Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4120
Peer-review started: April 17, 2023
First decision: May 12, 2023
Revised: May 19, 2023
Accepted: June 11, 2023
Article in press: June 11, 2023
Published online: July 14, 2023
Processing time: 84 Days and 0.6 Hours
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal disease with a significant impact on patients’ quality of life and a high socioeconomic burden. And the understanding of IBS has changed since the release of the Rome IV diagnosis in 2016. With the upcoming Rome V revision, it is necessary to review the results of IBS research in recent years. In this review of IBS, we can highlight future concerns by reviewing the results of IBS research on epidemiology, overlap disorders, pathophysiology, and treatment over the past decade and summarizing the latest research.
Core Tip: Irritable bowel syndrome (IBS) is a physical and mental illness that is becoming more prevalent, and its impact on society is expanding. Understanding of IBS has changed since the release of the Rome IV diagnosis in 2016, and this paper reviews the literature from the past decade to find that research around the brain-gut axis, diet, and gut microbiota are at the forefront of IBS. Moreover, as the research on the physiopathology of IBS has advanced, the treatment model has become more refined, which has important clinical implications.
- Citation: Huang KY, Wang FY, Lv M, Ma XX, Tang XD, Lv L. Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment. World J Gastroenterol 2023; 29(26): 4120-4135
- URL: https://www.wjgnet.com/1007-9327/full/v29/i26/4120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i26.4120
Irritable bowel syndrome (IBS) is a chronic functional disease, and the changes that it causes in bowel function and abdominal pain seriously affect the patient's normal life and work. It mainly affects young and female individuals, and it tends to overlap with other functional gastrointestinal diseases (FGIDs) and cause a huge burden to life and society's economy[1,2]. Prevalence varies greatly between countries because of differences in food, culture, and diagnosis. The Rome Foundation Global Study[3] coverage across the country reported that the overall prevalence of IBS was 3.8% in Rome IV and 10.1% in Rome III. The Rome IV criteria, based on symptoms that have undergone a change in dynasty, suggest that the pathogenesis of IBS is associated with gut-brain interactions, which may be an overlapping pathogenesis between FGIDs. Based on the results of Rome IV, many studies have been performed, so it is necessary to summarize their findings. The aim of this study is to summarize IBS from the perspectives of epidemiology, disease overlap, pathological mechanisms, diagnosis, and treatment, focusing on disease overlap, pathological mechanisms, and treatment.
The prevalence of IBS varies widely between different countries. In 2017, the Rome Foundation working group reviewed related work and showed that the prevalence of IBS varied from 1.1% (France and Iran) to 35.5% (Mexico), and the prevalence in Asia is also uneven[4-6]. It might be that many previous surveys did not use uniform diagnostic criteria or the same methodology, with geography, culture, and population being the reasons for different prevalences, and thus the included studies were heterogeneous. The goal of determining the global prevalence of IBS is still inaccurate[7]. Therefore, we discuss the epidemiology of IBS in different continents in recent years.
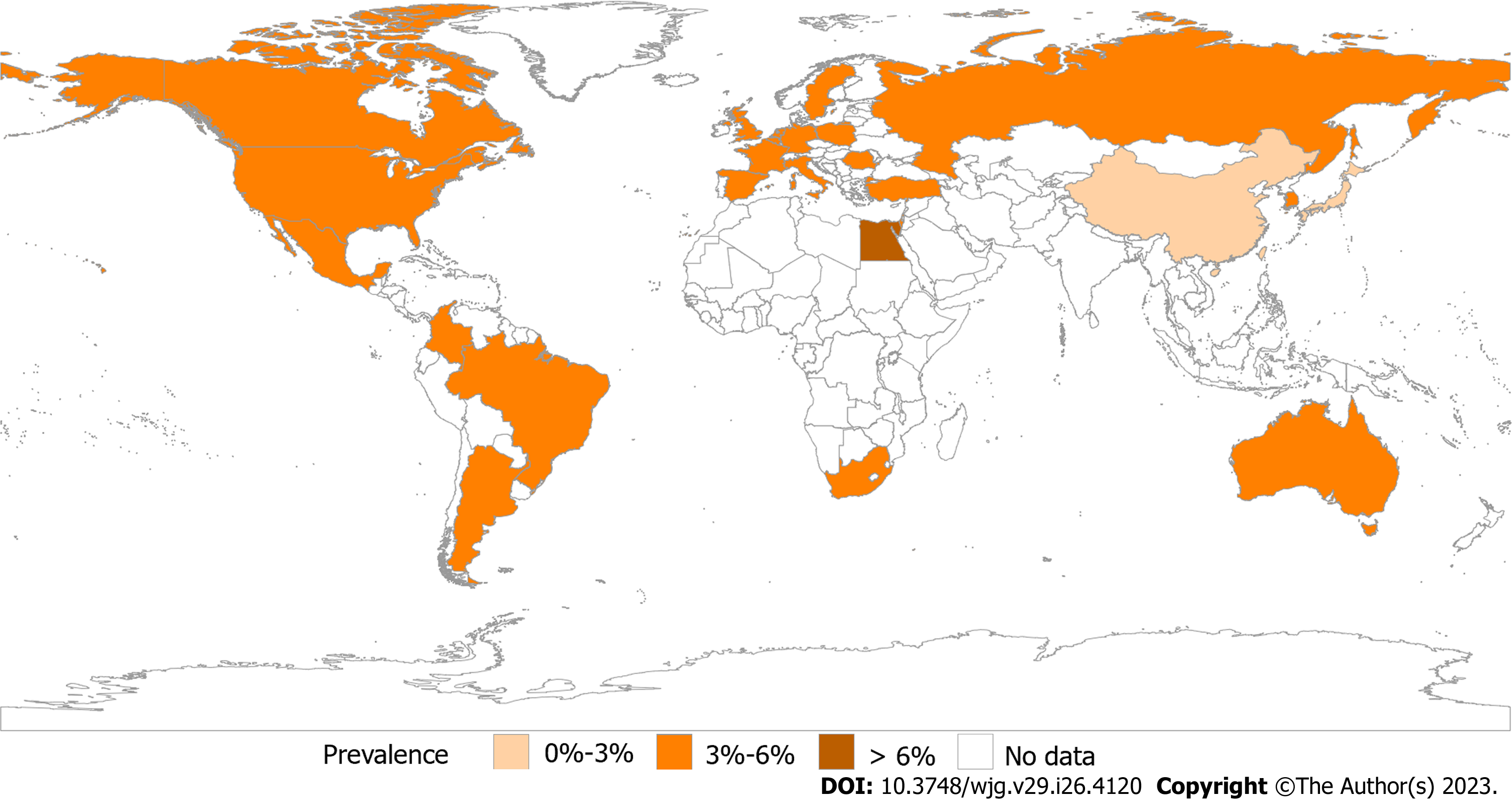
The Rome Foundation Global Epidemiological Study organized a study using Rome IV in 33 countries and Our analysis discovered that the prevalence rates in Europe and the United States were comparable, while those in Asia and Australia were marginally lower[7] (Figure 1). Egypt had the highest prevalence rate of internet surveyed countries[8]. As well as that, representative researches have also been carried out in various countries in recent years and reported that the prevalence of IBS was 5.2% (Rome IV), 5.9% (Rome III), and 6.98% (Rome IV) in Gibraltar, the United Kingdom[9], Hangzhou, China[10] and Latin America[11], respectively. Based on population survey data in the United States, Canada, and the United Kingdom, the results revealed the Rome III IBS rate was roughly twice as high as the Rome IV rate[12]. Overall, there is a clear predominance in the prevalence in Africa, and the prevalence based on Rome IV diagnosis is similar in the United States and Europe. However, prevalence varies widely between Europe and Asia, especially in Asian countries surveyed by using the internet and questionnaires. In the past, most studies have shown a higher prevalence of IBS in women[13]. Interestingly, IBS is equally common in men and women in Asia[14-16]. The highest prevalence was observed in the educated, the wealthy, students and younger individuals[17]. It also declined with age[1,18,19]. Through years of research and analysis, it was determined that estimating a pooled global prevalence of IBS was unlikely to be feasible, so regionalization should be emphasized in future research.
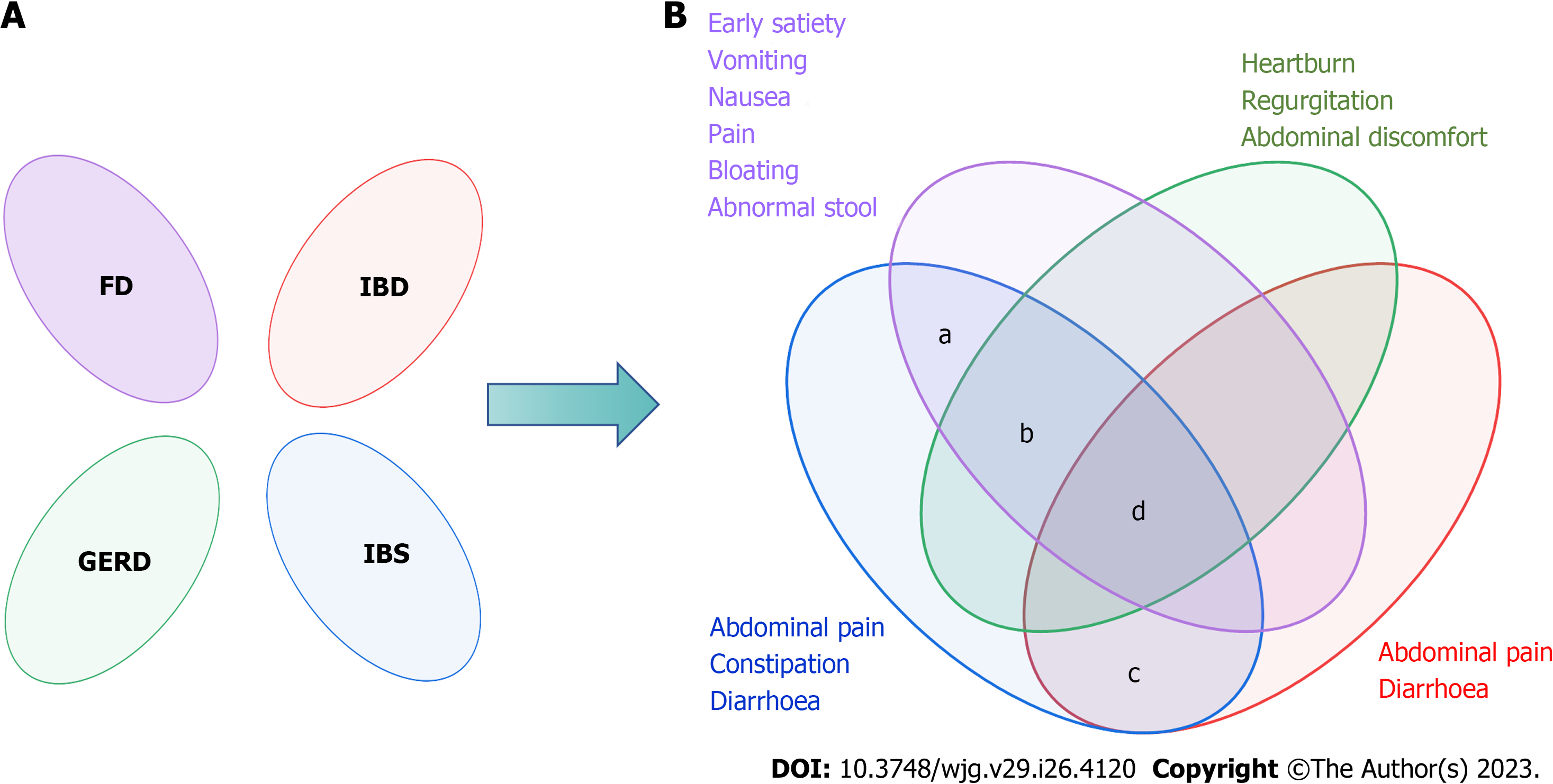
Rome criteria for IBS based on symptoms were the most recognized, and the overlap of FGIDs was gradually valued by Roman criteria over time (Figure 2). Now, Rome IV suggests that the pathologies exist in the gastrointestinal tract on a continuum instead of as separate disorders, and overlap may be a natural clinical symptom of FGIDs[20]. Likewise, 54127 adults from 26 countries participated in an internet survey and discovered that 68.3% had symptom overlap in both gastrointestinal regions and 2.3% had esophageal, gastroduodenal, bowel, and anorectal overlap[21]. Overall, by reviewing the overlap of IBS and other diseases, it was found that there was obvious overlap between IBS and FGIDs, and anxiety and depression were their common characteristics, which verified the vital position of the central nervous system and brain-gut axis in the pathological mechanism of FGIDs. Therefore, the overlapping pattern and pathology of FGIDs are something that should be studied in depth.
Functional dyspepsia (FD) and IBS are the most prevalent FGIDs, postprandial fullness, early satiation, epigastric pain, and epigastric burning are the main symptoms of FD. The global prevalence of FD varies from 10%-20%[22]. Clinical studies have identified not only overlap between FD and IBS[23-25], but also the most common overlapping characteristics. In the overlap between FD and IBS-D (diarrhea), abdominal pain, bloating, and diarrhea are prominent. However, in the overlap between FD and IBS-C (constipation), abdominal fullness and constipation are prominent.
In a longitudinal follow-up study published in 2022, 807 individuals (Rome IV) were included, 446 (55.3%) of whom had overlapping IBS and FD, which showed that patients with overlapping IBS and FD had more severe symptoms and were more likely to have depression and anxiety[26,27]. Furthermore, a prospective study in South Korea in 2019-2020 reported the same conclusion; moreover, women with overlap of IBS and FD experienced more severe gastrointestinal and depression symptoms than men. Interestingly, an Australian study showed no relationship between gender and overlap[25,28]. There seems to be a distinct overlap between IBS, FD, and gastroesophageal reflux disease (GERD), in which it is easier to merge psychological morbidity and sleep disturbance[29]. Although age, gender, and IBS subtype were not correlated with overlap[25], the pathogenesis analysis of IBS and FD indicates that psychological factors are linked to the overlap of IBS and FD. Therefore, the diagnosis of IBS or FD should be considered in terms of each other, especially when encountering some anxious, severe symptoms.
GERD is a condition in which stomach contents reflux and cause uncomfortable symptoms[30], which present with regurgitation, heartburn, or being asymptomatic. Then, GERD is divided into three phenotypic presentations: Nonerosive reflux disease, erosive esophagitis (RE), and Barrett’s esophagus (BE), with prevalence rates of 60%-70%, 30%, and 6%-8%, respectively[31]. Before Rome IV, a small number of studies had shown overlap between GERD and FGIDs[32], and IBS is a risk factor for GERD[33]. But now, the Rome Foundation considers overlap between FGIDs to be a trend. In patients with overlapping GERD and IBS, acid reflux and heartburn may present with abdominal pain or discomfort, and visceral hypersensitivity and gastrointestinal motility disorders may be coexisting mechanisms. However, the prevalence of patients with GERD and IBS (different criteria) varies greatly, and the overlap between IBS and GERD ranges from 3% to 79% based on the questionnaire and 10% to 74% when diagnosed by endoscopy[34]. In 2016, an Italian study with 697 heartburn patients found that cases of IBS overlapping with GERD/hypersensitive esophagus (HE) and overlapping functional heartburn (FH) were 147/454 (33%) and 187/243 (77%), respectively[35]. Besides, there is a higher risk of possible overlap between FGIDs.
Crohn’s disease (CD) and ulcerative colitis (UC) are common inflammatory bowel diseases (IBDs). CD is characterized by chronic or nocturnal diarrhea, abdominal pain, and weight loss, whereas UC is characterized by bloody diarrhea with rectal urgency and tenesmus[36,37]. Although some biomarkers are used to distinguish between IBS and IBD, there is also overlap between them. Patients with overlapping IBD and IBS are prone to diarrhea and abdominal pain, which can be serious. Besides, a 2020 meta-analysis showed that the pooled prevalence of IBS-type symptoms among patients with IBD was 32.5%[38]. IBS-D is related to gut infections, and the gut microbiome and the intestinal barrier are bridges that connect them. Thus, IBS-D is a common diagnosis in patients with chronic diarrhea following chronic infection[39,40]. Overall, IBD and IBS can be different stages of the same disease. Therefore, the overlapping disease characteristics of IBS and IBD should not be ignored when the patient has a history of intestinal infection. At the same time, it is necessary to prevent IBS when diagnosing IBD.
A follow-up study in the US performed an analysis of data from 655 adults to compare the degree of overlap between chronic overlapping pain conditions (COPCs). Surprisingly, IBS is the most common COPC other than headache. Furthermore, 63% of IBS cases have one or more COPCs, and 53% of IBS cases reported pain in ≥ 3 non abdominal areas[41]. Therefore, when there is chronic physical and abdominal pain, IBS overlap should not be ignored[42]. It was observed that IBS and nonceliac gluten sensitivity had significant symptom overlap, and their physiology and pathology were not clear[43]. Moreover, there is overlap between adolescents with endometriosis and IBS[44], and the overlap between IBS and endometriosis may have the same pathogenesis; specific mechanisms need to be further explored.
In the past, IBS was thought to be a functional disorder that could not be explained by organic disease or a clear etiology[45]. With the increasing research on IBS and the update of the Rome criteria, the view on the pathophysiological mechanisms of IBS has changed from functional to brain-gut interaction. The aim of this article is to review the pathophysiology from clinical studies and basic research on IBS after Rome IV.
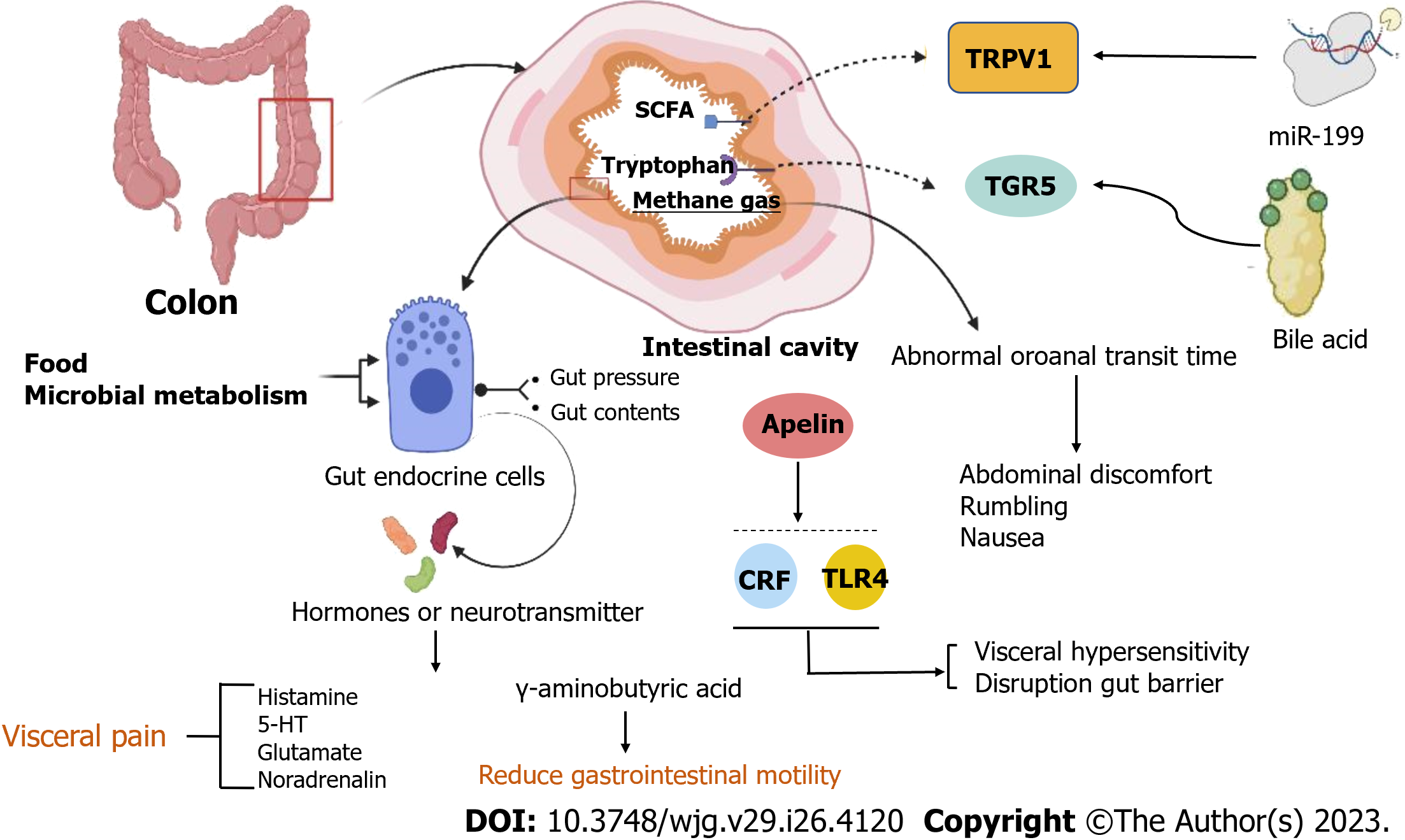
Recurrent abdominal discomfort, abdominal pain, and altered bowel habits are the core clinical symptoms of patients with IBS, and clinical studies on pathogenesis show that the microbiome, gastrointestinal endocrine cells, visceral hypersensitivity, and gastrointestinal motility disorders , are observed in IBS patients and are the direct causes of abdominal discomfort, abdominal pain, or diarrhea. It was discovered through experiments that the levels of colonic mucosal Takeda G protein-coupled receptor 5 protein expression, short-chain fatty acid (SCFA), fecal bile acids (FBA)[46,47], tryptophan (aryl hydrocarbon receptor kynurenine pathways), and methane gas production[47,48] were higher in patients with IBS than in healthy control (HC), and metabolites such as SCFA and bile acids are mainly associated with gastrointestinal malabsorption; there are differences between IBS subtypes, and neurotransmitters cause abdominal pain through the brain-gut axis and center. DuPont et al[49], recorded intestinal transport in 46 patients with IBS using a wireless pH/pressure recording capsule and found a delayed gastric emptying time in 35/46 (76%) IBS patients. And abnormal colonic transit and disorders of evacuation are important physiopathologies in patients with IBS, leading to constipation, bloating, and abdominal pain. Furthermore, abnormal oroanal transit time (OATT) was associated with hydrogen and methane concentrations, and more rapid OATT was associated with a higher severity of abdominal discomfort, rumbling, and nausea[48]. In addition, gut endocrine cells are scattered throughout the gastrointestinal tract and have sensory microvilli that sense gut pressure and gut contents[50-52], and when the gut lumen is stimulated by food[53], and microbial metabolism, the cells release hormones into the lamina propria to act mainly through paracrine and afferent and efferent synaptic transmission[54-56]. And studies found that histamine, 5-HT, glutamate, and noradrenalin strengthen visceral pain, and γ-aminobutyric acid reduces gastrointestinal motility.
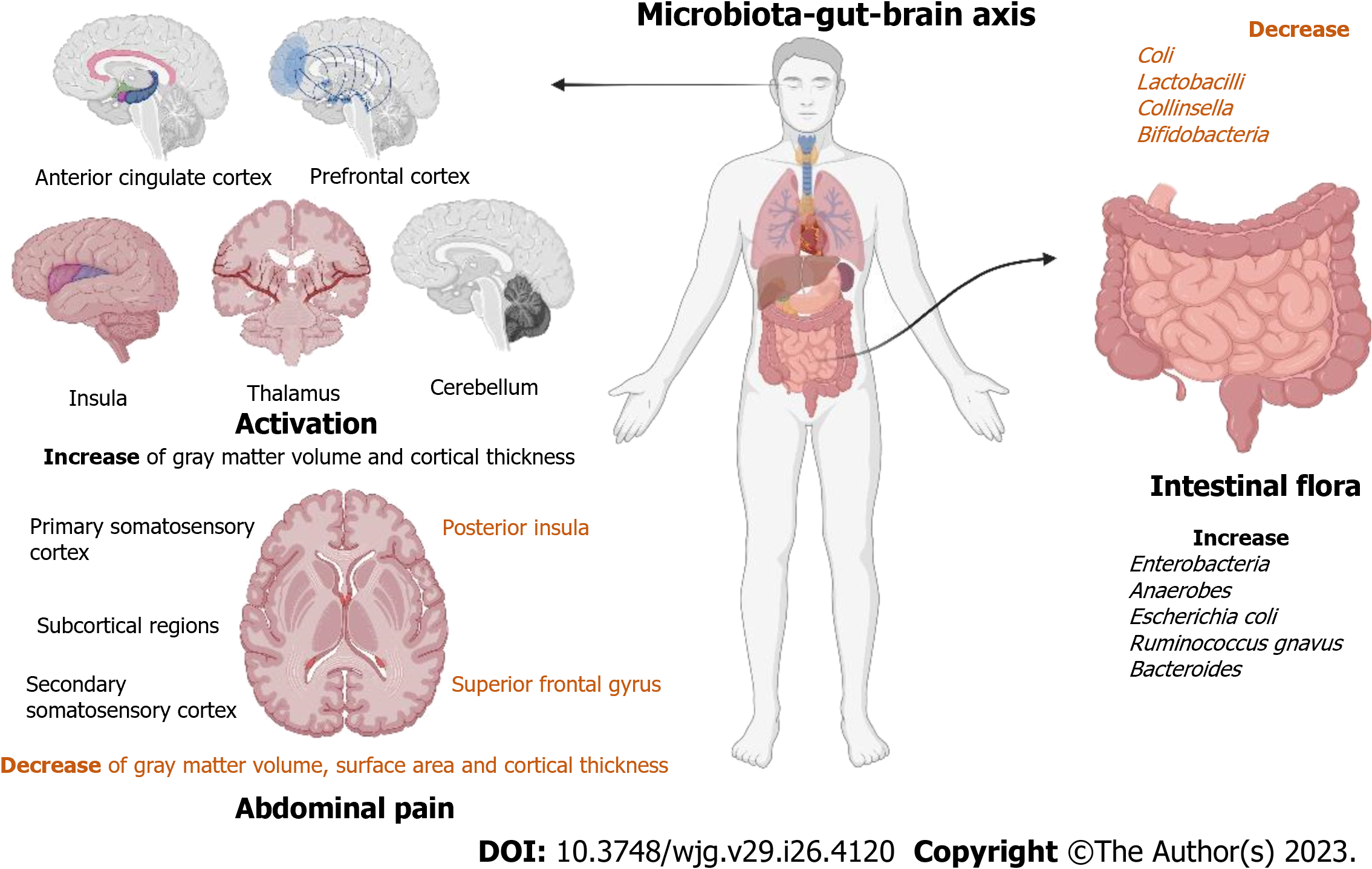
Abdominal pain in IBS patients has been shown to be associated with structural features of the brain. Rectal stimulation seems to activate the anterior cingulate cortex, prefrontal cortex, insula, thalamus, and cerebellum, and is higher in patients with IBS[57]. A study[58] of female IBS patients included 216 female IBS patients and 138 women serving as HC. In comparison to HC, patients with IBS had an increase in gray matter volume and cortical thickness in the primary and secondary somatosensory cortex and subcortical regions; however, the volume, surface, and cortical thickness of the gray matter in the posterior insula and superior frontal gyrus were reduced. Moreover, abdominal pain caused by rectal dilation is linked to the thicker left primary somatosensory cortex (Figure 3).
Visceral hypersensitivity and gut barrier disruption have been shown to be mediated via corticotropin-releasing factor (CRF)-Toll-like receptor 4 (TLR4)-proinflammatory cytokine signaling in animal experiments[59,60]. In addition, Nozu et al[61] conducted experiments based on IBS model rats and discovered that apelin activates CRF and TLR4, which may create a vicious cycle of proinflammatory cytokine signaling, which is a key pathway for the pathological mechanism of IBS. Then, the disruption of the gut barrier leads to an increase in lipopolysaccharides (LPS) and proinflammatory cytokines, which is a vital pathological mechanism that causes abdominal pain in patients with IBS[62]. And there are some new developments, such as a study using NanoString mRNA measurement of colonic neuroimmune gene expression and founding that the expression of the gene Trpv1 was higher in Gnotobiotic mice from patients with IBS and comorbid anxiety; moreover, it was associated with visceral hypersensitivity and anxiety[63]. Besides, decreasing miR-199 caused visceral hypersensitivity and augmented visceral pain in patients with IBS through translational upregulation of TRPV1[64]. Both activating BDNF-TrkB-PKMζ signaling in the thoracolumbar spinal cord of rats to increase synaptic activity and activating TLR4 trigger the release of pro-inflammatory cytokine afferent nerves and can cause visceral hypersensitivity[65,66]. Moreover, recent studies have shown that abnormal mast cell structure or function is a potential mechanism for visceral hypersensitivity in IBS[67], and post-IBS with gut microbial disorders leading to IBS and signaling pathways are also associated with visceral hypersensitivity[67,68]. In addition, an excellent review by Tozlu et al[69]. indicated that the number of mucosal eosinophils increased substantially more in patients with post-IBD IBS-D than in patients with active IBD, there was a reaction to the removal of allergic foods during treatment, and intestinal inflammation in patients with IBS was associated with food allergic reactions. Peptide YY (PYY) is localized in endocrine cells and regulates gut motility and visceral sensitivity by releasing and modulating serotonin[70] (Figure 4).
Koloski et al[71,72] discovered that higher baseline levels of anxiety and depression were significant predictors of developing IBS, and two prospective studies found that functional gastrointestinal symptoms preceded the mood disorder in two-thirds of patients. Dinan et al[73,74] have suggested that disturbances in the gut microbiota can affect brain function, behavior, and cognition, and the theory has developed into the microbiota-gut-brain axis, which is an important basis for the influence of gut microbes as well as neurotransmitters on IBS. The microbial diversity and abundance of stool in patients with IBS were altered compared to those in HC, with a decrease in Coli, Lactobacilli, Collinsella, and Bifidobacteria and an increase in Enterobacteria, Coli, anaerobes, Escherichia coli, Ruminococcus gnavus, and Bacteroides in patients with IBS. And a higher proportion of Bacteroides and Allisonella in patients with IBS-M[75,76]. In addition a study[77] used 16S rRNA metagenomic sequencing and performed phylogenetic investigation of communities by reconstruction of unobserved states to analyze fecal samples from control (n = 12) and IBS-D patients (n = 7) and reported that in patients with IBS, the abundances of Sutterellaceae, Acidaminococcaceae, and Desulfovibrionaceae were significantly increased, and those of Clostridiaceae, Leuconostocaceae, Enterococcaceae, Peptostreptococcaceae, and Lachnospiraceae were significantly decreased; moreover, secondary bile acid biosynthesis was decreased, and the citrate cycle was increased. Moreover, a study[78] used proton nuclear magnetic resonance spectroscopy and shotgun metagenomic sequencing to analyze fecal metabolites and the gut microbiome (IBS patients =142 and HC =120). It reported that the gut microbial diversity of IBS (Simpson’s evenness metric) was drastically lower than that of HC, and metabolomics found that the mechanism of IBS was related to 5-HT.
The diagnosis of IBS is based on symptoms ranging from the Manning criteria to the Rome criteria, and the most widely used diagnostic criteria are the Rome IV[79]. Research around Rome IV has revealed that there are many important biomarkers that guide the differential diagnosis and symptomatic treatment of IBS that may be taken into account. According to Vijayvargiya et al[80], FBA and fecal fat are potential biomarkers for IBS-D and IBS-C. Total FBA, chenodeoxycholic acid (CDCA), cholic acid (CA), and primary bile acids were significantly higher in patients with IBS-D than in healthy patients or patients with IBS-C. In contrast, deoxycholic acid (DCA) and combined DCA and CDCA (secretory) bile acids were significantly lower in patients with IBS-C than in HC and patients with IBS-D. Combining fasting serum 7α-hydroxy-4-cholesten-3-one and primary bile acids or fecal bile acid concentrations in stool samples is a simple, low-cost diagnostic for bile acid diarrhea (BAD). Circulating resolvin D1 (RvD1) and c-reactive protein (CRP) are inflammatory markers in patients with IBS-C; patients with IBS-C have higher CRP and lower RvD1 concentrations than HC[81]. Furthermore, radiopaque markers and scintigraphy can be used to assess transit function, and rectal sensation to balloon distension can be used to assess visceral hypersensitivity[82]. All of the ancillary tests listed above can be used to further identify the cause and guide medication use if the first-line medication is ineffective.
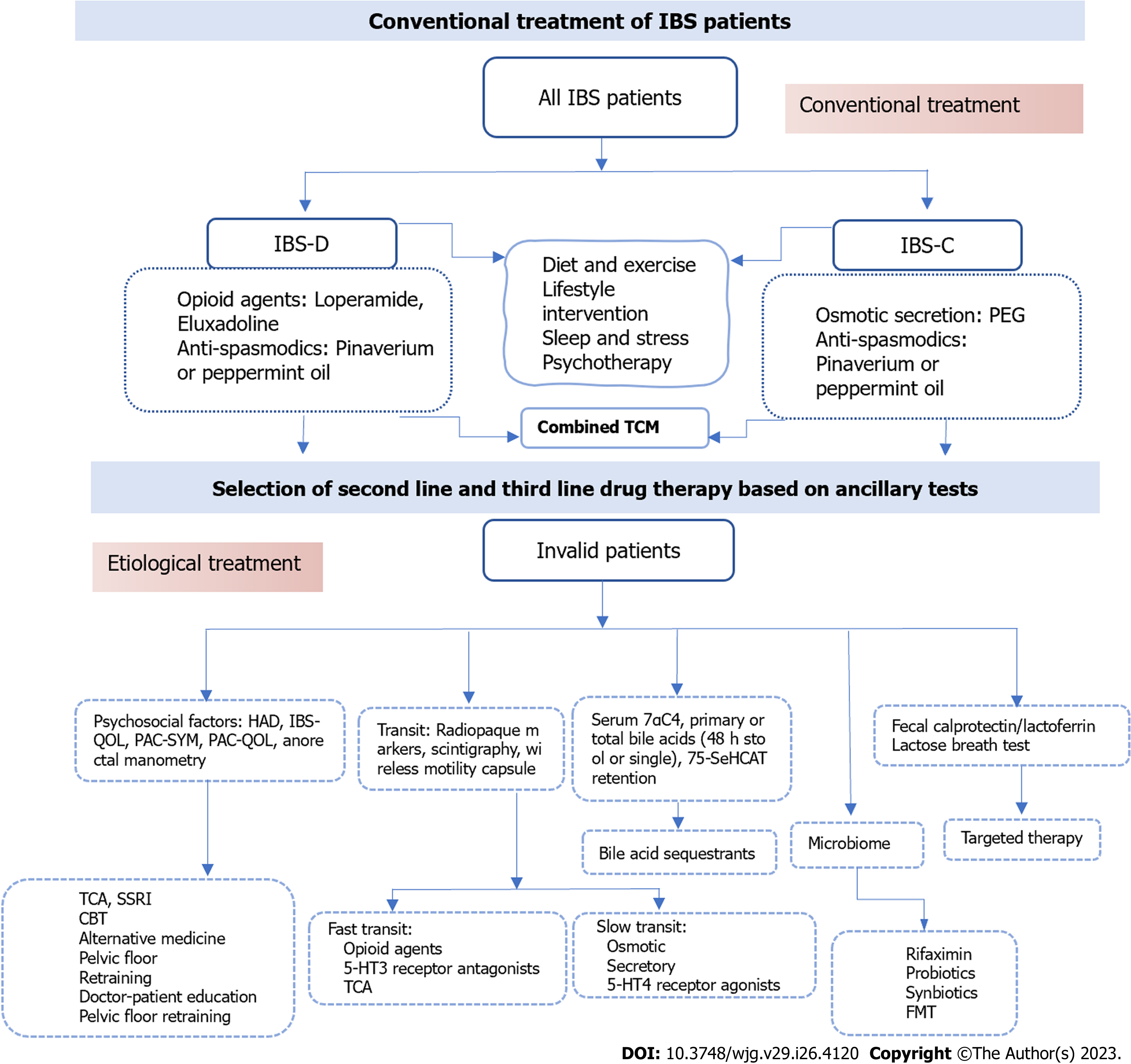
Patients with mild IBS first choose education, diet, and lifestyle interventions as prerequisites, combined with first-line therapeutic drugs. If first-line treatment is ineffective, clinical judgment combined with ancillary tests is required to select appropriate second-line drugs and non-pharmacological interventions (Figure 5). Furthermore, patients with psychological problems can be assessed using psychological questionnaires, emphasizing doctor-patient communication for emotional relief, and using tricyclic antidepressant (TCA) or selective serotonin reuptake inhibitor (SSRI) medications.
Stress reduction, appropriate exercise, and a special diet are the main non-pharmacological treatments for preventing induction; likewise, the publication of the British Gastroenterological Society guidelines[83] in 2021 and the updated guidelines from the American College of Gastroenterology (ACG) in 2022 emphasized that dietary counseling should be regarded as a first-line treatment option. A low-FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols, LFD) diet is currently the most recommended and effective diet for IBS intervention[84]. And FODMAP induces symptom generation in IBS based on the gut-brain axis[85]. The ACG suggests that the LFD diet be implemented in three steps: (1) A period of strict restriction (lasting no longer than 4-6 wk); (2) reintroduction of FODMAP foods; and (3) personalization based on reintroduction results[86]. The short-term efficacy and safety of LFD compared to a Western diet and conventional diet in relieving IBS patients are definite[87]; of course, a regular diet is the foundation. Gluten-free foods and dietary fiber are other currently approved diets for patients with IBS[88,89]. IBS-D patients benefit more from LFD than IBS-C patients, while fiber diets such as psyllium fiber are more effective in IBS-C patients[90]. Garg[91], professor, proposed the "FEED" method, in which ample daily psyllium fiber (25 g) and sufficient water (500 mL), along with elevation of the feet and exercises of the abdominal muscles while sitting on the toilet, can help IBS-D symptoms. In contrast, lactose, sorbitol, fructose, xylitol, mannitol, fat, alcohol, insoluble fibers, and fizzy drinks increase pain and flatulence and should be avoided by patients with IBS[92-94].
Since IBS is a gastrointestinal physical disorder that often fluctuates with stress, the Rome working team strongly recommends brain-gut axis behavior therapies as part of the treatment of DGBI disorders such as IBS[95]. Including hypnotherapy, dynamic psychotherapy, and relaxation therapy[96] can improve abdominal pain, standard of living, and psychological symptoms in patients with IBS and can reduce health care costs. Although some patients are unable to receive psychotherapy, recent studies have shown that cognitive behavioral treatment (CBT) of hypnotherapy is a potential and affordable treatment. A study included 436 patients with IBS (Rome III) who were followed up at 2 wk and 3, 6, 9 and 12 mo after the end of specific CBT treatment, and the results showed that not only did CBT improve symptoms, but the improvement usually extended up to 12 mo after treatment[97,98]. Additionally, gut-directed hypnotherapy (GHT) can also improve the symptoms of IBS by affecting gastrointestinal motility and visceral sensitivity[99,100]. Overall, most views support the ideal that it works because it is based on the brain-gut axis. GHT is beneficial in directly reducing the discomfort of IBS and refractory IBS as well as improving quality of life and health, and the efficacy is sustained. Moreover, it can reduce anxiety and depression, but its mechanism is largely unclear[101]. The mechanism of hypnotherapy is related to the brain-gut axis, but current research on the microbiome has not provided definitive results[102]. What is certain is that hypnotherapy works by regulating the autonomic nervous system (ANS). The vagus nerve is related to the brain-gut axis and can coordinate gastrointestinal functions, and there seems to be potential in studying the role of the vagus nerve[102,103].
IBS-D: The ACG published guidelines for IBS-D conditional recommendations in 2022[104] include the three drugs eluxadoline, rifaximin, and alosetron (moderate certainty), which can relieve or assist abdominal pain and stools, but there are adverse effects and contraindications. Loperamide (very low certainty) can relieve diarrhea, but there is no evidence that it improves abdominal discomfort. TCA and antispasmodics have low certainty. Moreover, SSRIs are recommended against use (low certainty) (Table 1).
| Type | Mechanism of action | Example | Appearing dose | Efficient | |
| IBS-D | |||||
| Opioid agents | Inhibits secretion, transit | Loperamide | 4 mg tid | Unknown for IBS; effective for diarrhea | First-line |
| Eluxadoline | 75 mg or 100 mg bid | Effective for FDA composite: 100 mg: OR, 0.87 (95%CI: 0.83-0.91); 75 mg: OR, 0.89 (95%CI: 0.84-0.94). RCTs: Effective for diarrhea and composite diarrhea + pain; not pain alone | |||
| Bile acid sequestrants | Bind to bile acids | Cholestyramine | 4 g bid | Unknown: effective in open-label studies; ineffective in 1, single-center RCT | |
| Colestipol | - | ||||
| Colesevelam | 32 mg bid | ||||
| Antibiotic | Anti-inflammatory | Rifaximin | 550 mg tid | Effective: In 2012 SRMA: Global: OR 1.57 (1.22 to 2.01); Bloating: OR 1.55 (1.23 to 1.96); In 2020 SRMA: FDA composite: OR: 0.92 (0.86 to 0.98) Global OR: 0.91 (0.77, 1.07) | Second-line |
| 5-HT3 | Delays colonic transit and reduces visceral pain | Alosetron | 0.5-1 mg qd or 1 mg bid | Effective: Global RR 1.60 (1.49 to 1.72); Pain RR 1.30 (1.22 to 1.39); FDA composite: OR 0.69 (0.60 to 0.80) | Third-line |
| Receptor antagonists | Ramosetron | 2.5 µg qd | |||
| IBS-C | |||||
| Osmotic | Osmotic secretion | PEG3350 | - | Effective: improves SBMs, CSBMs, consistency straining but not pain, bloating or incomplete evacuation | First-line |
| Secretory | Increased Cl- and water secretion | Lubiprostone | 8 µg bid | Effective: Lubiprostone 8 µg RR: 0.85 (0.78 to 0.96) for FDA endpoint | Second-line |
| Linaclotide | 290 mg qd | Effective: Adequate relief IBS: RR 1.95 (1.3 to 2.9); Abdo pain: RR 1.58 (1.02 to 2.46) RR 0.81 (0.76 to 0.86) for 290 µg for FDA endpoint | |||
| Plecanatide | 3 mg/6 mg qd | Effective: Using FDA endpoint 6 mg: RR 0.87 (0.81 to 0.94); 3 mg RR 0.88 (0.82 to 0.94) | |||
| Anti-absorptive | NHE3 inhibitor stimulates Na+, water secretion | Tenapanor | 15mg bid | Effective at 50 mg 2/d; NNT, 7-9 for complete SBM and combined complete SBM ≥ 30% pain reduction; 11 for abdominal pain reduction > 30% alone | Third-line |
| Pain | |||||
| Anti-spasmodics | Inhibition of muscarinic Ach receptors or block Ca++ channels, relaxation of GI smooth muscle | Pinaverium | 50 mg tid | May be effective: OR, 0.68 (95%CI: 0.57-0.71); overall NNT, 5 | First-line |
| Otilonium | 20/40/80 mg tid | ||||
| Hyoscine | - | ||||
| Peppermint Oil | 182 mg | Effective: OR, 0.43 (95%CI: 0.32-0.59); Global: RR 2.23 (95%CI: 1.78-2.81); overall NNT, 2.5; RCT of sustained release formulation: decrease pain, bloat, urgency but not total IBS scores | |||
| Antidepressants | Psychological, acting on the CNS | TCA | - | Effective: OR, 0.67 (95%CI: 0.58-0.77); for global: OR, 0.62 (95%CI: 0.43-0.88); NNT, 4 for abdominal pain | Second-line |
| SSRI | - | ||||
IBS-C: The first-line therapy for IBS-C are bulking agents and osmotic laxatives. The ACG published guidelines for IBS-C[105] and recommended them in 2022, including a strong recommendation for linaclotide (high certainty) and conditional recommendations for tenapanor, plecanatide, tegaserod, and lubiprostone (moderate certainty); polyethylene glycol laxatives, TCA, and antispasmodics have low certainty. The panel made a conditional recommendation against the use of SSRIs (low certainty). Chloride channel activators and guanylate cyclase activators are recommended for global IBS with constipation symptoms[106]. However, adverse effects of diarrhea may occur (Table 1).
Pain: Antispasmodics, including anticholinergic and calcium-blocking drugs, which can relieve pain and improve bowel movements, remain the first choice for abdominal pain in IBS[107]. Such as cimetropium/dicyclomine, peppermint oil, pinaverium, and trimebutine have clear benefits on abdominal pain and symptom scores[108]. By reviewing relevant RCTs[109-112], it was shown that most samples were small and of moderate quality. Overall, limited to short-term treatment Antidepressants can improve pain through central nervous system action, but clinical trials are scarce and the limitation of adverse events is uncertain. Although pinaverium was the most commonly used drug for the treatment of abdominal pain with a rapid onset of action and the improvement in abdominal pain was greater than that of bowel movements, its efficacy was less significant than that of placebo after one week. Whereas otilonium bromide (OB) significantly has a longer onset of action than pinaverium but is more suitable for patients with diarrhea. Moreover, drotaverine has a slow onset of action and is more suitable for the later stages of IBS. Although peppermint oil has been shown to be effective, it has many adverse events (heartburn or GERD symptoms, belching, headache, etc.). Finally, TCA and SSRIs have been shown to be effective, but SSRI adverse events are more numerous, and TCA is recommended for patients with significant anxiety or abdominal pain[113,114] (Table 1).
Traditional Chinese treatment: Traditional Chinese treatment (TCM) prescriptions Traditional Chinese medicine treatments, including prescriptions, acupuncture, and moxibustion, are the main treatments for IBS, although they are still complementary treatments that have been found to have great potential through research. Its possible mechanisms of action are mainly through regulating the enteric nervous system, improving gastrointestinal motility, reducing visceral hypersensitivity, regulating intestinal flora, and regulating the immune system to alleviate IBS[115].
A RCT designed in China, enrolling 216 patients with IBS who were assigned to the control group that took the Chang'an I Recipe or placebo group, reported that the Chang'an I Recipe outperformed the placebo in the treatment of IBS-D with no major side effects[116]. Furthermore, 60 IBS patients were enrolling in a study and were divided into the control group (n = 20) and the treatment group (n = 40), which were given oral pinaverium bromide tablets and Tongxie Yaofang decoction on the basis of conventional treatment, respectively. And the results reported that the flavored Tongxie Yaofang had a significant effect on the symptoms of patients with IBS-D[117], and improving the gut microbiome, alleviating visceral hypersensitivity, regulating 5-HT level in patients, and inhibiting colonic contraction are mechanisms for the treatment of IBS[118-120]. Moreover, Tongxie Anchang Decoction improves IBS by reducing visceral hypersensitivity, reversing mast cell infiltration, and regulating 5-HT[118,121]. And Xiang Sha Liu Jun Zi Decoction reduced the mean diarrhea score of IBS patients[122]. Finally, the Fuzi-Lizhong pill can impact bacterial diversity in the gut and regulate inflammation and immune system to treat IBS-D[123].
Acupuncture and moxibustion: The therapeutic effects of acupuncture are recognized worldwide, although its mechanisms of action are still being further explored. Acupuncture has a bright future in IBS and FGIDs, yet there remain controversies that need to be further explored. A randomized trial of 344 patients with IBS in the acupuncture group and 175 in the pinaverium bromide group reported that the acupuncture group was more effective than the control group, and the effect lasted up to 12 wk[124]. Besides, 126 patients with IBS-D (liver stagnation and spleen deficiency) were randomly assigned to one of three groups: A herb-separated moxibustion group (n = 42, applied to Jinsuo (GV 8)-eight-diagram points), a Western medication group (n = 42), and a Chinese herbal medication group (n = 42), and the results showed that the TCM symptom score, gastrointestinal symptom score, and IBS-SSS score were significantly reduced in the moxibustion group[125]. Overall, Pishu (BL 20), Zhongwan (RN 12), and Zusanli (ST 36) are acupuncture points commonly used in clinical practice, and acupuncture and moxibustion have few side effects[93].
Probiotics: Probiotics can relieve bloating, intestinal gas, and IBS symptoms, and in addition, studies have shown that probiotics (Lactobacillus, Bifidobacterium, Escherichia coli, and Streptococcus) can significantly relieve the overall symptoms of diarrhea and IBS-D[126,127]. However, the role of probiotics is controversial; a small number of studies have discovered that probiotics have no effect on bloating or abdominal pain[128,129]. A RCT included 389 patients with IBS. The control group was treated with oral probiotics for 6 wk. The final results showed that the treatment effect of probiotics was not superior to placebo when all IBS subtypes were included, but the analysis found a higher percentage of sustained responders in the probiotic group than in the placebo group in IBS-D[130]. Although there is a contradiction in the current evidence, analyzing it objectively resolved the contradictions and also demonstrated that probiotics have great potential for the treatment of IBS, especially in patients with IBS-D[131].
Prebiotics and synbiotics: Prebiotics and synbiotics, the collaboration of prebiotics and probiotics, become synbiotics, which have beneficial effects on the gastrointestinal tract by regulating the diversity and activity of intestinal microorganisms and protecting the integrity of the intestinal mucosa[132,133]. A RCT reported, that compared to placebo, synbiotics treatment over an 8-wk period (Lactobacillus and Bifidobacterium probiotic strains and short-chain fructooligosaccharides; colony-forming units (CFU) per sachet was five billion, bid) significantly improved overall symptoms of IBS, flatulence (P = 0.028), and bowel habits (P = 0.028). It is recommended to try probiotics for 12 wk and observe the efficacy[83], have benefits in improving IBS-D.
Fecal microbiota transplantation: Fecal microbiota transplantation (FMT) for Clostridium difficile infection has shown good efficacy in improving intestinal flora[134], and although studies on FMT for IBS are scarce and results remain controversial, the overall results suggest a positive trend for FMT for IBS. A RCT included 135 IBS patients randomly assigned to receive their own stool, 30 g FMT, or 60 g FMT, with response rates of 23.6%, 76.9%, and 89.1%, respectively[135]. Moreover, a recent study has found that FMT not only improves the symptoms of IBS patients but also improves depression and anxiety[93]. However, the studies of Madsen, A.M.A. et al[136] and Browne et al[137] reported that FMT capsules have no clinical benefit on abdominal pain, stool frequency, or stool form in IBS patients and have no benefit. Therefore, the clinical practice and clinical effect of FMT in the field of gut microbiomes and IBS need to be further verified and explored.
IBS-related studies have shown a downward trend in IBS dysfunction, and the brain-gut axis is gradually becoming more prominent in IBS research by reviewing the related research of the last decade. With the help of convenient laboratory tests, a new diagnosis and treatment model was formed based on the complex etiology and clinical combination of IBS, and the above has positive implications for a new understanding of IBS.
It is concluded that a pooled global prevalence of IBS is unlikely to be meaningful and that future research should focus more on regionalization. The definition of IBS has been updated with the discovery of overlapping symptoms and advances in research on IBS pathogenesis, and its definition tends to suggest that FGIDs are a group of disorders with the same pathogenesis, such as the brain-gut axis and visceral hypersensitivity. Therefore, in the future, we should pay more attention to the influence of the brain-gut axis and the central nervous system on the entire gastrointestinal tract and understand FGIDs as a whole. IBS is a dysfunctional disease, but in the absence of simple and inexpensive screening tests for many biological markers, patients such as those with BAD are still included in IBS-D. These technologies still need further validation and dissemination. It is possible that an updated Rome criteria will exclude them from the IBS diagnosis as technology advances. Furthermore, studies of IBS-related dietary interventions, such as LFD, special diets for IBS-C, and foods with gastrointestinal allergies, as well as the gut microenvironment and the brain-gut axis, are the hot spots of research on gut inflammation and the gut barrier.
Long-term treatment brings economic pressure and psychological burden, and for patients for whom conventional treatment is ineffective, further search for etiology should be done with the help of adjuvant examinations, and appropriate second-line treatment or psychotherapy should be chosen. In recent years, non-pharmacological treatment and Chinese medicine have been favored by IBS patients, but the current treatment should be further improved in order to facilitate the development of alternative medicine, with lifestyle, diet, and acupressure as routine interventions. It is important to note that lifestyle and CBT only relieve the symptoms and frequency of IBS; they do not improve the quality of life. Moreover, the involvement of microbiota in the brain-gut axis is widely recognized and studied, and RCTs related to intestinal flora have yielded encouraging results. However, current studies of microbiota are mostly related to IBS-D and have limitations. Let's look forward to more clarity on the treatment and management of IBS.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ertan A, United States; Garg P, India S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Yang W, Yang X, Cai X, Zhou Z, Yao H, Song X, Zhao T, Xiong P. The Prevalence of Irritable Bowel Syndrome Among Chinese University Students: A Systematic Review and Meta-Analysis. Front Public Health. 2022;10:864721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Roisinblit KC. Irritable bowel syndrome in women. J Midwifery Womens Health. 2013;58:15-24; quiz 116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 3. | Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99-114.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1190] [Article Influence: 297.5] [Reference Citation Analysis (0)] |
| 4. | Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 313] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 5. | Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M, Bolotin A, Friger M, Freud T, Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 6. | Rahman MM, Mahadeva S, Ghoshal UC. Epidemiological and clinical perspectives on irritable bowel syndrome in India, Bangladesh and Malaysia: A review. World J Gastroenterol. 2017;23:6788-6801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (6)] |
| 7. | Sperber AD. Epidemiology and Burden of Irritable Bowel Syndrome: An International Perspective. Gastroenterol Clin North Am. 2021;50:489-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 8. | Galica AN, Galica R, Dumitrascu DL. Epidemiology of Irritable Bowel Syndrome in Albania. J Gastrointestin Liver Dis. 2021;30:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kovács DB, Szekely A, Hubai AG, Palsson O. Prevalence, epidemiology and associated healthcare burden of Rome IV irritable bowel syndrome and functional dyspepsia in the adult population of Gibraltar. BMJ Open Gastroenterol. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Long Y, Huang Z, Deng Y, Chu H, Zheng X, Yang J, Zhu Y, Fried M, Fox M, Dai N. Prevalence and risk factors for functional bowel disorders in South China: a population based study using the Rome III criteria. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Pontet Y, Olano C. [Irritable bowel syndrome prevalence in Latin America]. Rev Gastroenterol Peru. 2021;41:144-149. [PubMed] |
| 12. | Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020;158:1262-1273.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 13. | Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (3)] |
| 15. | Gwee KA. Irritable bowel syndrome in developing countries--a disorder of civilization or colonization? Neurogastroenterol Motil. 2005;17:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. J Gastroenterol Hepatol. 2017;32:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Husain N, Chaudhry IB, Jafri F, Niaz SK, Tomenson B, Creed F. A population-based study of irritable bowel syndrome in a non-Western population. Neurogastroenterol Motil. 2008;20:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Devanarayana NM, Rajindrajith S, Pathmeswaran A, Abegunasekara C, Gunawardena NK, Benninga MA. Epidemiology of irritable bowel syndrome in children and adolescents in Asia. J Pediatr Gastroenterol Nutr. 2015;60:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 19. | AlButaysh OF, AlQuraini AA, Almukhaitah AA, Alahmdi YM, Alharbi FS. Epidemiology of irritable bowel syndrome and its associated factors in Saudi undergraduate students. Saudi J Gastroenterol. 2020;26:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1366] [Cited by in RCA: 1389] [Article Influence: 154.3] [Reference Citation Analysis (1)] |
| 21. | Sperber AD, Freud T, Aziz I, Palsson OS, Drossman DA, Dumitrascu DL, Fang X, Fukudo S, Ghoshal UC, Kellow J, Khatun R, Okeke E, Quigley EMM, Schmulson M, Simren M, Tack J, Whitehead WE, Whorwell P, Bangdiwala SI. Greater Overlap of Rome IV Disorders of Gut-Brain Interactions Leads to Increased Disease Severity and Poorer Quality of Life. Clin Gastroenterol Hepatol. 2022;20:e945-e956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 22. | Madisch A, Andresen V, Enck P, Labenz J, Frieling T, Schemann M. The Diagnosis and Treatment of Functional Dyspepsia. Dtsch Arztebl Int. 2018;115:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 449] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Whitehead WE, Gibbs NA, Li Z, Drossman DA. Is functional dyspepsia just a subset of the irritable bowel syndrome? Baillieres Clin Gastroenterol. 1998;12:443-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | von Wulffen M, Talley NJ, Hammer J, McMaster J, Rich G, Shah A, Koloski N, Kendall BJ, Jones M, Holtmann G. Overlap of Irritable Bowel Syndrome and Functional Dyspepsia in the Clinical Setting: Prevalence and Risk Factors. Dig Dis Sci. 2019;64:480-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 26. | Barberio B, Yiannakou Y, Houghton LA, Black CJ, Savarino EV, Ford AC. Overlap of Rome IV Irritable Bowel Syndrome and Functional Dyspepsia and Effect on Natural History: A Longitudinal Follow-Up Study. Clin Gastroenterol Hepatol. 2022;20:e89-e101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Choi YJ, Kim N, Yoon H, Shin CM, Park YS, Kim JW, Kim YS, Lee DH, Jung HC. Overlap between irritable bowel syndrome and functional dyspepsia including subtype analyses. J Gastroenterol Hepatol. 2017;32:1553-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Nam K, Kim N, Song HJ, Baik GH, Choi SC, Kim HJ, Lee JY, Park KS, Park SY, Park SJ. Gender difference in the overlap of irritable bowel syndrome and functional dyspepsia: a prospective nationwide multicenter study in Korea. J Gastroenterol. 2021;56:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Jones MP, Shah A, Walker MM, Koloski NA, Holtmann G, Talley NJ. Overlap of heartburn, functional dyspepsia, and irritable bowel syndrome in a population sample: Prevalence, temporal stability, and associated comorbidities. Neurogastroenterol Motil. 2022;34:e14349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 30. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-20; quiz 1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2368] [Cited by in RCA: 2454] [Article Influence: 129.2] [Reference Citation Analysis (2)] |
| 31. | Fass R, Boeckxstaens GE, El-Serag H, Rosen R, Sifrim D, Vaezi MF. Gastro-oesophageal reflux disease. Nat Rev Dis Primers. 2021;7:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 32. | Rasmussen S, Jensen TH, Henriksen SL, Haastrup PF, Larsen PV, Søndergaard J, Jarbøl DE. Overlap of symptoms of gastroesophageal reflux disease, dyspepsia and irritable bowel syndrome in the general population. Scand J Gastroenterol. 2015;50:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 33. | Lovell RM, Ford AC. Prevalence of gastro-esophageal reflux-type symptoms in individuals with irritable bowel syndrome in the community: a meta-analysis. Am J Gastroenterol. 2012;107:1793-801; quiz 1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | de Bortoli N, Tolone S, Frazzoni M, Martinucci I, Sgherri G, Albano E, Ceccarelli L, Stasi C, Bellini M, Savarino V, Savarino EV, Marchi S. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: common overlapping gastrointestinal disorders. Ann Gastroenterol. 2018;31:639-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | de Bortoli N, Frazzoni L, Savarino EV, Frazzoni M, Martinucci I, Jania A, Tolone S, Scagliarini M, Bellini M, Marabotto E, Furnari M, Bodini G, Russo S, Bertani L, Natali V, Fuccio L, Savarino V, Blandizzi C, Marchi S. Functional Heartburn Overlaps With Irritable Bowel Syndrome More Often than GERD. Am J Gastroenterol. 2016;111:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1799] [Article Influence: 224.9] [Reference Citation Analysis (111)] |
| 37. | Glick LR, Cifu AS, Feld L. Ulcerative Colitis in Adults. JAMA. 2020;324:1205-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Fairbrass KM, Costantino SJ, Gracie DJ, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:1053-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 39. | Müller M, Willén R, Stotzer PO. Colonoscopy and SeHCAT for investigation of chronic diarrhea. Digestion. 2004;69:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, Singh S, Grover M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 41. | Slade GD, Greenspan JD, Fillingim RB, Maixner W, Sharma S, Ohrbach R. Overlap of Five Chronic Pain Conditions: Temporomandibular Disorders, Headache, Back Pain, Irritable Bowel Syndrome, and Fibromyalgia. J Oral Facial Pain Headache. 2020;34:s15-s28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 42. | Üçüncü MZ, Çoruh Akyol B, Toprak D. The early diagnosis of fibromyalgia in irritable bowel syndrome patients. Med Hypotheses. 2020;143:110119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Rej A, Sanders DS. The overlap of irritable bowel syndrome and noncoeliac gluten sensitivity. Curr Opin Gastroenterol. 2019;35:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | DiVasta AD, Zimmerman LA, Vitonis AF, Fadayomi AB, Missmer SA. Overlap Between Irritable Bowel Syndrome Diagnosis and Endometriosis in Adolescents. Clin Gastroenterol Hepatol. 2021;19:528-537.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 371] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 46. | Waseem MR, Shin A, Siwiec R, James-Stevenson T, Bohm M, Rogers N, Wo J, Waseem L, Gupta A, Jarrett M, Kadariya J, Xu H. Associations of Fecal Short Chain Fatty Acids With Colonic Transit, Fecal Bile Acid, and Food Intake in Irritable Bowel Syndrome. Clin Transl Gastroenterol. 2023;14:e00541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 47. | Wei W, Wang H, Zhang Y, Niu B, Chen S, Zhang W, Yao S. Faecal bile acids and colonic bile acid membrane receptor correlate with symptom severity of diarrhoea-predominant irritable bowel syndrome: A pilot study. Dig Liver Dis. 2021;53:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Algera JP, Colomier E, Melchior C, Hreinsson JP, Midenfjord I, Clevers E, Simrén M, Törnblom H. Associations between postprandial symptoms, hydrogen and methane production, and transit time in irritable bowel syndrome. Neurogastroenterol Motil. 2023;35:e14482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 49. | DuPont AW, Jiang ZD, Harold SA, Snyder N, Galler GW, Garcia-Torres F, DuPont HL. Motility abnormalities in irritable bowel syndrome. Digestion. 2014;89:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Sandström O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech Ageing Dev. 1999;108:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Lee J, Cummings BP, Martin E, Sharp JW, Graham JL, Stanhope KL, Havel PJ, Raybould HE. Glucose sensing by gut endocrine cells and activation of the vagal afferent pathway is impaired in a rodent model of type 2 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R657-R666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 267] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 53. | Mazzawi T, El-Salhy M. Effect of diet and individual dietary guidance on gastrointestinal endocrine cells in patients with irritable bowel syndrome (Review). Int J Mol Med. 2017;40:943-952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 988] [Cited by in RCA: 1131] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 55. | Bertrand PP, Bertrand RL. Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci. 2010;153:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 56. | Bertrand PP. The cornucopia of intestinal chemosensory transduction. Front Neurosci. 2009;3:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Nisticò V, Rossi RE, D'Arrigo AM, Priori A, Gambini O, Demartini B. Functional Neuroimaging in Irritable Bowel Syndrome: A Systematic Review Highlights Common Brain Alterations With Functional Movement Disorders. J Neurogastroenterol Motil. 2022;28:185-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 58. | Grinsvall C, Ryu HJ, Van Oudenhove L, Labus JS, Gupta A, Ljungberg M, Törnblom H, Mayer EA, Simrén M. Association between pain sensitivity and gray matter properties in the sensorimotor network in women with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 59. | Nozu T, Okumura T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J Gastroenterol. 2015;50:819-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Nozu T, Miyagishi S, Nozu R, Takakusaki K, Okumura T. Lipopolysaccharide induces visceral hypersensitivity: role of interleukin-1, interleukin-6, and peripheral corticotropin-releasing factor in rats. J Gastroenterol. 2017;52:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Nozu T, Miyagishi S, Ishioh M, Takakusaki K, Okumura T. Peripheral apelin mediates visceral hypersensitivity and impaired gut barrier in a rat irritable bowel syndrome model. Neuropeptides. 2022;94:102248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Dlugosz A, Nowak P, D'Amato M, Mohammadian Kermani G, Nyström J, Abdurahman S, Lindberg G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 63. | Constante M, De Palma G, Lu J, Jury J, Rondeau L, Caminero A, Collins SM, Verdu EF, Bercik P. Saccharomyces boulardii CNCM I-745 modulates the microbiota-gut-brain axis in a humanized mouse model of Irritable Bowel Syndrome. Neurogastroenterol Motil. 2021;33:e13985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016;65:797-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 65. | Nozu T, Miyagishi S, Nozu R, Ishioh M, Takakusaki K, Okumura T. EMA401, an angiotensin II type 2 receptor antagonist blocks visceral hypersensitivity and colonic hyperpermeability in rat model of irritable bowel syndrome. J Pharmacol Sci. 2021;146:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Fan F, Tang Y, Dai H, Cao Y, Sun P, Chen Y, Chen A, Lin C. Blockade of BDNF signalling attenuates chronic visceral hypersensitivity in an IBS-like rat model. Eur J Pain. 2020;24:839-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Hasler WL, Grabauskas G, Singh P, Owyang C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol Motil. 2022;34:e14339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 68. | Chen Z, Liu Y, Wu X, Lin W, Liu Z, Huang Y, Chen Y, Tang Y, Chen A, Lin C. Spinal CircKcnk9 Regulates Chronic Visceral Hypersensitivity of Irritable Bowel Syndrome. J Pain. 2023;24:463-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Tozlu M, Cash B, Younes M, Ertan A. Dilemma in post-IBD patients with IBS-D symptoms: A 2020 overview. Expert Rev Gastroenterol Hepatol. 2021;15:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | El-Salhy M, Hatlebakk JG, Hausken T. Possible role of peptide YY (PYY) in the pathophysiology of irritable bowel syndrome (IBS). Neuropeptides. 2020;79:101973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 71. | Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain--gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 72. | Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. 2016;44:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 229] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 73. | Dinan TG, Cryan JF. Gut-brain axis in 2016: Brain-gut-microbiota axis - mood, metabolism and behaviour. Nat Rev Gastroenterol Hepatol. 2017;14:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 74. | Dinan TG, Cryan JF. Brain-Gut-Microbiota Axis and Mental Health. Psychosom Med. 2017;79:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 75. | Sabo CM, Dumitrascu DL. Microbiota and the irritable bowel syndrome. Minerva Gastroenterol (Torino). 2021;67:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Mishima Y, Ishihara S. Molecular Mechanisms of Microbiota-Mediated Pathology in Irritable Bowel Syndrome. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 77. | Lee SM, Kim N, Yoon H, Kim YS, Choi SI, Park JH, Lee DH. Compositional and Functional Changes in the Gut Microbiota in Irritable Bowel Syndrome Patients. Gut Liver. 2021;15:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 78. | Mujagic Z, Kasapi M, Jonkers DM, Garcia-Perez I, Vork L, Weerts ZZRM, Serrano-Contreras JI, Zhernakova A, Kurilshikov A, Scotcher J, Holmes E, Wijmenga C, Keszthelyi D, Nicholson JK, Posma JM, Masclee AA. Integrated fecal microbiome-metabolome signatures reflect stress and serotonin metabolism in irritable bowel syndrome. Gut Microbes. 2022;14:2063016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 79. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1897] [Article Influence: 210.8] [Reference Citation Analysis (3)] |
| 80. | Vijayvargiya P, Camilleri M, Burton D, Busciglio I, Lueke A, Donato LJ. Bile and fat excretion are biomarkers of clinically significant diarrhoea and constipation in irritable bowel syndrome. Aliment Pharmacol Ther. 2019;49:744-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 81. | Karatay E, Utku ÖG. Serum resolvin D1 levels as a marker of inflammation in constipation dominant irritable bowel syndrome. Turk J Gastroenterol. 2020;31:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Camilleri M, Boeckxstaens G. Irritable bowel syndrome: treatment based on pathophysiology and biomarkers. Gut. 2023;72:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 83. | Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, Agrawal A, Aziz I, Farmer AD, Eugenicos MP, Moss-Morris R, Yiannakou Y, Ford AC. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 84. | Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2022;71:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 192] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 85. | Wu J, Masuy I, Biesiekierski JR, Fitzke HE, Parikh C, Schofield L, Shaikh H, Bhagwanani A, Aziz Q, Taylor SA, Tack J, Van Oudenhove L. Gut-brain axis dysfunction underlies FODMAP-induced symptom generation in irritable bowel syndrome. Aliment Pharmacol Ther. 2022;55:670-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 86. | Chey WD, Hashash JG, Manning L, Chang L. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology. 2022;162:1737-1745.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 87. | Schumann D, Klose P, Lauche R, Dobos G, Langhorst J, Cramer H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition. 2018;45:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 88. | Zannini E, Arendt EK. Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: Lights and shadows. Food Res Int. 2018;110:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Ford AC, Moayyedi P, Chey WD, Harris LA, Lacy BE, Saito YA, Quigley EMM; ACG Task Force on Management of Irritable Bowel Syndrome. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2018;113:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 90. | Di Rosa C, Altomare A, Terrigno V, Carbone F, Tack J, Cicala M, Guarino MPL. Constipation-Predominant Irritable Bowel Syndrome (IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 91. | Garg P. Inflammation in Irritable Bowel Syndrome (IBS): Role of Psyllium Fiber Supplementation in Decreasing Inflammation and Physiological Management of IBS. Turk J Gastroenterol. 2021;32:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (14)] |
| 93. | Bonetto S, Fagoonee S, Battaglia E, Grassini M, Saracco GM, Pellicano R. Recent advances in the treatment of irritable bowel syndrome. Pol Arch Intern Med. 2021;131:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 94. | Sunderland R. Irritable bowel syndrome in adults: symptoms, treatment and management. Nurs Stand. 2017;31:52-63. [PubMed] |
| 95. | Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on Brain-Gut Behavior Therapies for Disorders of Gut-Brain Interaction. Gastroenterology. 2022;162:300-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 96. | Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114:21-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 259] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 97. | Lackner JM, Jaccard J, Radziwon CD, Firth RS, Gudleski GD, Hamilton F, Katz LA, Keefer L, Krasner SS, Ma CX, Sitrin MD, Brenner DM. Durability and Decay of Treatment Benefit of Cognitive Behavioral Therapy for Irritable Bowel Syndrome: 12-Month Follow-Up. Am J Gastroenterol. 2019;114:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 98. | Lalouni M, Ljótsson B, Bonnert M, Ssegonja R, Benninga M, Bjureberg J, Högström J, Sahlin H, Simrén M, Feldman I, Hedman-Lagerlöf E, Serlachius E, Olén O. Clinical and Cost Effectiveness of Online Cognitive Behavioral Therapy in Children With Functional Abdominal Pain Disorders. Clin Gastroenterol Hepatol. 2019;17:2236-2244.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Prior A, Colgan SM, Whorwell PJ. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut. 1990;31:896-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Whorwell PJ, Houghton LA, Taylor EE, Maxton DG. Physiological effects of emotion: assessment via hypnosis. Lancet. 1992;340:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 101. | Peter J, Fournier C, Keip B, Rittershaus N, Stephanou-Rieser N, Durdevic M, Dejaco C, Michalski M, Moser G. Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | De Benedittis G. Hypnobiome: A New, Potential Frontier of Hypnotherapy in the Treatment of Irritable Bowel Syndrome-A Narrative Review of the Literature. Int J Clin Exp Hypn. 2022;70:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 103. | DeBenedittis G, Cigada M, Bianchi A, Signorini MG, Cerutti S. Autonomic changes during hypnosis: a heart rate variability power spectrum analysis as a marker of sympatho-vagal balance. Int J Clin Exp Hypn. 1994;42:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Lembo A, Sultan S, Chang L, Heidelbaugh JJ, Smalley W, Verne GN. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology. 2022;163:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 105. | Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology. 2022;163:118-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 106. | Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116:17-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 468] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 107. | Qin D, Tao QF, Huang SL, Chen M, Zheng H. Eluxadoline Versus Antispasmodics in the Treatment of Irritable Bowel Syndrome: An Adjusted Indirect Treatment Comparison Meta-analysis. Front Pharmacol. 2022;13:757969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 108. | Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;2011:CD003460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 109. | Zheng L, Lai Y, Lu W, Li B, Fan H, Yan Z, Gong C, Wan X, Wu J, Huang D, Wang Y, Mei Y, Li Z, Jiang Z, Liu X, Ye J, Yang Y, Huang H, Xiao J. Pinaverium Reduces Symptoms of Irritable Bowel Syndrome in a Multicenter, Randomized, Controlled Trial. Clin Gastroenterol Hepatol. 2015;13:1285-1292.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 110. | Zheng L, Lu W, Xiao Q, Lai Y, Fan H, Sun Y, Huang D, Wang Y, Li Z, Jiang Z, Liu X, Zhang L, Zuo D, Shou Z, Tang Q, Huang H, Yang Y, Tang Z, Xiao J. Assessing the post-treatment therapeutic effect of pinaverium in irritable bowel syndrome: a randomized controlled trial. Sci Rep. 2021;11:13894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 111. | Chmielewska-Wilkoń D, Reggiardo G, Egan CG. Otilonium bromide in irritable bowel syndrome: a dose-ranging randomized double-blind placebo-controlled trial. World J Gastroenterol. 2014;20:12283-12291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 112. | Rai RR, Nijhawan S. Comparative evaluation of efficacy and safety of drotaverine versus mebeverine in irritable bowel syndrome: A randomized double-blind controlled study. Saudi J Gastroenterol. 2021;27:136-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Weerts ZZRM, Masclee AAM, Witteman BJM, Clemens CHM, Winkens B, Brouwers JRBJ, Frijlink HW, Muris JWM, De Wit NJ, Essers BAB, Tack J, Snijkers JTW, Bours AMH, de Ruiter-van der Ploeg AS, Jonkers DMAE, Keszthelyi D. Efficacy and Safety of Peppermint Oil in a Randomized, Double-Blind Trial of Patients With Irritable Bowel Syndrome. Gastroenterology. 2020;158:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 114. | Xie C, Tang Y, Wang Y, Yu T, Jiang L, Lin L. Efficacy and Safety of Antidepressants for the Treatment of Irritable Bowel Syndrome: A Meta-Analysis. PLoS One. 2015;10:e0127815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 115. | Chen GR, Xie XF, Peng C. Treatment of Irritable Bowel Syndrome by Chinese Medicine: A Review. Chin J Integr Med. 2023;29:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Tang XD, Lu B, Li ZH, Wei W, Meng LN, Li BS, Tang ZP, Gao R, Wang FY, Lu F, Bian LQ, Zhao YP, Wang P, Zhang YQ. Therapeutic Effect of Chang'an I Recipe ( I ) on Irritable Bowel Syndrome with Diarrhea: A Multicenter Randomized Double-Blind Placebo-Controlled Clinical Trial. Chin J Integr Med. 2018;24:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | He S, Lin Q, Huang J, Zheng L, Lai J, Chen C. Effect and Mechanism of Flavored Tongxie Yaofang Decoction for Diarrheal Irritable Bowel Syndrome under Intestinal Microecology. Evid Based Complement Alternat Med. 2022;2022:3904571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Tan X, Pei W, Xie C, Wang Z, Liu J, Cheng Y, Mao T, Shi L, Zhao X, Lu Q, Sun Z, Kou F, Jiang H, Li J. Tongxie Anchang Decoction Relieves Visceral Hypersensitivity in Diarrhea-Predominant Irritable Bowel Syndrome Rats by Regulating the NGF/TrkA Signaling Pathway. Evid Based Complement Alternat Med. 2021;2021:6679348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 119. | Yang C, Zhang SS, Li XL, Wang ZF, Zhao LQ. Inhibitory effect of TongXie-YaoFang formula on colonic contraction in rats. World J Gastroenterol. 2015;21:2912-2917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Li J, Cui H, Cai Y, Lin J, Song X, Zhou Z, Xiong W, Zhou H, Bian Y, Wang L. Tong-Xie-Yao-Fang Regulates 5-HT Level in Diarrhea Predominant Irritable Bowel Syndrome Through Gut Microbiota Modulation. Front Pharmacol. 2018;9:1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 121. | Tan X, Pei W, Xie C, Wang Z, Mao T, Zhao X, Kou F, Lu Q, Sun Z, Xue X, Li J. Network Pharmacology Identifies the Mechanisms of Action of Tongxie Anchang Decoction in the Treatment of Irritable Bowel Syndrome with Diarrhea Predominant. Evid Based Complement Alternat Med. 2020;2020:2723705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 122. | Shih YS, Tsai CH, Li TC, Lai HC, Wang KT, Liao WL, Hsieh CL. The effect of Xiang-Sha-Liu-Jun-Zi tang (XSLJZT) on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J Ethnopharmacol. 2019;238:111889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 123. | Zhen Z, Xia L, You H, Jingwei Z, Shasha Y, Xinyi W, Wenjing L, Xin Z, Chaomei F. An Integrated Gut Microbiota and Network Pharmacology Study on Fuzi-Lizhong Pill for Treating Diarrhea-Predominant Irritable Bowel Syndrome. Front Pharmacol. 2021;12:746923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 124. | Pei L, Geng H, Guo J, Yang G, Wang L, Shen R, Xia S, Ding M, Feng H, Lu J, Li J, Liu L, Shu Y, Fang X, Wu X, Wang X, Weng S, Ju L, Chen X, Shen H, Sun J. Effect of Acupuncture in Patients With Irritable Bowel Syndrome: A Randomized Controlled Trial. Mayo Clin Proc. 2020;95:1671-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 125. | Hao LJ, Shi ZM. [Therapeutic effect of herb-separated moxibustion at Jinsuo (GV 8)-eight-diagram points on diarrhea-type irritable bowel syndrome of liver stagnation and spleen deficiency]. Zhongguo Zhen Jiu. 2020;40:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 126. | Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, Hyuk J, Kyung Chang S, Kim K, Chung WS, Seo JG. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 127. | Dai C, Zheng CQ, Jiang M, Ma XY, Jiang LJ. Probiotics and irritable bowel syndrome. World J Gastroenterol. 2013;19:5973-5980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 128. | Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P, Yang XX, Gu X, Song LJ, Li Z, Zuo XL, Li YQ. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2018;8:2964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 129. | Wang Y, Chen N, Niu F, Li Y, Guo K, Shang X, E F, Yang C, Yang K, Li X. Probiotics therapy for adults with diarrhea-predominant irritable bowel syndrome: a systematic review and meta-analysis of 10 RCTs. Int J Colorectal Dis. 2022;37:2263-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 130. | Mack I, Schwille-Kiuntke J, Mazurak N, Niesler B, Zimmermann K, Mönnikes H, Enck P. A Nonviable Probiotic in Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study. Clin Gastroenterol Hepatol. 2022;20:1039-1047.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 131. | Jung K, Kim A, Lee JH, Cho D, Seo J, Jung ES, Kang HJ, Roh J, Kim W. Effect of Oral Intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1(TM)) on Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 132. | Gu Q, Yin Y, Yan X, Liu X, Liu F, McClements DJ. Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: A review. Adv Colloid Interface Sci. 2022;309:102781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 133. | You S, Ma Y, Yan B, Pei W, Wu Q, Ding C, Huang C. The promotion mechanism of prebiotics for probiotics: A review. Front Nutr. 2022;9:1000517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 134. | Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, Bakow B, Curran P, McKenney J, Tisch A, Reinert SE, Machan JT, Brandt LJ. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med. 2016;165:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 460] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 135. | El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 136. | Madsen AMA, Halkjær SI, Christensen AH, Günther S, Browne PD, Kallemose T, Hansen LH, Petersen AM. The effect of faecal microbiota transplantation on abdominal pain, stool frequency, and stool form in patients with moderate-to-severe irritable bowel syndrome: results from a randomised, double-blind, placebo-controlled study. Scand J Gastroenterol. 2021;56:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 137. | Browne PD, Cold F, Petersen AM, Halkjær SI, Christensen AH, Günther S, Hestbjerg Hansen L. Engraftment of strictly anaerobic oxygen-sensitive bacteria in irritable bowel syndrome patients following fecal microbiota transplantation does not improve symptoms. Gut Microbes. 2021;13:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |