Published online Sep 7, 2023. doi: 10.3748/wjg.v29.i33.4920
Peer-review started: April 10, 2023
First decision: June 17, 2023
Revised: July 21, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: September 7, 2023
Processing time: 143 Days and 13.9 Hours
Delayed passage of meconium or constipation during the perinatal period is traditionally regarded as a signal to initiate further work up to evaluate for serious diagnoses such as Hirschsprung’s disease (HD), meconium ileus due to Cystic Fibrosis, etc. The diagnosis of HD particularly warrants invasive testing to confirm the diagnosis, such as anorectal manometry or rectal suction biopsy. What if there was another etiology of perinatal constipation, that is far lesser known? Cow’s milk protein allergy (CMPA) is often diagnosed in infants within the first few weeks of life, however, there are studies that show that the CMPA allergen can be passed from mother to an infant in-utero, therefore allowing symptoms to show as early as day one of life. The presentation is more atypical, with perinatal constipation rather than with bloody stools, diarrhea, and vomiting. The diagnosis and management would be avoidance of cow's milk protein within the diet, with results and symptom improvement in patients immediately. Therefore, we discuss whether an alternative pathway to address perinatal constipation should be further discussed and implemented to potentially avoid invasive techniques in patients. This entails first ruling out CMPA with safe, noninvasive techniques with diet modification, and if unsu
Core Tip: Cow’s milk protein allergy (CMPA) is a far lesser known cause of perinatal constipation compared to more frequently considered diagnoses such as Hirschsprung’s, Cystic fibrosis related meconium ileus, etc. The presentation during the perinatal period is considered atypical caused by a non-immunoglobulin E (IgE) mechanism as opposed to the typical presentation caused by an IgE-mediated mechanism. The likelihood of CMPA is significant in the perinatal period, therefore should be considered more often. Here we discuss an alternative pathway for the workup of perinatal constipation focusing on CMPA as an etiology. The use of this pathway can avoid invasive tests among patients.
- Citation: Arakoni R, Kamal H, Cheng SX. Very early onset perinatal constipation: Can it be cow’s milk protein allergy? World J Gastroenterol 2023; 29(33): 4920-4926
- URL: https://www.wjgnet.com/1007-9327/full/v29/i33/4920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i33.4920
Delayed passage of meconium or constipation is defined as failure to pass stool within the first 48 h after birth[1]. With a positive history of delayed meconium passage, providers traditionally regard this as a signal towards serious diagnoses such as Hirschsprung’s disease (HD), meconium ileus (MI) due to cystic fibrosis (CF), or intestinal obstruction, and initiate work up such as barium enema, anorectal manometry, and or rectal suction biopsy per current recommendations[2]. We rarely consider cow's milk protein allergy (CMPA), even though it has been described to occur in the perinatal period[3,4] and causes disease that mimics HD[5-8]. Since there’s still no available diagnostic laboratory tests, the diagnosis of CMPA has to be clinical. We recently reported 3 cases of infants who had delayed passage of meconium with subsequent early-onset perinatal constipation that did not respond to conventional therapies and required rectal stimulation to defecate. The symptoms resolved when the milk protein component was withheld and recurred when milk proteins were reintroduced for the patients. The symptoms subsequently resolved again when switched to an extensively hydrolyzed or amino acid-based formula[9]. Thus, it had not only avoided more aggressive and invasive workup, but had also significantly improved the care of this very vulnerable young patient population. Unfortunately, per our recent survey, this early-onset atypical perinatal form of CMPA was rarely recognized by a large majority of care providers including gastroenterologists, contrasting the typical form of CMPA that occurs later in life. This is not surprising; it indicates that more presentations of this condition to gastroenterology communities are necessary. In fact, even for its typical, more known counterpart of CMPA, it has taken approximately 20 years of time before it became widely recognized by providers. CMPA was rarely diagnosed before 1950, but since 1970 the condition has been further documented[10]. This paper will discuss 4 burning questions regarding the atypical CMPA, typical CMPA, and HD.
A number of conditions can cause delayed passage of meconium or early-onset perinatal constipation. This includes HD, intestinal obstruction, MI, meconium plug syndrome, functional ileus, small left colon, drug effect, hypothyroidism, and megacystis-microcolon-intestinal hypoperistalsis syndrome[2]. However, these conditions might be much less common than we think when thinking about perinatal constipation. For example, a study conducted in 2008 looked at causes of meconium plug syndrome (radiological form of perinatal constipation) in the largest cohort of a patient population to that date, which actually indicated that only about 13% were due to HD, and none were caused by CF[11]. Table 1 below summarizes the incidences in pubmed of HD, meconium plug syndrome, MI, anorectal malformation, and CMPA in infants, whereas Table 2 lists the frequencies of HD relative to CMPA. As shown, the incidences of these congenital diagnoses are extremely low, less than 0.1%[2] for all four diagnoses individually, in contrast to CMPA, which is estimated to occur in 0.5%-17%[10,12]. Thus, it is possible that the majority of the cases of perinatal constipation are not caused by HD or other congenital etiologies, but by acquired CMPA. In accordance with this, we recently observed 25 neonates/young infants referred to our clinic for intractable perinatal constipation and found 23 responded to cow’s milk protein (CMP) avoidance, suggesting that this very early onset constipation is largely related to CMPA, specifically the atypical CMPA.
CMPA is an abnormal immunological response to CMP. According to time of onset, CMPA can be categorized into two forms: Atypical form that occurs early in life before, during, or shortly after birth, and typical form that commonly happens later in life, typically weeks or months after birth. Table 3 compares their differences in clinical presentation, way of allergen transmission, type of allergy reaction, and intestinal tissue and brief mechanism involved as well as their diagnosis, management, and relative awareness. The development of CMPA requires exposure followed by an immunological response to the milk allergen, which can take up to months to occur. Therefore, CMPA has been thought to most commonly occur within weeks or months after their first postnatal feed. However, cow’s milk allergens are not only transported postnatally via the oral route; they can also pass through the placenta and amniotic fluid to sensitize fetuses and cause allergy[3-4]. This indicates that the process of developing CMPA can occur prenatally. To support this, there are studies that show α-lactalbumin, -lactoglobulin, and α-casein were found in full-term neonates, which are indications of responses of cord blood lymphocytes to cow’s milk allergen[3,13]. Figure 1 demonstrates the passage of CMP from maternal ingestion through the placenta, affecting the infant in-utero, eventually leading to perinatal constipation. The typical, late-onset form of CMPA is now well known to care providers, however the early-onset form is often overlooked, particularly when this type of CMPA presents with atypical symptoms. Most pediatricians including pediatric gastroenterologists are not aware of the early-onset CMPA. As a result, many atypical cases of neonate infants CMPA were missed, leaving them undiagnosed or misdiagnosed. This resulted in delay of initiating the appropriate treatment for this very treatable condition or led to many unnecessary workup procedures.
| Atypical CMPA | Typical CMPA | |
| Time of onset | Perinatal period[3] | Early infancy[21,22] |
| Way of transmission of allergen | Vertically via placenta and amniotic fluid[3] | Vertically via breast feeding |
| Horizontally by oral ingestion[21,22] | ||
| Typical presentation | Intractable constipation[5,6] | Vomiting and diarrhea, bloody stools[21] |
| Type of allergy reaction | Non IgE mediated[5] | IgE mediated[5] |
| Tissues involved | Enteric neurons and smooth muscle[5-8] | Intestinal mucosa[5] |
| Mechanism involved | Immune mediated neuromuscular dysfunction leading to persistent spasm or failure to relax of the anorectum (HD like changes)[5-8] | Allergic enterocolitis[5] |
| Diagnosis | CMP avoidance and challenge. Both blood and skin allergy testings are of no diagnostic value[9] | CMP avoidance and challenge with or without blood and skin allergy testing[21] |
| Treatment | CMP avoidance[9] | CMP avoidance[9] |
| Awareness to providers | Rarely aware/reported | Well known/reported |
The exact mechanism by which CMPA causes perinatal constipation has remained incompletely understood. CMP can cause constipation in at least two ways: Nonspecifically through CMP constipating effect and specifically through CMPA. CMPA, rather than CMP constipation, is considered a more probable etiology for perinatal constipation, because for the CMP constipation to occur, patients will require a prolonged consumption of the CMP and this is unlikely to occur in this neonatal patient population, during the perinatal period. Then, how does CMPA lead to perinatal constipation? CMPA occurs by two primary mechanisms: an immunoglobulin E (IgE)-mediated immediate hypersensitivity reaction and a non-IgE-mediated delayed hypersensitivity reaction[9]. As shown in Table 3, the former is primarily seen in the typical CMPA, while the latter is speculated to cause the atypical CMPA. The IgE-mediated reaction is described as IgE antibodies that are secreted in response to the allergen and bind to the surface of mast cells and basophils, causing subsequent release of histamine and inflammatory mediators, leading to eosinophilia, allergic colitis and proctitis, and bloody/mucousy stools[5]. The non-IgE mediated reaction is not as well-known as compared to the IgE-mediated reaction, but hypotheses include milk antigens binding to immune complexes of immunoglobulin A or immunoglobulin G or directly binding and stimulating T cells, resulting in activation of an inflammatory cascade[14] that involves neuromodulation of the enteric nervous system[15], leading to alteration of the function of smooth muscle and intestinal motility that is functionally similar to that of HD[5], with increased anal pressure at rest[16] causing difficulties in stooling. As illustrated in one of our cases[9], early-onset constipation had normal appearing colorectal histology, with no evidence of lamina propria/muscularis mucosa eosinophilia nor increased mast cell infiltration, therefore the etiology of the perinatal constipation is likely not the IgE-mediated but more consistent with the non-IgE mediated mechanism. Also, all of our observed cases mentioned in the previous case series[9] and subsequent new cases did not respond to stool softeners but required rectal stimulation/rectal insertion for bowel movement, suggesting that the mechanism of constipation is likely not simply related to the hardness of the stool but the dysmotility of the muscle of the distal colon, either due to distal colon spasm or failure to relax (Figure 1).
How to effectively diagnose the infants with acquired condition from the infants with congenital disease as in HD and other anatomical obstructions without extensive testing remains clinically challenging.
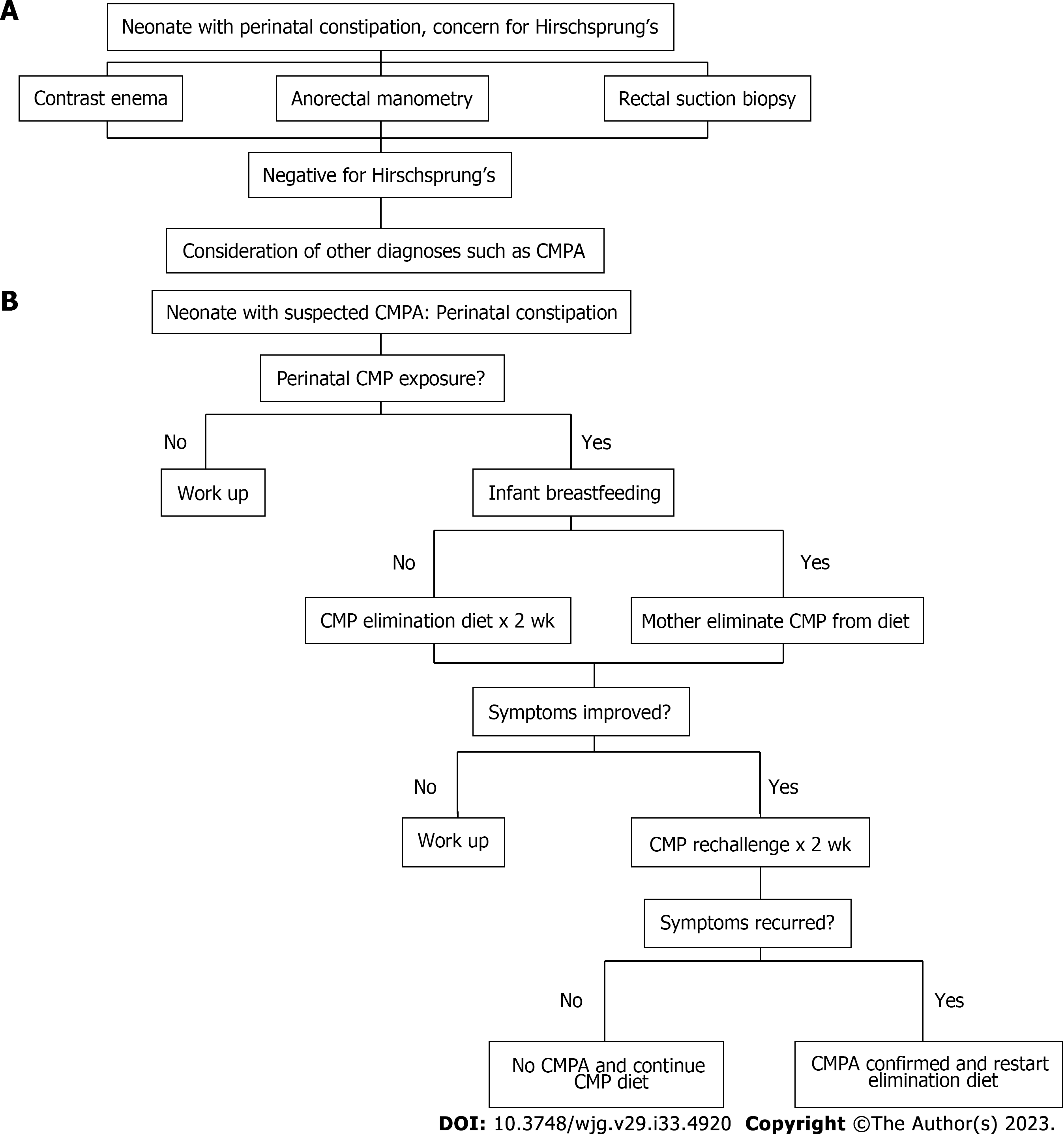
Current guidelines recommend a moderate level of suspicion for HD, although practice varies regarding the evaluation of these infants. At a minimum, they should be closely observed and evaluated promptly for HD if they develop symptoms of constipation even though a great majority of those patients are not due to HD[2]. We designate this management algorithm the “top-down” approach (Figure 2A).
Our practice has adopted a new management guideline in managing these patients[9] given that CMPA rather than HD causes most of these presentations. The new guideline has recommended performing a 2-week trial of CMP avoidance as the initial diagnostic procedure before performing a contrast enema, anorectal manometry, and or suction biopsy. We designate this new management algorithm the “bottom-up” approach (Figure 2B).
The top-down approach relies on highly sophisticated investigations (i.e., contrast enema, anorectal manometry or suction biopsy) to first rule out HD, which are rare and expensive; whereas the bottom-up protocol uses relatively simple food elimination diet to first rule in CMPA before rare entities are considered, which is common and inexpensive. With this new approach, we have promptly identified and successfully treated CMPA in 23 of 25 infants with intractable early-onset constipation without the need to go to any of the invasive testings (Cheng et al, unpublished observation). Thus, if this is validated by other clinical centers or practices, the new recommendation would largely help avert many unnecessary investigations, which are not only costly but are also invasive and expose patients to various potential complications.
Further understanding of the mechanism of CMPA makes it clear that infants can present with the manifestations immediately after birth. Therefore, shouldn’t we consider CMPA in our patients who present with perinatal constipation early on? There is no doubt that the serious diagnoses such as HD and CF related MI cannot be missed, however, if there is a way to eliminate invasive tests, we should, as providers, incorporate it into our medical practice. There are many unanswered questions that have yet to be further studied. For example, in our studies, all the mothers of patients with perinatal constipation consumed dairy products during pregnancy, however, this does not mean all mothers who consume dairy products during pregnancy will necessarily give birth to infants who will develop CMPA or symptoms of perinatal constipation. What factor(s) determines who develops and who does not develop sensitization and allergy remains unclear. Also unclear is the factor(s) that determines the type of CMPA, typical vs atypical. Similarly, although we know CMP allergens can pass the placenta and amniotic fluid to sensitize fetuses and cause allergy, we still do not know how long this sensitization process lasts and how long the allergens remain effective. The fact that our patients’ symptoms resolved following the 2-week elimination period of the allergen, however, seems to suggest that this could approximate the timeline of allergen effectiveness. Also, the overall prognosis of typical CMPA is good, with a total recovery of 56% at 1 year, 77% at 2 years, 87% at 3 years, 92% at 5 and 10 years and 97% at 15 years of age[17]. Whether this applies to atypical CMPA in the perinatal population requires further investigation. Regardless of what the type of CMPA is, our recommendation for treatment of CMPA is the same, that is, once a diagnosis of CMPA is confirmed, a milk-free diet will be continued until a new milk challenge has shown development of tolerance. All infants with CMPA will be rechallenged at 12 mo of age and, in the event of continued clinical sensitivity to CMP, controlled rechallenges will be performed every 6 mo up to 3 years of age; and thereafter every 12 mo until tolerance develops.
We thank Joseph Neu, MD, for discussions of the objectives of this manuscript and the feedback in regards to the alternative pathway when addressing delayed passage of meconium in the neonatal population. The artwork in Figure 1 is an original drawing by Alamelu Arakoni, B.Tech.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tang ST, China; Torres MRF, Brazil S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Clark DA. Times of first void and first stool in 500 newborns. Pediatrics. 1977;60:457-459. [PubMed] |
| 2. | Loening-Baucke V, Kimura K. Failure to pass meconium: diagnosing neonatal intestinal obstruction. Am Fam Physician. 1999;60:2043-2050. [PubMed] |
| 3. | Szépfalusi Z, Nentwich I, Gerstmayr M, Jost E, Todoran L, Gratzl R, Herkner K, Urbanek R. Prenatal allergen contact with milk proteins. Clin Exp Allergy. 1997;27:28-35. [PubMed] |
| 4. | Feiterna-Sperling C, Rammes S, Kewitz G, Versmold H, Niggemann B. A case of cow's milk allergy in the neonatal period--evidence for intrauterine sensitization? Pediatr Allergy Immunol. 1997;8:153-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Vitaliti G, Cimino C, Coco A, Praticò AD, Lionetti E. The immunopathogenesis of cow's milk protein allergy (CMPA). Ital J Pediatr. 2012;38:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Carroccio A, Iacono G. Review article: Chronic constipation and food hypersensitivity--an intriguing relationship. Aliment Pharmacol Ther. 2006;24:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Bloom DA, Buonomo C, Fishman SJ, Furuta G, Nurko S. Allergic colitis: a mimic of Hirschsprung disease. Pediatr Radiol. 1999;29:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Iacono G, Bonventre S, Scalici C, Maresi E, Di Prima L, Soresi M, Di Gesù G, Noto D, Carroccio A. Food intolerance and chronic constipation: manometry and histology study. Eur J Gastroenterol Hepatol. 2006;18:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Madala A, Lure AC, Cheng S, Cheng SX. Case Reports of Cow's Milk Protein Allergy Presenting as Delayed Passage of Meconium With Early Onset Infant Constipation. Front Pediatr. 2022;10:858476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Høst A. Cow's milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr Allergy Immunol. 1994;5:1-36. [PubMed] |
| 11. | Keckler SJ, St Peter SD, Spilde TL, Tsao K, Ostlie DJ, Holcomb GW 3rd, Snyder CL. Current significance of meconium plug syndrome. J Pediatr Surg. 2008;43:896-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, Sigurdardottir ST, Lindner T, Goldhahn K, Dahlstrom J, McBride D, Madsen C. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 846] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 13. | Kondo N, Kobayashi Y, Shinoda S, Kasahara K, Kameyama T, Iwasa S, Orii T. Cord blood lymphocyte responses to food antigens for the prediction of allergic disorders. Arch Dis Child. 1992;67:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Brill H. Approach to milk protein allergy in infants. Can Fam Physician. 2008;54:1258-1264. [PubMed] |
| 15. | Undem BJ, Taylor-Clark T. Mechanisms underlying the neuronal-based symptoms of allergy. J Allergy Clin Immunol. 2014;133:1521-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Miceli Sopo S, Arena R, Greco M, Bergamini M, Monaco S. Constipation and cow's milk allergy: a review of the literature. Int Arch Allergy Immunol. 2014;164:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow's milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Burge D, Drewett M. Meconium plug obstruction. Pediatr Surg Int. 2004;20:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Kubota A, Kawahara H, Okuyama H, Shimizu Y, Nakacho M, Ida S, Nakayama M, Okada A. Cow's milk protein allergy presenting with Hirschsprung's disease-mimicking symptoms. J Pediatr Surg. 2006;41:2056-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Van Leeuwen G, Glenn L, Woodruff C, Riley WC. Meconium plug syndrome with aganglionosis. Pediatrics. 1967;40:665-666. [PubMed] |
| 21. | Al-Beltagi M, Saeed NK, Bediwy AS, Elbeltagi R. Cow's milk-induced gastrointestinal disorders: From infancy to adulthood. World J Clin Pediatr. 2022;11:437-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (5)] |
| 22. | Martorell A, Plaza AM, Boné J, Nevot S, García Ara MC, Echeverria L, Alonso E, Garde J, Vila B, Alvaro M, Tauler E, Hernando V, Fernández M. Cow's milk protein allergy. A multi-centre study: clinical and epidemiological aspects. Allergol Immunopathol (Madr). 2006;34:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |