Published online Jul 21, 2023. doi: 10.3748/wjg.v29.i27.4344
Peer-review started: March 29, 2023
First decision: May 23, 2023
Revised: June 6, 2023
Accepted: July 6, 2023
Article in press: July 6, 2023
Published online: July 21, 2023
Processing time: 105 Days and 20.9 Hours
Right-sided ligamentum teres (RSLT) is often associated with portal venous anomalies (PVA) and is regarded as a concerning feature for hepatobiliary intervention. Most studies consider RSLT to be one of the causes of left-sided gallbladder (LGB), leading to the hypothesis that LGB must always be present with RSLT. However, some cases have shown that right-sided gallbladder (RGB) can also be present in livers with RSLT.
To highlight the rare variation that RSLT may not come with LGB and to determine whether ligamentum teres (LT) or gallbladder location is reliable to predict PVA.
This study retrospectively assessed 8552 contrast-enhanced abdominal computed tomography examinations from 2018 to 2021 [4483 men, 4069 women; mean age, 59.5 ± 16.2 (SD) years]. We defined the surrogate outcome as major PVAs. The cases were divided into 4 subgroups according to gallbladder and LT locations. On one hand, we analyzed PVA prevalence by LT locations using gallbladder location as a controlled variable (n = 36). On the other hand, we controlled LT location and computed PVA prevalence by gallbladder locations (n = 34). Finally, we investigated LT location as an independent factor of PVA by using propensity score matching (PSM) and inverse probability of treatment weighting (IPTW).
We found 9 cases of RSLT present with RGB. Among the LGB cases, RSLT is associated with significantly higher PVA prevalence than typical LT [80.0% vs 18.2%, P = 0.001; OR = 18, 95% confidence interval (CI): 2.92-110.96]. When RSLT is present, we found no statistically significant difference in PVA prevalence for RGB and LGB cases (88.9 % vs 80.0%, P > 0.99). Both PSM and IPTW yielded balanced cohorts in demographics and gallbladder locations. The RSLT group had a significantly higher PVA prevalence after adjusted by PSM (77.3% vs 4.5%, P < 0.001; OR = 16.27, 95%CI: 2.25-117.53) and IPTW (82.5% vs 4.7%, P < 0.001).
RSLT doesn't consistently coexist with LGB. RSLT can predict PVA independently while the gallbladder location does not serve as a sufficient predictor.
Core Tip: Right-sided ligamentum teres (RSLT) is often associated with intrahepatic anomalies. Most studies hypothesis that left-sided gallbladder (LGB) must exist with RSLT. However, our exploration reveals that RSLT doesn't consistently coexist with LGB. Our analysis further suggests that RSLT can predict poral venous anomalies (PVAs) independently while the gallbladder location does not serve as a sufficient predictor. Therefore, operators, interventional radiologists or interventional gastroenterologists should not use the gallbladder location as an alternative of ligamentum teres location for PVAs prediction.
- Citation: Lin HY, Lee RC, Chai JW, Hsu CY, Chou Y, Hwang HE, Liu CA, Chiu NC, Yen HH. Predicting portal venous anomalies by left-sided gallbladder or right-sided ligamentum teres hepatis: A large scale, propensity score-matched study. World J Gastroenterol 2023; 29(27): 4344-4355
- URL: https://www.wjgnet.com/1007-9327/full/v29/i27/4344.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i27.4344
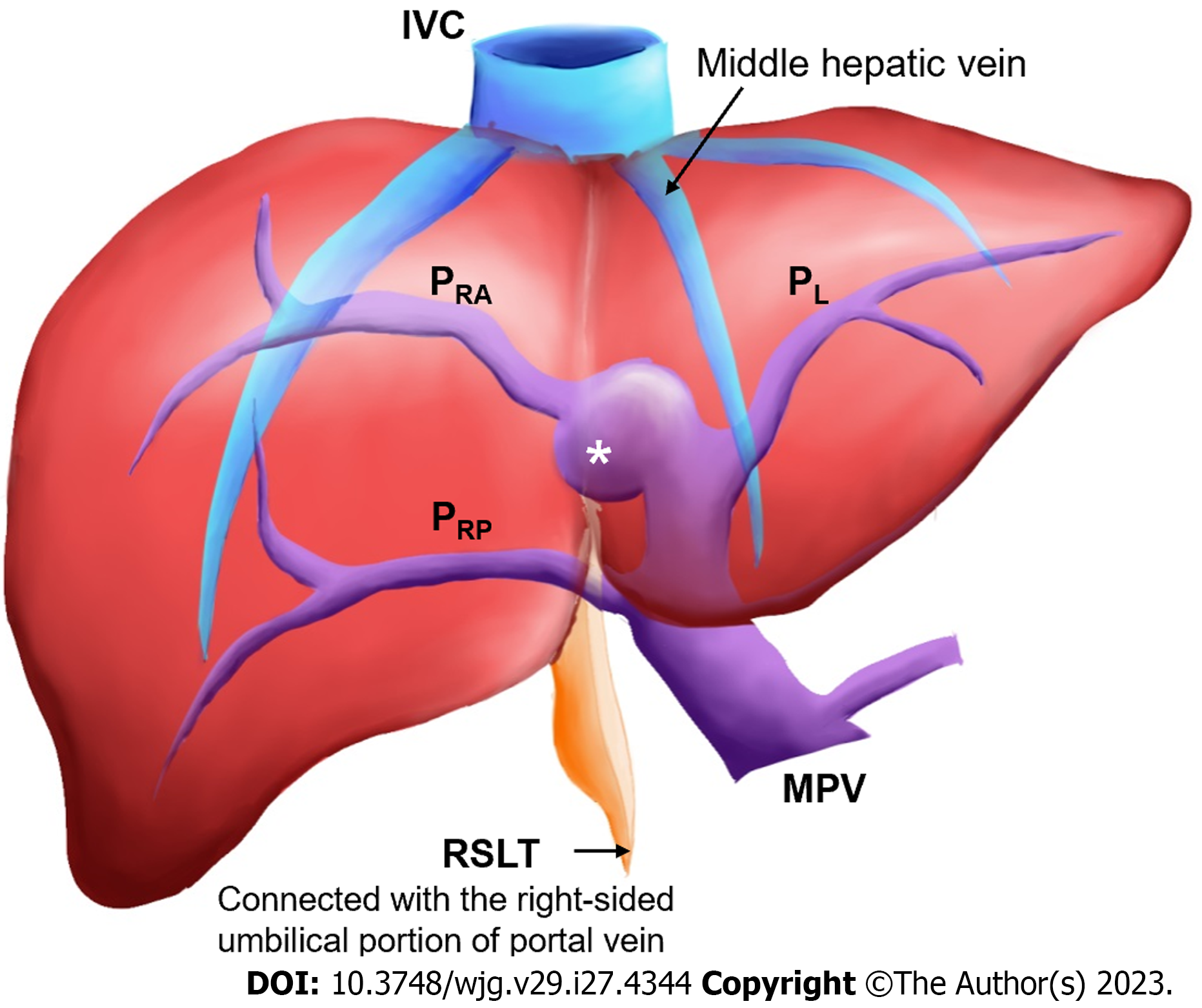
Right-sided ligamentum teres (RSLT) is a congenital anomaly caused by misconnection of the fetal umbilical vein to the right paramedian trunk of the portal vein (Figure 1). This anomaly was first reported by Matsumoto[1] in 1986. RSLT is associated with several intrahepatic vascular anomalies and anomalous biliary confluences[2-5]. Therefore, unawareness of RSLT before an intervention may result in serious morbidities[6-8]. Specifically, RSLT strongly correlates with portal venous anomalies (PVAs), a high interventional risk morbidity feature.
PVAs result in difficulties in liver surgery and lead to surgical complications[8,9], such as active bleeding, segmental devascularization, and hepatic failure due to misinterpretation and erroneous ligation of the portal venous branch. Therefore, accurately evaluating PVAs is crucial before any major hepatobiliary intervention. In this paper, we share a case of bleeding following an ultrasound-guided liver biopsy and unsuccessful first embolization as a result of PVAs not being identified before the intervention (Supplementary Figure 1). The bleeding was finally stopped through embolization once the PVAs had been recognized.
While RSLT is the key to PVA evaluation, the presence of a left-sided gallbladder (LGB) is a more commonly used anatomical anomaly in PVA screening. This is because: (1) Nagai et al[10] suggested that the variant gallbladder location is an outcome of RSLT, which has been accepted in many studies[2,5,11]; and (2) LGB is relatively easy to identify. However, Yamashita et al[3] and Lin et al[4] reported some cases in which RSLT could present with a GB in the typical right-side location, which conflicted with Nagai et al’s hypothesis[10]. Hence, the relationship between the location of the GB and ligamentum teres (LT) remains controversial and warrants further investigation.
In this paper, we present additional cases of RSLT together with a normal GB location, identified from a large data set. Then, to determine the key predictors of major PVA, we further investigate the association between GB location, LT location and PVA via a series of statistical analyses.
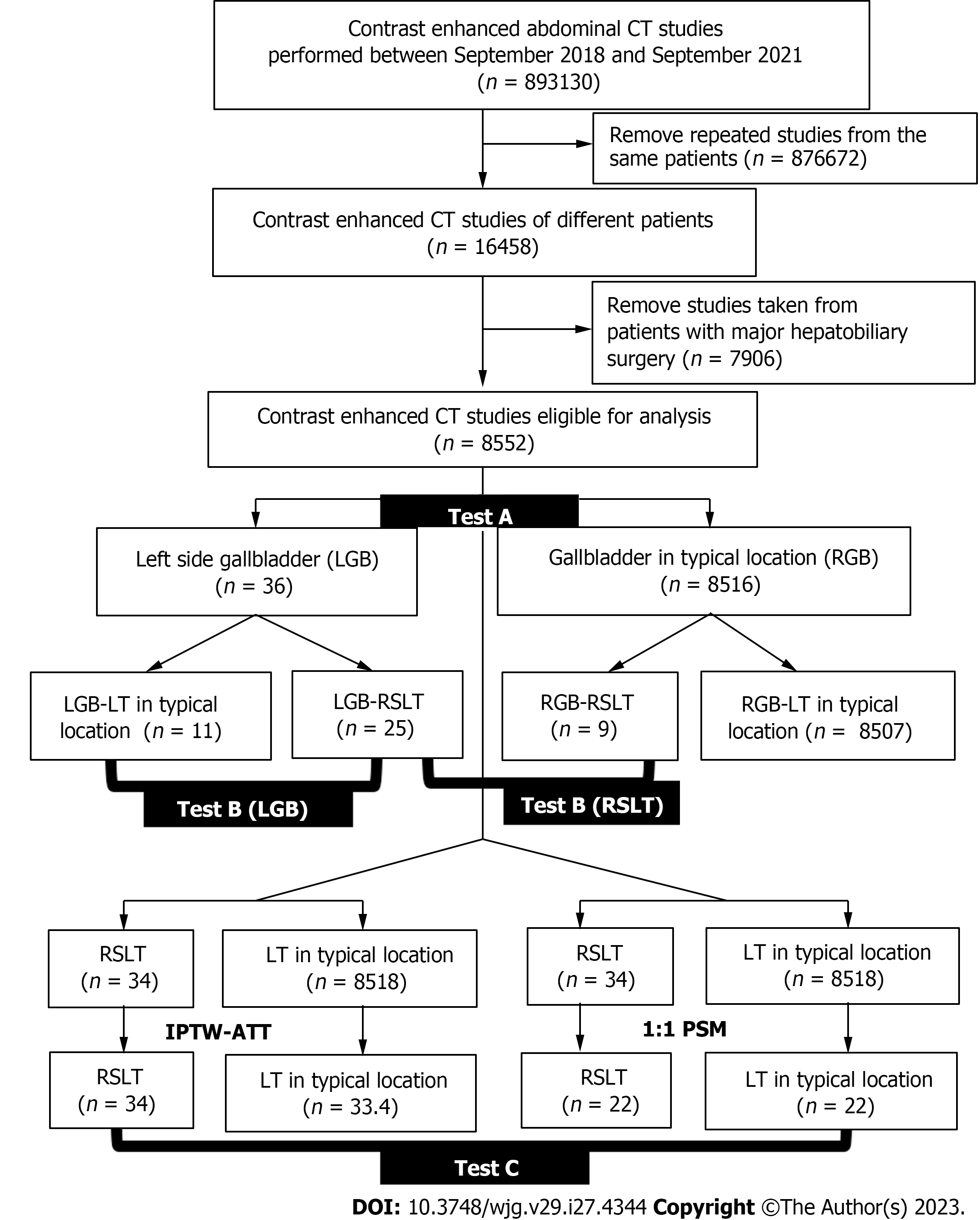
The institutional review board approved this retrospective study and waived the requirement for informed consent. We retrospectively reviewed 71822 contrast-enhanced multidetector computed tomography (MDCT) examinations conducted at the Radiology departments of Taipei Veterans General Hospital and Taichung Veterans General Hospital between September 2018 and September 2021. We excluded both repeat cases and patients who had undergone major hepatobiliary surgery. A total of 8552 cases were eligible for analysis (Figure 2). Although three of these cases have been previously reported[4], the report only highlights the existence of the rare variation. In this manuscript, we conducted a series of statistical analyses on a large number of patients. We also defined the surrogate outcome as the prevalence of major PVAs, which is the dominant risk factor for morbidity and mortality after hepatobiliary intervention.
Contrast-enhanced abdomen computed tomography (CT) studies were conducted using a Philips Brilliance iCT256, United States, or a Siemens Somatom Sensation 16 Slice CT, Germany. The examination parameters included a detector coverage of 128 × 0.625 (Philips Brilliance iCT256) or 16 × 0.75 (Siemens Somatom Sensation 16 Slice CT), a pitch of 0.91 (Philips Brilliance iCT256) or 1 (Siemens Somatom Sensation 16 Slice CT), a rotation time of 0.5 s, a section thickness of 5 mm, and a reconstruction interval of 5 mm for all images. Additional reconstructions were performed at a section thickness of 1 mm and a reconstruction interval of 0.7 mm for detailed interpretation. A total of 120 mL of nonionic iodinated contrast material with an iodine concentration of 350 mg/mL was injected. Scans were acquired in the portal venous phase by using a SmartPrep protocol, with the enhancement threshold set at 120 HU.
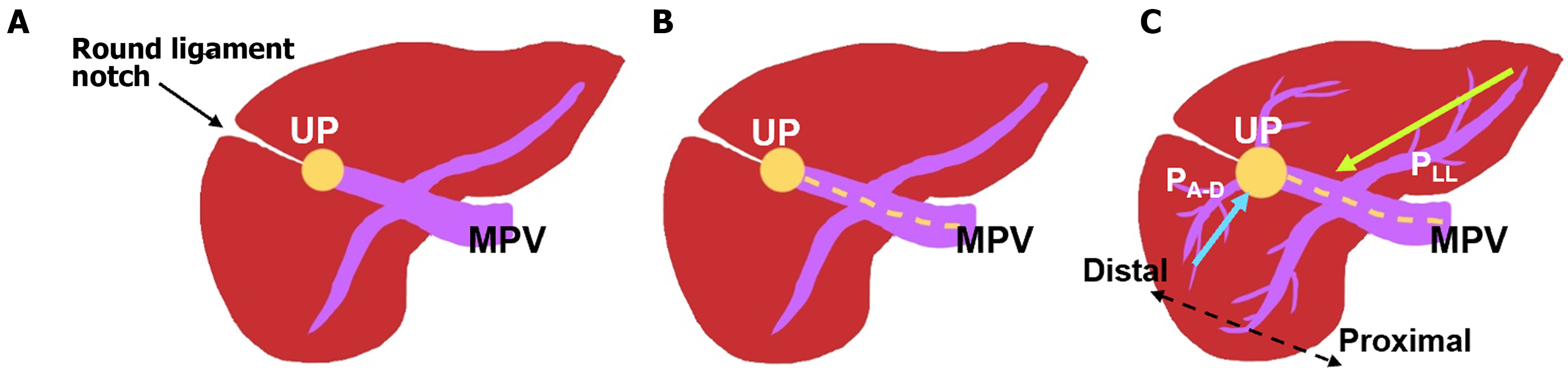
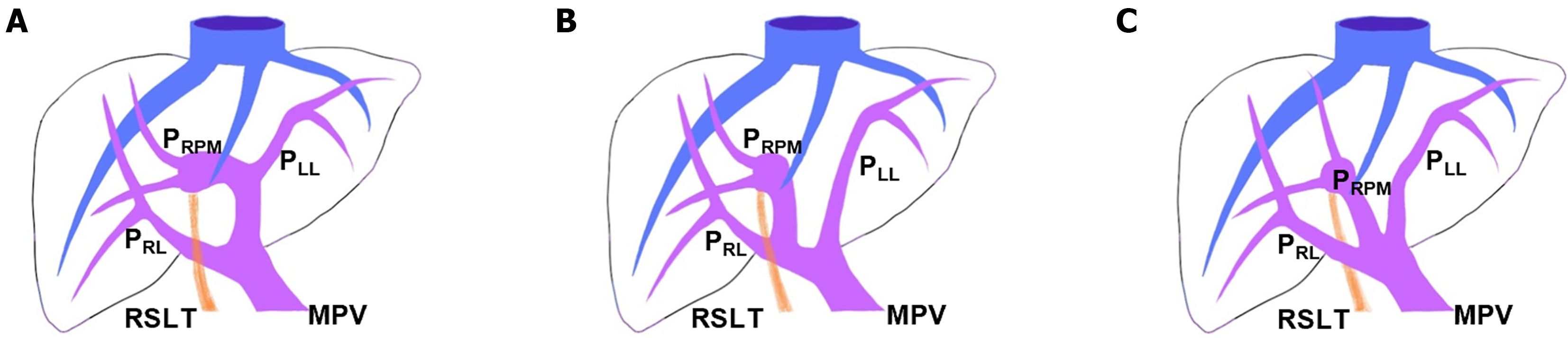
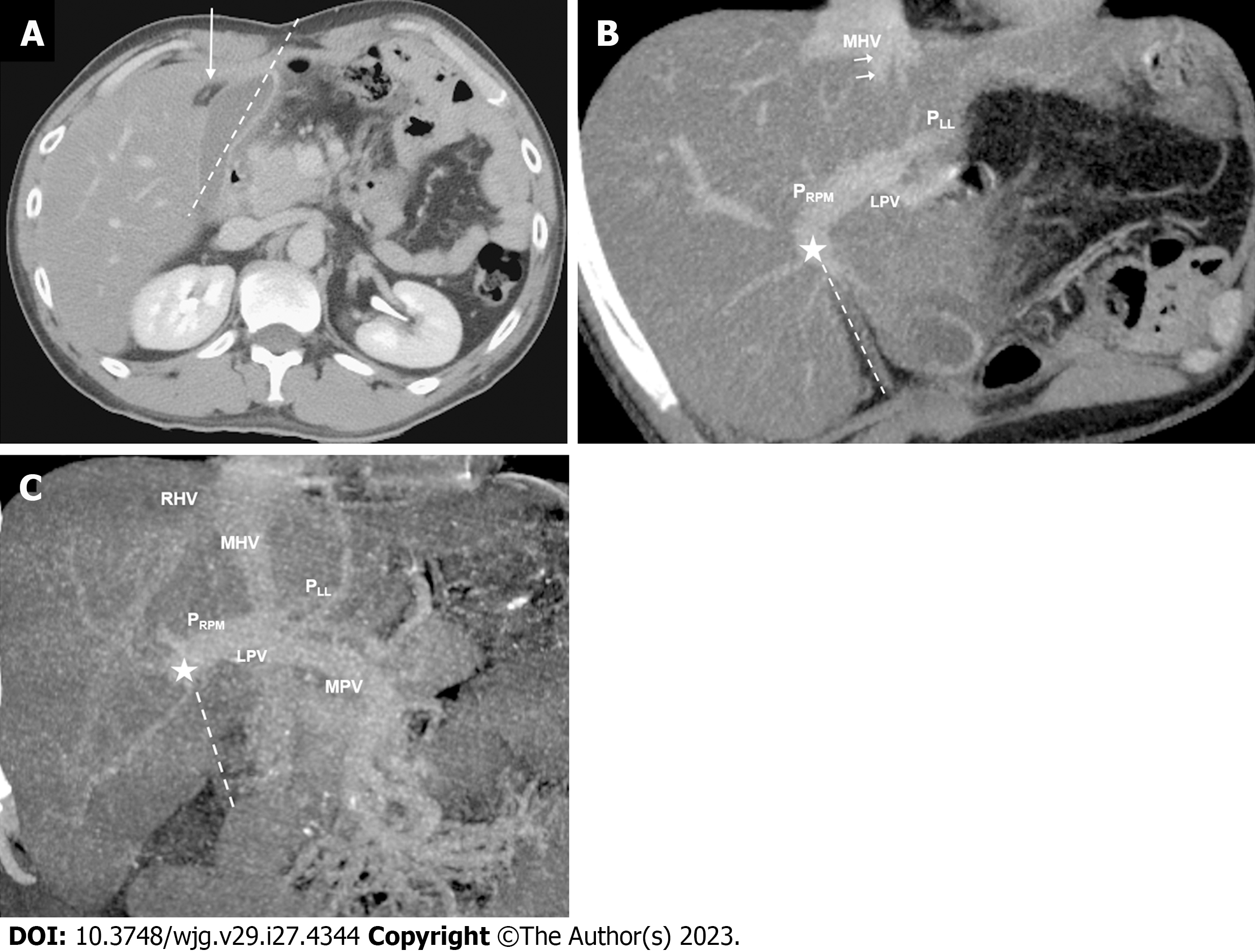
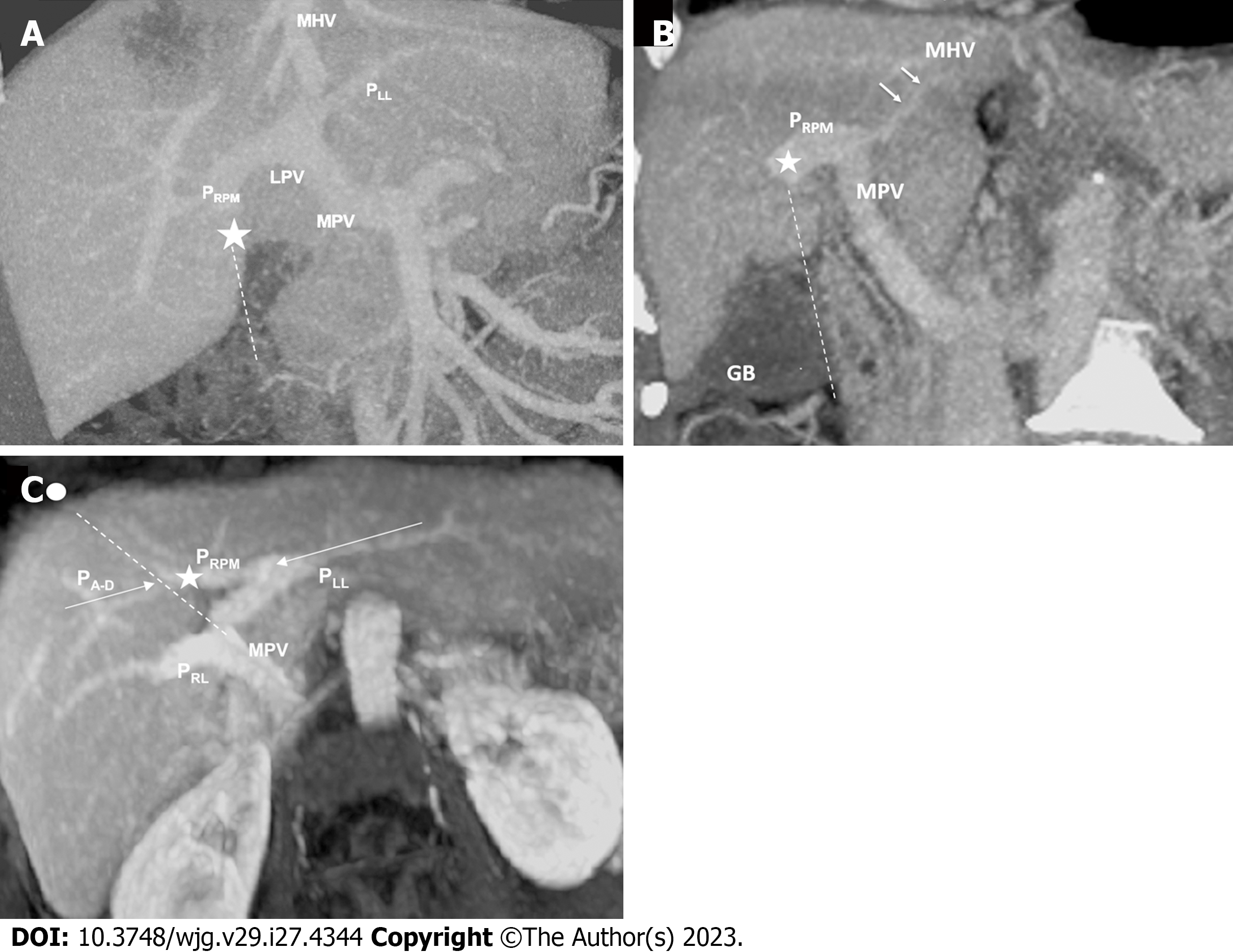
Coronal reconstructions were performed on all raw imaging data acquired from MDCT. The commercially available GE ADW 4.6 CT Workstation was used to process oblique axial multiplanar reformation (MPR), oblique coronal MPR, and maximum-intensity projection (MIP) images. These reconstructed images were then used for the discrimination of difficult portal vein ramifications and all cases involving PVAs. The images were independently analyzed by the following six radiologists: Lin HY, a radiologist with 2 years of experience; Hwang HE and Yen HH, radiologists with 4 years of experience; Chiu NC and Liu CA, radiologists with 15-20 years of experience; and Lee RC, a radiologist with more than 30 years of experience in interpreting CT and magnetic resonance imaging scans. Identification of RSLT was based on recognition of the round ligament (or LT) notch directly connected to the umbilical portion of the portal vein, which originates from the right portal branches (Figures 3 and 4). This technique followed the three-step method proposed by Yamashita et al[3] (Figure 3). In all cases, RSLT was positioned to the right of the middle hepatic vein (MHV), consistent with the definition of Shindoh et al[2] (Figures 5 and 6). Major PVA cases were predominantly identified as one of three types as defined by Shindoh et al[2] (Figure 4). However, cases other than these types were classified following the approach of Gallego et al[12] and Atri et al[13]. Single or subsegmental PVAs—such as separate segment VI branches from the right portal vein, as reported by Covey et al[14]—were not defined as major PVAs. GB location was defined by its long axis position relative to the umbilical fissure (LT notch) and MHV of the liver (Figures 5 and 6).
The surrogate outcome was defined as major PVAs on which three radiologists reached a consensus. To examine the relationship between GB location, LT location, and PVAs, three-step statistical tests were conducted, namely Test A, Test B, and Test C (Figure 2). First, we compared the prevalence of PVAs based on GB locations and referred to it as Test A. According to a previous study[11], GB location and PVAs are strongly correlated. However, Test A didn’t eliminate the confounding factor of LT location. To explore the effect of LT location, we divided our data into 4 subgroups based on not only GB location but also LT location and calculated PVAs prevalence for each subgroup, which was referred as Test B. Finally, we measured the PVAs prevalence per LT location as depicted in Test C. In Test C, we performed two analytical approaches: Propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) with average treatment effect in the treated (ATT), to cope with data imbalance and to minimize the confounding effects of GB location, sex, and age. Hence, the PVAs analyzed in Test C were solely conditioned on LT location.
A 1:1 PSM method was used to construct matched pairs between RSLT and LT at a typical location through the nearest neighbor approach. IPTW with ATT was then used to avoid missing rare variation data from PSM. Each patient was assigned an inverse weighting of the RSLT or LT at a typical location through calculated propensity scores and the following ATT weight equation:
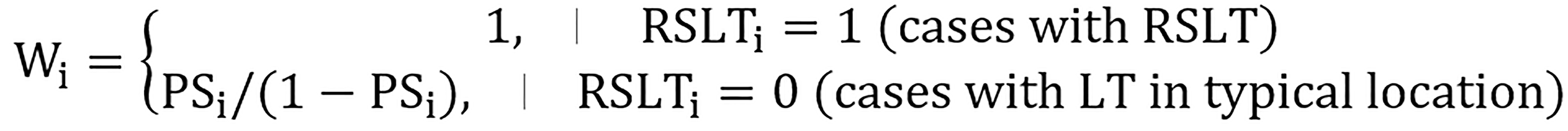
Therefore, the weighting applied on these two groups improved their comparability despite the imbalance in case numbers (Figure 2).
Continuous variables were expressed in the mean ± SD. Categorical variables were expressed in number and percentage. We used the Student’s t test to determine the differences between continuous variables and exploited the Chi-square or Fisher exact test for categorical variables. Logistic regression was performed to determine the odds ratio (OR) of the PVA prevalence. Standardized mean differences (SMDs) were calculated to diagnose the balance of matched data. Comparison of groups in matched data were performed by the paired t test for continuous variables and the McNemar’s test for categorical variables. A two-sided P value < 0.05 indicates statistical significance. All analyses were conducted using IBM SPSS Statistics version 25.0 (IBM, Armonk, NY, United States) and R software version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
The prevalence of LGB and RSLT were 0.42% (36 out of 8552 cases) and 0.40% (34 out of 8552 cases), respectively. Overall, trifurcation-type PVA was the most common anomaly. However, when LGB or RSLT was detected, “independent right lateral type” PVA was the dominant anomaly. Patient demographics for each subgroup were demonstrated in Table 1.
| Variables | RGB (n = 8516) | LGB (n = 36) | RSLT (n = 34) | typical LT (n = 8518) | |
| Age (yr)1 | 70.8 ± 15.9 | 59.6 ± 18.9 | 62.9 ± 17.7 | 79.5 ± 19.1 | |
| Sex, n (%) | No. of men | 4460 (52.4) | 23 (63.9) | 21 (61.8) | 4462 (52.4) |
| No. of women | 4056 (47.6) | 13 (36.1) | 13 (38.2) | 4056 (47.6) | |
| Chronic liver disease, n (%) | Hepatitis B virus | 962 (11.3) | 6 (16.7) | 6 (17.6) | 962 (11.3) |
| Hepatitis C virus | 281 (3.3) | 1 (2.8) | 0 (0.0) | 282 (3.3) | |
| Other diseases | 2520 (29.6) | 8 (22.2) | 8 (23.5) | 2520 (29.6) | |
| None | 4753 (55.8) | 21 (58.3) | 20 (58.8) | 4754 (55.8) | |
| Comorbidity, n (%) | Hepatocellular carcinoma | 911 (10.7) | 4 (11.1) | 2 (5.9) | 913 (10.7) |
| Non-HCC cancer | 4243 (49.8) | 18 (50.0) | 18 (52.9) | 4243 (49.8) | |
| Cardiovascular disease | 2127 (25.0) | 10 (27.8) | 8 (23.5) | 2129 (25.0) | |
| Chronic kidney disease | 845 (9.9) | 4 (11.1) | 5 (14.7) | 844 (9.9) | |
| Diabetes mellitus | 1899 (22.3) | 6 (16.7) | 9 (26.5) | 1896 (22.3) | |
| Cholelithiasis | 732 (8.6) | 3 (8.3) | 3 (8.8) | 732 (8.6) |
Test A investigated the population of PVA for each gallbladder location. The prevalence of major PVAs in the LGB group (n = 648, 61.1%) was higher than that in the right-sided GB (RGB) group (n = 22, 7.32%). A total of 670 PVA cases were identified, giving a prevalence of 7.83%, which is within the range reported in previous studies[15-19] (i.e., 6% to 16.5%) without considering single or subsegmental PVAs. However, the results of Test A did not exclude possible influencing factors, particularly LT location.
Test B investigated whether an LGB is sufficient for PVA prediction.
Test B (LGB): In this analysis, we categorized LGB cases by different LT locations. Among 36 patients with LGB, 25 had RSLT (69.4%), and 11 had typical LT (30.6%) as shown in Table 2. The prevalence of PVAs was 80.0% (20 out of 25 cases) for RSLT and 18.2% (2 out of 11 cases) for typical LT, which was significantly different based on Fisher’s exact test (P = 0.001). These differing results between LGB cases with different LT locations indicated that LGB alone was unreliable for predicting PVAs. To quantitatively measure the difference, we further performed logistic regression and obtained an OR of 18.00 [95% confidence interval (CI): 2.92-110.96] for RSLT over typical LT. No significant differences were observed in sex, age, and all the possible comorbidities.
| Variables | RSLT (n = 25) | Typical LT (n = 11) | P value | |
| Sex (men), n (%) | - | 17 (68.0) | 6 (54.5) | 0.439 |
| Age (yr) | - | 61.4 ± 18.2 | 55.5 ± 20.8 | 0.401 |
| Comorbidity, n (%) | Hepatitis B virus carrier | 5 (20.0) | 1 (9.1) | 0.643 |
| Hepatitis C virus carrier | 0 (0.0) | 1 (9.1) | 0.306 | |
| Hepatocellular carcinoma (HCC) | 2 (8.0) | 2 (18.2) | 0.570 | |
| Non-HCC malignancy | 13 (52.0) | 5 (45.5) | 0.717 | |
| Cardiovascular disease | 7 (28.0) | 3 (27.3) | 1.000 | |
| Chronic kidney disease | 4 (16.0) | 0 (0.0) | 0.290 | |
| Diabetes mellitus | 6 (24.0) | 0 (0.0) | 0.148 | |
| Outcomes | ||||
| Major portal vein anomalies1, n (%) | Overall | 20 (80.0) | 2 (18.2) | 0.0012 |
| Independent right lateral type | 13 (52.0) | 1 (9.1) | 0.025 | |
| Trifurcation | 6 (24.0) | 1 (9.1) | 0.400 | |
| Absence of R’t PV | 1 (4.0) | 0 (0.0) | > 0.99 | |
Test B (RSLT): On the other hand, we performed an analysis with the RSLT cases divided by GB location. This resulted in 9 RGB cases (26.5%) and 25 LGB cases (73.5%) from a total of 34 RSLT patients, as shown in Table 3. We detected PVAs in 8 of the 9 RGB cases (88.9%) and in 20 of the 25 LGB cases (80.0%); the difference was nonsignificant according to Fisher’s exact test (P > 0.99). These results indicated that GB location had no influence on the prevalence of PVAs given the existence of RSLT. The analysis showed no significant differences in demographics or confounding factors between the two subgroups.
| Variables | RGB (n =9) | LGB (n = 25) | P value | |
| Sex (men), n (%) | - | 4 (44.4) | 17 (68.0) | 0.254 |
| Age (yr) | - | 66.9 ± 16.6 | 61.4 ± 18.2 | 0.434 |
| Comorbidity, n (%) | Hepatitis B virus carrier | 1 (11.1) | 5 (20.0) | 0.293 |
| Hepatitis C virus carrier | 0 (0.0) | 0 (0.0) | NA | |
| Hepatocellular carcinoma (HCC) | 0 (0.0) | 2 (8.0) | > 0.99 | |
| Non-HCC malignancy | 5 (55.6) | 13 (52.0) | 0.855 | |
| Cardiovascular disease | 1 (11.1) | 7 (28.0) | 0.403 | |
| Chronic kidney disease | 1 (16.7) | 4 (16.0) | 1.000 | |
| Diabetes mellitus | 3 (33.3) | 6 (24.0) | 0.670 | |
| Outcomes | ||||
| Major PVA, n (%) | Overall | 8 (88.9) | 20 (80.0) | > 0.99 |
| Independent right lateral | 7 (87.5) | 13 (52.0) | 0.250 | |
| Trifurcation | 1 (12.5) | 6 (24.0) | 0.670 | |
| Absence of R’t PV | 0 (0.0) | 1 (4.0) | > 0.99 | |
According to the analysis results of Test B, we could conclude that GB location was not sufficient for PVA prediction. Furthermore, given the presence of RSLT, different GB locations even contributed to identical PVA prevalence.
Test C investigated the correlation between LT location and PVAs. We used PSM to handle unbalanced data and remove possible confounding factors, specifically GB location. As shown in Table 4, 22 patients of RSLT and typical LT were matched based on propensity score. Basic demographics and GB location were well balanced between the two groups, with SMDs less than 0.2. Also, both McNemar’s test and the paired-t test revealed that the confounding factors and demographics between matched cohorts were statistically similar. The prevalence of PVAs was 77.3% (17 out of 22) for the matched RSLT group and 4.5% (1 out of 5) for the matched typical LT group; the difference between these two groups was significant according to McNemar’s test (P < 0.001). Conditional logistic regression revealed an OR of 16.27 (95%CI: 2.25-117.53) for RSLT over typical LT. However, 12 rare variation data points in which RSLT coexisted with RGB were lost during the PSM process. Therefore, we implemented IPTW with ATT to prevent data exclusion from PSM and minimize the covariate imbalance between the groups with RSLT and typical LT. Following IPTW, the SMDs between the two cohorts were smaller than 0.1, indicating they are balanced for their demographics and GB locations. Compared with the typical LT cohort, the RSLT cohort had a significantly higher prevalence of PVAs after IPTW (4.7% vs 82.4%, P < 0.001; Table 5). As possible confounding effects were removed, the result suggests that LT location per se was effective for determining PVAs.
| Before propensity score matching | After propensity score matching | ||||||||
| Factors | RSLT (n = 34) | Typical LT (n = 8518) | P value | SMD1 | RSLT (n = 22) | Typical LT (n = 22) | P value | SMD1 | |
| Age | 62.9 ± 17.7 | 59.5 ± 16.2 | 0.2282 | 0.197 | 62.7 ± 17.1 | 59.9 ± 17.2 | 0.4113 | 0.156 | |
| Sex, n (%) | Men | 21 (61.8) | 4462 (52.4) | 0.3054 | 0.193 | 11 (50) | 11 (50) | 1.0002 | 0 |
| Women | 13 (38.2) | 4056 (47.6) | 11 (50) | 11 (50) | |||||
| GB, n (%) | LGB | 25 (7.35) | 28 (0.3) | < 0.0014 | 2.327 | 13 (59.1) | 13 (59.1) | 1.0002 | 0 |
| RGB | 9 (26.5) | 8490 (99.7) | 9 (40.9) | 9 (40.9) | |||||
| Outcomes | |||||||||
| Major PVA, n (%) | 17 (77.3) | 1 (4.5) | < 0.0015 | ||||||
| Before IPTW | After IPTW | ||||||||
| Factors | RSLT (n = 34) | Typical LT (n = 8518) | P value | SMD1 | RSLT (n = 34) | Typical LT (n = 34) | P value | SMD1 | |
| Age | 62.9 ± 17.7 | 59.5 ± 16.2 | 0.2282 | 0.197 | 62.9 ± 17.7 | 62.3 ± 14.6 | 0.879 | 0.034 | |
| Sex, n (%) | Men | 21 (61.8) | 4462 (52.4) | 0.3054 | 0.193 | 21 (61.8) | 19.4 (58.1) | 0.9533 | 0.076 |
| Women | 13 (38.2) | 4056 (47.6) | 13 (38.2) | 14 (41.9) | |||||
| GB, n (%) | LGB | 25 (7.35) | 28 (0.3) | < 0.0014 | 2.327 | 25 (73.5) | 24.4 (73.1) | 1.0003 | 0.011 |
| RGB | 9 (26.5) | 8490 (99.7) | 9 (26.5) | 9 (26.9) | |||||
| Outcomes | |||||||||
| Major PVA, n (%) | 28 (82.4) | 1.6 (4.7) | < 0.0014 | 2.514 | |||||
According to our statistical analysis results, RSLT is not consistently accompanied by LGB, and the key feature of predicting major PVAs is not LGB but rather RSLT. First, nine additional cases of RSLT without LGB were identified (Figure 2 and Table 3), indicating that the four cases reported by Yamashita et al[3] and Lin et al[4] were true existing variations and that RSLT does not consistently coexist with LGB. Second, the results of Test B indicated that the prevalence of PVAs is invariant regardless of the location of GB given RSLT was detected, which suggests that GB location is not sufficient for PVA prediction. Finally, the results of Test C concludes that the LT location is an independent risk factor of PVAs after possible confounding factors are eliminated in large propensity score–matched data sets.
Because GB location is an unreliable feature of PVAs, operators should not underestimate the risk of major vascular anomalies in livers with a normal GB location. As shown in Supplementary Figure 1, hepatobiliary interventions may be technically challenging and lead to complications if the PVAs are not recognized. Our results also suggest that LT location is strongly correlates with PVAs. Therefore, operators must be aware of the high surgical risk once RSLT has been detected. In addition to PVAs, RSLT also introduces nondeterministic anomalous arterial ramifications and biliary confluences, as reported by Nishitai et al[5] , hence, arterial and biliary patterns must be examined in preoperative imaging studies at the presence of RSLT.
Neglecting the aforementioned anomalies before an intervention may have life-threatening consequences, such as ischemic hepatic failure or bile leak[6-8]. Specifically, because independent ramification of the right lateral portal pedicle is the most common type of PVA in livers with RSLT, ligation of the left trunk of the portal vein during hepatobiliary surgery will disrupt portal flow in two-thirds of the entire liver if the common trunk of the left portal vein and the right paramedian pedicle are misinterpreted as the left portal vein. Multiple biliary complications during major hepatobiliary interventions in patients with RSLT have also been documented[6].
With the increasing popularity of three-dimensional magnetic resonance cholangiopancreatography (a low-risk examination that does not require the injection of contrast medium), biliary confluences in livers with RSLT should be comprehensively investigated during pre-interventional assessments, especially within the context of living donor liver transplantation.
This study has several limitations. First, a normal GB location in a liver with RSLT is a substantially rare variation that results in a remarkable loss of unmatched data when performing multivariate logistic regression. However, in the present study, this problem was addressed by preprocessing data with PSM and IPTW with ATT, yielding well-balanced cohorts and minimizing imbalances. Second, the diagnoses of all the variations were made by CT images without surgical or cadaveric anatomical proof. To compensate for this limitation and establish accurate image-based diagnoses, we explored several image-processing techniques, such as axial and coronal oblique MPR and MIP. We also followed the three-step method proposed by Yamashita et al[3] for diagnosing RSLT. In addition, the images were independently analyzed by six radiologists with 2-30 years of experience. To the best of our knowledge, this is the largest retrospective cohort study focusing on RSLTs. This is also the first article to highlight rare cases in which an RGB coexists with RSLT and further discuss the individual effects of LGB and RSLT on PVAs.
Understanding portal vein ramifications is crucial during preoperative planning of hepatobiliary interventions. We first observed that LGB is not consistently accompanied by RSLT and then performed a series of statistical analyses to evaluate the correlation between LGB, RSLT and PVAs. Our analysis results indicated that RSLT is an independent risk factor for PVAs, whereas GB location has no influence on PVAs if RSLT exists. Therefore, operators should avoid considering GB location, which is easily identified, as a surrogate feature of LT location.
The presence of right-sided ligamentum teres (RSLT) is often accompanied by portal venous anomalies (PVAs) and is considered a worrisome characteristic in hepatobiliary interventions. Most studies hypothesis that left-sided gallbladder (LGB) must exist with RSLT. However, in a reported study, right-sided gallbladder (RGB) was observed in livers with RSLT. Therefore, the relationship between the ligamentum teres hepatis (LT), gallbladder (GB), and PVAs is controversial and requires further investigation, despite the rarity of the anatomical variation of LT and GB, which can complicate statistical analysis.
To verify whether the RSLT coexists with a typical RGB, represent genuine existing variations or were merely misinterpreted and to determine the key predictors of major PVA, we conducted a comprehensive investigation. Additionally, to the best of our knowledge, all previous articles focusing on the RSLT had small sample sizes, not exceeding 1000.
First, to draw attention to the uncommon occurrence of the RSLT without the presence of the gallbladder (LGB), and secondly, to assess the reliability of both the LT and gallbladder location in predicting PVAs.
This retrospective study examined a total of 8552 contrast-enhanced abdominal computed tomography examinations conducted between 2018 and 2021, involving 4483 men and 4069 women, with a mean age of 59.5 ± 16.2 (SD) years. The primary focus was to assess major PVAs as a surrogate outcome. The cases were categorized into four subgroups based on the locations of the gallbladder and LT. On one hand, we analyzed the prevalence of PVAs based on LT locations while controlling for gallbladder location (n = 36). On the other hand, we controlled for LT location and determined the prevalence of PVAs based on gallbladder locations (n = 34). Lastly, we investigated the independent influence of LT location on PVA using propensity score matching (PSM) and inverse probability of treatment weighting (IPTW).
We identified a total of 9 cases where the RSLT coexisted with a typical RGB location. Among the cases with a LGB, the presence of RSLT was associated with a significantly higher prevalence of PVAs compared to those with a typical LT [80.0% vs 18.2%, P = 0.001; odds ratio (OR) = 18, 95% confidence interval (CI): 2.92-110.96]. However, when RSLT was present, there was no statistically significant difference in PVA prevalence between RGB and LGB cases (88.9% vs 80.0%, P > 0.99). We employed PSM and IPTW to ensure balanced cohorts in terms of demographics and gallbladder locations. After adjusting for these factors using PSM, the RSLT group still exhibited a significantly higher PVAs prevalence compared to the LT group (77.3% vs 4.5%, P < 0.001; OR = 16.27, 95%CI: 2.25-117.53). Similar results were observed when utilizing IPTW (82.5% vs 4.7%, P < 0.001).
RSLT doesn't always coexist with LGB. RSLT can predict PVA independently while the gallbladder location does not serve as a sufficient predictor.
Further investigation is needed to determine whether the existence of RSLT can predict the most predominant type of biliary or arterial anatomical variation.
We would like to express our special thanks to Chai JW, the head of the Department of Radiology at Taichung Veterans General Hospital in Taiwan, and to Lee RC, the head of the Division of Abdominal Radiology at Taipei Veterans General Hospital, for their invaluable assistance with technical support and assurance for the entire study. We would also like to thank Hsu CY from the Biostatistics Task Force and Department of Medical Research at Taichung Veterans General Hospital for kindly providing statistical advice for this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aseni P, Italy; Gad EH, Egypt; Wu SZ, China S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Matsumoto H. A newer concept of the segments of the liver. Jpn J Med Ultrasonics. 1986;13:551-552. |
| 2. | Shindoh J, Akahane M, Satou S, Aoki T, Beck Y, Hasegawa K, Sugawara Y, Ohtomo K, Kokudo N. Vascular architecture in anomalous right-sided ligamentum teres: three-dimensional analyses in 35 patients. HPB (Oxford). 2012;14:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Yamashita R, Yamaoka T, Nishitai R, Isoda H, Taura K, Arizono S, Furuta A, Ohno T, Ono A, Togashi K. Portal vein branching order helps in the recognition of anomalous right-sided round ligament: common features and variations in portal vein anatomy. Abdom Radiol (NY). 2017;42:1832-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Lin HY, Lee RC. Is right-sided ligamentum teres hepatis always accompanied by left-sided gallbladder? Case reports and literature review. Insights Imaging. 2018;9:955-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Nishitai R, Shindoh J, Yamaoka T, Akahane M, Kokudo N, Manaka D. Biliary architecture of livers exhibiting right-sided ligamentum teres: an indication for preoperative cholangiography prior to major hepatectomy. HPB (Oxford). 2016;18:929-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Hai S, Hatano E, Hirano T, Asano Y, Suzumura K, Sueoka H, Fujimoto J. Hepatectomy for Hilar Cholangiocarcinoma with Right-Sided Ligamentum Teres Using a Hepatectomy Simulation System. Case Rep Gastroenterol. 2017;11:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kaneoka Y, Yamaguchi A, Isogai M, Harada T. Hepatectomy for cholangiocarcinoma complicated with right umbilical portion: anomalous configuration of the intrahepatic biliary tree. J Hepatobiliary Pancreat Surg. 2000;7:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Koh MK, Ahmad H, Watanapa P, Jalleh RP, Habib NA. Beware the anomalous portal vein. HPB Surg. 1994;7:237-9; discussion 239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Sureka B, Patidar Y, Bansal K, Rajesh S, Agrawal N, Arora A. Portal vein variations in 1000 patients: surgical and radiological importance. Br J Radiol. 2015;88:20150326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Nagai M, Kubota K, Kawasaki S, Takayama T, BandaiY, Makuuchi M. Are left-sided gallbladders really located on the left side? Ann Surg. 1997;225:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Hsu SL, Chen TY, Huang TL, Sun CK, Concejero AM, Tsang LL, Cheng YF. Left-sided gallbladder: its clinical significance and imaging presentations. World J Gastroenterol. 2007;13:6404-6409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 12. | Gallego C, Velasco M, Marcuello P, Tejedor D, De Campo L, Friera A. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22:141-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Atri M, Bret PM, Fraser-Hill MA. Intrahepatic portal venous variations: prevalence with US. Radiology. 1992;184:157-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Covey AM, Brody LA, Getrajdman GI, Sofocleous CT, Brown KT. Incidence, patterns, and clinical relevance of variant portal vein anatomy. AJR Am J Roentgenol. 2004;183:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Hochstetter F. Anomalien der Pfortader und der Nabelvene in Verbindung mit Defekt oder Linkslage der Gallenblase. Arch Anat Entwick. 1886:369-384. |
| 16. | Idu M, Jakimowicz J, Iuppa A, Cuschieri A. Hepatobiliary anatomy in patients with transposition of the gallbladder: implications for safe laparoscopic cholecystectomy. Br J Surg. 1996;83:1442-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 17. | Maetani Y, Itoh K, Kojima N, Tabuchi T, Shibata T, Asonuma K, Tanaka K, Konishi J. Portal vein anomaly associated with deviation of the ligamentum teres to the right and malposition of the gallbladder. Radiology. 1998;207:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Fraser-Hill MA, Atri M, Bret PM, Aldis AE, Illescas FF, Herschorn SD. Intrahepatic portal venous system: variations demonstrated with duplex and color Doppler US. Radiology. 1990;177:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Soyer P, Bluemke DA, Choti MA, Fishman EK. Variations in the intrahepatic portions of the hepatic and portal veins: findings on helical CT scans during arterial portography. AJR Am J Roentgenol. 1995;164:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (1)] |