Published online Aug 7, 2023. doi: 10.3748/wjg.v29.i29.4481
Peer-review started: March 23, 2023
First decision: June 17, 2023
Revised: June 28, 2023
Accepted: July 17, 2023
Article in press: July 17, 2023
Published online: August 7, 2023
Processing time: 131 Days and 20.3 Hours
Tumor necrosis factor-α (TNF-α) antagonists, the first biologics approved for treating patients with inflammatory bowel disease (IBD), are effective for the induction and maintenance of remission and significantly improving prognosis. However, up to one-third of treated patients show primary nonresponse (PNR) to anti-TNF-α therapies, and 23%-50% of IBD patients experience loss of response (LOR) to these biologics during subsequent treatment. There is still no recognized predictor for evaluating the efficacy of anti-TNF drugs. This review summarizes the existing predictors of PNR and LOR to anti-TNF in IBD patients. Most predictors remain controversial, and only previous surgical history, disease mani
Core Tip: Tumor necrosis factor-α (TNF-α) antagonists play an essential role in the management of inflammatory bowel disease (IBD). However, a significant number of patients experience primary or secondary nonresponse to these drugs. Here, we summarize relevant predictors of anti-TNF nonresponse in IBD and discuss the next steps for treating patients with primary or secondary nonresponse to anti-TNF agents.
- Citation: Wang LF, Chen PR, He SK, Duan SH, Zhang Y. Predictors and optimal management of tumor necrosis factor antagonist nonresponse in inflammatory bowel disease: A literature review. World J Gastroenterol 2023; 29(29): 4481-4498
- URL: https://www.wjgnet.com/1007-9327/full/v29/i29/4481.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i29.4481
Inflammatory bowel disease (IBD), an immune-mediated inflammation of the gastrointestinal tract characterized by repeated remission and relapse, comprises Crohn's disease (CD) and ulcerative colitis (UC). Traditionally, IBD has been considered a disease of the Western world, but the newly industrialized countries of Asia, Africa, and South America are experiencing a rapid increase in incidence[1-3]; therefore, IBD has become a global disease[4,5].
IBD is a lifelong disease and is incurable. Currently, medical therapy for IBD mainly includes traditional therapeutics such as 5-aminosalicylates, thiopurines, and steroids, biologics such as antitumor necrosis factor (anti-TNF) therapy, vedolizumab and ustekinumab, and novel small-molecule drugs such as Janus kinase (JAK) inhibitors.
Anti-TNF therapies, the first biologics approved for the treatment of patients with IBD, are effective for the induction and maintenance of remission and significantly improve prognosis[6-8]. The development of anti-TNF therapies revolutionized the treatment of IBD and was a landmark event. Anti-TNF drugs are still the most commonly used biological agents in IBD at present[6]. Four TNF antagonists have been used in the treatment of IBD: infliximab, adalimumab, certolizumab, and golimumab[9]. However, up to one-third of treated patients show no primary response to anti-TNF-α therapies[10], and 23%-50% of IBD patients experience loss of response (LOR) to these biologics during subsequent treatment[11,12]. These patients not only fail to benefit from anti-TNF therapies but also suffer from the side effects of anti-TNF drugs, including increased susceptibility to infection, autoimmune diseases, and malignant tumors[13,14]. In addition, they face a serious financial burden. A retrospective study reported that direct healthcare expenditures increased significantly after the initiation of anti-TNF therapy and remained higher than preinitiation costs for up to 5 years[15].
Hence, it is important to assess the therapeutic response to anti-TNF agents in IBD before initiating treatment. In this review, we conducted a comprehensive search of studies to summarize relevant predictors of anti-TNF nonresponse in IBD and discuss the next steps of treatment for patients with primary or secondary nonresponse (SNR) to anti-TNF agents.
We conducted a search on PubMed and Web of Science. Keywords used include “inflammatory bowel disease”, “Crohn's disease”, “Ulcerative colitis”, “Tumor necrosis factor antagonists”, “anti-TNF”, “infliximab”, “adalimumab”, “certolizumab”, “golimumab”, “primary nonresponse”, “secondary nonresponse”, and “loss of response”. This review included articles, reviews and guidelines that investigated predictors of failure of TNF antagonists in IBD or optimized treatment (Supplementary Figure 1).
There is no consensus on the definition of primary nonresponse (PNR) in IBD patients as definitions vary across studies. Papamichael et al[11] defined PNR as a lack of objectively assessed improvement in baseline inflammatory signs after induction treatment in the presence of adequate concentrations of the drug and in the absence of antidrug antibodies (ADAs). In a cohort study, PNR was classified as treatment failure or use of corticosteroids (new prescription or previous dose not discontinued) or failure to reduce C-reactive protein (CRP) to 3 mg/L or less or to decrease by 50% or more from baseline and failure to decrease Harvey-Bradshaw Index score to 4 or less or by 3 or more from baseline before week 14[16]. In general, PNR refers to the absence of improvement in clinical symptoms or objective measures during the induction phase[17-19]. The incidence of PNR has been reported to range from 13%-40%[7,20,21].
SNR, also named LOR, describes the clinical phenomenon of patients who have an initial response to biologics but then subsequently lose this response[22]. Notably, the two features of the SNR are that the patient's symptoms improved during the initial treatment and that the recurrence of symptoms can only be due to the inflammatory response of IBD and not due to concurrent infection, fibrous stenosis, etc.[23]. SNR eventually occurs in 20%-50% of patients[12,24,25]. A recent meta-analysis found that the mean percentages of patients with SNR to infliximab, adalimumab, and certolizumab were 37.8%, 35.4%, and 43.3%, respectively[26].
Age: Real-world data suggest that elderly individuals with CD benefit less from infliximab and adalimumab at 12 wk[27]. In the precision-3 study, CD patients treated with certolizumab had a reduced probability of achieving a primary response as they aged[28]. However, several other studies have reported no correlation between age and PNR to anti-TNF in CD[21,29]. In UC patients, Arias et al[30] found that the benefit was greater when the baseline age was less than 40 years, whereas other studies did not show the impact of age on the efficacy[31,32]. Differences between the results may have originated from variations in designs and how outcomes were defined.
Gender: A single-center study in Britain involving CD patients reported that men were significantly less likely to PNR to infliximab[21]. Another Korean study showed that among CD patients, men benefited from clinical remission at week 14 more than women[29]. However, many researchers have not found an association between sex and PNR to anti-TNF therapy in CD[33-35]. Similarly, the influence of gender on anti-TNF therapy cannot be clearly defined in UC patients. Sandborn et al[36] reported that women responded better when assessing the efficacy of golimumab at week 6. Other studies did not report that sex could predict TNF antagonists response in UC[30,37].
Smoking: Smoking is an environmental risk factor for CD[38] and appears to be associated with nonresponse to anti-TNF therapy in CD patients. Analysis from the precision-3 study suggested that nonsmokers are more likely to achieve early clinical remission than smokers[28]. Zorzi et al[39] identified a positive association between smoking and anti-TNF nonresponse in CD patients by Cox proportional hazards regression. In addition, a meta-analysis published in 2021 revealed that when smoking status was defined smoking was significantly associated with a reduction in response to infliximab or adalimumab in patients with CD[40]. However, the negative effect of smoking on response was not found in another earlier meta-analysis[41]. Studies of UC have also reported inconsistent results. One Italian study found a significantly lower response to infliximab in ex-smokers[42], while others did not reach this conclusion[37,43]. The conflicting findings may be due to different definitions of smoking among the studies. In summary, smoking cessation is recommended for current smokers diagnosed with IBD[44].
Previous surgery: Although treatment strategies for IBD have changed, 17.4%-25% of patients with CD still require surgery[45-47]. Macaluso et al[27] used a logistic regression model to identify a history of previous surgery as an independent risk factor for PNR in CD patients. Another group reported similar results, showing that CD patients without previous surgery had a greater chance of achieving initial remission than patients with previous surgery, with a hazard ratio of 1.387[28]. CD patients with previous surgery had a lower response rate[48]. A study involving 201 CD patients also demonstrated that previous surgery was an independent predictor of PNR[34].
Disease duration: The analysis of pooled data from CD studies indicates that CD with a shorter disease duration is associated with a superior early response[49]. In the MODIFY study, patients who received early adalimumab achieved a higher clinical response and remission rate at week 26 than those who received delayed treatment[50]. This correlation has also been confirmed by a recent meta-analysis[51]. Studies have reported that among UC patients, a shorter disease duration is associated with a better response to anti-TNF drugs[32,52]. However, in general, authors did not find a positive association between long disease duration and anti-TNF nonresponse[31,48,53]. Although the current studies available cannot explain the underlying reasons for poorer response to anti-TNF in IBD with a longer disease duration, it is intuitive that a longer disease duration may contribute to the development of fibrosis, making earlier treatment attractive to patients[54].
Phenotype: The disease phenotype seems to be related to anti-TNF treatment response. In CD patients, isolated ileitis was inversely associated with the anti-TNF response, whereas the opposite was true for isolated colitis[29,48].
The pharmacokinetic (PK) of anti-TNF consists of four processes: absorption, distribution, metabolism, and elimination[55]. PK failures are characterized by undetectable or subtherapeutic drug concentrations associated with rapid nonimmune clearance or immunogenicity as well as the development of ADAs[56].
Drug concentration and antidrug antibodies: Several studies have demonstrated that subtherapeutic drug concentration is a predictor of PNR, with drug concentrations lower in IBD patients who failed to respond to anti-TNF therapy than in responders[16,57]. Post hoc analysis of data from the MUSIC trial data showed that CD patients with higher levels of certolizumab were more likely to achieve endoscopic response and remission at week 10[58]. Ding et al[17] suggested that low anti-TNF levels and the formation of ADAs could predict PNR in CD patients. The same results were reported in another study involving patients with UC[59].
Weight: Weight is a predictor of anti-TNF nonresponse. In a multicenter cohort study, high body mass index (BMI) at baseline in CD patients was associated with an increased risk of PNR[16]. Similar results were reported in another study[60]. In UC patients, Kurnool et al[61] reported that an increase in BMI had a negative impact on the response to anti-TNF drug therapy. The reason may be that, on the one hand, obesity induces a proinflammatory state[62], and on the other hand, the proteolytic clearance of immunoglobulins is usually related to weight, that is, the higher the weight is, the faster the clearance[63,64].
Serum albumin: Serum albumin levels predict the PK of anti-TNF therapy. A recent prospective study noted that low albumin levels at baseline in IBD patients predicted low infliximab concentrations at week 14[16]. Several other studies have reached similar conclusions[57,63]. One study of patients with UC found significantly higher serum albumin in responders than in primary nonresponders[65]. This effect occurs because albumin is the main transporter of drugs in blood, and serum albumin binds anti-TNF drugs to protect against degradation[66].
Fcγ receptor type IIIA: Single nucleotide substitutions within the Fcγ receptor type IIIA (FCGR3A) gene result in allelic variations, one valine (V) or one phenylalanine (F) at amino acid position 158. Functional polymorphisms in FCGR3A are significantly associated with response to anti-TNF therapy in CD patients[67]. Bek et al[67] used mono-compartmental population modeling to describe the PK of infliximab and found that the FCGR3A-158V/V genotype was associated with increased elimination of infliximab[67]. Further studies identified the FCGR3A VV phenotype as an independent predictor of ADAs generation and associated with a reduced clinical response in IBD patients at the end of induction[68].
Pharmacodynamic (PD) failure is associated with underlying non-TNF-driven inflammation characterized by no improvement in symptoms even at sufficient concentrations and without ADAs[56].
Pharmacokinetic/pharmacodynamic modeling: Kimura et al[69] developed Pharmacokinetic/pharmacodynamic (PK/PD) modeling to predict IBD response to infliximab during induction therapy. Another team of researchers in Japan developed a PK/PD model to calculate the Kanti-TNFα0/Kelse ratio to predict the PNR to TNF in IBD patients at the second administration[70]. The validity of this model remains to be tested in larger populations.
C-reactive protein: Several studies have investigated the relationship between CRP levels and anti-TNF responses. A multicenter retrospective study in Korea demonstrated that UC patients with CRP ≥ 3 mg/dL were more likely to achieve clinical remission at week 8[71]. This was also observed in CD treated with certolizumab[72]. However, opposite results were reported in another retrospective study of CD patients[73]. Presumably, high baseline CRP will exclude some patients with noninflammatory functional symptoms and predict a higher response, but it may also reflect a higher inflammatory load with increased drug loss[74].
Antineutrophil cytoplasmic antibody and anti-Saccharomycescerevisiae antibody: In a cohort study involving 90 UC patients, a greater proportion of antineutrophil cytoplasmic antibody (ANCA)-negative patients achieved clinical response during infliximab induction than ANCA-positive patients[75]. Another study in CD patients found that positive perinuclear ANCN (pANCA) is a predictor of anti-TNF nonresponse[76]. In a meta-analysis, pooled results showed that pANCA-negative patients with IBD had a nearly twofold higher response to anti-TNF therapy than pANCA -positive patients[77]. A single-center study evaluating pANCA and anti-Saccharomyces cerevisiae antibody (ASCA) simultaneously found that pANCA+/ASCA- serotypes significantly reduced early clinical response to infliximab in CD patients[78].
Fecal calprotectin: Fecal calprotectin (FC) is an indicator of gut inflammation and disease burden in IBD. Beltrán et al[79] noted that FC was higher in PNR patients with CD than in responders at weeks 0, 6, and 14, with a statistically significant difference only at week 0. Another study in UC patients also showed that early high FC was predictive of infliximab nonresponse[52]. Pavlidis et al[80] suggested that a decrease in FC of less than 70% after induction with anti-TNF drugs could predict PNR in patients with CD. However, some authors have not shown a relationship between FC and anti-TNF PNR in UC patients[81,82].
Fecal lactoferrin: Fecal lactoferrin (FL) can be used to monitor intestinal inflammation in IBD[83]. A retrospective study involving IBD demonstrated that dynamic monitoring of FL could distinguish responders from primary nonresponders, with two sustained drops in FL observed in responders during induction therapy[84].
TNF and TNF-receptor superfamily genes: Genetic polymorphisms associated with TNF and TNF receptors have been widely studied for their ability to predict the response to anti-TNF therapy. In a clinical trial studying CD, patients homozygous for the TNF/Lymphotoxin alpha (LTA) polymorphism, the LTA NcoI-TNFc-aa13L-aa26 haplotype 1-1-1-1, showed early nonresponse to infliximab[76]. Another study demonstrated that TNF-308 (rs1800629) was associated with response to anti-TNF therapy, and the presence of the minor allele (A) was associated with increased odds of nonresponse to anti-TNF therapy in IBD[85]. For TNF-receptor superfamilies (TNFRSF), Steenholdt et al[86] found that CD patients carrying the TNFRSF1B minor allele rs1061622 had a better response to infliximab induction therapy. In a Japanese study, TNFRSF1A (rs767455_G) and TNFRSF1B (rs1061624_A-rs3397_T) were associated with poor response in CD patients[87] and these results were replicated in another Spanish study[88]. Additionally, a meta-analysis indicated that TNFRSF1A (rs4149570) significantly improved anti-TNF responses in IBD[67].
Autophagy-related 16 like 1: Autophagy-related 16 like 1 (ATG16L1) is a risk factor for CD[89]. Koder et al[90] found a strong association between ATG16L1 (rs10210302) and response to adalimumab treatment in the CD population, with the TT genotype showing a better response after 12 wk of adalimumab treatment. Future studies on the relationship between ATG16L1 and the anti-TNF response are necessary to clarify these effects.
Apoptosis genes: Infliximab and adalimumab induce apoptosis by binding to membrane-bound TNF-α, which is the main mechanism of their efficacy[54]. An earlier study observed the strongest association between the Fas ligand -843 TT genotype and nonresponse to infliximab in CD patients[91]. Furthermore, Hlavaty et al[92] developed a novel apoptotic pharmacogenetic index based on three single nucleotide polymorphisms (Fas ligand-843 C/T, Fas-670 G/A, and Caspase9 93 C/T), with a higher score indicating a better response to anti-TNF therapy.
Nucleotide-binding oligomerization domain 2: Nucleotide-binding oligomerization domain 2 (NOD2) mutations predict an increased risk of complications related to CD[93]. Further studies showed that NOD2 mutations were less responsive to anti-TNF therapy than wild-type NOD2 in CD patients[94]. Another study demonstrated that CD patients with either NOD2 variants alone or in combination with ATG16L1 variants were more likely to receive intensive biologic therapy, which may indicate that NOD2 variants have a negative impact on response to biologic therapy[95]. However, this effect was not observed in another trial[96].
Interleukin: One study conducted in CD patients found that primary nonresponders had significantly higher interleukin-8 (IL-8) concentrations at baseline[97]. In addition, the level of IL-6 in responders was significantly lower than that in primary nonresponders at week 2 and week 6[97]. Another study of CD noted that IL17A and IL1B expression was significantly upregulated in anti-TNF refractory patients during anti-TNF therapy[98]. Oncostatin M (OSM), a member of the IL-6 cytokine family, has been shown to disrupt epithelial barrier function and drive intestinal inflammation[99]. An analysis of more than 200 IBD patients treated with anti-TNF therapy found higher baseline levels of OSM expression in those who failed anti-TNF therapy[100]. A cross-sectional study suggested that higher levels of OSM in the colon were associated with PNR to anti-TNF in patients with IBD[101].
Triggering receptor expressed on myeloid cells 1: Triggering receptor expressed on myeloid cells 1 (TREM1) expression has been proposed as a potential marker for predicting response to anti-TNF therapy in IBD patients. Gaujoux et al[102] demonstrated that TREM1 can be an ex-ante predictor of the anti-TNF response and that TREM1 Levels were downregulated in nonresponders, with a prediction accuracy of 94%. This phenomenon is also found in the inflamed mucosa.
Several studies have shown that gut microbes predict nonresponse to anti-TNF treatment in IBD. Magnusson et al[103] found that responders had lower dysbiosis indexes, a higher abundance of Faecalibacterium prausnitzii (F. prausnitzii) at baseline, and an increase in the abundance of F. prausnitzii during induction therapy compared with nonresponders. Another study found that high abundances of the genera Blautia, Faecalibacterium, Roseburia, and Negativibacillus at baseline were inversely associated with responsiveness to infliximab in CD[104]. In the same study, a high abundance of Sutterella, Roseburia, and Intenstinibacter appeared to predict response to infliximab in UC patients[104]. Alatawi et al[105] detected a reduction in the abundance of short-chain fatty acid-producing bacteria, including Anaerostipes, Coprococcus, Lachnospira, Roseburia, and Ruminococcus, in IBD patients unresponsive to anti-TNF therapy[105]. Nevertheless, a European multicenter study found no differences in the microbiota of anti-TNF therapy responders vs nonresponders in IBD[106]. Indicators that predict PNR to anti-TNF agents in patients with IBD are listed in Table 1.
| Predictor | Crohn’s disease | Ulcerative colitis |
| Clinical features | ||
| Age | Yes: Older[27,28]; No[21,29] | Yes: Older[30]; No[31,32] |
| Gender | Yes: Male[21], female[29]; No[33-35] | Yes: Male[36]; No[30,37] |
| Smoking | Yes: Smoker[28,39,40]; No[41] | Yes: Ex-smoker[42]; No[37,43] |
| Previous surgery | Yes[27,28,34,48] | |
| Disease duration | Yes: Longer[49-51]; No[48] | Yes: Longer[32,52]; No[30,53] |
| Phenotype | Yes: Isolated ileitis[29,48] | |
| Pharmacokinetic | ||
| Drug concentration | Yes: Low[16,17,57,58] | Yes: Low[57,59] |
| Antidrug antibodies | Yes[17] | Yes[59] |
| Weight | Yes: High[16,60] | Yes: High[61] |
| Serum albumin | Yes: Low[57] | Yes: Low[57,65] |
| FCGR3A | Yes: FCGR3A-158V/V[67,68] | Yes: FCGR3A-158V/V[68] |
| Pharmacodynamic | ||
| PK/PD model | Yes[69,70] | Yes[69,70] |
| Biologic markers | ||
| CRP | Yes: Low[72], High[73] | Yes: Low[71] |
| ANCA and ASCA | Yes: pANCA+[76,77] | Yes: ANCA+[75], pANCA+[77], pANCA+/ASCA-[78] |
| Fecal calprotectin | Yes: High[79] | Yes: High[52]; No[81,82] |
| Fecal lactoferrin | Yes: High[84] | Yes: High[84] |
| Genetic markers | ||
| TNF genes | Yes: Lymphotoxin alpha NcoI-TNFc-aa13L-aa26 haplotype 1-1-1-1[76], TNF-308A[85] | Yes: TNF-308A[85] |
| TNFRSF | Yes: TNFRSF1A (rs767455_G)[87], TNFRSF1B (rs1061624_A-rs3397_T)[87] | |
| ATG16L1 | Yes: ATG16L1 (rs10210302_CC)[90] | |
| Apoptosis genes | Yes: Fas ligand-843 TT genotype[91] | |
| NOD2 | Yes: NOD2 mutation[94,95] | |
| Cytokines | ||
| Interleukin | Yes: IL-8 (high)[97], IL-6 (low)[97], IL17A (high)[98], IL1B (high)[98], OSM (high)[100,101] | Yes: OSM (high)[100,101] |
| TREM1 | Yes: Low[102] | Yes: Low[102] |
| Gut microbes | Yes: Abundance of short-chain fatty acid-producing bacteria (decreased)[105] | Yes: Dysbiosis indexes (high)[103]; Abundance of short-chain fatty acid-producing bacteria(decreased)[105] |
Gender: A retrospective study identified that women were more likely to develop SNR to anti-TNF[107]. Another multicenter retrospective study found a similar result in the CD subgroup[108]. An earlier systematic review noted the male sex was a predictor of LOR in CD[109]. A single-center study demonstrated a significantly higher likelihood of SNR in men with UC[110]. However, no association has been reported between gender and SNR in most studies[21,30,31,37].
Smoking: Sandborn et al[28] found that current smoking was associated with LOR in individuals diagnosed with CD. This result was also validated in another single-center study[39]. Chaparro et al[111] reported that smoking was associated with the occurrence of LOR in CD.
Previous surgery: A Sicilian study of CD reported that previous surgery was associated with a low rate of clinical remission at 1 year[27]. However, many studies have not demonstrated a relationship between previous surgical history and SNR to anti-TNF therapy in CD[16,112].
Disease duration: Panaccione et al[49] showed that patients with CD whose duration was less than 1 year benefited more in maintaining remission. A retrospective cohort study reported that CD patients with a disease duration of more than 2 years had a significantly higher rate of SNR[113]. A subgroup analysis of the placebo-controlled CHARM trial also obtained a similar conclusion[114].
Phenotype: A recent study reported that accumulation of the upper digestive tract and the presence of fistulas at baseline were associated with SNR to adalimumab and infliximab in CD patients[27]. Another study involving 93 individuals verified that nonstructuring nonpenetrating CD was associated with sustained remission[39]. CD with concurrent fistula or stenosis had a lower clinical remission rate[115].
Drug concentration and antidrug antibodies: A multicenter cohort study confirmed that concentrations of infliximab < 7 mg/L and adalimumab < 12 mg/L were independently associated with SNR in CD patients[16]. A prospective study indicated that the trough level (TL) of infliximab < 5.5 µg/mL in patients with IBD was the best threshold to predict LOR[116]. Alternatively, the generation of ADAs, which in combination with circulating drugs also leads to a reduction in drug concentration, is associated with anti-TNF LOR in IBD[117].
Weight: Kennedy et al[16] found that obesity at baseline was associated with adalimumab treatment failure at week 54 in patients with CD. Another study also reported that IBD patients with a high BMI displayed a high rate of LOR[118]. In IBD patients treated with adalimumab, SNR was increased in those with BMI ≥ 30 kg/m2 compared with those with BMI < 30 kg/m2[119].
Serum albumin: In CD patients treated with certolizumab, low albumin predicted SNR[28]. Higher albumin levels were associated with lower LOR in IBD patients treated with infliximab[119]. A prospective study found that IBD patients with low albumin serum concentrations at baseline had a significantly increased risk for SNR to anti-TNF and that normalization of albumin levels during treatment did not reduce this risk[120].
Serum γ-globulin: A German study from IBD found a positive association between elevated serum γ-globulin concentrations and the risk of SNR to anti-TNF therapy[120]. Higher γ-globulin concentrations imply increased B-cell activity, resulting in increased ADAs production[120].
Matrix metalloproteinase 3: Matrix metalloproteinase 3 (MMP3) expression is significantly upregulated in inflamed colonic segments of IBD patients, suggesting the possible involvement of this enzyme in the inflammatory process[121,122]. A retrospective study from Italy showed that in IBD patients, MMP3 levels were significantly lower in responders (11.48 ng/mL) than in nonresponders (25.96 ng/mL) at week 52[123]. In the same study, MMP3 levels tended to be higher in patients without ADAs than in those with ADAs[123]. According to a previous report, MMP3 cleaved infliximab and adalimumab which may result in reduced drug efficacy[124].
Fcγ receptor type IIIA: A Spanish team found higher serum concentration levels of both infliximab and adalimumab in FCGR3A FF carriers than in FCGR3A VV carriers during maintenance therapy in IBD and found that the proportion of VV patients who developed ADAs was significantly higher than that of FF patients diagnosed with IBD[125].
Human leukocyte antigen: The value of human leukocyte antigen-DQA1*05 (HLA-DQA1*05) in predicting anti-TNF-ADAs production has been reported in several studies. A genome-wide analysis of 1240 subjects in the PANTS cohort revealed that approximately 40% of Europeans carried HLA-DQA1*05 and significantly increased rates of ADAs production[126]. Wilson et al[127], using genotypic analysis, showed that HLADQA1*05 was independently associated with LOR to infliximab and increased ADAs in IBD.
C-reactive protein: Post hoc analysis of ACCENT I, indicated that high levels of CRP before treatment predicted an increased likelihood of maintaining remission[128]. A study of IBD found that CRP > 5 mg/L was an independent predictor of SNR[116]. However, a Hungarian study reported that low levels of CRP at week 12 were associated with clinical remission at week 52 in CD patients on adalimumab[129]. Additionally, Angelison et al[82] did not find an association between CRP and SNR to anti-TNF agents in UC.
Antinuclear antibody: Among patients with IBD, those with positive antinuclear antibody (ANA) at baseline had higher odds of LOR to anti-TNF[130]. More studies are needed to investigate the relationship between ANA and response to anti-TNF therapy in the future.
Fecal calprotectin: Analyses from the 7-year PRECiSE 3 study revealed that an increase in FC implies an increased risk of LOR to anti-TNF[28]. However, Deshpande et al[131] reported that FC levels at week 14 could not predict the recurrence of CD one year later. Differences in the timing of FC measurement and sample size may have contributed to this discrepancy.
Fecal lactoferrin: Sorrentino et al[132] found that FL levels before and after anti-TNF treatment could be used to distinguish responders, partial responders, and nonresponders in IBD patients with suspected LOR[132]. In the same study, they proposed that responders had normal FL both before and after administration, partial responders had elevated FL before administration, partial FL decreased after administration but remained well above the normal threshold, and FL increased after LOR administration[132].
TNF and TNF-receptor superfamily genes: Currently, only a retrospective cohort study of CD has demonstrated that carrying the TNFRSF1B minor allele rs976881 was associated with LOR to infliximab[86]. More studies are urgently needed to explore the relationship between TNF and TNFRSF genes and SNR to anti-TNF therapy.
Interleukin: Higher baseline OSM in IBD patients with SNR to infliximab was found in a UK study[100]. Bertani et al[133] demonstrated that in CD patients treated with infliximab, those with low OSM levels at baseline and week 14 were more likely to achieve clinical remission at week 54[133]. Moreover, the level of OSM in patients with mucosal healing was significantly lower than that in patients without mucosal healing at week 54[133]. We summarize the predictors of SNR in Table 2.
| Predictor | Crohn’s disease | Ulcerative colitis |
| Clinical features | ||
| Gender | Yes: Female[107,108], male[109]; No[21] | Yes: Female[107], male[110]; No[31,37] |
| Smoking | Yes: Smoker[28,39,111]; No[41] | |
| Previous surgery | Yes[27]; No[16,112] | |
| Disease duration | Yes: Longer[49,113,114] | |
| Phenotype | Yes: Upper digestive tract[27], fistula[27,115], stenosis[115] | |
| Pharmacokinetic | ||
| Drug concentration | Yes: Low[16,116] | Yes: Low[116] |
| Antidrug antibodies | Yes[117] | Yes[117] |
| Weight | Yes: High[16,118,119] | Yes: High[118,119] |
| Serum albumin | Yes: Low[28,119,120] | Yes: Low[119,120] |
| Serum γ-globulin | Yes: High[120] | Yes: High[120] |
| MMP3 | Yes: High[123] | Yes: High[123] |
| FCGR3A | Yes: FCGR3A VV[125] | Yes: FCGR3A VV[125] |
| HLA | Yes: HLADQA1*05[127] | Yes: HLADQA1*05[127] |
| Biologic markers | ||
| CRP | Yes: Low[128], high[129] | No[82] |
| ANA | Yes: ANA+ [130] | Yes: ANA[130] |
| Fecal calprotectin | Yes: High[28]; No[131] | |
| Fecal lactoferrin | Yes: High[132] | Yes: High[132] |
| Genetic markers | ||
| TNFRSF | Yes: TNFRSF1B (rs976881)[86] | |
| Cytokines | ||
| Interleukin | Yes: OSM (high)[100,133] | Yes: OSM (high)[100] |
PNR or SNR to anti-TNF therapy was determined according to clinical symptoms, laboratory tests, endoscopy, imaging examinations, etc. It is worth noting that conditions such as poor adherence[134], improper drug storage medication storage[135], and co-infection[23] need to be excluded during assessment.
The British Society of Gastroenterology consensus defines therapeutic drug monitoring (TDM) as, the measurement of the drug (± ADAs) levels to assess compliance, drug metabolism, and immunogenicity with a view to guide dose adjustments or switch off therapy[136]. TDM can be used reactively or proactively. The American Gastroenterological Association recommends reactive TDM for adults who fail to respond to anti-TNF therapy[9]. A target TL of at least 5 μg/mL, 7.5 μg/mL, and 20.0 μg/mL for infliximab, adalimumab, and certolizumab, respectively, is suggested[9]. Papamichael et al[137] recommend a minimum drug concentration of at least 2.5 μg/mL at week 6 and a trough concentration of at least 1 μg/mL of golimumab during maintenance therapy. Several recent reviews showed that TDM was more beneficial than empirical strategies in terms of cost-effectiveness[138-140]. TDM plays an important role in optimizing anti-TNF therapy.
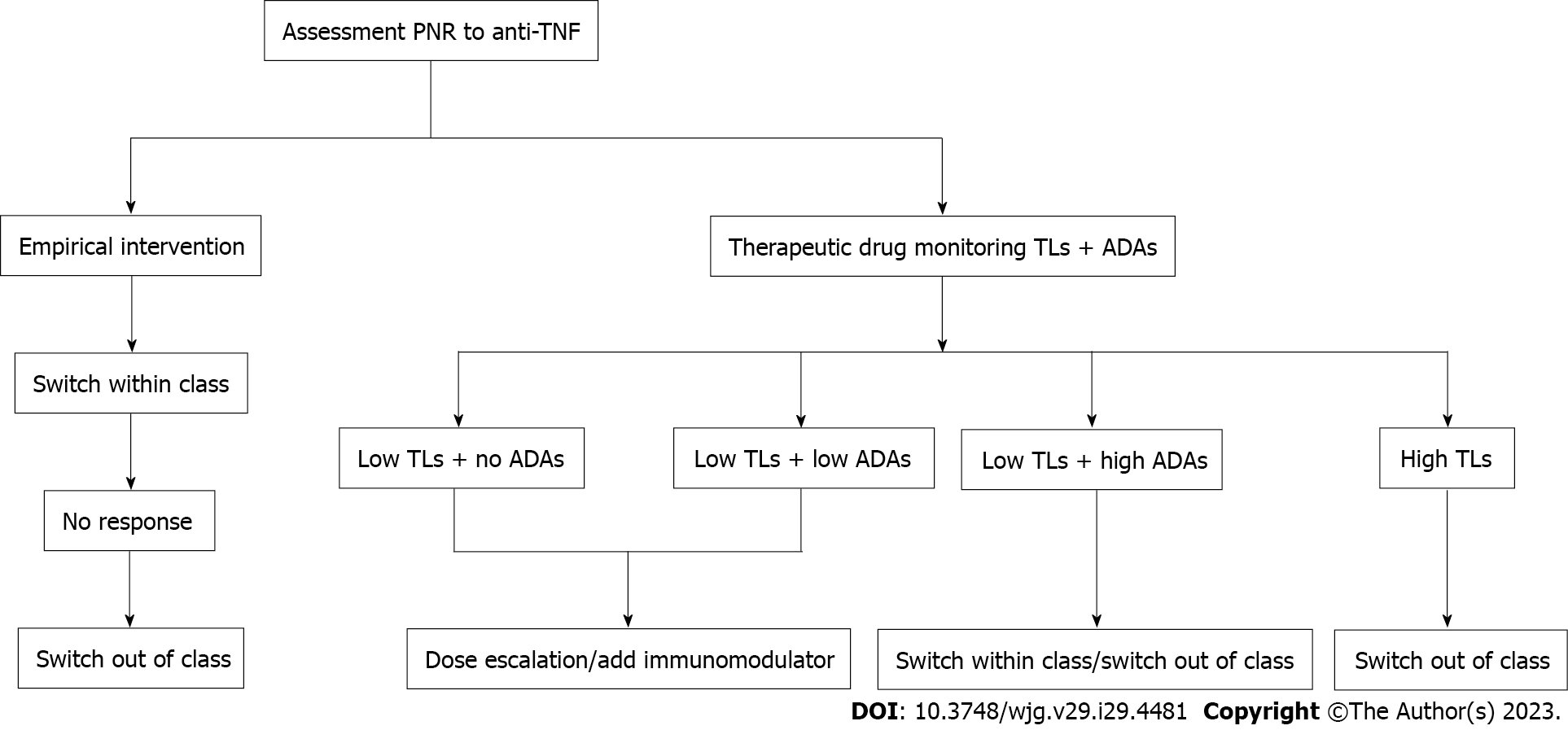
There is no consensus on the optimal management of PNR to TNF antagonists. A review proposed that the management of IBD patients with PNR to anti-TNF therapy consists of three major steps: prediction, prevention, and therapeutic intervention[11]. Clinical features, pharmacokinetics, genetic phenotypes, etc., can predict the development of PNR. Preventive measures to avoid PNR to anti-TNF include counseling patients to quit smoking, weight intervention, etc.[11,17]. For IBD patients with PNR, empirical intervention can be performed, switching to another TNF antagonist, or switching to a biological agent of a different mechanism, is desirable[141]. Ding et al[17] suggested that a second TNF antagonist be administered when the patient is PNR to the first TNF antagonist. If the treatment fails again, switching out of class should be considered.
Some scholars have also proposed that the medication of primary nonresponders can be adjusted according to TDM. With the help of TDM, rational and optimal treatment can be provided[136]. If patients have low TLs and no or low titer ADAs formation, dose optimization or the addition of an immunomodulator is recommended. When TLs are low and high-titer ADAs are detected, switching to another TNF antagonist or biologic agent with a different mechanism may be considered. For patients with therapeutic concentrations, switching out of class is suggested (Figure 1).
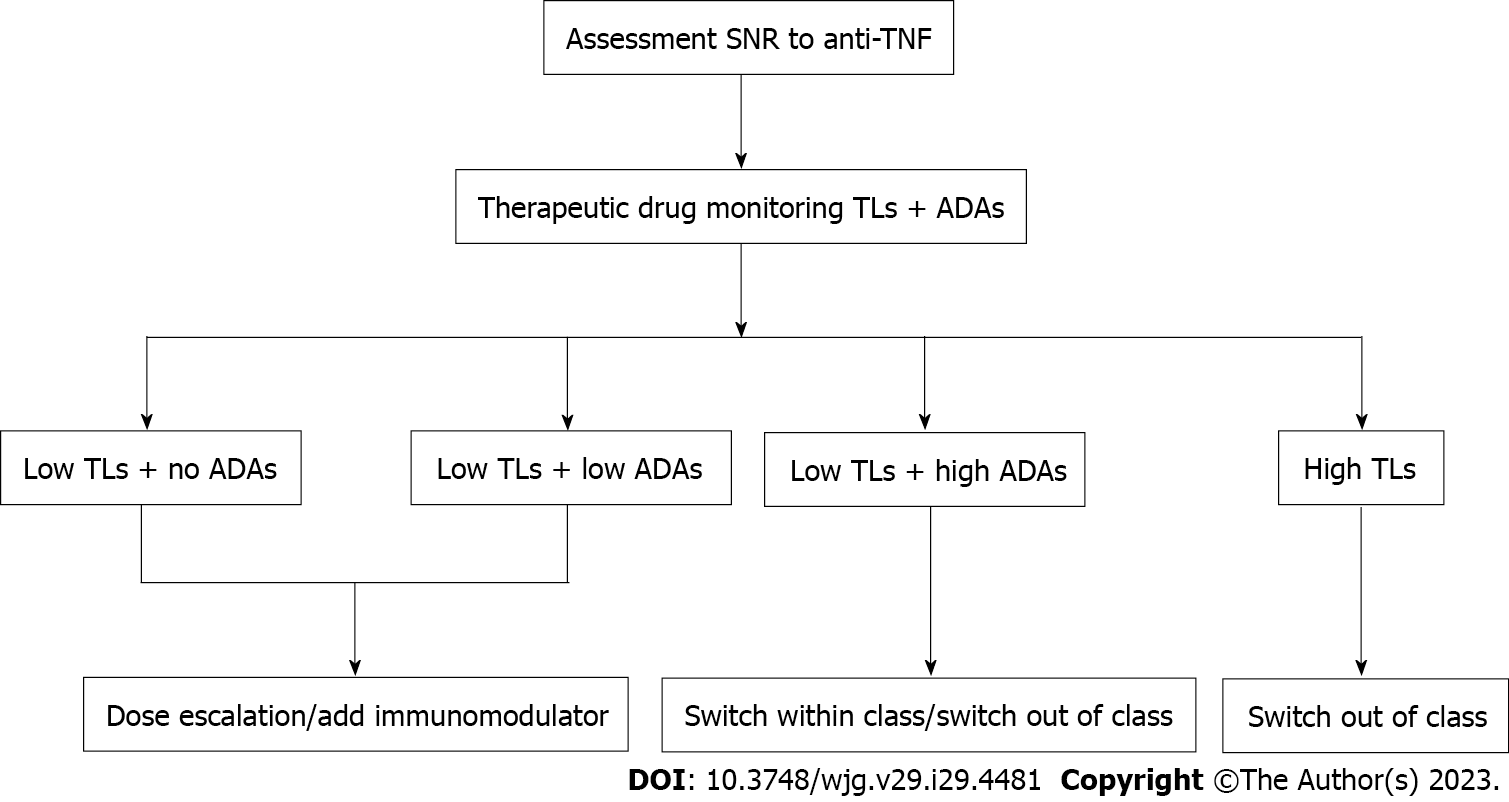
The detection of TNF antagonist and ADAs concentration is helpful to guide the next treatment of SNR (Figure 2).
Dose escalation: Dose intensification can reverse nonresponse to anti-TNF in IBD patients with subtherapeutic concentrations and no or low concentrations of ADAs. A meta-analysis reported a 34% need for anti-TNF dose escalation in CD at a median follow-up of 1 year, with pooled rates of 38%, 32%, and 2% for infliximab, adalimumab, and certolizumab, respectively[26]. A multicenter cohort study in Belgium found that 34% of CD patients treated with adalimumab required an increased dose to maintain clinical response, and clinical response was induced again in 67% of these patients[142]. Billioud et al[109] concluded that among CD patients who experienced LOR to adalimumab, 71.4% regained response and 39.9% achieved remission after dose optimization. Interestingly, a post hoc analysis of the TAXIT trial showed a significantly higher rate of clinical response with dose intensification, regardless of the presence of ADAs[143]. Meanwhile, Bodini et al[144] have suggested that, based on clinical need, anti-TNF doses can be increased, even in older patients of patients receiving combination therapy, with little risk of adverse reactions occurring.
Addition of an immunomodulator: The addition of an immunomodulator is a good option for IBD patients receiving anti-TNF therapy in whom subtherapeutic and no or low concentrations of ADAs are detected. For example, van Schaik et al[145] observed a significant increase in mean trough concentrations and a significant decrease in the incidence of ADAs in the infliximab combined with azathioprine group compared with infliximab alone in patients with IBD, whereas no differences were observed in the adalimumab combination vs monotherapy groups[145]. Another study involving patients with CD reported that, for both infliximab and adalimumab, combined immunomodulators reduced the risk of ADAs formation[16]. In the SONIC trial, the response rate in corticosteroid-free clinical remission at week 50 was significantly higher with infliximab adding immunomodulator than with monotherapy (55.6% vs 39.6%)[146]. In the UC-SUCCESS trial, infliximab plus an immunomodulator was also superior in achieving corticosteroid-free clinical remission[147]. In a 2-year cohort study of 46 patients with IBD, the addition of a low-dose immunomodulator, either azathioprine, methotrexate, or mycophenolate mofetil, reversed clinical response in approximately 50% of IBD patients who had failed to respond to anti-TNF monotherapy[148]. With regard to when to discontinue immunomodulators, Drobne et al[149] suggest that at least 6 mo of combination therapy is required. Mahmoud et al[150] compared different durations of combination therapy in relation to LOR and found no significant difference between durations of combination therapy (< 0.5 years, 0.5-1 year, 1-2 years, and > 2 years); however, durations of combination therapy longer than 2 years were associated with a lower risk of ADAs formation.
Switch within class: In the case of subtherapeutic concentrations with high titers of ADAs, switching within class to another anti-TNF agent should be considered. A retrospective study of IBD showed that when ADA titers of infliximab and adalimumab were > 9 μg/mL and 4 μg/mL, respectively, switching within class achieved a longer duration of response compared with dose intensification[151]. In another study of IBD, switching patients positive for ADAs to another anti-TNF agent achieved a response rate of 92%, whereas dose optimization achieved a response rate of 17%[152]. In cases where the first anti-TNF drug failed, switching to another drug achieved remission in approximately 50% of patients, an effect that has been reported in several other studies[153,154]. Moreover, a systematic review reported that switching to a second anti-TNF agent led to successful induction of remission in 46% of patients with IBD who had failed the first anti-TNF agent[155]. Of note, the previous generation of anti-TNF antibodies increases the risk of the generation of a second anti-TNF antibody in IBD[156]. Therefore, when switching to another anti-TNF agent, a combination of immunosuppressive agents is appropriate[136,157].
Switch out of class: If TL is sufficient with high ADAs, it is recommended that the patient switches to a drug that exerts its effects through another mechanism of action, considering that TNF-α is not the primary pathogenesis. Alternatively, for low TLs with high titers of ADAs, switching out of class is also effective. Subgroup analyses of trials investigating vedolizumab[158], ustekinumab[159,160], and tofacitinib[161] all showed that patients who had failed anti-TNF therapy benefited from treatment with a novel agent. One study involving 128 CD patients who had failed previous anti-TNF therapy reported that the corticosteroid-free clinical response rates of vedolizumab and ustekinumab treatment at weeks 12, 24, and 52 were 22.7%, 29.7%, 26.8% and 27.1%, 42.4%, 45.9% respectively[162]. Furthermore, propensity score matching concluded that patients who failed anti-TNF therapy benefited more from ustekinumab than vedolizumab[162].
IBD is incurable, and anti-TNF therapy plays an important role in IBD. Although existing studies have found that previous surgical history, disease manifestations, drug concentrations, ADAs, serum albumin, ANCA, p-ANCA, ANA, etc. have potential predictive effects, to date, there are no practically available indicators that can predict response to TNF antagonists in patients with IBD. Further research is needed to verify the accuracy of existing predictors or discover new biomarkers to achieve personalized treatment for patients with IBD.
TDM forms the core of an optimal strategy for treating IBD. It is recommended to optimize the dose or add immunomodulators when patients with low TLs and no or low titer ADAs. For nonresponders with low TLs and high titer ADAs, switching to another TNF antagonist or biologic agent with a different mechanism can be suggested. When TLs are sufficient, patients can consider switching to another biological agent. In the future, more large randomized controlled trials are needed to investigate the efficacy of different next-step therapies for IBD patients who do not respond to anti-TNF.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liakina V, Lithuania; Rodrigues AT, Brazil; Tantau AI, Romania S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Ng SC, Kaplan GG, Tang W, Banerjee R, Adigopula B, Underwood FE, Tanyingoh D, Wei SC, Lin WC, Lin HH, Li J, Bell S, Niewiadomski O, Kamm MA, Zeng Z, Chen M, Hu P, Ong D, Ooi CJ, Ling KL, Miao Y, Miao J, Janaka de Silva H, Niriella M, Aniwan S, Limsrivilai J, Pisespongsa P, Wu K, Yang H, Ng KK, Yu HH, Wang Y, Ouyang Q, Abdullah M, Simadibrata M, Gunawan J, Hilmi I, Lee Goh K, Cao Q, Sheng H, Ong-Go A, Chong VH, Ching JYL, Wu JCY, Chan FKL, Sung JJY. Population Density and Risk of Inflammatory Bowel Disease: A Prospective Population-Based Study in 13 Countries or Regions in Asia-Pacific. Am J Gastroenterol. 2019;114:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 2. | Park J, Cheon JH. Incidence and Prevalence of Inflammatory Bowel Disease across Asia. Yonsei Med J. 2021;62:99-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 3. | Mak WY, Zhao M, Ng SC, Burisch J. The epidemiology of inflammatory bowel disease: East meets west. J Gastroenterol Hepatol. 2020;35:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 413] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 4. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3527] [Article Influence: 271.3] [Reference Citation Analysis (5)] |
| 5. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4107] [Article Influence: 513.4] [Reference Citation Analysis (110)] |
| 6. | D'Haens GR, van Deventer S. 25 years of anti-TNF treatment for inflammatory bowel disease: lessons from the past and a look to the future. Gut. 2021;70:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2886] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 8. | Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 9. | Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 10. | Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 11. | Papamichael K, Gils A, Rutgeerts P, Levesque BG, Vermeire S, Sandborn WJ, Vande Casteele N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 191] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 12. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P; ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3055] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 13. | Beaugerie L, Rahier JF, Kirchgesner J. Predicting, Preventing, and Managing Treatment-Related Complications in Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18:1324-1335.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 14. | Cheon JH. Understanding the complications of anti-tumor necrosis factor therapy in East Asian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2017;32:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Targownik LE, Benchimol EI, Witt J, Bernstein CN, Singh H, Lix L, Tennakoon A, Zubieta AA, Coward S, Jones J, Kuenzig E, Murthy SK, Nguyen GC, Peña-Sánchez JN, Kaplan G. The Effect of Initiation of Anti-TNF Therapy on the Subsequent Direct Health Care Costs of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:1718-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 457] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 17. | Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 18. | Wong U, Cross RK. Primary and secondary nonresponse to infliximab: mechanisms and countermeasures. Expert Opin Drug Metab Toxicol. 2017;13:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 20. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-659, quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 21. | Sprakes MB, Ford AC, Warren L, Greer D, Hamlin J. Efficacy, tolerability, and predictors of response to infliximab therapy for Crohn's disease: a large single centre experience. J Crohns Colitis. 2012;6:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Marsal J, Barreiro-de Acosta M, Blumenstein I, Cappello M, Bazin T, Sebastian S. Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front Med (Lausanne). 2022;9:897936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Allez M, Karmiris K, Louis E, Van Assche G, Ben-Horin S, Klein A, Van der Woude J, Baert F, Eliakim R, Katsanos K, Brynskov J, Steinwurz F, Danese S, Vermeire S, Teillaud JL, Lémann M, Chowers Y. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis. 2010;4:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 24. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 403] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 25. | Zhang QW, Shen J, Zheng Q, Ran ZH. Loss of response to scheduled infliximab therapy for Crohn's disease in adults: A systematic review and meta-analysis. J Dig Dis. 2019;20:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Qiu Y, Chen BL, Mao R, Zhang SH, He Y, Zeng ZR, Ben-Horin S, Chen MH. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn's disease. J Gastroenterol. 2017;52:535-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 27. | Macaluso FS, Fries W, Privitera AC, Cappello M, Siringo S, Inserra G, Magnano A, Di Mitri R, Mocciaro F, Belluardo N, Scarpulla G, Magrì G, Trovatello A, Carroccio A, Genova S, Bertolami C, Vassallo R, Romano C, Citrano M, Accomando S, Ventimiglia M, Renna S, Orlando R, Rizzuto G, Porcari S, Ferracane C, Cottone M, Orlando A; Sicilian Network for Inflammatory Bowel Diseases [SN-IBD]. A Propensity Score-matched Comparison of Infliximab and Adalimumab in Tumour Necrosis Factor-α Inhibitor-naïve and Non-naïve Patients With Crohn's Disease: Real-Life Data From the Sicilian Network for Inflammatory Bowel Disease. J Crohns Colitis. 2019;13:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Sandborn WJ, Melmed GY, McGovern DP, Loftus EV Jr, Choi JM, Cho JH, Abraham B, Gutierrez A, Lichtenstein G, Lee SD, Randall CW, Schwartz DA, Regueiro M, Siegel CA, Spearman M, Kosutic G, Pierre-Louis B, Coarse J, Schreiber S. Clinical and demographic characteristics predictive of treatment outcomes for certolizumab pegol in moderate to severe Crohn's disease: analyses from the 7-year PRECiSE 3 study. Aliment Pharmacol Ther. 2015;42:330-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Choi CH, Song ID, Kim YH, Koo JS, Kim YS, Kim JS, Kim N, Kim ES, Kim JH, Kim JW, Kim TO, Kim HS, Kim HJ, Park YS, Park DI, Park SJ, Song HJ, Shin SJ, Yang SK, Ye BD, Lee KM, Lee BI, Lee SY, Lee CK, Im JP, Jang BI, Jeon TJ, Cho YK, Chang SK, Jeon SR, Jung SA, Jeen YT, Cha JM, Han DS, Kim WH; IBD Study Group of the Korean Association for the Study of the Intestinal Diseases. Efficacy and Safety of Infliximab Therapy and Predictors of Response in Korean Patients with Crohn's Disease: A Nationwide, Multicenter Study. Yonsei Med J. 2016;57:1376-1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Arias MT, Vande Casteele N, Vermeire S, de Buck van Overstraeten A, Billiet T, Baert F, Wolthuis A, Van Assche G, Noman M, Hoffman I, D'Hoore A, Gils A, Rutgeerts P, Ferrante M. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Taxonera C, Rodríguez C, Bertoletti F, Menchén L, Arribas J, Sierra M, Arias L, Martínez-Montiel P, Juan A, Iglesias E, Algaba A, Manceñido N, Rivero M, Barreiro-de Acosta M, López-Serrano P, Argüelles-Arias F, Gutierrez A, Busquets D, Gisbert JP, Olivares D, Calvo M, Alba C; Collaborators. Clinical Outcomes of Golimumab as First, Second or Third Anti-TNF Agent in Patients with Moderate-to-Severe Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:1394-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Bosca-Watts MM, Cortes X, Iborra M, Huguet JM, Sempere L, Garcia G, Gil R, Garcia M, Muñoz M, Almela P, Maroto N, Paredes JM. Short-term effectiveness of golimumab for ulcerative colitis: Observational multicenter study. World J Gastroenterol. 2016;22:10432-10439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Narula N, Kainz S, Petritsch W, Haas T, Feichtenschlager T, Novacek G, Eser A, Vogelsang H, Reinisch W, Papay P. The efficacy and safety of either infliximab or adalimumab in 362 patients with anti-TNF-α naïve Crohn's disease. Aliment Pharmacol Ther. 2016;44:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Billiet T, Papamichael K, de Bruyn M, Verstockt B, Cleynen I, Princen F, Singh S, Ferrante M, Van Assche G, Vermeire S. A Matrix-based Model Predicts Primary Response to Infliximab in Crohn's Disease. J Crohns Colitis. 2015;9:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Stein AC, Rubin DT, Hanauer SB, Cohen RD. Incidence and predictors of clinical response, re-induction dose, and maintenance dose escalation with certolizumab pegol in Crohn's disease. Inflamm Bowel Dis. 2014;20:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W, Gibson PR, Collins J, Järnerot G, Hibi T, Rutgeerts P; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85-95; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 683] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 37. | Gonzalez-Lama Y, Fernandez-Blanco I, Lopez-SanRoman A, Taxonera C, Casis B, Tabernero S, Bermejo F, Martinez-Silva F, Mendoza JL, Martinez-Montiel P, Carneros JA, Sanchez F, Maté J, Gisbert JP; Group for the Study of Inflammatory Bowel Diseases from Madrid. Open-label infliximab therapy in ulcerative colitis: a multicenter survey of results and predictors of response. Hepatogastroenterology. 2008;55:1609-1614. [PubMed] |
| 38. | Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology. 2019;157:647-659.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 505] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 39. | Zorzi F, Zuzzi S, Onali S, Calabrese E, Condino G, Petruzziello C, Ascolani M, Pallone F, Biancone L. Efficacy and safety of infliximab and adalimumab in Crohn's disease: a single centre study. Aliment Pharmacol Ther. 2012;35:1397-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Lee S, Kuenzig ME, Ricciuto A, Zhang Z, Shim HH, Panaccione R, Kaplan GG, Seow CH. Smoking May Reduce the Effectiveness of Anti-TNF Therapies to Induce Clinical Response and Remission in Crohn's Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2021;15:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Inamdar S, Volfson A, Rosen L, Sunday S, Katz S, Sultan K. Smoking and early infliximab response in Crohn’s disease: a meta-analysis. J Crohns Colitis. 2015;9:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Ribaldone DG, Dileo I, Pellicano R, Resegotti A, Fagoonee S, Vernero M, Saracco G, Astegiano M. Severe ulcerative colitis: predictors of response and algorithm proposal for rescue therapy. Ir J Med Sci. 2018;187:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Ferrante M, Vermeire S, Fidder H, Schnitzler F, Noman M, Van Assche G, De Hertogh G, Hoffman I, D'Hoore A, Van Steen K, Geboes K, Penninckx F, Rutgeerts P. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 44. | Ananthakrishnan AN, Kaplan GG, Bernstein CN, Burke KE, Lochhead PJ, Sasson AN, Agrawal M, Tiong JHT, Steinberg J, Kruis W, Steinwurz F, Ahuja V, Ng SC, Rubin DT, Colombel JF, Gearry R; International Organization for Study of Inflammatory Bowel Diseases. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022;7:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 45. | Jeuring SF, van den Heuvel TR, Liu LY, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, Masclee AA, Jonkers DM, Pierik MJ. Improvements in the Long-Term Outcome of Crohn's Disease Over the Past Two Decades and the Relation to Changes in Medical Management: Results from the Population-Based IBDSL Cohort. Am J Gastroenterol. 2017;112:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 46. | Rungoe C, Langholz E, Andersson M, Basit S, Nielsen NM, Wohlfahrt J, Jess T. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 47. | Ramadas AV, Gunesh S, Thomas GA, Williams GT, Hawthorne AB. Natural history of Crohn's disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 48. | Vermeire S, Louis E, Carbonez A, Van Assche G, Noman M, Belaiche J, De Vos M, Van Gossum A, Pescatore P, Fiasse R, Pelckmans P, Reynaert H, D'Haens G, Rutgeerts P; Belgian Group of Infliximab Expanded Access Program in Crohn's Disease. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn's disease. Am J Gastroenterol. 2002;97:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Panaccione R, Löfberg R, Rutgeerts P, Sandborn WJ, Schreiber S, Berg S, Maa JF, Petersson J, Robinson AM, Colombel JF. Efficacy and Safety of Adalimumab by Disease Duration: Analysis of Pooled Data From Crohn's Disease Studies. J Crohns Colitis. 2019;13:725-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Mantzaris GJ, Zeglinas C, Theodoropoulou A, Koutroubakis I, Orfanoudaki E, Katsanos K, Christodoulou D, Michalopoulos G, Tzouvala M, Moschovis D, Michopoulos S, Zampeli E, Soufleris K, Ilias A, Chatzievangelinou C, Kyriakakis A, Antachopoulou K, Karmiris K. The Effect of Early vs Delayed Initiation of Adalimumab on Remission Rates in Patients With Crohn's Disease With Poor Prognostic Factors: The MODIFY Study. Crohns Colitis 360. 2021;3:otab064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 51. | Hamdeh S, Aziz M, Altayar O, Olyaee M, Murad MH, Hanauer SB. Early vs Late Use of Anti-TNFa Therapy in Adult Patients With Crohn Disease: A Systematic Review and Meta-Analysis. Inflamm Bowel Dis. 2020;26:1808-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Beswick L, Rosella O, Rosella G, Headon B, Sparrow MP, Gibson PR, van Langenberg DR. Exploration of Predictive Biomarkers of Early Infliximab Response in Acute Severe Colitis: A Prospective Pilot Study. J Crohns Colitis. 2018;12:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Iborra M, Pérez-Gisbert J, Bosca-Watts MM, López-García A, García-Sánchez V, López-Sanromán A, Hinojosa E, Márquez L, García-López S, Chaparro M, Aceituno M, Calafat M, Guardiola J, Belloc B, Ber Y, Bujanda L, Beltrán B, Rodríguez-Gutiérrez C, Barrio J, Cabriada JL, Rivero M, Camargo R, van Domselaar M, Villoria A, Schuterman HS, Hervás D, Nos P; Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU). Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol. 2017;52:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Gisbert JP, Chaparro M. Predictors of Primary Response to Biologic Treatment [Anti-TNF, Vedolizumab, and Ustekinumab] in Patients With Inflammatory Bowel Disease: From Basic Science to Clinical Practice. J Crohns Colitis. 2020;14:694-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 55. | Vande Casteele N, Gils A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: Adding value to current practice. J Clin Pharmacol. 2015;55 Suppl 3:S39-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Fine S, Papamichael K, Cheifetz AS. Etiology and Management of Lack or Loss of Response to Anti-Tumor Necrosis Factor Therapy in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2019;15:656-665. [PubMed] |
| 57. | Buhl S, Dorn-Rasmussen M, Brynskov J, Ainsworth MA, Bendtzen K, Klausen PH, Bolstad N, Warren DJ, Steenholdt C. Therapeutic thresholds and mechanisms for primary non-response to infliximab in inflammatory bowel disease. Scand J Gastroenterol. 2020;55:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Colombel JF, Sandborn WJ, Allez M, Dupas JL, Dewit O, D'Haens G, Bouhnik Y, Parker G, Pierre-Louis B, Hébuterne X. Association between plasma concentrations of certolizumab pegol and endoscopic outcomes of patients with Crohn's disease. Clin Gastroenterol Hepatol. 2014;12:423-31.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 59. | Brandse JF, Mathôt RA, van der Kleij D, Rispens T, Ashruf Y, Jansen JM, Rietdijk S, Löwenberg M, Ponsioen CY, Singh S, van den Brink GR, D'Haens GR. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate With Response to Infliximab Induction Therapy in Patients With Moderate to Severe Ulcerative Colitis. Clin Gastroenterol Hepatol. 2016;14:251-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 60. | Assa A, Hartman C, Weiss B, Broide E, Rosenbach Y, Zevit N, Bujanover Y, Shamir R. Long-term outcome of tumor necrosis factor alpha antagonist's treatment in pediatric Crohn's disease. J Crohns Colitis. 2013;7:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 61. | Kurnool S, Nguyen NH, Proudfoot J, Dulai PS, Boland BS, Vande Casteele N, Evans E, Grunvald EL, Zarrinpar A, Sandborn WJ, Singh S. High body mass index is associated with increased risk of treatment failure and surgery in biologic-treated patients with ulcerative colitis. Aliment Pharmacol Ther. 2018;47:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 62. | Karagiannides I, Pothoulakis C. Obesity, innate immunity and gut inflammation. Curr Opin Gastroenterol. 2007;23:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, Ben Yehoyada M, Mould DR. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 64. | Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn's disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 65. | Morita Y, Bamba S, Takahashi K, Imaeda H, Nishida A, Inatomi O, Sasaki M, Tsujikawa T, Sugimoto M, Andoh A. Prediction of clinical and endoscopic responses to anti-tumor necrosis factor-α antibodies in ulcerative colitis. Scand J Gastroenterol. 2016;51:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 67. | Bek S, Nielsen JV, Bojesen AB, Franke A, Bank S, Vogel U, Andersen V. Systematic review: genetic biomarkers associated with anti-TNF treatment response in inflammatory bowel diseases. Aliment Pharmacol Ther. 2016;44:554-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 68. | Curci D, Lucafò M, Cifù A, Fabris M, Bramuzzo M, Martelossi S, Franca R, Decorti G, Stocco G. Pharmacogenetic variants of infliximab response in young patients with inflammatory bowel disease. Clin Transl Sci. 2021;14:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Kimura K, Yoshida A, Katagiri F, Takayanagi R, Yamada Y. Prediction of treatment failure during infliximab induction therapy in inflammatory bowel disease patients based on pharmacokinetic and pharmacodynamic modeling. Eur J Pharm Sci. 2020;150:105317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Yoshida A, Kimura K, Morizane T, Ueno F. Predictor of primary response to antitumor necrosis factor-α therapy for inflammatory bowel disease: a single-center observational study. Eur J Gastroenterol Hepatol. 2022;34:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 71. | Lee KM, Jeen YT, Cho JY, Lee CK, Koo JS, Park DI, Im JP, Park SJ, Kim YS, Kim TO, Lee SH, Jang BI, Kim JW, Park YS, Kim ES, Choi CH, Kim HJ; IBD study Group of Korean Association for the Study of Intestinal Diseases. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013;28:1829-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 72. | Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A; CDP870 Crohn's Disease Study Group. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 73. | Magro F, Rodrigues-Pinto E, Santos-Antunes J, Vilas-Boas F, Lopes S, Nunes A, Camila-Dias C, Macedo G. High C-reactive protein in Crohn's disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. 2014;8:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 74. | Kopylov U, Seidman E. Predicting durable response or resistance to antitumor necrosis factor therapy in inflammatory bowel disease. Therap Adv Gastroenterol. 2016;9:513-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 75. | Jürgens M, Laubender RP, Hartl F, Weidinger M, Seiderer J, Wagner J, Wetzke M, Beigel F, Pfennig S, Stallhofer J, Schnitzler F, Tillack C, Lohse P, Göke B, Glas J, Ochsenkühn T, Brand S. Disease activity, ANCA, and IL23R genotype status determine early response to infliximab in patients with ulcerative colitis. Am J Gastroenterol. 2010;105:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 76. | Taylor KD, Plevy SE, Yang H, Landers CJ, Barry MJ, Rotter JI, Targan SR. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn's disease. Gastroenterology. 2001;120:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 77. | Nguyen DL, Nguyen ET, Bechtold ML. pANCA positivity predicts lower clinical response to infliximab therapy among patients with IBD. South Med J. 2015;108:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Ferrante M, Vermeire S, Katsanos KH, Noman M, Van Assche G, Schnitzler F, Arijs I, De Hertogh G, Hoffman I, Geboes JK, Rutgeerts P. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 79. | Beltrán B, Iborra M, Sáez-González E, Marqués-Miñana MR, Moret I, Cerrillo E, Tortosa L, Bastida G, Hinojosa J, Poveda-Andrés JL, Nos P. Fecal Calprotectin Pretreatment and Induction Infliximab Levels for Prediction of Primary Nonresponse to Infliximab Therapy in Crohn's Disease. Dig Dis. 2019;37:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Pavlidis P, Gulati S, Dubois P, Chung-Faye G, Sherwood R, Bjarnason I, Hayee B. Early change in faecal calprotectin predicts primary non-response to anti-TNFα therapy in Crohn's disease. Scand J Gastroenterol. 2016;51:1447-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Dahlén R, Magnusson MK, Bajor A, Lasson A, Ung KA, Strid H, Öhman L. Global mucosal and serum cytokine profile in patients with ulcerative colitis undergoing anti-TNF therapy. Scand J Gastroenterol. 2015;50:1118-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Angelison L, Almer S, Eriksson A, Karling P, Fagerberg U, Halfvarson J, Thörn M, Björk J, Hindorf U, Löfberg R, Bajor A, Hjortswang H, Hammarlund P, Grip O, Torp J, Marsal J, Hertervig E; Swedish Organization for the Study of Inflammatory Bowel diseases (SOIBD). Long-term outcome of infliximab treatment in chronic active ulcerative colitis: a Swedish multicentre study of 250 patients. Aliment Pharmacol Ther. 2017;45:519-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Rubio MG, Amo-Mensah K, Gray JM, Nguyen VQ, Nakat S, Grider D, Love K, Boone JH, Sorrentino D. Fecal lactoferrin accurately reflects mucosal inflammation in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2019;10:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 84. | Sorrentino D, Nguyen VQ, Love K. Fecal Lactoferrin Predicts Primary Nonresponse to Biologic Agents in Inflammatory Bowel Disease. Dig Dis. 2021;39:626-633. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 85. | López-Hernández R, Valdés M, Campillo JA, Martínez-Garcia P, Salama H, Salgado G, Boix F, Moya-Quiles MR, Minguela A, Sánchez-Torres A, Miras M, Garcia A, Carballo F, Álvarez-López MR, Muro M. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet. 2014;41:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Steenholdt C, Enevold C, Ainsworth MA, Brynskov J, Thomsen OØ, Bendtzen K. Genetic polymorphisms of tumour necrosis factor receptor superfamily 1b and fas ligand are associated with clinical efficacy and/or acute severe infusion reactions to infliximab in Crohn's disease. Aliment Pharmacol Ther. 2012;36:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Matsukura H, Ikeda S, Yoshimura N, Takazoe M, Muramatsu M. Genetic polymorphisms of tumour necrosis factor receptor superfamily 1A and 1B affect responses to infliximab in Japanese patients with Crohn's disease. Aliment Pharmacol Ther. 2008;27:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Medrano LM, Taxonera C, Márquez A, Barreiro-de Acosta M, Gómez-García M, González-Artacho C, Pérez-Calle JL, Bermejo F, Lopez-Sanromán A, Martín Arranz MD, Gisbert JP, Mendoza JL, Martín J, Urcelay E, Núñez C. Role of TNFRSF1B polymorphisms in the response of Crohn's disease patients to infliximab. Hum Immunol. 2014;75:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2007] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 90. | Koder S, Repnik K, Ferkolj I, Pernat C, Skok P, Weersma RK, Potočnik U. Genetic polymorphism in ATG16L1 gene influences the response to adalimumab in Crohn's disease patients. Pharmacogenomics. 2015;16:191-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 91. | Hlavaty T, Pierik M, Henckaerts L, Ferrante M, Joossens S, van Schuerbeek N, Noman M, Rutgeerts P, Vermeire S. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn's disease. Aliment Pharmacol Ther. 2005;22:613-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 92. | Hlavaty T, Ferrante M, Henckaerts L, Pierik M, Rutgeerts P, Vermeire S. Predictive model for the outcome of infliximab therapy in Crohn's disease based on apoptotic pharmacogenetic index and clinical predictors. Inflamm Bowel Dis. 2007;13:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Adler J, Rangwalla SC, Dwamena BA, Higgins PD. The prognostic power of the NOD2 genotype for complicated Crohn's disease: a meta-analysis. Am J Gastroenterol. 2011;106:699-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 94. | Niess JH, Klaus J, Stephani J, Pflüger C, Degenkolb N, Spaniol U, Mayer B, Lahr G, von Boyen GB. NOD2 polymorphism predicts response to treatment in Crohn's disease--first steps to a personalized therapy. Dig Dis Sci. 2012;57:879-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Gutiérrez A, Scharl M, Sempere L, Holler E, Zapater P, Almenta I, González-Navajas JM, Such J, Wiest R, Rogler G, Francés R. Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn's disease. Gut. 2014;63:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 96. | Vermeire S, Louis E, Rutgeerts P, De Vos M, Van Gossum A, Belaiche J, Pescatore P, Fiasse R, Pelckmans P, Vlietinck R, Merlin F, Zouali H, Thomas G, Colombel JF, Hugot JP; Belgian Group of Infliximab Expanded Access Program and Fondation Jean Dausset CEPH, Paris, France. NOD2/CARD15 does not influence response to infliximab in Crohn's disease. Gastroenterology. 2002;123:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 97. | Billiet T, Cleynen I, Ballet V, Claes K, Princen F, Singh S, Ferrante M, Van Assche G, Gils A, Vermeire S. Evolution of cytokines and inflammatory biomarkers during infliximab induction therapy and the impact of inflammatory burden on primary response in patients with Crohn's disease. Scand J Gastroenterol. 2017;52:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Leal RF, Planell N, Kajekar R, Lozano JJ, Ordás I, Dotti I, Esteller M, Masamunt MC, Parmar H, Ricart E, Panés J, Salas A. Identification of inflammatory mediators in patients with Crohn's disease unresponsive to anti-TNFα therapy. Gut. 2015;64:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 99. | Verstockt S, Verstockt B, Vermeire S. Oncostatin M as a new diagnostic, prognostic and therapeutic target in inflammatory bowel disease (IBD). Expert Opin Ther Targets. 2019;23:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 100. | West NR, Hegazy AN, Owens BMJ, Bullers SJ, Linggi B, Buonocore S, Coccia M, Görtz D, This S, Stockenhuber K, Pott J, Friedrich M, Ryzhakov G, Baribaud F, Brodmerkel C, Cieluch C, Rahman N, Müller-Newen G, Owens RJ, Kühl AA, Maloy KJ, Plevy SE; Oxford IBD Cohort Investigators, Keshav S, Travis SPL, Powrie F. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017;23:579-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 619] [Cited by in RCA: 561] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 101. | Verstockt S, Verstockt B, Machiels K, Vancamelbeke M, Ferrante M, Cleynen I, De Hertogh G, Vermeire S. Oncostatin M Is a Biomarker of Diagnosis, Worse Disease Prognosis, and Therapeutic Nonresponse in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021;27:1564-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 102. | Gaujoux R, Starosvetsky E, Maimon N, Vallania F, Bar-Yoseph H, Pressman S, Weisshof R, Goren I, Rabinowitz K, Waterman M, Yanai H, Dotan I, Sabo E, Chowers Y, Khatri P, Shen-Orr SS; Israeli IBD research Network (IIRN). Cell-centred meta-analysis reveals baseline predictors of anti-TNFα non-response in biopsy and blood of patients with IBD. Gut. 2019;68:604-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 103. | Magnusson MK, Strid H, Sapnara M, Lasson A, Bajor A, Ung KA, Öhman L. Anti-TNF Therapy Response in Patients with Ulcerative Colitis Is Associated with Colonic Antimicrobial Peptide Expression and Microbiota Composition. J Crohns Colitis. 2016;10:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 104. | Dovrolis N, Michalopoulos G, Theodoropoulos GE, Arvanitidis K, Kolios G, Sechi LA, Eliopoulos AG, Gazouli M. The Interplay between Mucosal Microbiota Composition and Host Gene-Expression is Linked with Infliximab Response in Inflammatory Bowel Diseases. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 105. | Alatawi H, Mosli M, Saadah OI, Annese V, Al-Hindi R, Alatawy M, Al-Amrah H, Alshehri D, Bahieldin A, Edris S. Attributes of intestinal microbiota composition and their correlation with clinical primary non-response to anti-TNF-α agents in inflammatory bowel disease patients. Bosn J Basic Med Sci. 2022;22:412-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 106. | Vatn S, Carstens A, Kristoffersen AB, Bergemalm D, Casén C, Moen AEF, Tannaes TM, Lindstrøm J, Detlie TE, Olbjørn C, Lindquist CM, Söderholm JD, Gomollón F, Kalla R, Satsangi J, Vatn MH, Jahnsen J, Halfvarson J, Ricanek P; IBD-Character Consortium. Faecal microbiota signatures of IBD and their relation to diagnosis, disease phenotype, inflammation, treatment escalation and anti-TNF response in a European Multicentre Study (IBD-Character). Scand J Gastroenterol. 2020;55:1146-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Hong SW, Park J, Yoon H, Yang HR, Shin CM, Park YS, Kim N, Lee DH, Kim JS. Comparison of loss of response between anti-tumor necrosis factor alone and combined use with immunomodulators in patients with inflammatory bowel disease. Korean J Intern Med. 2021;36:S9-S17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Schultheiss JPD, Mahmoud R, Louwers JM, van der Kaaij MT, van Hellemondt BP, van Boeckel PG, Mahmmod N, Jharap B, Fidder HH, Oldenburg B. Loss of response to anti-TNFα agents depends on treatment duration in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2021;54:1298-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 109. | Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol. 2011;106:674-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 110. | Nasuno M, Miyakawa M, Tanaka H, Motoya S. Short- and Long-Term Outcomes of Infliximab Treatment for Steroid-Refractory Ulcerative Colitis and Related Prognostic Factors: A Single-Center Retrospective Study. Digestion. 2017;95:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Chaparro M, Panes J, García V, Mañosa M, Esteve M, Merino O, Andreu M, Gutierrez A, Gomollón F, Cabriada JL, Montoro MA, Mendoza JL, Nos P, Gisbert JP. Long-term durability of infliximab treatment in Crohn's disease and efficacy of dose "escalation" in patients losing response. J Clin Gastroenterol. 2011;45:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 112. | Miyoshi J, Hisamatsu T, Matsuoka K, Naganuma M, Maruyama Y, Yoneno K, Mori K, Kiyohara H, Nanki K, Okamoto S, Yajima T, Iwao Y, Ogata H, Hibi T, Kanai T. Early intervention with adalimumab may contribute to favorable clinical efficacy in patients with Crohn's disease. Digestion. 2014;90:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Ma C, Beilman CL, Huang VW, Fedorak DK, Kroeker KI, Dieleman LA, Halloran BP, Fedorak RN. Anti-TNF Therapy Within 2 Years of Crohn's Disease Diagnosis Improves Patient Outcomes: A Retrospective Cohort Study. Inflamm Bowel Dis. 2016;22:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 114. | Schreiber S, Reinisch W, Colombel JF, Sandborn WJ, Hommes DW, Robinson AM, Huang B, Lomax KG, Pollack PF. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn's disease. J Crohns Colitis. 2013;7:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 115. | Campos C, Perrey A, Lambert C, Pereira B, Goutte M, Dubois A, Goutorbe F, Dapoigny M, Bommelaer G, Hordonneau C, Buisson A. Medical Therapies for Stricturing Crohn's Disease: Efficacy and Cross-Sectional Imaging Predictors of Therapeutic Failure. Dig Dis Sci. 2017;62:1628-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Roblin X, Marotte H, Leclerc M, Del Tedesco E, Phelip JM, Peyrin-Biroulet L, Paul S. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015;9:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 117. | Ungar B, Chowers Y, Yavzori M, Picard O, Fudim E, Har-Noy O, Kopylov U, Eliakim R, Ben-Horin S; ABIRISK consortium. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut. 2014;63:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 118. | Scaldaferri F, D'Ambrosio D, Holleran G, Poscia A, Petito V, Lopetuso L, Graziani C, Laterza L, Pistone MT, Pecere S, Currò D, Gaetani E, Armuzzi A, Papa A, Cammarota G, Gasbarrini A. Body mass index influences infliximab post-infusion levels and correlates with prospective loss of response to the drug in a cohort of inflammatory bowel disease patients under maintenance therapy with Infliximab. PLoS One. 2017;12:e0186575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Chuck W, Shadbolt BF, Nordin F, Subramaniam K. BMI is important in predicting the loss of response in inflammatory bowel disease patients on tumour necrosis factor-α inhibitors. Eur J Gastroenterol Hepatol. 2022;34:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Schoenefuss F, Hoffmann P. Serum γ-globulin and albumin concentrations predict secondary loss of response to anti-TNFα in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2019;31:1563-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Lakatos G, Hritz I, Varga MZ, Juhász M, Miheller P, Cierny G, Tulassay Z, Herszényi L. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig Dis. 2012;30:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 122. | Meijer MJ, Mieremet-Ooms MA, van der Zon AM, van Duijn W, van Hogezand RA, Sier CF, Hommes DW, Lamers CB, Verspaget HW. Increased mucosal matrix metalloproteinase-1, -2, -3 and -9 activity in patients with inflammatory bowel disease and the relation with Crohn's disease phenotype. Dig Liver Dis. 2007;39:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 123. | Barberio B, D'Incà R, Facchin S, Dalla Gasperina M, Fohom Tagne CA, Cardin R, Ghisa M, Lorenzon G, Marinelli C, Savarino EV, Zingone F. Matrix Metalloproteinase 3 Predicts Therapeutic Response in Inflammatory Bowel Disease Patients Treated With Infliximab. Inflamm Bowel Dis. 2020;26:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 124. | Biancheri P, Brezski RJ, Di Sabatino A, Greenplate AR, Soring KL, Corazza GR, Kok KB, Rovedatti L, Vossenkämper A, Ahmad N, Snoek SA, Vermeire S, Rutgeerts P, Jordan RE, MacDonald TT. Proteolytic cleavage and loss of function of biologic agents that neutralize tumor necrosis factor in the mucosa of patients with inflammatory bowel disease. Gastroenterology. 2015;149:1564-1574.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 125. | Romero-Cara P, Torres-Moreno D, Pedregosa J, Vílchez JA, García-Simón MS, Ruiz-Merino G, Morán-Sanchez S, Conesa-Zamora P. A FCGR3A Polymorphism Predicts Anti-drug Antibodies in Chronic Inflammatory Bowel Disease Patients Treated With Anti-TNF. Int J Med Sci. 2018;15:10-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, Bewshea CM, Chanchlani N, Walker GJ, Perry MH, McDonald TJ, Lees CW, Cummings JRF, Parkes M, Mansfield JC, Irving PM, Barrett JC, McGovern D, Goodhand JR, Anderson CA, Ahmad T; PANTS Consortium. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn's Disease. Gastroenterology. 2020;158:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 127. | Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;51:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 128. | Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 129. | Kiss LS, Szamosi T, Molnar T, Miheller P, Lakatos L, Vincze A, Palatka K, Barta Z, Gasztonyi B, Salamon A, Horvath G, Tóth GT, Farkas K, Banai J, Tulassay Z, Nagy F, Szenes M, Veres G, Lovasz BD, Vegh Z, Golovics PA, Szathmari M, Papp M, Lakatos PL; Hungarian IBD Study Group. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn's disease. Aliment Pharmacol Ther. 2011;34:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 130. | Santos-Antunes J, Nunes AC, Lopes S, Macedo G. The Relevance of Vitamin D and Antinuclear Antibodies in Patients with Inflammatory Bowel Disease Under Anti-TNF Treatment: A Prospective Study. Inflamm Bowel Dis. 2016;22:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 131. | Deshpande AR, Strobel S. Prediction of Crohn's disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. 2011;34:586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 132. | Sorrentino D, Gray JM. Timely Monitoring of Inflammation by Fecal Lactoferrin Rapidly Predicts Therapeutic Response in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2021;27:1237-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 133. | Bertani L, Fornai M, Fornili M, Antonioli L, Benvenuti L, Tapete G, Baiano Svizzero G, Ceccarelli L, Mumolo MG, Baglietto L, de Bortoli N, Bellini M, Marchi S, Costa F, Blandizzi C. Serum oncostatin M at baseline predicts mucosal healing in Crohn's disease patients treated with infliximab. Aliment Pharmacol Ther. 2020;52:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 134. | van der Have M, Oldenburg B, Kaptein AA, Jansen JM, Scheffer RC, van Tuyl BA, van der Meulen-de Jong AE, Pierik M, Siersema PD, van Oijen MG, Fidder HH. Non-adherence to Anti-TNF Therapy is Associated with Illness Perceptions and Clinical Outcomes in Outpatients with Inflammatory Bowel Disease: Results from a Prospective Multicentre Study. J Crohns Colitis. 2016;10:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 135. | Rentsch C, Headon B, Ward MG, Gibson PR. Inadequate storage of subcutaneous biological agents by patients with inflammatory bowel disease: Another factor driving loss of response? J Gastroenterol Hepatol. 2018;33:10-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 136. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1567] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 137. | Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, Kozuch PL, Raffals LE, Baidoo L, Bressler B, Devlin SM, Jones J, Kaplan GG, Sparrow MP, Velayos FS, Ullman T, Siegel CA. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17:1655-1668.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (2)] |
| 138. | Marquez-Megias S, Nalda-Molina R, Sanz-Valero J, Más-Serrano P, Diaz-Gonzalez M, Candela-Boix MR, Ramon-Lopez A. Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review. Pharmaceutics. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 139. | Yao J, Jiang X, You JHS. A Systematic Review on Cost-effectiveness Analyses of Therapeutic Drug Monitoring for Patients with Inflammatory Bowel Disease: From Immunosuppressive to Anti-TNF Therapy. Inflamm Bowel Dis. 2021;27:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 140. | Ricciuto A, Dhaliwal J, Walters TD, Griffiths AM, Church PC. Clinical Outcomes With Therapeutic Drug Monitoring in Inflammatory Bowel Disease: A Systematic Review With Meta-Analysis. J Crohns Colitis. 2018;12:1302-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 141. | Gisbert JP, Chaparro M. Primary Failure to an Anti-TNF Agent in Inflammatory Bowel Disease: Switch (to a Second Anti-TNF Agent) or Swap (for Another Mechanism of Action)? J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 142. | Baert F, Glorieus E, Reenaers C, D'Haens G, Peeters H, Franchimont D, Dewit O, Caenepeel P, Louis E, Van Assche G; BIRD (Belgian IBD Research and Development). Adalimumab dose escalation and dose de-escalation success rate and predictors in a large national cohort of Crohn's patients. J Crohns Colitis. 2013;7:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 143. | Van Stappen T, Vande Casteele N, Van Assche G, Ferrante M, Vermeire S, Gils A. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 144. | Bodini G, Demarzo MG, Saracco M, Coppo C, De Maria C, Baldissarro I, Savarino E, Savarino V, Giannini EG. High anti-TNF alfa drugs trough levels are not associated with the occurrence of adverse events in patients with inflammatory bowel disease. Scand J Gastroenterol. 2019;54:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 145. | van Schaik T, Maljaars JP, Roopram RK, Verwey MH, Ipenburg N, Hardwick JC, Veenendaal RA, van der Meulen-de Jong AE. Influence of combination therapy with immune modulators on anti-TNF trough levels and antibodies in patients with IBD. Inflamm Bowel Dis. 2014;20:2292-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 146. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2376] [Article Influence: 158.4] [Reference Citation Analysis (1)] |
| 147. | Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, van Hoogstraten HJ, Chen AC, Zheng H, Danese S, Rutgeerts P. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392-400.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 695] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 148. | Macaluso FS, Sapienza C, Ventimiglia M, Renna S, Rizzuto G, Orlando R, Di Pisa M, Affronti M, Orlando E, Cottone M, Orlando A. The Addition of an Immunosuppressant After Loss of Response to Anti-TNFα Monotherapy in Inflammatory Bowel Disease: A 2-Year Study. Inflamm Bowel Dis. 2018;24:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Drobne D, Bossuyt P, Breynaert C, Cattaert T, Vande Casteele N, Compernolle G, Jürgens M, Ferrante M, Ballet V, Wollants WJ, Cleynen I, Van Steen K, Gils A, Rutgeerts P, Vermeire S, Van Assche G. Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:514-521.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 150. | Mahmoud R, Schultheiss HP, Louwers J, van der Kaaij M, van Hellemondt B, Mahmmod N, van Boeckel P, Jharap B, Fidder H, Oldenburg B. Immunomodulator Withdrawal From Anti-TNF Therapy Is Not Associated With Loss of Response in Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022;20:2577-2587.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Yanai H, Lichtenstein L, Assa A, Mazor Y, Weiss B, Levine A, Ron Y, Kopylov U, Bujanover Y, Rosenbach Y, Ungar B, Eliakim R, Chowers Y, Shamir R, Fraser G, Dotan I, Ben-Horin S. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13:522-530.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 152. | Afif W, Loftus EV Jr, Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 153. | R-Grau Mdel C, Chaparro M, Mesonero F, Barreiro-de Acosta M, Castro L, Castro M, Domènech E, Mancenido N, Pérez-Calle JL, Taxonera C, Barrio J, De Francisco R, Fernández-Salgado E, Luzón L, Merino O, Oltra L, Saro C, Bermejo F, García-Sánchez V, Ginard D, Gutiérrez A, Vera I, Antón R, Ber Y, Calvet X, Gisbert JP. Effectiveness of anti-TNFα drugs in patients with Crohn's disease who do not achieve remission with their first anti-TNFα agent. Dig Liver Dis. 2016;48:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 154. | Casanova MJ, Chaparro M, Mínguez M, Ricart E, Taxonera C, García-López S, Guardiola J, López-San Román A, Iglesias E, Beltrán B, Sicilia B, Vera MI, Hinojosa J, Riestra S, Domènech E, Calvet X, Pérez-Calle JL, Martín-Arranz MD, Aldeguer X, Rivero M, Monfort D, Barrio J, Esteve M, Márquez L, Lorente R, García-Planella E, de Castro L, Bermejo F, Merino O, Rodríguez-Pérez A, Martínez-Montiel P, Van Domselaar M, Alcaín G, Domínguez-Cajal M, Muñoz C, Gomollón F, Fernández-Salazar L, García-Sepulcre MF, Rodríguez-Lago I, Gutiérrez A, Argüelles-Arias F, Rodriguez C, Rodríguez GE, Bujanda L, Llaó J, Varela P, Ramos L, Huguet JM, Almela P, Romero P, Navarro-Llavat M, Abad Á, Ramírez-de la Piscina P, Lucendo AJ, Sesé E, Madrigal RE, Charro M, García-Herola A, Pajares R, Khorrami S, Gisbert JP. Effectiveness and Safety of the Sequential Use of a Second and Third Anti-TNF Agent in Patients With Inflammatory Bowel Disease: Results From the Eneida Registry. Inflamm Bowel Dis. 2020;26:606-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 155. | Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 251] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 156. | Chanchlani N, Lin S, Auth MK, Lee CL, Robbins H, Looi S, Murugesan SV, Riley T, Preston C, Stephenson S, Cardozo W, Sonwalkar SA, Allah-Ditta M, Mansfield L, Durai D, Baker M, London I, London E, Gupta S, Di Mambro A, Murphy A, Gaynor E, Jones KDJ, Claridge A, Sebastian S, Ramachandran S, Selinger CP, Borg-Bartolo SP, Knight P, Sprakes MB, Burton J, Kane P, Lupton S, Fletcher A, Gaya DR, Colbert R, Seenan JP, MacDonald J, Lynch L, McLachlan I, Shields S, Hansen R, Gervais L, Jere M, Akhtar M, Black K, Henderson P, Russell RK, Lees CW, Derikx LAAP, Lockett M, Betteridge F, De Silva A, Hussenbux A, Beckly J, Bendall O, Hart JW, Thomas A, Hamilton B, Gordon C, Chee D, McDonald TJ, Nice R, Parkinson M, Gardner-Thorpe H, Butterworth JR, Javed A, Al-Shakhshir S, Yadagiri R, Maher S, Pollok RCG, Ng T, Appiahene P, Donovan F, Lok J, Chandy R, Jagdish R, Baig D, Mahmood Z, Marsh L, Moss A, Abdulgader A, Kitchin A, Walker GJ, George B, Lim YH, Gulliver J, Bloom S, Theaker H, Carlson S, Cummings JRF, Livingstone R, Beale A, Carter JO, Bell A, Coulter A, Snook J, Stone H, Kennedy NA, Goodhand JR, Ahmad T; IMSAT study investigators. Implications for sequencing of biologic therapy and choice of second anti-TNF in patients with inflammatory bowel disease: results from the IMmunogenicity to Second Anti-TNF therapy (IMSAT) therapeutic drug monitoring study. Aliment Pharmacol Ther. 2022;56:1250-1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 157. | Roblin X, Williet N, Boschetti G, Phelip JM, Del Tedesco E, Berger AE, Vedrines P, Duru G, Peyrin-Biroulet L, Nancey S, Flourie B, Paul S. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: a prospective randomised trial. Gut. 2020;69:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 158. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1866] [Article Influence: 155.5] [Reference Citation Analysis (1)] |
| 159. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P, Ghosh S, de Villiers WJ, Panaccione R, Greenberg G, Schreiber S, Lichtiger S, Feagan BG; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 873] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 160. | Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P; UNITI–IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1345] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 161. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 162. | Biemans VBC, van der Woude CJ, Dijkstra G, van der Meulen-de Jong AE, Löwenberg M, de Boer NK, Oldenburg B, Srivastava N, Jansen JM, Bodelier AGL, West RL, de Vries AC, Haans JJL, de Jong D, Hoentjen F, Pierik MJ; Dutch Initiative on Crohn and Colitis (ICC). Ustekinumab is associated with superior effectiveness outcomes compared to vedolizumab in Crohn's disease patients with prior failure to anti-TNF treatment. Aliment Pharmacol Ther. 2020;52:123-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |