Published online Jul 21, 2023. doi: 10.3748/wjg.v29.i27.4271
Peer-review started: April 26, 2023
First decision: May 27, 2023
Revised: June 11, 2023
Accepted: June 27, 2023
Article in press: June 27, 2023
Published online: July 21, 2023
Processing time: 77 Days and 17.3 Hours
Hepatocellular carcinoma (HCC), the predominant type of liver cancer, is a major contributor to cancer-related fatalities across the globe. Diabetes has been identified as a significant risk factor for HCC, with recent research indicating that the hormone resistin could be involved in the onset and advancement of HCC in diabetic individuals. Resistin is a hormone that is known to be involved in inflammation and insulin resistance. Patients with HCC have been observed to exhibit increased resistin levels, which could be correlated with more severe disease stages and unfavourable prognoses. Nevertheless, the exact processes through which resistin influences the development and progression of HCC in diabetic patients remain unclear. This article aims to examine the existing literature on the possible use of resistin levels as a biomarker for HCC development and moni
Core Tip: Resistin, a hormone linked to the onset of insulin resistance and diabetes, could be involved in the development and advancement of hepatocellular carcinoma (HCC) in individuals with diabetes. Increased resistin levels have been observed in HCC patients and might be connected to a more severe disease stage and unfavourable prognosis. This review aims to assess the existing literature concerning the possible application of resistin as a biomarker for HCC development and monitoring while investigating the potential processes through which resistin influences HCC’s development and progression in diabetic patients. Gaining a better understanding of these processes may offer valuable insights for the prevention and therapy of this condition.
- Citation: Abdalla MMI. Serum resistin and the risk for hepatocellular carcinoma in diabetic patients. World J Gastroenterol 2023; 29(27): 4271-4288
- URL: https://www.wjgnet.com/1007-9327/full/v29/i27/4271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i27.4271
Liver cancer, particularly hepatocellular carcinoma (HCC), poses a significant global health challenge. As the sixth most prevalent cancer and the third leading cause of cancer-related deaths worldwide, it accounts for roughly one million fatalities each year[1-3]. HCC also ranks as the second primary factor contributing to premature cancer-related deaths[4], with projections indicating that annual liver cancer diagnoses will exceed one million by 2025[5]. Moreover, between 2020 and 2040, the number of liver cancer diagnoses is predicted to rise by 55.0%[6]. Although liver cancer prevalence and mortality have decreased in some East Asian countries, they have escalated in other parts of the world[7]. HCC represents the most frequent liver cancer variety, comprising 90% of cases[8]. It is commonly associated with chronic liver diseases (CLD), including viral hepatitis[9-12], alcoholic liver disease, liver cirrhosis[13-15] and “non-alcoholic fatty liver disease (NAFLD)”[16-18]. Because of the increased prevalence of obesity and type 2 diabetes mellitus (T2DM), the incidence of NAFLD and associated consequences, such as “non-alcoholic steatohepatitis (NASH)”, is rapidly increasing. NAFLD is currently the major cause of liver cirrhosis, which in turn, raises the probability of developing HCC[19]. The development of HCC is influenced by various factors, including oxidative stress[20], inflammation[21,22], and insulin resistance (IR)[22-25].
Diabetes is a chronic metabolic condition characterized by high blood sugar and IR, which is associated with an increased risk of several health complications, including cardiovascular disease, kidney disease, and fatty liver[26]. The most common form of diabetes, T2DM, is caused primarily by IR. The relationship between diabetes and HCC is complex and multifaceted. On the one hand, diabetes is a risk factor for the development of liver diseases, including liver fibrosis and HCC[27]. On the other hand, HCC can lead to diabetes due to IR, impaired glucose tolerance, and liver dysfunction[28,29].
Resistin, a hormone secreted by both adipocytes and macrophages, and linked to obesity and T2DM, has been connected to HCC development and progression[30-33]. High levels of resistin have been associated with IR, inflammation, and oxidative stress. All these factors are known risk factors for the development of HCC[32-34]. Studies have found that high resistin levels are correlated with a greater risk of getting HCC[30,32,35]. Nevertheless, the mechanisms by which resistin contributes to the initiation and progression of HCC in diabetic patients are not fully understood. Therefore, the purpose of this review is to assess the current literature on the potential use of resistin levels as a biomarker for the development and monitoring of HCC in diabetic patients. Additionally, this review aims to explore resistin’s role in HCC pathogenesis among this patient group and provide novel insights into the involved underlying mechanisms. These findings could help identify new targets for preventing and treating HCC in diabetic patients.
HCC is more prevalent in diabetic individuals than in those without diabetes. A thorough meta-analysis of 42 studies, including 17 case-control and 32 cohort studies, demonstrated that diabetic patients have a 2.31 times higher chance of developing HCC compared to non-diabetics. Furthermore, diabetic individuals experience a 2.43 times higher HCC mortality risk than their non-diabetic counterparts[36,37]. HCC patients also exhibit a higher prevalence of diabetes, with reported rates between 20% and 70%[38]. Additionally, a systematic review and meta-analysis of ten studies reported a 70% prevalence of liver cancer among those with elevated fasting blood glucose levels[39].
Numerous investigations have corroborated the heightened incidence of HCC in diabetic individuals. For instance, a population-based study in Taiwan discovered that diabetic patients had a 2-3 times greater risk of developing HCC than those without diabetes[40]. Similarly, a prospective cohort study of Chinese men and women in Singapore found a heightened HCC risk in diabetics[41]. Furthermore, an Italian hospital-based case-control study involving 224 HCC patients and 389 control subjects determined that the risk of HCC was significantly higher among patients with T2DM, especially those with longer disease durations[42]. Additionally, a Korean prospective cohort study using the “National Health Insurance Service-Health Screening Cohort” found a hazard ratio of 1.82, indicating an elevated HCC risk in diabetic patients[43].
Recent research has established that metabolic factors, such as diabetes mellitus, obesity, dyslipidemia, and metabolic syndromes, are substantial risk factors for HCC development[44,45]. In populations with low viral hepatitis prevalence, the overall influence of metabolic factors on HCC may be more substantial than that of viral hepatitis. A recent multicenter study in China found that 9.3% of hepatitis B virus (HBV)-infected patients undergoing curative resection for HCC had concomitant metabolic syndrome. During a median follow-up of 50.4 months, patients with metabolic syndrome had worse 5-year overall survival and recurrence-free survival rates, with increased overall recurrence rates, particularly after two years of surgery. Multivariate analyses revealed that metabolic syndrome was an independent risk factor for reduced overall survival and recurrence-free survival following curative resection for HCC. As a result, proper management of metabolic syndrome is essential for preventing post-hepatectomy recurrence. This information further emphasizes the importance of implementing a more rigorous surveillance program for recurrence in HBV-infected patients with concurrent metabolic syndrome, in addition to routine antiviral therapy[46].
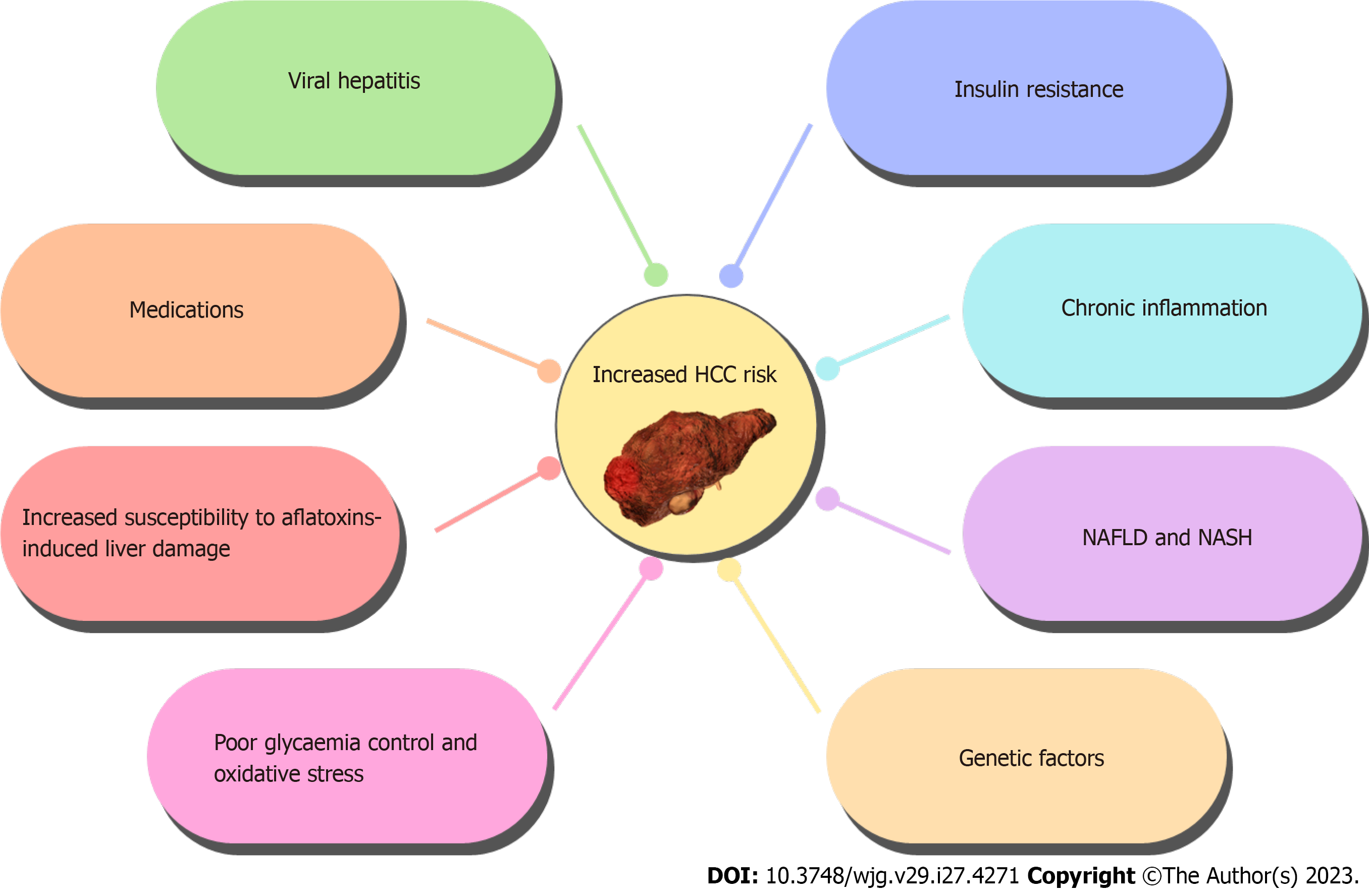
The elevated incidence of HCC in diabetic patients is attributable to a multitude of factors, such as IR, chronic inflammation, the administration of antidiabetic medications, and the progression of NAFLD and NASH[47-50]. IR and diminished glucose tolerance in diabetic individuals can result in hepatic fat accumulation, thereby promoting the onset of NAFLD[51,52]. NAFLD has been linked to a heightened risk of liver fibrosis and HCC[18,53,54]. Furthermore, the prevalence of diabetes has been recognized as a risk factor for the emergence of NASH and its subsequent progression to cirrhosis and HCC[50].
NAFLD is a multifaceted condition that can advance to severe fibrosis, cirrhosis, liver failure, and HCC[55]. In the United States, NAFLD contributes to roughly 20% of HCC cases and is associated with an increased risk of HCC development, especially in patients with metabolic syndrome, specific ethnic groups, and hepatic siderosis. The incidence of HCC in NASH-related cirrhosis varies considerably, ranging from 2.4% over seven years to 12.8% over three years, with some patients developing HCC de novo as a result of NASH[55]. Patients with T2DM have a higher risk of developing severe manifestations of NAFLD, such as cirrhosis and HCC[56-58].
The coexistence of diabetes and NASH may contribute to the elevated prevalence of HCC in diabetic individuals. A recent study found that the global burden of NASH-related liver cancer, attributable to increased fasting plasma glucose levels, has significantly risen over the past three decades, particularly in “low- and middle-income countries”. Consequently, effective prevention and management of high fasting plasma glucose levels are vital for reducing the worldwide burden of NASH-related liver cancer[59].
Besides NAFLD and NASH, other diabetes-associated complications may also contribute to the heightened HCC risk in diabetic patients. Such patients are more prone to chronic kidney disease, leading to the accumulation of uremic toxins and oxidative stress-both implicated in HCC development[60,61]. Diabetic patients are also more prone to hypertension[62], which has been linked to a higher HCC risk in some studies[44,63,64].
The consumption of antidiabetic medications, for instance, insulin and metformin, may also influence the HCC risk in diabetic patients[49]. Some studies suggest that IR and hyperinsulinemia could promote HCC development, potentially increasing the risk of HCC with insulin therapy[64-67]. Conversely, metformin has shown protective effects against HCC development in some studies, possibly due to its antineoplastic, anti-inflammatory, and antifibrotic properties[42,64,68-70].
Hyperglycemia and hyperinsulinemia are believed to promote HCC development and progression[71,72]. These conditions can activate various signaling pathways, such as the insulin-like growth factor-1 (IGF-1) pathway, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), mitogen-activated protein kinase (MAPK), and mTOR pathway, all involved in HCC development and progression[73-77].
Hyperinsulinemia can increase hepatic growth hormone receptor expression, leading to IGF-1 release and the activation of growth factor-like activity on hepatocytes. Insulin and IGF-1 inhibit cell proliferation and apoptosis, increasing the risk of HCC[78]. High glucose levels can also contribute to HCC development by generating advanced glycosylation end products, which activate inflammatory signaling pathways and produce reactive oxygen species that promote HCC development. IR might directly hasten the development of HCC by promoting the formation of new blood vessels in the liver[79,80].
Moreover, dyslipidemia, which is common in diabetic patients, may have a role in HCC initiation and progression[81,82]. Dyslipidemia can cause lipid accumulation in the liver, leading to liver damage and inflammation that promote HCC development[83]. Research has demonstrated a strong correlation between reduced total cholesterol levels and a heightened likelihood of HCC development[84-87]. Additionally, diabetic patients who exhibit high triglyceride levels and low high-density lipoprotein cholesterol levels, a pattern often seen in dyslipidemia, have been identified as being at a greater risk for HCC. However, the correlation between the levels of high-density lipoprotein cholesterol with HCC remains uncertain[85,88].
Furthermore, diabetic patients may experience weakened immune systems[89,90], which could increase the risk of developing chronic HBV or hepatitis C virus (HCV) infections, both of which are significant risk factors for HCC[91-95]. Although the connection between DM and HCC risk appears to be stronger in HCV than HBV, a United States study involving 52671 HCV-liver cirrhosis patients (including 7605 HCC cases) did not find a significant association between DM and HCC risk[96]. Moreover, immunosuppressive medications prescribed to manage diabetes-related complications, such as kidney and pancreas transplants, might also contribute to an elevated risk of HCC development[97-99].
Overall, the increased prevalence of HCC in diabetic patients is due to a combination of factors outlined in Figure 1. Further research is necessary to comprehensively understand the mechanisms linking diabetes and HCC and to devise effective strategies for preventing and treating HCC in diabetic patients.
Early detection of HCC is crucial for successful treatment. Although HCC can be diagnosed early in 30%-60% of cases, recurrences can still affect up to 80% of patients within five years, even after receiving curative treatments[100].
Screening for HCC in diabetic patients is challenging due to the high prevalence of coexisting liver illnesses such as NAFLD and NASH, and there is currently a lack of effective methods to monitor NAFLD-related HCC[101]. Although guidelines recommend regular HCC surveillance for high-risk individuals, which involves at least once every six months of liver ultrasonography and serum alpha-fetoprotein monitoring[102-106], the insidious onset of HCC often leads to late detection. Consequently, it is crucial to establish effective monitoring strategies and ensure early diagnosis and treatment to enhance patient outcomes.
The lack of official guidelines for NAFLD-related HCC diagnosis criteria results in clinical symptoms being the primary diagnostic tool, which can lead to late detection of the disease. Patients with NAFLD-related cirrhosis are considered high-risk subgroups for HCC, and ultrasonography is the primary surveillance test[107-109]. Imaging modalities such as ultrasound, computed tomography, and magnetic resonance imaging are commonly used for HCC screening in diabetic patients, and diagnostic accuracy can be improved by combining different imaging techniques[108-110].
The management of HCC in diabetic patients requires a multidisciplinary approach that considers the potential interactions between diabetes medications and cancer treatments, as well as their impact on glycaemic control[49,111-113]. Although surgical removal of the tumour and liver transplant are curative treatments for HCC, they may not be suitable for all diabetic patients due to the higher risk of surgical complications in this population. Furthermore, diabetic individuals with HCC are more likely to have advanced illnesses at the time of diagnosis, which may limit the effectiveness of these treatments[107-109].
Until recently, Sorafenib was the only medication approved by the United States Food and Drug Administration (FDA) for advanced HCC. Multi-kinase inhibitors like cabozantinib and ramucirumab have been approved as second-line treatments since 2017[114-116]. Nivolumab[117] and pembrolizumab[118], the checkpoint inhibitors have either received FDA approval or are currently being investigated. However, systemic therapies may pose significant challenges in managing side effects in patients with cirrhosis[119]. Moreover, the high cost of approved medications makes their usage difficult in low-income countries[120]. Thus, HCC prevention in high-risk individuals could be a viable alternative to HCC treatment since identifying high-risk individuals is possible, and the survival rates after diagnosis are low. Local therapies such as radiofrequency ablation, percutaneous ethanol injections, and transarterial chemoembolization (TACE) are available options for early-stage HCC[121-125]. Among these options, TACE is commonly used as a treatment approach. TACE involves the direct delivery of chemotherapy drugs into the blood vessels that supply the tumour, followed by the injection of embolic agents to obstruct the tumour’s blood flow. This targeted approach allows for the direct impact on the tumour while minimizing the systemic effects of chemotherapy. TACE is often recommended for patients with early-stage HCC who are not suitable candidates for surgery or liver transplantation. Additionally, it can serve as a bridge therapy prior to other definitive treatments or as a palliative measure to reduce tumor size and alleviate symptoms[124,125]. Systemic therapy with chemotherapy, targeted therapy, or immunotherapy may be used for advanced-stage HCC, but the choice of treatment should consider the potential interactions with diabetes medications and their impact on glycaemic control[126-128].
Preventing HCC development in diabetic patients is a crucial objective of diabetes management. Lifestyle adjustments, such as weight loss, exercise, and dietary changes, can enhance glycaemic control, diminish the risk of developing NAFLD and NASH, which are vital risk factors for HCC[101,109,111], and also minimize the risk of HCC[102,109,129]. Regular HCC screening for diabetic patients can facilitate early detection of the disease when curative therapies are more likely to succeed. The American Association for the Study of Liver Diseases recommends that diabetic patients with cirrhosis and advanced fibrosis undergo ultrasound screening every six months for HCC[107].
In addition, antidiabetic drugs may also aid in preventing HCC development in diabetic patients. Metformin, in particular, has demonstrated a protective effect against HCC development in some studies, potentially because of its anti-neoplastic, anti-inflammatory, and anti-fibrotic effects[49,64,68-70]. However, the use of other antidiabetic medications like insulin may raise the risk of HCC, though further investigation is necessary to completely comprehend the association between diabetes medications and HCC risk[67].
The timely diagnosis and detection of HCC is crucial for improving patient outcomes, which has led to an increased interest in identifying biomarkers for early detection. Resistin has emerged as a promising candidate in this regard. First identified in 2001, resistin is a hormone that is implicated in IR and is characterized by its pro-inflammatory properties. Resistin is predominantly synthesized by adipocytes in rodents. In contrast, in humans, while adipocytes have the capacity to synthesize resistin, the hormone is primarily produced by immune cells called macrophages, which are integral to immune responses and inflammation. The presence of resistin in human serum typically ranges within a physiological concentration of 7-22 ng/mL[130]. The RETN gene encodes resistin protein, which is also known as “adipocyte-specific secretory factor, Fizz3, RSTN, or cysteine-rich protein 1”[131-134].
Resistin contributes to IR by inducing persistent low-grade inflammation associated with obesity-induced macrophage infiltration in adipose tissues. Furthermore, resistin promotes p38 MAPK signaling, altering insulin signaling, modulating the cellular oxidative stress response, and enhancing cells proliferation by increasing the production of various inflammatory molecules such as interleukin (IL)-1β, IL-6, IL-8, IL-12, and tumor necrosis factor-alpha (TNF-α)[34,135-137]. Elevated resistin expression has been associated with inflammation, autoimmune illnesses, metabolic diseases, and malignant conditions, suggesting that it could be a reliable biomarker for HCC diagnosis, early detection, and prognosis[131,132,138,139].
Several clinical studies have investigated the potential of resistin as a diagnostic and prognostic biomarker for HCC[33,140-142]. Studies have shown that resistin is expressed in HCC tissue and is involved in the progression of HCC through its effects on cell proliferation, apoptosis, invasion, and angiogenesis[138,143-145]. Serum resistin levels have been found to be positively correlated with tumour size, TNM stage, and vascular invasion, highlighting that resistin might be a helpful HCC predictive biomarker[32]. Moreover, resistin has been assessed as a diagnostic biomarker for HCC in combination with other biomarkers, such as alpha-fetoprotein and des-gamma-carboxy prothrombin. The combination has been reported to exhibit higher diagnostic accuracy compared to any of the biomarkers alone[32,146,147].
Yagmur et al[139] investigated the clinical significance of resistin in CLD by measuring serum resistin levels in 82 CLD patients and 76 age and gender-matched healthy controls, and monitoring the patients for six years. The study found that resistin levels were significantly higher in liver cirrhosis patients compared to the healthy controls, with levels increasing as cirrhosis advanced. Resistin levels also showed a positive correlation with insulin secretion, a negative correlation with insulin sensitivity, and associations with inflammatory markers and clinical complications. The study concluded that patients with higher resistin levels had increased mortality within six years, suggesting that resistin could be a useful clinical biomarker for evaluating liver cirrhosis and its potential link to IR in patients with severe liver disease[140].
Da Silva et al[141] conducted a prospective cohort study to investigate potential factors associated with adiponectin and resistin levels in cirrhosis patients and their clinical significance. The study involved 122 cirrhosis patients from an outpatient clinic and a control group of 30 healthy subjects. The study found that patients with cirrhosis had higher adiponectin and resistin levels compared to the control group. Adiponectin levels, but not resistin well established levels, were significantly associated with the severity of liver dysfunction and a worse prognosis in patients with alcoholic liver disease, suggesting a potential role as a prognostic biomarker[142].
A recently published systematic review and meta-analysis investigated the correlation between serum resistin levels and NAFLD in adults. The review comprised 28 studies that included 4088 participants, which were analyzed using meta-analysis techniques. The study findings indicated that patients with NAFLD had considerably higher serum resistin levels when compared to healthy individuals. In contrast, patients with NASH had lower serum resistin levels than healthy controls. No significant difference was observed in serum resistin levels between patients with NAFLD and healthy controls or between patients with NAFLD and NASH. The study also suggested that serum resistin may be a potential biomarker for predicting the risk of developing NAFLD, a known risk factor for HCC, and could also differentiate between NAFLD and NASH. However, further research is necessary to support these findings and to comprehend the underlying mechanisms of this association[148].
A thorough search was executed on PubMed and Google Scholar databases, employing a set of keywords including “resistin and HCC”, “resistin and hepatocellular carcinoma”, “resistin and liver cancer”, “resistin as a biomarker for hepatocellular carcinoma”, and “resistin and hepatic cancer” with the objective of identifying research that investigates the role of resistin as a biomarker specifically for HCC. The search yielded four studies that delved into the clinical utility of resistin in the diagnosis and prognosis of HCC. The ensuing compilation of studies is presented in a chronological fashion, commencing with the pioneering study conducted in 2014 and culminating with the latest research from 2022.
Elbedewy et al[30] conducted a study to investigate whether serum resistin and IR could be considered as risk factors for HCC in HCV-cirrhotic patients with T2DM. The study involved 50 adult patients with HCV infection who were categorized into three groups based on their HCC status, and were subjected to routine tests for DM, HCV, liver cirrhosis, and HCC. The results revealed that patients with HCC and diabetes (group I) had significantly higher levels of homeostasis model assessment-IR (HOMA-IR) and resistin than diabetic patients with cirrhosis (group II) and control subjects (group III). The study concluded that HOMA-IR and serum resistin could potentially serve as novel biomarkers to identify HCV-cirrhotic patients with T2DM who are at a greater risk of developing HCC[30].
Furthermore, a prospective case-control study was conducted by Elsayed et al[33] to investigate the implications of IR and serum resistin as possible risk factors for HCC among individuals with HCV-related liver cirrhosis. The study involved 200 patients with HCV-related liver cirrhosis (100 with HCC and 100 without HCC) as well as 50 healthy controls. The study found that patients with HCC had significantly higher levels of resistin and HOMA-IR than cirrhotic patients and healthy controls. Patients with resistin levels more than or equal to 12 ng/mL and HOMA-IR values higher than or equal to 4 were 1.6 times more likely to experience HCC. These data imply that HOMA as well as serum resistin, may be useful in identifying HCV-cirrhotic individuals at high risk of developing HCC[33].
Mohamed et al[32] conducted a study to evaluate the potential of serum resistin levels as a biomarker for assessing response to therapy in individuals with hepatic cirrhosis and HCC. The study included 50 patients with HCV-related cirrhosis, 30 of whom had HCC and the remaining 20 did not. Patients with HCC had higher levels of serum resistin, which showed strong positive correlations with hepatic focal lesions, portal vein invasion, total bilirubin, international normalized ratio, and model of end-stage liver disease score. After one month of HCC intervention, serum resistin levels were significantly lower than before the intervention. These findings suggest that serum resistin could be used as a reliable biomarker for evaluating treatment response in HCC patients[32].
More recently, Ashour et al[31] conducted a case-control study to investigate the relationship between serum resistin levels and HCC in patients with liver cirrhosis. The study included 80 cirrhotic patients (40 with HCC and 40 without HCC). The results showed that serum resistin levels were significantly higher in the HCC group compared to the control group, with a strong positive correlation between resistin and total cholesterol and low-density lipoprotein. Additionally, the study found that resistin levels could be used as a diagnostic marker for HCC, with a sensitivity of 90% and specificity of 95% at a cutoff value of > 13.7 ng/mL. These findings support the use of serum resistin levels as a diagnostic biomarker for HCC in patients with liver cirrhosis[31]. Table 1 provides a summary of the above-mentioned studies.
| Study ID | Ref. | Study design | Number of participants (HCC/cirrhosis/control) | Age range or mean ± SD | Sex distribution | Resistin levels (ng/mL) (HCC) | Resistin levels (ng/mL) (cirrhosis) | Resistin levels (ng/mL) (control) | Main findings | Resistin as HCC biomarker |
| 1 | Elbedewy et al[30], 2014, Egypt | Prospective case-control study | 25 (HCC), 25 (cirrhosis), 25 (control) | HCC: Mean 53.92 ± 5.9 yr; range 43-65 yr. Cirrhosis: Mean 52.92 ± 7.371 yr; range 40-66 yr. Control: Mean 51.4 ± 6.028 yr; range 38-63 yr | HCC: 19 males/6 females. Cirrhosis: 17 males/8 females. Control: 20 males/5 females | 6.11 ± 1.6541 | 3.11 ± 1.5331 | 1.31 ± 0.31981 | Patients with HCC have significantly higher mean value of resistin than cirrhotic patients and control subjects | Promising biomarker for HCC |
| 2 | Elsayed et al[33], 2015, Egypt | Prospective case-control | 100 (HCC)/ 100 (cirrhosis)/ 50 (control) | 52.3 (HCC), 52.2 (cirrhosis), 51 (control) | 85% male (HCC), 66% male (cirrhosis) | 23.8 ± 7.81 | 9.9 ± 2.71 | 7.1 ± 1.81 | HCC patients had higher HOMA-IR and resistin levels; resistin and HOMA considered independent risk factors for HCC | Yes |
| 3 | Mohamed et al[32], 2018, Egypt | Prospective case-control | 50 (HCC)/-/25 (control) | 59.8 ± 9.6 (HCC), 57.6 ± 10.1 (control) | 37 males/13 females (HCC), 18 males/7 females (control) | 5.5 ± 1.71 (pre-treatment) | - | 3.3 ± 1.11 | Higher resistin levels in HCC patients compared to controls, and significant reduction in resistin levels after treatment | Yes |
| 4 | Ashour et al[31], 2022, Egypt | Case-control study | 80 (40 HCC/40 cirrhosis/0 control) | HCC: Median 62 yr (range 18-75). Cirrhosis: Median 59 yr (range 48-72) | HCC: 23 males/17 females. Cirrhosis: 25 males/15 females | 19.42 | 3.42 | N/A | Higher serum resistin levels in HCC patients compared to cirrhotic patients; resistin > 13.7 ng/mL able to diagnose HCC with 90% sensitivity and 95% specificity | Promising biomarker for HCC |
Resistin has been implicated in cancer development through various signaling pathways. Among these, the toll-like receptor 4 (TLR4), PI3K, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκβ) pathways are particularly noteworthy. These signaling cascades are instrumental in modulating various cellular processes that are crucial for cancer development and progression. In the context of different cancer types, these signaling pathways have been demonstrated to play an imperative role in fostering cellular proliferation. Notably, specific pathways are often selectively associated with distinct types of cancer. For instance, the AKT pathway, a downstream effector of PI3K, has been predominantly associated with prostate cancer, serving as a key regulator in promoting cell survival and growth[149]. Conversely, lung cancer demonstrates a more complex network of signaling pathways. Among these, PI3K, NFκβ, epidermal growth factor receptor, and TLR4 have been implicated[150]. These pathways collaboratively contribute to the progression of lung cancer through mechanisms such as cell proliferation, angiogenesis, and resistance to apoptosis.
Additionally, melanoma, a malignancy of melanocytes, has been found to be under the influence of distinct signaling axes, such as the phosphorylated AKT and Caveolin-1, which are critical in dictating the course of the disease[151].
Resistin’s influence extends beyond these pathways; it has been implicated in the activation of the IL-6 dependent signal transducer and activator of transcription 3 (STAT3) signaling pathway[152,153]. This pathway is especially noteworthy in breast cancer progression. Moreover, resistin has been linked to the progression of ovarian cancer through the modulation of microRNAs, such as “miR let-7a, miR-200c, and miR-186”[154].
Diving deeper into the IL-6/STAT3 axis, this signaling pathway has been linked with various aspects of cancer biology, including tumor progression, metastasis, and therapy resistance in diverse cancer types such as breast, colorectal, and HCC. The crux of this axis lies in the overexpression of IL-6 and the consequent hyperactivation of STAT3, a combination frequently associated with a grim prognosis. Resistin further exacerbates this by promoting the secretion of pro-inflammatory cytokines like IL-6, which activates STAT3. This sets into motion an autocrine loop intensifying STAT3 signaling, leading to aggressive tumor behavior[153,155,156].
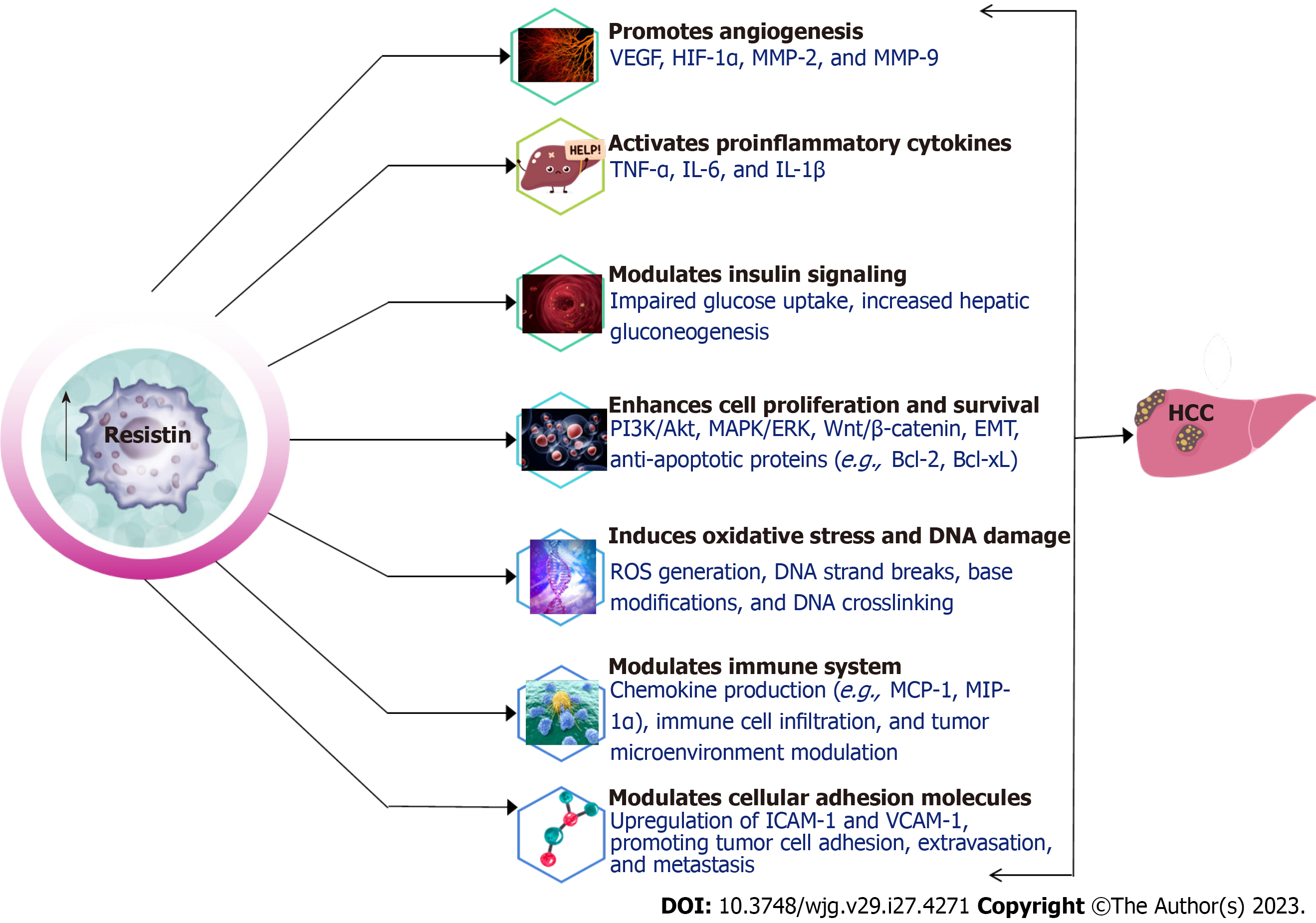
Intriguingly, Resistin dons yet another hat - that of a potential tumor suppressor. It has been shown to induce cell cycle arrest in colon cancer cells through the upregulation of suppressor of cytokine signaling 3 (SOCS3)[157]. SOCS3, part of the SOCS protein family, is integral in curbing cytokine signaling. This implies that resistin may play a role in keeping the proliferation of colon cancer cells in check, revealing its context-dependent pleiotropic nature. In summary, resistin orchestrates a plethora of pathways, including the activation of pro-inflammatory cytokines, fostering angiogenesis, modulating insulin signaling, and influencing cell proliferation and survival[155,156,158]. Its context-dependent roles in both promoting and potentially suppressing tumors accentuate the complexity of resistin’s function in cancer. This warrants a nuanced understanding and approach in considering resistin as a potential target for therapeutic interventions.
The role of resistin in activating proinflammatory cytokines has been well-established in various studies. These cytokines, including TNF-α, IL-6, and monocyte chemoattractant protein-1 are crucial in inflammation, cell proliferation, and apoptosis, all of which contribute to HCC development and progression[159-163].
Resistin has been demonstrated to directly stimulate TNF-α and IL-6 production in macrophages by binding to TLR4 and initiating downstream signaling pathways, such NFκβ[164,165]. The activation of the NFκB pathway results in the transcription of proinflammatory cytokines, perpetuating chronic inflammation associated with an increased risk of HCC[166].
In addition to direct stimulation, resistin activates other inflammatory pathways, notably the c-Jun N-terminal kinase and STAT3 pathways[167-170]. These pathways contribute to the production of proinflammatory cytokines and are implicated in the pathogenesis of HCC[168,169,171]. Moreover, resistin-induced cytokines have been linked to hepatic stellate cell activation, subsequently leading to liver fibrosis and cirrhosis, both of which are significant precursors to HCC[172-174]. In this regard, resistin’s activation of proinflammatory cytokines serves as a vital connection between resistin and the development of HCC in diabetic patients.
In addition to resistin’s proinflammatory effect, resistin has been implicated in macrophage activation and polarization of tumor-associated macrophages towards the M2 phenotype[175]. M2 macrophages are characterized by their pro-tumorigenic properties, such as promoting angiogenesis, immunosuppression, and tissue remodeling, which further advance HCC progression[176].
Additionally, resistin has been implicated in the modulation of the immune response through increased expression of the macrophage inflammatory protein-alpha (MIP-α), a chemokine also known as CC chemokine ligand 3[177]. It is a small signaling protein secreted by various immune cells, including macrophages, T cells, and dendritic cells. MIP-α plays a crucial role in immune response modulation by attracting and activating leukocytes, particularly monocytes and neutrophils, to the site of inflammation or infection. This chemokine is involved in various biological processes, including inflammation, immune cell activation, and the regulation of cell migration during an immune response. MIP-α has also been implicated in the progression of certain diseases, such as autoimmune disorders, chronic inflammatory conditions, and even cancer, due to its ability to modulate immune responses[178]. Furthermore, resistin has been reported to modulate the function of other immune cell populations, including natural killer cells and T lymphocytes, impairing their antitumor activities and allowing HCC tumor evasion[179,180]. Taken together, these findings highlight the multifaceted role of resistin in HCC pathogenesis through the modulation of the immune responses and tumour microenvironment.
Angiogenesis, the formation of new blood vessels from existing ones, is a pivotal process in tumor growth, invasion, and metastasis. Resistin promotes angiogenesis through various mechanisms, including the upregulation of vascular endothelial growth factor (VEGF), activation of the hypoxia-inducible factor-1α (HIF-1α) pathway, and modulation of other signaling pathways[181-184].
VEGF, which spurs endothelial cell proliferation, migration, and survival, is crucial for angiogenesis[185]. Studies have demonstrated that resistin boosts VEGF expression in different cell types, including cancer cells[186,187]. In a study by Tsai et al[178], resistin was found to upregulate VEGF expression in osteosarcoma via the activation of NFκβ signaling[181]. Furthermore, Chen et al[179] demonstrated that resistin enhanced VEGF formation in human chondrosarcoma cells through a PI3K/AKT-dependent mechanism[182]. Elevated VEGF expression in HCC tissue has been associated with a worse prognosis[188,189].
Resistin can also contribute to angiogenesis by stimulating the HIF-1α pathway[190]. Under hypoxic conditions, HIF-1α is stabilized and translocated to the nucleus, where it binds to hypoxia-response elements and promotes the transcription of various genes, including VEGF[191]. It has been demonstrated that resistin increased HIF-1α expression in human adipocytes under hypoxia, leading to enhanced VEGF expression[182].
Insulin signaling is critical for maintaining glucose homeostasis and is often disrupted in diabetes, obesity, and cancer. Resistin has been implicated in modulating insulin signaling, contributing to IR and affecting HCC development in diabetic patients[158].
Resistin has been demonstrated to disrupt insulin-driven glucose uptake in peripheral tissues like adipose tissue and skeletal muscle by interfering with insulin signaling[192,193]. Fu et al[191] found that resistin inhibited insulin-induced glucose uptake in 3T3-L1 adipocytes by reducing the activity of glucose transporters[194]. Similar results were observed in skeletal muscle cells, where resistin impaired insulin-stimulated glucose uptake by decreasing IRS-1-associated PI3K activity[195].
Resistin has been shown to affect liver glucose metabolism by enhancing hepatic gluconeogenesis[196]. In a study by Rajala et al[193], resistin administration in mice led to elevated liver glucose production and hyperglycemia. The researchers suggested that this effect occurs via activating the cAMP-protein kinase A pathway.
In L6 rat myotubes, resistin overexpression was shown to hinder insulin-mediated glucose uptake without altering glucose transporter type 4 (GLUT4) translocation, GLUT1 expression, or IRS signaling[197]. Moreover, resistin or RELMβ infusion in adult male Sprague Dawley rats led to hepatic IR, characterized by increased hepatic glucose production via AMP-activated protein kinase[198]. Lastly, resistin treatment in rat hepatocytes and mice with liver-specific resistin expression resulted in impaired hepatic insulin action by reducing phosphorylation of GSK3β at serine 9. This observation was made in a study conducted on C57BL/6 J mice[199].
The modulation of insulin signaling by resistin may indirectly contribute to HCC development by exacerbating hyperinsulinemia and IR in diabetic patients. Hyperinsulinemia has been associated with an increased risk of HCC, as insulin can promote cell proliferation and survival through the activation of mitogenic signaling pathways, such as the PI3K/Akt and Ras/MAPK pathways[200,201]. Furthermore, IR may lead to chronic inflammation and liver damage, which can promote HCC development[202,203]. Hence, resistin modulates insulin signaling by impairing insulin-stimulated glucose uptake and promoting hepatic gluconeogenesis. These effects may indirectly contribute to HCC development in diabetic patients by exacerbating hyperinsulinemia, IR, and inflammation.
Matrix metalloproteinases (MMPs) are enzymes responsible for the breakdown of extracellular matrix components, thereby aiding tumour invasion and metastasis[204]. Resistin has been found to increase MMP expression in various cell types, including endothelial cells and cancer cells[136,205]. In a study by Di Simone et al[203], resistin was shown to stimulate MMP-2 production in human endothelial cells, potentially contributing to angiogenesis and tumour invasion[206]. Similarly, Huang et al[207] demonstrated that resistin increased MMP-2 and MMP-9 expression in human colorectal cancer cells. Furthermore, a study by Tsai et al[178] revealed that resistin enhanced MMP-2 expression via the activation of the AMPK/p38 signaling pathway and downregulation of miR-519d, contributing to chondrosarcoma metastasis[208].
Moreover, resistin has been implicated in the promotion of HCC metastasis through the upregulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, both of which are critical mediators of cancer cell adhesion and migration. Evidence suggests that resistin stimulates the expression of these adhesion molecules in endothelial cells[206], thereby facilitating the adhesion and transendothelial migration of HCC cells. Taken together, the available evidence suggests that resistin contributes to HCC progression and metastasis through multiple mechanisms, including the promotion of angiogenesis, upregulation of VEGF, activation of the HIF-1α pathway, induction of MMP expression, and increased expression of cell adhesion molecules.
Resistin has been associated with the enhancement of cell proliferation as well as survival in various cell types, including cancer cells, which can contribute to HCC development and progression in diabetic patients. This is achieved through the activation of mitogenic signaling pathways like PI3K/AKT and MAPK/ERK[135,140,183,207,208]. These pathways have been found to mediate cell proliferation and HCC progression[207,208]. Additionally, resistin promotes cell survival by upregulating anti-apoptotic proteins like Bcl-2 and Bcl-xL[145]. For instance, a study by Pang et al[144] demonstrated that resistin increased Bcl-2 expression in human myeloma cell lines, contributing to improved cell survival and resistance to chemotherapy, potentially through the activation of the NFκβ signaling pathway.
Moreover, resistin impacts cell cycle regulation. Research revealed that resistin increased the expression of cyclin D1 in colon cancer patients[157]. Cyclin D1 is integral for cell cycle progression, particularly for the transition from the G1 to S phase. This upregulation of cyclin D1 was suggested to be a result of SOCS 3 upregulation, mediated by the activation of the ERK signaling pathway[157].
Overall, resistin contributes to cell proliferation and survival through various mechanisms, including the activation of mitogenic signaling pathways, induction of anti-apoptotic proteins, and modulation of cell cycle regulation. Figure 2 provides a schematic representation of the mechanisms through which resistin influences the development and metastasis of HCC.
Resistin, an adipokine implicated in obesity and IR, plays a crucial role in promoting HCC development and progression in diabetic patients. There is evidence supporting the potential use of resistin as a biomarker for HCC in diabetic patients. The potential mechanisms through which resistin promotes HCC include the activation of proinflammatory cytokines, promotion of angiogenesis, modulation of insulin signaling, and enhancement of cell proliferation and survival. These diverse effects highlight the need for further research to better understand the complex interplay between resistin and HCC development in diabetic patients.
This review, while offering a comprehensive overview of resistin’s role in HCC development among diabetic patients, possesses several limitations. First, the scope is bound to the literature available at the time, which may not encompass recent developments. Additionally, the multifaceted molecular mechanisms discussed are highly complex, and there may be aspects not extensively covered here. It is also imperative to differentiate between correlation and causation, as the review addresses associations but does not confirm causal relationships. Furthermore, the generalizability of the conclusion is uncertain, as genetic diversity, lifestyle factors, and additional health conditions could affect the interplay between resistin and HCC in different populations. Lastly, the reliance on studies that might contain biases or have their own limitations could inadvertently influence the conclusion drawn in this review. Recognizing these limitations is vital for a well-rounded understanding and highlights the importance of further studies.
Future studies should focus on identifying novel molecular targets for therapeutic intervention and developing strategies to counteract the deleterious effects of resistin on HCC development and progression. Understanding the molecular mechanisms underlying resistin’s role in HCC could lead to the development of novel diagnostic and therapeutic strategies for the management of HCC in diabetic patients. Additionally, the development of effective therapies targeting resistin and its associated signaling pathways may help mitigate the risk of HCC in diabetic patients, especially those with obesity and IR. Further research is required to assess the effectiveness and safety of such therapeutic strategies in preclinical and clinical settings.
Moreover, given the complexity of the molecular mechanisms underlying resistin’s role in HCC, a multidisciplinary approach involving collaboration between experts in endocrinology, oncology, and molecular biology is essential for advancing our understanding of this complex relationship. This collaborative effort may help identify potential biomarkers for early detection and prognosis of HCC in diabetic patients and facilitate the development of personalized medicine approaches.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Balbaa ME, Egypt; Yang L, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64265] [Article Influence: 16066.3] [Reference Citation Analysis (174)] |
| 2. | Chon YE, Park SY, Hong HP, Son D, Lee J, Yoon E, Kim SS, Ahn SB, Jeong SW, Jun DW. Hepatocellular carcinoma incidence is decreasing in Korea but increasing in the very elderly. Clin Mol Hepatol. 2023;29:120-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Oh JH, Jun DW. The latest global burden of liver cancer: A past and present threat. Clin Mol Hepatol. 2023;29:355-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 4. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2931] [Article Influence: 732.8] [Reference Citation Analysis (7)] |
| 5. | World Health Organization. International agency for research on cancer. [cited 15 February 2023]. Available from: https://www.iarc.who.int/. |
| 6. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1039] [Article Influence: 346.3] [Reference Citation Analysis (0)] |
| 7. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1219] [Article Influence: 243.8] [Reference Citation Analysis (0)] |
| 8. | Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: From diagnosis to treatment. Surg Oncol. 2016;25:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 333] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 9. | Askoura M, Abbas HA, Al Sadoun H, Abdulaal WH, Abu Lila AS, Almansour K, Alshammari F, Khafagy ES, Ibrahim TS, Hegazy WAH. Elevated Levels of IL-33, IL-17 and IL-25 Indicate the Progression from Chronicity to Hepatocellular Carcinoma in Hepatitis C Virus Patients. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 10. | Péneau C, Imbeaud S, La Bella T, Hirsch TZ, Caruso S, Calderaro J, Paradis V, Blanc JF, Letouzé E, Nault JC, Amaddeo G, Zucman-Rossi J. Hepatitis B virus integrations promote local and distant oncogenic driver alterations in hepatocellular carcinoma. Gut. 2022;71:616-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 131] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 11. | Luna-Cuadros MA, Chen HW, Hanif H, Ali MJ, Khan MM, Lau DT. Risk of hepatocellular carcinoma after hepatitis C virus cure. World J Gastroenterol. 2022;28:96-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 12. | Kudo M. Prioritized Requirements for First-Line Systemic Therapy for Hepatocellular Carcinoma: Broad Benefit with Less Toxicity. Liver Cancer. 2023;12:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 795] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 14. | Nakashima T, Kojiro M, Nakashima T. Hepatocellular carcinoma and liver cirrhosis. In: Hepatocellular Carcinoma: An Atlas of Its Pathology. Janpan: Springer Tokyo, 1987: 185-204. |
| 15. | Tillman HL, Choi SS. Risk for hepatocellular carcinoma in patients with alcoholic cirrhosis. Ann Intern Med. 2012;157:677-8; author reply 678. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Ioannou GN, Green P, Kerr KF, Berry K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J Hepatol. 2019;71:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 17. | Thomas CE, Diergaarde B, Kuipers AL, Adibi JJ, Luu HN, Chang X, Dorajoo R, Heng CK, Khor CC, Wang R, Jin A, Koh WP, Yuan JM. NAFLD polygenic risk score and risk of hepatocellular carcinoma in an East Asian population. Hepatol Commun. 2022;6:2310-2321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Shah PA, Patil R, Harrison SA. NAFLD-related hepatocellular carcinoma: The growing challenge. Hepatology. 2023;77:323-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 129] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 19. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1700] [Article Influence: 242.9] [Reference Citation Analysis (0)] |
| 20. | Brahma MK, Gilglioni EH, Zhou L, Trépo E, Chen P, Gurzov EN. Oxidative stress in obesity-associated hepatocellular carcinoma: sources, signaling and therapeutic challenges. Oncogene. 2021;40:5155-5167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 22. | Mohamed J, Nazratun Nafizah AH, Zariyantey AH, Budin SB. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16:e132-e141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 23. | Sakuma T, Nakamura M, Chiba T, Iwanaga T, Kan M, Kojima R, Ao J, Ma Y, Unozawa H, Fujita N, Kanayama K, Kanzaki H, Koroki K, Kobayashi K, Nakagawa R, Kanogawa N, Kiyono S, Kondo T, Saito T, Ogasawara S, Nakamoto S, Muroyama R, Kato J, Kishimoto T, Kato N. A diet-induced murine model for non-alcoholic fatty liver disease with obesity and insulin resistance that rapidly develops steatohepatitis and fibrosis. Lab Invest. 2022;102:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Guerra S, Mocciaro G, Gastaldelli A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur J Clin Invest. 2022;52:e13695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Tian Y, Wong VW, Wong GL, Yang W, Sun H, Shen J, Tong JH, Go MY, Cheung YS, Lai PB, Zhou M, Xu G, Huang TH, Yu J, To KF, Cheng AS, Chan HL. Histone Deacetylase HDAC8 Promotes Insulin Resistance and β-Catenin Activation in NAFLD-Associated Hepatocellular Carcinoma. Cancer Res. 2015;75:4803-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Matoori S. Diabetes and its Complications. ACS Pharmacol Transl Sci. 2022;5:513-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 513] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 28. | Nakatsuka T, Tateishi R. Development and prognosis of hepatocellular carcinoma in patients with diabetes. Clin Mol Hepatol. 2023;29:51-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 29. | Mossenta M, Busato D, Dal Bo M, Toffoli G. Glucose Metabolism and Oxidative Stress in Hepatocellular Carcinoma: Role and Possible Implications in Novel Therapeutic Strategies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Elbedewy MM, Ghazy MA, Elbedewy TA, Suliman GA. Serum Resistin and Insulin Resistance as Risk Factors for Hepatocellular Carcinoma in Cirrhotic Patients with Type 2 Diabetes Mellitus. Life Sci J. 2014;11:941-949. [DOI] [Full Text] |
| 31. | Ashour M, Maher F, Hussein M, Mohamed AE, Elnagar A. Assessment Of Serum Resistin Relation To Hepatocellular Carcinoma In Patient With Liver Cirrhosis. Neuroquantology. 2022;. [DOI] [Full Text] |
| 32. | Mohamed IEK, Rasmy HS, Aly WAE. Evaluation of serum resistin levels in patients with hepatocellular carcinoma before and after treatment. Egyptian Liver J. 2018;8:61-67. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Elsayed EY, Mosalam NA, Mohamed NR. Resistin and Insulin Resistance: A Link Between Inflammation and Hepatocarcinogenesis. Asian Pac J Cancer Prev. 2015;16:7139-7142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Chen C, Jiang J, Lü JM, Chai H, Wang X, Lin PH, Yao Q. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299:H193-H201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 35. | Duan XF, Tang P, Li Q, Yu ZT. Obesity, adipokines and hepatocellular carcinoma. Int J Cancer. 2013;133:1776-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 891] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 37. | Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 38. | Seyda Seydel G, Kucukoglu O, Altinbasv A, Demir OO, Yilmaz S, Akkiz H, Otan E, Sowa JP, Canbay A. Economic growth leads to increase of obesity and associated hepatocellular carcinoma in developing countries. Ann Hepatol. 2016;15:662-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 39. | Han H, Zhang T, Jin Z, Guo H, Wei X, Liu Y, Chen Q, He J. Blood glucose concentration and risk of liver cancer: systematic review and meta-analysis of prospective studies. Oncotarget. 2017;8:50164-50173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, Chen CJ. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 41. | Koh WP, Wang R, Jin A, Yu MC, Yuan JM. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Miele L, Bosetti C, Turati F, Rapaccini G, Gasbarrini A, La Vecchia C, Boccia S, Grieco A. Diabetes and Insulin Therapy, but Not Metformin, Are Related to Hepatocellular Cancer Risk. Gastroenterol Res Pract. 2015;2015:570356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: A prospective cohort study in Korea. Cancer. 2018;124:2748-2757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Simon TG, King LY, Chong DQ, Nguyen LH, Ma Y, VoPham T, Giovannucci EL, Fuchs CS, Meyerhardt JA, Corey KE, Khalili H, Chung RT, Zhang X, Chan AT. Diabetes, metabolic comorbidities, and risk of hepatocellular carcinoma: Results from two prospective cohort studies. Hepatology. 2018;67:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 45. | Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 46. | Wang MD, Shen F, Zeng YY, Yang T. ASO Author Reflections: Effect of Preoperative Metabolic Syndrome for Patients with Hepatocellular Carcinoma. Ann Surg Oncol. 2023;30:359-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 47. | Shi T, Kobara H, Oura K, Masaki T. Mechanisms Underlying Hepatocellular Carcinoma Progression in Patients with Type 2 Diabetes. J Hepatocell Carcinoma. 2021;8:45-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Ali Kamkar MM, Ahmad R, Alsmadi O, Behbehani K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-review. J Diabetes Metab Disord. 2014;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Plaz Torres MC, Jaffe A, Perry R, Marabotto E, Strazzabosco M, Giannini EG. Diabetes medications and risk of HCC. Hepatology. 2022;76:1880-1897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 50. | Mantovani A, Targher G. Type 2 diabetes mellitus and risk of hepatocellular carcinoma: spotlight on nonalcoholic fatty liver disease. Ann Transl Med. 2017;5:270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 51. | Cai X, Gao J, Hu J, Wen W, Zhu Q, Wang M, Liu S, Hong J, Wu T, Yang S, Tuerxun G, Li N. Dose-Response Associations of Metabolic Score for Insulin Resistance Index with Nonalcoholic Fatty Liver Disease among a Nonobese Chinese Population: Retrospective Evidence from a Population-Based Cohort Study. Dis Markers. 2022;2022:4930355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 52. | Saponaro C, Sabatini S, Gaggini M, Carli F, Rosso C, Positano V, Armandi A, Caviglia GP, Faletti R, Bugianesi E, Gastaldelli A. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 2022;42:2418-2427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 53. | Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, Teng M, Syn N, Lim G, Yong JN, Quek J, Xiao J, Dan YY, Siddiqui MS, Sanyal AJ, Muthiah MD, Loomba R, Huang DQ. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 198] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 54. | Yip TC, Lee HW, Chan WK, Wong GL, Wong VW. Asian perspective on NAFLD-associated HCC. J Hepatol. 2022;76:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 55. | Kumar R, Priyadarshi RN, Anand U. Non-alcoholic Fatty Liver Disease: Growing Burden, Adverse Outcomes and Associations. J Clin Transl Hepatol. 2020;8:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 56. | Zarghamravanbakhsh P, Frenkel M, Poretsky L. Metabolic causes and consequences of nonalcoholic fatty liver disease (NAFLD). Metabol Open. 2021;12:100149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 57. | Björkström K, Franzén S, Eliasson B, Miftaraj M, Gudbjörnsdottir S, Trolle-Lagerros Y, Svensson AM, Hagström H. Risk Factors for Severe Liver Disease in Patients With Type 2 Diabetes. Clin Gastroenterol Hepatol. 2019;17:2769-2775.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | Li Z, Yang N, He L, Wang J, Ping F, Li W, Xu L, Zhang H, Li Y. Estimates and trends of the global burden of NASH-related liver cancer attributable to high fasting plasma glucose in 1990-2019: analysis of data from the 2019 Global Burden of Disease Study. Diabetol Metab Syndr. 2023;15:6. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Fabrizi F, Cerutti R, Alfieri CM, Ridruejo E. An Update on Hepatocellular Carcinoma in Chronic Kidney Disease. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Lee CH, Hsieh SY, Lin JL, Liu MS, Yen TH. Hepatocellular carcinoma in patients with chronic kidney disease. World J Gastroenterol. 2013;19:2466-2472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Ramanathan RS. Correlation of duration, hypertension and glycemic control with microvascular complications of diabetes mellitus at a tertiary care hospital. J Integr Mol Med. 2017;4:1-4. [DOI] [Full Text] |
| 62. | Kasmari AJ, Welch A, Liu G, Leslie D, McGarrity T, Riley T. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am J Med. 2017;130:746.e1-746.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Cunha V, Cotrim HP, Rocha R, Carvalho K, Lins-Kusterer L. Metformin in the prevention of hepatocellular carcinoma in diabetic patients: A systematic review. Ann Hepatol. 2020;19:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 64. | Siddique A, Kowdley KV. Insulin resistance and other metabolic risk factors in the pathogenesis of hepatocellular carcinoma. Clin Liver Dis. 2011;15:281-296, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | De Minicis S, Agostinelli L, Rychlicki C, Sorice GP, Saccomanno S, Candelaresi C, Giaccari A, Trozzi L, Pierantonelli I, Mingarelli E, Marzioni M, Muscogiuri G, Gaggini M, Benedetti A, Gastaldelli A, Guido M, Svegliati-Baroni G. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS One. 2014;9:e97136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2010;30:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 68. | Tseng CH. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018;38:2018-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 69. | Casadei Gardini A, Faloppi L, De Matteis S, Foschi FG, Silvestris N, Tovoli F, Palmieri V, Marisi G, Brunetti O, Vespasiani-Gentilucci U, Perrone G, Valgiusti M, Granato AM, Ercolani G, Negrini G, Tamburini E, Aprile G, Passardi A, Santini D, Cascinu S, Frassineti GL, Scartozzi M. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer. 2017;86:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 70. | Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver Int. 2015;35:2203-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 71. | Maradagi T, Kumar R, Ponesakki G. Hyperglycaemia-induced human hepatocellular carcinoma (HepG2) cell proliferation through ROS-mediated P38 activation is effectively inhibited by a xanthophyll carotenoid, lutein. Diabet Med. 2022;39:e14713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Liu F, Sun Y, Liu B, Lu J, Li H, Zhu H, Gao H, Zhou X, Chang H. Insulin-like growth factor-1 induces epithelial-mesenchymal transition in hepatocellular carcinoma by activating survivin. Oncol Rep. 2018;40:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Zheng Y, Wu C, Yang J, Zhao Y, Jia H, Xue M, Xu D, Yang F, Fu D, Wang C, Hu B, Zhang Z, Li T, Yan S, Wang X, Nelson PJ, Bruns C, Qin L, Dong Q. Insulin-like growth factor 1-induced enolase 2 deacetylation by HDAC3 promotes metastasis of pancreatic cancer. Signal Transduct Target Ther. 2020;5:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 74. | Shi X, Teng F. Down-regulated miR-28-5p in human hepatocellular carcinoma correlated with tumor proliferation and migration by targeting insulin-like growth factor-1 (IGF-1). Mol Cell Biochem. 2015;408:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Li TT, Zhu D, Mou T, Guo Z, Pu JL, Chen QS, Wei XF, Wu ZJ. IL-37 induces autophagy in hepatocellular carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol Immunol. 2017;87:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Wu J, Zhu AX. Targeting insulin-like growth factor axis in hepatocellular carcinoma. J Hematol Oncol. 2011;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. J Clin Endocrinol Metab. 2000;85:4712-4720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Jabir NR, Ahmad S, Tabrez S. An insight on the association of glycation with hepatocellular carcinoma. Semin Cancer Biol. 2018;49:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Kaji K, Yoshiji H, Kitade M, Ikenaka Y, Noguchi R, Yoshii J, Yanase K, Namisaki T, Yamazaki M, Moriya K, Tsujimoto T, Kawaratani H, Akahane T, Uemura M, Fukui H. Impact of insulin resistance on the progression of chronic liver diseases. Int J Mol Med. 2008;22:801-808. [PubMed] |
| 80. | Filippatos T, Tsimihodimos V, Pappa E, Elisaf M. Pathophysiology of Diabetic Dyslipidaemia. Curr Vasc Pharmacol. 2017;15:566-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 81. | Shin HS, Jun BG, Yi SW. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin Mol Hepatol. 2022;28:773-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 46] [Reference Citation Analysis (0)] |
| 82. | Phan J, Ng V, Sheinbaum A, French S, Choi G, El Kabany M, Durazo F, Saab S, Tong M, Busuttil R, Han SH. Hyperlipidemia and Nonalcoholic Steatohepatitis Predispose to Hepatocellular Carcinoma Development Without Cirrhosis. J Clin Gastroenterol. 2019;53:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Osaki Y, Taniguchi S, Tahara A, Okamoto M, Kishimoto T. Metabolic syndrome and incidence of liver and breast cancers in Japan. Cancer Epidemiol. 2012;36:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Cho Y, Cho EJ, Yoo JJ, Chang Y, Chung GE, Jeong SM, Park SH, Han K, Shin DW, Yu SJ. Association between Lipid Profiles and the Incidence of Hepatocellular Carcinoma: A Nationwide Population-Based Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | Yi SW, Kim SH, Han KJ, Yi JJ, Ohrr H. Higher cholesterol levels, not statin use, are associated with a lower risk of hepatocellular carcinoma. Br J Cancer. 2020;122:630-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Sinn DH, Kang D, Cho SJ, Paik SW, Guallar E, Cho J, Gwak GY. Risk of hepatocellular carcinoma in individuals without traditional risk factors: development and validation of a novel risk score. Int J Epidemiol. 2020;49:1562-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Chiang CH, Lee LT, Hung SH, Lin WY, Hung HF, Yang WS, Sung PK, Huang KC. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology. 2014;59:2207-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 88. | Moutschen MP, Scheen AJ, Lefebvre PJ. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab. 1992;18:187-201. [PubMed] |
| 89. | Eibl N, Spatz M, Fischer GF, Mayr WR, Samstag A, Wolf HM, Schernthaner G, Eibl MM. Impaired primary immune response in type-1 diabetes: results from a controlled vaccination study. Clin Immunol. 2002;103:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 90. | Demir M, Serin E, Göktürk S, Ozturk NA, Kulaksizoglu S, Ylmaz U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eur J Gastroenterol Hepatol. 2008;20:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Zhang X, Zhu X, Ji Y, Li H, Hou F, Xiao C, Yuan P. Increased risk of hepatitis B virus infection amongst individuals with diabetes mellitus. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 92. | Fu SC, Huang YW, Wang TC, Hu JT, Chen DS, Yang SS. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with new onset diabetes: a nationwide cohort study. Aliment Pharmacol Ther. 2015;41:1200-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Tan Y, Wei S, Zhang W, Yang J, Yan L. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review. Cancer Manag Res. 2019;11:705-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 94. | Villar LM, Geloneze B, Vasques ACJ, Pires MLE, Miguel JC, da Silva EF, Marques VA, Scalioni LP, Lampe E. Prevalence of hepatitis B and hepatitis C among diabetes mellitus type 2 individuals. PLoS One. 2019;14:e0211193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Ioannou GN, Green P, Lowy E, Mun EJ, Berry K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS One. 2018;13:e0204412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Bhat M, Usmani SE, Azhie A, Woo M. Metabolic Consequences of Solid Organ Transplantation. Endocr Rev. 2021;42:171-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 97. | Wei Q, Gao F, Zhuang R, Ling Q, Ke Q, Wu J, Shen T, Zhang M, Xu X, Zheng S. A national report from China Liver Transplant Registry: steroid avoidance after liver transplantation for hepatocellular carcinoma. Chin J Cancer Res. 2017;29:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Bhat M, Mara K, Dierkhising R, Watt KDS. Immunosuppression, Race, and Donor-Related Risk Factors Affect De novo Cancer Incidence Across Solid Organ Transplant Recipients. Mayo Clin Proc. 2018;93:1236-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 99. | Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 100. | Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, Li L, Desiderio R, Thrift AP, Asch SM, Chu J, El-Serag HB. Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1828-1837.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 566] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 101. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6021] [Article Influence: 860.1] [Reference Citation Analysis (3)] |
| 102. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3224] [Article Influence: 460.6] [Reference Citation Analysis (1)] |
| 103. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 568] [Article Influence: 113.6] [Reference Citation Analysis (1)] |
| 104. | Korean Liver Cancer Association (KLCA); National Cancer Center (NCC), Goyang, Korea. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2019;20:1042-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 105. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2558] [Article Influence: 852.7] [Reference Citation Analysis (59)] |
| 106. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4932] [Article Influence: 704.6] [Reference Citation Analysis (9)] |
| 107. | Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2020;158:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (1)] |
| 108. | Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, Jang BK, Kim SG, Ahn SB, Kim H, Jun DW, Choi JI, Song DS, Kim W, Jeong SW, Kim MY, Koh H, Jeong S, Lee JW, Cho YK; Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2021;27:363-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 178] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 109. | Park HJ, Jang HY, Kim SY, Lee SJ, Won HJ, Byun JH, Choi SH, Lee SS, An J, Lim YS. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J Hepatol. 2020;72:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 110. | Singh MK, Das BK, Choudhary S, Gupta D, Patil UK. Diabetes and hepatocellular carcinoma: A pathophysiological link and pharmacological management. Biomed Pharmacother. 2018;106:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 111. | Kramer JR, Natarajan Y, Dai J, Yu X, Li L, El-Serag HB, Kanwal F. Effect of diabetes medications and glycemic control on risk of hepatocellular cancer in patients with nonalcoholic fatty liver disease. Hepatology. 2022;75:1420-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 112. | Arvanitakis K, Koufakis T, Kotsa K, Germanidis G. How Far beyond Diabetes Can the Benefits of Glucagon-like Peptide-1 Receptor Agonists Go? A Review of the Evidence on Their Effects on Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 113. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10244] [Article Influence: 602.6] [Reference Citation Analysis (2)] |
| 114. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1758] [Article Influence: 251.1] [Reference Citation Analysis (0)] |
| 115. | Zhu AX, Galle PR, Kudo M, Finn RS, Qin S, Xu Y, Abada P, Llovet J. A study of ramucirumab (LY3009806) vs placebo in patients with hepatocellular carcinoma and elevated baseline alpha-fetoprotein (REACH-2). J Clin Oncol. 2018;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 116. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3297] [Article Influence: 412.1] [Reference Citation Analysis (1)] |
| 117. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder VV, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox JJ, Daniele B, Ebbinghaus S, Chen E, Siegel AB, Zhu AX, Cheng AL. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 118. | Pinter M, Jain RK, Duda DG. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021;7:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (1)] |
| 119. | Zhang P, Yang Y, Wen F, He X, Tang R, Du Z, Zhou J, Zhang J, Li Q. Cost-effectiveness of sorafenib as a first-line treatment for advanced hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 120. | Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 121. | Rhim H, Lim HK. Radiofrequency ablation of hepatocellular carcinoma: pros and cons. Gut Liver. 2010;4 Suppl 1:S113-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Chen L, Sun J, Yang X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016;370:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 123. | Zhong BY, Jin ZC, Chen JJ, Zhu HD, Zhu XL. Role of Transarterial Chemoembolization in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol. 2023;11:480-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 124. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 149] [Article Influence: 74.5] [Reference Citation Analysis (1)] |
| 125. | Koulouris A, Tsagkaris C, Spyrou V, Pappa E, Troullinou A, Nikolaou M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J Hepatocell Carcinoma. 2021;8:387-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 126. | Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16:617-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 127. | Wu TC, Shen YC, Cheng AL. Evolution of systemic treatment for advanced hepatocellular carcinoma. Kaohsiung J Med Sci. 2021;37:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 128. | Islam MM, Poly TN, Walther BA, Yang HC, Jack Li YC. Statin Use and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 129. | Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 130. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3208] [Article Influence: 133.7] [Reference Citation Analysis (1)] |
| 131. | Al Hannan F, Culligan KG. Human resistin and the RELM of Inflammation in diabesity. Diabetol Metab Syndr. 2015;7:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 132. | Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276:11252-11256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 412] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 133. | Cao H, Hegele RA. Single nucleotide polymorphisms of the resistin (RSTN) gene. J Hum Genet. 2001;46:553-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 134. | Shen YH, Zhang L, Gan Y, Wang X, Wang J, LeMaire SA, Coselli JS, Wang XL. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727-7736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 135. | Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 136. | Codoñer-Franch P, Alonso-Iglesias E. Resistin: insulin resistance to malignancy. Clin Chim Acta. 2015;438:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 137. | Sudan SK, Deshmukh SK, Poosarla T, Holliday NP, Dyess DL, Singh AP, Singh S. Resistin: An inflammatory cytokine with multi-faceted roles in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 138. | Shi Y, Zhu N, Qiu Y, Tan J, Wang F, Qin L, Dai A. Resistin-like molecules: a marker, mediator and therapeutic target for multiple diseases. Cell Commun Signal. 2023;21:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 139. | Yagmur E, Trautwein C, Gressner AM, Tacke F. Resistin serum levels are associated with insulin resistance, disease severity, clinical complications, and prognosis in patients with chronic liver diseases. Am J Gastroenterol. 2006;101:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 140. | Elnagar MA. Assessment Of Serum Resistin Relation To Hepatocellular Carcinoma In Patient With Liver Cirrhosis. Neuroquantology. 2022;20:3295-3305. |
| 141. | da Silva TE, Costa-Silva M, Correa CG, Denardin G, Alencar MLA, Coelho MSPH, Muraro-Wildner L, Luiza-Bazzo M, González-Chica DA, Dantas-Correa EB, Narciso-Schiavon JL, Schiavon LL. Clinical Significance of Serum Adiponectin and Resistin Levels in Liver Cirrhosis. Ann Hepatol. 2018;17:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 142. | Gong WJ, Liu JY, Yin JY, Cui JJ, Xiao D, Zhuo W, Luo C, Liu RJ, Li X, Zhang W, Zhou HH, Liu ZQ. Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-κB pathway. Cancer Sci. 2018;109:2391-2400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 143. | Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, Park SH, Kim HS. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci Rep. 2016;6:18923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 144. | Pang J, Shi Q, Liu Z, He J, Liu H, Lin P, Cui J, Yang J. Resistin induces multidrug resistance in myeloma by inhibiting cell death and upregulating ABC transporter expression. Haematologica. 2017;102:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 145. | Sun H, Chua MS, Yang D, Tsalenko A, Peter BJ, So S. Antibody Arrays Identify Potential Diagnostic Markers of Hepatocellular Carcinoma. Biomark Insights. 2008;3:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Toniutto P, Fornasiere E, Fumolo E, Bitetto D. Risk factors for hepatocellular carcinoma recurrence after liver transplantation. Hepatoma Res. 2020;6:50. [DOI] [Full Text] |
| 147. | Han D, Chen J, Liu S, Zhang Z, Zhao Z, Jin W, Xin Y. Serum Resistin Levels in Adult Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. J Clin Transl Hepatol. 2021;9:484-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Kim HJ, Lee YS, Won EH, Chang IH, Kim TH, Park ES, Kim MK, Kim W, Myung SC. Expression of resistin in the prostate and its stimulatory effect on prostate cancer cell proliferation. BJU Int. 2011;108:E77-E83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 149. | Malvi P, Chaube B, Singh SV, Mohammad N, Vijayakumar MV, Singh S, Chouhan S, Bhat MK. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018;6:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 150. | Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC, Tsai CH, Chiu WC, Chu-Sung Hu S, Lu CW, Yang YF, Chiu CC, Ou-Yang F, Wang YM, Hou MF, Yuan SS. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2018;37:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 151. | Deshmukh SK, Srivastava SK, Bhardwaj A, Singh AP, Tyagi N, Marimuthu S, Dyess DL, Dal Zotto V, Carter JE, Singh S. Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget. 2015;6:11231-11241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 152. | Qiu L, Zhang GF, Yu L, Wang HY, Jia XJ, Wang TJ. Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition. Sci Rep. 2018;8:12522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 153. | Deb A, Deshmukh B, Ramteke P, Bhati FK, Bhat MK. Resistin: A journey from metabolism to cancer. Transl Oncol. 2021;14:101178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 154. | Singh S, Chouhan S, Mohammad N, Bhat MK. Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS3. FEBS Lett. 2017;591:1371-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 155. | Ashour MA, Wadea FM, Hussein NMM, Elnagar A-EMA. Insulin Resistance, Resistin Hormone and Hepatocellular Carcinoma Interplay: A Review Article. Egyptian J Hospital Med. 2023;90:2041-2044. [DOI] [Full Text] |
| 156. | Kelley RK, Greten TF. Hepatocellular Carcinoma - Origins and Outcomes. N Engl J Med. 2021;385:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 157. | Tan W, Luo X, Li W, Zhong J, Cao J, Zhu S, Chen X, Zhou R, Shang C, Chen Y. TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2019;40:446-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 158. | Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 159. | Shakiba E, Ramezani M, Sadeghi M. Evaluation of serum interleukin-6 levels in hepatocellular carcinoma patients: a systematic review and meta-analysis. Clin Exp Hepatol. 2018;4:182-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 160. | Pirvulescu MM, Gan AM, Stan D, Simion V, Calin M, Butoi E, Manduteanu I. Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling. Int J Biochem Cell Biol. 2014;50:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 161. | Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 454] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 162. | Tarkowski A, Bjersing J, Shestakov A, Bokarewa MI. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 163. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 2012] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 164. | Yung JHM, Giacca A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 165. | He G, Karin M. NF-κB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 948] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 166. | Lee C, Cheung ST. STAT3: An Emerging Therapeutic Target for Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 167. | Pirvulescu M, Manduteanu I, Gan AM, Stan D, Simion V, Butoi E, Calin M, Simionescu M. A novel pro-inflammatory mechanism of action of resistin in human endothelial cells: up-regulation of SOCS3 expression through STAT3 activation. Biochem Biophys Res Commun. 2012;422:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 168. | Sakamoto T, Kuboki S, Furukawa K, Takayashiki T, Takano S, Yoshizumi A, Ohtsuka M. TRIM27-USP7 complex promotes tumour progression via STAT3 activation in human hepatocellular carcinoma. Liver Int. 2023;43:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 169. | Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, Marra F. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 170. | Meroni M, Longo M, Erconi V, Valenti L, Gatti S, Fracanzani AL, Dongiovanni P. mir-101-3p Downregulation Promotes Fibrogenesis by Facilitating Hepatic Stellate Cell Transdifferentiation During Insulin Resistance. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 171. | Barry AE, Baldeosingh R, Lamm R, Patel K, Zhang K, Dominguez DA, Kirton KJ, Shah AP, Dang H. Hepatic Stellate Cells and Hepatocarcinogenesis. Front Cell Dev Biol. 2020;8:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 172. | Hamaidia M, Jamakhani M, Jacques JR, Fontaine A, Scherpereel A, Wasielewski E, Renaud L, Heinen V, Duysinx B, Willems L. Resistin mediates the immunoediting activity exerted by primary human macrophages towards mesothelioma cells. Cancer Res. 2023;83:663. [DOI] [Full Text] |
| 173. | Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 965] [Article Influence: 241.3] [Reference Citation Analysis (0)] |
| 174. | Zhao CW, Song WX, Liu B, Gao YH, Ding L, Huang YF, Qi X. Resistin induces chemokine and matrix metalloproteinase production via CAP1 receptor and activation of p38-MAPK and NF-κB signalling pathways in human chondrocytes. Clin Exp Rheumatol. 2022;40:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 175. | De la Fuente López M, Landskron G, Parada D, Dubois-Camacho K, Simian D, Martinez M, Romero D, Roa JC, Chahuán I, Gutiérrez R, Lopez-K F, Alvarez K, Kronberg U, López S, Sanguinetti A, Moreno N, Abedrapo M, González MJ, Quera R, Hermoso-R MA. The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol. 2018;40:1010428318810059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 176. | Bähr I, Jahn J, Zipprich A, Pahlow I, Spielmann J, Kielstein H. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. 2018;66:234-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 177. | Jahn J, Spielau M, Brandsch C, Stangl GI, Delank KS, Bähr I, Berreis T, Wrann CD, Kielstein H. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity (Silver Spring). 2015;23:2233-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 178. | Tsai HC, Cheng SP, Han CK, Huang YL, Wang SW, Lee JJ, Lai CT, Fong YC, Tang CH. Resistin enhances angiogenesis in osteosarcoma via the MAPK signaling pathway. Aging (Albany NY). 2019;11:9767-9777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 179. | Chen SS, Tang CH, Chie MJ, Tsai CH, Fong YC, Lu YC, Chen WC, Lai CT, Wei CY, Tai HC, Chou WY, Wang SW. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019;10:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 180. | Bougatef F, Quemener C, Kellouche S, Naïmi B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbé C, Menashi S, Mourah S. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood. 2009;114:5547-5556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 181. | Su CM, Hsu CJ, Tsai CH, Huang CY, Wang SW, Tang CH. Resistin Promotes Angiogenesis in Endothelial Progenitor Cells Through Inhibition of MicroRNA206: Potential Implications for Rheumatoid Arthritis. Stem Cells. 2015;33:2243-2255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 182. | Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3024] [Article Influence: 137.5] [Reference Citation Analysis (0)] |
| 183. | Pang L, Zhang Y, Yu Y, Zhang S. Resistin promotes the expression of vascular endothelial growth factor in ovary carcinoma cells. Int J Mol Sci. 2013;14:9751-9766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 184. | Su CM, Tang CH, Chi MJ, Lin CY, Fong YC, Liu YC, Chen WC, Wang SW. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem Pharmacol. 2018;154:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 185. | Huang GW, Tao YM, Ding X. Endocan expression correlated with poor survival in human hepatocellular carcinoma. Dig Dis Sci. 2009;54:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 186. | Choi SB, Han HJ, Kim WB, Song TJ, Choi SY. VEGF Overexpression Predicts Poor Survival in Hepatocellular Carcinoma. Open Med (Wars). 2017;12:430-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 187. | Theodoropoulos VE, Lazaris ACh, Sofras F, Gerzelis I, Tsoukala V, Ghikonti I, Manikas K, Kastriotis I. Hypoxia-inducible factor 1 alpha expression correlates with angiogenesis and unfavorable prognosis in bladder cancer. Eur Urol. 2004;46:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 175] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 188. | Xu J, Wang B, Xu Y, Sun L, Tian W, Shukla D, Barod R, Grillari J, Grillari-Voglauer R, Maxwell PH, Esteban MA. Epigenetic regulation of HIF-1α in renal cancer cells involves HIF-1α/2α binding to a reverse hypoxia-response element. Oncogene. 2012;31:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 189. | Fan HQ, Gu N, Liu F, Fei L, Pan XQ, Guo M, Chen RH, Guo XR. Prolonged exposure to resistin inhibits glucose uptake in rat skeletal muscles. Acta Pharmacol Sin. 2007;28:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 190. | Bełtowski J. Adiponectin and resistin--new hormones of white adipose tissue. Med Sci Monit. 2003;9:RA55-RA61. [PubMed] |
| 191. | Fu Y, Luo L, Luo N, Garvey WT. Proinflammatory cytokine production and insulin sensitivity regulated by overexpression of resistin in 3T3-L1 adipocytes. Nutr Metab (Lond). 2006;3:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 192. | Palanivel R, Maida A, Liu Y, Sweeney G. Regulation of insulin signalling, glucose uptake and metabolism in rat skeletal muscle cells upon prolonged exposure to resistin. Diabetologia. 2006;49:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 193. | Rajala MW, Qi Y, Patel HR, Takahashi N, Banerjee R, Pajvani UB, Sinha MK, Gingerich RL, Scherer PE, Ahima RS. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 194. | Moon B, Kwan JJ, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab. 2003;285:E106-E115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 195. | Wang YQ, Dong Y, Yao MH. Chromium picolinate inhibits resistin secretion in insulin-resistant 3T3-L1 adipocytes via activation of amp-activated protein kinase. Clin Exp Pharmacol Physiol. 2009;36:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 196. | Song R, Wang X, Mao Y, Li H, Li Z, Xu W, Wang R, Guo T, Jin L, Zhang X, Zhang Y, Zhou N, Hu R, Jia J, Lei Z, Irwin DM, Niu G, Tan H. Resistin disrupts glycogen synthesis under high insulin and high glucose levels by down-regulating the hepatic levels of GSK3β. Gene. 2013;529:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 197. | Gallagher EJ, LeRoith D. Hyperinsulinaemia in cancer. Nat Rev Cancer. 2020;20:629-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 198. | Olatunde A, Nigam M, Singh RK, Panwar AS, Lasisi A, Alhumaydhi FA, Jyoti Kumar V, Mishra AP, Sharifi-Rad J. Cancer and diabetes: the interlinking metabolic pathways and repurposing actions of antidiabetic drugs. Cancer Cell Int. 2021;21:499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 199. | Dongiovanni P, Rametta R, Meroni M, Valenti L. The role of insulin resistance in nonalcoholic steatohepatitis and liver disease development--a potential therapeutic target? Expert Rev Gastroenterol Hepatol. 2016;10:229-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 200. | Song C, Long X, He J, Huang Y. Recent evaluation about inflammatory mechanisms in nonalcoholic fatty liver disease. Front Pharmacol. 2023;14:1081334. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 201. | Itoh Y, Nagase H. Matrix metalloproteinases in cancer. Essays Biochem. 2002;38:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 202. | Parafiniuk K, Skiba W, Pawłowska A, Suszczyk D, Maciejczyk A, Wertel I. The Role of the Adipokine Resistin in the Pathogenesis and Progression of Epithelial Ovarian Cancer. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 203. | Di Simone N, Di Nicuolo F, Sanguinetti M, Castellani R, D'Asta M, Caforio L, Caruso A. Resistin regulates human choriocarcinoma cell invasive behaviour and endothelial cell angiogenic processes. J Endocrinol. 2006;189:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 204. | Wågsater D, Mumtaz M, Lofgren S, Hugander A, Dimberg J. Resistin in human colorectal cancer: increased expression independently of resistin promoter -420C > G genotype. Cancer Invest. 2008;26:1008-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 205. | Tsai CH, Tsai HC, Huang HN, Hung CH, Hsu CJ, Fong YC, Hsu HC, Huang YL, Tang CH. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget. 2015;6:258-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 206. | Hsu WY, Chao YW, Tsai YL, Lien CC, Chang CF, Deng MC, Ho LT, Kwok CF, Juan CC. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol. 2011;226:2181-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 207. | Huang T, Chen QF, Chang BY, Shen LJ, Li W, Wu PH, Fan WJ. TFAP4 Promotes Hepatocellular Carcinoma Invasion and Metastasis via Activating the PI3K/AKT Signaling Pathway. Dis Markers. 2019;2019:7129214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 208. | Zhou Y, Qiu J, Liu S, Wang P, Ma D, Zhang G, Cao Y, Hu L, Wang Z, Wu J, Jiang C. CFDP1 promotes hepatocellular carcinoma progression through activating NEDD4/PTEN/PI3K/AKT signaling pathway. Cancer Med. 2023;12:425-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |