Published online Aug 21, 2023. doi: 10.3748/wjg.v29.i31.4744
Peer-review started: April 27, 2023
First decision: July 9, 2023
Revised: July 23, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: August 21, 2023
Processing time: 113 Days and 3.1 Hours
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathological entity characterized by intrahepatic ectopic steatosis. As a consequence of increased consumption of high-calorie diet and adoption of a sedentary lifestyle, the incidence of NAFLD has surpassed that of viral hepatitis, making it the most common cause of chronic liver disease globally. Huangqin decoction (HQD), a Chinese medicinal formulation that has been used clinically for thousands of years, has beneficial outcomes in patients with liver diseases, including NAFLD. However, the role and mechanism of action of HQD in lipid metabolism disorders and insulin resistance in NAFLD remain poorly understood.
To evaluate the ameliorative effects of HQD in NAFLD, with a focus on lipid metabolism and insulin resistance, and to elucidate the underlying mechanism of action.
High-fat diet-induced NAFLD rats and palmitic acid (PA)-stimulated HepG2 cells were used to investigate the effects of HQD and identify its potential mechanism of action. Phytochemicals in HQD were analyzed by high-performance liquid chromatography (HPLC) to identify the key components.
Ten primary chemical components of HQD were identified by HPLC analysis. In vivo, HQD effectively prevented rats from gaining body and liver weight, improved the liver index, ameliorated hepatic histological aberrations, decreased transaminase and lipid profile disorders, and reduced the levels of pro-inflammatory factors and insulin resistance. In vitro studies revealed that HQD effectively alleviated PA-induced lipid accumulation, inflammation, and insulin resistance in HepG2 cells. In-depth investigation revealed that HQD triggers Sirt1/NF-κB pathway-modulated lipogenesis and inflammation, contributing to its beneficial actions, which was further corroborated by the addition of the Sirt1 antagonist EX-527 that compromised the favorable effects of HQD.
In summary, our study confirmed that HQD mitigates lipid metabolism disorders and insulin resistance in NAFLD by triggering the Sirt1/NF-κB pathway.
Core Tip: Huangqin decoction (HQD) has substantial therapeutic effects in liver diseases. We previously showed that HQD mitigates hepatic inflammation in a rat model of High-fat diet (HFD)-induced Nonalcoholic fatty liver disease (NAFLD) by inhibiting the TLR4/NF-κB/NLRP3 pathway. Here, we investigated the effects of HQD on lipid metabolism disorders and insulin resistance in NAFLD. Our results demonstrated that HQD effectively antagonizes hepatocyte steatosis and insulin resistance in HFD-fed rats and palmitic acid-challenged HepG2 cells by triggering Sirt1/NF-κB pathway-modulated lipogenesis and inflammation. These data will significantly promote the clinical application of HQD in the treatment of NAFLD.
- Citation: Yan BF, Pan LF, Quan YF, Sha Q, Zhang JZ, Zhang YF, Zhou LB, Qian XL, Gu XM, Li FT, Wang T, Liu J, Zheng X. Huangqin decoction alleviates lipid metabolism disorders and insulin resistance in nonalcoholic fatty liver disease by triggering Sirt1/NF-κB pathway. World J Gastroenterol 2023; 29(31): 4744-4762
- URL: https://www.wjgnet.com/1007-9327/full/v29/i31/4744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i31.4744
As lifestyles have been increasingly shifting to “eat more, move less”, overweight and obese cohorts is on a monumental rise. Consequently, the incidence of obesity-related chronic diseases, such as type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease (NAFLD) is on the rise. NAFLD is the most common liver disease characterized by excessive hepatocellular lipid build-up, which is caused by factors other than alcohol intake, drugs, viral infections, and autoimmunity[1]. The initial signs and symptoms of NAFLD are limited to benign steatosis[2]. However, if not managed, approximately 30% of NAFLD cases will progress to nonalcoholic steatohepatitis (NASH). Subsequently approximately 30%–40% of NASH cases will further progress to fibrosis and cirrhosis, and approximately 5%–25% of NASH patients will die from advanced liver diseases within ten years[2-5]. Additionally, epidemiological studies suggest that NAFLD patients are more prone to develop heart disease and die from heart attacks compared to healthy individuals[6]. Currently, NAFLD affects approximately 25% of the general population worldwide, and the associated morbidity in China has increased substantially from 18% to approximately 30% in recent decades[7]. It is estimated that more than 300 million cases of NAFLD will emerge in China by 2030, causing a tremendous strain on its healthcare and economy[8]. Unfortunately, no specific drug has been approved by the Food and Drug Administration for NAFLD; invasive bariatric surgery and lifestyle modifications are thought to be effective strategies[9]. Therefore, there is an urgent need to identify more efficacious agents for NAFLD treatment.
NAFLD is driven by multiple factors and its etiology remains complex and elusive. Several hypotheses have been proposed over the past few decades to explain its pathogenesis. According to the canonical “two-hit” hypothesis originally proposed by Day and James[10], obesity and insulin resistance-evoked excessive intrahepatic lipid accumulation constitutes the “first hit,” and ensuing oxidative stress and inflammation constitute the “second hit”. Subsequent studies found that hepatocyte regeneration and proliferation were impeded during the progression of NAFLD, which was termed as the “third-hit”[11]. Following further in-depth investigations, a more precise “multiple-hit” hypothesis was proposed that deems that hepatic steatosis increases the susceptibility of the liver to intra- and extrahepatic offenders, such as hepatic oxidative stress and inflammation, gut dysbiosis, and disturbed adipokines, and the multiple insults simultaneously and synergistically induce and advance NAFLD[12]. However, in both the hypotheses, hepatic steatosis is considered to be the paramount driver of NAFLD, and numerous studies have focused on lipid metabolism-associated targets (e.g., Sirt1) in an attempt to reduce intrahepatic fat[9]. Sirt1, a nicotinamide adenine dinucleotide-dependent lysine deacetylase, is a metabolic sensor that is highly expressed in metabolically active organs (e.g., the liver and skeletal muscles) and is extensively involved in glucose and lipid metabolism[9,13,14]. Clinical evidence indicates that patients with NAFLD exhibit defective Sirt1 expression in the liver[15]. Additionally, inhibition of Sirt1 exacerbated high-fat diet (HFD)-induced hepatic steatosis in mice, whereas activation of Sirt1 exerted anti-steatotic effect[16]. Importantly, it was reported that Sirt1 deacetylates lysine residues of NF-κB (RelA/p65 subunit), thereby affecting its transcriptional activity and restricting the expression of pro-inflammatory target genes[17]. Curative effects including anti-steatosis, anti-inflammation, and anti-apoptosis were noted when Sirt1/NF-κB pathway was triggered in methionine-choline-deficient diet-induced NASH mice, indicating the vital role of Sirt1/NF-κB pathway in defense against NAFLD[18]. In addition, recent studies suggest that NF-κB-stimulated pro-inflammatory factors activate hepatic insulin resistance, and thereby aggravate hepatic steatosis[17,19]. Therefore, triggering the Sirt1/NF-κB pathway represents a potential strategy for counteracting insulin resistance and hepatic steatosis in NAFLD.
Huangqin decoction (HQD) is a classical traditional Chinese medicine (TCM) formulation that was first mentioned in “Treatise on Febrile Diseases” by the medical sage Zhong-Jing Zhang. According to records, HQD harbors the efficacy of “clearing away heat and treating dysentery” and “regulating the stomach and relieving pain,” and has long been utilized to treat inflammatory diseases. It has a positive reputation as “the ancestral agent for curing dysentery in all ages” and “the first decoction for febrile diseases”[20]. Notably, HQD has been used to treat liver disorders (e.g., NAFLD) at the First Affiliated Hospital of Nanjing University of Chinese Medicine for decades with positive outcomes. HQD is composed of four botanical herbs, Scutellariae Radix (Huangqin in Chinese), Paeoniae Radix Alba (Baishao in Chinese), Glycyrrhizae Radix et Rhizome (Gancao in Chinese), and Jujubae Fructus (Dazao in Chinese), which are clinically combined in a 3:2:2:2 ratio. Previous studies reported that these herbs can alleviate NAFLD when used independently[21-23]. More importantly, in our recent study, the anti-hepatitis effects of HQD in HFD-induced NAFLD rats were found to be mediated via inhibition of the TLR4/NF-κB/NLRP3 pathway[24]. However, the regulatory mechanisms of HQD in hepatic steatosis and its ameliorative effects in insulin resistance remain undefined.
To address this gap, we used HFD-induced NAFLD rats and palmitic acid (PA)-induced HepG2 cells to investigate the ameliorative effects of HQD in lipid metabolism disorders and insulin resistance, and identify the potential molecular mechanisms by focusing on the Sirt1/NF-κB pathway.
Scutellariae Radix, Paeoniae Radix Alba, Glycyrrhizae Radix et Rhizome, and Jujubae Fructus were obtained from Wansheng Herbal Decoction Pieces Co. Ltd. (Bozhou, China) and authenticated by Professor Sheng-Jin Liu of the Nanjing University of Chinese Medicine (Nanjing, China). All voucher specimens (Voucher No. 220301, 220302, 220303, and 220304) were deposited at the Central Laboratory of Jiangsu Health Vocational College (Nanjing, China). Gallic acid, paeoniflorin, scutellarin, liquiritin, baicalin, scutellarein, wogonoside, baicalein, wogonin, and chrysin (purity ≥ 98%) were purchased from Yuanye Biotechnology Co. Ltd. (Shanghai, China). Fenofibrate (Fen) was obtained from SPGC Sine Pharmaceutical Co. Ltd. (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits for insulin, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β were obtained from Solarbio (Beijing, China). Commercial biochemical kits for triacylglycerol (TG), total cholesterol (TC), free fatty acids (FFA), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), alanine transaminase (ALT), and aspartate transaminase (AST) were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Hematoxylin and eosin (H&E) and Oil Red O staining kits were supplied by Beyotime (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), bovine serum albumin (BSA), and PA were purchased from Sigma-Aldrich (St. Louis, MO, United States). Penicillin-streptomycin solution was obtained from Thermo Fisher Scientific (Waltham, MA, United States). EX-527 was purchased from Selleck Chemicals (Houston, TX, United States). A colorimetric 2-deoxydglucose (2DG) uptake assay kit and antibodies against IL-1β, sterol regulatory element-binding protein (SREBP)-1, fatty acid synthase (FAS), cluster of differentiation 36 (CD36), Sirt1, NF-κB, p-NF-κB, IRS-2, p-IRS-2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Abcam (Cambridge, MA, United Kingdom). All the chemicals used were of analytical grade.
HQD was prepared using established protocols. Briefly, 300 g Scutellariae Radix, 200 g Paeoniae Radix Alba, 200 g Glycyrrhizae Radix et Rhizome, and 200 g Jujubae Fructus were combined in 10-fold volume of double-distilled water and extracted in boiling water for 1 h. Following filtration through gauze, the herbal residue was extracted again for 1 h at 100 °C with 8-fold volume of double-distilled water. Subsequently, the two extracts were pooled, concentrated under reduced pressure, and lyophilized to yield 173.1 g of the extract powder. The extract ratio of HQD was 19.23% (w/w). The lyophilized extract powder was stored at -80 °C for subsequent experiments.
An Agilent 1260 high-performance liquid chromatography (HPLC) separation system (Agilent, Santa Clara, CA, United States) equipped with a diode array detector was used to analyze the chemical composition of HQD. Briefly, the lyophilized powder (0.5 g) was dissolved in 20 mL of ultrapure water using ultrasonication. Following filtration through a 0.22 μm syringe filter, 20 μL of the supernatant was injected into the HPLC system and separated chromatographically through an Agilent ZORBAX SB-C18 column (4.6 mm × 150 mm, 5 μm). The mobile phase comprised of linear gradients of 0.1% (v/v) formic acid in water and acetonitrile. Gradient elution was performed as follows: 0–10 min, 5%–8% acetonitrile; 10–20 min, 8%–16% acetonitrile; 20–40 min, 16%–22% acetonitrile; 40–50 min, 22%–25% acetonitrile; 50–60 min, 25%–40% acetonitrile; 60–70 min, 40%–60% acetonitrile; 70–75 min, 60%–5% acetonitrile; 75–80 min, 5% acetonitrile. Programmable parameters for flow rate, injection volume, and detector wavelength were 1 mL/min, 5 μL, and 270 nm, respectively.
Male Sprague-Dawley rats (seven weeks old, weighing 180–200 g) were obtained from the Hangzhou Medical College (Hangzhou, China). The rats were housed in a conventional habitat (40%–50% humidity, 25 ± 1 °C, and 12 h light/dark) at the Laboratory Animal Center of the Jiangsu Health Vocational College. The experimental protocols strictly complied with the European Community criteria and were authorized by the Animal Ethics Committee of the Jiangsu Health Vocational College (Permission No. JHVC-IACUC-2022-B007).
Following one week of acclimatization, the rats were randomly allocated to five groups (n = 8/group): Normal, HFD, HFD plus Fen (30 mg/kg), and HFD plus HQD (400 and 800 mg/kg). Rats in the normal group were fed a normal chow diet (10% calories from fat), whereas the rats in the other groups were fed a HFD (60% calories from fat) for 16 wk to establish the NAFLD model[25]. Both diets were provided by Jiangsu Xietong Pharmaceutical Bioengineering Co. Ltd. (Nanjing, China). Starting from the 8th week, the rats were orally administered Fen or HQD once daily for intervention, whereas the rats in the normal and HFD groups received an equal volume of 0.5% sodium carboxymethylcellulose (CMC-Na) orally as the control. Body weights of the animals were monitored weekly during the experimental period. At the end of the experiment, the rats were fasted overnight prior to being anesthetized with 1% pentobarbital sodium. To obtain serum, blood samples were collected from the abdominal aorta and centrifuged at 3500 rpm for 15 min. The rat livers were swiftly resected and flushed with phosphate buffered saline. Liver weights were measured and the liver indices (liver weight/body weight) were calculated. Portions of the liver were fixed in 4% paraformaldehyde for histological analysis and the remaining liver specimen was snap-frozen and stored at -80 °C for future molecular biology analyses.
Two days prior to the termination of the experiment, the mice were fasted for 12 h and then orally administered 2 g/kg glucose. Blood was drawn from the tail vein before or at the indicated time points (30, 60, 90, and 120 min) after gavage. Blood glucose levels were measured using a glucometer (Yuwell, Inc., Zhenjiang, China). The oral glucose tolerance test (OGTT) curve and area under the curve (AUC) of the OGTT were drawn and calculated using GraphPad Prism 8.0. At the end of the experiment, fasting blood glucose (FBG) and serum insulin levels were determined using a glucometer and an insulin ELISA kit, respectively. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: (FBG × fasting serum insulin)/22.5.
HepG2 cells were purchased from the Chinese Academy of Cell Resource Center (Shanghai, China) and cultured in DMEM (supplemented with 10% FBS and 1% penicillin-streptomycin) at 37 °C in a humid environment containing 5% CO2. PA stock solution (5 mmol/L) was dissolved in 5% BSA by incubating at 55 °C for 15 min. Subsequently, the PA-BSA solution was incorporated into the medium at a final concentration of 125 μM. HQD-mediated cytotoxicity was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. For the interventions, HepG2 cells (70% confluent and serum-starved for 12 h) were treated with insulin (1 IU/mL), various doses of HQD (0, 25, 75, or 125 μg/mL) or a combination of HQD (100 μg/mL) and EX-527 (10 μM) under PA (125 μM) challenge for 24 h. HepG2 cells grown in serum-free media with 5% BSA served as the blank control.
HepG2 cells were seeded in 96-well plates (1×104 cells/well) and cultured overnight, followed by exposure to different concentrations of HQD (0, 25, 50, 75, 100, 120, 150, 175, and 200 μg/mL) with or without 125 μM PA for 24 h. MTT solution (10 μL of 5 mg/mL) was added to each well and incubated at 37 °C for 4 h. Dimethyl sulfoxide (150 μL) was added to dissolve the formazan crystals with vigorously shaking for 5 min. Finally, a Multiskan MK3 microplate reader (Thermo Fisher Scientific) was used to measure the absorbance at 490 nm. Cell viability (% of control) was calculated using the following formula: [Optical density (OD) 490 (sample-blank)/OD 490 (control-blank)] × 100%.
HepG2 cells were cultured overnight in 96-well plates (5 × 103 cells/well) and then starved for 12 h. The cells were stimulated without or with 1 µM insulin for 20 min to activate the glucose transporters. A colorimetric 2DG uptake test was performed according to the manufacturer’s protocol to evaluate glucose absorption by HepG2 cells in response to insulin.
TNF-β, IL-6, and IL-1β levels in the liver and cell supernatants, as well as serum insulin levels, were measured using the corresponding ELISA kits following the manufacturer’s instructions. Biochemical parameters in the serum, liver, and HepG2 cells, including TG, TC, LDL-c, HDL-c, FFA, ALT, and AST were determined using commercial kits following the manufacturer's instructions.
Fixed liver tissues were embedded in paraffin, cut into 5 μm slices, and stained with H&E according to the standard procedure. Immunohistochemical analyses were performed as described previously[25]. Briefly, primary antibodies against IL-1β, SREBP-1, Sirt1, and p-NF-κB were diluted 200-fold and then incubated with the tissue samples overnight at 4 °C. Following that, 100 μL of secondary antibody (diluted 400-fold) was added dropwise to each section. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H + L) was used for IL-1β and SREBP-1. HRP- or Alexa Fluor594-conjugated goat anti-rabbit IgG (H + L) was used for Sirt1 and p-NF-κB. Subsequently, 4',6-diamidino-2-phenylindole (DAPI) or 3,3'-diaminobenzidine substrate solution was sequentially added to each section. Images were captured using either a DM2500 optical microscope (Leica, Wetzlar, Germany) or an LSM 700 confocal laser microscope (Zeiss, Oberkochen, Germany). Finally, the data were quantified using the Image-Pro Plus software (version 6.0; Media Cybernetics, Rockville, MD, United States). For immunofluorescence analysis, HepG2 cells were immobilized in 4% paraformaldehyde solution for 10 min, blocked with 5% BSA, and incubated overnight with primary antibodies against Sirt1 and NF-κB (both diluted 200-fold). Thereafter, the cells were probed with Alexa Fluor 488-conjugated secondary antibodies (diluted 500-fold) and counterstained with DAPI to visualize the nuclei. Fluorescence images were acquired using an LSM 700 confocal laser microscope (Zeiss).
Paraffin-embedded liver tissues were cut into 5 μm sections, followed by staining with Oil Red O dye according to standard procedure. HepG2 cells were fixed in 4% paraformaldehyde for 15 min, rinsed with distilled water, and stained with Oil Red O dye. Images were captured using a DM2500 optical microscope (Leica).
Pre-chilled radio immunoprecipitation assay buffer (containing a protease inhibitor cocktail) was used to lyse liver samples. A commercial bicinchoninic acid assay kit was used to quantify total proteins. Immunoblot analysis was performed as previously described[25]. Specific primary antibodies against SREBP-1c, FAS, CD36, Sirt1, NF-κB, p-NF-κB, IRS-2, p-IRS-2, and GAPDH were used at 1000-fold dilution. The protein bands were visualized and semi-quantified using Azure Biosystems C600 (Azure Biosystems Inc., Dublin, CA, United States) and ImageJ software (NIH, Bethesda, MD, United States), respectively.
Data were analyzed using IBM SPSS software (version 21.0; Armonk, NY, United States) and expressed as mean ± SD. One-way analysis of variance followed by a least significant difference test for multiple comparisons was used to assess significant differences among groups. Statistical significance was set at P ≤ 0.05.
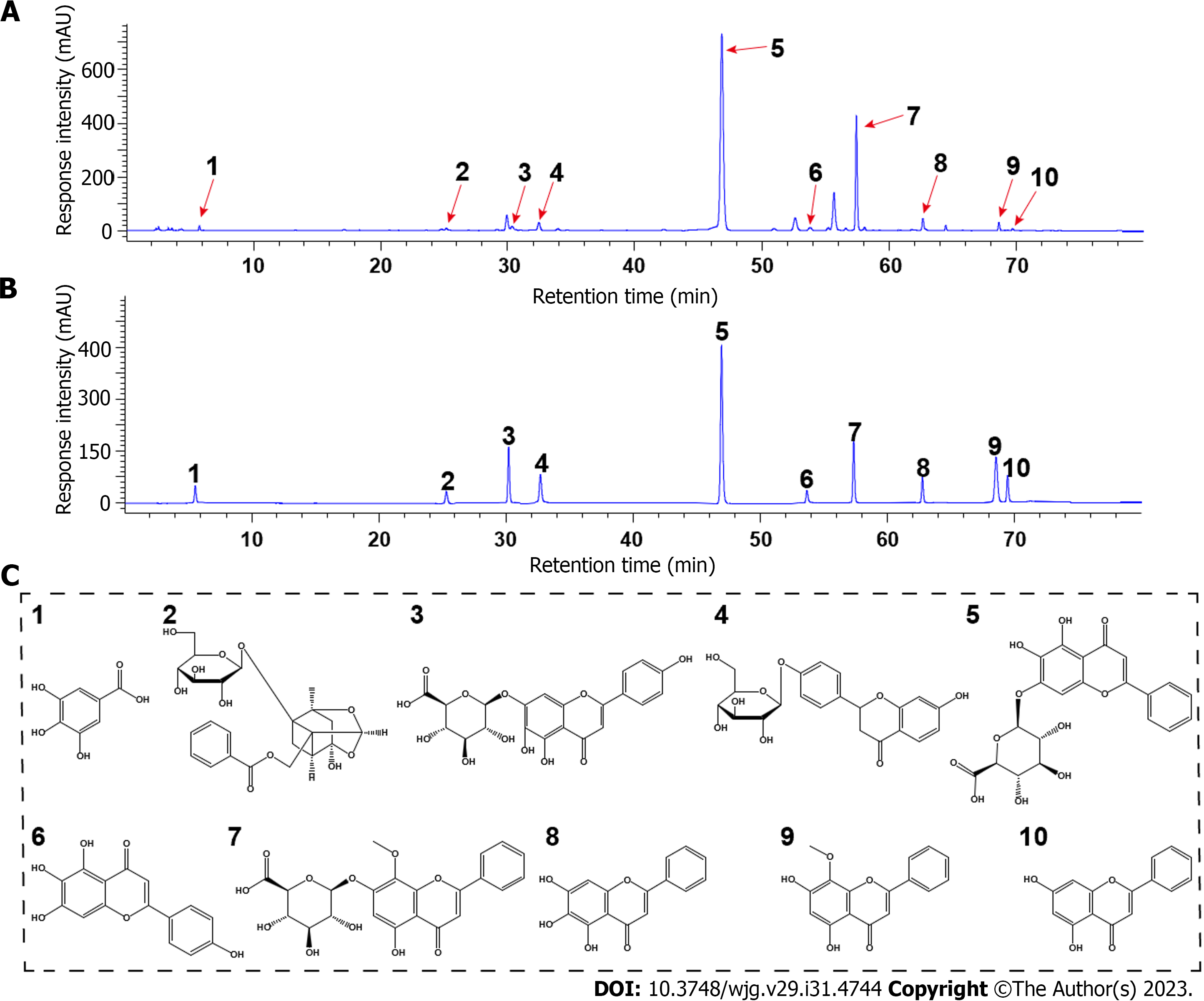
The multifaceted effectiveness of TCMs stem from their multi-component trait. First, we sought to identify the primary chemical components of HQD using HPLC analysis. As shown in Figure 1, ten major monomeric components were identified in HQD by comparing the retention times of the standards. Based on the standards, the concentrations of: (1) Gallic acid, (2) paeoniflorin, (3) scutellarin, (4) liquiritin, (5) baicalin, (6) scutellarein, (7) wogonoside, (8) baicalein, (9) wogonin, and (10) chrysin were determined to be 0.709, 2.005, 1.417, 1.626, 60.092, 0.916, 13.471, 1.905, 2.088, and 0.559 mg/g, respectively.
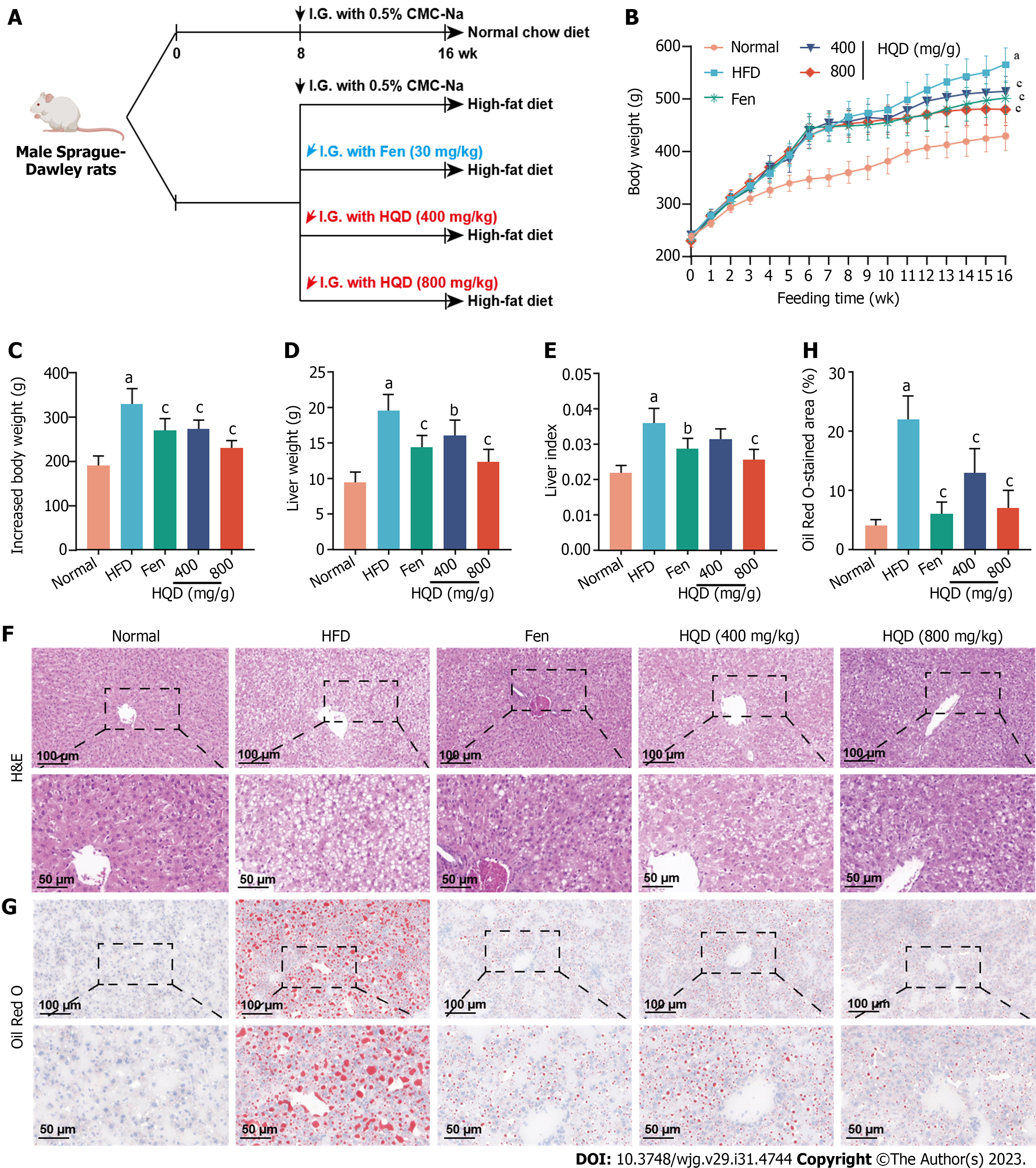
Patients with NAFLD frequently present with increased body and liver weight. Fen, a lipid-lowering medication that is clinically used for NAFLD treatment, was chosen as the positive control drug. The experimental scheme is indicated in Figure 2A. As shown in Figure 2B-E, HQD treatment (400 and 800 mg/kg) significantly attenuated HFD-induced increase in body and liver weight, as well as the liver index. As hepatic steatosis is the primary hallmark of NAFLD, we performed histological analysis to assess the effect of HQD on hepatic steatosis. H&E staining indicated that rats in the HFD group presented with severe structural disruption of the hepatic lobules, steatosis, and hepatocellular ballooning degeneration, whereas oral administration of HQD mitigated these pathological alterations in a dose-dependent manner, as evidenced by relatively intact hepatic lobules and smaller fat vacuoles (Figure 2F). Additionally, Oil Red O staining showed numerous lipid droplets in the HFD group that reduced significantly following HQD treatment (Figure 2G and H). Notably, high-dose HQD exerted anti-steatosis effects comparable to those of Fen.
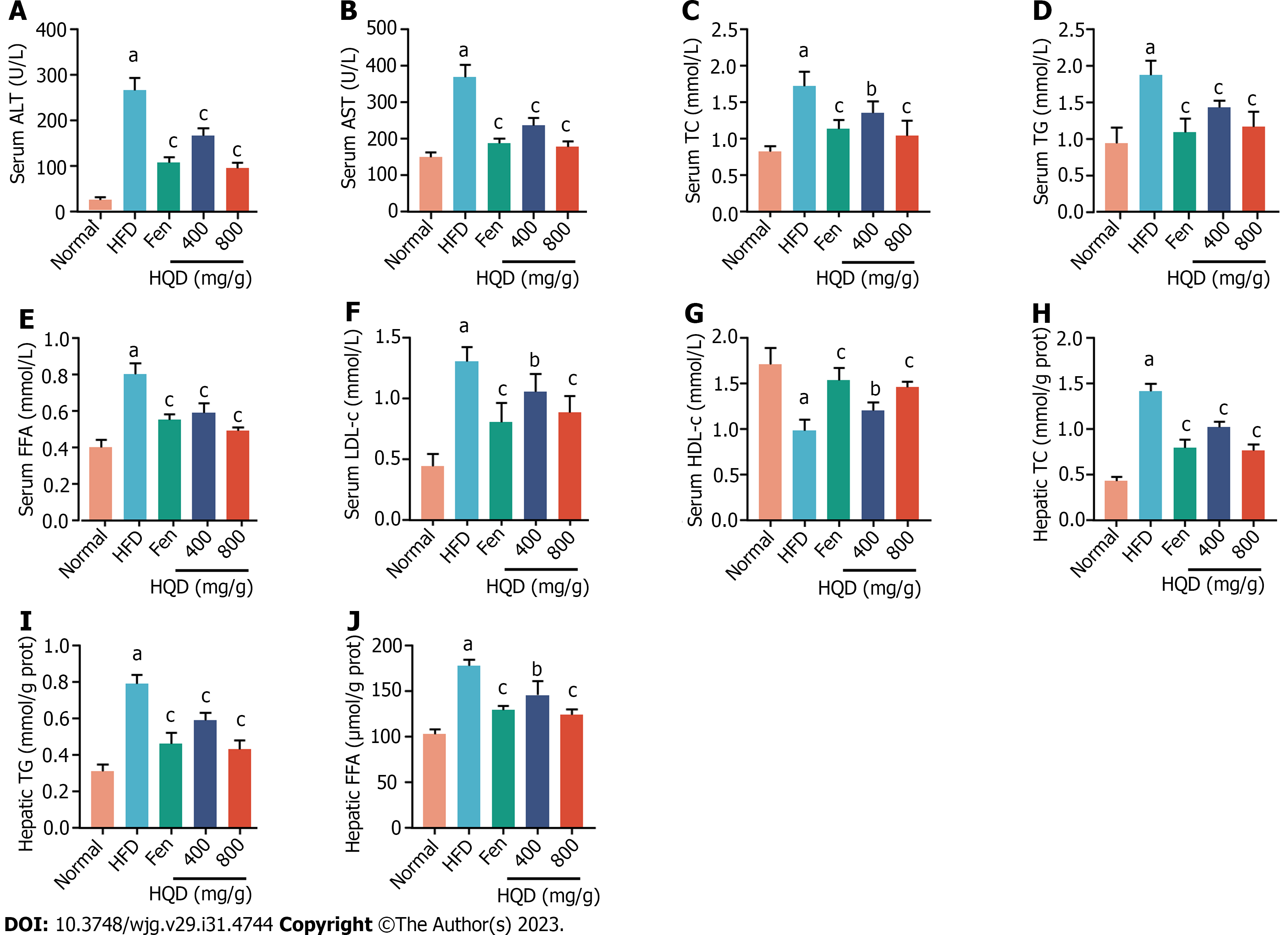
Biochemical parameters of liver damage and lipid metabolism disorders, including ALT, AST, TC, TG, FFA, LDL-c, and HDL-c, are robust diagnostic markers for NAFLD. As shown in Figure 3, serum levels of ALT, AST, TC, TG, FFA, and LDL-c, as well as hepatic TC, TG, and FFA in the HFD group were noticeably higher than those in the control group. These levels were markedly reduced following HQD treatment. In contrast, the HFD-challenged group exhibited lower serum HDL-c level than the normal group, which was markedly restored following HQD treatment. In addition, the therapeutic efficacy of a high dose of HQD was equivalent to that of Fen.
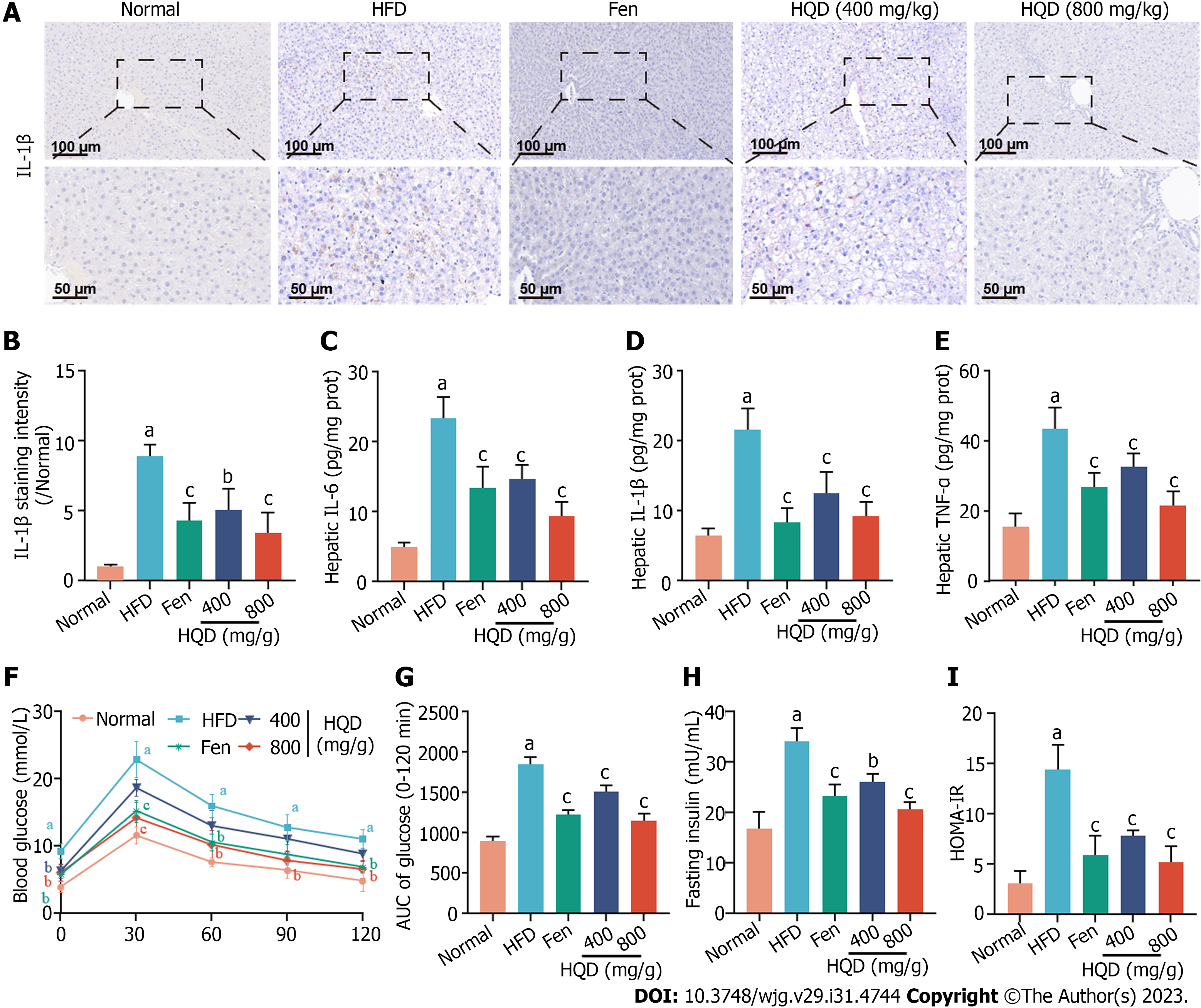
Liver inflammation and insulin resistance are pivotal concomitant factors that facilitate NAFLD progression. Inflammation exacerbates insulin resistance, and both form a vicious cycle with each promoting the other and accelerating NAFLD progression[26]. As shown in Figure 4A and B, IL-1β-positive area in the liver of the HFD group was significantly higher relative to that in the control group, indicating extensive inflammatory response in the liver, which was further supported by markedly elevated levels of IL-6, IL-1β, and TNF-α in the liver (Figure 4C-E). However, all these alterations were reversed following HQD interventions. To ascertain the effect of HQD on glucose tolerance, an OGTT was performed. As shown in Figure 4F and G, higher levels of blood glucose (0–120 min) were noted in the HFD group following intragastric administration of glucose, which was robustly attenuated by HQD treatment. This was quantitatively verified by the AUC of the OGTT. Moreover, the HFD-induced elevation in serum insulin levels also decreased markedly following HQD treatment (Figure 4H). As shown in Figure 4I, the HOMA-IR value in HFD-challenged rats was significantly higher than that in normal rats, and was effectively reduced following HQD intervention. Notably, high-dose HQD showed curative efficacy similar to that of Fen.
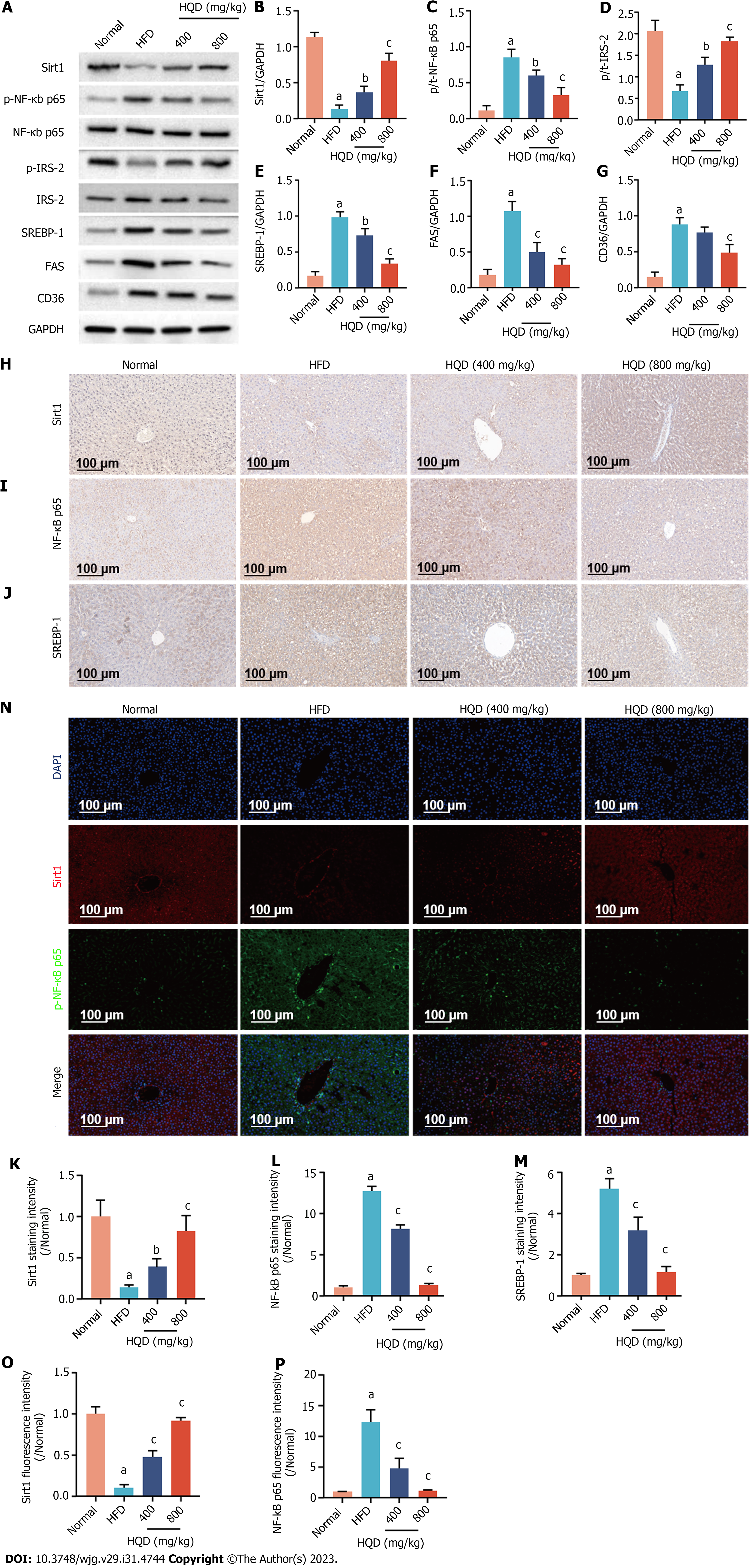
Sirt1/NF-κB pathway-modulated insulin resistance and hepatic steatosis play an important role in the progression of NAFLD. Therefore, protein levels of Sirt1, NF-κB, p-NF-κB, and the downstream insulin signal transduction- and lipid metabolism-related proteins (i.e., IRS-2, p-IRS-2, SREBP-1, FAS, and CD36) in the liver were examined. As shown in Figure 5A-G, Sirt1 and p-IRS-2 protein levels were significantly lower in the HFD group relative to that in the normal group, whereas those of p-NF-κB, SREBP-1, FAS, and CD36 were clearly elevated in response to HFD challenge. Oral supplementation with HQD markedly reversed these changes in a dose-dependent manner. To further validate these findings, the levels of Sirt1, p-NF-κB, and SREBP-1 were evaluated immunohistochemically. Consistent with the previous data, mild staining intensity of Sirt1 (brown/fluorescence) and strong staining intensities of p-NF-κB (brown/fluorescence) and SREBP-1 (brown) were observed in the HFD group, indicating the up-regulation of these proteins and activation of Sirt1/NF-κB pathway (Figure 5H-P). However, HQD administration substantially reduced the increased expression noted in immunohistochemical analyses. Overall, these findings suggest that the therapeutic effects of HQD on HFD-induced disorders in insulin signaling and lipid metabolism are closely associated with the Sirt1/NF-κB pathway.
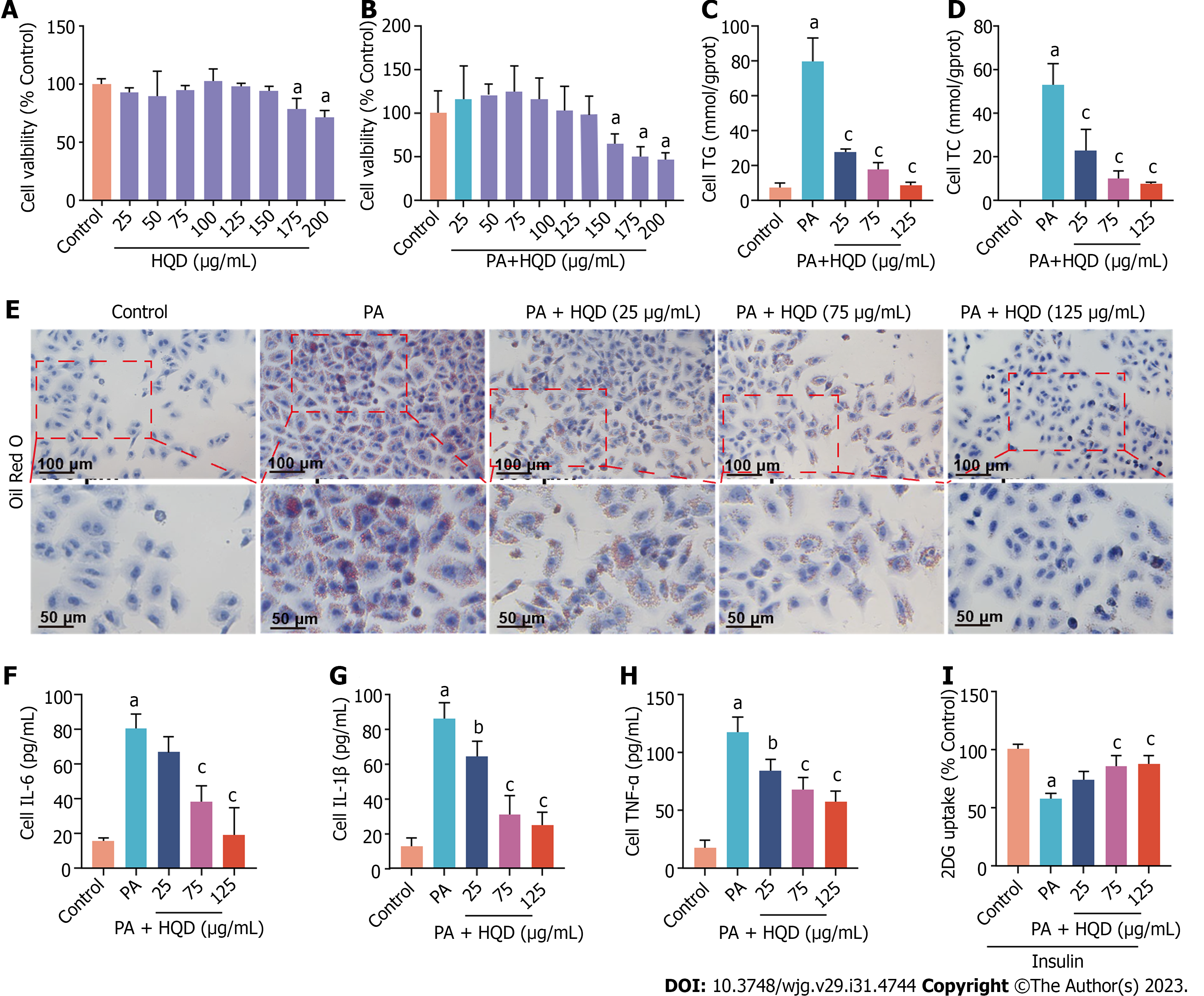
The direct exposure of HepG2 cells to PA is a commonly used approach to mimic the symptoms of NAFLD in vitro[27,28]. PA-induced HepG2 cells were used to investigate the therapeutic effects of HQD in NAFLD in vitro. Initially, MTT assay was used to examine the cytotoxicity of HQD in HepG2 cells. As shown in Figure 6A and B, HQD presented no obvious cytotoxic effect in HepG2 cells at doses ≤ 150 μg/mL, and the safe concentration of HQD when co-incubated with PA (125 μM) was determined to be ≤ 125 μg/mL. Accordingly, HQD at doses of 25, 75, and 125 μg/mL were selected for the treatment of PA-induced HepG2 cells in subsequent experiments. As shown in Figure 6C and D, the levels of neutral lipids (TC and TG) in PA-challenged HepG2 cells were noticeably increased relative to that in the control cells, but markedly reduced following HQD intervention. This was further supported by the results of Oil Red O staining, which showed reduced lipid droplets in the HQD-treated groups (Figure 6E). As inflammation is a major cause of NAFLD and is strongly associated with insulin resistance, we examined the effects of HQD on the release of inflammatory factors in PA-challenged HepG2 cells. Figure 6F-H shows that HQD attenuated the PA-induced increase in the levels of IL-6, IL-1β, and TNF-β in a dose-dependent manner. A 2DG assay was performed to assess insulin resistance. The results showed that 2DG uptake was significantly lower in PA-treated HepG2 cells, whereas it increased markedly following HQD treatment (Figure 6I), indicative of the ameliorative effect of HQD on insulin resistance.
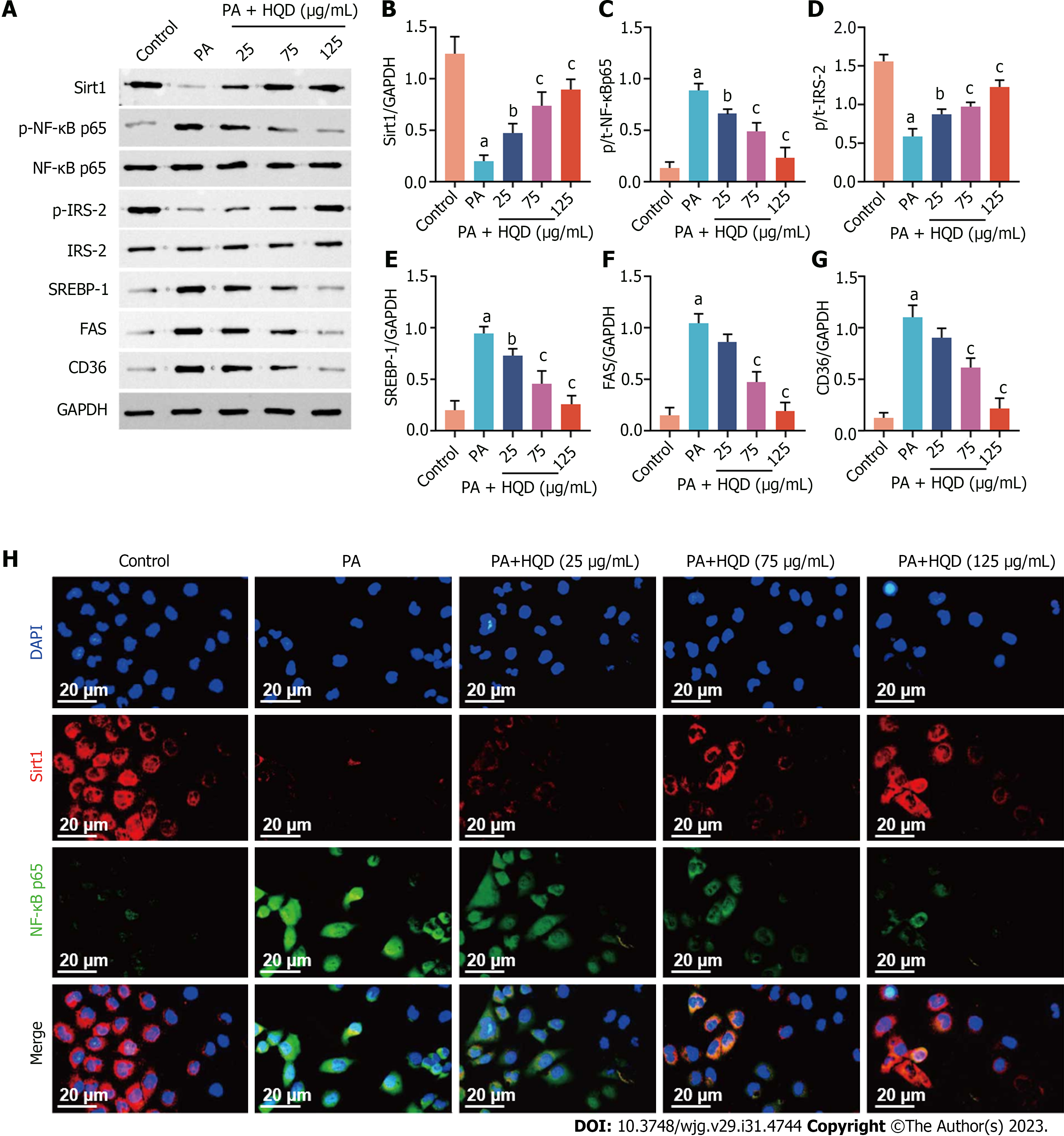
Consistent with the in vivo results, we noted that PA-stimulated HepG2 cells showed markedly decreased expression of Sirt1 and p-IRS-2, and increased expression of p-NF-κB, SREBP-1, FAS, and CD36 compared with that in the control cells (Figure 7A-G), indicative of inhibition of the Sirt1/NF-B pathway. However, these changes were restored following HQD treatment. To further determine the expression and distribution of Sirt1 and NF-κB, immunofluorescence analyses were performed. Consistently, the intensity of Sirt1 staining in the PA group was clearly lower compared to that in the control group. Furthermore, significantly increased NF-κB nuclear translocation was noted in PA-induced HepG2 cells, which was conspicuously reversed following HQD treatment (Figure 7H). Collectively, these data suggest that HQD protects hepatocytes against PA-induced insults via the Sirt1/NF-κB pathway.
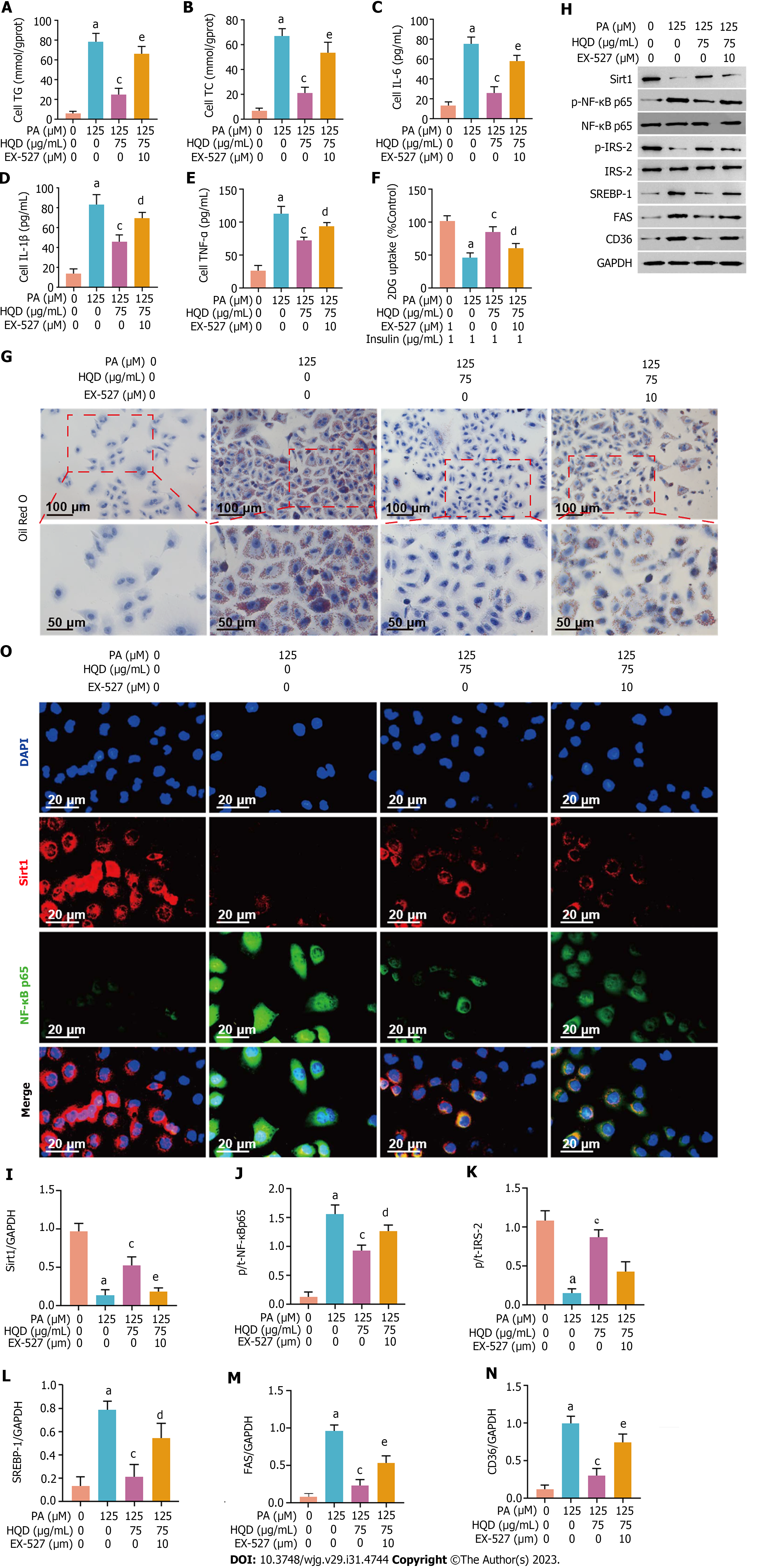
To further confirm that the HQD-mediated mitigatory effects on insulin resistance and lipid accumulation occurs via the Sirt1/NF-κB pathway, we used a Sirt1 inhibitor, EX-527 to block the pathway. As shown in Figure 8A-G, the ameliorative effects of HQD on lipid accumulation, inflammation, and 2DG uptake were significantly compromised following co-treatment with EX-527. More importantly, EX-527 abolished the regulatory effects of HQD on Sirt1/NF-κB pathway and its downstream-modulated insulin signal transduction- and lipid metabolism-related proteins (Figure 8H-O). In summary, our data indicate that the protective effects of HQD against hepatic lipid accumulation, inflammation, and insulin resistance are mediated through triggering of the Sirt1/NF-κB pathway.
NAFLD is the most prevalent chronic liver disease for which no specific effective drug has been approved, making its treatment a top research priority[29]. Unlike synthetic drugs, whose efficacy depends mainly on their action on one target, the efficacy of TCM depends on its holistic effects on multiple targets and pathways. Therefore, TCMs are promising complementary and auxiliary agents for metabolic diseases with multifactorial pathogeneses, such as NAFLD[30-32]. HQD, a well-known TCM formula, has emerged as a potentially effective modality for the clinical treatment of liver diseases, including NAFLD. In our study, several constituents of HQD, including gallic acid, paeoniflorin, scutellarin, liquiritin, baicalin, scutellarein, wogonoside, baicalein, wogonin, and chrysin were identified by HPLC analysis. The beneficial effects of these compounds in NAFLD have been reported both in vivo and in vitro[33-36], which provide the basis for the potential application of HQD in NAFLD therapy. In the present study, using both in vivo and in vitro experiments, we demonstrated that HQD mitigates insulin resistance and hepatic steatosis by modulating the Sirt1/NF-κB pathway.
Overconsumption of HFD, which is associated with a modern lifestyle, plays a role in the progression of NAFLD[37]. Currently, NAFLD murine models are most commonly developed by long-term consumption of an HFD, resulting in symptoms similar to those observed in patients with NAFLD[37]. PA is an essential fatty acid for TG synthesis in the liver and increased concentrations of PA in hepatocytes accelerate the progression[38]. Typically, the exogenous addition of PA induces hepatocellular lipid accumulation to mimic the pathological process of NAFLD in vitro. We used HFD-fed rats and PA-treated HepG2 cells to study the therapeutic effects of HQD on NAFLD. Our results showed that the HFD-fed rats had higher body and liver weights. Histological analysis revealed hepatic steatosis and injuries, which were further supported by aberrant levels of TC, TG, ALT, and AST in the serum and liver. Furthermore, abnormal levels of serum FFA, LDL-c, HDL-c, OGTT, insulin, and HOMA-IR were observed in the HFD-fed rats, indicating the onset of dyslipidemia and insulin resistance. Consistent with these findings, PA-stimulated HepG2 cells exhibited a marked increase in lipid accumulation, intracellular TC and TG levels, and glucose uptake. Notably, all the above mentioned NAFLD symptoms were dose-dependently reversed following HQD treatment, indicating that HQD mitigates lipid metabolism disorders and insulin resistance in NAFLD.
Compromised lipid metabolism contributes to hepatic steatosis, thereby triggering the onset of NAFLD[39,40]. Several studies have confirmed that the activation of de novo lipogenesis (DNL) promotes FFA generation and esterification into TG, which are crucial driving factors contributing to lipid metabolism dysfunction[41]. A previous clinical trial revealed that individuals with NAFLD had higher nighttime plasma FFA levels and a threefold increase in DNL[42]. SREBP-1c is a key transcription factor that controls hepatic DNL and lipolysis by modulating the activities of lipogenic enzymes (FAS) and fatty acid importer proteins (CD36) as downstream targets[43-45]. The active state of SREBP-1c directly influences FLD progression in fatty liver[43]. In the present study, rats with HFD-induced NAFLD and PA-treated HepG2 cells showed elevated SREBP-1, FAS, and CD36 Levels, indicating increased DNL and fatty acid uptake. However, oral HQD supplementation effectively reversed these changes. Sirt1 plays a critical role in cellular energy metabolism and deacetylates and inhibits SREBP-1c activity in the regulation of hepatic lipid metabolism; however, it is downregulated in patients with NAFLD[15]. In the rat liver and HepG2 cells, we observed deactivation of Sirt1 following exposure to HFD or PA, whereas HQD treatment increased Sirt1 levels, resulting in NAFLD mitigation.
Insulin resistance is the initial driver of hepatic steatosis in NAFLD[10,11,46]. Moreover, recent studies have suggested that obesity-induced chronic low-grade inflammation exacerbates insulin resistance[26,47]. Specifically, hypertrophic adipocytes promote macrophage infiltration and secrete pro-inflammatory factors including IL-6, IL-1β, and TNF-α, which downregulate insulin signaling molecules by inhibiting the phosphorylation of IRS-2, ultimately promoting insulin resistance[48-50]. NF-κB acts as an inducible transcription factor and induces the production of pro-inflammatory factors[51]. A previous study reported that FFA-induced insulin resistance is strongly associated with the activation of the pro-inflammatory NF-κB pathway[52]. More importantly, Sirt1 can inactivate NF-κB by deacetylating lysine 310, impeding the expression of pro-inflammatory target genes, whereas patients with NAFLD are more susceptible to inactivation of the Sirt1/NF-κB pathway[17,53]. In both, in vivo and in vitro NAFLD models, phosphorylation of NF-κB was upregulated and accompanied by increased levels of pro-inflammatory factors (IL-1β, TNF-α, and IL-6), whereas phosphorylation of IRS-2 was downregulated, suggesting that Sirt1/NF-κB pathway-mediated insulin resistance occurred in NAFLD. In contrast, the HQD intervention clearly restored these changes. Furthermore, treatment with the Sirt1 inhibitor, EX-527, abolished all HQD-mediated favorable effects in PA-challenged HepG2 cells, confirming that HQD mitigated hepatic steatosis and insulin resistance via the Sirt1/NF-κB pathway.
Fen, a peroxisome proliferator-activated receptor α (PPARα) agonist, offers potential therapeutic efficacy against NAFLD by inducing fatty acid oxidation and inhibiting inflammatory gene expression in the liver[54,55]. Clinical studies have shown that Fen may have positive therapeutic outcomes in NAFLD/NASH[56,57]. Herein, we found that HQD achieved therapeutic effects comparable to those of Fen by activating Sirt1. Several studies have shown that Sirt1, a key regulator of PPARα signaling, can trigger PPARα-mediated fatty acid oxidation in the liver, showing favorable effects in NAFLD[9,58,59]. Although HQD and Fen act on different targets, their therapeutic effects could be interpreted from the upstream and downstream regulatory relationships between Sirt1 and PPARα make.
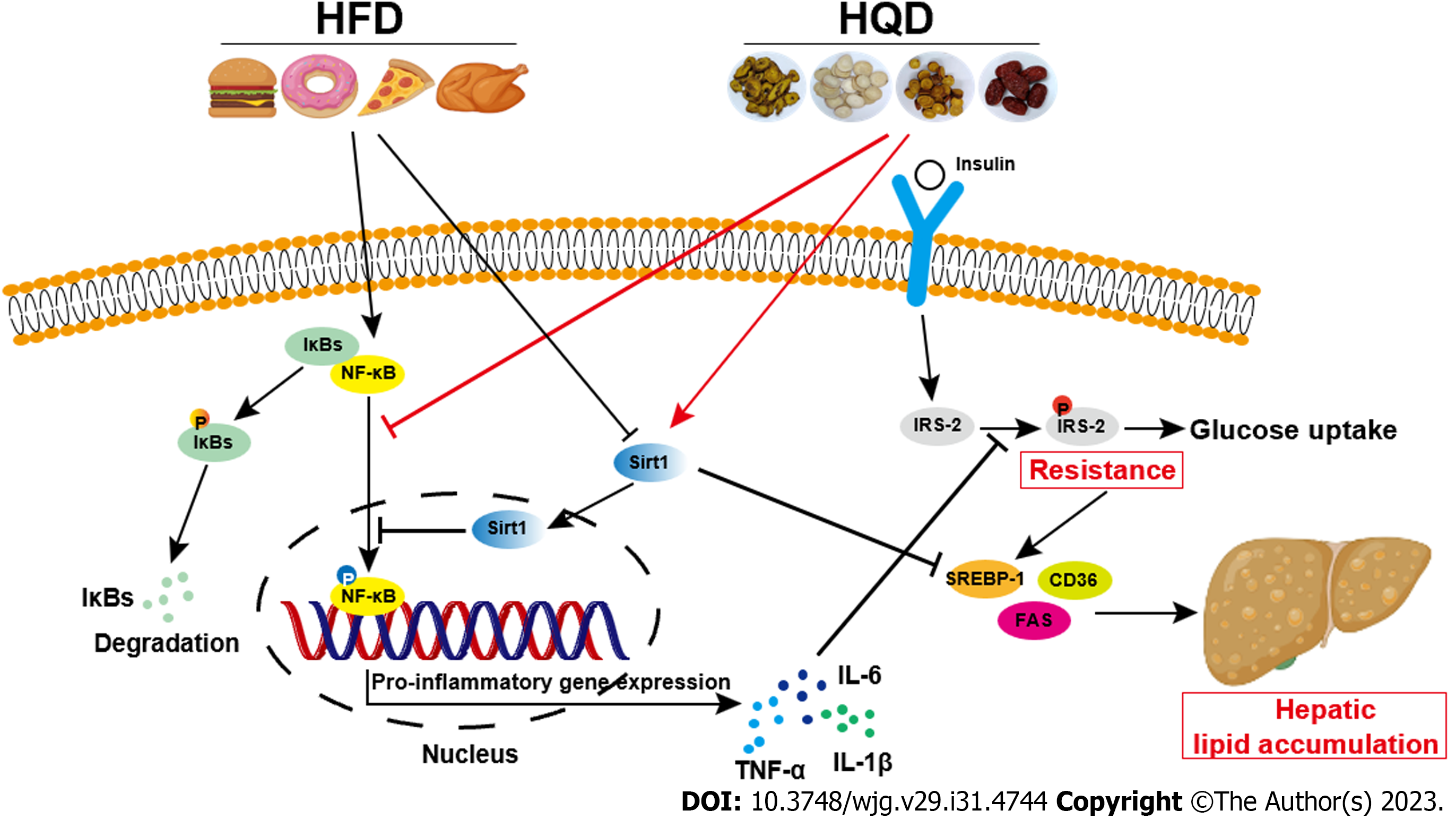
In summary, we have demonstrated the mitigating effects of HQD on lipid metabolism disorders and insulin resistance. Our investigations revealed that HQD mediates these beneficial effects by modulating lipogenesis and inflammatory responses which trigger the Sirt1/NF-κB pathway (Figure 9). Our study provides strong evidence supporting HQD as a promising agent for NAFLD treatment.
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease, for which no effective therapeutic drugs have been approved. Huangqin decoction (HQD), a traditional Chinese medicine, has been widely used to treat liver diseases in clinical practice. Our study confirmed that HQD effectively mitigates high-fat diet (HFD)-induced hepatic inflammation. However, whether HQD has ameliorative effects on lipid metabolism disorders and insulin resistance, as well as the underlying mechanisms, remains unclear.
Convincing evidence for HQD in the clinical treatment of NAFLD is required.
This study aimed to investigate the therapeutic efficacy of HQD on lipid metabolism disorders and insulin resistance and explore the possible molecular mechanisms by focusing on the Sirt1/NF-κB pathway.
High-performance liquid chromatography was used to identify the key components of HQD. HFD-induced NAFLD rats and palmitic acid-induced HepG2 cells were treated with HQD to investigate the mitigating effects of HQD on lipid metabolism disorders and insulin resistance. Finally, molecular biology techniques, including western blotting, immunohistochemistry, and immunofluorescence, were applied to explore the protein expression in the Sirt1/NF-κB pathway.
Ten key components of HQD were identified. The results showed that HQD effectively prevented lipid metabolism disorders in rats and HepG2 cells, inflammatory responses, and insulin resistance. Mechanistic exploration revealed that triggering Sirt1/NF-κB pathway-modulated lipogenesis and inflammation contributed to the favorable effects of HQD, which was further validated by the addition of EX-527 (a Sirt1 antagonist).
Supported by our previous findings, we strongly believe that HQD is a promising complementary or auxiliary agent for the clinical treatment of NAFLD.
HQD has been used to treat liver diseases in clinical practice for decades, with positive outcomes. More in-depth and precise research on the molecular mechanisms of HQD could guide and promote the clinical treatment of NAFLD more effectively.
The authors are grateful to Yue-Tao He (Leishenhe Mechanical and Electrical Equipment Co. Ltd.) for kindly providing high-quality reagents.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Poland; Dietrich CG, Germany S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Seval GC, Kabacam G, Yakut M, Seven G, Savas B, Elhan A, Cinar K, Idilman R. The natural course of non-alcoholic fatty liver disease. Hepatol Forum. 2020;1:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 5. | Yan T, Yan N, Wang P, Xia Y, Hao H, Wang G, Gonzalez FJ. Herbal drug discovery for the treatment of nonalcoholic fatty liver disease. Acta Pharm Sin B. 2020;10:3-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 6. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1487] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 7. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3792] [Article Influence: 541.7] [Reference Citation Analysis (2)] |
| 8. | Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, Eguchi Y, Geier A, Kondili LA, Kroy DC, Lazarus JV, Loomba R, Manns MP, Marchesini G, Nakajima A, Negro F, Petta S, Ratziu V, Romero-Gomez M, Sanyal A, Schattenberg JM, Tacke F, Tanaka J, Trautwein C, Wei L, Zeuzem S, Razavi H. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1308] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 9. | Zheng X, Zhang XG, Liu Y, Zhu LP, Liang XS, Jiang H, Shi GF, Zhao YY, Zhao ZW, Teng Y, Pan K, Zhang J, Yin ZQ. Arjunolic acid from Cyclocarya paliurus ameliorates nonalcoholic fatty liver disease in mice via activating Sirt1/AMPK, triggering autophagy and improving gut barrier function. J Funct Foods. 2021;86:104686. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 3129] [Article Influence: 115.9] [Reference Citation Analysis (36)] |
| 11. | Mouzaki M, Loomba R. An update on the role of the microbiome in non-alcoholic fatty liver disease pathogenesis, diagnosis, and treatment. Curr Treat Options Gastroenterol. 2020;18:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2118] [Article Influence: 235.3] [Reference Citation Analysis (1)] |
| 13. | Cao Y, Jiang X, Ma H, Wang Y, Xue P, Liu Y. SIRT1 and insulin resistance. J Diabetes Complications. 2016;30:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Li X. SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai). 2013;45:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Wu T, Liu YH, Fu YC, Liu XM, Zhou XH. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann Clin Lab Sci. 2014;44:410-418. [PubMed] |
| 16. | Zhang Y, Geng C, Liu X, Li M, Gao M, Fang F, Chang Y. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol Metab. 2017;6:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | de Gregorio E, Colell A, Morales A, Marí M. Relevance of SIRT1-NF-κB Axis as Therapeutic Target to Ameliorate Inflammation in Liver Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Komeili-Movahhed T, Bassirian M, Changizi Z, Moslehi A. SIRT1/NFκB pathway mediates anti-inflammatory and anti-apoptotic effects of rosmarinic acid on in a mouse model of nonalcoholic steatohepatitis (NASH). J Recept Signal Transduct Res. 2022;42:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Cobbina E, Akhlaghi F. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev. 2017;49:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 20. | Liu SH, Cheng YC. Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J Ethnopharmacol. 2012;140:614-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Chen Q, Liu M, Yu H, Li J, Wang S, Zhang Y, Qiu F, Wang T. Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med. 2018;72:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Luo Z, Liu Y, Han X, Yang W, Wang G, Wang J, Jiang X, Sen M, Li X, Yu G, Shi Y. Mechanism of Paeoniae Radix Alba in the Treatment of Non-alcoholic Fatty Liver Disease Based on Sequential Metabolites Identification Approach, Network Pharmacology, and Binding Affinity Measurement. Front Nutr. 2021;8:677659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Hajiaghamohammadi AA, Ziaee A, Samimi R. The efficacy of licorice root extract in decreasing transaminase activities in non-alcoholic fatty liver disease: a randomized controlled clinical trial. Phytother Res. 2012;26:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Yan BF, Wang Y, Wang WB, Ding XJ, Wei B, Liu SJ, Fu TM, Chen L, Zhang JZ, Liu J, Zheng X. Huangqin decoction mitigates hepatic inflammation in high-fat diet-challenged rats by inhibiting TLR4/NF-κB/NLRP3 pathway. J Ethnopharmacol. 2023;303:115999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Yan BF, Chen X, Chen YF, Liu SJ, Xu CX, Chen L, Wang WB, Wen TT, Zheng X, Liu J. Aqueous extract of Paeoniae Radix Alba (Paeonia lactiflora Pall.) ameliorates DSS-induced colitis in mice by tunning the intestinal physical barrier, immune responses, and microbiota. J Ethnopharmacol. 2022;294:115365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Zhu Y, Wan N, Shan X, Deng G, Xu Q, Ye H, Sun Y. Celastrol targets adenylyl cyclase-associated protein 1 to reduce macrophages-mediated inflammation and ameliorates high fat diet-induced metabolic syndrome in mice. Acta Pharm Sin B. 2021;11:1200-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Wang Y, Zhao H, Li X, Li N, Wang Q, Liu Y, Liang Q, Shao Z, Zhang N, Zhao T, Peng L, Li P. Tangshen Formula Alleviates Hepatic Steatosis by Inducing Autophagy Through the AMPK/SIRT1 Pathway. Front Physiol. 2019;10:494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Xiao Q, Zhang S, Yang C, Du R, Zhao J, Li J, Xu Y, Qin Y, Gao Y, Huang W. Ginsenoside Rg1 Ameliorates Palmitic Acid-Induced Hepatic Steatosis and Inflammation in HepG2 Cells via the AMPK/NF-κB Pathway. Int J Endocrinol. 2019;2019:7514802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Badolati N, Masselli R, Sommella E, Sagliocchi S, Di Minno A, Salviati E, Campiglia P, Dentice M, Tenore GC, Stornaiuolo M, Novellino E. The Hepatoprotective Effect of Taurisolo, a Nutraceutical Enriched in Resveratrol and Polyphenols, Involves Activation of Mitochondrial Metabolism in Mice Liver. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Shi H, Dong C, Wang M, Liu R, Wang Y, Kan Z, Wang L, Si G. Exploring the mechanism of Yizhi Tongmai decoction in the treatment of vascular dementia through network pharmacology and molecular docking. Ann Transl Med. 2021;9:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Huo M, Ma L, Liu G. Exploring the mechanism of Yixinyin for myocardial infarction by weighted co-expression network and molecular docking. Sci Rep. 2021;11:22567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Liu W, Shang J, Deng Y, Han X, Chen Y, Wang S, Yang R, Dong F, Shang H. Network pharmacology analysis on mechanism of Jian Pi Qing Gan Yin decoction ameliorating high fat diet-induced non-alcoholic fatty liver disease and validated in vivo. J Ethnopharmacol. 2022;295:115382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Chao J, Cheng HY, Chang ML, Huang SS, Liao JW, Cheng YC, Peng WH, Pao LH. Gallic Acid Ameliorated Impaired Lipid Homeostasis in a Mouse Model of High-Fat Diet-and Streptozotocin-Induced NAFLD and Diabetes through Improvement of β-oxidation and Ketogenesis. Front Pharmacol. 2020;11:606759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Li YC, Qiao JY, Wang BY, Bai M, Shen JD, Cheng YX. Paeoniflorin Ameliorates Fructose-Induced Insulin Resistance and Hepatic Steatosis by Activating LKB1/AMPK and AKT Pathways. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 35. | Zhang X, Huo Z, Luan H, Huang Y, Shen Y, Sheng L, Liang J, Wu F. Scutellarin ameliorates hepatic lipid accumulation by enhancing autophagy and suppressing IRE1α/XBP1 pathway. Phytother Res. 2022;36:433-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Hu Q, Zhang W, Wu Z, Tian X, Xiang J, Li L, Li Z, Peng X, Wei S, Ma X, Zhao Y. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol Res. 2021;165:105444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 37. | Zheng X, Zhao MG, Jiang CH, Sheng XP, Yang HM, Liu Y, Yao XM, Zhang J, Yin ZQ. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates insulin resistance and hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine. 2020;66:153130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, Poniachik J. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond). 2004;106:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 525] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 39. | Park JC, Jeong WJ, Seo SH, Choi KY. WDR76 mediates obesity and hepatic steatosis via HRas destabilization. Sci Rep. 2019;9:19676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 707] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 41. | Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 713] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 42. | Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 799] [Article Influence: 72.6] [Reference Citation Analysis (0)] |
| 43. | Song Z, Xiaoli AM, Yang F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 300] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 44. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 1747] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 45. | Manzoor M, Muroi M, Ogawa N, Kobayashi H, Nishimura H, Chen D, Fasina OB, Wang J, Osada H, Yoshida M, Xiang L, Qi J. Isoquercitrin from Apocynum venetum L. produces an anti-obesity effect on obese mice by targeting C-1-tetrahydrofolate synthase, carbonyl reductase, and glutathione S-transferase P and modification of the AMPK/SREBP-1c/FAS/CD36 signaling pathway in mice in vivo. Food Funct. 2022;13:10923-10936. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 46. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 47. | Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 238] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 48. | Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929-2935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 49. | Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5334] [Cited by in RCA: 5424] [Article Influence: 169.5] [Reference Citation Analysis (0)] |
| 50. | Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 477] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 51. | Zhang JN, Ma Y, Wei XY, Liu KY, Wang H, Han H, Cui Y, Zhang MX, Qin WD. Remifentanil Protects against Lipopolysaccharide-Induced Inflammation through PARP-1/NF-κB Signaling Pathway. Mediators Inflamm. 2019;2019:3013716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458-3465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 397] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 53. | Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1942] [Cited by in RCA: 2188] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 54. | Yoo J, Jeong IK, Ahn KJ, Chung HY, Hwang YC. Fenofibrate, a PPARα agonist, reduces hepatic fat accumulation through the upregulation of TFEB-mediated lipophagy. Metabolism. 2021;120:154798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 55. | Mahmoudi A, Moallem SA, Johnston TP, Sahebkar A. Liver Protective Effect of Fenofibrate in NASH/NAFLD Animal Models. PPAR Res. 2022;2022:5805398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | El-Haggar SM, Mostafa TM. Comparative clinical study between the effect of fenofibrate alone and its combination with pentoxifylline on biochemical parameters and liver stiffness in patients with non-alcoholic fatty liver disease. Hepatol Int. 2015;9:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson PA, Moris L, Nilsson PM, Eriksson JW, Lind L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J Clin Lipidol. 2018;12:1390-1403.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 58. | Huang K, Du M, Tan X, Yang L, Li X, Jiang Y, Wang C, Zhang F, Zhu F, Cheng M, Yang Q, Yu L, Wang L, Huang D, Huang K. PARP1-mediated PPARα poly(ADP-ribosyl)ation suppresses fatty acid oxidation in non-alcoholic fatty liver disease. J Hepatol. 2017;66:962-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Kim TH, Yang YM, Han CY, Koo JH, Oh H, Kim SS, You BH, Choi YH, Park TS, Lee CH, Kurose H, Noureddin M, Seki E, Wan YY, Choi CS, Kim SG. Gα12 ablation exacerbates liver steatosis and obesity by suppressing USP22/SIRT1-regulated mitochondrial respiration. J Clin Invest. 2018;128:5587-5602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |