Published online Aug 21, 2023. doi: 10.3748/wjg.v29.i31.4797
Peer-review started: June 15, 2023
First decision: July 14, 2023
Revised: July 21, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: August 21, 2023
Processing time: 64 Days and 1.8 Hours
The relationship between copeptin and the severity of circulatory dysfunction and systemic stress response in patients with chronic liver disease (CLD) has been established. Nevertheless, the potential of serum copeptin levels to predict the prognosis of CLD patients remains unclear.
To conduct a systematic review and meta-analysis to investigate the correlation between serum copeptin and transplant-free survival (TFS) in this population.
To achieve the objective of the meta-analysis, PubMed, Embase, the Cochrane Library, and the Web of Science were searched to identify observational studies with longitudinal follow-up. The Cochrane Q test was utilized to assess between-study heterogeneity, and the I2 statistic was estimated. Random-effects models were employed to combine the outcomes, taking into account the potential influence of heterogeneity.
Ten datasets including 3133 patients were involved. The follow-up durations were 1 to 48 mo (mean: 12.5 mo). Overall, it was shown that a high level of serum copeptin was associated with a poor TFS [risk ratio (RR): 1.82, 95% confidence interval: 1.52-2.19, P < 0.001; I2 = 0%]. In addition, sensitivity analysis by omitting one dataset at a time showed consistent results (RR: 1.73-2.00, P < 0.05). Finally, subgroup analyses according to study country, study design, patient diagnosis, cutoff of copeptin, follow-up duration, and study quality score also showed similar results (P for subgroup difference all > 0.05).
Patients with CLD who have high serum copeptin concentrations may be associated with a poor clinical prognosis.
Core Tip: Serum copeptin has been related to the severity of circulatory dysfunction and systemic stress response in patients with chronic liver disease (CLD). However, little is known about the relationship between serum copeptin and the prognosis of patients with CLD. In this systematic review and meta-analysis, evidence from ten datasets including 3133 patients were integrated. The results showed that a high level of serum copeptin was associated with a poor transplant-free survival in these patients. These findings support the use of serum copeptin as a prognostic biomarker for patients with CLD.
- Citation: Tan HQ, Zhao M, Huang Z, Liu Y, Li H, Ma LH, Liu JY. Circulating copeptin level and the clinical prognosis of patients with chronic liver disease. World J Gastroenterol 2023; 29(31): 4797-4808
- URL: https://www.wjgnet.com/1007-9327/full/v29/i31/4797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i31.4797
Chronic liver disease (CLD) patients are more likely to develop complications associated with the progression of the disease, such as portal hypertension, ascites, spontaneous bacterial peritonitis, gastroesophageal varices, and hepatic encephalopathy[1,2]. For those with advanced CLD (ACLD), the clinical prognosis is generally poor, which has become a substantial cause of morbidity and mortality worldwide[3,4]. Accumulating evidence suggests that patients with ACLD may have multiple features of circulatory dysfunction, such as incremental intrahepatic vascular resistance, decrease of portal blood flow, and a reduced systemic vascular resistance[5,6], all of which could subsequently activate the neuro
Throughout the process of planning, conducting, and reporting the study, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[15,16] and Cochrane Handbook[17] were followed.
We searched electronic databases including PubMed, Embase, the Cochrane Library, and Web of Science, starting from inception and ending April 21st, 2023, for studies that had been published up to that date. The search was performed with terms related to our study including: (1) “Copeptin” OR “C-terminal provasopressin”; and (2) “cirrhosis” OR “cirrhotic” OR “liver” OR “hepatic” OR “hepatitis”. There was no limitation on the language of the publication in the search for human studies. As part of our manual screening process, references from relevant original and review articles were screened for possible relevant studies.
Inclusion criteria were developed in accordance with PICOS recommendations and according to the aim of the meta-analysis.
P (patients): Adult patients (18 years or older) with a confirmed diagnosis of CLD; I (exposure): Patients with a high serum concentration of copeptin at baseline. Methods for measuring serum copeptin and cutoffs for defining high serum copeptin were consistent with those of the original studies; C (control): Patients with a low serum concentration of copeptin at baseline; O (outcomes): Incidence of transplant-free survival (TFS) compared between CLD patients with high vs low serum levels of copeptin at baseline. S (study design): Studies with longitudinal follow-up, including cohort and case-control studies, as well as post-hoc analyses of clinical trials.
Excluded from the meta-analysis were reviews, editorials, preclinical studies, and studies that did not involve patients with CLD, failed to measure serum copeptin, or did not report the relevant outcome. In instances where there was a patient population overlap, the study with the greatest sample size was incorporated into the meta-analysis.
Two of the authors conducted literature searches, data collection, and assessments of study quality independently. In instances where discrepancies arose, a third author was consulted for discussion, and a consensus was reached. The analysis of studies included the collection of information pertaining to study information, design characteristics, patient diagnosis, demographic factors, measuring methods, serum copeptin cutoffs, follow-up durations, and adjusted variables for the evaluation of the association between serum copeptin levels and TFS in patients with CLD. In terms of quality, the study was scored using the Newcastle-Ottawa Scale[18] based on the criteria for participant selection, the comparability of the groups, and the validity of the outcomes. There were nine stars on the scale, with a larger number of stars representing a higher quality study.
Risk ratios (RRs) and corresponding 95% confidence interval (CI) were used as the variables to indicate the association between serum concentration of copeptin and the survival of patients with CLD. A logarithmical transformation was performed on the RR and its corresponding standard error from each study to stabilize and normalize its variance[19]. In order to estimate between-study heterogeneity, the Cochrane Q test and the I2 statistic[20] were used. An I2 > 50% indicates that there is significant heterogeneity between studies. The utilization of a random-effects model was employed to amalgamate the findings, as it has been acknowledged to encompass the impact of potential heterogeneity[17]. In order to assess the impact of individual studies on the meta-analysis outcomes, sensitivity analyses were conducted by eliminating one dataset at a time[21]. To ascertain the effect of study characteristics on the outcome, subgroup analyses were executed based on the study country, design, patient diagnosis, cutoffs of copeptin, follow-up duration, and study quality scores. The subgroups were defined based on the medians of continuous variables. A funnel plot was used to estimate publication bias based on visual judgments of symmetry, along with Egger’s regression asymmetry test[22]. The statistical analyses were carried out with RevMan (Version 5.1; Cochrane Collaboration, Oxford, United Kingdom) and Stata software (version 12.0; Stata Corporation, College Station, TX, United States).
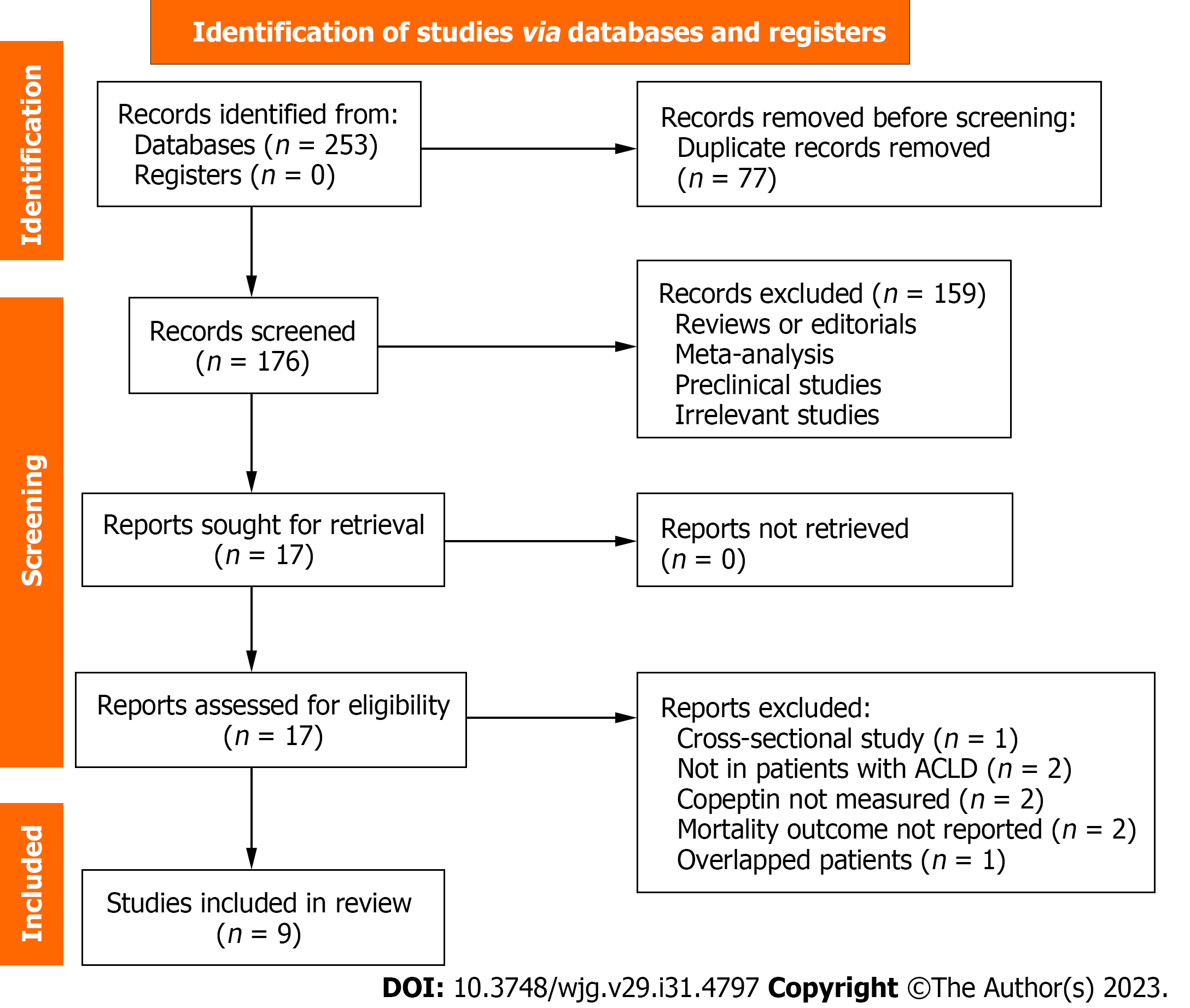
Figure 1 shows the process of the literature search and study retrieval. Initially, 253 records were obtained from the database, and 77 duplicate entries were removed. After screening the titles and abstracts, a further 159 studies were removed as they did not fit the meta-analysis’ objectives. Following full-text reviews of 17 studies, eight were excluded because of the reasons listed in Figure 1. Accordingly, 9 studies were obtained for subsequent meta-analysis[23-31].
One of the studies reported two datasets from two cohort studies[26], and these datasets were independently incorporated into the meta-analysis. Overall, ten datasets from nine cohort studies[23-31], which included 3133 patients with CLD, were used for the meta-analysis. The characteristics of the included studies are summarized in Table 1. These studies were published between 2013 and 2021, and performed in European and Asian countries. As for the study design, four cohorts were prospective[23,25,26,29], and six were retrospective[24,26-28,30,31]. Patients with cirrhosis were included in six cohorts of the included studies[23-27], while patients with various CLD were included in the other four cohorts[28-31]. The mean ages of the patients were 49.0 years to 67.3 years, and the proportions of men were 58.2% to 83.4%. Serum copeptin was measured with the Kryptor immunoassay in eight cohorts[23-28,30], while other methods such as enzyme-linked immunosorbent assay and the automated copeptin immunofluorescent assay were used in the other two studies[29,31]. A high serum level of copeptin was defined according to receiver operating characteristic analysis in four cohorts[24,25,27,31], medians in three cohorts[26,29], tertiles in two cohorts[23,28], and the upper limit of normal level in one cohort[30]. The follow-up durations were 1 mo to 48 mo (mean: 12.5 mo). Multivariate regression analyses were applied in all of the included studies when the association between serum copeptin and TFS of patients with CLD were estimated, and factors including age, sex, and scores for hepatic dysfunction were adjusted, such as the Child-Pugh score, the Model for End-Stage Liver Disease score, and the albumin-bilirubin score. Among the included studies, all had quality scores between seven and nine stars, indicating that they were of good quality (Table 2).
| Ref. | Country | Design | Diagnosis | Patient number | Mean age (yr) | Male (%) | CP class C (%) | Methods for copeptin measuring | Copeptin analysis | Median follow-up duration (mo) | Variables adjusted |
| Moreno et al[23], 2013 | France | PC | Cirrhosis (alcohol 84.2%) | 125 | 58 | 69 | 33.7 | Kryptor immunoassay | 13 pmol/L, T3:T1-2 | 11 | Age, sex, CP Class, and CRP |
| Kerbert et al[24], 2015 | The Netherlands | RC | Cirrhosis (alcohol 34.4%, viral 18%) | 61 | 54 | 75.4 | 31.1 | Kryptor immunoassay | 21.9 pmol/L, ROC analysis | 12 | Age, sex, and MELD score |
| Sola 2016-original | Spain | PC | Cirrhosis (alcohol 43%, HCV 34%) | 265 | 60 | 66 | NR | Kryptor immunoassay | 14 pmol/L, median | 3 | Age, sex, MELD score, and leukocyte count |
| Sola 2016-validation | Multiple European countries | RC | Cirrhosis (alcohol 51%, HCV 19%) | 120 | 57 | 72 | NR | Kryptor immunoassay | 19 pmol/L, median | 3 | Age, sex, and MELD score |
| Kerbert et al[25], 2016 | The Netherlands and France | PC | Cirrhosis (alcohol 70.1%, viral 11.4%) | 184 | 55.7 | 70.7 | 21.2 | Kryptor immunoassay | 12.3 pmol/L, ROC analysis | 12 | Age, sex, CP class, and CRP |
| Kerbert et al[27], 2017 | Multiple European countries | RC | Acute decompensated cirrhosis (alcohol 61%, HCV 31.9%, and HBV 5.7%) | 779 | 58 | 65.7 | NR | Kryptor immunoassay | 13.6 pmol/L, ROC analysis | 3 | Age, sex, WBC, sodium, and MELD score |
| Schneider et al[28], 2019 | Germany | RC | End-stage liver disease (alcohol 63.4%, viral 8.5%) | 615 | 57.2 | 61.9 | NR | Kryptor immunoassay | 16.3 pmol/L, T3:T1-2 | 9.3 | Age, sex, and MELD-Na score |
| Zhao et al[29], 2019 | China | PC | HBV related ACLF | 151 | 49 | 83.4 | NR | ELISA | 18.7 pmol/L, median | 1 | Age, sex, and MELD score |
| Hartl et al[30], 2021 | Austria | RC | ACLD (alcohol 36.2%, viral 35.9%) | 663 | 56.6 | 68.2 | 16.5 | Kryptor immunoassay | 11.4 pmol/L, ULN | 26.2 | Age, sex, MELD score, HVPG, albumin, sodium, and presence of HCC |
| Shigefuku et al[36], 2021 | Japan | RC | CLD (alcohol 45.9%, viral 57.0%), cirrhosis 66.5% | 170 | 67.3 | 58.2 | NR | Automated copeptin immunofluorescent assay | 4.8 pmol/L, ROC analysis | 48 | Age, sex, eGFR, ALBI score, and presence of HCC |
| Ref. | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age and sex | Control for other confounding factors | Assessment of outcome | Suitable follow-up duration | Adequacy of follow-up of cohorts | Total |
| Moreno et al[23], 2013 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Kerbert et al[24], 2015 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Sola 2016-original | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Sola 2016-validation | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Kerbert et al[25], 2016 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kerbert et al[27], 2017 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Schneider et al[28], 2019 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Zhao et al[29], 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 8 |
| Hartl et al[30], 2021 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Shigefuku et al[36], 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
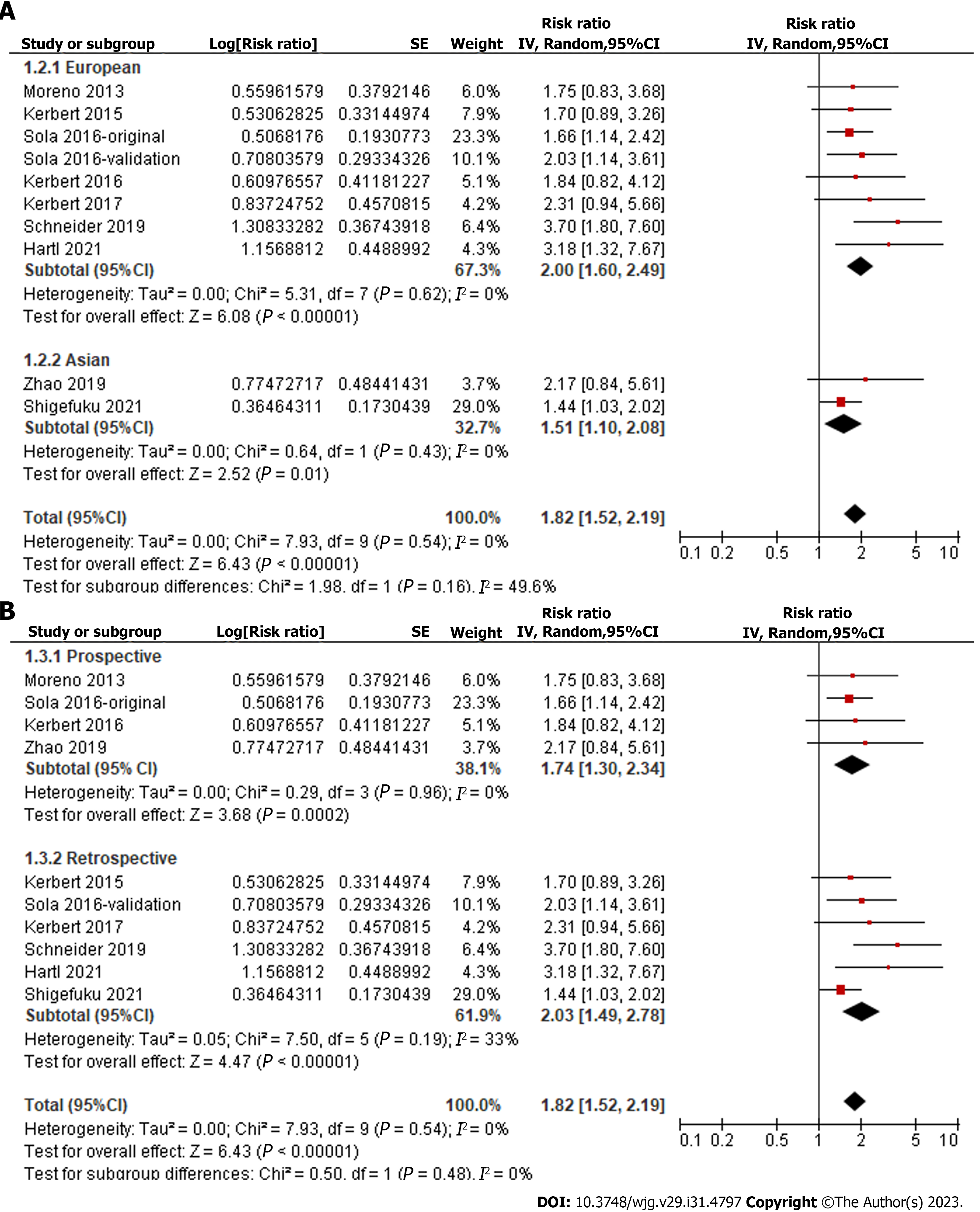
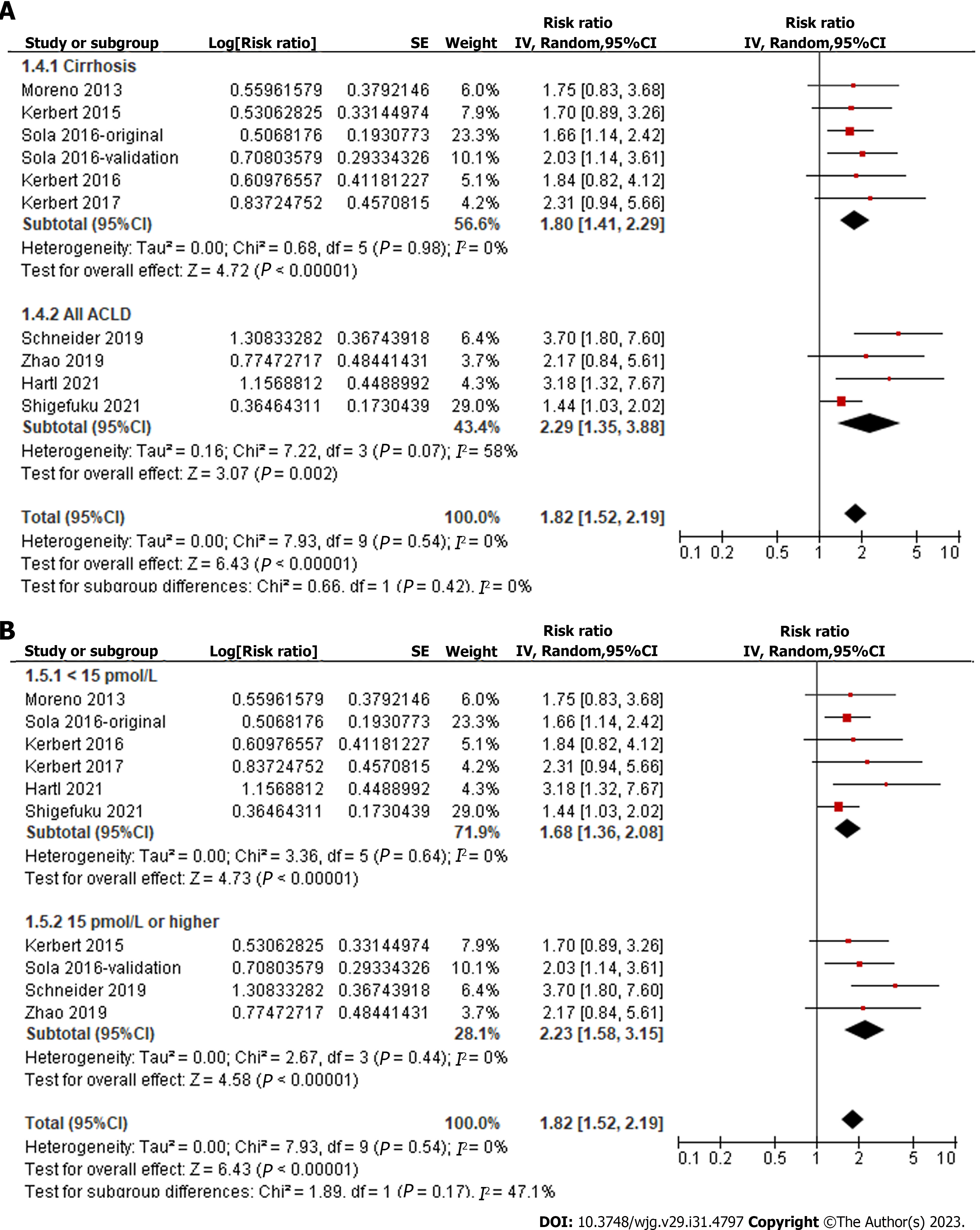
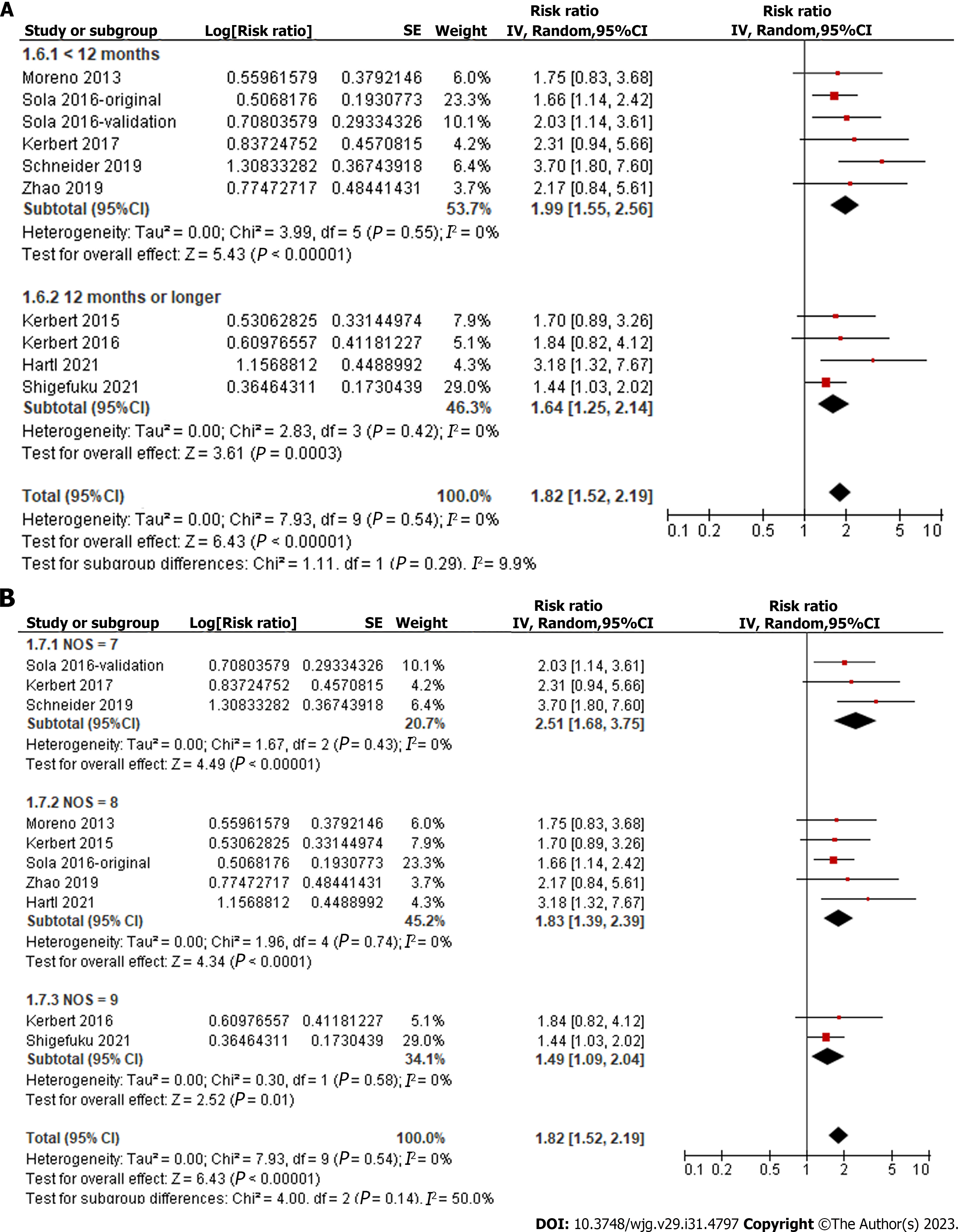
Overall, pooled results of ten datasets from nine cohort studies showed that a high level of serum copeptin at baseline was associated with a poor TFS (RR: 1.82, 95%CI: 1.52-2.19, P < 0.001; Figure 2) with no evidence of significant heterogeneity (P for Cochrane Q test = 0.54, I2 = 0%). In addition, sensitivity analysis by omitting one dataset at a time showed consistent results (RR: 1.73-2.00, P < 0.05). Finally, subgroup analyses showed that the association between serum copeptin and poor TFS was consistent between European and Asian studies (P for subgroup difference = 0.16, Figure 3A), between prospective and retrospective cohorts (P for subgroup difference = 0.48, Figure 3B), between studies of patients with cirrhosis and all CLD (P for subgroup difference = 0.42, Figure 4A), between studies with cutoffs for copeptin < and ≥ 15 pmol/L (P for subgroup difference = 0.17, Figure 4B), between studies with mean follow-up durations < and ≥ 1 year (P for subgroup difference = 0.29, Figure 5A), and between studies with different quality scores (P for subgroup difference = 0.14, Figure 5B).
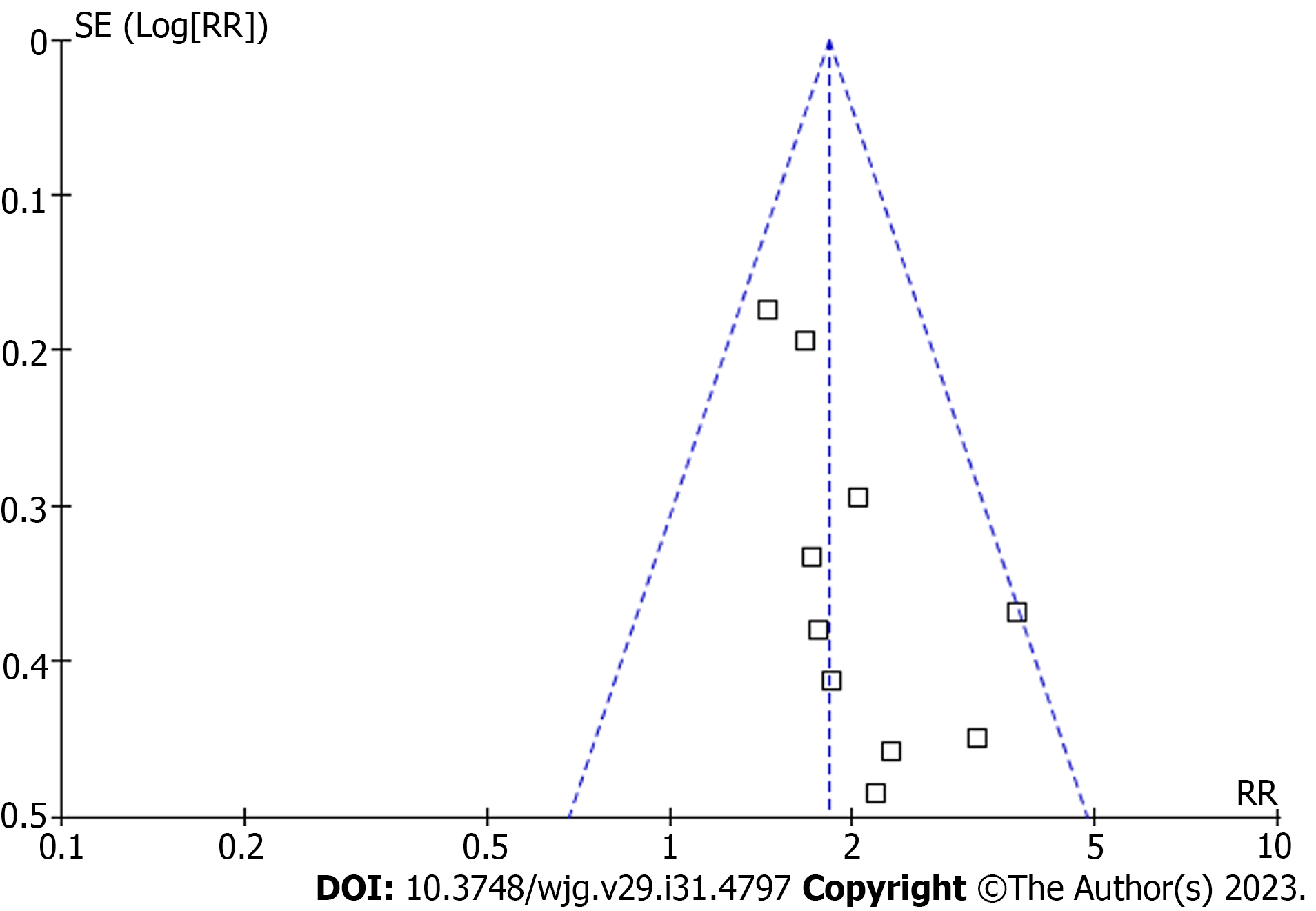
The funnel plots for the meta-analysis of the association between copeptin and TFS in patients with CLD are shown in Figure 6. Based on visual examination, the plots are symmetrical, suggesting that publication bias is low. Additionally, Egger’s regression tests indicated a low likelihood of publication bias (P = 0.47).
This systematic review and meta-analysis synthesized data from ten cohort studies to investigate the association between serum copeptin concentration and poor TFS in patients with CLD. Our findings indicate that patients with a high serum copeptin level at baseline are at a greater risk of experiencing poor TFS during follow-up, compared to those with a low serum copeptin concentration. The robustness of our results was confirmed through sensitivity analyses that excluded individual datasets and subgroup analyses based on various study characteristics, including country, design, patient diagnosis, copeptin cutoffs, follow-up duration, and study quality scores. These findings demonstrate that a high serum copeptin level may be a useful index which is associated with poor prognosis for patients with CLD.
To the best of our knowledge, this is the first meta-analysis investigating the potential role of serum copeptin concentration as a prognostic factor of patients with CLD. Several advantages in meta-analysis methodologies deserve to be noticed. For example, we performed a comprehensive literature search in four commonly used databases, which could provide current evidence regarding the relationship of serum copeptin and TFS of patients with CLD. Furthermore, it is noteworthy that all of the studies incorporated in this analysis were cohort studies, implying a potential longitudinal correlation between elevated serum copeptin levels and diminished transplant-free survival among individuals with CLD. Additionally, the utilization of multivariate regression analysis in all of the studies included in this review indicates that the relationship between heightened serum copeptin levels and reduced TFS in this population may be autonomous of potential confounding factors, such as age, gender, and hepatic dysfunction scores. Ultimately, multiple sensitivity and subgroup analyses were executed, and the uniform outcomes reinforced the durability and stability of the conclusions. Collectively, these conclusions substantiate that elevated serum levels of copeptin in individuals with CLD may serve as an indicator of unfavorable prognosis.
There may be multiple mechanisms underlying the relationship between a high serum copeptin level and a poor survival outcome in patients with CLD. Previous studies have shown that a high copeptin level in patients with cirrhosis was correlated to the risk of various complications that may lead to a poor prognosis of these patients, such as gastrointestinal hemorrhage due to portal hypertension, hepatorenal syndrome, hepatic encephalopathy, and larger amounts of ascites[32,33]. Pathophysiologically, as a surrogate marker of AVP, increased copeptin may reflect the enhanced systemic release of AVP in these patients[34]. A high AVP in patients with cirrhosis may deteriorate the status of vasoconstriction, water retention, and hyponatremia, which have all been recognized as key risk factors for poor survival of these patients[35]. The role of copeptin in the pathogenesis and progression of CLD should be further investigated, either as a simple biomarker or an active participant in the disease.
This study is subject to certain limitations. Firstly, the meta-analysis results were predominantly influenced by studies involving patients with ACLD, including those with decompensated cirrhosis and other end-stage liver diseases. The efficacy of copeptin as a prognostic factor in patients with early CLD requires further validation in future research. Additionally, despite the utilization of multivariate regression analyses across all the studies included, the potential for residual factors to confound the association between copeptin and TFS cannot be entirely ruled out. For example, serum copeptin may predict the response to tolvaptan in patients with decompensated cirrhosis[36]. Accordingly, use of treatments such as tolvaptan may confound the association between copeptin and TFS. Furthermore, the determination of an optimal serum copeptin cutoff for predicting the survival of patients with CLD remains elusive, necessitating further investigation. Moreover, the absence of a causal association between elevated copeptin levels and unfavorable TFS in CLD patients is attributable to the reliance on observational studies in the meta-analysis.
The findings of the meta-analysis indicate that an elevated serum copeptin concentration in individuals with CLD is linked to unfavorable TFS. The assessment of serum copeptin levels may hold significance in the stratification of risk among CLD patients. Furthermore, it is imperative to investigate whether the reduction of copeptin levels in these patients is correlated with a better clinical outcome, particularly in those with ACLD.
Patients with chronic liver disease (CLD) will develop various complications with the progression of the disease. Upregulated systemic arginine vasopressin (AVP) has been observed in patients with advanced CLD. However, measuring AVP is clinically challenging due to the short half-life. Copeptin is a C-terminus of AVP precursor, which may be of importance for prognostic prediction in patients with CLD.
Identifying biomarkers that predict the prognosis of patients with CLD is clinically important. Although there are pilot studies aiming to correlate copeptin with survival of patients with CLD, the results are not always consistent. In this regard, a systematic review with meta-analysis is particularly useful.
To investigate the correlation between serum copeptin and transplant-free survival (TFS) in patients with CLD with a systematic review and meta-analysis.
Studies were obtained by search of PubMed, Embase, the Cochrane Library, and Web of Science. Two authors independently screened the studies, assessed the study quality with Newcastle-Ottawa Scale, and extracted the data. Risk ratios and corresponding 95% confidence intervals were used as the variables to indicate the association between serum concentration of copeptin and the survival of patients with CLD. The RevMan and Stata software were used for the statistical analyses.
This meta-analysis enrolled ten datasets involving 3133 patients, who were followed for 1 to 48 mo (mean: 12.5 mo). We found that a high level of serum copeptin was associated with a poor TFS, with a risk ratio of 1.82. Additionally, sensitivity analysis retrieved similar results by omitting one dataset at a time. The robustness of the finding was further evidenced by consistent results of subgroup analyses according to study country, study design, patient diagnosis, cutoff of copeptin, follow-up duration, and study quality score.
High serum concentration of copeptin may be associated with a poor clinical prognosis in patients with CLD. These findings were not significantly affected by either of the included studies and were not influenced by multiple study characteristics within the subgroup analysis.
In view of the standard methods for the measuring copeptin in clinical practice, as well as the finding of the meta-analysis, evaluating serum copeptin may be considered at the initial management of patients with CLD, which may provide prognostic significance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt; Rodrigues AT, Brazil S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Br VK, Sarin SK. Acute-on-chronic liver failure: Terminology, mechanisms and management. Clin Mol Hepatol. 2023;29:670-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 2. | Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023;20:388-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 350] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 3. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 4. | Younossi ZM, Wong G, Anstee QM, Henry L. The Global Burden of Liver Disease. Clin Gastroenterol Hepatol. 2023;21:1978-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 207] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 5. | Engelmann C, Clària J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75 Suppl 1:S49-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 218] [Article Influence: 54.5] [Reference Citation Analysis (1)] |
| 6. | Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018;38:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 7. | Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA. 2023;329:1589-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 155] [Article Influence: 77.5] [Reference Citation Analysis (33)] |
| 8. | Wagener G, Bakker J. Vasopressin in cirrhosis and sepsis: physiology and clinical implications. Minerva Anestesiol. 2015;81:1377-1383. [PubMed] |
| 9. | Rondon-Berrios H, Velez JCQ. Hyponatremia in Cirrhosis. Clin Liver Dis. 2022;26:149-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 10. | Leng G, Sabatier N. Measuring Oxytocin and Vasopressin: Bioassays, Immunoassays and Random Numbers. J Neuroendocrinol. 2016;28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 11. | Bankir L, Bichet DG, Morgenthaler NG. Vasopressin: physiology, assessment and osmosensation. J Intern Med. 2017;282:284-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 12. | Szmygin H, Szydełko J, Matyjaszek-Matuszek B. Copeptin as a novel biomarker of cardiometabolic syndrome. Endokrynol Pol. 2021;72:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Di Martino V, Weil D, Cervoni JP, Thevenot T. New prognostic markers in liver cirrhosis. World J Hepatol. 2015;7:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Mookerjee RP. Prognosis and Biomarkers in Acute-on-Chronic Liver Failure. Semin Liver Dis. 2016;36:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 15. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4682] [Article Influence: 1170.5] [Reference Citation Analysis (0)] |
| 16. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40521] [Article Influence: 10130.3] [Reference Citation Analysis (2)] |
| 17. | Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. 2021. [cited 5 July 2023]. Available from: https://training.cochrane.org/handbook. |
| 18. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010. [cited 5 July 2023]. Available from: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf. |
| 19. | Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. [cited 5 July 2023]. Available from: http://handbook-5-1.cochrane.org/. |
| 20. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25802] [Article Influence: 1121.8] [Reference Citation Analysis (0)] |
| 21. | Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 846] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 22. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 23. | Moreno JP, Grandclement E, Monnet E, Clerc B, Agin A, Cervoni JP, Richou C, Vanlemmens C, Dritsas S, Dumoulin G, Di Martino V, Thevenot T. Plasma copeptin, a possible prognostic marker in cirrhosis. Liver Int. 2013;33:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Kerbert AJ, Verbeke L, Chiang FW, Laleman W, van der Reijden JJ, van Duijn W, Nevens F, Wolterbeek R, van Hoek B, Verspaget HW, Coenraad MJ. Copeptin as an Indicator of Hemodynamic Derangement and Prognosis in Liver Cirrhosis. PLoS One. 2015;10:e0138264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Kerbert AJ, Weil D, Verspaget HW, Moréno JP, van Hoek B, Cervoni JP, Di Martino V, Coenraad MJ, Thevenot T. Copeptin is an independent prognostic factor for transplant-free survival in cirrhosis. Liver Int. 2016;36:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Solà E, Kerbert AJ, Verspaget HW, Moreira R, Pose E, Ruiz P, Cela R, Morales-Ruiz M, López E, Graupera I, Solé C, Huelin P, Navarro AA, Ariza X, Jalan R, Fabrellas N, Benten D, de Prada G, Durand F, Jimenez W, van der Reijden JJ, Fernandez J, van Hoek B, Coenraad MJ, Ginès P. Plasma copeptin as biomarker of disease progression and prognosis in cirrhosis. J Hepatol. 2016;65:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Kerbert AJC, Verspaget HW, Navarro ÀA, Jalan R, Solà E, Benten D, Durand F, Ginès P, van der Reijden JJ, van Hoek B, Coenraad MJ; CANONIC Study Investigators of the EASL-CLIF Consortium. Copeptin in acute decompensation of liver cirrhosis: relationship with acute-on-chronic liver failure and short-term survival. Crit Care. 2017;21:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Schneider C, Remmler J, Netto J, Seehofer D, Engelmann C, Berg T, Thiery J, Kaiser T. Copeptin - a biomarker of short-term mortality risk (7 days) in patients with end-stage liver disease. Clin Chem Lab Med. 2019;57:1897-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Zhao R, Wu W, Zhou Z, Zheng X, Sun W, Shi Y, Yu H, Wang F, Zhao H, Sun S, Jin L, Sheng J. Prognostic utility of novel biomarkers in acute-on-chronic liver failure (ACLF) associated with hepatitis B: A multicenter prospective study. Hepatol Res. 2019;49:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Hartl L, Jachs M, Desbalmes C, Schaufler D, Simbrunner B, Paternostro R, Schwabl P, Bauer DJM, Semmler G, Scheiner B, Bucsics T, Eigenbauer E, Marculescu R, Szekeres T, Peck-Radosavljevic M, Kastl S, Trauner M, Mandorfer M, Reiberger T. The differential activation of cardiovascular hormones across distinct stages of portal hypertension predicts clinical outcomes. Hepatol Int. 2021;15:1160-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Shigefuku R, Iwasa M, Eguchi A, Tamai Y, Yoshikawa K, Sugimoto R, Takei Y. Serum copeptin level is a biomarker associated with ascites retention and the formation of a portosystemic shunt in chronic liver disease. J Gastroenterol Hepatol. 2021;36:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Tawfik AK, Helmy A, Yousef M, Abou-Saif S, Kobtan A, Asaad E, Abd-Elsalam S. Copeptin as a novel marker predicting prognosis of liver cirrhosis and its major complications. Hepat Med. 2018;10:87-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Abudeif A, Hashim MS, Ahmed NM, Ahmed AO. Serum copeptin is associated with major complications of liver cirrhosis and spontaneous bacterial peritonitis. Clin Exp Hepatol. 2023;9:71-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Glavaš M, Gitlin-Domagalska A, Dębowski D, Ptaszyńska N, Łęgowska A, Rolka K. Vasopressin and Its Analogues: From Natural Hormones to Multitasking Peptides. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 35. | Alukal JJ, John S, Thuluvath PJ. Hyponatremia in Cirrhosis: An Update. Am J Gastroenterol. 2020;115:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 36. | Shigefuku R, Iwasa M, Eguchi A, Tempaku M, Tamai Y, Suzuki T, Takei Y. Serum Copeptin and Zinc-α2-glycoprotein Levels Are Novel Biomarkers of Tolvaptan Treatment in Decompensated Cirrhotic Patients with Ascites. Intern Med. 2021;60:3359-3368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |