Published online Sep 14, 2023. doi: 10.3748/wjg.v29.i34.5038
Peer-review started: March 23, 2023
First decision: June 17, 2023
Revised: July 15, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: September 14, 2023
Processing time: 169 Days and 6.8 Hours
Hepatocellular carcinoma (HCC) is a common clinical condition with a poor prognosis and few effective treatment options. Potent anticancer agents for treating HCC must be identified. Epigenetics plays an essential role in HCC tumorigenesis. Suberoylanilide hydroxamic acid (SAHA), the most common histone deacetylase inhibitor agent, triggers many forms of cell death in HCC. However, the underlying mechanism of action remains unclear. Family with sequence similarity 134 member B (FAM134B)-induced reticulophagy, a selective autophagic pathway, participates in the decision of cell fate and exhibits anti
To elucidate potential roles and underlying molecular mechanisms of reticulophagy in SAHA-induced HCC cell death.
The viability, apoptosis, cell cycle, migration, and invasion of SAHA-treated Huh7 and MHCC97L cells were measured. Proteins related to the reticulophagy pathway, mitochondria-endoplasmic reticulum (ER) contact sites, intrinsic mitochondrial apoptosis, and histone acetylation were quantified using western blotting. ER and lysosome colocalization, and mitochondrial Ca2+ levels were characterized via confocal microscopy. The level of cell death was evaluated through Hoechst 33342 staining and propidium iodide colocalization. Chromatin immunoprecipitation was used to verify histone H4 lysine-16 acetylation in the FAM134B promoter region.
After SAHA treatment, the proliferation of Huh7 and MHCC97L cells was significantly inhibited, and the migration and invasion abilities were greatly blocked in vitro. This promoted apoptosis and caused G1 phase cells to increase in a concentration-dependent manner. Following treatment with SAHA, ER-phagy was activated, thereby triggering autophagy-mediated cell death of HCC cells in vitro. Western blotting and chromatin immunoprecipitation assays confirmed that SAHA regulated FAM134B expression by enhancing the histone H4 lysine-16 acetylation in the FAM134B promoter region. Further, SAHA disturbed the Ca2+ homeostasis and upregulated the level of autocrine motility factor receptor and proteins related to mitochondria-endoplasmic reticulum contact sites in HCC cells. Additionally, SAHA decreased the mitochondrial membrane potential levels, thereby accelerating the activation of the reticulophagy-mediated mitochondrial apoptosis pathway and promoting HCC cell death in vitro.
SAHA stimulates FAM134B-mediated ER-phagy to synergistically enhance the mitochondrial apoptotic pathway, thereby enhancing HCC cell death.
Core Tip: Family with sequence similarity 134 member B (FAM134B) is considered to be a tumor suppressor protein that can play a pivotal role in inhibiting hepatocellular carcinoma (HCC) cells. In addition, FAM134B acts as a putative reticulophagy receptor in the regulation of the reticulophagy process. Furthermore, suberoylanilide hydroxamic acid (SAHA) upregulates FAM134B expression in HCC cells and promotes apoptosis and autophagy-mediated cell death. Thus, FAM134B-mediated reticulophagy synergizes with SAHA to induce HCC cell death. Our findings offer novel insights into the mechanism underlying SAHA-induced HCC cell death.
- Citation: Li JY, Tian T, Han B, Yang T, Guo YX, Wu JY, Chen YS, Yang Q, Xie RJ. Suberoylanilide hydroxamic acid upregulates reticulophagy receptor expression and promotes cell death in hepatocellular carcinoma cells. World J Gastroenterol 2023; 29(34): 5038-5053
- URL: https://www.wjgnet.com/1007-9327/full/v29/i34/5038.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i34.5038
Hepatocellular carcinoma (HCC) represents the most well-known and prevalent primary liver cancer in China. The mortality rates with HCC have consistently increased annually[1]. Immune checkpoint therapies have recently emerged as noteworthy treatments for HCC[2-4]. A considerable proportion of patients, approximately 70% with advanced HCC, fail to derive benefits from immunotherapy[5]. Thus, pursuing more potent anticancer medications to combat HCC must persist. Owing to its high degree of malignancy, poor prognosis, and relatively limited range of treatment strategies, it is necessary to seek more powerful anticancer agents for treating HCC. In the past decade, accumulating evidence has validated the role that epigenetics plays in HCC tumorigenesis[6,7]. Epigenetic regulation changes the transcriptional activity of key genes without altering DNA sequences[8,9].
Epigenetic regulation occurs primarily through DNA methylation, post-translational histone modification, chromatin remodeling, and non-coding RNA-mediated gene silencing[10]. Current therapies targeting epigenetic modifications to cancer mainly include DNA methyltransferases and histone deacetylases (HDACs) as well as microRNAs (miRNAs). Due to the widespread existence of DNA methylation variation in HCC, a variety of corresponding regulators of DNA methyltransferase have been developed[11]. At the same time, miRNAs such as miRNA-148a are used in anti-HCC therapy by combining with oncolytic viruses.
Histone acetylation modification, induced by HDACs and histone acetyltransferases, is a prominent mode of epigenetic regulation[12]. The histone acetylation/deacetylation balance is dynamically regulated to maintain global chromatin structure[13]. Therefore, any dysregulation may contribute to altered gene expression, leading to pathological conditions, such as HCC. Suberoylanilide hydroxamic acid (SAHA) represents the most typical HDAC inhibitor (HDACi) and was the first of its kind to be approved for human treatment. So far, SAHA has been found to induce the differentiation of malignant tumor cells and accelerate apoptosis in vitro and in vivo[14]. According to our previous results, SAHA may act as a potential initiator of endoplasmic reticulum (ER) stress-associated apoptosis in HepG2 hepatoma cells by activating ER stress-related apoptotic pathways[15]. However, it is still unknown whether SAHA utilizes a new mechanism to induce HCC cell death through some different therapeutic targets.
Acetylation of histone H4 lysine 16 (H4K16ac) is important for gene initiation[16]. Recently, researchers have found that H4K16ac is closely associated with autophagy induction and significantly correlated with autophagy regulation. Moreover, deacetylase inhibitors can promote the upregulation of H4K16ac and lead to autophagic death of cancer cells[17]. However, the regulatory mechanism underlying the induction of H4K16ac-mediated reticulophagy is unclear.
Family with sequence similarity 134 member B (FAM134B) has been proposed as a cancer suppressor gene[18,19]. Numerous researchers have demonstrated that in colorectal carcinoma, the presence of FAM134B limits the overgrowth and suppresses the proliferation of cancer cells[20,21]. In addition, FAM134B acts as a putative reticulophagy receptor in regulating ER turnover and maintaining calcium homeostasis by remodeling ER[22,23]. Recent findings have also identified that FAM134B-mediated ER-phagy may regulate ER-mitochondrion interaction[24]. As the largest cellular organelle, the ER can interact with mitochondria through multiple contact sites, termed mitochondria-ER contact sites (MERCS).
Many ER-related and mitochondria-related proteins have been discovered at MERCS, including the inositol 1, 4, 5-trisphosphate receptor type 1 (IP3R1)/glucose-regulated protein 75 (GRP75)/voltage-dependent anion channel 1 (VDAC1) complex, which is a central component of MERCS that contributes to calcium exchange regulation[25,26]. Recent research has suggested that Ca2+ deregulation between ER and mitochondria by MERCS led to mitochondrial calcium overload, thereby activating the mitochondria-associated apoptotic pathway[27]. In the present study, we verified that SAHA treatment augmented FAM134B expression and facilitated Huh7 and MHCC97L HCC cell apoptosis; however, the regulatory mechanisms underlying this effect remain unknown. In our study, we elucidated potential roles and underlying molecular mechanisms of reticulophagy in SAHA-induced HCC cell death. Our findings may offer new perspectives for clinical trials of HCC.
The human Huh7 and MHCC97L cell lines were derived from the cell bank of the Chinese Academy of Sciences (Shanghai, China). Both cell lines were cultivated at 37 °C in a 5% CO2-supplemented atmosphere and maintained in high-glucose Dulbecco’s modified Eagle’s medium (ESscience, Shanghai, China) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, United States) and 1% penicillin-streptomycin (BioInd, Beit-Haemek, Israel). SAHA (Abcam, Cambridge, United Kingdom) was dissolved in dimethyl sulfoxide as a 5 mmol/L stock solution and then diluted with the complete medium to achieve ultimate concentrations of 0-24 μmol/L. Both cell lines were exposed to SAHA or vehicle treated with 0.1% dimethyl sulfoxide.
Cell counting kit-8 (CCK-8) assay was applied to evaluate the anti-proliferative effects of SAHA. Briefly, MHCC97L and Huh7 cells (5 × 103) were plated onto 96-well plates and given 24 h to adhere before being treated with SAHA. The cells were then exposed to various doses of SAHA (0, 0.5, 1, 3, 6, 9, 12, 18, and 24 μmol/L) for 48 h. Subsequently, 10 μL CCK-8 reagent (Solarbio, Beijing, China) was added to each well and incubated for 4 h. Absorbance at 450 nm was recorded using a spectrophotometer (BioRad, Hercules, United States). The IC50 of SAHA was calculated using GraphPad Prism software (v9.0.0, GraphPad Software, La Jolla, CA, United States).
The data regarding the apoptosis and cell cycle of HCC cells treated with 0, 1, 3, 6, and 12 μmol/L SAHA were obtained via standard flow cytometry (NovoCyte, Agilent, Santa Clara, CA, United States). The apoptosis assay was carried out using a cellular apoptosis detection kit (KeyGEN BioTECH, Nanjing, China) following the manufacturer’s protocol. Briefly, Huh7 and MHCC97L cells were plated onto 6-well plates at 1 × 105 cells per well before adding SAHA solution. Then, each group of cells was harvested, rinsed with chilled phosphate buffered saline (PBS), and loaded with binding buffer. The cells were labelled with Annexin V-FITC/propidium iodide (PI) solution, and the stained cells were calculated using the flow cytometer. Similarly, cell cycle analysis was conducted using the flow cytometer following the manufacturer’s instructions. Each group of cells was collected and washed thrice with chilled PBS. The cells were fixed with 70% ethanol and stained with PI/RNase I solution (KeyGEN BioTECH), and the percentages of cells in the G1, S, and G2 stages were measured using the flow cytometer. The inbuilt software NovoExpress® 1.4.1 was used for statistical analysis.
The wound healing assay was monitored to evaluate the effect of SAHA on HCC cell migration. Briefly, Huh7 and MHCC97L cells (3 × 104) were planted onto 6-well plates, grown until they formed an optically confluent monolayer, and then wounded with a sterile 200 μL micropipette tip. Upon treatment with 0 μmol/L or 3 μmol/L SAHA for 48 h, HCC cells were photographed using a microscope (× 40).
The cell invasion and migration assay in vitro was carried out using 24 transwell plates divided into upper and lower chambers using sterile polycarbonate with 8 μm pore size (Corning Life Science, Corning, NY, United States). The sterile polycarbonate covered 200 μL Matrigel (BD Biosciences, San Jose, CA, United States) in the cell invasion assay but not in the cell migration assay. Upon treatment with 0 μmol/L or 3 μmol/L SAHA for 48 h, cells that passed through the pore were stained with crystal violet, photographed, and quantified.
Huh7 and MHCC97L cells were exposed to 0, 1, 3, 6, and 12 μmol/L SAHA for 48 h. Then, the cells were rinsed with pre-chilled PBS, lysed in 100 μL RIPA lysis buffer with protease inhibitor (Solarbio), and collected with cell scrapers. Protein samples were boiled for 5 min, and total protein extracts were subjected to standard sodium-dodecyl sulfate gel electrophoresis and subsequently removed to polyvinylidene difluoride membranes (Merck Millipore, Burlington, MA, United States). The membranes were blocked with rapid blocking solution and stained overnight with the corresponding primary antibodies, including FAM134B (Proteintech; Wuhan, China 1:1500), CCPG1 (Proteintech; 1:1500), LC3 (Abcam; 1:2000), ATG12 (Cell Signaling Technology; Danvers, MA, United States 1:1500), H4 (Proteintech; 1:2000), total acH4 (Proteintech; 1:2000), H3K27ac (Cell Signaling Technology; 1:6000), H4K5ac (Cell Signaling Technology; 1:6000), H4K12ac (Cell Signaling Technology; 1:6000), H4K16ac (Abcam; 1:10000), GRP75 (Abcam; 1:2000), VDAC1 (Abcam; 1:2000), IP3R (Abcam; 1:1000), autocrine motility factor receptor (AMFR) (Proteintech; 1:500), cyt c (Cell Signaling Technology; 1:1500), cleaved caspase-3 (Cell Signaling Technology; 1:1500), Bax (Cell Signaling Technology; 1:1500), Bcl-2 (Cell Signaling Technology; 1;1500), and β-actin (Abcam; 1:1000), followed by incubation with the corresponding secondary antibodies (1:8000). Polyvinylidene fluoride membranes carrying proteins were treated with enhanced chemiluminescence reagent (Solarbio), and ImageLab software was used to observe the protein bands.
ER-trackers and Lyso-trackers (Beyotime; Nanjing, China), the specific organelle dyes, were applied to stain and locate ER and lysosomes, respectively. Huh7 and MHCC97L cells were exposed to 0, 1, 3, 6, and 12 μmol/L SAHA for 48 h. Then, the cells were coincubated with the two tracking dyes at 37 °C for 45 min and rinsed thrice with PBS. The stained cells were viewed at × 200 magnification under a confocal microscope (Olympus, Tokyo, Japan) and immediately imaged.
Huh7 and MHCC97L cells (3 × 104) were seeded in confocal laser Petri dishes. The cells were pretreated with 0.5 nmol/L bafilomycin A1 (MedChemexpress, NJ, United States) for 12 h and then exposed with 12 μmol/L SAHA for 48 h. Subsequently, Hoechst 33342/PI double fluorescent chromatin staining assay was conducted using a Viastain™ Hoechst 33342/PI viability kit (Beyotime). Cells were stained with Hoechst 33342 and PI for 30 min at 25 °C in the dark. The labelled cells were observed with a confocal microscope under × 400 magnification.
Rhod-2 AM Red (Abcam), a specific Ca2+ indicator, was applied to detect the level of mitochondrial Ca2+. Huh7 and MHCC97L cells were exposed to 0, 1, 3, 6, and 12 μmol/L SAHA for 48 h and rinsed thrice with Hank’s balanced salt solution. The treated cells were labelled with a mixture of Mito-Tracker Green (Beyotime) and Rhod-2 AM Red (Beyotime) and incubated for 50 min. The labelled cells were observed with a confocal microscope under × 400 magnification.
Chromatin immunoprecipitation (ChIP) assay of Huh7 cells was conducted as described previously[28]. Huh7 cells treated with 0 μmol/L or 6 μmol/L SAHA were subjected to ChIP assay using a ChIP Kit (Thermo Fisher Scientific, Waltham, MA, United States). In brief, the Huh7 cells were cross-linked with 1% formaldehyde and lysed with sodium-dodecyl sulfate lysis buffer. The lysate was sonicated and centrifuged (9000 × g/min) to harvest chromatin fragments (200-1000 bp). Immunoprecipitation was conducted with the following antibodies: anti-H4K16ac (1:50); rabbit IgG (1:50); and anti-RNA polymerase II (1:50). A no-antibody sample was used as input control. Input DNA and ChIP DNA were detected via quantitative polymerase chain reaction.
The JC-1 fluorescence mitochondrial imaging technique was applied to examine the mitochondrial membrane potential of HCC cells treated with 0, 1, 3, 6, and 12 μmol/L SAHA. Briefly, Huh7 and MHCC97L cells (1 × 105) were plated onto 6-well plates before adding SAHA solution and incubated with JC-1 fluorescence solution in an incubator for 20 min. Analysis was performed using a flow cytometer.
Data were processed and analyzed, and experimental graphs were prepared using GraphPad Prism software. All experiments were conducted in biological triplicate. All data are shown as means ± standard deviation. One-way analysis of variance was conducted for multigroup comparisons. P values less than 0.05 were considered to indicate significance.
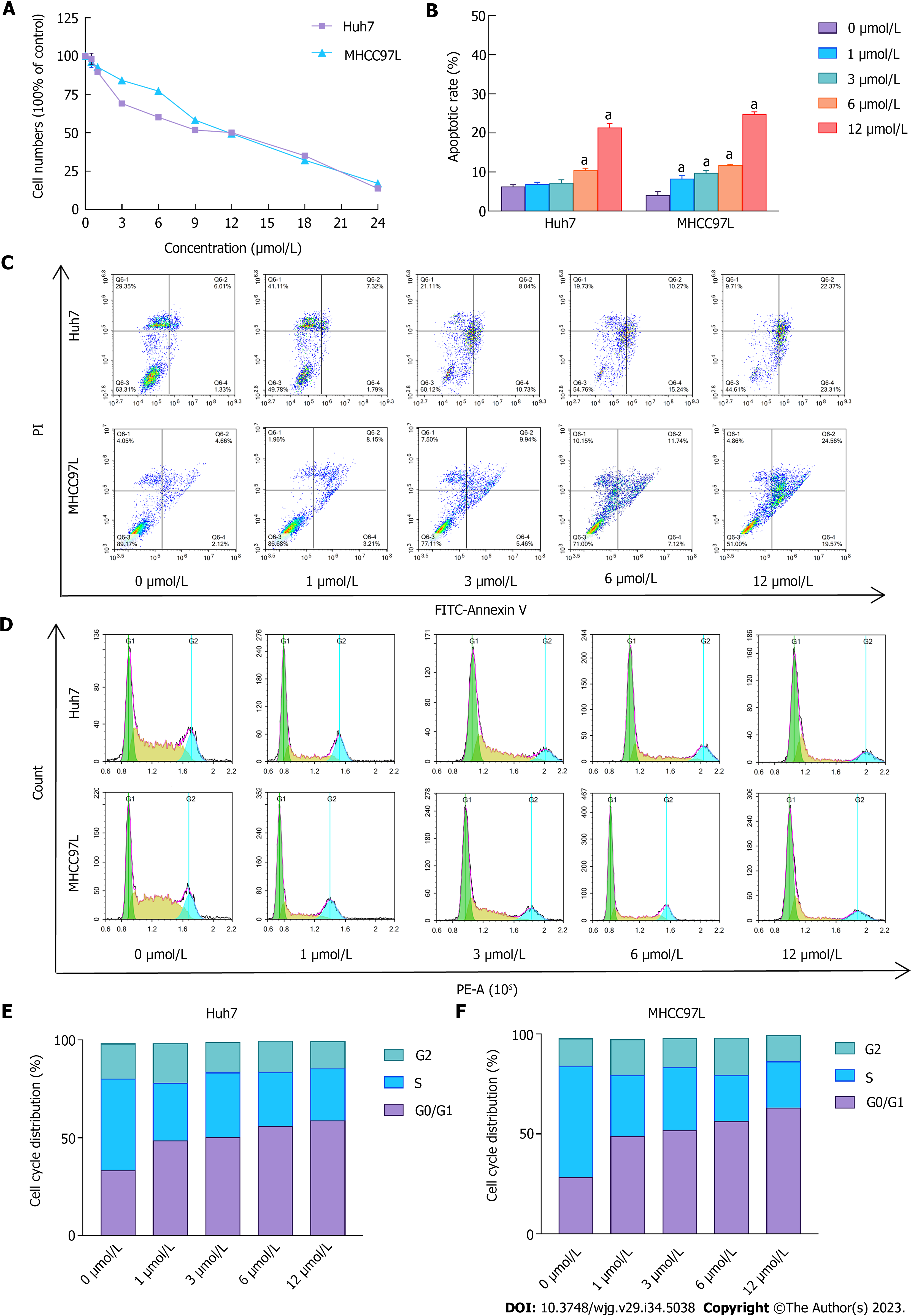
In our previous study, we confirmed that SAHA suppressed the overgrowth of HepG2 cells and mediated apoptosis by promoting the ER stress-associated apoptotic pathway[15]. However, this was not demonstrated in Huh7 and MHCC97L cells. As presented in Figure 1A, HCC cell growth was notably suppressed in SAHA-treated cells; the semi-lethal dose was 12 μmol/L. Apoptosis in Huh7 and MHCC97L cells was determined using flow cytometry with different SAHA concentrations. Consistent with previous studies, SAHA induced apoptosis in Huh7 and MHCC97L cells (Figures 1B and C). The percentage of specific cell populations that were early apoptotic and late apoptotic throughout the apoptotic stage is shown in Figure 1C. In addition, cell cycle assay results showed that an increasing number of cells were blocked in the G1 phase (Figures 1D-F).
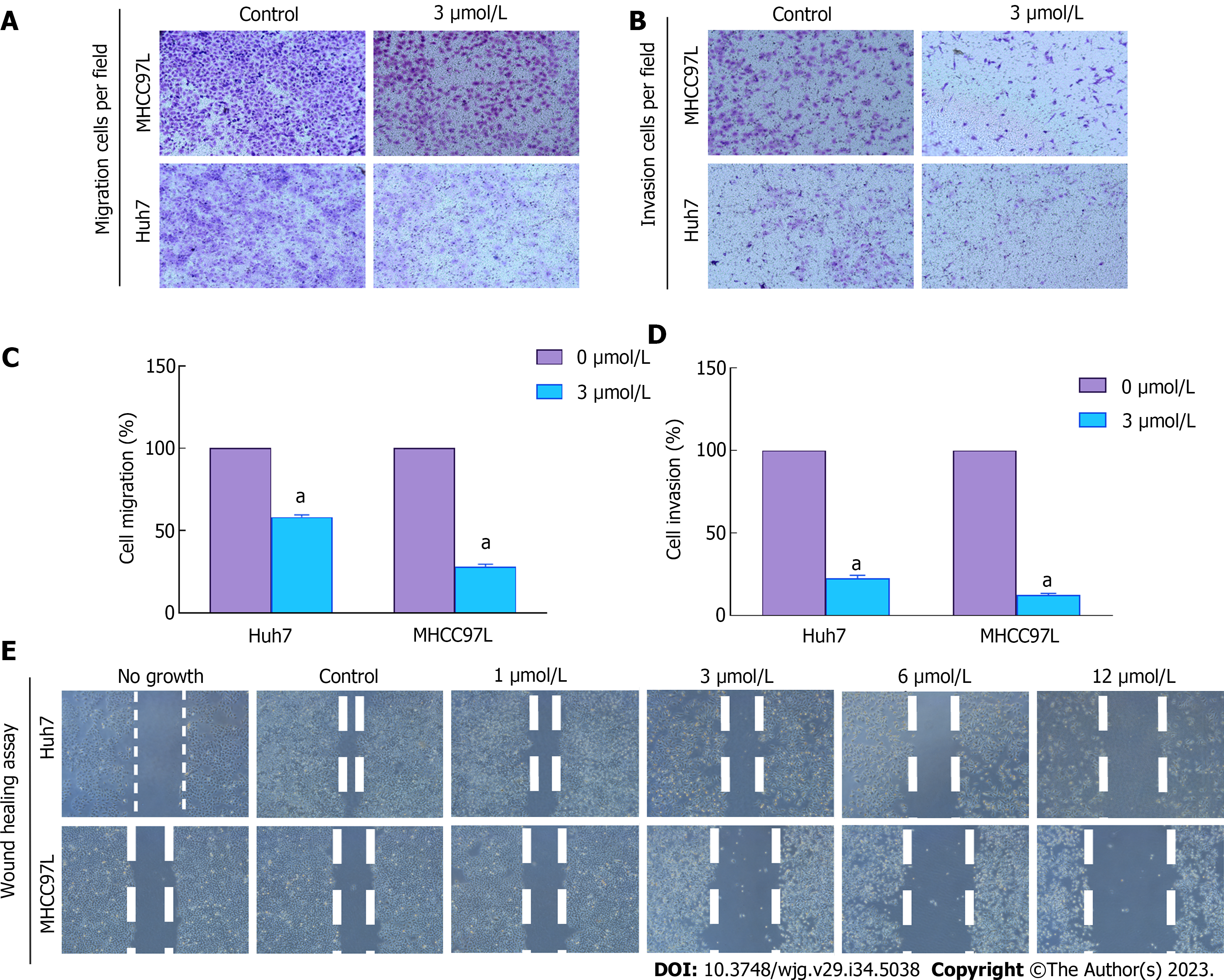
Both Huh7 and MHCC97L cells were tested under various treatment conditions (0 μmol/L or 3 μmol/L SAHA) to assess their ability to cross a membrane from low to high nutrient solution in vitro. Serum was used as an inducer, mimicking the process of tumor cell invasion into adjacent tissues in vivo. Cells invading through the semiporous membrane into the underlying medium containing a high serum concentration were photographed under an optical microscope (Figures 2A and B) and quantitatively analyzed (Figures 2C and D). The model cells were compared with the control sample, which was assumed to represent 100% invasion without any treatment. Huh7 and MHCC97L cells showed 78% and 68% lower migration and 44% and 30% lower invasion following treatment with 3 μmol/L SAHA, respectively. Further wound-healing tests were used to verify how cancer cells interact and move. The results showed a gradual decrease in cell migration distance after SAHA treatment (Figure 2E). Overall, SAHA resulted in reduced cell invasion and migration in HCC cells.
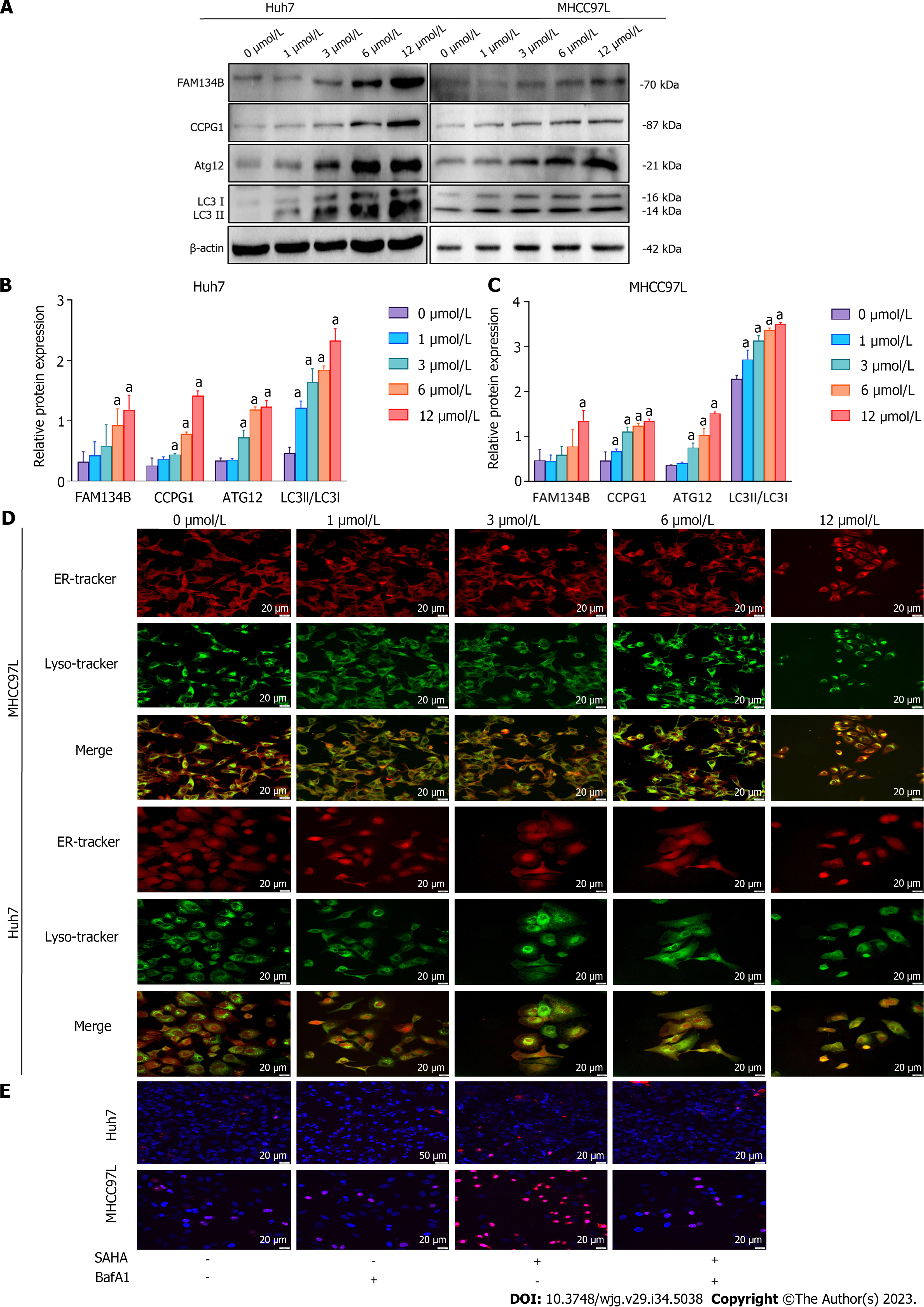
The effect of SAHA on FAM134B-mediated ER-phagy was assessed using western blotting. In HCC cells, SAHA increases the expression of proteins linked to the reticulophagy-related signaling pathway. We found increases in the expression of FAM134B, CCPG1, and autophagy-related protein Atg12 and the LC3-II/LC3-I ratio (Figures 3A-C). In the process of reticulophagy, ER fragments were delivered to lysosomes for final degradation. To detect the final state of ER autophagic lysosome formation, we used organelle markers that could trace the ER and lysosomes to detect the colocalization of both. We found that colocalization coverage of the ER and lysosomes increased in Huh7 and MHCC97L cells under SAHA treatment (Figure 3D). The above results suggested that SAHA could enhance the level of ER-phagy in HCC cells.
Previous research has reported that appropriate autophagy is a protective response under cellular stress conditions, but uncontrolled autophagy leads to autophagy-mediated cell death[29]. To further verify whether autophagic death mode exists in HCC cells under SAHA treatment, Huh7 and MHCC97L cells were pretreated with BafA1, a specific inhibitor of the late phase of autophagy that restrains autophagosomal fusion with lysosomes, 12 h before SAHA treatment. Nuclear double staining with Hoechst 33342 and PI was conducted to observe the level of cell death in the treated cells. The results showed that HCC cells treated with 12 μmol/L SAHA for 48 h after pretreatment with BafA1 exhibited significantly lower cell death rates compared with cells treated with 12 μmol/L SAHA alone, indicating that autophagy-mediated cell death was involved in SAHA-induced HCC cell death (Figure 3E).
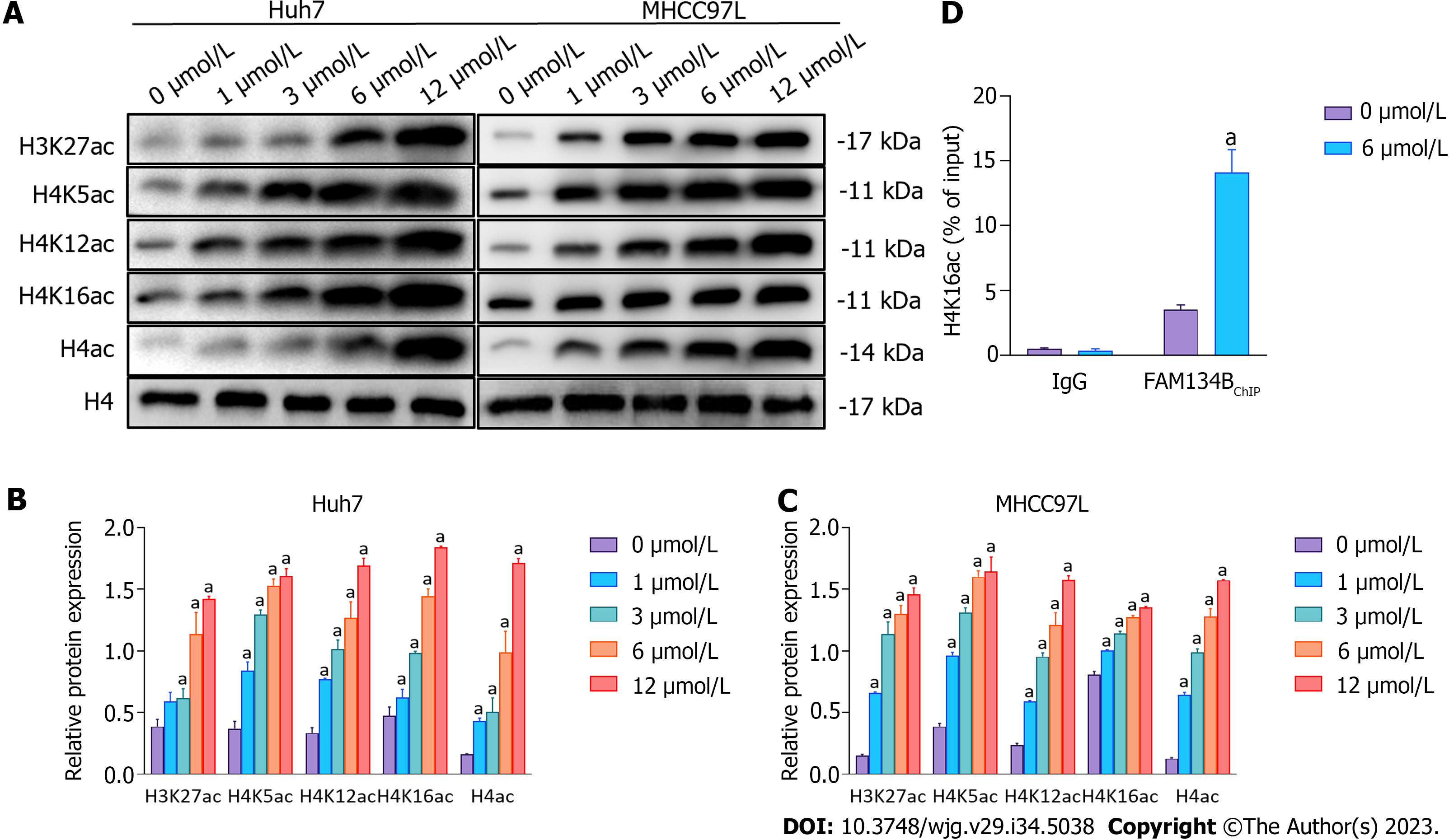
After SAHA treatment, the levels of H3K27ac, total H4ac, H4K5ac, H4K12ac, and H4K16ac in Huh7 and MHCC97L cells were determined using western blotting. We found that various doses of SAHA could upregulate the levels of these proteins (Figures 4A-C). Histone hyperacetylation results in gene transcription activation, and recent findings have shown that H4K16ac is linked to the regulation of autophagy-related genes; therefore, we further focused on the regulatory role of H4K16ac in gene expression. We conducted ChIP assays to confirm whether the regulation of FAM134B transcription by SAHA was mediated by H4K16ac upregulation. Our results showed that H4K16ac in the FAM134B promoter region was significantly increased in Huh7 cells (Figure 4D).
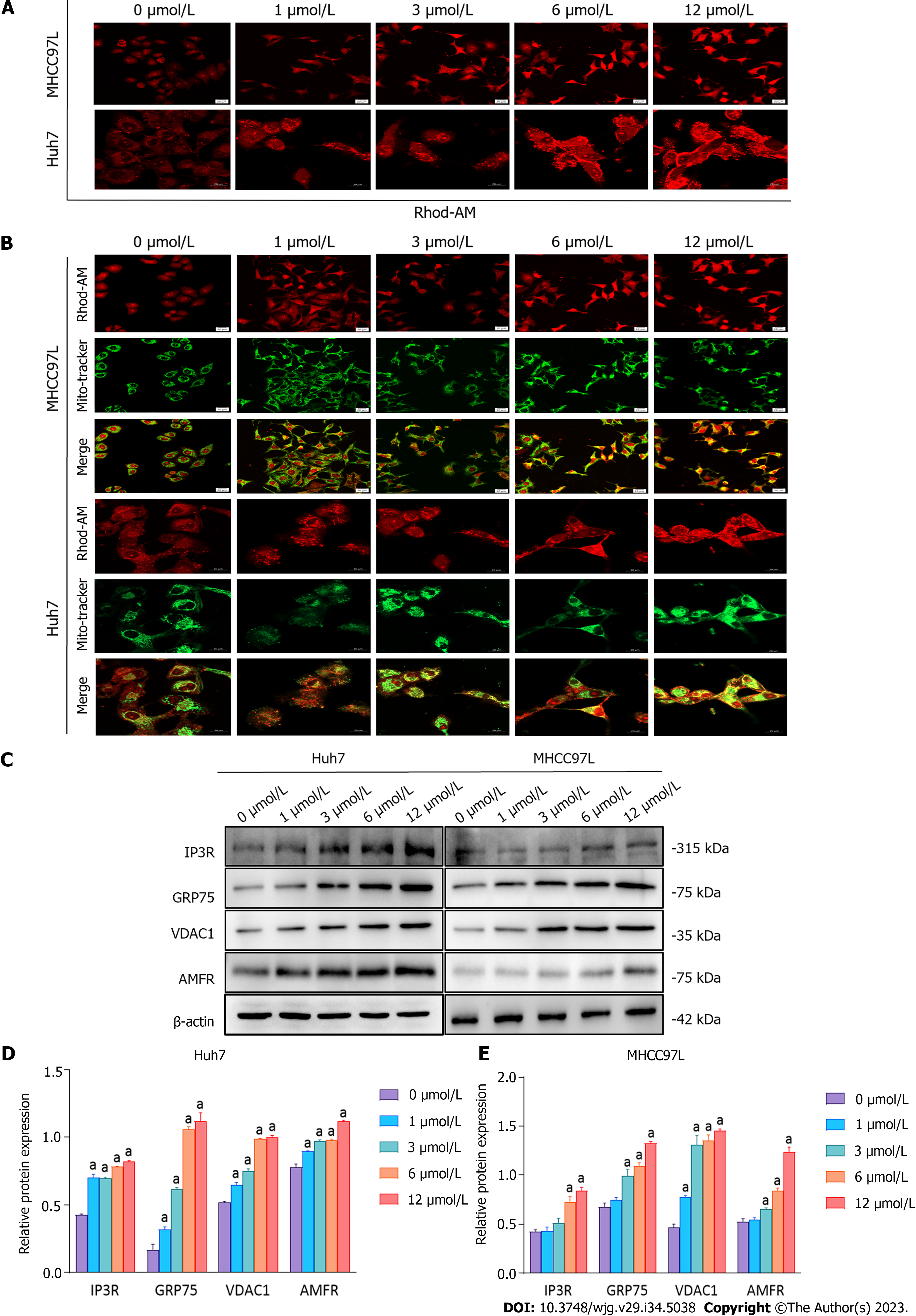
After SAHA treatment, the level of cytosolic Ca2+ in Huh7 and MHCC97L cells was determined using Rhod-2 AM Red staining, and mitochondrial Ca2+ was colocalized with Mito-Tracker Green and Rhod-2 AM Red. The results showed that SAHA markedly elevated cytosolic and mitochondrial Ca2+ levels (Figures 5A and B). Classical papers reported that increased cytosolic Ca2+ could increase the expression of AMFR, which targets the outer mitochondrial membrane (OMM) for ubiquitination and degradation[30-32]. We evaluated the protein level of AMFR in Huh7 and MHCC97L cells and found that SAHA could considerably increase AMFR protein levels. The IP3R-GRP75-VDAC1 complex is one of the most significant components of MERCS, which regulates ER-mitochondrial calcium flux. To determine the altered expression of MERCS in response to SAHA, the levels of IP3R, GRP75, and VDAC1 were examined using protein blots. Similar to our hypothesis, SAHA administration raised the protein levels of the above three proteins relative to the control group (Figures 5C-E). These findings showed that SAHA contributed to calcium buildup in the mitochondria by enhancing the interaction between the ER and mitochondria.
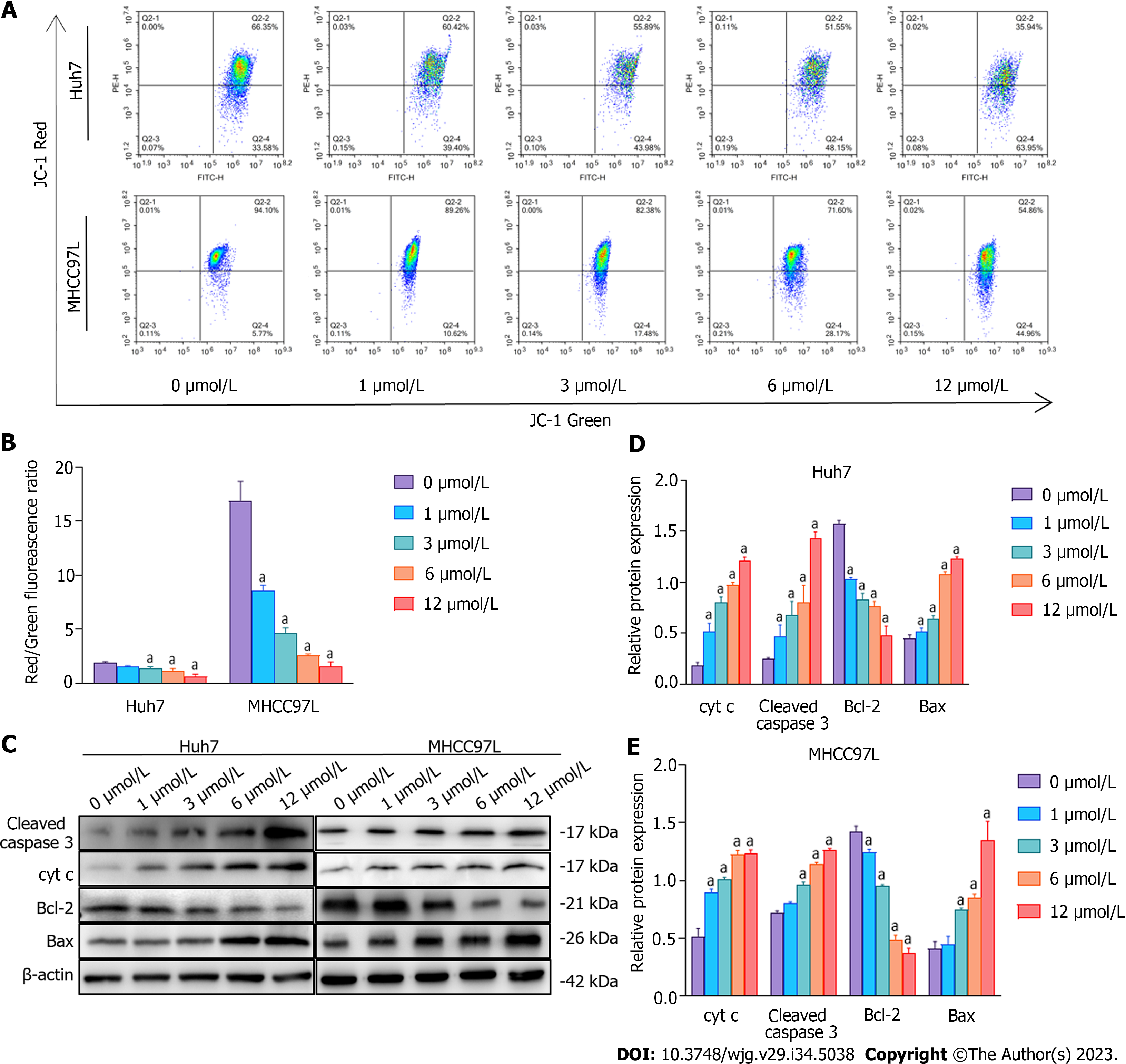
After SAHA treatment, mitochondrial membrane potential was monitored via JC-1 staining. We found that SAHA treatment reduced mitochondrial membrane potential (Figures 6A and B). To investigate the particular SAHA-induced apoptotic processes, the levels of cyt c, caspase-3, and proteins related to the Bcl-2 family were detected using western blotting. The experimental results revealed that SAHA upregulated the expression of cyt c, cleaved caspase-3, and Bax and downregulated the expression of Bcl-2 (Figures 6C-E). These results clearly indicated that the mitochondrial membrane potential was at a lower level, and the mitochondrial apoptotic pathway was activated under the action of SAHA.
SAHA, also known as vorinostat, is an HDACi that has shown tumor-suppressive properties in clinical trials[33]. Findings have revealed that SAHA could suppress cell proliferation and accelerate apoptosis in multiple malignant tumor cells. Scholars have demonstrated that SAHA increased the expression of death receptor 5 in liver cancer cells, which led to the initiation of the death receptor-mediated apoptosis pathway[34]. Furthermore, SAHA contributed to the initiation of the mitochondria-related apoptosis pathway by enhancing the protein level of Bim and Bax[35]. Our previous study found that SAHA could initiate the ER stress-related apoptotic pathway to foster apoptosis in liver cancer cells by upregulating the protein level of CHOP, a transcription factor that accelerates proapoptotic gene transcription[15]. In the present study, we found that SAHA suppressed the proliferation and induced cell cycle arrest in Huh7 and MHCC97L cells. Moreover, SAHA initiated Huh7 and MHCC97L cell apoptosis.
We also indicated that SAHA could augment the expression of FAM134B, a putative cancer suppressor gene. Studies have uncovered the pivotal role of FAM134B in multiple malignancies. Lee et al[20] reported that FAM134B may exert anticancer effects by influencing mitochondrial function and inducing cell cycle arrest in colon cancer. FAM134B also acts as a cancer suppressor in breast carcinoma. Upregulating FAM134B expression was significantly correlated with a higher survival in patients with breast carcinoma[36]. Zhong[37] confirmed that FAM134B was decreased in liver cancer, and its decreased expression was correlated with malignant liver cancer. In contrast, upregulation of FAM134B inhibited HepG2 cell proliferation and enhanced cell apoptosis. FAM134B was proposed as the first discovered mammalian receptor of reticulophagy[38,39], a type of selective phagocytosis. As an ER-anchored protein, FAM134B mediates the sequestration of ER fragments into phagophore membranes through its LC3-interacting region, which binds the autophagy modifier protein LC3[40-42]. In the present study, we detected the proteins related to ER-phagy in Huh7 and MHCC97L cells treated with various doses of SAHA. We found that the expression levels of FAM134B, Atg12, and LC3II/LC3I were considerably upregulated. Moreover, SAHA augmented the colocalization of ER and lysosomes in Huh7 and MHCC97L cells. The above results indicated that SAHA treatment enhanced the level of ER-phagy.
ER-phagy is a protective response under cellular stress conditions, but uncontrolled autophagy leads to the depletion of organelles and key proteins, which in turn lead to caspase-independent cell death, also known as autophagy-mediated cell death[43]. Recent findings have revealed that SAHA could induce autophagy-mediated cell death in malignant tumor cells[44,45]. In fact, a decrease in cell viability detected using CCK-8 was not the same as apoptosis induction, as revealed in this study. We found that 12 μmol/L SAHA resulted in markedly reduced Huh7 and MHCC97L cell proliferation levels (Figure 1), with proliferation being inhibited by approximately 50%; however, the rate of early apoptosis was only about 25%. This phenomenon reveals that SAHA may also elicit other types of cell death to suppress the proliferation of Huh7 and MHCC97L cells, such as autophagy-mediated cell death.
To confirm this hypothesis, we investigated cells with or without pretreatment with BafA1, a specific inhibitor of the late phase of autophagy that restrains autophagosomal fusion with lysosomes. Notably, BafA1-mediated autophagy inhibition reduced SAHA-induced cell death. These results confirmed that SAHA could enhance autophagy-mediated cell death, potentially by promoting FAM134B-mediated ER-phagy. However, the specific mechanism by which SAHA regulated FAM134B expression in Huh7 and MHCC97L cells remains elusive. As an HDACi, the essential role of SAHA is the enhancement of histone acetylation. In previous research, we revealed that SAHA markedly augmented the acetylated histones H4K5 and H4K12 in HepG2 cells[15]. In the present study, SAHA was shown to augment H4K16ac. In addition, Füllgrabe et al[17] reported that H4K16ac was linked to altered gene expression, including the modulation of autophagy-related genes; however, it was unclear whether H4K16ac regulated FAM134B transcription and its associated ER-phagy. We used ChIP to detect H4K16ac in the FAM134B promoter region. Our findings revealed that FAM134B promoter H4K16ac was considerably elevated in Huh7 cells exposed to SAHA, which enhanced the FAM134B transcription.
As a key organelle in eukaryotic cells, the ER contributes to protein synthesis and maintenance of calcium homeostasis[46,47]; hence, ER dysfunction results in the agglomeration of protein aggregates and disturbance of calcium homeostasis in the ER. Moreover, the ER contributes to organelle communication[27,48]. For example, the ER can interact with mitochondria through MERCS, as the short distance (15-20 nm) between the ER and OMM enables ER-anchored proteins to interact with OMM proteins[49]. The IP3R1-GRP75-VDAC1 complex is a central component of MERCS, which is involved in regulating calcium flow[50]. Emerging evidence has indicated that the imbalance of Ca2+ between the ER and mitochondria by MERCS leads to calcium overload in the mitochondria, which activates the mitochondria-associated apoptotic pathway[51].
Furthermore, under ER stress conditions, increased cytosolic Ca2+ would elevate the level of ER E3 ligase AMFR, which targets the OMM for ubiquitination and degradation[32]. High AMFR levels accelerate OMM degradation, which brings the inner mitochondrial membrane closer to the ER, thus promoting interplay between the ER and mitochondria[30]. In the present study, we revealed that SAHA upregulated the expression of MERCS-related proteins, including IP3R1, GRP75, and VDAC1, thereby enhancing the exchange of Ca2+ from the ER to the mitochondria, along with mitochondrial Ca2+ overload. Additionally, SAHA augmented cytosolic Ca2+ and increased AMFR expression, thus decreasing mitochondrial membrane potential and elevating mitochondrial membrane permeability, resulting in the release of proapoptotic proteins. As expected, we found that SAHA upregulated the expression level of mitochondria-dependent apoptotic proteins, including cytochrome c, cleaved caspase-3, and Bax but downregulated the expression of Bcl-2. The above results revealed that SAHA treatment enhanced the interplay between the ER and mitochondria and promoted Ca2+ transmission from the ER to the mitochondria, thereby activating the mitochondria-related apoptotic pathway.
This study had a few limitations. Despite the presence of numerous reticulophagy receptors, we focused on only FAM134B in this manuscript. For comprehensive knowledge, our team will verify multiple receptors in subsequent laboratory studies. Knocking down the gene encoding FAM134B could verify if it is a key gene in the pathway leading to HCC death. In this experiment, H4K16ac, a highly relevant acetylation site, was selected for the study. However, SAHA acts as a broad-spectrum deacetylase inhibitor influencing numerous acetylation sites, which need to be further verified in subsequent experiments. Results of this study indicated that SAHA can inhibit the proliferation of liver cancer cells in vitro; however, in vivo analyses could confirm the consistency of its effectiveness. Basic medical research serves the clinic, and the clinical verification of various aspects is more persuasive. Our study provided basic information that aids ongoing and prospective in vivo experiment.
The HDACi SAHA initiated apoptosis and autophagy-mediated cell death in Huh7 and MHCC97L cells to exert antitumor activity in HCC. Our results underscore a crucial link between the induction of ER-phagy and H4K16ac-linked FAM134B gene expression, which facilitates FAM134B-mediated ER-phagy. Moreover, we presented evidence that suggested that SAHA induced the mitochondria-associated apoptotic pathway in Huh7 and MHCC97L cells by enhancing the interplay between the ER and mitochondria and promoting Ca2+ exchange. In summary, SAHA promoted FAM134B-mediated ER-phagy, which acted synergistically with the mitochondrial apoptotic pathway to promote HCC cell death.
Suberoylanilide hydroxamic acid (SAHA) has been demonstrated to trigger multiple forms of cell death in hepatocellular carcinoma (HCC). Family with sequence similarity 134 member B (FAM134B), a reticulophagy receptor, has been recognized as a cancer suppressor protein in multiple tumors, including HCC. However, few researchers have focused on the relationship between reticulophagy and SAHA-induced HCC cell death.
Reticulophagy is involved in a variety of human cancer pathologies. However, its specific function in the modulation of SAHA-initiated HCC cell death remains unproven.
To validate the potential regulatory mechanisms of the FAM134B-mediated reticulophagy in SAHA-induced HCC cell death.
The proliferation, apoptosis, and cell cycle of SAHA-treated Huh7 and MHCC97L cells were quantified using cell counting kit-8 and flow cytometry. The migration and invasion of Huh7 and MHCC97L cells were measured using the transwell assay. Proteins related to the reticulophagy pathway, mitochondria-endoplasmic reticulum contact sites, intrinsic mitochondrial apoptosis, and histone H4K16 acetylation were detected using western blotting. ER and lysosome co-localization, and mitochondrial Ca2+ levels were observed via confocal microscopy. Autophagy-mediated cell death was validated through Hoechst33342 staining and propidium iodide colocalization. The enrichment of histone H4 lysine 16 acetylation in the FAM134B promoter region was determined using chromatin immunoprecipitation.
SAHA treatment augmented the expression of proteins related to the reticulophagy pathway and enhanced the level of reticulophagy in HCC cells. Chromatin immunoprecipitation experiments confirmed that SAHA regulated FAM134B expression by increasing the histone H4 lysine 16 acetylation in the FAM134B promoter region. SAHA interfered with Ca2+ homeostasis in HCC cells and upregulated the expression of autocrine motility factor receptor-related and mitochondria-endoplasmic reticulum contact sites-related proteins. Furthermore, SAHA reduced mitochondrial membrane potential and aggravated the activation of the reticulophagy-mediated mitochondrial apoptosis pathway and HCC cell death.
SAHA stimulated excessive reticulophagy and induced autophagy-mediated cell death, which acted synergistically with the mitochondria-dependent apoptotic pathway to facilitate HCC cell death.
FAM134B-induced reticulophagy may further provide a novel avenue for more effective interventions in HCC treatment. Our results confirmed that reticulophagy participates in SAHA-induced apoptosis and autophagy-mediated cell death in HCC cells, where SAHA-induced regulation of FAM134B expression via histone H4 lysine 16 is the key to HCC cell death.
The authors thank Mr. Yao Mu and Mrs. Yao Ran from the Guizhou Provincial Key Laboratory of Pathogenesis and Drug Research on Common Chronic Diseases, Guizhou Medical University (Guizhou, China) for their technical advice for using the confocal microscope and flow cytometer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Franci G, Italy; Rizzo A, Italy S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Cai YX
| 1. | Wang W, Wei C. Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis. 2020;7:308-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 2. | Santoni M, Rizzo A, Kucharz J, Mollica V, Rosellini M, Marchetti A, Tassinari E, Monteiro FSM, Soares A, Molina-Cerrillo J, Grande E, Battelli N, Massari F. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72:1365-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 116] [Reference Citation Analysis (0)] |
| 3. | Di Federico A, Rizzo A, Carloni R, De Giglio A, Bruno R, Ricci D, Brandi G. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 4. | Rizzo A, Ricci AD, Brandi G. Atezolizumab in advanced hepatocellular carcinoma: good things come to those who wait. Immunotherapy. 2021;13:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? Expert Rev Gastroenterol Hepatol. 2022;16:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | He B, Dai L, Zhang X, Chen D, Wu J, Feng X, Zhang Y, Xie H, Zhou L, Zheng S. The HDAC Inhibitor Quisinostat (JNJ-26481585) Supresses Hepatocellular Carcinoma alone and Synergistically in Combination with Sorafenib by G0/G1 phase arrest and Apoptosis induction. Int J Biol Sci. 2018;14:1845-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Ji H, Zhou Y, Zhuang X, Zhu Y, Wu Z, Lu Y, Li S, Zeng Y, Lu QR, Huo Y, Shi Y, Bu H. HDAC3 Deficiency Promotes Liver Cancer through a Defect in H3K9ac/H3K9me3 Transition. Cancer Res. 2019;79:3676-3688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1920] [Cited by in RCA: 2277] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 9. | Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 10. | Chianese A, Santella B, Ambrosino A, Stelitano D, Rinaldi L, Galdiero M, Zannella C, Franci G. Oncolytic Viruses in Combination Therapeutic Approaches with Epigenetic Modulators: Past, Present, and Future Perspectives. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Zhao P, Malik S, Xing S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front Oncol. 2021;11:677926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 12. | Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 2022;23:329-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 480] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 13. | Shen Y, Wei W, Zhou DX. Histone Acetylation Enzymes Coordinate Metabolism and Gene Expression. Trends Plant Sci. 2015;20:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 14. | Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 643] [Cited by in RCA: 895] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 15. | Yu L, Xie R, Tian T, Zheng L, Tang L, Cai S, Ma Z, Yang T, Han B, Yang Q. Suberoylanilide hydroxamic acid upregulates histone acetylation and activates endoplasmic reticulum stress to induce apoptosis in HepG2 liver cancer cells. Oncol Lett. 2019;18:3537-3544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Samata M, Alexiadis A, Richard G, Georgiev P, Nuebler J, Kulkarni T, Renschler G, Basilicata MF, Zenk FL, Shvedunova M, Semplicio G, Mirny L, Iovino N, Akhtar A. Intergenerationally Maintained Histone H4 Lysine 16 Acetylation Is Instructive for Future Gene Activation. Cell. 2020;182:127-144.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 17. | Füllgrabe J, Lynch-Day MA, Heldring N, Li W, Struijk RB, Ma Q, Hermanson O, Rosenfeld MG, Klionsky DJ, Joseph B. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature. 2013;500:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Islam F, Gopalan V, Lam AK. RETREG1 (FAM134B): A new player in human diseases: 15 years after the discovery in cancer. J Cell Physiol. 2018;233:4479-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Kasem K, Sullivan E, Gopalan V, Salajegheh A, Smith RA, Lam AK. JK1 (FAM134B) represses cell migration in colon cancer: a functional study of a novel gene. Exp Mol Pathol. 2014;97:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Lee KT, Islam F, Vider J, Martin J, Chruścik A, Lu CT, Gopalan V, Lam AK. Overexpression of family with sequence similarity 134, member B (FAM134B) in colon cancers and its tumor suppressive properties in vitro. Cancer Biol Ther. 2020;21:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Islam F, Chaousis S, Wahab R, Gopalan V, Lam AK. Protein interactions of FAM134B with EB1 and APC/beta-catenin in vitro in colon carcinoma. Mol Carcinog. 2018;57:1480-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Jiang X, Wang X, Ding X, Du M, Li B, Weng X, Zhang J, Li L, Tian R, Zhu Q, Chen S, Wang L, Liu W, Fang L, Neculai D, Sun Q. FAM134B oligomerization drives endoplasmic reticulum membrane scission for ER-phagy. EMBO J. 2020;39:e102608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Zhu L, Wang X, Wang Y. Roles of FAM134B in diseases from the perspectives of organelle membrane morphogenesis and cellular homeostasis. J Cell Physiol. 2021;236:7242-7255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Chen W, Ouyang X, Chen L, Li L. FAM134B-mediated ER-phagy regulates ER-mitochondria interaction through MAMs. Acta Biochim Biophys Sin (Shanghai). 2022;54:412-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Bao FX, Shi HY, Long Q, Yang L, Wu Y, Ying ZF, Qin DJ, Zhang J, Guo YP, Li HM, Liu XG. Mitochondrial Membrane Potential-dependent Endoplasmic Reticulum Fragmentation is an Important Step in Neuritic Degeneration. CNS Neurosci Ther. 2016;22:648-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Missiroli S, Patergnani S, Caroccia N, Pedriali G, Perrone M, Previati M, Wieckowski MR, Giorgi C. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018;9:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 27. | Sterea AM, El Hiani Y. The Role of Mitochondrial Calcium Signaling in the Pathophysiology of Cancer Cells. Adv Exp Med Biol. 2020;1131:747-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Liang C, Xie RJ, Wang JL, Zhang YW, Zhang JY, Yang Q, Han B. Roles of C/EBP-homologous protein and histone H3 lysine 4 methylation in arsenic-induced mitochondrial apoptosis in hepatocytes. Toxicol Ind Health. 2022;38:745-756. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 559] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 30. | Watanabe H, Carmi P, Hogan V, Raz T, Silletti S, Nabi IR, Raz A. Purification of human tumor cell autocrine motility factor and molecular cloning of its receptor. J Biol Chem. 1991;266:13442-13448. [PubMed] |
| 31. | Mo J, Chen J, Zhang B. Critical roles of FAM134B in ER-phagy and diseases. Cell Death Dis. 2020;11:983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Joshi V, Upadhyay A, Kumar A, Mishra A. Gp78 E3 Ubiquitin Ligase: Essential Functions and Contributions in Proteostasis. Front Cell Neurosci. 2017;11:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Park SJ, Kim SM, Moon JH, Kim JH, Shin JS, Hong SW, Shin YJ, Lee DH, Lee EY, Hwang IY, Kim JE, Kim KP, Hong YS, Lee WK, Choi EK, Lee JS, Jin DH, Kim TW. SAHA, an HDAC inhibitor, overcomes erlotinib resistance in human pancreatic cancer cells by modulating E-cadherin. Tumour Biol. 2016;37:4323-4330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Carlisi D, Lauricella M, D'Anneo A, Emanuele S, Angileri L, Di Fazio P, Santulli A, Vento R, Tesoriere G. The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Eur J Cancer. 2009;45:2425-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Jiang X, Tsang YH, Yu Q. c-Myc overexpression sensitizes Bim-mediated Bax activation for apoptosis induced by histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) through regulating Bcl-2/Bcl-xL expression. Int J Biochem Cell Biol. 2007;39:1016-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Chipurupalli S, Ganesan R, Martini G, Mele L, Reggio A, Esposito M, Kannan E, Namasivayam V, Grumati P, Desiderio V, Robinson N. Cancer cells adapt FAM134B/BiP mediated ER-phagy to survive hypoxic stress. Cell Death Dis. 2022;13:357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Zhong L. [The Expression Significance and Function of FAM134B in Hepatocellular Carcinoma Tissue]. 2020. |
| 38. | Chino H, Mizushima N. ER-Phagy: Quality Control and Turnover of Endoplasmic Reticulum. Trends Cell Biol. 2020;30:384-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 39. | Grumati P, Dikic I, Stolz A. ER-phagy at a glance. J Cell Sci. 2018;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 40. | Ferro-Novick S, Reggiori F, Brodsky JL. ER-Phagy, ER Homeostasis, and ER Quality Control: Implications for Disease. Trends Biochem Sci. 2021;46:630-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 41. | Hübner CA, Dikic I. ER-phagy and human diseases. Cell Death Differ. 2020;27:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 42. | Wilkinson S. ER-phagy: shaping up and destressing the endoplasmic reticulum. FEBS J. 2019;286:2645-2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Liao Y, Duan B, Zhang Y, Zhang X, Xia B. Excessive ER-phagy mediated by the autophagy receptor FAM134B results in ER stress, the unfolded protein response, and cell death in HeLa cells. J Biol Chem. 2019;294:20009-20023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Hrzenjak A, Kremser ML, Strohmeier B, Moinfar F, Zatloukal K, Denk H. SAHA induces caspase-independent, autophagic cell death of endometrial stromal sarcoma cells by influencing the mTOR pathway. J Pathol. 2008;216:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Lee YJ, Won AJ, Lee J, Jung JH, Yoon S, Lee BM, Kim HS. Molecular mechanism of SAHA on regulation of autophagic cell death in tamoxifen-resistant MCF-7 breast cancer cells. Int J Med Sci. 2012;9:881-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Kania E, Pająk B, Orzechowski A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed Res Int. 2015;2015:352794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 47. | Puzianowska-Kuznicka M, Kuznicki J. The ER and ageing II: calcium homeostasis. Ageing Res Rev. 2009;8:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Thoudam T, Ha CM, Leem J, Chanda D, Park JS, Kim HJ, Jeon JH, Choi YK, Liangpunsakul S, Huh YH, Kwon TH, Park KG, Harris RA, Park KS, Rhee HW, Lee IK. PDK4 Augments ER-Mitochondria Contact to Dampen Skeletal Muscle Insulin Signaling During Obesity. Diabetes. 2019;68:571-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 49. | Means RE, Katz SG. Yes, MAM! Mol Cell Oncol. 2021;8:1919473. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Zhang SS, Zhou S, Crowley-McHattan ZJ, Wang RY, Li JP. A Review of the Role of Endo/Sarcoplasmic Reticulum-Mitochondria Ca(2+) Transport in Diseases and Skeletal Muscle Function. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Xu H, Guan N, Ren YL, Wei QJ, Tao YH, Yang GS, Liu XY, Bu DF, Zhang Y, Zhu SN. IP(3)R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018;19:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |