Published online Sep 14, 2023. doi: 10.3748/wjg.v29.i34.5082
Peer-review started: April 19, 2023
First decision: May 27, 2023
Revised: June 9, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: September 14, 2023
Processing time: 142 Days and 5 Hours
Neuroendocrine tumors (NET) are rare heterogeneous tumors that arise from neuroendocrine cells throughout the body. Acromegaly, a rare and slowly progressive disorder, usually results from a growth hormone (GH)-secreting pituitary adenoma.
We herein describe a 38-year-old patient who was initially diagnosed with diabetes. During colonoscopy, two bulges were identified and subsequently removed through endoscopic submucosal dissection. Following the surgical intervention, the excised tissue samples were examined and confirmed to be grade 2 NET. 18F-ALF-NOTATATE positron emission tomography-computed tomography (PET/CT) and 68Ga-DOTANOC PET/CT revealed metastases in the peri-intestinal lymph nodes, prompting laparoscopic low anterior resection with total mesorectal excision. The patient later returned to the hospital because of hyperglycemia and was found to have facial changes, namely a larger nose, thicker lips, and mandibular prognathism. Laboratory tests and magnetic resonance imaging (MRI) suggested a GH-secreting pituitary adenoma. The pituitary adenoma shrunk after treatment with octreotide and was neuroendoscopically resected via a trans-sphenoidal approach. Whole-exome sequencing analysis revealed no genetic abnormalities. The patient recovered well with no evidence of recurrence during follow-up.
18F-ALF-NOTATE PET/CT and MRI with pathological analysis can effectively diagnose rare cases of pituitary adenomas complicated with rectal NET.
Core Tip: We herein present a rare case of rectal dual-source grade 2 neuroendocrine tumors with a pituitary growth hormone-secreting tumor that caused acromegaly and diabetes. The rarity of this combination makes an accurate diagnosis difficult to achieve. The correct diagnosis can be obtained by using 18F-ALF-NOTATATE-positron emission tomography–computed tomography and magnetic resonance imaging combined with pathological analysis.
- Citation: Li JY, Chen J, Liu J, Zhang SZ. Simultaneous rectal neuroendocrine tumors and pituitary adenoma: A case report and review of literature. World J Gastroenterol 2023; 29(34): 5082-5090
- URL: https://www.wjgnet.com/1007-9327/full/v29/i34/5082.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i34.5082
Neuroendocrine tumors (NETs) are a heterogeneous group of rare tumors that exhibit complex clinical behaviors. They typically originate from peptidergic neurons and neuroendocrine cells throughout the body, with gastroenteropancreatic NETs being the predominant form. The rectum is the most common location of these tumors, accounting for 37.4% of cases[1,2]. Acromegaly, another rare disorder that often goes undiagnosed, is a chronic, progressive endocrine and metabolic disease with an inconspicuous onset. The main cause of acromegaly is excessive production of growth hormone (GH), and > 95% of patients with enlarged limbs have pituitary adenomas that secrete GH[3]. GH stimulates the liver to produce insulin-like growth factor-1 (IGF-1), and excessive proliferation of soft tissue, bone, and cartilage can occur secondary to long-term excessive secretion of GH and IGF-1. This results in the typical symptom of enlarged limbs and can have a significant impact on various organ systems, such as the respiratory, cardiovascular, digestive, and glucose metabolism systems. Compression or invasion of pituitary adenomas can result in headaches, visual impairment, and adenohypophysis. The median age at diagnosis of acromegaly ranges from 40.5 years to 47.0 years, with delays in diagnosis lasting from 4.5 years to 9.0 years. Delayed diagnosis can significantly increase the incidence of complications and difficulty in treating patients with large limbs. In this case report, we describe a 38-year-old woman who was diagnosed with two concurrent grade 2 (G2) rectal NETs and a pituitary adenoma on the basis of 18F-ALF-NOTATATE positron emission tomography-computed tomography (PET/CT) and 18F-fluorodeoxyglucose (18F-FDG) PET/CT findings. To the best of our knowledge, the combination of simultaneous rectal NETs and a GH-secreting pituitary adenoma has not been previously reported. To diagnose this disorder, patients may undergo both 18F-ALF-NOTATATE PET/CT and
A 38-year-old woman was admitted to our hospital for evaluation of long-standing chronic constipation and polydipsia.
Upon admission to the hospital, the patient underwent colonoscopy, which revealed two masses. The masses were confirmed to be G2 NETs after endoscopic submucosal dissection and pathological examination. Peri-intestinal lymph node metastasis was detected using 18F-ALF-NOTATATE PET/CT and 18F-FDG PET/CT, leading to treatment by laparoscopic low anterior resection with total mesenterectomy. After surgery, the patient exhibited symptoms of poor blood glucose control, and further examination revealed the presence of a pituitary macroadenoma. The patient received three cycles of octreotide acetate before undergoing surgical intervention for the pituitary macroadenoma.
The patient was diagnosed with diabetes on the basis of polydipsia and abnormal blood glucose concentrations.
She had no family history of NETs or psychological or genetic disorders.
All vital signs were stable and physical examination revealed no notable abnormalities.
Laboratory examinations showed that the patient had a high GH concentration at 47.79 ng/mL (reference range, 0.126-9.88 ng/mL) and high IGF-1 concentration at 497 ng/mL (reference range, 111-284 ng/mL). However, she had normal serum concentrations of sex hormones, adrenocorticotropic hormone (ACTH), thyrotropin, free thyroid hormone, and prolactin. Blood samples were collected at Peking Union Medical College Hospital 0, 30, 60, 120, and 180 min after a standard 75-g glucose test; at each of these time points, her plasma GH concentration was 29.1, 20.9, 19.2, 23.2, and 28.4 ng/mL, respectively.
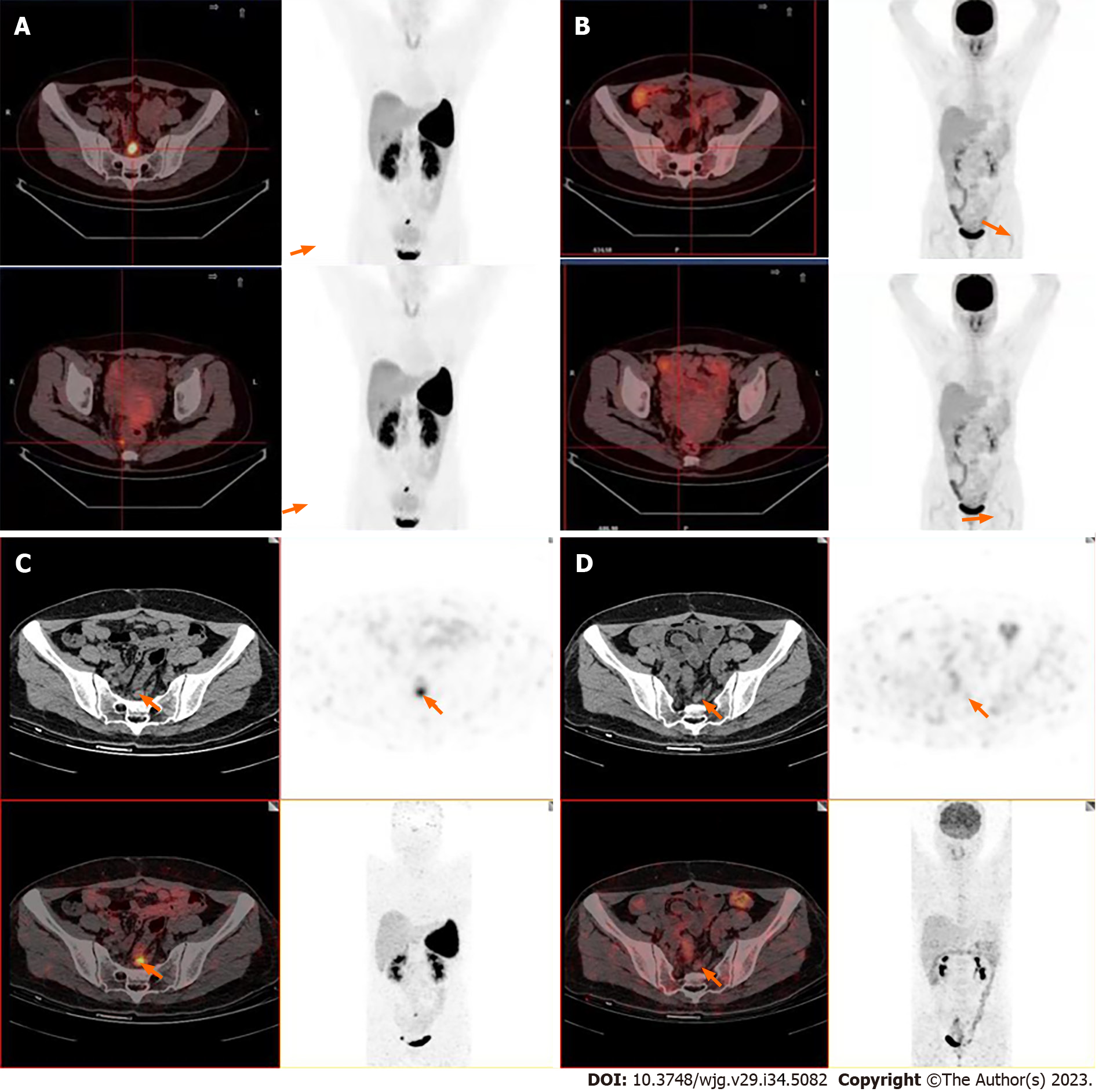
18F-ALF-NOTATATE PET/CT and 18F-FDG PET/CT were performed to detect metastases (Figure 1A and B). 18F-ALF-NOTATATE PET/CT clearly revealed metastases and high uptake in three peri-rectal and pre-sacral lymph nodes with a maximum standardized uptake value (SUV) of 13.24, 7.31, and 5.55, respectively, whereas these findings were not depicted on 18F-FDG PET/CT. The patient then underwent 68Ga-DOTANOC PET/CT at the First Affiliated Hospital of Sun Yat-Sen University. The 68Ga-DOTANOC PET/CT findings were consistent with the 18F-ALF-NOTATATE PET/CT findings, showing that the largest affected lymph node had a maximum SUV of 4.8 (Figure 1C and D).
The patient was diagnosed with rectal NETs with peripheral lymph node metastasis. The final pathology report listed rectal NETs, G2, stage T1N1M0 with 4/15 lymph node metastases. The patient also had acromegaly and secondary diabetes caused by a GH-secreting pituitary adenoma.
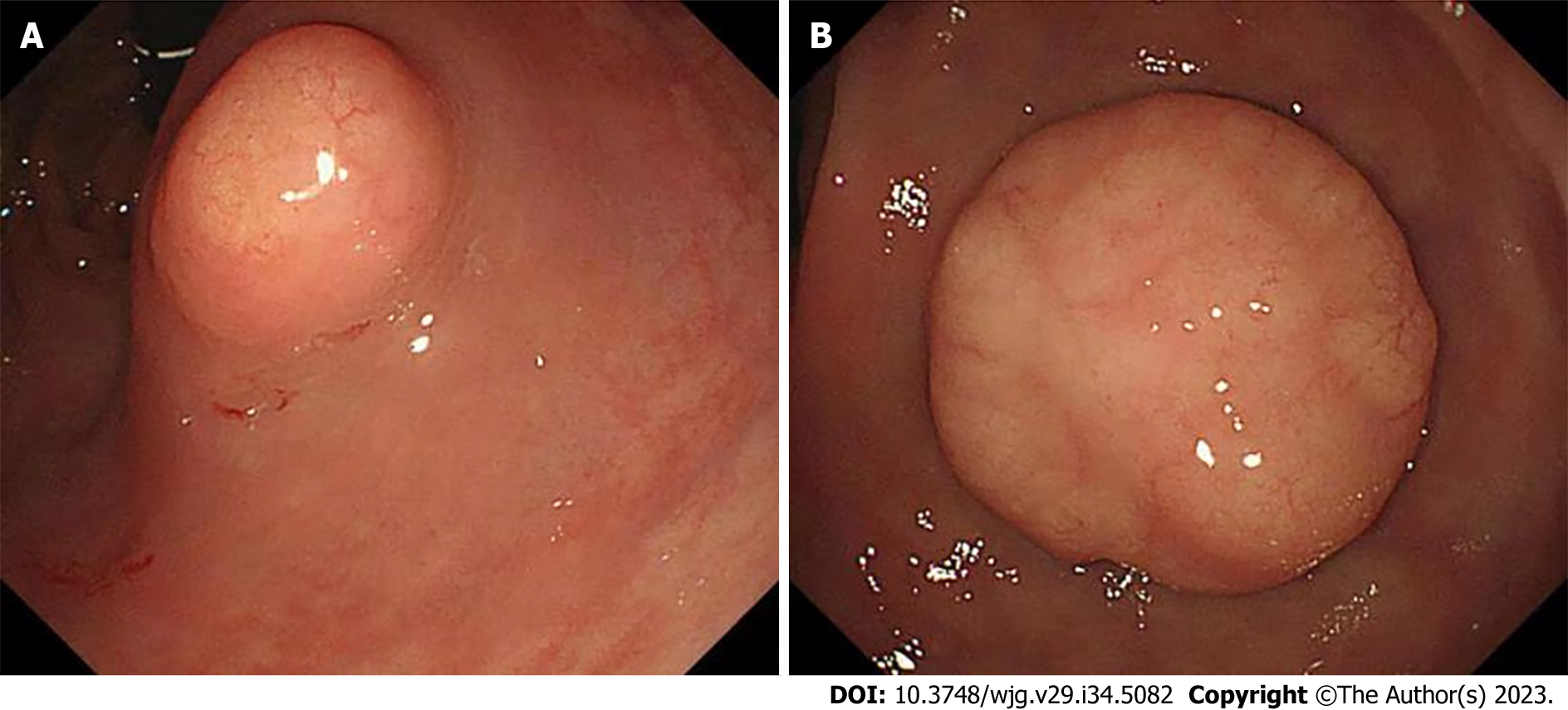
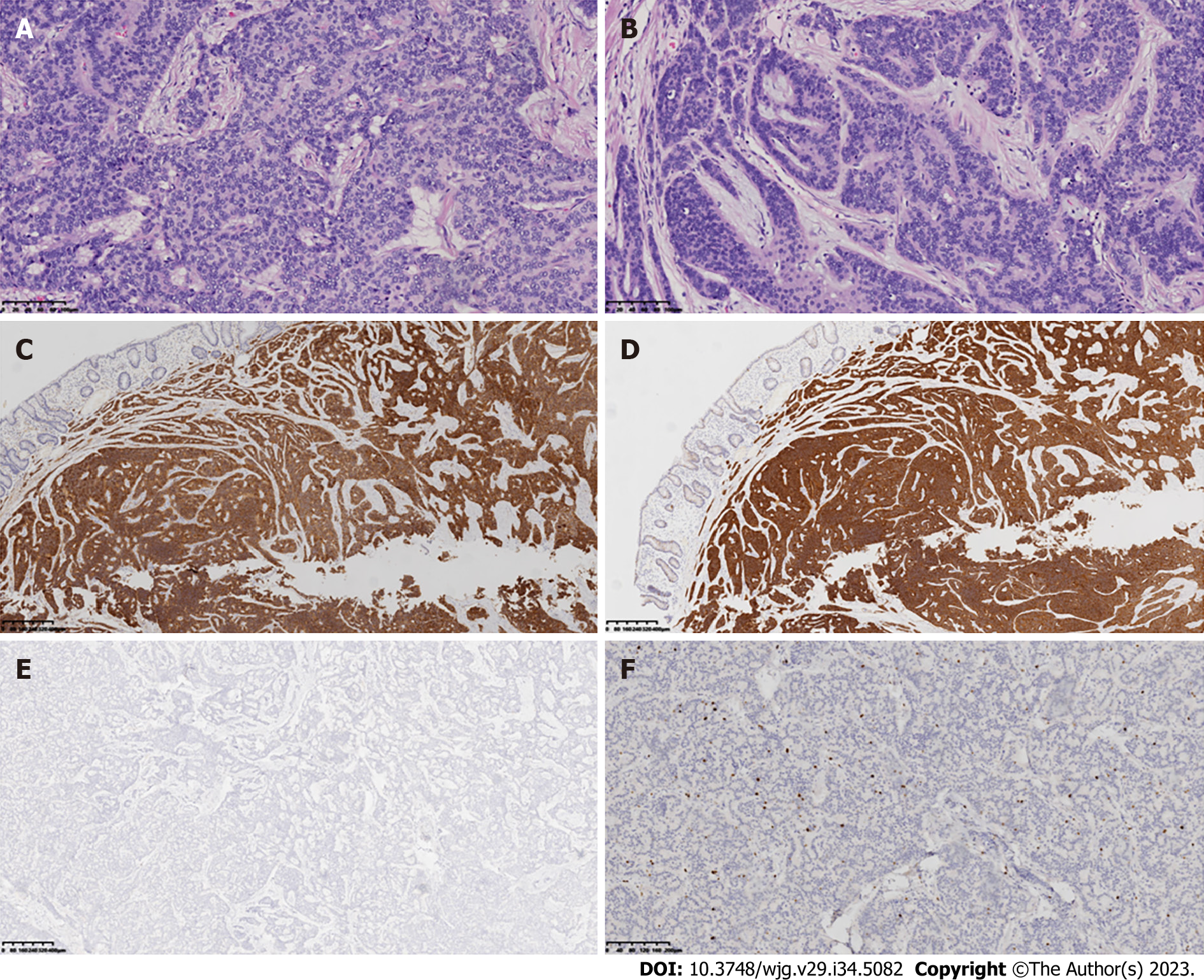
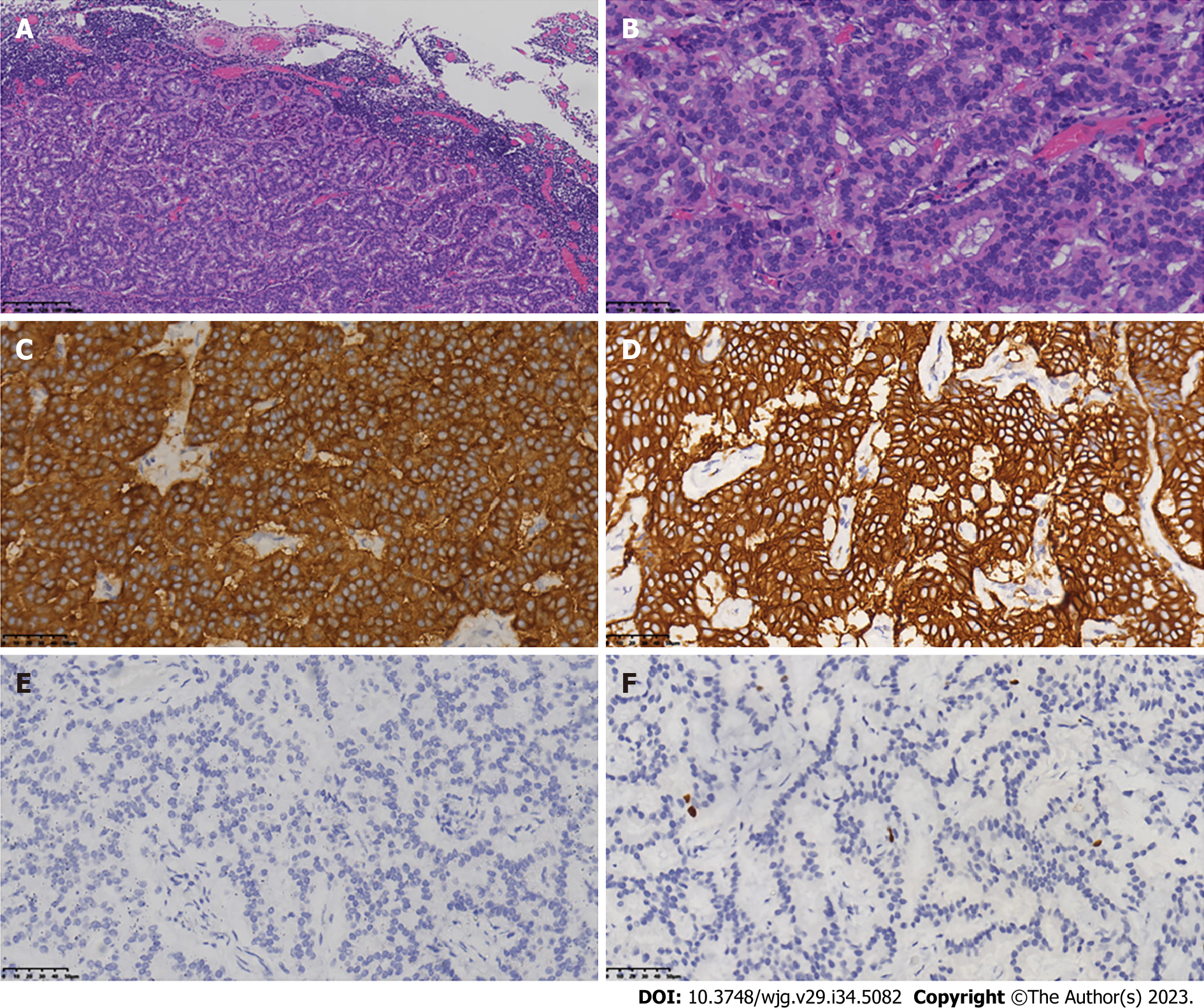
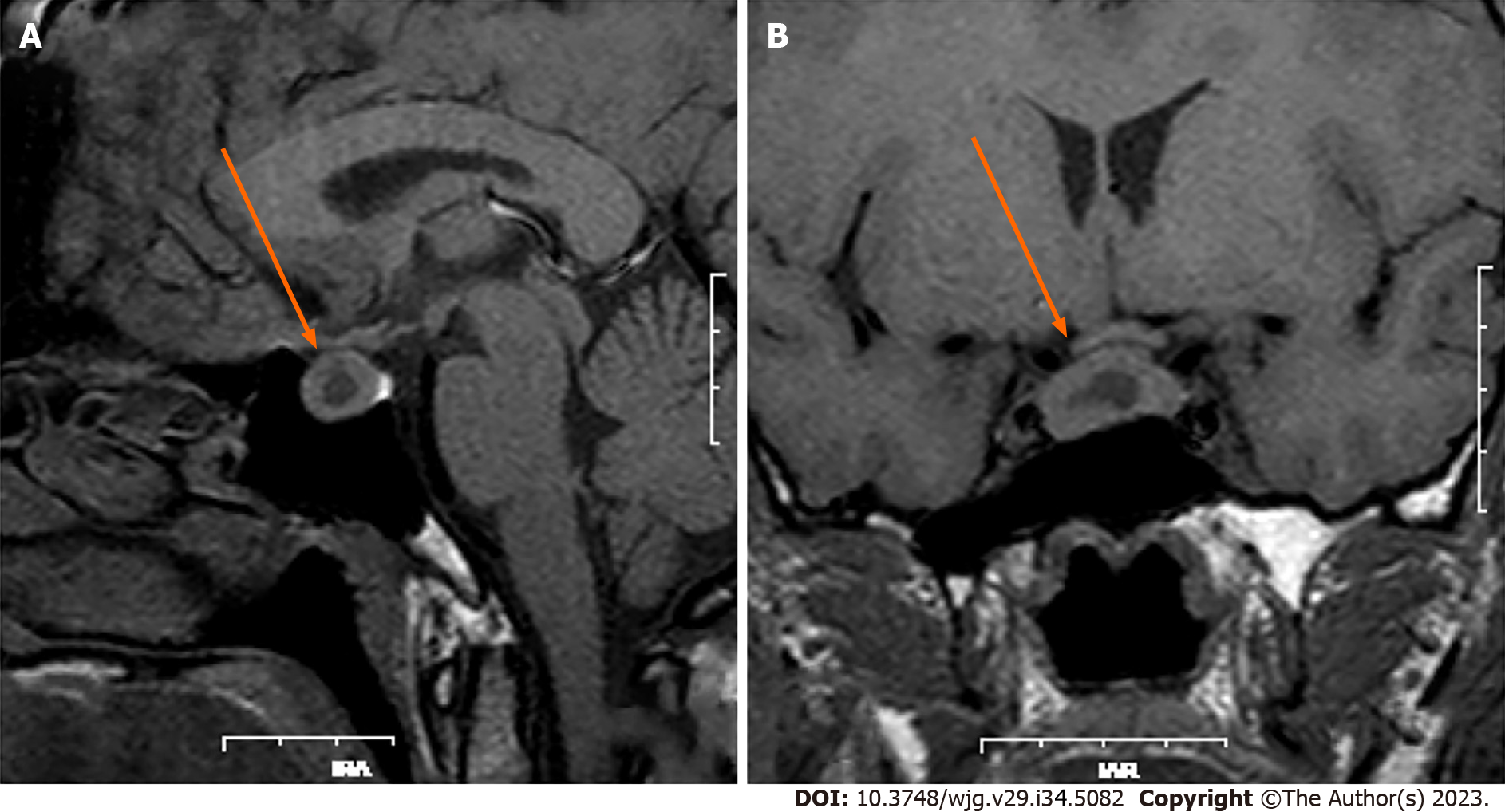
Because of chronic constipation, colonoscopy was performed and revealed two lesions of 1.0 cm and 1.8 cm in diameter located 3.0 cm from the anus (Figure 2). These rectal lesions were resected by minimally invasive endoscopic submucosal dissection on 27 June 2019. Pathological examination of the two rectal specimens suggested histologic G2 NETs that were positive for synaptophysin and CD56 but negative for chromogranin A (Figure 3). The Ki67 index was 7% for the 1.8-cm-diameter tumor and 4% for the 1.0-cm-diameter tumor. Strong expression of SSTR2 (+++) suggested a predominance of the SSTR2 subtype. Only a few scattered cells were phosphorylated histone H3-positive. The larger lesion was 0.1 cm from the circumferential margin, whereas the smaller lesion was adjacent to the excision edge. 18F-ALF-NOTATATE PET/CT and 18F-FDG PET/CT scans in our hospital showed metastasis and high uptake in three perirectal and presacral lymph nodes. Laparoscopic low anterior resection with total mesorectal excision was performed 5 mo after diagnosis of the rectal lesions. Histological analysis of the operative specimens showed that the lymph nodes contained neuroendocrine cells. Immunohistochemical staining was positive for synaptophysin and CD56 but negative for chromogranin A. The Ki67 index was 3% (Figure 4). Four months after the rectal lesion was diagnosed by pathological examination, the patient returned to the local hospital because of hyperglycemia, and examination revealed facial changes including an enlarged nose, thickened lips, and mandibular kyphosis. A 2.4 cm × 1.3 cm × 1.3 cm pituitary adenoma was revealed by contrast-enhanced magnetic resonance imaging (MRI) (Figure 5). The GH-secreting pituitary macroadenoma was diagnosed by pathological examination. The patient was treated with long-acting octreotide (20 mg) at an interval of 28 d, and the lesion was reduced to 1.16 cm × 0.61 cm × 1.27 cm after three courses of treatment. The patient then underwent successful neuroendoscopic pituitary surgery via a trans-sphenoidal approach with sellar base reconstruction. Postoperative pathological examination confirmed the diagnosis of a pituitary adenoma, which was positive for GH and CAM5.2, was negative for ACTH, and had a Ki67 index of < 1%. The patients’ skin condition significantly improved, becoming smoother and more refined after undergoing neuroendoscopic pituitary gland surgery. Moreover, her biochemical indicators consistently remained within the reference range postoperatively, especially with regard to her blood glucose control, which normalized without the need for hypoglycemic drugs. Given that the patients’ GH-secreting pituitary tumor was a macroadenoma, direct surgery was challenging and complete removal was difficult. However, the tumor gradually decreased in size with the three cycles of octreotide acetate microsphere treatment, allowing successful R0 resection through timely surgical intervention. Thereafter, follow-up head MRI findings remained normal and the serum GH and IGF-1 concentrations consistently stayed within the reference range.
The patient returned for follow-up at 4, 13, and 16 mo after her pituitary surgery. No evidence of recurrence was found, and she remained asymptomatic.
Rectal NETs can cause symptoms such as bleeding or changes in bowel habits; however, many patients are asymptomatic. The incidence of gastrointestinal NETs, including rectal NETs, is increasing, with a reported incidence of 1.04 per 100000 individuals per year according to the surveillance, epidemiology and end results database[4]. This increase is likely due to improved diagnostic endoscopy rather than a genuine increase in incidence.
Approximately 80% of rectal NETs are small (< 1 cm). There is typically no invasion or metastasis at the time of initial diagnosis. A strong correlation reportedly exists between the tumor size and spread, with only 2% of tumors under 1 cm having metastasized. The incidence of metastasis of rectal NETs ranges from 10% to 15% for 1- to 2-cm tumors but increases to 60% to 80% for tumors larger than 2 cm[5-7]. Treatment for localized G1 and G2 rectal NETs can be purely endoscopic, whereas low anterior resection of the rectum and removal of lymph nodes is necessary for advanced, localized rectal NETs. Most rectal NETs are located in the mid-section of the rectum, approximately 5 cm to 10 cm from the anus[5,8]. In the present case, concurrent rectal G2 NETs were detected in the distal rectum approximately 3 cm from the anus. 18F-ALF-NOTATATE PET/CT showed evidence of lymph node metastases, making this the first patient to be diagnosed with NETs by a combination of 18F-ALF-NOTATATE PET/CT and 18F-FDG PET/CT in our hospital. To confirm these findings, we referred the patient to the First Affiliated Hospital of Sun Yat-Sen University for a 68Ga-DOTANOC PET/CT scan, the results of which were consistent with our initial findings. The 18F-based method is promising, with 18F-octreotide being a potential alternative to 68Ga-DOTA peptides. 18F-AlF-NOTA-octreotide (18F-OC) is a peptide imaging agent that can be rapidly synthesized and demonstrates strong uptake by tumors[9,10]. The combination of 18F-FDG and 18F-OC PET/CT has the potential to enhance the staging and management of neuroendocrine neoplasms[11]. At our hospital, more than 200 patients have undergone combined 18F-ALF-NOTATATE PET/CT and 18F-FDG PET/CT scanning to detect rectal NETs.
Pituitary adenomas are among the most common primary central nervous system tumors; their estimated prevalence is 17% of all such tumors[12-14]. Approximately half (46%-64%) of these tumors are hormone-secreting (i.e. functional). Frequently secreted hormones include prolactin, GH, thyrotropin, and ACTH. GH-secreting adenomas account for 13% to 20% of hormone-secreting pituitary adenomas. They cause gigantism before and acromegaly after closure of the epiphyseal growth plates[15-17]. GH hypersecretion leads to acral enlargement (77%), coarse facial features (54%), profuse sweating (52%), and insulin resistance (15%). A diagnosis of acromegaly is often confirmed by a high serum concentration of IGF-1. When the IGF-1 concentration is equivocal, an oral glucose tolerance test may be performed, and the absence of GH suppression to < 1 ng/mL is indicative of acromegaly[18]. MRI of the pituitary gland is used to assess the size and location of an adenoma. Our patient was diagnosed with a pituitary adenoma resulting in acromegaly and diabetes mellitus. Despite being histologically benign, hormone-secreting pituitary adenomas are associated with significant morbidity resulting from their direct impact on surrounding neurovascular structures and/or excessive hormone secretion, both of which may lead to a shortened lifespan[19-21]. Early diagnosis and effective management are critical for reducing morbidity and minimizing mortality.
The global prevalence of acromegaly, a rare and slowly progressive disorder, is approximately 60 per million. Acromegaly occurs when the pituitary gland produces too much GH[22-24]. Pituitary adenomas, which are responsible for approximately 90% of cases of acromegaly, are often very large at the time of detection because of delays in diagnosis. We have herein reported a very rare case. Although familial hereditary syndromes can be linked to the disease, our patient had no known family history of NETs, and whole-exome sequencing with an illumina genome analyzer (Illumina, San Diego, CA, United States) at Jinyu Medicine revealed no genetic abnormalities.
Krug et al[25] described a patient with acromegaly that was caused not by a pituitary adenoma but instead by a sporadic pulmonary NET[26]. Here, we have reported the simultaneous occurrence of rectal NETs with a GH-secreting pituitary adenomas; to the best of our knowledge, this combination of tumors has not been previously reported. The increased risk of colonic polyposis in individuals with acromegaly is well-recognized[21]. Several studies have shown that patients with acromegaly have a 2 to 14-fold increased risk of colon cancer compared with the normal population[27-30]. Furthermore, nodular thyroid disease is frequently seen in patients with acromegaly[31], some of whom have thyroid malignancies. The co-occurrence of rectal NETs and a GH-secreting pituitary adenoma in the present patient may have been coincidental. There is emerging evidence of an association between the GH/IGF-1 endocrine axis and cancer progression[32]. Excessive secretion of GH and IGF-1 by the pituitary adenoma may have stimulated the growth of our patients’ rectal NETs. Surgical resection of the adenoma is the preferred treatment option for patients diagnosed with a GH-secreting pituitary adenoma. This approach can effectively eliminate or reduce the adenoma and decrease the levels of GH and IGF-1. The transsphenoidal approach is the primary method utilized to perform surgical interventions on pituitary adenomas, whereas craniotomy is only required in rare cases. Despite significant advancements in surgical techniques, there are still inherent risks associated with major extremity surgery, such as olfactory disturbance; hypopituitarism; temporary or permanent central diabetes insipidus; damage to vital nerves, blood vessels, brain tissue, and the blood supply of the skull base, resulting in postoperative cranial nerve dysfunction such as optic nerve impairment; cerebrospinal fluid rhinorrhea; meningitis; bacteremia; sepsis; hypothalamic syndrome; and even death. Patients with GH-secreting pituitary adenomas are at significantly higher risk when undergoing general anesthesia than are patients with other types of pituitary adenomas. These risks include abnormal cardiopulmonary function, increased perioperative risk, and difficulty in tracheal intubation and extubation because of soft tissue hyperplasia, making the operation more challenging for surgeons. The incidence and risk of rectal NETs in patients with acromegaly resulting from GH-secreting pituitary adenomas are unknown. Further research is necessary to elucidate the underlying mechanisms and to determine the clinical significance of this association.
We have herein presented a rare case of concurrent rectal G2 NETs combined with a pituitary adenoma. Pituitary adenomas lead to acromegaly and diabetes mellitus. Accurate diagnosis of this patients’ condition was difficult. 18F-ALF-NOTATATE PET/CT and pathological analysis achieved the correct diagnoses in this case.
We sincerely appreciate the patient and her family for their cooperation in information acquisition, treatment, and follow-up.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasan A, Egypt; Lykoudis PM, United Kingdom S-Editor: Qu XL L-Editor: Filipodia P-Editor: Yu HG
| 1. | Fang C, Wang W, Zhang Y, Feng X, Sun J, Zeng Y, Chen Y, Li Y, Chen M, Zhou Z, Chen J. Clinicopathologic characteristics and prognosis of gastroenteropancreatic neuroendocrine neoplasms: a multicenter study in South China. Chin J Cancer. 2017;36:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M. Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis. 2021;53:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | AlDallal S. Acromegaly: a challenging condition to diagnose. Int J Gen Med. 2018;11:337-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2491] [Article Influence: 311.4] [Reference Citation Analysis (4)] |
| 5. | Hrabe J. Neuroendocrine Tumors of the Appendix, Colon, and Rectum. Surg Oncol Clin N Am. 2020;29:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Maione F, Chini A, Milone M, Gennarelli N, Manigrasso M, Maione R, Cassese G, Pagano G, Tropeano FP, Luglio G, De Palma GD. Diagnosis and Management of Rectal Neuroendocrine Tumors (NETs). Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Li YW, He YP, Liu FQ, Peng JJ, Cai SJ, Xu Y, Wang MH. Grade G2 Rectal Neuroendocrine Tumor Is Much More Invasive Compared With G1 Tumor. Front Oncol. 2021;11:646536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Phan AT, Oberg K, Choi J, Harrison LH Jr, Hassan MM, Strosberg JR, Krenning EP, Kocha W, Woltering EA, Maples WJ; North American Neuroendocrine Tumor Society (NANETS). NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the thorax (includes lung and thymus). Pancreas. 2010;39:784-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Laverman P, McBride WJ, Sharkey RM, Eek A, Joosten L, Oyen WJ, Goldenberg DM, Boerman OC. A novel facile method of labeling octreotide with (18)F-fluorine. J Nucl Med. 2010;51:454-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 10. | Laverman P, D'Souza CA, Eek A, McBride WJ, Sharkey RM, Oyen WJ, Goldenberg DM, Boerman OC. Optimized labeling of NOTA-conjugated octreotide with F-18. Tumour Biol. 2012;33:427-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Long T, Yang N, Zhou M, Chen D, Li Y, Li J, Tang Y, Liu Z, Li Z, Hu S. Clinical Application of 18F-AlF-NOTA-Octreotide PET/CT in Combination With 18F-FDG PET/CT for Imaging Neuroendocrine Neoplasms. Clin Nucl Med. 2019;44:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;14 Suppl 5:v1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1260] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 13. | Mehta GU, Lonser RR. Management of hormone-secreting pituitary adenomas. Neuro Oncol. 2017;19:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Shih RY, Schroeder JW, Koeller KK. Primary Tumors of the Pituitary Gland: Radiologic-Pathologic Correlation. Radiographics. 2021;41:2029-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Tjörnstrand A, Gunnarsson K, Evert M, Holmberg E, Ragnarsson O, Rosén T, Filipsson Nyström H. The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur J Endocrinol. 2014;171:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Agustsson TT, Baldvinsdottir T, Jonasson JG, Olafsdottir E, Steinthorsdottir V, Sigurdsson G, Thorsson AV, Carroll PV, Korbonits M, Benediktsson R. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol. 2015;173:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Daly AF, Beckers A. The Epidemiology of Pituitary Adenomas. Endocrinol Metab Clin North Am. 2020;49:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 18. | Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA; Endocrine Society. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:3933-3951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1130] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 19. | Ritvonen E, Löyttyniemi E, Jaatinen P, Ebeling T, Moilanen L, Nuutila P, Kauppinen-Mäkelin R, Schalin-Jäntti C. Mortality in acromegaly: a 20-year follow-up study. Endocr Relat Cancer. 2016;23:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Dekkers OM, Biermasz NR, Pereira AM, Romijn JA, Vandenbroucke JP. Mortality in acromegaly: a metaanalysis. J Clin Endocrinol Metab. 2008;93:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 370] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 22. | McCabe J, Ayuk J, Sherlock M. Treatment Factors That Influence Mortality in Acromegaly. Neuroendocrinology. 2016;103:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N. Epidemiology of acromegaly: review of population studies. Pituitary. 2017;20:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 24. | Crisafulli S, Luxi N, Sultana J, Fontana A, Spagnolo F, Giuffrida G, Ferraù F, Gianfrilli D, Cozzolino A, Cristina De Martino M, Gatto F, Barone-Adesi F, Cannavò S, Trifirò G. Global epidemiology of acromegaly: a systematic review and meta-analysis. Eur J Endocrinol. 2021;185:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Krug S, Boch M, Rexin P, Pfestroff A, Gress T, Michl P, Rinke A. Acromegaly in a patient with a pulmonary neuroendocrine tumor: case report and review of current literature. BMC Res Notes. 2016;9:326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic Complications of Acromegaly and the Impact of the Current Treatment Landscape: An Update. Endocr Rev. 2019;40:268-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Dworakowska D, Grossman AB. Colonic Cancer and Acromegaly. Front Endocrinol (Lausanne). 2019;10:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Kasuki L, Maia B, Gadelha MR. Acromegaly and Colorectal Neoplasm: An Update. Front Endocrinol (Lausanne). 2022;13:924952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Patel GS, Grossmann I, Rodriguez K, Soni M, Joshi PK, Patel SC, Shreya D, Zamora DI, Sange I. Acromegaly and the Colon: Scoping Beyond the Pituitary. Cureus. 2021;13:e20018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Terzolo M, Puglisi S, Reimondo G, Dimopoulou C, Stalla GK. Thyroid and colorectal cancer screening in acromegaly patients: should it be different from that in the general population? Eur J Endocrinol. 2020;183:D1-D13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Uchoa HB, Lima GA, Corrêa LL, Vidal AP, Cavallieri SA, Vaisman M, Buescu A, Gadelha MR. Prevalence of thyroid diseases in patients with acromegaly: experience of a Brazilian center. Arq Bras Endocrinol Metabol. 2013;57:685-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Werner H, Laron Z. Role of the GH-IGF1 system in progression of cancer. Mol Cell Endocrinol. 2020;518:111003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |