INTRODUCTION
Recent studies support that inflammatory bowel disease (IBD) can be categorized as a “microbial dysbiosis disease,” because of its progression synchronizing the dysbacteriosis of gut microbiota[1]. Host physiology such as barrier function, metabolism, immune responses, and homeostasis involves microbiome-induced cell signaling, proliferation, and neurotransmitter biosynthesis[2]. In IBD patients, intestinal bacterial diversity decreases and the bacterial community structure changes[3]. In dextran sodium sulphate (DSS)-induced colitis in mice, some probiotics, including Lactbacillus and Bifidobacterium, are significantly reduced[4]. New evidence indicates that IBD is not merely a consequence of chronic inflammation, but also of disruption of the gut microbiome and destruction of the intestinal epithelial barrier[5].
Gut microbiota play a role in inflammation-related activities. Fecal microbiota transplantation (FMT) has shown high efficacy and safety in treating ulcerative colitis (UC)[6,7] due to its immunomodulatory and anti-inflammatory functions[8]. Our previous study showed that FMT can counter DSS-induced colitis in mice by increasing the relative abundance of Lactobacillus[9]. FMT has also shown therapeutic potential for a range of other diseases, such as hepatic disorders and metabolic syndrome[10]. Recent studies have demonstrated that Toll-like receptor 4 (TLR4) is exploited by FMT in treating many diseases such as spleen deficiency diarrhea[11], Parkinson’s disease[12,13], developmental arsenic neurotoxicity[14], fluorosis[15], and acute lung injury[16]. Previous studies have indicated that FMT intervention can inhibit activation of the nuclear factor kappa B (NF-κB) signaling pathway[17], which is downstream of TLR4. However, there have been limited studies investigating the role of TLR4 in FMT for UC.
As a class of transmembrane proteins that recognize invading microbes and activate immune cells, Toll-like receptors (TLRs) regulate gene transcription and the acquired intestinal immune response[18]. In the etiology of IBD, microbes in the intestinal lumen induce abnormal immune responses, along with excessive leakage of bacterial antigens into the mucosa[19]. TLR4, an important immune activator, is highly expressed in the intestinal epithelial cells and lamina propria cells of UC patients[20]. It binds to ligands to activate cytokine signaling, recruit inflammatory cells, and damage intestinal mucosal barrier, all of which aggravate intestinal inflammatory lesions. More importantly, substantial evidence supports a pro-inflammatory role of the TLR4 signaling pathway in UC. Expression levels of TLR4 are positively correlated with disease activity indices (DAIs), endoscopy scores, and histopathological scores[21]. DSS-induced colitis deteriorates in mice with TLR4 overexpression[22,23], but is stably maintained in TLR4-deficient mice[24,25]. Multiple experiments have shown that inhibiting the TLR4 signaling pathway can prevent DSS-induced colitis[26,27]. While TLR4 plays a crucial role in intestinal injury and repair, its role in shaping colonic bacterial homeostasis and microbiota-related immunity remains poorly understood.
Our previous studies confirmed the efficacy FMT on IBD, but the mechanism has not been reported[9]. Therefore, we explored the role of TLR4 in the mechanism by which FMT treats DSS-induced colitis in the mice.
MATERIALS AND METHODS
Animals
Wild-type (WT) C57BL/10J mice and TLR4-knockout (KO) mice on the C57BL/10J background (female; 6 to 8 wk of age; weighing 18-20 g; specific pathogen-free (SPF) grade) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). All mice were reared in an SPF condition at the experimental animal center of the Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University. Throughout the acclimatization and study periods, all mice were maintained in a 12 h-light/12 h-dark cycle (21 °C ± 2 °C with a relatively constant humidity of 45% ± 10%) and had access to food and water ad libitum. All mice were group-housed and reared in a standard cage, with TLR4 KO mice kept separately from C57BL/10J mice in different cages.
DSS-induced colitis
DSS (36-50 kDa) was purchased from MP Biomedicals LLC (Irvine, CA, United States) and dissolved in distilled water. Experimental colitis was induced as previously described with minor changes[9]. For different groups, the mice were administered 2.5% (w/v) DSS in drinking water for 7 d. Mice in the KO (DSS + FMT) group were fed fecal microbiota from healthy WT mice from day 8 (once every 2 d) until the end of the experiment, while mice in the KO (DSS + water) group were fed normal saline at the same time. The mice were evaluated daily by scoring via the disease activity index (DAI)[28]. The DAI score was calculated on a 0-4 scale as previously described[29].
Fecal preparation and transplantation
The process of FMT was performed as previously described[9]. Briefly, feces from donor mice (healthy WT mice) were collected and resuspended in sterile normal saline at 0.125 g/mL. Then 0.2 mL of this suspension was administered to mice once every 2 d by oral gavage. This process lasted 7 d.
Histopathology
Mice were euthanized by cervical dislocation, and their abdominal cavity was opened immediately. The colon tissue was dissected; colons were measured for colon length, and tissues were examined for gross macroscopic appearance and stool consistency. The distal colon segment was placed in 10% neutral buffered formalin for 24 h, embedded in paraffin, and cut into sections 4 μm in thickness. Then the sections were stained with hematoxylin and eosin (H&E). H&E-stained sections were examined for inflammation and tissue damage by an experienced pathologist in a blinded manner. Tissue histology was scored by summing the scores of the following parameters according to a previous study[30]: Extent of inflammation, aberrant crypt foci, lymphocyte infiltration, and aberrant colon wall.
Fecal DNA extraction and 16S ribosomal RNA sequencing
Fecal DNA extraction and 16S ribosomal RNA (rRNA) sequencing were performed as previously reported[9]. The V3-V4 hypervariable region of the bacterial 16S-rRNA gene was amplified with primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAA T-3’) with the ABI GeneAmp® 9700 PCR thermocycler (Applied Biosystems, Foster City, CA, United States)[9]. All PCR products were extracted from a 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States)[31]. Purified amplicons were sequenced on the Illumina MiSeq PE300 platform (Illumina, San Diego, CA, United States). The raw 16S rRNA gene sequencing reads were demultiplexed, quality-filtered by fastp version 0.20.0, and merged by FLASH version 1.2.7. Operational taxonomic units (OTUs) with 97% similarity cutoff[32] were clustered using UPARSE version 7.1[33], and chimeric sequences were identified and removed. Bacterial alpha-diversity was determined by sampling-based OTU analysis. Analysis of species accumulation curves was performed to assess the rationality and efficiency of the sequencing depth. Principal component analysis (PCA) was implemented in R programming. Using the Wilcoxon rank-sum test, the bacterial taxonomic analysis was performed for comparison at the bacterial phylum, class, order, family, genus levels between two groups. Based on the matrix of normalized relative abundance, bacteria with significantly different abundances between assigned taxa were determined by linear discriminant analysis effect size (LEfSe) with the Kruskal-Wallis rank-sum test (P < 0.05). LDA was used to assess the effect size of each feature (LDA score [log10] = 3 as the cut-off value).
Transcriptome analysis
Total RNA was extracted from inflammatory colonic tissue. For sequencing, a 1 cm colon tissue was sampled from the site about 2 cm from the anus, regardless of whether there was visible inflammation. The tissue samples with minimum and maximum histological scores were removed. Then the colon samples from four randomly chosen animals in each group was used for sequencing. Methods for amplifying and sequencing followed those previously published[9,29]. Briefly, 2 μg RNA per sample was used to sequence on the Illumina Hiseq 4000 platform. Differential expression analysis was performed using the DESeq R package (1.10.1) according to the manufacturer’s protocol. Then, to explore the potential function of the differentially expressed genes (DEGs), GOseq R package[34] and KOBAS software[35] were used to test the enrichment of DEGs in Gene Ontology (GO) functional annotations[36] and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways[37].
Correlation analysis for gut microbiota and transcriptome
We used Metastats software to confirm the difference in the relative abundance of microbiota among the samples (P ≤ 0.05). We used DEG sequencing to carry out transcriptome difference analysis (the threshold was Padj < 0.05 & |log2FC| > 1). Finally, R psych software package was used to analyze the Spearman association between the transcriptome and intestinal microflora. Those with |R| > 0.8 and P < 0.05 (strong correlation) were screened for mapping.
Statistical analysis
Differences were analyzed using the t-test with Graphpad Prism 8.0 software (GraphPad Software Inc., La Jolla, CA, United States). Results are shown as the mean ± standard error of the mean. P < 0.05 was considered statistically significant.
RESULTS
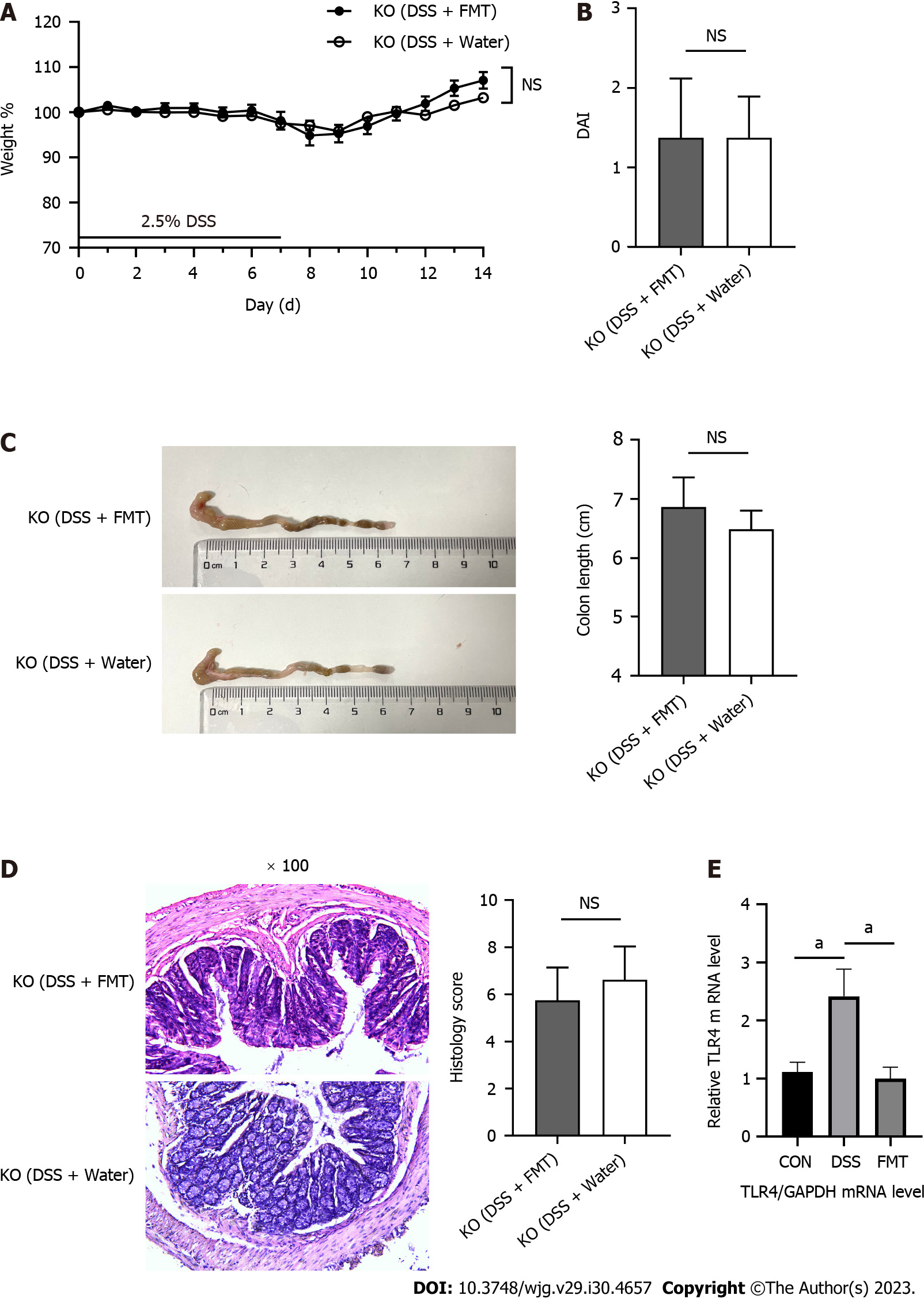
FMT does not improve acute colitis induced by DSS in TLR4-KO mice
In our previous experiment, we found that FMT is effective to treat colitis[9]. We further explored whether this efficacy is related to the TLR4 pathway. Acute DSS-induced colitis was induced in eight animals per group using 2.5% DSS in the drinking water. After gavage with fecal microbiota from the WT mice, mice in the KO (DSS + water) and KO (DSS + FMT) groups displayed indistinguishable body weight loss, colon length, DAI score, and histology score (Figure 1A-D). Beyond our expectation, FMT had no effect on colonic inflammation in TLR4-KO mice. We compared the expression of TLR4 gene in the intestine of WT mice before and after FMT. The transcriptome sequence data indicated that DSS increased, but FMT effectively decreased the expression of TLR4 (Figure 1E).
Figure 1 Fecal microbiota transplantation did not alleviate acute colitis induced by dextran sodium sulphate in Toll-like receptor 4 knockout mice.
A: Body weight of mice during the course of colitis; B: The bar chart represents the disease activity index (DAI) score of mice on day 14; C: Representative images of colons from mice (left) and statistical analysis of colon length (right); D: Representative hematoxylin and eosin staining of colon tissues, original magnification 100 ×, and histological scores (right); E: Relative quantification of the transcription level of Toll-like receptor 4 (TLR4) among groups. aP < 0.05. DSS: Dextran sodium sulphate; FMT: Fecal microbiota transplantation; KO: Knockout.
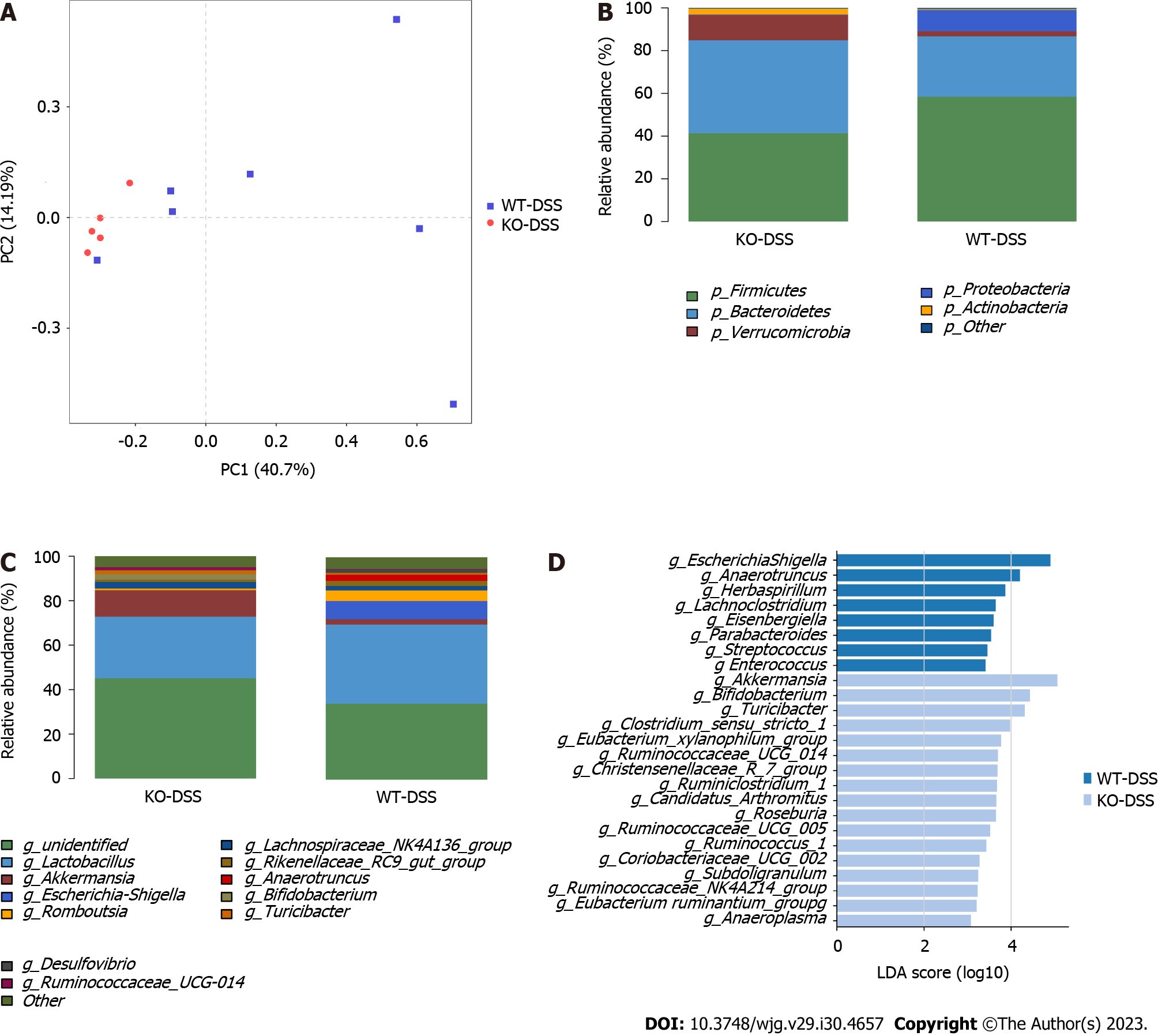
FMT changes the intestinal flora of TLR4-KO mice
We investigated whether FMT changed the composition of gut microbiota in the KO (DSS + water) and KO (DSS + FMT) groups. We employed LEfSe to evaluate the bacterial taxa (at genus level) in the two groups (Figure 2A). The dominating taxa in KO (DSS + FMT) group were enriched in Lactobacillus, which indicated that we had successfully transplanted the gut microbiota of healthy WT mice. Meanwhile, the KO (DSS + FMT) group had a lower abundance of Akkermansia, indicating that FMT could alter the relative abundance of Akkermansia in KO mice (Figure 2B).
Figure 2 Fecal microbiota transplantation changed the gut microbiota of Toll-like receptor 4 knockout mice.
A: Linear discriminant analysis (LDA) effect size (LEfSe) analysis in two groups with an LDA score > 3.0; B: The relative abundance of Akkermansia in Toll-like receptor 4 knockout mice. aP < 0.05, bP < 0.01. DSS: Dextran sodium sulphate; FMT: Fecal microbiota transplantation.
TLR4 KO alleviates DSS-induced colitis
We used TLR4-deficient mice and WT mice to determine whether TLR4 may protect mice from DSS-induced colitis. Mice in the KO-DSS (n = 8) and WT-DSS (n = 7) groups were given distilled drinking water containing 2.5% DSS for 7 d (Figure 3A). Compared with WT mice, KO mice showed lower susceptibility to DSS, as manifested by their much smaller body weight loss (Figure 3B), lower DAI (Figure 3C), and longer colons (Figure 3D). Compared to the WT-DSS group, mice in the KO-DSS group exhibited a more intact colon structure, less severe crypt damage, and reduced inflammatory infiltration (Figure 3E). In summary, KO mice showed increased tolerance to DSS-induced colitis.
Figure 3 Toll-like receptor 4 knockout alleviated dextran sodium sulphate-induced inflammation in the colon.
A: Scheme of the animal experimental design; B: The change in body weight of mice from days 0 to 7 during the disease course (knockout-dextran sodium sulphate [KO-DSS]: n = 8; wild type [WT]-DSS: n = 7); C: The bar chart represents the disease activity index (DAI) score on day 7; D: Representative colons (left) and statistical analysis (right) of colonic length; E: Representative hematoxylin and eosin staining of colon tissues (left), original magnification 100 ×, and histological scores (right). aP < 0.05, bP < 0.01, and cP < 0.001 were considered statistically significant. FMT: Fecal microbiota transplantation.
TLR4 deficiency influences the diversity and composition of gut microbiota
We further investigated whether the protection against DSS-induced colitis was due to TLR4 KO or microbiota re-composition. We detected the gut microbiota of WT and KO mice in the basal and DSS-treated states. We analyzed the beta-diversity of microbiota based on PCA. An evident clustering separation between OTUs revealed the different community structures between each two groups, suggesting that these communities are distinct in terms of their compositional structure (Figure 4A and 5A).
Figure 4 Diversities and compositions of gut microbiota in knockdown-control and wild type-control groups.
A: β-diversity evaluated using the weighted UniFrac-based PCA (knockdown-control [KO-CON]: n = 7; wild type [WT]-CON: n = 7); B and C: Bar graphs showing the relative abundances of different bacteria at the phylum and genus levels; D: Linear discriminant analysis (LDA) effect size analysis in groups with an LDA score > 3.0 between two groups.
Figure 5 Diversities and compositions of gut microbiota in knockout-dextran sodium sulphate and wild type-dextran sodium sulphate groups.
A: Multiple sample principal component analysis (knockout-dextran sodium sulphate [KO-DSS]: n = 5; wild type [WT]-DSS: n = 7); B and C: Bar graphs showing the relative abundances of different bacteria at the phylum and genus levels; D: Linear discriminant analysis (LDA) effect size (LEfSe) analysis in groups with an LDA score > 3.0.
At the phylum level, TLR4 deficiency decreased the abundance of Bacteroidetes and increased the abundances of Actinobacteria and Verrucomicrobia (P < 0.05; Figure 4B), compared to those in WT mice. After DSS induction, a significant increase of phylum Proteobacteria was observed in the WT-DSS group compared to the KO-DSS group (P < 0.05; Figure 5B). Verrucomicrobia was the most abundant phylum among those with significant differences (P < 0.05). At the genus level, Akkermansia abundance was significantly higher in KO mice than in WT mice either healthy or diseased (P < 0.05; Figure 4C and 5C). To further investigate the potential effect of microbiota composition on DSS-induced colitis, we used the LEfSe to detect the marked differences in the dominant bacterial communities between the two groups (Figure 4D and 5D). Specifically, Lactobacillus and Peptococcus were enriched in the WT-CON group (Figure 4D), while Escherichia Shigella and Anaerotruncus were enriched in the WT-DSS group (Figure 5D). Interestingly, Akkermansia and Bifidobacterium were enriched either in healthy and diseased KO mice (Figure 4D and 5D). The collective results of our study indicated clear differences in the intestinal microbiome between WT mice and KO mice, both in healthy conditions and during illness. These findings highlight the important role of TLR4 in shaping the composition and diversity of the intestinal microbiota.
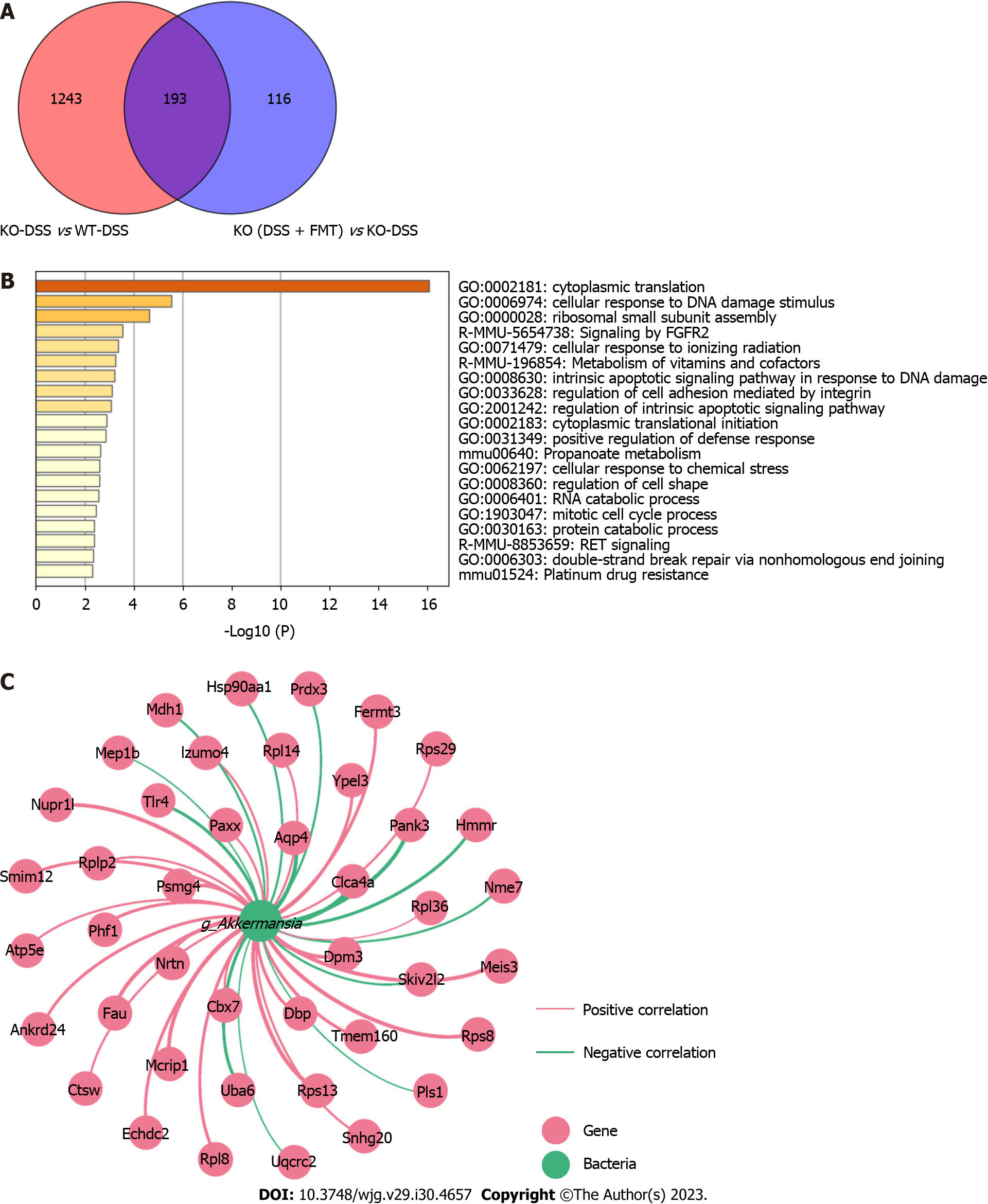
TLR4-KO-shaped microbiota affect the transcriptome in the colon of mice
To further explore whether FMT can change the gene expression related to TLR4, we investigated the DEGs between groups. Compared to those in the WT-DSS group, 1436 genes were differentially expressed in the KO-DSS group, and 309 genes in the KO (DSS + FMT) group. Furthermore, 193 DEGs were found among the KO-DSS group, WT-DSS group, and KO (DSS + FMT) group (Figure 6A). GO enrichment analysis showed that these DEGs were mainly involved in cytoplasmic translation and cellular response to DNA damage stimulus (Figure 6B). According to 16S rRNA sequencing analysis, we found that Akkermansia was dominant in the KO group. To characterize potential gene-microbe interactions, we computed gene-microbe correlations with Spearman correlation efficients (Figure 6C). The top nine genes correlating with Akkermansia included Aqp4, Clca4a, Dpm3, Fau, Mcrip1, Meis3, Nupr1 L, Pank3, and Rps13 (|R| > 0.9, P < 0.01).
Figure 6 Colonic transcriptome profile and gene-microbe correlation.
A: Venn diagram illustrates genes regulated by fecal microbiota transplantation (FMT) and Toll-like receptor 4 knockout (KO); B: The top 20 Gene Ontology terms enriched in these 193 differentially expressed genes (DEGs); C: Network visualizing 193 DEGs associated with Akkermansia (|R| > 0.8, P < 0.05). CON: Control; DSS: Dextran Sodium Sulphate; WT: Wild type.
DISCUSSION
Researchers have found that patients with active UC can benefit from FMT[38]. Moreover, our previous study also verified that FMT can treat colitis in mice. In the present study, the expression of TLR4 was upregulated by DSS, and downregulated after FMT. It therefore stands to reason that, by inhibiting TLR4, a protective effect from intestinal inflammation will be induced. Considering the ubiquitous involvement of the TLR4 signaling pathway in the activities of the mucosa, we designed this animal study to elucidate its interaction with FMT in UC. In this study, TLR4 KO significantly alleviated the clinical and histological manifestations of DSS-induced colitis. Notably, the increased relative abundance of the predominant Akkermansia species contributed to the heightened resistance against colon inflammation. Through further investigation, we discovered that genetic KO of TLR4 significantly impacted the structure and composition of the gut microbiota, resulting in a shift towards an anti-inflammatory configuration. This shift plays a crucial role in promoting enhanced resistance and tolerance to colitis.
At the phylum level, DSS changed the relative abundances of Bacteroidetes, Actinobacteria, and Verrucomicrobia in TLR4-KO mice compared to WT mice. Possibly, the high abundance of anti-inflammatory Akkermansia in the gut microbiota curbs the aggravation of colitis, despite the absence of TLR4 signaling. Akkermansia was the dominant genus in healthy KO mice, while after the treatment of FMT, their level decreased. Compared with that in the WT group, the status of colitis in the KO group was not significantly attenuated by FMT, suggesting that the therapeutic effect of FMT on colitis is closely related to the TLR4 signaling pathway and Akkermansia.
In the gut, the expression of TLRs changes with the composition of microbiota[39], as well as the activity of the intestinal epithelium such as inflammation[40]. In the present study, we observed the difference in microbial composition between WT-DSS and KO-DSS groups. At the phylum level, the KO-DSS group had a higher relative abundance of Actinobacteria and Verrucomicrobia, while WT-DSS had a higher relative abundance of Proteobacteria. In addition, Verrucomicrobia demonstrated the most significant difference at the phylum level. Lo Sasso et al[41] analyzed the composition of gut microbiota in UC patients via fecal microbiota whole-genome sequencing, finding increased abundance of Proteobacteria and decreased abundance of Verrucomicrobia. In addition, one study characterized the mucosal microbiome of pediatric UC patients, noting a significant decrease in the phylum Verrucomicrobia at the phylum level[42]. It has been reported that the abundance of Proteobacteria increases in UC mice[43]. Moreover, the relative abundance of Proteobacteria in DSS-induced mice rises remarkably, compared with that in WT mice, which can be restored to normal after Lizhong therapy[44]. Consistently, this study proves that DSS can raise the abundance of Proteobacteria in WT mice, rather than KO mice.
In particular, we found that the abundance of Akkermansia increased in the KO-DSS group, but then dropped notably after FMT, indicating its role in the effect of FMT on UC. As previously reported, the abundance of Akkermansia decreases in UC patients[45], but it is unclear whether this is a cause or consequence of UC. Akkermansia can protect intestinal barrier function and reduce the production of inflammatory cytokines[46]. On the other hand, Akkermansia can increase the production of short-chain fatty acids and antioxidant enzymes, indicating that Akkermansia may proliferate to alleviate colitis[47]. According to our experiment, the relative abundance of Akkermansia was negatively correlated with the severity of colitis in our animal models. Akkermansia bear great therapeutic potential for colitis. Studies on human and mice have revealed that the injection of beneficial bacteria such as Lactobacillus, Akkermansia, and Bifidobacterium can alleviate the inflammation in UC patients[48-50]. In a systematic review of three studies, the abundance of Akkermansia decreased in all UC patients[51]. A high abundance of Akkermansia can modulate host metabolism to prevent seizures[52]. Several Akkermansia species have demonstrated the ability to modulate immune responses and protect barrier function[53].
Despite the widely recognized beneficial properties of Akkermansia as a potential probiotic, it is crucial to take into account the potential occurrence of adverse effects. Patients with colorectal cancer have a higher abundance of A. muciniphila[3]. A prior study demonstrated that the genetic deletion of TLR4 exacerbates the severity of colon inflammation, resulting in the decreased abundance of Akkermansia[54]. This conflicting conclusion may be explained by various factors, such as the different mouse species and different experimental models used. When the equilibrium of the gut microbiota is disturbed, beneficial microbes have the potential to shift towards virulent species, leading to adverse effects on the host. Studies have suggested a potential link between Akkermansia and TLR4 signaling. A study demonstrated that the administration of anthocyanins extracted from Lycium ruthenicum (ACs) increases the abundance of Akkermansia, thus inhibiting the lipopolysaccharide/NF-κB/TLR4 pathway to improve intestinal function[55]. It has also been observed that inhibition of the TLR4 signaling pathway can increase the abundance of Akkermansia[56]. Akkermansia promotes the integrity of the intestinal barrier and regulates immune homeostasis, potentially by interacting with TLR4[57,58]. In this study, the composition and structure of gut microbiota presented a significant difference between KO-DSS mice and WT-DSS mice. Based on the above results, we advocate that Akkermansia can increase resistance to acute colitis in TLR4-KO mice. However, more in-depth investigations are needed to determine if Akkermansia negatively associated with TLR4 are a potential target of FMT in treating UC.
TLR4 is differentially expressed in patients with early and advanced UC, indicating a close correlation between TLR4 and UC[59]. Inhibition of TLR4 significantly decreases the expression of cell cycle regulatory genes. Furthermore, TLR4 signaling in colonic epithelial cells promotes the recruitment of inflammatory cells through microRNA 155-mediated posttranscriptional regulation[60]. In the current study, our results showed that FMT downregulated the expression of genes related to the TLR4/myosin light chain kinase signaling pathway in WT mice, highlighting the importance of TLR4 in the effectiveness of FMT. Functional analysis revealed that most DEGs were enriched in cytoplasmic translation and cellular response to DNA damage stimulus. The top nine DEGs strongly related to Akkermansia were primarily associated with cell cycle regulation, transcriptional control, apoptosis, stress responses, and inflammatory responses. Their functions aligned with the main processes identified in GO analysis, indicating their involvement in crucial biological pathways. These functions highlight its potential role in modulating various cellular activities. Aquaporin 4 (AQP4), a water channel protein that facilitates transmembrane water movement, has the strongest correlation[61]. AQPs are widely distributed in mammals’ secretory and absorptive epithelial cells and are responsible for transport and trafficking processes. In colonic inflammation, AQP4 is abundantly expressed in the basolateral membrane of colonic epithelial cells in humans and mice. The permeability of cell membranes is positively correlated with AQP4 expression[62]. AQP4 overexpression facilitates the entry of water into cytes, thereby contributing to cytotoxic edema[63-65]. AQP4 deficiency alleviates experimental colitis in the mice[66]. Although we did not use the same mouse KO model in the present study, the effect of AQP4 on colonic inflammation is consistent with that of TLR4. Activating the high mobility group box 1 protein/TLR4/NF-κB pathway can increase the expression of AQP4[67,68]. Furthermore, lipopolysaccharide, a potent TLR4 agonist, significantly increases the mRNA level of AQP4 expression through TLR4 signaling in the cortex and astrocytes[62]. We speculate that TLR4 deficiency can protect against colitis by increasing the abundance of Akkermansia and reducing the expression of AQP4. As shown by previous results, FMT can relieve colitis in WT mice[9]. However, in this study, FMT did not exert effects on colonic inflammation in TLR4-KO mice. It was intriguing to determine that the abundance of Akkermansia, which was dominant in TLR4-KO mice, was significantly decreased after FMT. This may be related to the decreased relative abundance of Akkermansia. While the DEGs mentioned above may have roles in immune regulation, inflammation, or cellular processes that can intersect with TLR4 signaling, their specific relationships with TLR4 are not extensively characterized. Notwithstanding, further studies are needed to determine whether FMT also targets Akkermansia to regulate the expression of related DEGs in countering colon inflammation.
In this study, we assessed the microbial diversity and composition in DSS-induced mice. The bacteria inhabited in the mucosa may play major roles in the development of IBD. So it is necessary to explore the function of microbiota in mucosal tissues in future study. However, animal studies have certain limitations in evaluating the mechanism of TLR4. Therefore, clinical studies should be designed to unveil the interplay among TLR4, gut microbiota, and UC.
ARTICLE HIGHLIGHTS
Research background
It is well known that microbiota dysbiosis contributes to the occurrence of inflammatory bowel disease (IBD). Fecal microbiota transplantation (FMT) has shown promising therapeutic effects on both clinical and basic studies of ulcerative colitis (UC). Substantial evidence supports a negative pro-inflammatory role of Toll-like receptor 4 (TLR4) signaling pathway in IBD. However, it remains unknown whether this modulation is also involved in the treatment of FMT on UC.
Research motivation
FMT treats other diseases by regulating the TLR4 signaling pathway. Previous studies have shown that the expression of TLR4 is higher in the intestinal mucosa of patients with effective FMT and lower in patients with poor FMT. We speculate that the TLR4 signaling pathway may be involved in the therapeutic mechanism of FMT on IBD.
Research objectives
To clarify the necessity of TLR4 signaling pathway in FMT on regulating gut microbiota in dextran sodium sulphate (DSS)-induced colitis.
Research methods
Experimental colitis was constructed in wild-type (WT) and TLR4-knockout (KO) mice and fecal microbiota was transplanted by gavage. Colon inflammation severity in mouse model was measured by disease activity index (DAI) score and hematoxylin and eosin (H&E) staining. Gut microbiota alteration was analyzed through 16S ribosomal RNA sequencing. The difference of gene expression in mouse colon was obtained by transcriptome sequencing of colon tissue.
Research results
In KO mice treated with FMT or water, these two groups displayed indistinguishable body weight loss, colon length, DAI score, and histology score, which showed that FMT could hardly alter the disease progress in KO mice. Next, compared with WT mice, the scores of DAI and colon histology clearly decreased in the KO-DSS group. KO mice experienced enhanced resistibility to DSS-induced colitis. There was a significant difference in the microbiota structure between KO and WT mice. Akkermansia was the dominant genus in healthy KO mice. But unexpectedly, after treatment with FMT, the relative abundance of Akkermansia decreased, while the level of Lactobacillus in the intestine of mice was maintained. The ineffectiveness in KO mice after FMT was related to the decrease of Akkermansia. GO enrichment analysis showed that DEGs between each group were mainly involved in cytoplasmic translation and cellular response to DNA damage stimulus. Finally, we listed the top nine genes related to Akkermansia.
Research conclusions
FMT may ameliorate DSS-induced colitis by regulating the TLR4 signaling pathway.
Research perspectives
This study provides new insights into the underlying mechanisms of FMT as a treatment for UC, which greatly helps to optimize FMT treatment in the future.