Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4873
Peer-review started: May 21, 2023
First decision: July 10, 2023
Revised: July 20, 2023
Accepted: August 9, 2023
Article in press: August 9, 2023
Published online: August 28, 2023
Processing time: 95 Days and 21.7 Hours
The albumin-bilirubin (ALBI) score is an index of liver function recently deve
To investigate the ALBI score for identifying decompensation risk at the 3-year follow-up in patients with compensated cirrhosis.
One-hundred and twenty-three patients with compensated cirrhosis without HCC in King Chulalongkorn Memorial Hospital diagnosed by imaging were retros
Among 123 cirrhotic patients enrolled, 13.8% (n = 17) developed decompensating events at a median time of 25 [95% confidence interval (CI): 17-31] mo. Median baseline ALBI score in compensated cirrhosis was significantly lower than that of patients who developed decompensation events [-2.768 (-2.956 to -2.453) vs -2.007 (-2.533 to -1.537); P = 0.01]. Analysis of decompensation risk at 3 years showed that ALBI score had a time-dependent area under the curve (tAUC) of 0.86 (95%CI: 0.78-0.92), which was significantly better than that of ALBI-Fibrosis-4 (ALBI-FIB4) score (tAUC = 0.77), MELD score (tAUC = 0.66), Child-Pugh score (tAUC = 0.65), and FIB-4 score (tAUC = 0.48) (P < 0.05 for all). The 3-year cumulative incidence of decompensation was 3.1%, 22.6%, and 50% in the low-, middle-, and high-risk groups, respectively (P < 0.001). The odds ratio for decompensation in patients of the high-risk group was 23.33 (95%CI: 3.88-140.12, P = 0.001).
The ALBI score accurately identifies decompensation risk at the 3-year follow-up in patients with compensated cirrhosis. Those cirrhotic patients with a high-risk grade of ALBI score showed a 23 times greater odds of decom
Core Tip: The albumin-bilirubin (ALBI) score has been successfully applied to the prediction of survival in patients with non-malignant liver diseases of various etiologies. This study demonstrated that the ALBI score can accurately identify decompensation risk at the 3-year follow-up in patients with compensated cirrhosis. The ALBI score is a simple and ready-to-use tool to help clinicians monitor and make appropriate treatment strategies in patients with compensated cirrhosis.
- Citation: Navadurong H, Thanapirom K, Wejnaruemarn S, Prasoppokakorn T, Chaiteerakij R, Komolmit P, Treeprasertsuk S. Validation of the albumin-bilirubin score for identifying decompensation risk in patients with compensated cirrhosis. World J Gastroenterol 2023; 29(32): 4873-4882
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4873.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4873
Cirrhosis is the end stage of chronic liver disease which is currently the 11th leading cause of death and 15th leading cause of morbidity across the world, accounting for 2.2% of deaths and 1.5% of disability-adjusted life years worldwide[1]. The disease evolves from an asymptomatic phase as compensated cirrhosis to a symptomatic phase as decompensated cirrhosis[2]. Decompensated cirrhosis is defined by the presence of variceal bleeding, encephalopathy, ascites, hepato-renal syndrome, and/or jaundice[3]. Transition from a compensated to a decompensated stage occurs at a rate of 5%-7% per year[4]. Median survival of patients with compensated cirrhosis is 12 years, while that of decompensated patients is less than 2 years[5]. Once decompensation has occurred, mortality without transplant is as high as 85% over 5 years[4].
For over 60 years, the best predictor of decompensation in cirrhotic patients has been the hepatic venous pressure gradient (HVPG)[6]. HVPG has a greater discriminative ability to predict clinical decompensation in patients with compensated cirrhosis than either the model for end-stage liver disease (MELD) or Child-Pugh score. Research shows that patients with a HVPG < 10 mmHg have a 90% probability of not developing clinical decompensation in a median follow-up of 4 years[6]. However, HVPG measurement is invasive, requires specialized healthcare personnel, and is often unavailable in many healthcare systems. The appearance of noninvasive tests, most notably, transient elastography, has provided a staging tool for prognostic markers of portal hypertension[7]. Recently, Baveno VII criteria were developed using transient elastography for liver stiffness measurements and platelet counts to define clinically significant portal hypertension and prognosis, risk stratification, and indication to start beta-blocker therapy in compensated advanced chronic liver disease and compensated cirrhosis patients[8]. Within a median follow-up of 40 mo, 7.2% of the 1159 compensated advanced chronic liver disease and compensated cirrhosis patients developed an initial decompensation event[8].
Well-known prognostic scoring systems that are currently used such as the MELD score and Child-Pugh score were primarily established to predict mortality in patients with cirrhosis. The Child-Pugh score was originally developed to assess the survival of cirrhotic patients undergoing shunt surgery to relieve portal hypertension in order to treat variceal bleeding[9,10]. The MELD score was developed to more precisely evaluate 3-mo mortality for patients with cirrhosis in order to prioritize liver donor allocation[11]. The MELD score is considered more reproducible than the Child-Pugh score because it does not include subjective variables such as ascites and encephalopathy. However, the MELD score has not been shown to be superior to the Child-Pugh score in terms of predictive accuracy in different cirrhotic populations[12].
Many studies have attempted to evaluate or develop a prognostic scoring system for predicting the risk of decom
The ALBI score was recently created and validated to specifically assess hepatocellular carcinoma (HCC) liver functional reserve for predicting survival of HCC patients receiving various treatment modalities[16]. The ALBI grade was calculated using albumin and bilirubin levels. Its application has been increasingly expanded to chronic liver disease in general and has proven remarkably accurate in terms of prognosis[17]. Many publications have shown that the ALBI score is highly prognostic in cirrhotic patients and has shown the ability to correlate to HVPG levels[18], predicting the presence of gastroesophageal varices and stratifying bleeding risk[19], and severe portopulmonary hypertension[20].
Since the utility of ALBI score in predicting decompensation risk in patients with compensated cirrhosis has yet been fully investigated, we aimed to evaluate the ALBI score’s ability to identify decompensation risk at 3 years follow-up in patients with compensated cirrhosis.
Patients with compensated cirrhosis receiving care at King Chulalongkorn Memorial Hospital from January 2016 to December 2020 were enrolled retrospectively. The diagnosis of cirrhosis was made by imaging with ultrasonography, multiphasic contrast-enhanced computed tomography, or gadoxetic acid-enhanced magnetic resonance imaging. Patients with missing data, HCC at baseline, or a history of hepatic decompensation at the time of diagnosis were excluded. Baseline characteristics of patients including age, sex, and etiologies of cirrhosis were obtained from medical records. Laboratory data including serum creatinine, albumin, bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), platelets, and international normalized ratio (INR) were collected.
Decompensation events, defined as ascites development, variceal bleeding, or grade 3 or 4 hepatic encephalopathy, were ascertained by clinicians in charge of their care and supported with either endoscopy reports, abdominal imaging, or medical reports. Time to decompensation was calculated from date of study entry until date of first recorded decom
The ALBI score at baseline was calculated using the equation (log10 bilirubin in µmol/L × 0.66) + [albumin in g/L × (-0.085)][16] and validated to categorize decompensation risk into low-, middle-, and high-risk groups classified by ALBI grades 1, 2, and 3. The cut points of ALBI grades were similar to those in HCC patients: ALBI grade 1: ≤ -2.60; grade 2: > -2.60 but ≤ -1.39; grade 3: > -1.39.
For baseline characteristics, continuous variables with a normal distribution are presented as the mean ± SD, while those with a non-normal distribution are presented as median and interquartile range (IQR). The Mann-Whitney U test was used to compare differences in continuous variables while Fisher’s exact test was used to assess for significant differences in binomial variables. Time to decompensation according to baseline ALBI grade and overall survival following the first decompensation were examined by Kaplan-Meier graphs and compared using the log-rank test. Cox proportional hazards analysis was used to identify ALBI score and other potential factors associated with decompensation. Significant factors identified in the univariate analysis were included in the multivariate analysis. The odds ratio (OR) calculated by logistic regression analysis provided estimates of the change in decompensation odds at each ALBI grade at baseline. The time-dependent area under the curve (tAUC) was estimated to evaluate the ability of each prognostic score to predict decompensation. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics version 22.
A total of 123 compensated cirrhotic patients were enrolled in our study. Table 1 summarizes their baseline characteristics. Mean age was 63.9 years (SD: 12.3), and 72 (58.5%) patients were male. Mean body mass index was 24.5 kg/m2 (SD: 3.7). Viral hepatitis B was the most common etiology of cirrhosis (n = 43, 35%), followed by viral hepatitis C (n = 30, 24.4%), nonalcoholic steatohepatitis (NASH) (n = 29, 23.6%), alcohol liver disease (n = 19, 15.4%), and autoimmune hepatitis (n = 2, 1.6%). All patients with viral hepatitis B or viral hepatitis C received antiviral treatment with a sustained virological response. For patients with NASH, 17 (58.6%) patients had diabetes mellitus and 15 (51.7%) patients were obese. At baseline, 113 (91.9%) patients had Child-Pugh class A with 91 (80.5%) and 22 (19.5%) patients having a Child-Pugh score of 5 and 6, respectively, and 10 (8.1%) had Child-Pugh class B. For ALBI grade at baseline, 64 (52%) patients had ALBI grade 1, 53 (43.1%) had ALBI grade 2, and 6 (4.9%) had ALBI grade 3. Median prognostic scores predicting first decompensation at baseline were: MELD score (8.7; IQR: 7.8-10.1), ALBI score (-2.63; IQR: -2.91 to -2.06), ALBI-FIB4 score (-2.79; IQR: -3.28 to -1.93), and FIB-4 score (3.2; IQR: 1.8-5.3).
| Variable | n = 123 |
| Age, yr, mean (SD) | 63.9 (12.3) |
| Male | 72 (58.5) |
| Body mass index, kg/m2, mean (SD) | 24.5 (3.7) |
| Obesity | 54 (43.9) |
| Diabetes | 33 (26.8) |
| Etiology of disease | |
| HBV | 43 (35) |
| HCV | 30 (24.4) |
| NASH | 29 (23.6) |
| Alcohol | 19 (15.4) |
| Autoimmune hepatitis | 2 (1.6) |
| Laboratory data | |
| Creatinine, mg/dL, median (IQR) | 0.8 (0.7-0.9) |
| Albumin, g/dL, median (IQR) | 4 (3.4-4.3) |
| Bilirubin, mg/dL, median (IQR) | 0.9 (0.6-1.5) |
| AST, U/L, median (IQR) | 44 (28-66) |
| ALT, U/L, median (IQR) | 33 (24-58) |
| Platelets, × 109/L, median (IQR) | 142 (104-200) |
| INR, median (IQR) | 1.1 (1-1.2) |
| Child-Pugh grade | |
| A | 113 (91.9) |
| B | 10 (8.1) |
| Decompensation event | 17 (13.8) |
| Variceal bleeding | 8 (47) |
| Ascites development | 5 (29.4) |
| Grade 3 or 4 hepatic encephalopathy | 4 (23.6) |
| MELD, median (IQR) | 8.7 (7.8-10.1) |
| ALBI score, median (IQR) | -2.63 (-2.91 to -2.06) |
| ALBI grade | |
| 1 | 64 (52) |
| 2 | 53 (43.1) |
| 3 | 6 (4.9) |
| ALBI-FIB4 score, median (IQR) | -2.79 (-3.28 to -1.93) |
| FIB-4 score, median (IQR) | 3.2 (1.8-5.3) |
During a median follow-up of 36 (IQR: 35-36) mo, 17 (13.8%) patients developed an initial decompensation event within 3 years follow-up at a median time of 25 [95% confidence interval (CI): 17-31] mo. Events included variceal bleeding in eight (47%) patients, ascites in five (29.4%), and grade 3 or 4 hepatic encephalopathy in four (23.6%). Among the 17 patients who experienced decompensating events, the most common precipitants of hepatic decompensation were gastrointestinal bleeding (n = 8, 47%), followed by infection (n = 1, 6%). However, in eight (47%) of the patients who developed decompensating events, no specific cause of decompensation could be identified. The eight patients who experienced variceal bleeding received a combination of endoscopic treatment, intravenous octreotide, and antibiotic prophylaxis. Treatment for ascites in the five affected patients involved a combination of spironolactone and furosemide, with one patient requiring abdominal paracentesis due to tense ascites. All the four patients with grade 3 or 4 hepatic encephalopathy were treated with lactulose. Additionally, the patient who experienced decompensation due to infection received intravenous antibiotic therapy. Overall survival following the first decompensation was 82.4% at 3 years. The median overall survival of patients who developed the first decompensation was 29.9 (95%CI: 23.7-36.0) mo.
In compensated cirrhotic patients who developed an initial decompensation, albumin, bilirubin, ALT, ALBI, MELD, ALBI-FIB4, and Child-Pugh scores were found to be associated with initial decompensation, with an hazard ratio (HR) of 0.10 (95%CI: 0.03-0.26, P < 0.001), 1.21 (95%CI: 1.02-1.43, P = 0.02), 1.01 (95%CI: 1.00-1.02, P = 0.01), 8.31 (95%CI: 3.48-19.85, P < 0.001), 1.11 (95%CI: 1.02-1.21, P = 0.01), 2.30 (95%CI: 1.60-3.31, P < 0.001), and 1.98 (95%CI: 1.15-3.39, P = 0.01), respec
| Variable | Univariate | Multivariate | ||
| Hazard ratio (95%CI) | P value | Adjusted hazard ratio (95%CI) | P value | |
| Age | 1.01 (0.97-1.05) | 0.47 | 1.01 (0.97-1.06) | 0.56 |
| Male | 0.58 (0.22-1.50) | 0.26 | 0.55 (0.18-1.69) | 0.29 |
| Creatinine | 1.20 (0.83-1.74) | 0.31 | ||
| Albumin | 0.10 (0.03-0.26) | < 0.001 | ||
| Bilirubin | 1.21 (1.02-1.43) | 0.02 | ||
| AST | 1.00 (0.99-1.00) | 0.34 | ||
| ALT | 1.01 (1.00-1.02) | 0.01 | ||
| Platelets | 0.99 (0.98-1.00) | 0.27 | ||
| INR | 1.58 (0.11-22.29) | 0.73 | ||
| ALBI score | 8.31 (3.48-19.85) | < 0.001 | 4.18 (1.40-12.53) | 0.01 |
| MELD score | 1.11 (1.02-1.21) | 0.01 | 1.07 (0.92-1.24) | 0.34 |
| ALBI-FIB4 score | 2.30 (1.60-3.31) | < 0.001 | 1.73 (0.82-3.64) | 0.15 |
| FIB-4 score | 1.02 (0.92-1.13) | 0.67 | ||
| Child-Pugh score | 1.98 (1.15-3.39) | 0.01 | 1.26 (0.61-2.58) | 0.54 |
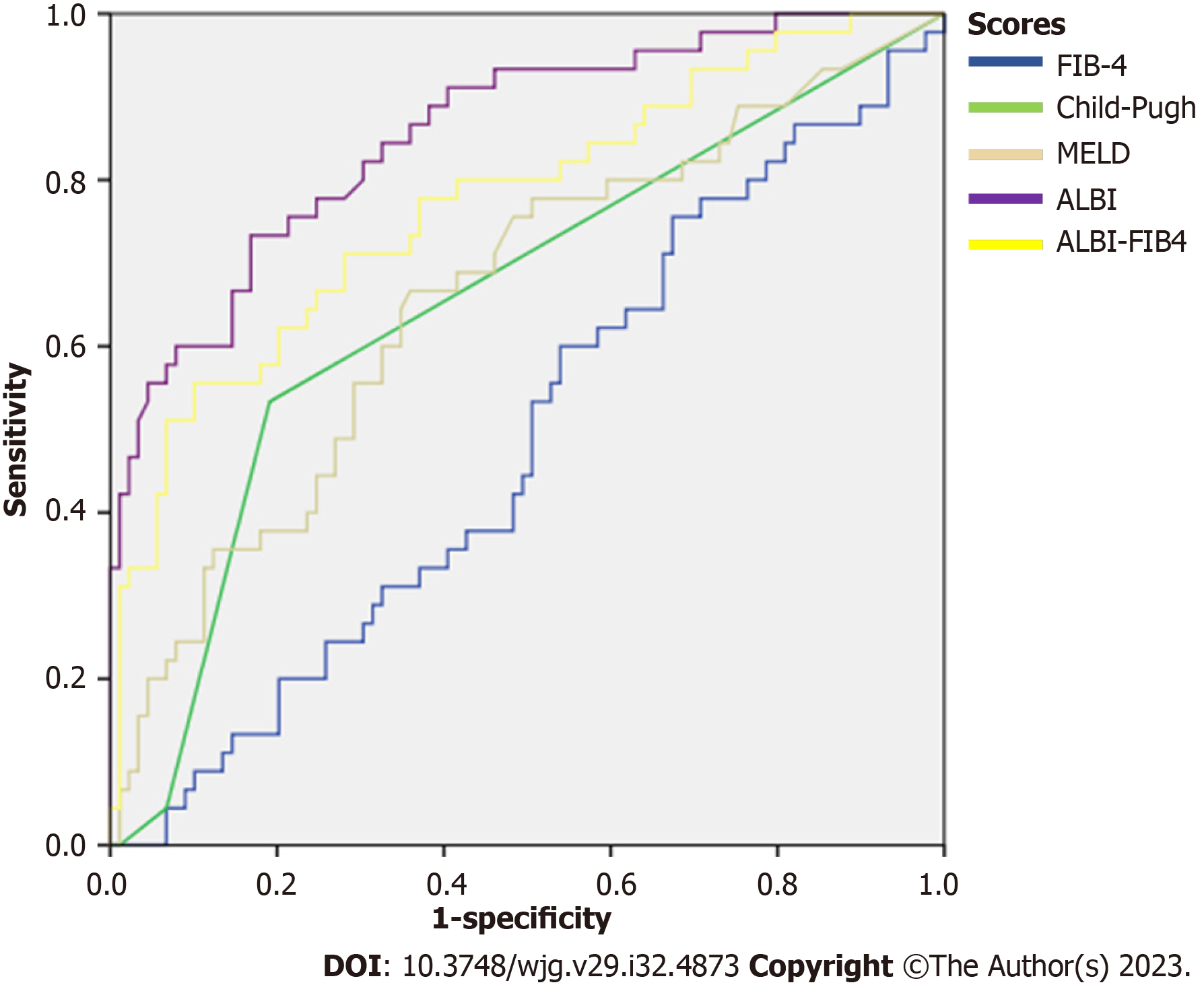
An analysis of decompensation risk at the 3-year follow-up demonstrated that ALBI score had an tAUC of 0.86 (95%CI: 0.78-0.92), which performed significantly better than ALBI-FIB4 (tAUC = 0.77), MELD (tAUC = 0.66), Child-Pugh (tAUC = 0.65), or FIB-4 scores (tAUC = 0.48) (P < 0.05 for all) (Table 3 and Figure 1).
| Prognostic score | tAUC | P value vs ALBI score |
| ALBI | 0.86 (0.78-0.92) | Reference |
| MELD | 0.66 (0.56-0.75) | < 0.001 |
| ALBI-FIB4 | 0.77 (0.68-0.86) | 0.04 |
| FIB-4 | 0.48 (0.38-0.58) | < 0.001 |
| Child-Pugh | 0.65 (0.55-0.75) | < 0.001 |
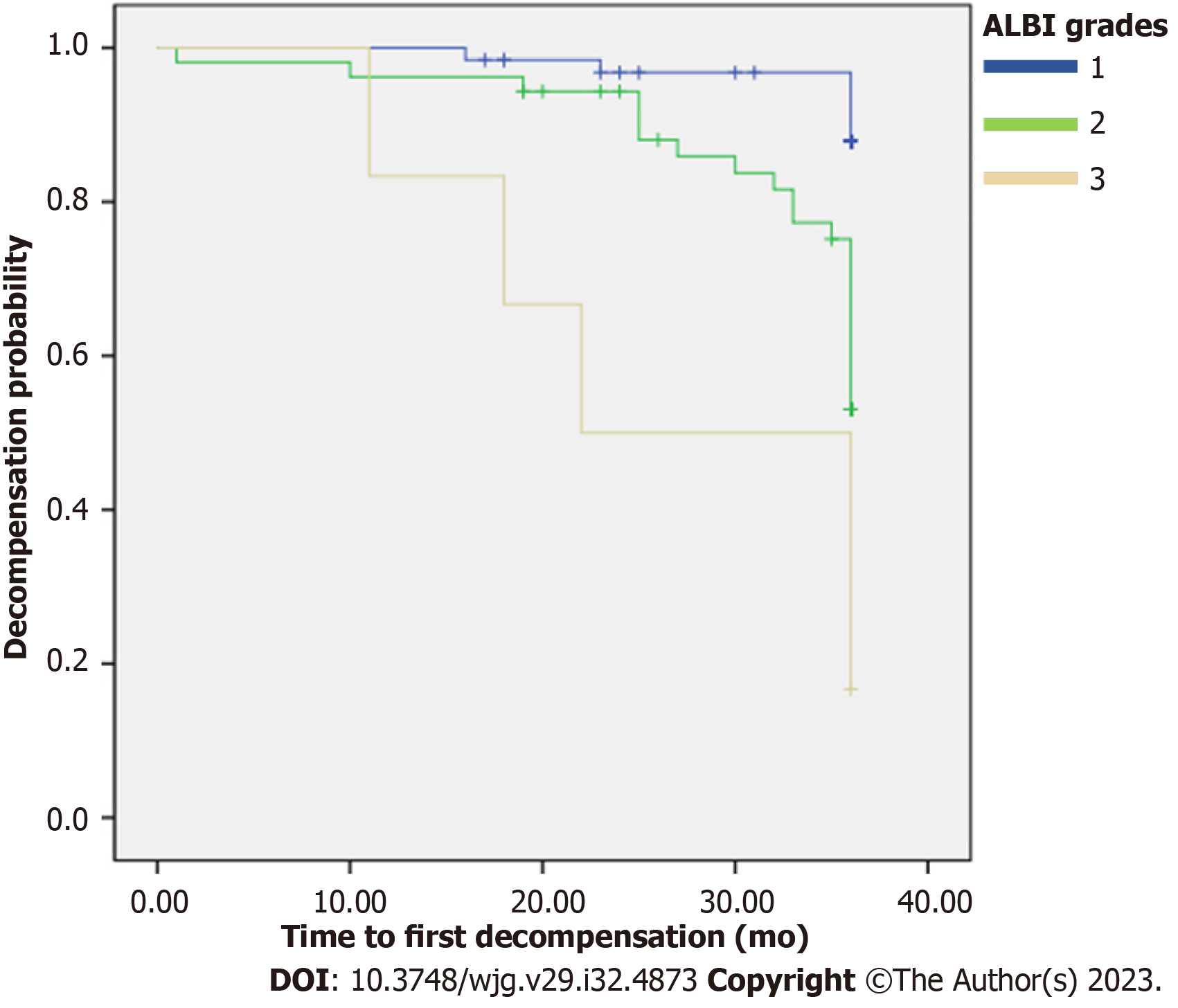
In patients who developed a decompensation event, the majority were in the middle-risk group (n = 12, 70.6%), two in low-risk group (11.8%), and three in high-risk group (17.6%) according to the ALBI grade (Table 4). Median baseline ALBI score in the decompensated cirrhosis group was significantly higher than that of the compensated cirrhosis group [-2.768 (-2.956 to -2.453) vs -2.007 (-2.533 to -1.537), P = 0.01]. The cumulative incidence of decompensation at 3 years was 3.1% in the low-risk group, 22.6% in the middle-risk group, and 50% in the high-risk group (P = 0.003 and P < 0.001, respectively) (Table 4). The OR for decompensation in patients in the high-risk and middle-risk groups was 23.33 (95%CI: 3.88-140.12, P = 0.001) and 7.83 (95%CI: 1.75-35.01, P = 0.007), respectively (Table 4). Patients in the high-risk group exhibited a significantly shorter time to the initial decompensation compared to those in both the middle-risk and low-risk groups [26.5 mo (95%CI: 18.5-34.5), 33.2 mo (95%CI: 31.5-35.1), and 35.5 mo (95%CI: 34.7-36.2), respectively (P < 0.001)] (Figure 2).
| ALBI grade | Decompensation at 3-yr (n, %) | P value | OR (95%CI) | P value |
| 1 | 2/64 (3.1) | - | 1.0 (reference) | - |
| 2 | 12/53 (22.6) | 0.003 | 7.83 (1.75-35.01) | 0.007 |
| 3 | 3/6 (50) | < 0.001 | 23.33 (3.88-140.12) | 0.001 |
Regarding the etiology of liver disease within each decompensation risk group, viral hepatitis was found in a significantly higher number of patients within the low-risk group compared to the middle and high-risk groups, with 45 (61%), 24 (32.9%), and 4 (5.5%) patients, respectively (P = 0.02). However, there was no statistically significant difference in the prevalence of NASH, alcohol-related liver disease, or autoimmune hepatitis among the decompensation risk groups.
This study validated the ALBI score as an accurate prognostic tool to stratify patients with compensated cirrhosis for the risk of decompensation at the 3-year follow-up. The ALBI grade identified high-risk patients more effectively than either MELD, Child-Pugh, ALBI-FIB4, or FIB-4 score.
Novel scoring systems have been developed for prognostic stratification risk of decompensation among patients with compensated cirrhosis over a medium- or long-term follow-up period. One novel scoring system focusing on liver stiffness and measured by transient elastography, presence of gastroesophageal varices from endoscopic screening, albumin, and platelets, has shown excellent accuracy in predicting risk of decompensation at the 3-year follow-up with a tAUC of 0.89. This performance was significantly higher than that of ALBI-FIB-4, Baveno VII criteria, or MELD score. ALBI grade score maintained a tAUC of over 0.8 throughout the 5-year follow-up period[15]. Other novel scoring systems, which consisted of simple and routinely performed serum marker-based scores such as AST, ALT, albumin, bilirubin, and platelets, have also shown an effective ability to identify high-risk patients for the risk of decompensation. The tAUC ranged from 0.69-0.80 using these scoring systems, which was significantly higher than that of the MELD or Child-Pugh score[14,21].
The ALBI score was recently developed to assess liver functional reserve and prognosis among HCC patients[16]. It offers a simple, evidence-based, objective, and discriminatory method that has been extensively tested with an international cohort and enables more detailed prognostic classification than the Child-Pugh grade[16]. Due to the fact that ALBI is simple to calculate needing only albumin and bilirubin measures, application of ALBI has been increasingly extended to other chronic liver diseases including decompensation for liver cirrhosis[17]. Several studies reported that ALBI might be comparable to MELD for predicting short-term mortality, but better than MELD in predicting longer-term mortality in patients with decompensated cirrhosis[22-25]. Recently, one study that evaluated the correlation between the ALBI score and portal pressure in cirrhotic patients showed that ALBI had a better correlation with HVPG compared to MELD, Child-Pugh, FIB-4, and aminotransferase/platelet ratio index scores with a tAUC of 0.72 (P < 0.001)[18]. This study also showed that ALBI grade 3 was able to predict early mortality in patients with a MELD score lower than 14. Based on the pathophysiology of decompensated cirrhosis, which involves an elevation in portal pressure, it has been observed that when the HVPG surpasses 10 mmHg, it correlates with the occurrence of decompensation[26], Therefore, the ALBI score exhibits potential in predicting decompensation by virtue of its correlation with HVPG. However, this study had a higher median MELD score at enrollment than our study (13 vs 8.7). By using ALBI grade 3 to predict decompensation stemming from increases in portal pressure, our study may need more patients with a higher MELD score at enrollment to evaluate the performance of ALBI to predict decompensation due to an increase in HVPG.
Our study found that the odds of decompensation in patients of the high-risk group was 23.33 times higher compared to patients in the lower risk group. The small sample size of the high-risk group and the high dispersion of ALBI score causes the precision of the OR in our study to be low. Thus, we need a greater number of high-risk patients for quanti
Although our study cohort was enrolled at a single-centered tertiary care hospital in Thailand, baseline characteristics of our patients were similar to those of cohorts used to validate other newly developed scoring systems in different countries and continents. In a cohort comprised of an Asian population[15], the most common etiology of cirrhosis was viral hepatitis B at 37.1% compared to 35% in our study cohort. Baseline MELD score and Child-Pugh score in our study cohort were similar to those of cohorts used to validate other scoring systems, where 90% of patients had Child-Pugh class A with a median MELD score ranging from 7-9[14,15,21]. The rate of decompensation in our study cohort, at 13.8%, was found to be lower compared to those of other cohorts utilizing different scoring systems, where the decompensation rates ranged between 19.3% and 26.9%[14,15,21]. This discrepancy in decompensation rates could potentially be attri
Viral hepatitis accounted for 59% of patients in our cohort, all of whom received antiviral treatment resulting in a sustained virological response. Among patients with viral hepatitis, 70.3% were classified as belonging to the low-risk group. We observed a significant increase in the number of patients with viral hepatitis in the low-risk group compared to the middle and high-risk groups (P = 0.02). Consequently, 52% (n = 64) of patients in our cohort were categorized as belonging to the low-risk group, while only 4.9% (n = 6) were classified as high-risk. This distribution can primarily be attributed to the prevalence of viral hepatitis as the underlying etiology of liver disease in our study population.
The strength of this study was that we provided the first evidence that the ALBI score accurately identified decompensation risk at the 3-year follow-up in patients with compensated cirrhosis. The ALBI score is a useful tool to help select high-risk patients to guide treatment to reduce the risk of decompensation. This study represents the ability of the ALBI score to assess liver function and liver disease progression with the advantage of being simple to calculate using only serum albumin and bilirubin levels.
This study has several limitations. First, the cohort in our study was retrospectively completely only at a single-center tertiary care hospital in Thailand. A large multi-center prospective cohort study is required to validate the ALBI score. Second, most of patients had Child-Pugh class A, suggesting that the number of patients with decompensated cirrhosis is relatively low. Thus, our findings may not be readily applicable to a population predominantly with advanced cirrhosis. Third, comparisons to other novel scoring systems that require predictors besides laboratory variables such as transient elastography and gastroesophageal varices from endoscopic findings, could not be performed due to the lack of this information in our study cohort. Inclusion of patients with prompt predictor variables to validate is required. Finally, additional data of the ALBI score including changes in annual ALBI grading or changing of ALBI grades between compensation and decompensation may give new information for the prediction of a decompensation event.
This study has documented the excellent performance of the ALBI score to accurately identify decompensation risk at the 3-year follow-up in patients with compensated cirrhosis. The ALBI score is a simple and ready-to-use tool to help cli
The albumin-bilirubin (ALBI) score is an index of liver function recently developed to assess prognosis in patients with hepatocellular carcinoma (HCC). It has been successfully applied to the prediction of survival in patients with non-malignant liver diseases of various etiologies.
The utility of ALBI score in predicting decompensation risk in patients with compensated cirrhosis has yet been fully investigated.
The objective of this study was to investigate the ALBI score for identifying decompensation risk at the 3-year follow-up in patients with compensated cirrhosis.
One-hundred and twenty-three patients with compensated cirrhosis without HCC in King Chulalongkorn Memorial Hospital diagnosed by imaging were retrospectively enrolled from January 2016 to December 2020. The ALBI score was calculated and validated to classify decompensation risk into low-, middle-, and high-risk groups using three ALBI grade ranges (ALBI grade 1: ≤ -2.60; grade 2: > -2.60 but ≤ -1.39; grade 3: > -1.39). Decompensation events were defined as ascites development, variceal bleeding, or grade 3 or 4 hepatic encephalopathy.
Among 123 cirrhotic patients enrolled, 13.8% (n = 17) developed decompensating events at a median time of 25 [95% confidence interval (CI): 17-31] mo. Analysis of decompensation risk at 3 years showed that ALBI score had a time-dependent area under the curve (tAUC) of 0.86 (95%CI: 0.78-0.92) which was significantly better than that of ALBI-Fibrosis-4 (ALBI-FIB4) score (tAUC = 0.77), model for end-stage liver disease score (tAUC = 0.66), Child-Pugh score (tAUC = 0.65), or FIB-4 score (tAUC = 0.48) (P < 0.05 for all). The 3-year cumulative incidence of decompensation was 3.1%, 22.6% and 50% in the low-, middle-, and high-risk groups, respectively (P < 0.001). The odds ratio for decom
The ALBI score accurately identifies decompensation risk at the 3-year follow-up in patients with compensated cirrhosis. Those patients with a high-risk grade of ALBI score showed a 23 times greater odds of decompensation.
The ALBI score represents an outstanding non-invasive scoring system, enabling clinicians to make precise decisions regarding the monitoring and guidance of treatment for patients with compensated cirrhosis.
This work was supported by funding from the Fatty Liver Research Grant, Division of Gastroenterology, Faculty of Medicine Foundation, Chulalongkorn University. We would like to thank all the clinical and research staff from the Division of Gastroenterology, King Chulalongkorn Memorial Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Grgurevic I, Croatia; Gupta R, India; Reshetnyak VI, Russia; Yang XR, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Cheemerla S, Balakrishnan M. Global Epidemiology of Chronic Liver Disease. Clin Liver Dis (Hoboken). 2021;17:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 320] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 2. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 856] [Article Influence: 214.0] [Reference Citation Analysis (1)] |
| 3. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2133] [Article Influence: 112.3] [Reference Citation Analysis (3)] |
| 4. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 5. | Samonakis DN, Koulentaki M, Coucoutsi C, Augoustaki A, Baritaki C, Digenakis E, Papiamonis N, Fragaki M, Matrella E, Tzardi M, Kouroumalis EA. Clinical outcomes of compensated and decompensated cirrhosis: A long term study. World J Hepatol. 2014;6:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 810] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 7. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1935] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 8. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1488] [Article Influence: 496.0] [Reference Citation Analysis (2)] |
| 9. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 10. | Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 219] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 12. | Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Yu Q, Xu C, Li Q, Ding Z, Lv Y, Liu C, Huang Y, Zhou J, Huang S, Xia C, Meng X, Lu C, Li Y, Tang T, Wang Y, Song Y, Qi X, Ye J, Ju S. Spleen volume-based non-invasive tool for predicting hepatic decompensation in people with compensated cirrhosis (CHESS1701). JHEP Rep. 2022;4:100575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 14. | Guha IN, Harris R, Berhane S, Dillon A, Coffey L, James MW, Cucchetti A, Harman DJ, Aithal GP, Elshaarawy O, Waked I, Stewart S, Johnson PJ. Validation of a Model for Identification of Patients With Compensated Cirrhosis at High Risk of Decompensation. Clin Gastroenterol Hepatol. 2019;17:2330-2338.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Liu C, Cao Z, Yan H, Wong YJ, Xie Q, Hirooka M, Enomoto H, Kim TH, Hanafy AS, Liu Y, Huang Y, Li X, Kang N, Koizumi Y, Hiasa Y, Nishimura T, Iijima H, Jung YK, Yim HJ, Guo Y, Zhang L, Ma J, Kumar M, Jindal A, Teh KB, Sarin SK, Qi X. A Novel SAVE Score to Stratify Decompensation Risk in Compensated Advanced Chronic Liver Disease (CHESS2102): An International Multicenter Cohort Study. Am J Gastroenterol. 2022;117:1605-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2012] [Article Influence: 201.2] [Reference Citation Analysis (0)] |
| 17. | Toyoda H, Johnson PJ. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022;4:100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (1)] |
| 18. | Hsieh YC, Lee KC, Wang YW, Yang YY, Hou MC, Huo TI, Lin HC. Correlation and prognostic accuracy between noninvasive liver fibrosismarkers and portal pressure in cirrhosis: Role of ALBI score. PLoS One. 2018;13:e0208903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Miyamoto Y, Enomoto H, Nishikawa H, Nishimura T, Iwata Y, Nishiguchi S, Iijima H. Association of the Modified ALBI Grade With Endoscopic Findings of Gastroesophageal Varices. In Vivo. 2021;35:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Kawaguchi T, Honda A, Sugiyama Y, Nakano D, Tsutsumi T, Tahara N, Torimura T, Fukumoto Y. Association between the albumin-bilirubin (ALBI) score and severity of portopulmonary hypertension (PoPH): A data-mining analysis. Hepatol Res. 2021;51:1207-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Schneider ARP, Schneider CV, Schneider KM, Baier V, Schaper S, Diedrich C, Coboeken K, Mayer H, Gu W, Trebicka J, Blank LM, Burghaus R, Lippert J, Rader DJ, Thaiss CA, Schlender JF, Trautwein C, Kuepfer L. Early prediction of decompensation (EPOD) score: Non-invasive determination of cirrhosis decompensation risk. Liver Int. 2022;42:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Zou D, Qi X, Zhu C, Ning Z, Hou F, Zhao J, Peng Y, Li J, Deng H, Guo X. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Fragaki M, Sifaki-Pistolla D, Orfanoudaki E, Kouroumalis E. Comparative evaluation of ALBI, MELD, and Child-Pugh scores in prognosis of cirrhosis: is ALBI the new alternative? Ann Gastroenterol. 2019;32:626-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Oikonomou T, Goulis L, Doumtsis P, Tzoumari T, Akriviadis E, Cholongitas E. ALBI and PALBI Grades Are Associated with the Outcome of Patients with Stable Decompensated Cirrhosis. Ann Hepatol. 2019;18:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 25. | Wan SZ, Nie Y, Zhang Y, Liu C, Zhu X. Assessing the Prognostic Performance of the Child-Pugh, Model for End-Stage Liver Disease, and Albumin-Bilirubin Scores in Patients with Decompensated Cirrhosis: A Large Asian Cohort from Gastroenterology Department. Dis Markers. 2020;2020:5193028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (1)] |