Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4214
Peer-review started: March 2, 2023
First decision: May 16, 2023
Revised: May 23, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: July 14, 2023
Processing time: 129 Days and 18.5 Hours
Deep angiomyxoma (DAM) is a very rare tumor type. Magnetic resonance imaging (MRI) is considered the best imaging modality for diagnosing DAM. Computed tomography (CT) is used mainly to assess the invasion range of DAM. The value of ultrasonography in the diagnosis of DAM is still controversial. Through a literature review, we summarized the current state of ultrasonic examination for DAM and reported for the first time the contrast-enhanced ultrasound (CEUS) features of DAM seen using a biplane transrectal probe.
A 37-year-old woman presented with a sacrococcygeal mass that had gradually increased in size over the previous 6 mo. MRI and CT examinations failed to allow a definite diagnosis to be made. Transperineal core needle biopsy (CNB) guided by transrectal ultrasound and CEUS was suggested after a multidisciplinary discussion. Grayscale ultrasound of the lesion showed a layered appea
Transrectal CEUS can show the layered perfusion characteristics of the contrast agent, guiding subsequent transperineal CNB of the enhanced area within the DAM.
Core Tip: Deep angiomyxoma (DAM) is a very rare tumor. Imaging examinations play an important role in the diagnosis of DAM. Magnetic resonance imaging is considered the best imaging modality for diagnosing DAM. Computed tomography is used mainly to assess the invasion range of DAM. The value of ultrasonography in the diagnosis of DAM is still controversial. We reported for the first time the contrast-enhanced ultrasound (CEUS) features of DAM seen using a biplane transrectal probe. Transrectal CEUS can provide more abundant diagnostic information in terms of the blood perfusion characteristics of DAM, guiding subsequent transperineal puncture of the enhanced area within the tumor.
- Citation: Zhang Q, Yan HL, Lu Q, Luo Y. Value of contrast-enhanced ultrasound in deep angiomyxoma using a biplane transrectal probe: A case report. World J Gastroenterol 2023; 29(26): 4214-4221
- URL: https://www.wjgnet.com/1007-9327/full/v29/i26/4214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i26.4214
Deep angiomyxoma (DAM) is a very rare tumor that mainly occurs in the pelvis and perineum of women of reproductive age[1]. DAM is classified as a "tumor of uncertain differentiation" in the World Health Organization classification. Since it was first described in 1983, DAM has been described in multiple single case reports and some case series reports[1-3]. Imaging examinations play an important role in the diagnosis of DAM and in evaluating the scope of lesion invasion. Magnetic resonance imaging (MRI) is considered the best imaging modality for the diagnosis of DAM; computed tomography (CT) is mainly used to evaluate the invasion range of DAM, and the value of ultrasound in the diagnosis of DAM is still controversial. This article reports for the first time the contrast-enhanced ultrasound (CEUS) features of DAM seen using a biplane transrectal probe and reviews the imaging features of DAM in the literature.
A 37-year-old woman presented with a sacrococcygeal mass that had been increasing in size over the previous 6 mo.
The patient inadvertently found a sacrococcygeal mass 6 mo prior, which was approximately the size of a pigeon egg at first, without pain or other discomfort. The mass gradually increased in size, accompanied by lumbosacral distension. No definite diagnosis was made after an examination at the local hospital; thus, the patient was transferred to our hospital for further diagnosis and treatment.
The patient had no previous medical history.
The patient and family histories were negative.
A digital rectal examination revealed a large solid mass in the anterior rectal wall, with a hard texture and fixed position. The mass boundary could not be palpated.
The results of laboratory examinations were normal, including routine blood analysis, carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigen (CA) 19-9, and CA125 results.
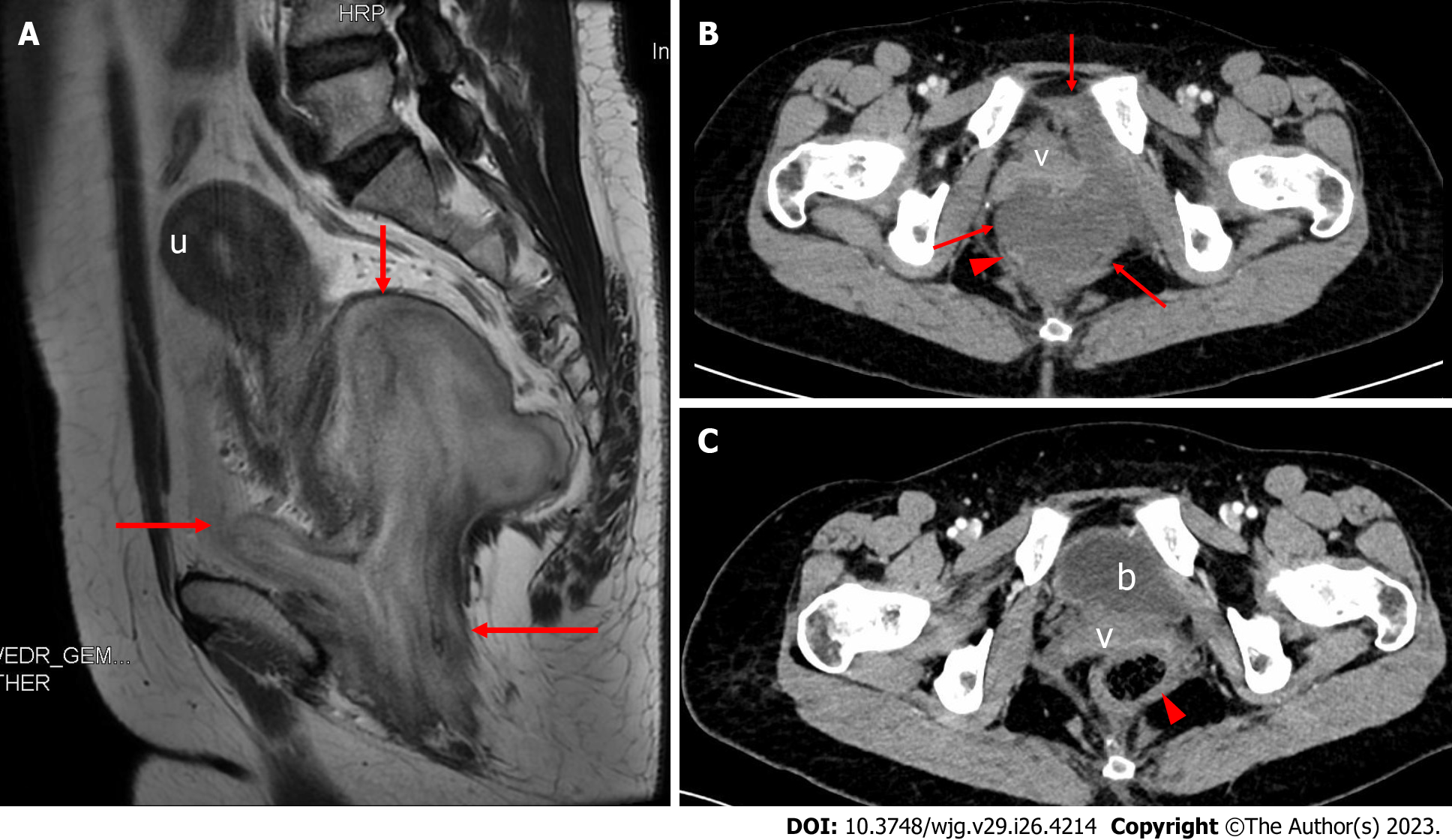
Preoperative MRI showed a paracervical mass in the pelvis, measuring approximately 9.5 cm × 8.1 cm in size (Figure 1A). T2-weighted imaging (T2WI) presented stratification changes with alternating high and low signals that were clearly demarcated from the surrounding tissue, and the uterus was pushed and displaced cephalad. Preoperative contrast-enhanced CT (CECT) demonstrated a pelvic mass between the cervix and rectum with nonenhanced areas (Figure 1B). The mass pushed against and displaced the anterior vagina and the right posterior rectum.
Preoperative transrectal ultrasound (TRUS) was performed with the MyLab Twice ultrasound system (Esaote, Genoa, Italy) equipped with a biplane endoscopic probe (TRT33, linear frequency of 4-13 MHz, convex frequency of 3-9 MHz). Grayscale ultrasound showed that the left pelvic mass had an irregular shape (Figure 2A). The internal echo of the mass presented a layered appearance with alternating hyperechoic and hypoechoic patterns, and the boundary between the mass and rectum wall was clear. Color Doppler flow imaging (CDFI) showed some blood vessels scattered in the tumor (Figure 2B), and the dispersed intratumoral blood vessels were consistent with the layered appearance of the tumor on grayscale ultrasound. Then, transrectal CEUS was performed with a bolus injection of 2.4 mL of SonoVue (Bracco, Milan, Italy) through the elbow vein (Figure 2C). A laminated distribution of the contrast agent was observed within the mass, which was also consistent with the layered appearance of the tumor on grayscale ultrasound.
Transperineal core needle (16G) biopsy (CNB) was performed under the guidance of TRUS (Figure 2D). The probe was switched to linear mode, and puncture sampling was performed under real-time monitoring by TRUS, avoiding the nonenhanced areas of the tumor. No complications occurred during the biopsy.
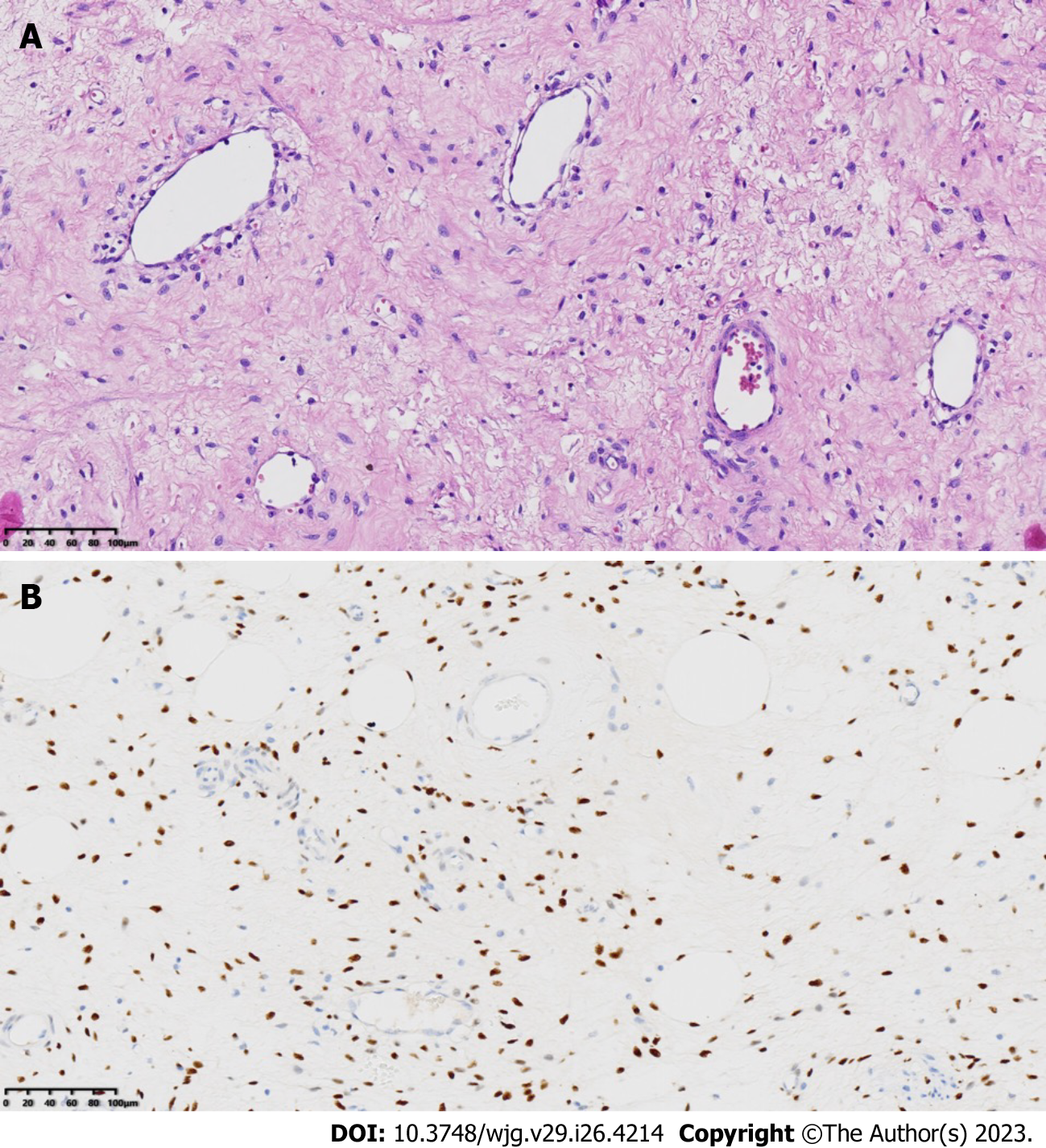
Postoperative immunohistochemical staining showed the following results: CD34 (vascular +), CK (pan) (-), ER (+), PR (+), Desmin (+), S-100 (-), SMA (+), CDK4 (-), and MDM2 (±). FISH revealed an unbalanced translocation of the HMGA2 gene. The final diagnosis was a DAM in the deep pelvis (Figure 3).
A preoperative examination was completed. The patient underwent laparoscopic-assisted transabdominal surgery combined with transperineal surgery for large pelvic tumor resection and pelvic floor peritoneal reconstruction on February 10, 2022.
During surgery, the tumor was found to be located below the pelvic peritoneal reflex plane, behind the cervix, and in front of the left side of the rectum. It was irregular in shape, with a smooth capsule, and contained both cystic and solid components. The postoperative pathological report was consistent with DAM.
The patient recovered well after surgery and underwent regular outpatient follow-up examinations. CECT of the pelvic cavity was performed on August 12, 2022 (Figure 1C), six months after resection, and indicated no obvious signs of tumor recurrence. No recurrence or metastasis was found by the last follow-up on November 9, 2022.
DAM is a very rare type of tumor that most commonly occurs in the pelvis and perineum of women of reproductive age[1]. Histologically, the typical morphology of DAM is an infiltrating, uniformly hypocellular tumor, consisting of small-sized spindled or stellate cells scattered randomly in the myxoid stroma and containing many small to medium/Large blood vessels, without nuclear atypia[1] (Figure 3). Clinically, DAM presents as a slowly growing painless mass. DAM is characterized by locally aggressive growth, with recurrence and metastasis occurring in some cases[2,3]. Accurate preoperative diagnosis and complete resection of the lesion are key to the treatment and prevention of recurrence[4]. Imaging examinations play an important role in the diagnosis of DAM and in assessing the extent of lesion invasion[5]. Commonly used imaging examinations include MRI, CT, and ultrasound[6].
CT is mainly used to evaluate the invasion range of DAM and perform follow-up observations, but its value in displaying typical signs such as a laminated or swirled appearance of the DAM tissue is limited[5-7]. MRI is considered the best imaging modality for the diagnosis of DAM[6,8-10]. T2WI can reveal the swirled or layered appearance of the mass, which has been reported in at least 83% of cases[6]. MRI is also critical to determining the range of tumor extension into the surrounding space and the mode of tumor invasion into surrounding organs[6,9]. Retrospective analysis of the T2WI MRI data in our case showed a typical laminated and swirled appearance consistent with the reports in the literature[8-10] (Figure 1A). However, we failed to make a definitive diagnosis, mainly because DAM is rare; we had insufficient experience in diagnosing this tumor. Some experts believe that it is difficult to accurately diagnose DAM before surgery, and these tumors are often misdiagnosed as other soft tissue tumors[11-13], such as Bartholin cyst, lipoma, and cellular angiofibroma. The misdiagnosis rate is as high as 82%[11].
Although MRI has many advantages in the diagnosis of DAM, the cost of MRI is relatively high, and patients with metal in the body cannot be examined by MRI. Similar to MRI, ultrasound can also be applied to avoid radiation exposure. Because of its low cost, convenient operation, and noninvasiveness, ultrasound can be used for multiple examinations in cases of DAM and follow-up examinations after surgery. In addition, ultrasound is not limited by the presence of metal objects such as heart stents and is also the preferred choice for patients with claustrophobia.
However, the literature related to ultrasound for DAM is scarce[14], and the value and role of ultrasound have not been systematically summarized. Through a literature review, we found that the previous ultrasonography methods related to DAM mainly consisted of examinations with transabdominal convex array probes[10,15], intracavity convex array probes[9,14,16,17], and transperineal linear array probes[18,19]. Among the 53 reports related to DAM, only 2[9,15] reported a typical laminated or swirled appearance on grayscale ultrasound. In all reports, CDFI did not show swirled or layered blood vessel distribution characteristics corresponding to the grayscale ultrasound findings, and CEUS examination was not performed in any of the previous reports, as shown in Table 1.
| No. | Ultrasonographic approach and probe | No. of tumors | Sex | Age (yr) | Location | Maximal diameter (cm) | Internal echogenicity | No. of cystic component | Laminated/swirled pattern in 2D |
| 1 | Transabdominal convex array probe | 4 | F | 18-50 | Pelvis | 3-38.8 (mean: 16.2 ± 9.4) | Hypoechoic or isoechoic internal components | 3 | No |
| 2 | Transabdominal convex array probe | 8 | 7F; 1M | 35-64 (median 39) | Vulva, pelvis, perineal region, spermatic cord, and scrotum | 7-21 | Heterogeneous, isoechoic internal components | 2 | Yes |
| 3 | Transvaginal end-fire probe | 1 | F | 40 | Pelvis extending to the perineum | 11.8 | Mixed echogenicity | 0 | Yes |
| 4 | Transvaginal end-fire probe | 1 | F | 54 | Behind the uterus | 18 | Heterogeneous hypoechoic internal components | 1 | No |
| 5 | Transvaginal end-fire probe | 1 | F | 44 | Left pelvis | 12 | Hypoechoic to isoechoic internal components | 0 | No |
| 6 | Transrectal radial probe | 1 | F | 61 | Left perirectal mass | 10.7 | Hypoechoic to isoechoic internal components | NA | No |
| 7 | Transperineal linear probe | 36 | M | 1-81 (mean 48.3 ± 20.6) | Epididymis, testes, spermatic cord, scrotum | 1.6-25 (mean: 8.36) | Heterogeneous hypoechoic internal components | NA | No |
| 8 | Transperineal linear probe | 1 | F | 28 | Vulva | 4.8 | Heterogeneous hypoechoic internal components | 0 | No |
We report for the first time the CEUS features of DAM in the pelvic cavity seen using a biplane transrectal probe. In this case, transrectal grayscale ultrasound showed a laminated appearance with alternating hyperechoic and hypoechoic patterns inside the mass (Figure 2A), demonstrating the same details as MRI and excellent resolution, which confirms the high sensitivity of transrectal high-frequency ultrasound in detecting the characteristic histological structure of DAM. We also observed that the distribution of scattered blood vessels within the tumor on CDFI was consistent with the layered appearance of the tumor on grayscale ultrasound (Figure 2B). This layered distribution of vascular features has not been previously reported in the literature[9-11,15,18,19], which may be related to the fact that more anatomical details of the tumor can be displayed by high-frequency ultrasound with a biplane transrectal probe[9,20,21]; however, this is yet to be verified by subsequent cases. Reportedly, CEUS may be of great value in the diagnosis of DAM[15], as this modality can better show the characteristics of DAM and help to determine the role of ultrasound in tumor treatment. However, to our knowledge, the diagnosis of DAM by CEUS has not been reported. In our case, the mass was examined by transrectal CEUS (Figure 2C). The characteristic laminated perfusion of the contrast agent was observed within the mass, which was also consistent with the layered appearance of the tumor on grayscale ultrasound.
We believe that the advantages of ultrasound with a biplane transrectal probe and CEUS in the diagnosis of DAM in the deep pelvic cavity are as follows:
First, the biplane transrectal high-frequency probe can enter the pelvic cavity along the anal canal and rectum and clearly display the laminated or swirled appearance of DAM, as well as the boundary of the mass and its relationships with adjacent structures, allowing this modality to be comparable to MRI. Typically, transperineal ultrasound and transabdominal ultrasound cannot simultaneously meet the requirements of satisfactory detection depth and high resolution of images when examining deep pelvic lesions. Additionally, the biplane transrectal probe effectively overcomes the obvious limitation of the observation angle of the convex end-fire probe.
Second, the laminated perfusion characteristics inside the mass can be displayed in real time by CEUS, which is helpful for the diagnosis and differential diagnosis of DAM.
Third, the gold standard for the diagnosis of DAM depends on histopathology and immunohistochemistry[18]. However, the proportion of cystic components in DAM is as high as 75%[10], and these components appeared as unenhanced areas on transrectal CEUS, which were easy to distinguish from enhanced areas of the mass, guiding subsequent precise and effective puncture of the enhanced area (Figure 2D). After transrectal CEUS, our patient immediately underwent transperineal CNB[22,23] guided by TRUS at the examination bed, and a clear pathological diagnosis was obtained, which provided a basis for formulating the surgical plan and reducing the patient's waiting time before surgery.
Fourth, compared with MRI and CT, TRUS is convenient, rapid, radiation-free, and economical. TRUS can dynamically display the internal and adjacent structures of the lesion in real time and can be used for repeated monitoring of the lesion before surgery.
Fifth, for patients with cardiac pacemakers, internal metal stents, or claustrophobia, ultrasound with a biplane transrectal probe and CEUS can be used instead of MRI.
Sixth, long-term follow-up is necessary due to the high recurrence rate of DAM[24]. Due to the above advantages, ultrasound with a biplane transrectal probe and CEUS are ideal tools for the postoperative follow-up of pelvic DAM patients. In summary, ultrasound with a biplane transrectal probe and CEUS can be one of the first choices for the diagnosis and follow-up of DAM patients.
In conclusion, pelvic DAM is a rare tumor. In our case, the typical laminated appearance of DAM could be observed by ultrasound with a biplane transrectal probe and CEUS. Moreover, CNB of the enhanced area inside the tumor could be accurately guided by transrectal CEUS, enabling the pathological gold standard for the diagnosis of DAM; thus, this modality is expected to be one of the preferred methods for the diagnosis of DAM in the pelvic cavity.
The authors thank Dr. Wang WY (Department of Pathology, West China Hospital, Sichuan University) for providing the pathological data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Martino A, Italy; Naganuma H, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol. 1983;7:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 371] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Gay F, Champigneulle J, Tortuyaux JM, Cuny T, Régent D, Laurent-Croisé V. Aggressive angiomyxoma. Diagn Interv Imaging. 2013;94:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Giraudmaillet T, Mokrane FZ, Delchier-Bellec MC, Motton S, Cron C, Rousseau H. Aggressive angiomyxoma of the pelvis with inferior vena cava involvement: MR imaging features. Diagn Interv Imaging. 2015;96:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Song M, Glasgow M, Murugan P, Rivard C. Aggressive Angiomyxoma of the Vulva and Bladder. Obstet Gynecol. 2017;130:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Outwater EK, Marchetto BE, Wagner BJ, Siegelman ES. Aggressive angiomyxoma: findings on CT and MR imaging. AJR Am J Roentgenol. 1999;172:435-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 117] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Surabhi VR, Garg N, Frumovitz M, Bhosale P, Prasad SR, Meis JM. Aggressive angiomyxomas: a comprehensive imaging review with clinical and histopathologic correlation. AJR Am J Roentgenol. 2014;202:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Jeyadevan NN, Sohaib SA, Thomas JM, Jeyarajah A, Shepherd JH, Fisher C. Imaging features of aggressive angiomyxoma. Clin Radiol. 2003;58:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Srinivasan S, Krishnan V, Ali SZ, Chidambaranathan N. "Swirl sign" of aggressive angiomyxoma-a lesser known diagnostic sign. Clin Imaging. 2014;38:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Tariq R, Hasnain S, Siddiqui MT, Ahmed R. Aggressive angiomyxoma: swirled configuration on ultrasound and MR imaging. J Pak Med Assoc. 2014;64:345-348. [PubMed] |
| 10. | Kumar N, Goyal A, Manchanda S, Sharma R, Kumar A, Bansal VK. Aggressive pelvic angiomyxoma and its mimics: can imaging be the guiding light? Br J Radiol. 2020;93:20200255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bai HM, Yang JX, Huang HF, Cao DY, Chen J, Yang N, Lang JH, Shen K. Individualized managing strategies of aggressive angiomyxoma of female genital tract and pelvis. Eur J Surg Oncol. 2013;39:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Smith HO, Worrell RV, Smith AY, Dorin MH, Rosenberg RD, Bartow SA. Aggressive angiomyxoma of the female pelvis and perineum: review of the literature. Gynecol Oncol. 1991;42:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Zou R, Xu H, Shi Y, Wang J, Wang S, Zhu L. Retrospective analysis of clinicopathological features and prognosis for aggressive angiomyxoma of 27 cases in a tertiary center: a 14-year survey and related literature review. Arch Gynecol Obstet. 2020;302:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Yang X, Zhang L, Zhao W, Zhang Y, Yu J. Invasive angiomyxoma diagnosed by transvaginal ultrasound: a case report. Ann Palliat Med. 2021;10:5870-5874. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Zhao CY, Su N, Jiang YX, Yang M. Application of ultrasound in aggressive angiomyxoma: Eight case reports and review of literature. World J Clin Cases. 2018;6:811-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Hajjar R, Alharthi M, Richard C, Gougeon F, Loungnarath R. Pelvic Aggressive Angiomyxoma: Major Challenges in Diagnosis and Treatment. Cureus. 2019;11:e4419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Huang CC, Sheu CY, Chen TY, Yang YC. Aggressive angiomyxoma: a small palpable vulvar lesion with a huge mass in the pelvis. J Low Genit Tract Dis. 2013;17:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Sun J, Lian PH, Ye ZX, Dong X, Ji ZG, Wen J, Li HZ. Aggressive Angiomyxoma in the Scrotum: A Case Series and Literature Review. Front Surg. 2022;9:762212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Ota H, Otsuki K, Ichihara M, Ishikawa T, Okai T. A case of aggressive angiomyxoma of the vulva. J Med Ultrason (2001). 2013;40:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Wenig BM, Vinh TN, Smirniotopoulos JG, Fowler CB, Houston GD, Heffner DK. Aggressive psammomatoid ossifying fibromas of the sinonasal region: a clinicopathologic study of a distinct group of fibro-osseous lesions. Cancer. 1995;76:1155-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Granter SR, Nucci MR, Fletcher CD. Aggressive angiomyxoma: reappraisal of its relationship to angiomyofibroblastoma in a series of 16 cases. Histopathology. 1997;30:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Zhao JY, Zhuang H, Lu CY, Yao J, Luo Y, Yu YY. Transperineal core-needle biopsy of a rectal subepithelial lesion guided by endorectal ultrasound after contrast-enhanced ultrasound: A case report. World J Gastroenterol. 2021;27:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Nuernberg D, Saftoiu A, Barreiros AP, Burmester E, Ivan ET, Clevert DA, Dietrich CF, Gilja OH, Lorentzen T, Maconi G, Mihmanli I, Nolsoe CP, Pfeffer F, Rafaelsen SR, Sparchez Z, Vilmann P, Waage JER. EFSUMB Recommendations for Gastrointestinal Ultrasound Part 3: Endorectal, Endoanal and Perineal Ultrasound. Ultrasound Int Open. 2019;5:E34-E51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Draeger DL, Protzel C, Hakenberg OW. Aggressive Angiomyxoma as a Rare Differential Diagnosis of Enlargement of the Scrotum. Clin Genitourin Cancer. 2016;14:e237-e239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |