Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4883
Peer-review started: May 20, 2023
First decision: June 22, 2023
Revised: July 6, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: August 28, 2023
Processing time: 96 Days and 15.3 Hours
Approximately 40% of colorectal cancer (CRC) cases are linked to Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations. KRAS mutations are associated with poor CRC prognosis, especially KRAS codon 12 mutation, which is associated with metastasis and poorer survival. However, the clinicopathological characteristics and prognosis of KRAS codon 13 mutation in CRC remain unclear.
To evaluate the clinicopathological characteristics and prognostic value of codon-specific KRAS mutations, especially in codon 13.
This retrospective, single-center, observational cohort study included patients who underwent surgery for stage I-III CRC between January 2009 and December 2019. Patients with KRAS mutation status confirmed by molecular pathology reports were included. The relationships between clinicopathological characteristics and individual codon-specific KRAS mutations were analyzed. Survival data were analyzed to identify codon-specific KRAS mutations as recurrence-related factors using the Cox proportional hazards regression model.
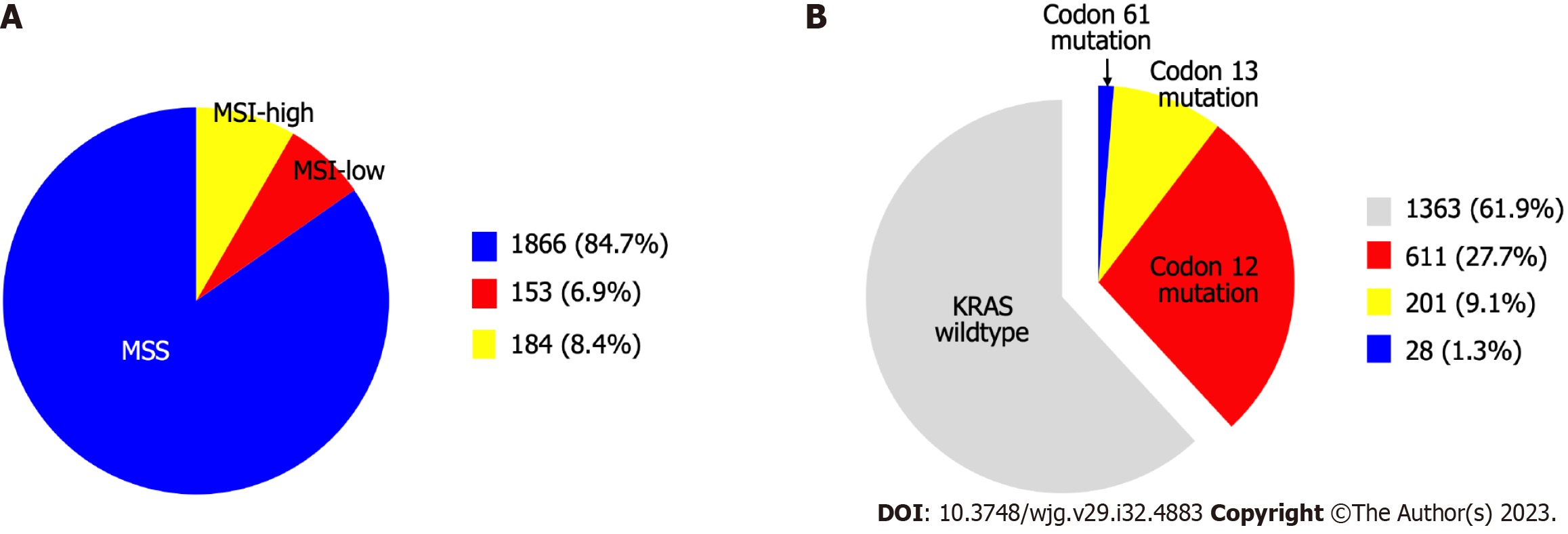
Among the 2203 patients, the incidence of KRAS codons 12, 13, and 61 mutations was 27.7%, 9.1%, and 1.3%, respectively. Both KARS codons 12 and 13 mutations showed a tendency to be associated with clinical characteristics, but only codon 12 was associated with pathological features, such as stage of primary tumor (T stage), lymph node involvement (N stage), vascular invasion, perineural invasion, tumor size, and microsatellite instability. KRAS codon 13 mutation showed no associations (77.2% vs 85.3%, P = 0.159), whereas codon 12 was associated with a lower 5-year recurrence-free survival rate (78.9% vs 75.5%, P = 0.025). In multivariable analysis, along with T and N stages and vascular and perineural invasion, only codon 12 (hazard ratio: 1.399; 95% confidence interval: 1.034-1.894; P = 0.030) among KRAS mutations was an independent risk factor for recurrence.
This study provides evidence that KRAS codon 13 mutation is less likely to serve as a prognostic biomarker than codon 12 mutation for CRC in a large-scale cohort.
Core Tip: Based on a large-scale cohort of patients with stage I-III colorectal cancer (CRC), Kirsten rat sarcoma viral oncogene homolog (KRAS) codon 13 mutation is less pathogenic and recurrent. Moreover, focusing on the biological effects of codon-specific KRAS mutations and minimizing interference with various medical therapies, previous in vivo studies demonstrating that KRAS codon 13 mutation is less aggressive were translated into clinical outcomes in this study. This may influence many oncologists to consult with patients on their prognosis after surgery. We propose that KRAS codon 13 mutation is less likely to serve as a prognostic factor of CRC, compared with codon 12.
- Citation: Ahn HM, Kim DW, Oh HJ, Kim HK, Lee HS, Lee TG, Shin HR, Yang IJ, Lee J, Suh JW, Oh HK, Kang SB. Different oncological features of colorectal cancer codon-specific KRAS mutations: Not codon 13 but codon 12 have prognostic value. World J Gastroenterol 2023; 29(32): 4883-4899
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4883.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4883
Kirsten rat sarcoma viral oncogene homolog (KRAS) is one of the downstream molecules of the epidermal growth factor receptor (EGFR) associated with cell proliferation, anti-apoptosis, and survival[1-3]. Abnormal activation of KRAS, a well-known oncogene, triggers uncontrolled tumor cell proliferation regardless of the initiating molecular signal from EGFR[4]. Mutations in KRAS promote the development of cancer in a variety of organs including the breast, prostate, lung, pancreas, colon, and rectum[1,2]. According to previous reports, approximately 40% of colorectal cancer (CRC) cases are linked to KRAS mutations[5-7], which occur more frequently in the proximal rather than in the distal colon[4,8,9]. Clinically, KRAS mutations are associated with resistance to anti-EGFR therapy and poor CRC prognosis[10,11].
CRC-related point mutations in KRAS occur at different codon locations. In most cases, KRAS mutations are detected in codon 12 or 13, whereas mutations in codon 61 or 146 have been reported only in a minority of patients with CRC[12]. Several clinical studies have indicated that KRAS codon 12 mutations are associated with metastasis and poor survival in advanced CRC[8,12-14]. In-vitro studies comparing cells with KRAS codon 12 and 13 mutations have demonstrated stronger transforming activity and resistance to apoptosis in cells with mutations in KRAS codon 12 than codon 13[15,16]. Most reports have concluded that KRAS codon 12 mutation is a poor prognostic factor following CRC resection. However, the oncological role of KRAS codon 13 mutation is controversial. KRAS codon 13 mutation has been linked to advanced-stage or lymph node metastasis and has been considered predictive of a higher likelihood of death in several studies[17,18]. In contrast, other investigators have shown no association between KRAS codon 13 mutations and tumor progression or CRC prognosis[4,19].
In addition to the controversial prognostic significance of KRAS codon 13 mutations, limited information is available regarding the clinical characteristics of codon-specific KRAS mutations in CRC. The incidence of codon-specific KRAS mutations other than those involving codon 12 (including codon 13) is low. Owing to the infrequency of KRAS abnormalities, the pathological features of codon-specific mutations at sites other than codon 12 remain unclear. Owing to the small cohort sizes of previous studies[4,8,12,14,20], the clinical roles of codon-specific KRAS mutations in CRC, including codons 12 and 13, are yet to be validated. Moreover, studies on the oncological effects of codon-specific KRAS mutations, particularly regarding abnormalities located within minor codons, are limited.
This study was designed to elucidate the clinicopathological characteristics associated with codon-specific KRAS mutations in CRC, including codons 12, 13, and 61. The main objective of this study was to determine whether KRAS codon 13 mutation could serve as a prognostic biomarker for CRC in a relatively large cohort of individuals.
This retrospective observational cohort study was registered at ClinicalTrials.gov (NCT05657210) and reviewed 3144 patients who underwent surgery for CRC between January 2009 and December 2019, with available clinical data on recurrence and survival. All patients underwent routine colon or rectal resection and lymph node dissection according to the tumor location, with or without diverting ileostomies or colostomies. The surgical specimens were submitted to the laboratory for pathological evaluation. Patients with confirmed molecular pathology reports of KRAS mutation status were included, whereas those with incomplete data on KRAS mutations (n = 368) or microsatellite instability (MSI) status (n = 232) were excluded. Patients with dual or triple KRAS mutations (within more than one codon) from pathology reports (n = 2) were excluded. Additionally, to understand the biological importance and minimize the potential influence of systemic therapeutic factors on the prognosis of codon-specific KRAS mutations, we excluded patients with stage IV metastatic CRC (n = 339). Finally, data from 2203 eligible patients were collected separately for statistical analysis. This study was approved by the Institutional Review Board (IRB No. B-2203-742-101) of Seoul National University Bundang Hospital and the requirement for informed consent was waived.
All patients who underwent colorectal surgery for curative purposes were recommended adjuvant therapy according to the pathological stage of the cancer. Patients with pathological stage III and high-risk stage II colon cancer are recommended adjuvant chemotherapy. In rectal cancer, patients with pathological stages II and III are treated with adjuvant chemotherapy after surgery. However, in patients with clinical T4 or positive nodes without distant metastasis, preoperative chemoradiation therapy is recommended with long-course radiotherapy (dose of 5040 cGy of radiation over 5 wk; 28 fractions) combined with chemotherapy with 5-fluorouracil/Leucovorin or capecitabine.
According to the cancer monitoring protocol after curative surgery at our facility, patients were evaluated regularly one month after surgery, then every 3 mo for the first 2 years, every 6 mo for the next 3 years, and every 12 mo thereafter for a total of 5 years. Monitoring included measurements of serum carcinoembryonic antigen (CEA) levels every 3 mo; imaging modalities, including computed tomography (CT) (abdomen, pelvis, and chest) every 6 mo; and annual colonoscopy. Cancer recurrence was confirmed histologically or radiologically. The assigned research nurse constantly updated the data on recurrence and death. Information about deaths was double-checked by comparison with the database of the National Health Insurance Service, Korea, which lists the life and death records of Korean people. The registry data were constantly updated and managed by an assigned research nurse in the colorectal surgery department of our hospital.
Basic patient clinical information [age, sex, height, weight, and American Society of Anesthesiologists (ASA) score] was collected. Cancer-related clinical characteristics such as primary tumor location, preoperative CEA level, and diverting stoma were included. Data on pathological features were collected based on pathology reports of surgical specimens. The following variables were statistically analyzed: T and N stages, tumor size, lymphatic invasion, vascular invasion, perineural invasion, number of harvested lymph nodes, number of metastatic lymph nodes, MSI status, and KRAS mutation status. Codon-specific KRAS mutation status was examined for codons 12, 13, and 61.
KRAS mutations were identified from formalin-fixed, paraffin-embedded cancerous tissue obtained from surgical specimens. After deoxyribonucleic acid (DNA) extraction from the tissue, the exons 2 and 3 of the KRAS gene were separately amplified by polymerase chain reaction (PCR) using optimized PCR reagents and primers. Codon-specific KRAS mutations were identified by pyrosequencing (PyroMark Q24 Mdx, QIAGEN, Hilden, Germany). MSI status was also evaluated using formalin-fixed tissues during surgery. PCR with five markers (BAT26, BAT25, D5S346, D17S250, and D2S123) followed by fragmentation assay (ABI-3130xl, Thermo Fisher Scientific, MA, United States) was performed to identify the MSI status.
Descriptive statistics were used to identify the basic clinicopathological characteristics of the patients, including MSI status frequency and KRAS mutations. The differences between wild-type and mutant KRAS as well as the mean values of continuous variables, were compared using either the independent t-test or the Mann-Whitney U test according to the results of the Kolmogorov-Smirnov test. Chi-squared or Fisher’s exact tests were used to compare categorical variables. Overall survival (OS) and recurrence-free survival (RFS) were calculated from the date of surgery and compared using the Kaplan-Meier method and the log-rank test. For the analysis of risk factors for tumor recurrence, the Cox proportional hazards regression model was used, with the covariance input criterion set at P < 0.1. Patients were subdivided based on the primary tumor location (colon vs rectum) and MSI status [microsatellite stable (MSS)/MSI-low versus MSI-high]. Each subgroup was analyzed for recurrence-related factors using a Cox proportional hazards regression model. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 25.0, for Windows (SPSS, IBM). Descriptive results of continuous variables are expressed as mean ± SD. P value < 0.05 were considered statistically significant.
The present study included 2203 patients who underwent CRC surgery. The clinicopathological characteristics of the patients are shown in Table 1. In terms of MSI status, 1866 patients (84.7%) were identified as MSS, 153 (6.9%) as MSI-low, and 184 (8.4%) as MSI-high (Figure 1A). KRAS mutations were detected in 840 patients (38.1%) patients. The incidence of KRAS codons 12, 13, and 61 substitutions was 27.7%, 9.1%, and 1.3%, respectively (Figure 1B).
| Clinical characteristics (n = 2203) | Value1 |
| Age (yr) | 64.7 ± 12.2 |
| Sex | |
| Male | 1264 (57.4) |
| Female | 939 (42.6) |
| Body mass index (kg/m2) | 23.9 ± 3.3 |
| ASA score | |
| 1 | 575 (26.1) |
| 2 | 1412 (64.1) |
| 3 | 211 (9.6) |
| 4 | 5 (0.2) |
| Cancer location | |
| Cecum | 46 (2.1) |
| Ascending colon | 386 (17.5) |
| Hepatic flexure | 88 (4.0) |
| Transverse colon | 115 (5.2) |
| Splenic flexure | 18 (0.8) |
| Descending colon | 79 (3.6) |
| Sigmoid colon | 771 (35.0) |
| Rectum | 700 (31.7) |
| Preoperative CEA (ng/mL) | 7.7 ± 42.3 |
| Diverting stoma | |
| Ileostomy | 435 (19.7) |
| Colostomy | 62 (2.8) |
| T stage | |
| 0 | 18 (0.8) |
| 1 | 275 (12.5) |
| 2 | 383 (17.4) |
| 3 | 1282 (58.2) |
| 4 | 245 (11.1) |
| N stage | |
| 0 | 1286 (58.4) |
| 1 | 639 (29.0) |
| 2 | 278 (12.6) |
| Tumor size (cm) | 4.4 ± 2.4 |
| Lymphatic invasion | 597 (27.1) |
| Vascular invasion | 469 (21.3) |
| Perineural invasion | 934 (42.4) |
| Harvested lymph nodes | 45.3 ± 21.2 |
| Metastatic lymph nodes | 1.4 ± 2.9 |
| Adjuvant/Neoadjuvant therapy | |
| Colon | |
| Adjuvant therapy-stage II | 274 (51.4) |
| Adjuvant therapy-stage III | 575 (90.0) |
| Rectum | |
| Neoadjuvant therapy | 200 (28.6) |
| Operative first-Adjuvant therapy | 264 (52.8) |
Among the clinical characteristics, female sex, lower ASA score, right-sided colon cancer, higher preoperative CEA levels, and low rates of diverting stoma formation were associated with KRAS mutations in codons 12, 13, and 61. Most pathological features, including T stage, N stage, tumor size, lymphatic invasion, perineural invasion, and number of harvested lymph nodes, were associated with KRAS mutations, along with molecular features such as MSI status (Table 2).
| KRAS overall2 | KRAS Codon 12 | KRAS Codon 13 | KRAS Codon 61 | |||||||||
| WT (%) | MT (%) | P value | WT (%) | MT (%) | P value | WT (%) | MT (%) | P value | WT (%) | MT (%) | P value | |
| Age | 0.418 | 0.734 | 0.698 | 0.246 | ||||||||
| < 65 yr | 644 (62.8) | 382 (37.2) | 745 (72.6) | 281 (27.4) | 936 (91.1) | 91 (8.9) | 1016 (99.0) | 10 (1.0) | ||||
| ≥ 65 yr | 719 (61.1) | 458 (38.9) | 847 (72.0) | 330 (28.0) | 1067 (90.7) | 110 (9.3) | 1160 (98.5) | 18 (1.5) | ||||
| Sex | < 0.001 | < 0.001 | 0.006 | 0.238 | ||||||||
| Male | 851 (67.3) | 413 (32.7) | 961 (76.0) | 303 (24.0) | 1167 (92.3) | 97 (7.7) | 1251 (99.0) | 13 (1.0) | ||||
| Female | 512 (54.5) | 427 (45.5) | 631 (67.2) | 308 (32.8) | 836 (88.9) | 104 (11.1) | 924 (98.4) | 15 (1.6) | ||||
| BMI | 0.098 | 0.347 | 0.485 | 0.104 | ||||||||
| < 25 kg/m2 | 853 (60.6) | 555 (39.4) | 1008 (71.6) | 400 (28.4) | 1275 (90.6) | 133 (9.4) | 1386 (98.4) | 22 (1.6) | ||||
| ≥ 25 kg/m2 | 510 (64.2) | 285 (35.8) | 584 (73.5) | 211 (26.5) | 727 (91.4) | 68 (8.6) | 789 (99.2) | 6 (0.8) | ||||
| ASA score | 0.010 | 0.002 | 0.942 | 0.347 | ||||||||
| 1-2 | 1212 (61.0) | 775 (39.0) | 1417 (71.3) | 570 (28.7) | 1806 (90.9) | 181 (9.1) | 1963 (98.8) | 24 (1.2) | ||||
| 3-4 | 151 (69.9) | 65 (30.1) | 175 (81.0) | 41 (19.0) | 196 (90.7) | 20 (9.3) | 212 (98.1) | 4 (1.9) | ||||
| Cancer location (1)3 | < 0.001 | 0.002 | < 0.001 | 0.219 | ||||||||
| Right-sided | 329 (51.8) | 306 (48.2) | 429 (67.6) | 206 (32.4) | 546 (86.0) | 89 (14.0) | 624 (98.3) | 11 (1.7) | ||||
| Left-sided | 1034 (65.9) | 534 (34.1) | 1163 (74.2) | 405 (25.8) | 1456 (92.9) | 112 (7.1) | 1552 (98.9) | 17 (1.1) | ||||
| Cancer location (2)4 | 0.092 | 0.405 | 0.117 | 0.966 | ||||||||
| Colon | 912 (60.7) | 591 (39.3) | 1078 (71.7) | 425 (28.3) | 1356 (90.2) | 147 (9.8) | 1484 (98.7) | 19 (1.3) | ||||
| Rectum | 451 (64.4) | 249 (35.6) | 514 (73.4) | 186 (26.6) | 646 (92.3) | 54 (7.7) | 691 (98.7) | 9 (1.3) | ||||
| Preoperative CEA | < 0.001 | < 0.001 | 0.037 | 0.301 | ||||||||
| < 5.0 ng/mL | 1131 (64.7) | 616 (35.3) | 1299 (74.4) | 448 (25.6) | 1599 (91.5) | 148 (8.5) | 1727 (98.9) | 20 (1.1) | ||||
| ≥ 5.0 ng/mL | 232 (50.9) | 224 (49.1) | 293 (64.3) | 163 (35.7) | 403 (88.4) | 53 (11.6) | 448 (98.2) | 8 (1.8) | ||||
| Diverting stoma | 0.001 | 0.071 | 0.029 | 0.131 | ||||||||
| No | 1024 (60.0) | 682 (40.0) | 1217 (71.3) | 489 (28.7) | 1538 (90.2) | 168 (9.8) | 1681 (98.5) | 25 (1.5) | ||||
| Yes | 339 (68.2) | 158 (31.8) | 375 (75.5) | 122 (24.5) | 464 (93.4) | 33 (6.6) | 494 (99.4) | 3 (0.6) | ||||
| T stage | 0.008 | 0.003 | 0.488 | 0.139 | ||||||||
| T0-2 | 446 (66.0) | 230 (34.0) | 517 (76.5) | 159 (23.5) | 610 (90.2) | 66 (9.8) | 671 (99.3) | 5 (0.7) | ||||
| T3-4 | 917 (60.1) | 610 (39.9) | 1075 (70.4) | 452 (29.6) | 1392 (91.2) | 135 (8.8) | 1504 (98.5) | 23 (1.5) | ||||
| N stage | 0.012 | 0.046 | 0.617 | 0.094 | ||||||||
| N0 | 824 (64.1) | 462 (35.9) | 950 (73.9) | 336 (26.1) | 1172 (91.1) | 114 (8.9) | 1274 (99.1) | 12 (0.9) | ||||
| N1-2 | 539 (58.8) | 378 (41.2) | 642 (70.0) | 275 (30.0) | 830 (90.5) | 87 (9.5) | 901 (98.3) | 16 (1.7) | ||||
| MSI status | 0.003 | 0.001 | 0.458 | 0.973 | ||||||||
| MSS | 1138 (61.0) | 728 (39.0) | 1327 (71.1) | 539 (28.9) | 1701 (91.2) | 165 (8.8) | 1842 (98.7) | 24 (1.3) | ||||
| MSI-low | 90 (58.8) | 63 (41.2) | 110 (71.9) | 43 (28.1) | 135 (88.2) | 18 (11.8) | 151 (98.7) | 2 (1.3) | ||||
| MSI-high | 135 (73.4) | 49 (26.6) | 155 (84.2) | 29 (15.8) | 166 (90.2) | 18 (9.8) | 182 (98.9) | 2 (1.1) | ||||
| Tumor size (cm) | 4.3 ± 2.4 | 4.6 ± 2.3 | 0.005 | 4.3 ± 2.5 | 4.6 ± 2.1 | 0.001 | 4.4 ± 2.3 | 4.6 ± 2.7 | 0.837 | 4.4 ± 2.4 | 4.5 ± 2.1 | 0.708 |
| Lymphatic invasion | 0.005 | 0.099 | 0.080 | 0.302 | ||||||||
| No | 1022 (63.6) | 584 (36.4) | 1176 (73.2) | 430 (26.8) | 1470 (91.5) | 136 (8.5) | 1589 (98.9) | 18 (1.1) | ||||
| Yes | 341 (57.1) | 256 (42.9) | 416 (69.7) | 181 (30.3) | 532 (89.1) | 65 (10.9) | 587 (98.3) | 10 (1.7) | ||||
| Vascular invasion | 0.090 | 0.047 | 0.614 | 0.061 | ||||||||
| No | 1057 (61.0) | 677 (39.0) | 1236 (71.3) | 498 (28.7) | 1573 (90.7) | 161 (9.3) | 1716 (99.0) | 18 (1.0) | ||||
| Yes | 306 (65.2) | 163 (34.8) | 356 (75.9) | 113 (24.1) | 429 (91.5) | 40 (8.5) | 459 (97.9) | 10 (2.1) | ||||
| Perineural invasion | 0.003 | 0.027 | 0.387 | 0.048 | ||||||||
| No | 819 (64.5) | 450 (35.5) | 940 (74.1) | 329 (25.9) | 1159 (91.3) | 110 (8.7) | 1258 (99.1) | 11 (0.9) | ||||
| Yes | 544 (58.2) | 390 (41.8) | 652 (69.8) | 282 (30.2) | 843 (90.3) | 91 (9.7) | 917 (98.2) | 17 (1.8) | ||||
| Harvested LN | 44.5 ± 20.6 | 46.5 ± 22.1 | 0.040 | 45.0 ± 21.1 | 45.9 ± 21.6 | 0.500 | 45.0 ± 20.9 | 47.9 ± 24.1 | 0.079 | 45.2 ± 21.3 | 47.7 ± 16.6 | 0.208 |
| Metastatic LN | 1.4 ± 3.1 | 1.3 ± 2.6 | 0.420 | 1.4 ± 3.1 | 1.3 ± 2.4 | 0.406 | 1.4 ± 2.9 | 1.4 ± 3.1 | 0.832 | 1.4 ± 2.9 | 1.4 ± 1.9 | 0.149 |
Analysis of the codon-specific KRAS mutational status revealed significant associations of both clinical and pathological characteristics with KRAS codon 12 mutations, including female sex, lower ASA score, right-sided colon cancer, preoperative CEA level above the normal range (≥ 5.0 ng/mL), T stage, N stage, MSI status, tumor size, vascular invasion, and perineural invasion. In contrast, only female sex, right-sided colon cancer, high preoperative CEA levels, diverting stoma formation, and no pathological features were significantly correlated with KRAS codon 13 mutations. Other than perineural invasion, no clinical characteristics or pathological features were associated with KRAS codon 61 mutations (Table 2).
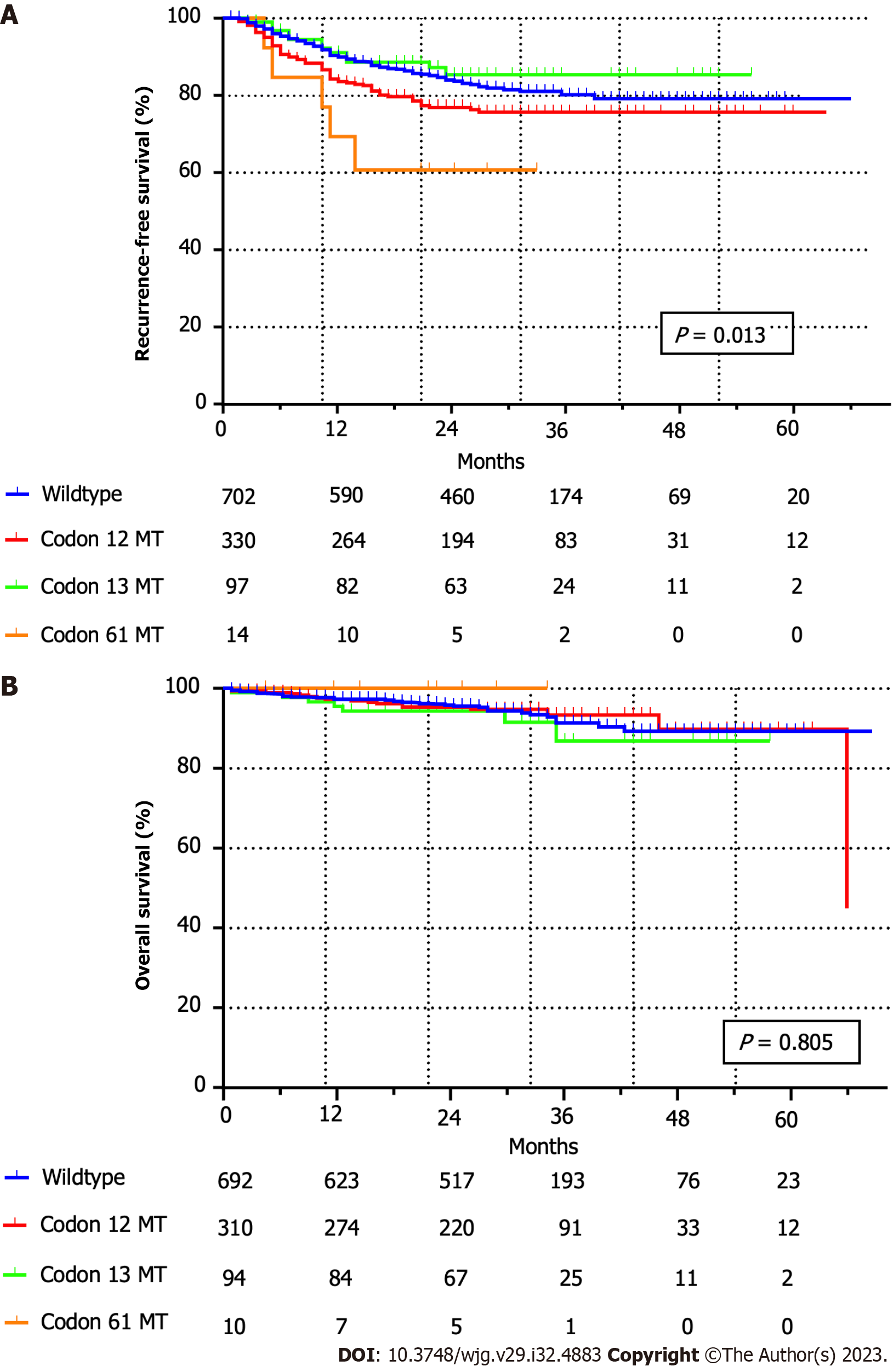
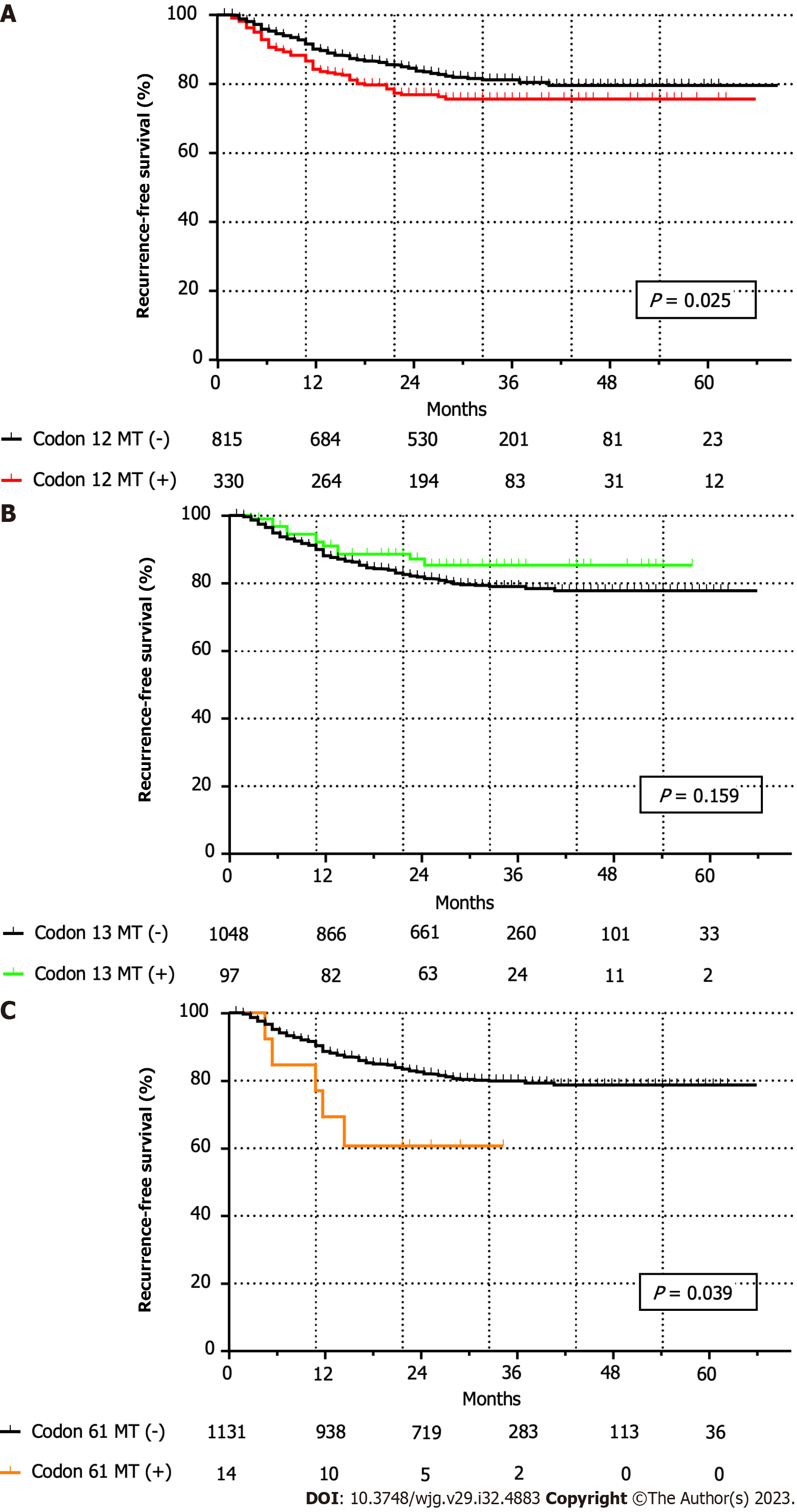
At a mean ± SD follow-up duration of 29.7 mo ± 14.3 mo, and a median of 29 (0-85) months, recurrence within 5 years of curative surgery was observed in 205 (9.3%) among the 2203 patients. Five-year RFS (78.3% vs 77.4%, P = 0.130) and OS (89.0% vs 89.5%, P = 0.971) rates did not differ significantly between the wild-type and KRAS mutant CRC groups. Notably, the 5-year RFS for all codon-specific KRAS mutations was statistically different (wild-type, codon 12, and codon 13 mutations: 78.4%, 75.5%, and 85.3%, respectively; P = 0.013; Figure 2A), but the 5-year OS rates were comparable (wild-type, codon 12, and codon 13 mutations: 89.2%, 89.8%, and 86.9%, respectively; P = 0.805; Figure 2B). The 5-year RFS rate of the KRAS codon 12 mutation group was significantly lower than that of the patients without codon 12 mutations (78.9% vs 75.5%, P = 0.025; Figure 3A). The 5-year RFS rate of the KRAS codon 13 mutation group was higher than that of the patients without codon 13 mutations; however, the difference was not statistically significant (77.2% vs 85.3%, P = 0.159; Figure 3B). The RFS of the KRAS codon 61 mutation group was significantly lower than that of the patients without codon 61 mutations (78.2% vs 60.6%, P = 0.039; Figure 3C); however, all cases of recurrence occurred within 2 years of surgery.
In the univariate analysis of recurrence-related factors, cancer location (colon or rectum), preoperative CEA level, diverting stoma, T stage, N stage, MSI status, tumor size, lymphatic invasion, vascular invasion, perineural invasion, number of metastatic lymph nodes, and KRAS codon 12 mutations were associated with recurrence. In multivariable analysis, most pathological features, including higher T stage [hazard ratio (HR): 2.620; 95% confidence intervals (CI): 1.479-4.641; P = 0.001], higher N stage (HR: 2.001; 95%CI: 1.399-2.861; P < 0.001), vascular invasion (HR: 1.578; 95%CI: 1.164-2.139; P = 0.003), perineural invasion (HR: 1.684; 95%CI: 1.194-2.376; P = 0.003), and mutation of KRAS codon 12 (HR: 1.399; 95%CI: 1.034-1.894; P = 0.030) were identified as independent risk factors of recurrence in multivariable analysis. Among the clinical characteristics, only the presence of a diverting stoma (HR: 1.874; 95%CI: 1.260-2.787; P = 0.002) was independently correlated with recurrence (Table 3).
| Recurrence | Multivariable Cox regression analysis2 | ||||||
| Absent1 (n = 1998) | Present1 (n = 205) | P value | HR | 95%CI | P value | ||
| Lower | Upper | ||||||
| Age (yr) | 0.716 | ||||||
| < 65 | 933 (46.7) | 93 (45.4) | - | - | - | - | |
| ≥ 65 | 1065 (53.3) | 112 (54.6) | - | - | - | ||
| Sex | 0.616 | ||||||
| Male | 1143 (57.2) | 121 (59.0) | - | - | - | - | |
| Female | 855 (42.8) | 84 (41.0) | - | - | - | ||
| BMI | 0.094 | ||||||
| < 25 kg/m2 | 1266 (63.4) | 142 (69.3) | - | - | - | - | |
| ≥ 25 kg/m2 | 732 (36.6) | 63 (30.7) | - | - | - | ||
| ASA score | 0.980 | ||||||
| 1-2 | 1802 (90.2) | 185 (90.2) | - | - | - | - | |
| 3-4 | 196 (9.8) | 20 (9.8) | - | - | - | ||
| Cancer location (1)3 | 0.860 | ||||||
| Right-sided | 577 (28.9) | 58 (28.3) | - | - | - | - | |
| Left-sided | 1421 (71.1) | 147 (71.7) | - | - | - | ||
| Cancer location (2)4 | |||||||
| Colon | 1376 (68.9) | 127 (62.0) | 0.043 | 1.000 | |||
| Rectum | 622 (31.1) | 78 (38.0) | 1.053 | 0.718 | 1.545 | 0.791 | |
| Preoperative CEA | < 0.001 | ||||||
| < 5.0 ng/mL | 1607 (80.4) | 140 (68.3) | 1.000 | ||||
| ≥ 5.0 ng/mL | 391 (19.6) | 65 (31.7) | 1.158 | 0.849 | 1.579 | 0.354 | |
| Diverting stoma | < 0.001 | ||||||
| No | 1568 (78.5) | 138 (67.3) | 1.000 | ||||
| Yes | 430 (21.5) | 67 (32.7) | 1.874 | 1.260 | 2.787 | 0.002 | |
| T stage | < 0.001 | ||||||
| T0-2 | 659 (33.0) | 17 (8.3) | 1.000 | ||||
| T3-4 | 1339 (67.0) | 188 (91.7) | 2.620 | 1.479 | 4.641 | 0.001 | |
| N stage | < 0.001 | ||||||
| N0 | 1230 (61.6) | 56 (27.3) | 1.000 | ||||
| N1-2 | 768 (38.4) | 149 (72.7) | 2.001 | 1.399 | 2.861 | < 0.001 | |
| MSI status | 0.037 | ||||||
| MSS | 1680 (84.1) | 186 (90.7) | 0.855 | 0.342 | 2.138 | 0.738 | |
| MSI-low | 143 (7.2) | 10 (4.9) | 1.284 | 0.643 | 2.566 | 0.479 | |
| MSI-high | 175 (8.8) | 9 (4.4) | 1.000 | ||||
| Tumor size (cm) | 4.3 ± 2.4 | 4.9 ± 2.1 | < 0.001 | 0.997 | 0.927 | 1.074 | 0.944 |
| Lymphatic invasion | < 0.001 | ||||||
| No | 1493 (74.7) | 113 (55.1) | 1.000 | ||||
| Yes | 505 (25.3) | 92 (44.9) | 1.324 | 0.977 | 1.793 | 0.070 | |
| Vascular invasion | < 0.001 | ||||||
| No | 1615 (80.8) | 119 (58.0) | 1.000 | ||||
| Yes | 383 (19.2) | 86 (42.0) | 1.578 | 1.164 | 2.139 | 0.003 | |
| Perineural invasion | < 0.001 | ||||||
| No | 1211 (60.6) | 58 (28.3) | 1.000 | ||||
| Yes | 787 (39.4) | 147 (71.7) | 1.684 | 1.194 | 2.376 | 0.003 | |
| Harvested LN | 45.3 ± 21.2 | 44.9 ± 21.4 | 0.705 | - | - | - | - |
| Metastatic LN | 1.2 ± 2.6 | 3.3 ± 4.5 | < 0.001 | 1.028 | 0.995 | 1.061 | 0.095 |
| KRAS Codon 12 | |||||||
| Wild-type | 1459 (73.0) | 133 (64.9) | 0.013 | 1.000 | |||
| Mutation | 539 (27.0) | 72 (35.1) | 1.399 | 1.034 | 1.894 | 0.030 | |
| KRAS Codon 13 | |||||||
| Wild-type | 1809 (90.5) | 193 (94.1) | 0.088 | 1.000 | |||
| Mutation | 189 (9.5) | 12 (5.9) | 0.637 | 0.350 | 1.160 | 0.140 | |
| KRAS Codon 61 | |||||||
| Wild-type | 1975 (98.8) | 200 (97.6) | 0.176 | 1.000 | |||
| Mutation | 23 (1.2) | 5 (2.4) | 1.950 | 0.790 | 4.812 | 0.147 | |
Tumor size (HR: 1.100; 95%CI: 1.011-1.198; P = 0.027), vascular invasion (HR: 1.981; 95%CI: 1.362-2.880; P < 0.001), perineural invasion (HR: 1.793; 95%CI: 1.200-2.679; P = 0.004), the presence of metastatic lymph nodes (HR: 1.048; 95%CI: 1.014-1.083; P = 0.006), and KRAS codon 12 mutation (HR: 1.496; 95%CI: 1.019-2.196; P = 0.040) were determined as independent risk factors for cancer recurrence when the primary tumor location was in the colon. Perineural invasion (HR: 3.358; 95%CI: 1.885-5.983; P < 0.001), and the presence of metastatic lymph nodes (HR: 1.095; 95%CI: 1.017-1.178; P = 0.016) were independently associated with cancer recurrence when the primary tumor was in the rectum. No codon-specific KRAS mutations were associated with recurrent rectal cancer (Table 4).
| Colon (n = 1503) | Rectum (n = 700) | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Preoperative CEA ≥ 5.0 ng/mL | 1.290 (0.860-1.933) | 0.218 | 1.215 (0.705-2.220) | 0.444 |
| Diverting stoma (+) | 0.903 (0.394-2.067) | 0.809 | 1.249 (0.755-2.065) | 0.386 |
| T3-4 stage (vs T0-2) | 1.211 (0.737-1.991) | 0.450 | 1.079 (0.601-1.939) | 0.799 |
| N1-2 stage (vs N0) | 1.241 (0.863-1.784) | 0.244 | 1.126 (0.649-1.954) | 0.674 |
| Tumor size (cm) | 1.100 (1.011-1.198) | 0.027 | 1.077 (0.948-1.223) | 0.256 |
| Lymphatic invasion | 1.342 (0.919-1.960) | 0.128 | 0.971 (0.562-1.676) | 0.915 |
| Vascular invasion | 1.981 (1.362-2.880) | < 0.001 | 1.401 (0.841-2.334) | 0.195 |
| Perineural invasion | 1.793 (1.200-2.679) | 0.004 | 3.358 (1.885-5.983) | < 0.001 |
| Metastatic LN | 1.048 (1.014-1.083) | 0.006 | 1.095 (1.017-1.178) | 0.016 |
| KRAS Codon 12 mutation | 1.496 (1.019-2.196) | 0.040 | 1.492 (0.902-2.466) | 0.119 |
| KRAS Codon 13 mutation | 0.831 (0.412-1.678) | 0.606 | 0.481 (0.146-1.578) | 0.227 |
| KRAS Codon 61 mutation | 2.385 (0.730-7.795) | 0.150 | 2.270 (0.511-10.088) | 0.282 |
Among MSS/MSI-low CRC patients, tumor size (HR: 1.117; 95%CI: 1.038-1.202; P = 0.003), vascular invasion (HR: 1.740; 95%CI: 1.282-2.363; P < 0.001), perineural invasion (HR: 2.335; 95%CI: 1.663-3.279; P < 0.001), number of metastatic lymph nodes (HR: 1.050; 95%CI: 1.020-1.081; P = 0.001), and KRAS codon 12 mutation (HR: 1.467; 95%CI: 1.077-1.998; P = 0.015) were independent risk factors for cancer recurrence. In contrast, only a high preoperative CEA level (HR: 8.321; 95%CI: 1.387-49.920; P = 0.020) was associated with recurrence in MSI-high CRC. In cases of MSI-high CRC, the KRAS codon 12 mutation was statistically irrelevant regarding cancer recurrence, and there were no cases of recurrence during the study period among patients with KRAS-mutant CRC involving codons 13 and 61 (Table 5).
| MSS/MSI-low (n = 2019) | MSI-high (n = 184)1 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Preoperative CEA ≥ 5.0 ng/mL | 1.178 (0.839-1.653) | 0.344 | 8.321 (1.387-49.920) | 0.020 |
| Rectal cancer (vs colon cancer) | 1.238 (0.850-1.804) | 0.266 | - | - |
| Diverting stoma (+) | 1.069 (0.707-1.617) | 0.752 | 2.431 (0.139-42.442) | 0.543 |
| T3-4 stage (vs T0-2) | 1.175 (1.038-1.202) | 0.407 | 0.284 (0.020-4.032) | 0.353 |
| N1-2 stage (vs N0) | 1.190 (0.879-1.610) | 0.260 | 1.000 (0.151-6.643) | 1.000 |
| Tumor size (cm) | 1.117 (1.038-1.202) | 0.003 | 0.991 (0.713-1.379) | 0.960 |
| Lymphatic invasion | 1.242 (0.909-1.698) | 0.174 | 1.154 (0.149-8.923) | 0.891 |
| Vascular invasion | 1.740 (1.282-2.363) | < 0.001 | 0.009 (0.000-29.277) | 0.255 |
| Perineural invasion | 2.335 (1.663-3.279) | < 0.001 | 0.538 (0.049-5.909) | 0.613 |
| Metastatic LN | 1.050 (1.020-1.081) | 0.001 | 1.442 (0.865-2.402) | 0.160 |
| KRAS Codon 12 mutation | 1.467 (1.077-1.998) | 0.015 | 2.508 (0.406-15.510) | 0.323 |
| KRAS Codon 13 mutation | 0.713 (0.390-1.301) | 0.270 | - | - |
| KRAS Codon 61 mutation | 2.265 (0.915-5.605) | 0.077 | - | - |
Among the 2203 patients who underwent curative surgery for stage I-III CRC, the incidence of codon-specific KRAS abnormalities was, respectively, 27.7%, 9.1%, and 1.3% for patients with KRAS codon 12, 13, and 61 mutations. Only 9.3% (205/2203) recurrences were observed during the 5-year follow-up period. To our knowledge, this study is based on the largest scaled cohort that has ever analyzed not only the oncological impact but also the clinicopathological characteristics of codon-specific KRAS mutations in patients with CRC. Most previous studies have reported similar results for KRAS codon 12 mutations, but not codon 13, in CRC as a poor oncological factor[4,8,12,14,20]. Despite the minimal oncological effects of minor KRAS mutations, such as in codon 61, the data obtained were sufficient to gain statistical power, supporting previous findings that KRAS codon 61 mutation is not associated with the clinicopathological features of CRC[21]. An earlier study in a Japanese cohort also identified KRAS codon 12, but not codon 13, as an independent risk factor for tumor recurrence in stage I-III CRC. While their results supported the utility of KRAS codon 12 mutation as a poor prognostic factor, the correlation between codon-specific KRAS mutations and clinicopathological characteristics could not be validated because of the small sample size[20]. In the present study, we analyzed the largest sample group of patients, which provided not only results complementing earlier studies on KRAS mutations in CRC, but also additional information on correlations with clinicopathological characteristics and prognostic factors for individual codon-specific KRAS mutations.
In addition to resistance to anti-EGFR therapies, such as cetuximab and panitumumab[22], KRAS codon 12 mutation in CRC has been established as a poor prognostic factor of survival associated with aggressive behavior[23]. However, the role of KRAS codon 13 mutation in CRC remains unclear. Several studies have suggested that KRAS codon 13 mutations are associated with advanced-stage disease and metastasis of CRC and potentially serve as a predictive factor for a higher likelihood of death[17,18]. An earlier meta-analysis reported a lower overall survival in patients with KRAS codon 13 mutant CRC with no exposure to anti-EGFR therapy than in those treated with targeted therapy[24]. Other studies have demonstrated that KRAS codon 13 mutations are not associated with CRC progression[4,19]. Another meta-analysis of metastatic CRC with mutated KRAS codon 13 revealed a more significant response to cetuximab than that in patients with other codon-specific KRAS mutations[25]. To ascertain the correlation between codon-specific KRAS mutations and clinical oncological outcomes throughout the stages of CRC, therapeutic options such as chemotherapy, radiotherapy, and targeted therapy, along with inevitable resistance mechanisms, should be considered[5,26,27].
The survival analysis showed that CRC recurrence, but not overall survival, was associated with codon-specific KRAS mutations. Analysis of individual codons showed that KRAS codon 12 mutation is an independent risk factor for recurrence, while KRAS codon 13 and 61 mutations appeared to be statistically irrelevant. In earlier in vivo molecular biology studies, cells with KRAS codon 12 and 13 mutations displayed similar morphological changes, but only codon 12 mutants induced anchorage-independent growth, implying a lower aggressiveness of KRAS codon 13 mutations[15]. Another in vitro study reported that KRAS codon 12 mutant cells were more resistant to apoptosis and exhibited enhanced anti-apoptotic molecular signaling relative to codon 13 mutant cells, consistent with the finding that the codon 13 mutation is less aggressive[16]. These in vivo results were translated into the clinical outcomes of our study, demonstrating that KRAS codon 13 mutation is less aggressive and less likely to serve as a poor prognostic factor for CRC compared with KRAS codon 12 mutation.
Interestingly, the prognosis of KRAS codon 12 mutant CRC varied based on the primary tumor location in either the colon or rectum. The majority of experiments on tumor location were stratified into right- or left-sided colorectum based on the splenic flexure[28,29]. Even the definition of ‘left-sided’ differs among studies according to the involvement of the rectum[30,31]. Thus, in the present study, recurrence-related factors were analyzed by subgrouping the tumors into colon and rectum. In the subgroup of tumors located in the colon, patients with KRAS codon 12 mutations were estimated to be at a 1.5-fold higher risk of CRC recurrence than those without codon 12 mutations. In contrast, in the rectum, all codon-specific KRAS mutations were not linked to recurrence. To the best of our knowledge, this is the first study to investigate the oncological impact of codon-specific KRAS mutations based on tumor location (colon or rectum). Our findings support the theory that KRAS codon 12 mutation is a poor prognostic factor for colon cancer, but not for rectal cancer.
Previous studies have shown that the combination of KRAS mutations and MSI status is a potential prognostic factor in various stages of CRC [26,32-36]. In addition, since MSI status is associated with chemoresistance[37,38], the MSS/MSI-low and MSI-high subgroups were analyzed separately to eliminate the effect of MSI status on prognosis. Interestingly, in the MSS/MSI-low patient subgroup, only KRAS codon 12 mutation was statistically related to recurrence, whereas there was no association between codon-specific KRAS mutations and recurrence among MSI-high tumors. It is well known that poor oncological outcomes including disease-free and overall survival were reported within MSS tumors combined with KRAS mutation[33-36]. To the best of our knowledge, analysis results of codon-specific KRAS mutations in MSS/MSI-low and MSI-high tumors have never been reported. Based on our subgroup analysis, KRAS codon 12 mutations may be associated with the location of colon and MSS tumors, and not all CRC patients with KRAS codon 12 mutations have poor outcomes.
Clarifying the effects of codon-specific KRAS mutations on the prognosis of stage IV CRC is a complex issue[5,26,27,39]. A recent study on KRAS mutations in CRC with liver metastasis reported that KRAS codon 12 mutations were associated with poorer overall survival, while codon 13 was not; however, they also pointed out the exclusion of perioperative management such as anti-epidermal growth factor receptor agents[12]. Among the patients diagnosed with stage IV CRC who underwent surgery in our hospital during the period of the present study, 48.4% had KRAS mutations. However, only about half of them (53.1%) underwent surgery with curative intent, whereas the others underwent palliative treatment. Additionally, there is a wide range of variations in the metastatic burden and forms of treatment for these patients. Therefore, in the present study, we excluded stage IV disease to focus on the biological importance and prognostic impact of codon-specific KRAS mutations in stage I-III CRC.
In two patients in our cohort, KRAS mutations were detected at two or more codon sites. The first patient was a 75-year-old male who underwent surgery for descending colon cancer and was pathologically diagnosed with stage III (pT3N1M0) colon cancer with codon 12 and 13 KRAS mutations. The second patient was a 60-year-old female who underwent surgery for sigmoid colon cancer diagnosed as stage I (pT1N0M0) with both codon 12 and 61 KRAS mutations. Both patients survived for more than 5 years after surgery with no recurrence or metastasis. In a previous study, 12 patients with two or more codon mutations among 505 CRC KRAS mutation cases were reported but were eventually excluded from the analysis[21]. For the same reason, these two patients were excluded from the current study despite our intellectual curiosity.
The present study had several limitations. First, BRAF mutation, a biomarker related to the prognosis of CRC after surgery, was omitted from our analysis. According to previous studies on CRC biomarkers, both BRAF and MSI status have an important prognostic impact on recurrence and survival[34,40]. Unfortunately, a large amount of data was collected without knowledge of the BRAF mutation status because of alterations in routine molecular examinations by our facility during the study period. Second, since KRAS mutations were evaluated using postoperative specimens for both colon and rectal cancer, it may be audacious to conclude that the KRAS codon 12 mutation is a prognostic factor in rectal cancer. In advanced rectal cancer, trimodality therapy comprises chemoradiation followed by surgery, which takes at least 1-2 mo. This delay may affect the oncological outcome; therefore, the prognostic value of codon-specific KRAS mutations according to the primary tumor site should be carefully interpreted. Third, uncontacted patients without follow-up could have missing data on recurrence and survival despite constantly updating the clinical data by the assigned research nurses in our department. The refusal to revisit after a few follow-ups could have produced missing data in our cohort, and double-checking with the National Health Insurance database might have reduced the error as much as possible. Unfortunately, these efforts could not separate other causes of death from cancer-related ones. Fourth, this study had a retrospective and single-center design, which could have led to selection bias. Despite this, the present study was based on a large-scale cohort with a relatively well-organized CRC registry of patients who underwent surgery, and is the largest cohort study ever that analyzed codon-specific KRAS mutations.
Most of the KRAS mutations in our study involved KRAS codons 12 and 13. Notably, KRAS codon 12 mutation was significantly associated with pathological features closely related to cancer recurrence and had a poor prognostic impact in patients with MSS tumors, or those located in the colon but not in the rectum. Given its irrelevance to pathological features and recurrence, we propose that KRAS codon 13 mutation is less likely to serve as a prognostic factor for CRC.
Abnormal activation of Kirsten rat sarcoma viral oncogene homolog (KRAS), a well-known oncogene, triggers uncontrolled tumor cell proliferation. Approximately 40% of colorectal cancer (CRC) are linked to KRAS mutations. CRC -related point mutations in KRAS occur at different codon locations. KRAS codon 12 or 13 mutations are detected in a majority of CRC patients, whereas mutations in codon 61 or 146 have been reported only in a minority.
KRAS mutations are associated with poor CRC prognosis, especially KRAS codon 12 mutation, which is associated with metastasis and poorer survival. However, the clinicopathological characteristics and prognosis of KRAS codon 13 mutation in CRC remain controversial.
This study aimed to evaluate the clinicopathological characteristics and prognostic value of codon-specific KRAS mutations, especially in codon 13.
This retrospective, single-center, observational cohort study included patients who underwent surgery for stage I-III CRC. The relationships between clinicopathological characteristics and individual codon-specific KRAS mutations were analyzed. By using the Cox proportional hazards regression model, survival analysis were performed to identify codon-specific KRAS mutations as recurrence-related factors.
Both KARS codons 12 and 13 mutations showed a tendency to be associated with clinical characteristics, but only codon 12 was associated with pathological features. KRAS codon 13 mutation showed no associations, whereas codon 12 was associated with a lower 5-year recurrence-free survival rate. In multivariable analysis, only codon 12 (HR: 1.399; 95% confidence interval: 1.034-1.894; P = 0.030) among KRAS mutations was an independent risk factor for recurrence. This may influence many oncologists to consult with patients on their prognosis after surgery.
KRAS codon 12 mutation was significantly associated with pathological features closely related to cancer recurrence and had a poor prognostic impact in patients with microsatellite stable tumors, or those located in the colon but not in the rectum. On the other hand, KRAS codon 13 mutation is irrelevant to pathological features and recurrence, which consider less likely to serve as a prognostic factor for CRC.
Focusing on the biological effects of codon-specific KRAS mutations, KRAS codon 13 mutation is less pathogenic and recurrent, Based on a large-scale cohort of patients with stage I-III CRC. This study’s results may influence not only the prognosis but also the management of CRC patients individually. Therefore, the therapeutic usage and needs of codon-specific KRAS mutation in CRC should be considered in future studies.
The authors would like to thank the staff involved in the operating room and pathology laboratory of Seoul National University Bundang Hospital. The authors thank the Division of Statistics at the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Malekpour A, Iran; Wang XL, China; Xie Q, China S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Cicenas J, Tamosaitis L, Kvederaviciute K, Tarvydas R, Staniute G, Kalyan K, Meskinyte-Kausiliene E, Stankevicius V, Valius M. KRAS, NRAS and BRAF mutations in colorectal cancer and melanoma. Med Oncol. 2017;34:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | Kerk SA, Papagiannakopoulos T, Shah YM, Lyssiotis CA. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat Rev Cancer. 2021;21:510-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 3. | Wan XB, Wang AQ, Cao J, Dong ZC, Li N, Yang S, Sun MM, Li Z, Luo SX. Relationships among KRAS mutation status, expression of RAS pathway signaling molecules, and clinicopathological features and prognosis of patients with colorectal cancer. World J Gastroenterol. 2019;25:808-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Li W, Liu Y, Cai S, Yang C, Lin Z, Zhou L, Liu L, Cheng X, Zeng W. Not all mutations of KRAS predict poor prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2019;12:957-967. [PubMed] |
| 5. | Er TK, Chen CC, Bujanda L, Herreros-Villanueva M. Clinical relevance of KRAS mutations in codon 13: Where are we? Cancer Lett. 2014;343:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Kodaz H, Hacibekiroglu I, Erdogan B, Turkmen E, Tozkir H, Albayrak D, Uzunoglu S, Cicin I. Association between specific KRAS mutations and the clinicopathological characteristics of colorectal tumors. Mol Clin Oncol. 2015;3:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Fan JZ, Wang GF, Cheng XB, Dong ZH, Chen X, Deng YJ, Song X. Relationship between mismatch repair protein, RAS, BRAF, PIK3CA gene expression and clinicopathological characteristics in elderly colorectal cancer patients. World J Clin Cases. 2021;9:2458-2468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Jones RP, Sutton PA, Evans JP, Clifford R, McAvoy A, Lewis J, Rousseau A, Mountford R, McWhirter D, Malik HZ. Specific mutations in KRAS codon 12 are associated with worse overall survival in patients with advanced and recurrent colorectal cancer. Br J Cancer. 2017;116:923-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Rosty C, Young JP, Walsh MD, Clendenning M, Walters RJ, Pearson S, Pavluk E, Nagler B, Pakenas D, Jass JR, Jenkins MA, Win AK, Southey MC, Parry S, Hopper JL, Giles GG, Williamson E, English DR, Buchanan DD. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod Pathol. 2013;26:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 1699] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 11. | Asawa P, Bakalov V, Kancharla P, Abel S, Chahine Z, Monga DK, Kirichenko AV, Wegner RE. The prognostic value of KRAS mutation in locally advanced rectal cancer. Int J Colorectal Dis. 2022;37:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Margonis GA, Kim Y, Spolverato G, Ejaz A, Gupta R, Cosgrove D, Anders R, Karagkounis G, Choti MA, Pawlik TM. Association Between Specific Mutations in KRAS Codon 12 and Colorectal Liver Metastasis. JAMA Surg. 2015;150:722-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | He K, Wang Y, Zhong Y, Pan X, Si L, Lu J. KRAS Codon 12 Mutation is Associated with More Aggressive Invasiveness in Synchronous Metastatic Colorectal Cancer (mCRC): Retrospective Research. Onco Targets Ther. 2020;13:12601-12613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Li W, Qiu T, Zhi W, Shi S, Zou S, Ling Y, Shan L, Ying J, Lu N. Colorectal carcinomas with KRAS codon 12 mutation are associated with more advanced tumor stages. BMC Cancer. 2015;15:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Guerrero S, Casanova I, Farré L, Mazo A, Capellà G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750-6756. [PubMed] |
| 16. | Guerrero S, Figueras A, Casanova I, Farré L, Lloveras B, Capellà G, Trias M, Mangues R. Codon 12 and codon 13 mutations at the K-ras gene induce different soft tissue sarcoma types in nude mice. FASEB J. 2002;16:1642-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193-1197. [PubMed] |
| 18. | Bazan V, Migliavacca M, Zanna I, Tubiolo C, Grassi N, Latteri MA, La Farina M, Albanese I, Dardanoni G, Salerno S, Tomasino RM, Labianca R, Gebbia N, Russo A. Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype. Ann Oncol. 2002;13:1438-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Imamura Y, Morikawa T, Liao X, Lochhead P, Kuchiba A, Yamauchi M, Qian ZR, Nishihara R, Meyerhardt JA, Haigis KM, Fuchs CS, Ogino S. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res. 2012;18:4753-4763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 209] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Hayama T, Hashiguchi Y, Okamoto K, Okada Y, Ono K, Shimada R, Ozawa T, Toyoda T, Tsuchiya T, Iinuma H, Nozawa K, Matsuda K. G12V and G12C mutations in the gene KRAS are associated with a poorer prognosis in primary colorectal cancer. Int J Colorectal Dis. 2019;34:1491-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Imamura Y, Lochhead P, Yamauchi M, Kuchiba A, Qian ZR, Liao X, Nishihara R, Jung S, Wu K, Nosho K, Wang YE, Peng S, Bass AJ, Haigis KM, Meyerhardt JA, Chan AT, Fuchs CS, Ogino S. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature review. Mol Cancer. 2014;13:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Bellio H, Fumet JD, Ghiringhelli F. Targeting BRAF and RAS in Colorectal Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, Benamouzig R, Jullian E, Laurent-Puig P, Olschwang S, Muller O, Hoffmann I, Rabes HM, Zietz C, Troungos C, Valavanis C, Yuen ST, Ho JW, Croke CT, O'Donoghue DP, Giaretti W, Rapallo A, Russo A, Bazan V, Tanaka M, Omura K, Azuma T, Ohkusa T, Fujimori T, Ono Y, Pauly M, Faber C, Glaesener R, de Goeij AF, Arends JW, Andersen SN, Lövig T, Breivik J, Gaudernack G, Clausen OP, De Angelis PD, Meling GI, Rognum TO, Smith R, Goh HS, Font A, Rosell R, Sun XF, Zhang H, Benhattar J, Losi L, Lee JQ, Wang ST, Clarke PA, Bell S, Quirke P, Bubb VJ, Piris J, Cruickshank NR, Morton D, Fox JC, Al-Mulla F, Lees N, Hall CN, Snary D, Wilkinson K, Dillon D, Costa J, Pricolo VE, Finkelstein SD, Thebo JS, Senagore AJ, Halter SA, Wadler S, Malik S, Krtolica K, Urosevic N. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer. 2001;85:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 666] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Kwak MS, Cha JM, Yoon JY, Jeon JW, Shin HP, Chang HJ, Kim HK, Joo KR, Lee JI. Prognostic value of KRAS codon 13 gene mutation for overall survival in colorectal cancer: Direct and indirect comparison meta-analysis. Medicine (Baltimore). 2017;96:e7882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Chen J, Ye Y, Sun H, Shi G. Association between KRAS codon 13 mutations and clinical response to anti-EGFR treatment in patients with metastatic colorectal cancer: results from a meta-analysis. Cancer Chemother Pharmacol. 2013;71:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Cionca FL, Dobre M, Dobrea CM, Iosif CI, Comănescu MV, Ardeleanu CM. Mutational status of KRAS and MMR genes in a series of colorectal carcinoma cases. Rom J Morphol Embryol. 2018;59:121-129. [PubMed] |
| 27. | Tsilimigras DI, Ntanasis-Stathopoulos I, Bagante F, Moris D, Cloyd J, Spartalis E, Pawlik TM. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence. Surg Oncol. 2018;27:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388-2394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 387] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H; Colon/Rectum Carcinomas (Primary Tumor) Study Group. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 553] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 30. | Brulé SY, Jonker DJ, Karapetis CS, O'Callaghan CJ, Moore MJ, Wong R, Tebbutt NC, Underhill C, Yip D, Zalcberg JR, Tu D, Goodwin RA. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 31. | Elnatan J, Goh HS, Smith DR. C-KI-RAS activation and the biological behaviour of proximal and distal colonic adenocarcinomas. Eur J Cancer. 1996;32A:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Hu J, Yan WY, Xie L, Cheng L, Yang M, Li L, Shi J, Liu BR, Qian XP. Coexistence of MSI with KRAS mutation is associated with worse prognosis in colorectal cancer. Medicine (Baltimore). 2016;95:e5649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Nash GM, Gimbel M, Cohen AM, Zeng ZS, Ndubuisi MI, Nathanson DR, Ott J, Barany F, Paty PB. KRAS mutation and microsatellite instability: two genetic markers of early tumor development that influence the prognosis of colorectal cancer. Ann Surg Oncol. 2010;17:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Lin CC, Lin JK, Lin TC, Chen WS, Yang SH, Wang HS, Lan YT, Jiang JK, Yang MH, Chang SC. The prognostic role of microsatellite instability, codon-specific KRAS, and BRAF mutations in colon cancer. J Surg Oncol. 2014;110:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Taieb J, Le Malicot K, Shi Q, Penault-Llorca F, Bouché O, Tabernero J, Mini E, Goldberg RM, Folprecht G, Luc Van Laethem J, Sargent DJ, Alberts SR, Emile JF, Laurent Puig P, Sinicrope FA. Prognostic Value of BRAF and KRAS Mutations in MSI and MSS Stage III Colon Cancer. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 36. | Nazemalhosseini-Mojarad E, Kishani Farahani R, Mehrizi M, Baghaei K, Yaghoob Taleghani M, Golmohammadi M, Peyravian N, Ashtari S, Pourhoseingholi MA, Asadzadeh Aghdaei H, Zali MR. Prognostic Value of BRAF and KRAS Mutation in Relation to Colorectal Cancer Survival in Iranian Patients: Correlated to Microsatellite Instability. J Gastrointest Cancer. 2020;51:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Lee SY, Kim DW, Lee J, Park HM, Kim CH, Lee KH, Oh HK, Kang SB, Kim HR. Association between microsatellite instability and tumor response to neoadjuvant chemoradiotherapy for rectal cancer. Ann Surg Treat Res. 2022;103:176-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Lee SY, Kim DW, Lee HS, Ihn MH, Oh HK, Min BS, Kim WR, Huh JW, Yun JA, Lee KY, Kim NK, Lee WY, Kim HC, Kang SB. Low-Level Microsatellite Instability as a Potential Prognostic Factor in Sporadic Colorectal Cancer. Medicine (Baltimore). 2015;94:e2260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Tonello M, Baratti D, Sammartino P, Di Giorgio A, Robella M, Sassaroli C, Framarini M, Valle M, Macrì A, Graziosi L, Coccolini F, Lippolis PV, Gelmini R, Deraco M, Biacchi D, Santullo F, Vaira M, Di Lauro K, D'Acapito F, Carboni F, Giuffrè G, Donini A, Fugazzola P, Faviana P, Sorrentino L, Scapinello A, Del Bianco P, Sommariva A. Microsatellite and RAS/RAF Mutational Status as Prognostic Factors in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann Surg Oncol. 2022;29:3405-3417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 40. | Formica V, Sera F, Cremolini C, Riondino S, Morelli C, Arkenau HT, Roselli M. KRAS and BRAF Mutations in Stage II and III Colon Cancer: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2022;114:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |