Published online Sep 7, 2023. doi: 10.3748/wjg.v29.i33.4991
Peer-review started: June 14, 2023
First decision: July 7, 2023
Revised: July 22, 2023
Accepted: August 21, 2023
Article in press: August 21, 2023
Published online: September 7, 2023
Processing time: 78 Days and 9.6 Hours
The increased prevalence of inflammatory bowel disease (IBD) among patients with obesity and type 2 diabetes suggests a causal link between these diseases, potentially involving the effect of hyperglycemia to disrupt intestinal barrier integrity.
To investigate whether the deleterious impact of diabetes on the intestinal barrier is associated with increased IBD severity in a murine model of colitis in mice with and without diet-induced obesity.
Mice were fed chow or a high-fat diet and subsequently received streptozotocin to induce diabetic-range hyperglycemia. Six weeks later, dextran sodium sulfate was given to induce colitis. In select experiments, a subset of diabetic mice was treated with the antidiabetic drug dapagliflozin prior to colitis onset. Endpoints included both clinical and histological measures of colitis activity as well as histochemical markers of colonic epithelial barrier integrity.
In mice given a high-fat diet, but not chow-fed animals, diabetes was associated with significantly increased clinical colitis activity and histopathologic markers of disease severity. Diabetes was also associated with a decrease in key components that regulate colonic epithelial barrier integrity (colonic mucin layer content and epithelial tight junction proteins) in diet-induced obese mice. Each of these effects of diabetes in diet-induced obese mice was ameliorated by restoring normoglycemia.
In obese mice, diabetes worsened clinical and pathologic outcomes of colitis via mechanisms that are reversible with treatment of hyperglycemia. Hyperglycemia-induced intestinal barrier dysfunction offers a plausible mechanism linking diabetes to increased colitis severity. These findings suggest that effective diabetes management may decrease the clinical severity of IBD.
Core Tip: Metabolic syndrome affects many patients with inflammatory bowel disease (IBD). This study used mouse models of colitis to investigate how diabetes and obesity interact to impair intestinal barrier function and exacerbate IBD outcomes, highlighting the deleterious impact of sustained hyperglycemia on intestinal barrier integrity. We showed that diabetic hyperglycemia impairs the colonic mucin barrier and tight junction protein abundance in the setting of diet-induced obesity, which corresponds to worse clinical and histopathological IBD outcomes. These findings are important because as more patients with IBD are affected by obesity and/or diabetes, it is imperative to understand how these disease processes interact.
- Citation: Francis KL, Alonge KM, Pacheco MC, Hu SJ, Krutzsch CA, Morton GJ, Schwartz MW, Scarlett JM. Diabetes exacerbates inflammatory bowel disease in mice with diet-induced obesity. World J Gastroenterol 2023; 29(33): 4991-5004
- URL: https://www.wjgnet.com/1007-9327/full/v29/i33/4991.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i33.4991
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), and type 2 diabetes (T2D) are among the most challenging and costly medical disorders in modern society. Each is a chronic condition with no permanent medical cure that is increasing in prevalence globally and is associated with significant patient morbidity and economic cost[1,2]. IBD is an autoimmune condition affecting the gastrointestinal tract. The pathogenesis of IBD is multifactorial, involving genetic predisposition, immunologic abnormalities, alterations in gut microbiota, and environmental factors, particularly exposure to a Western diet[3-5]. The pathogenesis of T2D involves insulin resistance and loss of pancreatic β cell function related to risk factors including obesity, visceral adiposity, and exposure to high-fat and high-sugar diets[6,7].
There is increasing evidence that these two disease processes may be linked. Recent national cohort studies have shown that patients with IBD are at increased risk of developing T2D, even after controlling for multiple risk factors including steroid exposure, age, and body mass index[8,9]. Furthermore, the development of T2D in patients with IBD is a predictor of poor disease-related outcomes, with several studies showing higher rates of IBD-related hospitalizations in patients with IBD[10,11] as well as disease flares in patients with either CD or UC and increased IBD-related surgeries in patients with CD[11].
Obesity also constitutes a potential link between T2D and IBD. T2D is strongly associated with obesity, with the majority of T2D patients being either overweight or obese[12,13]. Obesity also affects up to 40% of adult IBD patients, with an additional 20%-40% of patients being overweight[14]. Comorbid obesity in IBD patients has been associated with higher rates of surgical complications and more severe disease that may be less responsive to standard medical therapies[15,16]. Consumption of obesity-inducing high-fat diets (HFD) is a well-known risk factor for developing IBD[17-19], and in preclinical rodent studies, diet-induced obesity (DIO) caused by consuming a HFD worsens clinical and histological IBD outcomes[20,21]. However, the mechanisms underlying the relationship among DIO, T2D, and the development of IBD are unknown.
Notably, both IBD and metabolic syndrome (of which T2D and DIO comprise two of the principle components) are associated with an altered gut microbiome, chronic systemic inflammation, and intestinal barrier dysfunction[4,22,23]. Specifically, the role of increased gut permeability leading to enhanced influx of microbial products from the gut lumen into systemic circulation has been implicated in the pathogenesis of metabolic syndrome and its many complications[22]. Increased gut permeability is also associated with active IBD, as confocal laser endomicroscopic assessment of fluorescent leakage across the intestinal barrier is increased in symptomatic patients with IBD (both CD and UC) compared to healthy controls and asymptomatic IBD patients[24].
Currently, the mechanisms that link intestinal barrier dysfunction in diabetes, obesity, and IBD remain poorly understood. Recent work indicates that diabetic hyperglycemia may drive intestinal barrier impairments resulting in increased risk for enteric infections[25]. However, the extent to which intestinal barrier dysfunction contributes to the increased risk of IBD complications in patients with comorbid T2D and what role DIO may play in this effect is unknown.
The current work was undertaken to determine the pathogenic role of diabetes to exacerbate intestinal inflammation in a mouse model of IBD with or without coexisting DIO, highlighting intestinal barrier disruption as a mechanism that links diabetes and IBD.
Eight-week-old C57BL/6J male mice were purchased from Jackson Laboratory (Bar Harbor, ME, United States). All animals were group-housed under specific pathogen-free conditions in a temperature-controlled environment (14:10 h lights on/off cycle; lights on at 7:00 am) in cages containing a maximum of five animals. Mice were either fed standard laboratory chow (5053 PicoLab® Rodent Diet 20; LabDiet, St. Louis, MO, United States) throughout the study or placed on an HFD (D12492; Research Diets, New Brunswick, NJ, United States) at 8 wk of age for a period of 9 subsequent weeks to induce DIO. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Washington. Body weight (BW) and food intake were recorded at least twice weekly throughout the experiment.
Mice were randomly chosen to be placed on a HFD for 9 wk to induce DIO (n = 30) or maintained on standard chow (n = 20) and then received five consecutive daily intraperitoneal injections of streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO, United States) at a low dose (40 mg/kg BW) to induce diabetes-range hyperglycemia (STZ-diabetes; random blood glucose levels ≥ 250 mg/dL) or sodium citrate vehicle control (Veh) at an equivalent dose (40 mg/kg)[26]. Mice were chosen randomly for the treatment groups, anesthetized with isoflurane during STZ injections, and placed immediately back into their home cage afterwards. Following STZ administration, random blood glucose levels were recorded at least twice weekly throughout the experiment.
Six weeks following STZ or Veh administration, 2% dextran sodium sulfate (DSS) (36-50 kDa; MP Biochemicals, Santa Ana, CA, United States) dissolved in autoclaved water or untreated autoclaved water (Veh) was provided in drinking water for 7 d, with 4-8 animals per experimental group. Animals were acclimated to the medicated water delivery system for 1 wk prior to DSS course in their home cage, with daily water and food intake recorded. To control for the observation that diabetic mice consumed more water leading up to the DSS course, paired water administration was performed during the DSS course. Disease activity index (DAI) scores were calculated daily based on the sum of the percentage of BW lost (0-4), degree of rectal bleeding (0-4), and consistency of stools (0-4)[27].
A subset of C57BL/6J male mice (n = 23) was placed on an HFD to induce DIO and then received either STZ or sodium citrate Veh as described above. After 6 wk of sustained hyperglycemia, the sodium-glucose cotransporter-2 inhibitor (SGLT2i) dapagliflozin was added to the drinking water for randomly selected groups at a dose of 25 mg/kg based on ideal BW (0.03 kg) to ameliorate hyperglycemia for 3 wk in total[28]. During the last week, 2% DSS was added to drinking water for select experimental groups as detailed above. Results were compared to mice that received drinking water without dapagliflozin. There were 7-8 animals in each experimental group.
Mice were sacrificed on day 7 of the DSS course by euthanasia with an anesthetic overdose. Blood, colon, small intestine, liver, mesenteric fat, spleen, and fecal samples were collected. The spleen weight was recorded. Sections of liver, spleen, and mesenteric fat were stored either fresh frozen at -80 °C or placed into 10% zinc-buffered formalin (ZBF) for fixation and histological processing. The entire colon was removed from the surrounding mesentery and excised, and the length was measured from the end of the cecum to the end of the rectum. The colon was flushed with 0.1 M phosphate-buffered saline (PBS). A small section of proximal colon was cut and flash frozen at -80 °C, and the remaining colon was fixed in ZBF for histological examination. The small intestine was divided in half and flushed with PBS. Pieces of the proximal and distal small bowel were flash frozen at -80 °C, and the remaining tissue was placed into ZBF. Tissue collection and assessment of intestinal barrier integrity were performed 7 wk after STZ administration, limiting any direct toxic effect of intraperitoneal injection of STZ on the colon.
After the 3 d fixation in ZBF, the intestines were cut longitudinally, swiss-rolled, pinned, and placed in 70% ethanol. Colon, proximal small bowel, and distal small bowel were paraffin embedded, sectioned at 4 µm thickness, deparaffinized, and stained with either hematoxylin and eosin or Alcian blue (AB) to stain mucins by the University of Washington Diabetes Research Center Cellular and Molecular Imaging Core. Using a modified protocol from Wirtz et al[29], histopathologic scoring of the extent of tissue damage was performed by a blinded pathologist, taking into account distribution of tissue damage [0 = none, 1 = focal (less than 3 sites), 2 = moderate (3-5 sites), 3 = diffuse (> 5 sites)], severity of tissue damage (0 = none, 1 = isolated focal epithelial damage, 2 = mucosal erosions and ulcerations, 3 = extensive damage deep into the bowel wall), lamina propria inflammatory cell infiltration (0 = infrequent, 1 = increased, some neutrophils, 2 = submucosal presence of inflammatory cell clusters, 3 = transmural cell infiltrations), and presence of chronic or active inflammation (0 = none, 1 = active, 2 = chronic).
Brightfield microscopy of AB-stained colonic tissues was performed using the Keyence BZ-X800 microscope (Keyence Corp. of America, Itasca, IL, United States). Four random images at × 20 magnification were taken from each animal of intact colonic tissue, avoiding any ulceration of the epithelium, by a blinded independent investigator. Percent area of AB staining was performed using Fiji open source imaging software specific for AB imaging analysis (Color Deconvolution, Vector: AB & H, Color 1). Threshold limits were set (0, 207) and used to measure the percent area of staining across the region of interest selected (four representative crypts per image). This process was repeated for four distinct images per animal, and values were averaged per animal.
Colon sections mounted on slides were deparaffinized with xylene, and antigen-retrieval in 10 mmol/L sodium citrate, pH 6.0 was performed. Sections were incubated in 0.1 M PBS followed by 0.2% Triton X-100 in PBS, then blocked in 2% donkey serum in 0.05% Triton X-100 at 37 °C for 1 h, and finally incubated overnight with rabbit anti-E-cadherin (24E10; Cell Signaling Technology, Danvers, MA, United States). Sections were washed, incubated for 2 h with Alexa 555-conjugated donkey anti-rabbit antibody, and then stained with DAPI.
Immunofluorescence images were captured using the Keyence BZ-X800 microscope, and four random images of intact colonic tissue at × 20 magnification were taken from each animal. Percent area of E-cadherin staining in the epithelium was performed using Fiji open source imaging software, building on prior published immunohistochemistry protein quantification methods[30]. Fluorescent images (E-cadherin: 555) were converted to 8-bit images, threshold staining limits were set (E-cadherin: 9, 255), and the region of interest outlining the entire epithelium captured in the × 20 image was selected. Percent area values were recorded for each image, repeated for a total of four images per animal, and then averaged.
Data from individual experiments including blood glucose, DAI, colon length, and spleen length data were shown as dot plots representing data from individual animals, and bar graphs represented mean ± standard error of the mean. AB and E-cadherin data were also presented as mean values ± standard error of the mean. Student’s t test was used to compare the means in two groups, and one-way analysis of variance was used to compare multiple groups, using GraphPad Prism 5 (GraphPad software, La Jolla, CA, United States). Animals were not excluded from the study unless otherwise indicated. All differences were considered statistically significant at P < 0.05.
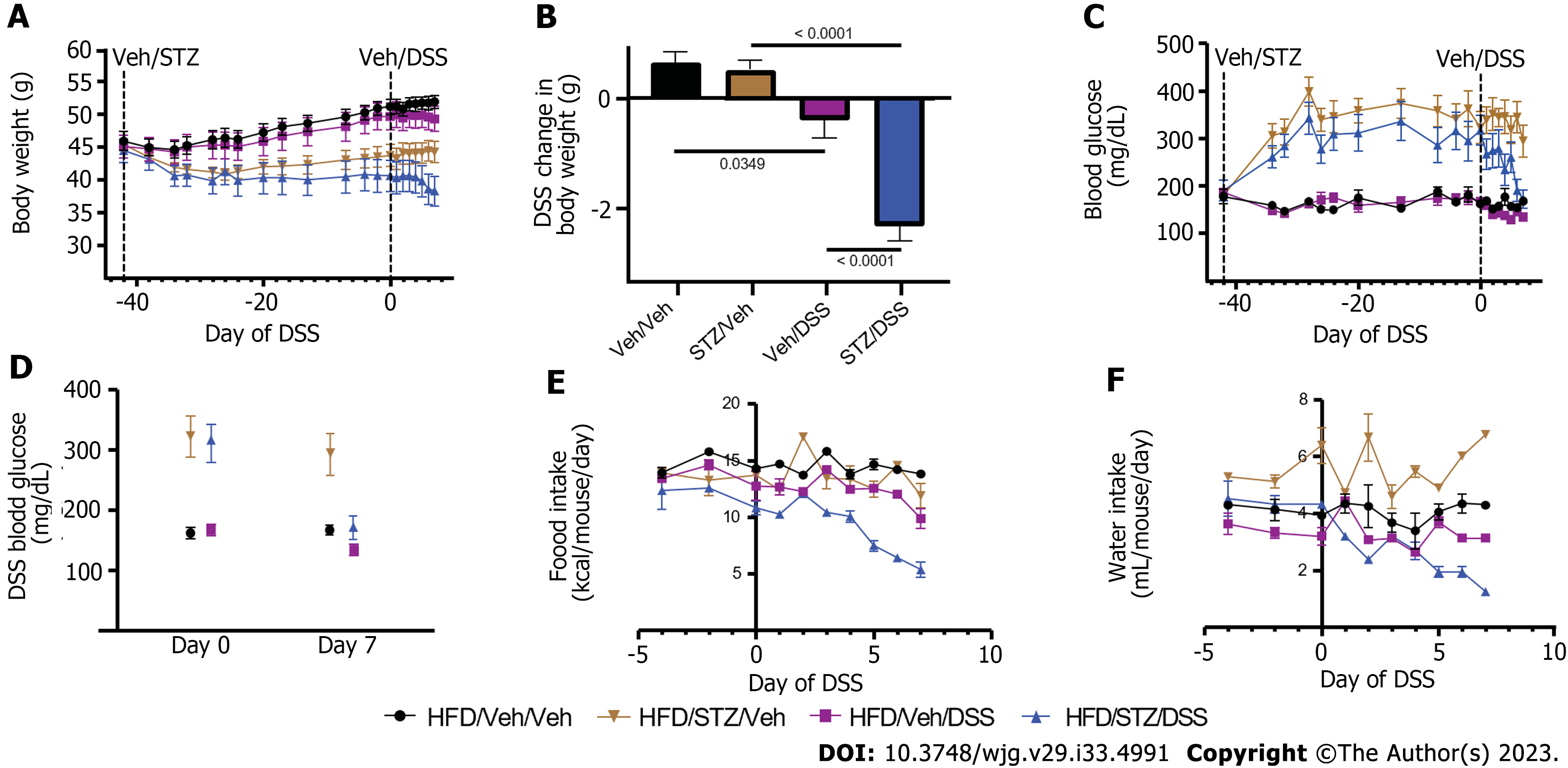
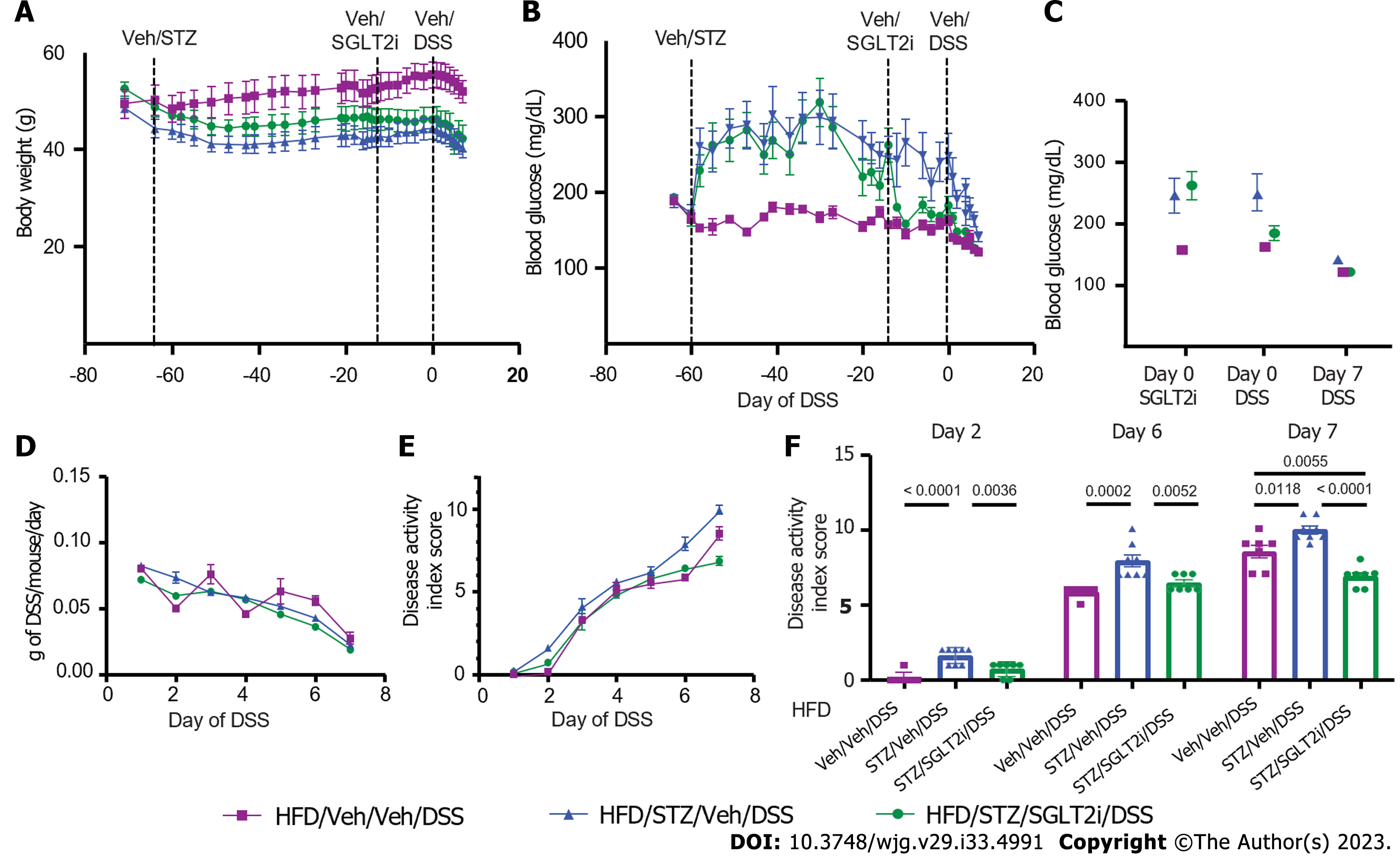
As a first step, we characterized the effects of STZ and DSS administration either alone or in combination on BW, blood glucose, food intake, and water intake in DIO mice. Mice were fed an HFD for 9 wk to induce DIO and then given either Veh or STZ to induce diabetes. After 6 wk, both STZ and Veh groups were subsequently exposed to either DSS or control drinking water (Veh), generating four study groups (Veh/Veh, STZ/Veh, Veh/DSS, and STZ/DSS). In terms of BW, all mice developed DIO after 9 wk of a HFD leading up to STZ administration, with no significant differences in BW between each group (mean BW pre-STZ: Veh/Veh 45.9 ± 3.9 g, Veh/STZ 45.4 ± 1.6 g, Veh/DSS 45.02 ± 5.0 g, STZ/DSS 44.4 ± 5.2 g; P = 0.924) (Figure 1A). Following STZ administration, BW decreased relative to Veh-treated controls but stabilized prior to DSS administration (Figure 1A). While BW was maintained in Veh-treated non-diabetic (Veh/Veh) and STZ-diabetic (STZ/Veh) mice, BW declined in both Veh/DSS and STZ/DSS groups throughout the 7-d DSS course but to a greater extent in STZ/DSS mice (Figure 1A and B).
As expected, STZ administration induced diabetes-range hyperglycemia across all groups relative to Veh-treated controls (30-d pre-DSS random blood glucose mean level: STZ 333 ± 31 mg/dL vs Veh 167 ± 10 mg/dL; P < 0.0001) (Figure 1C). Notably, there was a significant reduction in the blood glucose level during DSS course in the STZ/DSS group (Figure 1C and D), which was associated with a reduction in food and water intake during DSS administration (Figure 1E and F). This reduction in blood glucose during DSS course was less pronounced in non-diabetic DSS-treated mice (Veh/DSS, Figure 1C and D), as the decrease in food and water intake during the DSS course was similarly less significant in these mice (Figure 1E and F). Paired water administration between Veh/DSS-treated and STZ/DSS-treated groups ensured that hyperglycemic mice did not consume more DSS-treated water (Figure 1F).
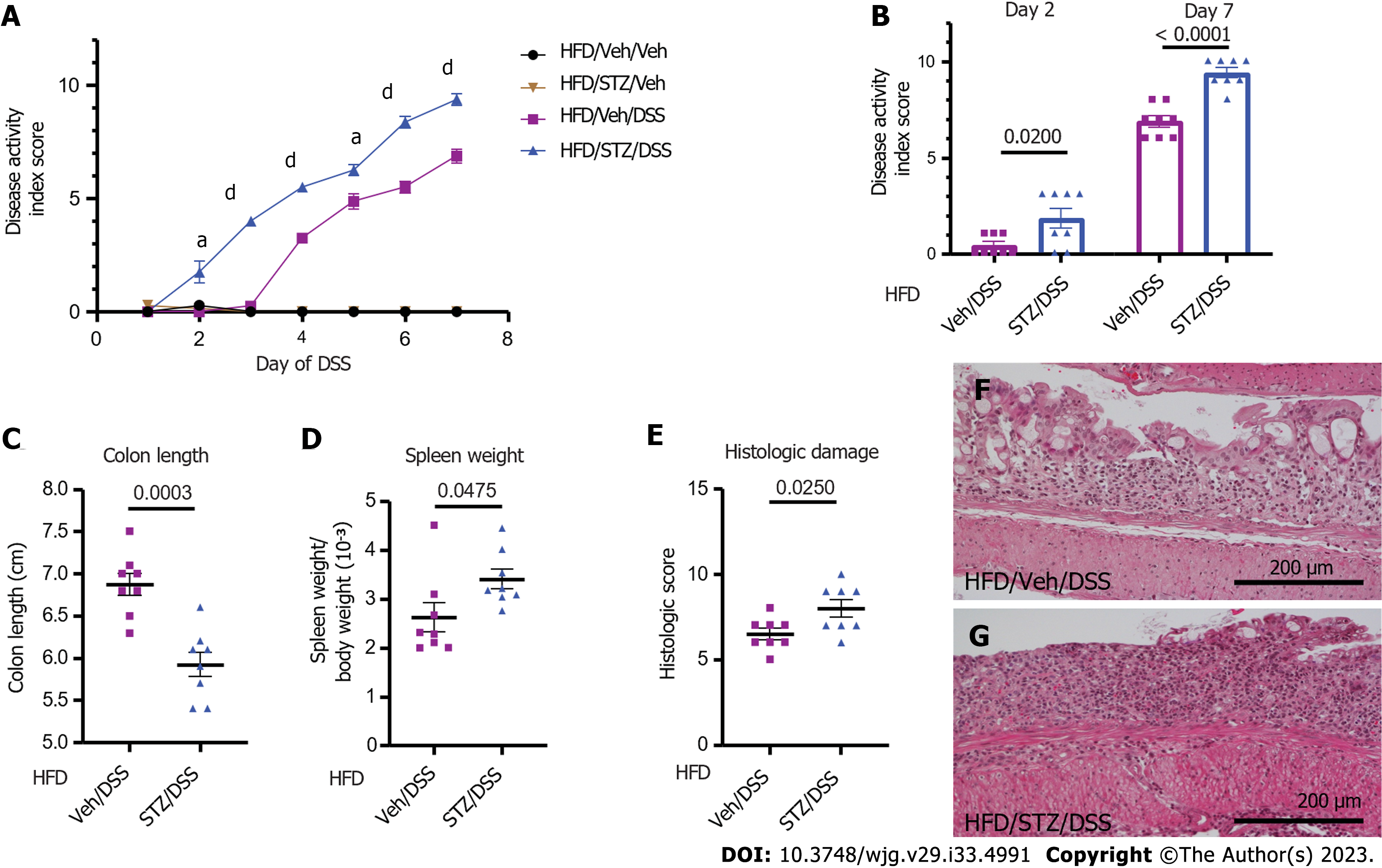
We next determined the effects of STZ-hyperglycemia on clinical and histopathological outcomes of DSS colitis in DIO mice. Diabetic STZ/DSS mice had a more rapid onset of DSS colitis, preceding the onset of clinical colitis symptoms in Veh/DSS mice by 2 d (day 2 vs day 4 following DSS) (Figure 2A and B), and their DAI scores were significantly higher throughout the entire course of DSS (from days 2-7; Figure 2A and B, P < 0.05 on days 2 and 5, P < 0.0001 on days 3, 4, 6, and 7), such that by the end of the DSS course (day 7), the mean DAI score was significantly higher in diabetic STZ/DSS mice compared to normoglycemic Veh/DSS controls (Figure 2B). As disease activity was undetectable in groups that did not receive DSS (Veh/Veh and STZ/Veh), we concluded that STZ-diabetes does not independently cause colitis symptoms (Figure 2A).
In murine models of IBD, colonic length serves as a pathologic marker of disease severity, as it shortens in response to mural inflammation in DSS colitis[20]. In STZ/DSS mice, the mean colon length at the time of sacrifice on day 7 was significantly shorter compared to Veh/DSS mice (Figure 2C). Spleen weight serves as another pathologic marker of disease severity in DSS colitis, as its weight increases in response to systemic inflammation[20]. Our finding that the spleen-to-BW ratio was significantly higher in STZ/DSS mice than Veh/DSS mice (Figure 2D) indicated greater systemic inflammation in the former group. Consistent with this interpretation, histologic damage scores assessing the degree of colonic inflammation and tissue injury were also significantly higher in STZ/DSS mice than in Veh/DSS mice (Figure 2E-G). Collectively, these findings indicated that in DIO mice, STZ-diabetes both hastens the onset of and worsens the clinical and histopathological severity of DSS-induced colitis.
To investigate the mechanism by which STZ-hyperglycemia mitigates IBD outcomes in the setting of DIO, we examined the effect of STZ with and without DSS exposure on intestinal barrier function. The mucous layer of the colon and the tight junction proteins in the colonic epithelial layer are key components of the barrier that defend against pathogen entry into the bloodstream. The former is composed of mucins secreted by goblet cells in colonic crypts. By coating the colonic epithelial layer, it limits exposure of the epithelium to luminal contents[31,32]. When this mucous layer is thinned in disease states, its protective properties are diminished, placing the epithelium at greater risk of injury[31,32].
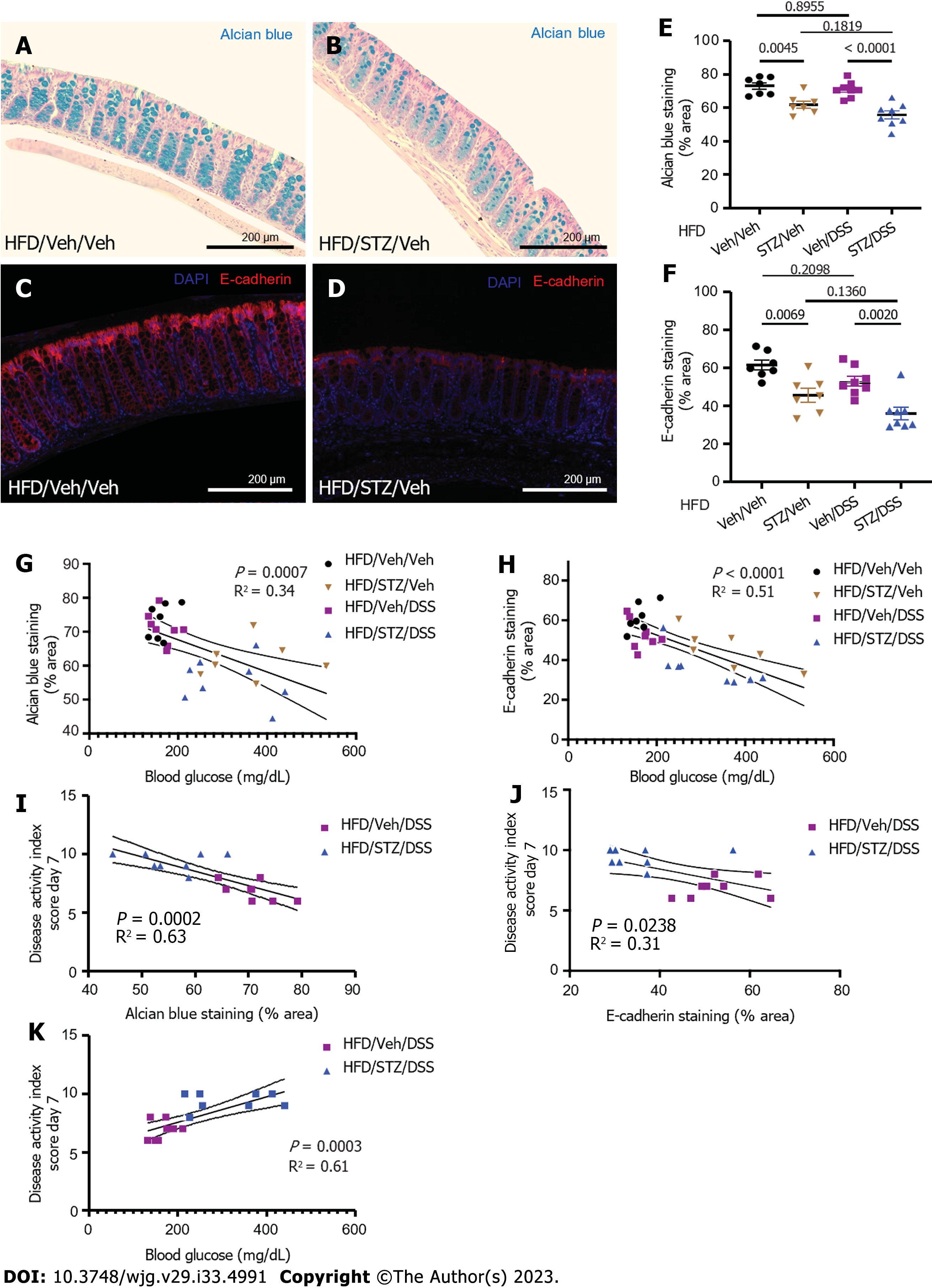
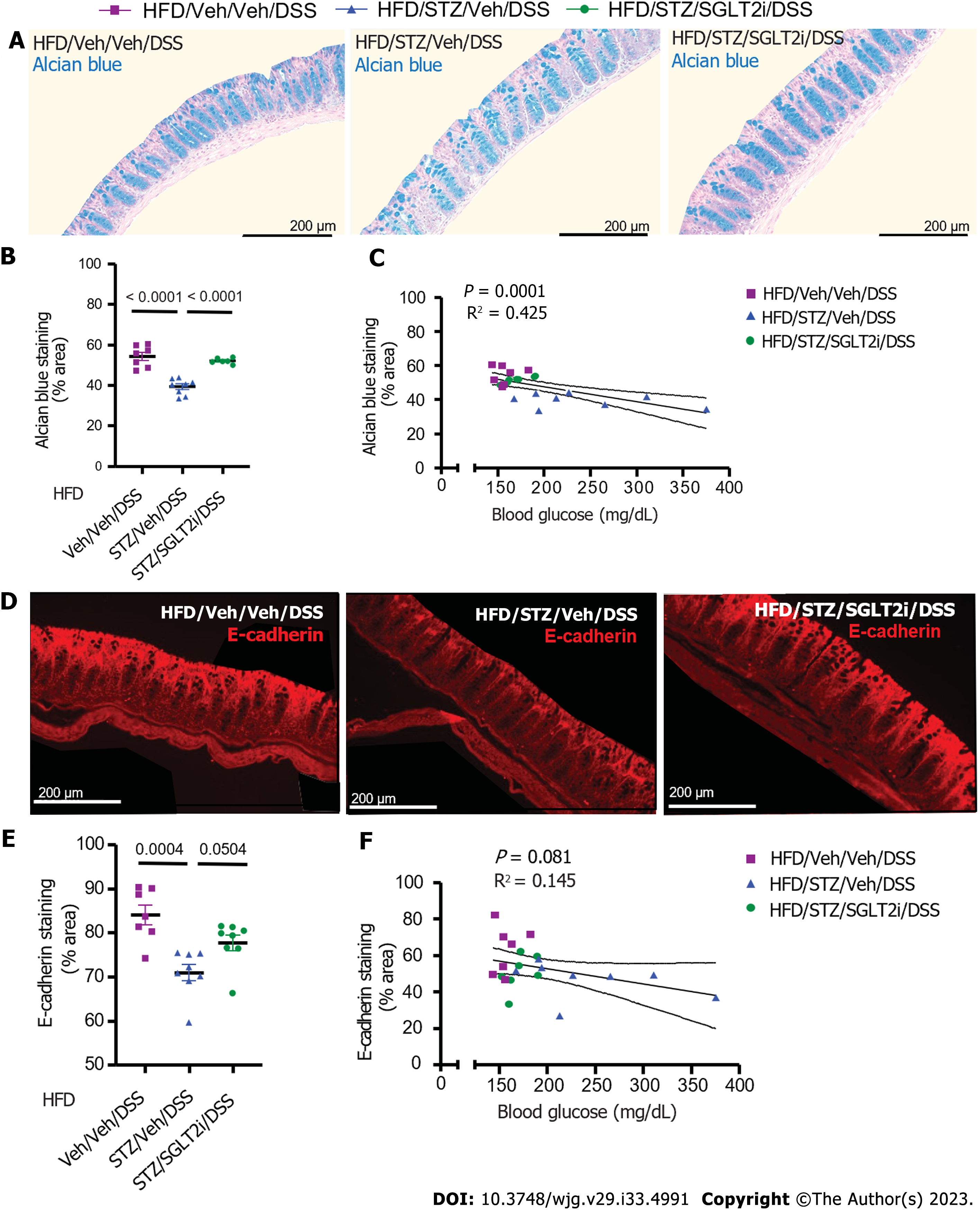
To assess the degree of mucous layer thinning, we stained postmortem colonic tissue for mucins and goblet cells with AB. Our findings showed that in DIO mice, hyperglycemia (STZ/Veh) was associated with significantly reduced AB staining compared to non-diabetic controls (Veh/Veh) (Figure 3A-C). We further showed that at the end of the DSS course in DSS-treated mice, STZ-diabetes was associated with significantly decreased colonic AB staining and sampled from areas of the colon with intact epithelium (Figure 3C). Therefore, diabetes was independently found to impair the colonic mucin barrier, with no significant effect of DSS alone when looking at areas of intact bowel without active colonic inflammation. Loss of the colonic mucin barrier, therefore, could play a causal role in the effect of diabetes to increase the rapidity of onset and severity of DSS colitis in the setting of DIO.
Tight junction proteins are an additional key component of the protective barrier of the colon, as they help to regulate the permeability of the epithelial layer[33]. Changes in the composition and concentration of tight junction proteins that occur in many disease states including IBD are associated with a more permeable epithelial barrier[33-35]. In the setting of DIO, we found that the abundance of the colonic epithelial tight junction protein E-cadherin, a key determinant of epithelial barrier integrity, was significantly decreased in hyperglycemic mice (STZ/Veh)[36] compared to non-diabetic controls (Veh/Veh) (Figure 3D-F). In the presence of DSS exposure, STZ-diabetes was also associated with a significant decrease of colonic E-cadherin staining (Figure 3F) when superimposed on DIO. Combined with the decrease of the protective mucous layer noted above, these data suggest that epithelial barrier integrity is impaired by diabetic hyperglycemia. Importantly, the degree of hyperglycemia correlated inversely with both AB staining and E-cadherin staining in all DIO mice regardless of exposure to STZ or DSS (Figure 3G and H). Furthermore, in DSS-treated groups, the amount of AB and E-cadherin staining correlated inversely with DAI scores on day 7 (Figure 3I and J). Finally, the degree of hyperglycemia correlated directly with IBD activity (Figure 3K). Taken together, these findings supported a mechanism whereby hyperglycemia increased the onset and severity of DSS colitis by decreasing the expression of key tight junction proteins and diminishing the colonic mucins that help maintain gut epithelial barrier integrity.
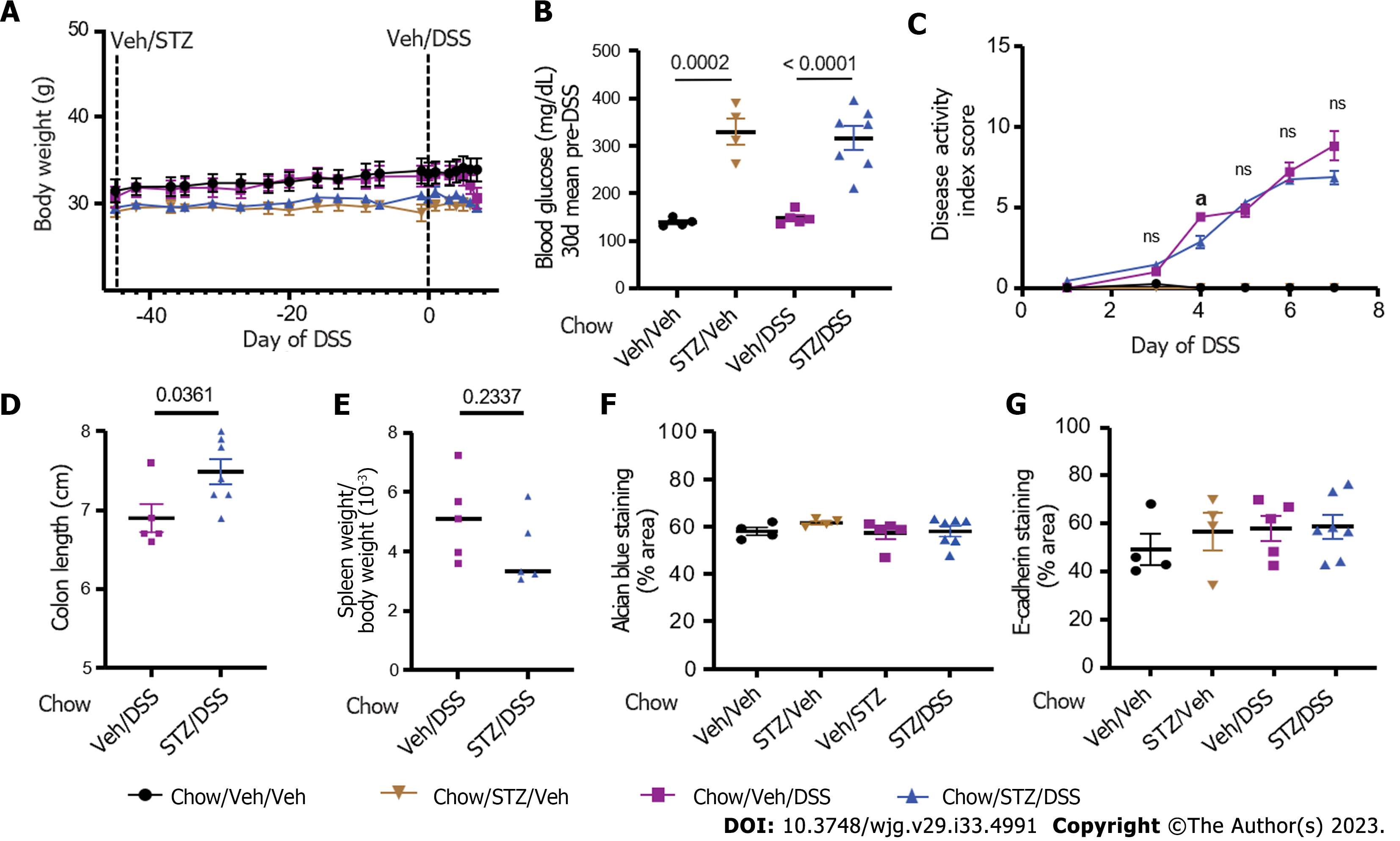
To investigate the contribution made by DIO to the deleterious effects of STZ-diabetes on IBD outcomes in mice, we repeated the above experiments in chow-fed mice. Mice were given low-dose STZ (or Veh) to induce T2D-range hyperglycemia 6 wk prior to DSS. In contrast to HFD-fed mice (mean BW 45.1 ± 4.1 g) in the above experiments, chow-fed mice maintained normal BWs leading up to STZ administration (Figure 4A; mean BW 30.1 ± 2.1 g). STZ administration induced hyperglycemia leading up to the DSS course, which was comparable to that observed in mice fed an HFD (Figure 4B). During the course of DSS, in contrast to STZ-diabetic DIO mice, STZ-diabetic chow-fed mice (Chow/STZ/DSS) did not exhibit a more rapid onset or greater degree of severity of colitis following DSS administration compared to chow-fed mice without STZ-diabetes (Chow/Veh/DSS) (Figure 4C). Chow/Veh/DSS mice had a slightly shorter colon length on day 7 of DSS compared to Chow/STZ/DSS mice (Figure 1D), but there were no significant differences in spleen weight between both DSS-treated groups (Figure 1E).
Notably, neither AB mucin staining (Chow/Veh/Veh vs Chow/STZ/Veh, P = 0.761; Chow/Veh/DSS vs Chow/STZ/DSS, P = 0.994) (Figure 4) nor E-cadherin tight junction protein staining (Chow/Veh/Veh vs Chow/STZ/Veh, P = 0.863; Chow/Veh/DSS vs Chow/STZ/DSS, P = 0.999) (Figure 4G) was impacted by STZ administration among chow-fed mice, irrespective of DSS administration. Thus, non-obese, chow-fed mice were protected from the deleterious effects of STZ-diabetes on both colitis severity and markers of gut epithelial permeability. These findings indicated that hyperglycemia exacerbates DSS-induced colitis only in the setting of DIO.
To determine the impact of hyperglycemia per se, independent of other elements of the diabetic state generated by STZ, on intestinal barrier function and colitis outcomes, we repeated the study with DIO mice treated with STZ and DSS, with the additional step of administering the SGLT2i dapagliflozin at a dose that normalizes glycemia to a subgroup of these mice prior to DSS administration[28]. As before, mice were given an HFD to induce DIO (mean BW 49.7 ± 6.2 g) (Figure 5A) and were then given either Veh or STZ to induce diabetic-range hyperglycemia (mean post-STZ blood glucose levels: Veh 173.6 ± 15.8 vs STZ 282.5 ± 68.6; P = 0.0007) (Figure 5B). After 6 wk of sustained hyperglycemia post-STZ treatment, the SGLT2i dapagliflozin (or Veh) was added to the drinking water, which resulted in euglycemic blood glucose levels equivalent to non-STZ treated control levels (mean 10 d pre-DSS blood glucose levels: Veh/Veh/DSS 156.8 ± 11.7 mg/dL vs STZ/SGLT2i/DSS 172.8 ± 17.5 mg/dL; P = 0.793) (Figure 5B and C) and significantly lower than STZ-treated mice who did not receive dapagliflozin (STZ/Veh/DSS 242.1 ± 72.8mg/dL vs STZ/SGLT2i/DSS 172.8 ± 17.5 mg/dL; P = 0.022).
After 2 wk of SGLT2i (or Veh) exposure, DSS was added to drinking water for the last week to induce colitis in each group. Similar to our earlier observations in DIO mice (Figure 1), DSS administration caused a significant reduction in BW (Figure 5A) and blood glucose levels in all groups (Figure 5B and C). Paired DSS water administration was performed, and total DSS consumption did not differ significantly between groups (Figure 5D). As previously noted (Figure 2A), the onset of colitis symptoms was accelerated in hyperglycemic STZ/Veh/DSS mice with DIO compared to normoglycemic Veh/Veh/DSS mice, becoming evident by day 2 compared to day 3 (Figure 5E and F). This more rapid onset of symptoms was mitigated by SGLT2i treatment, as STZ/SGLT2i/DSS mice manifested clinical colitis on day 3 instead of day 2 (Figure 5E and F). While symptoms of clinical colitis severity were comparable between groups from days 3-5 (Figure 5E), STZ/Veh/DSS mice demonstrated significantly worse DAI scores on both days 6 and 7 of DSS administration than either non-STZ treated mice (Veh/Veh/DSS) or STZ-treated mice receiving SGLT2i (STZ/SGLT2i/DSS) (Figure 5F).
Similar outcomes were observed when histochemical parameters of epithelial barrier were assessed in areas of intact colon in DSS-treated mice. The effect of untreated hyperglycemia to decrease AB mucins in DIO mice (compared to non-diabetic controls) in intact areas of colonic epithelium was fully reversed by dapagliflozin treatment (Figure 6A and B). Moreover, the amount of colonic AB staining correlated inversely with the degree of hyperglycemia across the three groups (based on 1-wk average blood glucose levels prior to DSS) (Figure 6C). E-cadherin abundance similarly was decreased by STZ treatment and was partially reversed by SGLT2i administration, although this did not achieve statistical significance (Figure 6D and E, P = 0.050). The abundance of the colonic tight junction protein E-cadherin reached near significance with an inverse correlation to blood glucose levels as well (Figure 6F). These findings strengthened the conclusion that in DIO mice, hyperglycemia is the primary driver of both STZ-induced colonic epithelial barrier disruption and colitis severity.
In the current work, we determined whether diabetic hyperglycemia exacerbates colitis severity in a murine model of IBD. Our findings demonstrated that diabetic hyperglycemia both accelerates the onset and worsens the clinical and pathological outcomes of DSS colitis. Interestingly, each of these effects of diabetes on IBD outcomes was detected in mice with DIO but not in chow-fed, non-obese mice. Furthermore, we show that the severity of IBD increases directly in relation to the degree of hyperglycemia and that reversal of hyperglycemia with an antidiabetic medication eliminated this effect of diabetes. Taken together, these findings support a model whereby the combination of obesity and hyperglycemia predisposes to more severe outcomes of intestinal inflammation in IBD.
As a first step towards understanding the mechanisms by which diabetes and DIO influence IBD outcomes, we sought to investigate the effect of diabetes on the intestinal barrier, focusing in particular on the colonic mucin layer and tight junction proteins. Previous work had suggested that diabetic hyperglycemia impairs intestinal barrier function and increases intestinal permeability, although how this might impact IBD pathology had not been studied[25]. Importantly, it has been reported that symptomatic patients with both UC and CD have increased in vivo intestinal permeability[24] and decreased intestinal mucins and goblet cell depletion compared to healthy controls[37]. Additionally, in IBD colon organoid cultures, tight junction proteins are significantly reduced[38]. In our current work, we reported that STZ-induced diabetic hyperglycemia significantly impairs these two components of intestinal barrier integrity in the setting of DIO. Furthermore, the amount of mucins and tight junction protein staining correlated inversely with colitis disease activity. This supported a model in which the diabetic state acts injuriously on the intestinal barrier, making the intestines more susceptible to DSS-induced colitis. It is notable that diabetes on its own is insufficient to cause IBD pathology, but when exposed to a chemical colitic agent, the presence of diabetes and associated impaired intestinal barrier function significantly worsens IBD outcomes.
Our next goal was to determine the contribution of DIO on the effect of diabetes to exacerbate DSS-induced intestinal inflammation. Obesity is present in the vast majority of T2D patients[12,13], and patients with IBD have similar rates of obesity compared to the general population[39]. Comorbid obesity has been associated with higher hospitalization rates, more active disease, and a higher prevalence of perianal disease in patients with CD[40]. In patients with UC, elevated body mass index is associated with an increased risk of biologic therapy treatment failure[41]. Furthermore, HFD exposure exacerbates IBD outcomes in preclinical rodent models[20,21]. Conversely, severe hyperglycemia seen in uncontrolled, insulin-deficient T1D has a deleterious effect on intestinal barrier function independent of obesity[25]. To determine the contribution of DIO on the ability of a more modest, physiologic degree of hyperglycemia mimicking T2D to impact intestinal barrier pathology and IBD outcomes, we tested whether low-dose STZ would worsen DSS colitis in DIO mice fed an HFD compared to non-obese, chow-fed mice. We reported here that the effect of modest hyperglycemia to impair intestinal barrier function and therefore influence IBD outcomes is dependent on coexisting DIO. One potential explanation for these findings involves an effect of the HFD exposure on the gut microbiome, shifting its composition to a more mucin-degrading and proinflammatory profile[42]. When superimposed on this change of gut flora and the state of chronic inflammation in obesity, we observed clear-cut, deleterious effects of diabetes on both intestinal permeability and colitis disease activity. Further studies are warranted to determine the exact contribution that the HFD consumption makes to worsen IBD outcomes independent of obesity.
Next, we determined the specific contribution made by hyperglycemia
Reversal of hyperglycemia after STZ treatment with an SGLT2i resulted in significant improvement in the colonic mucin barrier, with no difference between non-STZ treated normoglycemic mice and mice treated with STZ who then received SGLT2i. The abundance of tight junction proteins also tended to improve with normalization of the blood glucose level, although it did not reach statistical significance. From these findings, we inferred that the effect of diabetes to influence intestinal barrier pathology and IBD outcomes appears to be dependent, or at least heavily reliant, on hyperglycemia. This conclusion was further supported by our findings that the degree of intestinal barrier dysfunction and colitis disease severity varied directly with the degree of hyperglycemia. These findings heightened the importance of studies to evaluate the impact of effective diabetes treatment in patients with IBD, especially given recent evidence that patients with IBD are at increased risk of developing T2D[8,9], that comorbid T2D predicts poorer IBD outcomes[11], and that high-fat, obesogenic diets are associated with a higher incidence of IBD[17-19].
The growing patient population affected by T2D, obesity, and IBD creates a compelling rationale for continued efforts to understand shared mechanisms between these disease processes, particularly in light of evidence that comorbid T2D or obesity negatively affects IBD outcomes in patients[10,11,40,41]. It is also imperative to understand how treatments for each of these conditions affect the others, particularly as corticosteroids are a mainstay of treatment to induce remission in both UC and CD and are known to both exacerbate hyperglycemia in patients with pre-existing T2D and to precipitate hyperglycemia in patients with no prior diabetes diagnosis[43]. These considerations underscore the need for clinicians to consider how these disease processes and their respective treatments affect their patients and highlight the need to investigate the potential role of antidiabetic medications in IBD management prior to hyperglycemia onset or exacerbation among those at risk.
In mice with DIO, diabetic hyperglycemia disrupts the intestinal barrier integrity and is associated with more severe clinical and pathological outcomes of colitis, highlighting the potential translational importance of ensuring optimal diabetes management in IBD patients.
Emerging epidemiologic evidence links type 2 diabetes (T2D) and obesity to inflammatory bowel disease (IBD). However, evidence to determine the exact mechanisms by which obesity and/or diabetes influence IBD outcomes is limited. This study uses mouse models of colitis to investigate how diabetes and obesity interact to impair intestinal barrier function and exacerbate IBD outcomes, highlighting the deleterious impact of sustained hyperglycemia on intestinal barrier integrity.
Patients with IBD are at an increased risk of developing T2D, which serves as a predictor of poor outcomes in IBD. The rates of comorbid obesity in IBD are increasing as well, and obesity is related to a more severe IBD phenotype. As more patients with IBD are affected by obesity and/or T2D, it is imperative to understand how these disease processes interact and how treatments for each condition may impact the other.
In this study, we used murine models of colitis to determine the effect of T2D-range hyperglycemia on IBD outcomes and intestinal barrier function with and without coexisting diet-induced obesity (DIO).
Mice were fed standard chow or a high-fat diet to induce DIO and then given streptozotocin (STZ) to induce sustained T2D-range hyperglycemia. Mice were then given dextran sodium sulfate (DSS) to induce colitis. Body weight and blood glucose levels were compared as well as clinical colitis scores and histopathologic assessment of intestinal injury. The effects of hyperglycemia and DIO on intestinal barrier function were interrogated by comparing colonic mucins and tight junction protein abundance. To highlight the role of hyperglycemia itself, a sodium-glucose cotransporter-2 inhibitor was subsequently used to selectively reverse hyperglycemia prior to DSS course.
In the setting of DIO, STZ-diabetes significantly worsened clinical and histopathological outcomes of DSS colitis in mice. This effect was associated with a significant reduction in the colonic mucin barrier and tight junction protein abundance and was ameliorated by the use of a sodium-glucose cotransporter-2 inhibitor to reverse hyperglycemia prior to colitis onset. Together, these findings highlighted the deleterious effect of diabetic hyperglycemia on the intestinal barrier as a mechanism by which diabetes and obesity interact to affect IBD outcomes.
This study reported the novel finding that diabetic hyperglycemia disrupted intestinal barrier integrity in the setting of DIO and exacerbated DSS colitis outcomes in mice. Given the increased prevalence of T2D in patients with IBD and the negative impact of comorbid obesity on IBD outcomes, it is imperative to understand how these disease processes interact.
These findings have significant translational relevance, and future research can expand on them by determining whether strict glycemic control in patients with T2D and IBD is associated with improved IBD outcomes.
The authors are grateful for the technical assistance provided by Vincent Damian, Bao Anh Phan, Tammy Doan, and Renad Sehat. The authors also thank the University of Washington Department of Comparative Medicine Histology and Imaging Core and the University of Washington Diabetes Research Center Cellular and Molecular Imaging Core for assistance with tissue processing and staining.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American Gastroenterological Association, No. 1327177; Crohn’s and Colitis Foundation, No. 8-13167744; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition, No. 17997.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Duan SL, China; Losurdo G, Italy S-Editor: Fan JR L-Editor: Filipodia P-Editor: Cai YX
| 1. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1451] [Article Influence: 290.2] [Reference Citation Analysis (0)] |
| 2. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4377] [Article Influence: 291.8] [Reference Citation Analysis (4)] |
| 3. | Guan Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J Immunol Res. 2019;2019:7247238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 628] [Article Influence: 104.7] [Reference Citation Analysis (1)] |
| 4. | Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 904] [Article Influence: 113.0] [Reference Citation Analysis (3)] |
| 5. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3351] [Article Influence: 186.2] [Reference Citation Analysis (11)] |
| 6. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3640] [Article Influence: 202.2] [Reference Citation Analysis (0)] |
| 7. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3395] [Article Influence: 485.0] [Reference Citation Analysis (0)] |
| 8. | Jess T, Jensen BW, Andersson M, Villumsen M, Allin KH. Inflammatory Bowel Diseases Increase Risk of Type 2 Diabetes in a Nationwide Cohort Study. Clin Gastroenterol Hepatol. 2020;18:881-888.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Kang EA, Han K, Chun J, Soh H, Park S, Im JP, Kim JS. Increased Risk of Diabetes in Inflammatory Bowel Disease Patients: A Nationwide Population-based Study in Korea. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Fuschillo G, Celentano V, Rottoli M, Sciaudone G, Gravina AG, Pellegrino R, Marfella R, Romano M, Selvaggi F, Pellino G. Influence of diabetes mellitus on inflammatory bowel disease course and treatment outcomes. A systematic review with meta-analysis. Dig Liver Dis. 2023;55:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (1)] |
| 11. | Kumar A, Teslova T, Taub E, Miller JD, Lukin DJ. Comorbid Diabetes in Inflammatory Bowel Disease Predicts Adverse Disease-Related Outcomes and Infectious Complications. Dig Dis Sci. 2021;66:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Iglay K, Hannachi H, Joseph Howie P, Xu J, Li X, Engel SS, Moore LM, Rajpathak S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 316] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 13. | Leitner DR, Frühbeck G, Yumuk V, Schindler K, Micic D, Woodward E, Toplak H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies - EASO Can Lead the Way. Obes Facts. 2017;10:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 14. | Singh S, Dulai PS, Zarrinpar A, Ramamoorthy S, Sandborn WJ. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 302] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 15. | Swanson SM, Harper J, Zisman TL. Obesity and inflammatory bowel disease: diagnostic and therapeutic implications. Curr Opin Gastroenterol. 2018;34:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Weissman S, Patel K, Kolli S, Lipcsey M, Qureshi N, Elias S, Walfish A, Swaminath A, Feuerstein JD. Obesity in Inflammatory Bowel Disease Is Associated with Early Readmissions Characterised by an Increased Systems and Patient-level Burden. J Crohns Colitis. 2021;15:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017;152:398-414.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 18. | Owczarek D, Rodacki T, Domagała-Rodacka R, Cibor D, Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol. 2016;22:895-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 207] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (2)] |
| 19. | Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 218] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Cheng L, Jin H, Qiang Y, Wu S, Yan C, Han M, Xiao T, Yan N, An H, Zhou X, Shao Q, Xia S. High fat diet exacerbates dextran sulfate sodium induced colitis through disturbing mucosal dendritic cell homeostasis. Int Immunopharmacol. 2016;40:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J Nutr. 2013;143:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 359] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 23. | Michielan A, D'Incà R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015;2015:628157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 489] [Article Influence: 48.9] [Reference Citation Analysis (1)] |
| 24. | Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired Intestinal Permeability Contributes to Ongoing Bowel Symptoms in Patients With Inflammatory Bowel Disease and Mucosal Healing. Gastroenterology. 2017;153:723-731.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 25. | Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 26. | Scarlett JM, Rojas JM, Matsen ME, Kaiyala KJ, Stefanovski D, Bergman RN, Nguyen HT, Dorfman MD, Lantier L, Wasserman DH, Mirzadeh Z, Unterman TG, Morton GJ, Schwartz MW. Central injection of fibroblast growth factor 1 induces sustained remission of diabetic hyperglycemia in rodents. Nat Med. 2016;22:800-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 27. | Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23:6016-6029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 621] [Cited by in RCA: 580] [Article Influence: 72.5] [Reference Citation Analysis (15)] |
| 28. | Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 389] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 29. | Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 1040] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 30. | Crowe AR, Yue W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 515] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 31. | Hansson GC. Mucus and mucins in diseases of the intestinal and respiratory tracts. J Intern Med. 2019;285:479-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 32. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 975] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 33. | Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Ciclitira PJ, Al-Hassi HO. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22:3117-3126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (4)] |
| 34. | Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 360] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 36. | Daulagala AC, Bridges MC, Kourtidis A. E-cadherin Beyond Structure: A Signaling Hub in Colon Homeostasis and Disease. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 37. | Kang Y, Park H, Choe BH, Kang B. The Role and Function of Mucins and Its Relationship to Inflammatory Bowel Disease. Front Med (Lausanne). 2022;9:848344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 38. | d'Aldebert E, Quaranta M, Sébert M, Bonnet D, Kirzin S, Portier G, Duffas JP, Chabot S, Lluel P, Allart S, Ferrand A, Alric L, Racaud-Sultan C, Mas E, Deraison C, Vergnolle N. Characterization of Human Colon Organoids From Inflammatory Bowel Disease Patients. Front Cell Dev Biol. 2020;8:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 39. | Losurdo G, La Fortezza RF, Iannone A, Contaldo A, Barone M, Ierardi E, Di Leo A, Principi M. Prevalence and associated factors of obesity in inflammatory bowel disease: A case-control study. World J Gastroenterol. 2020;26:7528-7537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 41. | Kurnool S, Nguyen NH, Proudfoot J, Dulai PS, Boland BS, Vande Casteele N, Evans E, Grunvald EL, Zarrinpar A, Sandborn WJ, Singh S. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 42. | Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, Darfeuille-Michaud A, Barnich N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 397] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 43. | Elena C, Chiara M, Angelica B, Chiara MA, Laura N, Chiara C, Claudio C, Antonella F, Nicola G. Hyperglycemia and Diabetes Induced by Glucocorticoids in Nondiabetic and Diabetic Patients: Revision of Literature and Personal Considerations. Curr Pharm Biotechnol. 2018;19:1210-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |