Published online Aug 7, 2023. doi: 10.3748/wjg.v29.i29.4580
Peer-review started: June 7, 2023
First decision: June 16, 2023
Revised: June 21, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: August 7, 2023
Processing time: 56 Days and 0.2 Hours
Osteoporosis is an extrahepatic complication of primary biliary cholangitis (PBC) that increases the risk of fractures and mortality. However, Epidemiological studies of osteoporosis in patients with PBC in China and the Asia-Pacific region is lack.
To assess the prevalence and clinical characteristics of osteoporosis in Chinese patients with PBC.
This retrospective analysis included consecutive patients with PBC from a tertiary care center in China who underwent bone mineral density (BMD) assessment using dual-energy X-ray absorptiometry between January 2013 and December 2021. We defined subjects with T-scores ≤ -2.5 in any sites (L1 to L4, femoral neck, or total hip) as having osteoporosis. Demographic, serological, clinical, and histological data were collected. Independent risk factors for osteoporosis were identified by multivariate logistic regression analysis.
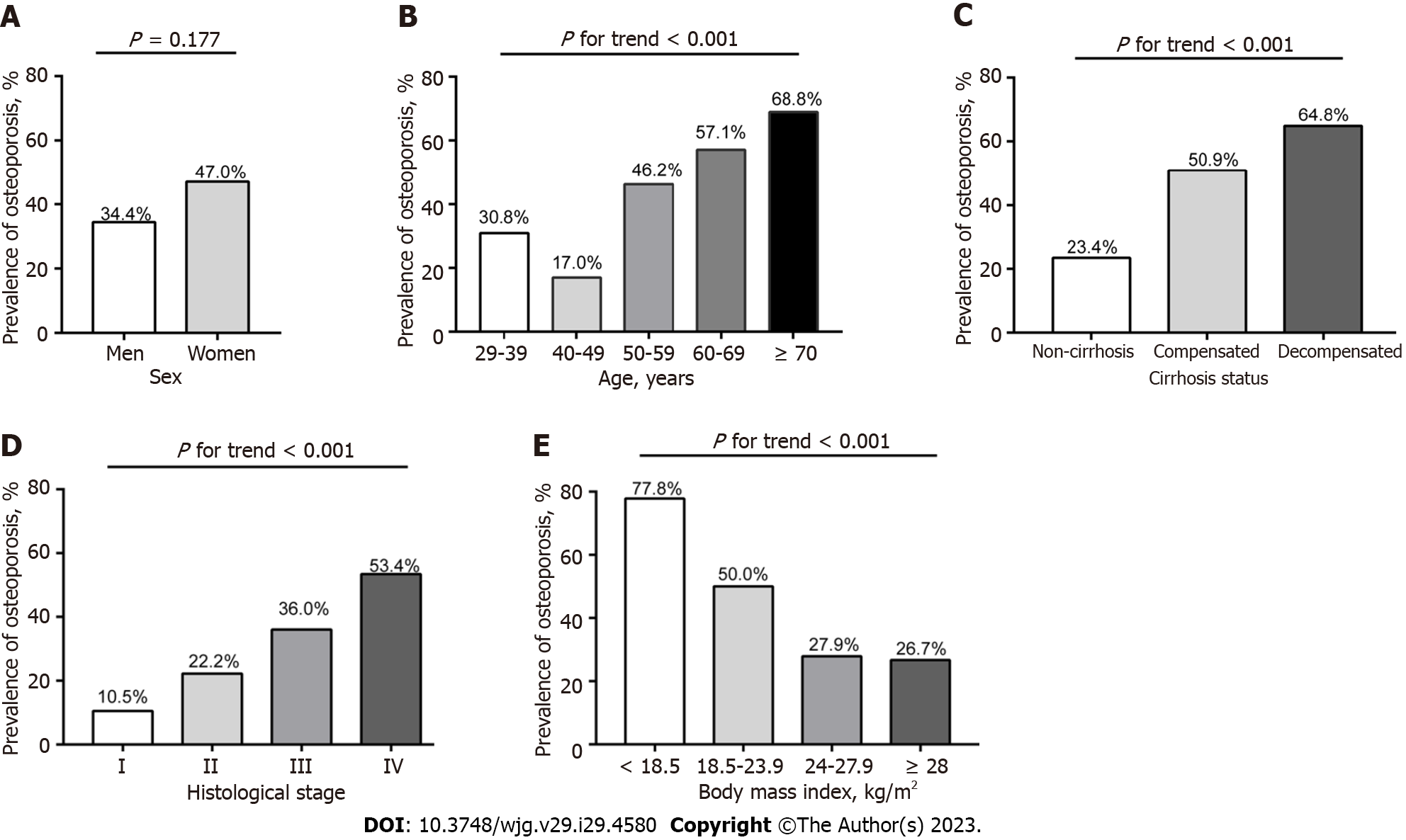
A total of 268 patients with PBC [236 women (88.1%); mean age, 56.7 ± 10.6 years; 163 liver biopsies (60.8%)] were included. The overall prevalence of osteoporosis in patients with PBC was 45.5% (122/268), with the prevalence of osteoporosis in women and men being 47.0% and 34.4%, respectively. The prevalence of osteoporosis in postmenopausal women was significantly higher than that in premenopausal women (56.3% vs 21.0%, P < 0.001). Osteoporosis in patients with PBC is associated with age, fatigue, menopausal status, previous steroid therapy, body mass index (BMI), splenomegaly, gastroesophageal varices, ascites, Mayo risk score, histological stage, alanine aminotransferase, albumin, bilirubin, platelet and prothrombin activity. Multivariate regression analysis identified that older age, lower BMI, previous steroid therapy, higher Mayo risk score, and advanced histological stage as the main independent risk factors for osteoporosis in PBC.
Osteoporosis is very common in Chinese patients with PBC, allowing for prior screening of BMD in those PBC patients with older age, lower BMI, previous steroid therapy and advanced liver disease.
Core Tip: In this paper, we reported for the first large-sample study to explore the prevalence and potential risk factors for osteoporosis in Chinese patients with primary biliary cholangitis (PBC). The prevalence of osteoporosis in Chinese patients with PBC was 45.5%. Osteoporosis in PBC is strongly associated with older age, lower body mass index, previous steroid use, the severity of liver disease, and advanced histological stage. This study provides reference information for future PBC-related guideline development and public policy formulation in China and the Asia-Pacific region.
- Citation: Chen JL, Liu Y, Bi YF, Wang XB. Prevalence and risk factors of osteoporosis detected by dual-energy X-ray absorptiometry among Chinese patients with primary biliary cholangitis. World J Gastroenterol 2023; 29(29): 4580-4592
- URL: https://www.wjgnet.com/1007-9327/full/v29/i29/4580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i29.4580
Primary biliary cholangitis (PBC, also known as primary biliary cirrhosis) is a chronic immune-mediated, progressive cholestatic liver disease characterized by nonsuppurative destructive intrahepatic cholangitis focused on the small bile ducts, which mainly affects middle-aged women over 40 years old, typically manifested as fatigue, pruritus and even metabolic bone disease[1,2]. Currently, ursodeoxycholic acid (UDCA) is its first-line treatment drug, and untreated PBC may eventually lead to cirrhosis and liver failure, requiring liver transplantation[3]. Moreover, PBC affects all races and ethnicities with significant regional differences[1,4]. It is estimated that at least 1 in 1000 women older than 40 years globally has PBC[5], and the prevalence and incidence of PBC in Europe and the United States are higher than that in the Asia-Pacific region[6]. However, the prevalence of PBC in China ranks second only to Japan in the Asia-Pacific region and is increasing[6].
Osteoporosis is a disease characterized by decreased bone density or bone loss, leading to an increased risk of fracture[7]. The prevalence of osteoporosis in patients with PBC is at least three times that of age- or sex-matched controls[8,9]. Thus, osteoporosis is considered an extrahepatic complication of PBC[10]. Unlike increased bone resorption in postmenopausal osteoporosis, osteoporosis in PBC is mainly caused by decreased bone formation[11]. Moreover, recently, a large cohort study indicated that the risk of fracture and post-fracture mortality of PBC patients were significantly higher than those of the control group in the general population[12]. Prevention and timely diagnosis of osteoporosis are key to reducing the associated complications for patients with PBC. With the increasing prevalence of PBC, most likely due to the improvement of diagnosis and awareness, it is likely that the incidence and prevalence of PBC-related osteoporosis will also increase[11]. According to previous studies from Europe, Africa and North America, the prevalence of osteo
Therefore, this study aimed to investigate the prevalence and clinical features of osteoporosis and independent risk factors associated with osteoporosis using bone mineral density (BMD) detected by dual-energy X-ray absorptiometry (DEXA) in a large series of Chinese patients with PBC.
We carried out a retrospective observational study of all consecutive PBC patients between January 2013 and December 2021 from Beijing Ditan Hospital, China. The baseline date was the first admission with a diagnosis of PBC and completion of a BMD examination in the hospital. The study protocol was approved by the Ethics Committee of Beijing Ditan Hospital, Beijing, China (No. DTEC-KT2022-010-01).
The inclusion criteria were as follows: (1) Age greater than 18 years old; (2) at least two of the following: Elevated γ-glutamyl transpeptidase (GGT) or alkaline phosphatase (ALP), positive antimitochondrial antibody (AMA) or gp210, sp100, and pathological features of non-suppurative cholangitis or small bile duct destruction[19]; and (3) complete BMD examination using DEXA method at baseline. The exclusion criteria were as follows: (1) Alcoholic liver disease, non-alcoholic fatty liver disease, viral hepatitis, drug-induced liver injury, or inherited liver disease; (2) liver transplantation, liver cancer, or other malignant lesions; (3) evidence of intrahepatic or extrahepatic biliary obstruction; (4) severe cardiac or renal insufficiency; (5) previous or current hormone replacement therapy; and (6) pregnancy or breast-feeding.
A history of UDCA or steroid treatment, prior fractures, and comorbidities were recorded. Symptoms of chronic cholestasis, such as fatigue and pruritus, and physical signs of liver disease, such as splenomegaly, hepatomegaly, edema and ascites, were collected. Blood was collected for hematological, biochemical and immunological tests after an overnight fast and tested at the Laboratory of Beijing Ditan Hospital using standard methods. These laboratory parameters include serum bilirubin, albumin, ALP, GGT, calcium, phosphorus, creatinine, 25-hydroxyvitamin D, aspartate aminotransferase, alanine aminotransferase (ALT), prothrombin activity (PTA), platelet count (PLT), immunoglobulin G, serum immunoglobulin M, and autoantibodies [AMA, antinuclear antibody (ANA), anti-centromere antibody (ACA), gp210, and sp100] were measured at the time of the first BMD examination. Age, smoking status, body mass index (BMI), menopausal status, duration of PBC, histological stage, and cirrhosis status were recorded. Esophagogastroscopy and abdominal ultrasonography were also evaluated. The Mayo risk score (MRS) was calculated using the previous algorithm[20]. The liver histological stage was determined according to Ludwig’s criteria[21].
Certified technicians measured BMD at the lumbar spine (L1 to L4), femoral neck, and total hip using a DEXA scanner (Lunar, GE Healthcare, United States). The diagnoses of osteopenia and osteoporosis were based on the World Health Organization thresholds: T-score is between -1.0 and -2.5 and ≤ -2.5, respectively[22]. T-score were presented as absolute values (g/cm2) and the number of SD lower than the average peak value of young sex-matched normal individuals. Z-score are also presented as the number of SD from normal values corrected for sex and age. We defined patients with T-scores ≤ -2.5 in any sites (L1 to L4, femoral neck, or total hip) as having osteoporosis[23].
All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, United States). Quantitative data are expressed as mean ± SD or median and interquartile range, and categorical data are expressed as frequencies with percentages. Student t-test or Mann-Whitney U test was used to analyse differences in continuous variables. Chi-squared or Fisher’s exact tests were used for categorical variables. The independent risk factor of significant variable associated with osteoporosis with (P value < 0.05) in univariate analyses was determined using multivariate logistic regression with the backward stepwise selection method (the criteria for entering and removing variables were P < 0.05 and > 0.10, respectively). Statistical significance was defined as a two-tailed P value < 0.05.
From January 2013 to December 2021, we retrospectively enrolled 268 subjects in the final analysis who had undergone BMD examination by DEXA scanner from 1272 patients with PBC. Figure 1 summarizes the enrolment process. The demographic, clinical, serological, histological, and BMD characteristics of all patients with PBC are shown in Table 1. The mean age of the overall patients was 56.7 ± 10.6 years (range 29-94 years). The ratio of women:men was 7.38:1. Liver biopsies were performed in 60.8% of patients. Among the women, 73.7% were postmenopausal. Prior fractures had occurred in 11 patients, including six vertebral fractures and five peripheral fractures. Compared with women, men PBC patients had more smoker, UDCA-treated patients, fewer ACA positive, lower serum calcium, and higher serum creatinine as well as BMD absolute value (all P < 0.05). However, there was no difference between men and women in the T- and Z-score (P > 0.05).
| Characteristics | Overall (n = 268) | Men (n = 32) | Women (n = 236) | P value |
| Age (years) (range) | 56.7 ± 10.6 (29-94) | 60.0 ± 9.3 (38-79) | 56.3 ± 10.7 (29-94) | 0.059 |
| Age group (years), n (%) | 0.201 | |||
| 29-39 | 13 (4.9) | 1 (3.1) | 12 (5.1) | |
| 40-49 | 53 (19.8) | 2 (6.3) | 51 (21.6) | |
| 50-59 | 93 (34.7) | 11 (34.4) | 82 (34.7) | |
| 60-69 | 77 (28.7) | 12 (37.5) | 65 (27.5) | |
| ≥ 70 | 32 (11.9) | 6 (18.8) | 26 (11.0) | |
| Duration of PBC (years) | 3.0 (1.0-6.0) | 4.0 (1.6-6.8) | 3.0 (1.0-6.0) | 0.246 |
| Smoking, n (%) | 20 (7.5) | 17 (53.1) | 3 (1.3) | < 0.001 |
| Postmenopausal, n (%) | 174 (73.7) | 174 (73.7) | ||
| Pruritus, n (%) | 45 (16.8) | 4 (12.5) | 41 (17.4) | 0.489 |
| Fatigue, n (%) | 91 (34.0) | 10 (31.3) | 81 (34.3) | 0.731 |
| Hepatomegaly, n (%) | 9 (3.4) | 1 (3.1) | 8 (3.4) | 1.000 |
| Splenomegaly, n (%) | 187 (69.8) | 20 (62.5) | 167 (70.8) | 0.340 |
| Gastroesophageal varices, n (%) | 110 (41.0) | 14 (43.8) | 96 (40.7) | 0.740 |
| Ascites, n (%) | 103 (38.4) | 11 (34.4) | 92 (39.0) | 0.615 |
| Prior fractures, n (%) | 11 (4.1) | 0 (0.0) | 11 (4.7) | 0.371 |
| BMI (kg/m2) | 22.6 ± 3.3 | 22.1 ± 3.0 | 22.7 ± 3.4 | 0.367 |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 46 (17.2) | 8 (25.0) | 38 (16.1) | 0.210 |
| Hypertension, n (%) | 58 (21.6) | 7 (21.9) | 51 (21.6) | 0.973 |
| Hashimoto's thyroiditis, n (%) | 66 (24.6) | 7 (21.9) | 59 (25.0) | 0.700 |
| Rheumatoid arthritis, n (%) | 8 (3.0) | 0 (0.0) | 8 (3.4) | 0.602 |
| Sicca syndrome, n (%) | 18 (6.7) | 1 (3.1) | 17 (7.2) | 0.706 |
| Previous medication, n (%) | ||||
| UDCA use | 155 (57.8) | 24 (75.0) | 131 (55.5) | 0.036 |
| Steroid use | 32 (11.9) | 3 (9.4) | 29 (12.3) | 0.633 |
| Cirrhosis status, n (%) | 0.661 | |||
| Non-cirrhosis | 107 (39.9) | 15 (46.9) | 92 (39.0) | |
| Compensated | 53 (19.8) | 5 (15.6) | 48 (20.3) | |
| Decompensated | 108 (40.3) | 12 (37.5) | 96 (40.7) | |
| PBC-AIH overlap syndrome, n (%) | 42 (15.7) | 5 (15.6) | 37 (15.7) | 0.994 |
| Mayo risk score | 5.2 ± 1.4 | 5.4 ± 1.4 | 5.1 ± 1.5 | 0.301 |
| Histological stage, n (%)1 | 0.630 | |||
| Ⅰ-Ⅱ | 65 (39.9) | 6 (46.2) | 59 (39.3) | |
| Ⅲ-Ⅳ | 98 (60.1) | 7 (53.8) | 91 (60.7) | |
| Laboratory data | ||||
| ALT (U/L) | 36.2 (22.2-70.0) | 42.8 (29.0-74.4) | 35.8 (21.3-69.7) | 0.236 |
| AST (U/L) | 46.4 (29.2-88.7) | 39.2 (30.1-62.2) | 48.1 (29.0-87.7) | 0.642 |
| Bilirubin (mg/dL) | 1.1 (0.7-1.9) | 0.9 (0.7-1.5) | 1.1 (0.7-2.0) | 0.708 |
| Albumin (g/L) | 37.2 ± 6.6 | 35.5 ± 6.0 | 37.4 ± 6.6 | 0.124 |
| ALP (U/L) | 157.2 (101.7-263.1) | 166.3 (102.8-315.8) | 156.5 (101.2-262.4) | 0.747 |
| GGT (U/L) | 118.2 (48.0-276.3) | 146.3 (52.3-321.2) | 117.0 (47.6-276.3) | 0.568 |
| PTA (%) | 93.0 ± 20.6 | 92.6 ± 19.9 | 93.0 ± 20.8 | 0.922 |
| PLT (× 109/L) | 140.2 ± 79.4 | 140.7 ± 80.1 | 136.2 ± 74.4 | 0.761 |
| Calcium (mmol/L) | 2.22 ± 0.14 | 2.17 ± 0.16 | 2.23 ± 0.14 | 0.018 |
| Phosphorous (mmol/L) | 1.14 ± 0.24 | 1.09 ± 0.17 | 1.15 ± 0.25 | 0.201 |
| 25-hydroxyvitamin D (ng/mL)2 | 14.3 ± 6.9 | 17.2 ± 6.6 | 13.6 ± 6.9 | 0.224 |
| Creatinine (μmol/L) | 56.4 (49.5-65.9) | 67.1 (60.2-86.6) | 55.0 (48.6-63.6) | < 0.001 |
| IgM (g/L) | 2.86 (1.60-4.40) | 2.55 (1.16-4.14) | 2.87 (1.64-4.49) | 0.240 |
| IgG (g/L) | 15.3 (12.0-19.1) | 15.0 (11.9-19.3) | 15.6 (12.0-19.1) | 0.674 |
| gp210 (+), n (%) | 99 (36.9) | 10 (31.3) | 89 (37.7) | 0.477 |
| Sp100 (+), n (%) | 38 (14.2) | 1 (12.5) | 34 (14.4) | 0.772 |
| ACA, n (%) | 54 (20.1) | 2 (6.3) | 52 (22.0) | 0.037 |
| ANA, n (%) | 165 (61.6) | 21 (65.6) | 144 (61.0) | 0.615 |
| Lumbar spine BMD (L1-L4), g/cm2 | 0.828 ± 0.142 | 0.893 ± 0.146 | 0.818 ± 0.139 | 0.005 |
| T-score | -2.04 ± 1.27 | -1.81 ± 1.31 | -2.1 ± 1.27 | 0.267 |
| Z-score | -0.929 ± 1.182 | -1.18 ± 1.33 | -0.89 ± 1.16 | 0.198 |
| BMD classification, n (%) | 0.536 | |||
| Osteoporosis | 108 (40.3) | 10 (31.3) | 98 (41.5) | |
| Osteopenia | 96 (35.8) | 13 (40.6) | 83 (35.2) | |
| Normal | 64 (23.9) | 9 (28.1) | 55 (23.3) | |
| Femoral neck BMD, g/cm2 | 0.654 ± 0.128 | 0.714 ± 0.139 | 0.646 ± 0.125 | 0.005 |
| T-score | -1.80 ± 1.12 | -1.59 ± 1.03 | -1.83 ± 1.13 | 0.262 |
| Z-score | -0.73 ± 1.02 | -0.63 ± 0.99 | -0.74 ± 1.03 | 0.590 |
| BMD classification, n (%) | 0.374 | |||
| Osteoporosis | 78 (29.1) | 7 (21.9) | 71 (30.1) | |
| Osteopenia | 123 (45.9) | 14 (43.8) | 109 (46.2) | |
| Normal | 67 (25.0) | 11 (34.4) | 56 (23.7) | |
| Total Hip BMD, g/cm2 | 0.784 ± 0.153 | 0.855 ± 0.148 | 0.774 ± 0.152 | 0.005 |
| T-score | -1.42 ± 1.06 | -1.19 ± 0.98 | -1.45 ± 1.07 | 0.198 |
| Z-score | -0.64 ± 0.98 | -0.70 ± 0.96 | -0.63 ± 1.00 | 0.714 |
| BMD classification, n (%) | 0.601 | |||
| Osteoporosis | 45 (16.8) | 5 (15.6) | 40 (16.9) | |
| Osteopenia | 127 (47.4) | 13 (40.6) | 114 (48.3) | |
| Normal | 96 (35.8) | 14 (43.8) | 82 (34.7) | |
| Osteoporosis lumbar or neck or hip | 122 (45.5) | 11 (34.4) | 111 (47.0) | 0.177 |
There were significant differences in the BMD value, T-score, and Z-score at any sites (L1 to L4, femoral neck, or total hip) in patients with osteoporosis compared to those without (all P < 0.001) (Supplementary Table 1). The prevalence of osteoporosis and osteopenia in the lumbar spine (L1 to L4), femoral neck and total hip were 40.3% and 35.8%, 29.1% and 45.9%, 16.8% and 47.4%, respectively (Table 1). Overall, the prevalence of osteoporosis was 45.5% (122/268) in all PBC patients, considering the lowest BMD values at the lumbar spine, femoral neck and total hip (Table 1 and Sup
Table 2 shows the results of univariate analysis of the association between osteoporosis and potential risk factors in patients with PBC. In the univariate analysis, osteoporosis was correlated with older age, fatigue, postmenopausal status, previous steroids use, splenomegaly, gastroesophageal varices, ascites, advanced histological stage (Ⅲ or Ⅳ), higher MRS and bilirubin levels, and lower BMI, ALT, albumin, PTA, and PLT regardless of overall or women patients (all P < 0.05). However, there was no association between osteoporosis and other biochemical parameters or immunological indicators such as immunoglobulin, gp210, sp100, ACA, PBC-autoimmune hepatitis (AIH) overlap syndrome, or extrahepatic autoimmune diseases.
| Variables | Overall patients (n = 268) | P value | Women patients (n = 236) | P value | ||
| Osteoporosis (n = 122) | No osteoporosis (n = 146) | Osteoporosis (n = 111) | No osteoporosis (n = 125) | |||
| Women, n (%) | 111 (91.0) | 125 (85.6) | 0.177 | 111 (100) | 125 (100) | |
| Age (year) | 60.5 ± 10.5 | 53.6 ± 9.7 | < 0.001 | 60.4 ± 10.7 | 52.6 ± 9.4 | < 0.001 |
| Duration of PBC (year) | 3.0 (1.0-7.0) | 2.3 (1.0-5.0) | 0.125 | 3.0 (1.0-7.0) | 2.0 (1.0-5.0) | 0.217 |
| Smoking, n (%) | 9 (7.4) | 11 (7.5) | 0.961 | 2 (1.8) | 1 (1.6) | 0.602 |
| Postmenopausal, n (%) | 98 (88.3) | 76 (60.8) | < 0.001 | 98 (88.3) | 76 (60.8) | < 0.001 |
| Pruritus, n (%) | 22 (18.0) | 23 (15.8) | 0.619 | 21 (18.9) | 20 (16.0) | 0.555 |
| Fatigue, n (%) | 50 (41.0) | 41 (28.1) | 0.026 | 48 (43.2) | 33 (26.4) | 0.007 |
| Hepatomegaly, n (%) | 2 (1.6) | 7 (4.8) | 0.188 | 2 (1.8) | 6 (4.8) | 0.204 |
| Splenomegaly, n (%) | 103 (84.4) | 84 (57.5) | < 0.001 | 96 (86.5) | 71 (56.8) | < 0.001 |
| Gastroesophageal varices, n (%) | 72 (59.0) | 38 (26.0) | < 0.001 | 66 (59.5) | 30 (24.0) | < 0.001 |
| Ascites, n (%) | 67 (54.9) | 36 (24.7) | < 0.001 | 61 (55.0) | 31 (24.8) | < 0.001 |
| Prior fractures, n (%) | 8 (5.0) | 3 (2.1) | 0.064 | 8 (7.2) | 3 (2.4) | 0.080 |
| BMI (kg/m2) | 21.6 ± 3.2 | 23.4 ± 3.3 | < 0.001 | 21.8 ± 3.1 | 23.4 ± 3.4 | < 0.001 |
| Comorbidities | ||||||
| Diabetes mellitus, n (%) | 23 (18.9) | 23 (15.8) | 0.503 | 20 (18.0) | 18 (14.4) | 0.450 |
| Hypertension, n (%) | 28 (23.0) | 30 (20.5) | 0.634 | 26 (23.4) | 25 (20.0) | 0.524 |
| Hashimoto's thyroiditis, n (%) | 27 (22.1) | 39 (26.7) | 0.386 | 26 (23.4) | 33 (26.4) | 0.598 |
| Rheumatoid arthritis, n (%) | 4 (3.3) | 4 (2.7) | 0.796 | 4 (3.6) | 4 (3.2) | 0.864 |
| Sicca syndrome, n (%) | 8 (6.6) | 10 (6.8) | 0.924 | 8 (7.2) | 9 (7.2) | 0.998 |
| Previous medication | ||||||
| UDCA use, n (%) | 78 (63.9) | 77 (52.7) | 0.065 | 69 (62.2) | 62 (49.6) | 0.053 |
| Steroid use, n (%) | 23 (18.9) | 9 (6.2) | 0.001 | 21 (18.9) | 8 (6.4) | 0.003 |
| PBC-AIH overlap syndrome, n (%) | 17 (13.9) | 25 (17.1) | 0.475 | 17 (15.3) | 20 (16.0) | 0.885 |
| Mayo risk score | 5.7 ± 1.5 | 4.7 ± 1.3 | < 0.001 | 5.7 ± 1.4 | 4.6 ± 1.2 | < 0.001 |
| Histological stage, n (%)1 | < 0.001 | < 0.001 | ||||
| Ⅰ-Ⅱ | 10 (17.2) | 55 (52.4) | 9 (16.1) | 50 (53.2) | ||
| Ⅲ-Ⅳ | 48 (82.8) | 50 (47.6) | 47 (83.9) | 44 (46.8) | ||
| ALT (U/L) | 32.0 (20.8-54.8) | 43.7 (23.7-84.4) | 0.007 | 31.2 (20.5-31.2) | 46.3 (29.1-84.4) | 0.013 |
| AST (U/L) | 44.0 (29.1-83.7) | 49.5 (29.2-91.5) | 0.782 | 46.3 (29.1-84.4) | 50.9 (28.7-91.5) | 0.899 |
| Bilirubin (mg/dL) | 1.2 (0.8-2.40 | 0.9 (0.7-1.7) | 0.002 | 1.2 (0.8-2.5) | 0.9 (0.7-1.6) | 0.001 |
| Albumin (g/L) | 35.5 ± 6.9 | 38.7 ± 5.9 | < 0.001 | 35.7 ± 6.9 | 39.0 ± 5.9 | < 0.001 |
| ALP (U/L) | 164.2 (110.8-266.3) | 149.3 (94.4-261.5) | 0.180 | 164.3 (112.3-265.0) | 144.5 (93.6-257.4) | 0.175 |
| GGT (U/L) | 107.7 (47.9-237.1) | 143.1 (47.7-307.3) | 0.320 | 108.5 (45.4-242.5) | 129.1 (48.6-303.3) | 0.432 |
| PTA (%) | 87.9 ± 20.2 | 97.2 ± 20.1 | < 0.001 | 87.6 ± 20.2 | 97.8 ± 20.1 | < 0.001 |
| PLT (× 109/L) | 119.3 ± 71.4 | 157.7 ± 81.7 | < 0.001 | 116.4 ± 67.4 | 162.4 ± 84.5 | < 0.001 |
| Calcium (mmol/L) | 2.20 ± 0.15 | 2.24 ± 0.13 | 0.065 | 2.21 ± 0.14 | 2.24 ± 0.13 | 0.100 |
| Phosphorous (mmol/L) | 1.15 ± 0.30 | 1.14 ± 0.17 | 0.703 | 1.15 ± 0.31 | 1.15 ± 0.17 | 0.869 |
| 25-hydroxyvitamin D (ng/mL)2 | 14.0 ± 7.8 | 14.5 ± 6.2 | 0.823 | 14.1 ± 8.0 | 13.4 ± 5.8 | 0.748 |
| Creatinine (μmol/L) | 58.4 (47.2-67.5) | 56.2 (50.6-65.4) | 0.724 | 55.9 (46.9-67.5) | 54.9 (49.9-60.7) | 0.710 |
| IgM (g/L) | 2.80 (1.53-4.39) | 2.87 (1.64-4.63) | 0.497 | 2.87 (1.61-4.49) | 2.93 (1.65-4.49) | 0.776 |
| IgG (g/L) | 15.7 (12.0-19.5) | 15.2 (11.9-18.9) | 0.453 | 15.7 (12.3-19.6) | 15.3 (11.8-18.6) | 0.420 |
| gp210 (+), n (%) | 48 (39.3) | 51 (34.9) | 0.456 | 45 (40.5) | 44 (35.2) | 0.398 |
| Sp100 (+), n (%) | 19 (15.6) | 19 (13.0) | 0.550 | 18 (16.2) | 16 (12.8) | 0.456 |
| ACA, n (%) | 30 (24.6) | 24 (16.4) | 0.098 | 29 (26.1) | 23 (18.4) | 0.153 |
| ANA, n (%) | 74 (60.7) | 91 (62.3) | 0.779 | 69 (62.2) | 75 (60.0) | 0.734 |
In multivariate analysis, older age [odds ratio (OR), 1.80; 95%CI (confidence interval): 1.33-2.44, P < 0.001] per 10 years, gastroesophageal varices (OR, 2.11; 95%CI: 1.14-3.92, P = 0.018), lower BMI (OR, 0.85; 95%CI: 0.77-0.93, P < 0.001), previous steroid use (OR, 4.19; 95%CI: 1.66-10.56, P = 0.002), higher MRS (OR, 1.36; 95%CI: 1.08-1.71, P = 0.009) were the independent risk factors associated with the presence of osteoporosis in all patients with PBC (Table 3). In addition, when the histological stage was included in the multivariate analysis, higher bilirubin (OR, 1.20; 95%CI: 1.0-1.42, P = 0.044) and advanced histological stage (OR, 3.74; 95%CI: 1.60-8.77, P = 0.002) gained statistical significance, but the effects of gastroesophageal varices, previous steroid use, and higher MRS were removed. When menopausal status was included in the multivariate analysis (only for female patients), the splenomegaly (OR, 2.62; 95%CI: 1.18-5.80, P = 0.018) and postmenopausal (OR, 2.92; 95%CI: 1.02-8.38, P = 0.046) gained statistical significance. When both menopausal status and histological stage were included in the multivariate analysis, advanced histological stage, older age, lower BMI, previous steroid use, and higher MRS were identified as independent risk factors for osteoporosis. However, menopausal status and other variables, such as bilirubin, were not entered as independent factors of osteoporosis in the final model.
| Variables | Overall patients (n = 268) | Overall patients underwent liver biopsy (n = 163) | Women patients (n = 236) | Women patients underwent liver biopsy (n = 150) | ||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age, per 10 years | 1.80 (1.33-2.44) | < 0.001 | 1.95 (1.30-2.92) | 0.001 | 1.63 (1.04-2.57) | 0.035 | 2.02 (1.27-3.20) | 0.003 |
| Fatigue | NS | NS | NS | NS | ||||
| Splenomegaly | NS | NS | 2.62 (1.18-5.80) | 0.018 | NS | |||
| Gastroesophageal varices | 2.11 (1.14-3.92) | 0.018 | NS | NS | NS | |||
| Ascites | NS | NS | NS | NS | ||||
| BMI | 0.85 (0.77-0.93) | < 0.001 | 0.87 (0.77-0.98) | 0.023 | 0.83 (0.75-0.92) | < 0.001 | 0.85 (0.74-0.97) | 0.014 |
| Previous steroid use | 4.19 (1.66-10.56) | 0.002 | NS | 4.01 (1.42-11.31) | 0.009 | 3.99 (1.24-12.87) | 0.020 | |
| Mayo risk score | 1.36 (1.08-1.71) | 0.009 | NS | 1.61 (1.22-2.14) | 0.001 | 1.64 (1.12-2.41) | 0.011 | |
| ALT | NS | NS | NS | NS | ||||
| Bilirubin | NS | 1.20 (1.01-1.42) | 0.044 | NS | NS | |||
| Albumin | NS | NS | NS | NS | ||||
| PTA | NS | NS | NS | NS | ||||
| PLT | NS | NS | NS | NS | ||||
| Postmenopausal | 2.92 (1.02-8.38) | 0.046 | NS | |||||
| Histological stage Ⅲ or Ⅳ | 3.74 (1.60-8.77) | 0.002 | 3.02 (1.13-8.06) | 0.027 | ||||
To our knowledge, this observational study is the largest population study on BMD in PBC among currently published studies, whether in China, the Asia-Pacific region, or globally. Moreover, this is the first large-sample study to explore the prevalence and potential risk factors for osteoporosis in Asian patients with PBC. PBC patients with advanced stage (Ludwig Ⅲ/Ⅳ) had more than 2-fold increased risk of osteoporosis compared to patients with early stage (Ludwig Ⅰ/Ⅱ). The main independent risk factors identified for osteoporosis include older age, lower BMI, previous steroid use, liver disease severity determined by the MRS, and advanced histological stage. These factors are consistent with those of previous studies in Europe and the United States[8,9].
A recent meta-analysis indicated that the risk of osteoporosis increased by 1.8 times in PBC patients compared with non-PBC participants[24]. Similar to this result, our study indicated a 2.3-time increased risk of osteoporosis in PBC patients aged 40 years or older, accounting for 95% of the total patients, compared to age-matched controls in China[23]. However, by searching the literature, we found that since 2001, the prevalence of PBC osteoporosis evaluated by DEXA has been verified in European, African, and American populations but not in Asian populations (Supplementary Table 3). As is well known, genetic factors play a crucial role in the pathogenesis of PBC[1], it is imperative to explore the preva
It has been recognized that glucocorticoid administration can significantly increase the risk of osteoporosis and bone fragility[25]. Consistently, our study found that the osteoporosis rate of PBC patients previously treated with steroid was significantly higher than that of patients without steroid treatment (71.9% vs 41.0%, P = 0.001). However, although many patients in our study were treated with UDCA (64%), it had no effect on the prevalence of osteoporosis. Previous studies have also shown that the treatment of PBC itself has not been shown to improve BMD[10]. In addition, PBC-related osteoporosis is strongly correlated with the severity of liver disease[8,9,14]. Our study results also showed that the more severe liver disease determined by MRS and histological stage in PBC, the more prone the individual is to osteoporosis. Moreover, the univariate analysis in this study demonstrated that osteoporosis in PBC was related to fatigue, higher bilirubin, lower albumin, PTA, and features of portal hypertension, including splenomegaly, gastroesophageal varices, ascites and thrombocytopenia, which are also common clinical indicators of liver disease severity. Moreover, although the pathogenesis of hepatic osteodystrophy has not been clarified, it is generally believed that chronic cholestasis itself may lead to bone loss in PBC patients[14]. Bilirubin inhibits the function of osteoblasts in vitro, which may be related to the low bone formation rate of PBC patients[26]. In our study, bilirubin levels were also statistically significant when the histological stage was considered as a variable in multivariate analysis. Similarly, Menon et al[9] found that higher baseline bilirubin level rather than the histological stage was the only variable independently related to bone loss rate after 3 years of follow up[9].
In addition, lower BMI and older age are recognized risk factors for osteoporosis in postmenopausal women as well as in the general population[27]. Our study also verified the association between lower BMI and older age in PBC and osteoporosis, thus further proving that this may be associated with similar pathogenesis in the general population. However, although the menopausal status was not statistically significant when including histological stage in our multivariate analysis, it was selected as an independent indicator of osteoporosis when excluding histological stage from the model, indicating that histological stage captured the impact of menopausal status when the two variables competed in the model. Interestingly, lower ALT levels were also found to be related to a higher osteoporosis rate in the current univariate analysis, which is similar to our previous study that indicated that the biochemical response rate of PBC patients with lower ALT levels at baseline were worse than that of patients with higher ALT levels[28]. However, the ALT level was not an independent factor of osteoporosis in multivariate analysis.
In addition, one advantage of our study was that it explored the correlation between immune indicators and PBC osteoporosis, which has not been discussed in previous studies. Anti-gp210 and sp100 antibodies are two specific ANAs for PBC diagnosis[2]. Previous studies showed that gp210-positive is associated with poor prognosis in PBC patients[29]. Meanwhile, PBC is an immune-mediated cholangitis with complex pathogenesis, which often occurs concomitantly with PBC-AIH overlap syndrome and other extrahepatic autoimmune diseases such as sicca syndrome, rheumatoid arthritis, and Hashimoto's thyroiditis[2,30]. However, our study showed that osteoporosis in PBC patients was not related to these immunological features.
Up to now, the pathogenesis of PBC osteoporosis is still unclear. Most experts believed that it seems to be mainly caused by reduced bone formation, although increased bone resorption may play a role in certain situations, such as in post-menopausal women and patients with hypogonadism[10]. Osteoblast mediated bone formation and osteoclast dependent bone resorption are two opposite processes that affect bone mass: when absorption exceeds formation, bone mass will inevitably decrease, and this negative balance will lead to bone loss and osteoporosis[31]. Several studies assessing bone histomorphometry have shown that most of the osteoporosis patients with PBC had reduced tetracycline double labeling, bone formation rate, osteoblasts numbers, and reduced serum osteocalcin level, all of which indicate that osteoblast dysfunction and bone formation deficiency are the core of the pathogenesis of PBC-related osteoporosis[32-34]. In addition, other changes, increased levels of bilirubin and bile salts, and production of fibronectin may also reduce bone formation by inhibiting the proliferation and survival of osteoblasts in PBC or cholestasis[26,35]. Other conditions of PBC patients, including increased formation of osteoclast, low vitamin D levels, calcium malabsorption and sarcopenia, may be contributing factors to the panorama of PBC osteopathy[31,33,36,37].
Nevertheless, our study has several limitations. First, despite being the largest DEXA-based BMD measurement cohort of PBC to date, the sample size was relatively small, especially for men. Thus, it would be interesting and necessary to explore the same objective for larger sample size with a fairly balanced number of women and men. Second, this was a single-center, retrospective study. As a tertiary care center in China, our patients come from different regions of China and may not be representative of those at primary or secondary medical institutions. Prospective studies in Chinese populations may validate our findings. Third, a history of use of anti-osteoporosis treatments such as bisphosphonate, Vitamin D, and calcium supplementation was not included in this study for the analysis of the factors influencing osteoporosis. However, data on osteoporosis therapies related to PBC are insufficient and controversial, and the overall quality of evidence is low[11]. Therefore, we do not think that receiving anti-osteoporosis treatments in the past affected our results. In the future, it is necessary to conduct high-quality research and explore PBC specific therapies focused on improving bone formation.
In summary, we found a significantly higher prevalence of osteoporosis in Chinese patients with PBC. Osteoporosis in PBC is strongly associated with older age, lower BMI, previous steroid use, the severity of liver disease, and advanced histological stage. Thus, this study contributes to identifying PBC patients who require early screening for BMD, and potential interventions to diminish the risk of osteoporosis and fractures. This study may help to provide reference information for the development and formulation of future PBC-related guideline and public health policy in China and the Asia-Pacific region.
Primary biliary cholangitis (PBC) is a chronic immune-mediated, progressive cholestatic liver disease. Osteoporosis is an extrahepatic complication of PBC that increases the risk of fractures and mortality.
Although the prevalence of osteoporosis in PBC is high in Europe and North America, relevant epidemiological studies of osteoporosis in patients with PBC in China and the Asia-Pacific region is lack.
To assess the prevalence and clinical characteristics of osteoporosis in Chinese patients with PBC.
We performed a retrospective observational study to evaluation the prevalence and risk factors of osteoporosis in Chinese patients with PBC from a tertiary care center who underwent bone mineral density (BMD) assessment using dual-energy X-ray absorptiometry between January 2013 and December 2021. Demographic, serological, clinical, and histological data were collected. Independent risk factors for osteoporosis were identified by multivariate logistic regression analysis.
The prevalence of osteoporosis in Chinese patients with PBC was 45.5%. Osteoporosis in PBC is strongly associated with older age, lower body mass index (BMI), previous steroid use, the severity of liver disease, and advanced histological stage.
Osteoporosis is very common in Chinese patients with PBC, allowing for prior screening of BMD in those PBC patients with older age, lower BMI, previous steroid therapy, and advanced liver disease.
This study provides reference information for future PBC-related guideline development and public policy formulation in China and the Asia-Pacific region.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jennane R, France; Kuroki H, Japan; Lee MK, South Korea S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet. 2020;396:1915-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 412] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 3. | Levy C, Manns M, Hirschfield G. New Treatment Paradigms in Primary Biliary Cholangitis. Clin Gastroenterol Hepatol. 2023;21:2076-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Jepsen P, Grønbæk L, Vilstrup H. Worldwide Incidence of Autoimmune Liver Disease. Dig Dis. 2015;33 Suppl 2:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Zeng N, Duan W, Chen S, Wu S, Ma H, Ou X, You H, Kong Y, Jia J. Epidemiology and clinical course of primary biliary cholangitis in the Asia-Pacific region: a systematic review and meta-analysis. Hepatol Int. 2019;13:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 1458] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 8. | Guañabens N, Parés A, Ros I, Caballería L, Pons F, Vidal S, Monegal A, Peris P, Rodés J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Menon KV, Angulo P, Weston S, Dickson ER, Lindor KD. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001;35:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Danford CJ, Trivedi HD, Papamichael K, Tapper EB, Bonder A. Osteoporosis in primary biliary cholangitis. World J Gastroenterol. 2018;24:3513-3520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Trivedi HD, Danford CJ, Goyes D, Bonder A. Osteoporosis in Primary Biliary Cholangitis: Prevalence, Impact and Management Challenges. Clin Exp Gastroenterol. 2020;13:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Schönau J, Wester A, Schattenberg JM, Hagström H. Risk of fractures and postfracture mortality in 3980 people with primary biliary cholangitis: A population-based cohort study. J Intern Med. 2023;294:164-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Wariaghli G, Mounach A, Achemlal L, Benbaghdadi I, Aouragh A, Bezza A, El Maghraoui A. Osteoporosis in chronic liver disease: a case-control study. Rheumatol Int. 2010;30:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Guañabens N, Cerdá D, Monegal A, Pons F, Caballería L, Peris P, Parés A. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology. 2010;138:2348-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Floreani A, Mega A, Camozzi V, Baldo V, Plebani M, Burra P, Luisetto G. Is osteoporosis a peculiar association with primary biliary cirrhosis? World J Gastroenterol. 2005;11:5347-5350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12:1390-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Benetti A, Crosignani A, Varenna M, Giussani CS, Allocca M, Zuin M, Podda M, Battezzati PM. Primary biliary cirrhosis is not an additional risk factor for bone loss in women receiving regular calcium and vitamin D supplementation: a controlled longitudinal study. J Clin Gastroenterol. 2008;42:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Boulton-Jones JR, Fenn RM, West J, Logan RF, Ryder SD. Fracture risk of women with primary biliary cirrhosis: no increase compared with general population controls. Aliment Pharmacol Ther. 2004;20:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, Duan W, Chen S, Kong Y, Zhang D, Wei L, Wang FS, Lin HC, Yang JM, Tanwandee T, Gani RA, Payawal DA, Sharma BC, Hou J, Yokosuka O, Dokmeci AK, Crawford D, Kao JH, Piratvisuth T, Suh DJ, Lesmana LA, Sollano J, Lau G, Sarin SK, Omata M, Tanaka A, Jia J. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol Int. 2022;16:1-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 20. | Dickson ER, Grambsch PM, Fleming TR, Fisher LD, Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 523] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 528] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 2366] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 23. | Wang L, Yu W, Yin X, Cui L, Tang S, Jiang N, Zhao N, Lin Q, Chen L, Lin H, Jin X, Dong Z, Ren Z, Hou Z, Zhang Y, Zhong J, Cai S, Liu Y, Meng R, Deng Y, Ding X, Ma J, Xie Z, Shen L, Wu W, Zhang M, Ying Q, Zeng Y, Dong J, Cummings SR, Li Z, Xia W. Prevalence of Osteoporosis and Fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw Open. 2021;4:e2121106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 308] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 24. | Fan J, Wang Q, Sun L. Association between primary biliary cholangitis and osteoporosis: meta-analysis. Clin Rheumatol. 2017;36:2565-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 793] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 26. | Janes CH, Dickson ER, Okazaki R, Bonde S, McDonagh AF, Riggs BL. Role of hyperbilirubinemia in the impairment of osteoblast proliferation associated with cholestatic jaundice. J Clin Invest. 1995;95:2581-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Kröger H, Tuppurainen M, Honkanen R, Alhava E, Saarikoski S. Bone mineral density and risk factors for osteoporosis--a population-based study of 1600 perimenopausal women. Calcif Tissue Int. 1994;55:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 139] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Chen J, Xue D, Gao F, Tao L, Li Y, Zhang Q, Wang R, Sun L, Yang X, Liu Y, Zhu B, Niu S, Wang X. Influence factors and a predictive scoring model for measuring the biochemical response of primary biliary cholangitis to ursodeoxycholic acid treatment. Eur J Gastroenterol Hepatol. 2018;30:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yang F, Yang Y, Wang Q, Wang Z, Miao Q, Xiao X, Wei Y, Bian Z, Sheng L, Chen X, Qiu D, Fang J, Tang R, Gershwin ME, Ma X. The risk predictive values of UK-PBC and GLOBE scoring system in Chinese patients with primary biliary cholangitis: the additional effect of anti-gp210. Aliment Pharmacol Ther. 2017;45:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 897] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 31. | Pugliese N, Arcari I, Aghemo A, Lania AG, Lleo A, Mazziotti G. Osteosarcopenia in autoimmune cholestatic liver diseases: Causes, management, and challenges. World J Gastroenterol. 2022;28:1430-1443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (6)] |
| 32. | Hodgson SF, Dickson ER, Wahner HW, Johnson KA, Mann KG, Riggs BL. Bone loss and reduced osteoblast function in primary biliary cirrhosis. Ann Intern Med. 1985;103:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 145] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85:1356-1362. [PubMed] |
| 34. | Guichelaar MM, Malinchoc M, Sibonga J, Clarke BL, Hay JE. Bone metabolism in advanced cholestatic liver disease: analysis by bone histomorphometry. Hepatology. 2002;36:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Kawelke N, Bentmann A, Hackl N, Hager HD, Feick P, Geursen A, Singer MV, Nakchbandi IA. Isoform of fibronectin mediates bone loss in patients with primary biliary cirrhosis by suppressing bone formation. J Bone Miner Res. 2008;23:1278-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Olivier BJ, Schoenmaker T, Mebius RE, Everts V, Mulder CJ, van Nieuwkerk KM, de Vries TJ, van der Merwe SW. Increased osteoclast formation and activity by peripheral blood mononuclear cells in chronic liver disease patients with osteopenia. Hepatology. 2008;47:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Saeki C, Oikawa T, Kanai T, Nakano M, Torisu Y, Sasaki N, Abo M, Saruta M, Tsubota A. Relationship between osteoporosis, sarcopenia, vertebral fracture, and osteosarcopenia in patients with primary biliary cholangitis. Eur J Gastroenterol Hepatol. 2021;33:731-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |