Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.3964
Peer-review started: March 27, 2023
First decision: May 12, 2023
Revised: May 22, 2023
Accepted: June 6, 2023
Article in press: June 6, 2023
Published online: July 7, 2023
Processing time: 92 Days and 9.2 Hours
The estimated world prevalence of hepatitis B virus (HBV) infection is 316 million. HBV infection was identified in 1963 and nowadays is a major cause of cirrhosis and hepatocellular carcinoma (HCC) despite universal vaccination programs, and effective antiviral therapy. Long-term administration of nucleos(t)ide analogues (NA) has been the treatment of choice for chronic hepatitis B during the last decades. The NA has shown a good safety profile and high efficacy in controlling viral replication, improving histology, and decreasing the HCC incidence, decompensation, and mortality. However, the low probability of HBV surface antigen seroclearance made necessary an indefinite treatment. The knowledge, in recent years, about the different phases of the viral cycle, and the new insights into the role of the immune system have yielded an increase in new therapeutic approaches. Consequently, several clinical trials evaluating combinations of new drugs with different mechanisms of action are ongoing with promising results. This integrative literature review aims to assess the knowledge and major advances from the past of hepatitis B, the present of NA treatment and with
Core Tip: Treatment for chronic hepatitis B has been used for decades, showing a good safety profile and high virological and clinical efficacy, decreasing hepatocellular carcinoma, clinical decompensation, and mortality. However, the low probability of hepatitis B virus surface antigen seroclearance with therapy made necessary indefinite treatment in a majority of patients. With the new insights about the immune system role in hepatitis B virus infection and the knowledge of the viral cycle phases, there has been in recent years increased activity in new therapeutic approaches. This review focuses on the past, present, and future of chronic hepatitis B therapy.
- Citation: Broquetas T, Carrión JA. Past, present, and future of long-term treatment for hepatitis B virus. World J Gastroenterol 2023; 29(25): 3964-3983
- URL: https://www.wjgnet.com/1007-9327/full/v29/i25/3964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i25.3964
The hepatitis B virus (HBV) was identified in 1963 when the modern research history of viral hepatitis began. The Nobel Prize winner Baruch S. Blumberg (1925-2011) discovered, 60 years ago, an enigmatic serum protein named “Australia” antigen (AuAg)[1]. Some years later, the recognition of the HBV surface antigen (HBsAg) allowed for the first-time screening of blood donors. The HBsAg was the first marker assessed by a highly sensitive immune analysis, the HBV genome the first identified by deoxyribonucleic acid (DNA), the antibody against HBV core (anti-HBc) the first evaluated by the anti-μ capture technique, and the HBV vaccine the first produced by gene technology[2].
Infection by HBV may lead to acute or chronic hepatitis. Chronic hepatitis B (CHB) infection is defined as HBsAg serum detection for at least six months. The estimated world prevalence of CHB was 316 million in 2019, a major cause of cirrhosis and hepatocellular carcinoma (HCC)[3]. The treatment of choice during the last decades has been the long-term administration of nucleos(t)ide analogues (NA) with a high barrier to resistance. However, the low probability of HBsAg seroclearance made necessary an indefinite therapy.
This integrative literature review aims to review the major advances from the past of hepatitis B, the present of NA treatment and withdrawal, and the future perspectives for the new combined molecules to achieve a functional cure.
In the 1960s, virology was a young science, and techniques for the diagnosis of most viral diseases were complicated and suboptimal. The most often used method was the complement fixation reaction, which required four complex biological component mixtures from four different animal species. The first hint came from the American physician and geneticist Baruch S. Blumberg who used an immunological approach in 1967[1]. Blumberg’s co-worker, Harvey J. Alter, discovered a new antigen in Australian aborigines named the AuAg.
Dane et al[4] were inspecting AuAg immune complexes under the electron microscope and identified virus-like particles of 42 nm in size. In 1971, Almeida et al[5] were able to free the core particles from the “Dane” particle and showed that patients formed, against this core antigen (HBcAg), antibodies (anti-HBc) suggesting that the Dane particle was the cause of hepatitis B, and the AuAg was the surface antigen of the HBV envelope (HBsAg)[5].
Many scientists, including Blumberg, recognized that the HBsAg did not allow to assess the severity of the disease. Magnius et al[6] discovered an additional marker when looking for HBsAg subtypes, the HB e antigen (HBeAg), which helped to distinguish highly infectious from less infectious HBV carriers. However, direct detection of the nucleic acid within HBV was not possible at that time since the HBV still could not be grown in cell cultures, and patient sera contained a few nanograms of Dane particles/mL. It was in 1974 when Robinson et al[7] identified the polymerase activity, and the HBV-DNA. The nucleic acid of HBV was a small circular double-stranded DNA but not covalently closed such as the polyoma- or papillomaviruses. Therefore, the ability of the HBV-DNA polymerase to transcribe both RNA and DNA was similar to retroviruses[2,8].
After more than half a century, current international guidelines have recommended using these three viral markers, HBsAg, HBeAg, and HBV-DNA for characterizing HBV infection[9-11].
HBV is a partially double-stranded virus that belongs to the Hepadnaviridae family[12]. The virus replicates in the host’s hepatocytes. The first step is viral cell entry via the bile acid transporter sodium taurocholate cotransporting polypeptide (NTCP)[13,14]. The HBV is transported to the nucleus to release the relaxed circular DNA (rcDNA) genome[15] and becomes a covalently closed circular DNA (cccDNA) that uses the host-cell DNA repair mechanism and can serve as a template for all viral transcripts that are translated into viral proteins. The HBV genome can be integrated into the host genome with the risk of hepatocyte transformation and carcinogenesis[16]. The pre-genomic RNA (pgRNA) is packaged and reversely transcribed to begin replication. The pgRNA is also enveloped and secreted. The HBV-integrated DNA encodes three HBsAg proteins: Large (L), middle (M), and small (S) HBs which are especially important in HBeAg-negative patients[17]. The viral capsids will be enveloped with the HBsAg and released as infectious virions, non-infectious sub-viral spherical or filamentous particles[18], empty virions (HBsAg and core proteins), and RNA virions[19]. The role of subviral particles in the pathogenesis of chronic HBV infection is not fully understood, but they could act as an immune evasion mechanism by blocking the host’s neutralizing antibodies (anti-HBs) and promoting the spread and persistence of the infection[20,21].
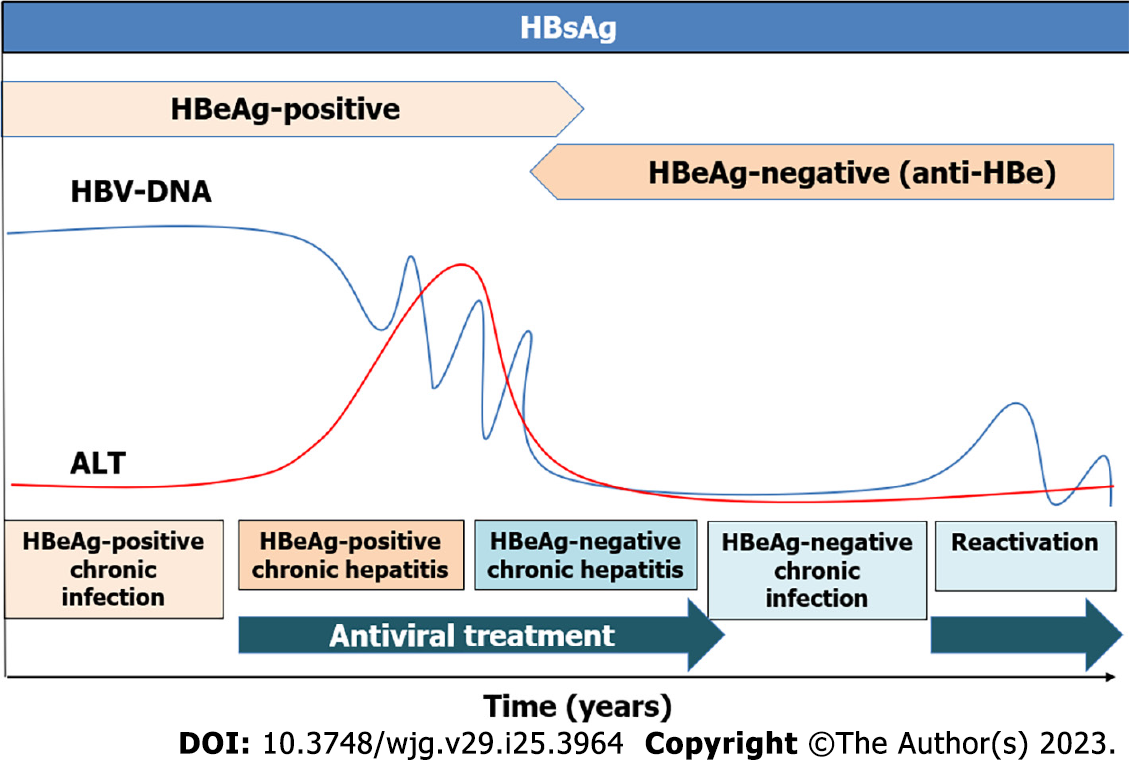
The first step in HBV infection is a highly replicative phase before immune recognition begins, after weeks or months of delay. A vigorous cellular immune response suppresses viral replication and eliminates most of the HBV-infected hepatocytes resulting in acute hepatitis. During acute hepatitis B, HBsAg disappears within six months. If HBsAg persist longer than this period, is considered a CHB infection. CHB is a dynamic interaction between HBV and the host’s immune system.
Infection of newborns or infants results in a persistent infection because of an ineffective immune response. After a long anergic phase, immune defense emerges and leads to the selection of escape mutants. The cellular immune responses against HBcAg appear and HBeAg loses its immunomodulatory function. Therefore, the HBeAg-positive chronic infection usually occurs in younger patients during the first years. Patients have a high viral load with normal liver function tests. The HBeAg-negative chronic infection is characterized by low levels of HBV-DNA with normal liver function and without significant fibrosis. Generally, treatment is not recommended in these two phases, but guidelines advocate treating older than 30-40 years HBeAg-positive patients[9-11]. Chronic hepatitis, defined by the elevation of transaminases and high viral load, induces liver fibrosis progression to cirrhosis and risk of developing HCC, being antiviral treatment recommended in these phases (Figure 1).
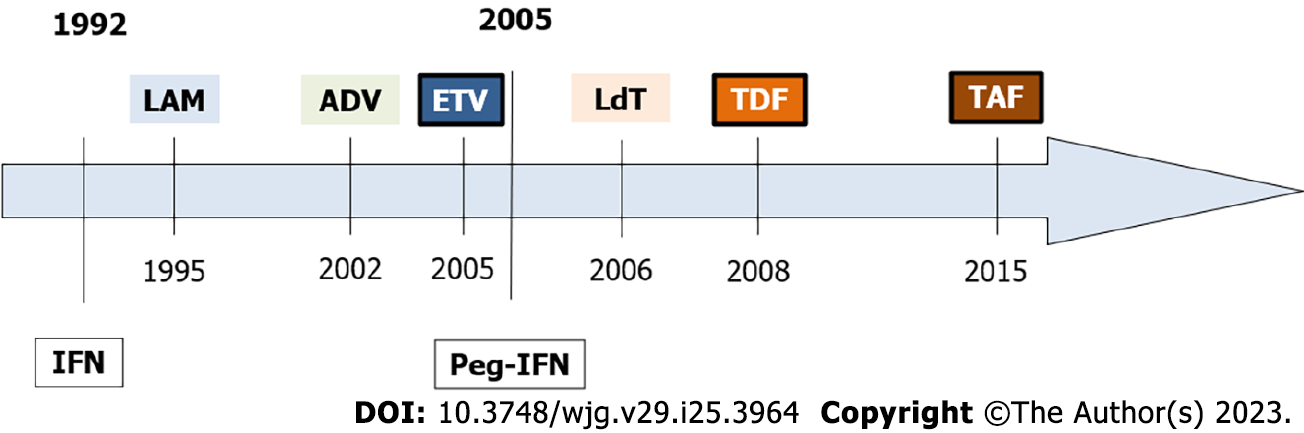
In 1976, William Robinson and Thomas Merigan reported that interferon alpha suppressed HBV replication and cured some patients suffering from CHB[22]. However, further clinical studies showed that only a minority of the patients achieved the clearance of HBsAg while the majority showed a viral breakthrough under treatment or a relapse after the end of therapy (EoT). Conventional interferon alpha was approved in 1992 and one year later Wong et al[23] demonstrated an HBeAg loss rate of 33%. Efficacy in HBeAg-negative patients was very limited and only 15% to 25% showed a sustained biochemical response after 12 mo of treatment[24]. In 2005, pegylated interferon (Peg-IFN) replaced the standard form due to its improved pharmacokinetics and prolonged half-life. In HBeAg-positive patients, 12 mo of therapy achieved the sustained response (HBeAg loss with HBV-DNA < 2000 IU/mL, 6 mo after therapy) in 20% to 30% and the HBsAg loss in 3% to 7%[25]. The Peg-IFN therapy has important disadvantages, its high variability of response and its unfavorable safety profile with a significant number of patients ineligible or unwilling.
In 1995, Benhamou et al[26] reported that HIV-co-infected patients with HBV who received the HIV drug named lamivudine (LAM) lost their HBV-DNA and improved their hepatitis (Figure 2). The same year Jules Dienstag showed the efficacy and safety of LAM in HBV-mono-infected patients[27]. LAM was well tolerated, but resistance soon developed in cases with high replication. After 5 years of therapy, 75% of the LAM-treated patients developed resistant HBV variants. The acyclic nucleotide analog adefovir (ADV) was approved in 2002 for LAM-resistant CHB patients. Unfortunately, its activity was relatively low and was rapidly replaced by the newer drug named tenofovir (TDF). This very similar drug was approved in 2001 for HIV and in 2008 for HBV[28]. The guanosine analog entecavir (ETV) was originally developed against the herpes simplex virus. In 1997, ETV showed its strong activity against HBV in the HepG2.2.15 cell line. In 2001, showed its efficacy in LAM-resistant CHB patients, and in 2005, it was approved over LAM[29]. In 2006, a synthetic thymidine nucleoside analogue named telbivudine (LdT) was approved but its activity was relatively low and was rapidly replaced. In 2015, the prodrug of TDF, tenofovir alafenamide (TAF), was approved for older patients with lower eGFR or lower bone mineral density[30,31].
The safety profile of NA is very good even in HBV-infected patients with decompensated liver disease, liver transplants, extrahepatic manifestations, acute hepatitis, or severe exacerbation[32,33]. However, NA inhibits the HBV polymerase activity only during the time of administration. Long-term therapy with NA can produce a decline in bone mineral density and renal tubular dysfunction[34].
TDF and ETV have renal metabolism and must be adjusted if patients have an estimated glomerular filtration rate (eGFR) of less than 50 mL/min per 1.73 m2. A multicenter retrospective study including 6189 CHB patients treated with TDF (n = 2482) or ETV (n = 3707) showed that TDF was associated with a higher risk of worsening renal function (adjusted HR 1.26, 95%CI 1.11-1.43)[35]. A systematic review and meta-analysis showed that TDF and ETV can affect renal function and patients with cirrhosis under TDF had a higher risk of renal damage and hypophosphatemia[36]. The effects of TDF on bone mineral density are suspected to be related to an increased tubular phosphate metabolism, but with few clinical implications as they appear to be reversible after withdrawal[34]. Recently, it has been shown an improvement in bone density and renal function in patients previously treated with TDF after switching to TAF[37]. However, TAF is not approved in very low eGFR (below 15 mL/min per 1.73 m2).
Even if side effects of NA are scarce, CHB patients usually start treatment at young ages, and with NA treatment, they may be treated for decades. Therefore, questions on NA treatment duration and withdrawal have been gaining interest in recent years.
Classically, three viral markers have been used to characterize HBV infection: (1) The qualitative HBsAg; (2) the HBeAg; and (3) the HBV-DNA. However, most patients under NA treatment have undetectable HBV-DNA and normal alanine aminotransferase (ALT). Therefore, other biomarkers are needed to monitor antiviral activity. The quantified HBsAg is one of the most extensively studied biomarkers because HBsAg negativization, spontaneously or with treatment, has been related to a good prognosis[38]. It has been shown that the decline in HBsAg levels during NA therapy is very slow (around 0.1 Log IU/mL per year)[39-41] suggesting that it would take decades, even long life, to achieve the HBsAg loss[42,43]. Importantly, some patients have shown a faster and larger HBsAg decline during NA therapy achieving low HBsAg levels[41,43]. Importantly, low HBsAg levels after NA withdrawal (below 1000 in Caucasian and below 100 in Asian patients) can be predictors of HBsAg seroclearance[44-47].
The NA can be classified as low (LAM, ADV, and LdT) or high (ETV, TDF, and TAF) barrier of resistance. Current international guidelines recommend the use of NA with a high barrier of resistance to prevent the progression of liver disease, decompensation of cirrhosis, the need for liver transplantation, the development of HCC and to improve survival[9-11].
The suppression of HBV-DNA to undetectable levels (virological response) is normally associated with a normalization of transaminases (biochemical response) and an improvement in survival. In the presence of cirrhosis, all patients with detectable HBV-DNA should be treated[9-11]. Indications for HBeAg-positive and HBeAg-negative CHB patients’ treatment are generally based on the combination of three main criteria: Liver disease severity, HBV-DNA units, and ALT levels[9-11].
In HBeAg-positive CHB patients, long-term NA therapy can induce HBeAg loss and seroconversion, leading to a low replicative phase with partial immune control[9-11]. In these patients, antiviral treatment with ETV has demonstrated a 5 years cumulative probability with a virological response of 99%, HBeAg loss of 53%, but HBsAg loss of only 1.4%[48]. Similarly, TDF for 10 years has demonstrated an HBeAg loss of 52% but only a 4.9% of HBsAg loss[33].
In HBeAg-negative patients, the 5-year cumulative probability with ETV of virological response was 96%, although HBsAg loss was only achieved in 4.6%[32]. In the TDF registry study with patients treated for 10 years, 100% achieved a virological response, and 83% biochemical response, but only 3.4% succeeded in the HBsAg loss[33].
Similar rates of virological response have been described for TAF in HBeAg-positive[30] and HBeAg-negative patients[31].
This high efficacy in controlling viral replication causes histological and elastographic improvement. The long-term treatment with NA has shown a significant regression of liver fibrosis and even cirrhosis. A study with paired liver biopsies, after a median time of 6 years, in 57 patients receiving ETV[49] showed histological improvement (decrease ≥ 2 points in the Knodell necroinflammatory score) in 96% of patients, and fibrosis improvement (decrease ≥ 1 point in the Ishak fibrosis score) in 88% of them. In a trial with TDF[50], including 348 patients with paired liver biopsies (at baseline and week 240), 87% showed histological improvement and 51% fibrosis regression (decrease ≥ 1 point in the Ishak fibrosis score). Among the 96 patients with cirrhosis at baseline, 74% achieved some degree of fibrosis regression.
A decrease in liver stiffness measurement (LSM) after long-term treatment with NA has been described[51]. After treatment initiation, the LSM decline is faster mainly related to the improvement in necroinflammatory activity. After 6 mo of therapy, the following LSM decrease is slower and could be associated with a true improvement of fibrosis[52,53].
The most important outcome of antiviral therapy is to improve survival. Studies comparing untreated and ETV-treated patients demonstrated that antiviral therapy reduced the incidence of liver-related complications, HCC, and mortality[54,55]. Similarly, a multicenter study in TDF-treated patients with cirrhosis showed a reduced risk of developing, clinical decompensation, HCC or liver transplantation, and death compared to untreated patients[56]. However, patients treated with NA, are still in danger of developing HCC, and high-risk patients stratified by risk scores such as REACH-B or PAGE-B, should continue HCC surveillance[57].
In recent years there has been a debate about the effect of the two main NA on HCC risk. Some data suggest that TDF is associated with significantly lower HCC risk compared to ETV in Asian patients[58]. A Chinese study including 29350 patients, showed a reduced HCC incidence in patients receiving TDF. However, there were differences in HCC risk factors between groups: TDF-treated patients were younger, and more frequently HBeAg-positive, females, without cirrhosis, and without diabetes. Thus, only 1% of patients with cirrhosis had received TDF[59]. Similarly, a recent meta-analysis of individual data including 42939 patients receiving TDF (n = 6979) or ETV (n = 35960) monotherapy, showed a lower risk of HCC with TDF, especially in patients older ≥ than 50, males, HBeAg-positive, and non-diabetic subgroups[60]. In contrast, other studies have failed to demonstrate this association[61,62]. A large study including 1935 Caucasian patients from the PAGE-B cohort did not find differences in 5-year cumulative HCC incidence between ETV (5.4%) or TDF (6.0%) after a median follow-up of 7.5 years[62].
The “functional” cure defined as the HBsAg loss, with or without seroconversion of antibody against HBsAg (anti-HBs), is considered the optimal goal for antiviral treatments[10,38]. However, even if third-generation NA are drugs with a high barrier to HBV resistance, and high efficacy, achieving in most patients a virological and biochemical response, the HBsAg seroclearance is anecdotic[63,64]. Table 1 summarizes the efficacy of available treatments to achieve the HBsAg loss.
| Ref. | Treatment (duration) | HBeAg | Ethnicity | Follow-up | HBsAg loss (%) |
| Current antiviral treatments | |||||
| Lau et al[84], 2005 | Peg-IFNα (1 yr) | + | Mainly Asian | 6 mo | 2.9 |
| Marcellin et al[85], 2013 | Peg-IFNα (1 yr) | - | Asian | 60 mo | 12.0 |
| Chang et al[48], 2010 | ETV (5 yr) | + | Asian | - | 1.4 |
| Ahn et al[32], 2016 | ETV (5 yr) | - | Mixed | - | 4.6 |
| Marcellin et al[33], 2019 | TDF (10 yr) | + | Mixed | - | 4.9 |
| Marcellin et al[33], 2019 | TDF (10 yr) | - | Mixed | - | 3.4 |
| Chan et al[30], 2016 | TAF (3 yr) | + | Mixed | - | 4.0 |
| Buti et al[31], 2016 | TAF (3 yr) | - | Mixed | - | 3.0 |
| Combined treatments | |||||
| Marcellin et al[86], 2016 | Peg-IFN + TDF (1 yr) | +/- | Mixed | 18 mo | 9.1 |
| Bourlière et al[87], 2017 | ETV, TDF, ADV, and LAM (1 yr) | - | Caucasian | 12 mo | 7.8 |
| Lim et al[88], 2022 | NA add-on Peg-IFN (1 yr) | +/- | Asian | 6 mo | 12.1 |
| Mo et al[89], 2022 | NAs (HBsAg < 1500 IU/mL, 1 yr) | - | Asian | 48 mo | 33.2 |
| Lim et al[88], 2022 | NA switch to Peg-IFN (1 yr) | +/- | Asian | 6 mo | 9.7 |
| NA discontinuation | |||||
| Chen et al[90], 2018 | 2.7 yr | + | Asian | 96 mo | 19.6 |
| Song et al[91], 2021 | 2.9 yr | + | Asian | 73 mo | 9.5 |
| Kuo et al[92], 2019 | 3.1 yr | + | Asian | 24 mo | 4.7 (ETV); 0 (TDF) |
| Jeng et al[72], 2018 | 2.9 yr | - | Asian | 36 mo | 13 |
| Chen et al[90], 2018 | 2.9 yr | - | Asian | 96 mo | 33.1 |
| Kuo et al[92], 2019 | 3.1 yr | - | Asian | 36 mo | 10 (ETV); 15 (TDF) |
| Chen et al[93], 2020 | 3.2 yr | - | Asian | 58 mo | 20.8 |
| Song et al[91], 2021 | 2.7 yr | - | Asian | 73 mo | 14.6 |
| Hirode et al[47], 2022 | 3.0 yr | - | Mixed | 17 mo | 15.0 |
In patients without advanced fibrosis, the HBsAg loss is associated with an improvement in survival, and minimal risk of developing cirrhosis, decompensation, or HCC[38]. A recent study of 1972 patients with HBsAg loss, showed an HCC annual incidence of 0.38 per 100 person-years (median follow-up of 5.6 years). The study did not show differences between patients who achieved the functional cure spontaneously or with NA[65]. A systematic review showed lower HCC incidence in patients with functional cure (1.86%) compared to those who did not achieve it (6.56%) (P < 0.001). Cirrhosis, age ≥ 50, and male gender were the major risks for developing HCC after HBsAg seroclearance[66]. However, the 10-year HBsAg loss rate described in a large multicenter cohort (n = 4769) treated with ETV or TDF was only 2.1% with an annual incidence of 0.22%[64].
International guidelines of the main scientific societies recommend different treatment duration with NA. In non-cirrhotic HBeAg-positive CHB patients, NA treatment can be discontinued when HBeAg seroconversion is achieved and after one year of consolidation[9-11]. However, in non-cirrhotic HBeAg-negative patients, the NA treatment duration has been a matter of debate in recent years[39-43,67].
In 2008 the APASL guideline suggested the possibility of discontinuing NA therapy in HBeAg-negative patients after at least 2 years with undetectable HBV-DNA documented on three separate occasions 6 mo apart[68]. This approach in Asian patients was mostly driven by economics and reimbursement policies. Despite this, in 2012, Hadziyannis et al[69] reported in 33 Caucasian patients a high rate of HBsAg seroclearance after stopping ADV (39% at 5.5 years after withdrawal). In 2017, the first randomized controlled trial, the FINITE study, confirmed that the strategy of stopping therapy increased the HBsAg loss rate compared to continuing treatment (19% vs 0%)[70]. Based on these studies, in 2017 EASL guideline introduced the possibility of treatment withdrawal in non-cirrhotic HBeAg-negative CHB patients after 3 years of viral suppression[10]. In the last years, several studies have evaluated this strategy with different results (Table 1). Studies in Asian patients have shown a lower HBsAg loss rate compared to Caucasians. The different distribution of HBV-genotypes (especially genotype D), the inclusion or not of patients with advanced liver disease, and the different retreatment rules could explain these dissimilar results[45,71-73].
One of the important points to be considered before stopping treatment is the selection of the best candidates. The initial stopping rules proposed were based on the duration of treatment and virological suppression[9,10]. It has been suggested that NA withdrawal can be successful, only if the transcriptional activity of cccDNA has been silenced during treatment[74]. Additionally, it has been reported that long-term suppression of HBV-DNA can improve CD8 + T cell functions and restore the capacity of immune control by decreasing NK cell killing of HBV-specific T cells and increasing serum cytokines[44,75-77]. This immune restoration can exert control of viral replication after treatment discontinuation, and a large proportion of patients remain as inactive carriers. Moreover, the recurrence of HBV replication after NA discontinuation can represent a trigger for the immune response[78] that can induce an accelerated HBsAg decline and may be beneficial to eradicate residual cccDNA-containing hepatocytes. Our group has recently observed that HBsAg decline was faster during the first year after NA cessation compared to the on-treatment decline[46].
HBsAg and hepatitis B core-related antigen (HBcrAg) levels have been evaluated as surrogate markers to select the patients with a better outcome after NA cessation. It has been shown that not only HBsAg levels at the EoT but also on-treatment HBsAg kinetics are good predictors of HBsAg loss[79,80]. In a study performed by our group, we showed that on-treatment HBsAg kinetics had a high accuracy to predict the HBsAg loss one year after withdrawal[46]. Patients with an HBsAg decline > 1 Log IU/mL during treatment had a probability of 50% of achieving HBsAg loss one year after discontinuation. A large multicenter multiethnic study (n = 1552), the RETRACT-B study, showed different cut-offs of HBsAg at EoT in Asian and Caucasian patients. HBsAg levels at EoT < 100 IU/mL in Asian and < 1000 IU/mL in Caucasian patients were associated with a 4-year HBsAg loss probability of 33% and 41%, respectively[47]. Regarding levels of HBcrAg, the CREATE study, including 572 patients with NA discontinuation, showed lower HBcrAg levels at EoT in those with a virological response and HBsAg loss. Patients with undetectable HBcrAg (< 2 Log U/mL) at EoT showed a higher virological response and HBsAg loss rates one year after withdrawal (65% and 12%, respectively). The same group confirmed in 1216 patients that non-Asian ethnicity, HBsAg levels < 100 IU/mL, and undetectable HBcrAg were associated with the highest HBsAg loss rate[81].
Another important point is to define standardized re-treatment criteria. Some authors recommend delaying retreatment, to allow patients with a “beneficial” flare to achieve a functional cure[82]. However, other authors suggested that re-treatment should be initiated in response to significant increases in serum HBV-DNA, regardless of other liver parameters as cases of fulminant hepatitis have been reported[83]. Table 1 summarizes the efficacy of NA withdrawal to achieve the HBsAg loss[84-93].
The safety of treatment withdrawal is under discussion because on one hand hepatitis flare after NA cessation can induce hepatic decompensation but on the other hand early initiation of NA retreatment can inhibit the beneficial effect of flare-associated immune activation[47,72,94,95]. A study from Taiwan including 691 patients (44.6% with cirrhosis) showed a clinical relapse in 60.6% (7 developed a hepatic decompensation and 3 died)[72]. Similarly, the RETRACT-B study showed hepatic decompensation in only 1.22% (4.3% in patients with cirrhosis and 0.8% in those without cirrhosis; P < 0.01)[47]. In this study, 7 patients died (0.45%) by hepatic decompensation and 4 (0.25%) due to hepatitis B-associated flare.
Another important issue is the risk of developing HCC. The study of Jeng et al[72] included patients with (n = 308) and without cirrhosis (n = 383). The HCC incidence at 1, and 3 years after treatment discontinuation (0.15% and 1.00% for non-cirrhotic vs 1.30% and 4.00% for patients with cirrhosis) was similar to those during NA therapy (0.08% and 0.30% for non-cirrhotic vs 1.50% and 3.40% for patients with cirrhosis). Similarly, in the RETRACT-B study, the HCC incidence at 48 mo after NA withdrawal was 2.2% in patients with cirrhosis and 0.7% in those without cirrhosis[47]. Therefore, NA withdrawal does not seem to increase HCC rates. However, the tolerable level of circulating HBV DNA during long-term follow-up post-NA withdrawal has not been yet elucidated.
The evidence of increased HBsAg loss rates after NA cessation in some HBeAg-negative CHB patients must be balanced with safety. NA withdrawal should be performed only in centers with expertise in CHB management and in adherent patients willing stricter control after carefully discussing the pros and cons[74,96,97].
Some recent studies have evaluated, with contradictory results, the use of NA in patients who do not meet strict criteria for treatment. On one hand, two retrospective studies in HBeAg-positive HBV-infected patients from Korea showed a lower risk of HCC and cirrhosis in those treated compared to untreated controls, suggesting a benefit of starting NA treatment at earlier phases[98,99]. Similarly, another Asian study including “minimally active” carriers showed a high risk of HCC (HR 9.9; 95%CI, 1.239-76.923; P = 0.031) when compared to inactive carriers[100]. But, the study included HBeAg-positive patients and those with a LSM > 9 kPa[101].
On the other hand, some European studies have not demonstrated significant benefits. A study from Italy, including patients without treatment with normal ALT and DNA < 20000 IU/mL, showed a benign course during a follow-up longer than 4 years[102]. Another study including Caucasian patients with HBeAg-negative chronic infection showed a high rate of HBsAg loss (15%) and low transition to CHB (6.3%) in patients with normal ALT and DNA < 2000 IU/mL or in the “grey zone” (HBV-DNA < 2000 IU/mL and ALT 40-80 U/L or, HBV-DNA 2000-20000 IU/mL and ALT < 40 U/L or ALT 40-80 U/L) after a median follow-up of 8.2 years[103]. However, a recent study from the United States has shown that patients without treatment indications, according to AASLD guidelines, had twice the risk of developing cirrhosis and/or HCC when compared to treated patients[104].
Simplifying indications for treatment initiation could be cost-effective and associated with survival improvement[105]. Therefore, it is plausible that antiviral treatment criteria will be expanded in the coming years to achieve the Global Hepatitis Elimination Goals, especially when new therapies with higher HBsAg loss rates will be available[106].
The persistence of the cccDNA in the hepatocytes perpetuates HBV infection. So, detection and measurement of cccDNA levels would be the most precise tool to recognize HBV eradication. However, cccDNA can only be measured in liver biopsy samples by non-standardized or semi-automated techniques[107]. In recent years, there has been an effort to identify new biomarkers that could serve as cccDNA transcriptional activity surrogate markers.
The hepatitis B virus core-related antigen (HBcrAg) is an interesting biomarker composed of the hepatitis B core antigen (HBcAg), the HBeAg, and the 22-KDa pre-core protein (p22cr)[108]. These protein components are translated from cccDNA transcripts reflecting the cccDNA transcriptional activity. The HBcrAg is detected by automated chemiluminescence techniques using specific monoclonal antibodies with a limited sensitivity of 3.0 Log U/mL. The HBcrAg kinetics has been analyzed during NA therapy in a cohort of 222 CHB patients (132 HBeAg-negative and 90 HBeAg-positive) receiving ETV[109]. The decline of HBcrAg levels was 0.244 Log U/mL per year. HBcrAg levels were lower in HBeAg-negative patients compared to HBeAg-positive, but no differences were found between TDF or ETV[109,110]. On the other hand, the persistence of detectable HBcrAg at EoT has been related to severe aminotransferase flares after NA treatment cessation[111]. The main limitations of HBcrAg are its low sensitivity and its low levels after a long-treatment period in HBeAg-negative patients. Therefore, a great proportion of treated HBeAg-negative patients can show undetectable HBcrAg[112]. Recently, a new ultrasensitive assay has been described to improve the HBcrAg sensitivity, but further studies are needed to consider it in clinical practice[113].
The HBV-RNA is a newer marker under evaluation. The pgRNA is packaged in nucleocapsids and serves as the template for reverse transcription to HBV-DNA. In patients not receiving antiviral therapy, serum HBV-RNA levels are much lower than serum HBV-DNA levels. It can be detected in serum samples as pgRNA and spliced pgRNA variants produced by transcription of cccDNA as a marker of intrahepatic cccDNA transcriptional activity[114]. However, serum HBV-RNA is usually quantified by in-house assays and there are no commercial tests available for routine clinical practice. During NA therapy, reverse transcription of pgRNA to HBV-DNA is blocked, but cccDNA transcription to pgRNA persists, and HBV-RNA may be detected in the serum of patients with undetectable HBV-DNA. Therefore, the HBV-RNA could be useful to monitor NA-treated patients[115,116]. Recently, it has been shown that serum HBV-RNA reduced after treatment initiation from 1.46 Logs at week 48 to 1.77 Logs at week 96[117]. On one hand, serum pgRNA levels at wk 4 after NA initiation were correlated with low HBsAg levels (≤ 100 IU/mL) and seroclearance during follow-up[118]. On the other hand, high levels of pgRNA have been associated with viral relapse after NA withdrawal[119].
The recognition of HBsAg composition could be an interesting tool for following CHB patients because it changes across the stages of HBV infection. The main HBsAg component is S-HBs. The L-HBs and M-HBs proportion is lower in the inactive carrier stage compared to those in HBeAg-negative or HBeAg-positive CHB patients[120,121]. Interestingly, L-HBs and M-HBs proportions decrease prior to total HBsAg loss in patients receiving antiviral treatment[122].
Nowadays, the quantitative HBsAg is the most used and widely available biomarker as a consequence of its standardized and commercial tests. It includes the HBsAg produced not only from cccDNA but also by the integrated DNA that is the main source of the HBsAg in HBeAg-negative CHB patients, being more representative of the transcriptional activity. Moreover, quantitative HBsAg has shown a better predictive capacity compared to HBrcAg and HBV-RNA[123].
However, despite the potential utility of these new biomarkers for monitoring NA treatment, their usefulness should still be established for monitoring the new treatment strategies.
After several years without major advances in novel therapies for CHB, currently, a multitude of new molecules are emerging, individually or in combination, as future treatment strategies. The last part of this review summarizes the main groups of evolving molecules evaluated in clinical trials (Table 2) and the combinations that are showing higher rates of sustained HBsAg loss (Table 3).
| Treatment class | Mechanism of action | Types |
| Drugs targeting HBV life cycle | ||
| Entry inhibitors | Blockage of liver-specific bile acid transporter (NTCP) | Inhibitors of NTCP[124]; NMAb[125] |
| Capsid assembly modulators | Interfere with capsid formation and disrupt the encapsidation of pgRNA | CAMs[126] |
| Post-transcriptional control inhibitors | Post-transcriptional gene silencing by inhibition of the translation of viral proteins | SiRNA[127-129]; ASOs[130,131] |
| HBsAg release inhibitors | Intracellular degradation of HBsAg via proteasomal and lysosomal degradation | NAPs[132,133] |
| Immunomodulators | ||
| Innate immune activator | Stimulation of innate immunity through TLRs and RIG-I | TLRs agonist[134-137]; RIG-I agonists[138,139] |
| Adaptive immune activator | Blocking the PD-1/PD-L1 pathway to reverse T-cell exhaustion; stimulation of host’s immune response to generate CD4 and CD8 HBV-specific T cells | Checkpoint inhibitors[140,141]; therapeutic vaccines[142,143] |
| Drug class | Drug | Patients | Time therapy (wk) | Efficacy | Safety |
| NA +/- CAM | NA vs NA + JNJ-6379 (bersacapavir) | 232 | 24-48 | HBsAg decline 0.25 log IU/mL vs 0.41 log IU/mL | No major AE |
| SiRNA +/- NA | AB-729 vs NA + AB-729 | 43 | 8 | HBsAg decline 2.03 log IU/mL monotherapy vs 2.16 log IU/mL combination | Injection site reactions; ALT flares |
| ASO + NA | ASO-GSK3228836 (bepirovirsen) ± NA | 457 | 12-24 | HBsAg < LoQ in 28%-29% and HBsAg loss in 9%-10% after 24 wk of EoT | Injection site reactions; few cases of grade 3-4 ALT flares |
| Inhibitor of NTCP + Peg-IFN | Bulevirtide + Peg-IFN in HDV-HBV co-infection | 90 | 48 | HBsAg loss 26.7% in one arm vs 0% in the other | Related to Peg-IFN; injection site reactions |
| NA + TLR agonists | NA + TLR7 agonist (vesatolimod, GS-9620) | 162 | 24 | No changes in HBsAg | Some grade 3 AE with higher doses (few treatment discontinuations) |
| NA + TLR agonists | NA + TLR8 agonist (selgantolimod) | 48 | 24 | HBsAg loss 5% at week 48 | Mild and transient gastrointestinal AE |
| NA + checkpoint inhibitors | NA + PD-1 inhibitor (nivolumab) | 12 | 1 dose (24 follow-up) | HBsAg reduction 0.48 log IU/mL (HBsAg loss in 5%) | No major AE |
| NA + checkpoint inhibitors | NA + PD-L1 inhibitor (ASC22, Menvafolimab) | 48 | 24 | HBsAg decline 0.38 log IU/mL (HBsAg loss in 19%) | Grade 1 and 2 ALT flares |
| NA + SiRNA +/- CAM | NA + JNJ-3989 (siRNA) + NA ± JNJ-6379 (CAM) | 117 | 48 | HBsAg decline 2.1 log IU/mL in double vs 1.8 log IU/mL in triple combination | No major AE |
| NAP + NA + Peg-IFN | REP2139 or REP 2165 + NA + Peg-IFN | 40 | 48 | HBsAg loss in 35% and HBsAg < 100 IU/mL in 75% | Related to Peg-IFN |
| SiRNA + NA +/- Peg-IFN | VIR 2218 (siRNA) + NA +/- Peg-IFN | 80 | 24 | HBsAg decline 2.03 log IU/mL in dual arm vs 2.55 log IU/mL in triple arm (HBsAg < 100 IU/mL in 95% and HBsAg < 10 IU/mL in 55%) | Related to Peg-IFN |
Several drugs are being evaluated in phase II clinical trials. These new molecules can be classified into two main groups according to their mechanism of action: (1) Molecules targeting different steps in the HBV life cycle; and (2) those acting as immunomodulators.
Molecules targeting different steps in the HBV life cycle are summarized according to their treatment class, mechanism of action, and type in Table 2[124-143].
HBV entry inhibitors protect naïve hepatocytes from infection. However, they do not directly target cccDNA. The recent identification of liver-specific bile acid transporter, the NTCP, has allowed exploring new therapeutics to block viral entry and reduce viral spread[144]. Bulevirtide consists of the preS1 domain of the large surface protein, which inhibits NTCP and prevents viral entry. In the phase 2 study, Bulevirtide monotherapy was evaluated in patients with CHB co-infected with the hepatitis D virus (HDV)[124]. HBV-DNA declined by > 1 Log in 32% of patients, although HBsAg was not affected. Its low effect on HBsAg levels made most studies focus on long-term treatment for HBV/HDV co-infected patients.
The primary mechanism of action of nucleocapsid assembly modulators (CAMs) is to drive the nucleocapsid misassemble. These molecules interfere with capsid formation and disrupt the encapsidation of pgRNA. They have been classified into two main families (Class I and Class II). These agents have been shown to decrease serum HBV-DNA and RNA levels, although declines in HBsAg levels are negligible. Moreover, it has been shown HBV-RNA and HBcrAg rebounded after discontinuation of treatment[126]. Due to these limitations, to date, the role of CAMs in future treatments for CHB must be defined.
RNA molecules, both pgRNA, and mRNAs encoding viral proteins, are essential for HBV replication. Small interfering RNA (SiRNA) is a specific post-transcriptional gene silencing mechanism that can inhibit the translation of viral proteins needed for cccDNA formation. SiRNA is 21-23 nucleotide duplexes processed from large double-stranded RNAs[145]. Chemically synthesized short RNA duplexes were shown to be capable of gene silencing in vitro without inducing an interferon response[146]. Different anti-HBV siRNA are being tested in clinical trials. Available clinical data are promising and show that siRNAs are safe and well-tolerated[127]. A study of siRNA therapy that targeted the common 3’ ends of all HBV transcripts showed that while the treatment markedly reduced HBsAg levels in HBeAg-positive patients, it had minimal effects in HBeAg-negative patients[128].
Antisense nucleotides (ASOs) are single-stranded DNAs (8-10 nucleotides) that target HBV mRNA sequences, forming a DNA-RNA hybrid that is rapidly degraded by cytoplasmic ribonuclease-H[147]. Early results of ASOs, alone or in combination, have shown a profound HBsAg decline[130]. The B-Clear Study, a phase 2b clinical trial evaluating bepirovirsen 300 mg subcutaneously once weekly for 24 wk, showed undetectable HBsAg in 9% of patients who received bepirovirsen plus NA and in 10% who received bepirovirsen alone[131].
HBsAg plays a major role in HBV infection and is the most abundant HBV antigen. Therefore, blocking its release could potentially prevent the release of new virions, decline the number of subviral particles and restore the HBV-specific immune response[148]. Nucleic acid polymers (NAPs) are amphipathic phosphorothioate oligonucleotides. The antiviral effect of NAPs against HBV infection is the inhibition of HBsAg release, although the exact mechanism is unknown, resulting in rapid clearance of HBsAg[132]. In clinical studies, NAP monotherapy was accompanied by rapid HBsAg decline and seroconversion. Recently, NAP-based combination therapy with TDF and Peg-IFN achieved a functional cure in 35% of patients[133] Interestingly, in HBeAg-negative patients, HBsAg produced by integrated DNA was lost. However, more than 90% of patients experienced immune-mediated transaminase flares during therapy[132].
Immunomodulator molecules are summarized according to their treatment class, mechanism of action, and type in Table 2.
The impaired immune response is one of the main barriers to eliminating HBV. T cell immune exhaustion due to the presence of high amounts of HBsAg and a defective response of the innate immune response has a role in HBV persistence. Thus, treatment strategies to restore HBV-specific immune responses with immunomodulatory therapies have been evaluated to achieve viral clearance.
Interferon was the first drug approved for CHB treatment. Despite the long use of this treatment, the exact mechanism of action is still unclear. It has been suggested that Peg-IFN has two main effects, acting on the transcriptional activity of cccDNA and also an immunomodulatory effect, enhancing both innate and adaptive immunity[149,150]. Due to its hypothetical immunomodulatory effects, the synergistic effects of combination with NA and Peg-IFN have been evaluated.
A recent meta-analysis found that NA and Peg-IFN combination improved HBsAg seroclearance rates (RR: 4.52, 95%CI: 1.95-10.47 for adding-on and RR: 12.15, 95%CI 3.99-37.01 for switching-to) compared to NA monotherapy. One of the strongest predictors of HBsAg seroclearance was the HBsAg level at the start of IFN therapy[151]. Our group also showed that the addition of Peg-IFN to NA produced a faster and larger decrease in HBsAg levels compared to NA monotherapy, especially in those patients with IL28CC polymorphism[112]. A better Peg-IFN response in patients with IL28CC polymorphism has been described especially in those infected with genotype D[152,153]. More recently, a Chinese real-world study (n = 3988), the Everest Project (NCT04035837), has reported that the addition of Peg-IFN alpha 2b to NA in HBeAg-negative patients achieved a 4-year HBsAg loss rate of 33%[89].
The main concerns on the use of Peg-IFN are side effects, poor tolerability, and major contraindication such as decompensated cirrhosis. These explain the preference to use NA in routine clinical practice. However, in selected patients with good tolerance, the adding-on or switching-to strategy may improve the HBsAg seroclearance. Indeed, some of the phase 2 trials evaluating new antivirals, have included concomitant treatment with Peg-IFN and have shown better outcomes.
HBV can be recognized by receptors such as toll-like receptors (TLRs)[154] and retinoic acid-inducible gene-1 (RIG-1)[155] TLRs are receptors that initiated intracellular pathways and induce antiviral molecules such as interferons, and cytokines. It has been reported that the activation of TLR-mediated pathways results in the suppression of HBV replication. An oral TLR 7 (TLR7) agonist, GS9620, has shown a decrease in serum HBV-DNA and HBsAg in chimpanzees[134]. However, a phase IIb trial in humans, showed a minimal decrease in HBsAg levels, despite the increase in cytokines and NK cell activity[135,136]. A more recent trial has shown that the combination of selgantolimod, a TLR8 agonist, with a NA resulted in a minimal reduction of HBsAg levels[137]. RIG-I constitutes an intracytoplasmic receptor that interacts with RNA viruses. Once activated, it leads to signal transduction through protein kinase complexes and activation of transcription factors that migrate to the nucleus and activate interferon-stimulable genes leading to the production of IFN[138]. Inarigivir activates the RIG-I pathway. Although early studies reported a reduction in HBV-DNA levels, there were cases of severe hepatotoxicity, with multiple cases of acute liver failure and one death, that lead to its discontinuation[139].
The immune system of HBV-infected patients shows high levels of programmed death receptor 1 (PD-1)-expressing CD8 T cells and high levels of programmed cell death ligand 1 (PD-L1) expression. This suggests that PD-L1 blockade could be a suitable strategy to treat chronic HBV infection. Furthermore, anti-PD-L1 blockade increases the number of IFN-γ-producing T-cells and the amount of IFN-γ produced per cell[156]. Checkpoint inhibitors against PD-1 and PD-L1 have been shown to be effective in several malignancies by reversing T-cell exhaustion and restoring immunity. However, the efficacy to achieve HBsAg clearance has been disappointing. In the CheckMate 040 trial, nivolumab increased HBV-DNA 1 Log from baseline in 10% of HBsAg-positive patients[140]. PD-1 blockade showed limited antiviral activity, and no patient exhibited HBsAg seroconversion[141].
HBV entry can be interrupted by small compounds such as neutralizing monoclonal antibodies (NMAb). In a phase 2 study, it has been evaluated the combination of NMAb VIR-3434 with small interfering RNA (SiRNA) VIR-2218 for up to 20 wk. Combination treatment was generally well tolerated with no serious adverse events. The majority of patients achieved low levels of HBsAg (< 10 IU/mL) but any patient lost the HBsAg during the study[125].
These immunogenic molecules are developed to stimulate the host immune response generating CD4 and CD8 HBV-specific T cells to suppress viral replication and HBsAg loss. Early studies in humans showed a small decline in HBsAg[142]. However, several new therapeutic vaccines are in development probably to be used in combination with other drugs such as SiRNA[143].
In recent years, a better understanding of the immune response to HBV has emerged with new therapeutic possibilities. T cell receptor (TCR)- redirected T cell and chimeric antigen receptor (CAR) T cell involve genetic engineering to confer specificity in front of HBV. CAR-T cells are transduced with an antibody-like receptor to recognize HBsAg on the surface of infected hepatocytes whereas TCR-redirected T cells respond to HBV peptides[157]. However, the investigation in this field is still under development and is being tested in Phase I studies evaluating HBV-infected patients with HCC[158,159].
None of the new previously described molecules can achieve significant sustained HBsAg loss if used alone. Therefore, the most advisable strategy seems to be the combination of molecules with different mechanisms of action[160]. Table 3 summarizes the efficacy of the evolving combinations to achieve sustained HBsAg loss.
Long-term treatment for CHB has been used for decades, showing a good safety profile and high efficacy in controlling viral replication, improving histology, and decreasing the incidence of HCC, clinical decompensation, and mortality. However, the persistence of cccDNA and the low probability of HBsAg seroclearance with NA therapy made necessary indefinite treatment in a vast majority of patients. With the new insights into the role of the immune system in HBV infection persistence and the knowledge of the different viral cycle phases, there has been an increase in new therapeutic approaches in recent years. Moreover, several clinical trials evaluating new drugs with different mechanisms of action are ongoing with promising results. It is expected that in the coming years, there will be a paradigm shift in the treatment of CHB, as we have already seen in chronic hepatitis C, and functional cure in an important number of patients seems closer than ever. Meanwhile, an effort to improve diagnostic rates, and to assure access to treatment for all patients who need it must be urgently done.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, India; Korkmaz P, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Millman I, Loeb LA, Bayer ME, Blumberg BS. Australia antigen (a hepatitis-associated antigen): purification and physical properties. J Exp Med. 1970;131:1190-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013;10:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 3. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 415] [Article Influence: 138.3] [Reference Citation Analysis (0)] |
| 4. | Dane DS, Cameron CH, Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970;1:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 560] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Almeida JD, Rubenstein D, Stott EJ. New antigen-antibody system in Australia-antigen-positive hepatitis. Lancet. 1971;2:1225-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 178] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Magnius LO, Espmark A. A new antigen complex co-occurring with Australia antigen. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Robinson WS, Clayton DA, Greenman RL. DNA of a human hepatitis B virus candidate. J Virol. 1974;14:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | lumberg BS. Hepatitis B: The Hunt for a Killer Virus: Princeton: Princeton University Press, 2003. |
| 9. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 10. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 11. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2844] [Article Influence: 406.3] [Reference Citation Analysis (0)] |
| 12. | Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 539] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 13. | Colpitts CC, Verrier ER, Baumert TF. Targeting Viral Entry for Treatment of Hepatitis B and C Virus Infections. ACS Infect Dis. 2015;1:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Verrier ER, Colpitts CC, Sureau C, Baumert TF. Hepatitis B virus receptors and molecular drug targets. Hepatol Int. 2016;10:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Rabe B, Vlachou A, Panté N, Helenius A, Kann M. Nuclear import of hepatitis B virus capsids and release of the viral genome. Proc Natl Acad Sci U S A. 2003;100:9849-9854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 16. | Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64:S84-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 710] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 17. | Tong S, Revill P. Overview of hepatitis B viral replication and genetic variability. J Hepatol. 2016;64:S4-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 18. | Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 625] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 19. | Hu J, Liu K. Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 214] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 20. | Zoulim F, Testoni B, Lebossé F. Kinetics of intrahepatic covalently closed circular DNA and serum hepatitis B surface antigen during antiviral therapy for chronic hepatitis B: lessons from experimental and clinical studies. Clin Gastroenterol Hepatol. 2013;11:1011-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Lebossé F, Testoni B, Fresquet J, Facchetti F, Galmozzi E, Fournier M, Hervieu V, Berthillon P, Berby F, Bordes I, Durantel D, Levrero M, Lampertico P, Zoulim F. Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol. 2017;66:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Greenberg HB, Pollard RB, Lutwick LI, Gregory PB, Robinson WS, Merigan TC. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med. 1976;295:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 320] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Wong DK, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 705] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 24. | Manesis EK, Hadziyannis SJ. Interferon alpha treatment and retreatment of hepatitis B e antigen-negative chronic hepatitis B. Gastroenterology. 2001;121:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, Liaw YF, Xie Q, Heathcote EJ, Chan HL, Janssen HL. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 26. | Benhamou Y, Dohin E, Lunel-Fabiani F, Poynard T, Huraux JM, Katlama C, Opolon P, Gentilini M. Efficacy of lamivudine on replication of hepatitis B virus in HIV-infected patients. Lancet. 1995;345:396-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 28. | van Bömmel F, Wünsche T, Mauss S, Reinke P, Bergk A, Schürmann D, Wiedenmann B, Berg T. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Petersen J, Buti M. Considerations for the long-term treatment of chronic hepatitis B with nucleos(t)ide analogs. Expert Rev Gastroenterol Hepatol. 2012;6:683-93; quiz 694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Chan HL, Fung S, Seto WK, Chuang WL, Chen CY, Kim HJ, Hui AJ, Janssen HL, Chowdhury A, Tsang TY, Mehta R, Gane E, Flaherty JF, Massetto B, Gaggar A, Kitrinos KM, Lin L, Subramanian GM, McHutchison JG, Lim YS, Acharya SK, Agarwal K; GS-US-320-0110 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 31. | Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL, Acharya SK, Flaherty JF, Massetto B, Cathcart AL, Kim K, Gaggar A, Subramanian GM, McHutchison JG, Pan CQ, Brunetto M, Izumi N, Marcellin P; GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 32. | Ahn J, Lee HM, Lim JK, Pan CQ, Nguyen MH, Ray Kim W, Mannalithara A, Trinh H, Chu D, Tran T, Min A, Do S, Te H, Reddy KR, Lok AS. Entecavir safety and effectiveness in a national cohort of treatment-naïve chronic hepatitis B patients in the US - the ENUMERATE study. Aliment Pharmacol Ther. 2016;43:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Marcellin P, Wong DK, Sievert W, Buggisch P, Petersen J, Flisiak R, Manns M, Kaita K, Krastev Z, Lee SS, Cathcart AL, Crans G, Op den Brouw M, Jump B, Gaggar A, Flaherty J, Buti M. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liver Int. 2019;39:1868-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 34. | Casado JL, Santiuste C, Vazquez M, Bañón S, Rosillo M, Gomez A, Perez-Elías MJ, Caballero C, Rey JM, Moreno S. Bone mineral density decline according to renal tubular dysfunction and phosphaturia in tenofovir-exposed HIV-infected patients. AIDS. 2016;30:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Mak LY, Hoang J, Jun DW, Chen CH, Peng CY, Yeh ML, Kim SE, Huang DQ, Jeong JY, Yoon E, Oh H, Tsai PC, Huang CF, Ahn SB, Trinh H, Xie Q, Wong GLH, Enomoto M, Shim JJ, Lee DH, Liu L, Kozuka R, Cho YK, Jeong SW, Kim HS, Trinh L, Dao A, Huang R, Hui RW, Tsui V, Quek S, Khine HHTW, Ogawa E, Dai CY, Huang JF, Cheung R, Wu C, Chuang WL, Lim SG, Yu ML, Yuen MF, Nguyen MH. Longitudinal renal changes in chronic hepatitis B patients treated with entecavir versus TDF: a REAL-B study. Hepatol Int. 2022;16:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: A systematic review and Meta-analysis. Int Immunopharmacol. 2017;42:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Fong TL, Lee BT, Tien A, Chang M, Lim C, Ahn A, Bae HS. Improvement of bone mineral density and markers of proximal renal tubular function in chronic hepatitis B patients switched from tenofovir disoproxil fumarate to tenofovir alafenamide. J Viral Hepat. 2019;26:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, Donaldson E, Westman G, Buchholz S, Miller V, Hansen BE. Association Between Seroclearance of Hepatitis B Surface Antigen and Long-term Clinical Outcomes of Patients With Chronic Hepatitis B Virus Infection: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 39. | Boglione L, D'Avolio A, Cariti G, Gregori G, Burdino E, Baietto L, Cusato J, Ghisetti V, De Rosa FG, Di Perri G. Kinetics and prediction of HBsAg loss during therapy with analogues in patients affected by chronic hepatitis B HBeAg negative and genotype D. Liver Int. 2013;33:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Zoulim F, Carosi G, Greenbloom S, Mazur W, Nguyen T, Jeffers L, Brunetto M, Yu S, Llamoso C. Quantification of HBsAg in nucleos(t)ide-naïve patients treated for chronic hepatitis B with entecavir with or without tenofovir in the BE-LOW study. J Hepatol. 2015;62:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Broquetas T, Garcia-Retortillo M, Hernandez JJ, Puigvehí M, Cañete N, Coll S, Cabrero B, Giménez MD, Solà R, Carrión JA. Quantification of HBsAg to predict low levels and seroclearance in HBeAg-negative patients receiving nucleos(t)ide analogues. PLoS One. 2017;12:e0188303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Chevaliez S, Hézode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 43. | Seto WK, Wong DK, Fung J, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen levels and hepatitis B surface antigen seroclearance in chronic hepatitis B patients receiving 10 years of nucleoside analogue therapy. Hepatology. 2013;58:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | García-López M, Lens S, Pallett LJ, Testoni B, Rodríguez-Tajes S, Mariño Z, Bartres C, García-Pras E, Leonel T, Perpiñán E, Lozano JJ, Rodríguez-Frías F, Koutsoudakis G, Zoulim F, Maini MK, Forns X, Pérez-Del-Pulgar S. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2021;74:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 45. | Sonneveld MJ, Chiu SM, Park JY, Brakenhoff SM, Kaewdech A, Seto WK, Tanaka Y, Carey I, Papatheodoridi M, van Bömmel F, Berg T, Zoulim F, Ahn SH, Dalekos GN, Erler NS, Höner Zu Siederdissen C, Wedemeyer H, Cornberg M, Yuen MF, Agarwal K, Boonstra A, Buti M, Piratvisuth T, Papatheodoridis G, Chen CH, Maasoumy B; CREATE study group. Probability of HBsAg loss after nucleo(s)tide analogue withdrawal depends on HBV genotype and viral antigen levels. J Hepatol. 2022;76:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 46. | Broquetas T, Hernandez JJ, Garcia-Retortillo M, Canillas L, Puigvehí M, Cañete N, Coll S, Viu A, Garrido E, Mico M, Bessa X, Carrión JA. On-therapy HBsAg kinetics can predict HBsAg loss after nucleos(t)ide analogues interruption in HBeAg-negative patients. The cup is half full and half empty. Dig Liver Dis. 2022;54:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Hirode G, Choi HSJ, Chen CH, Su TH, Seto WK, Van Hees S, Papatheodoridi M, Lens S, Wong G, Brakenhoff SM, Chien RN, Feld J, Sonneveld MJ, Chan HLY, Forns X, Papatheodoridis GV, Vanwolleghem T, Yuen MF, Hsu YC, Kao JH, Cornberg M, Hansen BE, Jeng WJ, Janssen HLA; RETRACT-B Study Group. Off-Therapy Response After Nucleos(t)ide Analogue Withdrawal in Patients With Chronic Hepatitis B: An International, Multicenter, Multiethnic Cohort (RETRACT-B Study). Gastroenterology. 2022;162:757-771.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 127] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 48. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, Zhang H, Tenney DJ, Tamez R, Iloeje U. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 49. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 50. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1369] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 51. | Facciorusso A, Garcia Perdomo HA, Muscatiello N, Buccino RV, Wong VW, Singh S. Systematic review with meta-analysis: Change in liver stiffness during anti-viral therapy in patients with hepatitis B. Dig Liver Dis. 2018;50:787-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Dong XQ, Wu Z, Li J, Wang GQ, Zhao H; China HepB-Related Fibrosis Assessment Research Group. Declining in liver stiffness cannot indicate fibrosis regression in patients with chronic hepatitis B: A 78-week prospective study. J Gastroenterol Hepatol. 2019;34:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Kong Y, Sun Y, Zhou J, Wu X, Chen Y, Piao H, Lu L, Ding H, Nan Y, Jiang W, Xu Y, Xie W, Li H, Feng B, Shi G, Chen G, Zheng H, Cheng J, Wang T, Liu H, Lv F, Shao C, Mao Y, Sun J, Chen T, Han T, Han Y, Wang L, Ou X, Zhang H, Jia J, You H. Early steep decline of liver stiffness predicts histological reversal of fibrosis in chronic hepatitis B patients treated with entecavir. J Viral Hepat. 2019;26:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, Chan HY, Wong VW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 396] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 55. | Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, Wang CC, Su WW, Chen MY, Peng CY, Chien RN, Huang YW, Wang HY, Lin CL, Yang SS, Chen TM, Mo LR, Hsu SJ, Tseng KC, Hsieh TY, Suk FM, Hu CT, Bair MJ, Liang CC, Lei YC, Tseng TC, Chen CL, Kao JH; C-TEAM study group and the Taiwan Liver Diseases Consortium. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 56. | Liu K, Choi J, Le A, Yip TC, Wong VW, Chan SL, Chan HL, Nguyen MH, Lim YS, Wong GL. Tenofovir disoproxil fumarate reduces hepatocellular carcinoma, decompensation and death in chronic hepatitis B patients with cirrhosis. Aliment Pharmacol Ther. 2019;50:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 57. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 58. | Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of Hepatocellular Carcinoma in Patients Treated With Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019;5:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 59. | Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir Is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology. 2020;158:215-225.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 60. | Choi WM, Yip TC, Wong GL, Kim WR, Yee LJ, Brooks-Rooney C, Curteis T, Cant H, Chen CH, Chen CY, Huang YH, Jin YJ, Jun DW, Kim JW, Park NH, Peng CY, Shin HP, Shin JW, Yang YH, Lim YS. Hepatocellular carcinoma risk in patients with chronic hepatitis B receiving tenofovir- vs. entecavir-based regimens: Individual patient data meta-analysis. J Hepatol. 2023;78:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 61. | Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, Nam SW, Bae SH, Choi JY, Yoon SK, Han NI, Jang JW. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020;69:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 62. | Papatheodoridis GV, Dalekos GN, Idilman R, Sypsa V, Van Boemmel F, Buti M, Calleja JL, Goulis J, Manolakopoulos S, Loglio A, Papatheodoridi M, Gatselis N, Veelken R, Lopez-Gomez M, Hansen BE, Savvidou S, Kourikou A, Vlachogiannakos J, Galanis K, Yurdaydin C, Esteban R, Janssen HLA, Berg T, Lampertico P. Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B. J Hepatol. 2020;73:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 63. | Grossi G, Viganò M, Loglio A, Lampertico P. Hepatitis B virus long-term impact of antiviral therapy nucleot(s)ide analogues (NUCs). Liver Int. 2017;37 Suppl 1:45-51. [PubMed] |
| 64. | Hsu YC, Yeh ML, Wong GL, Chen CH, Peng CY, Buti M, Enomoto M, Xie Q, Trinh H, Preda C, Liu L, Cheung KS, Yeo YH, Hoang J, Huang CF, Riveiro-Barciela M, Kozuka R, Istratescu D, Tsai PC, Accarino EV, Lee DH, Wu JL, Huang JF, Dai CY, Cheung R, Chuang WL, Yuen MF, Wong VW, Yu ML, Nguyen MH. Incidences and Determinants of Functional Cure During Entecavir or Tenofovir Disoproxil Fumarate for Chronic Hepatitis B. J Infect Dis. 2021;224:1890-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 65. | Choi J, Yoo S, Lim YS. Comparison of Long-Term Clinical Outcomes Between Spontaneous and Therapy-Induced HBsAg Seroclearance. Hepatology. 2021;73:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Kuang XJ, Jia RR, Huo RR, Yu JJ, Wang JJ, Xiang BD, Li LQ, Peng Z, Zhong JH. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat. 2018;25:1026-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Wong DK, Seto WK, Fung J, Ip P, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency. Clin Gastroenterol Hepatol. 2013;11:1004-10.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S; Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 69. | Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629-636.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 70. | Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, Eisenbach C, Welzel TM, Zachoval R, Felten G, Schulze-Zur-Wiesch J, Cornberg M, Op den Brouw ML, Jump B, Reiser H, Gallo L, Warger T, Petersen J; FINITE CHB study investigators [First investigation in stopping TDF treatment after long-term virological suppression in HBeAg-negative chronic hepatitis B]. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 71. | Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, Petersen J. Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology. 2016;63:1481-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 72. | Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 73. | Chien RN, Liaw YF. Re-treatment for severe hepatitis flare in HBeAg-negative chronic hepatitis B: An appraisal with combined HBsAg/ALT kinetics. J Viral Hepat. 2020;27:544-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Berg T, Lampertico P. The times they are a-changing - A refined proposal for finite HBV nucleos(t)ide analogue therapy. J Hepatol. 2021;75:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 75. | Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, Colombo M, Missale G, Ferrari C. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963-73.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 76. | Höner Zu Siederdissen C, Rinker F, Maasoumy B, Wiegand SB, Filmann N, Falk CS, Deterding K, Port K, Mix C, Manns MP, Herrmann E, Wedemeyer H, Kraft AR, Cornberg M. Viral and Host Responses After Stopping Long-term Nucleos(t)ide Analogue Therapy in HBeAg-Negative Chronic Hepatitis B. J Infect Dis. 2016;214:1492-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 77. | Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, Becht E, Hansi NK, Foster GR, Su TH, Tseng TC, Lim SG, Kao JH, Newell EW, Kennedy PT, Bertoletti A. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest. 2018;128:668-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 78. | Rinker F, Zimmer CL, Höner Zu Siederdissen C, Manns MP, Kraft ARM, Wedemeyer H, Björkström NK, Cornberg M. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 79. | Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, Lee CM. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 80. | Liu J, Li T, Zhang L, Xu A. The Role of Hepatitis B Surface Antigen in Nucleos(t)ide Analogues Cessation Among Asian Patients With Chronic Hepatitis B: A Systematic Review. Hepatology. 2019;70:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 81. | Sonneveld MJ, Park JY, Kaewdech A, Seto WK, Tanaka Y, Carey I, Papatheodoridi M, van Bömmel F, Berg T, Zoulim F, Ahn SH, Dalekos GN, Erler NS, Höner Zu Siederdissen C, Wedemeyer H, Cornberg M, Yuen MF, Agarwal K, Boonstra A, Buti M, Piratvisuth T, Papatheodoridis G, Maasoumy B; CREATE Study Group. Prediction of Sustained Response After Nucleo(s)tide Analogue Cessation Using HBsAg and HBcrAg Levels: A Multicenter Study (CREATE). Clin Gastroenterol Hepatol. 2022;20:e784-e793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 82. | van Bömmel F, Berg T. Risks and Benefits of Discontinuation of Nucleos(t)ide Analogue Treatment: A Treatment Concept for Patients With HBeAg-Negative Chronic Hepatitis B. Hepatol Commun. 2021;5:1632-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 83. | Agarwal K, Lok J, Carey I, Shivkar Y, Biermer M, Berg T, Lonjon-Domanec I. A case of HBV-induced liver failure in the REEF-2 phase II trial: Implications for finite treatment strategies in HBV 'cure'. J Hepatol. 2022;77:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 84. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N; Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1173] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 85. | Marcellin P, Bonino F, Yurdaydin C, Hadziyannis S, Moucari R, Kapprell HP, Rothe V, Popescu M, Brunetto MR. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013;7:88-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 86. | Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou L, Martins EB, Dinh P, Lin L, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HL; Study 149 Investigators. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology. 2016;150:134-144.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 87. | Bourlière M, Rabiega P, Ganne-Carrie N, Serfaty L, Marcellin P, Barthe Y, Thabut D, Guyader D, Hezode C, Picon M, Causse X, Leroy V, Bronowicki JP, Carrieri P, Riachi G, Rosa I, Attali P, Molina JM, Bacq Y, Tran A, Grangé JD, Zoulim F, Fontaine H, Alric L, Bertucci I, Bouvier-Alias M, Carrat F; ANRS HB06 PEGAN Study Group. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 88. | Lim SG, Yang WL, Ngu JH, Chang J, Tan J, Ahmed T, Dan YY, Lim K, Lee YM, Lee GH, Tan PS, Wai KL, Phyo WW, Khine HHTW, Lee C, Tay A, Chan E. Switching to or Add-on Peginterferon in Patients on Nucleos(t)ide Analogues for Chronic Hepatitis B: The SWAP RCT. Clin Gastroenterol Hepatol. 2022;20:e228-e250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 89. | Mo Z, Xie D, Fu L, Zhang W, Wei J, Li G, Yang J, Chen X, Li J, Shang J, Guan Y, Jiang Y, Guo Y, Zeng Y, Chang J, Peng Y, Lin M, Huang G, Gu S, Geng J, Gao Z. Functional cure based on pegylated interferon α-2b therapy in nucleoside analog-suppressed HBeAg negative chronic hepatitis B: a multicenter real-world study (Everest Project in china)-4 years data update. Hepatology. 2022;76:S24-25. |
| 90. | Chen CH, Hung CH, Wang JH, Lu SN, Hu TH, Lee CM. Long-term incidence and predictors of hepatitis B surface antigen loss after discontinuing nucleoside analogues in noncirrhotic chronic hepatitis B patients. Clin Microbiol Infect. 2018;24:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 91. | Song DS, Jang JW, Yoo SH, Kwon JH, Nam SW, Bae SH, Choi JY, Yoon SK. Improving the Prediction of Relapse After Nucleos(t)ide Analogue Discontinuation in Patients With Chronic Hepatitis B. Clin Infect Dis. 2021;73:e892-e903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Kuo MT, Hu TH, Hung CH, Wang JH, Lu SN, Tsai KL, Chen CH. Hepatitis B virus relapse rates in chronic hepatitis B patients who discontinue either entecavir or tenofovir. Aliment Pharmacol Ther. 2019;49:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 93. | Chen CH, Hu TH, Wang JH, Lai HC, Hung CH, Lu SN, Peng CY. Comparison of HBsAg changes between HBeAg-negative patients who discontinued or maintained entecavir therapy. Hepatol Int. 2020;14:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Seto WK, Hui AJ, Wong VW, Wong GL, Liu KS, Lai CL, Yuen MF, Chan HL. Treatment cessation of entecavir in Asian patients with hepatitis B e antigen negative chronic hepatitis B: a multicentre prospective study. Gut. 2015;64:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 174] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 95. | Liaw YF, Jeng WJ, Chang ML. HBsAg Kinetics in Retreatment Decision for Off-Therapy Hepatitis B Flare in HBeAg-Negative Patients. Gastroenterology. 2018;154:2280-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 96. | Liaw YF. Finite nucleos(t)ide analog therapy in HBeAg-negative chronic hepatitis B: an emerging paradigm shift. Hepatol Int. 2019;13:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 97. | Chien RN, Liaw YF. Current Trend in Antiviral Therapy for Chronic Hepatitis B. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 98. | Chang Y, Choe WH, Sinn DH, Lee JH, Ahn SH, Lee H, Shim JJ, Jun DW, Park SY, Nam JY, Cho EJ, Yu SJ, Lee DH, Lee JM, Kim YJ, Kwon SY, Paik SW, Yoon JH. Nucleos(t)ide Analogue Treatment for Patients With Hepatitis B Virus (HBV) e Antigen-Positive Chronic HBV Genotype C Infection: A Nationwide, Multicenter, Retrospective Study. J Infect Dis. 2017;216:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, Lee HC, Lee YS. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 100. | Lee HW, Kim SU, Baatarkhuu O, Park JY, Kim DY, Ahn SH, Han KH, Kim BK. Progression of Untreated Minimally Active Chronic HBV Infection Compared to Inactive Infection. Clin Gastroenterol Hepatol. 2019;17:2808-2810.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Bonacci M, Forns X, Lens S. The HBeAg-Negative "Gray Zone" Phase: A Frequent Condition With Different Outcomes in Western and Asian Patients? Clin Gastroenterol Hepatol. 2020;18:263-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Oliveri F, Surace L, Cavallone D, Colombatto P, Ricco G, Salvati N, Coco B, Romagnoli V, Gattai R, Salvati A, Moriconi F, Yuan Q, Bonino F, Brunetto MR. Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis B virus infection: Benign course towards HBsAg clearance. Liver Int. 2017;37:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Bonacci M, Lens S, Mariño Z, Londoño MC, Rodríguez-Tajes S, Mas A, García-López M, Pérez-Del-Pulgar S, Sánchez-Tapias JM, Forns X. Anti-viral therapy can be delayed or avoided in a significant proportion of HBeAg-negative Caucasian patients in the Grey Zone. Aliment Pharmacol Ther. 2018;47:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 104. | Huang DQ, Lee DH, Le MH, Le A, Yeo YH, Trinh HN, Chung M, Nguyen V, Johnson T, Zhang JQ, Wong C, Li J, Cheung R, Nguyen MH. Liver Complications in Untreated Treatment-Ineligible versus Treated Treatment-Eligible Patients with Hepatitis B. Dig Dis. 2023;41:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 105. | Lim YS, Ahn SH, Shim JJ, Razavi H, Razavi-Shearer D, Sinn DH. Impact of expanding hepatitis B treatment guidelines: A modelling and economic impact analysis. Aliment Pharmacol Ther. 2022;56:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 106. | Jeng WJ, Lok AS. Should Treatment Indications for Chronic Hepatitis B Be Expanded? Clin Gastroenterol Hepatol. 2021;19:2006-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 107. | Xia Y, Stadler D, Ko C, Protzer U. Analyses of HBV cccDNA Quantification and Modification. Methods Mol Biol. 2017;1540:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol. 2019;70:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 217] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 109. | Lam YF, Seto WK, Wong D, Cheung KS, Fung J, Mak LY, Yuen J, Chong CK, Lai CL, Yuen MF. Seven-Year Treatment Outcome of Entecavir in a Real-World Cohort: Effects on Clinical Parameters, HBsAg and HBcrAg Levels. Clin Transl Gastroenterol. 2017;8:e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 110. | Mak LY, Wong DK, Cheung KS, Seto WK, Fung J, Yuen MF. First-line oral antiviral therapies showed similar efficacies in suppression of serum HBcrAg in chronic hepatitis B patients. BMC Gastroenterol. 2021;21:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 111. | Carey I, Gersch J, Wang B, Moigboi C, Kuhns M, Cloherty G, Dusheiko G, Agarwal K. Pregenomic HBV RNA and Hepatitis B Core-Related Antigen Predict Outcomes in Hepatitis B e Antigen-Negative Chronic Hepatitis B Patients Suppressed on Nucleos(T)ide Analogue Therapy. Hepatology. 2020;72:42-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 112. | Broquetas T, Garcia-Retortillo M, Micó M, Canillas L, Puigvehí M, Cañete N, Coll S, Viu A, Hernandez JJ, Bessa X, Carrión JA. Hepatitis B surface antigen and hepatitis B core-related antigen kinetics after adding pegylated-interferon to nucleos(t)ids analogues in hepatitis B e antigen-negative patients. World J Hepatol. 2020;12:1076-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Inoue T, Kusumoto S, Iio E, Ogawa S, Suzuki T, Yagi S, Kaneko A, Matsuura K, Aoyagi K, Tanaka Y. Clinical efficacy of a novel, high-sensitivity HBcrAg assay in the management of chronic hepatitis B and HBV reactivation. J Hepatol. 2021;75:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 114. | Giersch K, Allweiss L, Volz T, Dandri M, Lütgehetmann M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol. 2017;66:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 115. | van Bömmel F, Bartens A, Mysickova A, Hofmann J, Krüger DH, Berg T, Edelmann A. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology. 2015;61:66-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 116. | Butler EK, Gersch J, McNamara A, Luk KC, Holzmayer V, de Medina M, Schiff E, Kuhns M, Cloherty GA. Hepatitis B Virus Serum DNA andRNA Levels in Nucleos(t)ide Analog-Treated or Untreated Patients During Chronic and Acute Infection. Hepatology. 2018;68:2106-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 117. | Mak LY, Cloherty G, Wong DK, Gersch J, Seto WK, Fung J, Yuen MF. HBV RNA Profiles in Patients With Chronic Hepatitis B Under Different Disease Phases and Antiviral Therapy. Hepatology. 2021;73:2167-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 118. | Mak LY, Wong D, Kuchta A, Hilfiker M, Hamilton A, Chow N, Mao X, Seto WK, Yuen MF. Hepatitis B virus pre-genomic RNA and hepatitis B core-related antigen reductions at week 4 predict favourable hepatitis B surface antigen response upon long-term nucleos(t)ide analogue in chronic hepatitis B. Clin Mol Hepatol. 2023;29:146-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 119. | Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, Zhang R, Chen R, Li T, Zhang T, Yuan Q, Li PC, Huang Q, Colonno R, Jia J, Hou J, McCrae MA, Gao Z, Ren H, Xia N, Zhuang H, Lu F. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol. 2016;65:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 120. | Pfefferkorn M, Böhm S, Schott T, Deichsel D, Bremer CM, Schröder K, Gerlich WH, Glebe D, Berg T, van Bömmel F. Quantification of large and middle proteins of hepatitis B virus surface antigen (HBsAg) as a novel tool for the identification of inactive HBV carriers. Gut. 2018;67:2045-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 121. | Peiffer KH, Kuhnhenn L, Jiang B, Mondorf A, Vermehren J, Knop V, Susser S, Walter D, Dietz J, Carra G, Finkelmeier F, Zeuzem S, Sarrazin C, Hildt E. Divergent preS Sequences in Virion-Associated Hepatitis B Virus Genomes and Subviral HBV Surface Antigen Particles From HBV e Antigen-Negative Patients. J Infect Dis. 2018;218:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 122. | Pfefferkorn M, Schott T, Böhm S, Deichsel D, Felkel C, Gerlich WH, Glebe D, Wat C, Pavlovic V, Heyne R, Berg T, van Bömmel F. Composition of HBsAg is predictive of HBsAg loss during treatment in patients with HBeAg-positive chronic hepatitis B. J Hepatol. 2021;74:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 123. | Lim SG, Phyo WW, Ling JZJ, Cloherty G, Butler EK, Kuhns MC, McNamara AL, Holzmayer V, Gersch J, Yang WL, Ngu JH, Chang J, Tan J, Ahmed T, Dan YY, Lee YM, Lee GH, Tan PS, Huang DQ, Khine HTW, Lee C, Tay A, Chan E. Comparative biomarkers for HBsAg loss with antiviral therapy shows dominant influence of quantitative HBsAg (qHBsAg). Aliment Pharmacol Ther. 2021;53:172-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 124. | Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, Lehr T, Lempp FA, Wedemeyer H, Haag M, Schwab M, Haefeli WE, Blank A, Urban S. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 125. | Gane E, Jucov A, Dobryanska M, Yoon KT, Lim TH, Arizpe A, Cloutier D, L Shen, Gupta SV, Lau AH, Hwang C, Lim YS. Safety, tolerability, and antiviral activity of the siRNA VIR-2218 in combination with the investigational neutralizing monoclonal antibody VIR-3434 for the treatment of chronic hepatitis B virus infection: preliminary results from the Phase 2 MARCH trial. Hepatology. 2022;76:S18. |
| 126. | Taverniti V, Ligat G, Debing Y, Kum DB, Baumert TF, Verrier ER. Capsid Assembly Modulators as Antiviral Agents against HBV: Molecular Mechanisms and Clinical Perspectives. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 127. | Hui RW, Mak LY, Seto WK, Yuen MF. RNA interference as a novel treatment strategy for chronic hepatitis B infection. Clin Mol Hepatol. 2022;28:408-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 128. | Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, Ferrari C, Given BD, Hamilton J, Kanner SB, Lai CL, Lau JYN, Schluep T, Xu Z, Lanford RE, Lewis DL. RNAi-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 371] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 129. | Ghany MG, Lok AS. Functional cure of hepatitis B requires silencing covalently closed circular and integrated hepatitis B virus DNA. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 130. | Yuen MF, Heo J, Kumada H, Suzuki F, Suzuki Y, Xie Q, Jia J, Karino Y, Hou J, Chayama K, Imamura M, Lao-Tan JY, Lim SG, Tanaka Y, Xie W, Yoon JH, Duan Z, Kurosaki M, Park SJ, Labio ME, Kumar R, Kweon YO, Yim HJ, Tao Y, Cremer J, Elston R, Davies M, Baptiste-Brown S, Han K, Campbell FM, Paff M, Theodore D. Phase IIa, randomised, double-blind study of GSK3389404 in patients with chronic hepatitis B on stable nucleos(t)ide therapy. J Hepatol. 2022;77:967-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 131. | Yuen MF, Lim SG, Plesniak R, Tsuji K, Janssen HLA, Pojoga C, Gadano A, Popescu CP, Stepanova T, Asselah T, Diaconescu G, Yim HJ, Heo J, Janczewska E, Wong A, Idriz N, Imamura M, Rizzardini G, Takaguchi K, Andreone P, Arbune M, Hou J, Park SJ, Vata A, Cremer J, Elston R, Lukić T, Quinn G, Maynard L, Kendrick S, Plein H, Campbell F, Paff M, Theodore D; B-Clear Study Group. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N Engl J Med. 2022;387:1957-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 132. | Boulon R, Blanchet M, Lemasson M, Vaillant A, Labonté P. Characterization of the antiviral effects of REP 2139 on the HBV lifecycle in vitro. Antiviral Res. 2020;183:104853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 133. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 134. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508-1517, 1517.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 315] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 135. | Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Yoshida EM, Ahn SH, Tsai NCS, Fung S, Gane EJ. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 136. | Boni C, Vecchi A, Rossi M, Laccabue D, Giuberti T, Alfieri A, Lampertico P, Grossi G, Facchetti F, Brunetto MR, Coco B, Cavallone D, Mangia A, Santoro R, Piazzolla V, Lau A, Gaggar A, Subramanian GM, Ferrari C. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology. 2018;154:1764-1777.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 137. | Gane EJ, Kim HJ, Visvanathan K, Kim YJ, Nguyen AH, Wallin JJ, Chen DY, McDonald C, Arora P, Tan SK, Gaggar A, Roberts SK, Lim YS. Safety, Pharmacokinetics, and Pharmacodynamics of the Oral TLR8 Agonist Selgantolimod in Chronic Hepatitis B. Hepatology. 2021;74:1737-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 138. | Alexopoulou A, Vasilieva L, Karayiannis P. New Approaches to the Treatment of Chronic Hepatitis B. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 139. | Yuen MF, Chen CY, Liu CJ, Jeng WJ, Elkhashab M, Coffin CS, Kim W, Greenbloom S, Ramji A, Lim YS, Kim YJ, Fung SK, Kim DJ, Jang JW, Lee KS, Iyer RP, Macfarlane C, Jackson K, Locarnini SA, Chan HLY, Afdhal NH. A phase 2, open-label, randomized, multiple-dose study evaluating Inarigivir in treatment-naïve patients with chronic hepatitis B. Liver Int. 2023;43:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 140. | Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Cruz CD, Kudo M. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 141. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3310] [Article Influence: 413.8] [Reference Citation Analysis (1)] |
| 142. | Lok AS, Pan CQ, Han SH, Trinh HN, Fessel WJ, Rodell T, Massetto B, Lin L, Gaggar A, Subramanian GM, McHutchison JG, Ferrari C, Lee H, Gordon SC, Gane EJ. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 143. | Michler T, Kosinska AD, Festag J, Bunse T, Su J, Ringelhan M, Imhof H, Grimm D, Steiger K, Mogler C, Heikenwalder M, Michel ML, Guzman CA, Milstein S, Sepp-Lorenzino L, Knolle P, Protzer U. Knockdown of Virus Antigen Expression Increases Therapeutic Vaccine Efficacy in High-Titer Hepatitis B Virus Carrier Mice. Gastroenterology. 2020;158:1762-1775.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 144. | Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;205-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 145. | van den Berg F, Limani SW, Mnyandu N, Maepa MB, Ely A, Arbuthnot P. Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 146. | Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6971] [Cited by in RCA: 7022] [Article Influence: 292.6] [Reference Citation Analysis (0)] |
| 147. | Wong GLH, Gane E, Lok ASF. How to achieve functional cure of HBV: Stopping NUCs, adding interferon or new drug development? J Hepatol. 2022;76:1249-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 158] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 148. | Phillips S, Jagatia R, Chokshi S. Novel therapeutic strategies for chronic hepatitis B. Virulence. 2022;13:1111-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 149. | Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, Remouchamps C, Chou WM, Thasler WE, Hüser N, Durantel D, Liang TJ, Münk C, Heim MH, Browning JL, Dejardin E, Dandri M, Schindler M, Heikenwalder M, Protzer U. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 741] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 150. | Nishio A, Bolte FJ, Takeda K, Park N, Yu ZX, Park H, Valdez K, Ghany MG, Rehermann B. Clearance of pegylated interferon by Kupffer cells limits NK cell activation and therapy response of patients with HBV infection. Sci Transl Med. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 151. | Liu J, Wang T, Zhang W, Cheng Y, He Q, Wang FS. Effect of combination treatment based on interferon and nucleos(t)ide analogues on functional cure of chronic hepatitis B: a systematic review and meta-analysis. Hepatol Int. 2020;14:958-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 152. | Lampertico P, Viganò M, Cheroni C, Facchetti F, Invernizzi F, Valveri V, Soffredini R, Abrignani S, De Francesco R, Colombo M. IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis B. Hepatology. 2013;57:890-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 153. | Boglione L, Cusato J, Allegra S, Esposito I, Patti F, Cariti G, Di Perri G, D'Avolio A. Role of IL28-B polymorphisms in the treatment of chronic hepatitis B HBeAg-negative patients with peginterferon. Antiviral Res. 2014;102:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 154. | Du K, Liu J, Broering R, Zhang X, Yang D, Dittmer U, Lu M. Recent advances in the discovery and development of TLR ligands as novel therapeutics for chronic HBV and HIV infections. Expert Opin Drug Discov. 2018;13:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 155. | Chan YK, Gack MU. Viral evasion of intracellular DNA and RNA sensing. Nat Rev Microbiol. 2016;14:360-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 338] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 156. | Ferrando-Martinez S, Huang K, Bennett AS, Sterba P, Yu L, Suzich JA, Janssen HLA, Robbins SH. HBeAg seroconversion is associated with a more effective PD-L1 blockade during chronic hepatitis B infection. JHEP Rep. 2019;1:170-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 157. | Maini MK, Burton AR. Restoring, releasing or replacing adaptive immunity in chronic hepatitis B. Nat Rev Gastroenterol Hepatol. 2019;16:662-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 158. | Meng F, Zhao J, Tan AT, Hu W, Wang SY, Jin J, Wu J, Li Y, Shi L, Fu JL, Yu S, Shen Y, Liu L, Luan J, Shi M, Xie Y, Zhou CB, Wong RW, Lu-En W, Koh S, Bertoletti A, Wang T, Zhang JY, Wang FS. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol Int. 2021;15:1402-1412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 159. | Yang F, Zheng X, Koh S, Lu J, Cheng J, Li P, Du C, Chen Y, Chen X, Yang L, Chen W, Wong RW, Wai LE, Wang T, Zhang Q. Messenger RNA electroporated hepatitis B virus (HBV) antigen-specific T cell receptor (TCR) redirected T cell therapy is well-tolerated in patients with recurrent HBV-related hepatocellular carcinoma post-liver transplantation: results from a phase I trial. Hepatol Int. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 160. | Lim SG, Baumert TF, Boni C, Gane E, Levrero M, Lok AS, Maini MK, Terrault NA, Zoulim F. The scientific basis of combination therapy for chronic hepatitis B functional cure. Nat Rev Gastroenterol Hepatol. 2023;20:238-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 88] [Reference Citation Analysis (0)] |