Published online Sep 14, 2023. doi: 10.3748/wjg.v29.i34.5054
Peer-review started: May 16, 2023
First decision: July 10, 2023
Revised: July 19, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 14, 2023
Processing time: 115 Days and 2.1 Hours
Di (2-ethylhexyl) phthalate (DEHP) is a common plasticizer known to cause liver injury. Green tea is reported to exert therapeutic effects on heavy metal exposure-induced organ damage. However, limited studies have examined the therapeutic effects of green tea polyphenols (GTPs) on DEHP-induced liver damage.
To evaluate the molecular mechanism underlying the therapeutic effects of GTPs on DEHP-induced liver damage.
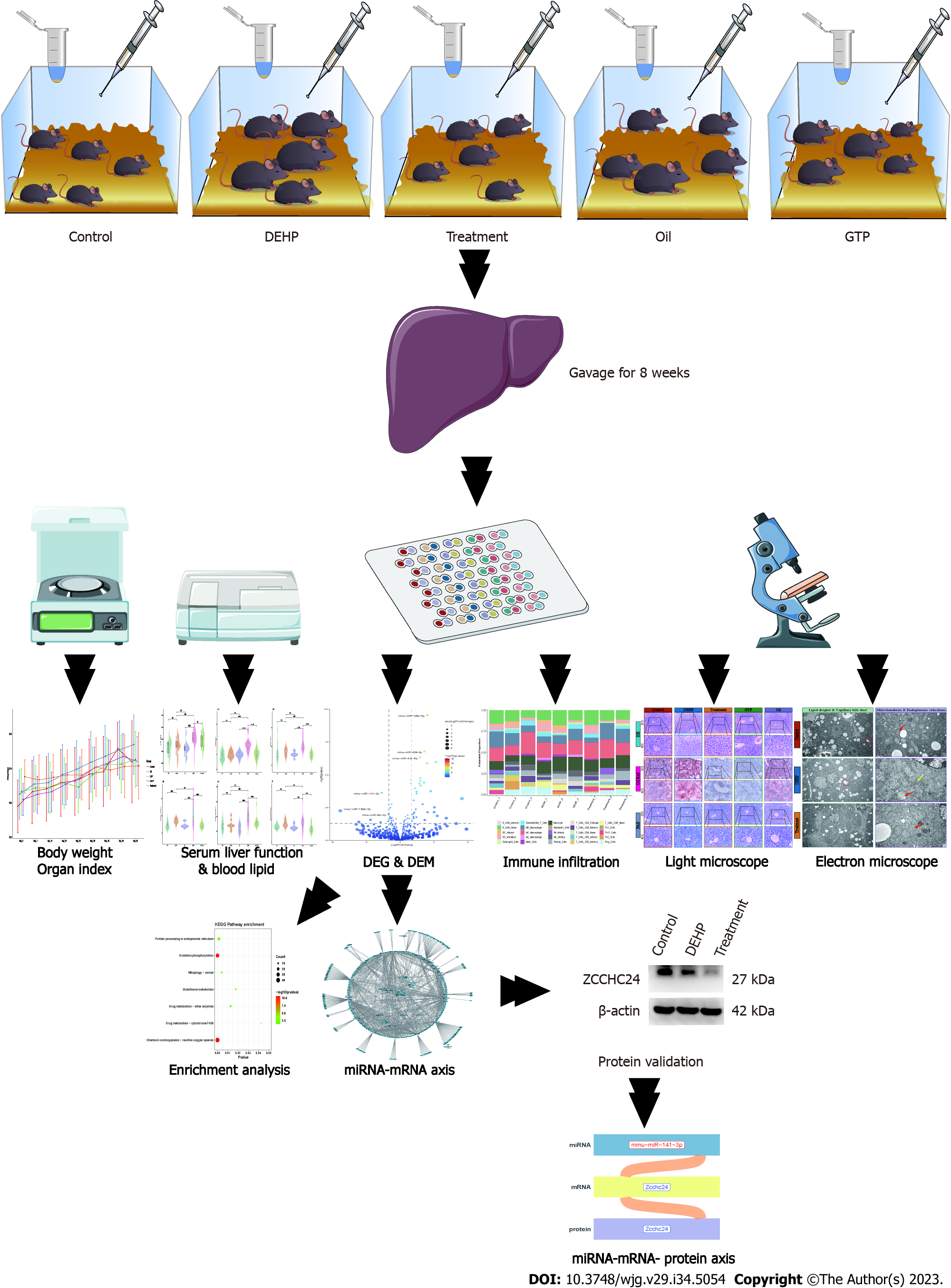
C57BL/6J mice were divided into the following five groups: Control, model [DEHP (1500 mg/kg bodyweight)], treatment [DEHP (1500 mg/kg bodyweight) + GTP (70 mg/kg bodyweight), oil, and GTP (70 mg/kg bodyweight)] groups. After 8 wk, the liver function, blood lipid profile, and liver histopathology were examined. Differentially expressed micro RNAs (miRNAs) and mRNAs in the liver tissues were examined using high-throughput sequencing. Additionally, functional enrichment analysis and immune infiltration prediction were performed. The miRNA-mRNA regulatory axis was elucidated using the starBase database. Protein expression was evaluated using immunohistochemistry.
GTPs alleviated DHEP-induced liver dysfunction, blood lipid dysregulation, fatty liver disease, liver fibrosis, and mitochondrial and endoplasmic reticulum lesions in mice. The infiltration of macrophages, mast cells, and natural killer cells varied between the model and treatment groups. mmu-miR-141-3p (a differentially expressed miRNA), Zcchc24 (a differentially expressed mRNA), and Zcchc24 (a differentially expressed protein) constituted the miRNA-mRNA-protein regulatory axis involved in mediating the therapeutic effects of GTPs on DEHP-induced liver damage in mice.
This study demonstrated that GTPs mitigate DEHP-induced liver dysfunction, blood lipid dysregulation, fatty liver disease, and partial liver fibrosis, and regulate immune cell infiltration. Additionally, an important miRNA-mRNA-protein molecular regulatory axis involved in mediating the therapeutic effects of GTPs on DEHP-induced liver damage was elucidated.
Core Tip: Green tea polyphenols (GTPs) alleviated Di (2-ethylhexyl) phthalate (DEHP)-induced liver dysfunction, blood lipid dysregulation, fatty liver disease, liver fibrosis, and mitochondrial and endoplasmic reticulum lesions in mice. The infiltration of macrophages, mast cell, and natural killer cells varied between the model and treatment groups. mmu-miR-141-3p (a differentially expressed miRNA), Zcchc24 (a differentially expressed mRNA), and Zcchc24 (a differentially expressed protein) constituted the miRNA-mRNA-protein regulatory axis involved in mediating the therapeutic effects of GTPs on DEHP-induced liver damage in mice.
- Citation: Shi H, Zhao XH, Peng Q, Zhou XL, Liu SS, Sun CC, Cao QY, Zhu SP, Sun SY. Green tea polyphenols alleviate di-(2-ethylhexyl) phthalate-induced liver injury in mice. World J Gastroenterol 2023; 29(34): 5054-5074
- URL: https://www.wjgnet.com/1007-9327/full/v29/i34/5054.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i34.5054
Di (2-ethylhexyl) phthalate (DEHP), which is the most widely used representative phthalic acid ester, can non-covalently bind to polyolefin plastics and predominantly serves as a plasticizer, increasing the flexibility, transparency, durability, and longevity of plastics. Additionally, DEHP is extensively detected in various daily-life products (including baby toys, food packaging, and cosmetics) and several surgical and medical devices[1]. Furthermore, DEHP can be continuously released into the environment (air, soil, water, food, etc.), enter living organisms via ingestion, inhalation, or skin contact, and subsequently exert toxic effects on health[2]. A previous study reported that DEHP and its metabolites were detected in 100% of tested human urine samples, indicating persistent exposure to DEHP[3]. The DEHP exposure range of the general population is estimated to be 5.8-19 μg/kg/d, while DEHP exposure in medical environments may exceed 167.9 mg/d[4]. The development of modern chemical agriculture has increased the severity of DEHP pollution. DEHP exposure in the Pearl River Delta region of Guangdong Province, China, can reach up to 61 μg/kg/d, which is higher than the tolerable intake of DEHP[5]. Additionally, DEHP undergoes rapid degradation upon ingestion in humans. According to the United States Environmental Protection Agency, the average half-life of DEHP in the human body is 12 h. DEHP and its active metabolite mono-(2-ethylhexyl) phthalate have been detected in various human tissues, including the liver, blood, placenta, amniotic fluid, and early-pregnancy chorionic villus sample[6]. Therefore, evaluating the effect of DEHP on the environment and human health has piqued the interest of the scientific community.
The liver, an important organ involved in the synthesis, metabolism, and detoxification processes, is highly susceptible to acute or chronic damage induced by various drugs[7]. Recent studies have demonstrated that DEHP adversely affects multiple systems in the body. In particular, epidemiological and animal studies have demonstrated the hepatoxicity of DEHP[8]. The mechanism underlying the hepatotoxic effects of DEHP mainly involves oxidative stress, cell apoptosis, and signaling pathway activation. DEHP induces apoptosis in healthy human liver cells through the mitochondrial signaling pathway and/or the caspase-mediated death receptor pathway[9]. Additionally, DEHP may adversely affect gap junctional intercellular communication, peroxisome beta-oxidation activity, and DNA replication synthesis, leading to the formation of liver tumors[10]. Currently, the most extensively studied mechanism of DEHP-induced liver damage is oxidative stress in which the regulation of reactive oxygen species (ROS) production plays a critical role[11]. Excessive ROS production leads to peroxidation of the polyunsaturated fatty acids in the cell membrane. DEHP promotes lipid peroxidation in the liver, primarily through the downregulation of superoxide dismutase and catalase activity and the upregulation of malondialdehyde (MDA) concentrations[12]. Additionally, DEHP mediates the pathogenesis of non-alcoholic fatty liver disease in a high-fat diet-fed animal model by promoting lipid peroxidation[8].
Green tea is the second most widely consumed beverage worldwide after water[13]. The harvested tea leaves are steamed at high temperatures to inactivate the polyphenol oxidase in green tea, preserving most of the vitamins in tea leaves. Thus, green tea exhibits enhanced antioxidant activity. Epidemiological and clinical studies have reported that phenol compounds in tea extracts, such as green tea polyphenols (GTPs), exert a wide range of beneficial effects on human health, including anti-aging, neuroprotective[14], and therapeutic or preventive effects on various diseases, such as cancer[15], cardiovascular disease[16], and obesity[17]. Moreover, GTPs mitigate the adverse effects of poisoning with various heavy metals[18]. However, limited studies have examined the therapeutic effects of GTPs on DEHP-induced liver diseases.
Micro RNAs (miRNAs), a class of endogenous non-coding small RNAs encoded by mRNA, regulate gene expression by modulating mRNA stability. Several miRNAs are reported to play important roles in the pathogenesis of liver fibrosis[19], cirrhosis[20], and hepatocellular carcinoma[21]. Some miRNAs are diagnostic markers for drug-induced liver injury[22]. This study aimed to identify key protein nodes that may affect the expression level of miRNAs in the regulatory network of miRNA and target genes by analyzing the miRNA regulation network. Hence, this study will provide a theoretical basis for future studies on the functions and regulatory mechanisms underlying the therapeutic effects of GTPs on DEHP exposure-induced liver damage.
DEHP was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). GTPs were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Corn oil was purchased from Hebei Pin Research Biotechnology Co., Ltd. (Baoding, China). Anti-ZCCHC24 antibodies were purchased from Biorbyt Ltd. (Cambridge, United Kingdom).
Animal experiments were performed at the specific pathogen-free grade animal laboratory of the Medical College of Jinan University. C57BL/6J mice (n = 50) were purchased from Guangdong Yaokang Biotechnology Co., Ltd. And allowed to acclimatize to the laboratory environment for 1 wk. The animals were maintained under the following conditions: Room temperature, 20 ℃-24 ℃; relative humidity, 50%-65%; circadian cycle, 12-h light-dark cycle; access to food and water, ad libitum; diet, regular mouse chow. This study was approved by the Institutional Animal Care and Use Committee of Jinan University (ethics approval number: IACUC-20210630-15). All experimental procedures were performed according to the regulations established by the ethical committee.
Previous studies[23,24] have reported that DEHP is soluble in corn oil. Hence, this study used corn oil as the solvent for DEHP. After 1 wk of acclimatization, 50 mice were randomly assigned into the following five groups (10 mice/group): Control group, administered with 0.2 mL distilled water; model group, administered with 0.2 mL corn oil and DEHP (1500 mg/kg bodyweight); treatment group, administered with 0.2 mL corn oil and DEHP (1500 mg/kg bodyweight), followed by administration of 0.2 mL GTPs (70 mg/kg bodyweight) after 1 h; oil group, administered with 0.2 mL corn oil; GTP group, administered with 0.2 mL GTP (70 mg/kg bodyweight). Based on our previous study, DEHP[25] and GTPs[26] were gavaged. The doses were adjusted weekly based on the bodyweight of the mice. The mice were subjected to daily gavage for 8 wk. At the end of the experimental period, the blood and liver samples were obtained under anesthesia after the mice were allowed to fast overnight. Liver index = (liver weight/bodyweight) × 100%.
The blood from mice was collected using the retro-orbital venous plexus method. Next, the whole blood was placed in a 1.5-mL centrifuge tube and left undisturbed at room temperature for 2 h. The sample was centrifuged at 5 °C and 3000 rpm for 15 min using a high-speed refrigerated centrifuge to obtain the serum. The serum levels of liver function markers [aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin (TBIL)] and lipids [low-density lipoprotein (LDL), total cholesterol, and triglycerides (TGs)] were analyzed using a fully automated biochemical analyzer.
The tissue sections were fixed in 4% neutral buffered formalin, embedded in paraffin, and sectioned into 4 μm-thick sections. The sections were deparaffinized using xylene, rehydrated using a series of graded ethanol solutions, stained with hematoxylin for 5–10 min, washed with distilled water, and stained with eosin for 2-7 min. Next, the sections were dehydrated using a series of graded ethanol solutions, cleared with xylene, mounted with mounting medium, and covered with a cover slip.
The frozen sections were fixed in 4% neutral buffered formalin, rinsed with 60% isopropanol, and allowed to dry. The sections were then stained with oil red O for 15-30 min, rinsed with 60% isopropanol, counterstained with hematoxylin for 1-2 min, washed with distilled water, dehydrated, mounted with a mounting medium, and covered with a coverslip.
The tissue sections were deparaffinized using xylene, rehydrated using a series of graded ethanol solutions, and oxidized with a periodic acid solution for 10-15 min. Next, the sections were rinsed with distilled water, stained with Schiff reagent for 30-60 min in the dark, counterstained with hematoxylin, washed with distilled water, dehydrated, mounted using a mounting medium, and covered with a cover slip.
The tissue sections were deparaffinized using xylene, rehydrated using a series of graded ethanol solutions, stained with Weigert’s iron hematoxylin for 10 min, rinsed with distilled water, stained with Biebrich scarlet-acid fuchsin solution for 5-10 min, washed with distilled water, and incubated with phosphotungstic-phosphomolybdic acid solution for 5-10 min. Next, the sections were counterstained with aniline blue for 5-10 min, washed with distilled water, dehydrated, mounted with a mounting medium, and covered with a cover slip.
The tissue sections were deparaffinized using xylene, rehydrated using a series of graded ethanol solutions, stained with Sirius red solution for 1 h, washed with distilled water, dehydrated, and cleared with xylene. Finally, the sections were mounted with a mounting medium and covered with a cover slip.
One mouse from the control, treatment, and model groups was randomly selected for electron microscopy. Liver sections with a size of approximately 1 mm were treated with 2.5% glutaraldehyde (Scientific Phygene, Fuzhou, China) and rinsed thrice with phosphate-buffered saline (PBS) (PH = 7.4). The samples were fixed with 1% osmium tetroxide (Ted Pella, Redding, United States) for 2 h, rinsed thrice with PBS, and dehydrated using alcohol and acetone gradients as follows: 50% ethanol for 30 min, 70% ethanol for 30 h, 80% acetone for 30 min, and 90% acetone for 30 h. Next, the samples were washed thrice with 100% acetone and embedded in epoxy resin (Ted Pella, Redding, United States). Ultrathin sections (7 nm) were prepared using a microtome (LKB, Bromma, Sweden). The sections were stained with uranyl acetate (EMS, Hatfield, United States) for 30 min and 3% lead citrate (Ted Pella, Redding, United States) for 15 min. The target structures were observed using a transmission electron microscope (JEM-1400, Japan Electron Optics Laboratory Co., Ltd. Tokyo, Japan).
Liver samples (n = 3 per group) from the control, treatment, and model groups stored in liquid nitrogen were randomly selected and sent to Huada Genomics (Wuhan, China, http://www.genomics.cn) for high-throughput sequencing. For specific sequencing steps, refer to the Supplementary material.
The mRNA and miRNA expression levels were measured using fragments per kilobase of transcript per million mapped reads (FPKM) values. Sequencing data were subjected to quality control. Differential expression between groups was estimated using the Limma R package (version: 3.52.2) based on the generalized linear model. To comparatively analyze the expression levels between multiple groups, the control-model and model-treatment expression profiles were regressed using the Limma package in R software (version: 4.2.1, https://posit.co/). A loose threshold was set to avoid excessive filtering of differentially expressed mRNAs and miRNAs (log fold-change > 1; P < 0.05). Differentially expressed mRNAs and miRNAs were identified.
The mRNA expression data were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses to identify the enrichment of mRNAs in the GO terms biological process (BP), cellular component (CC), and molecular function (MF) and the KEGG signaling pathways. The clusterprofiler R package (version: 4.4.4) was used for enrichment analyses with the reference files based on the org.Mm.eg.db R package (version 3.1.0) for mouse enrichment analysis. Enrichment was considered significant at P < 0.05.
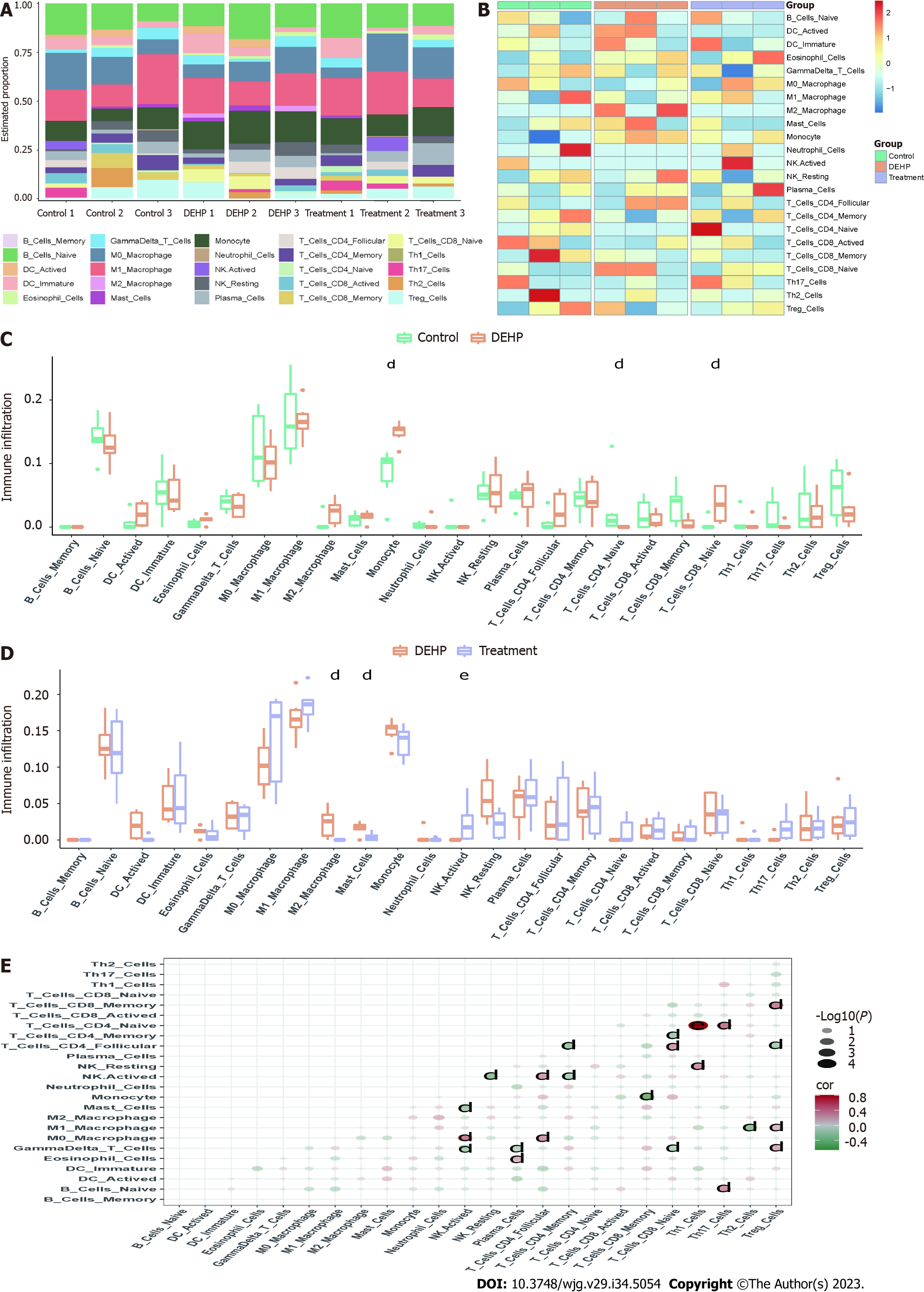
CIBERSORT is a gene expression-based algorithm for the accurate detection of immune cell infiltration. The “CIBERSORT” R package (CIBERSORTR script v1.03; http://cibersort.stanford.edu/) developed by Newman et al[27] was used to successfully quantify 22 types of immune cells, including B cells, regulatory T cells, CD4+ T, CD8+ T, natural killer (NK) cells, mast cells, plasma cells, dendritic cells, neutrophils, eosinophils, and macrophages. The reference dataset of mouse immune cells was obtained from Chen et al[28]. The mRNA expression data of the control, model, and treatment groups were examined using the CIBERSORT algorithm with a perm of the deconvolution algorithm set to 1000.
miRNA, a type of non-coding RNA, negatively regulates gene expression at the posttranscriptional level. To generate a preliminary regulatory network of miRNA-mRNA in the model and treatment groups, the upregulated miRNAs or mRNAs and downregulated mRNAs or miRNAs were selected and analyzed using Cytoscape software (version: 3.7.1, https://cytoscape.org/).
The Sun Yat-sen University research team developed the starBase database (https://starbase.sysu.edu.cn/starbase2/) to analyze interaction networks between long non-coding RNA, miRNA, competitive endogenous RNAs, RNA-binding proteins, and mRNAs using cross-linked immunoprecipitation sequencing (CLIP-seq) (high-throughput sequencing of RNA using CLIP, photoactivable ribonucleoside-enhanced CLIP, individual nucleotide resolution ultraviolet CLIP, and cross-linking ligation and sequencing of hybrids) data. To obtain the final miRNA-mRNA regulatory network, the initially selected network was intersected with the mouse interaction network obtained from the starBase database. Gene expression was verified using immunohistochemical analyses. This study aimed to establish the miRNA-mRNA-protein regulatory axis.
The paraffin sections (4 μm) of mouse liver tissue were deparaffinized, rehydrated, and subjected to antigen retrieval in a buffer solution (pH 9.0). After blocking endogenous peroxidase activity and non-specific binding, the sections were incubated with anti-ZCCHC24 antibodies (1:100) at 4 °C overnight. The sections were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Immunoreactive signals were developed using diaminobenzidine. The sections were counterstained with hematoxylin and observed under a microscope.
The average optical density (AOD) (integrated optical density/area) of positive reactions was calculated using Image-Pro Plus 6.0 software. Data were analyzed using the dplyr R package (v. 1.0.10) and visualized using the ggplot2 R package (v. 3.3.6). Categorical variables were analyzed using the Chi-squared test. Continuous variables were represented as mean ± SD. Continuous variables between two groups were compared using the Wilcoxon signed-rank test, while those between more than two groups were compared using the Kruskal-Wallis test. Differences were considered significant at P < 0.05.
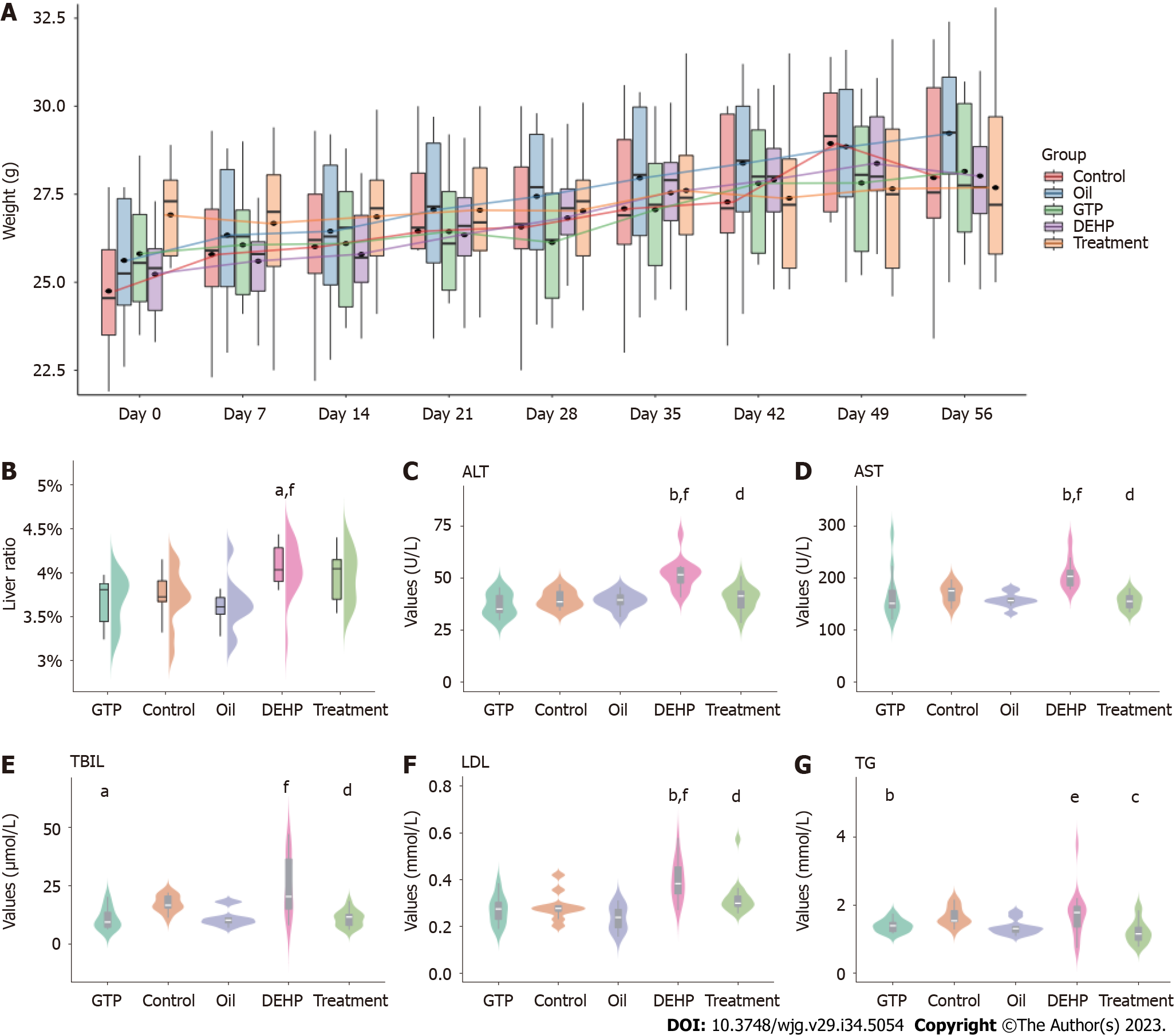
The flowchart of the study is shown in Figure 1. Mice in all groups survived during the experiment and did not exhibit aberrant behaviors in urination, defecation, food intake, or water consumption. As shown in Supplementary Table 1 and Figure 2A, the bodyweight of mice in all groups increased with time and was not significantly different between the groups. The liver index (Supplementary Table 2 and Figure 2B) values in the model group were significantly higher than those in the control group (P = 0.016) but were not significantly different between the treatment and model groups (P = 0.51). Additionally, the liver index values in the oil and GTP groups were not significantly different from those in the control group (P > 0.05).
The serum levels of ALT, AST, TBIL, LDL, and TGs were analyzed. As shown in Figure 2C-G, the serum levels of ALT, AST, TBIL, LDL, and TGs in the model group were significantly higher than those in the control group (P < 0.001). Compared with those in the model group, the serum levels of liver function markers and lipids were significantly downregulated in the treatment group (P < 0.001). The ALT, AST, and LDL levels were not significantly different between the oil, control, and GTP groups (P > 0.05).
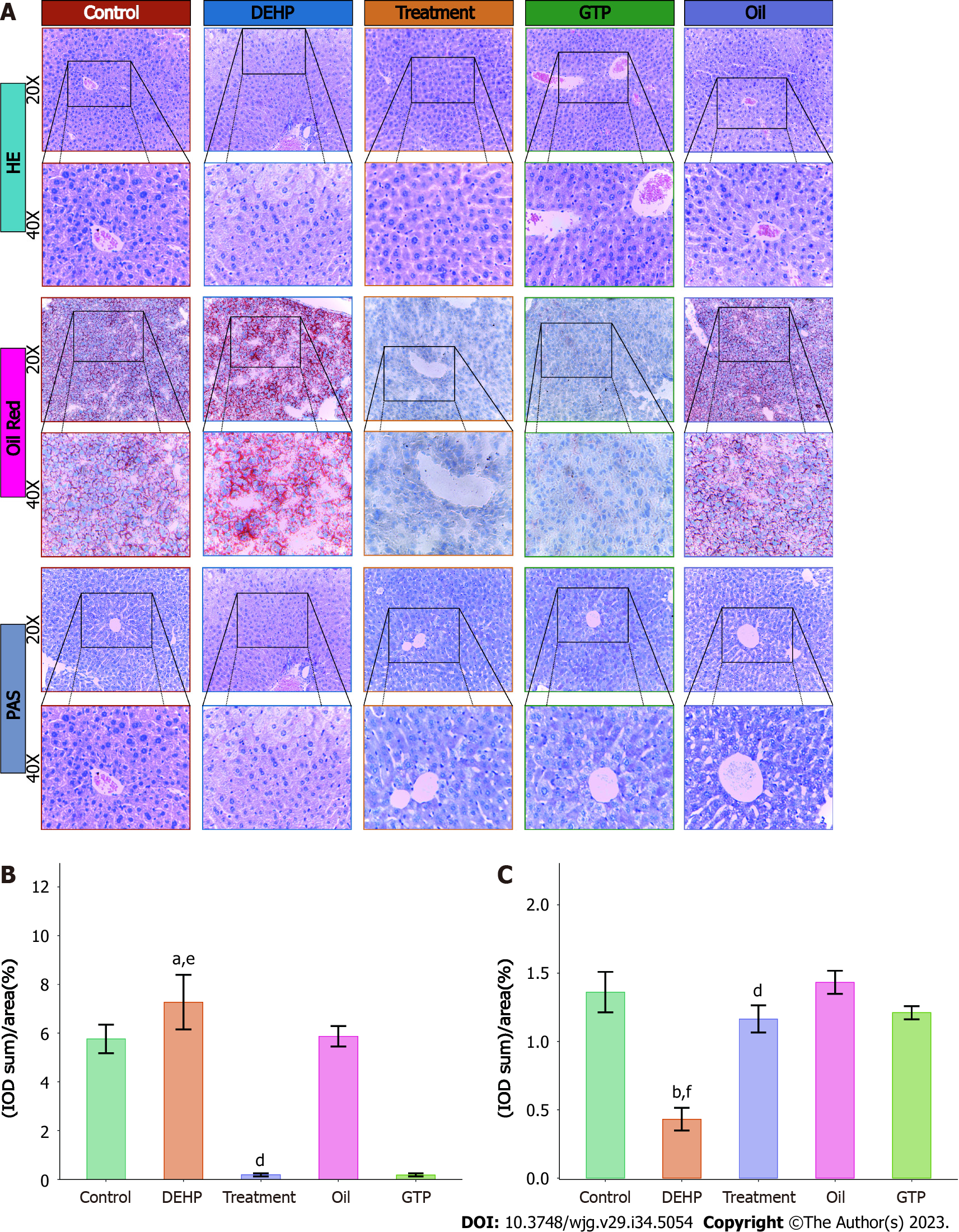
The results of liver hematoxylin and eosin staining are shown in Figure 3A. Mice in the model group exhibited significant hepatocyte ballooning degeneration, whereas those in the control, oil, and GTP groups did not exhibit hepatocyte ballooning degeneration.
Oil red O staining was performed to further analyze the severity of fatty liver in different groups. As shown in Figure 3A, the model group exhibited the highest red color staining intensity in the liver, followed by the oil and control groups. Meanwhile, the liver of the treatment and GTP groups exhibited the lowest red staining intensity. Five positive staining sites in the liver of the model and treatment groups were selected to calculate the AOD. As shown in Figure 3B, the AOD in the model group (7.270 ± 1.120%) was significantly higher than that in the treatment (0.185 ± 0.061%) (P = 0.037) and control groups (5.760 ± 0.586%) (P < 0.001).
The results of Periodic acid-Schiff staining (Figure 3A) revealed the lack of red-stained areas in the liver of the model group, while the red-stained areas were upregulated in the control, oil, treatment, and GTP groups. Five positive staining sites in the liver of the model and treatment groups were selected to calculate the AOD. As shown in Figure 3C, the AOD in the model group (0.431 ± 0.083%) was significantly lower than that in the treatment (1.170 ± 0.099%) and control groups (1.360 ± 0.148%) (P < 0.001).
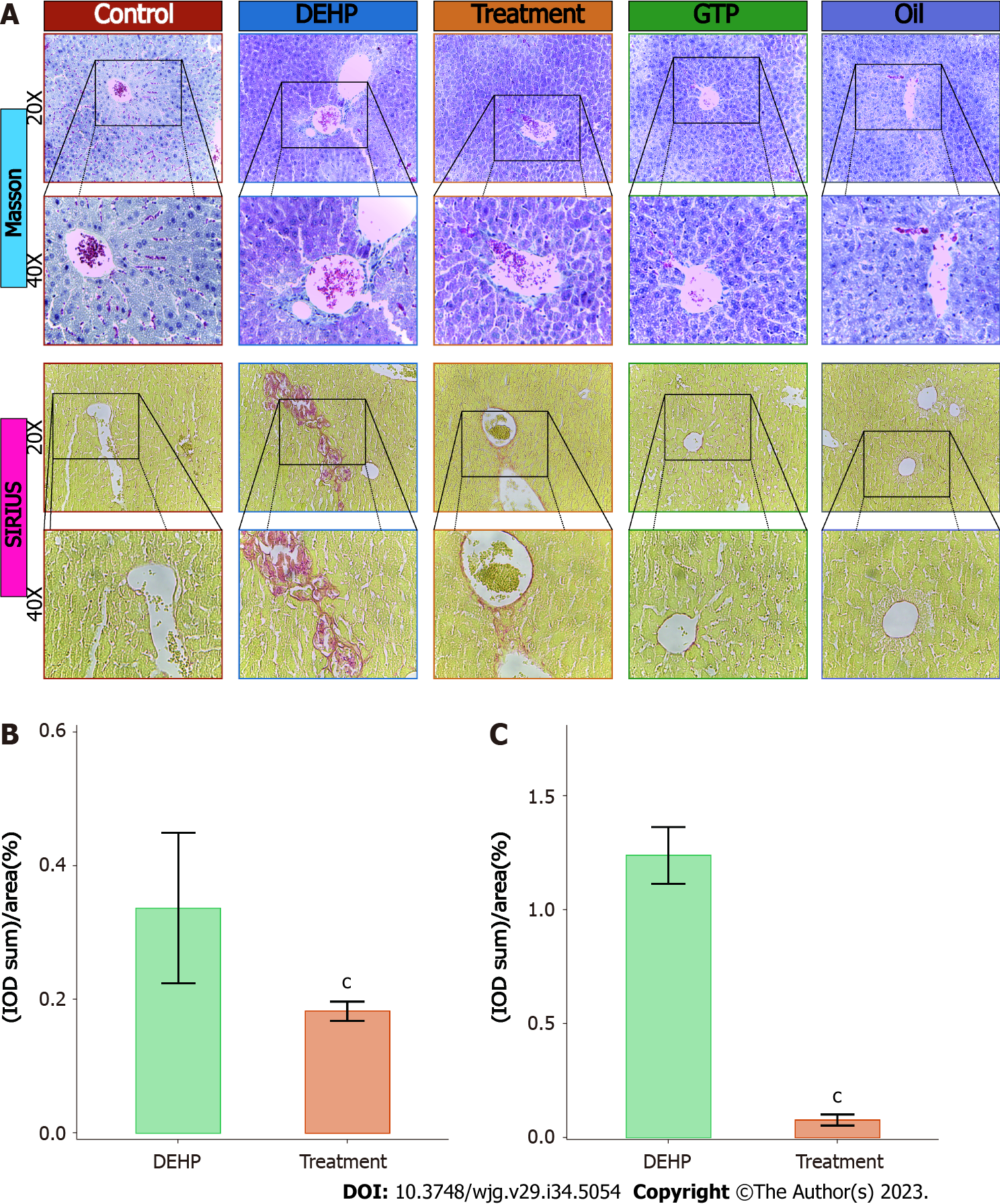
The results of Masson’s trichrome staining (Figure 4A) revealed blue-stained fibrous tissue around the liver blood vessels in the model group. In contrast, the area of blue-stained fibrous tissue around the liver blood vessels in the treatment group was lower than that in the model group. Fibrous tissue was not detected around the liver blood vessels in the control, oil, and GTP groups. Five positive staining sites in the liver of the model and treatment groups were selected to calculate the AOD. As shown in Figure 4B, the AOD in the model group (0.337 ± 0.113%) was significantly higher than that in the treatment group (0.183 ± 0.014%) (P = 0.02).
In Sirius red staining, type I collagen fibers exhibit strong orange-yellow or bright red colors under a polarized light microscope, whereas type III collagen fibers exhibit green color. In this study, the results of Sirius red staining (Figure 4A) revealed red-stained fibrous tissue around the blood vessels in the model group. The red-stained area around the blood vessels in the treatment group was significantly lower than that in the model group. Red-stained fibrous tissue was not detected around the blood vessels of the control, oil, and GTP groups. Next, semiquantitative analysis was performed by selecting five positive staining sites in the liver of the model and treatment groups to calculate the AOD. The AOD in the model group (1.240 ± 0.125%) was significantly higher than that in the treatment group (0.080 ± 0.025%) (P = 0.012) (Figure 4C).
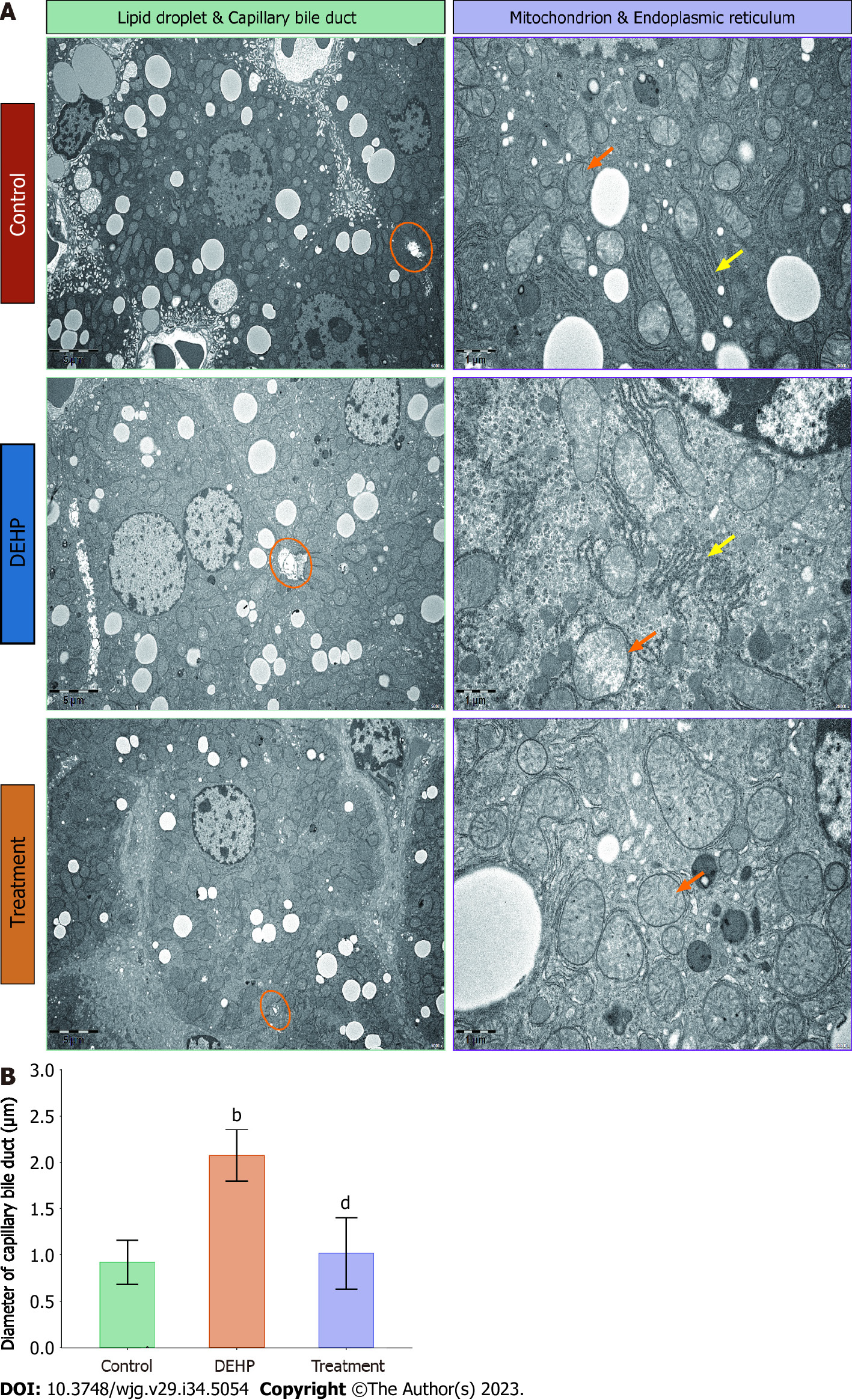
The liver tissues of the control, model, and treatment groups were subjected to transmission electron microscopy. The number and size of lipid droplets in the liver were upregulated and the capillary bile ducts were significantly dilated in the model group (Figure 5A). Additionally, the mitochondria were slightly swollen, and the arrangement of the rough endoplasmic reticulum was disordered. In contrast, the number of lipid droplets in the liver was downregulated in the treatment group, while the capillary bile ducts exhibited physiological structure. Additionally, the swelling of the mitochondria was alleviated. The results of quantitative analysis of the diameter of small bile ducts (Figure 5B) were consistent with the observations in Figure 5A. However, these findings must be carefully interpreted. Additionally, further studies with large sample sizes are needed to confirm and generalize the results.
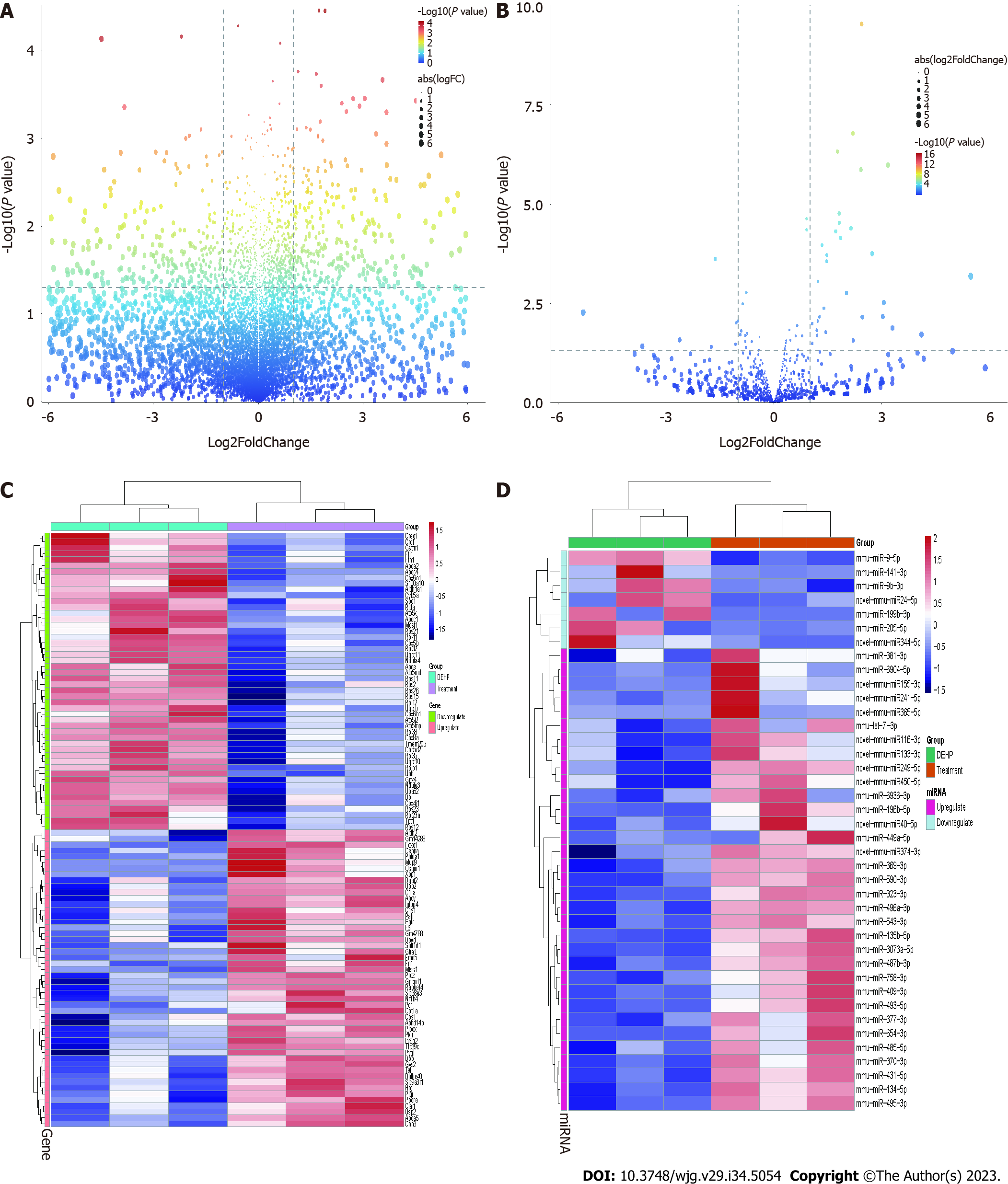
The liver samples from the control, treatment, and model groups were subjected to high-throughput screening with 3 replicate samples for each group. The mRNA and miRNA expression data were subjected to quality control analysis. The differences in the expression levels of miRNA and mRNA were minimal between the groups (Supplementary Figure 1A and B). Next, principal component analysis was performed (Supplementary Figure 1C and D). The mRNA and miRNA profiles of the control, model, and treatment groups exhibited distinct separation. Differential analysis revealed that compared with those in the model group, the number of upregulated mRNAs and miRNAs was 377 and 33, respectively, while the number of downregulated mRNAs and miRNAs was 583 and 7, respectively (Figure 6A and B). The expression levels of the significantly upregulated and downregulated mRNAs and miRNAs are shown in Figure 6C and D.
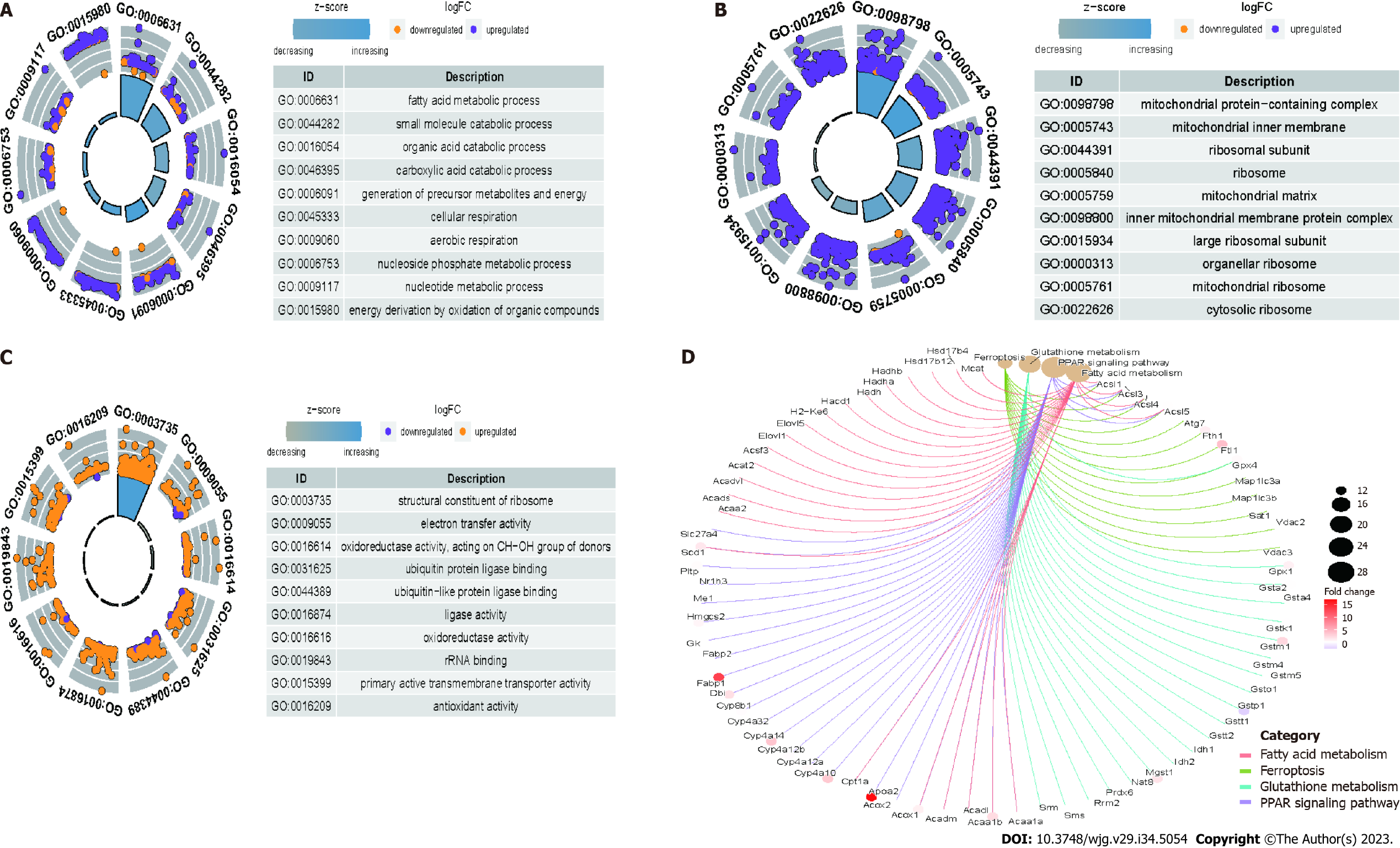
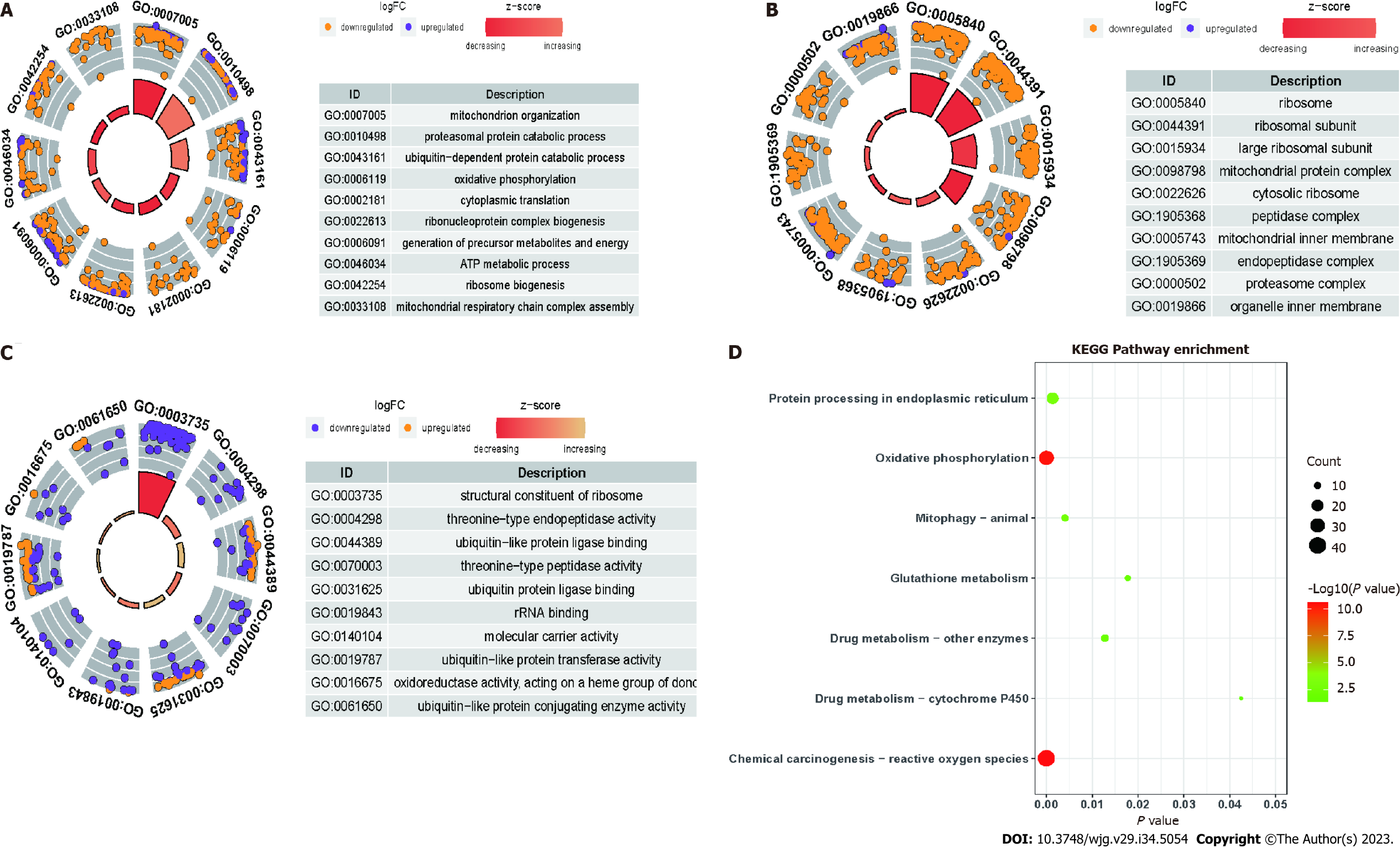
Compared with those in the control group, the differentially expressed mRNAs in the model group were enriched in the following GO terms: BP term: Fatty acid metabolic process, small molecule catabolic process, organic acid catabolic process, and carboxylic acid catabolic process (Figure 7A); CC term: Mitochondrial protein-containing complex, mitochondrial inner membrane, ribosome subunit, and ribosome (Figure 7B); MF term: Ribosome structure, electron transfer activity, oxidoreductase activity, and ubiquitin protein ligase binding (Figure 7C). Additionally, the differentially expressed mRNAs in the model group were enriched in the following KEGG pathways: Fatty acid metabolism, ferroptosis, glutathione metabolism, and PPAR signaling pathway (Figure 7D). Compared with those in the model group, the differentially expressed mRNAs in the treatment group were enriched in the following GO terms: BP term: Mitochondrial organization, proteasome protein catabolic process, and oxidative phosphorylation (Figure 8A); CC term: Ribosome, ribosomal subunit, and large ribosomal subunit (Figure 8B); MF term: Structural constituent of ribosome and molecular carrier activity (Figure 8C). Additionally, the differentially expressed mRNAs in the treatment group were enriched in the following KEGG pathways: Mitochondrial autophagy, glutathione metabolism, oxidative phosphorylation, and drug metabolism (Figure 8D).
The proportions and infiltration levels of immune cells in the control, model, and treatment groups are shown in Figure 9A and B. Compared with those in the control group, the infiltration levels of monocytes and immature CD8+ T cells were significantly upregulated and the infiltration levels of immature CD4+ T cells were significantly downregulated in the model group (Figure 9C). Meanwhile, compared with those in the model group, the infiltration levels of mast cells and active NK cells were significantly upregulated and the infiltration levels of M2 macrophages were significantly downregulated in the treatment group (Figure 9D). The correlation analysis results of various immune cells are shown in Figure 9E.
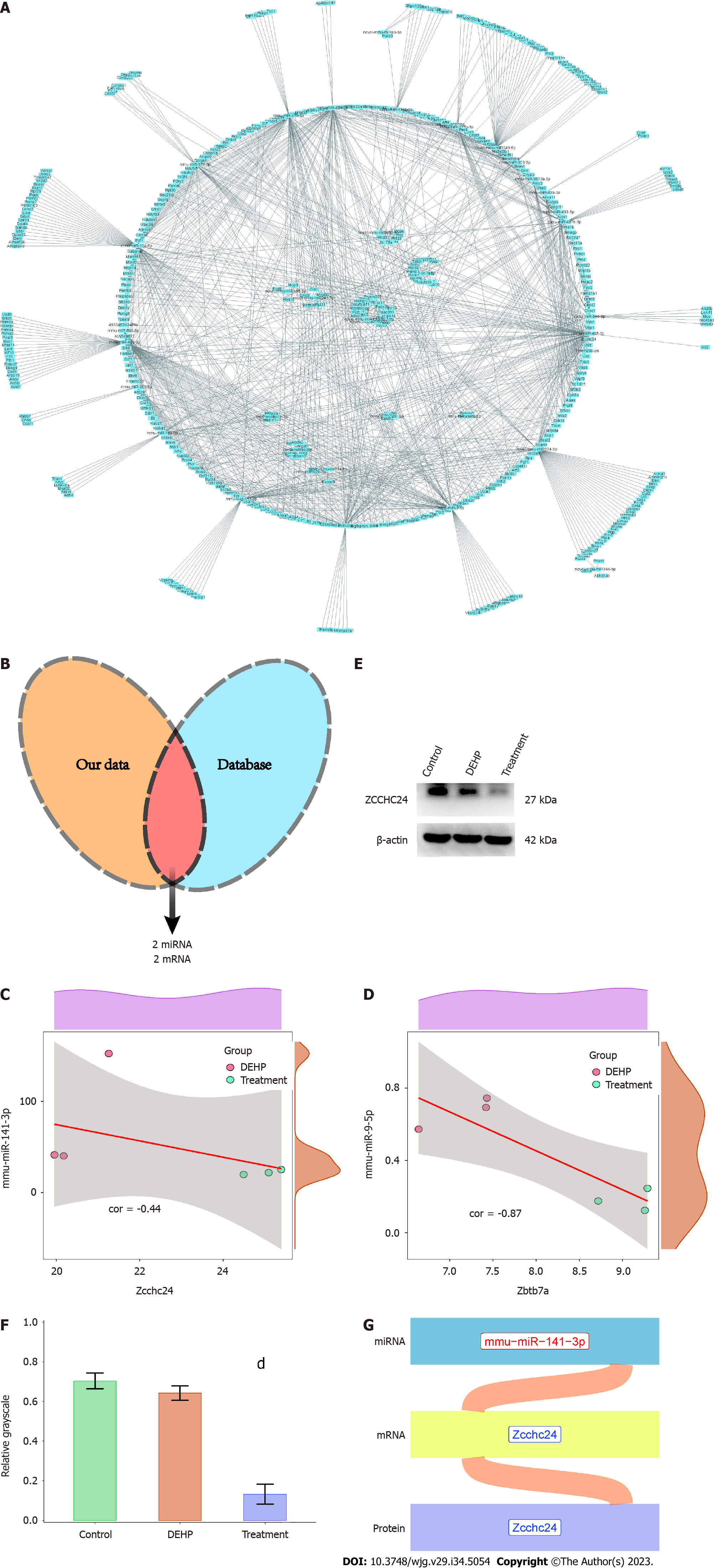
Network diagrams (Figure 10A) of upregulated miRNAs and downregulated mRNAs, as well as that of downregulated miRNAs and upregulated mRNAs, in the treatment group were constructed. The data were matched with the miRNA-mRNA regulatory axis in the starBase database to obtain the mmu-miR-141-3p/Zcchc24 and mmu-miR-9-5p/Zbtb7a axes (Figure 10B). Correlation analysis (Figure 10C and D) revealed that the correlation coefficients of mmu-miR-141-3p/Zcchc24 and mmu-miR-9-5p/Zbtb7a axes were -0.44 and -0.87, respectively. To validate these regulatory axes, the liver tissues of the control, model, and treatment groups were subjected to western blotting (Figure 10E). Compared with that in the model group, the average gray value of Zcchc24 protein was significantly downregulated in the treatment group (Figure 10F), indicating that protein expression changes were consistent with mRNA expression changes. However, Zbtb7a protein expression could not be validated using western blotting or immunohistochemical analysis due to low expression levels. These findings indicate that the mmu-miR-141-3p/Zcchc24 (mRNA)/Zcchc24 (protein) regulatory axis plays an important role in the protective effects of GTPs on DEHP-induced liver injury in mice (Figure 10G).
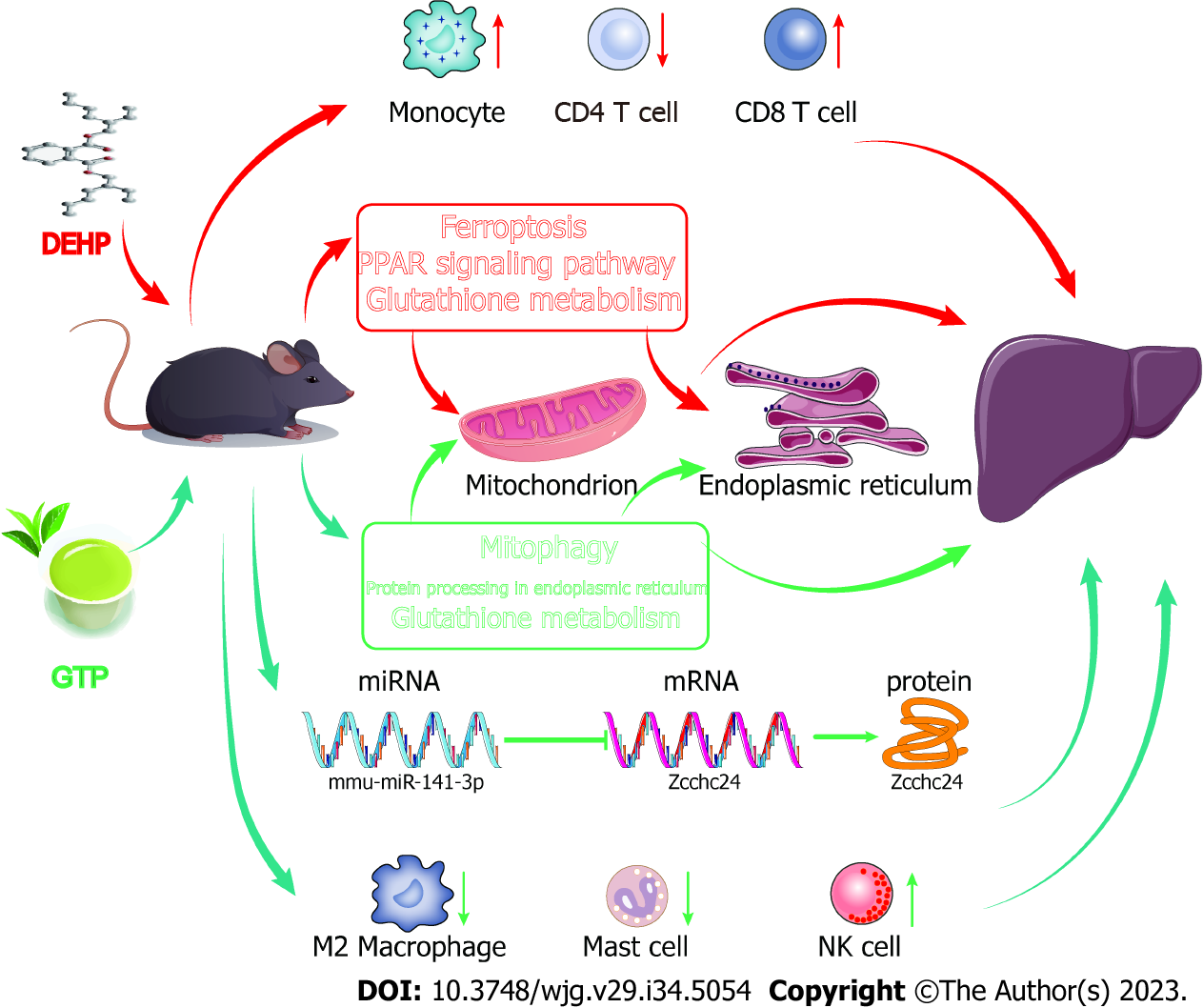
This study aimed to investigate the therapeutic mechanism of GTPs in alleviating DEHP-induced liver dysfunction, blood lipid dysregulation, non-alcoholic fatty liver, and liver fibrosis. Additionally, the role of a miRNA-mRNA-protein regulatory axis in the therapeutic effects of GTPs on DEHP-induced liver damage was elucidated (Figure 11). Thus, the findings of this study provide valuable insights into the potential therapeutic application of GTPs in DEHP-induced liver damage.
Histopathological analysis revealed that GTPs are effective in mitigating DEHP-induced non-alcoholic fatty liver disease and liver fibrosis in mice. This finding is consistent with that of previous studies, which reported the beneficial effects of GTP on liver health. For example, a meta-analysis[29] of 20 randomized controlled trials with 1536 participants revealed that green tea decreases the levels of total cholesterol and LDL. Zhao et al[30] reported that DEHP exposure significantly promotes inflammation, necrosis, and fibrosis in the liver and upregulates the expression of proteins associated with the development of liver inflammation and fibrosis. Kim et al[31] suggested that GTPs downregulate the expression of collagen content and type 1 collagen, and consequently alleviate liver fibrosis. Additionally, animal studies[32] have indicated that supplementation of green tea effectively prevented excessive accumulation of visceral and liver lipids, elevated blood glucose levels, and alleviated aberrant blood lipid levels, liver dysfunction, and hepatic steatosis in male C57BL/6 mice fed on a high-fat diet for six weeks as evidenced by the analysis of serum biochemical parameters, histological changes, lipid accumulation, inflammatory cytokines, and related indices. These findings further indicated the therapeutic potential of GTPs in liver-related conditions.
In this study, GTPs were found to ameliorate the DEHP-induced pathological damage to the liver microstructures, including the mitochondria, endoplasmic reticulum, and capillary bile ducts. These findings are consistent with those of previous studies, which reported the adverse effects of DEHP on liver microstructures. For instance, an animal study[33] indicated that DEHP induced mitochondrial and endoplasmic reticulum ultrastructural damages, characterized by increased fission and decreased fusion. Furthermore, Sun et al[34] suggested that DEHP promoted mitochondrial-associated endoplasmic reticulum membrane disruption, potentially through endoplasmic reticulum unfolded protein response, to induce endoplasmic reticulum stress. Consistent with the results of this study, in vitro and in vivo experimental studies[35] have demonstrated that GTPs effectively alleviate acetaminophen-induced liver damage and mitochondrial dysfunction. These studies have shown that GTPs possess antioxidant capacity and can prevent liver mitochondrial damage. Specifically, GTPs have been found to enhance the membrane potential and activity of liver mitochondrial respiratory chain complexes, thereby protecting against mitochondrial dysfunction. However, further research is needed to fully elucidate the underlying mechanisms and to explore the clinical applications of GTPs in protecting liver microstructures from DEHP-induced damage.
In this study, we aimed to analyze the signaling pathways involved in DEHP-induced liver damage and the hepatoprotective effects of GTPs using high-throughput sequencing. Ferroptosis, a recently discovered non-apoptotic cell death process, is induced by intracellular iron-dependent lipid peroxidation damage. Consistent with the findings of this study, Yin et al[36] investigated the acute toxicity of DEHP exposure in marine medaka. They conducted transcriptome analysis on the liver of DEHP-exposed medaka and reported that females were more sensitive to the immune response than males under acute DEHP exposure conditions. Furthermore, they found that DEHP exposure promoted iron depletion, leading to iron overload, increased levels of MDA and lipid peroxidation, and decreased levels of glutathione. These findings suggest that DEHP can rapidly alter certain molecular regulatory patterns and induce cell death through ferroptosis. In addition, we discussed the PPAR signaling pathway, which is a classic pathway associated with hyperlipidemia, regulates lipid metabolism and blood lipid levels by mediating various biological functions, such as the synthesis and decomposition of cholesterol and the oxidation of fatty acids[37]. Previous studies[38,39] have revealed that DEHP exposure dysregulates blood lipid levels and hepatic lipid metabolism in mice through the PPAR signaling pathway.
In this study, we utilized the CIBERSORT algorithm to identify specific alterations in immune cells. Consistent with the findings of this study, Yang et al[40] investigated the effect of DEHP on the immune system of male C57Bl/6 mice and reported significant atrophy of the thymus and spleen with the proportions of immature CD4+ and CD8+ populations exhibiting the highest downregulation. Additionally, they found a downregulation in the number of T and B cells in the spleen. A previous study[41] reported that green tea extract promotes macrophage phagocytic activity at a dosage of 5 mg/kg bodyweight and enhances NK cell activity and T cell proliferation at a dosage of 40 mg/kg bodyweight, supporting the potential immunomodulatory effects of GTPs demonstrated in this study.
This study identified the mmu-miR-141-3p/Zcchc24 (mRNA)/Zcchc24 (protein) regulatory axis, which exerted its function through Zcchc24. Zcchc24 is reported to be involved in cell differentiation in mice. Previous studies[42] have suggested that Zcchc24 can serve as a key gene in liver cancer prediction models, which are used to predict patient survival time. Additionally, Zcchc24 is one of the major selective splicing factors that plays a critical role in cell fate transition, development, and disease progression.
This study has several limitations. In this study, three samples per group were analyzed using high-throughput sequencing, which may have yielded biased results. Additionally, the levels of immune cells in each group were determined using the CIBERSORT algorithm. However, immune cell infiltration was not verified using alternate methods, such as single-cell sequencing. Furthermore, the importance of Zcchc24 was not verified using knockdown or overexpression experiments.
In conclusion, this study demonstrated that GTPs can alleviate the DEHP-induced changes in liver function and lipid profiles, improve fatty liver disease and partial liver fibrosis, and regulate immune cell infiltration. Additionally, an important miRNA-mRNA-protein molecular regulation axis that plays a crucial role in the therapeutic effects of GTPs on DEHP-induced liver damage was validated.
Di (2-ethylhexyl) phthalate (DEHP) is a widely used plasticizer that has been shown to cause liver injury. Previous studies have reported the therapeutic effects of green tea on organ damage caused by heavy metal exposure. However, there is limited research on the therapeutic effects of green tea polyphenols (GTPs) specifically on DEHP-induced liver damage.
Despite the known therapeutic effects of green tea on heavy metal exposure-induced organ damage, there is a lack of studies investigating the specific therapeutic effects of GTPs on DEHP-induced liver damage.
The research objectives of this study were to evaluate the molecular mechanism underlying the therapeutic effects of GTPs on DEHP-induced liver damage.
In this study, C57BL/6J mice were divided into different groups and treated with DEHP and GTPs. After 8 wk, various assessments were conducted, including examination of liver function, blood lipid profile, and liver histopathology. High-throughput sequencing was used to analyze differentially expressed miRNAs and mRNAs in the liver tissues. Functional enrichment analysis and immune infiltration prediction were performed, and the miRNA-mRNA regulatory axis was elucidated using the starBase database. Protein expression was evaluated using immunohistochemistry.
The results of this study showed that GTPs had beneficial effects on DEHP-induced liver damage in mice. GTPs alleviated liver dysfunction, blood lipid dysregulation, fatty liver disease, liver fibrosis, and mitochondrial and endoplasmic reticulum lesions. The infiltration of immune cells, such as macrophages, mast cells, and natural killer cells, varied between the model and treatment groups. Furthermore, the study identified specific miRNAs, mRNAs, and proteins that constituted a regulatory axis involved in mediating the therapeutic effects of GTPs on DEHP-induced liver damage.
The findings of this study indicate that GTPs have a therapeutic effect on DEHP-induced liver damage. GTPs were shown to alleviate liver dysfunction, blood lipid dysregulation, fatty liver disease, and partial liver fibrosis. Additionally, GTPs were found to regulate immune cell infiltration. The study also identified a significant miRNA-mRNA-protein regulatory axis involved in mediating the therapeutic effects of GTPs on DEHP-induced liver damage.
Further studies are needed to investigate the long-term effects of GTPs on DEHP-induced liver damage and to explore the potential mechanisms underlying the regulation of immune cell infiltration. Additionally, future research should focus on optimizing the dosage and administration of GTPs to maximize their therapeutic effects and minimize potential side effects. Furthermore, clinical trials are warranted to evaluate the efficacy and safety of GTPs as a potential therapeutic intervention for DEHP-induced liver damage in humans.
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript. We also thank Figraw (www.figdraw.com) for the materials provided in the scientific research drawing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kuznietsova H, Ukraine; Scarfì S, Italy S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Liu Y, Guo Z, Zhu R, Gou D, Jia PP, Pei DS. An insight into sex-specific neurotoxicity and molecular mechanisms of DEHP: A critical review. Environ Pollut. 2023;316:120673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Erythropel HC, Maric M, Nicell JA, Leask RL, Yargeau V. Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol. 2014;98:9967-9981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Silva MJ, Wong LY, Samandar E, Preau JL, Calafat AM, Ye X. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch Toxicol. 2017;91:3287-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-octyl phthalate. Reprod Toxicol. 2002;16:721-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Chen L, Zhao Y, Li L, Chen B, Zhang Y. Exposure assessment of phthalates in non-occupational populations in China. Sci Total Environ. 2012;427-428:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Matsumoto M, Hirata-Koizumi M, Ema M. Potential adverse effects of phthalic acid esters on human health: a review of recent studies on reproduction. Regul Toxicol Pharmacol. 2008;50:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Jadeja RN, Urrunaga NH, Dash S, Khurana S, Saxena NK. Withaferin-A Reduces Acetaminophen-Induced Liver Injury in Mice. Biochem Pharmacol. 2015;97:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Chen H, Zhang W, Rui BB, Yang SM, Xu WP, Wei W. Di(2-ethylhexyl) phthalate exacerbates non-alcoholic fatty liver in rats and its potential mechanisms. Environ Toxicol Pharmacol. 2016;42:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Yang G, Zhang W, Qin Q, Wang J, Zheng H, Xiong W, Yuan J. Mono(2-ethylhexyl) phthalate induces apoptosis in p53-silenced L02 cells via activation of both mitochondrial and death receptor pathways. Environ Toxicol. 2015;30:1178-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Isenberg JS, Kamendulis LM, Smith JH, Ackley DC, Pugh G Jr, Lington AW, Klaunig JE. Effects of Di-2-ethylhexyl phthalate (DEHP) on gap-junctional intercellular communication (GJIC), DNA synthesis, and peroxisomal beta oxidation (PBOX) in rat, mouse, and hamster liver. Toxicol Sci. 2000;56:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3074] [Article Influence: 236.5] [Reference Citation Analysis (1)] |
| 12. | Santhosh A, Nair KG, Arun P, Deepadevi KV, Manojkumar V, Lakshmi LR, Kurup PA. Effect of DEHP [di-(2-ethyl hexyl) phthalate] on lipid peroxidation in liver in rats and in primary cultures of rat hepatocytes. Indian J Med Res. 1998;108:17-23. [PubMed] |
| 13. | Ghosh J, Das J, Manna P, Sil PC. Hepatotoxicity of di-(2-ethylhexyl)phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF-κB pathway activation. Free Radic Biol Med. 2010;49:1779-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Afzal M, Safer AM, Menon M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology. 2015;23:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 15. | Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698S-702S; discussion 1703S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 531] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 16. | Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring). 2007;15:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 17. | Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68:1075-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Zwolak I. Epigallocatechin Gallate for Management of Heavy Metal-Induced Oxidative Stress: Mechanisms of Action, Efficacy, and Concerns. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Zhao Z, Lin CY, Cheng K. siRNA- and miRNA-based therapeutics for liver fibrosis. Transl Res. 2019;214:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | El-Maraghy SA, Adel O, Zayed N, Yosry A, El-Nahaas SM, Gibriel AA. Circulatory miRNA-484, 524, 615 and 628 expression profiling in HCV mediated HCC among Egyptian patients; implications for diagnosis and staging of hepatic cirrhosis and fibrosis. J Adv Res. 2020;22:57-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Lin Q, Zhou CR, Bai MJ, Zhu D, Chen JW, Wang HF, Li MA, Wu C, Li ZR, Huang MS. Exosome-mediated miRNA delivery promotes liver cancer EMT and metastasis. Am J Transl Res. 2020;12:1080-1095. [PubMed] |
| 22. | Sanjay S, Girish C. Role of miRNA and its potential as a novel diagnostic biomarker in drug-induced liver injury. Eur J Clin Pharmacol. 2017;73:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Hou P, Dai W, Jin Y, Zhao F, Liu J, Liu H. Maternal exposure to di-2-ethylhexyl phthalate (DEHP) depresses lactation capacity in mice. Sci Total Environ. 2022;837:155813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | XueXia L, YaNan L, Zi T, YuSheng Z, ZeLin W, Peng Z, MeiNa X, FuJun L. Di-2-ethylhexyl phthalate (DEHP) exposure induces sperm quality and functional defects in mice. Chemosphere. 2023;312:137216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Zhou X, Zhang Z, Shi H, Liu Q, Chang Y, Feng W, Zhu S, Sun S. Effects of Lycium barbarum glycopeptide on renal and testicular injury induced by di(2-ethylhexyl) phthalate. Cell Stress Chaperones. 2022;27:257-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Abdelrazek HM, Helmy SA, Elsayed DH, Ebaid HM, Mohamed RM. Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase- 3. Reprod Biol. 2016;16:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4763] [Cited by in RCA: 8921] [Article Influence: 892.1] [Reference Citation Analysis (0)] |
| 28. | Chen Z, Huang A, Sun J, Jiang T, Qin FX, Wu A. Inference of immune cell composition on the expression profiles of mouse tissue. Sci Rep. 2017;7:40508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 29. | Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:823-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Zhao ZB, Ji K, Shen XY, Zhang WW, Wang R, Xu WP, Wei W. Di(2-ethylhexyl) phthalate promotes hepatic fibrosis by regulation of oxidative stress and inflammation responses in rats. Environ Toxicol Pharmacol. 2019;68:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 31. | Kim HK, Yang TH, Cho HY. Antifibrotic effects of green tea on in vitro and in vivo models of liver fibrosis. World J Gastroenterol. 2009;15:5200-5205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Zhou J, Yu Y, Ding L, Xu P, Wang Y. Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Zhao Y, Cui JG, Zhang H, Li XN, Li MZ, Talukder M, Li JL. Role of mitochondria-endoplasmic reticulum coupling in lycopene preventing DEHP-induced hepatotoxicity. Food Funct. 2021;12:10741-10749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Sun X, Lin Y, Huang Q, Shi J, Qiu L, Kang M, Chen Y, Fang C, Ye T, Dong S. Di(2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J Cell Mol Med. 2015;19:581-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Lin Y, Huang J, Gao T, Wu Y, Huang D, Yan F, Weng Z. Preliminary Study on Hepatoprotective Effect and Mechanism of (-)-Epigallocatechin-3-gallate against Acetaminophen-induced Liver Injury in Rats. Iran J Pharm Res. 2021;20:46-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 36. | Yin X, Zeb R, Wei H, Cai L. Acute exposure of di(2-ethylhexyl) phthalate (DEHP) induces immune signal regulation and ferroptosis in oryzias melastigma. Chemosphere. 2021;265:129053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Giordano Attianese GM, Desvergne B. Integrative and systemic approaches for evaluating PPARβ/δ (PPARD) function. Nucl Recept Signal. 2015;13:e001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Zhang Y, Ge S, Yang Z, Li Z, Gong X, Zhang Q, Dong W, Dong C. Disturbance of di-(2-ethylhexyl) phthalate in hepatic lipid metabolism in rats fed with high fat diet. Food Chem Toxicol. 2020;146:111848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Xu M, Li Y, Wang X, Zhang Q, Wang L, Zhang X, Cui W, Han X, Ma N, Li H, Fang H, Tang S, Li J, Liu Z, Yang H, Jia X. Role of Hepatocyte- and Macrophage-Specific PPARγ in Hepatotoxicity Induced by Diethylhexyl Phthalate in Mice. Environ Health Perspect. 2022;130:17005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 40. | Yang Q, Xie Y, Depierre JW. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin Exp Immunol. 2000;122:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Huang AC, Cheng HY, Lin TS, Chen WH, Lin JH, Lin JJ, Lu CC, Chiang JH, Hsu SC, Wu PP, Huang YP, Chung JG. Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In Vivo. 2013;27:627-634. [PubMed] |
| 42. | Apizi A, Wang L, Wusiman L, Song E, Han Y, Jia T, Zhang W. Establishment and verification of a prognostic model of liver cancer by RNA-binding proteins based on the TCGA database. Transl Cancer Res. 2022;11:1925-1937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |