Published online Jan 21, 2023. doi: 10.3748/wjg.v29.i3.536
Peer-review started: October 9, 2022
First decision: November 18, 2022
Revised: November 29, 2022
Accepted: January 3, 2023
Article in press: January 3, 2023
Published online: January 21, 2023
Processing time: 95 Days and 6.6 Hours
Multiple linear stapler firings during double stapling technique (DST) after laparoscopic low anterior resection (LAR) are associated with an increased risk of anastomotic leakage (AL). However, it is difficult to predict preoperatively the need for multiple linear stapler cartridges during DST anastomosis.
To develop a deep learning model to predict multiple firings during DST anas
We collected 9476 MR images from 328 mid-low rectal cancer patients undergoing LAR with DST anastomosis, which were randomly divided into a training set (n = 260) and testing set (n = 68). Binary logistic regression was adopted to create a clinical model using six factors. The sequence of fast spin-echo T2-weighted MRI of the entire pelvis was segmented and analyzed. Pure-image and clinical-image integrated deep learning models were constructed using the mask region-based convolutional neural network segmentation tool and three-dimensional convolutional networks. Sensitivity, specificity, accuracy, positive predictive value (PPV), and area under the receiver operating characteristic curve (AUC) was calculated for each model.
The prevalence of ≥ 3 linear stapler cartridges was 17.7% (58/328). The prevalence of AL was statistically significantly higher in patients with ≥ 3 cartridges compared to those with ≤ 2 cartridges (25.0% vs 11.8%, P = 0.018). Preoperative carcinoembryonic antigen level > 5 ng/mL (OR = 2.11, 95%CI 1.08-4.12, P = 0.028) and tumor size ≥ 5 cm (OR = 3.57, 95%CI 1.61-7.89, P = 0.002) were recognized as independent risk factors for use of ≥ 3 linear stapler cartridges. Diagnostic performance was better with the integrated model (accuracy = 94.1%, PPV = 87.5%, and AUC = 0.88) compared with the clinical model (accuracy = 86.7%, PPV = 38.9%, and AUC = 0.72) and the image model (accuracy = 91.2%, PPV = 83.3%, and AUC = 0.81).
MRI-based deep learning model can predict the use of ≥ 3 linear stapler cartridges during DST anastomosis in laparoscopic LAR surgery. This model might help determine the best anastomosis strategy by avoiding DST when there is a high probability of the need for ≥ 3 linear stapler cartridges.
Core Tip: Multiple linear stapler firings during double stapling technique (DST) anastomosis are associated with an increased risk of anastomotic leakage after laparoscopic low anterior resection. This retrospective study developed a deep learning model to predict the use of ≥ 3 linear stapler cartridges during DST anastomosis. With the help of the artificial intelligence to identify and extract information from pelvic magnetic resonance imaging, we developed a clinical-image integrated model with satisfactory accuracy. This model might help preoperatively to determine the anastomosis strategy for rectal cancer patients (suggesting not to perform DST when the risk for ≥ 3 firings is high).
- Citation: Cai ZH, Zhang Q, Fu ZW, Fingerhut A, Tan JW, Zang L, Dong F, Li SC, Wang SL, Ma JJ. Magnetic resonance imaging-based deep learning model to predict multiple firings in double-stapled colorectal anastomosis. World J Gastroenterol 2023; 29(3): 536-548
- URL: https://www.wjgnet.com/1007-9327/full/v29/i3/536.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i3.536
Anastomotic leakage (AL) is the most common postoperative complication after laparoscopic low anterior resection (LAR) for mid and low rectal cancer[1]. The consequences of AL include higher mortality, need for remedial re-operation, unplanned stoma, delay before adjuvant therapy, and compromised long-term oncological outcomes[2-4]. Although several techniques have been designed to prevent AL[5-9], the prevalence of this complication has hardly improved over the past 20 years[10,11].
Of these techniques, the double stapling technique (DST) has facilitated bowel reconstruction but failed to eliminate AL[2]. During this procedure, the distal margin of the tumor-bearing specimen is transected by one or more linear stapler firings to create the rectal stump. Several publications have identified multiple linear stapler firings as an independent risk factor for AL[1,6,12-16]. Both the Chinese Expert Consensus Statement on the Diagnostic, Prevention and Treatment of the AL for Rectal Cancer (2019) and the United States Food and Drug Administration have suggested limiting the number of stapler firings to two in the DST procedure[17,18]. A recent review of DST suggested that alternative anastomotic techniques to avoid multiple firings on the rectal stump might lower the AL rate[11].
If the number of stapler cartridges used during surgery were predictable before operation, we could predetermine whether DST would be the ideal method for reconstruction. Several studies have reported the association between pelvimetry findings and the technical difficulties (including the use of ≥ 3 linear stapler cartridges) in LAR for mid-low rectal cancer[19-21]. However, previous studies only considered the dimension of pelvic bone landmarks in pelvimetry but ignored mesorectum thickness, tumor size, or tumoral infiltration to nearby organs (prostate, seminal vesicle, uterus). Based on our subjective experience, we speculated that the narrow (male) pelvis, thick mesorectum, aggressive tumor infiltration, and low transection margin might be associated with the need for ≥ 3 linear stapler cartridges to close the rectal stump. Besides, a simple comparison of one or several measurements of pelvimetry is insufficient to reveal the difficulty of the pelvic procedure. For a lean female patient or a heavy male patient, the same interspinous distance has a vast difference in clinical significance. Furthermore, manual measurement of pelvimetry indicators is time-consuming and labor-intensive.
These shortcomings of existing predictive methods prompted us to design and develop a new model to predict more precisely and effectively the need for ≥ 3 linear stapler firings during DST. Pelvic magnetic resonance imaging (MRI), a routine and first-choice tool for preoperative staging of rectal cancer[22], can capture mesorectal or nearby tissue infiltration characteristics in addition to bony structures. On the other hand, machine learning and deep learning models have been widely applied in health care because of their high ability to predict and make decisions[23]. Owing to the recent technological development[24-25], image-reading artificial intelligence (AI) programs can be used to recognize target features, and then interpret images or provide diagnoses based on these target features[26-30].
In this study, we aimed to create a deep learning pre-warning model for the use of multiple linear stapler cartridges during DST anastomosis by adopting AI to identify, extract and integrate image information from pelvic MRI.
We retrospectively analyzed the records of 328 patients who underwent laparoscopic LAR for mid-low rectal cancer at Ruijin Hospital, Shanghai, China, between 2016 and 2021. Clinicopathological data were collected from our prospective institutional database and the study was approved by Ruijin Hospital Ethics Committee (Approval No. 2019-82). Informed consent was waived by the committee because of the retrospective nature of the study. The study was registered at clinicaltrials.gov with the registry number: NCT05498506.
The inclusion criteria were: (1) Rectal carcinoma confirmed by histopathological evaluation; (2) Tumor located in the mid-low rectum (< 10 cm from the anal verge); (3) Performance of DST anastomosis; and (4) Pelvic MRI obtained within 14 d before surgery.
The exclusion criteria were: (1) Other anastomotic techniques (e.g., trans-anal rectal excision); (2) Hartmann’s operation or other procedures without anastomosis; (3) Robotic surgery; and (4) The number of linear stapler cartridges was not traceable in the operative report. By using an unbiased random sampling method with a split ratio of 4:1, the patients were divided into a training set (n = 260) and testing set (n = 68).
Laparoscopic LAR was performed by one operating team who treated > 200 cases of rectal cancer per year. The surgical procedure followed the national guidelines for laparoscopic radical resection of colorectal cancer (2018 edition). Distal rectal transection was performed with an endoscopic linear stapler (Endo-GIA™ Ultra Universal Stapler Reload with Tri-staple™ Technology; Covidien Limited Liability Company, Minneapolis, MN, USA), fired manually through the right lower quadrant 12-mm trocar. The 60-mm purple cartridges containing three different staple heights (3.0 mm, 3.5 mm, and 4.0 mm) were routinely used. However, the 45-mm purple cartridges could be used when the stapler could not be placed perpendicularly to the rectum with the 60-mm cartridges.
We collected and analyzed baseline characteristics [sex, age, body mass index (BMI)], laboratory analysis [hemoglobin, albumin, carcinoembryonic antigen (CEA)], and tumor features [distance from the anal verge, circumferential resection margin (CRM), tumor size, tumor stage]. For the clinical model, we created a multivariate binary logistic regression model based on clinical variables that might be associated with the number of linear stapler cartridges during surgery: Three binary variables [sex (male, female), CEA level [normal, elevated (> 5 ng/mL)], and CRM (positive, negative)] and three continuous variables (BMI, distance from the anal verge, and tumor size).
Pelvic MRI was performed by a Philips INGENIA™ MR scanner with a field strength of 3.0 T and the patient in the supine position. The scanning parameters included: Repetition time = 3565 ms; echo time = 80 ms; layer thickness = 5 mm; image matrix = 312 ´ 357, field of view = 250 ´ 340 ´ 166 mm.
The sequence of fast spin-echo (FSE) T2-weighted MRI with a large field of view with fat suppression obtained in the axial plane of the entire pelvis was retrieved from the Picture Archiving and Communication System for image segmentation. A total of 9476 T2-weighted MR images were collected from the enrolled patients. Fifteen patients in the training set were randomly selected by random number tables and 367 images from these patients served for manual labeling. A radiological expert with > 15 years of experience in pelvic MRI labeled three target regions (pelvis, mesorectum, and tumor body) on each of the consecutive T2-weighted images. These regions were represented by drab, yellow, and green, respectively (Supplementary Figure 1), using an open annotation tool named Labelme (available at labelme.csail.mit.edu)[31]. Data were transformed into the Common Objects in Context (COCO) dataset format[32].
Mask region-based convolutional neural network (Mask R-CNN)[24] was used to detect and segment the three target regions (Supplementary Figure 2).
The entire Mask R-CNN network was trained on the training set, and the performance of the testing set was evaluated using the mean Average Precision (mAP). When mAP was > 50, we considered the segmentation model to have performed well[24].
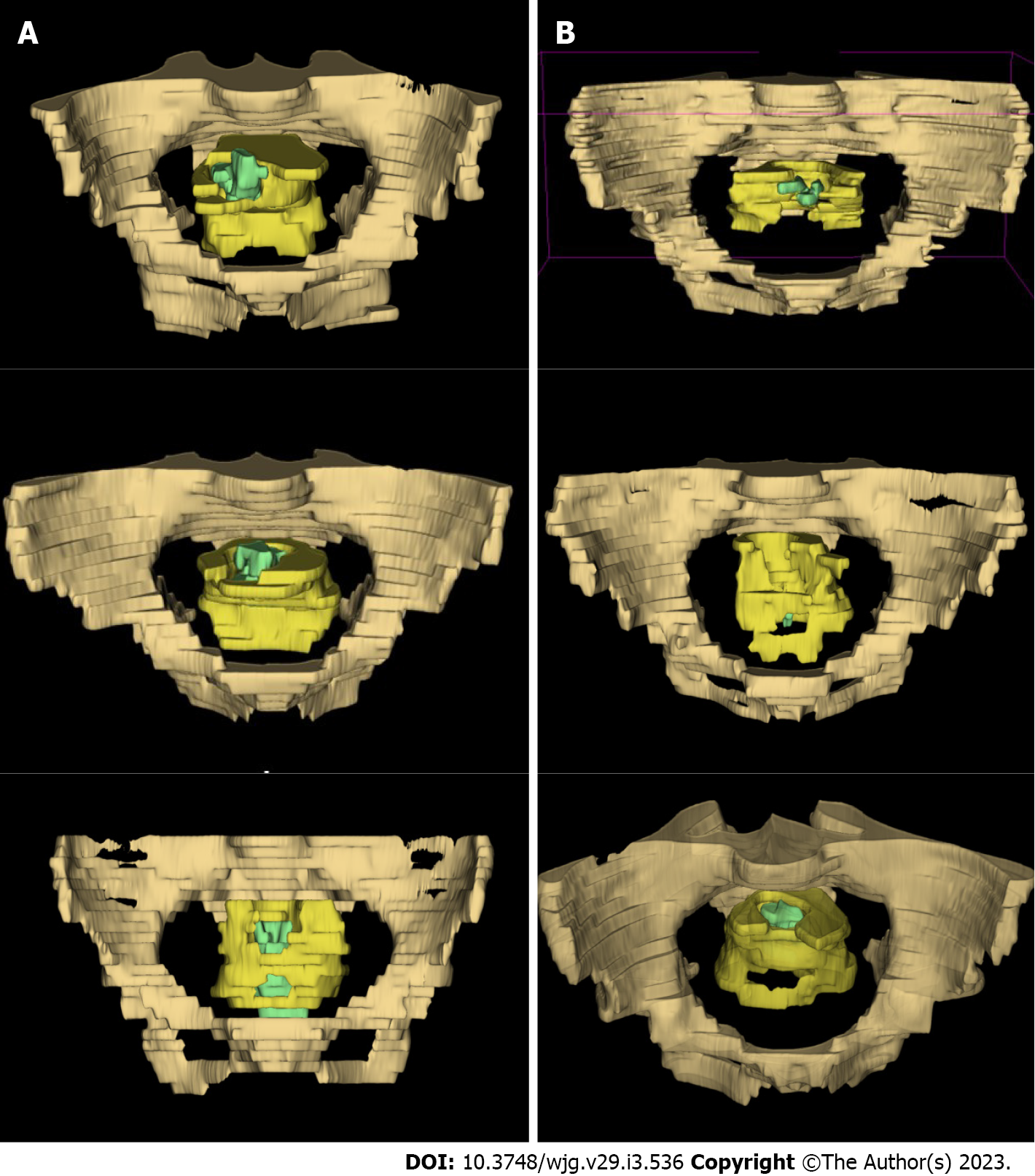
To visualize intuitively the segmentation of the target region, 3D Slicer software (available at www.slicer.org) was adopted to reconstruct a three-dimensional visualization model for each patient (Figure 1).
A three-dimensional convolutional networks (C3D)-based model was used to generate the probability of multiple linear stapler cartridges after segmentation[25]. We used all the images of one patient as the input whereas the output was the probability of ≥ 3 linear stapler cartridges. When the probability was greater than a preset threshold (set to 0.5 empirically), the sample was judged as positive. We trained the C3D network on the training set for 100 epochs and obtained the final C3D model.
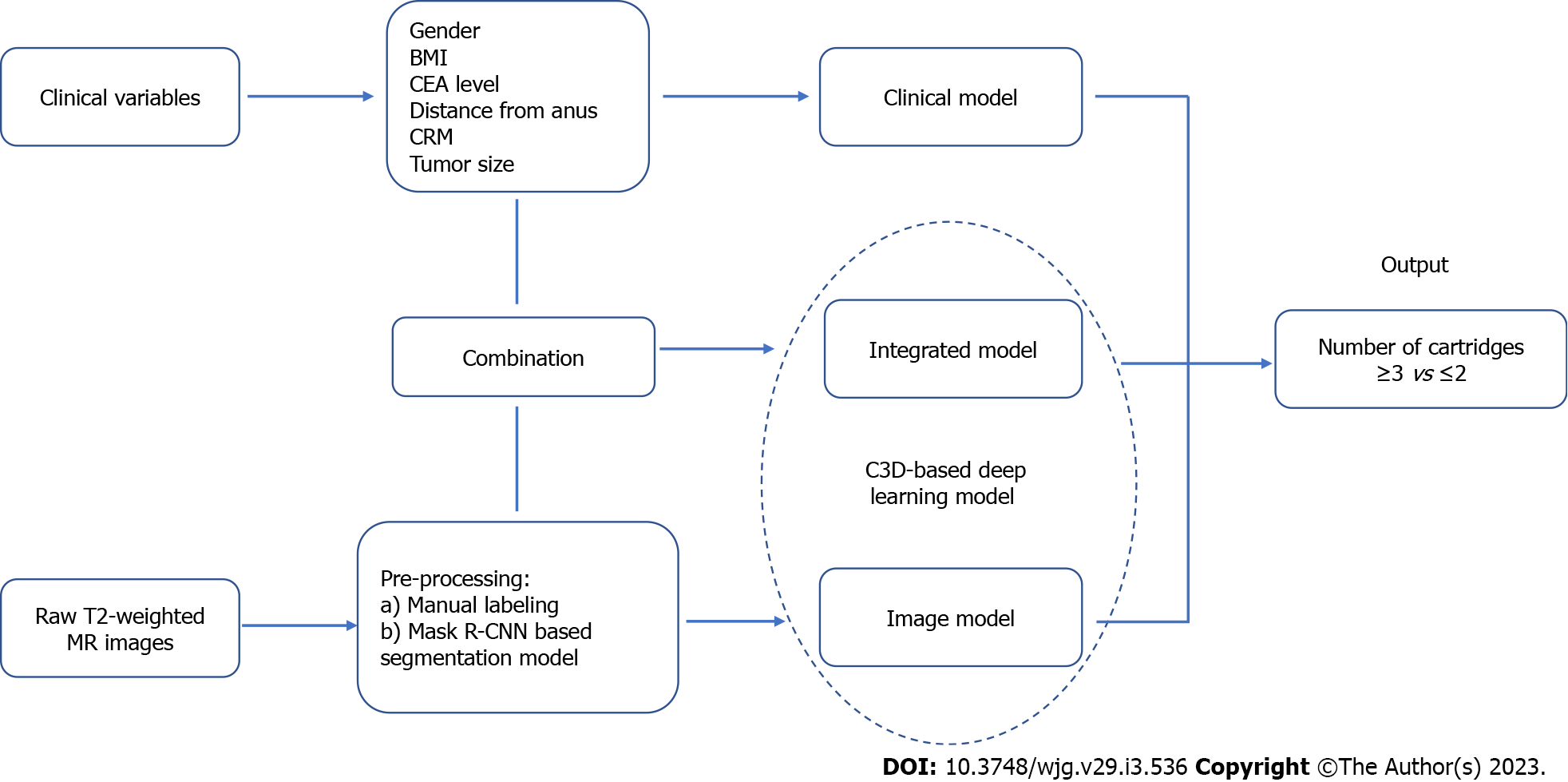
Two deep learning models were used in our study, a pure image model using only T2-weighted MR images segmented by Mask R-CNN and an integrated model using MR images as well as six above-mentioned clinical variables. The flow chart of the design of these pre-warning models is shown in Figure 2. Our source code is publicly available (https://github.com/suli609/MRI-DST).
Finally, one clinical model and two deep learning models were evaluated on the testing set. A receiver operating characteristic (ROC) curve was plotted for each model. Sensitivity, specificity, accuracy, positive predictive value (PPV), and area under the curve (AUC) were calculated for each curve. AUC > 0.70 indicated an acceptable model.
Statistical Package for the Social Sciences (SPSS 13.0, Chicago, IL, USA) was used for statistical analysis. The statistical methods of this study were reviewed by Shuang Wu from China Novartis Institutes for BioMedical Research Co. Ltd. Numerical variables were examined by non-parametric Wilcoxon rank-sum test. Pearson’s Chi-Square or Fisher’s exact test was adopted to analyze categorical data. Multivariate analysis was performed by binary logistic regression model. The difference was considered statistically significant if two-sided P values were < 0.05.
The entire study population included 328 patients, 227 male and 101 female with a median age of 63 (range 24 - 87) years. The prevalence of use of ≥ 3 linear stapler cartridges was 17.7% (58/328). The training set (n = 260) consisted of 48 cases with ≥ 3 cartridges and 212 cases with ≤ 2 cartridges. The testing set (n = 68) consisted of 10 cases with ≥ 3 cartridges and 58 cases with ≤ 2 cartridges.
When clinicopathological characteristics were compared between the patients with ≥ 3 cartridges and those with ≤ 2 cartridges in the training set (Table 1), there was no statistically significant difference between the two groups with respect to sex, age, BMI, diabetes mellitus, preoperative CEA serum level, and the percentage of patients undergoing neoadjuvant chemoradiotherapy. No statistically significant difference was found in the distance from tumor to the anal verge, tumor size, tumor stage, operation time, or insufficient distal resection margin (≤ 5 mm). The incidence of AL was statistically significantly higher in the patients with ≥ 3 cartridges compared to those with ≤ 2 cartridges (P = 0.018).
| Number of linear stapler cartridges | ≥ 3 | ≤ 2 | P value |
| n = 48 (18.5%) | n = 212 (81.5%) | ||
| Sex, n (%) | 0.125 | ||
| Male | 38 (79.2) | 144 (67.9) | |
| Female | 10 (20.8) | 68 (32.1) | |
| Age (y), median (quartile) | 62 (55-71) | 63 (55-68) | 0.749 |
| BMI (Kg/m2), median (quartile) | 23.5 (21.1-25.3) | 22.9 (21.3-25.1) | 0.942 |
| Diabetes mellitus, n (%) | 0.801 | ||
| Yes | 7 (14.6) | 28 (13.2) | |
| No | 41 (85.4) | 184 (86.8) | |
| Hemoglobin (g/L), median (quartile) | 136 (124-143) | 133 (124-144) | 0.540 |
| Albumin (g/L), median (quartile) | 39 (36-41) | 40 (37-42) | 0.015 |
| CEA (ng/mL), median (quartile) | 4.27 (2.11-7.08) | 3.05 (2.11-5.61) | 0.147 |
| nCRT, n (%) | 0.865 | ||
| Yes | 13 (27.1) | 60 (28.3) | |
| No | 35 (72.9) | 152 (71.7) | |
| Distance from anus (cm), median (quartile) | 7.2 (5.9-8.4) | 7.0 (5.6-8.7) | 0.842 |
| CRM evaluated by MRI, n (%) | 0.103 | ||
| Positive | 16 (33.3) | 47 (22.2) | |
| Negative | 32 (66.7) | 165 (77.8) | |
| Operation time (min), median (quartile) | 139 (111-180) | 143 (116-175) | 0.526 |
| Length of cartridges used, n (%) | 0.113 | ||
| Only 60 mm | 42 (87.5) | 200 (94.3) | |
| 45 mm ± 60 mm | 6 (12.5) | 12 (5.7) | |
| Anastomotic leakage, n (%) | 0.018 | ||
| Yes | 12 (25.0) | 25 (11.8) | |
| No | 36 (75.0) | 187 (88.2) | |
| Tumor size (cm), median (quartile) | 3.7 (3.1-5.1) | 3.5 (2.9-4.2) | 0.091 |
| T stage, n (%) | 0.213 | ||
| T ≤ 2 | 11 (22.9) | 68 (32.1) | |
| T 3-4 | 37 (77.1) | 144 (67.9) | |
| N stage, n (%) | 0.879 | ||
| N0 | 25 (52.1) | 113 (53.3) | |
| N+ | 23 (47.9) | 99 (46.7) | |
| DRM, n (%) | 0.395 | ||
| ≤ 5 mm | 4 (8.3) | 27 (12.7) | |
| > 5 mm | 44 (91.7) | 185 (87.3) |
Univariate and multivariate analysis revealed two independent risk factors for use of ≥ 3 Linear stapler cartridges: Preoperative CEA level > 5 ng/mL (OR = 2.11, 95%CI 1.08-4.12, P = 0.028) and tumor size ≥ 5 cm (OR = 3.57, 95%CI 1.61-7.89, P = 0.002) (Table 2). All these clinicopathological features were comparable between the training set and testing set (Table 3).
| Factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Sex (M/F) | 0.56 (0.26, 1.18) | 0.125 | NA | NA |
| Age (yr) (≥ 70/< 70) | 1.60 (0.76, 3.29) | 0.205 | NA | NA |
| BMI (Kg/m2) (≥ 25/ < 25) | 1.24 (0.63, 2.44) | 0.542 | NA | NA |
| Diabetes mellitus (Y/N) | 1.12 (0.46, 2.75) | 0.801 | NA | NA |
| Albumin (g/L) (< 35/≥ 35) | 2.42 (0.92, 6.37) | 0.074 | NA | NA |
| CEA (ng/mL) (> 5/≤ 5) | 1.99 (1.04, 3.81) | 0.038 | 2.11 (1.08, 4.12) | 0.028 |
| nCRT (Y/N) | 0.94 (0.47, 1.90) | 0.865 | NA | NA |
| Distance from anus (cm) (< 5/≥ 5) | 0.60 (0.20, 1.79) | 0.358 | NA | NA |
| CRM evaluated by MRI (+/-) | 1.76 (0.89, 3.47) | 0.103 | NA | NA |
| Length of cartridges (mm) (45/60) | 0.42 (0.15, 1.18) | 0.113 | NA | NA |
| Tumor size (cm) (≥ 5/< 5) | 3.38 (1.55, 7.37) | 0.002 | 3.57 (1.61, 7.89) | 0.002 |
| Testing set | Training set | P value | |
| n = 68 | n = 260 | ||
| Sex, n (%) | 0.543 | ||
| Male | 45 (66.2) | 182 (70.0) | |
| Female | 23 (33.8) | 78 (30.0) | |
| Age (yr), median (quartile) | 63 (57-71) | 63 (55-68) | 0.322 |
| BMI (Kg/m2), median (quartile) | 23.7 (22.0-25.0) | 22.9 (21.3-25.1) | 0.248 |
| Diabetes mellitus, n (%) | 0.303 | ||
| Yes | 6 (8.8) | 35 (13.5) | |
| No | 62 (91.2) | 225 (86.5) | |
| Albumin (g/L), median (quartile) | 39 (36-41) | 40 (37-42) | 0.111 |
| CEA (ng/mL), n (%) | (Missing=5) | 0.863 | |
| > 5 | 21 (30.9) | 76 (29.8) | |
| ≤ 5 | 47 (69.1) | 179 (70.2) | |
| nCRT, n (%) | 0.081 | ||
| Yes | 12 (17.6) | 73 (28.1) | |
| No | 56 (82.4) | 187 (71.9) | |
| Distance from anus (cm), median (quartile) | 7.1 (5.8-8.7) | 7.0 (5.6-8.7) | 0.828 |
| CRM evaluated by MRI, n (%) | 0.051 | ||
| Positive | 9 (13.2) | 63 (24.2) | |
| Negative | 59 (86.8) | 197 (75.8) | |
| Tumor size (cm), n (%) | 0.340 | ||
| ≥ 5 | 6 (8.8) | 34 (13.1) | |
| < 5 | 62 (91.2) | 226 (86.9) | |
| Number of linear stapler cartridges, n (%) | 0.470 | ||
| ≥ 3 | 10 (14.7) | 48 (18.5) | |
| ≤ 2 | 58 (85.3) | 212 (81.5) | |
| Length of cartridges used, n (%) | 0.603 | ||
| Only 60 mm | 62 (91.2) | 242 (93.1) | |
| 45 mm ± 60 mm | 6 (8.8) | 18 (6.9) | |
| Anastomotic leakage, n (%) | 0.686 | ||
| Yes | 11 (16.2) | 37 (14.2) | |
| No | 57 (83.8) | 223 (85.8) |
Of the three-dimensional reconstruction models presented in Figure 1, those in Figure 1A, 1C, and 1E were models from patients with the use of ≥ 3 linear stapler cartridges while those in Figure 1B, 1D, and 1F were models from patients with the use of ≤ 2 cartridges. Characteristics potentially relevant to the use of ≥ 3 cartridges were narrow pelvis (Figure 1A, drab part), thick mesorectum (Figure 1C, yellow part), and large tumor size with low distal margin (Figure 1E, green part), as can be seen in the models in the left column.
The mAP of the segmentation model was 57.2 for the object detection task and 53.7 for the instance segmentation task.
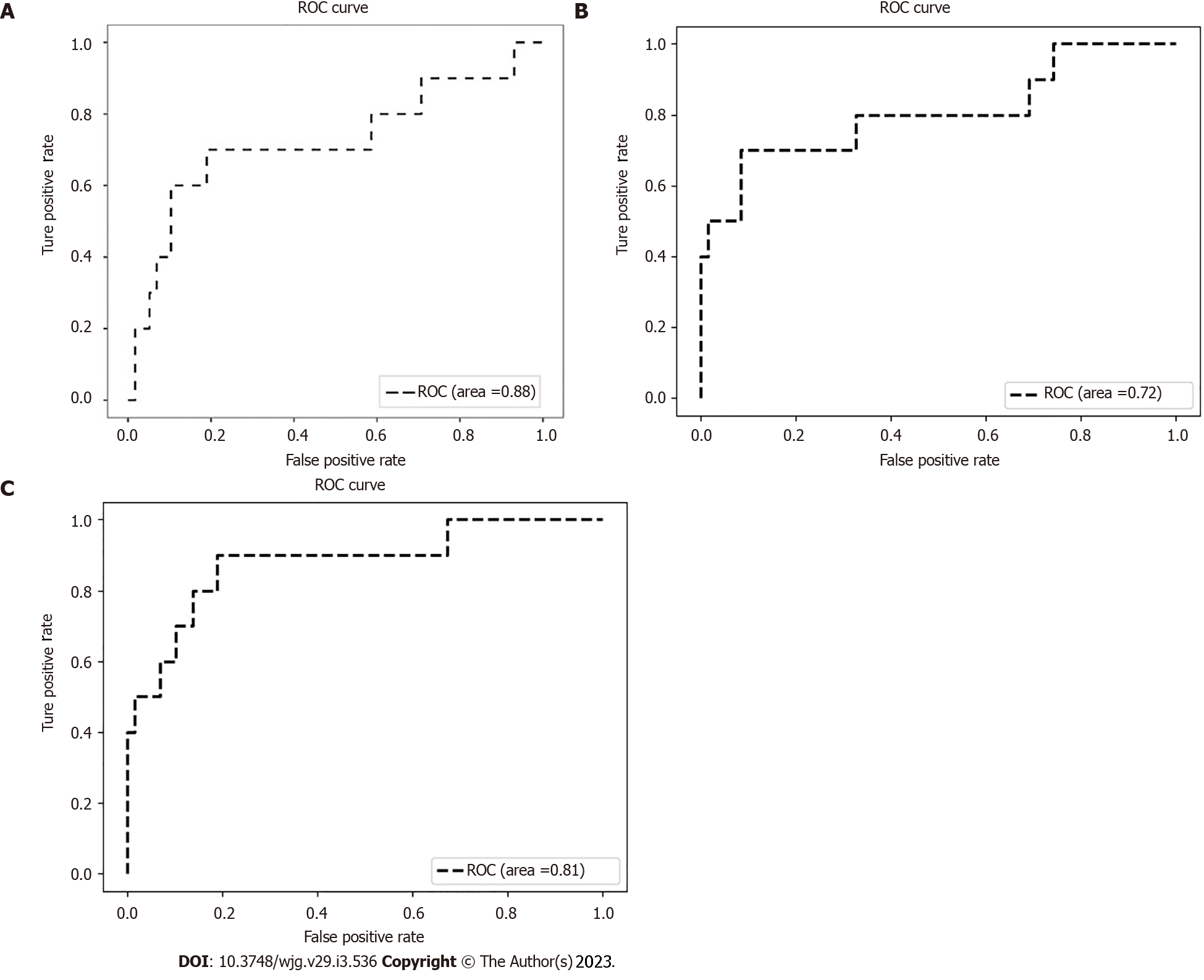
The sensitivity, specificity, and accuracy of the clinical model were 70.0%, 81.0%, and 79.4%, respectively (Youden index = 0.51, PPV = 38.9%). The relevant technical indicators of the image model were as follows: Sensitivity = 50.0%, specificity = 98.3%, accuracy = 91.2%, Youden index = 0.48, and PPV = 83.3%. The integrated model showed the best pre-warning performance: Sensitivity = 70.0%, specificity = 98.3%, accuracy = 94.1%, Youden index = 0.68, and PPV = 87.5%. Finally, the AUC was 0.72, 0.81, and 0.88 for the clinical model, the image model, and the integrated model, respectively (Figure 3).
Our deep learning model can predict the probability of using ≥ 3 linear stapler cartridges in the DST anastomosis during laparoscopic LAR surgery. Compared with the clinical model and the pure image model, the integrated model, which combined both the clinical variables and pelvic MR images, had a better Youden index (0.68) and AUC (0.88). Our results suggest that clinical or imaging information alone is insufficient to predict the use of ≥ 3 cartridges during surgery and an MRI-based integrated deep learning model might help determine the best anastomotic strategy for mid-low rectal cancer patients.
The safety, feasibility, and oncological outcomes of laparoscopic LAR surgery for mid-low rectal cancer have been confirmed by a series of high-quality randomized controlled trials[33,34]. During laparoscopic LAR, the DST method is considered to be difficult in some patients because the size and angle of linear staplers are limited in laparoscopy[14,35]. Consequently, multiple stapler firings are often needed. Two mechanisms might give rise to AL: Either space is left between two adjacent staple lines, or crossing the staple line with another row of staples or crushing the first staple line with the jaws can dislodge, break or deform the staples[6,12,13,18].
This has prompted surgeons to modify anastomosis techniques, which have been described as follows: Transanal transection of the rectal stump with transanal anastomosis[36,37]; intra-luminal transection of the rectal stump with manual purse-string sutures (e.g., trans-anal total mesorectal excision technique)[37,38]; vertical rectal division using a linear stapler after making an additional skin incision above the pubic symphysis[6]; transverse rectal division using a Contour® stapler during laparoscopic surgery[7]; lateralization of the stump by Nelaton catheter pulling method[8]; side-to-end anastomosis (Baker technique)[9]; trans-anal reinforcement of anastomosis[39]; or removing the “dog ears”/ crossing staple lines[40,41].
Thus, if there is a high probability of using ≥ 3 cartridges according to preoperative data, one of these other anastomosis methods might be more suitable than the DST method. Foo et al[21] reported a pre-warning model to predict the likelihood of transecting the rectum with ≥ 3 stapler cartridges, which included the following parameters: Sex, pelvic inlet, interspinous distance, intertuberous distance, and tumor height. Two other studies investigated the technical difficulty in LAR surgery with DST anastomosis but they used other indicators, such as operative time, pelvic operative time, blood loss, conversion rate, complications, or specimen quality[42,43]. The factors associated with technical difficulty were BMI, tumor height, interspinous distance, intertubercle distance, pelvic inlet, and pubic tubercle height. The similarity of these studies with ours is that we combined clinical information with pelvic anatomical factors and the pelvimetry was conducted in pelvic MRI. However, the strengths of our pre-warning model are mainly featured as follows: (1) By using AI-based segmentation of images, the pelvimetry is recognized as a whole instead of isolated measurements; (2) All parameters considered in the above-mentioned clinical models (sex, pelvic measurement, BMI, tumor size/height/stage) were synthesized in our image-reading models. This is why we performed segmentation of three different target regions (bony, fatty, and tumoral) in our study; and (3) This AI-based pre-warning model can shorten the prediction time to 100ms. The only data needed are six clinical factors and the sequence of FSE T2-weighted MR images.
Compared with other segmentation algorithms, such as faster R-CNN, the implementation process of Mask R-CNN is simpler, and the segmentation accuracy is higher. The mAP achieved by our model met the needs of most application scenarios[24]. The actual segmentation effect is close to the target regions manually segmented by radiologists (Supplementary Figure 2). The C3D network structure has good versatility, and the overheads of training the model are small, which is suitable for scenarios with limited training samples[25,44].
Our study had several limitations. First, the small sample size in the testing set lowered the statistical power of our analysis. With this sample size, the statistical difference between the three ROC curves might have been underestimated. Second, the lack of cases made it impossible to validate this model in an external set. Further prospective multi-center studies are needed to verify the validity of this model. Third, deep learning was only conducted on FSE T2-weighted sequences with specific scanning parameters. Further studies could focus on other MRI sequences or contrast-enhanced MRI. Fourth, the number of cartridges was not the only factor involved in AL. The intersection of staple lines[45], the precompression before stapler firings[2], and the distance between the linear staple line and the circular end-to-end anastomosis[35] might also have been implicated in addition to the number of firings. However, we could not include these factors in our analysis because of the retrospective nature of our study. Finally, apart from those factors mentioned above, the number of linear stapler cartridges depended on other factors that were difficult to assess, such as the proper lateralization of the intestinal tube[8] and the precise placement of the trocar through which the linear stapler was fired[2,35]. Thus, none of our three models achieved 100% accuracy in the testing set. However, the PPV increased to 87.5% in the integrated model compared with 38.9% in the clinical model, indicating that the trans-abdominal DST method would be unsuitable for positive cases predicted by the integrated model.
With the goal of predicting the use of ≥ 3 linear stapler cartridges during DST anastomosis in laparoscopic LAR surgery, our pelvic MRI-based deep learning model might be helpful in the preoperative determination of the best anastomosis strategy for mid-low rectal cancer patients, and, in particular, in avoiding the DST technique when there is a high probability of the need for ≥ 3 linear stapler cartridges. In this setting, another anastomotic technique without staple line crossing should be chosen. Larger studies are needed to validate its clinical value and determine if this strategy can help lower the AL rate.
The need for multiple (≥ 3) linear stapler firings during double stapling technique (DST) is associated with an increased risk of anastomotic leakage (AL) after laparoscopic low anterior resection (LAR).
Current methods using clinical data cannot predict precisely the use of ≥ 3 linear stapler firings before surgery.
This study aimed to develop a pelvic magnetic resonance imaging (MRI)-based deep learning model to predict the multiple firings during DST anastomosis.
Clinical data and 9476 MR images from 328 mid-low rectal cancer patients undergoing LAR with DST anastomosis were retrospectively collected. A pure-image model and a clinical-image integrated model were constructed using image-reading deep learning technologies, respectively.
The clinical-image integrated model showed better predictive performance compared with the clinical model and the pure image model with the highest accuracy (94.1%) and area under the curve (0.88).
Our deep learning model might help determine the anastomosis strategy for mid-low rectal cancer patients (suggesting not to perform the DST when the risk for ≥ 3 linear stapler firings is high).
The clinical value of this clinical-image integrated model will be validated in further prospective studies. The incidence of AL is expected to be decreased with this strategy.
We express our sincere gratitude to Shuang Wu (Statistical programmer) for her technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mijwil MM, Iraq; Shahria MT, United States; Sun D, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 324] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 2. | Kawada K, Sakai Y. Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol. 2016;22:5718-5727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Colon Cancer Laparoscopic or Open Resection Study Group; Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, Lacy A, Bonjer HJ. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1053] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 4. | Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, Fürst A, Lacy AM, Haglind E, Tuynman JB, Daams F, Bonjer HJ; COLOR COLOR II study group. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer: Increased Risk of Local Recurrence. Ann Surg. 2022;275:e420-e427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 5. | Katsuno H, Shiomi A, Ito M, Koide Y, Maeda K, Yatsuoka T, Hase K, Komori K, Minami K, Sakamoto K, Saida Y, Saito N. Comparison of symptomatic anastomotic leakage following laparoscopic and open low anterior resection for rectal cancer: a propensity score matching analysis of 1014 consecutive patients. Surg Endosc. 2016;30:2848-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis. 2008;23:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Ishii Y, Hasegawa H, Nishibori H, Endo T, Kitajima M. The application of a new stapling device for open surgery (Contour Curved Cutter Stapler) in the laparoscopic resection of rectal cancer. Surg Endosc. 2006;20:1329-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hotta T, Takifuji K, Yokoyama S, Matsuda K, Oku Y, Hashimoto T, Yamamoto N, Yamaue H. Rectal transection by the Nelaton catheter pulling method during a laparoscopic low anterior resection. Dis Colon Rectum. 2011;54:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Nakada I, Kawasaki S, Sonoda Y, Watanabe Y, Tabuchi T. Abdominal stapled side-to-end anastomosis (Baker type) in low and high anterior resection: experiences and results in 69 consecutive patients at a regional general hospital in Japan. Colorectal Dis. 2004;6:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Shearer R, Gale M, Aly OE, Aly EH. Have early postoperative complications from laparoscopic rectal cancer surgery improved over the past 20 years? Colorectal Dis. 2013;15:1211-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Emile SH, Barsom SH, Elfallal AH, Wexner SD. Comprehensive literature review of the outcome, modifications, and alternatives to double-stapled low pelvic colorectal anastomosis. Surgery. 2022;172:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Braunschmid T, Hartig N, Baumann L, Dauser B, Herbst F. Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc. 2017;31:5318-5326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 13. | Kawada K, Hasegawa S, Hida K, Hirai K, Okoshi K, Nomura A, Kawamura J, Nagayama S, Sakai Y. Risk factors for anastomotic leakage after laparoscopic low anterior resection with DST anastomosis. Surg Endosc. 2014;28:2988-2995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Kim CW, Baek SJ, Hur H, Min BS, Baik SH, Kim NK. Anastomotic Leakage After Low Anterior Resection for Rectal Cancer Is Different Between Minimally Invasive Surgery and Open Surgery. Ann Surg. 2016;263:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. 2009;209:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Degiuli M, Elmore U, De Luca R, De Nardi P, Tomatis M, Biondi A, Persiani R, Solaini L, Rizzo G, Soriero D, Cianflocca D, Milone M, Turri G, Rega D, Delrio P, Pedrazzani C, De Palma GD, Borghi F, Scabini S, Coco C, Cavaliere D, Simone M, Rosati R, Reddavid R; collaborators from the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Risk factors for anastomotic leakage after anterior resection for rectal cancer (RALAR study): A nationwide retrospective study of the Italian Society of Surgical Oncology Colorectal Cancer Network Collaborative Group. Colorectal Dis. 2022;24:264-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 17. | Chinese Society of Colorectal Surgery. Chinese Expert Consensus Statement on the Diagnostic, Prevention and Treation of the Anastomotic Leakage for Rectal Cancer (article in Chinese). Zhonghua Weichang Waike Zazhi. 2019;22: 201-206. [RCA] [DOI] [Full Text] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 18. | US. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Surgical Staplers and Staples for Internal Use - Labeling Recommendations. [Internet] [accessed 8 October 2021]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/surgical-staplers-and-staples-internal-use-labeling-recommendations. |
| 19. | Zhou XC, Su M, Hu KQ, Su YF, Ye YH, Huang CQ, Yu ZL, Li XY, Zhou H, Ni YZ, Jiang YI, Lou Z. CT pelvimetry and clinicopathological parameters in evaluation of the technical difficulties in performing open rectal surgery for mid-low rectal cancer. Oncol Lett. 2016;11:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc. 2010;24:2974-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Foo CC, Hung HT, Ho YC, Lam WWM, Law WL. Predicting the level of difficulty of the double-stapling technique in laparoscopic total mesorectal excision. Surg Endosc. 2020;34:3382-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;39:367-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 23. | Aggarwal K, Mijwil MM, Sonia, Al-Mistarehi A, Alomari S, Gök M, Alaabdin AMZ, Abdulrhman SH. Has the Future Started? Iraqi Journal For Computer Science and Mathematics. 3:115-123. [DOI] [Full Text] |
| 24. | He K, Gkioxari G, Dollar P, Girshick R. Mask r-cnn. Proceedings of the IEEE international conference on computer vision. Venice, Italy: IEEE, 2017: 2961-2969. [DOI] [Full Text] |
| 25. | Tran D, Bourdev L, Fergus R, Torresani L, Paluri M. Learning spatiotemporal features with 3d convolutional networks. Proceedings of the IEEE international conference on computer vision. IEEE, 2015: 4489-4497. [DOI] [Full Text] |
| 26. | Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5683] [Cited by in RCA: 5332] [Article Influence: 666.5] [Reference Citation Analysis (0)] |
| 27. | Nam JG, Park S, Hwang EJ, Lee JH, Jin KN, Lim KY, Vu TH, Sohn JH, Hwang S, Goo JM, Park CM. Development and Validation of Deep Learning-based Automatic Detection Algorithm for Malignant Pulmonary Nodules on Chest Radiographs. Radiology. 2019;290:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 328] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 28. | Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, van der Laak JAWM; the CAMELYON16 Consortium, Hermsen M, Manson QF, Balkenhol M, Geessink O, Stathonikos N, van Dijk MC, Bult P, Beca F, Beck AH, Wang D, Khosla A, Gargeya R, Irshad H, Zhong A, Dou Q, Li Q, Chen H, Lin HJ, Heng PA, Haß C, Bruni E, Wong Q, Halici U, Öner MÜ, Cetin-Atalay R, Berseth M, Khvatkov V, Vylegzhanin A, Kraus O, Shaban M, Rajpoot N, Awan R, Sirinukunwattana K, Qaiser T, Tsang YW, Tellez D, Annuscheit J, Hufnagl P, Valkonen M, Kartasalo K, Latonen L, Ruusuvuori P, Liimatainen K, Albarqouni S, Mungal B, George A, Demirci S, Navab N, Watanabe S, Seno S, Takenaka Y, Matsuda H, Ahmady Phoulady H, Kovalev V, Kalinovsky A, Liauchuk V, Bueno G, Fernandez-Carrobles MM, Serrano I, Deniz O, Racoceanu D, Venâncio R. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA. 2017;318:2199-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1871] [Cited by in RCA: 1547] [Article Influence: 193.4] [Reference Citation Analysis (0)] |
| 29. | Stanzione A, Verde F, Romeo V, Boccadifuoco F, Mainenti PP, Maurea S. Radiomics and machine learning applications in rectal cancer: Current update and future perspectives. World J Gastroenterol. 2021;27:5306-5321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (4)] |
| 30. | Mijwil MM. Skin cancer disease images classification using deep learning solutions. Multimed Tools Appl. 2021;80:26255-26271. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Russell BC, Torralba A, Murphy K P, Freeman WT. LabelMe: a database and web-based tool for image annotation. International journal of computer vision. 77:157-173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1878] [Cited by in RCA: 742] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 32. | Lin TY, Maire M, Belongie S, Hays J, Perona P, Ramanan D, Dollar P, Zitnick CL. Microsoft coco: Common objects in context. European conference on computer vision. Springer, Cham, 2014: 740-755. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6197] [Cited by in RCA: 2778] [Article Influence: 252.5] [Reference Citation Analysis (0)] |
| 33. | Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 924] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 34. | Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB, Choi HS, Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, Kang GH, Chie EK, Kim SY, Sohn DK, Kim DH, Kim JS, Lee HS, Kim JH, Oh JH. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 640] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 35. | Kuroyanagi H, Oya M, Ueno M, Fujimoto Y, Yamaguchi T, Muto T. Standardized technique of laparoscopic intracorporeal rectal transection and anastomosis for low anterior resection. Surg Endosc. 2008;22:557-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Nakagoe T, Ishikawa H, Sawai T, Tsuji T, Takeshita H, Nanashima A, Akamine S, Yamaguchi H, Yasutake T. Oncological outcome of ultra-low anterior resection with total mesorectal excision for carcinoma of the lower third of the rectum: Comparison of intrapelvic double-stapled anastomosis and transanal coloanal anastomosis. Hepatogastroenterology. 2005;52:1692-1697. [PubMed] |
| 37. | Spinelli A, Foppa C, Carvello M, Sacchi M, De Lucia F, Clerico G, Carrano FM, Maroli A, Montorsi M, Heald RJ. Transanal Transection and Single-Stapled Anastomosis (TTSS): A comparison of anastomotic leak rates with the double-stapled technique and with transanal total mesorectal excision (TaTME) for rectal cancer. Eur J Surg Oncol. 2021;47:3123-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 38. | Kim HJ, Choi GS, Park JS, Park SY. Comparison of intracorporeal single-stapled and double-stapled anastomosis in laparoscopic low anterior resection for rectal cancer: a case-control study. Int J Colorectal Dis. 2013;28:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Baek SJ, Kim J, Kwak J, Kim SH. Can trans-anal reinforcing sutures after double stapling in lower anterior resection reduce the need for a temporary diverting ostomy? World J Gastroenterol. 2013;19:5309-5313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Chen ZF, Liu X, Jiang WZ, Guan GX. Laparoscopic double-stapled colorectal anastomosis without "dog-ears". Tech Coloproctol. 2016;20:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Crafa F, Megevand J, Romano G, Sileri P. New double-stapled anastomotic technique to avoid crossing staple lines. Tech Coloproctol. 2015;19:319-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Miyata S, Yamaguchi T. Factors affecting the difficulty of laparoscopic total mesorectal excision with double stapling technique anastomosis for low rectal cancer. Surgery. 2009;146:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Hong JS, Brown KGM, Waller J, Young CJ, Solomon MJ. The role of MRI pelvimetry in predicting technical difficulty and outcomes of open and minimally invasive total mesorectal excision: a systematic review. Tech Coloproctol. 2020;24:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Carreira J, Zisserman A. Quo vadis. Action recognition? a new model and the kinetics dataset. Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. 2017: 6299-6308. [DOI] [Full Text] |
| 45. | Lee S, Ahn B, Lee S. The Relationship Between the Number of Intersections of Staple Lines and Anastomotic Leakage After the Use of a Double Stapling Technique in Laparoscopic Colorectal Surgery. Surg Laparosc Endosc Percutan Tech. 2017;27:273-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |