Published online Aug 28, 2023. doi: 10.3748/wjg.v29.i32.4831
Peer-review started: May 3, 2023
First decision: June 14, 2023
Revised: July 14, 2023
Accepted: August 7, 2023
Article in press: August 7, 2023
Published online: August 28, 2023
Processing time: 114 Days and 0 Hours
Non-alcoholic fatty liver disease (NAFLD) causes significant global disease burden and is a leading cause of mortality. NAFLD induces a myriad of aberrant changes in hepatocytes at both the cellular and molecular level. Although the disease spectrum of NAFLD is widely recognised, the precise triggers for disease progression are still to be fully elucidated. Furthermore, the propagation to cirrhosis is poorly understood. Whilst some progress in terms of treatment options have been explored, an incomplete understanding of the hepatic cellular and molecular alterations limits their clinical utility. We have therefore reviewed some of the key pathways responsible for the pathogenesis of NAFLD such as innate and adaptative immunity, lipotoxicity and fibrogenesis, and highlighted current trials and treatment options for NAFLD patients.
Core Tip: Non-alcoholic fatty liver disease (NAFLD) is a significant global disease burden and a leading cause of mortality causing aberrant changes in hepatocytes. Although the disease spectrum is widely recognised, precise triggers for disease progression remain poorly understood. Whilst some progress has evolved in terms of treatment, there are still no approved pharmacological therapies for NAFLD treatment due to the incomplete understanding of the hepatic cellular and molecular alterations in disease pathogenesis.
- Citation: Petagine L, Zariwala MG, Patel VB. Non-alcoholic fatty liver disease: Immunological mechanisms and current treatments. World J Gastroenterol 2023; 29(32): 4831-4850
- URL: https://www.wjgnet.com/1007-9327/full/v29/i32/4831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i32.4831
Non-alcoholic fatty liver disease (NAFL) (NAFLD) is the most common cause of liver disease globally which occurs due to an excessive accumulation of fat in the liver in the absence of secondary causes or other liver disease aetiologies[1]. NAFLD encompasses the spectrum of disease from simple NAFL, non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis, and in many cases hepatocellular carcinoma (HCC)[1]. Due to increasing trends of sedentary lifestyles and dietary choices the prevalence of NAFLD continues to rise[1].
NAFLD has a significant global disease burden (882 million cases in 2017)[2], with cases likely to be higher due to poor screening of high risk asymptomatic populations. Furthermore, inaccurate disease progression predication markers and a lack of available licenced therapeutics hinders the treatment of NAFLD. Management of NAFLD is largely based on lifestyle modifications, but many newer therapies are being evaluated (discussed later). Further understanding into the mechanisms of disease progression, diagnostic biomarkers and therapeutic intervention should help mitigate burden of disease. Finally, liver disease combination therapies are becoming increasingly popular. This review will highlight some of the recent developments in NAFLD and aims to define the pathological features associated with disease progression including the immunological mechanisms as well as the current therapeutic interventions used for NAFLD.
Global reports relating to the epidemiology of NAFLD have estimated a global prevalence of NAFLD between 25% and 35%[3-5], with Europe as high as 30%[6], 35% in South American countries[6,7], and 35% in North America[6]. During the period of 1991 to 2019, trend analysis shown that increasing global prevalence increased yearly by 0.7%, rising from 21.9% in 1991 to 37.3% in 2019[5].
NAFLD has a global impact on health care systems due to its high rates of morbidity and mortality[8]. NASH is a particularly prevalent cause of chronic liver disease which in turn leads to cirrhosis and HCC, whereas NAFLD has been noted as the biggest cause of HCC in the United States, France, and the United Kingdom, with NAFLD-related HCC predicted to increase globally alongside the rise in obesity[3]. In the United States, by 2030, almost 49% of the total population is projected to be obese[3].
Globally, the phenotype of patients with NAFLD appears to be men of a mean age of 51.7 years old, with obesity and/or type 2 diabetes[6]. A linear increase prevalence of NAFLD, diabetes and metabolic disorders have also been documented[9,10], and especially occurs in those with central obesity, diabetes and metabolic syndrome[11,12]. Metabolic comorbidities include hypertension (37%) and metabolic syndrome (40%)[6]. NAFLD prevalence in type-2 diabetes mellitus (T2DM) is as high as 70%[13], and patients with T2DM also have a twofold increased risk of all-cause mortality[4,14]. Prevalence of NAFLD in patients with morbid obesity also rises to 90%[4]. Therefore, prevalence of NAFLD poses a significant global health burden requiring clinical attention.
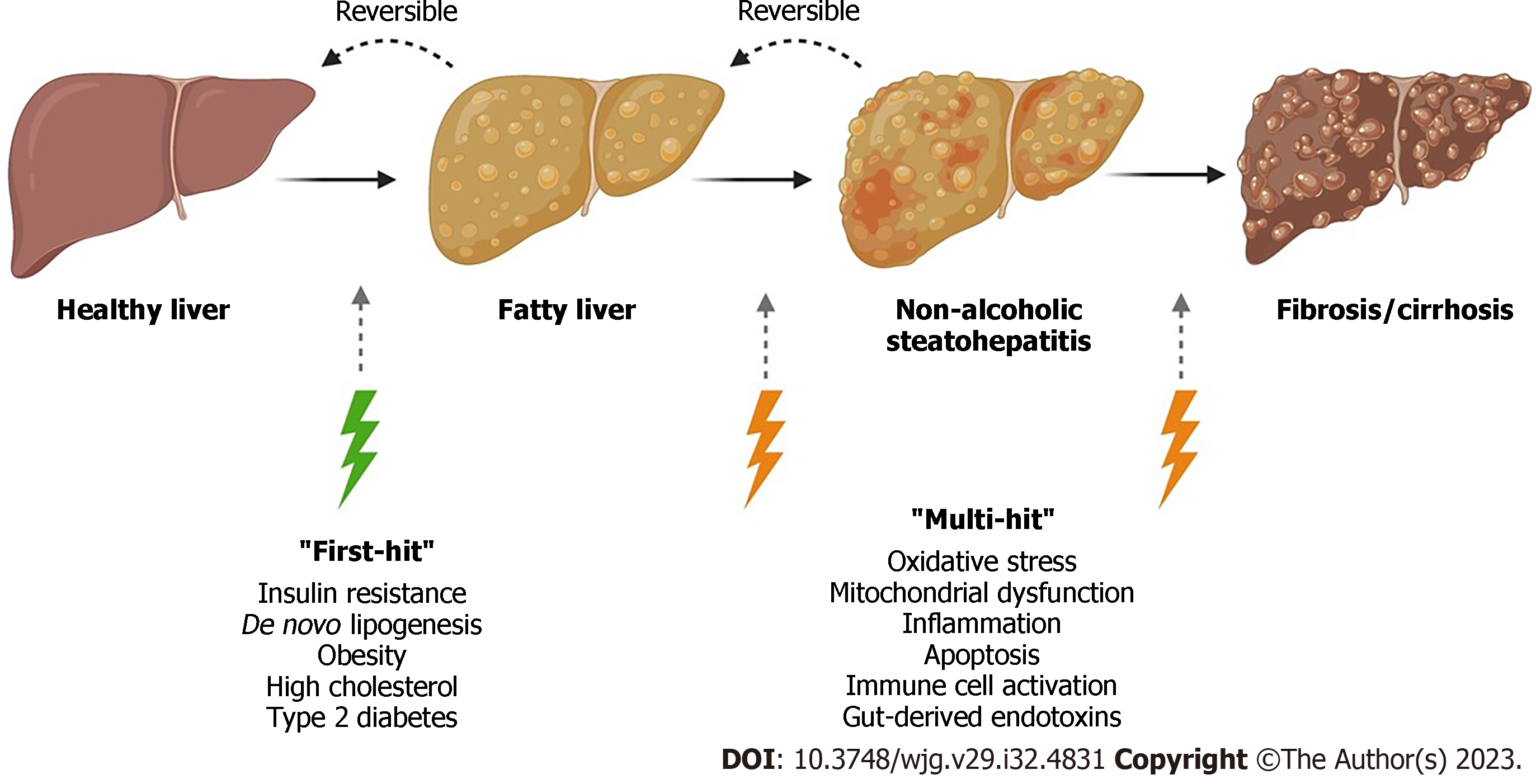
NAFLD is the most common cause of liver dysfunction with a high association for obesity and insulin resistance (IR). The spectrum of NAFLD can lead to progressive NASH, fibrosis, and lastly HCC and liver failure. NAFLD is a complex disease which results from environmental causes as well as polygenic background and risk factors (Figure 1). Although the molecular mechanisms underlying disease progression are complex, the histological spectrum of disease has been well described[15].
NAFLD is defined as a disease of the liver which is characterised by macrovesicular fat deposition and storage (> 5% of the hepatocytes) due to dysregulation in the mechanisms of fat synthesis and utilisation by the liver[7,16]. Steatosis is predominantly graded on a four-step scale, from 0 to 3. Grade 0 is defined as a normal liver containing fat in < 5% of hepatocytes[17,18]; grade 1 occurs when fat deposits occur in < 33% of hepatocytes, and grade 2 when fat occurs in 33%-66%[17,18]; grade 3 is the final stage in the spectrum of steatosis which occurs when > 66% hepatocytes contain fat[17,18]. The most important histological feature of NASH is hepatocyte ballooning and lobular inflammation as well as a steatotic liver. Hepatocyte ballooning is the second histological feature of NASH. Hepatocyte ballooning is defined by a clear, flocculent, not vacuolar cytoplasm with a ballooned shape. Inflammation occurs in a lobular pattern in NASH, containing Kupffer cells (KC), aggregates of neutrophils, and Mallory-Denk bodies[15]. NASH is defined as a chronic state of inflammation whereby hepatic stellate cells (HSCs) transform into myofibroblasts[19]. These transformed cells produce extra-cellular collagen matrix[19]. Normally fibrogenesis is a wound healing process. However, in NAFLD, sustained and progressive insults occurring over many years causes unregulated fibrogenesis[19]. Initially collagen deposits form in the perisinusoidal space. As collagen bundles form, architectural remodelling occurs which can lead to cirrhosis and HCC. Fibrosis stage 1 (F1) occurs when mild perisinusoidal/pericellular fibrosis is documented without septa[20]. Stage two occurs when fibrosis occurs in perisinusoidal/pericellular and portal/periportal regions with few septa (F2)[20]. Stage three (F3) is seen when numerous septa are documented, also known as bridging fibrosis[20]. Lastly, stage four is a cirrhotic liver[20]. An overview of NAFLD progression is shown in Figure 1.
Progression of the fibrosis stage to a cirrhotic scarred liver can vary between individuals, with cirrhosis occurring up to 15 to 20 years after initial diagnosis[19,21]. The median survival rate for patients with compensated cirrhosis is approximately 9 to 12 years, however, patients with decompensated cirrhosis have a significantly lower median survival rate of approximately 2 years[22]. Patients in the compensated stage are frequently asymptomatic, and often remain undiagnosed[22]. Therefore, early detection of cirrhotic patients who are still in the compensated stage is crucial, as early diagnosis could prevent or slow down disease progression. The onset of decompensated cirrhosis occurs when symptoms such as ascites, hepatic encephalopathy, and/or gastroesophageal variceal haemorrhage are found[22]. Histologically, cirrhosis is diagnosed when a disconnection of the hepatocytes from the central vein occurs as well as capillarisation of the liver sinusoids[19]. These modifications that occur to the liver structure and integrity lead to elevated intravascular resistance within the portal system and decreased hepatic perfusion, ultimately causing a loss of liver function[19]. Hence, timely diagnosis and management of cirrhosis is required to improve patient outcomes.
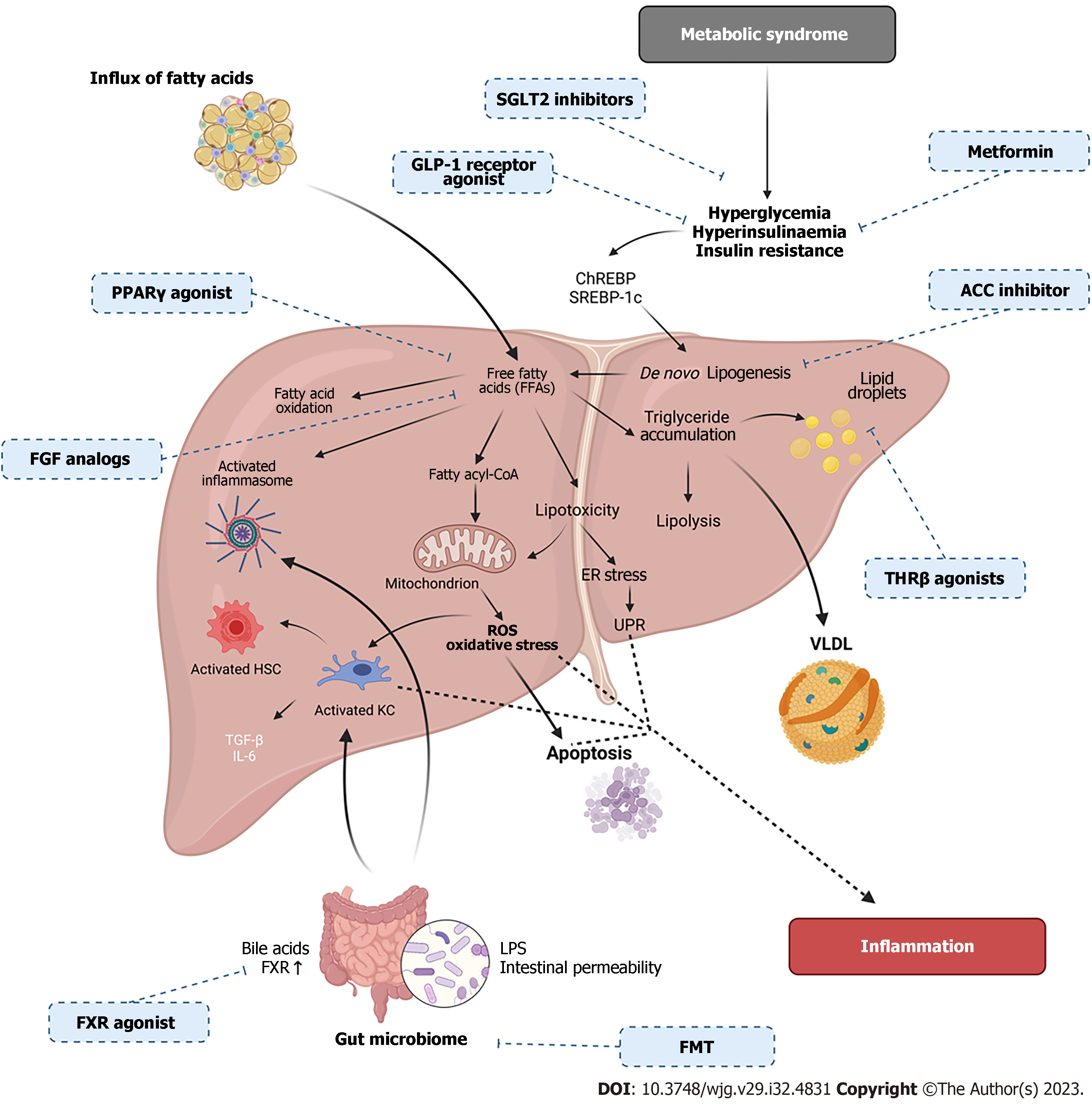
Many studies have shown that metabolic dysfunction and IR play a significant role in the development of NAFLD. Various animal and clinical studies suggest that chronic low grade inflammation may play a role in the development of IR and NAFLD as well as extrahepatic complications such as cardiovascular diseases, T2DM, and renal dysfunction[23]. IR is a complex state by which the skeletal muscle, liver, and adipose tissue become less sensitive to insulin and its metabolic effects[24]. IR is related to obesity, hypertension, hyperglycaemia and metabolic syndrome. During a carbohydrate-rich diet, excess glucose is converted into fatty acids via lipogenesis, using acetyl-CoA which is generated from glycolysis-driven pyruvate[25]. These fatty acids are then incorporated into very low-density lipoproteins (VLDL) for transport to white adipose tissue for storage[25]. Accumulation of lipids in adipocytes trigger downstream signalling of pathways including c-Jun N-terminal kinase (JNK) and nuclear factor-kappa B (NF-κB), leading to production of proinflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-6[26,27]. JNK regulates the production of proinflammatory cytokines, karyomitosis, and cellular apoptosis, and therefore, contributes to inflammation and IR. Research has proposed activation of JNK also accelerates lipid accumulation, thus, exacerbating liver injury. JNK-1 deficiency in adipose tissue shows protection from development of hepatic steatosis and promotes glucose intolerance, insulin clearance, IR, and hepatic steatosis[24]. Kluwe et al[28] also showed that in a mouse model, inhibition of JNK lead to modulation of fibrosis in hepatocytes. In a high fat diet (HFD) model of NAFLD, IR, liver injury and increased autophagy were documented[29]. However, JNK inhibition decreased autophagy and IR[29]. Therefore, JNK signalling plays a significant role in NAFLD progression.
The hormone adiponectin is secreted by adipocytes and is associated with improved insulin sensitivity. Adiponectin-mediated signalling is also able to stimulate fatty acid β-oxidation, glucose utilisation and uptake, as well as suppression of fatty acid synthesis[30]. Adiponectin can also inhibit the glycerol 3 phosphate (G3P) pathway; however, research has shown that during NAFLD serum levels of adiponectin are reduced[30]. Another metabolite of the G3P pathway is fatty acyl-CoA which is involved in mitochondrial β-oxidation. In patients with NAFLD, an increase in mitochondrial β-oxidation can lead to a state of oxidative stress due to increased substrate delivery to the mitochondrial electron transport chain (ETC), increasing reactive oxygen species (ROS) and damaging mitochondrial DNA[30,31]. Although β-oxidation is upregulated, ATP levels are decreased in NAFLD due to reduced activity of ETC complexes I and IV[32], and have been correlated to levels of disease progression. Therefore, modifications to mitochondrial function can exacerbate disease progression in NAFLD. Hyperinsulinemia alongside increased levels of ROS also leads to an imbalance between mitochondrial fission and fusion proteins such as dynamin-related protein 1 (Drp1) and mitofusin-2 proteins (Mfn2)[33]. It is possible that an excessive mitochondrial fission in the liver plays an important role in NAFLD progression. Increases in the Drp1-to-Mfn2 ratio causes enhanced mitochondrial fission which in turn causes reduced endoplasmic reticulum association, decreases oxidative phosphorylation capacity, and elevated mitochondrial ROS production[33], which can exacerbate disease state. Therefore, it is plausible that a reduction in mitochondrial oxidative capacity as well as the impairment of metabolic fuels provides a plausible relationship between mitochondrial dysfunction, lipotoxicity and IR. Therefore, metabolic health and mitochondrial function are fundamental to progression of NAFLD (Figure 2).
The inflammasome pathway and macrophages are known to play a significant role in the development of IR. Production of IL-1β and IL-18 can be regulated by the inflammasome pathway, which contains complexes including nucleotide-binding oligomerization domain (NOD)-, leucine-rich repeats-and pyrin domain-containing protein 3 (NLRP3) and activation of caspase-1[34]. In NAFLD, inflammasome formation can be activated upon a variety of stress stimuli such as damage-associated molecular patterns, pathogen-associated molecular patterns, for example, lipopolysaccharide (LPS) from the gut-liver axis, mitochondrial ROS, endoplasmic reticulum stress, and ROS[34-36]. Activation of the NLPR3 inflammasome has shown to contribute to disease progression from steatosis to NASH[34,37]. MRNA levels of NLRP3 inflammasome components such as NLRP3, caspase-1, IL-1β, and IL-18 were also found to be significantly upregulated in NAFLD patients[37-39]. Therefore, targeted modulation of the inflammasome will lead to improved insulin signalling and mitochondrial function.
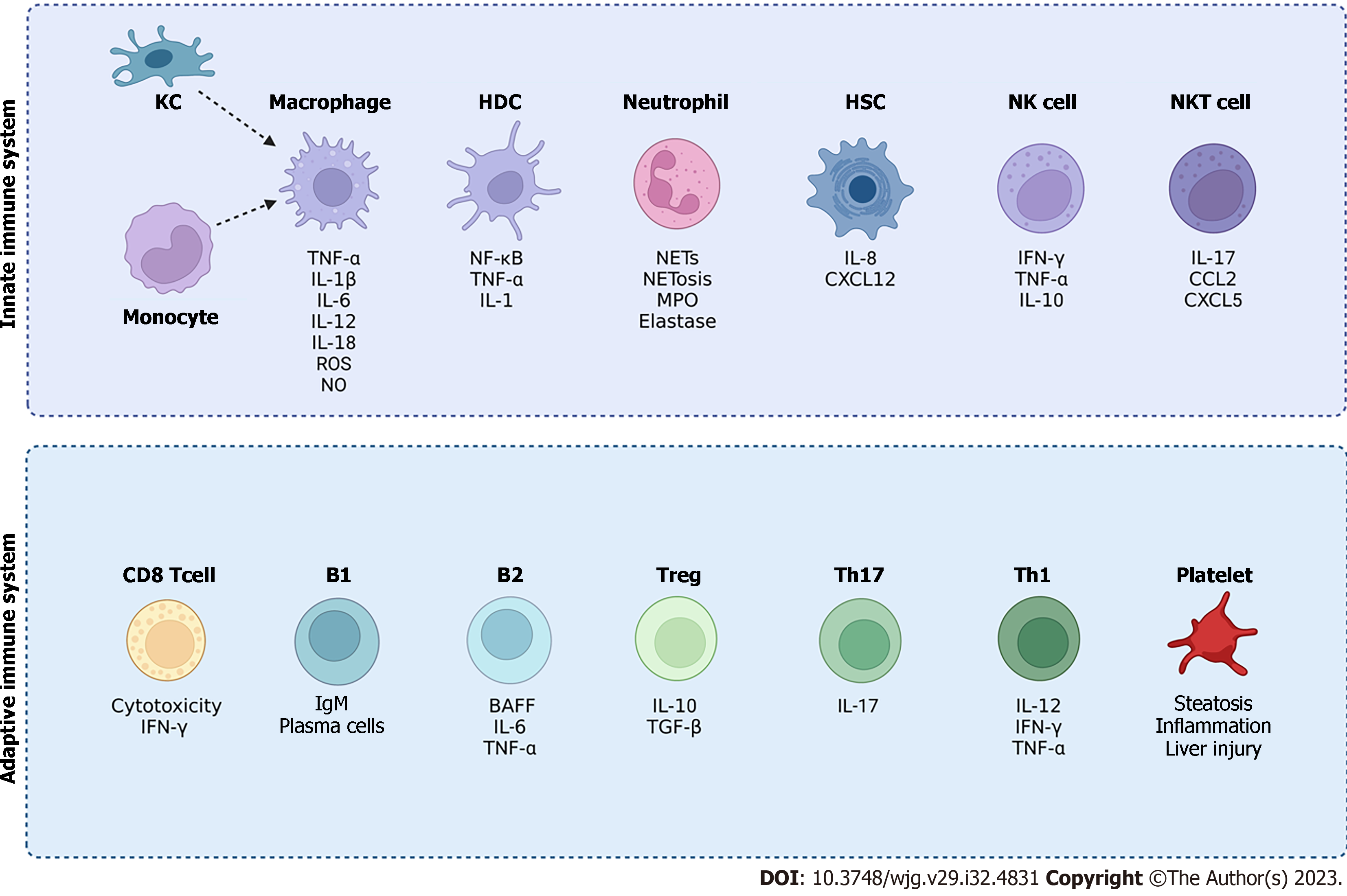
In chronic liver disease, the activation of the immune response can both restore tissue function as well as cause tissue injury. Therefore, an amplified immune response may lead to organ dysfunction. The following section describes the involvement of various immune cell types in the pathogenesis of NAFLD (Figure 3).
Innate immune system in NAFLD: During NAFLD, the innate immune system is involved in the activation of resident KCs, with recruitment of innate immune cells such as neutrophils, monocytes, natural killer (NK) cells and natural killer T (NKT) cells[40], and inflammation is further exacerbated via the production of cytokines, (e.g. TNF-α, IL-1β, IL-6, IL-12, IL-18), chemokines, nitric oxide (NO) and ROS[40-42].
Macrophages: The main role of macrophages in the liver is predominantly for immunoregulatory and detoxifying functions, however, alterations in macrophage phenotypes and dynamics have been reported to be involved in the pathogenesis of NAFLD[43,44]. Liver resident KCs are a large population of macrophages as well as other subsets of macrophages such as monocyte-derived macrophages and liver capsular macrophages[45]. M1 macrophages have a pro-inflammatory phenotype and are induced by mediators such as LPS and interferon-γ (IFN-γ) and their activation causes secretion of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α[46,47]. On the other hand, M2 macrophages have an anti-inflammatory phenotype and are induced by Th2 cytokines such as IL-4 and IL-13, causing secretion of anti-inflammatory factors such as IL-10 and transforming growth factor-β (TGF-β)[46,47]. M2-type macrophages have also been reported to induce M1-type KCs apoptosis decreasing disease progression[48]. Therefore, the balance between M1 and M2 macrophages is important for homeostasis in the liver.
During NAFLD, macrophages can recognise and respond to stimuli through pattern recognition receptors such as membrane-bound toll-like receptors (TLRs), such as TLR4 and TLR9, and cytoplasmic NOD-like receptors [43,45,49]. LPS translocated from the gut can activate TRL4 and TLR9, and this recognition of stimuli can then result in inflammation via secretion of proinflammatory cytokines such as TNF-α, IL-1β, IL-6, IL-12, and IL-18, as well as molecules such as ROS and NO. Activation of TLR9 can induce the release of IL-1β from KCs, which occurs during processes such as lipid accumulation, fibrinogenesis and cell death[50]. In NAFLD patients, TLR4 expression is often correlated with hepatic inflammation and fibrosis[51]. There are also many advantages to the activation of macrophages due to their immunosuppressive effects and secretion of anti-inflammatory cytokines, however, they also have pro-fibrinogenic effects. Activation of M2-type macrophages can release TGF-β1 and IL-13, leading to a fibrotic response of liver remodelling and tissue repair[52].
During IR, circulating free fatty acids (FFA) can also directly activate KCs causing activation of the stress-response kinases (JNK1 and JNK2) producing pro-inflammatory cytokines and extracellular vesicles (EVs), stimulating macrophage activation. EVs have been studied in their contribution to pathobiology of NAFLD, and have been found to mediate inflammation[53,54]. Hepatocytes can release EVs containing cargoes which activate signalling pathways[53,54]. Some EVs can carry mitochondrial DNA, which in mice and humans have been shown to activate macrophages via TLR9 [53,54]. Activation of TLR9 then causes inflammation though resultant downstream activation of NF-κB-dependent pro-inflammatory cytokines in macrophages[53,54]. Macrophage activation and inflammation may also occur via EV formation in response to lipid-associated toxicity[55]. Therefore, both harmful and beneficial macrophage phenotypes can co-exist during NAFLD, and the balance between these phenotypes must be considered for therapeutic strategies. Both cellular and molecular macrophage targets for therapy may provide a new perspective as well as the adoptive transfer therapies.
Neutrophils: Neutrophil infiltration during NASH has been documented in both patients and in mouse models. In the early stages of NAFLD, recruitment of neutrophils occurs via chemokines such as C-X-C motif chemokine ligand (CXCL) 1, IL-8, and CXCL2[56]. Research has shown that various neutrophil specific components are released in NAFLD. Neutrophil elastase is a major inflammatory protease which can be released by neutrophils. In a mouse model of NAFLD, elastase suppression has been shown to improve disease severity[57]. As well as elastase, neutrophil proteinase-3 has also been reported as elevated in NASH and both the levels of proteinase-3 and elastase were correlated with liver fibrosis[58]. Elastase has also been shown to play a role in metabolic dysfunction and IR, and therefore, targeting or improving IR and metabolic disease may improve inflammation in NAFLD. Myeloperoxidase (MPO), a neutrophil derived enzyme has been shown to become increased in NASH. It is thought that both neutrophils and MPO may cause activation of HSCs inducing liver fibrosis and inflammation in NASH[59,60]. Plasma levels of MPO have been found to be correlated with fibrosis in NAFLD patients[61]. More research is required to determine how neutrophils contribute to disease pathogenesis in NAFLD.
NKT cells: NKT cells provide a bridge between the innate and adaptive immune system and express both NK cell and T cell markers[40]. NKT cells become activated upon antigen presentation by CD1d which can be expressed by a variety of cells such as hepatocytes, macrophages, dendritic cells, and B cells[40]. Pro-inflammatory cytokines such as IL-4 and IFN-γ are secreted after NKT activation causing further tissue injury. Although there is conflicting evidence, during steatosis levels of NKT cells become reduced, whereas in NASH and fibrosis they are increased[40]. This has been shown by in vivo studies where NKT cells were elevated in the blood and liver of patients with moderate-to-severe steatosis[62]. During early-stage NAFLD such at fatty liver, the phenotype of NKT cells demonstrate a pro-inflammatory Th1 cytokines profile such as IFN-γ, whereas, in advanced end stage disease, they have a profibrotic role[40].
NK cells: NK cells have a cytotoxic role and can attack cells via perforin-mediated pathways or cell-cell interactions, for example Fas. NK cells can also act as regulatory cells by releasing various cytokines and chemokines, such as IFN-γ, TNF-α, and IL-10 as well as growth factors[40]. NK cell-associated cytotoxic ligands, such as TNF-related apoptosis-inducing ligand (TRAIL), NK group 2 member D, and major histocompatibility (MHC) class I chain-related protein A and B mRNAs, have been reported to be elevated in obese NASH patients[63]. NK cells therefore may possibly promote inflammation and hepatocyte apoptosis via TRAIL secretion[63], leading to progression of fibrosis.
Dendritic cells: Hepatic dendritic cells (HDCs), which highly express MHC-class II molecules and CD45 are important immune cells in the liver which have migratory capabilities as well as production of cytokines such as TNF-α, IFN-γ, IL-2, IL-4, and IL-6[64]. Three distinct subsets of HDCs have been described in experimental models of HDCs: Lymphoid, myeloid and plasmacytoid[64]. They play a crucial role in the progression of metabolic steatohepatitis bridging lipid metabolism and inflammation. HDCs can shift from a tolerant state to an active state, triggering an inflammatory process[65]. In an immune tolerant state, immature HDCs secrete IL-10, and TGF-β, as well as limit T cell expansion[64]. In a tolerant state they supress inflammasome activation, maintaining homeostasis in the liver. This regulatory role of HDC in NASH can therefore restrict inflammation as well as clear apoptotic cells and necrotic debris[65]. Additionally, they are involved in lipid storage within the liver and are important for antigen presentation and induction of inflammatory pathways[64]. Recent studies suggest that HDCs have antifibrogenic effects by activating metalloproteinases, such as matrix metallopeptidase 9 which has been found to be involved in regression of fibrosis as well as remodelling of the extracellular matrix.
On the other hand, in an active state HDCs cause inflammation and liver damage as well as contribute to fibrosis. In an active or inflammatory state, mature recruit macrophages to the liver and can activate the NF-κB pathway as well as produce pro-inflammatory cytokines such as TNF-α and IL-1, contributing to the inflammatory microenvironment[64]. In humans, a subset of HDCs (CD11C + cDC2) have been shown to play an important role in development of fibrosis and have been positively correlated with metabolic steatohepatitis[64]. HDC’s in a mouse model of metabolic associated fatty liver disease (MAFLD) have shown that in a matured form they produce more inflammatory cytokines and may be responsible for proinflammatory responses[65]. HDC may therefore play dual roles in both the suppression and progression of disease state.
Adaptive immune system in NAFLD: The adaptive immune system is defined by antigenic specificity and immunological memory and includes predominantly B and T lymphocytes. Experimental research has suggested that sustained immune responses activated by oxidative stress-related antigens can affect the pathogenesis of disease via activation of CD4+ T-cells, which, in turn, stimulate macrophage M1 responses and liver CD8+ T- and NKT cell recruitment[66]. Experimental in vitro data as well as in vivo data supports adaptive immunity in NAFLD disease progression.
T cells: Conventional T cells have been well studied in their involvement in the pathogenesis of NAFLD. The main CD4+ T-cell are divided into Th1, Th2, and Th17 populations characterised by production of specific cytokines. Recruitment of CD4+ T-cell in the liver have been reported to be increased in individuals diagnosed with NASH[67-71], as well as in animal models fed a high calorie diet[72]. Upon presentation to inflammatory stimuli, CD4+ T cells can differentiate into Th17 cells[73], a subset of pro-inflammatory T helper cells defined by their ability to produce IL-17. In animal models of NAFLD, in the liver it has been documented that the Th-17 phenotype was favoured, promoting inflammation[74]. Human studies in obese and overweight patients have also shown an increase in the Th17 population[74]. The IL-17 family of cytokines have been implicated in the progression of fatty liver disease through interference of the insulin signalling pathway[75]. In mouse models, deficiency of IL-17A, IL-17F or IL-17A receptor (IL-17RA) results in increased steatosis but reduced steatohepatitis[76,77]. In HFD mouse model, neutralisation or IL-17RA deficiency as well as treatment with anti-IL-17mAb therapy has shown to protect mice from diet-induced liver injury via improvement of lipid accumulation, suppressing KCs activation, decreased pro-inflammatory cytokines levels and inhibition downstream of NF-κB signalling[40,78]. IL-17 has also been shown to activate the signal transducer and activator of transcription 3 pathway in HSCs causing progression of fibrosis in the liver[79]. These studies indicate the involvement of IL-17 in the pathogenesis of NAFLD.
Regulatory T cells (Tregs), a subset of cells which promote immune tolerance and facilitate tissue repair and express the transcription factor forkhead box P3. Although there is limited data available, the number of hepatic Tregs have been documented to be decreased in in animal models of NAFLD[80]. In humans, the levels of Tregs in the liver and the circulation of patients with NAFLD is also reported to be decreased[80]. Also, in a NASH mouse model, induced by a HFD and endotoxin challenge, a decrease in liver Tregs was documented, however, transfer of Tregs into mice showed reduced liver injury and inflammation, through decreased expression of TNF-α[81].
B cells: Until recently, the role of B cells is the pathogenesis of NAFLD was less understood. B cells are highly specific antibody producing cells of the adaptive immune system. B cells contribute to approximately 6% of intrahepatic cells[40,82]. B cells produce the B cell activating factor (BAFF), a cytokine controlling the process of B cell survival and maturation[83,84]. Liver biopsies from patients with NASH have been shown to contain both B and T cells in the inflammatory infiltrates [73,85,86].
In mouse models, B cells have been shown to become activated in NASH, concomitantly with the onset of steatohepatitis and thus, maturing to plasma blasts and plasma cells[73,86]. Another study has also shown that BAFF signalling increased IR in an NAFLD model as well as promoting fatty liver[87]. Whereas, in mice models of NASH, B2 cell responses upregulate BAFF[73]. Levels of BAFF in patients with NASH appear to occur at a higher level than with patients with fatty liver, therefore it has been proposed BAFF levels correlate with severity of steatohepatitis fibrosis[88]. BAFF receptor-deficient mice showed an improvement in HFD-induced obesity and IR, as well as a reduction in the number of B cells and a decrease in serum immunoglobulin G level[40]. The involvement of B cells in the progression of liver disease can also be due to production of pro-inflammatory mediators and antigen-presentation[89,90]. In patients with NASH, MHC class II molecules become upregulated causing inflammatory infiltration and recruitment of CD4+ and CD8+ T cells to the liver[73], whereby Th1 CD4 + T cell activation and IFN-γ production occurs[86]. Therefore, this research suggests B cells and BAFF play an important role in NAFLD pathogenesis and can contribute to pathogenesis of NAFLD via autoimmune hepatitis and liver fibrogenesis [87,90,91].
Serum levels of IgA has been found to be associated with patients with NASH and levels of IgA can predict advanced liver disease progression[92]. Patients with HCC have also been documented to have higher serum IgA[93-95]. In both mouse models and human studies, liver resident IgA accumulation in hepatic cells has been associated with chronic inflammation and fibrosis[93]. Regression of HCC has also been documented when IgA is inhibited, causing reactivation of CD8+ T cell function[93,94].
B cell deficiency also has been shown to improve development of NASH. The mechanisms and signalling of B cell activation in NASH is via myeloid differentiation early response protein 88[40,96]. Studies have shown that elimination of myeloid differentiation early response protein 88 on B cells reduced hepatic T cell-mediated inflammation and fibrosis, but had no effects on steatosis[40,96]. Therefore, B cells show involvement in the pathogenesis of NAFLD, and manipulation on B cells may provide a therapeutic opportunity for NAFLD treatment. An overview of innate and adaptive immune cells involved in NAFLD is shown in Figure 3.
Most commonly a combination of clinical history, laboratory findings, biopsies and radiological testing are used in the diagnosis of NAFLD. The clinical diagnosis of NAFLD is usually considered when aminotransferases become elevated or imaging on the liver detects high hepatic fat[97]. Both the American and European guidelines agree that suspicion of fibrosis warrants a confirmatory liver biopsy[98].
Current biomarkers: Liver function tests, particularly alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, can indicate liver damage in NAFLD/NASH[12,99]. These levels may be elevated by two to four times the normal limit. Mild elevations in aminotransferase levels may be present in lower stages of the disease, but routine tests may appear normal. Alkaline phosphatase and gamma-glutamyl transferase (GGT) levels may be up to three times the upper limit even in the absence of advanced disease[100]. A serum GGT cut-off value of 96.5 can predict advanced fibrosis with a sensitivity of 83% and specificity of 69%[101].
Several different biomarker panels have been described in their use to assess liver fat. These include the liver fat score (LFS) (sensitivity 86%, specificity 71%), hepatic steatosis index (sensitivity 92.5%, specificity 92.4%), fatty liver index (FLI) (sensitivity 87%, specificity 86%) and the steatotest (sensitivity 95.5%, positive predictive value 97.0%)[102]. LFS is calculated using a patients serum AST/ALT ratio, fasting serum AST level, fasting serum insulin level, any presence of metabolic syndrome and diagnosed diabetes mellitus. Studies have shown that a score greater than -0.640 can predict NAFLD with a sensitivity of 86% and specificity of 71%. The FLI uses waist circumference, body mass index (BMI), triglyceride, and GGT and this was initially developed to detect fatty liver in western countries.
Screening tools: The fibrosis-4 scoring system uses a patients age, platelet count, AST, and ALT to determine a liver fibrosis score and can help to predict advanced fibrosis. A score < 1.45 defines a negative predictive value or low probability of advanced liver fibrosis[103]. Scores of A score > 3.25 indicated a higher likelihood and has a positive predictive value of 65%, and specificity of 97% at predicting advanced fibrosis[103]. Recently, a new FAST™ screening tool has been used which compromises a combination of FibroScan® parameters such as liver stiffness measurement and controlled attenuation parameter as well as AST. This screening tool has been shown to have diagnostic accuracy when predicting those at risk of NASH. The FAST™ screening tool may provide a better non-invasive algorithm for diagnosis of NASH. FAST™ performed better than other non-invasive algorithms for the diagnosis of at-risk NASH[104,105]. Results have shown that the FAST score had the highest area under curve for the most high-risk NASH criteria as well as liver stiffness showing a consistently acceptable performance in predicting NASH[106].
Emerging biomarkers: Various non-invasive biomarkers for NAFLD have been developed and validated over the last 20 years, however, there is a need to develop and validate new biomarkers for NAFLD which encompass IR, inflammation and fibrogenesis[107-109].
Cell Death: As well as current markers such as cytokeratin 18, necroptosis is a form of cell death which is characterised by changes such as organelle swelling, plasma membrane damage, and release of cellular contents[110]. Proteins such as receptor-interacting protein kinase (RIPK) family members such as RIPK1 and RIPK3, and mixed lineage kinase domain-like (MLKL) are involved in the process[111]. During NAFLD, TLR4 ligands such as LPS cause activation of the TLR4 receptor causing activation of proteins such as RIPK3 and MLKL leading to necroptosis[112]. In patients with MAFLD, necroptosis components such as RIPK3 have been shown to be upregulated, therefore, it may be important to consider markers of necroptosis in NAFLD.
MicroRNAs (miRNAs): miRNAs are non-coding RNAs which play a role in gene expression regulation[113] and have been implicated as potential biomarkers in NAFLD. Research has shown that miRNAS such as miR-21, miR-34a, miR-122, and miR-451 have been found to be upregulated in NAFLD patients[114-117]. Various studies have also shown that profiles of miRNAs in NAFLD patients can differentiate between disease stage. In NAFLD and NASH, miR-122, miR-192, miR-19a, miR-19b, miR-125b, and miR-375 have been found to be increased greater than 2-fold, whereas continuous upregulated expression of miR-122, miR-192, and miR-375 was found in NASH patients[118,119]. Furthermore, higher expression of miR-122 and miR-21 have been reported in people with high fasting blood glucose, obesity and fatty liver infiltrations[119,120]. Therefore, these findings suggest that miRNAs may be promising biomarkers for NAFLD and metabolic disease, in particular miR-122, miR-192, and miR-34a, which have been shown to be correlated with severity of NAFLD.
EVs: Growing evidence suggests that EVs play a significant role pathological disease including those associated with obesity and metabolic syndrome. Obesity, IR, T2DM and NAFLD have all been linked to changes in the abundance and phenotype of circulating EVs[121] and therefore circulating EVs and their composition may be a candidate biomarker for NAFLD. EVs contain substances such as genetic material including miRNAs[122]. It has been documented that in humans, the number of AD-EVs is correlated with IR in overweight people[123], as well as visceral AD-EV number correlated with liver injury measured by ALT and AST and metabolic syndrome[124]. Circulating EVs have also been shown to become elevated during the progression of NASH and have reported to be correlated with histological findings[125]. Research has also shown that EVs from a NAFLD model in mice contained miR-122 and miR-192[125]. In cirrhotic patients, plasma hepatocyte-EVs were found to contain elevated levels of CK-18[126]. EVs from visceral adipose tissue can exacerbate disease in NAFLD by causing further inflammation, fibrosis and IR. Both pro- and anti-fibrotic EVs have been documented in the liver and therefore it is plausible to investigate the differences in the pro- and anti-fibrotic phenotypes to track disease progression and likelihood of fibrosis development. Future research should consider the use of EVs in monitoring metabolic dysfunction and IR in NAFLD, with focus on EV phenotypes, cargo and cell specific markers.
Despite NAFLD being an extremely prevalent liver disease, no specific pharmacological interventions are currently Food and Drug Administration approved for treatment. Some therapeutic agents used as anti-diabetics, antilipidemic and natural bile treatments have previously been evaluated in their ability to treat liver disease, although they have limitations. Predominantly, lifestyle interventions including diet and exercise are most commonly used to treat NAFLD. The current therapeutic interventions used for NAFLD are outlined below including future potential agents such as sirtuins, antioxidants and vitamins.
Lifestyle modifications: A substantial amount of research indicated that changes to lifestyle are a primary approach for the treatment of NAFLD[127]. Diet changes and weight loss can reverse liver disease. Weight loss in the range of 5%-10% can provide beneficial effects to NAFLD patients via a reduction in NAFLD activity score (NAS)[128,129]. A weight reduction greater than 10% has also been shown to reduce the severity of fibrosis as well as resolution of NASH[12,129,130].
European Association for the Study of the Liver (EASL), National Institute for Health and Care Excellence and American Association for the Study of Liver Diseases guidelines provide recommendations in terms of diet and physical activity for the management of NAFLD. These bodies recommend caloric restriction with a calorie deficit of 500-1000 kcal a day, as well as limiting the consumption of alcohol, fats and coffee. EASL also favours the Mediterranean diet, with studies indicating a reduction in liver fat in NAFLD patients[131]. Lifestyle modifications in terms of physical exercise can also positively effect liver fat content. Both resistance/weight training, high-intensity interval training and aerobic exercise have all equally been shown to reduce liver fat content; however, in men with NAFLD, high-intensity interval training was the most effective in restoring hepatic fat, reducing hepatic stiffness and improving Kupffer cell function; key features of NASH[132].
Evidence has suggested that the ketogenic diet (KD) is an effective treatment for NAFLD. Ketogenesis is a metabolic process resulting in the production of ketone bodies, namely acetoacetate, beta-hydroxybutyrate (BHB), and acetone, which act as alternative energy sources[33,133]. The KD has been shown to change hepatic mitochondrial fluxes and redox state as well as significantly reducing liver fat content and hepatic IR[134]. These changes were found to be accompanied by an increase in the net hydrolysis of liver triglycerides, a decrease in endogenous glucose production, and lower serum insulin levels[134]. BHB has also been shown to interact with inflammasomes[135,136] and neutralise ROS[137], leading to a reduction in inflammatory cytokines and oxidative damage via its antioxidant capacity. These findings suggest a KD can contribute to the reversal of NAFLD through improvement of IR and cellular redox function.
Bariatric surgery: Bariatric surgery been shown to be an effective treatment for NAFLD improving overall liver health via facilitating weight loss, improving insulin sensitivity, and subsiding inflammation[138,139]. Roux-en-Y gastric bypass (RYGB) and laparoscopic sleeve gastrectomy (LSG) are the most prevalent bariatric procedures, which lead to significant weight loss and metabolic health improvements[140].
Several research studies have investigated the impact of bariatric surgery in NAFLD and have shown positive results. Studies have indicated that bariatric surgery resulted in significant improvements in liver enzymes and histology, with a decrease in liver fat and fibrosis. Results from two meta-analyses have shown that treatment of NAFLD using bariatric surgery resulted in a biopsy-confirmed resolution of steatosis (56%-66%), inflammation (45%-50%), ballooning degeneration (49%-76%), and fibrosis (25%-40%), as well as showing a decrease in NAS scoring[140-142]. Bariatric surgery has therefore proven to be effective in ameliorating NAFLD, however, it is important to clarify which type of surgery is most effective. A study by Baldwin et al[143] compared RYGB and LSG against its effectiveness at improving AST and ALT concentration, NAS and NAFLD fibrosis score. Overall, both procedures reduce AST and ALT levels, however, LSG showed slightly more favourable results[139]. Another study has shown NAS scoring reduced significantly in patients who underwent both surgery types 12-mo after the surgery[139]. RYGB patients had a more significantly decreased steatosis and superior improvement in plasma lipid profile[144]. Furthermore, bariatric surgery has been demonstrated to lower the risk of liver-related complications and death in individuals with NAFLD. Bariatric surgery can therefore be considered as a promising treatment option for those with NAFLD who are overweight or obese.
Insulin sensitising agents: IR is believed to play an essential role in the development and progression of NAFLD. In recent years, various insulin sensitizers such as biguanides, thiazolidinediones (TZDs), glucagon-like peptide-1 receptor agonists (GLP-1), and dipeptidyl peptidase 4 inhibitors have been investigated as potential therapeutic targets for NAFLD[145]. However, there are safety concerns associated with long-term use of these targets[145].
Biguanides: As the development of NAFLD is closely associated with IR, diabetes, hyperglycaemia and hyperlipidaemia, antidiabetic drugs are often utilised in the treatment of NAFLD[145]. Metformin is considered the first-line treatment for T2DM due to its ability to improve insulin sensitivity and promote weight loss without causing hypoglycaemia[145,146]. Although its mechanisms of action are not fully understood, it works by lowering hepatic glucose production[145]. Previous open-label studies have suggested that metformin may have a positive impact on hepatic steatosis and necroinflammation, although excessive weight loss may have confounded these results[30]. However, some studies have shown that metformin does not significantly improve the histological response in NAFLD[30,147], but improves liver function and BMI[148]. In a mouse model, metformin treatment showed improvements to the gut-liver axis via attenuation of the loss of tight junction proteins in the small intestine as well as reducing the increase of endotoxin levels in the portal circulation[149].
GLP-1: GLP-1 receptor agonists can alter IR by promoting weight loss via suppressing appetite and delaying gastric emptying[150]. In NAFLD patients, research has shown GLP-1 receptor agonists improve hepatic and adipose tissue IR, suppress de novo lipogenesis and oxidative stress as well as increased clearance of VLDL[30,150]. GLP-1 may also modulate the immune system via the reprogramming of macrophages to the M2 phenotype. In the exenatide study of cardiovascular event lowering trial, GLP-1 receptor agonists reduced cardiovascular risk[151] and visceral fat, improved glucose tolerance, body fat percentage and resting energy rate (NCT01144338). The trial semaglutide unabated sustainability in treatment of type 2 diabetes (NCT02054897) are a series of phase III clinical trials which suggest treatment with semaglutide has a higher effectiveness than other GLP-1 therapeutics in the reduction of HbA1c in patients with T2DM. In a phase 2 trial with patients diagnosed with NASH receiving semaglutide treatment of 0.1 mg (80 patients), 0.2 mg (78 patients), or 0.4 mg (82 patients), patients resulted in a significantly higher NASH resolution when compared to the placebo (NCT02970942)[152]. Although NASH resolution was significantly improved, no significant difference was shown in the fibrosis stage[152].
Fibroblast growth factor (FGF) based therapeutics: FGF analogs have been proposed to target steps of disease pathogenesis. FGF-21 treatments are currently in clinical development for the treatment of NASH[153,154] and data suggests that FGF21 is anti-fibrotic and has the potential to improve the metabolic syndrome and is effective in treating NASH. The BALANCED trial (NCT03976401) evaluated the effects of efruxifermin, a long-acting Fc-FGF21 fusion protein[155]. In this study, 80 patients were given either placebo (n = 21) or efruxifermin 28 mg (n = 19), efruxifermin 50 mg (n = 20) or efruxifermin 70 mg (n = 20) via weekly subcutaneous injection for 16 wk[155]. Treatment with efruxifermin was found to significantly reduced hepatic fat fraction (HFF) in patients with F1-F3 stage NASH[155]. FGF based compounds which are currently in phase II are efruxifermin (FGF-21), pegbelfirmin (FGF-21), aldafermin (FGF-19), pegozafermin (FGF-21) and BFK8588A (FGF-21), which have been shown to achieve a reduction in ALT levels[109].
Thyroid hormone receptor-β (THRβ) 1 agonists: Currently, several different THRβ specific agonists have been shown to produce positive therapeutic effects in both animal models and clinical trials for treatment of NAFLD. Treatment with TG68, a novel THRβ agonist, has positive effects on resolution of NAFLD via reduction in liver weight, hepatic steatosis, serum transaminases, and circulating triglycerides in a NAFLD model[156]. Resmetirom (MGL-3196) has also been found to significantly reduce hepatic lipid content and improve liver enzyme levels and plasma lipid levels in NASH patients[157], however, glucose or insulin levels remained unchanged. Another THRβ agonist, VK2809 has been shown to reduce hepatic steatosis in a mouse model and in a phase II clinical trial reduced liver lipid content[158,159].
Currently there are many ongoing clinical trials studying the effects of THRβ agonists in NAFLD. The VOYAGE study is assessing VK2809 to determine its efficacy in the resolution of biopsy proven NASH (NCT04173065). The DUET study is also assessing the effects of orally administered TERN-501 and TERN-101 in presumed NASH (NCT05415722), whilst the LIFT study is assessing TERN-101 alone in NASH patients (NCT04328077)[160]. In a NASH mouse model, TERN-501 reduced steatosis as well as reducing serum total cholesterol, triglyceride and ALT levels[161].
Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists: PPAR-γ is a ligand-activated nuclear receptor and its activation causes insulin sensitization and enhances glucose metabolism. TZDs are a group of insulin sensitisers used to treat T2DM by action on PPAR-γ. PPAR agonists have been shown to modulate the innate immune response. PPAR-δ can promote anti-inflammatory polarization of macrophages and modulate their activation[162], whilst PPAR-γ ligands can inhibit the activation of macrophages and cytokine production (TNF-α, IL-6, and IL-1β), thus, reducing inflammation[163]. Activated PPARs can also regulate immune cells (macrophages, DCs, T cells, and B cells) as well as decrease inflammatory cytokine production[163].
Pioglitazone, a TZD, has been used for treatment of NASH with its effects improving steatohepatitis, ballooning degeneration and lobular inflammation[164]. Pioglitazone (45 mg/d) for 6 mo has been shown in patients with prediabetes or T2DM to improve the fibrosis stage (NCT00227110)[165]. Elafibranor is a dual PPAR-α/δ agonist. PPAR-δ functions to regulates peroxisomal β-oxidation of FFA as well as improve insulin sensitivity, lipid and glucose homeostasis[30]. Several clinical trials have assessed the effects of elafibronor in improving histology in NASH patients such as RESOLVE-IT (NCT02704403), and the GOLDEN trial[166]. In both trials elafibranor showed no effect.
Sodium glucose cotransporter 2 (SGLT2) Inhibitors: Another class of drugs lower serum glucose levels via inhibition of the SGLT2 and promote weight loss. Studies have shown that SGLT2 inhibitors reduce ALT levels correlating with changes in bodyweight and glycaemic control[30], although further studies are required to identify whether SGLT2 inhibitors can prevent progression of NAFLD/NASH.
Current clinical trials are underway to assess the efficacy and effectiveness of SGLT2 inhibitors. A randomised clinical trial aims to compare the effect of the pioglitazone and empagliflozin combination on liver fat mass (NCT04976283). Another trial is investigating dapagliflozin in NASH (NCT05254626).
Farnesoid X receptor (FXR) agonists: The FXR is a nuclear receptor which is activated by bile acids. FXR inhibits the expression sterol regulatory element binding protein-1 and carbohydrate-responsive element-binding protein. FXR also enhances the clearance of high-density lipoprotein and VLDL in the liver and well as promoting hepatic regeneration. However, little is documented regarding the immune modulation in this class of drugs.
Obeticholic acid (OCA) is a synthetic bile acid which acts as a FXR agonist. The use of OCA for NASH is still under investigation, due to reported side effects. The FLINT trial (NCT01265498) was a multicentre, randomized, double-blind, placebo-controlled phase IIb study assessing the effects of 25 mg of OCA for 72 wk. Treatment with OCA was linked with a significant improvement fibrosis stage in the treated group (35% vs. 19%; P = 0.004), although there was no difference in rates of NASH resolution. A phase III trial (REGENERATE) is currently active to further assess the treatment of OCA in patients with NASH (NCT02548351).
Other FXR agonists, such as tropifexor, have been studied in the treatment of NASH in the FLIGHT-FXR study (NCT02855164). This 48-wk study using tropifexor found sustained decreases in ALT and liver fat content (measured by HFF using magnetic resonance imaging-estimated proton density fat fraction) during the therapy duration[167]. A Phase IIa (LIFT trial) studying TERN-101, a FXR agonist, showed that in a 12-wk controlled trial, significant improvements in cT1, a marker of fibro-inflammation was observed[160].
Other treatment approaches: Modulation of the gut microbiome: Gut dysbiosis is a common feature of NAFLD[168]. Whilst pre- and pro- biotics are being evaluated, faecal microbiota transplant (FMT) may provide an alternative approach. HFD-fed mice which received FMT from healthy donors showed a significant reduction in intracellular hepatic lipid and proinflammatory cytokines concentration (IFN-γ and IL-17)[169]. Small intestinal microbiota transplants from healthy lean individuals to obese individuals have reported improvements in insulin sensitivity in those patients with metabolic syndrome[170]. A phase I pilot study is currently underway to study FMT in patients with NASH (NCT02469272). FMT as a treatment for NASH patients seems to be both a safe and efficient treatment, although, more high quality studies, trials and follow-ups are required to verify its therapeutic potential[168].
Modulation of the immune system: Methods for modulating the immune system as potential therapies for NAFLD are currently under investigation. Various pleiotropic effects of platelets have recently been discovered in liver homeostasis and disease as platelets are also involved in inflammatory regulation. Anti-platelet therapy (APT) has been shown to reduce NASH pathogenesis in rats[171]. Evidence has shown that APT may have a protective effect in patients with NAFLD[172]. In the liver, platelet and neutrophils can interact leading to neutrophil extracellular trap formation[173]. APT has been shown to reduce both NASH and HCC development[174]. It has been observed that APT reduced platelet accumulation in hepatocytes as well as reducing immune cell interaction, which led to decreases in cytokine and chemokine release, thus attenuating macrovesicular steatosis and liver damage[174].
It is well documented that NASH can progress to HCC. Immunotherapy, including programmed cell death 1 (PD-1) treatment, has been approved for the treatment of HCC[175]. PD-1 can interfere with the immune response and contributes to the growth and expansion of cancer. It has been documented that in NASH there is a progressive accumulation of exhausted, unconventionally activated CD8+ PD1 + T cells[175] and data has shown that in HCC, immune surveillance was impaired[175]. Several pharmaceutical agents have been developed to target PD-1 receptors. Pembrolizumab has shown significant enhancement in overall survival and progression-free survival although statistical significance was not met[176]. However, it is also thought that NASH-derived HCC may be less responsive to immune modulated therapy due to NASH-related aberrant T cell activation, causing damage to tissues[175,177,178]. A summary of current clinical trials is shown in Table 1; and an overview of the metabolic and molecular mechanisms occurring in NAFLD pathogenesis with therapeutic targets in Figure 4.
| Drug name | Condition | Target | Clinical trial Number | Phase | Status | Primary endpoint |
| GH509 | NASH NAFLD | NCT05784779 | Phase Ib/II | Recruiting | Change in liver fat content assessed by MRI-PDFF | |
| LUM-201 | NASH NAFLD | Lipid accumulation | NCT05364684 | Phase II | Recruiting | Change in intrahepatic lipid content measured by proton magnetic resonance spectroscopy |
| Choline | NAFLD | Choline deficiency | NCT05200156 | N/A | Recruiting | Change in Thiobarbituric acid reactive substances serum level |
| Dasatinib and quercetin | Fibrosis | Senescence | NCT05506488 | Phase I | Recruiting | Improvement of fibrosis NAFLD score based on histology after 21 wk |
| GSK4532990 | NAFLD fibrosis | 17 β-HSD | NCT05583344 | Phase II | Recruiting | Improvement of fibrosis measured by clinical research network scoring |
| Lisinopril | NASH HCC | ACE inhibitor | NCT04550481 | Phase II | Recruiting | Changes in fibrosis marker PRO-C3 |
| Rencofilstat | NASH Fibrosis NAFLD | Cyclophilin inhibitor | NCT05402371 | Phase II | Recruiting | Improvement in fibrosis score CRN or NASH resolution |
| Lactobacillus reuteri GMNL-263 and GMNL-89 and lactobacillus rhamnosus GMNL-74 | NAFLD | Gut microbiome | NCT05402449 | N/A | Recruiting | Changes in serum ALT levels |
| Lubiprostone | NAFLD | Type 2 chloride channel activator | NCT05768334 | Phase III | Recruiting | Changes in liver fat by measured by MRI-PDFF |
| Bacillus coagulans TCI711 | NAFLD | Gut microbiome | NCT05635474 | N/A | Recruiting | Changes measured by fibroscan |
| TVB-2640 | NAFLD | FASN inhibitor | NCT04906421 | Phase III | Active, not recruiting | Improvement in NAS and CRN scoring |
| ASC41 | NASH NAFLD | THRβ agonist | NCT05462353 | Phase II | Recruiting | Improvement in NAS score |
| Ketohexokinase inhibition | NAFLD | Ketohexokinase | NCT05463575 | Phase II | Recruiting | Insulin-mediated suppression of endogenous glucose production |
| PF-06865571/ PF-05221304 | NAFLD NASH with fibrosis | DGAT2 inhibitor/ACC inhibitor | NCT04321031 | Phase II | Active, not recruiting | Resolution of NASH |
| ZED1227 | NAFLD fibrosis | TG2 inhibitor | NCT05305599 | Phase II | Recruiting | Relative change of serum PRO-C3 levels |
| MK-3655 | NASH | FGF21 agonist | NCT04583423 | Phase II | Active, not recruiting | Resolution of NASH |
| MXP22 (probiotic and antioxidant capsule) | NAFLD | Gut microbiome | NCT05808049 | N/A | Recruiting | Changes in steatosis measure by fibroscan |
| TERN 501/TERN-101 | NASH | THRβ agonist/ FXR agonist | NCT05415722 | Phase II | Active, not recruiting | Relative change in liver fat content (MRI-PDFF) |
| MET642 | NASH | FXR agonist | NCT04773964 | Phase II | Active, not recruiting | Safety study to measure adverse events |
| VK2809 | NASH | THRβ agonist | NCT04173065 | Phase II | Recruiting | Relative change in liver fat content (MRI-PDFF) |
NAFLD is a prevalent and progressive disease that can lead to liver damage and is strongly associated with obesity, IR, and metabolic syndrome. Current treatments for NAFLD include lifestyle modifications such as diet and exercise, surgery, as well as medications to sensitise insulin. However, there is a need for effective and safe interventions that can directly target the underlying mechanisms of NASH/NAFLD in relation to IR, the gut microbiome and immunological mechanisms.
There is a growing body of evidence that NAFLD and metabolic syndrome are closely linked, and it is crucial for future research to prospectively evaluate interventions and therapeutics which both target improvement to liver outcomes as well as comorbidities associated with NAFLD (including cardiovascular diseases, T2DM, renal dysfunction). It is also essential to develop more refined and early risk stratification tools and biomarkers to identify individuals at the highest risk for NAFLD, considering that this condition affects a substantial portion of the world. Understanding the implications of metabolic signatures, chronic insulin signalling, mitochondrial dysfunction and cellular redox, is important for accurate prognosis and potential therapeutics.
Significant progress in the field has been made using bioinformatics to integrate intra- and extra-hepatic signals including gut and adipose interactions as well as patient information regarding lifestyle, nutrition, and comorbidities. The interplay between this multitude of factors provides promise for advancing therapeutics targeting immune regulation and mitochondrial function during progression of NAFLD. Advancements to the field should consider multidisciplinary approaches for the prevention, diagnosis, treatment, and care of patients with NAFLD.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sun H, China; Zhang Y, China S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis. 2016;20:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (3)] |
| 2. | Ge X, Zheng L, Wang M, Du Y, Jiang J. Prevalence trends in non-alcoholic fatty liver disease at the global, regional and national levels, 1990-2017: a population-based observational study. BMJ Open. 2020;10:e036663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 1212] [Article Influence: 303.0] [Reference Citation Analysis (0)] |
| 4. | Alexander M, Loomis AK, Fairburn-Beech J, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Avillach P, Egger P, Kendrick S, Waterworth DM, Sattar N, Alazawi W. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 5. | Teng ML, Ng CH, Huang DQ, Chan KE, Tan DJ, Lim WH, Yang JD, Tan E, Muthiah MD. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2023;29:S32-S42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 327] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 6. | Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 400] [Article Influence: 133.3] [Reference Citation Analysis (2)] |
| 7. | Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 8. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3797] [Article Influence: 542.4] [Reference Citation Analysis (2)] |
| 9. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 454] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 10. | Kanwar P, Kowdley KV. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clin Liver Dis. 2016;20:225-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2695] [Article Influence: 128.3] [Reference Citation Analysis (3)] |
| 12. | Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, Singhal R, Mahawar K, Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 455] [Article Influence: 151.7] [Reference Citation Analysis (0)] |
| 13. | Ali A, Amin MJ, Ahmed MU, Taj A, Aasim M, Tabrez E. Frequency of non-alcoholic fatty liver disease (NAFLD) and its associated risk factors among Type-2 diabetics. Pak J Med Sci. 2022;38:28-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 14. | Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: A 20 year-community study. Hepatology. 2018;67:1726-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 15. | Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu Rev Pathol. 2016;11:451-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 16. | Bedossa P. Histological Assessment of NAFLD. Dig Dis Sci. 2016;61:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Nassir F, Rector RS, Hammoud GM, Ibdah JA. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol Hepatol (N Y). 2015;11:167-175. [PubMed] |
| 18. | Qayyum A, Nystrom M, Noworolski SM, Chu P, Mohanty A, Merriman R. MRI steatosis grading: development and initial validation of a color mapping system. AJR Am J Roentgenol. 2012;198:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Heyens LJM, Busschots D, Koek GH, Robaeys G, Francque S. Liver Fibrosis in Non-alcoholic Fatty Liver Disease: From Liver Biopsy to Non-invasive Biomarkers in Diagnosis and Treatment. Front Med (Lausanne). 2021;8:615978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 121] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 20. | El-Kamary SS, Mohamed MM, El-Raziky M, Shardell MD, Shaker OG, ElAkel WA, Esmat G. Liver fibrosis staging through a stepwise analysis of non-invasive markers (FibroSteps) in patients with chronic hepatitis C infection. Liver Int. 2013;33:982-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4120] [Article Influence: 206.0] [Reference Citation Analysis (3)] |
| 22. | Shetty A, Jun Yum J, Saab S. The Gastroenterologist's Guide to Preventive Management of Compensated Cirrhosis. Gastroenterol Hepatol (N Y). 2019;15:423-430. [PubMed] |
| 23. | Petrescu M, Vlaicu SI, Ciumărnean L, Milaciu MV, Mărginean C, Florea M, Vesa ȘC, Popa M. Chronic Inflammation-A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 24. | Chen L, Chen R, Wang H, Liang F. Mechanisms Linking Inflammation to Insulin Resistance. Int J Endocrinol. 2015;2015:508409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 352] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 25. | Han HS, Kang G, Kim JS, Choi BH, Koo SH. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48:e218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 508] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 26. | Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753-4761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 642] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 27. | Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 28. | Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 29. | Yan H, Gao Y, Zhang Y. Inhibition of JNK suppresses autophagy and attenuates insulin resistance in a rat model of nonalcoholic fatty liver disease. Mol Med Rep. 2017;15:180-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Khan RS, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 299] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 31. | Delli Bovi AP, Marciano F, Mandato C, Siano MA, Savoia M, Vajro P. Oxidative Stress in Non-alcoholic Fatty Liver Disease. An Updated Mini Review. Front Med (Lausanne). 2021;8:595371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 168] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 32. | Gusdon AM, Song KX, Qu S. Nonalcoholic Fatty liver disease: pathogenesis and therapeutics from a mitochondria-centric perspective. Oxid Med Cell Longev. 2014;2014:637027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Cooper ID, Brookler KH, Kyriakidou Y, Elliott BT, Crofts CAP. Metabolic Phenotypes and Step by Step Evolution of Type 2 Diabetes: A New Paradigm. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Yu L, Hong W, Lu S, Li Y, Guan Y, Weng X, Feng Z. The NLRP3 Inflammasome in Non-Alcoholic Fatty Liver Disease and Steatohepatitis: Therapeutic Targets and Treatment. Front Pharmacol. 2022;13:780496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Lalor P F. The Impact of the NLRP3 Pathway in the Pathogenesis of Non-Alcoholic Fatty Liver Disease and Alcohol-Related Liver Disease. Livers 2021. . [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Xu GX, Wei S, Yu C, Zhao SQ, Yang WJ, Feng YH, Pan C, Yang KX, Ma Y. Activation of Kupffer cells in NAFLD and NASH: mechanisms and therapeutic interventions. Front Cell Dev Biol. 2023;11:1199519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 37. | Wan X, Xu C, Yu C, Li Y. Role of NLRP3 Inflammasome in the Progression of NAFLD to NASH. Can J Gastroenterol Hepatol. 2016;2016:6489012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 38. | Zhu X, Lin X, Zhang P, Liu Y, Ling W, Guo H. Upregulated NLRP3 inflammasome activation is attenuated by anthocyanins in patients with nonalcoholic fatty liver disease: A case-control and an intervention study. Clin Res Hepatol Gastroenterol. 2022;46:101843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl). 2014;92:1069-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 40. | Moayedfard Z, Sani F, Alizadeh A, Bagheri Lankarani K, Zarei M, Azarpira N. The role of the immune system in the pathogenesis of NAFLD and potential therapeutic impacts of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2022;13:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 42. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2920] [Article Influence: 417.1] [Reference Citation Analysis (1)] |
| 43. | Kazankov K, Jørgensen SMD, Thomsen KL, Møller HJ, Vilstrup H, George J, Schuppan D, Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 670] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 44. | Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD - more than inflammation. Nat Rev Endocrinol. 2022;18:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 45. | Xu L, Liu W, Bai F, Xu Y, Liang X, Ma C, Gao L. Hepatic Macrophage as a Key Player in Fatty Liver Disease. Front Immunol. 2021;12:708978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1460] [Cited by in RCA: 1789] [Article Influence: 137.6] [Reference Citation Analysis (0)] |
| 47. | Xiao Z, Liu M, Yang F, Liu G, Liu J, Zhao W, Ma S, Duan Z. Programmed cell death and lipid metabolism of macrophages in NAFLD. Front Immunol. 2023;14:1118449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 48. | Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, Pavoine C. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 438] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 49. | Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate Immunity and Inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 383] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 50. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-34.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 51. | Nati M, Chung KJ, Chavakis T. The Role of Innate Immune Cells in Nonalcoholic Fatty Liver Disease. J Innate Immun. 2022;14:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Schuppan D, Surabattula R, Wang XY. Determinants of fibrosis progression and regression in NASH. J Hepatol. 2018;68:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 372] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 53. | Srinivas AN, Suresh D, Santhekadur PK, Suvarna D, Kumar DP. Extracellular Vesicles as Inflammatory Drivers in NAFLD. Front Immunol. 2020;11:627424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 54. | Dorairaj V, Sulaiman SA, Abu N, Abdul Murad NA. Extracellular Vesicles in the Development of the Non-Alcoholic Fatty Liver Disease: An Update. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Schattenberg JM, Lee MS. Extracellular Vesicles as Messengers Between Hepatocytes and Macrophages in Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 56. | Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Zang S, Wang L, Ma X, Zhu G, Zhuang Z, Xun Y, Zhao F, Yang W, Liu J, Luo Y, Liu Y, Ye D, Shi J. Neutrophils Play a Crucial Role in the Early Stage of Nonalcoholic Steatohepatitis via Neutrophil Elastase in Mice. Cell Biochem Biophys. 2015;73:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Mirea AM, Toonen EJM, van den Munckhof I, Munsterman ID, Tjwa ETTL, Jaeger M, Oosting M, Schraa K, Rutten JHW, van der Graaf M, Riksen NP, de Graaf J, Netea MG, Tack CJ, Chavakis T, Joosten LAB. Increased proteinase 3 and neutrophil elastase plasma concentrations are associated with non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes. Mol Med. 2019;25:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 59. | Pulli B, Ali M, Iwamoto Y, Zeller MW, Schob S, Linnoila JJ, Chen JW. Myeloperoxidase-Hepatocyte-Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid Redox Signal. 2015;23:1255-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 60. | Rensen SS, Slaats Y, Nijhuis J, Jans A, Bieghs V, Driessen A, Malle E, Greve JW, Buurman WA. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 61. | Alkhouri N, Li L, Hanouneh I, Feldstein A, Hazen S. Plasma Myeloperoxidase Levels Correlate with the Presence of Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Am J Gastroenterol 2012; 107: S181. [DOI] [Full Text] |
| 62. | Adler M, Taylor S, Okebugwu K, Yee H, Fielding C, Fielding G, Poles M. Intrahepatic natural killer T cell populations are increased in human hepatic steatosis. World J Gastroenterol. 2011;17:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Kahraman A, Fingas CD, Syn WK, Gerken G, Canbay A. Role of stress-induced NKG2D ligands in liver diseases. Liver Int. 2012;32:370-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Méndez-Sánchez N, Córdova-Gallardo J, Barranco-Fragoso B, Eslam M. Hepatic Dendritic Cells in the Development and Progression of Metabolic Steatohepatitis. Front Immunol. 2021;12:641240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Henning JR, Graffeo CS, Rehman A, Fallon NC, Zambirinis CP, Ochi A, Barilla R, Jamal M, Deutsch M, Greco S, Ego-Osuala M, Bin-Saeed U, Rao RS, Badar S, Quesada JP, Acehan D, Miller G. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology. 2013;58:589-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 66. | Sutti S, Jindal A, Bruzzì S, Locatelli I, Bozzola C, Albano E. Is there a role for adaptive immunity in nonalcoholic steatohepatitis? World J Hepatol. 2015;7:1725-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 68. | Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW, Hubscher SG, Reynolds GM, Aalto K, Anstee QM, Jalkanen S, Salmi M, Smith DJ, Day CP, Adams DH. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest. 2015;125:501-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 69. | Inzaugarat ME, Ferreyra Solari NE, Billordo LA, Abecasis R, Gadano AC, Cherñavsky AC. Altered phenotype and functionality of circulating immune cells characterize adult patients with nonalcoholic steatohepatitis. J Clin Immunol. 2011;31:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, Lorentzen A, Einer C, Schulz S, Clavel T, Protzer U, Thiele C, Zischka H, Moch H, Tschöp M, Tumanov AV, Haller D, Unger K, Karin M, Kopf M, Knolle P, Weber A, Heikenwalder M. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26:549-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 71. | Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59:886-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 72. | Hirsova P, Bamidele AO, Wang H, Povero D, Revelo XS. Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Front Endocrinol (Lausanne). 2021;12:760860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 73. | Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 74. | Chackelevicius CM, Gambaro SE, Tiribelli C, Rosso N. Th17 involvement in nonalcoholic fatty liver disease progression to non-alcoholic steatohepatitis. World J Gastroenterol. 2016;22:9096-9103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 75. | Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L, Qiu D, Li Z, Ma X. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 76. | Giles DA, Moreno-Fernandez ME, Stankiewicz TE, Cappelletti M, Huppert SS, Iwakura Y, Dong C, Shanmukhappa SK, Divanovic S. Regulation of Inflammation by IL-17A and IL-17F Modulates Non-Alcoholic Fatty Liver Disease Pathogenesis. PLoS One. 2016;11:e0149783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 77. | Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, Sheridan R, Xanthakos SA, Steinbrecher KA, Sartor RB, Kohli R, Karp CL, Divanovic S. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830-1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 207] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 78. | Xu R, Tao A, Zhang S, Zhang M. Neutralization of interleukin-17 attenuates high fat diet-induced non-alcoholic fatty liver disease in mice. Acta Biochim Biophys Sin (Shanghai). 2013;45:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, Österreicher CH, Stickel F, Ley K, Brenner DA, Kisseleva T. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-776.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 563] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 80. | Van Herck MA, Weyler J, Kwanten WJ, Dirinck EL, De Winter BY, Francque SM, Vonghia L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front Immunol. 2019;10:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 81. | Rau M, Schilling AK, Meertens J, Hering I, Weiss J, Jurowich C, Kudlich T, Hermanns HM, Bantel H, Beyersdorf N, Geier A. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J Immunol. 2016;196:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 82. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1620] [Article Influence: 115.7] [Reference Citation Analysis (1)] |
| 83. | Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 84. | Tsiantoulas D, Sage AP, Mallat Z, Binder CJ. Targeting B cells in atherosclerosis: closing the gap from bench to bedside. Arterioscler Thromb Vasc Biol. 2015;35:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 85. | Grohmann M, Wiede F, Dodd GT, Gurzov EN, Ooi GJ, Butt T, Rasmiena AA, Kaur S, Gulati T, Goh PK, Treloar AE, Archer S, Brown WA, Muller M, Watt MJ, Ohara O, McLean CA, Tiganis T. Obesity Drives STAT-1-Dependent NASH and STAT-3-Dependent HCC. Cell. 2018;175:1289-1306.e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 86. | Bruzzì S, Sutti S, Giudici G, Burlone ME, Ramavath NN, Toscani A, Bozzola C, Schneider P, Morello E, Parola M, Pirisi M, Albano E. B2-Lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD). Free Radic Biol Med. 2018;124:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 87. | Kawasaki K, Abe M, Tada F, Tokumoto Y, Chen S, Miyake T, Furukawa S, Matsuura B, Hiasa Y, Onji M. Blockade of B-cell-activating factor signaling enhances hepatic steatosis induced by a high-fat diet and improves insulin sensitivity. Lab Invest. 2013;93:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Miyake T, Abe M, Tokumoto Y, Hirooka M, Furukawa S, Kumagi T, Hamada M, Kawasaki K, Tada F, Ueda T, Hiasa Y, Matsuura B, Onji M. B cell-activating factor is associated with the histological severity of nonalcoholic fatty liver disease. Hepatol Int. 2013;7:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 89. | Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 456] [Cited by in RCA: 435] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 90. | DiLillo DJ, Horikawa M, Tedder TF. B-lymphocyte effector functions in health and disease. Immunol Res. 2011;49:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Béland K, Marceau G, Labardy A, Bourbonnais S, Alvarez F. Depletion of B cells induces remission of autoimmune hepatitis in mice through reduced antigen presentation and help to T cells. Hepatology. 2015;62:1511-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 92. | McPherson S, Henderson E, Burt AD, Day CP, Anstee QM. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 93. | Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 425] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 94. | Gregory SN, Perati SR, Brown ZJ. Alteration in immune function in patients with fatty liver disease. Hepatoma Res 2022; 8: 31. [DOI] [Full Text] |
| 95. | Sung PS, Park DJ, Roh PR, Mun KD, Cho SW, Lee GW, Jung ES, Lee SH, Jang JW, Bae SH, Choi JY, Choi J, Ahn J, Yoon SK. Intrahepatic inflammatory IgA(+)PD-L1(high) monocytes in hepatocellular carcinoma development and immunotherapy. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 96. | Barrow F, Khan S, Fredrickson G, Wang H, Dietsche K, Parthiban P, Robert S, Kaiser T, Winer S, Herman A, Adeyi O, Mouzaki M, Khoruts A, Hogquist KA, Staley C, Winer DA, Revelo XS. Microbiota-Driven Activation of Intrahepatic B Cells Aggravates NASH Through Innate and Adaptive Signaling. Hepatology. 2021;74:704-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 97. | Nassir F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 98. | Ando Y, Jou JH. Nonalcoholic Fatty Liver Disease and Recent Guideline Updates. Clin Liver Dis (Hoboken). 2021;17:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 99. | Piazzolla VA, Mangia A. Noninvasive Diagnosis of NAFLD and NASH. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 100. | Sanyal D, Mukherjee P, Raychaudhuri M, Ghosh S, Mukherjee S, Chowdhury S. Profile of liver enzymes in non-alcoholic fatty liver disease in patients with impaired glucose tolerance and newly detected untreated type 2 diabetes. Indian J Endocrinol Metab. 2015;19:597-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 101. | Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 102. | Zeng Y, He H, An Z. Advance of Serum Biomarkers and Combined Diagnostic Panels in Nonalcoholic Fatty Liver Disease. Dis Markers. 2022;2022:1254014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Kumar R, Teo EK, How CH, Wong TY, Ang TL. A practical clinical approach to liver fibrosis. Singapore Med J. 2018;59:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Woreta TA, Van Natta ML, Lazo M, Krishnan A, Neuschwander-Tetri BA, Loomba R, Mae Diehl A, Abdelmalek MF, Chalasani N, Gawrieh S, Dasarathy S, Vuppalanchi R, Siddiqui MS, Kowdley KV, McCullough A, Terrault NA, Behling C, Kleiner DE, Fishbein M, Hertel P, Wilson LA, Mitchell EP, Miriel LA, Clark JM, Tonascia J, Sanyal AJ; NASH Clinical Research Network. Validation of the accuracy of the FAST™ score for detecting patients with at-risk nonalcoholic steatohepatitis (NASH) in a North American cohort and comparison to other non-invasive algorithms. PLoS One. 2022;17:e0266859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 105. | Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, Yilmaz Y, Czernichow S, Zheng MH, Wong VW, Allison M, Tsochatzis E, Anstee QM, Sheridan DA, Eddowes PJ, Guha IN, Cobbold JF, Paradis V, Bedossa P, Miette V, Fournier-Poizat C, Sandrin L, Harrison SA. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 556] [Cited by in RCA: 550] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 106. | Lee JS, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Jang JY, Park SY, Lee CK, Kim SU. Comparison of FibroScan-Aspartate Aminotransferase (FAST) Score and Other Non-invasive Surrogates in Predicting High-Risk Non-alcoholic Steatohepatitis Criteria. Front Med (Lausanne). 2022;9:869190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | Reinson T, Buchanan RM, Byrne CD. Noninvasive serum biomarkers for liver fibrosis in NAFLD: current and future. Clin Mol Hepatol. 2023;29:S157-S170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 108. | Singh SP, Barik RK. NonInvasive Biomarkers in Nonalcoholic Fatty Liver Disease: Are We There Yet? J Clin Exp Hepatol. 2020;10:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 109. | Dufour JF, Anstee QM, Bugianesi E, Harrison S, Loomba R, Paradis V, Tilg H, Wong VW, Zelber-Sagi S. Current therapies and new developments in NASH. Gut. 2022;71:2123-2134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 110. | Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 111. | Zhao J, Hu Y, Peng J. Targeting programmed cell death in metabolic dysfunction-associated fatty liver disease (MAFLD): a promising new therapy. Cell Mol Biol Lett. 2021;26:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 112. | Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268-31279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 860] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 113. | O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2111] [Cited by in RCA: 3374] [Article Influence: 482.0] [Reference Citation Analysis (0)] |
| 114. | Salvoza NC, Klinzing DC, Gopez-Cervantes J, Baclig MO. Association of Circulating Serum miR-34a and miR-122 with Dyslipidemia among Patients with Non-Alcoholic Fatty Liver Disease. PLoS One. 2016;11:e0153497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 115. | Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, Sugimoto K, Ohashi K, Teradaira R, Inoue T, Hamajima N, Hashimoto S. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 116. | Celikbilek M, Baskol M, Taheri S, Deniz K, Dogan S, Zararsiz G, Gursoy S, Guven K, Ozbakır O, Dundar M, Yucesoy M. Circulating microRNAs in patients with non-alcoholic fatty liver disease. World J Hepatol. 2014;6:613-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 117. | Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 467] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 118. | Liu XL, Pan Q, Zhang RN, Shen F, Yan SY, Sun C, Xu ZJ, Chen YW, Fan JG. Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population. World J Gastroenterol. 2016;22:9844-9852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (2)] |
| 119. | Sun Y, Shen Y, Liang X, Zheng H, Zhang Y. MicroRNAs as Biomarkers and Therapeutic Targets for Nonalcoholic Fatty Liver Disease: A Narrative Review. Clin Ther. 2023;45:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 120. | Pillai SS, Lakhani HV, Zehra M, Wang J, Dilip A, Puri N, O'Hanlon K, Sodhi K. Predicting Nonalcoholic Fatty Liver Disease through a Panel of Plasma Biomarkers and MicroRNAs in Female West Virginia Population. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Eguchi A, Iwasa M, Nakagawa H. Extracellular vesicles in fatty liver disease and steatohepatitis: Role as biomarkers and therapeutic targets. Liver Int. 2023;43:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 122. | Wu D, Zhu H, Wang H. Extracellular Vesicles in Non-alcoholic Fatty Liver Disease and Alcoholic Liver Disease. Front Physiol. 2021;12:707429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 123. | Kranendonk ME, Visseren FL, van Balkom BW, Nolte-'t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014;22:1296-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 124. | Kranendonk ME, Visseren FL, van Herwaarden JA, Nolte-'t Hoen EN, de Jager W, Wauben MH, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 2014;22:2216-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 125. | Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 126. | Garcia-Martinez I, Alen R, Rada P, Valverde AM. Insights Into Extracellular Vesicles as Biomarker of NAFLD Pathogenesis. Front Med (Lausanne). 2020;7:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Moore MP, Cunningham RP, Dashek RJ, Mucinski JM, Rector RS. A Fad too Far? Dietary Strategies for the Prevention and Treatment of NAFLD. Obesity (Silver Spring). 2020;28:1843-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 128. | Mantovani A, Dalbeni A. Treatments for NAFLD: State of Art. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 129. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-78.e5; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1627] [Article Influence: 162.7] [Reference Citation Analysis (1)] |
| 130. | Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, Chan FK, Sung JJ, Woo J, Chan HL. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. 2013;59:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 131. | Mascaró CM, Bouzas C, Tur JA. Association between Non-Alcoholic Fatty Liver Disease and Mediterranean Lifestyle: A Systematic Review. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 132. | Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, Okamoto Y, Tanaka K, Shoda J. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci Rep. 2017;7:43029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 133. | Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21:e13024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 134. | Luukkonen PK, Dufour S, Lyu K, Zhang XM, Hakkarainen A, Lehtimäki TE, Cline GW, Petersen KF, Shulman GI, Yki-Järvinen H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2020;117:7347-7354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 135. | Achanta LB, Rae CD. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem Res. 2017;42:35-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 136. | Wu Y, Teng Y, Zhang C, Pan Y, Zhang Q, Zhu X, Liu N, Su X, Lin J. The ketone body β-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J Nanobiotechnology. 2022;20:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 137. | Miller VJ, Villamena FA, Volek JS. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J Nutr Metab. 2018;2018:5157645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 138. | O'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, Crosthwaite G, Brown W. Long-Term Outcomes After Bariatric Surgery: a Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes Surg. 2019;29:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 481] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 139. | Głuszyńska P, Lemancewicz D, Dzięcioł JB, Razak Hady H. Non-Alcoholic Fatty Liver Disease (NAFLD) and Bariatric/Metabolic Surgery as Its Treatment Option: A Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 140. | Lee KC, Wu PS, Lin HC. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin Mol Hepatol. 2023;29:77-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 101] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 141. | Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:1040-1060.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 142. | Fakhry TK, Mhaskar R, Schwitalla T, Muradova E, Gonzalvo JP, Murr MM. Bariatric surgery improves nonalcoholic fatty liver disease: a contemporary systematic review and meta-analysis. Surg Obes Relat Dis. 2019;15:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 143. | Baldwin D, Chennakesavalu M, Gangemi A. Systematic review and meta-analysis of Roux-en-Y gastric bypass against laparoscopic sleeve gastrectomy for amelioration of NAFLD using four criteria. Surg Obes Relat Dis. 2019;15:2123-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 144. | Pedersen JS, Rygg MO, Serizawa RR, Kristiansen VB, Albrechtsen NJW, Gluud LL, Madsbad S, Bendtsen F. Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Non-Alcoholic Fatty Liver Disease: A 12-Month Follow-Up Study with Paired Liver Biopsies. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 145. | Ozturk ZA, Kadayifci A. Insulin sensitizers for the treatment of non-alcoholic fatty liver disease. World J Hepatol. 2014;6:199-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 146. | Pinyopornpanish K, Leerapun A, Pinyopornpanish K, Chattipakorn N. Effects of Metformin on Hepatic Steatosis in Adults with Nonalcoholic Fatty Liver Disease and Diabetes: Insights from the Cellular to Patient Levels. Gut Liver. 2021;15:827-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 147. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 148. | Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 149. | Brandt A, Hernández-Arriaga A, Kehm R, Sánchez V, Jin CJ, Nier A, Baumann A, Camarinha-Silva A, Bergheim I. Metformin attenuates the onset of non-alcoholic fatty liver disease and affects intestinal microbiota and barrier in small intestine. Sci Rep. 2019;9:6668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 150. | Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, Rinaldi L, Marfella R, Sasso FC. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 128] [Reference Citation Analysis (0)] |
| 151. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1494] [Article Influence: 186.8] [Reference Citation Analysis (0)] |
| 152. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1216] [Article Influence: 304.0] [Reference Citation Analysis (0)] |
| 153. | Raptis DD, Mantzoros CS, Polyzos SA. Fibroblast Growth Factor-21 as a Potential Therapeutic Target of Nonalcoholic Fatty Liver Disease. Ther Clin Risk Manag. 2023;19:77-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 154. | Tian H, Zhang S, Liu Y, Wu Y, Zhang D. Fibroblast Growth Factors for Nonalcoholic Fatty Liver Disease: Opportunities and Challenges. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 155. | Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, Behling CA, Hu C, Fong E, de Temple B, Tillman EJ, Rolph TP, Cheng A, Yale K. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021;27:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 156. | Caddeo A, Kowalik MA, Serra M, Runfola M, Bacci A, Rapposelli S, Columbano A, Perra A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 157. | Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, Bansal MB, Baum S, Neuschwander-Tetri BA, Taub R, Moussa SE. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 524] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 158. | Zhou J, Waskowicz LR, Lim A, Liao XH, Lian B, Masamune H, Refetoff S, Tran B, Koeberl DD, Yen PM. A Liver-Specific Thyromimetic, VK2809, Decreases Hepatosteatosis in Glycogen Storage Disease Type Ia. Thyroid. 2019;29:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 159. | Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 160. | Nelson CH, Jones C, Klucher K, Jin F, Marmon T, Chung D et al. Liver-distributed farnesoid X receptor agonist TERN-101 demonstrates potent target engagement with a favorable exposure-response profile in non-alcoholic steatohepatitis patients. J Hepatol. 2022;77:S716. [DOI] [Full Text] |
| 161. | Kirschberg T, Jones C, Xu Y, Wang Y, Fenaux M, Klucher K. TERN-501, a potent and selective agonist of thyroid hormone receptor beta, strongly reduces histological features and biomarkers of non-alcoholic steatohepatitis associated pathology in rodent models. J Hepatol. 2020;73:S684. |
| 162. | Wiering L, Tacke F. Treating inflammation to combat non-alcoholic fatty liver disease. J Endocrinol. 2023;256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 163. | Ortiz-López N, Fuenzalida C, Dufeu MS, Pinto-León A, Escobar A, Poniachik J, Roblero JP, Valenzuela-Pérez L, Beltrán CJ. The immune response as a therapeutic target in non-alcoholic fatty liver disease. Front Immunol. 2022;13:954869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 164. | Ndakotsu A, Vivekanandan G. The Role of Thiazolidinediones in the Amelioration of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus. 2022;14:e25380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 165. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 166. | Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A; GOLDEN-505 Investigator Study Group. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology. 2016;150:1147-1159.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 167. | Sanyal AJ, Lopez P, Lawitz EJ, Lucas KJ, Loeffler J, Kim W, Goh GBB, Huang JF, Serra C, Andreone P, Chen YC, Hsia SH, Ratziu V, Aizenberg D, Tobita H, Sheikh AM, Vierling JM, Kim YJ, Hyogo H, Tai D, Goodman Z, Schaefer F, Carbarns IRI, Lamle S, Martic M, Naoumov NV, Brass CA. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat Med. 2023;29:392-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 168. | Abenavoli L, Maurizi V, Rinninella E, Tack J, Di Berardino A, Santori P, Rasetti C, Procopio AC, Boccuto L, Scarpellini E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 169. | Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW, Fan JG. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep. 2017;7:1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 170. | Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, Hermes G, Bouter KE, Koopen AM, Holst JJ, Knop FK, Blaak EE, Zhao J, Smidt H, Harms AC, Hankemeijer T, Bergman JJGHM, Romijn HA, Schaap FG, Olde Damink SWM, Ackermans MT, Dallinga-Thie GM, Zoetendal E, de Vos WM, Serlie MJ, Stroes ESG, Groen AK, Nieuwdorp M. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017;26:611-619.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 681] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 171. | Fujita K, Nozaki Y, Wada K, Yoneda M, Endo H, Takahashi H, Iwasaki T, Inamori M, Abe Y, Kobayashi N, Kirikoshi H, Kubota K, Saito S, Nagashima Y, Nakajima A. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut. 2008;57:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 172. | Thongtan T, Deb A, Vutthikraivit W, Laoveeravat P, Mingbunjerdsuk T, Islam S, Islam E. Antiplatelet therapy associated with lower prevalence of advanced liver fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Indian J Gastroenterol. 2022;41:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 173. | Hilscher MB, Shah VH. Small but Mighty: Platelets in NASH and Other Chronic Liver Diseases. Hepatology. 2020;71:1501-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 174. | Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, Peiseler M, Surewaard BGJ, Rath D, Ali A, Wolf MJ, Drescher H, Healy ME, Dauch D, Kroy D, Krenkel O, Kohlhepp M, Engleitner T, Olkus A, Sijmonsma T, Volz J, Deppermann C, Stegner D, Helbling P, Nombela-Arrieta C, Rafiei A, Hinterleitner M, Rall M, Baku F, Borst O, Wilson CL, Leslie J, O'Connor T, Weston CJ, Chauhan A, Adams DH, Sheriff L, Teijeiro A, Prinz M, Bogeska R, Anstee N, Bongers MN, Notohamiprodjo M, Geisler T, Withers DJ, Ware J, Mann DA, Augustin HG, Vegiopoulos A, Milsom MD, Rose AJ, Lalor PF, Llovet JM, Pinyol R, Tacke F, Rad R, Matter M, Djouder N, Kubes P, Knolle PA, Unger K, Zender L, Nieswandt B, Gawaz M, Weber A, Heikenwalder M. Platelet GPIbα is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 175. | Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, Friebel E, Engleitner T, Lenggenhager D, Moncsek A, Heide D, Stirm K, Kosla J, Kotsiliti E, Leone V, Dudek M, Yousuf S, Inverso D, Singh I, Teijeiro A, Castet F, Montironi C, Haber PK, Tiniakos D, Bedossa P, Cockell S, Younes R, Vacca M, Marra F, Schattenberg JM, Allison M, Bugianesi E, Ratziu V, Pressiani T, D'Alessio A, Personeni N, Rimassa L, Daly AK, Scheiner B, Pomej K, Kirstein MM, Vogel A, Peck-Radosavljevic M, Hucke F, Finkelmeier F, Waidmann O, Trojan J, Schulze K, Wege H, Koch S, Weinmann A, Bueter M, Rössler F, Siebenhüner A, De Dosso S, Mallm JP, Umansky V, Jugold M, Luedde T, Schietinger A, Schirmacher P, Emu B, Augustin HG, Billeter A, Müller-Stich B, Kikuchi H, Duda DG, Kütting F, Waldschmidt DT, Ebert MP, Rahbari N, Mei HE, Schulz AR, Ringelhan M, Malek N, Spahn S, Bitzer M, Ruiz de Galarreta M, Lujambio A, Dufour JF, Marron TU, Kaseb A, Kudo M, Huang YH, Djouder N, Wolter K, Zender L, Marche PN, Decaens T, Pinato DJ, Rad R, Mertens JC, Weber A, Unger K, Meissner F, Roth S, Jilkova ZM, Claassen M, Anstee QM, Amit I, Knolle P, Becher B, Llovet JM, Heikenwalder M. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 845] [Article Influence: 211.3] [Reference Citation Analysis (1)] |
| 176. | Jeong S, Shin WY, Oh YH. Immunotherapy for NAFLD and NAFLD-related hepatocellular carcinoma. Front Endocrinol (Lausanne). 2023;14:1150360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 177. | Kudo M. Impaired Response to Immunotherapy in Non-Alcoholic Steatohepatitis-Related Hepatocellular Carcinoma? Liver Cancer. 2021;10:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | T-cell Dysfunction Limits Immunotherapy Efficacy in NASH-Induced Liver Cancer. Cancer Discov. 2021;11:OF31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |