Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4860
Peer-review started: May 31, 2023
First decision: July 8, 2023
Revised: July 15, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: August 28, 2023
Processing time: 86 Days and 4.5 Hours
Resistance to antibiotics is one the main factors constraining the treatment and control of Helicobacter pylori (H. pylori) infections. Therefore, there is an urgent need to develop new antimicrobial agents to replace antibiotics. Our previous study found that linolenic acid-metronidazole (Lla-Met) has a good antibacterial effect against H. pylori, both antibiotic-resistant and sensitive H. pylori. Also, H. pylori does not develop resistance to Lla-Met. Therefore, it could be used for preparing broad-spectrum antibacterial agents. However, since the antibacterial mechanism of Lla-Met is not well understood, we explored this phenomenon in the present study.
To understand the antimicrobial effect of Lla-Met and how this could be applied in treating corresponding infections.
H. pylori cells were treated with the Lla-Met compound, and the effect of the compound on the cell morphology, cell membrane permeability, and oxidation of the bacteria cell was assessed. Meanwhile, the differently expressed genes in H. pylori in response to Lla-Met treatment were identified.
Lla-Met treatment induced several changes in H. pylori cells, including roughening and swelling. In vivo experiments revealed that Lla-Met induced oxidation, DNA fragmentation, and phosphatidylserine ectropionation in H. pylori cells. Inhibiting Lla-Met with L-cysteine abrogated the above phenomena. Transcriptome analysis revealed that Lla-Met treatment up-regulated the expression of superoxide dismutase SodB and MdaB genes, both anti-oxidation-related genes.
Lla-Met kills H. pylori mainly by inducing oxidative stress, DNA damage, phosphatidylserine ectropionation, and changes on cell morphology.
Core Tip: The clarithromycin resistant Helicobacter pylori (H. pylori) is listed by the World Health Organization as the priority bacteria in urgent need of developing new antibiotics. Our previous research found that linolenic acid-metronidazole has a good antibacterial effect on H. pylori and is not easy to develop drug resistance. Therefore, we further explored its antibacterial mechanism against H. pylori. It was found that it mainly kills H. pylori by inducing oxidative stress, DNA damage, phosphatidylserine ectropionation, and changes on cell morphology. This study may provide a theoretical basis for the development and application of new anti H. pylori lead compound.
- Citation: Zhou WT, Dai YY, Liao LJ, Yang SX, Chen H, Huang L, Zhao JL, Huang YQ. Linolenic acid-metronidazole inhibits the growth of Helicobacter pylori through oxidation. World J Gastroenterol 2023; 29(32): 4860-4872
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4860.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4860
Helicobacter pylori (H. pylori) is the main pathogen that causes upper digestive diseases, such as chronic gastritis, peptic ulcer, and gastric cancer[1-4]. At present, the treatment options for H. pylori infections include standard triple therapy, bismuth-containing quadruple therapy, and sequential therapy[5,6]. Due to the overuse and misuse of antibiotics, the drug resistance rate of H. pylori, including multi-drug resistance, is gradually increasing, negatively impacting the control and treatment of H. pylori infections[7-10]. Therefore, there is an urgent need to develop new anti-H. pylori agents[11].
Due to the long period and significant investment required for developing new antibiotics, the transformation or modification of the existing drugs is more efficient in shortening the drug research and development cycle. Modifying existing drugs could improve their efficacy while reducing the development of antimicrobial resistance. Zinc linolenic acid and liposome linolenic acid are linolenic acid derivatives effective at increasing the sensitivity of drug-resistant H. pylori. Resistance against zinc linolenic acid and liposome linolenic acid is minimal[12,13]. Although metronidazole is a widely used and cost-effective drug, its clinical application for H. pylori infection treatment is limited by resistance development.
Our previous study found that the minimum inhibitory concentration (MIC) of linolenic acid-metronidazole (Lla-Met) against six strains of the drug-resistant H. pylori was 2-4 μg/mL. Additionally, the H. pylori strains did not develop resistance against this compound. Therefore, Lla-Met would serve as promising antibiotics. However, its antibacterial mechanism is poorly understood[14], this study explored this mechanism.
H. pylori strain G27 (Courtesy of Prof. Bi Hongkai, Nanjing Medical University), calf serum, a Columbia blood agar base, a brain heart infusion (BHI,OXOID) medium, L-cysteine (L-cys) (AR 99%, MACKLIN), a fluorescence orthomicroscope (OLYMPUS, Tokyo, Japan), reactive oxygen species (ROS) detection kits (Beyotime), cell apoptosis 4’,6-diamidino-2-phenylindole (DAPI) detection kits (Beyotime), apoptosis detection kits (Beyotime), reverse transcription kits (Monad), reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) kits (Monad), a Lightcycler96 fluorescence ration PCR instrument (Roche, Germany), and a scanning electron microscope were used in the present study.
Standard H. pylori strain G27 stored at -80 °C were thawed and centrifuged to remove the preservation solution (Glycerin:BHI:serum = 3:6:1). The bacteria were inoculated on a Columbia agar medium, or a brain heart infusion medium supplemented with 10% calf serum and cultured in a microaerophilic environment.
The effect of the Lla-Met on H. pylori morphology was observed by scanning electron microscopy[15-18]. H. pylori was treated with 4 and 8 μg/mL of Lla-Met and incubated for 24 h in a three-gas incubator. The bacteria were pelleted by centrifugation and fixed overnight with 2.5% glutaraldehyde. The bacteria suspension was centrifuged to remove glutaraldehyde before dehydration with 30%, 50%, 70%, 90%, and 100% ethanol. The pellet was dried through refrigeration in a vacuum. After that, the H. pylori morphology was observed and photographed under a KYKY-EM8100 scanning electron microscope (KYKY, Beijing).
The H. pylori cells were stained as previously described by Hwang et al[19]. Briefly, the G27 bacterial suspension (1 × 108 CFU/mL) at the logarithmic phase was treated with Lla-Met for 2 h at the rate of 16 μg/mL. The cell suspension was centrifuged at 12000 r/min for 2 min to pellet the cells. The medium was poured out, and the harvested cells were suspended in phosphate buffered saline (PBS). The cells were stained with a propidium iodide solution (PI, 10 μg/mL, Thermo Fisher) at 37 °C protected from light 30 min and thereafter centrifuged at 12000 r for 5 min. The dye unbound to the harvested cells was washed away with sterile PBS. Thereafter, the cells were suspended in PBS and immediately observed under a fluorescence microscope (Olympus Corporation, Tokyo, Japan).
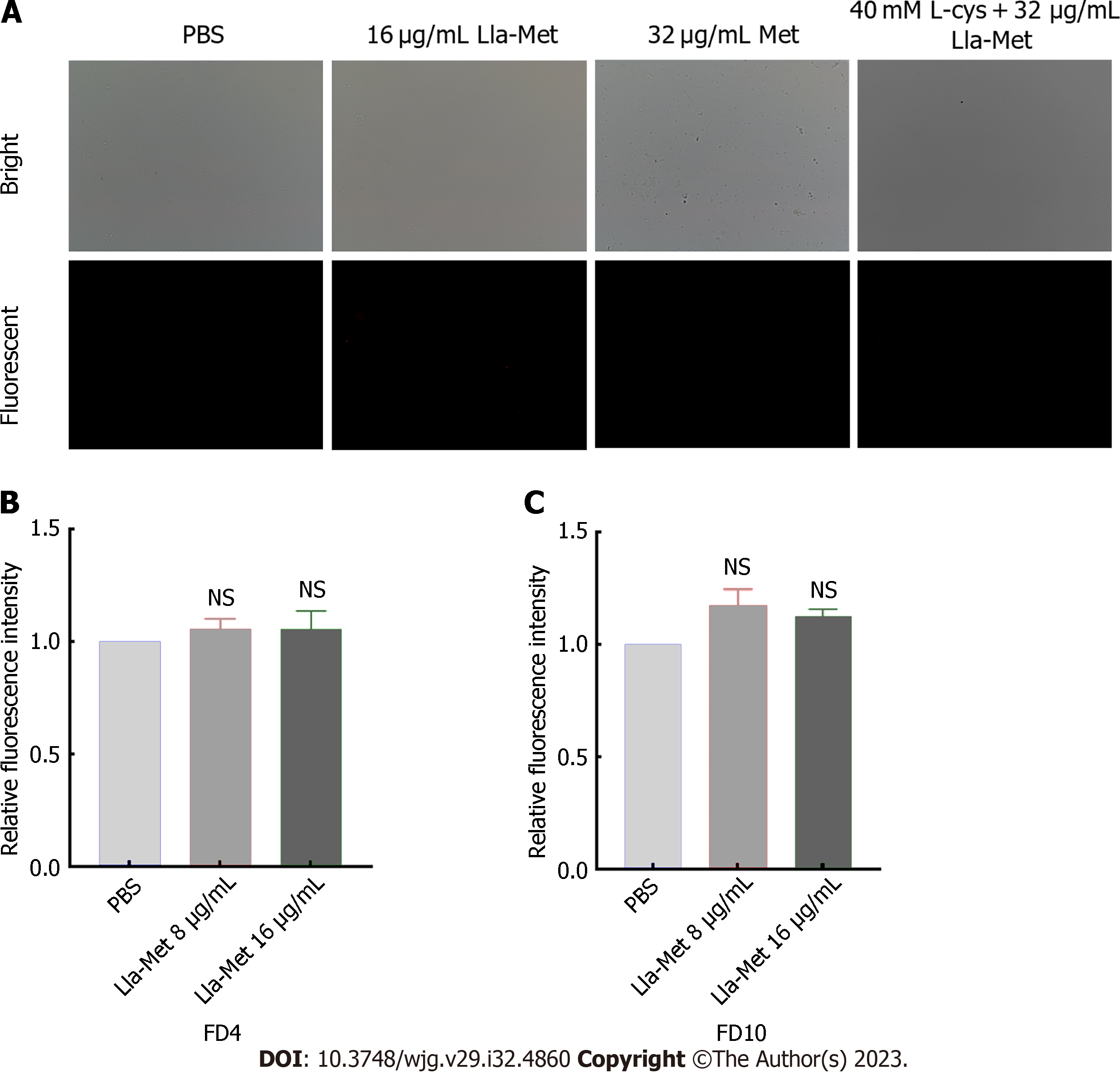
FITC-FD was mainly used to evaluate the degree of H. pylori cell membrane damage after treatment with Lla-Met. The process was performed as previously described[20]. Briefly, G27 bacterial suspension (1 × 108 CFU/mL) at the logarithmic phase was incubated with Lla-Met (16 μg/ML) for 2 h, centrifuged, and the pellet was suspended in sterile PBS. The cell suspension was protected from light with FIFC-labeled glucan FD4, about 4.0 kDa, with a diameter of 1.4 nm (Sigma, United States) and FD10, about 10.1 kDa, with a diameter of 2.3 nm (Sigma, United States), both at a final concentration of 100 μg/mL. After 30 min of incubation at 37 °C, the unbound fluorescent dye was washed off with sterile PBS. The cells were suspended in sterile PBS. Finally, the fluorescence influx of FD4 and FD10 was detected at the excitation and emission wavelengths of 495 nm and 520 nm, respectively, by a multimode reader (BioTek, America).
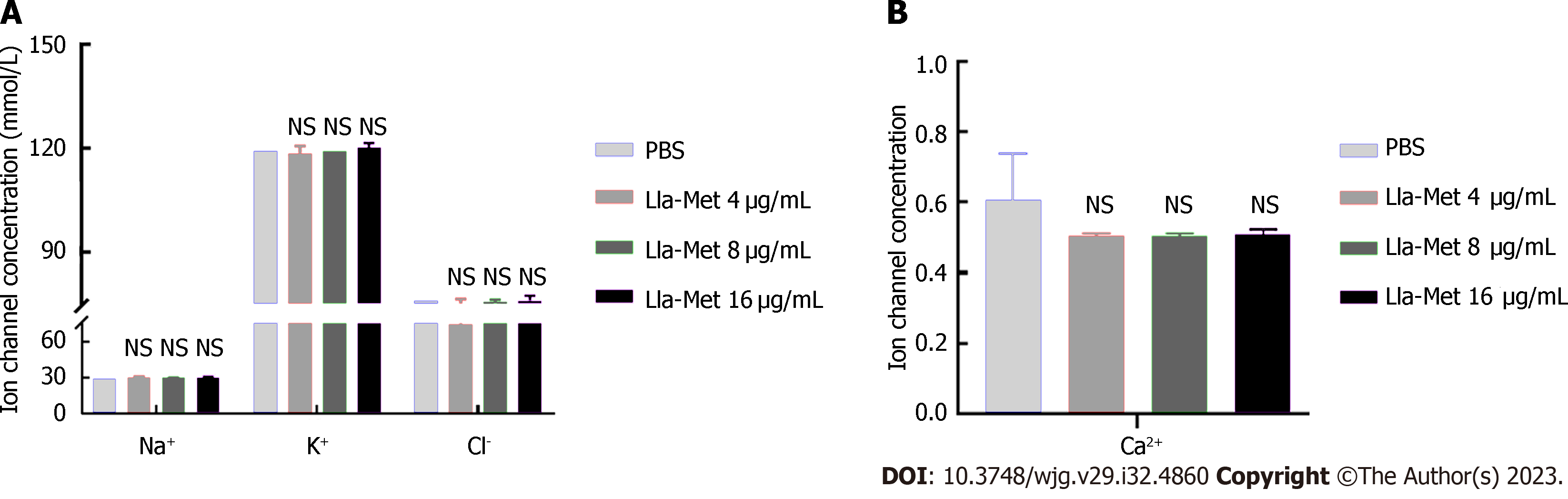
The G27 bacterial suspension (1 × 108 CFU/mL) at the logarithmic phase was incubated with Lla-Met (16 μg/mL) for 2 h and centrifuged at 12000 r/min for 2 min. The changes in concentrations of extracellular K+, Na+, Cl-, and Ca2+ were determined by ion-selective electrodes. Three biological repeats were performed for each experiment.
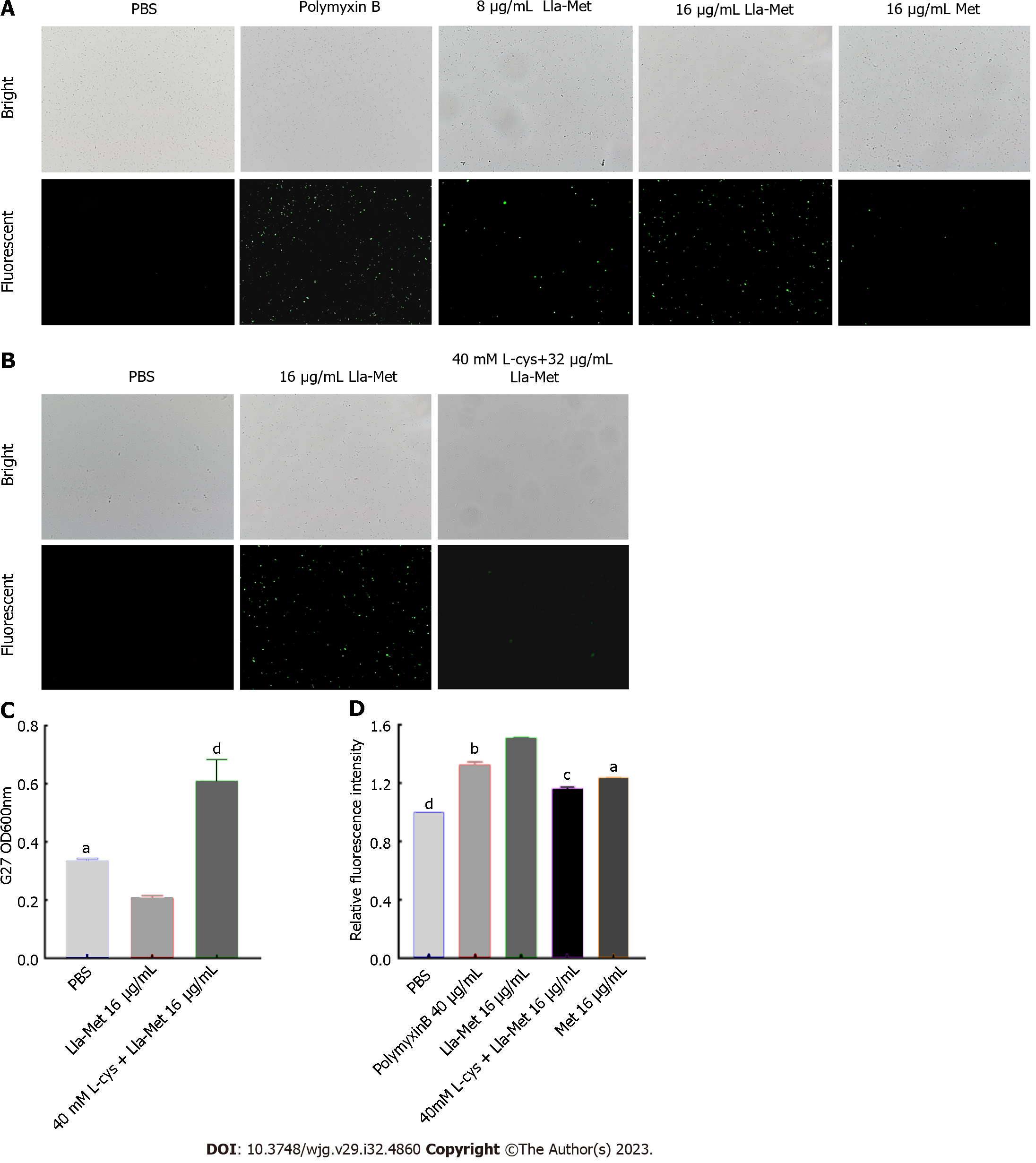
The level of intracellular ROS in the H. pylori cell was detected by the DC-FDH probe, as described by Akhtar et al[21-22]. Briefly, G27 bacterial suspension (1 × 108 CFU/mL) was treated with Lla-Met (8 μg/mL and 16 μg/mL) for 2 h and centrifuged to remove the supernatant. The harvested cells were protected from light DCF-DA (10 μM) for 30 min and centrifuged at 12000 r for 2 min. The excess probe was washed off with sterile PBS. Finally, the cells were resuspended in PBS solution, and the fluorescence intensity was analyzed using a multifunctional microplate reader (BioTek, America) at the excitation and emission wavelengths of 485 nm and 520 nm, respectively. The fluorescence intensity was also analyzed using a fluorescent microscope (OLYMPUS, Tokyo, Japan). PBS and polymyxin were the controls.
The G27 bacterial suspension was incubated with L-cys, a ROS scavenger, to evaluate the effect of ROS on the viability of G27 bacteria. Briefly, G27 bacterial suspension (1 × 108 CFU/mL) at the logarithmic phase was incubated with or without 40 mmol/L L-cys (which did not affect the viability of G27 cells) for 1 h and thereafter with Lla-Met (16 μg/mL) for 8 h. The optical density values were measured at OD600 nm using a multifunctional microplate reader (BioTek, America). In addition, the fluorescence intensity was analyzed using fluorescence microscopy (OLYMPUS, Tokyo, Japan). G27 bacterial suspension treated with or without 40 mmol/L L-cys was incubated for 1 h. The suspension was treated with Lla-Met (16 μg/mL) for 2 h and centrifuged to remove the supernatant. The harvested bacteria were incubated with DCF-DA (10 μM) for 30 min and washed with sterile PBS.
When DAPI passes through the intact cell membrane, it binds to bacterial DNA. The bacterial DNA thus stains blue. Damaged DNA appears as dots. Therefore, the fragmentation of H. pylori DNA after Lla-Met treatment was detected using the DAPI staining[23]. Briefly, the G27 bacterial suspension (1 × 108 CFU/mL) was incubated with or without 40 mmol/L L-cys for 1 h and thereafter with Lla-Met (16 μg/mL) for 2 h. Thereafter, the suspension was centrifuged to remove the supernatant and treated with DAPI (1 μg/mL) for 30 min. The unbound dye was washed off with PBS, and the cells were resuspended in PBS. Fluorescence intensity was determined at excitation and emission wavelengths of 358 nm and 460 nm, respectively (OLYMPUS, Tokyo, Japan), with PBS as a control.
Phosphatidylserine (PS) is usually located on the inner side of the cell membrane. At the early stage of apoptosis, PS is translocated to the cell surface. Annexin-V, a Ca2+-dependent phospholipid binding protein, bind to PS with high affinity[24]. The G27 bacterial suspension in the logarithmic phase (1 × 108 CFU/mL) was incubated with or without 40 mmol/L L-cys for 1 h and thereafter with Lla-Met (16 μg/mL) for 2 h. Thereafter, the suspension was centrifuged to remove the supernatant, and the pellet was incubated with Annexin-V and incubated for 30 min. The unbound dye was washed off with PBS, and the cells were resuspended in PBS. The fluorescence intensity was analyzed at excitation and emission wavelengths of 490 nm and 520 nm, respectively, using a multifunctional microplate reader (BioTek, America) and a fluorescence microscope (OLYMPUS, Tokyo, Japan), with PBS as a control.
G27 bacterial suspension (1 × 108 CFU/mL) (OD600 = 0.3) was incubated with 2, 4, 8, and 10 μg/mL Lla-Met for 0 h, 2 h, and 8 h, and the ODs were measured at 600 nm. Three biological repeats were performed for each experiment. When the OD remained constant (0.3), the bacterial RNA was extracted for transcriptome sequencing, which was performed by Nanjing Fengzi Bio-pharm Technology. Three biological repeats were performed for each experiment.
The sequencing was performed using Illumina PE150 technology. The alignment and transcript assembly were performed using Boetie2 and the Rockhhoper software. All genes were quantitatively analyzed, and the differentially expressed genes (DEGs) were identified. The biological processes and pathways regulated by the DEGs were then identified. Principal component analysis (PCA) demonstrates principal component analysis, analyzing the composition of different samples can respond to the differences and distances between samples, the more similar the sample composition, the closer the distance in the PCA graph.
Total bacterial RNA was extracted using a Novizan RNA kit, and the expression of mRNA was analyzed by a real-time fluorescent quantitative PCR instrument (Lightcycler96 fluorescent quantitative PCR instrument, Roche, Germany). The 16s was used as the reference gene. The sequences of primers used in this study are shown Table 1. Three biological repeats were performed for each experiment.
| Name | Forward primers | Reverse primers |
| 16s | AGGATCAAGGTTTAAGGATT | CTGGAGACTAAGCCCTCC |
| MdaB | AGGCTATGAACACGCTCAAGAAGTG | TTTCACAATCCAAGGCTCTCCCATC |
| SodB | AAGCGACTGCCTTAAGCGATGAG | TCCAGCCAGAGCCAAACAAAGTG |
Statistical analysis was performed using the SPSS software, Version 26.0. Continuous data were expressed as mean ± SD. Differences between groups were analyzed using the t-test, while multiple groups were compared using the single factor variance analysis. P < 0.05 was considered statistically significant.
The impact of the Lla-Met compound on the morphology of H. pylori was observed using scanning electron microscopy. H. pylori in the control group was found to have a smooth and homogenous cell surface (Figures 1A and D). The surface of H. pylori in the treatment group (4 μg/mL and 8 μg/mL) was rough and swollen, and the cell damage worsened with the Lla-Met concentration (Figures 1B, C, E, and F).
PI penetrates through a damaged cell membrane, where it binds and stains the DNA. Therefore, an influx of intracellular PI represents the integrity of the bacterial cell membrane. The fluorescence intensity of PI in the G27 treatment group (16 μg/mL Lla-Met) was weaker than in the control group, though statistically insignificant (Figure 2A).
FITC-labelled glucans of different pore sizes (FD4 and FD10) were detected using a multifunctional microplate detector to examine damage to the H. pylori cell membrane after treatment with Lla-Met. Linolenic acid treatment had no significant effect on the permeability of H. pylori cells (Figures 2B and C).
To further investigate whether Lla-Met compound penetrated the cells via ion channels, the concentrations of K+, Na+, Cl-, and Ca2+ ions in the supernatant were measured after treating H. pylori with Lla-Met. There was no statistically significant difference in the concentrations of the aforementioned ions between the treatment and the control group after G27 was applied with 16 μg/mL compound (Figures 3A and B).
DC-FDA fluorescent probes can be used to detect whether Lla-Met compounds can accelerate intracellular oxidation reactions. Compared with the control group, 8 μg/mL and 16 μg/mL Lla-Met compound increased the intracellular oxidation in H. pylori cell. Moreover, 8 μg/mL Lla-Met was more potent than 16 μg/mL metronidazole (Figure 4A); The relative fluorescence intensity of H. pylori treated with 16 μg/mL Lla-Met was stronger than that treated with 40 μg/mL polymyxin B (P < 0.01, Figure 4D). However, L-cys treatment abrogated the effect of Lla-Met (Figures 4B and D, P < 0.01), implying that L-cys abolished ROS generated by Lla-Met in H. pylori. In addition, L-cys pretreatment increased the cell viability from 20.5% to 57.7% (Figure 4C).
In the early stage of apoptosis, PS translocates to the cell surface, where it could be bound by Ca2+-dependent phospholipid-binding protein. Thus, the phospholipid-binding protein could be used for analyzing cell apoptosis in prokaryotes. In the present study, we found that the fluorescence intensity of H. pylori treated with 16 μg/mL Lla-Met was higher than that of the untreated group and the L-cys pretreatment group (Figure 5A). The multifunctional microplate labelling instrument results showed that the relative fluorescence intensity of Lla-Met-compound treatment group was significantly stronger than that of the PBS group and L-cys pretreatment group (Figure 5C, P < 0.0001). These results indicated that linolenic-acid-metronidazole caused PS eversion, but L-cys pretreatment inhibited this phenomenon.
Subsequently, cellular DNA fragmentation serves as a marker of late apoptosis. The DAPI staining evaluated whether Lla-Met compound could cause the fragmentation of H. pylori DNA. The results showed that 16 μg/mL Lla-Met caused the fragmentation of bacterial H. pylori DNA (Figure 5B red circles represent the fragmented DNA). However, DNA fragmentation was inhibited in the L-cys treatment group (Figure 5D, P < 0.0001). These findings suggested that Lla-Met caused the fragmentation of H. pylori DNA by inducing the accumulation of ROS.
The half inhibitory concentration of Lla-Met was used for the oxidation analysis (Figure 6A). The OD values were unchanged after H. pylori was dosed with 8 μg/mL Lla-Met compound for 0, 4, and 8 h. The RNA-seq data for H. pylori in different treatment groups (Figure 6B). The closer the Pearson correlation coefficient approaches 1, the higher the similarity of events. The PCA is in Figure 6C. The difference and distance between samples are illustrated. The closer the similarity between samples, the closer the distance in the PCA diagram. The Venn diagram, which shows the DEGs in each group. A total of 1130 DEGs were detected between A_M_1 and A_M_2, of which 575 were up-regulated and 555 were down-regulated. A total of 1016 DEGs were detected between A_M_1 and A_M_3, of which 488 were up-regulated, and 528 were down-regulated. A total of 533 DEGs were detected between A_M_2 and A_M_3, including 265 up-regulated genes and 268 down-regulated genes. Among them, 344 genes were co-expressed in A_M_1, A_M_2, and A_M_3 (Figure 6D). The gene set enrichment analysis and Gene Ontology of the DEGs. The DEGs were divided into three main categories: Those that regulate biological processes, secretion of cellular components, and molecular function. The differential genes between the groups are primarily concentrated in tRedox pathways, metabolic processes and other pathways. Gene set enrichment analysis revealed that the DEGs regulated the REDOX and the metabolism pathway (Figure 6E). Lla-Met is inducing production of ROS in H. pylori, and therefore an increased expression of MdaB and SodB, both of which are associated with protection against the oxidative stress (Table 2). RT-qPCR and transcriptome sequence analyses revealed comparable findings (Figure 6F).
| Gene | Name | Log2 fold change | Description | Enrichment pathway |
| MdaB | HPG27_RS03065 | 4.962 | Flavodoxin family protein | Oxidoreductase activity |
| SodB | HPG27_RS05265 | 4.2287 | Superoxide dismutase | Oxidoreductase activity |
Lla-Met compound is synthesized from linolenic acid and metronidazole. Linolenic acid is an essential fatty acid with broad-spectrum antibacterial spectrum and antioxidant activities and capability to overcome H. pylori resistance to antibiotic treatment. In addition, with more functional groups, it can react with various substances to form related derivatives, which are widely used in the anti-infection treatment. Obonyo et al[25] suggested that the antibacterial mechanism of linolenic acid liposome against H. pylori is mainly to cause damage to the bacterial cell membrane. Huang et al[26] used linolenic acid and zinc to synthesize zinc linolenic acid with an MIC of 4-8 μg/mL to drug-resistant H. pylori strains. Its antibacterial mechanism is mainly to destroy cell membrane and cause accumulation of ROS, which finally leads to the death of bacteria. In this experiment, the damage caused by Lla-Met compound to the cell membrane was detected by PI, FD4, FD10 and lactate dehydrogenase activity determination. There was no change in cell membrane permeability after H. pylori was treated with 16 μg/mL Lla-Met compound. This result suggested that Lla-Met compound did not inhibit H. pylori by damaging the cell membrane.
The accumulation of intracellular ROS activates eukaryotic cell apoptosis[27]. This process produces dying cells with typical morphological features, including cell shrinkage, membrane blistering, chromatin condensation, DNA fragmentation, and PS ectropion[28]. Studies have shown that apoptosis also occurs in prokaryotic cells, and is characterized with similar morphological characteristics as those in eukaryotes, such as the destruction of bacterial membrane integrity, DNA fragmentation, and PS ectropion[22,29]. Therefore, in the present study, we investigated whether Lla-Met compound could inhibit H. pylori growth by causing oxidative damage. In the experiment, 8 μg/mL Lla-Met compound was found to produce a stronger fluorescence signal compared with the control group. In addition, at a higher dose of 16 μg/mL Lla-Met, H. pylori produced a stronger fluorescence signal compared with the positive control group. Indicated that Lla-Met compound could increase accumulation of ROS in H. pylori in a dose-and time-dependent manner. This experiment also investigated whether intracellular ROS accumulation could affect H. pylori viability. The results showed that excessive accumulation of ROS could affect viability of H. pylori by reducing it to 20.5%, which increased to 57.7% when H. pylori was treated with 40 mmol/L L-cys. Interestingly, the accumulation of intracellular ROS was also found to significantly decrease after H. pylori was treated with L-cys. This result suggested that Lla-Met compound could cause excessive accumulation of intracellular ROS, leading to a decrease in cell viability, and that ROS accumulation could be reversed by L-cys treatment. In addition, after treatment with Lla-Met compound at different concentrations for 24 h, H. pylori surface became rough and swollen compared with the control group. As previously demonstrated, Lla-Met compound caused no damage to the cell membrane of H. pylori. This suggested that the death of H. pylori was due to the accumulation of intracellular ROS caused by Lla-Met compound.
Oxidation can cause both prokaryotic and eukaryotic cell death. In the present study, treatment with 16 μg/mL Lla-Met for 2 h was found to cause H. pylori DNA fragmentation and PS ectropion. Meanwhile, these effects were found to be reversed after H. pylori was treated with L-cys. DNA damage and membrane depolarization are characteristic changes in eukaryotic cell apoptosis[30]. Our experimental results showed that H. pylori cell death is similar to eukaryotic apoptosis, and ROS accumulation could induce prokaryotic cell-like death. However, compared with other studies, significant damage to the cell membrane was not found in the present study. This result indicated that damage to the integrity of the cell membrane might not be as necessary in the apoptosis of prokaryotic cells as slight DNA fragmentation and PS ectropion.
These results indicated that Lla-Met compound can promote intracellular ROS-generation reaction in H. pylori and effectively inhibit its growth. However, since several enzymes are involved in ROS-generating reaction, we used RT-qRCR to identify and verify key enzymes involved in this process and detect transcriptome changes. The results revealed that superoxide dismutase MdaB and SodB genes were found to play an important role. Under normal circumstances, the intracellular oxidative system and antioxidant system are in a dynamic balance. However, after treatment of with linolonic acid-metronidazole compound, superoxide dismutase MdaB and SodB genes were found to be highly expressed in H. pylori, and intracellular ROS was found to accumulate excessively, thereby damaging DNA and causing PS ectropion.
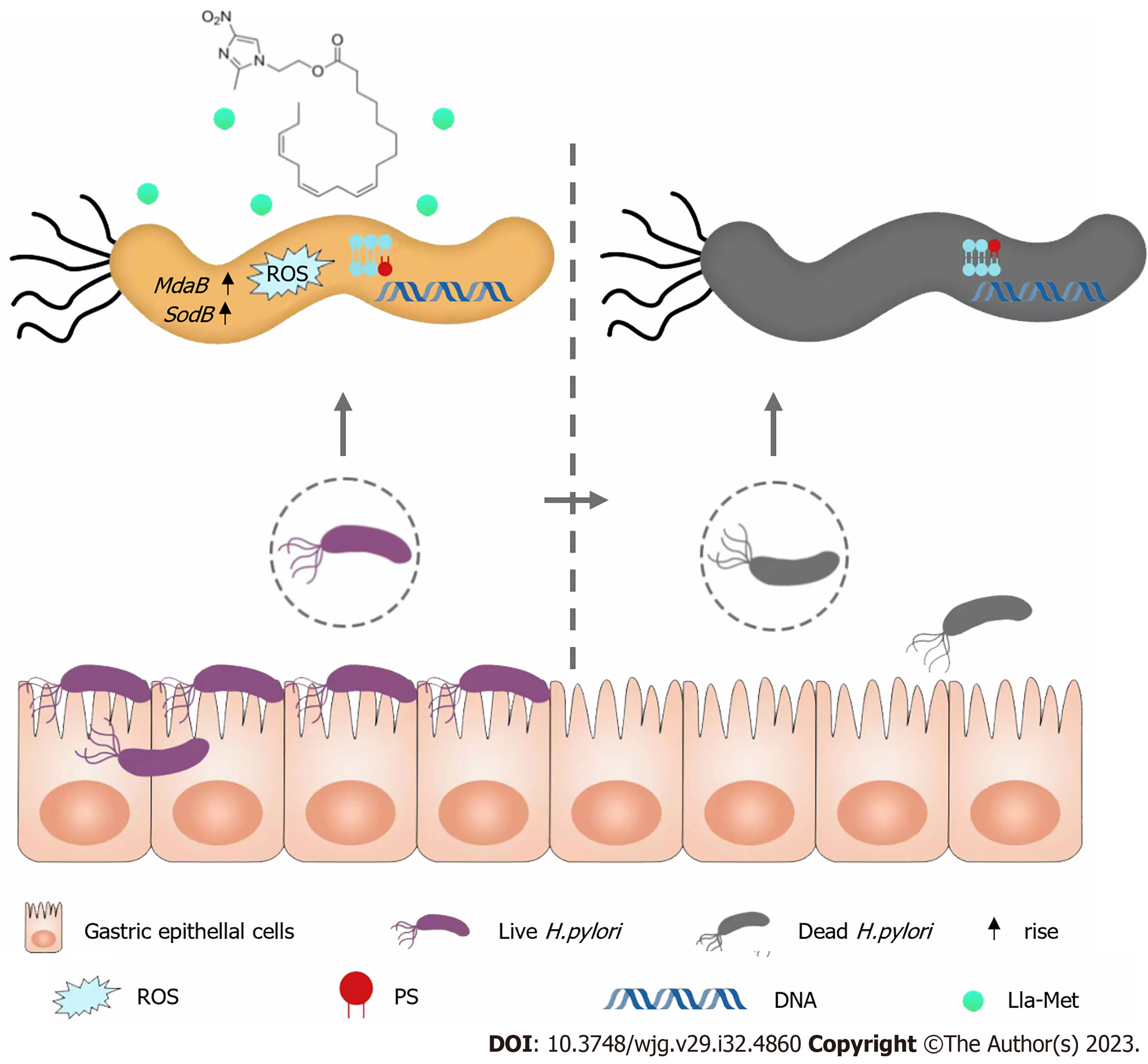
In this paper, the mechanism of linoleic-metronidazole compound was demonstrated to involve inhibiting H. pylori growth by inducing excessive ROS accumulation, resulting in excessive superoxide dismutase MdaB and SodB genes expression (Figure 7). Besides, this study further proves the antibacterial effect of Lla-Met on H. pylori at the molecular level, providing theoretical support for further research and development of Lla-Met as an anti-H. pylori drug to help overcome H. pylori resistance to current antibiotic drugs.
Helicobacter pylori (H. pylori) is recognized as an important human pathogen associated with superficial gastritis, atrophic gastritis, gastric cancer, etc., each of which has become a serious threat to human health and survival. The rate of drug resistance is increasing due to the wide use of antibiotics and high rates of resistance to clarithromycin, metronidazole, and levofloxacin are associated with the failure of H. pylori eradication. At present, the mechanism of antibiotic resistance of H. pylori is not completely understood. It is very difficult to prevent drug resistance and improve the rate of eradication of the target, thus warranting exploration of the mechanism of drug resistance to H. pylori, and provision of an experimental basis for the prevention and treatment of drug resistance.
Currently, there is a serious drug resistance situation in H. pylori and new antibiotics are urgently needed; however, antibiotic research and development are very difficult. If we can understand the antibacterial mechanism of linolenic acid-metronidazole (Lla-Met), we can better apply it to antimicrobial treatment and solve the problem of antibiotic resistance.
The objectives of this study were to confirm the antibacterial effect of Lla-Met on H. pylori, and to provide theoretical support for further research and development of Lla-Met as an anti-H. pylori drug, and to help overcome the resistance of H. pylori to existing antibiotic drugs.
H. pylori cells were treated with the Lla-Met compound, and the effect of the compound on the cell morphology, cell membrane permeability, and oxidation of the bacteria cell was assessed by scanning electron microscope, propidium iodide staining, FIFC-FD, detection of ion channels, detection of intracellular reactive oxygen species, and detection of phosphatidylserine ectropion. Meanwhile, the differently expressed genes in H. pylori in response to Lla-Met treatment were identified by transcriptome sequencing and quantitative real-time polymerase chain reaction.
The expression of both SodB and MdaB genes was up-regulated after treatment with Lla-Met, and both genes are associated with antioxidants. Lla-Met inhibits the growth of H. pylori through oxidation.
The mechanism of linoleic-metronidazole compound was demonstrated to involve inhibiting H. pylori growth by inducing excessive reactive oxygen species accumulation, resulting in excessive superoxide dismutase MdaB and SodB genes expression.
This study proves the antibacterial effect of Lla-Met on H. pylori at the molecular level, providing theoretical support for further research and development of Lla-Met as an anti-H. pylori drug to help overcome H. pylori resistance to current antibiotic drugs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Krzyzek P, Poland; Maurya DK, India S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (5)] |
| 2. | Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1997;11 Suppl 1:71-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 3. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 269] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 4. | Yamaoka Y. How to eliminate gastric cancer-related death worldwide? Nat Rev Clin Oncol. 2018;15:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Couturier MR, Marshall BJ, Goodman KJ, Mégraud F. Helicobacter pylori diagnostics and treatment: could a lack of universal consensus be the best consensus? Clin Chem. 2014;60:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Kanizaj TF, Kunac N. Helicobacter pylori: future perspectives in therapy reflecting three decades of experience. World J Gastroenterol. 2014;20:699-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 7. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 549] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 8. | Hu Y, Zhu Y, Lu NH. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front Cell Infect Microbiol. 2017;7:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Fallone CA, Moss SF, Malfertheiner P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology. 2019;157:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 10. | Suzuki S, Esaki M, Kusano C, Ikehara H, Gotoda T. Development of Helicobacter pylori treatment: How do we manage antimicrobial resistance? World J Gastroenterol. 2019;25:1907-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 11. | Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 12. | Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 824] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 13. | Thamphiwatana S, Gao W, Obonyo M, Zhang L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc Natl Acad Sci U S A. 2014;111:17600-17605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Dai YY, Qin C, Huang GR, Qin YC, Huang YY, Huang YQ, Zhao LJ. Linolenic Acid-Metronidazole: a Compound Relieving Drug Resistance and Inhibiting Helicobacter pylori. Antimicrob Agents Chemother. 2022;66:e0007322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Hakansson AP, Roche-Hakansson H, Mossberg AK, Svanborg C. Apoptosis-like death in bacteria induced by HAMLET, a human milk lipid-protein complex. PLoS One. 2011;6:e17717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Nissenkorn I, Sluzker D, Jaffee A, Servadio C. [Life with an ileal conduit: a long-term follow-up]. Harefuah. 1986;111:6-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Dai G, Cheng N, Dong L, Muramatsu M, Xiao S, Wang MW, Zhu DX. Bactericidal and morphological effects of NE-2001, a novel synthetic agent directed against Helicobacter pylori. Antimicrob Agents Chemother. 2005;49:3468-3473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Jiamboonsri P, Pithayanukul P, Bavovada R, Chomnawang MT. The inhibitory potential of Thai mango seed kernel extract against methicillin-resistant Staphylococcus aureus. Molecules. 2011;16:6255-6270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Hwang JH, Jin Q, Woo ER, Lee DG. Antifungal property of hibicuslide C and its membrane-active mechanism in Candida albicans. Biochimie. 2013;95:1917-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Choi H, Lee DG. Antifungal activity and pore-forming mechanism of astacidin 1 against Candida albicans. Biochimie. 2014;105:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Akhtar F, Khan AU, Misba L, Akhtar K, Ali A. Antimicrobial and antibiofilm photodynamic therapy against vancomycin resistant Staphylococcus aureus (VRSA) induced infection in vitro and in vivo. Eur J Pharm Biopharm. 2021;160:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Lee H, Lee DG. Gold nanoparticles induce a reactive oxygen species-independent apoptotic pathway in Escherichia coli. Colloids Surf B Biointerfaces. 2018;167:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Kim H, Lee DG. Lupeol-induced nitric oxide elicits apoptosis-like death within Escherichia coli in a DNA fragmentation-independent manner. Biochem J. 2021;478:855-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Pérez-Lara Á, Thapa A, Nyenhuis SB, Nyenhuis DA, Halder P, Tietzel M, Tittmann K, Cafiso DS, Jahn R. PtdInsP(2) and PtdSer cooperate to trap synaptotagmin-1 to the plasma membrane in the presence of calcium. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Obonyo M, Zhang L, Thamphiwatana S, Pornpattananangkul D, Fu V. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol Pharm. 2012;9:2677-2685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Huang Y, Hang X, Jiang X, Zeng L, Jia J, Xie Y, Li F, Bi H. In Vitro and In Vivo Activities of Zinc Linolenate, a Selective Antibacterial Agent against Helicobacter pylori. Antimicrob Agents Chemother. 2019;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 3758] [Article Influence: 341.6] [Reference Citation Analysis (0)] |
| 28. | Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1729] [Cited by in RCA: 1925] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 29. | Roche-Hakansson H, Vansarla G, Marks LR, Hakansson AP. The human milk protein-lipid complex HAMLET disrupts glycolysis and induces death in Streptococcus pneumoniae. J Biol Chem. 2019;294:19511-19522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Erental A, Kalderon Z, Saada A, Smith Y, Engelberg-Kulka H. Apoptosis-like death, an extreme SOS response in Escherichia coli. mBio. 2014;5:e01426-e01414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |