Published online Aug 7, 2023. doi: 10.3748/wjg.v29.i29.4542
Peer-review started: May 6, 2023
First decision: May 17, 2023
Revised: May 24, 2023
Accepted: July 5, 2023
Article in press: July 5, 2023
Published online: August 7, 2023
Processing time: 87 Days and 17.7 Hours
Gastric carcinoma (GC) is the third most frequent cause of cancer-related death, highlighting the pressing need for novel clinical treatment options. In this regard, microRNAs (miRNAs) have emerged as a promising therapeutic strategy. Studies have shown that miRNAs can regulate related signaling pathways, acting as tumor suppressors or tumor promoters.
To explore the effect of miR-204-3p on GC cells.
We measured the expression levels of miR-204-3p in GC cells using quantitative real-time polymerase chain reaction, followed by the delivery of miR-204-3p overexpression and miR-204-3p knockdown vectors into GC cells. CCK-8 was used to detect the effect of miR-204-3p on the proliferation of GC cells, and the colony formation ability of GC cells was detected by the clonal formation assay. The effects of miR-204-3p on GC cell cycle and apoptosis were detected by flow cytometry. The BABL/c nude mouse subcutaneous tumor model using MKN-45 cells was constructed to verify the effect of miR-204-3p on the tumorigenicity of GC cells. Furthermore, the study investigated the effects of miR-204-3p on various proteins related to the MAPK signaling pathway, necroptosis signaling pathway and apoptosis signaling pathway on GC cells using Western blot techniques.
Firstly, we found that the expression of miR-204-3p in GC was low. When treated with the lentivirus overexpression vector, miR-204-3p expression significantly increased, but the lentivirus knockout vector had no significant effect on miR-204-3p. In vitro experiments confirmed that miR-204-3p overexpression inhibited GC cell viability, promoted cell apoptosis, blocked the cell cycle, and inhibited colony formation ability. In vivo animal experiments confirmed that miR-204-3p overexpression inhibited subcutaneous tumorigenesis ability in BABL/c nude mice. Simultaneously, our results verified that miR-204-3p overexpression can inhibit GC cell proliferation by inhibiting protein expression levels of KRAS and p-ERK1/2 in the MAPK pathway, as well as inhibiting protein expression levels of p-RIP1 and p-MLK1 in the necroptosis pathway to promote the BCL-2/BAX/Caspase-3 apoptosis pathway.
MiR-204-3p overexpression inhibited GC cell proliferation by inhibiting the MAPK pathway and necroptosis pathway to promote apoptosis of GC cells. Thus, miR-204-3p may represent a new potential therapeutic target for GC.
Core Tip: Gastric carcinoma (GC) is a global health problem that seriously endangers human life; therefore, it is important to identify effective treatment targets. In this regard, microRNAs (miRNAs) have emerged as a promising therapeutic strategy. Studies have shown that miRNAs regulated signaling pathways, acting as tumor suppressors or tumor promoters. In this study, we first verified the inhibitory effect of miR-204-3p overexpression on GC cells through in vitro and in vivo experiments. Simultaneously, miR-204-3p overexpression induced GC cell apoptosis by inhibiting the MAPK pathway and the necroptosis pathway. Thus, miR-204-3p may represent a new potential therapeutic target for GC.
- Citation: Li X, Tibenda JJ, Nan Y, Huang SC, Ning N, Chen GQ, Du YH, Yang YT, Meng FD, Yuan L. MiR-204-3p overexpression inhibits gastric carcinoma cell proliferation by inhibiting the MAPK pathway and RIP1/MLK1 necroptosis pathway to promote apoptosis. World J Gastroenterol 2023; 29(29): 4542-4556
- URL: https://www.wjgnet.com/1007-9327/full/v29/i29/4542.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i29.4542
Gastric carcinoma (GC) is a gastrointestinal tumor and the third major cause of cancer-related death[1,2]. Early clinical symptoms are mild or asymptomatic, resulting in difficult diagnosis and a low patient survival rate[3,4]. Currently, the clinical therapy of GC primarily consists of radiotherapy, chemotherapy, and surgical excision, but the therapeutic effect is unsatisfactory[5,6]. Therefore, feasible targeted therapies are particularly important for GC patients. As research has progressed, molecular targets have been found to have a role in the occurrence and development of GC.
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression by binding to the 3'-untranslated region of target mRNA[7]. With further study in genetic engineering, it has been confirmed that miRNAs regulate different signaling pathways to take part in important cellular processes[8]. miRNAs can act as tumor promoters or inhibitors to target mRNA to regulate GC proliferation, metastasis, angiogenesis, and drug resistance[9-11]. Conse
MiR-204-3p has demonstrated efficacy in treating various pathologies, including retinopathy, diabetes, and cancer[12-15]. Crucially, miR-204-3p is underexpressed in melanoma, thyroid carcinoma, glioma and bladder carcinoma, and is related to patient prognosis[16-19]. However, the mechanism of miR-204-3p in GC remains unclear.
Our team's previous research confirmed that the expression of miR-204-3p in GC tissue is low and is associated with poor prognosis in GC patients. In addition, we also verified that KRAS is its direct target[20]. However, the anti-GC effect of miR-204-3p still requires further research to support the possibility of miR-204-3p becoming a new target for the treatment of GC. In this study, we focused on determining the impact of miR-204-3p on GC cells phenotype and its anti-GC molecular mechanism, to provide theoretical support for the treatment of GC by miR-204-3p.
A normal human gastric epithelial cell line (GES-1) and three GC cell lines (HGC-27, AGS and MKN-45) were acquired from BNCC (Beijing, China). MKN-45 and HGC-27 cells were cultured in RPIM-1640 medium, AGS cells in DMEM/F-12 medium and GES-1 cells in DMEM medium. All culture media were purchased from Gibco (United States), and were supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum. Genechem (Shanghai, China) provided the green fluorescent protein-labeled miR-204-3p overexpression lentiviral vector (OE group), miR-204-3p knockdown lentiviral vector (KD group) and empty lentiviral vector (NC group), which were then transfected into HGC-27, MKN-45 and AGS cells using the tool virus user manual as a guide.
Cell viability was evaluated using the CCK-8 assay. Specifically, lentivirus-transfected AGS and HGC-27 cells were seeded into 96-well plates (5000 cells/well) and cultured for 24 h, 48 h and 72 h, respectively. Following this step, each well was treated with 10 μL CCK-8 reagent (MedChemExpress, United States), and a microplate reader was used to measure the value at 450 nm following incubation for 2 h at 37°C.
Lentivirus-transfected AGS and HGC-27 cells were inoculated into 6-well plates (500 cells/well), and fixed with 4% paraformaldehyde for 30 min after 2 wk of continuous culture. Next, the fixing solution was washed off and the cells were stained using 0.5% crystal violet for 10 min. Finally, the cell clones were photographed and statistically analyzed based on clone sizes (diameter > 1 mm).
Cell cycle was confirmed using the cell cycle kit (KeyGEN BioTECH, China). Lentivirus-transfected HGC-27 and AGS cells were collected at 1 × 106 cells/mL, and then fixed overnight at 37℃ with 4% paraformaldehyde. On the second day, the fixing solution was washed off with PBS and 500 μL cell cycle detection working solution (Rnase A:PI = 1:9) was added. The distribution of various groups of cells in the cell cycle was detected after they had reacted for 30 min.
The Annexin V-APC/7-AAD double staining kit (KeyGEN BioTECH, China) was used to confirm apoptosis (early apoptosis and late apoptosis) in each group. Lentivirus-transfected AGS and HGC-27 cells were collected, and 500 μL Binding Buffer, 5 μL Annexin V-APC and 5 μL 7-AAD were added sequentially and gently mixed. Apoptosis was observed after the cells had reacted for 10 min.
The expression of related proteins in lentivirus-transfected AGS and HGC-27 cells was detected. Firstly, total protein was extracted with RIPA (Epizyme Biotech, China) from GC cells and their content was confirmed using the BCA assay (Epizyme Biotech, China). Next, the proteins were isolated and transferred onto a polyvinylidene fluoride membrane, which was sealed with 5% skim milk powder, soaked in primary antibody and incubated overnight. On the second day, it was washed with TBST and soaked in HRP-linked secondary antibody (1:1000, 7074/7076, CST, United States) for 1 h. Finally, protein bands were visualized with ECL reagent (KeyGEN BioTECH, China), and the gray values of the protein bands were analyzed using Image J. GAPDH or β-tubulin was used as an internal control to standardize target proteins. p-ERK1/2 (1:5000, 4370), ERK1/2 (1:5000, 9102), RIP1 (1:1000, 3493), p-RIP1 (1:1000, 65746), MLK1 (1:1000, 5029) and p-MLK1 (1:1000, 91689) antibodies were purchased from CST. BAX (1:2000, ab32503), Caspase-3 (1:1000, ab13847), BCL-2 (1:5000, ab182858) and KRAS (1:1000, ab275876) antibodies were purchased from Abcam.
The related genes expressed in each group of GC cells were determined using quantitative real-time polymerase chain reaction (qRT-PCR). Briefly, total RNA was extracted from GC cells using TRIzol reagent (Invitrogen, United States), then cDNA was synthesized (Takara Bio, Japan) and gene expression levels were measured (Takara Bio, Japan). The primer sequences are shown in Table 1. U6 or GAPDH was used as a housekeeping gene, and target genes were calculated using the 2-ΔΔCt method.
| Primer | Forward sequence (5’-3’) | Reverse sequence (5’-3’) |
| KRAS | TGTGGACGAATATGATCCAACA | GCAAATACACAAAGAAAGCCCT |
| ERK1 | ATGTCATCGGCATCCGAGAC | GGATCTGGTAGAGGAAGTAGCA |
| ERK2 | TACACCAACCTCTCGTACATCG | ATGTCTGAAGCGCAGTAAGATT |
| GAPDH | CACCCACTCCTCCACCTTTGA | TCTCTCTTCCTCTTGTGCTCTCTTGC |
| miR-204-3p | CAAGTCGCTGGGAAGGCAA | CAGTGCAGGGTCCGAGGT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
BABL/c nude mice (male, 4 wk, SPF) were provided by the Animal Laboratory Center of Ningxia Medical University. The animal protocol (IACUC-NYLAC-2022-251) was approved by the Institutional Animal Care and Use Committee of Ningxia Medical University. Following 7 d of adaptive feeding in BALB/c nude mice, lentivirus-transfected MKN-45 cells were cultured, and the cell concentration adjusted to 5 × 107 cells/mL. A suspension containing 100 μL cells was slowly subcutaneously injected into the back of nude mice, which were then returned to their cage for feeding. Growth of the subcutaneous tumor and mouse body weight were observed daily. The tumor volume (V) was calculated by V = (W2 × L)/2 (long diameter: L; short diameter: W). When the tumor on the back grew to an appropriate size and conformed to animal ethics, the animals were killed by CO2 and photographed.
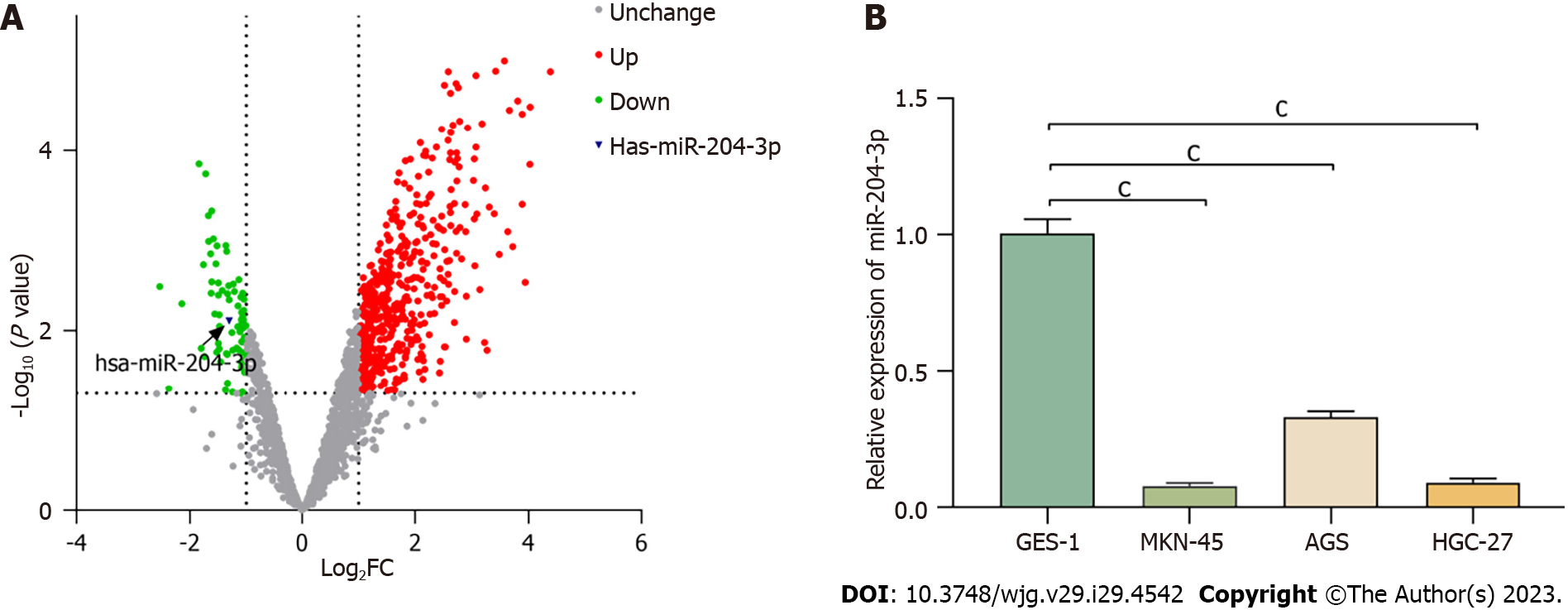
We used the GEO database (https://www.ncbi.nlm.nih.gov/geo/) to screen differentially expressed miRNAs. We searched the keywords " miRNAs" and "gastric carcinoma, and downloaded Differential expression microarrays of miRNAs (series: GES79973). Then adjusted for P < 0.05, |log2 (fold change)| > 1, and analyzed significant miRNAs.
The statistical methods used in this study were reviewed by Li-Qun Wang from the Department of Epidemiology, Department of Medical Statistics, Institute of Public Health and Management, Ningxia Medical University. All data represent the mean ± SD of at least three independent samples. Statistical analysis was conducted using SPSS 27.0 and GraphPad Prism 8.0. One-way analysis of variance was used to analyze the differences between groups. P < 0.05 indicated a statistically significant difference.
In our previous study, we analyzed 40 pairs of tissue samples and discovered that miR-204-3p expression was lower in GC tissues compared to normal tissues[20]. To further validate this finding, we utilized the GEO database to identify differentially expressed miRNAs in GC tissues and paracancerous tissues, which confirmed the downregulation of miR-204-3p in GC tissues (Figure 1A). Subsequently, we investigated miR-204-3p expression between GC cell lines (AGS, MKN-45 and HGC-27) and the normal gastric epithelial cell line GES-1. The results revealed that miR-204-3p was underexpressed in MKN-45, AGS, and HGC-27 cells compared to GES-1 cells (Figure 1B).
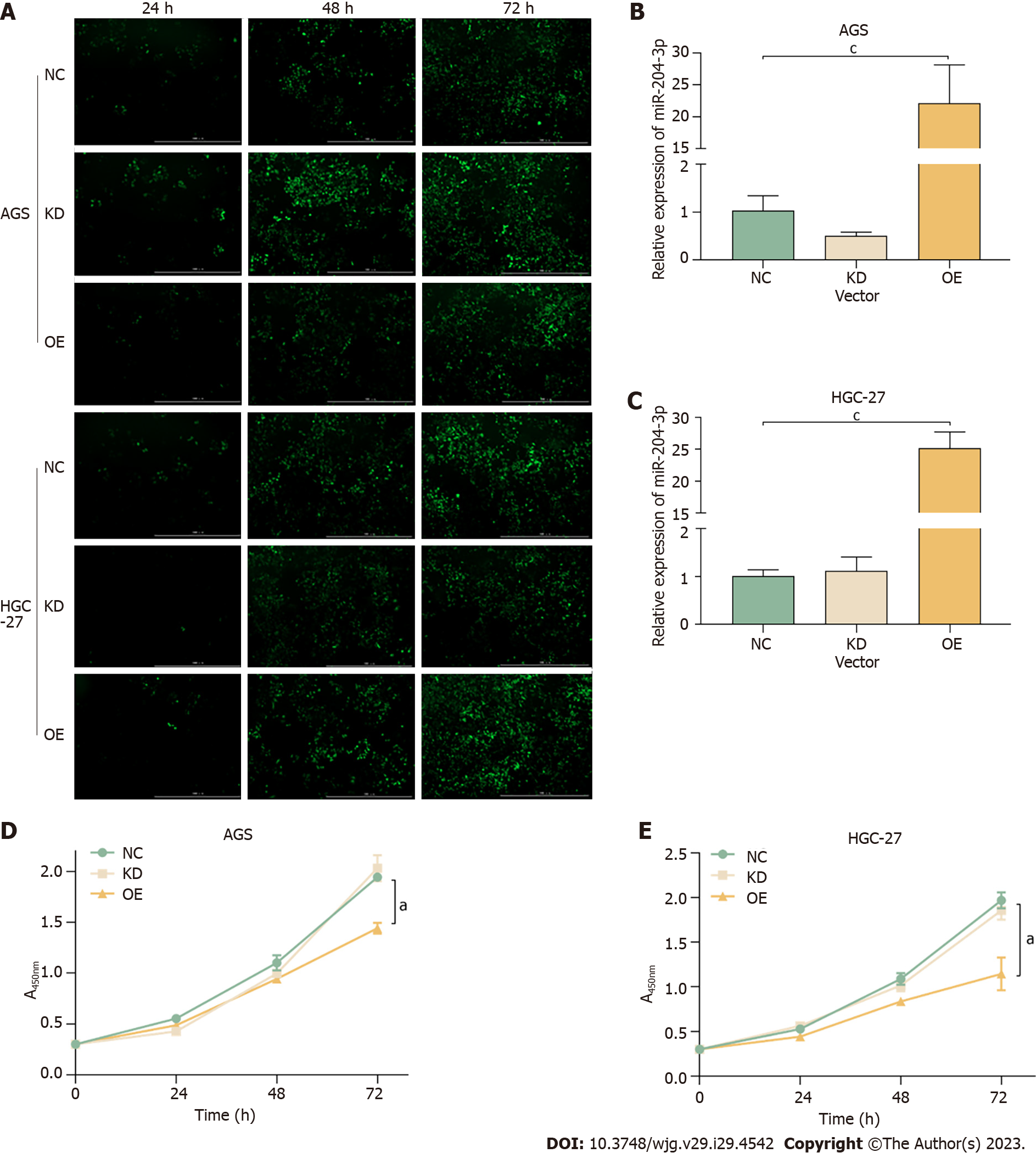
Lentivirus transfection caused miR-204-3p overexpression and miR-204-3p knockdown in AGS and HGC-27 cells. The transfection rate was found to be approximately 90% after 72 h, indicating high transfection efficiency (Figure 2A). The qRT-PCR results demonstrated that the miR-204-3p overexpression lentivirus significantly upregulated its expression compared to the NC group in AGS and HGC-27 cells, but the miR-204-3p knockdown lentivirus did not result in downregulation of its expression (Figure 2B and C). These findings indicated that miR-204-3p overexpression in GC cells was highly satisfactory, while miR-204-3p knockdown does not yield meaningful outcomes. Subsequently, the cell viability assay showed that miR-204-3p overexpression significantly inhibited GC cell viability (Figure 2D and E).
The impact of miR-204-3p on the colony forming ability of GC cells was evaluated. The crystal violet positive staining in the miR-204-3p overexpression group decreased (Figure 3A and B), and simultaneously, the number of clones formed in AGS and HGC-27 cells was distinctly reduced compared to the NC group (Figure 3C and D). This indicated that miR-204-3p overexpression inhibited the colony forming ability of GC cells.
We evaluated apoptosis by collecting cells from each group (Figure 4). The apoptosis rates of AGS cells were 2.53 ± 0.12%, 3.73 ± 0.83% and 10.6 ± 0.70% in the NC, KD and OE groups, respectively (Figure 4A and C). Among HGC-27 cells, the apoptosis rates were 9.47 ± 0.58%, 7.87 ± 1.63% and 18.40 ± 1.27% in the NC, KD and OE groups, respectively (Figure 4B and D). The findings revealed that HGC-27 and AGS cells with miR-204-3p overexpression had a notably higher apoptosis rate, which indicated that miR-204-3p overexpression stimulated GC cell apoptosis.
We analyzed cell cycle distribution to investigate whether miR-204-3p inhibited GC cell proliferation by mediating the cell cycle (Figure 5). Among AGS cells, the G0/G1 phase percentages were 44.91 ± 1.15%, 45.36 ± 0.70% and 49.30 ± 0.41% in the NC, KD and OE groups, respectively (Figure 5A and C). Among HGC-27 cells, the G0/G1 phase percentages were 29.36 ± 0.29%, 29.57 ± 1.11% and 41.03 ± 0.47% in the NC, KD and OE groups, respectively (Figure 5B and D). These results revealed that AGS and HGC-27 cells with miR-204-3p overexpression had a notably higher number of cells in G0/G1 phase, which indicated that miR-204-3p overexpression blocked GC cell cycle in the G0/G1 phase.
A subcutaneous tumor formation experiment was conducted in BABL/c nude mice using lentivirus-transfected MKN-45 cells to observe the changes in tumor size and body weight. The results revealed that the OE group had smaller tumors compared to the NC group (Figure 6A and B). According to tumor growth data, it was found that back tumor growth was notably slower in the OE group compared to the NC group (Figure 6C). Additionally, the weight of BABL/c nude mice in the OE group increased significantly (Figure 6D), which indicated that miR-204-3p overexpression suppressed subcutaneous tumorigenesis in BABL/c nude mice.
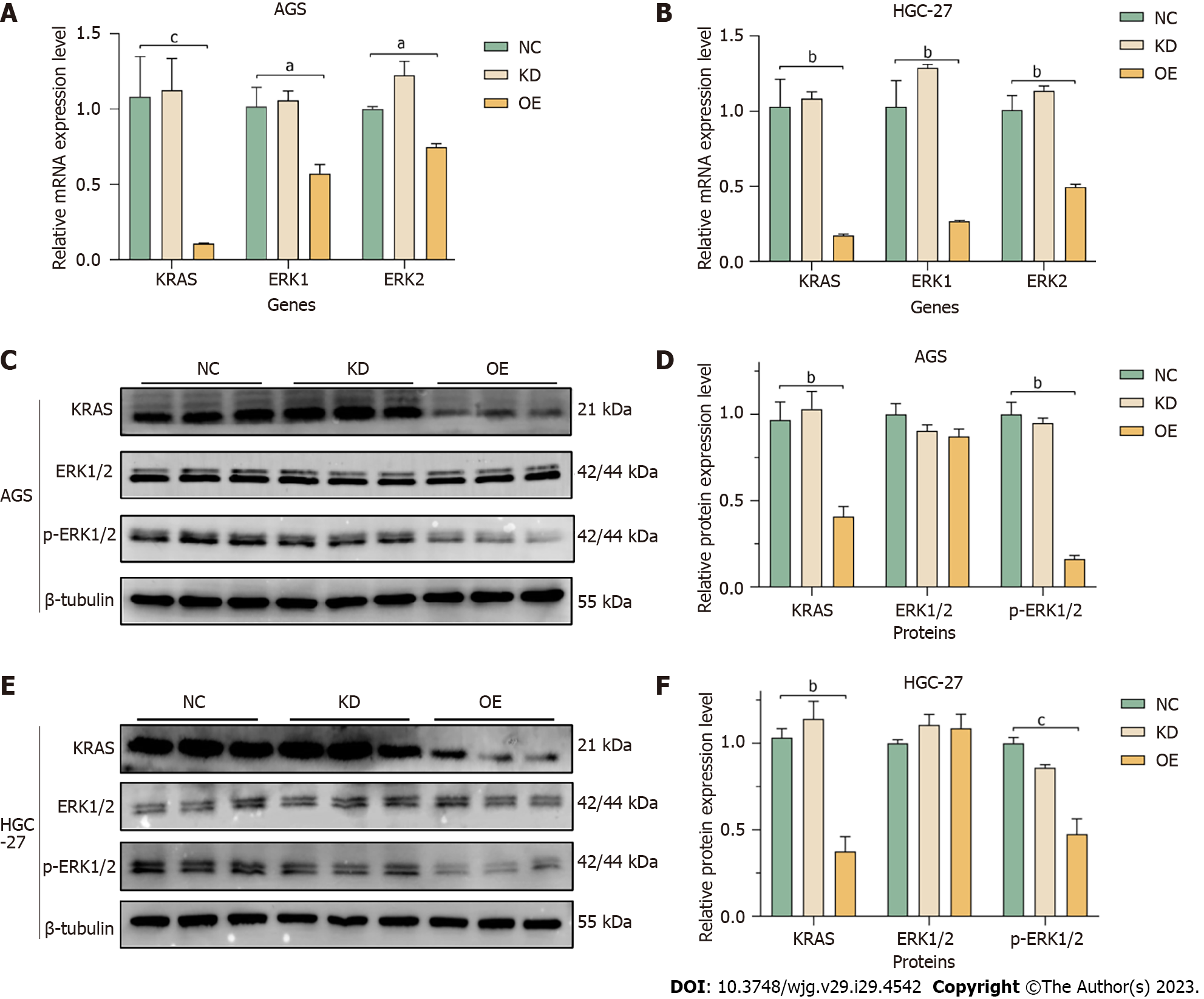
We investigated the effect of miR-204-3p on the MAPK signaling pathway. Firstly, the impact of miR-204-3p on the mRNA levels of KRAS, ERK1 and ERK2 was detected using qRT-PCR. The results revealed that miR-204-3p overexpression resulted in a significant decrease in KRAS, ERK1 and ERK2 in AGS and HGC-27 cells (Figure 7A and B). We further investigated the impact of miR-204-3p on the MAPK signaling pathway-related proteins, including KRAS, ERK1/2, and p-ERK1/2. The results showed that in HGC-27 and AGS cells, miR-204-3p overexpression caused noteworthy lower levels of KRAS and p-ERK1/2, but no significant difference was observed in ERK1/2 (Figure 7C-F). These findings indicated that miR-204-3p overexpression effectively inhibited GC cell proliferation, and this effect was achieved through the inhibition of KRAS, which subsequently prevented the phosphorylation of downstream effector protein ERK1/2 in the MAPK signaling pathway.
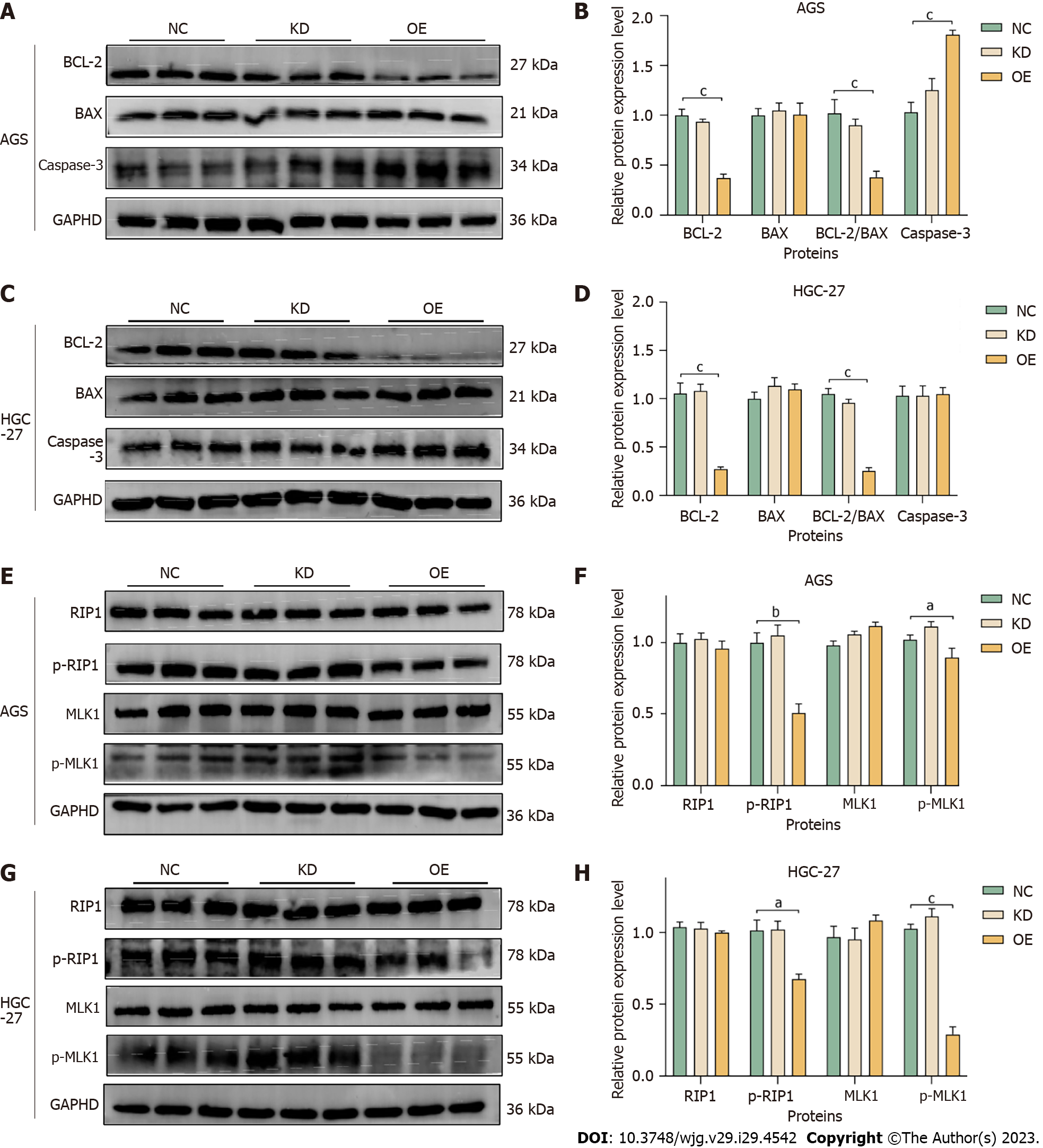
The effect of miR-204-3p on apoptosis-related proteins in GC cells was investigated. The results indicated a reduction in BCL-2 and the BCL-2/BAX ratio, as well as an increase in Caspase-3 in the OE group (Figure 8A-D), which confirmed that miR-204-3p overexpression stimulated GC cell apoptosis via the BCL-2/BAX signaling pathway.
We examined the protein changes in RIP1, p-RIP1, MLK1 and p-MLK1 during necroptosis and investigated the impact of miR-204-3p on GC cells necroptosis. The results revealed that miR-204-3p overexpression significantly inhibited the protein expression levels of p-RIP1 and p-MLK1 (Figure 8E-H), which confirmed that miR-204-3p overexpression inhibited GC cells necroptosis via the RIP1/MLK1 signaling pathway.
MiR-204-3p plays a crucial role in various diseases. Some studies have confirmed that miR-204-3p upregulation can be targeted to inhibit Nox4 to reduce memory deficits[21]. Moreover, its overexpression inhibited high glucose induced lens epithelial cells migration and epithelial-mesenchymal transition (EMT), and inhibited high glucose induced podocytes apoptosis and dysfunction[13,15]. Notably, miR-204-3p upregulation inhibited malignant melanoma migration, invasion and EMT progression by targeting inhibition of PAX2[19], its upregulation also inhibited hepatocellular carcinoma cell proliferation by targeting inhibition of FN1[22]. MiR-204-3p was found to be underexpressed in bladder cancer tissues and was related to poor prognosis, it regulated bladder cancer cell proliferation by targeting LDHA mediated glycolysis[16]. Apoptosis of glioma cells is induced by miR-204-3p targeting IGFBP2[23]. Furthermore, LINC00963 was overexpressed in osteosarcoma tissues and was related to poor prognosis, miR-204-3p reversed LINC00963 in promoted osteosarcoma cell proliferation and inhibited migration and invasion[24]. LINC00514 was upregulated in GC tissues, its overexpression stimulated GC cell growth and inhibited EMT by sponging miR-204-3p/KRAS[20]. These results suggest that miR-204-3p may be a new target for cancer therapy.
Firstly, we found that the expression of miR-204-3p was low in GC cells, and its overexpression resulted in the inhibition of cell proliferation, colony formation ability, and the cell cycle, while promoting apoptosis. In vivo tumor formation experiments in 4-week-old BABL/c nude mice verified that miR-204-3p overexpression inhibited subcutaneous tumor growth. Thus, both in vitro and in vivo experiments demonstrated the inhibitory influence of miR-204-3p overexpression on GC cells.
Apoptosis, a programmed cell death, is a natural barrier against tumorigenesis. However, in cancer, abnormal exp
In tumors, the MAPK pathway is frequently activated to control apoptosis, cell growth, and cell division[28]. Signal transmission of the MAPK signaling pathway follows a three-step enzyme-linked reaction. KRAS, as an upstream activation protein, is activated when bound to GTP. This change causes the recruitment of KRAS to RAF on the cell membrane and promotes RAF activation. Activated RAF phosphorylates and activates MEK, while MEK phosphorylates and further activates ERK, which is located at the end of the signaling pathway and can transfer into the nucleus and bind to transcription factors, thereby regulating transcription programs and mediating cell growth, migration and differentiation[29-31]. Previously, we established that miR-204-3p targeted KRAS[20]. The current study confirmed that miR-204-3p upregulation can inhibit KRAS and p-ERK1/2, which suggested that miR-204-3p overexpression could inhibit the MAPK signaling pathway.
Necroptosis is a newly discovered mechanism of programmed cell death that has the potential to regulate tumorigenesis[32]. This process is primarily regulated by three proteins: RIP1, RIP3 and MLK1. Specifically, RIP1 is activated through phosphorylation, which then recruits RIP3[33]. Once activated, phosphorylated RIP3 can oligomerize MLK1 and transfer it to the plasma membrane, ultimately resulting in necroptosis characterized by cell swelling and organelle damage[34,35]. Interestingly, necroptosis has been found to both promote and inhibit cancer growth. As a form of cell death, necroptosis inhibits the development of tumors, yet it may also incite an inflammatory reaction that encourages cancer metastasis and immunosuppression. Research has revealed that glioblastoma, pancreatic cancer, and lung cancer can be impacted by the upregulation of RIP1, RIP3, and MLK1[36-38]. It was found that downregulation of MLK1 inhibited tumor cell growth and increased sensitivity to radiotherapy in both GC and ovarian cancer[39,40]. We detected necroptosis-related proteins and discovered that miR-204-3p overexpression decreased p-RIP1 and p-MLK1. These findings suggest that miR-204-3p overexpression can inhibit necroptosis through the RIP1/MLK1 pathway, ultimately inhibiting GC cell proliferation.
To sum up, our study verified that miR-204-3p is underexpressed in GC, and that its overexpression inhibits GC cell proliferation, promotes apoptosis, arrests the cell cycle in the G0/G1 phase, inhibits cell colony formation and the formation of subcutaneous tumors. Necroptosis is typically initiated by tumor necrosis factor (TNF) stimulation[41]. RIP1 binds to FADD, which then recruits caspase-8. The activation of caspase-8 promotes the process of RIP1-dependent apoptosis[32]. Additionally, RIP1 activates ERK to regulate the MAPK signaling pathway[42-44]. In the MAPK signaling pathway, phosphorylation of ERK can activate BCL-2, which in turn stimulates the apoptosis pathway and accelerates the process of apoptosis[45,46]. We verified that miR-204-3p overexpression can inhibit GC cell proliferation by inhibiting the MAPK signaling pathway and inhibiting the RIP1/MLK1 necroptosis pathway to promote the BCL-2/BAX/Caspase-3 apoptosis pathway (Figure 9).
MiR-204-3p overexpression inhibited GC cell proliferation by inhibiting the MAPK pathway and necroptosis pathway to promote GC cell apoptosis. Thus, miR-204-3p may represent a new potential therapeutic target for GC.
Gastric carcinoma (GC) is a common gastrointestinal malignancy worldwide. Based on the cancer-related mortality, the current prevention and treatment strategies for GC still show poor clinical results. Therefore, it is important to find effective treatment targets.
At present, the main treatment for GC is surgery, chemotherapy and radiotherapy, but the therapeutic effect is not ideal.
To explore the effect of miR-204-3p on GC cells.
We determined the expression level of miR-204-3p in GC, and then used an miR-204-3p overexpression vector and an miR-204-3p knockdown vector in GC cells. The influence of miR-204-3p on the changes in cell phenotype and tumorigenicity in vivo was assessed. Furthermore, the effects of miR-204-3p on various proteins related to the MAPK signaling pathway, necroptosis signaling pathway and apoptosis signaling pathway in GC cells were investigated.
It was found that miR-204-3p was underexpressed in GC, and miR-204-3p overexpression inhibited GC cell viability, promoted cell apoptosis, blocked the cell cycle, inhibited colony formation ability and inhibited tumorigenicity in vivo. It was also verified that miR-204-3p overexpression can promote apoptosis by inhibiting the MAPK pathway and the necroptosis pathway, thus inhibiting GC cell proliferation.
MiR-204-3p overexpression inhibited GC cell proliferation by inhibiting the MAPK pathway and the necroptosis pathway to promote GC cell apoptosis.
MiR-204-3p can be used for targeted therapy of GC, and can also be used as a new biomarker for GC.
The authors would like to acknowledge Li-Qun Wang for statistical analysis assistance. Thanks to the Key Laboratory of Hui Ethnic Medicine Modernization of Ministry of Education for providing experimental equipment and space.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta S, Brazil; Wang L, China S-Editor: Fan JR L-Editor: Webster JR P-Editor: Yu HG
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2858] [Article Influence: 571.6] [Reference Citation Analysis (5)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64637] [Article Influence: 16159.3] [Reference Citation Analysis (176)] |
| 3. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (1)] |
| 4. | Sung JK. Diagnosis and management of gastric dysplasia. Korean J Intern Med. 2016;31:201-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 5. | Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 3074] [Article Influence: 512.3] [Reference Citation Analysis (0)] |
| 6. | Liu H, Xu J, Yao Q, Zhang Z, Guo Q, Lin J. Rab7 Is Associated with Poor Prognosis of Gastric Cancer and Promotes Proliferation, Invasion, and Migration of Gastric Cancer Cells. Med Sci Monit. 2020;26:e922217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 8. | Saliminejad K, Khorram Khorshid HR, Soleymani FS, Ghaffari SH. An overview of mi-croRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451-5465. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1335] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 9. | Matsuo M, Nakada C, Tsukamoto Y, Noguchi T, Uchida T, Hijiya N, Matsuura K, Moriyama M. MiR-29c is downregulated in gastric carcinomas and regulates cell proliferation by targeting RCC2. Mol Cancer. 2013;12:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Shin JY, Kim YI, Cho SJ, Lee MK, Kook MC, Lee JH, Lee SS, Ashktorab H, Smoot DT, Ryu KW, Kim YW, Choi IJ. MicroRNA 135a suppresses lymph node metastasis through down-regulation of ROCK1 in early gastric cancer. PLoS One. 2014;9:e85205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Yang M, Shan X, Zhou X, Qiu T, Zhu W, Ding Y, Shu Y, Liu P. miR-1271 regulates cisplatin resistance of human gastric cancer cell lines by targeting IGF1R, IRS1, mTOR, and BCL2. Anticancer Agents Med Chem. 2014;14:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Gao L, Zhao C, Li S, Dou Z, Wang Q, Liu J, Ren W, Zhi K. circ-PKD2 inhibits carcinogenesis via the miR-204-3p/APC2 axis in oral squamous cell carcinoma. Mol Carcinog. 2019;58:1783-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Han X, Li Q, Wang C, Li Y. MicroRNA-204-3p Attenuates High Glucose-Induced MPC5 Podocytes Apoptosis by Targeting Braykinin B2 Receptor. Exp Clin Endocrinol Diabetes. 2019;127:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Liu X, Guo JW, Lin XC, Tuo YH, Peng WL, He SY, Li ZQ, Ye YC, Yu J, Zhang FR, Ma MM, Shang JY, Lv XF, Zhou AD, Ouyang Y, Wang C, Pang RP, Sun JX, Ou JS, Zhou JG, Liang SJ. Macrophage NFATc3 prevents foam cell formation and atherosclerosis: evidence and mechanisms. Eur Heart J. 2021;42:4847-4861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Li X, Sun M, Cheng A, Zheng G. LncRNA GAS5 regulates migration and epithelial-to-mesenchymal transition in lens epithelial cells via the miR-204-3p/TGFBR1 axis. Lab Invest. 2022;102:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Guo J, Zhao P, Liu Z, Li Z, Yuan Y, Zhang X, Yu Z, Fang J, Xiao K. MiR-204-3p Inhibited the Proliferation of Bladder Cancer Cells via Modulating Lactate Dehydrogenase-Mediated Glycolysis. Front Oncol. 2019;9:1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Guo Q, Fan Y, Wang Q, Li B, Qiu W, Qi Y, Pan Z, Zhang S, Zhao S, Yang K, Xu H, Li M, Gao Z, Xu J, Wang H, Wang S, Tang Q, Qiu J, Guo X, Deng L, Zhang P, Zhao R, Xue H, Wang C, Li G. Glioblastoma upregulates SUMOylation of hnRNP A2/B1 to eliminate the tumor suppressor miR-204-3p, accelerating angiogenesis under hypoxia. Cell Death Dis. 2023;14:147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 18. | Ma H, Shi Q, Fang J, Wang R, Zhao J, Lin S, Dong J, Zhang Y, Shen X, Chen J, Zhong Q. Long non-coding RNA AFAP1-AS1 promotes thyroid cancer progression by sponging miR-204-3p and upregulating DUSP4. J Biochem. 2022;171:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Song J, Chen X, Zhang L, Song D, Xiong H. MicroRNA-204-3p modulates epithelial-mesenchymal transition by targeting paired box gene 2 in human melanoma A-375 cells. Transl Cancer Res. 2019;8:2032-2043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Yuan L, Li J, Yang Y, Chen Y, Bu Y, Ye M, Mao X, Ma T, Yu L, Nan Y. LINC00514 promotes gastric cancer cell growth and EMT progression via miR-204-3p/KRAS. Aging (Albany NY). 2021;13:12007-12015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Tao W, Yu L, Shu S, Liu Y, Zhuang Z, Xu S, Bao X, Gu Y, Cai F, Song W, Xu Y, Zhu X. miR-204-3p/Nox4 Mediates Memory Deficits in a Mouse Model of Alzheimer's Disease. Mol Ther. 2021;29:396-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Cui ZH, Shen SQ, Chen ZB, Hu C. Growth inhibition of hepatocellular carcinoma tumor endothelial cells by miR-204-3p and underlying mechanism. World J Gastroenterol. 2014;20:5493-5504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Chen PH, Chang CK, Shih CM, Cheng CH, Lin CW, Lee CC, Liu AJ, Ho KH, Chen KC. The miR-204-3p-targeted IGFBP2 pathway is involved in xanthohumol-induced glioma cell apoptotic death. Neuropharmacology. 2016;110:362-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Zhou Y, Yin L, Li H, Liu LH, Xiao T. The LncRNA LINC00963 facilitates osteosarcoma proliferation and invasion by suppressing miR-204-3p/FN1 axis. Cancer Biol Ther. 2019;20:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Holdenrieder S, Stieber P. Apoptotic markers in cancer. Clin Biochem. 2004;37:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8:603-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 1118] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 27. | Pan LL, Wang AY, Huang YQ, Luo Y, Ling M. Mangiferin induces apoptosis by regulating Bcl-2 and Bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac J Cancer Prev. 2014;15:7065-7068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Buchegger K, Silva R, López J, Ili C, Araya JC, Leal P, Brebi P, Riquelme I, Roa JC. The ERK/MAPK pathway is overexpressed and activated in gallbladder cancer. Pathol Res Pract. 2017;213:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 753] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 30. | Whelan JT, Hollis SE, Cha DS, Asch AS, Lee MH. Post-transcriptional regulation of the Ras-ERK/MAPK signaling pathway. J Cell Physiol. 2012;227:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 32. | Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X, Liu C. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 754] [Cited by in RCA: 735] [Article Influence: 122.5] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, Cai Q, Yang ZH, Huang D, Wu R, Han J. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 34. | Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1146] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 35. | Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1601] [Cited by in RCA: 2191] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 36. | Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, Torres-Hernandez A, Pergamo M, Ochi A, Zambirinis CP, Pansari M, Rendon M, Tippens D, Hundeyin M, Mani VR, Hajdu C, Engle D, Miller G. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature. 2016;532:245-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 37. | Wang Q, Chen W, Xu X, Li B, He W, Padilla MT, Jang JH, Nyunoya T, Amin S, Wang X, Lin Y. RIP1 potentiates BPDE-induced transformation in human bronchial epithelial cells through catalase-mediated suppression of excessive reactive oxygen species. Carcinogenesis. 2013;34:2119-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Park S, Hatanpaa KJ, Xie Y, Mickey BE, Madden CJ, Raisanen JM, Ramnarain DB, Xiao G, Saha D, Boothman DA, Zhao D, Bachoo RM, Pieper RO, Habib AA. The receptor interacting protein 1 inhibits p53 induction through NF-kappaB activation and confers a worse prognosis in glioblastoma. Cancer Res. 2009;69:2809-2816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | He L, Peng K, Liu Y, Xiong J, Zhu FF. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Onco Targets Ther. 2013;6:1539-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui W, Chuangqi C, Changjiang Q, Sile C, Yulong H, Shirong C. Prognostic value of mixed lineage kinase domain-like protein expression in the survival of patients with gastric caner. Tumour Biol. 2016;37:13679-13685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3:re4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 461] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 42. | Liu Y, Liu T, Lei T, Zhang D, Du S, Girani L, Qi D, Lin C, Tong R, Wang Y. RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review). Int J Mol Med. 2019;44:771-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Obitsu S, Sakata K, Teshima R, Kondo K. Eleostearic acid induces RIP1-mediated atypical apoptosis in a kinase-independent manner via ERK phosphorylation, ROS generation and mitochondrial dysfunction. Cell Death Dis. 2013;4:e674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Filipczak PT, Thomas C, Chen W, Salzman A, McDonald JD, Lin Y, Belinsky SA. TSC2 Deficiency Unmasks a Novel Necrosis Pathway That Is Suppressed by the RIP1/RIP3/MLKL Signaling Cascade. Cancer Res. 2016;76:7130-7139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Vitagliano O, Addeo R, D'Angelo V, Indolfi C, Indolfi P, Casale F. The Bcl-2/Bax and Ras/Raf/MEK/ERK signaling pathways: implications in pediatric leukemia pathogenesis and new prospects for therapeutic approaches. Expert Rev Hematol. 2013;6:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, Franklin R, McCubrey JA. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review). Int J Oncol. 2003;22:469-480. [PubMed] |