Published online Jul 21, 2023. doi: 10.3748/wjg.v29.i27.4236
Peer-review started: March 24, 2023
First decision: May 13, 2023
Revised: May 25, 2023
Accepted: June 21, 2023
Article in press: June 21, 2023
Published online: July 21, 2023
Processing time: 110 Days and 22.7 Hours
Decreased muscle mass and function, also known as sarcopenia, is common in patients with cirrhosis and is associated with a poor prognosis. Although the pathogenesis of this disorder has not been fully elucidated, a disordered gut-muscle axis probably plays an important role. Decreased barrier function of the gut and liver, gut dysbiosis, and small intestinal bacterial overgrowth (SIBO) can lead to increased blood levels of ammonia, lipopolysaccharides, pro-inflammatory mediators, and myostatin. These factors have complex negative effects on muscle mass and function. Drug interventions that target the gut microbiota (long-term use of rifaximin, lactulose, lactitol, or probiotics) positively affect most links of the compromised gut-muscle axis in patients with cirrhosis by decreasing the levels of hyperammonemia, bacterial translocation, and systemic inflammation and correcting gut dysbiosis and SIBO. However, although these drugs are promising, they have not yet been investigated in randomized controlled trials specifically for the treatment and prevention of sarcopenia in patients with cirrhosis. No data exist on the effects of fecal transplantation on most links of gut-muscle axis in cirrhosis; however, the results of animal experimental studies are promising.
Core Tip: Sarcopenia is common in patients with cirrhosis and is associated with a poor prognosis. Although the pathogenesis of this disorder has not been fully elucidated, a disordered gut-muscle axis probably plays an important role. Drugs that target the gut microbiota positively affect most links of the compromised gut-muscle axis in patients with cirrhosis. However, they have not yet been investigated in randomized controlled trials specifically for the treatment and prevention of sarcopenia in patients with cirrhosis.
- Citation: Maslennikov R, Alieva A, Poluektova E, Zharikov Y, Suslov A, Letyagina Y, Vasileva E, Levshina A, Kozlov E, Ivashkin V. Sarcopenia in cirrhosis: Prospects for therapy targeted to gut microbiota. World J Gastroenterol 2023; 29(27): 4236-4251
- URL: https://www.wjgnet.com/1007-9327/full/v29/i27/4236.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i27.4236
Sarcopenia is defined as the loss of muscle mass, strength, and physical function[1]. Sarcopenia has been detected in 14%-55% of patients with cirrhosis[2-6] and attracts the attention of researchers increasingly[7-10]. Sarcopenia was found in 68.5% of patients with cirrhosis who were admitted to the intensive care unit[11]. Recent studies have shown that sarcopenia in patients with cirrhosis is associated with poor short- and long-term prognoses[3,6,11,12]. The decrease in muscle mass in patients with cirrhosis over a year also has an unfavorable prognostic value[13]. A recent meta-analysis of 22 studies confirmed that sarcopenia is an independent predictor of increased mortality in patients with cirrhosis[14].
Sarcopenia in patients with cirrhosis is associated with a decrease in the quality of life[15], increased hepatic venous pressure gradient[12], portal hypertension-related complications (ascites and upper gastrointestinal varices), infections (urinary tract infection and spontaneous peritonitis), hepatic encephalopathy, increased risk of hepatocellular carcinoma, longer hospital stay, higher 30-d readmission rate, lower body mass index and serum albumin levels, longer prothrombin time, higher total bilirubin concentrations, and higher Child-Pugh score[16-20]. In addition, patients with cirrhosis have an increased amount of fat in the muscles (myosteatosis), which is also an unfavorable prognostic factor[21].
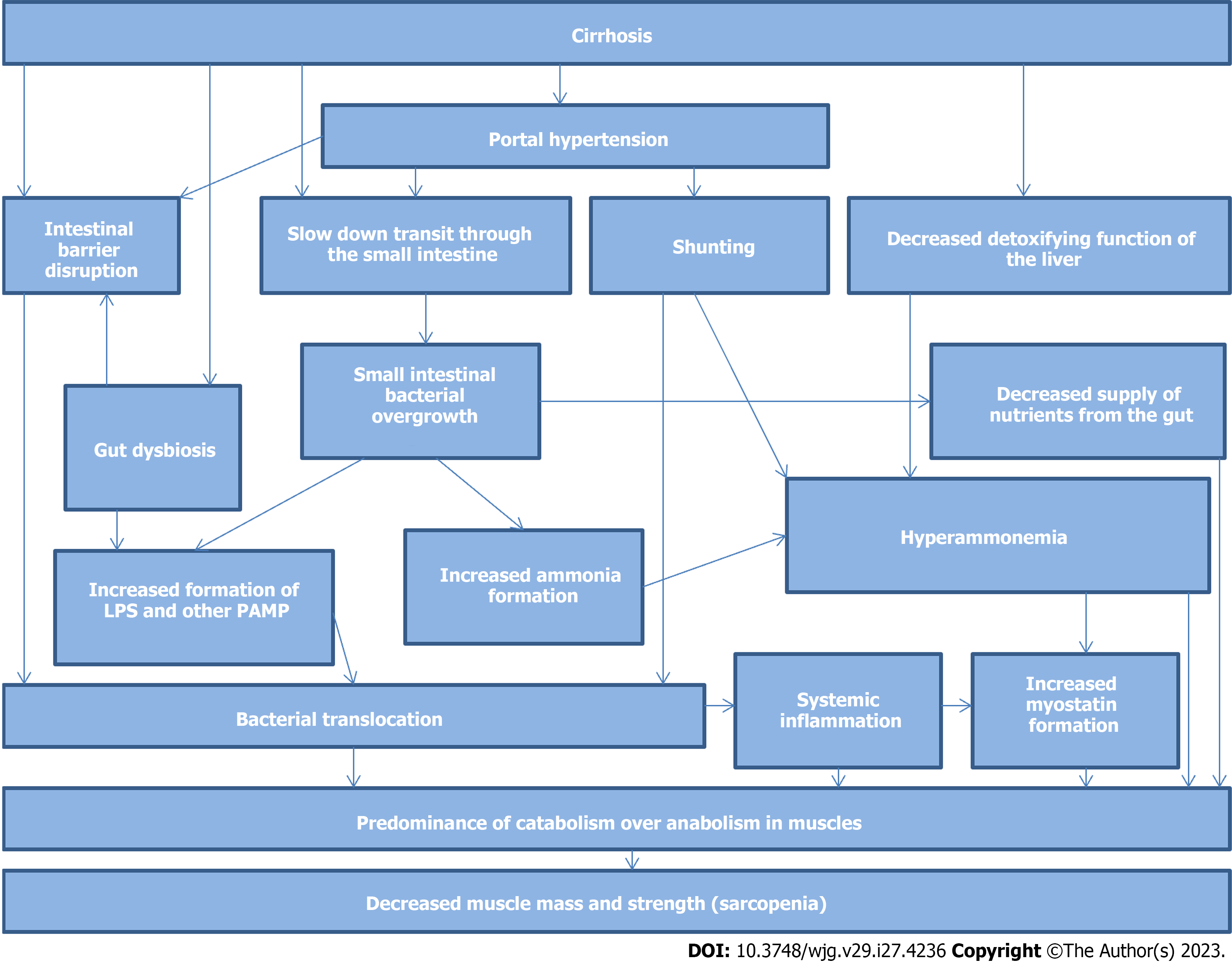
The exact mechanisms underlying the development of sarcopenia in patients with cirrhosis have not yet been established. Among the factors contributing to the development of sarcopenia in cirrhosis, are disorders of the metabolic function of the liver, decreased appetite[22], increased muscle autophagy[2], increased serum myostatin (a protein that blocks muscle growth)[6], catabolic effects of systemic inflammation induced by bacterial translocation from the gut[23-24], and low testosterone levels[25] were found.
Cirrhosis is associated with a disturbed composition of the gut microbiota, or dysbiosis[26-29]. Despite some inconsistencies, most studies have shown that in patients with cirrhosis, there was an abundance of harmful bacteria of taxa Bacilli, Streptococcaceae, Enterococcaceae, Proteobacteria, and Enterobacteriaceae, and there was a decrease in beneficial gram-positive bacteria of families Ruminococcaceae and Lachnospiraceae[30-42]. The harmful bacteria are facultative anaerobes; therefore, they can survive in oxygenated tissues, penetrate them, and spread throughout the body. This process was termed bacterial translocation and is believed to be an important contributor to the progression of cirrhosis, including the development of a systemic inflammatory response[26-29]. In addition, harmful Enterobacteriaceae of the phylum Proteobacteria, which are abundant in the gut microbiota of patients with cirrhosis, have an active endotoxin [lipopolysaccharide (LPS)]. Since cirrhosis increases the permeability of the intestinal barrier[43-46], LPS can penetrate the body (molecular bacterial translocation), leading to a systemic inflammatory response. The production of short-chain fatty acids (SCFA) by beneficial bacteria also decreases in patients with cirrhosis[30]. These SCFAs are used as a source of energy by intestinal epithelial cells, strengthen the intestinal barrier, and act on specific receptors to regulate human body functions[47]. The metabolism of bile acids, which also affect many human metabolic pathways through their special receptors[48], depends on the gut bacteria and disturbs in cirrhosis[42].
The values of the MELD scale that used to assess the risk of death in patients with cirrhosis are directly correlated with the abundance of harmful gut bacteria from the families Staphylococcaceae, Enterococcaceae, and Enterobacteriaceae, and inversely correlated with the abundance of beneficial bacteria from the families Lachnospiraceae and Ruminococcaceae[36]. Patients with acute-on-chronic liver failure have a lower abundance of beneficial gram-positive bacteria[36]. The Child–Turcotte–Pugh score, used to assess cirrhosis severity, correlates positively with the abundance of Streptococcaceae and negatively with the abundance of Lachnospiraceae[41]. Severe dysbiosis is associated with higher C-reactive protein (CRP) levels, lower serum albumin and cholinesterase levels, and poorer long-term prognosis[40]. The abundance of Lachnospiraceae is negatively correlated with the risk of hospitalization of cirrhosis patients in the intensive care unit[49]. Abundant Enterobacteriaceae, Enterococcaceae, and Streptococcaceae are associated with hepatic encephalopathy, circulatory failure, and respiratory failure within 30 d of hospitalization of these patients, respectively[49].
Serum LPS levels are positively correlated with the abundance of Enterobacteriaceae and negatively correlated with the abundance of Lachnospiraceae and Ruminococcaceae[36]. Most of the bacterial genetic material found in the blood and ascitic fluid of patients with cirrhosis is from harmful Proteobacteria and Bacilli instead of the beneficial Lachnospiraceae and Ruminococcaceae that are dominant in the gut[50,51]. The serum level of pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α) is positively correlated with the serum LPS level and the risk of cirrhosis decompensation, spontaneous bacterial peritonitis, and acute-on-chronic liver failure[52].
Therefore, gut dysbiosis in patients with cirrhosis is associated with bacterial translocation, systemic inflammation, decompensation of liver function, and poor short- and long-term prognoses.
Small intestinal bacterial overgrowth (SIBO) is an increase in the content of bacteria in the small intestine[53] and has been detected in almost half of patients with cirrhosis[54]. The prevalence of SIBO increases with increasing severity of cirrhosis[54]. Patients with cirrhosis and SIBO have ascites, minimal and overt hepatic encephalopathy, and spontaneous bacterial peritonitis more often than those without SIBO[54-55]. No relationship has been found between SIBO on the one hand and hypocoagulation, alanine aminotransferase, glutamyl transpeptidase activity, white blood cell, platelet counts, hemoglobin, ascitic fluid albumin levels, and esophageal varices on the other hand in cirrhosis[54]. In patients with cirrhosis, SIBO is associated with the presence of bacterial DNA in peripheral blood[56], higher serum LPS[57] and CRP[58] levels, splanchnic vasodilation, and hyperdynamic circulation[58]. Gut microbiota changes in patients with both cirrhosis and SIBO do not correspond to cirrhosis-associated gut dysbiosis. Therefore, gut dysbiosis and SIBO are most likely separate disorders[59] and SIBO is an independent factor associated with bacterial translocation, systemic inflammation, and the development of several complications in cirrhosis.
The pathology of the gut microbiota (gut dysbiosis and SIBO) influences the development of sarcopenia (gut-muscle axis) in patients with cirrhosis.
Bacterial translocation-induced systemic inflammation causes protein catabolism and decreases muscle mass. This is evidenced by the association of sarcopenia with biomarkers of bacterial translocation (LPS) and systemic inflammation (TNF-α) in cirrhosis[23-24]. TNF-α reduced the formation of proteins in muscles[60]. The injection of LPS into the bodies of experimental animals had a similar effect[61].
The second mechanism of harmful effect of the gut microbiota disorders on the muscle tissue in cirrhosis is based on the fact that an excess amount of bacteria in SIBO competes with the human body for nutrients that enter the intestine with food. In support of this mechanism, an association was found between SIBO and malnutrition in cirrhosis[62].
A third mechanism linking the pathology of the gut microbiota with sarcopenia in cirrhosis is associated with an increase in the formation of myostatin, a protein that enhances protein catabolism in muscles, and inhibits their growth[63-65]. Serum myostatin levels are elevated in patients with cirrhosis and are correlated with the severity of sarcopenia[6]. Increased myostatin formation in patients with cirrhosis is likely associated with hyperammonemia that led to an increase in the myostatin level as well as a decrease in muscle mass and strength by an NF-κB-mediated mechanism in experimental animals[66]. However, such effects were not observed in mice with knockout of the myostatin gene[66]. The muscles are normally one of the main sources of ammonia in the body. Therefore, through negative feedback, hyperammonemia may reduce muscle mass to reduce the formation of ammonia by muscles. However, in patients with cirrhosis, the main source of ammonia for systemic circulation is the gut microbiota, which catabolize dietary and mucous proteins. Normally, gut microbiota-derived ammonia is neutralized by the liver; however, in patients with cirrhosis, the detoxifying function of the liver is reduced and portocaval shunts work around it, which leads to a dysfunction of this mechanism for regulating the volume of muscle mass[67-70]. In addition, hyperammonemia may lead to the development of sarcopenia in other ways[69], including increased autophagy[71] and inhibition of muscle protein anabolism[72].
The increased serum TNF-α levels are associated with the increased myostatin gene expression and the decreased muscle mass in experimental cirrhosis, suggesting that systemic inflammation can lead to sarcopenia increasing myostatin production among other mechanisms[24].
Several recent studies have reported changes in the composition of the gut microbiota associated with sarcopenia[73-76]. The abundances of Bacteroidaceae, Eggerthella, Escherichia coli, Fusobacterium, Micrococcaceae, Rothia, Veillonella, Weissella, and other bacteria are associated with a decrease in muscle mass, while the abundances of Acidaminococcus, Akkermansia, Anaerotruncus, Anaerostipes, Barnesiellaceae, Bifidobacterium catenulatum, Catenibacterium, Collinsella, Coprococcus, Coriobacteriaceae, Desulfovibrio, Dialister, Dorea, Erysipelatoclostridiaceae, Granulicella, Intestinimonas, Lachnospiraceae, Megasphaera, Methanobrevibacter, Oscillospira, Prevotella, Ruminococcus flavefaciens, Senegalimassilia, and other bacteria showed an association with normal muscle mass in patients with cirrhosis. The pronounced heterogeneity of these results were obtained that may be caused by different methods used to assess the muscle mass of patients. Patients with sarcopenia had higher serum levels of CRP, TNF-α, interleukin 1-beta, 2 and 6, granulocyte-macrophage-colony-stimulating factor, fibroblast growth factor, and C-X-C motif chemokine ligand 10 than patients without it[73]. Although serum LPS and myostatin levels in this study were higher in patients with sarcopenia than in those without, this increase did not reach the limit of significance[73]. There was no significant difference in zonilin and other cytokines levels and reported manifestations of cirrhosis between patients with and without sarcopenia[73]. Patients with sarcopenia were more likely to have ascites, higher Child-Pugh scores[76], and lower albumin levels[75] than patients with normal muscle mass. No other significant associations were reported in these studies on associations between gut microbiota and muscle mass in cirrhosis.
Disorders of the gut-muscle axis in cirrhosis are shown simplified in Figure 1.
Therefore, it is assumed that the condition of the gut microbiota plays an important role in the development of sarcopenia in patients with cirrhosis and therapy aimed at the gut microbiota (antibiotics, prebiotics, probiotics, and fecal transplantation) may be useful in the treatment and prevention of sarcopenia.
Rifaximin is a poorly-absorbed antibiotic used against harmful intestinal bacteria. Recent meta-analyses have shown that rifaximin effectively eliminates SIBO[77,78] in patients with irritable bowel syndrome[79-82], cystic fibrosis[83], Crohn's disease[84], diabetes[85], celiac disease[86], acromegaly[87], uncomplicated diverticular disease[88,89], systemic sclerosis[90], rosacea[91], and ulcerative colitis[92]. However, rifaximin was ineffective against SIBO in patients after gastrectomy[93]. The meta-analyses also noted that rifaximin is generally well-tolerated and has a low incidence of side effects[77,78]. Other antibiotics, such as chlortetracycline[94], neomycin, doxycycline, amoxicillin/clavulanate, ciprofloxacin[95], and metronidazole[96] were less effective than rifaximin in eradicating SIBO.
Rifaximin also improved intestinal barrier function (reduced blood levels of diamine oxidase and D-lactic acid), mitigated systemic inflammation (decreased serum CRP level and erythrocyte sedimentation rate)[92], and accelerated orocecal transit[85].
Rifaximin increases the abundance of beneficial bacteria (Akkermansia, Bifidobacterium, Faecalibacterium prausnitzii, and Ruminococcaceae) and decreases the number of harmful ones (Alphaproteobacteria, Eggerthella, Enterococcus, and Streptococcaceae) in several studies (Table 1)[79,97-106].
| Disease | Taxa that increase in the abundance after rifaximin | Taxa that decrease in the abundance after rifaximin |
| Alzheimer's disease[97] | Anaerostipes, Blautia, Erysipelotrichaceae, Erysipelatoclostridium, Faecalitalea, Lactobacillus, and Ruminiclostridum | |
| Irritable bowel syndrome[79,98-100] | Acidimicrobiales, Acidobacteria, Alteromonas, Arthrobacter, Bacillus, Bacteroidaceae, Butyricimonas, Chloroflexi, Cytophagia, Coprobacillus, Bifidobacterium, Deinococcales, Devosia, Dyella, Faecalibacterium prausnitzii, Frankiales, Gordonibacter, Holdemania, Kocuria, Methylophilales, Micrococcales, Micromonosporales, Nitriliruptorales, Parabacteroides, Prevotellaceae, Propionibacteriales, Rhizobiales, Rhodobacterales, Sphingomonadales, and Streptomycetales | Alphaproteobacteria, Anaerotruncus, Blautia luti, Butyricimonas, Cronobacter, Escherichia, Eubacterium ventriosum, Rhodospirillales, Romboutsia, Roseburia inulinivorans, Streptococcaceae, and Tyzzerella |
| Symptomatic uncomplicated diverticular disease[101-102] | Akkermansia, Bacteroidaceae, Citrobacter, Coprococcus, Dialister Ruminococcaceae, and Veillonellaceae | Anaerotruncus, Anaerostipes, Blautia, Christensenellaceae, Dehalobacteriaceae, Eggerthella lenta, Haemophilus parainfluenzae, Mogibacteriaceae, and Pasteurellaceae |
| Chronic Kidney Disease[103] | - | Anaerotruncus, Clostridium, and Turicibacter |
| Gut diseases[104] | Faecalibacterium | Ruminococcus and Roseburia |
| Ulcerative colitis[105] | Bacteroides and Bifidobacterium | Enterococcus and Lactobacillus |
| Crohn's disease[106] | Bifidobacterium, Atopobium, and Faecalibacterium prausnitzii |
Meta-analyses have shown that rifaximin is effective in the primary and secondary prevention of spontaneous bacterial peritonitis, a clinical manifestation of bacterial translocation in patients with cirrhosis; rifaximin was superior to norfloxacin and other antibiotics in this respect[107-110]. Rifaximin also reduces the risk of mortality and need for liver transplantation in patients with cirrhosis[111]. In addition, it is effective in the management of hepatic encephalopathy, which is associated with hyperammonemic conditions such as sarcopenia, and significantly reduces blood ammonium levels in patients with cirrhosis[111-113]. One study reported that rifaximin eradicated SIBO in 76% of patients with cirrhosis-associated minimal hepatic encephalopathy, and this was associated with a more prominent decrease in blood ammonium levels than in patients with cirrhosis without SIBO that were also treated with rifaximin[114].
Rifaximin decreased the blood LPS levels in cirrhosis[115-121], and this was associated with a decrease in serum ammonia levels[118]. Rifaximin also reduces circulating neutrophil expression of TLR-4, which is the main endotoxin receptor[122]. However, the effects of rifaximin on various biomarkers of systemic inflammation in cirrhosis have been inconsistent across studies.
Importantly, rifaximin does not alter the overall resistome[123]; i.e., their intake does not lead to the development of antibacterial resistance. Rifaximin also reduces the rate of all bacterial infections[124] and variceal bleeding in patients with cirrhosis[125-126], prevents the development of hepatorenal syndrome[127-128] and refractory ascites[129-130]. The use of rifaximin in patients with cirrhosis led to a decrease in the abundance of harmful Streptococcus, Eggerthella, and Veillonella (Table 2)[42,117-120,122,130,131].
| Ref. | Taxa that increase in the abundance after rifaximin | Taxa that decrease in the abundance after rifaximin |
| Bajaj et al[120] | Eubacteriaceae | Veillonellaceae |
| Kaji et al[117] | Streptococcus, Veillonella | |
| Kaji et al[118] | Streptococcus, Veillonella | |
| Kakiyama et al[42] | Veillonellaceae | |
| Kawaguchi et al[131] | Lactobacillus, Streptococcus, Veillonella | |
| Lv et al[130] | Bacteroidetes vulgatus | Bacteroides uniformis, Eggerthella lenta, Haemophilus, Prevotella, Roseburia |
| Patel et al[122] | Akkermansia, Hungatella, Streptococcus, Veillonella | |
| Zeng et al[119] | Bacteroidaceae | Veillonellaceae |
Overall, the literature suggests that rifaximin does positive affect the main pathogenetic links of the disordered gut-muscle axis in cirrhosis, in particular SIBO, hyperammonemia, and bacterial translocation. Rifaximin also modulates the composition of the gut microbiota reducing the number of bacterial taxa has been associated with muscle loss in patients with cirrhosis. This drug has shown excellent safety and tolerability for long-term use in patients with cirrhosis[127,132,133]. It is very important, since the prevention and treatment of sarcopenia require long-term therapy. This suggests that rifaximin may be effective and safe for these purposes in cirrhosis.
Although other antibiotics have also had positive effects on the prevention of spontaneous bacterial peritonitis, hepatic encephalopathy, and the reduction of bacterial translocation and hyperammonemia in patients with cirrhosis[111,134-136], most of them have poorer safety profiles and fewer number of studies. Thereby, recommendations to investigate their long-term effects on the prevention and treatment of sarcopenia in patients with cirrhosis seem premature. However, this does not exclude their potential use when new data on the efficacy and safety of their long-term use become available.
Rifaximin prevents skeletal muscle atrophy and weakness by decreasing intramuscular myostatin and pro-inflammatory cytokine levels in experimental cirrhosis[24,137]. An uncontrolled study showed that the long-term use of rifaximin in patients with cirrhosis improved nutritional status and allowed patients to maintain a constant amount of muscle mass[138]. Randomized placebo-controlled studies on the effects of long-term rifaximin use on muscle mass, serum myostatin, and other biomarkers of gut-muscle axis disorders in cirrhosis are required to verify this data.
Lactulose and lactitol are artificial disaccharides that are not broken down by human digestive enzymes and are utilized by the intestinal microbiota. These drugs are primarily used as osmotic laxatives for conditions that involve constipation (including irritable bowel syndrome)[139-141]. They also used for the prevention and treatment of hepatic encephalopathy[142,143], because the abundance of these carbohydrates switches the metabolism of gut bacteria from proteolytic to saccharolytic. This leads to a decrease in the formation of ammonium and other neurotropic metabolites of amino acids[144], and their laxative effects accelerate the removal of these harmful products from the intestine.
Meta-analyses have shown that lactulose and lactitol are effective against hepatic encephalopathy and that they reduce blood ammonia levels. They also decrease the risk of other serious liver-related adverse events, such as liver failure, variceal bleeding, hepatorenal syndrome, spontaneous bacterial peritonitis and other serious infections. These drugs also have excellent safety profiles and have been shown to reduce mortality[111,145-148].
Lactulose and lactitol can be safely taken for six months or more[149-151]. However, they may cause steatorrhea, which can worsen the nutritional status of patients[152].
We were unable to find any data on the effects of these disaccharides on SIBO. Two factors should be considered in this case. First, since these drugs are growth factors for bacteria, they can provoke the development of SIBO. However, SIBO can be eliminated by accelerating intestinal motility caused by these drugs. Further research is required to determine which effect outweighs the other.
The intake of lactitol was accompanied by an increase in the abundance of Bifidobacterium, Lactobacillus, Veillonella, Enterobacter, Sutterella, Haemophilus, and Aggregatibacter, and a decrease in the abundance of Bacteroides, Clostridium, Eubacterium, Klebsiella, Pseudoflavonifractor, and coliform bacteria (Table 3)[153-157]. In addition, there was a decrease in the LPS concentration in the blood of patients with chronic viral hepatitis[158].
| Ref. | Disease | Taxa that increase in the abundance after disaccharide | Taxa that decrease in the abundance after disaccharide |
| Lactitol | |||
| Riggio et al[153] | Cirrhosis | Lactobacilli | Enterobacteria and Enterocicci |
| Ballongue et al[154] | Healthy persons | Bifidobacterium, Lactobacillus and Streptococcus | Bacteroides, Clostridium, coliforms, and Eubacterium |
| Li et al[155] | Chronic constipation | Actinobacteria, Bifidobacteriales, Bifidobacteriaceae, Anaerostipes, and Bifidobacterium | - |
| Tarao et al[156] | Cirrhosis | Bifidobacterium and Lactobacillus | Bacteroides and Clostridium |
| Lu et al[157] | Cirrhosis | Bifidobacterium, Veillonella, Enterobacter, Sutterella, Haemophilus, Aggregatibacter, Lactobacillus salivarius, L. fermentium, and L. oris | Klebsiella Pseudoflavonifractor, and others |
| Chen et al[158] | Chronic viral hepatitis | Bifidobacterium and Lactobacillus | Clostridium perfringens |
| Lactulose | |||
| Riggio et al[153] | Cirrhosis | Lactobacilli | - |
| Ballongue et al[154] | Healthy persons | Bifidobacterium, Lactobacillus and Streptococcus | Bacteroides, Clostridium, coliforms and Eubacterium |
| Ziada et al[159] | Minimal hepatic encephalopathy | Bifidobacterium, Lactobacillus, and Bacteroidaceae | Enterobacteriaceae and Enterococcus |
The use of lactulose is associated with an increase in the levels of Bifidobacterium and Lactobacillus and a decrease in the abundance of Clostridium, Eubacterium, Enterococcus, and Enterobacteriaceae in patients with cirrhosis (Table 3)[153,154,159,160]. However, other studies have not shown an effect of lactulose on the composition of gut microbiota[161,162]. Several studies demonstrated that the use of lactulose in patients with minimal hepatic encephalopathy led to a decrease in the blood levels of LPS, bacterial DNA, and pro-inflammatory cytokines (TNF-α, IL-6 and IL-18)[160,163,164].
The use of lactulose and lactitol has also been associated with a decrease in the amount of bacterial proteolysis products such as cresol, skatol, phenol, and indole in the feces, as well as an increase in the amount of lactate in the feces. Lactate can acidify the feces[154] and convert neutral ammonia to ammonium ions which, unlike ammonia, do not pass through the intestinal barrier because of the presence of an electric charge. Thus, these drugs not only decrease the bacterial catabolism of amino acids with the formation of ammonia, but also decrease ammonia absorption from the intestine. These drugs significantly reduce hyperammonemia after an amino acid load[143].
Thereby, these prebiotic disaccharides reduce the severity of the following adverse factors of the disordered gut-muscle axis in cirrhosis: hyperammonemia, bacterial translocation, and systemic inflammation. Most studies have shown positive effects of these drugs on the composition of the gut microbiota. However, their impact on SIBO is yet to be explored. These drugs are considered safe for long-term use. However, their effects on myostatin levels and muscle mass in patients with cirrhosis and other diseases have not been studied.
Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts[165]. They can be used to treat liver diseases[165-166].
Meta-analyses have shown that probiotics are effective in the prevention and treatment of hepatic encephalopathy and that they decrease the blood levels of LPS and ammonia. However, they do not have a significant effect on mortality or the incidence of spontaneous bacterial peritonitis in patients with cirrhosis[111,167-170]. A meta-analysis did not show that probiotics have a significant effect on the serum level of pro-inflammatory cytokines (TNA-alpha and IL-6)[169]. Another meta-analysis revealed that prebiotics do not significantly affect CRP levels, but they lead to a decrease in TNF-α levels[171]. Among the studies not included in these meta-analyses, the following described the effect of probiotics on inflammatory biomarkers in cirrhosis. The probiotic fungus Saccharomyces boulardii decreases serum CRP levels and ameliorates hyperdynamic circulation in patients with decompensated cirrhosis[172]. Escherichia coli Nissle 1917 reduced the serum levels of ІL-6, ІL-8, and interferon-γ in patients with hepatic encephalopathy[160]. The probiotics that included Clostridium butyricum and Bifidobacterium infantis strengthen the intestinal barrier[173].
Probiotics have been shown to be safe when used for six months in patients with cirrhosis[174].
The use of probiotics in cirrhosis leads to an increase in the numbers of Bifidobacterium, Lactobacillus, Lachnospiraceae, Bacteroidaceae, Clostridiales incertae sedis XIV, Faecalibacterium prausnitzii, Syntrophococcus sucromutans, Bacteroidetes vulgatus, Prevotella, Alistipes shahii, Clostridium cluster I, Clostridium coccoides, and Eubacterium cylindroides and a decrease in the numbers of Enterobacteriaceae, Enterococcus, Proteus hauseri, Citrobacter sp., and Morganella (Table 4)[159,160,175-178].
| Probiotic | Taxa that increase in the abundance after the probiotic | Taxa that decrease in the abundance after the probiotic |
| Lactobacillus acidophilus[159] | Bifidobacterium, Lactobacillus, and Bacteroidaceae | Enterobacteriaceae and Enterococcus |
| Escherichia coli Nissle 1917[160] | Bifidobacterium and Lactobacillus | Pathogenic enterobacteria |
| Escherichia coli Nissle 1917[175] | Bifidobacterium and Lactobacillus | Proteus hauseri, Citrobacter, and Morganella |
| Yakult 400[176] | Clostridium coccoides and Eubacterium cylindroides | Enterobacteriaceae |
| Lactobacillus GG[177] | Clostridiales Incertae Sedis XIV and Lachnospiracea | Enterobacteriaceae |
| Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58[178] | Faecalibacterium prausnitzii, Syntrophococcus sucromutans, Bacteroides vulgatus, Alistipes shahii, and Prevotella |
Meta-analyses have also shown that probiotics effectively eliminate SIBO[179-180]. This has been observed in patients with cirrhosis[181], chronic liver disease[182], secondary lactase deficiency[183], diarrhea-predominant irritable bowel syndrome[184-186], gastric and colorectal cancer[187], systemic sclerosis[188], and bariatric surgery[189].
The meta-analysis showed that probiotics enhance both muscle mass and muscle strength in patients with sarcopenia that was not associated with cirrhosis[190]. The probiotic that included Akkermansia muciniphila and Faecalibacterium prausnitzii decreased myostatin levels in a mouse model of muscular atrophy[191].
Although probiotics have been shown to be effective against several pathological links of the disturbed gut-muscle axis in patients with cirrhosis (SIBO, gut dysbiosis, hyperammonemia, and bacterial translocation), improve muscle function, and increase muscle mass in sarcopenia of another origin, no study has evaluated the effect of probiotics on muscle mass and function in cirrhosis.
Fecal transplantation involves the transfer of intestinal microbiota from the donor to the intestine of a recipient. This procedure is effective in hepatic encephalopathy[192-194] and leads to an increase in the abundance of Ruminococcaceae and Bifidobacteriaceae and a decrease in the abundance of Streptococcaceae and Veillonellaceae in patients with cirrhosis[193].
No data exist on the effects of this procedure on the severity of hyperammonemia, bacterial transplantation, systemic inflammation, myostatin levels, muscle mass and strength, or SIBO in patients with cirrhosis. No human studies have been conducted on the effect of fecal transplantation on muscle mass and strength in patients with sarcopenia of any origin; however, the results of animal experimental studies are promising[195].
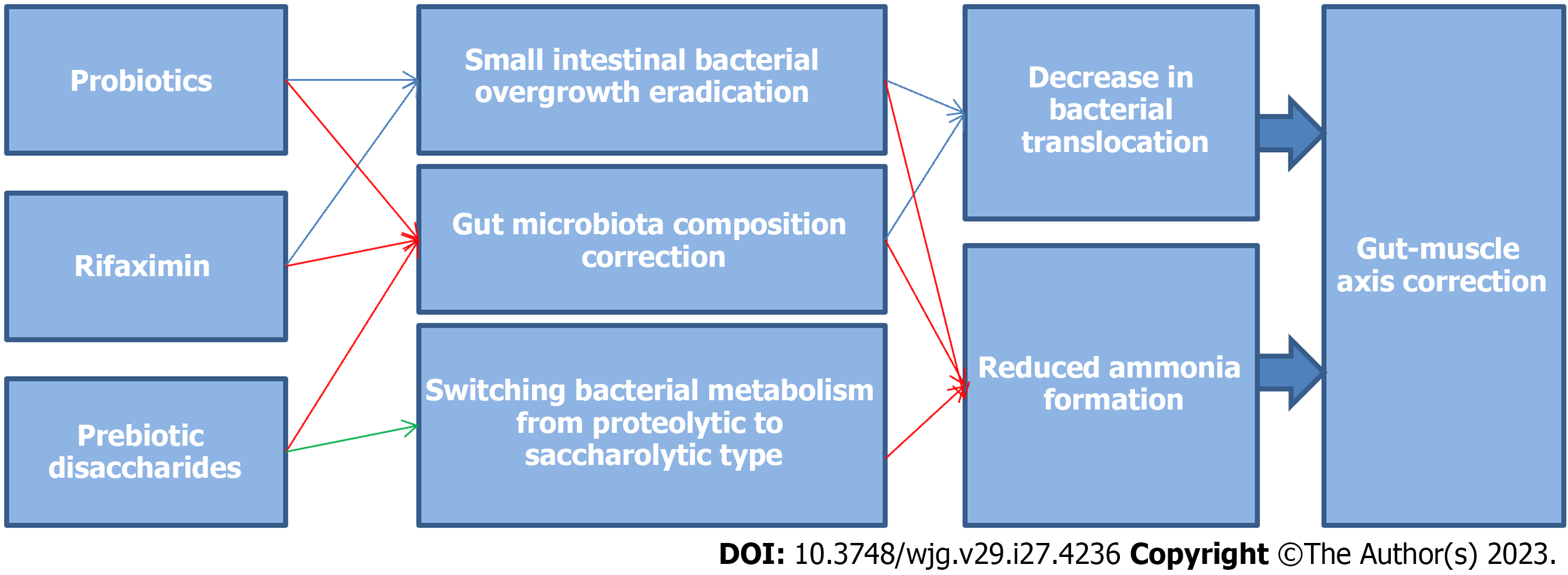
Drug interventions targeting the gut microbiota positively affect most links of the compromised gut-muscle axis in cirrhosis (Table 5, Figure 2). Therefore, they are promising for the treatment and prevention of sarcopenia in cirrhosis, but have not yet been investigated in randomized controlled trials for these purposes. There are no data on the effect of fecal transplantation on most links of the gut-muscle axis and sarcopenia in cirrhosis, but the results of animal experimental studies are promising.
| Intervention | SIBO | Gut dysbiosis | Hyperammonemia | Bacterial translocation | Systemic inflammation |
| Rifaximin | + | + | + | + | CR |
| Prebiotic disaccharides | ND | + | + | + | + |
| Probiotics | + | + | + | + | CR |
| Fecal transplantation | ND | + | ND | ND | ND |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dai K, China; Li C, China; Zhang Y, China S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Hari A. Muscular abnormalities in liver cirrhosis. World J Gastroenterol. 2021;27:4862-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 2. | Anand A, Nambirajan A, Kumar V, Agarwal S, Sharma S, Mohta S, Gopi S, Kaushal K, Gunjan D, Singh N, Madhusudhan KS, Chauhan SS, Sharma MC, Bansal VK, Saraya A. Alterations in Autophagy and Mammalian Target of Rapamycin (mTOR) Pathways Mediate Sarcopenia in Patients with Cirrhosis. J Clin Exp Hepatol. 2022;12:510-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 3. | Guo G, Li C, Hui Y, Mao L, Sun M, Li Y, Yang W, Wang X, Yu Z, Fan X, Jiang K, Sun C. Sarcopenia and frailty combined increases the risk of mortality in patients with decompensated cirrhosis. Ther Adv Chronic Dis. 2022;13:20406223221109651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 4. | Hernández-Conde M, Llop E, Gómez-Pimpollo L, Blanco S, Rodríguez L, Fernández Carrillo C, Perelló C, López-Gómez M, Martínez-Porras JL, Fernández-Puga N, Van Den Brule E, Royuela A, Calleja JL. A nomogram as an indirect method to identify sarcopenia in patients with liver cirrhosis. Ann Hepatol. 2022;27:100723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 5. | De A, Kumari S, Kaur A, Singh A, Kalra N, Singh V. Hand-grip strength as a screening tool for sarcopenia in males with decompensated cirrhosis. Indian J Gastroenterol. 2022;41:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 6. | Boga S, Yildirim AE, Ucbilek E, Koksal AR, Sisman ST, Durak I, Sen I, Dogu B, Serin E, Ucbilek AB, Yildirim MO, Erturk SM, Alkim H, Alkim C. The effect of sarcopenia and serum myokines on prognosis and survival in cirrhotic patients: a multicenter cross-sectional study. Eur J Gastroenterol Hepatol. 2022;34:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 7. | Warner Ii ER, Satapathy SK. Sarcopenia in the Cirrhotic Patient: Current Knowledge and Future Directions. J Clin Exp Hepatol. 2023;13:162-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 8. | Kuchay MS, Martínez-Montoro JI, Llamoza-Torres CJ, Fernández-García JC, Ramos-Molina B. Liver cirrhosis and sarcopenia: a dreadful combination. Hepatobiliary Surg Nutr. 2022;11:729-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Kumar R, Prakash SS, Priyadarshi RN, Anand U. Sarcopenia in Chronic Liver Disease: A Metabolic Perspective. J Clin Transl Hepatol. 2022;10:1213-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Fox R, Stenning K, Slee A, Macnaughtan J, Davies N. Sarcopenia in liver cirrhosis: Prevalence, pathophysiology and therapeutic strategies. Anal Biochem. 2022;647:114581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Khan S, Benjamin J, Maiwall R, Tripathi H, Kapoor PB, Shasthry V, Saluja V, Agrawal P, Thapar S, Kumar G. Sarcopenia is the independent predictor of mortality in critically ill patients with cirrhosis. J Clin Transl Res. 2022;8:200-208. [PubMed] |
| 12. | Matsui T, Nagai H, Watanabe G, Mouri K, Yoshimine N, Amanuma M, Kobayashi K, Ogino Y, Mukozu T, Matsukiyo Y, Daidou Y, Wakui N, Nakano S, Momiyama K, Matsuda T, Igarashi Y. Measurement of skeletal muscle volume is useful for predicting prognosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2022;34:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Kim TH, Jung YK, Yim HJ, Baik JW, Yim SY, Lee YS, Seo YS, Kim JH, Yeon JE, Byun KS. Impacts of muscle mass dynamics on prognosis of outpatients with cirrhosis. Clin Mol Hepatol. 2022;28:876-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 14. | Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, Engelmann C, Zhang P, Jeong JY, van Vugt JLA, Xiao H, Deng H, Gao X, Ye Q, Zhang J, Yang L, Cai Y, Liu N, Li Z, Han T, Kaido T, Sohn JH, Strassburg C, Berg T, Trebicka J, Hsu YC, IJzermans JNM, Wang J, Su GL, Ji F, Nguyen MH. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;76:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 243] [Article Influence: 81.0] [Reference Citation Analysis (1)] |
| 15. | Shanavas N, Devadas K, Nahaz N, Varghese J, Cyriac R, Mathew D. Association of Sarcopenia with Health Related Quality of Life in Cirrhotics. J Assoc Physicians India. 2021;69:11-12. [PubMed] |
| 16. | Topan MM, Sporea I, Dănilă M, Popescu A, Ghiuchici AM, Lupuşoru R, Şirli R. Impact of Sarcopenia on Survival and Clinical Outcomes in Patients With Liver Cirrhosis. Front Nutr. 2021;8:766451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Zeng X, Shi ZW, Yu JJ, Wang LF, Luo YY, Jin SM, Zhang LY, Tan W, Shi PM, Yu H, Zhang CQ, Xie WF. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. 2021;12:1948-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Nardelli S, Riggio O, Gioia S, Merli M, Spagnoli A, di Martino M, Pelle G, Ridola L. Risk factors for hepatic encephalopathy and mortality in cirrhosis: The role of cognitive impairment, muscle alterations and shunts. Dig Liver Dis. 2022;54:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Miwa T, Hanai T, Nishimura K, Maeda T, Ogiso Y, Imai K, Suetsugu A, Takai K, Shiraki M, Shimizu M. Handgrip strength stratifies the risk of covert and overt hepatic encephalopathy in patients with cirrhosis. JPEN J Parenter Enteral Nutr. 2022;46:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Dajti E, Renzulli M, Ravaioli F, Marasco G, Vara G, Brandi N, Rossini B, Colecchia L, Alemanni LV, Ferrarese A, Vestito A, Tamè M, Azzaroli F, Festi D, Golfieri R, Colecchia A. The interplay between sarcopenia and portal hypertension predicts ascites and mortality in cirrhosis. Dig Liver Dis. 2023;55:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Ebadi M, Tsien C, Bhanji RA, Dunichand-Hoedl AR, Rider E, Motamedrad M, Mazurak VC, Baracos V, Montano-Loza AJ. Skeletal Muscle Pathological Fat Infiltration (Myosteatosis) Is Associated with Higher Mortality in Patients with Cirrhosis. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 22. | Ishizu Y, Ishigami M, Honda T, Imai N, Ito T, Yamamoto K, Yokoyama S, Ishikawa T, Kawashima H. Decreased appetite is associated with the presence of sarcopenia in patients with cirrhosis. Nutrition. 2022;103-104:111807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Sato S, Namisaki T, Murata K, Fujimoto Y, Takeda S, Enomoto M, Shibamoto A, Ishida K, Ogawa H, Takagi H, Tsuji Y, Kaya D, Fujinaga Y, Furukawa M, Inoue T, Sawada Y, Nishimura N, Kitagawa K, Ozutsumi T, Takaya H, Kaji K, Shimozato N, Kawaratani H, Moriya K, Akahane T, Mitoro A, Yoshiji H. The association between sarcopenia and endotoxin in patients with alcoholic cirrhosis. Medicine (Baltimore). 2021;100:e27212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Han JW, Kim DI, Nam HC, Chang UI, Yang JM, Song DS. Association between serum tumor necrosis factor-α and sarcopenia in liver cirrhosis. Clin Mol Hepatol. 2022;28:219-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Sinclair M, Grossmann M, Angus PW, Hoermann R, Hey P, Scodellaro T, Gow PJ. Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol. 2016;31:661-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Gut-liver axis in cirrhosis: Are hemodynamic changes a missing link? World J Clin Cases. 2021;9:9320-9332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (3)] |
| 27. | Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, Thalheimer U. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol. 2014;20:16795-16810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 28. | Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 29. | Gómez-Hurtado I, Such J, Sanz Y, Francés R. Gut microbiota-related complications in cirrhosis. World J Gastroenterol. 2014;20:15624-15631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Jin M, Kalainy S, Baskota N, Chiang D, Deehan EC, McDougall C, Tandon P, Martínez I, Cervera C, Walter J, Abraldes JG. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019;39:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | Zeng Y, Chen S, Fu Y, Wu W, Chen T, Chen J, Yang B, Ou Q. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. 2020;27:143-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 32. | Kajihara M, Koido S, Kanai T, Ito Z, Matsumoto Y, Takakura K, Saruta M, Kato K, Odamaki T, Xiao JZ, Sato N, Ohkusa T. Characterisation of blood microbiota in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, Zhang X, Xu H, Ren J. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Zheng R, Wang G, Pang Z, Ran N, Gu Y, Guan X, Yuan Y, Zuo X, Pan H, Zheng J, Wang F. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020;9:4232-4250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Lapidot Y, Amir A, Nosenko R, Uzan-Yulzari A, Veitsman E, Cohen-Ezra O, Davidov Y, Weiss P, Bradichevski T, Segev S, Koren O, Safran M, Ben-Ari Z. Alterations in the Gut Microbiome in the Progression of Cirrhosis to Hepatocellular Carcinoma. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 36. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 838] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 37. | Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Sci Rep. 2016;6:26800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 38. | Liu Y, Jin Y, Li J, Zhao L, Li Z, Xu J, Zhao F, Feng J, Chen H, Fang C, Shilpakar R, Wei Y. Small Bowel Transit and Altered Gut Microbiota in Patients With Liver Cirrhosis. Front Physiol. 2018;9:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Inoue T, Nakayama J, Moriya K, Kawaratani H, Momoda R, Ito K, Iio E, Nojiri S, Fujiwara K, Yoneda M, Yoshiji H, Tanaka Y. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin Infect Dis. 2018;67:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 40. | Maslennikov R, Ivashkin V, Efremova I, Alieva A, Kashuh E, Tsvetaeva E, Poluektova E, Shirokova E, Ivashkin K. Gut dysbiosis is associated with poorer long-term prognosis in cirrhosis. World J Hepatol. 2021;13:557-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (3)] |
| 42. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 621] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 43. | Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, Gasbarrini A, Miele L, Grieco A. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25:4814-4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (4)] |
| 44. | Lian XX, Sun YP, Guo XX. [Correlation between intestinal mucosal permeability and prognosis in patients with liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Choi Y, Jeon WK, Hwang SJ, Kim BI, Sohn CI, Park DI, Cho YK, Kim HJ, Park JH. The role of the gut barrier function in the pathophysiology of viral liver cirrhosis. Hepatogastroenterology. 2011;58:1244-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, Spiliopoulou I, Kaltezioti V, Charonis A, Nikolopoulou VN, Scopa CD, Thomopoulos KC. Altered intestinal tight junctions' expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. Eur J Clin Invest. 2012;42:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 47. | Bach Knudsen KE, Lærke HN, Hedemann MS, Nielsen TS, Ingerslev AK, Gundelund Nielsen DS, Theil PK, Purup S, Hald S, Schioldan AG, Marco ML, Gregersen S, Hermansen K. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 366] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 48. | Shulpekova Y, Zharkova M, Tkachenko P, Tikhonov I, Stepanov A, Synitsyna A, Izotov A, Butkova T, Shulpekova N, Lapina N, Nechaev V, Kardasheva S, Okhlobystin A, Ivashkin V. The Role of Bile Acids in the Human Body and in the Development of Diseases. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 49. | Bajaj JS, Vargas HE, Reddy KR, Lai JC, O'Leary JG, Tandon P, Wong F, Mitrani R, White MB, Kelly M, Fagan A, Patil R, Sait S, Sikaroodi M, Thacker LR, Gillevet PM. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:756-765.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 50. | Bruns T, Reuken PA, Stengel S, Gerber L, Appenrodt B, Schade JH, Lammert F, Zeuzem S, Stallmach A. The prognostic significance of bacterial DNA in patients with decompensated cirrhosis and suspected infection. Liver Int. 2016;36:1133-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Alvarez-Silva C, Schierwagen R, Pohlmann A, Magdaleno F, Uschner FE, Ryan P, Vehreschild MJGT, Claria J, Latz E, Lelouvier B, Arumugam M, Trebicka J. Compartmentalization of Immune Response and Microbial Translocation in Decompensated Cirrhosis. Front Immunol. 2019;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Wang Z, Wang A, Gong Z, Biviano I, Liu H, Hu J. Plasma claudin-3 is associated with tumor necrosis factor-alpha-induced intestinal endotoxemia in liver disease. Clin Res Hepatol Gastroenterol. 2019;43:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Ivashkin VT, Maev IV, Abdulganieva DI, Alekseeva OP, Alekseenko SA, Zolnikova OYu, Korochanskaya NV, Medvedev OS, Poluektova EA, Simanenkov VI, Trukhmanov AS, Khlynov IB, Tsukanov VV, Shifrin OS, Ivashkin KV, Lapina TL, Maslennikov RV, Fadeeva MV, Ulyanin AI. Practical Recommendation of the Scientific Сommunity for Human Microbiome Research (CHMR) and the Russian Gastroenterological Association (RGA) on Small Intestinal Bacterial Overgrowth in Adults. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2022;32:68-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Feng X, Li X, Zhang X, Chen W, Tian Y, Yang Q, Yang Y, Pan H, Jiang Z. Hepatic Encephalopathy in Cirrhotic Patients and Risk of Small Intestinal Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Biomed Res Int. 2022;2022:2469513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 56. | Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Wang J, Chen M, Sun G, Et Al. Small bowel bacterial overgrowth and endotoxemia in cirrhosis. Zhonghua Nei Ke Za Zhi. 2002;41:459-461. [PubMed] |
| 58. | Maslennikov R, Pavlov C, Ivashkin V. Is small intestinal bacterial overgrowth a cause of hyperdynamic circulation in cirrhosis? Turk J Gastroenterol. 2019;30:964-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Kudryavtseva A, Krasnov G. Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis. World J Gastroenterol. 2022;28:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 60. | Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-α impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336-E347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Lang CH, Frost RA, Jefferson LS, Kimball SR, Vary TC. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol Endocrinol Metab. 2000;278:E1133-E1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Yao J, Chang L, Yuan L, Duan Z. Nutrition status and small intestinal bacterial overgrowth in patients with virus-related cirrhosis. Asia Pac J Clin Nutr. 2016;25:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 63. | Esposito P, Picciotto D, Battaglia Y, Costigliolo F, Viazzi F, Verzola D. Myostatin: Basic biology to clinical application. Adv Clin Chem. 2022;106:181-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 64. | Sharma M, McFarlane C, Kambadur R, Kukreti H, Bonala S, Srinivasan S. Myostatin: expanding horizons. IUBMB Life. 2015;67:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 65. | Dschietzig TB. Myostatin - From the Mighty Mouse to cardiovascular disease and cachexia. Clin Chim Acta. 2014;433:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 66. | Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C, Stark GR, Welle S, Naga Prasad SV, Dasarathy S. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162-18167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 67. | Richardson AJ, McKain N, Wallace RJ. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013;13:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 68. | Walker V. Ammonia metabolism and hyperammonemic disorders. Adv Clin Chem. 2014;67:73-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 69. | Jindal A, Jagdish RK. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin Mol Hepatol. 2019;25:270-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 70. | Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 71. | Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G, McDonald C, Almasan A, Hazen SL, Naga Prasad SV, Dasarathy S. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983-E993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 72. | Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, Hatzoglou M, Dasarathy S. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J Hepatol. 2016;65:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Ponziani FR, Picca A, Marzetti E, Calvani R, Conta G, Del Chierico F, Capuani G, Faccia M, Fianchi F, Funaro B, Josè Coelho-Junior H, Petito V, Rinninella E, Paroni Sterbini F, Reddel S, Vernocchi P, Cristina Mele M, Miccheli A, Putignani L, Sanguinetti M, Pompili M, Gasbarrini A; GuLiver study group. Characterization of the gut-liver-muscle axis in cirrhotic patients with sarcopenia. Liver Int. 2021;41:1320-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 74. | Ren X, Hao S, Yang C, Yuan L, Zhou X, Zhao H, Yao J. Alterations of intestinal microbiota in liver cirrhosis with muscle wasting. Nutrition. 2021;83:111081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 75. | Maslennikov R, Ivashkin V, Alieva A, Poluektova E, Kudryavtseva A, Krasnov G, Zharkova M, Zharikov Y. Gut dysbiosis and body composition in cirrhosis. World J Hepatol. 2022;14:1210-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 76. | Lee PC, Lee KC, Yang TC, Lu HS, Cheng TY, Chen YJ, Chiou JJ, Huang CW, Yang UC, Chia-Hui Tan E, Chou SH, Kuo YL, Schnabl B, Huang YH, Hou MC. Sarcopenia-related gut microbial changes are associated with the risk of complications in people with cirrhosis. JHEP Rep. 2023;5:100619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 77. | Wang J, Zhang L, Hou X. Efficacy of rifaximin in treating with small intestine bacterial overgrowth: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15:1385-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther. 2017;45:604-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 79. | Zhuang X, Tian Z, Li L, Zeng Z, Chen M, Xiong L. Fecal Microbiota Alterations Associated With Diarrhea-Predominant Irritable Bowel Syndrome. Front Microbiol. 2018;9:1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 80. | Liu ZJ, Wei H, Duan LP, Zhu SW, Zhang L, Wang K. [Clinical features of irritable bowel syndrome with small intestinal bacterial overgrowth and a preliminary study of effectiveness of Rifaximin]. Zhonghua Yi Xue Za Zhi. 2016;96:1896-1902. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Bae S, Lee KJ, Kim YS, Kim KN. Determination of rifaximin treatment period according to lactulose breath test values in nonconstipated irritable bowel syndrome subjects. J Korean Med Sci. 2015;30:757-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with Rifaximin. World J Gastroenterol. 2009;15:2628-2631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Furnari M, De Alessandri A, Cresta F, Haupt M, Bassi M, Calvi A, Haupt R, Bodini G, Ahmed I, Bagnasco F, Giannini EG, Casciaro R. The role of small intestinal bacterial overgrowth in cystic fibrosis: a randomized case-controlled clinical trial with rifaximin. J Gastroenterol. 2019;54:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | Biancone L, Vernia P, Agostini D, Ferrieri A, Pallone F. Effect of rifaximin on intestinal bacterial overgrowth in Crohn's disease as assessed by the H2-Glucose Breath Test. Curr Med Res Opin. 2000;16:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Cuoco L, Montalto M, Jorizzo RA, Santarelli L, Arancio F, Cammarota G, Gasbarrini G. Eradication of small intestinal bacterial overgrowth and oro-cecal transit in diabetics. Hepatogastroenterology. 2002;49:1582-1586. [PubMed] |
| 86. | Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, Ferone D. Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92:2119-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 88. | Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Assessment of small intestinal bacterial overgrowth in uncomplicated acute diverticulitis of the colon. World J Gastroenterol. 2005;11:2773-2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | D'Incà R, Pomerri F, Vettorato MG, Dal Pont E, Di Leo V, Ferronato A, Medici V, Sturniolo GC. Interaction between rifaximin and dietary fibre in patients with diverticular disease. Aliment Pharmacol Ther. 2007;25:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Parodi A, Sessarego M, Greco A, Bazzica M, Filaci G, Setti M, Savarino E, Indiveri F, Savarino V, Ghio M. Small intestinal bacterial overgrowth in patients suffering from scleroderma: clinical effectiveness of its eradication. Am J Gastroenterol. 2008;103:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 91. | Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, Parodi A, Savarino V. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 92. | Yang C, Zhang X, Wang S, Huo X, Wang J. Small intestinal bacterial overgrowth and evaluation of intestinal barrier function in patients with ulcerative colitis. Am J Transl Res. 2021;13:6605-6610. [PubMed] |
| 93. | Pérez Aisa A, García Gavilán MC, Alcaide García J, Méndez Sánchez IM, Rivera Irigoin R, Fernández Cano F, Pereda Salguero T, Rivas Ruiz F. Small intestinal bacterial overgrowth is common after gastrectomy but with little impact on nutritional status. Gastroenterol Hepatol. 2019;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 94. | Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Rifaximin versus chlortetracycline in the short-term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2000;14:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci. 2008;53:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Lauritano EC, Gabrielli M, Scarpellini E, Ojetti V, Roccarina D, Villita A, Fiore E, Flore R, Santoliquido A, Tondi P, Gasbarrini G, Ghirlanda G, Gasbarrini A. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13:111-116. [PubMed] |
| 97. | Suhocki PV, Ronald JS, Diehl AME, Murdoch DM, Doraiswamy PM. Probing gut-brain links in Alzheimer's disease with rifaximin. Alzheimers Dement (NY). 2022;8:e12225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Liu Z, Zhu S, He M, Li M, Wei H, Zhang L, Sun Q, Jia Q, Hu N, Fang Y, Song L, Zhou C, Tao H, Kao JY, Zhu H, Owyang C, Duan L. Patients with breath test positive are necessary to be identified from irritable bowel syndrome: a clinical trial based on microbiomics and rifaximin sensitivity. Chin Med J (Engl). 2022;135:1716-1727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 99. | Li Y, Hong G, Yang M, Li G, Jin Y, Xiong H, Qian W, Hou X. Fecal bacteria can predict the efficacy of rifaximin in patients with diarrhea-predominant irritable bowel syndrome. Pharmacol Res. 2020;159:104936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 100. | Soldi S, Vasileiadis S, Uggeri F, Campanale M, Morelli L, Fogli MV, Calanni F, Grimaldi M, Gasbarrini A. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol. 2015;8:309-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 101. | De Vincentis A, Santonico M, Del Chierico F, Altomare A, Marigliano B, Laudisio A, Reddel S, Grasso S, Zompanti A, Pennazza G, Putignani L, Guarino MPL, Cicala M, Antonelli Incalzi R. Gut Microbiota and Related Electronic Multisensorial System Changes in Subjects With Symptomatic Uncomplicated Diverticular Disease Undergoing Rifaximin Therapy. Front Med (Lausanne). 2021;8:655474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 102. | Ivashkin V, Shifrin O, Maslennikov R, Poluektova E, Korolev A, Kudryavtseva A, Krasnov G, Benuni N, Barbara G. Eubiotic effect of rifaximin is associated with decreasing abdominal pain in symptomatic uncomplicated diverticular disease: results from an observational cohort study. BMC Gastroenterol. 2023;23:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 103. | Kimber C, Zhang S, Johnson C, West RE 3rd, Prokopienko AJ, Mahnken JD, Yu AS, Hoofnagle AN, Ir D, Robertson CE, Miyazaki M, Chonchol M, Jovanovich A, Kestenbaum B, Frank DN, Nolin TD, Stubbs JR. Randomized, Placebo-Controlled Trial of Rifaximin Therapy for Lowering Gut-Derived Cardiovascular Toxins and Inflammation in CKD. Kidney360. 2020;1:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 104. | Ponziani FR, Scaldaferri F, De Siena M, Mangiola F, Matteo MV, Pecere S, Petito V, Sterbini FP, Lopetuso LR, Masucci L, Cammarota G, Sanguinetti M, Gasbarrini A. Increased Faecalibacterium abundance is associated with clinical improvement in patients receiving rifaximin treatment. Benef Microbes. 2020;11:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Brigidi P, Swennen E, Rizzello F, Bozzolasco M, Matteuzzi D. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother. 2002;14:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, Calanni F, Brigidi P, Gibson GR, Costabile A. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65:2556-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 107. | Pimentel R, Gregório C, Figueiredo P. Antibiotic prophylaxis for prevention of spontaneous bacterial peritonitis in liver cirrhosis: systematic review. Acta Gastroenterol Belg. 2021;84:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 108. | Faust N, Yamada A, Haider H, Komaki Y, Komaki F, Micic D, Sakuraba A. Systemic review and network meta-analysis: Prophylactic antibiotic therapy for spontaneous bacterial peritonitis. World J Hepatol. 2020;12:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 109. | Soni H, Kumar-M P, Sharma V, Bellam BL, Mishra S, Mahendru D, Mandavdhare HS, Medhi B, Dutta U, Singh V. Antibiotics for prophylaxis of spontaneous bacterial peritonitis: systematic review & Bayesian network meta-analysis. Hepatol Int. 2020;14:399-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 110. | Kamal F, Khan MA, Khan Z, Cholankeril G, Hammad TA, Lee WM, Ahmed A, Waters B, Howden CW, Nair S, Satapathy SK. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Dhiman RK, Thumburu KK, Verma N, Chopra M, Rathi S, Dutta U, Singal AK, Taneja S, Duseja A, Singh M. Comparative Efficacy of Treatment Options for Minimal Hepatic Encephalopathy: A Systematic Review and Network Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:800-812.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 112. | Eltawil KM, Laryea M, Peltekian K, Molinari M. Rifaximin vs. conventional oral therapy for hepatic encephalopathy: a meta-analysis. World J Gastroenterol. 2012;18:767-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 113. | Zhu GQ, Shi KQ, Huang S, Wang LR, Lin YQ, Huang GQ, Chen YP, Braddock M, Zheng MH. Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Aliment Pharmacol Ther. 2015;41:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 114. | Zhang Y, Feng Y, Cao B, Tian Q. Effects of SIBO and rifaximin therapy on MHE caused by hepatic cirrhosis. Int J Clin Exp Med. 2015;8:2954-2957. [PubMed] |
| 115. | Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 116. | Takaya H, Namisaki T, Sato S, Kaji K, Tsuji Y, Kaya D, Fujinaga Y, Sawada Y, Shimozato N, Kawaratani H, Moriya K, Akahane T, Mitoro A, Yoshiji H. Increased Endotoxin Activity Is Associated with the Risk of Developing Acute-on-Chronic Liver Failure. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Kaji K, Saikawa S, Takaya H, Fujinaga Y, Furukawa M, Kitagawa K, Ozutsumi T, Kaya D, Tsuji Y, Sawada Y, Kawaratani H, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin Alleviates Endotoxemia with Decreased Serum Levels of Soluble CD163 and Mannose Receptor and Partial Modification of Gut Microbiota in Cirrhotic Patients. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 118. | Kaji K, Takaya H, Saikawa S, Furukawa M, Sato S, Kawaratani H, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yoshiji H. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol. 2017;23:8355-8366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 119. | Zeng X, Tang XJ, Sheng X, Ni W, Xin HG, Chen WZ, Jiang CF, Lin Y, Shi J, Shi B, Chen YX, Yuan ZL, Xie WF. Does low-dose rifaximin ameliorate endotoxemia in patients with liver cirrhosis: a prospective study. J Dig Dis. 2015;16:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 120. | Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8:e60042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 121. | Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liver Int. 2012;32:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | Patel VC, Lee S, McPhail MJW, Da Silva K, Guilly S, Zamalloa A, Witherden E, Støy S, Manakkat Vijay GK, Pons N, Galleron N, Huang X, Gencer S, Coen M, Tranah TH, Wendon JA, Bruce KD, Le Chatelier E, Ehrlich SD, Edwards LA, Shoaie S, Shawcross DL. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J Hepatol. 2022;76:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 133] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 123. | Yu X, Jin Y, Zhou W, Xiao T, Wu Z, Su J, Gao H, Shen P, Zheng B, Luo Q, Li L, Xiao Y. Rifaximin Modulates the Gut Microbiota to Prevent Hepatic Encephalopathy in Liver Cirrhosis Without Impacting the Resistome. Front Cell Infect Microbiol. 2021;11:761192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 124. | Mariani M, Zuccaro V, Patruno SF, Scudeller L, Sacchi P, Lombardi A, Vecchia M, Columpsi P, Marone P, Filice G, Bruno R. The impact of rifaximin in the prevention of bacterial infections in cirrhosis. Eur Rev Med Pharmacol Sci. 2017;21:1151-1158. [PubMed] |
| 125. | Zeng X, Sheng X, Wang PQ, Xin HG, Guo YB, Lin Y, Zhong JW, He CZ, Yin J, Liu TT, Ma WJ, Xiao X, Shi PM, Yuan ZL, Yang L, Ma X, Xu JM, Shen XZ, Yang CQ, Zhu X, Lv NH, Xie WF. Low-dose rifaximin prevents complications and improves survival in patients with decompensated liver cirrhosis. Hepatol Int. 2021;15:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 126. | Flamm SL, Mullen KD, Heimanson Z, Sanyal AJ. Rifaximin has the potential to prevent complications of cirrhosis. Therap Adv Gastroenterol. 2018;11:1756284818800307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 127. | Ibrahim ES, Alsebaey A, Zaghla H, Moawad Abdelmageed S, Gameel K, Abdelsameea E. Long-term rifaximin therapy as a primary prevention of hepatorenal syndrome. Eur J Gastroenterol Hepatol. 2017;29:1247-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 128. | Dong T, Aronsohn A, Gautham Reddy K, Te HS. Rifaximin Decreases the Incidence and Severity of Acute Kidney Injury and Hepatorenal Syndrome in Cirrhosis. Dig Dis Sci. 2016;61:3621-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 129. | Wang Z, Hou W, Zhang W, Wu HX, Zheng SJ, Hu ZJ. [Rifaximin improves clinical symptoms and short-term survival in cirrhotic patients with refractory type ascites]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:1170-1174. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 130. | Lv XY, Ding HG, Zheng JF, Fan CL, Li L. Rifaximin improves survival in cirrhotic patients with refractory ascites: A real-world study. World J Gastroenterol. 2020;26:199-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 131. | Kawaguchi T, Suzuki F, Imamura M, Murashima N, Yanase M, Mine T, Fujisawa M, Sato I, Yoshiji H, Okita K, Suzuki K. Rifaximin-altered gut microbiota components associated with liver/neuropsychological functions in patients with hepatic encephalopathy: An exploratory data analysis of phase II/III clinical trials. Hepatol Res. 2019;49:404-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 132. | Yokoyama K, Fukuda H, Yamauchi R, Higashi M, Miyayama T, Higashi T, Uchida Y, Shibata K, Tsuchiya N, Fukunaga A, Umeda K, Takata K, Tanaka T, Shakado S, Sakisaka S, Hirai F. Long-Term Effects of Rifaximin on Patients with Hepatic Encephalopathy: Its Possible Effects on the Improvement in the Blood Ammonia Concentration Levels, Hepatic Spare Ability and Refractory Ascites. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 133. | Kawaratani H, Kondo Y, Tatsumi R, Kawabe N, Tanabe N, Sakamaki A, Okumoto K, Uchida Y, Endo K, Kawaguchi T, Oikawa T, Ishizu Y, Hige S, Takami T, Terai S, Ueno Y, Mochida S, Takikawa Y, Torimura T, Matsuura T, Ishigami M, Koike K, Yoshiji H. Long-Term Efficacy and Safety of Rifaximin in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 134. | Glal KAM, Abd-Elsalam SM, Mostafa TM. Nitazoxanide versus rifaximin in preventing the recurrence of hepatic encephalopathy: A randomized double-blind controlled trial. J Hepatobiliary Pancreat Sci. 2021;28:812-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Gómez-Hurtado I, Gimenez P, García I, Zapater P, Francés R, González-Navajas JM, Manichanh C, Ramos JM, Bellot P, Guarner F, Such J. Norfloxacin is more effective than Rifaximin in avoiding bacterial translocation in an animal model of cirrhosis. Liver Int. 2018;38:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 136. | Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol. 1991;23:175-178. [PubMed] |
| 137. | Murata K, Kaji K, Nishimura N, Enomoto M, Fujimoto Y, Takeda S, Tsuji Y, Fujinaga Y, Takaya H, Kawaratani H, Namisaki T, Akahane T, Yoshiji H. Rifaximin enhances the Lcarnitinemediated preventive effects on skeletal muscle atrophy in cirrhotic rats by modulating the gutlivermuscle axis. Int J Mol Med. 2022;50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 138. | Ishikawa T, Endo S, Imai M, Azumi M, Nozawa Y, Sano T, Iwanaga A, Honma T, Yoshida T. Changes in the Body Composition and Nutritional Status after Long-term Rifaximin Therapy for Hyperammonemia in Japanese Patients with Hepatic Encephalopathy. Intern Med. 2020;59:2465-2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Miller LE, Tennilä J, Ouwehand AC. Efficacy and tolerance of lactitol supplementation for adult constipation: a systematic review and meta-analysis. Clin Exp Gastroenterol. 2014;7:241-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 140. | Maev IV, Kazyulin AN, Kucheryavy YuA, Cheryomushkin SV, Goncharenko AYu, Gilyuk АV. Diagnosis of Functional Gastrointestinal Disorders and Choice of Treatment Regimen in Constipation Patients. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2021;31:7-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Ivashkin VT, Maev IV, Shelygin YuA, Baranskaya EK, Belous SS, Belousova EA, Beniashvili AG, Vasilyev SV, Veselov AV, Grigoryev EG, Kostenko NV, Kashnikov VN, Kulikovskiy VF, Loranskaya ID, Lyashenko OS, Poluektova EA, Rumyantsev VG, Timerbulatov VM, Fomenko OYu, Khubezov DA, Chashkova EYu, Chibisov GI, Shapina MV, Sheptulin AA, Shifrin OS, Trukhmanov AS, Alekseeva OP, Alekseenko SA, Baranovsky AYu, Zolnikova OYu, Korochanskaya NV, Mammayev SN, Khlynov IB, Tsukanov VV. Diagnosis and Treatment of Irritable Bowel Syndrome: Clinical Recommendations of the Russian Gastroenterological Association and Association of Coloproctologists of Russia. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2021;31:74-95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 142. | Morgan MY. Current state of knowledge of hepatic encephalopathy (part III): non-absorbable disaccharides. Metab Brain Dis. 2016;31:1361-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 143. | Sharma P, Sharma BC. Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 144. | Masini A, Efrati C, Merli M, Attili AF, Amodio P, Ceccanti M, Riggio O. Effect of lactitol on blood ammonia response to oral glutamine challenge in cirrhotic patients: evidence for an effect of nonabsorbable disaccharides on small intestine ammonia generation. Am J Gastroenterol. 1999;94:3323-3327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 145. | Blanc P, Daures JP, Rouillon JM, Peray P, Pierrugues R, Larrey D, Gremy F, Michel H. Lactitol or lactulose in the treatment of chronic hepatic encephalopathy: results of a meta-analysis. Hepatology. 1992;15:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 146. | Gluud LL, Vilstrup H, Morgan MY. Nonabsorbable disaccharides for hepatic encephalopathy: A systematic review and meta-analysis. Hepatology. 2016;64:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 147. | Gluud LL, Vilstrup H, Morgan MY. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. Cochrane Database Syst Rev. 2016;2016:CD003044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 148. | Sharma P, Sharma BC, Agrawal A, Sarin SK. Primary prophylaxis of overt hepatic encephalopathy in patients with cirrhosis: an open labeled randomized controlled trial of lactulose versus no lactulose. J Gastroenterol Hepatol. 2012;27:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 149. | Agrawal A, Sharma BC, Sharma P, Sarin SK. Secondary prophylaxis of hepatic encephalopathy in cirrhosis: an open-label, randomized controlled trial of lactulose, probiotics, and no therapy. Am J Gastroenterol. 2012;107:1043-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 150. | Riggio O, Balducci G, Ariosto F, Merli M, Tremiterra S, Ziparo V, Capocaccia L. Lactitol in the treatment of chronic hepatic encephalopathy--a randomized cross-over comparison with lactulose. Hepatogastroenterology. 1990;37:524-527. [PubMed] |
| 151. | Riggio O, Balducci G, Ariosto F, Merli M, Pieche U, Pinto G, Tremiterra S, Ziparo V, Capocaccia L. Lactitol in prevention of recurrent episodes of hepatic encephalopathy in cirrhotic patients with portal-systemic shunt. Dig Dis Sci. 1989;34:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 152. | Merli M, Caschera M, Piat C, Pinto G, Diofebi M, Riggio O. The effect of lactulose and lactitol administration on fecal fat excretion in patients with liver cirrhosis. J Clin Gastroenterol. 1992;15:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 153. | Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, Romiti A, Candiani C, Capocaccia L. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol. 1990;12:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 154. | Ballongue J, Schumann C, Quignon P. Effects of lactulose and lactitol on colonic microflora and enzymatic activity. Scand J Gastroenterol Suppl. 1997;222:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 137] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 155. | Li XQ, Zhang XM, Wu X, Lan Y, Xu L, Meng XC, Li JN. Beneficial effects of lactitol on the composition of gut microbiota in constipated patients. J Dig Dis. 2020;21:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 156. | Tarao K, Tamai S, Ito Y, Okawa S, Hayashi M. [Effects of lactitol on fecal bacterial flora in patients with liver cirrhosis and hepatic encephalopathy]. Nihon Shokakibyo Gakkai Zasshi. 1995;92:1037-1050. [PubMed] |
| 157. | Lu H, Chen L, Pan X, Yao Y, Zhang H, Zhu X, Lou X, Zhu C, Wang J, Li L, Wu Z. Lactitol Supplementation Modulates Intestinal Microbiome in Liver Cirrhotic Patients. Front Med (Lausanne). 2021;8:762930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 158. | Chen C, Li L, Wu Z, Chen H, Fu S. Effects of lactitol on intestinal microflora and plasma endotoxin in patients with chronic viral hepatitis. J Infect. 2007;54:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 159. | Ziada DH, Soliman HH, El Yamany SA, Hamisa MF, Hasan AM. Can Lactobacillus acidophilus improve minimal hepatic encephalopathy? A neurometabolite study using magnetic resonance spectroscopy. Arab J Gastroenterol. 2013;14:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 160. | Manzhalii E, Moyseyenko V, Kondratiuk V, Molochek N, Falalyeyeva T, Kobyliak N. Effect of a specific Escherichia coli Nissle 1917 strain on minimal/mild hepatic encephalopathy treatment. World J Hepatol. 2022;14:634-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 161. | Sarangi AN, Goel A, Singh A, Sasi A, Aggarwal R. Faecal bacterial microbiota in patients with cirrhosis and the effect of lactulose administration. BMC Gastroenterol. 2017;17:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 162. | Wang JY, Bajaj JS, Wang JB, Shang J, Zhou XM, Guo XL, Zhu X, Meng LN, Jiang HX, Mi YQ, Xu JM, Yang JH, Wang BS, Zhang NP. Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J Dig Dis. 2019;20:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 163. | Jain L, Sharma BC, Srivastava S, Puri SK, Sharma P, Sarin S. Serum endotoxin, inflammatory mediators, and magnetic resonance spectroscopy before and after treatment in patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol. 2013;28:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 164. | Moratalla A, Ampuero J, Bellot P, Gallego-Durán R, Zapater P, Roger M, Figueruela B, Martínez-Moreno B, González-Navajas JM, Such J, Romero-Gómez M, Francés R. Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver Int. 2017;37:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Ivashkin VT, Maev IV, Abdulganieva DI, Alekseenko SA, Gorelov AV, Zakharova IN, Zolnikova OYu, Ivashkina NYu, Korochanskaya NV, Mammayev SN, Poluektova EA, Trukhmanov AS, Usenko DV, Uspensky YuP, Tsukanov VV, Shifrin OS, Berezhnaya IV, Ivashkin KV, Lapina TL, Maslennikov RV, Nikolaeva SV, Sugyan NG, Ulyanin AI. Practical Recommendations of Scientific Society for the Study of Human Microbiome and the Russian Gastroenterological Association on Use of Probiotics, Prebiotics, Synbiotics and Functional Foods in Treatment and Prevention of Gastroenterological Diseases in Children and Adults. Russian Journal of Gastroenterology, Hepatology, Coloproctology. 2021;31:65-91. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 166. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Probiotics in hepatology: An update. World J Hepatol. 2021;13:1154-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 167. | Cao Q, Yu CB, Yang SG, Cao HC, Chen P, Deng M, Li LJ. Effect of probiotic treatment on cirrhotic patients with minimal hepatic encephalopathy: A meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 168. | Viramontes Hörner D, Avery A, Stow R. The Effects of Probiotics and Symbiotics on Risk Factors for Hepatic Encephalopathy: A Systematic Review. J Clin Gastroenterol. 2017;51:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 169. | Huang L, Yu Q, Peng H, Zhen Z. Alterations of gut microbiome and effects of probiotic therapy in patients with liver cirrhosis: A systematic review and meta-analysis. Medicine (Baltimore). 2022;101:e32335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 170. | Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 171. | Kazemi A, Soltani S, Ghorabi S, Keshtkar A, Daneshzad E, Nasri F, Mazloomi SM. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials. Clin Nutr. 2020;39:789-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 172. | Maslennikov R, Efremova I, Ivashkin V, Zharkova M, Poluektova E, Shirokova E, Ivashkin K. Effect of probiotics on hemodynamic changes and complications associated with cirrhosis: A pilot randomized controlled trial. World J Hepatol. 2022;14:1667-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 173. | Xia X, Chen J, Xia J, Wang B, Liu H, Yang L, Wang Y, Ling Z. Role of probiotics in the treatment of minimal hepatic encephalopathy in patients with HBV-induced liver cirrhosis. J Int Med Res. 2018;46:3596-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 174. | Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, Khattri A, Malhotra S, Duseja A, Chawla YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327-37.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 175. | Lata J, Novotný I, Príbramská V, Juránková J, Fric P, Kroupa R, Stibůrek O. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol. 2007;19:1111-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 176. | Koga H, Tamiya Y, Mitsuyama K, Ishibashi M, Matsumoto S, Imaoka A, Hara T, Nakano M, Ooeda K, Umezaki Y, Sata M. Probiotics promote rapid-turnover protein production by restoring gut flora in patients with alcoholic liver cirrhosis. Hepatol Int. 2013;7:767-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 177. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, Luketic V, Stravitz RT, Siddiqui MS, Fuchs M, Thacker LR, Wade JB, Daita K, Sistrun S, White MB, Noble NA, Thorpe C, Kakiyama G, Pandak WM, Sikaroodi M, Gillevet PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (1)] |
| 178. | Horvath A, Durdevic M, Leber B, di Vora K, Rainer F, Krones E, Douschan P, Spindelboeck W, Durchschein F, Zollner G, Stauber RE, Fickert P, Stiegler P, Stadlbauer V. Changes in the Intestinal Microbiome during a Multispecies Probiotic Intervention in Compensated Cirrhosis. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 179. | Zhong C, Qu C, Wang B, Liang S, Zeng B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J Clin Gastroenterol. 2017;51:300-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 180. | Nickles MA, Hasan A, Shakhbazova A, Wright S, Chambers CJ, Sivamani RK. Alternative Treatment Approaches to Small Intestinal Bacterial Overgrowth: A Systematic Review. J Altern Complement Med. 2021;27:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 181. | Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2014;12:1003-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 182. | Kwak DS, Jun DW, Seo JG, Chung WS, Park SE, Lee KN, Khalid-Saeed W, Lee HL, Lee OY, Yoon BC, Choi HS. Short-term probiotic therapy alleviates small intestinal bacterial overgrowth, but does not improve intestinal permeability in chronic liver disease. Eur J Gastroenterol Hepatol. 2014;26:1353-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 183. | Ruchkina IN, Fadeeva NA. [The role of a combined probiotic in the treatment of lactase deficiency]. Ter Arkh. 2021;93:431-434. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 184. | Luo M, Liu Q, Xiao L, Xiong LS. Golden bifid might improve diarrhea-predominant irritable bowel syndrome via microbiota modulation. J Health Popul Nutr. 2022;41:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 185. | Bustos Fernández LM, Man F, Lasa JS. "Impact of Saccharomyces boulardii CNCM I-745 on bacterial overgrowth and composition of intestinal microbiota in IBS-D patients: results of a randomized pilot study". Dig Dis. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 186. | Lee SH, Joo NS, Kim KM, Kim KN. The Therapeutic Effect of a Multistrain Probiotic on Diarrhea-Predominant Irritable Bowel Syndrome: A Pilot Study. Gastroenterol Res Pract. 2018;2018:8791916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 187. | Liang S, Xu L, Zhang D, Wu Z. Effect of probiotics on small intestinal bacterial overgrowth in patients with gastric and colorectal cancer. Turk J Gastroenterol. 2016;27:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 188. | García-Collinot G, Madrigal-Santillán EO, Martínez-Bencomo MA, Carranza-Muleiro RA, Jara LJ, Vera-Lastra O, Montes-Cortes DH, Medina G, Cruz-Domínguez MP. Effectiveness of Saccharomyces boulardii and Metronidazole for Small Intestinal Bacterial Overgrowth in Systemic Sclerosis. Dig Dis Sci. 2020;65:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 189. | Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, Morton JM. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13:1198-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 190. | Prokopidis K, Giannos P, Kirwan R, Ispoglou T, Galli F, Witard OC, Triantafyllidis KK, Kechagias KS, Morwani-Mangnani J, Ticinesi A, Isanejad M. Impact of probiotics on muscle mass, muscle strength and lean mass: a systematic review and meta-analysis of randomized controlled trials. J Cachexia Sarcopenia Muscle. 2023;14:30-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 191. | Byeon HR, Jang SY, Lee Y, Kim D, Hong MG, Lee D, Shin JH, Seo JG. New Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii are Effective for Improving the Muscle Strength of Mice with Immobilization-Induced Muscular Atrophy. J Med Food. 2022;25:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 192. | Hong AS, Tun KM, Hong JM, Batra K, Ohning G. Fecal Microbiota Transplantation in Decompensated Cirrhosis: A Systematic Review on Safety and Efficacy. Antibiotics (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 193. | Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, Fagan A, Hayward M, Holtz ML, Matherly S, Lee H, Osman M, Siddiqui MS, Fuchs M, Puri P, Sikaroodi M, Gillevet PM. Fecal Microbial Transplant Capsules Are Safe in Hepatic Encephalopathy: A Phase 1, Randomized, Placebo-Controlled Trial. Hepatology. 2019;70:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 194. | Tun KM, Hong AS, Batra K, Naga Y, Ohning G. A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplantation in the Treatment of Hepatic Encephalopathy and Clostridioides difficile Infection in Patients With Cirrhosis. Cureus. 2022;14:e25537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 195. | Chen H, Xu C, Zhang F, Liu Y, Guo Y, Yao Q. The gut microbiota attenuates muscle wasting by regulating energy metabolism in chemotherapy-induced malnutrition rats. Cancer Chemother Pharmacol. 2020;85:1049-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |