16.8) years old. In both groups (microlithiasis + other-AP), the majority of patients were male [24/36 (66.7%) and 46/81 (56.8%), respectively], resem
| Variable | Microlithiasis (n = 36) | Other (n = 81) | Total (n = 117) | P value |
| Age (yr) | 0.162 | |||
| mean ± SD | 60.1 ± 18.4 | 55.3 ± 16.8 | 56.8 ± 17.4 | |
| Range | 23-93 | 21-87 | 21-93 | |
| Sex | 0.315 | |||
| Female | 12/36 (33.3%) | 35/81 (43.2%) | 47/117 (40.2%) | |
| Male | 24/36 (66.7%) | 46/81 (56.8%) | 70/117 (59.8%) | |
| Alkaline phosphatase | 0.032 | |||
| N-Miss | 1/36 (2.7%) | 12/81 (14.8%) | 13/117 (11.1%) | |
| ULN | 23/36 (63.8%) | 30/81 (37%) | 53/117 (45.2%) | |
| WL | 12/36 (33.2%) | 39/81 (48.1%) | 51/117 (43.5%) | |
| Total bilirubin | 0.003 | |||
| ULN | 21/36 (58.3%) | 24/81 (29.6%) | 45/117 (38.5%) | |
| WL | 15/36 (41.7%) | 57/81 (70.4%) | 72/117 (61.5%) | |
| Creatine kinase | 0.498 | |||
| N-Miss | 8/36 (22.2%) | 32/81 (39.5%) | 40/117 (34.1%) | |
| ULN | 5/36 (13.8%) | 6/81 (7.4%) | 11/117 (9.4%) | |
| WL | 23/36 (63.8%) | 43/81 (53%) | 66/117 (56.4%) | |
| CRP | 0.199 | |||
| ULN | 32/36 (88.9%) | 64/81 (79%) | 96/117 (88.8%) | |
| WL | 4/36 (11.1%) | 17/81 (21%) | 21/117 (17.9%) | |
| Total protein | 0.405 | |||
| N-Miss | 31/36 (86.1%) | 73/81 (90.1%) | 104/117 (88.8%) | |
| WL | 5/36 (13.8%) | 8/81 (9.8%) | 13/117 (11.1%) | |
| Erythrocytes | 0.650 | |||
| N-Miss | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| LLN | 10/36 (27.7%) | 25/81 (30.8%) | 35/117 (29.9%) | |
| ULN | 4/36 (11.1%) | 5/81 (6.1%) | 9/117 (7.6%) | |
| WL | 22/36 (61.1%) | 50/81 (61.7%) | 72/117 (61.5%) | |
| Gamma-GT | 0.082 | |||
| N-Miss | 0/36 (0%) | 2/81 (2.4%) | 2/117 (1.7%) | |
| ULN | 32/36 (88.8%) | 59/81 (72.8%) | 91/117 (77.7%) | |
| WL | 4/36 (11.1%) | 20/81 (24.6%) | 24/117 (20.5%) | |
| AST/GOT | 0.079 | |||
| N-Miss | 0/36 (0%) | 4/81 (4.9%) | 4/117 (3.4%) | |
| ULN | 28/36 (77.8%) | 47/81 (58%) | 75/117 (64.1%) | |
| WL | 8/36 (22.2%) | 30/81 (37%) | 38/117 (32.4%) | |
| ALT/GPT | 0.052 | |||
| N-Miss | 0/36 (0%) | 2/81 (2.4%) | 2/117 (1.7%) | |
| ULN | 28/36 (77.8%) | 43/81 (53%) | 71/117 (60.6%) | |
| WL | 8/36 (22.2%) | 30/81 (37%) | 38/117 (32.4%) | |
| Urea | ||||
| N-Miss | 7/36 (19.4%) | 20/81 (24.6%) | 27/117 (23%) | |
| LLN | 13/36 (36.1%) | 41/81 (50.6%) | 54/117 (46.1%) | |
| ULN | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| WL | 16/36 (44.4%) | 19/81 (23.4%) | 35/117 (29.9%) | |
| Hematocrit | < 0.001 | |||
| N-Miss | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| ULN | 36/36 (100%) | 80/81 (98.7%) | 116/117 (99.1%) | |
| Haemoglobin | 0.725 | |||
| N-Miss | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| LLN | 9/36 (25%) | 18/81 (22.2%) | 27/117 (23%) | |
| ULN | 3/36 (8.3%) | 4/81 (4.9%) | 7/117 (5.9%) | |
| WL | 32/36 (88.9%) | 64/81 (79.0%) | 96/117 (82%) | |
| INR | 0.440 | |||
| ULN | 2/36 (5.5%) | 8/81 (9.8%) | 10/117 (8.5%) | |
| WL | 34/36 (94.4%) | 73/81 (90.1%) | 107/117 (91.4%) | |
| Potassium | 0.985 | |||
| LLN | 2/36 (5.5%) | 4/81 (4.9%) | 6/117 (5.1%) | |
| ULN | 1/36 (2.7%) | 2/81 (2.4%) | 3/117 (2.5%) | |
| WL | 33/36 (91.6%) | 75/81 (92.5%) | 108/117 (92.3%) | |
| Serum creatinine | 0.909 | |||
| LLN | 2/36 (5.5%) | 6/81 (7.4%) | 8/117 (6.8%) | |
| ULN | 7/36 (19.4%) | 14/81 (17.2%) | 21/117 (17.9%) | |
| WL | 27/36 (75%) | 61/81 (75.3%) | 88/117 (75.2%) | |
| LDH | 0.018 | |||
| N-Miss | 6/36 (16.6%) | 28/81 (34.5%) | 34/117 (29%) | |
| ULN | 26/36 (72.2%) | 33/81 (40.7%) | 59/117 (50.4%) | |
| WL | 4/36 (11.1%) | 20/81 (24.6%) | 24/117 (20.5%) | |
| Leukocytes | 0.143 | |||
| N-Miss | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| LLN | 1/36 (2.7%) | 0/81 (0%) | 1/117 (0.8%) | |
| ULN | 16/36 (44.4%) | 47/81 (58%) | 63/117 (53.8%) | |
| WL | 19/36 (52.7%) | 33/81 (40.7%) | 52/117 (44.4%) | |
| Lipase | 0.237 | |||
| N-Miss | 0/36 (0%) | 2/81 (2.4%) | 2/117 (1.7%) | |
| ULN | 32/36 (88.9%) | 75/81 (92.5%) | 107/117 (91.4%) | |
| WL | 4/36 (11.1%) | 4/81 (4.9%) | 8/117 (6.8%) | |
| MCV | 0.315 | |||
| N-Miss | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| LLN | 3/36 (8.3%) | 7/81 (8.6%) | 10/117 (8.5%) | |
| ULN | 1/36 (2.7%) | 9/81 (11.1%) | 10/117 (8.5%) | |
| WL | 32/36 (88.9%) | 64/81 (79%) | 96/117 (82%) | |
| Triglycerides | 0.582 | |||
| N-Miss | 26/36 (72.2%) | 43/81 (53%) | 69/117 (58.9%) | |
| ULN | 3/36 (8.3%) | 15/81 (18.5%) | 18/117 (15.3%) | |
| WL | 7/36 (19.4%) | 23/81 (28.3%) | 30/117 (25.6%) | |
| RDW | ||||
| N-Miss | 36/36 (100%) | 81/81 (100%) | 117/117 (100%) | |
| False | 0/36 (0%) | 0/81 (0%) | 0/117 (0%) | |
| True | 0/36 (0%) | 0/81 (0%) | 0/117 (0%) | |
| Sodium | 0.154 | |||
| LLN | 2/36 (5.5%) | 12/81 (14.8%) | 14/117 (11.9%) | |
| WL | 34/36 (94.4%) | 69/81 (85.1%) | 103/117 (88%) | |
| Quick’s value | 0.130 | |||
| LLN | 2/36 (5.5%) | 6/81 (7.4%) | 8/117 (6.8%) | |
| ULN | 13/36 (36.1%) | 44/81 (54.3%) | 57/117 (48.7%) | |
| WL | 21/36 (58.3%) | 31/81 (38.2%) | 52/117 (44.4%) | |
| Thrombocytes | 0.627 | |||
| N-Miss | 22/36 (61.1%) | 51/81 (62.9%) | 73/117 (62.3%) | |
| LLN | 2/36 (5.5%) | 3/81 (3.7%) | 5/117 (4.2%) | |
| ULN | 2/36 (5.5%) | 2/81 (2.4%) | 4/117 (3.4%) | |
| WL | 10/36 (27.7%) | 25/81 (30.8%) | 35/117 (29.9%) | |
| TSH | 0.773 | |||
| N-Miss | 6/36 (16.6%) | 13/81 (16%) | 19/117 (16.2%) | |
| LLN | 0/36 (0%) | 1/81 (1.2%) | 1/117 (0.8%) | |
| ULN | 1/36 (2.7%) | 3/81 (3.7%) | 4/117 (3.4%) | |
| WL | 29/36 (80.5%) | 64/81 (79%) | 93/117 (79.4%) |
Previous and more recent studies on idiopathic pancreatitis still report a proportion of idiopathic pancreatitis stably at 20%-30%[12,13]. However, it has been suspected for decades and is increasingly supported by evidence that a large proportion of pancreatitis patients classified primarily as idiopathic actually suffer from a biliary aetiology and that detecting these patients during the first episode of pancreatitis is restricted due to the lack of availability of timely and high quality EUS exams[14]. Furthermore, there is a lack of reliable data on when, during an inpatient stay of an IAP-labeled patient, an EUS could detect biliary microconcrements as the trigger for pancreatitis without causing an unne
Our study has several limitations. First, the retrospective study approach did not allow us to generate a uniform definition of the two entities microlithiasis and sludge. Even after extensive literature research, we were unable to delineate a uniform but distinct definition of biliary microlithiasis and sludge. We thus decided to use the terms as synonyms between the endoscopy centers of the three participating university hospitals. This might impose a significant bias. The macrolithiasis, which was again clearly listed in the endoscopy findings across the universities, ensured quality of EUS. Likewise, the patient cohort declared as other-AP in terms of aetiology varied greatly between the participating centers (Supplementary Table 1). Ultimately, this probably reflects the individual diagnostic scope and the question of whether EUS can generate added value in the context of the individual patient. Also, due to the retrospective study design, no attempt could be made to increase the degree of purity of biliary (microlithiasis and sludge) triggered pancreatitis by uniformly fulfilling laboratory chemistry tests prior to EUS. This resulted in a proportion of patients of 36.6% with, for example, missing calcium values in the laboratory chemistry pancreatitis workup.
Our study is convincing in presenting for the first time a robust ML-based and externally validated prediction model for pancreatitis patients declared idiopathic early in the diagnostic workup and may be helpful as a noninvasive decision tool by combining simple and widely used laboratory values to decide for or against EUS. In order to make the micro
We present for the first time an ML-based tool, externally validated in two sets of data from tertiary pancreatic referral centers, to predict the presence of biliary sludge and microlithiasis in patients with an initial label of idiopathic pancreatitis with an accuracy of 0.7607 (95%CI: 0.673-0.8347), PPV of 0.7573, and NPV of 0.7857. Upon prospective validation, the prediction score will aid in decision-making on which patient to subject to EUS for diagnostic workup at a first epi
About 30% of acute pancreatitis (AP) cases classified as idiopathic actually have a biliary and thus monocausally treatable origin.
To date, there is no predictive score to differentiate between idiopathic and sludge- and microlithiasis-triggered acute biliary pancreatitis. Undiagnosed biliary pancreatitis aetiology poses the risk of overdiagnosis and additional patient burden. AP triggered by small biliary concrements (microlithiasis and sludge) is a particularly challenging diagnosis.
The aim of this study was to develop a machine-learning based prediction score for the presence of microlithiasis and sludge in AP patients. External score validation was performed at two university pancreas centres.
The clinical and laboratory parameters of 218 AP patients were used to calculate a machine-learning based prediction model for the presence of sludge and microlithiasis. Forty-seven patients with endosonographic evidence of sludge and microlithiasis (and no other possible underlying pancreatitis aetiology) were used in the identification cohort and compared with 171 AP patients without endosonographic evidence of sludge and microlithiasis. We trained supervised machine learning classifiers using H2O.ai automatically selecting the best suitable predictor model to predict micro
The score, constructed from a total of 28 simple variables to be collected in the early phase of pancreatitis-associated hospitalisation and validated externally at two university pancreas centres, can predict the presence of biliary sludge and microlithiasis with an accuracy of 0.7607 (95% confidence interval: 0.673-0.8347), positive predictive value of 0.7573, and negative predictive value of 0.7857.
For the first time, we present a machine-learning based prediction score to differentiate between sludge- and micro
Upon prospective validation, the prediction score will aid in decision-making on which patient to subject to endosonography for diagnostic workup at a first episode of pancreatitis specifically to differentiate between sludge/microlithiasis-triggered and idiopathic AP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Liu C, China; Zhao CF, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 363] [Article Influence: 121.0] [Reference Citation Analysis (1)] |
| 2. | Lee JK, Enns R. Review of idiopathic pancreatitis. World J Gastroenterol. 2007;13:6296-6313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 245] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 4. | Hallensleben ND, Umans DS, Bouwense SA, Verdonk RC, Romkens TE, Witteman BJ, Schwartz MP, Spanier MB, Laheij R, van Santvoort HC, Besselink MG, van Hooft JE, Bruno MJ; Dutch Pancreatitis Study Group. The diagnostic work-up and outcomes of 'presumed' idiopathic acute pancreatitis: A post-hoc analysis of a multicentre observational cohort. United European Gastroenterol J. 2020;8:340-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Umans DS, Rangkuti CK, Sperna Weiland CJ, Timmerhuis HC, Bouwense SAW, Fockens P, Besselink MG, Verdonk RC, van Hooft JE; Dutch Pancreatitis Study Group. Endoscopic ultrasonography can detect a cause in the majority of patients with idiopathic acute pancreatitis: a systematic review and meta-analysis. Endoscopy. 2020;52:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Lee YS, Kang BK, Hwang IK, Kim J, Hwang JH. Long-term Outcomes of Symptomatic Gallbladder Sludge. J Clin Gastroenterol. 2015;49:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Chebli JM, Duarte Gaburri P, Meirelles de Souza AF, de Castro Ferreira LE, Andrade Chebli L, Ferrari AP Jr, Martins das Neves M. "Idiopathic" acute pancreatitis due to biliary sludge: prevention of relapses by endoscopic biliary sphincterotomy in high-risk patients. Am J Gastroenterol. 2000;95:3008-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, Irwig L, Levine D, Reitsma JB, de Vet HC, Bossuyt PM. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1579] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 9. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1042] [Article Influence: 86.8] [Reference Citation Analysis (6)] |
| 10. | Beyer G, Hoffmeister A, Michl P, Gress TM, Huber W, Algül H, Neesse A, Meining A, Seufferlein TW, Rosendahl J, Kahl S, Keller J, Werner J, Friess H, Bufler P, Löhr MJ, Schneider A, Lynen Jansen P, Esposito I, Grenacher L, Mössner J, Lerch MM, Mayerle J; Collaborators:. S3-Leitlinie Pankreatitis – Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) – September 2021 – AWMF Registernummer 021-003. Z Gastroenterol. 2022;60:419-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 11. | Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 892] [Cited by in RCA: 1216] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 12. | Kim HJ, Kim MH, Bae JS, Lee SS, Seo DW, Lee SK. Idiopathic acute pancreatitis. J Clin Gastroenterol. 2003;37:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Del Vecchio Blanco G, Gesuale C, Varanese M, Monteleone G, Paoluzi OA. Idiopathic acute pancreatitis: a review on etiology and diagnostic work-up. Clin J Gastroenterol. 2019;12:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Umans DS, Timmerhuis HC, Hallensleben ND, Bouwense SA, Anten MG, Bhalla A, Bijlsma RA, Boermeester MA, Brink MA, Hol L, Bruno MJ, Curvers WL, van Dullemen HM, van Eijck BC, Erkelens GW, Fockens P, van Geenen EJM, Hazen WL, Hoge CV, Inderson A, Kager LM, Kuiken SD, Perk LE, Poley JW, Quispel R, Römkens TE, van Santvoort HC, Tan AC, Thijssen AY, Venneman NG, Vleggaar FP, Voorburg AM, van Wanrooij RL, Witteman BJ, Verdonk RC, Besselink MG, van Hooft JE; Dutch Pancreatitis Study Group. Role of endoscopic ultrasonography in the diagnostic work-up of idiopathic acute pancreatitis (PICUS): study protocol for a nationwide prospective cohort study. BMJ Open. 2020;10:e035504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Oría A, Alvarez J, Chiapetta L, Fontana JJ, Iovaldi M, Paladino A, Bianchi R, Frider B. Risk factors for acute pancreatitis in patients with migrating gallstones. Arch Surg. 1989;124:1295-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | ASGE Standards of Practice Committee; Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, Ikenberry SO, Jain R, Khan K, Krinsky ML, Strohmeyer L, Dominitz JA. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 335] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 17. | Phillip V, Huber W, Hagemes F, Lorenz S, Matheis U, Preinfalk S, Schuster T, Lippl F, Saugel B, Schmid RM. Incidence of acute pancreatitis does not increase during Oktoberfest, but is higher than previously described in Germany. Clin Gastroenterol Hepatol. 2011;9:995-1000.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Umans DS, Hallensleben ND, Verdonk RC, Bouwense SAW, Fockens P, van Santvoort HC, Voermans RP, Besselink MG, Bruno MJ, van Hooft JE; Dutch Pancreatitis Study Group. Recurrence of idiopathic acute pancreatitis after cholecystectomy: systematic review and meta-analysis. Br J Surg. 2020;107:191-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Yin M, Zhang R, Zhou Z, Liu L, Gao J, Xu W, Yu C, Lin J, Liu X, Xu C, Zhu J. Automated Machine Learning for the Early Prediction of the Severity of Acute Pancreatitis in Hospitals. Front Cell Infect Microbiol. 2022;12:886935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 20. | Jin X, Ding Z, Li T, Xiong J, Tian G, Liu J. Comparison of MPL-ANN and PLS-DA models for predicting the severity of patients with acute pancreatitis: An exploratory study. Am J Emerg Med. 2021;44:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Choi HW, Park HJ, Choi SY, Do JH, Yoon NY, Ko A, Lee ES. Early Prediction of the Severity of Acute Pancreatitis Using Radiologic and Clinical Scoring Systems With Classification Tree Analysis. AJR Am J Roentgenol. 2018;211:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Zarnescu NO, Costea R, Zarnescu Vasiliu EC, Neagu S. Clinico-biochemical factors to early predict biliary etiology of acute pancreatitis: age, female gender, and ALT. J Med Life. 2015;8:523-526. [PubMed] |