Published online Sep 14, 2023. doi: 10.3748/wjg.v29.i34.5075
Peer-review started: June 4, 2023
First decision: June 21, 2023
Revised: July 15, 2023
Accepted: August 25, 2023
Article in press: August 25, 2023
Published online: September 14, 2023
Processing time: 96 Days and 4.8 Hours
Primary biliary cholangitis (PBC) is a chronic progressive autoimmune cholestatic disease. The main target organ of PBC is the liver, and nonsuppurative inflammation of the small intrahepatic bile ducts may eventually develop into cirrhosis or liver fibrosis.
To explore the clinical characteristics of early-stage PBC, identify PBC in the early clinical stage, and promptly treat and monitor PBC.
The data of 82 patients with PBC confirmed by pathology at Tianjin Second People’s Hospital from January 2013 to November 2021 were collected, and the patients were divided into stage I, stage II, stage III, and stage IV according to the pathological stage. The general data, serum biochemistry, immunoglobulins, and autoimmune antibodies of patients in each stage were retrospectively analyzed.
In early-stage (stages I + II) PBC patients, 50.0% of patients had normal alanine aminotransferase (ALT) levels, and 37.5% had normal aspartate aminotransferase (AST) levels. For the remaining patients, the ALT and AST levels were mildly elevated; all of these patients had levels of < 3 times the upper limit of normal values. The AST levels were significantly different among the three groups (stages I + II vs stage III vs stage IV, P < 0.05). In the early stage, 29.2% of patients had normal alkaline phosphatase (ALP) levels. The remaining patients had different degrees of ALP elevation; 6.3% had ALP levels > 5 times the upper limit of normal value. Moreover, γ-glutamyl transferase (GGT) was more robustly elevated, as 29.2% of patients had GGT levels of > 10 times the upper limit of normal value. The ALP values among the three groups were significantly different (P < 0.05). In early stage, the jaundice index did not increase significantly, but it gradually increased with disease progression. However, the above indicators were significantly different (P < 0.05) between the early-stage group and the stage IV group. With the progression of the disease, the levels of albumin and albumin/globulin ratio tended to decrease, and the difference among the three groups was statistically significant (P < 0.05). In early-stage patients, IgM and IgG levels as well as cholesterol levels were mildly elevated, but there were no significant differences among the three groups. Triglyceride levels were normal in the early-stage group, and the differences among the three groups were statistically significant (P < 0.05). The early detection rates of anti-mitochondria antibody (AMA) and AMA-M2 were 66.7% and 45.8%, respectively. The positive rate of anti-sp100 antibodies was significantly higher in patients with stage IV PBC. When AMA and AMA-M2 were negative, in the early stage, the highest autoantibody was anti-nuclear antibody (ANA) (92.3%), and in all ANA patterns, the highest was ANA centromere (38.5%).
In early-stage PBC patients, ALT and AST levels are normal or mildly elevated, GGT and ALP levels are not elevated in parallel, GGT levels are more robustly elevated, and ALP levels are normal in some patients. When AMA and AMA-M2 are negative, ANA especially ANA centromere positivity suggests the possibility of early PBC. Therefore, in the clinic, significantly elevated GGT levels with or without normal ALP levels and with ANA (particularly ANA centromere) positivity (when AMA and AMA-M2 are negative) may indicate the possibility of early PBC.
Core Tip: This is a retrospective study of the characteristics of early-stage primary biliary cholangitis (PBC) patients, in which we found the suggestive role of γ-glutamyl transferase as an indicator of cholestasis in the early diagnosis of PBC. We also found that when anti-mitochondria antibody (AMA) and AMA-M2 were negative, positivity for anti-nuclear antibody (ANA) especially ANA centromere indicates early-stage PBC.
- Citation: Zhu YJ, Li J, Liu YG, Jiang Y, Cheng XJ, Han X, Wang CY, Li J. Role of biochemical markers and autoantibodies in diagnosis of early-stage primary biliary cholangitis. World J Gastroenterol 2023; 29(34): 5075-5081
- URL: https://www.wjgnet.com/1007-9327/full/v29/i34/5075.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i34.5075
Primary biliary cholangitis (PBC) is a chronic progressive autoimmune cholestatic disease. The main target organ of PBC is the liver, and nonsuppurative inflammation of the small intrahepatic bile ducts may eventually develop into cirrhosis or liver fibrosis[1]. Currently, patients who are diagnosed and treated early respond well to ursodeoxycholic acid (UDCA) and have an estimated survival rate similar to that of the general population. Based on this, we retrospectively analyzed the data of patients with PBC in our hospital, aiming to analyze the biochemical parameters and autoimmune antibody characteristics of patients with early PBC to help identify early-stage PBC, actively treat this condition, and delay disease progression.
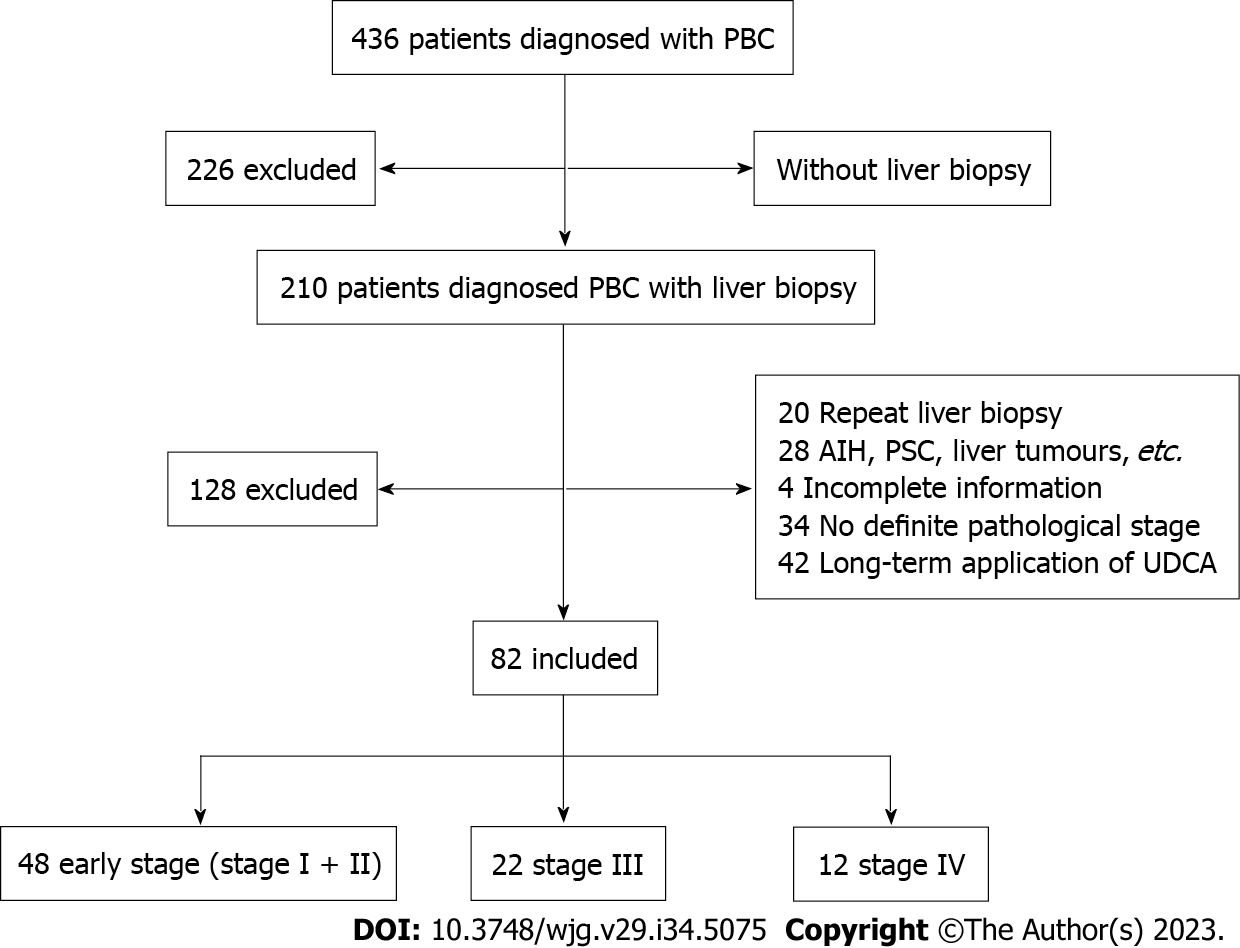
This retrospective observational study was conducted at the Clinical School of the Second People's Hospital. Patients were included if they met the following criteria: (1) Confirmed PBC (detected by “APASL clinical practice guidance: The diagnosis and management of patients with primary biliary cholangitis”). The diagnosis of PBC can be established when two or more of the following three criteria are met: (a) Biochemical evidence of cholestasis based mainly on the elevation of alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT) with the exclusion of extrahepatic biliary obstruction by imaging studies; (b) presence of anti-mitochondria antibody (AMA) or other PBC-specific anti-nuclear antibodies (ANAs) including anti-sp100 or anti-gp210; and (c) histologic evidence of non-suppurative destructive cholangitis mainly affecting the interlobular bile ducts; (2) underwent liver pathology biopsy and had a clear pathological stage; (3) initially diagnosed with PBC; and (4) were not previously treated with UDCA. Exclusion was based on the following criteria: (1) Various liver tumors; (2) other autoimmune diseases; (3) alcoholic liver disease, nonalcoholic fatty liver disease, and drug-induced liver injury; (4) active viral hepatitis; (5) obstructive cholestasis; (6) repeated liver biopsy; (7) incomplete information; (8) no defined pathological stage; (9) long-term application of UDCA; and (10) rejected liver biopsy (Figure 1).
For the detection of ANA, a Hep-2 kit was used, according to standard indirect immunofluorescence (IIF) protocols. Each serum sample was investigated at a starting dilution of 1:100 and titrated to zero positive; samples with positive IIF at a titer ≥ 1:100 were deemed positive. Line immunoassay was used to detect AMA (M2), anti-sp100, and anti-gp210. According to Hep-2 cell patterns, ANAs were divided into nuclear (including homogeneous, cytoplasmic speckled, nucleolar, centromere, discrete, and nuclear) and cytoplasmic (including fibrillar, cytoplasmic speckled, and reticular)[2]. Pathology readings were performed by two experienced pathologists and at least one chief pathologist.
The data were analyzed with SPSS 25.0 software. Normally distributed data were analyzed by ANOVA and are expressed as the mean ± SD. Nonnormally distributed data were analyzed by the rank sum test and are expressed as medians and ranges. Count data are expressed as the number of subjects and percentages and were compared using the chi-square test. A difference was considered statistically significant at P < 0.05.
The age distribution of PBC patients ranged from 33-71 years, with an average age of 53.7 ± 8.9 years. The mean age in the three stage groups (stages I + II, stage III, and stage IV) was 52.4 ± 9.2 years, 57.7 ± 7.3 years, and 51.5 ± 8.7 years, respectively. The 82 patients with PBC were predominantly female (68/82), and the number of females in the three stage groups was 38, 20 and 10, respectively. There were no statistically significant differences in the mean age or sex proportion among the groups (P > 0.05).
In early-stage patients (n = 48), 18 (37.5%) patients did not have significant symptoms. The major symptoms of patients were gastrointestinal symptoms (including abdominal discomfort, pain, bloating, and nausea), fatigue, yellow urine, and pruritus.
The alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were normal or mildly increased in the early-stage PBC patients, and an increasing trend with the progression of the pathological stage was observed. Furthermore, the AST levels were significantly different among the three groups (P < 0.05). There were significant differences in total bilirubin (TBIL), direct bilirubin (DBIL), and total bile acid (TBA) levels among the three groups of PBC patients (P < 0.05), and the difference between the early stage group and stage IV group was statistically significant (P = 0.002, 0.003, and 0.009, respectively). With pathological progression, a decreasing trend for albumin and albumin/globulin levels was observed, and there were statistically significant differences among the three groups and between the early stage group and stage IV group (P = 0.005 and 0.001, respectively). ALP and GGT levels were trending toward an increase. While 29.2% of patients had normal ALP levels, the remaining patients had different degrees of elevation. For 6.3% of the patients, the ALP levels were elevated to > 5 times the upper limit of normal value. The elevation in GGT levels was more robust, as 29.2% of patients had elevated GGT levels of > 10 times the upper limit of normal value. The difference of ALP levels among the three groups was statistically significant (P < 0.05), and there was a statistically significant difference between the early stage group and stage IV group (P = 0.006). Triglyceride levels gradually decreased with the progression of the disease, and the differences among the different groups were statistically significant (P < 0.05). Cholesterol (CHO) levels showed an increasing tendency. CHO levels gradually increased but were not significantly different among the three groups. IgG, IgA, and IgM all showed an increasing trend with disease progression. IgG and IgM were predominantly elevated, while IgA and complement (C)3 and C4 were normal in the early stage. The IgA levels were significantly different among the three groups (P < 0.05) (Table 1).
| Early-stage (stages I + II; n = 48) | Stage III (n = 22) | Stage IV (n = 12) | P value | |
| ALT | 53.5 (29.5-124.5) | 54.0 (30.0-127.6) | 80.0 (42.8-127.4) | 0.639 |
| AST | 53.5 (34.0-97.3)a | 68.5 (39.6-114.1) | 112.2 (81.1-136.3) | 0.009 |
| TBA | 9.0 (3.6-36.4)a | 18.0 (7.0-45.1) | 28.2 (18.0-111.5) | 0.011 |
| TBIL | 15.4 (11.2-27.8)a | 16.7 (13.7-24.2)a | 61.2 (28.1-114.3) | 0.002 |
| DBIL | 5.2 (3.7-16.0)a | 8.7 (2.5-12.4)a | 42.3 (15.8-67.3) | 0.003 |
| ALB | 42.1 (38.3-44.8)a | 39.3 (36.5-42.5) | 35.8 (30. 5-41.0) | 0.005 |
| A/G | 1.2 (1.1-1.4)a | 1.0 (0.9-1.3) | 0.9 (0.7-1.1) | 0.001 |
| ALP | 200.4 (117.8-414.0)a | 292.5 (162.0-421.3) | 463.5 (329.6-770.5) | 0.008 |
| GGT | 289.0 (133.0-668.8) | 532.0 (326. 5-764.3) | 336.5 (264.0-648.0) | 0.070 |
| CHO | 5.8 (4.6-6.9) | 6.2 (5.1-7.5) | 6.8 (4.9-7.0) | 0.509 |
| TG | 1.3 (1.0-1.8) | 1.6 (1.2-2.1)a | 1.1 (0.8-1.5) | 0.019 |
| IgG | 16.7 ± 4.6 | 17.6 ± 6.7 | 20.6 ± 7.9 | 0.056 |
| IgA | 2.9 (2.1-3.3) | 3.5 (2.5-4.7) | 3.4 (2.4-4.4) | 0.031 |
| IgM | 3.4 (1.8-5.1) | 3.7 (2.1-5.0) | 3.7 (1.9-6.8) | 0.967 |
| C3 | 1.4 (1.2-1.6) | 1.6 (1.3-1.8) | 1.2 (1.0-1.7) | 0.126 |
| C4 | 0.2 (0.2-0.3) | 0.2 (0.2-0.3) | 0.2 (0.2-0.3) | 0.963 |
The positive rates of AMA, AMA-M2, anti-sp100, and anti-gp210 were 52 (63.4%), 40 (48.8%), 16 (19.5%), and 13 (15.9%), respectively. The positive rate of ANA was 62 (75.6%). Cytoplasmic speckled pattern had the highest rate of 48.8%, and this was followed by nuclear pattern at 23.2% and centromere pattern at 15.9%. The positive rate of AMA-M2 significantly differed among the three groups (P = 0.045), but the remaining autoantibodies were not significantly different (P > 0.05). In early-stage PBC patients, 32 patients were positive for AMA, 22 positive for AMA-M2, 8 positive for anti-sp100, and 9 positive for anti-gp210 (Table 2). Thirty-four patients were positive for ANA, of which 24 had cytoplasmic speckled pattern, 10 had nuclear pattern, and 6 had centromere pattern (Table 3).
| Autoantibody | AMA | AMA-M2 | Anti-sp100 | Anti-gp210 | Anti-SSA/Ro52 | Anti-SSA/Ro60 | ANA |
| n | 32 | 22 | 8 | 9 | 8 | 2 | 34 |
| % | 66.7 | 45.8 | 16.7 | 18.8 | 16.7 | 4.2 | 70.8 |
| ANA | Cytoplasmic speckled | Fibrillar | Reticular | Discrete | Homogeneous | Centromere | Nuclear speckled | Nucleolar | Nuclear |
| n | 24 | 2 | 6 | 7 | 1 | 6 | 5 | 0 | 10 |
| % | 50 | 4.2 | 12.5 | 14.6 | 2.1 | 12.5 | 10.4 | 0 | 20.8 |
There were 25 PBC patients negative for both AMA and AMA-M2. These included 13 patients in the early stage, 6 patients in stage III, and 6 patients in stage IV. The autoantibody positive rates among the three groups were not significantly different (P > 0.05). In early-stage AMA- and AMA-M2-negative patients, the positive rate was highest for ANA (92.3%), followed by anti-SSA/RO52 (23.1%) and anti-sp100 (15.3%). In all ANA patterns, the highest was ANA centromere (38.5%).
PBC occurs mainly in middle-aged women, and the common clinical presentations are fatigue, splenomegaly, jaundice, and pruritus[1]. PBC may take 10-15 years to develop from the onset of illness to the symptomatic stage. Early diagnosis and treatment can improve the survival and quality of life of patients. In the early stage of PBC, patients have no obvious symptoms, but most patients have liver fibrosis or cirrhosis when they are diagnosed. Therefore, to improve PBC patient prognosis, early diagnosis and treatment are essential.
In this study, we found that GGT levels were significantly elevated in early-stage patients (29.2% of patients had elevated levels of > 10 times the upper limit of normal value) and tended to increase with the progression of illness. A study from Switzerland found elevated GGT levels in 15 of 24 PBC patients with normal ALP levels and liver biopsy results, and GGT levels were elevated in 13 patients who had early Nakanura stage histology. These findings suggest that elevated GGT levels have potential diagnostic value in PBC patients with normal ALP levels[3]. Reportedly, elevated GGT levels are closely related to the inflammation caused by fat deposition[4]. GGT is a key factor in maintaining the activity of reduced glutathione (GSH), which is an important antioxidant in the body. GGT is also involved in the production of GSH, which plays a cytoprotective role in inflammation and when an inflammatory response occurs[5]. In contrast to the levels of GGT, the levels of the cholestasis indicator ALP are not significantly elevated, and some PBC patients have normal ALP levels. Elevated ALP levels may be due to increased intracapillary pressure or to bile acids dissolving ALP from the lipid membrane into the blood[6]. A study of 67 patients with normal ALP levels and AMA positivity who underwent liver puncture biopsies found that 55 of these cases had pathology consistent with characteristic PBC presentation. Of these 55 patients, 50 were in the early stage[7]. In our study, we found that the elevation of GGT levels was more pronounced in patients with early-stage disease, which may be related to early chronic inflammation, excessive GSH depletion, and a compensatory increase in GGT.
We found that most patients had normal or mildly elevated ALT and AST levels in the early stage, and these levels increased with disease progression. These findings suggest that the degree of hepatocellular damage gradually increases. Alternatively, TBA, TBIL, and DBIL levels were not significantly elevated in the early stage but gradually increased with disease progression. In stage IV, TBIL and DBIL levels were significantly elevated, and DBIL levels were robustly elevated. This may be due to most of the hepatic parenchyma being damaged. In this study, IgM levels were predominantly elevated in early-stage PBC patients, which is consistent with previous studies[8]. A rise in IgM levels may be caused by a strong IgM secretory capacity and cellular autophagy[9].
AMA and AMA-M2 may be detectable in serum when patients are symptom-free and liver tests are normal, and they are highly specific autoantibodies for PBC. In recent years, with the development of detection methods, anti-sp100 and anti-gp210 have already been identified as PBC-specific autoantibodies. In a retrospective study of 4371 patients from Italy, the specificity of anti-sp100 and anti-gp210 for PBC was confirmed, especially in AMA-negative patients[10]. However, the reported positive rates vary widely across regions. The differences in antibody positive rates among regions could be due to different patient selection criteria and differences in technical and genetic backgrounds. In our study, the autoantibody rates of all patients were as follows: AMA, 63.4%; AMA-M2, 48.8%; and anti-gp210, 15.9%. These rates are lower than those reported in previous studies[11,12]. We found that the positive rate of anti-sp100 was 19.5%, and the positive rate of ANA was 75.6%. These rates are higher than those in other reports. One possible explanation for this is that our study did not adopt random sampling, but only patients with PBC confirmed by liver biopsy were included. This may be the main reason why the autoantibody positive rates in this study are lower than those in other studies. The positive rates of AMA, AMA-M2, and anti-gp210 in early-stage patients with PBC were 66.7%, 45.8%, and 18.8%, respectively, which were similar to the rates of all PBC patients. The positive rate of anti-sp100 was significantly higher in stage IV PBC patients.
The number of AMA- and AMA-M2-negative PBC patients was 25, of which 13 (86.7%) were in the early stage. In early-stage AMA- and AMA-M2-negative patients, the positive rate of ANA was highest (92.3%). In all ANA patterns, the highest was ANA centromere (38.5%), which decreased with the progression of the disease. However, there were no significant differences in this parameter among the three groups (P > 0.05). In this article, we summarized available data on the characteristics of ANA patterns. We should be aware of the possibility of unexplained elevations in serum markers for cholestasis in AMA(M2)-negative patients, but ANA, especially centromere pattern, was positive in early-stage patients. Although those ANAs are not specific for PBC diagnostic purposes, they can still be used as auxiliary markers to reduce the rate of missed diagnoses when AMA(M2) is negative. A previous study reported that ANA centromere was almost exclusively limited to PBC patients. Thus, this antibody can still be used as an auxiliary marker to reduce the rate of missed diagnoses when AMA(M2) is negative[12].
In summary, in early-stage PBC patients, ALT and AST levels are normal or mildly elevated, GGT and ALP levels are not elevated in parallel, GGT levels are more robustly elevated, and ALP levels are normal in some patients. When AMA and AMA-M2 are negative, ANA (especially ANA centromere) positivity suggests the possibility of early PBC. Therefore, in the clinic, significantly elevated GGT levels with or without normal ALP levels and with ANA (particularly ANA centromere) positivity (when AMA and AMA-M2 are negative) may indicate the possibility of early PBC.
This study is a retrospective study with limited inclusion. Thus, it adopted nonrandom sampling with some bias. Further studies should be conducted in the future to expand the sample size and to follow up on the prognosis of patients with early-stage PBC after treatment with UDCA.
The long course and insidious symptoms of primary biliary cholangitis (PBC) make it difficult to diagnose in the early stage.
To analyze clinical features and autoantibodies in patients with early-stage PBC.
To improve the diagnosis rates of early-stage PBC.
We included 82 patients with PBC diagnosed by liver pathology with clear pathologic stage and divided them into three groups to compare their laboratory parameters and autoantibody positivity.
In early-stage PBC patients, alanine aminotransferase and aspartate aminotransferase levels were normal or mildly elevated, gamma glutamyl transferase (GGT) and alkaline phosphatase (ALP) levels were not elevated in parallel, GGT levels were more robustly elevated, and ALP levels were normal in some patients. When anti-mitochondria antibody (AMA) and AMA-M2 were negative, anti-nuclear antibody (ANA) (especially ANA centromere) positivity suggests the possibility of early-stage PBC.
We found that GGT is elevated significantly and earlier in the early-stage PBC group. ANA and associated-ANA subtypes can be used as second-line markers for the diagnosis of early-stage PBC (particularly when specific autoantibodies are negative).
We hope that this study will increase the rates of diagnosis of early-stage PBC by clinicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Filipec Kanizaj T, Croatia; Granito A, Italy S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, Duan W, Chen S, Kong Y, Zhang D, Wei L, Wang FS, Lin HC, Yang JM, Tanwandee T, Gani RA, Payawal DA, Sharma BC, Hou J, Yokosuka O, Dokmeci AK, Crawford D, Kao JH, Piratvisuth T, Suh DJ, Lesmana LA, Sollano J, Lau G, Sarin SK, Omata M, Tanaka A, Jia J. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol Int. 2022;16:1-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, Fritzler MJ, Garcia-De La Torre I, Herold M, Mimori T, Satoh M, von Mühlen CA, Andrade LE. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014-2015. Front Immunol. 2015;6:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 3. | Terziroli Beretta-Piccoli B, Stirnimann G, Mertens J, Semela D, Zen Y, Mazzucchelli L, Voreck A, Kolbus N, Merlo E, Di Bartolomeo C, Messina P, Cerny A, Costantini S, Vergani D, Mieli-Vergani G; Swiss PBC Cohort Study Group. Primary biliary cholangitis with normal alkaline phosphatase: A neglected clinical entity challenging current guidelines. J Autoimmun. 2021;116:102578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Haring R, Baumeister SE, Völzke H, Dörr M, Kocher T, Nauck M, Wallaschofski H. Prospective inverse associations of sex hormone concentrations in men with biomarkers of inflammation and oxidative stress. J Androl. 2012;33:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Chen LW, Huang MS, Shyu YC, Chien RN. Gamma-glutamyl transpeptidase elevation is associated with metabolic syndrome, hepatic steatosis, and fibrosis in patients with nonalcoholic fatty liver disease: A community-based cross-sectional study. Kaohsiung J Med Sci. 2021;37:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Poupon R. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology. 2015;61:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 7. | Sun C, Xiao X, Yan L, Sheng L, Wang Q, Jiang P, Lian M, Li Y, Wei Y, Zhang J, Chen Y, Li B, Huang B, Peng Y, Chen X, Fang J, Qiu D, Hua J, Tang R, Leung P, Gershwin ME, Miao Q, Ma X. Histologically proven AMA positive primary biliary cholangitis but normal serum alkaline phosphatase: Is alkaline phosphatase truly a surrogate marker? J Autoimmun. 2019;99:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Lleo A, Liao J, Invernizzi P, Zhao M, Bernuzzi F, Ma L, Lanzi G, Ansari AA, Coppel RL, Zhang P, Li Y, Zhou Z, Lu Q, Gershwin ME. Immunoglobulin M levels inversely correlate with CD40 ligand promoter methylation in patients with primary biliary cirrhosis. Hepatology. 2012;55:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Lian C, Zhao Y, Sun J, Zhao L, Zhang F. Role of cell autophagy in the generation of IgM and hepatic fibrosis in primary biliary cholangitis. Clin Rheumatol. 2020;39:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Granito A, Muratori P, Muratori L, Pappas G, Cassani F, Worthington J, Guidi M, Ferri S, DE Molo C, Lenzi M, Chapman RW, Bianchi FB. Antinuclear antibodies giving the 'multiple nuclear dots' or the 'rim-like/membranous' patterns: diagnostic accuracy for primary biliary cirrhosis. Aliment Pharmacol Ther. 2006;24:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 12. | Granito A, Muratori P, Quarneti C, Pappas G, Cicola R, Muratori L. Antinuclear antibodies as ancillary markers in primary biliary cirrhosis. Expert Rev Mol Diagn. 2012;12:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |