Published online Jan 21, 2023. doi: 10.3748/wjg.v29.i3.450
Peer-review started: September 30, 2022
First decision: November 17, 2022
Revised: November 18, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 21, 2023
Processing time: 104 Days and 2.7 Hours
Seronegative spondyloarthropathy (SpA) usually starts in the third decade of life with negative rheumatoid factor, human leukocyte antigen-B27 genetic marker and clinical features of spinal and peripheral arthritis, dactylitis, enthesitis and extra-articular manifestations (EAMs). Cases can be classified as ankylosing spondylitis, psoriatic arthritis, reactive arthritis, enteropathic arthritis, or juvenile-onset spondyloarthritis. Joint and gut inflammation is intricately linked in SpA and inflammatory bowel disease (IBD), with shared genetic and immunopathogenic mechanisms. IBD is a common EAM in SpA patients, while extraintestinal manifestations in IBD patients mostly affect the joints. Although individual protocols are available for the management of each disease, the standard the
Core Tip: Seronegative spondyloarthropathy (SpA) with negative rheumatoid factor has spinal and peripheral arthritis, dactylitis, enthesitis and extra-articular manifestations (EAMs). It can be classified into ankylosing spondylitis, psoriatic arthritis, reactive arthritis, enteropathic arthritis, and juvenile-onset spondyloarthritis. Inflammatory bowel disease (IBD) is a common EAM in SpA, whereas extraintestinal manifestations in IBD mostly affect the joints. Anti-tumor necrosis factor monoclonal antibodies are effective medications with indicated use in SpA and IBD, a drug of choice for treating SpA-associated IBD. A tight collaboration between gastroenterologists and rheumatologists with mutual referral from early accurate diagnosis to prompt therapy is required in this complex clinical scenario.
- Citation: Wang CR, Tsai HW. Seronegative spondyloarthropathy-associated inflammatory bowel disease. World J Gastroenterol 2023; 29(3): 450-468
- URL: https://www.wjgnet.com/1007-9327/full/v29/i3/450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i3.450
Spondyloarthropathy (SpA) usually starts in the third decade of life with a shared genetic marker human leukocyte antigen (HLA)-B27 and clinical features including spinal and peripheral arthritis, dactylitis (sausage-like swelling of the digits), enthesitis (inflammation at the attachment of ten
| Category | AS | PsA | ReA | EnA | JSpA |
| Demographic | |||||
| Sex, M:F | 3:1 | 1:1 | 5-10:1 | 1:1 | ERA 3:1, JPsA 1:2 |
| Age, yr | 20-40 | 35-45 | Any | 20-40 | < 16 |
| Laboratory | |||||
| HLA-B27 | > 90% | Axial 50%-70% | 60%-80% | Axial 50%-70% | ERA 40%-70% |
| Peripheral 20% | Peripheral 20% | JPsA 10% | |||
| Clinical | |||||
| Affected joints | Spine, sacroiliitis | Any area | Peripheral, sacroiliitis | Peripheral | Peripheral, sacroiliitis |
| Peripheral | 30%, lower | Common, upper | Common, lower | Common, lower | Common, lower |
| Sacroiliitis | 100% | 50% | 30% in urogenital | 20% | 40%-60% in ERA |
| Dactylitis | Uncommon | Common | Common | Uncommon | 20% in JPsA |
| Enthesitis | Common | Common | Common | Uncommon | Uncommon |
| EAM common | Intestine, skin, uveitis | Intestine, skin, uveitis | Skin, uveitis | Intestine, skin, uveitis | Intestine, skin, uveitis |
| Treatment | Spinal physical therapy, NSAIDs/cDMARDs for peripheral SpA, biologics, JAKi | NSAIDs, avoid CS, cDMARDs for peripheral SpA, biologics, JAKi, PDE4i | NSAIDs, antibiotics for chlamydia-induced ReA, cDMARDs for peripheral SpA | Coxibs/cDMARDs for peripheral SpA, biologics, JAKi | Spinal physical therapy, NSAIDs/cDMARDs for peripheral SpA, biologics |
| Prognosis | Life-threatening EAMs with heart, intestine or neurological involvement | Comorbidities associated with more severe disease activity | Usually a self-limited disease | Rarely grave EnA in controlled intestinal activity | More spinal deformity and THR as compared with adult SpA or other JIA subtypes |
IBDs, mainly Crohn’s disease (CD) and ulcerative colitis (UC), are chronic idiopathic inflammatory disorders of the intestinal tract with progressive disease course[10-12]. CD features chronic granulomatous transmural inflammation with discontinuous lesions involving any part of the intestine, ileum, and colon in particular, complicated by intestinal granuloma, obstruction, stricture, and fistula[11], whereas UC is characterized by continuous mucosal inflammation extending from the rectum toward the colon without the above complications[12]. Extraintestinal manifestations (EIMs) occur in 25% to 40% of IBD patients and mostly affect the joints, followed by the skin, eyes, and hepatobiliary tract[10,13]. Primary sclerosing cholangitis (PSC) is the most frequently observed hepatobiliary manifestation[13,14]. IBD has been identified in 60% to 80% of PSC patients. Up to 5% of UC patients have PSC, while it is less frequent in CD patients. Furthermore, CD and UC patients have an increased risk of intestinal malignancies, such as colorectal cancer[10,15]. IBD was initially thought to be a rare disease in Asia, contrary to the West[16]. Recent population-based data have revealed a rapidly rising incidence in eastern countries while plateauing or even declining in western nations[17]. The epidemiological evolution in IBD is supposedly linked to the Westernized lifestyle and industrialization, including dietary changes, antibiotics use, hygienic status, microbial exposure and pollution, as all are potential environmental risk factors. Furthermore, increased disease awareness, advances in diagnosis, and improved healthcare access can also contribute to the increasing trend of IBD incidence[17,18]. Table 2 shows the demographic, clinical, laboratory, therapeutic, and prognostic characters of the two main types of IBD.
| Category | Ulcerative colitis | Crohn’s disease |
| Demographic | ||
| Sex, M:F | 1:1 | 1:1 |
| Age at onset in yr | 30-50 | 10-40 |
| Laboratory | ||
| ANCA | Common | Rare |
| ASCA | Rare | Common |
| Clinical | ||
| Origin/Location | Rectum/colon, rectum | Terminal ileum/any part |
| Distribution | Continuous | Skip lesions |
| Pathology | ||
| Inflamed thickness | Mucosa, submucosa | Transmural |
| Crypt abscess | Common | Uncommon |
| Granuloma | Rare | Common |
| Fissure | Uncommon | Common |
| Fibrosis | Rare | Common |
| Treatment | ASA, CS, IS, biologics, JAKi, S1PR modulator, surgery for refractory medical disease or malignancy | CS, IS, biologics, surgery for refractory medical disease, complication or malignancy |
| Prognosis | Complete remission in most patients, low surgical requirement | Prolonged remission in about 20% of patients, 10-yr surgical resection risk near 50% |
An individual susceptibility to IBD is strongly conditioned by the interaction between intestinal microbiota and the host immune response[19]. Westernized lifestyle-associated dysbiosis, an individual loss of diversity in microbiome composition, has been observed in IBD, and there is a trend toward restored intestinal eubiosis in such patients responding to anti-tumor necrosis factor (TNF) therapy[20]. Accumulating evidence indicates that intestinal inflammation is linked to dysbiosis occurring in rheumatic diseases[21]. The interaction between dysbiosis and the intestinal immune system can lead to the aberrant activation of immune cells that can recirculate from the gut to the EIM sites as observed in SpA[19,21]. Subclinical gut inflammation in SpA patients represents the repertoire in which immune cells are activated, and is correlated with the severity of spinal inflammation[22]. Genetic risk factors are shared between SpA and IBD, and changes in the composition of the intestinal microbiota are observed in both diseases, indicating that joint and gut inflammation is intricately linked in SpA[19,23].
Since SpA and IBD patients share common genetic and immunopathogenic mechanisms[23], SpA patients have an up to four-fold increased risk of IBD compared to the general population. Different forms of SpA can be associated with variable frequencies of intestinal involvement, whereas articular involvement is frequently observed in IBD. Nevertheless, the chronic medication history of patients’ needs to be considered to appropriately evaluate gastrointestinal symptoms in SpA. In addition to direct gastrointestinal adverse reactions, it is necessary to rule out infectious complications with a detailed microbiological survey due to potential immunosuppressive effects. Furthermore, it has been suggested that SpA patients should be evaluated by gastroenterologists when suspected IBD symptoms are present, including rectal bleeding, perianal disease, and chronic diarrhea with organic characteristics[24]. Although individual protocols for managing each disease are available, the standard therapeutic guidelines of seronegative SpA-associated IBD patients remain to be established. In particular, some therapeutic options used to manage one disease might have a negative impact on another disease[25].
Herein, we provide a thorough overview on coexisting IBD in different subtypes of seronegative SpA patients.
AS is a chronic autoimmune disease mainly involving spinal and sacroiliac as well as peripheral joints, with up to 50% of cases mainly affecting the hips and knees[1,2]. There is a similar pooled prevalence of 0.25% and 0.20% in AS from Caucasian-dominant Europe and North America, respectively[1,26]. Furthermore, this disorder has a prevalence of 0.25% and 0.20% in Taiwan and China, respectively, both with a Han Chinese-dominant population[27,28]. In the EAMs of AS patients, frequencies of about 30% have been found for anterior uveitis in both Caucasian and Han Chinese populations[29,30]. Typical attacks are abrupt and unilateral, with pain, photophobia and visual impairment, frequently alternating from one eye to another[1,2]. PsO occurs in more than 10% of Caucasians, more common than in Han Chinese patients[1,29]. There is a 5% to 10% incidence of IBD in AS patients from western countries[29], whereas frequencies of only 0.4% to 0.6% have been identified for IBD in Han Chinese AS populations[30,31]. In comparison with earlier years, there is a sharply increasing current incidence of IBD, without changes in AS prevalence from East Asia[16]. Despite a progressively narrowing gap between Asia and West, the prevalence of IBD remains much higher in Western countries compared to that in Asian nations. In the 21st century, the pooled prevalence of IBD in North America and Europe is estimated to be about 0.3% of the general population[17,18], whereas in the Han Chinese population, the recent prevalence of UC and CD per 100000 individuals has risen to 12.8 and 3.9 in Taiwan and 24.5 and 18.6 in Hong Kong, respectively[32,33]. Although genome-wide association studies have demonstrated shared risk alleles between the two disorders, the above-mentioned clinical observations suggest that ethnicity can be an important factor causing inconsistency in the coexistent frequencies of IBD in AS between Caucasian and Han Chinese populations. Further investigations in the gut-joint axis of inflammation in SpA should consider the issue of disconnection between the occurrence of IBD in AS on the basis of ethnicity, i.e., Han Chinese or other races[23].
In addition to pharmacotherapy, physical therapy and regular exercise in AS patients, either with active or stable axial SpA, can improve the symptoms and functions by maintaining posture and spinal flexibility[34]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are the drug of choice for initial therapy for axial SpA. There are frustrated outcomes regarding axial symptoms, spinal pain in particular, in AS patients receiving conventional disease-modifying anti-rheumatic drug (cDMARD) therapy, including methotrexate (MTX) and sulfasalazine (SAZ). Nevertheless, clinical evidence supports the use of cDMARDs for controlling peripheral arthritis in AS patients. With advances in the understanding of immunopathogenesis in AS[1,2,23], there are increasing numbers of novel medications, including biologics targeting TNF or interleukin (IL)-17 and small-molecule agents, Janus kinase inhibitors (JAKis)[34]. Such therapies have been associated with substantial improvements in disease activity and quality of life.
IBD manifestations in AS represent a clinical challenge by increasing the disease burden with difficulties in managing such patients[35]. Nevertheless, the introduction of new therapeutics targeting both articular and intestinal manifestations, TNF inhibitor (TNFi) in particular, has revolutionized the treatment of patients not responding to conventional medications[1,2,36,37]. In Table 3, the English-language literature is summarized for published reports related to the occurrences of IBD, flare-up or new-onset, in AS patients under the treatment of different TNF blockades, including adalimumab (ADA), certolizumab pegol (CZP), etanercept (ETA), golimumab (GOL), and infliximab (IFX)[38-62]. Notably, most of the enrolled cases were predominantly Caucasian. Since the dosages of TNFi for IBD therapy are higher than those used in AS, new-onset or flare events of IBD can occur in such patients during the therapeutic period, indicating the potential inefficacy of particular TNF blockade in the AS-associated IBD manifestation. Notably, monoclonal antibodies (mAbs) have better protective effects than recombinant soluble TNF receptor fusion proteins. Despite the lack of observed IBD events in AS patients during three GLO randomized clinical trials (RCTs), four cases were reported to have a flare at 2 mo to 5 mo after starting treatment[63].
| No. | Clinical trials, n | Countries involved in clinical trials | Cases, n | TNFi or JAKi | IBD manifestation events, flare-up and new-onset | IBD manifestation events per 100 patient-yr1 | Ref. |
| 1 | 7 | Canada, Germany, Netherlands | 366 | IFX | 1 CD | 0.2 | [38-44] |
| 2 | 9 | European nations, United Kingdom, United States | 724 | ETA | 14 (8 CD, 6 UC) | 2.0 | [45-52] |
| 3 | 5 | France, Germany, Netherlands, United States, etc. | 2026 | ADA | 14 | 0.7 | [53-55] |
| 4 | 3 | Canada, Germany, Netherlands, United States, etc. | 837 | GOL | 0 | 0 | [56-58] |
| 5 | 1 | Belgium, Canada, France, Germany, Netherlands, United States | 121 | CZP | 1 CD | 0.2 | [59,60] |
| 6 | 1 | Australia, Canada, European nations, United States, etc. | 133 | TOF | 0 | 0 | [61] |
| 7 | 1 | Australia, Canada, European nations, Israel, United States, etc. | 211 | UPA | 1 CD | 1.8 | [62] |
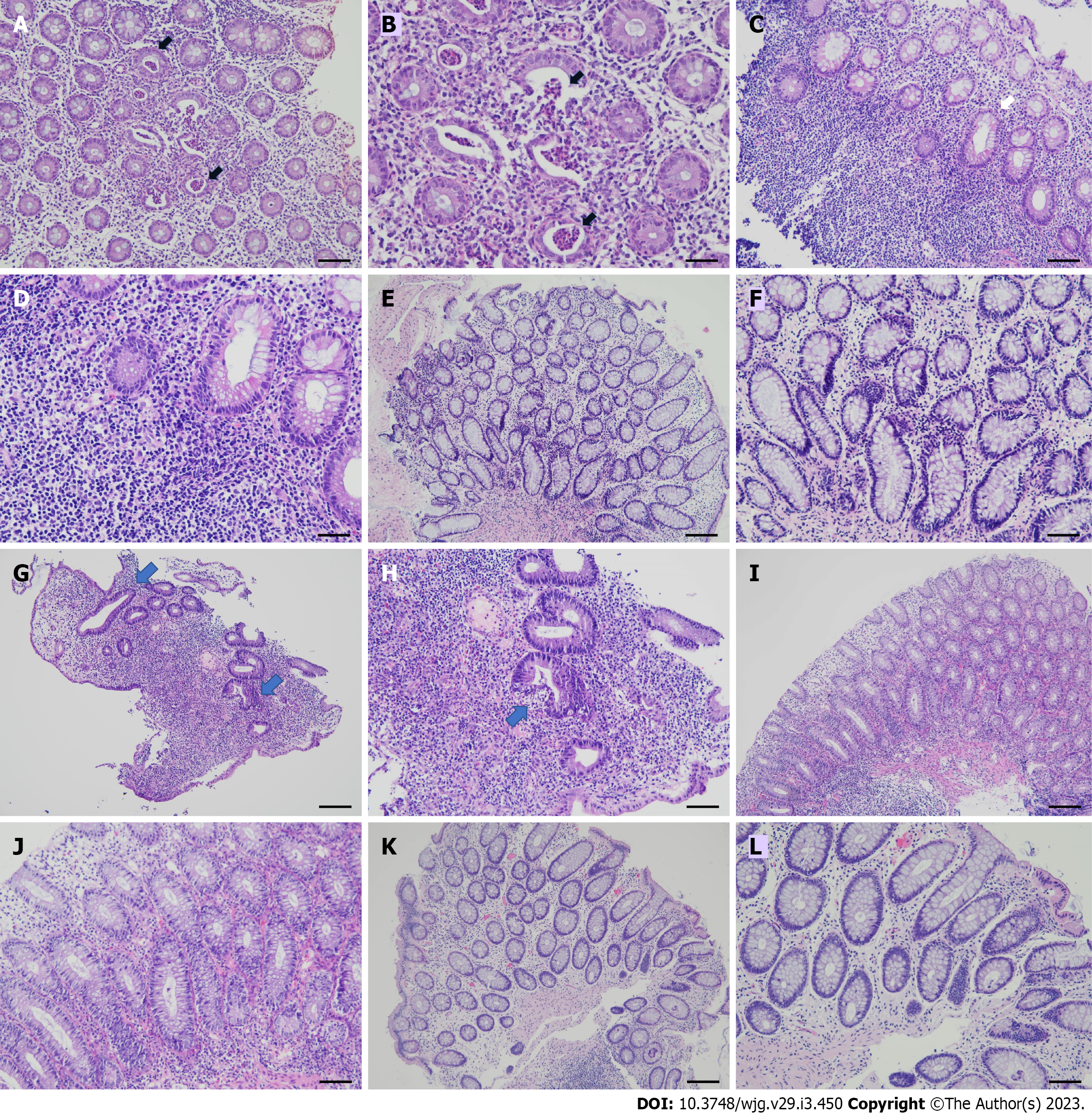
Table 4 shows the demographic, clinical, laboratory, medication, course and outcome profiles in 4 AS-associated IBD patients, 3 UC patients, and 1 ulcerative proctitis (UP) patient with moderate to severe activity. All received endoscopic biopsy with characteristic histopathological changes (Figure 1A, B, G, and H). This 5-year observation enrolled 878 (86% male) Han Chinese AS patients by the authors. There was a 0.5% occurrence of IBD. At IBD onset, there was a long disease period (12 years to 25 years, 16.5 ± 5.8) with high-activity treated with NSAIDs and cDMARDs. For IBD therapy, corticosteroids (CSs) were prescribed in the acute stage with topical and systemic high-dose for case No. 1 and others, respectively, followed by aminosalicylate (ASA) or plus low-dose CS for maintenance. Nevertheless, all experienced a disease relapse, while case No. 3 had colonic perforation that required surgical intervention. Repeated endoscopic biopsy in case No. 1 showed chronic active rectitis (Figure 1C and D). Due to refractory activity, all started ADA injection with 40 mg biweekly. A relapse occurred in case No. 2 under the tapered dosage of 40 mg every 4 wk (Figure 1I and J); however, there were no more flares for 4.8 years after resuming a biweekly regimen. Altogether, all had no IBD flares under ADA 40 mg biweekly injection without CS, cDMARD, or immunosuppressants for 4.3 years to 5.8 years (5.1 ± 0.7). All had clinical IBD remission and only mild non-specific lymphocytic infiltration (Figure 1E, F, K, and L). Despite histopathological changes more resistant to resolution than clinical remission in IBD[64], whether microscopic healing provides additional outcome benefits remains to be determined.
| No. | Age in yr and sex | 1AS period in yr | Affected joints | Other EA | 3BASDAI/2AS medication | HLA-B27/3ESR | IBD clinical manifestation | IBD entity/6severity | 4IBD medication | Disease course, under ADA 40 mg q2w SCI | Final outcome |
| 1 | 42, F | 12 | SI, spine, hip | Uveitis | 7.6/NSAIDs | Positive/38 | Rectal bleeding, BWL, anemia | UP/ moderate, MS 9 | CS, mSAZ, ADA 40 mg q2w | No IBD relapse for 4.3 yr | AS in low activity with BASDAI 2.0-2.5, IBD in remission, MS 0 |
| 2 | 35, M | 15 | SI, spine, hip | Uveitis | 8.8/NSAIDs, SAZ | Positive/80 | Bloody diarrhea, BWL, fever, anemia | UC/severe, MS 12 | CS, mSAZ, ADA 40 mg q4 to q2w | No IBD relapse for 4.8 yr | AS in low activity with BASDAI 2.5-3.0, IBD in remission, MS 1 |
| 3 | 45, M | 14 | SI, spine, hip | Nil | 8.4/NSAIDs, SAZ | Positive/42 | Bloody diarrhea, BWL, anemia, 5colon perforation | UC/severe, MS 11 | CS, SAZ, ADA 40 mg q2w | No IBD relapse for 5.8 yr | AS in low activity with BASDAI 2.5-3.0, IBD in remission, MS 2 |
| 4 | 45, F | 25 | SI, spine, shoulder hip | Nil | 8.1/NSAIDs, SAZ, MTX | Positive/35 | Bloody diarrhea, BWL, anemia | UC/severe, MS 11 | CS, SAZ, ADA 40 mg q4 to q2w | No IBD relapse for 5.3 yr | AS in low activity with BASDAI 2.5-3.0, IBD in remission, MS 1 |
Reactivation or development of IBD in AS patients receiving ETA therapy is thought to be caused by particular structure, administration mode, neutralizing effect, and/or pharmacokinetic characteristics of ETA[65]. Despite the indirect evidence based on the risks of IBD among AS patients during biologics therapy, the American College of Rheumatology (ACR)/Spondylitis Association of America/ Spondyloarthritis Research and Treatment Network have recommended the treatment with anti-TNF mAbs over other biologics in adults with AS and coexisting IBD[66]. Notably, AS patients under IL-17 blockade therapy have increased risks of IBD development or exacerbation compared with the placebo-controlled group[67,68]. Moreover, according to the management recommendations of axial SpA from Assessment of Spondyloarthritis International Society/European League Against Rheumatism for the EAMs, anti-TNF mAbs are effective in IBD therapy and in the prevention of uveitis recurrence, whereas ETA has no effects on treating IBD and contradictory outcomes in uveitis prevention[69]. The use of a special mAb can be made in consultation with gastroenterologists due to different indications of mAbs in the IBD subtype, ADA and IFX for CD or UC, CZP for CD, and GOL for UC.
In the Han Chinese population, ADA is an effective biologic agent in controlling the articular activities in AS[70]. For ADA therapy in IBD, higher remission and response rates have been observed in China compared with those in Western countries[71]. Furthermore, its efficacy has also been demonstrated in moderate to severe IBD patients in Taiwan with more rigorous prescription criteria than in the West[72]. Interestingly, contradictory to our favorable therapeutic results without any UC flares in Han Chinese AS-associated IBD patients (Table 4), in an RCT of AS patients (97% Caucasian) under 40 mg ADA biweekly injection for 24 wk, 2 cases experienced a UC flare, 1.9 events per 100 patient-years vs none in the placebo group[54]. In systemic rheumatic disorders, clinical outcomes under the similar immunosuppressant treatment can be variable in different racial populations[73], while the ethnic factor has been considered to be involved in therapeutic responses to biologics therapy[74]. Further international collaborations in large-scale RCTs enrolling more ethnic groups might be needed to evaluate such an issue in AS-associated IBD.
UP patients with inflammation limited in the rectum can manifest as tenesmus, urgency, and rectal bleeding[75]. Such patients might fail to improve and require additional medications despite the beneficent effects of ASA and CS. Medical therapy in UP refractory to the standard treatment is challenging due to no evidence-based large-scale data of other medications[76]. In addition, UC patients limited to the rectum are usually excluded from the RCT on biologics therapy. Nevertheless, a recent referral cohort with 118 cases followed for up to 20 years revealed that UP resistant to conventional therapies could have clinical responses to anti-TNF mAbs[77]. Furthermore, long-term outcome in UP patients receiving biologics therapy was superior to azathioprine treatment, consistent with the results demonstrating beneficent efficacy of refractory UP under anti-TNF therapy from a retrospective cohort with 104 cases[77,78]. In our 5-year observation, a UP case (No. 1 in Table 4) resistant to ASA use showed a clinical remission under ADA therapy for more than 4 years.
A better understanding of the complex IBD pathogenesis has brought about a therapeutic approach focusing on clinical and histopathological remission with precise molecular targeting of inflammatory cascades. Since the successful results on the use of IFX in CD patients in 1997, three additional anti-TNF mAbs, two anti-integrin mAbs, three small-molecule agents including a sphingosine-1-phosphate receptor modulator and two JAKis, and two mAbs targeting the p40 subunit of IL-12/IL-23 and the p19 unit of IL-23 have been approved by the United States Food and Drug Administration (FDA), expanding the options for IBD treatment[79].
The signaling pathway of JAK-signal transducer and activator of transcription (STAT), including JAKs 1-3, STATs 1-6, and tyrosine kinase 2, can regulate miscellaneous cytokine receptors and has pathogenic roles in various autoimmune and inflammatory disorders[80]. Furthermore, individual cytokine receptors can recruit their own combined JAKs and STATs to activate distinct processes in different targeted cells, while antagonizing a specific JAK can inhibit diverse cytokine pathway, expanding the effects of JAKi on cytokine-targeted therapy[81]. Tofacitinib (TOF), a pan-JAKi targeting JAKs 1-3, and upadacitinib (UPA), a selective JAK1 inhibitor, have been approved by the FDA in adult UC with moderately to severely activity with intolerance or poor responses to TNF mAbs in 2018 and 2022, respectively, overcoming the challenges of using biologics to avoid immunogenicity induction and parenteral administration[79]. Furthermore, TOF and UPA have received an indication in adult AS with an inadequate response or intolerance to TNFi in 2021 and 2022, respectively (Table 3). In a recent phase III RCT enrolling 136 AS patients with more than 80% Caucasians, there were no observed IBD events under TOF 5 mg bid therapy for 16 wk[61], validating its expected effects for UC manifestation in TNFi-refractory AS patients. Nevertheless, there was an observed new-onset CD event under UPA 15 mg once daily treatment for 14 wk in another phase III RCT enrolling 209 AS patients dominant in Caucasians (1.8 events per 100 patient-years)[62].
IL-12 helps naïve T cells differentiate into type 1 T helper (Th1) cells secreting IL-6, interferon (IFN)-γ and TNF, while IL-23 stimulates Th17 cells to express IL-17, IFN-γ, TNF, granulocyte-macrophage colony-stimulating factor, and IL-21, all of which promote mucosal inflammation in IBD patients[79,82]. IL-12 is encoded by two separate genes, IL-12A (p35) and IL-12B (p40), to form an active heterodimer following protein synthesis with p35 and p40 chains, while IL-12 p40 chain can dimerize with IL-23 p19 chain to form IL-23[82]. Ustekinumab (UST), a p40 chain mAb, was approved for the treatment of moderately to severely active CD patients who failed or were intolerant to treatment with anti-TNF therapy in 2016, and for moderately to severely active UC in 2019[79]. Moreover, risankizumab (RIS), a p19 chain mAb, was approved for the treatment of moderately to severely active CD patients who failed or were intolerant to treatment with TNF blockers in 2022. Nevertheless, both UST and RIS have no indication for treating TNFi-refractory AS patients. In a national cohort study evaluating the long-term UST effects in 152 CD patients including 17 associated with AS, efficacy was not identified in SpA symptoms[83].
PsA, a chronic inflammatory arthritis with impaired function and reduced quality of life, develops in up to 30% of PsO patients[84]. Both axial and peripheral joints can be involved with five clinical patterns not mutually exclusive, including the most commonly observed asymmetric oligoarticular, symmetric polyarticular, distal interphalangeal joint-predominant, axial/SpA-predominant, and the rarely identified deforming/destructive subtype, i.e., arthritis mutilans[4,84]. Cutaneous lesions can be found in most cases at the time of articular presentation; however, in up to 15% of PsA patients, arthritis can antedate the appearance of skin disease, i.e., PsA sine PsO[85]. Dactylitis or enthesitis has been reported in up to 50% of patients. In addition, about 40% to 50% of patients are positive for HLA-B27, higher in axial than the peripheral-only type[84]. The prevalence of PsA is between 0.3% and 1.0% in the United States[84], whereas it is much lower in Han Chinese, with the prevalence ranging from 0.01% to 0.1%[86,87].
Uveitis has been identified in 8% of PsA patients, affecting the anterior and posterior poles of the eyes[84]. Comorbidities in PsA are associated with more severe disease activities, including diabetes, hypertension, hyperlipidemia, metabolic syndrome and fatty liver, while there is an increased risk of cardiovascular events[88]. It is estimated that 9.6% of CD patients have PsO (2.2% in the general population), while 0.5% of PsO patients have CD (0.2% in general population)[89]. Despite there being a lower occurrence than in CD, there is a similar trend between patients with UC and PsO[90]. In com
Based on high-quality, evidence-based, domain-focused recommendations for medication selection in PsA, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis updated treatment recommendations for such patients in 2021[95]. Choice of therapy for an individual patient should ideally address all active disease domains, related EAMs, and comorbidities. For patients with axial involvement not responding to NSAID use, initiation of a targeted therapy is strongly recommended, including TNFi, IL-17i, and JAKi. For peripheral arthritis, cDMARDs such as MTX and SAZ can be used as first-line therapy. In dactylitis, enthesitis, nail or topicals-unresponsive PsO, cDMARD-refractory peripheral arthritis, recent evidence supports the use of IL17i (ixekizumab, SEK), IL23i (guselkumab, RIS), JAKi (TOF, UPA), phosphodiesterase 4 inhibitors (PDE4is, apremilast), TNFi (ADA, CZP, ETA, GOL, IFX), and UST. For PsA-related EAMs, MTX or TNF mAbs can be used for the treatment of anterior uveitis. TNF mAbs and UST have demonstrated their therapeutic efficacy in CD and UC. TOF and UPA are effective in treating UC, while RIS has efficacy in CD therapy. Notably, IL17i can increase the risk of IBD onset or exacerbation, and their use should be avoided in IBD, even in disease remission[25,96]. Since comorbidities are associated with greater PsA activity and reduced therapeutic responses, their recognition and monitoring with appropriate management is important for health-care providers caring for such patients[91,95].
ReA has a sterile, transient nature, typically with an asymmetric oligoarthritis of the lower limbs fol
Eye involvement including anterior uveitis and conjunctivitis preceding arthritis occurs in one-fifth of patients[100], while up to half of cases have mucocutaneous lesions with characteristic keratoderma blennorhagicum and circinate balanitis[97-99]. Upon colonoscopic biopsies of the terminal ileum and colon, histological alterations mimicking IBD with the features of acute enterocolitis or early CD were found in two-thirds of ReA patients despite an asymptomatic condition in most cases[101]. Notably, there are no known reports of increased IBD occurrences in ReA patients.
Although the disease course of post-dysentery ReA is unaltered by antibiotics use, such therapy is indicated for the identification of C. trachomatis infection[98,99]. Due to a self-limited nature in most ReA patients, NSAIDs are prescribed as first-line therapy. In patients not responding to NSAIDs or with chronic ReA, cDMARDs are indicated with SAZ as the drug of choice and MTX as an alternative. In patients refractory to cDMARD treatment, off-label use of ETA has shown beneficial effects[102].
Musculoskeletal conditions with articular, periarticular, muscular, and skeletal manifestations are frequently observed, with an up to 50% frequency in IBD patients[103]. Rheumatological EIMs are associated with HLA-A2, DR1, and DQw5 alleles in CD, and with DRB1*0103, B27, and B58 alleles in UC[104]. Arthritis is the most common EIM in IBD involving axial (spondylitis, sacroiliitis), peripheral joints, or a combination. The prevalence of arthritis decreases with increasing age in IBD patients[105]. It occurs equally in both sexes, more commonly in CD with colon involvement than in UC, and can precede, be concomitant with, or follow the onset of IBD[106]. Peripheral arthritis can be classified into two entities: Type 1 pauciarticular and type 2 polyarticular (Table 5)[107-109]. Type 1 arthropathy is often acute, asymmetrical, and affecting less than five joints, commonly involving the large knee joint. It is usually related to IBD activity and is self-limiting, with a duration of no more than 10 wk. Treatment of the underlying intestinal inflammation is usually associated with improvement of arthritis. Type 2 arthropathy is a symmetrical arthritis involving five or more joints, commonly involving the small metacarpophalangeal joint. It is not related to IBD activity and may persist for years with articular erosion and destruction. There is an association of type 1 arthropathy with erythema nodosum, uveitis and HLA-DRB1*0103, B35 and B24, and type 2 arthropathy with uveitis and HLA-B44[108]. Notably, such a categorization of peripheral arthritis can be related more to the duration and progression of articular presentation, while the patients with a polyarticular manifestation can begin their clinical course with an oligoarticular involvement[104].
| Category | Type 1 pauciarticular | Type 2 polyarticular |
| Prevalence | 4% to 5% in IBD, higher in CD than UC | 3% in IBD, higher in CD than UC |
| Joint manifestation | ||
| Involved numbers | < 5 | ≥ 5 |
| Articular distribution | Large joint, asymmetric | Mainly small joint |
| Involved area with the decreasing frequencies | Knee, ankle, wrist, elbow, MCP, hip, shoulder, MTP, PIP | MCP, knee, PIP, wrist, ankle, elbow, hip, shoulder, MTP |
| Erosion/destruction | Absent | Present |
| Clinical course | Early in IBD disease course, acute and self-limiting (mostly under 10 wk) | Arthritis for months, episodic exacerbation for yr |
| Disease characters | ||
| IBD activity | Parallel with activity | Independent of activity |
| Other EIM | EN, uveitis | Uveitis |
| HLA association | HLA-B27, B35, DR*0103 | HLA-B44 |
| Treatment | Control of IBD activity, coxibs, CS, cDMARDs (SAZ 1st choice), TNF mAbs for refractory cases, JAKi for anti-TNF failure | Coxibs, CS, cDMARDs (SAZ 1st choice), TNF mAbs for refractory cases, JAKi for anti-TNF failure |
Axial involvement can be a part of IBD but independent of gut pathology. It is more common in CD than in UC, with an up to 25% frequency[110]. Most IBD patients with axial spondylitis are HLA-B27-positive despite a lower association rate than idiopathic AS, 50% to 70% vs more than 90%. There is a 5% to 10% occurrence of AS in IBD with a 1:1 sex ratio and a development at any age, rather than a 3:1 male to female ratio and onset before 40 years of age in idiopathic AS[111]. Although sacroiliitis can be detected by magnetic resonance imaging (MRI) in IBD patients, most of them are asymptomatic, HLA-B27-negative and without progression into AS[112]. The prevalence of symptomatic sacroiliitis is estimated to be less than 10%.
Dactylitis, enthesitis, and tenosynovitis also occur in IBD patients as musculoskeletal EIMs. Enthesitis presenting with Achilles tendinitis, plantar fasciitis, and chest wall pain can lead to structural changes of underlying bones with functional disability[109]. Ultrasonography or MRI examination of the affected area can help in earlier detection missed by clinical inspection[113].
NSAIDs are suggested as initial therapy for peripheral and axial SpA. Nevertheless, their use is controversial in IBD due to an association with the development of intestinal ulcerations and flares of IBD[114]. Although the safety of cyclooxygenase 2 inhibitors have been investigated[115,116], their use should be limited to a short course during the IBD remission. Systemic CS can be helpful for peripheral arthritis despite ineffectivity in controlling axial SpA and enthesitis, while intra-articular CS injection may be effective in cases with limited numbers of joint involvement[106,109]. SAZ, a formulation of ASA available in intestinal therapy, is an effective cDMARD in improving peripheral arthritis, but not axial arthritis or sacroiliitis in IBD patients[117]. MTX is an alternative cDMARD recommended for the treatment of IBD-associated peripheral SpA[118]. Furthermore, TNFi can be reserved for patients with IBD-associated axial or peripheral SpA not responsive to conventional therapies[119]; however, ETA should be avoided due to its inefficacy for IBD treatment and a potential clinical exacerbation[120]. Since the doses of TNF blockade for IBD therapy is higher than those used in treating SpA, it is recommended that high-dose regimen is preferred in IBD-associated SpA during active intestinal disease[121]. Two JAKis have been approved for treating TNFi-refractory UC and AS/PsA patients. Despite the lack of an evidence-based indication, such therapy might be considered in UC-related EnA patients lacking therapeutic responses to anti-TNF therapy. Although UST use is indicated for IBD and PsA therapy, it is only effective in CD/UC-associated peripheral rather than axial SpA[122].
At least 5% of IBD patients, more frequently in CD than UC, experience ocular EIM, with uveitis as the commonest manifestation, particularly in those cases associated with arthritis[123,124]. Anterior uveitis in patients with IBD is initially treated with CS eye drops, followed by systemic CS or IS if unsuccessful[104]. Anti-TNF mAbs have shown efficacy in IBD-associated uveitis, while their use can be considered in cases refractory to the aforementioned treatment[125].
JSpA, a distinct disease to adult SpA, constitutes up to one-third of juvenile idiopathic arthritis (JIA), and usually affects males and starts in early adolescence (before the age 16)[126-129]. This disease primarily affects children fulfilling the criteria for JIA categories of enthesitis-related arthritis (ERA) and juvenile psoriatic arthritis (JPsA) as well as undifferentiated arthritis with either features[127,130]. An approximate 20% prevalence of JSpA was found in JIA cohort studies[131], while approximately 10% of adult AS patients have an onset of disease in childhood[1,3]. There is HLA-B27 positivity detected in 40% to 60% of ERA, whereas only 10% of JPsA patients show HLA-B27 positivity[129]. JSpA commonly manifests with peripheral arthritis, usually asymmetric, oligoarticular, involving joints of the lower extremities including the hip, knee, ankle, and midfoot. Tender entheses are commonly present at insertions of the patellar ligament at the inferior patella, plantar fascia at the calcaneus, and the Achilles tendon[132]. About 40% to 60% of ERA cases have sacroiliitis, an early sign of axial involvement, in their disease course[129]. Nevertheless, children are known to have silent sacroiliitis without inflammatory back pain[127]. Dactylitis can be observed in 30% of patients with JPsA[133]. JSpA has a poorer outcome with more spinal deformity and need for total hip replacement, as compared with cases of other forms of JIA and their adult counterparts[127].
Similar to the adult-onset disease, common EAMs in JSpA include skin, eye, and bowel involvement[126-129]. The overall prevalence of uveitis, more common with acute anterior uveitis, is approximately 10%[134]. Two-thirds of children with SpA have been reported to have gastrointestinal symptoms[135]. Intestinal inflammation on ileocolonic biopsy has been identified in JSpA[136], while ERA with sacroiliitis had increased levels of fecal calprotectin, a gastrointestinal inflammation marker[137]. IBD in children might begin with arthritis before clinically evident intestine inflammation, while difficult-to-control arthritis, longstanding, vague gastrointestinal complaints and anemia might be helpful clues for earlier diagnosis[138]. In a large-scale survey of 3071 JIA patients, 11 with 4 JSpA had IBD (8 CD, 3 UC); furthermore, there was 1.31 case per 1000 patient-years, higher than the annual incidences of 10 cases per 100000 in pediatric populations of western countries[139]. In another large-scale investigation with 8942 JIA patients, 48 had IBD (22 CD, 13 UC, 13 indeterminate), showing a prevalence of 0.54%, much higher than the reported 0.02% in a Western pediatric population[140]. Furthermore, the occurrences of IBD were identified in 2% to 6% of ERA patients and 0.3% to 0.5% of JPsA patients[133,140,141].
According to the 2019 ACR guidelines for JIA treatment[142], initial therapy with a cDMARD is recommended over NSAID monotherapy, while MTX is suggested over other cDMARDs. Oral CS is only recommended as bridging therapy, with a limited course of less than 3 mo. Furthermore, initial biologic therapy (ADA, ETA, GOL, abatacept, tocilizumab) may be considered for patients with risk factors (seropositivity, articular damage), involvement of high-risk joints (cervical spine, wrist, hip), or high disease activity. For sacroiliitis and enthesopathy, NSAID therapy is recommended, while TNFi is suggested for refractory cases. Notably, UST and SEC have been approved for use in JPsA and ERA/JPsA patients, respectively, as an option for TNFi-resistant patients[143,144]. Although ADA and IFX are both approved in treating pediatric CD and UC, the incidence of IBD in JIA patients was increased in those receiving IFX but not ADA therapy[140]. Furthermore, IFX use is not approved in JIA patients. ADA appears to be a drug of choice for treating patients with JSpA-associated IBD. Neve
Finally, Table 6 lists current FDA-approved indications of biologics and small molecules for sero
| Category | AS | PsA | JIA1 | UC | CD |
| Biologics/TNFi | |||||
| Etanercept | X | X | X | ||
| Infliximab | X | X | X | X | |
| Adalimumab | X | X | X | X | X |
| Golimumab | X | X | X | X | |
| Certolizumab pegol | X | X | X | ||
| Biologics/IL-17i | |||||
| Ixekizumab | X | X | |||
| Secukinumab | X | X | X | ||
| Biologics/IL-12/23i | |||||
| Ustekinumab | X | X | X | X | |
| Biologics/IL-23i | |||||
| Guselkumab | X | ||||
| Risankizumab | X | X | |||
| Biologics/IL-1i | |||||
| Canakinumab | X | ||||
| Biologics/IL-6i | |||||
| Tocilizumab | X | ||||
| Biologics/anti-integrin mAb | |||||
| Natalizumab | X | ||||
| Vedolizumab | X | X | |||
| Biologics/anti-CTLA-4 mAb | |||||
| Abatacept | X | X | |||
| Small molecules/JAKi | |||||
| Tofacitinib | X | X | X | X | |
| Upadacitinib | X | X | X | ||
| Small molecules/PDE4i | |||||
| Apremilast | X | ||||
| Small molecules/S1PR modulator | X |
Seronegative SpA usually starts in the third decade of life with the HLA-B27 genetic marker and clinical features of spinal and peripheral arthritis, dactylitis, enthesitis and EAMs. This group of patients who have negative rheumatoid factor can be classified into AS, PsA, ReA, EnA and JSpA cases. Joint and gut inflammation are intricately linked in SpA and IBD, with shared genetic and immunopathogenic mechanisms. IBD is a common EAM in SpA patients, while EIMs in IBD patients mostly affect the joints. Although individual protocols for managing each disease have been established, the standard ther
The authors are indebted to Dr. Kang JW, Division of Gastroenterology and Hepatology, for his valuable comments, and to other doctors at the National Cheng Kung University Hospital involved in the diagnosis and management of reported patients. The institutional review board of National Cheng Kung University Hospital approved this study (No. B-ER-105-108).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandit R, United States; Triantafillidis J, Greece; Wang LH, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. N Engl J Med. 2016;374:2563-2574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 523] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 2. | Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 906] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 3. | Bakland G, Nossent HC. Epidemiology of spondyloarthritis: a review. Curr Rheumatol Rep. 2013;15:351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Sieper J, Braun J, Dougados M, Baeten D. Axial spondyloarthritis. Nat Rev Dis Primers. 2015;1:15013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 143] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Moll JM, Haslock I, Macrae IF, Wright V. Associations between ankylosing spondylitis, psoriatic arthritis, Reiter's disease, the intestinal arthropathies, and Behcet's syndrome. Medicine (Baltimore). 1974;53:343-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 329] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Khan MA. Update on spondyloarthropathies. Ann Intern Med. 2002;136:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 320] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Healy PJ, Helliwell PS. Classification of the spondyloarthropathies. Curr Opin Rheumatol. 2005;17:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Duba AS, Mathew SD. The Seronegative Spondyloarthropathies. Prim Care. 2018;45:271-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Zeidler H, Mau W, Khan MA. Undifferentiated spondyloarthropathies. Rheum Dis Clin North Am. 1992;18:187-202. [PubMed] |
| 10. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2202] [Article Influence: 137.6] [Reference Citation Analysis (6)] |
| 11. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1806] [Article Influence: 225.8] [Reference Citation Analysis (111)] |
| 12. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2486] [Article Influence: 310.8] [Reference Citation Analysis (2)] |
| 13. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 14. | Wang CR, Tsai HW. Autoimmune liver diseases in systemic rheumatic diseases. World J Gastroenterol. 2022;28:2527-2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Broomé U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Chi KR. Epidemiology: Rising in the East. Nature. 2016;540:S100-S102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020;35:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 18. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4107] [Article Influence: 513.4] [Reference Citation Analysis (110)] |
| 19. | Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol. 2019;25:2162-2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 93] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (7)] |
| 20. | Ribaldone DG, Caviglia GP, Abdulle A, Pellicano R, Ditto MC, Morino M, Fusaro E, Saracco GM, Bugianesi E, Astegiano M. Adalimumab Therapy Improves Intestinal Dysbiosis in Crohn's Disease. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Macaluso F, Guggino G, Rizzo A, Ferrante A, Ciccia F. Histopathology of the gut in rheumatic diseases. Reumatismo. 2018;70:178-186. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Rizzo A, Guggino G, Ferrante A, Ciccia F. Role of Subclinical Gut Inflammation in the Pathogenesis of Spondyloarthritis. Front Med (Lausanne). 2018;5:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Gracey E, Vereecke L, McGovern D, Fröhling M, Schett G, Danese S, De Vos M, Van den Bosch F, Elewaut D. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol. 2020;16:415-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 24. | Sanz Sanz J, Juanola Roura X, Seoane-Mato D, Montoro M, Gomollón F; Grupo de Trabajo del proyecto PIIASER. Screening of Inflammatory Bowel Disease and Spondyloarthritis for Referring Patients Between Rheumatology and Gastroenterology. Reumatol Clin (Engl Ed). 2018;14:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Ben Nessib D, Ferjani H, Maatallah K, Rahmouni S, Kaffel D, Hamdi W. Update on therapeutic management of spondyloarthritis associated with inflammatory bowel disease. Clin Rheumatol. 2020;39:3543-3553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Stolwijk C, van Onna M, Boonen A, van Tubergen A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res (Hoboken). 2016;68:1320-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 27. | Chou CT, Pei L, Chang DM, Lee CF, Schumacher HR, Liang MH. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol. 1994;21:302-306. [PubMed] |
| 28. | Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford). 2014;53:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 450] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 29. | Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 30. | Wang CR, Weng CT, Lee CT, Huang KY, Hsu SM, Liu MF. Rare occurrence of inflammatory bowel disease in a cohort of Han Chinese ankylosing spondylitis patients- a single institute study. Sci Rep. 2017;7:13165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Lai SW, Kuo YH, Liao KF. Incidence of inflammatory bowel disease in patients with ankylosing spondylitis. Ann Rheum Dis. 2021;80:e144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Ng SC, Leung WK, Shi HY, Li MK, Leung CM, Ng CK, Lo FH, Hui YT, Tsang SW, Chan YK, Loo CK, Chan KH, Hui AJ, Chow WH, Harbord M, Ching JY, Lee M, Chan V, Tang W, Hung IF, Ho J, Lao WC, Wong MT, Sze SF, Shan EH, Lam BC, Tong RW, Mak LY, Wong SH, Wu JC, Chan FK, Sung JJ. Epidemiology of Inflammatory Bowel Disease from 1981 to 2014: Results from a Territory-Wide Population-Based Registry in Hong Kong. Inflamm Bowel Dis. 2016;22:1954-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Yen HH, Weng MT, Tung CC, Wang YT, Chang YT, Chang CH, Shieh MJ, Wong JM, Wei SC. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide populationbased study. Intest Res. 2019;17:54-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 34. | Danve A, Deodhar A. Treatment of axial spondyloarthritis: an update. Nat Rev Rheumatol. 2022;18:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 35. | Lubrano E, Luchetti MM, Benfaremo D, Mauro D, Ciccia F, Perrotta FM. Inflammatory bowel disease manifestations in spondyloarthritis: considerations for the clinician. Expert Rev Clin Immunol. 2021;17:1199-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ. 2017;357:j2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Yeshi K, Ruscher R, Hunter L, Daly NL, Loukas A, Wangchuk P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 38. | Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider M, Sörensen H, Zeidler H, Thriene W, Sieper J. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet. 2002;359:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 882] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 39. | Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Golder W, Gromnica-Ihle E, Kellner H, Schneider M, Sörensen H, Zeidler H, Reddig J, Sieper J. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum. 2003;48:2224-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 40. | Braun J, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sörensen H, Zeidler H, Sieper J. Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis. Ann Rheum Dis. 2005;64:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 41. | Braun J, Baraliakos X, Brandt J, Listing J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sörensen H, Zeidler H, Sieper J. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology (Oxford). 2005;44:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, Braun J; Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005;52:582-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 612] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 43. | Braun J, Sieper J, Geusens P, Dijkmans B, Han J, Xu, W. Efficacy and safety of infliximab in patients with ankylosing spondylitis: 102 wk results of the ASSERT trial. Ann Rheum Dis. 2006;65 Suppl II:87. |
| 44. | Stone M, Salonen D, Lax M, Payne U, Lapp V, Inman R. Clinical and imaging correlates of response to treatment with infliximab in patients with ankylosing spondylitis. J Rheumatol. 2001;28:1605-1614. [PubMed] |
| 45. | Brandt J, Khariouzov A, Listing J, Haibel H, Sörensen H, Grassnickel L, Rudwaleit M, Sieper J, Braun J. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48:1667-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 46. | Davis JC Jr, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, Kivitz A, Fleischmann R, Inman R, Tsuji W; Enbrel Ankylosing Spondylitis Study Group. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230-3236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 543] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 47. | Davis J Jr, Webb A, Lund S, Sack K. Results from an open-label extension study of etanercept in ankylosing spondylitis. Arthritis Rheum. 2004;51:302-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Davis JC, van der Heijde DM, Braun J, Dougados M, Cush J, Clegg D, Inman RD, Kivitz A, Zhou L, Solinger A, Tsuji W. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann Rheum Dis. 2005;64:1557-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 49. | Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 529] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 50. | Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, Mola EM, Salvarani C, Sanmartí R, Sany J, Sibilia J, Sieper J, van der Linden S, Veys E, Appel AM, Fatenejad S. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis. 2004;63:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 232] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Baraliakos X, Brandt J, Listing J, Haibel H, Sörensen H, Rudwaleit M, Sieper J, Braun J. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept: clinical and magnetic resonance imaging data. Arthritis Rheum. 2005;53:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | van der Heijde D, Da Silva JC, Dougados M, Geher P, van der Horst-Bruinsma I, Juanola X, Olivieri I, Raeman F, Settas L, Sieper J, Szechinski J, Walker D, Boussuge MP, Wajdula JS, Paolozzi L, Fatenejad S; Etanercept Study 314 Investigators. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:1572-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Haibel H, Rudwaleit M, Brandt HC, Grozdanovic Z, Listing J, Kupper H, Braun J, Sieper J. Adalimumab reduces spinal symptoms in active ankylosing spondylitis: clinical and magnetic resonance imaging results of a fifty-two-week open-label trial. Arthritis Rheum. 2006;54:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, Dougados M, Reveille JD, Wong RL, Kupper H, Davis JC Jr; ATLAS Study Group. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 646] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 55. | Elewaut D, Braun J, Anderson JK, Arikan D, Chen S, Hojnik M, De Craemer AS, Curtis JR. Low Incidence of Inflammatory Bowel Disease Adverse Events in Adalimumab Clinical Trials Across Nine Different Diseases. Arthritis Care Res (Hoboken). 2021;73:289-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Inman RD, Davis JC Jr, Heijde Dv, Diekman L, Sieper J, Kim SI, Mack M, Han J, Visvanathan S, Xu Z, Hsu B, Beutler A, Braun J. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58:3402-3412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 57. | Deodhar A, Braun J, Inman RD, van der Heijde D, Zhou Y, Xu S, Han C, Hsu B. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 5-year results of the GO-RAISE study. Ann Rheum Dis. 2015;74:757-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 58. | Reveille JD, Deodhar A, Caldron PH, Dudek A, Harrison DD, Kim L, Lo KH, Leu JH, Hsia EC. Safety and Efficacy of Intravenous Golimumab in Adults with Ankylosing Spondylitis: Results through 1 Year of the GO-ALIVE Study. J Rheumatol. 2019;46:1277-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, Reveille JD, Rudwaleit M, van der Heijde D, Stach C, Hoepken B, Fichtner A, Coteur G, de Longueville M, Sieper J. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 373] [Cited by in RCA: 359] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 60. | van der Heijde D, Dougados M, Landewé R, Sieper J, Maksymowych WP, Rudwaleit M, Van den Bosch F, Braun J, Mease PJ, Kivitz AJ, Walsh J, Davies O, Bauer L, Hoepken B, Peterson L, Deodhar A. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology (Oxford). 2017;56:1498-1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler LS, Fleishaker D, Wang L, Wu J, Menon S, Wang C, Dina O, Fallon L, Kanik KS, van der Heijde D. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 62. | van der Heijde D, Baraliakos X, Sieper J, Deodhar A, Inman RD, Kameda H, Zeng X, Sui Y, Bu X, Pangan AL, Wung P, Song IH. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis. 2022;81:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 63. | Bawany MZ, Rafiq E, Thotakura R, Lay R, Silverman AL, Nawras A. Golimumab may induce exacerbation of inflammatory bowel disease when it is used for the treatment of ankylosing spondylitis: a case report with a review of literature. Am J Ther. 2014;21:e26-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Moss AC. The meaning of low-grade inflammation in clinically quiescent inflammatory bowel disease. Curr Opin Gastroenterol. 2014;30:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Haraoui B, Krelenbaum M. Emergence of Crohn's disease during treatment with the anti-tumor necrosis factor agent etanercept for ankylosing spondylitis: possible mechanisms of action. Semin Arthritis Rheum. 2009;39:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 66. | Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, Haroon N, Borenstein D, Wang R, Biehl A, Fang MA, Louie G, Majithia V, Ng B, Bigham R, Pianin M, Shah AA, Sullivan N, Turgunbaev M, Oristaglio J, Turner A, Maksymowych WP, Caplan L. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019;71:1599-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 67. | Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, Mpofu S, Richards HB; MEASURE 1 Study Group; MEASURE 2 Study Group. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med. 2015;373:2534-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 780] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 68. | Schreiber S, Colombel JF, Feagan BG, Reich K, Deodhar AA, McInnes IB, Porter B, Das Gupta A, Pricop L, Fox T. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78:473-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 69. | van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, Regel A, Ciurea A, Dagfinrud H, Dougados M, van Gaalen F, Géher P, van der Horst-Bruinsma I, Inman RD, Jongkees M, Kiltz U, Kvien TK, Machado PM, Marzo-Ortega H, Molto A, Navarro-Compàn V, Ozgocmen S, Pimentel-Santos FM, Reveille J, Rudwaleit M, Sieper J, Sampaio-Barros P, Wiek D, Braun J. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1038] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 70. | Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, Wu H, Wang G, Shi Q, Andhivarothai N, Anderson J, Pangan AL. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis. 2014;73:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 71. | Wu KC, Ran ZH, Gao X, Chen M, Zhong J, Sheng JQ, Kamm MA, Travis S, Wallace K, Mostafa NM, Shapiro M, Li Y, Thakkar RB, Robinson AM. Adalimumab induction and maintenance therapy achieve clinical remission and response in Chinese patients with Crohn's disease. Intest Res. 2016;14:152-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Chang CW, Wei SC, Chou JW, Hsu TC, Chuang CH, Lin CP, Hsu WH, Yen HH, Lin JK, Fang YJ, Wang HY, Lin HH, Wu DC, Ni YH, Wang CY, Wong JM. Safety and Efficacy of Adalimumab for Patients With Moderate to Severe Crohn's Disease: The Taiwan Society of Inflammatory Bowel Disease (TSIBD) Study. Intest Res. 2014;12:287-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | González LA, Toloza SM, Alarcón GS. Impact of race and ethnicity in the course and outcome of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40:433-454, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Leone A, Sciascia S, Kamal A, Khamashta M. Biologicals for the treatment of systemic lupus erythematosus: current status and emerging therapies. Expert Rev Clin Immunol. 2015;11:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Wu XR, Liu XL, Katz S, Shen B. Pathogenesis, diagnosis, and management of ulcerative proctitis, chronic radiation proctopathy, and diversion proctitis. Inflamm Bowel Dis. 2015;21:703-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Gecse KB, Lakatos PL. Ulcerative proctitis: an update on the pharmacotherapy and management. Expert Opin Pharmacother. 2014;15:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Dubois E, Moens A, Geelen R, Sabino J, Ferrante M, Vermeire S. Long-term outcomes of patients with ulcerative proctitis: Analysis from a large referral centre cohort. United European Gastroenterol J. 2020;8:933-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Pineton de Chambrun G, Amiot A, Bouguen G, Viennot S, Altwegg R, Louis E, Collins M, Fumery M, Poullenot F, Armengol L, Buisson A, Abitbol V, Laharie D, Seksik P, Nancey S, Blanc P, Bouhnik Y, Pariente B, Peyrin-Biroulet L; PROTECT-GETAID study group. Efficacy of Tumor Necrosis Factor Antagonist Treatment in Patients With Refractory Ulcerative Proctitis. Clin Gastroenterol Hepatol. 2020;18:620-627.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 79. | Baumgart DC, Le Berre C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N Engl J Med. 2021;385:1302-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 80. | Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;17:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 81. | Tanaka Y, Luo Y, O'Shea JJ, Nakayamada S. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18:133-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 308] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 82. | Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 988] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 83. | Liefferinckx C, Verstockt B, Gils A, Noman M, Van Kemseke C, Macken E, De Vos M, Van Moerkercke W, Rahier JF, Bossuyt P, Dutré J, Humblet E, Staessen D, Peeters H, Van Hootegem P, Louis E, Franchimont D, Baert F, Vermeire S; Belgian Inflammatory Bowel Disease Research and Development Group [BIRD group]. Long-term Clinical Effectiveness of Ustekinumab in Patients with Crohn's Disease Who Failed Biologic Therapies: A National Cohort Study. J Crohns Colitis. 2019;13:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 84. | Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376:957-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 957] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 85. | Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)--an analysis of 220 patients. Q J Med. 1987;62:127-141. [PubMed] |
| 86. | Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R, Le Chen S, Zhang NZ. Rheumatic diseases in China. Arthritis Res Ther. 2008;10:R17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 280] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 87. | Chen YT, Wu CY, Li YL, Chen LY, Chiou HY. Time Trends in Psoriasis and Psoriatic Arthritis Incidence from 2002 to 2016 in Taiwan: An Age-Period-Cohort Analysis. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Whitlock SM, Enos CW, Armstrong AW, Gottlieb A, Langley RG, Lebwohl M, Merola JF, Ryan C, Siegel MP, Weinberg JM, Wu JJ, Van Voorhees AS. Management of psoriasis in patients with inflammatory bowel disease: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2018;78:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 89. | Ogdie A, Schwartzman S, Husni ME. Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol. 2015;27:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 90. | Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn's disease. J Eur Acad Dermatol Venereol. 2009;23:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 91. | Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: Review and update. Clin Immunol. 2020;214:108397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 92. | Charlton R, Green A, Shaddick G, Snowball J, Nightingale A, Tillett W, Smith CH, McHugh N; PROMPT study group. Risk of uveitis and inflammatory bowel disease in people with psoriatic arthritis: a population-based cohort study. Ann Rheum Dis. 2018;77:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Manos CK, Xiao R, Brandon TG, Ogdie A, Weiss PF. Risk Factors for Arthritis and the Development of Comorbid Cardiovascular and Metabolic Disease in Children with Psoriasis. Arthritis Rheumatol. 2017;69 suppl 10:2309. |
| 94. | Chia AYT, Ang GWX, Chan ASY, Chan W, Chong TKY, Leung YY. Managing Psoriatic Arthritis With Inflammatory Bowel Disease and/or Uveitis. Front Med (Lausanne). 2021;8:737256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Coates LC, Soriano ER, Corp N, Bertheussen H, Callis Duffin K, Campanholo CB, Chau J, Eder L, Fernández-Ávila DG, FitzGerald O, Garg A, Gladman DD, Goel N, Helliwell PS, Husni ME, Jadon DR, Katz A, Laheru D, Latella J, Leung YY, Lindsay C, Lubrano E, Mazzuoccolo LD, Mease PJ, O'Sullivan D, Ogdie A, Olsder W, Palominos PE, Schick L, Steinkoenig I, de Wit M, van der Windt DA, Kavanaugh A; GRAPPA Treatment Recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 328] [Article Influence: 109.3] [Reference Citation Analysis (1)] |
| 96. | Mehta P, Lawrence A, Aggarwal A. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 98. | Schmitt SK. Reactive Arthritis. Infect Dis Clin North Am. 2017;31:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 99. | Jubber A, Moorthy A. Reactive arthritis: a clinical review. J R Coll Physicians Edinb. 2021;51:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 100. | Kvien TK, Gaston JS, Bardin T, Butrimiene I, Dijkmans BA, Leirisalo-Repo M, Solakov P, Altwegg M, Mowinckel P, Plan PA, Vischer T; EULAR. Three month treatment of reactive arthritis with azithromycin: a EULAR double blind, placebo controlled study. Ann Rheum Dis. 2004;63:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Cuvelier C, Barbatis C, Mielants H, De Vos M, Roels H, Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987;28:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 103] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Flagg SD, Meador R, Hsia E, Kitumnuaypong T, Schumacher HR Jr. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum. 2005;53:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:235-241. [PubMed] |
| 104. | Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology. 2021;161:1118-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 105. | Karreman MC, Luime JJ, Hazes JMW, Weel AEAM. The Prevalence and Incidence of Axial and Peripheral Spondyloarthritis in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2017;11:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 106. | Felice C, Pugliese D, Papparella LG, Pizzolante F, Onori E, Gasbarrini A, Rapaccini GL, Guidi L, Armuzzi A. Clinical management of rheumatologic conditions co-occurring with inflammatory bowel diseases. Expert Rev Clin Immunol. 2018;14:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Orchard TR, Wordsworth BP, Jewell DP. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut. 1998;42:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 324] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 108. | Orchard TR, Thiyagaraja S, Welsh KI, Wordsworth BP, Hill Gaston JS, Jewell DP. Clinical phenotype is related to HLA genotype in the peripheral arthropathies of inflammatory bowel disease. Gastroenterology. 2000;118:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 109. | Amarnani A, Thakker S, Panush RS. Reflecting on the immunopathology of arthritis associated with inflammatory bowel disease: what do we know and what should we know? Clin Rheumatol. 2022;41:2581-2588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 110. | Greenstein AJ, Janowitz HD, Sachar DB. The extra-intestinal complications of Crohn's disease and ulcerative colitis: a study of 700 patients. Medicine (Baltimore). 1976;55:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 693] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 111. | Rodríguez-Reyna TS, Martínez-Reyes C, Yamamoto-Furusho JK. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol. 2009;15:5517-5524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 112. | Su CG, Judge TA, Lichtenstein GR. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:307-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Schett G, Lories RJ, D'Agostino MA, Elewaut D, Kirkham B, Soriano ER, McGonagle D. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol. 2017;13:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 114. | Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 115. | El Miedany Y, Youssef S, Ahmed I, El Gaafary M. The gastrointestinal safety and effect on disease activity of etoricoxib, a selective cox-2 inhibitor in inflammatory bowel diseases. Am J Gastroenterol. 2006;101:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 116. | Kefalakes H, Stylianides TJ, Amanakis G, Kolios G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol. 2009;65:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 117. | Olivieri I, Cantini F, Castiglione F, Felice C, Gionchetti P, Orlando A, Salvarani C, Scarpa R, Vecchi M, Armuzzi A. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014;13:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 118. | Dougados M. Methotrexate in peripheral spondyloarthritis including psoriatic arthritis: a need for further evaluation. Rheumatology (Oxford). 2012;51:1343-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F; European Crohn’s and Colitis Organisation. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 546] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 120. | Bieber A, Fawaz A, Novofastovski I, Mader R. Antitumor Necrosis Factor-α Therapy Associated with Inflammatory Bowel Disease: Three Cases and a Systematic Literature Review. J Rheumatol. 2017;44:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 121. | Armuzzi A, Felice C, Lubrano E, Cantini F, Castiglione F, Gionchetti P, Orlando A, Salvarani C, Scarpa R, Marchesoni A, Vecchi M, Olivieri I; Italian SpA-IBD Expert Panel Group. Multidisciplinary management of patients with coexisting inflammatory bowel disease and spondyloarthritis: A Delphi consensus among Italian experts. Dig Liver Dis. 2017;49:1298-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Guillo L, D'Amico F, Danese S, Peyrin-Biroulet L. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J Crohns Colitis. 2021;15:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 123. | Orchard TR, Chua CN, Ahmad T, Cheng H, Welsh KI, Jewell DP. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology. 2002;123:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 124. | Taleban S, Li D, Targan SR, Ippoliti A, Brant SR, Cho JH, Duerr RH, Rioux JD, Silverberg MS, Vasiliauskas EA, Rotter JI, Haritunians T, Shih DQ, Dubinsky M, Melmed GY, McGovern DP. Ocular Manifestations in Inflammatory Bowel Disease Are Associated with Other Extra-intestinal Manifestations, Gender, and Genes Implicated in Other Immune-related Traits. J Crohns Colitis. 2016;10:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 125. | S Mehta N, Emami-Naeini P. A Review of Systemic Biologics and Local Immunosuppressive Medications in Uveitis. J Ophthalmic Vis Res. 2022;17:276-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 126. | Tse SM, Laxer RM. New advances in juvenile spondyloarthritis. Nat Rev Rheumatol. 2012;8:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 127. | Gmuca S, Weiss PF. Juvenile spondyloarthritis. Curr Opin Rheumatol. 2015;27:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Weiss PF, Colbert RA. Juvenile Spondyloarthritis: A Distinct Form of Juvenile Arthritis. Pediatr Clin North Am. 2018;65:675-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 129. | Srinivasalu H, Sikora KA, Colbert RA. Recent Updates in Juvenile Spondyloarthritis. Rheum Dis Clin North Am. 2021;47:565-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 130. | Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392. [PubMed] |
| 131. | Saurenmann RK, Rose JB, Tyrrell P, Feldman BM, Laxer RM, Schneider R, Silverman ED. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum. 2007;56:1974-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 132. | Weiss PF, Klink AJ, Behrens EM, Sherry DD, Finkel TH, Feudtner C, Keren R. Enthesitis in an inception cohort of enthesitis-related arthritis. Arthritis Care Res (Hoboken). 2011;63:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 133. | Zisman D, Gladman DD, Stoll ML, Strand V, Lavi I, Hsu JJ, Mellins ED; CARRA Legacy Registry Investigators. The Juvenile Psoriatic Arthritis Cohort in the CARRA Registry: Clinical Characteristics, Classification, and Outcomes. J Rheumatol. 2017;44:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 134. | Marino A, Weiss PF, Brandon TG, Lerman MA. Juvenile Spondyloarthritis: focus on uveitis. Pediatr Rheumatol Online J. 2020;18:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 135. | Weiss PF, Roth J. Juvenile-Versus Adult-Onset Spondyloarthritis: Similar, but Different. Rheum Dis Clin North Am. 2020;46:241-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Mielants H, Veys EM, Cuvelier C, De Vos M, Goemaere S, Maertens M, Joos R. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy--a prospective study. J Rheumatol. 1993;20:1567-1572. [PubMed] |
| 137. | Lamot L, Miler M, Vukojević R, Vidović M, Lamot M, Trutin I, Gabaj NN, Harjaček M. The Increased Levels of Fecal Calprotectin in Children With Active Enthesitis Related Arthritis and MRI Signs of Sacroiliitis: The Results of a Single Center Cross-Sectional Exploratory Study in Juvenile Idiopathic Arthritis Patients. Front Med (Lausanne). 2021;8:650619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 138. | Akin E. Juvenile spondyloarthropathies: inflammation in disguise. Minn Med. 2009;92:45-47. [PubMed] |
| 139. | Barthel D, Ganser G, Kuester RM, Onken N, Minden K, Girschick HJ, Hospach A, Horneff G. Inflammatory Bowel Disease in Juvenile Idiopathic Arthritis Patients Treated with Biologics. J Rheumatol. 2015;42:2160-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 140. | van Straalen JW, Krol RM, Giancane G, Panaviene V, Ailioaie LM, Doležalová P, Cattalini M, Susic G, Sztajnbok FR, Maritsi D, Constantin T, Sawhney S, Rygg M, Oliveira SK, Nordal EB, Saad-Magalhães C, Rubio-Perez N, Jelusic M, de Roock S, Wulffraat NM, Ruperto N, Swart JF. Increased incidence of inflammatory bowel disease on etanercept in juvenile idiopathic arthritis regardless of concomitant methotrexate use. Rheumatology (Oxford). 2022;61:2104-2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 141. | Pagnini I, Scavone M, Maccora I, Mastrolia MV, Marrani E, Bertini F, Lamot L, Simonini G. The Development of Extra-Articular Manifestations in Children With Enthesitis-Related Arthritis: Natural Course or Different Disease Entity? Front Med (Lausanne). 2021;8:667305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, Colbert RA, Feldman BM, Ferguson PJ, Gewanter H, Guzman J, Horonjeff J, Nigrovic PA, Ombrello MJ, Passo MH, Stoll ML, Rabinovich CE, Schneider R, Halyabar O, Hays K, Shah AA, Sullivan N, Szymanski AM, Turgunbaev M, Turner A, Reston J. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Rheumatol. 2019;71:846-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 143. | Mohanakrishnan R, Beier S, Deodhar A. IL-23 inhibition for the treatment of psoriatic arthritis. Expert Opin Biol Ther. 2022;22:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Brunner HI, Foeldvari I, Alexeeva E, Ayaz NA, Calvo Penades I, Kasapcopur O, Chasnyk VG, Hufnagel M, Żuber Z, Schulert G, Ozen S, Rakhimyanova A, Ramanan A, Scott C, Sozeri B, Zholobova E, Martin R, Zhu X, Whelan S, Pricop L, Martini A, Lovell D, Ruperto N; Paediatric Rheumatology INternational Trials Organization (PRINTO) and Pediatric Rheumatology Collaborative Study Group (PRCSG). Secukinumab in enthesitis-related arthritis and juvenile psoriatic arthritis: a randomised, double-blind, placebo-controlled, treatment withdrawal, phase 3 trial. Ann Rheum Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 145. | Cardile S, Romano C. Current issues in pediatric inflammatory bowel disease-associated arthropathies. World J Gastroenterol. 2014;20:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |