Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4912
Peer-review started: May 15, 2023
First decision: July 10, 2023
Revised: July 18, 2023
Accepted: August 1, 2023
Article in press: August 1, 2023
Published online: August 28, 2023
Processing time: 101 Days and 22.5 Hours
Interleukin-17 (IL-17) inhibitors are known to cause exacerbation or new onset of inflammatory bowel disease upon administration. However, few reports have described characteristic endoscopic and histopathologic findings, and no small intestinal lesions have been reported so far.
A woman in her 60s with psoriasis was administered ixekizumab (IXE), an anti-IL-17A antibody, for the treatment of psoriasis. Twenty months after commencing treatment, the patient visited our hospital because of persistent diarrhea. Blood tests performed at the time of the visit revealed severe inflammation, and colonoscopy revealed multiple round ulcers throughout the colon. A tissue biopsy of the ulcer revealed infiltration of inflammatory cells and granuloma-like findings in the submucosal layer. Capsule endoscopy revealed multiple jejunal erosions. After the withdrawal of IXE, the symptoms gradually improved, and ulcer reduction and scarring of the colon were endoscopically confirmed.
To the best of our knowledge, 17 reports have documented IL-17 inhibitor-induced entero-colitis with endoscopic images, endoscopic findings, and pathological characteristics, including the present case. Nine of these cases showed diffuse loss of vascular pattern, coarse mucosa/ulcer formation in the left colon, and endoscopic findings similar to those of ulcerative colitis. In the remaining eight cases, discontinuous erosions and ulcerations from the terminal ileum to the rectum were seen, with endoscopic findings similar to those of Crohn’s disease. In this case, the findings were confirmed by capsule endoscopy, which has not been previously reported.
Core Tip: While Interleukin-17 (IL-17) inhibitors are effectively used in the treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis, they are ineffective in patients with Crohn’s disease (CD) and can worsen their condition. To the best of our knowledge, we present capsule endoscopic images of IL-17 inhibitor-induced entero-colitis for the first time, suggesting that IL-17-induced inflammatory lesions may be distributed in the proximal small bowel, unlike CD lesions. We also compared the endoscopic and pathological features of IL-17 inhibitor-induced entero-colitis with those previously reported.
- Citation: Saito K, Yoza K, Takeda S, Shimoyama Y, Takeuchi K. Drug-induced entero-colitis due to interleukin-17 inhibitor use; capsule endoscopic findings and pathological characteristics: A case report. World J Gastroenterol 2023; 29(32): 4912-4919
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4912.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4912
Interleukin-17 (IL-17) inhibitors, such as ixekizumab (IXE) and secukinumab, are a class of molecular-targeted therapies used to treat psoriasis, psoriatic arthritis, and ankylosing spondylitis. IL-17 is a type of inflammatory cytokine produced by helper T cells and is known not only to induce local inflammation in the human body but also to be involved in host infection defense against pathogens in the skin and intestinal epithelium[1]. In patients with both psoriasis and Crohn’s disease (CD), biopsy specimens of lesions express high levels of IL-17[2,3]. Therefore, IL-17 inhibitors were hypothesized to be effective in treating psoriasis and CD. However, IL-17 inhibitors are only effective in psoriasis; in patients with CD, IL-17 inhibitors are ineffective and exacerbate the disease[4]. Furthermore, in clinical trials of IL-17 inhibitors in inflammatory bowel disease (IBD), rheumatic diseases, and dermatological diseases, exacerbations or new-onset IBD have been reported at a frequency of 0.4%[5]. The mechanism underlying this seemingly contradictory adverse reaction remains unclear.
A woman in her 60s with diarrhea and anorexia.
Gastrointestinal symptoms appeared 24 mo after IXE was started for the treatment of psoriasis.
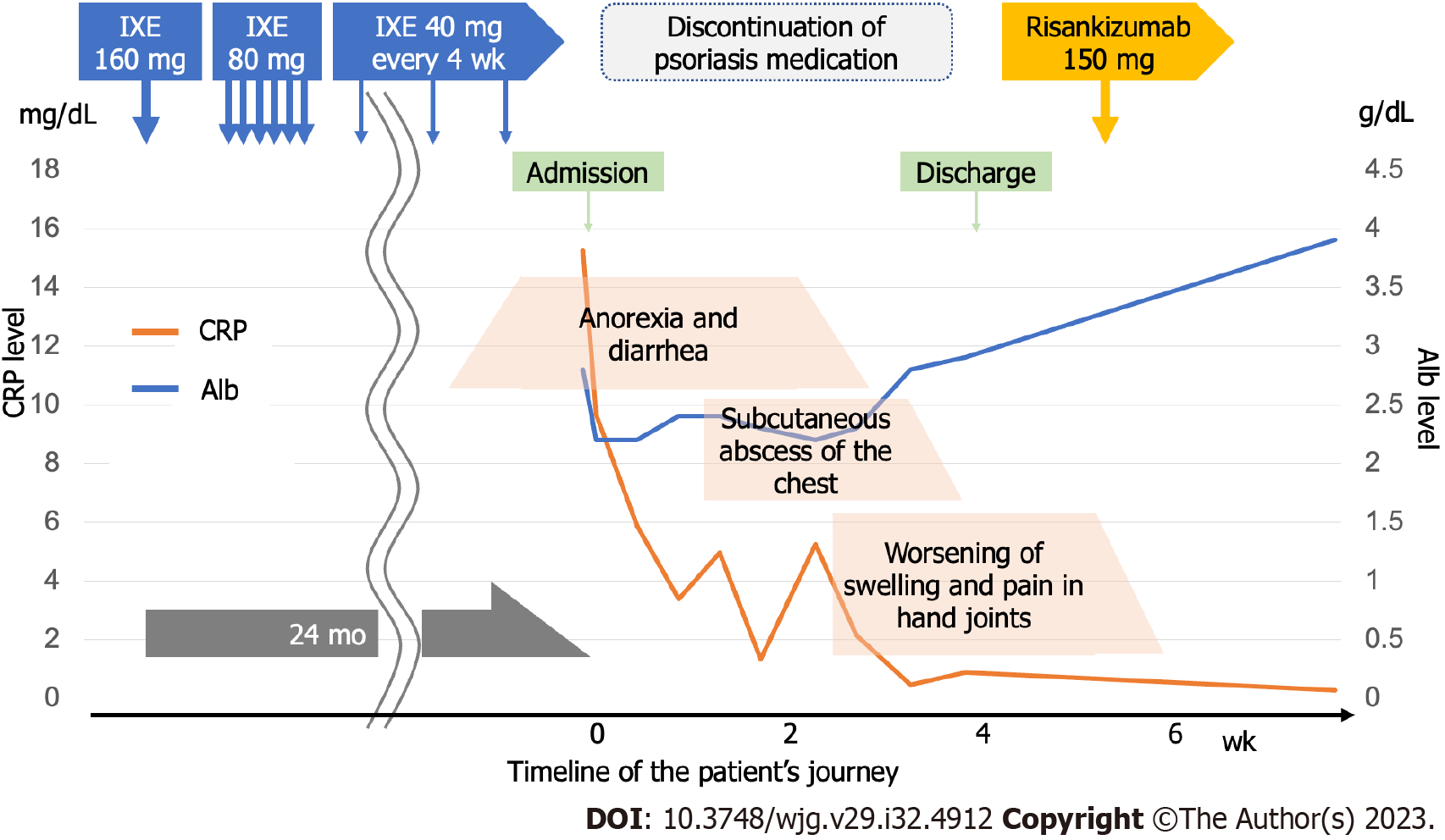
The patient was diagnosed with psoriatic arthritis by her family physician and started on IXE. However, anorexia and diarrhea appeared 20 mo after treatment initiation. After conservative treatment by her family doctor, her symptoms did not improve, and she visited our hospital 24 mo after IXE initiation for a close examination and treatment.
Her medical history included type 1 diabetes at the age of 35 and hypothyroidism at the age of 50 years, each of which was medically managed by her family physician. No family history of IBD was reported; her father had gastric cancer, and her mother had diabetes. The injectable medications used were insulin and IXE for diabetes and psoriasis, respectively.
The patient was conscious but noticeably emaciated, appeared weakened, and walked with a limp. She had a body temperature of 36.0 ℃ and 114/52 mmHg of blood pressure. The skin of the upper extremities was fragile, with epidermal exfoliation of the right forearm. Multiple scars were observed on the upper arm and mild deformities and swelling of the hand joints.
On admission, blood biochemistry tests showed anemia with a hemoglobin level of 10.4 g/dL and hypoalbuminemia with an albumin level of 2.8 g/dL. She was also dehydrated, with a blood urea nitrogen level of 28.2 mg/dL and creatinine of 0.8 mg/dL and had high inflammation with a C-reactive protein level of 15.3 mg/dL. Leucine-rich alpha-2 glycoprotein level was 44.1 μg/mL and fecal calprotectin level was also high at 7357 mg/kg, suggesting strong intestinal inflammation.
Computed tomography revealed edematous wall thickening of the intestinal tract, continuous from the ascending colon to the rectum.
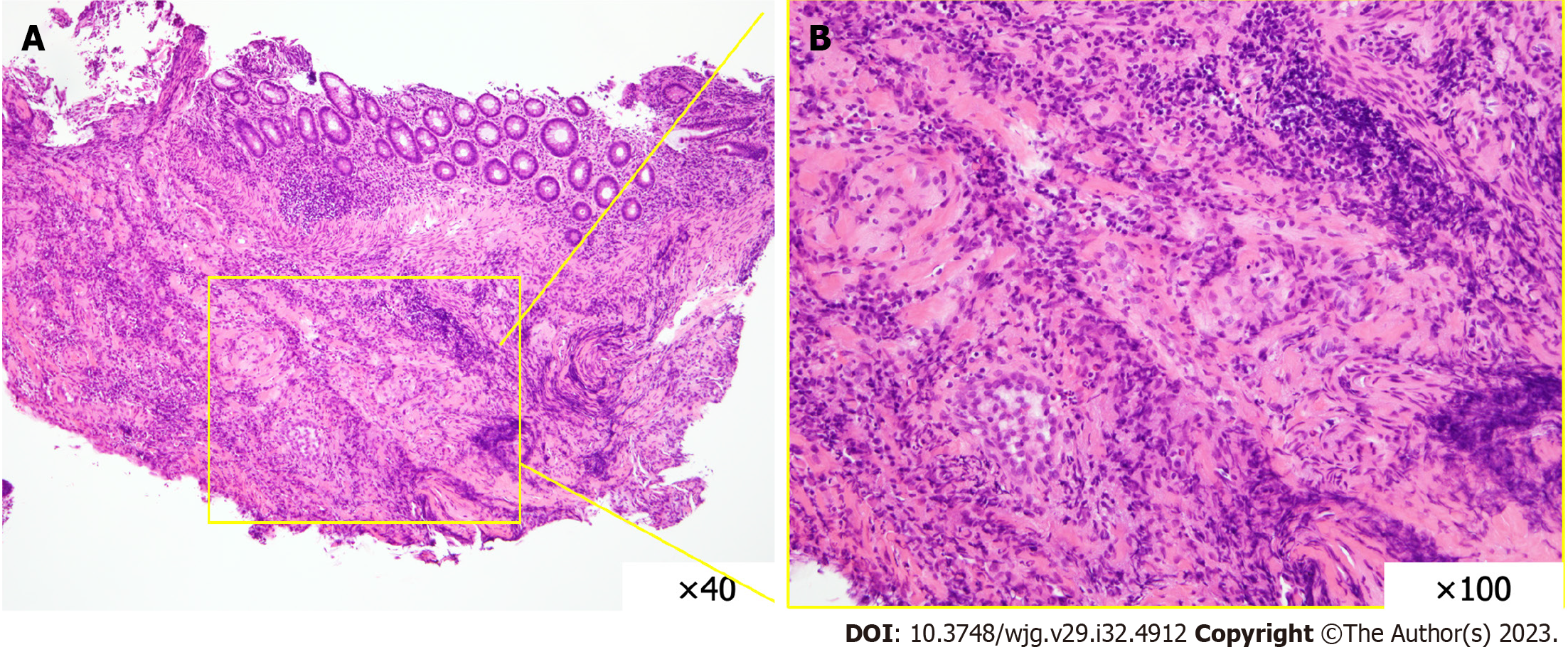
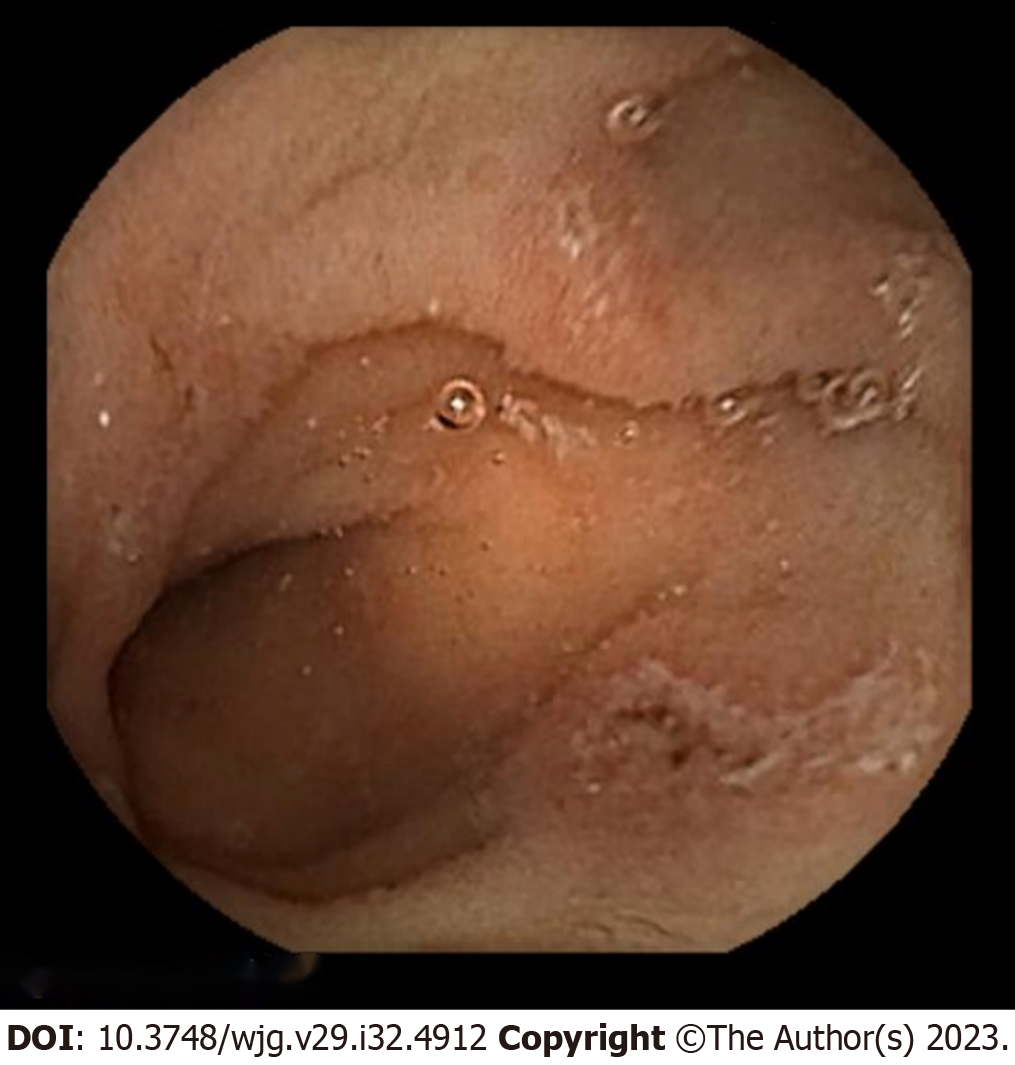
Colonoscopy revealed multiple round, punched-out ulcers with a longitudinal trend from the cecum to the rectum (Figure 1). The intervening mucosa of the ulcer was nearly normal, and the ulcer did not coincide with the mesenteric attachment. A biopsy of the ulcer showed mucosal erosion, lymphocyte-dominated inflammatory cell infiltration, and regenerative epithelial growth (Figure 2). Submucosal fibroblast and collagen fiber proliferation were also present-a granuloma-like finding similar to that of CD. An upper gastrointestinal endoscopy revealed reflux esophagitis and chronic gastritis. The gastric mucosa exhibited scattered erythema and erosions; however, no specific abnormalities were observed in the duodenum. Capsule endoscopy revealed multiple jejunal erosions (Figure 3). The erosions were scattered on the proximal side of the jejunum; each erosion was shallow and < 1 cm in size, and hematin adhesions were visible on the surface. However, stool culture and Clostridioides difficile toxin tests were negative, as were cytomegalovirus antigen and polymerase chain reaction tests and interferon-gamma release assays.
As no episodes of irradiation or introduction of other new drugs occurred, we suspected drug-induced due to IXE.
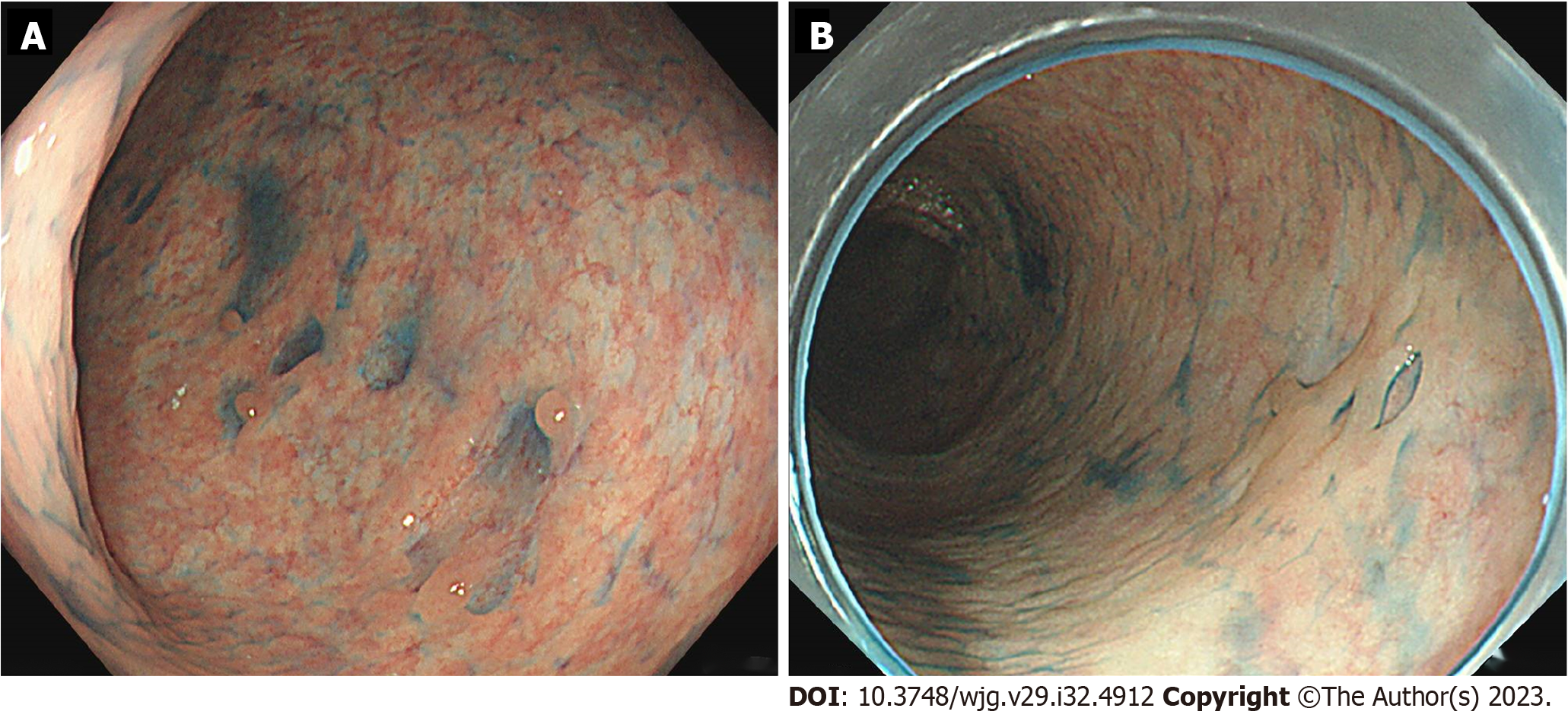
First, we monitored the patients’ progress during drug withdrawal and intestinal rest after fasting. Multiple erosions in the upper jejunum were also observed; therefore, the patient commenced on bonoprazan fumarate, a potassium-competitive acid blocker. Due to the lack of improvement in symptoms, steroid administration was considered, and a gradual improvement in abdominal symptoms was observed. Three weeks after withdrawal, endoscopy revealed shrinkage of the ulcer and scarring (Figure 4A). Although her abdominal symptoms resolved, her skin and joint symptoms worsened, and she was started on Risankizumab by her family physician for psoriasis treatment.
The abdominal, skin, and joint symptoms remained stable, and endoscopy performed 4 mo after IXE withdrawal confirmed the disappearance of all ulcers and scarring (Figure 4B). As abdominal symptoms improved, capsule endoscopy was not performed again due to a lack of patient consent, and bonoprazan fumaric acid was also discontinued. The clinical course from IXE initiation to the present is illustrated in Figure 5.
Drug-induced entero-colitis caused by IL-17 inhibitors is well known in the field of dermatology; however, very few reports have described all the endoscopic images, endoscopic features, and pathological characteristics of this condition. To the best of our knowledge, only 16 cases have been reported thus far[5-20]; we reviewed 17 cases, including our own (Table 1). Nine reported cases showed ulcerative colitis (UC)-like findings characterized by circumferential loss of vascular pattern and coarse mucosa/ulceration in the left colon, and eight reported cases with CD-like findings characterized by discontinuous erosion/ulceration from the terminal ileum to the rectum. All patients who presented with CD-like endoscopic findings after IXE administration had granulomas. In almost all the reports, the disease prognosis appeared to be good, with improvement in abdominal symptoms after the administration of steroids or molecularly targeted drugs. Only one patient with UC-like endoscopic findings after IXE administration required surgery because of a lack of improvement with drug administration.
| Year | Ref. | Age | Sex | Primary disease | Drug | Time to onset | IBD | Endoscopic findings | Pathological findings | Treatment and course |
| 2017 | Shiga et al[5] | 56 | M | Psoriasis | SEC | 8 wk | CD | Longitudinal ulcer of the ileum and round ulcer of the esophagus | Nonspecific inflammatory cell infiltration | Improved with prednisolone 40 mg/d |
| 2018 | Philipose et al[6] | 31 | M | Psoriasis | IXE | 3 mo | UC | Loss of vascular permeability throughout the sigmoid colon, erythematous coarse mucosa, ulcer | Lymphoplasmacytic infiltration | Mesalamine and methylprednisolone did not improve, but IFX administration improved |
| 2018 | Wang et al[7] | 41 | F | Psoriasis | SEC | 1 wk | UC | Coarse mucosa and deep-burrowing ulceration of the entire sigmoid colon | Cryptitis, erosions, lymhoplasmacytic infiltration | Improved with methylprednisolone 40 mg/d and cyclosporine 2 mg/kg |
| 2018 | Ehrlich et al[8] | 42 | M | Ankylosing spondylitis | SEC | 6 wk | UC | Deep ulcers and fragile mucosa of the transverse and sigmoid colon | Cryptitis, crypt abscess, loss of crypts | No improvement with solumedrol, improved after introduction of IFX |
| 2019 | Smith et al[9] | 42 | M | Psoriasis | IXE | 12 wk | CD | Deep rounded punctate ulcers of the transverse and descending colon | Pancolitis with rare granuloma | No improvement with solumedrol, improved after introduction of IFX |
| 2019 | Uchida et al[10] | 41 | F | Psoriasis | SEC | 4 mo | UC | Easy bleeding edematous mucosa of rectum to sigmoid colon, erosions, ulcers | High degree of inflammatory cell infiltration into the stroma and crypt abscess | Improved with mesalazine 2400 mg/d |
| 2019 | Achufusi et al[11] | 39 | M | Psoriasis | SEC | 6 mo | UC | Ulceration of the splenic flexure, moderate to severe active colitis, ulceration at 30 cm, and active colitis in the rectum | Atrophy of the crypts, decreased goblet cells, cryptitis, crypt abscess | No improvement with steroids, improved after introduction of IFX |
| 2019 | Johnston and Veettil[12] | 27 | M | Ankylosing spondylitis | SEC | 4 mo | UC | Multiple ulcers and moderate inflammation, sigmoid colon | Crypt abscess | No improvement with mesalazine and hydrocortisone, improvement with introduction of IFX |
| 2019 | Haidari et al[13] | 69 | M | Psoriatic arthritis | SEC | 18 mo | CD | Multiple ulcers of the terminal ileum | Neutrophil infiltration of the epithelium of the crypts, no granuloma | Originally asymptomatic |
| 2020 | Nazarian et al[14] | 48 | F | Psoriasis | IXE | 12 wk | CD | Mild erythema and punctate ulcerations in the terminal ileum | Active inflammation with the presence of granuloma | Improved with budesonide administration |
| 2020 | Varga et al[15] | 52 | M | Psoriasis | SEC | 2 wk | UC | Loss of vascular permeability of sigmoid colon, ulcer | Lymphocytic infiltration of lamina propria, cryptitis, crypt abscess | Improved with prednisone 60 mg/d and mesalazine 3200 mg |
| 2020 | Gallego et al[16] | 42 | M | Psoriasis | IXE | 2 wk | CD | Aphthous erosions and patchy ulcers of the rectum to cecum and terminal ileum | Cryptitis, crypt abscess, non-caseating granuloma | Improved with systemic corticosteroid administration |
| 2021 | Ali et al[20] | 70 | F | Psoriasis | SEC | 1 mo | UC | Ulcerated and edematous mucosa in sigmoid colon | Acutely and chronically inflamed granulation tissue with extensive plasma cell infiltrate | Intravenous methylprednisolone |
| 2022 | Kakizoe et al[17] | 65 | M | Psoriasis | SEC | 15 mo | CD | Deep ulcers of the cecum and transverse colon | No description | Hematochezia persisted after drug discontinuation and improved after induction of ADA |
| 2022 | Morosanu et al[19] | 42 | F | Psoriasis | IXE | 1 wk | UC | Continuous congestive, friable rectal and colonic mucosa, spontaneously bleeding, deep and large ulcerations | Neutrophilic inflammatory infiltrate disposed irregularly, edema and congestion, decrease of the crypts mucosecretion and crypt’s abscesses | Total colectomy with ileostoma and rectum preservation |
| 2023 | Khouri et al[18] | 38 | F | Psoriatic arthritis | SEC | 1 mo | CD | Small ulcerations throughout the entire lumen of the terminal ileum and the cecum | Minimal architecture distortion in the large bowel mucosa, along with focal acute colitis | Initiated with prednisone and SEC was switched to IFX |
| 2022 | Our case | 69 | F | Psoriatic arthritis | IXE | 21 mo | CD | Multiple round punctate ulcers throughout the colon. Capsule endoscopy shows multiple erosions in the jejunum | Inflammatory cell infiltrate, predominantly lymphocytes. Granulomatous fibroblasts and collagen fibers in the submucosa | Improvement only with drug discontinuation and fasting bowel rest |
Another case similar to CD with multiple ulcers of a similar round shape, as in the present case, has also been reported. However, in the present case, the ulcers tended to be longitudinally arranged and did not coincide with the mesenteric attachment side, which is atypical of CD. Furthermore, no reports have indicated improvement in abdominal symptoms with drug discontinuation alone, as in this case. In the present case, the various test results allowed us to promptly identify IXE as the suspected drug, and we surmised that excessive therapeutic intervention could be avoided. It should be noted that the introduction of a new drug may be necessary to manage the primary disease after drug withdrawal, and close communication with the dermatologist is important.
The association between psoriasis and IBD should be investigated in future studies. It has been reported that 1%-2% of patients with psoriasis have IBD[21]. Coincidentally-timed events during the initiation or administration of IL-17 inhibitors highlighted that IBD cannot be excluded.
At the very least, we should always check for IBD-related symptoms and family history before administering IL-17 inhibitors and suggest a screening colonoscopy if possible. Here, we report, for the first time, the capsule endoscopic findings of IL-17 inhibitor-induced entero-colitis. We also compared the endoscopic and pathological features of IL-17 inhibitor-induced entero-colitis with those previously reported. We believe that these findings will be useful for dermatologists and gastroenterologists in clinical practice.
The authors express their profound gratitude to the doctors, nurses, endoscopy staff, and medical personnel involved in the treatment of this patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dai YC, China; Fries W, Italy; Tang ST, China S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1086] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 2. | Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, Isobe K, Hibi T. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 3. | Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, Bowman EP, Krueger JG. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 798] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 4. | Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, Karczewski J, Pezous N, Bek S, Bruin G, Mellgard B, Berger C, Londei M, Bertolino AP, Tougas G, Travis SP; Secukinumab in Crohn's Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1198] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 5. | Shiga H, Fukuda S, Iijima K. Interleukin-17A Inhibitor-induced Crohn's Disease/Behçet's Disease-like Lesions. Inflamm Bowel Dis. 2017;23:E38-E39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Philipose J, Ahmed M, Idiculla PS, Mulrooney SM, Gumaste VV. Severe de novo Ulcerative Colitis following Ixekizumab Therapy. Case Rep Gastroenterol. 2018;12:617-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Wang J, Bhatia A, Krugliak Cleveland N, Gupta N, Dalal S, Rubin DT, Sakuraba A. Rapid Onset of Inflammatory Bowel Disease after Receiving Secukinumab Infusion. ACG Case Rep J. 2018;5:e56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Ehrlich D, Jamaluddin N, Pisegna J, Padua D. A Challenging Case of Severe Ulcerative Colitis following the Initiation of Secukinumab for Ankylosing Spondylitis. Case Rep Gastrointest Med. 2018;2018:9679287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Smith MK, Pai J, Panaccione R, Beck P, Ferraz JG, Jijon H. Crohn's-like disease in a patient exposed to anti-Interleukin-17 blockade (Ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019;19:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Uchida S, Oiso N, Komeda Y, Kudo M, Kawada A. Paradoxical ulcerative colitis during treatment with secukinumab for psoriasis. Eur J Dermatol. 2019;29:444-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Achufusi TG, Harnee PS, Rawlins S. A Rare Case of New-Onset Ulcerative Colitis following Initiation of Secukinumab. Case Rep Med. 2019;2019:2975631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Johnston DN, Veettil R. A case of new onset ulcerative colitis following secukinumab treatment. Br J Hosp Med (Lond). 2019;80:544-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Haidari W, Al-Naqshabandi S, Ahn CS, Bloomfeld RS, Feldman SR. Asymptomatic Crohn's disease identified in a patient being treated with secukinumab: A case report. SAGE Open Med Case Rep. 2019;7:2050313X19893580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Nazarian A, Grin A, Wijeratne DT. Ixekizumab Associated New-Onset Inflammatory Bowel Disease. ACG Case Rep J. 2020;7:e00316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Fernández-de la Varga M, Del Pozo-Del Valle P, Béjar-Serrano S, Garrido-Marín A, Bastida Paz G. Secukinumab-induced ulcerative colitis: opening Pandora's box of immunity. Gastroenterol Hepatol. 2020;43:358-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Merino Gallego E, Gómez Torres K, Martínez Amate E. Debut Of Inflammatory Bowel Disease Associated To Ixekizumab In Patient With Moderate, Difficult -To-Manage Psoriasis. Gastroenterol Hepatol. 2020;43:622-623. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Kakizoe K, Fujioka S, Noda M, Torisu T. IBD-like Lesions in a Secukinumab-treated Patient. Intern Med. 2022;61:2077-2078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Khouri A, Moreno C, Niland B. New-Onset Crohn's Disease following Initiation of Secukinumab: A Case Report and Review of the Role of IL-17 in the Pathogenesis of Crohn's Disease. Case Rep Gastrointest Med. 2023;2023:1769290. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Morosanu AM, Mihai IR, Rezus II, Gavrilescu O, Dranga M, Prelipcean CC, Mihai C. New onset severe ulcerative colitis following Ixekizumab therapy. Arch Clin Cases. 2022;9:173-176. [PubMed] [DOI] [Full Text] |
| 20. | Ali AK, Torosian A, Porter C, Bloomfeld RS, Feldman SR. New onset inflammatory bowel disease in patient treated with secukinumab: Case report and review of literature. Dermatol Ther. 2021;34:e15151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Eppinga H, Poortinga S, Thio HB, Nijsten TEC, Nuij VJAA, van der Woude CJ, Vodegel RM, Fuhler GM, Peppelenbosch MP. Prevalence and Phenotype of Concurrent Psoriasis and Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |