Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.3932
Peer-review started: December 28, 2022
First decision: February 1, 2023
Revised: February 15, 2023
Accepted: April 30, 2023
Article in press: April 30, 2023
Published online: July 7, 2023
Processing time: 181 Days and 20.2 Hours
Glycogen storage diseases (GSDs), also referred to as glycogenoses, are inherited metabolic disorders of glycogen metabolism caused by deficiency of enzymes or transporters involved in the synthesis or degradation of glycogen leading to aberrant storage and/or utilization. The overall estimated GSD incidence is 1 case per 20000-43000 live births. There are over 20 types of GSD including the subtypes. This heterogeneous group of rare diseases represents inborn errors of carbohydrate metabolism and are classified based on the deficient enzyme and affected tissues. GSDs primarily affect liver or muscle or both as glycogen is particularly abundant in these tissues. However, besides liver and skeletal muscle, depending on the affected enzyme and its expression in various tissues, multiorgan involvement including heart, kidney and/or brain may be seen. Although GSDs share similar clinical features to some extent, there is a wide spectrum of clinical phenotypes. Currently, the goal of treatment is to maintain glucose homeostasis by dietary management and the use of uncooked cornstarch. In addition to nutritional interventions, pharmacological treatment, physical and supportive therapies, enzyme replacement therapy (ERT) and organ transplan
Core Tip: Glycogen storage diseases are multisystemic diseases that can present at any age. Primarily affected organs are liver and skeletal muscle, but heart, central nervous system, kidneys, intestines, and other organs may also be affected. As the initial presenting symptoms can occur in adulthood, it is a group of rare diseases that should be recognized and managed by not only pediatricians but also physicians taking care of adults.
- Citation: Gümüş E, Özen H. Glycogen storage diseases: An update. World J Gastroenterol 2023; 29(25): 3932-3963
- URL: https://www.wjgnet.com/1007-9327/full/v29/i25/3932.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i25.3932
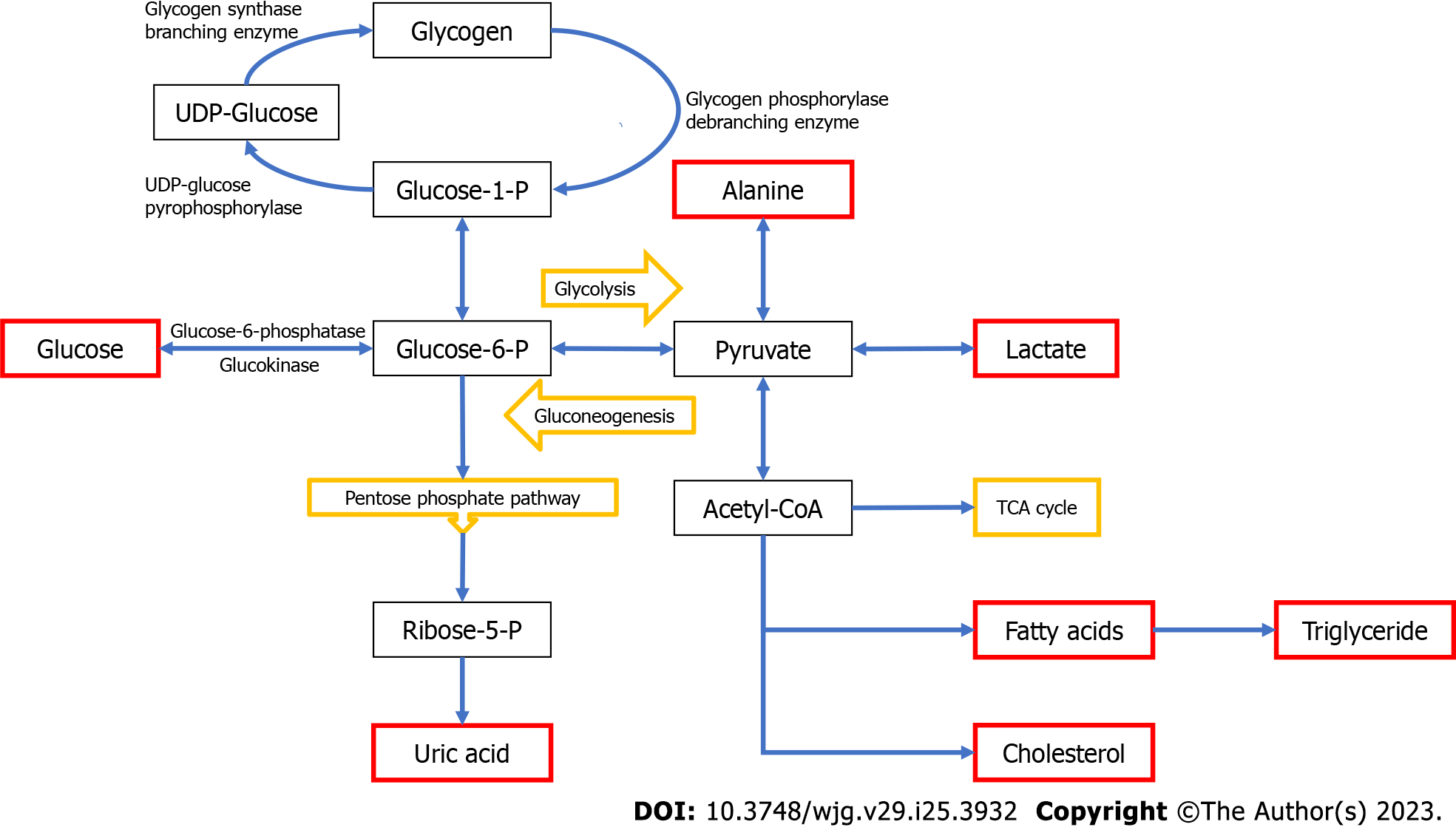
Glycogen storage diseases (GSDs), also referred to as glycogenoses, are inherited metabolic disorders of glycogen metabolism caused by deficiency of enzymes or transporters involved in the synthesis or degradation of glycogen[1]. Disturbances in glycogen metabolism result in aberrant storage and/or utilization of glycogen. Both glycogen formation and breakdown involve several enzymatic reactions and are strictly dependent on hormone regulation (Figure 1)[2]. After a meal, insulin stimulates glycogen storage in muscle and liver by simultaneously promoting glycogen synthesis and inhibiting glycogen breakdown. During exercise or between meals, glucagon and cathecolamines inhibit glycogen synthesis while promoting glycogen breakdown[3]. Hepatic glycogen serves as a depot source of glucose to maintain euglycemia during fasting periods while glycogen in muscle provides glucose to produce necessary energy during high-intensity exertion.
GSDs are multisystemic diseases that can present at any age from the neonatal period to adulthood. The overall GSD incidence is approximately 1 case per 20000-43000 live births and 80% of hepatic GSDs are caused by types I, III, and IX[4,5]. This heterogeneous group of rare diseases represents inborn errors of carbohydrate metabolism and are classified based on the deficient enzyme and affected tissues (Table 1). GSDs primarily affect liver or muscle or both as glycogen is particularly abundant in these tissues. However, besides liver and skeletal muscle, depending on the affected enzyme and its expression in various tissues, multiorgan involvement including heart, kidney and/or brain may be seen[6]. Although GSDs share similar clinical features to some extent, there is a wide spectrum of clinical phenotypes. Hypoglycemia is the hallmark of hepatic GSDs. Hepatomegaly is also a cardinal manifestation of GSDs with liver involvement except for GSD-0. Muscle GSDs, on the other hand, may present with exercise intolerance, muscle cramps/pain, rhabdomyolysis, and muscle weakness and in the case of cardiac involvement, cardiomyopathy[7]. Since the initial presenting symptoms can occur in adulthood, it is a group of rare diseases that should be recognized and managed by not only pediatricians but also physicians taking care of adults. Being multisystemic diseases, GSDs are best managed by a cross-disciplinary approach to achieve good metabolic control, improve the quality of life of patients, and reduce morbidity and mortality[7]. It is recommended that a medical professional with expertise in treating such conditions (e.g., a metabolic disorders specialist, a biochemical geneticist, an endocrinologist, or a hepatologist) should lead and coordinate the patient’s care together with a metabolic dietician. Nephrologists, hematologists, genetic counselors, cardiologists, gastroenterologists, neurologists, physical therapists, social workers, and transplant specialists may also be required in the management of a GSD depending on the specific manifestations, complications, and type of the disease. In this article, we aim to update the review published in 2007[1] based on new data and provide a comprehensive review of GSDs. This review provides general characteristics of all types of GSDs with a focus on those with liver involvement.
| GSD type (eponym) | OMIM# | Defective enzyme or transporter | Gene/inheritance | Gene location | Primary tissue involvement | Distinctive features |
| GSD-0 | GSD-0a: 240600 | Liver glycogen synthase | GYS2/AR | 12p12.1 | Liver | No hepatomegaly. Postprandial hyperglycemia, glycosuria, and hyperlactatemia. Extremely low amount of glycogen in liver tissue |
| GSD-0b: 611556 | Muscle glycogen synthase | GYS1/AR | 19q13.33 | Muscle | Cardiac involvement, risk of sudden cardiac arrest | |
| GSD-I (von Gierke) | GSD-Ia: 232200 | Glucose-6-phosphatase | G6PC/AR | 17q.21 | Liver | Coagulopathy, anemia, osteopenia, osteoporosis, renal dysfunction, HA, HCC |
| GSD-Ib: 232220 | Glucose-6-phosphatase transporter | SLC37A4/AR | 11q23.3 | Liver | Neutropenia, neutrophil dysfunction, recurrent infections, oral and intestinal mucosal ulcers, IBD, autoimmunity | |
| GSD-II (Pompe) | 232300 | Acid α-glucosidase | GAA/AR | 17q25.3 | Muscle | Cardiomyopathy, infantile-onset form. Muscle weakness, late-onset form |
| Danon disease (formerly GSD-IIb) | 300257 | Lysosome-associated membrane protein-2 | LAMP2/XLD | Xq24 | Muscle | Skeletal and cardiac myopathy, arrhythmia, intellectual disability |
| GSD of heart | 600858 | AMP-activated protein kinase, γ-2 regulatory subunit | PRKAG2/AD | 7q36.1 | Muscle | Severe ventricular hypertrophy. Electrocardiographic preexcitation and conduction system disease. Premature sudden cardiac death (< 40 yr) |
| GSD of heart, lethal congenital | 261740 | AMP-activated protein kinase, γ-2 noncatalytic subunit | PRKAG2/AD | 7q36.1 | Muscle | Some mutations (R531Q, R384T) cause more severe phenotype. Fetal onset, extreme cardiomegaly, death in infancy |
| GSD-III (Cori/Forbes) | IIIa/IIIb: 232400 | Glycogen debrancher enzyme | AGL/AR | 1p21.2 | IIIa: Liver + muscle; IIIb: Liver | Liver fibrosis, cirrhosis, HA, HCC (as a complication of cirrhosis). IIIa: Elevated CK, motor developmental delay, myopathy, cardiomyopathy |
| GSD-IV (Andersen) | 232500 | Glycogen branching enzyme | GBE1/AR | 3p12.2 | Liver | Classical hepatic form (rapidly progressive liver disease, HSM, cirrhosis, HCC). Non-progressive hepatic form. Neuromuscular presentation (perinatal, congenital, childhood and adult forms). Myopathy, cardiomyopathy, neuropathy, CNS involvement, APBD. Amylopectin aggregations in liver |
| GSD-V (McArdle) | 232600 | Muscle glycogen phosphorylase | PYGM/AR | 11q13.1 | Muscle | Exercise intolerance, muscle cramps, rhabdomyolysis, myoglobinuria, “second wind” phenomenon |
| GSD-VI (Hers) | 232700 | Liver glycogen phosphorylase | PYGL/AR | 14q22.1 | Liver | Phenotypic variability (overlap with GSD-IX). Severe hepatic involvement reported. Mild hypotonia and cardiopathy reported. Excessive glycogen accumulation with structurally normal glycogen in liver tissue. Enzyme deficiency in erythrocytes, leukocytes |
| GSD-VII (Tarui) | 232800 | Muscle phosphofructokinase | PFKM/AR | 12q13.11 | Muscle | Exertional myopathy, exercise intolerance, muscle cramps, hemolytic anemia. Rapidly progressive infantile form (multisystem involvement, seizures, cardiomyopathy) |
| GSD-IX | GSD-IXa1 (XLG-1): 306000 | Phosphorylase kinase, α-subunit, liver | PHKA2/XLR | Xp22.13 | Liver | The most common subtype. Symptomatic female carriers due to X chromosome inactivation. Clinical symptoms and laboratory abnormalities gradually disappear with age. Severe phenotypes reported |
| GSD-IXb: 261750 | Phosphorylase kinase, β-subunit | PHKB/AR | 16q12.1 | Liver | Marked accumulation of glycogen in both liver and muscle. Muscle symptoms are generally mild or absent | |
| GSD-IXc: 613027 | Phosphorylase kinase, γ-subunit | PHKG2/AR | 16p11.2 | Liver | More severe phenotype with increased risk for liver fibrosis and cirrhosis | |
| GSD-IXd: 300559 | Phosphorylase kinase, α-subunit, muscle | PHKA1/XLR | Xq13.1 | Muscle | Muscle weakness and muscle cramps during exercise. Mostly in adults | |
| GSD-X | 261670 | Muscle phosphoglycerate mutase | PGAM2/AR | 7p13 | Muscle | Exercise intolerance, muscle cramps and pain, rhabdomyolysis, myoglobinuria |
| Fanconi-Bickel syndrome (formerly GSD-XI) | 227810 | Glucose transporter 2 | SLC2A2/AR | 3q26.2 | Liver | Hepatorenal involvement. Proximal renal tubular dysfunction. Osteoporosis/rickets. Different patterns of dysglycemia. Postprandial hyperglycemia and hypergalactosemia |
| GSD-XI | 612933 | Lactate dehydrogenase A | LDHA/AR | 11p15.1 | Muscle | Exertional myoglobinuria, easy fatigability, exercise induced myalgia, erythematosquamous skin lesions on the extensor surfaces of the extremities |
| GSD-XII | 611881 | Fructose-1,6-bisphosphate aldolase A | ALDOA/AR | 16p11.2 | Muscle | Rhabdomyolysis induced by fever and/or exercise, hemolytic anemia with or without myopathy or cognitive dysfunction |
| GSD-XIII | 612932 | Enolase 3 (β-enolase) | ENO3/AR | 17p13.2 | Muscle | Exercise intolerance, exercise induced myalgia, muscle weakness |
| GSD-XV | 613507 | Glycogenin-1 | GYG1/AR | 3q24 | Muscle | Ventricular arrhythmogenic cardiomyopathy, progressive muscle weakness |
There are two types of glycogen synthase (GYS) encoded at different genetic loci; muscle GYS (GYS1; 19q13.33) and liver GYS (GYS2; 12p12.1)[8]. In 1963, GSD-0 was initially reported as glycogen synthetase deficiency in the liver[9]. GSD-0 is distinct from other hepatic GSDs due to the marked decrease in liver glycogen content, thereby making its classification questionable as a genuine GSD. However, since the disease exhibits a phenotype like that of the classic glycogenoses due to unavailability of glycogen during periods of fasting, it is classified as a GSD. GSD-0 is an autosomal recessive genetic disease[10]. The disease is caused by homozygous or compound heterozygous mutations in the GYS2 gene which was mapped to 12p12.2 in 1994[11]. Liver GYS, the hepatic isoform, is responsible for catalyzing the rate-limiting step in hepatic glycogen synthesis. GYS deficiency in liver leads to a marked reduction in hepatic glycogen stores. The inability to synthase glycogen inevitably leads to conversion of dietary carbohydrate to lactate rather than being stored as glycogen in the liver. Postprandial hyperglycemia, glycosuria, and lactic acidemia are replaced by ketotic hypoglycemia during fasting[12]. There is often ketosis after a routine overnight fast.
There are wide phenotypical variations[13]. Fasting hypoglycemia usually manifests in late infancy when overnight feedings are discontinued. Hypoglycemia typically occurs early in the morning prior to having breakfast. Hypoglycemia is responsible for the symptoms observed in GSD-0, which encompasses lethargy, pallor, nausea, vomiting, and, in some cases, seizures. Although some children may display developmental delay, most are neurologically normal. Some patients may remain asymptomatic or experience only mild symptoms[14]. Notably, liver enlargement is not a feature of GSD-0. GSD-0 is the only hepatic GSD that is not typically associated with hepatomegaly[15]. Short stature and osteopenia are frequently observed in GSD-0, but other long-term complications commonly seen in other GSDs have not been documented[16]. Hyperglycemia and glycosuria are rare presentations in GSD-0 but may pose diagnostic difficulties when observed[17]. Postprandial hyperglycemia and glycosuria when taken together with a normal sized liver may mistakenly indicate early stages of diabetes. GSD-0 is underdiagnosed due to the lack of physical findings and milder phenotype[16,18].
Symptoms in GSD-0 are rapidly alleviated by frequent intake of protein-rich meals and bedtime consumption of uncooked cornstarch (UCCS), a slow-release glucose source. The preservation of gluconeogenesis and fatty acid oxidation pathways explains the less severe clinical course of GSD-0 compared to other types of hepatic GSDs. Increased protein intake during meals provides necessary substrates for gluconeogenesis and shows a protective effect against overweight/obesity and insulin resistance[19]. Extended periods of fasting can result in severe hyperketonemia and elevated plasma free fatty acid levels, which in turn leads to the inhibition of alanine release from skeletal muscle causing a reduction in the availability of gluconeogenic substrates, thereby exacerbating hypoglycemia[16]. While fasting is associated with hypoglycemia, hyperketonemia, and low alanine concentrations, feeding causes hyperglycemia and hyperlactatemia. Simple carbohydrates should be limited, and low-glycemic-index complex carbohydrates should be included in the diet to minimize postprandial hyperglycemia and hyperlactatemia. Patients are generally fed more frequently during the daytime to prevent hypoglycemia.
The administration of glucose or galactose to patients with GSD-0 results in elevated levels of serum lactate and lipids and can be used as a diagnostic test[2,17]. Traditional methods of diagnosis, such as liver biopsy to confirm extremely low hepatic glycogen levels and low to absent GYS activity, have been replaced by non-invasive mutation analysis of the GYS2 gene.
Browner et al[20] discovered that muscle GYS, which is distinct from liver GYS, is expressed in both muscle and heart. The defect may be inherited or acquired. Enzyme activity is decreased in patients with type 2 diabetes. Muscle GYS deficiency causes cardiomyopathy and exercise intolerance in affected patients[8]. Histologic examination of muscle shows lack of glycogen and mitochondrial proliferation.
The disease was first described by Gierke[21] in 1929 based on autopsy results showing excessive glycogen storage in the livers and kidneys of two patients. In 1952, Cori and Cori[22] discovered the deficiency of glucose-6-phosphatase (G6Pase) as the causative defect in patients with similar disease phenotype. After more than two decades, in 1978, Narisawa et al[23] described the deficiency of glucose-6-phosphate translocase (G6PT), the transporter protein of G6Pase complex (G6PC).
The deficiency of either G6Pase or G6PT activity causes GSD type I (GSD-I). The G6PT/G6PC functions as a multicomponent system and is responsible for glucose production by catalyzing the terminal step of both the glycogenolysis and gluconeogenesis pathways[24]. G6PT translocates G6P into the endoplasmic reticulum, wherein G6Pase converts G6P into free glucose and inorganic phosphate[25]. Two major subtypes of GSD-I are defined according to which part of the complex is defective. Deficiency of the catalytic subunit of G6Pase causes GSD-Ia while deficiency of G6PT activity results in GSD-Ib. Approximately 80% of cases with GSD-I are type Ia while the remaining 20% are type Ib. The presence of further subtypes (GSD-Ic and GSD-Id) is controversial. The majority, if not all, of typical cases of GSD-I are attributed to mutations in the genes encoding G6Pase and G6PT. Additionally, it has been noted that only two subtypes of GSD-I (namely, GSD-Ia and GSD-Ib) have been confirmed in clinical practice, and the existence of other forms of GSD-I requires further substantiation. Because both glycogenolysis and gluconeogenesis are affected due to inability in converting G6P to free glucose the main metabolic derangement of both subtypes is fasting hypoglycemia.
GSD-I is inherited in an autosomal recessive manner. The overall incidence of the disease is approximately 1:100000[26]. The estimated carrier rate in the general population is 1:150. The disease may be more prevalent in people of Ashkenazi Jewish (c.247C>T), Mexican-Hispanic (c.379_380dupTA) and Japanese heritage (c.648G>T) due to the increased frequency of mentioned pathogenic variants. The carrier frequency for the c.247C>T variant among Ashkenazi Jews has been reported to be as high as 1:63[27].
In 1952, Cori and Cori[22] identified the first specific enzyme deficiency associated with an inherited disorder through demonstration of G6Pase deficiency. Subsequently, in 1995, the gene that encodes the catalytic subunit of the G6PC was identified on chromosome 17q21[28]. Later, its molecular and biochemical characteristics were described in detail[29].
While some neonates may exhibit severe hypoglycemia and lactic acidosis, infants who do not receive any treatment typically present between 3-6 mo of age (at a median age of 6 mo) coinciding with prolonged feeding intervals, increased sleeping time through the night or onset of an intercurrent illness disrupting normal patterns of feeding[30]. The onset of symptoms can be soon after birth, and episodes typically remain unresponsive to glucagon therapy. Symptoms mainly include difficulties with feeding, tremors, pallor, excessive sweating, hyperventilation, cyanosis, apnea, irritability, seizures, somnolence, and cerebral edema/dysfunction, with exacerbations typically occurring in the morning or prior to feedings. Severe episodes of ketotic hypoglycemia, if untreated, may eventually lead to coma and sudden infant death[31]. Older infants may exhibit certain physical characteristics, such as doll-like facies with full cheeks and relatively thin extremities along with frequent lethargy, difficulty in waking from sleep, tremors, an insatiable appetite, growth retardation, and a prominent abdomen resulting from pronounced enlargement of the liver and kidneys. In some cases, xanthomas may appear on extensor surfaces, such as the elbows, knees, or buttocks. During an infection, symptoms of severe hypoglycemia are more prevalent owing to diminished appetite and/or gastrointestinal symptoms (e.g., vomiting and diarrhea) both preventing adequate oral intake. Delayed motor development can be seen but cognitive development is generally normal unless there is cerebral damage due to prolonged or recurrent neuroglycopenia[1,32].
Impaired platelet function, especially in individuals with inadequate metabolic control predisposes patients to nose bleeding[33]. Diminished glucose uptake into platelets due to chronic hypoglycemia and subsequent intracellular ATP deficiency have been proposed as potential causes of platelet dysfunction in GSD-Ia[34]. Additionally, decreased plasma concentration of von Willebrand factor antigen indicating an acquired von Willebrand disease was reported for patients with GSD-Ia[35]. In addition, epistaxis, easy bruising, menorrhagia, intrahepatic adenoma hemorrhage, and excessive bleeding during surgical procedures can also occur[36,37].
Patients with GSD-I have hypovitaminosis D despite adequate supplementation[38]. Low bone mineral density is a long-term complication of GSD-I particularly in those with poor metabolic control[39,40]. Osteoporosis may arise as a consequence of poor nutrition, chronic lactic acidosis and hypogonadism[41]. Anemia is a common complication in both subtypes of GSD-I with a reported prevalence ranging from 17% to 60% across different age groups[30]. The etiology of anemia in GSD-I is complex and involves various factors, including the restrictive nature of the diet, altered iron absorption due to excessive intake of UCCS, chronic lactic acidosis, chronic kidney disease, bleeding diathesis, chronic illness, suboptimal metabolic control, hepatic adenomas, and inflammatory bowel disease. The prevalence and pathophysiology appear to differ in individuals with GSD-Ia and those with GSD-Ib. A multicenter study involving 202 subjects with GSD-I (Ia/Ib: 163/39 subjects) showed that anemia is more common in patients with GSD-Ib compared to GSD-Ia (71.8% vs 41.7%, respectively). In addition, the prevalence of severe anemia is also increased in GSD-Ib in comparison to patients with GSD-Ia (41% vs 4.9%, respectively)[42]. Severe anemia in GSD-Ia appears to be related to large hepatic adenomas, while in GSD-Ib it is often associated with enterocolitis[42]. Development of severe anemia during the course of the disease warrants further evaluation for hepatic adenomas and inflammatory bowel disease in GSD-Ia and GSD-Ib, respectively.
Patients diagnosed with GSD-Ia or GSD-Ib may experience intermittent diarrhea which seems to deteriorate with age[43]. Diarrhea was reported in 35% of the GSD-Ia and in 55% of the GSD-Ib patients[30]. However, the cause of diarrhea remains unknown. Intolerance to UCCS and inflammatory bowel disease are possible causes of diarrhea in this population. Inflammatory bowel disease is a well characterized feature in individuals with GSD-Ib. Neutropenia and impaired neutrophil function are the underlying causes of inflammatory bowel disease in GSD-Ib[44]. However, inflammatory bowel disease was also recently reported in adult patients with GSD-Ia as a new, long-term complication of the disease[45]. The prevalence of symptomatic inflammatory bowel disease in adults with GSD-Ia also seems to be higher than the general population[45]. The authors speculated that inflammatory bowel disease in GSD-Ia may be caused by chronic UCCS therapy, which could be altering the microbiota of the gastrointestinal tract leading to inflammation. More recently, very early onset inflammatory bowel disease was reported in a child with GSD-Ia at the age of 42 mo[46].
A notable finding among the majority of patients with this condition during childhood is growth retardation, while short stature is commonly observed in affected adults[5,47,48]. In the absence of effective treatment, a range of long-term complications may arise in individuals with GSD-I, including delayed puberty, liver adenomas, hepatocellular carcinoma, renal dysfunction, chronic kidney disease, chronic renal failure, urolithiasis, arterial and pulmonary hypertension, osteopenia/osteoporosis, polycystic ovary syndrome, and gout. Cognitive delay and epilepsy due to repeated or severe hypoglycemic events may occur[31]. Hyperlipidemia may cause xanthomas, pancreatitis, and cholelithiasis[30,49]. Acute pancreatitis may develop secondary to very high serum triglycerides in GSD-I and necessitate plasmapheresis[50].
Systemic metabolic perturbations and glycogen deposition in the kidneys result in glomerular and proximal and distal renal tubular injury. Renal manifestations may occur in childhood but often are not noticed without proper diagnostic work-up. The prevalence of renal involvement tends to rise as patients age[51]. Glomerular hyperfiltration, whose underlying mechanism is not yet fully understood, is typically the initial manifestation of renal involvement. Possible etiologies have been suggested including activation of the renin-angiotensin system, persistent oxidative stress, profibrotic cytokines such as transforming growth factor-β, and changes in energy reserves of renal tubular epithelial cells[52-54]. Glomerular hyperfiltration then progresses to microalbuminuria, proteinuria, glomerular scarring and interstitial fibrosis, and end-stage renal disease in adult patients[48,55]. Hypercalciuria and hypocitraturia due to proximal and distal tubular dysfunction cause nephrocalcinosis and/or urolithiasis[56,57]. This may increase the risk of urinary tract infections causing further renal parenchymal damage. Hypertension and hematuria are other findings[55,56]. Systemic hypertension may develop early in childhood but is seen more often in adults with GSD-I[58]. Renal cysts have also been described in individuals with GSD-I[59]. Gout can develop due to persistent hyperuricemia as gouty attacks, gouty tophi, and kidney stones.
In GSD-Ia patients, various types of liver lesions, including hepatic adenoma, hepatocellular carcinoma, hepatoblastoma, focal fatty infiltration, focal fatty sparing, peliosis hepatis, and focal nodular hyperplasia have been reported, with hepatic adenomas being the most prevalent among them[37]. The prevalence of hepatic adenomas was reported to vary between 22% to 75%, and they usually manifest during or after puberty, particularly in the second or third decade of life. The median age of adenoma presentation is 15 years[30]. Although the prevalence of hepatic adenomas increases with age in GSD-I, they may be seen in younger children[60]. Progression in size and/or number of hepatic adenomas occurs in half of patients[30]. Inadequate metabolic control appears to play a central role in hepatic adenoma formation. The degree of hyperlipidemia is associated with development of hepatic adenomas[61]. However, the pathophysiological mechanisms are yet to be fully understood and factors other than metabolic control may also be responsible for adenoma formation. In a recent study by Cho et al[62], in addition to mitochondrial dysfunction and metabolic alterations caused by G6Pase deficiency, persistent autophagy impairment and activation of multiple tumor-promoting pathways were reported as contributing factors to hepatic adenoma/hepatocellular carcinoma development in GSD-I. Chromosomal and genetic alterations may also play a role in hepatocellular carcinoma associated with GSD-I[63]. Hepatic adenomas have the potential to transform into hepatocellular carcinoma over an extended period, with reports of malignant transformation occurring as long as 28 years after initial diagnosis[64,65]. A rapid increase in size or number of adenomas is associated with an increased risk of adenoma to hepatocellular carcinoma transformation and should be evaluated carefully.
The link between GSD-I and risk for cardiovascular disease is controversial. Although GSD-Ia patients have elevated levels of triglycerides, very low density lipoprotein and low density lipoprotein, the occurrence of endothelial vascular dysfunction and atherosclerosis is uncommon. It has been suggested that the increased serum levels of apoE may offset the elevated risk of atherosclerosis associated with dyslipidemia[66]. Moreover, the reduced von Willebrand factor antigen and density of individual oligomers found in 60% of GSD-Ia patients may also contribute to protection against vascular complications[35]. In addition, an increase in serum levels of antioxidative factors may contribute as a protective mechanism[67,68]. There are conflicting data regarding whether patients with GSD-I are at increased risk for atherosclerosis[69,70]. Pulmonary hypertension is a rare long-term complication of GSD-I with few cases reported. Patients with a concomitant predisposing condition for pulmonary arterial hypertension are at increased risk[37].
The main neurological impact of GSD is related to hypoglycemia. Patients with GSD-I may suffer from brain damage, which may be caused by recurrent severe hypoglycemia[71]. Studies have found a significant correlation between the frequency of hospital admissions for hypoglycemia and abnormalities in both performance ability tests and brainstem auditory evoked potentials. In addition, electroencephalography abnormalities were found to be correlated with dietary compliance. The magnetic resonance imaging abnormalities observed in GSD-I patients were the dilatation of occipital horns and/or hyperintensity of subcortical white matter in the occipital lobes[71]. Brain imaging abnormalities were more frequent among GSD-I patients with early symptom onset, frequent and longer hospital admissions, and poor metabolic control including elevated levels of uric acid, lactate, and triglyceride[32,72].
Some females may have polycystic ovaries and irregular menstrual cycles with normal fertility[73]. Women with GSD-Ia may have pregnancies and deliveries without complications[74]. In consideration of the risk of development of hepatic adenomas in GSD-I patients, estrogen-containing contraceptives should be avoided whenever possible[75]. In addition to hypoglycemia, the most prominent laboratory abnormalities observed in patients with GSD-I include lactic acidosis, hyperlipidemia (especially hypertriglyceridemia but also hypercholesterolemia), and hyperuricemia (Figure 1). Mild elevation in transaminase levels is usually detected[30]. Ultrasonographic examination may reveal enlarged kidneys in affected patients of all ages. Serum biotinidase activity is increased in GSD-Ia patients[76-79]. Biotinidase activity was reported to be positively correlated with hypertriglyceridemia in subjects with GSD-I while severe fibrosis and cirrhosis were related to reduced enzyme activity[80]. There may also be hypercalciuria[5]. There is little or no increase in blood glucose concentration in response to administration of glucagon and this may even lead to worsening of the metabolic acidosis. Histopathological examination of the liver in patients with GSD-Ia typically reveals a mosaic pattern with pale-staining and swollen hepatocytes. Other observed features include steatosis and nuclear hyperglycogenation. Periodic acid-Schiff (PAS)-positive and diastase sensitive glycogen is evenly dispersed throughout the cytoplasm. Glycogen accumulation may be within the normal range or exhibit only a mild increase. While fibrosis is not as prominent in GSD-I as in GSD types III, IV, and VI, it may still be present in some affected individuals[5,81-83]. GSD-Ia is usually suspected based on a set of clinical (e.g., hepatomegaly) and biochemical features (e.g., hypoglycemia, lactic acidosis, hypercholesterolemia, hypertriglyceridemia, and hyperuricemia). The definitive diagnosis is confirmed by a mutation analysis or a liver biopsy and an enzyme assay. If a liver biopsy is performed, diagnosis can be confirmed by measuring G6Pase enzyme activity on a liver biopsy specimen; however, it should be kept in mind that measurement of G6Pase enzyme activity will not detect GSD-Ib. When the specific mutation in the index case is known, prenatal diagnosis via chorionic villus sampling can be performed for GSD-I[84].
The mainstay of treatment is to prevent hypoglycemia by avoiding prolonged fasting[85]. Continuously providing a dietary supply of glucose during the day and night by frequent feedings, frequent ingestion of UCCS or nocturnal enteral tube feeding are possible feeding strategies. Infants and children should be fed frequently, not allowing fasting periods longer than 3-4 h. In adolescents and adults, fasting more than 5-6 h should be avoided. Small, frequent meals with balanced macronutrient content and use of UCCS are recommended. Continuous intragastric feeding through a nasogastric or gastrostomy tube can be used overnight allowing the patients to sleep through the night[37]. To ensure adequate glucose supply, a glucose infusion rate of 8-10 mg/kg/min should be maintained for infants, while a rate of 4-8 mg/kg/min is recommended for older children. UCCS can be introduced as early as 6-12 mo of age. For the administration of UCCS in GSD-I patients, the recommended dose is 1-1.5 g of UCCS per kilogram of ideal body weight every 3-4 h for young children and 1.5-2 g of UCCS per kilogram of body weight every 4-5 h for older children, adolescents, and adults[85]. Digestion of UCCS is slow, enabling a sustained release of glucose, thereby achieving a more stable glycemic profile over an extended duration, in contrast to other carbohydrate sources. The administration of UCCS has been shown to achieve adequate glycemia for a median duration of 4.25 h (ranging between 2.5-6 h)[86]. Glycosade®, a modified, waxy maize extended-release cornstarch, is available as a single-dose overnight treatment[87].
In GSD-I, intake of fructose and galactose, which cannot be metabolized to glucose via G6P, further contributes to the metabolic derangement. Lactose (galactose and glucose), fructose and sucrose (fructose and glucose) should be restricted in all age groups. Restricting the intake of fruits, vegetables, juices, and dairy products renders the diet inadequate. Micronutrients, vitamins, and minerals should be supplemented to avoid nutritional deficiencies. The recommended dietary plan is to provide 60%-70% of calories from complex carbohydrates, such as whole-grain breads, pastas, legumes, and rice, with a portion of the carbohydrates coming from cornstarch. Additionally, 10%-15% of calories should come from protein and 25%-30% from fat. Effective dietary management is essential to minimize the metabolic derangement associated with GSD-I and to reduce the development of long-term complications[37,85]. However, caution must be exercised to avoid overtreatment. Overtreatment with UCCS has many consequences including obesity, increased glycogen storage in the liver, worsening lactic acidosis, increased gastrointestinal disturbances, hyperinsulinemia, and insulin resistance[88].
If there is anemia, the causes must be evaluated (e.g., nutritional deficiencies, liver adenomas, enterocolitis, menorrhagia in females, and occult blood loss from the gastrointestinal tract) and appropriate treatment should be started. In the case of severe anemia, hepatic adenomas in GSD-Ia and enterocolitis in GSD-Ib should be investigated[42]. To prevent gout in the presence of hyperuricemia, allopurinol is typically administered at a dosage of 10 mg/kg/d, divided into three doses. If acidosis is present, indicated by a blood base excess of less than -5 mmol/L or a blood bicarbonate level below 20 mmol/L, bicarbonate or potassium citrate should be prescribed, with a recommended dose of 1 to 2 mmol/kg/d divided into four doses and 5 to 10 mEq every 8-12 h, respectively[85]. Angiotensin converting enzyme inhibitors or angiotensin receptor blockers should be used to delay the progression of renal damage[53,89-91]. Evidence of hyperfiltration (sustained estimated glomerular filtration rate > 140 mL/min/1.73 m2), persistent microalbuminuria and frank proteinuria should prompt initiation of angiotensin converting enzyme inhibitors or angiotensin receptor blockers[37]. If serum triglyceride levels remain high despite optimizing dietary treatment, the administration of lipid-lowering drugs, such as 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors and fibrates, may be necessary to decrease the risk of atherosclerosis, cholelithiasis, and pancreatitis. For adults with persistently elevated cholesterol levels, statins may be considered as a treatment option[85]. The positive effect of medium-chain triglycerides on lowering serum cholesterol and triglyceride levels has been reported[92,93].
Recommendations regarding perioperative management of patients with GSD-I are available[37,94]. Close monitoring of blood glucose, electrolytes, and lactate levels is crucial during the peri-operative period. The patient should be admitted to the hospital 24 h before the surgery, continuous intravenous supply with 10% dextrose should be provided and continued until oral feeding is re-established. The administration of Ringer lactate solution should be avoided in GSD-I patients, as it may exacerbate lactic acidosis and worsen metabolic decompensation[37]. Bleeding time must be normalized before elective surgical interventions by 24-h continuous gastric drip feeding for one week or by intravenous glucose infusion over 24 to 48 h[85].
In 1968, after realizing that in vitro G6Pase activity was normal despite glucose not being released from G6P in vivo, a second subtype of GSD-I was identified[95]. In 1975, it was elucidated that a transport system specific to G6P exists and is responsible for transporting G6P from the cytoplasm to the endoplasmic reticulum[96]. The responsible gene, SLC37A4 (the solute carrier family 37 member 4), has been cloned and located on chromosome 11q23[97,98].
GSD-Ib is characterized by distinctive features such as recurrent infections, neutropenia, and neutrophil dysfunction, in addition to the clinical symptoms and findings observed in GSD-Ia. While not all GSD-Ib patients have neutropenia and neutrophil dysfunction, these conditions are common and predispose patients to severe infections and inflammatory bowel disease[44]. Patients with GSD-Ib may have normal neutrophil counts in the first year of life. G6PT gene, unlike G6Pase, is also expressed in hematopoietic progenitor cells, which may be responsible for neutropenia and recurrent infections in GSD-Ib[99]. The neutrophil dysfunction in GSD-Ib includes both impaired motility and respiratory burst[100,101]. Impaired glucose transport across the cell membrane of polymorphonuclear leukocytes may be responsible for neutrophil dysfunction in GSD-Ib. Microsomal transport of G6P has a potential role in the antioxidant protection of neutrophils. Dysfunction of this transporter due to genetic defects in G6PT may impair cellular functions and induce apoptosis, contributing to the neutrophil dysfunction seen in GSD-Ib[102]. Some individuals with GSD-Ib do not develop neutropenia. It has been suggested that this could be due to residual transporter activity of some G6PT mutations[103]. GSD-Ib patients with neutropenia and neutrophil/monocyte dysfunction are at an increased risk for severe infectious complications due to impaired immune function. Young children with GSD-Ib may experience frequent otitis, gingivitis, periodontal disease, dental caries, and skin abscesses. Oral and genital ulcerations and intestinal mucosal ulcers may occur[43,104]. Individuals with GSD-Ib may experience recurrent episodes of diarrhea. The underlying cause of this symptom appears to be inflammation of the intestinal mucosa, as evidenced by elevated fecal α1-antitrypsin excretion and colonic inflammation in colonoscopic biopsies[44]. There is no established association between the specific genetic mutations causing GSD-Ib and the occurrence of neutropenia, bacterial infections, and other systemic complications in affected individuals[105]. Patients with GSD-Ib may require liver transplantation. Although hypoglycemia, lactic acidosis and dyslipidemia improve after liver transplantation, neutropenia generally continues to be present as it is primarily attributable to an intrinsic defect in the neutrophils[106-108].
Another characteristic clinical finding of GSD-Ib is the occurrence of Crohn disease-like colitis[109,110]. The enterocolitis observed in GSD-Ib patients has been found to have histological features similar to those seen in inflammatory bowel disease/Crohn disease, characterized by transmural inflammatory changes and the formation of granulomas[111]. Accompanying findings and symptoms include fever, diarrhea, and perioral and anal ulcers. Interestingly, the severity of the primary disorder does not appear to be correlated with the occurrence or severity of intestinal symptoms[109,110]. Manifestations of inflammatory bowel disease may improve with granulocyte colony-stimulating factor (G-CSF) treatment[112]. Enteral nutrition with a polymeric formula enriched in the anti-inflammatory cytokine transforming growth factor-β is recommended as a first-line treatment of digestive complications in GSD-Ib[113]. Inflammatory bowel disease may require treatment with anti-inflammatory and immunosuppressive medications[113]. Successful treatment of inflammatory bowel disease with biologics including infliximab and adalimumab in GSD-Ib patients refractory to conventional treatment has been reported[114,115].
GSD-Ib is characterized by an increased risk for developing autoimmune disorders like thyroid autoimmunity and myasthenia gravis[116]. GSD-Ib patients have a higher likelihood of developing thyroid autoimmunity and hypothyroidism, while GSD-Ia patients show little indication of thyroid pathologies[117,118]. Based on the slightly elevated levels of thyrotropin, even in patients with overt hypothyroidism, it could be postulated that there is concomitant damage occurring at the hypothalamus or pituitary gland[118]. Recently, predisposition to autoimmunity in GSD-Ib patients was linked with a profound defect in conventional T cells and regulatory T cells caused by defective engagement of glycolysis in T cells due to G6PT deficiency[119]. Although a rare outcome of GSD-Ib, patients may develop terminal kidney disease, which may necessitate kidney transplantation[106].
Nutritional management of GSD-Ib is similar to that of GSD-Ia. Neutropenic patients with GSD-Ib should be treated with G-CSF. G-CSF therapy may normalize the number of neutrophils and restore myeloid functions[120-122]. The implementation of a combined therapeutic approach including both dietary management and G-CSF treatment improves the prognosis of patients by significantly mitigating metabolic and myeloid abnormalities. G-CSF administration is associated with not only an elevation of peripheral neutrophil counts, but also a reduction in the incidence of febrile episodes and infections, as well as improvement in enterocolitis in patients with GSD-Ib[123]. In conjunction with other therapies (aminosalicylates, mesalamine, and corticosteroids), G-CSF ameliorates inflammatory bowel disease symptoms[124]. To prevent complications such as splenomegaly, hypersplenism, hepatomegaly, and bone pain, it is recommended that the lowest effective dose of G-CSF is used. Caution must be exercised regarding the development of splenomegaly and myeloid malignancy[124,125]. Vitamin E has been reported to be effective in reducing the frequency of infections and improving neutropenia[126].
Liver transplantation is the ultimate therapy for hepatic metabolic disease related to GSD-I. There is no possibility of the recurrence of GSD-I within the allograft. Liver transplantation is warranted in various situations, such as hepatic adenomas with a high risk of malignant transformation, rapid progression in size and/or number of hepatic adenomas, development of hepatocellular carcinoma, poor metabolic control despite medical therapy, and growth failure[127]. Liver transplantation corrects all liver related biochemical abnormalities including hypoglycemia, lactic acidosis, hyperuricemia, and hyperlipidemia, but its potential to reverse and/or prevent renal disease remains uncertain[107,128-130]. Recently, an unusual post-transplant finding of two siblings with persistent hyperuricemia requiring allopurinol treatment has been reported[131]. Moreover, chronic renal failure is a well-known complication that may arise as a consequence of liver transplantation in individuals with GSD-Ia, and progression to renal failure within a few years of transplantation was reported[128]. It is uncertain whether post-transplantation renal failure is related to disease progression, toxicity from immunosuppressants used after liver transplantation, a secondary reaction to poor metabolic control, or a combination of these factors. Renal transplantation in GSD-I, on the other hand, corrects only renal abnormalities[132]. Conflicting results have been reported in different studies regarding whether catch-up growth is achieved or not following liver transplantation in children with GSD-I[133,134].
Despite improved survival and growth, long-term complications of GSD-I like progressive renal failure and development of hepatic adenomas do not respond completely to dietary treatment. Although liver transplantation corrects metabolic derangement and improves the quality of life of these patients, it is not without complications[128]. These findings suggest that novel therapeutic approaches with higher success and lower complication rates are warranted. A recent advance in the treatment of neutropenia and neutrophil dysfunction in individuals with GSD-Ib is repurposing empagliflozin, a sodium-glucose co-transporter-2 (SGLT2) inhibitor that is approved to treat type 2 diabetes in adults, to improve neutrophil number and function. A study conducted by Veiga-Da-Cunha et al[135] revealed the crucial function of glucose-6-phosphate transporter in neutrophils, which clarifies the pathophysiology of neutropenia in GSD-Ib patients. In addition to G6P, G6PT transports the G6P structural analog 1,5-anhydroglucitol-6-phosphate (1,5AG6P). Neutrophils lacking G6PT activity cannot transport 1,5AG6P from the cytosol into the endoplasmic reticulum, where it is normally dephosphorylated by G6PC3, a phosphatase in the membrane of the endoplasmic reticulum. Cytosolic accumulation of 1,5AG6P inhibits glucose phosphorylation by hexokinases that catalyzes the first step of glycolysis. As glycolysis is the sole energy source for mature neutrophils, depletion of intracellular G6P leads to a deficit in energy production which in turn results in neutrophil dysfunction and subsequent apoptosis. Empagliflozin inhibits renal SGLT2 leading to increased urinary excretion of 1,5AG. This leads to a reduction in the concentration of 1,5AG in the blood, thereby decreasing the cellular accumulation of toxic 1,5AG6P in neutrophils[136]. Following the first report of successful repurposing of empagliflozin to treat neutropenia and neutrophil dysfunction in 4 patients with GSD-Ib, several case reports and case series have shown beneficial effects of this treatment approach on neutrophil number and function, inflammatory bowel disease, recurrent infections[137-139], oral and urogenital mucosal lesions, skin abscesses, anemia, wound healing, and dose reduction or even cessation of G-CSF therapy in GSD-Ib patients[140-144]. A recent international multicenter study examining the clinical experience of 112 patients with GSD-Ib treated with empagliflozin reported improvements in neutrophil counts in the majority of patients, leading to the cessation of regular G-CSF injections in 55% of the participants[145]. Despite a favorable safety profile in patients with GSD-Ib, there is a risk of hypoglycemia with SGLT2 inhibitors. A low dose at treatment initiation with careful titration to optimal dosing is recommended[141]. Growing evidence suggests that empagliflozin is a candidate for first-line treatment of neutropenia and neutrophil dysfunction related symptoms in GSD-Ib patients.
Another promising novel therapeutic strategy is gene therapy by using recombinant adeno-associated virus vectors. The use of a viral vector to administer G6Pase and hepatocyte transplantation are being investigated as potential treatments for GSD-I. Various animal models have shown an increase in hepatic G6Pase and G6PT activity, as well as improvements in metabolic parameters[146-150]. Multiple approaches have been explored for the integration of the G6Pase transgene into the host genome[151,152]. The successful correction of metabolic imbalances in animal models through gene therapy shows promising potential for future applications of gene therapy in humans. A phase I/II clinical trial using a recombinant adeno-associated virus vector expressing a codon-optimized human G6Pase-α or G6PC for treatment of human GSD-Ia (NCT 03517085) has just been completed and the results are pending.
Glycogen debrancher enzyme has two independent catalytic activities; alpha-glucanotransferase and amylo-1,6-glucosidase, with the two catalytic sites being separated on the same polypeptide. Both catalytic activities are required for complete debranching enzyme activity[153]. Deficient activity of these catalytic sites results in accumulation of glycogen with short outer chains, previously defined as limit-dextrins. Deficiency in glycogen debranching enzyme due to biallelic pathogenic variants in the AGL gene results in the harmful accumulation of abnormal glycogen in hepatocytes. The AGL gene was mapped to the chromosomal locus 1p21, and its nucleotide sequence was determined, revealing the existence of multiple tissue-specific isoforms[154,155]. GSD-III is inherited in an autosomal recessive manner.
GSD-III makes up about 24% of all GSDs, and its estimated incidence is approximately 1 case per 83000 live births in Europe, and 1 in 100000 live births in North America[156]. Certain populations have an increased prevalence due to a founder effect. The highest known GSD-III prevalence occurs in Inuit population in Nunavik (about 1:2500, c.4456delT variant), the Faroese population of the Faroe Islands (about 1:3600, c.1222C>T variant) and North African Jews from Israel (about 1:5400, c.4456delT variant)[156-158]. There is currently limited evidence supporting a correlation between disease severity and pathogenic variants in the AGL gene, except for specific exon 3 variants (c.18_19delGA and c.16C>T) which have been found to be associated with GSD-IIIb (liver involvement only). It was suggested that in muscle isoforms of the AGL gene, alternative exon or translation initiation may not require exon 3, thereby resulting in normal enzyme activity in the muscle tissues of patients with GSD-IIIb who harbor an exon 3 deletion[159,160]. Recent evidence suggests that the presence of frameshift, nonsense, and splice site variants may lead to severe phenotypes. Differences in tissue expression of the deficient enzyme is responsible for the phenotypic variability observed in GSD-III patients[153].
GSD-III is characterized by heterogeneous involvement of the liver, skeletal muscle, and cardiac muscle, leading to variable clinical presentations. Various subtypes are defined by the extent of tissue involvement. Two major subtypes of GSD-III have been identified. GSD-IIIa affects both the liver and the muscle (skeletal and cardiac) and is the most prevalent subtype accounting for approximately 85% of cases. Meanwhile, GSD-IIIb primarily affects only the liver and comprises approximately 15% of all GSD-III cases[48,159]. In a limited number of cases, it has been demonstrated that there is a selective loss of either glucosidase activity (resulting in muscle involvement, referred to as GSD-IIIc) or transferase activity (resulting in both muscle and liver involvement, referred to as GSD-IIId)[161,162].
Hepatomegaly, ketotic hypoglycemia, growth retardation and dyslipidemia (hypertriglyceridemia) are the dominant features of hepatic involvement in infancy and childhood. As gluconeogenesis is intact in GSD-III, fasting hypoglycemia tends to be milder than that seen in GSD-I. During infancy, serum hepatic transaminases are markedly elevated. Uric acid and lactate concentrations are relatively normal[163]. Symptoms and laboratory findings related with liver involvement often improve with age and usually disappear after puberty[164,165]. However, liver disease can also be progressive resulting in liver fibrosis, cirrhosis, hepatic failure, and end-stage liver disease[107,165]. Hepatic fibrosis may occur as early as 1 year of age[166]. Overt liver cirrhosis is not common and occurs rarely[153,165]. Hepatocellular carcinoma can develop as a long-term complication of liver cirrhosis, rather than transformation of an adenoma to carcinoma, as seen in GSD-I[167,168]. The prevalence of hepatic adenomas has been reported to range from 4% to as high as 25%[169]. A recent descriptive, retrospective, international, multi-center cohort study revealed that the overall prevalence of severe hepatic complications (hepatic cirrhosis, hepatic adenomas and/or hepatocellular carcinoma) was 11%[170]. Liver transplantation for cirrhosis and/or hepatocellular carcinoma have been reported[107,168]. Children with failure to thrive often catch-up in height in adulthood with optimized, individualized dietary management.
Muscle symptoms associated with GSD-III can manifest concurrently with liver disease or long after hepatic disorders or even after the resolution of hepatic symptoms during childhood. An elevation in creatine kinase (CK) level is observed in 81% to 94% of cases with muscle involvement, serving as a useful indicator of muscle pathology[171]. Nonetheless, a normal CK level does not entirely exclude the possibility of an underlying muscular disease[172,173]. The median age of onset of CK elevation was reported to be 10 years[170]. Although muscle involvement becomes clinically more obvious later in life, mild muscle weakness on physical examination, motor developmental delay (delayed sitting, delayed standing upright, delayed onset of walking), exercise intolerance, and hypotonia were reported in the majority of pediatric patients with GSD-III[174-176]. Muscle weakness and wasting may slowly progress and become severe by the third or fourth decade of life[165,173].
In a subset of adult patients with GSD-III, muscle symptoms can present in the absence of any clinical or previous evidence of liver dysfunction[165,177]. Muscle weakness, although minimal during childhood, is slowly progressive in nature and may become the predominant feature with significant permanent muscle weakness in adults with type IIIa disease[171]. Although myopathy generally progresses slowly and is not severely debilitating, some patients may have severe muscle involvement leading to loss of ambulation[170]. Myopathy can be proximal, distal, or more generalized. Exercise intolerance with muscle fatigue, cramps and pain are evident in more than half of patients[170,174,175]. Bulbar or respiratory dysfunctions are rarely seen in GSD-III patients while no clinical involvement of facial or ocular muscles has been described in the literature[178].
Cardiac involvement in GSD-III is variable. Cardiac involvement is present in most patients, with varying degrees of severity ranging from ventricular hypertrophy detected on electrocardiography to clinically apparent cardiomegaly[179]. Left ventricular hypertrophy, right ventricular hypertrophy, interventricular septal hypertrophy, QT prolongation, sinus tachycardia, and pulmonary hypertension were among electrocardiographic and/or echocardiographic findings of cardiac involvement[170,180]. According to International Study on Glycogen Storage Disease data presented by Sentner et al[170], 58% of patients with GSD-IIIa showed cardiac hypertrophy mostly presented by electrocardiographic and/or echocardiographic signs of left ventricular hypertrophy. In the same cohort, only 15% of all patients developed hypertrophic cardiomyopathy. Mogahed et al[175] reported that cardiac muscle involvement is less common and mostly subclinical in the pediatric age group. However, a more recent study reported that 91% of patients showed cardiac involvement at a median age of 2.6 years, 86% of cases being under 2 years of age[181]. Moreover, 56% of the patients presented with a symptomatic cardiomyopathy at some point during the follow-up period indicating a more severe cardiac phenotype especially in those on a diet with insufficient caloric and protein intake and suboptimal UCCS treatment[181]. Cardiomyopathy usually presents with asymptomatic left ventricular hypertrophy but can progress to hypertrophic cardiomyopathy with decreased left ventricular function and/or arrhythmias, severe cardiac dysfunction, or congestive heart failure[181,182]. Sudden death has occasionally been reported[183].
Patients with GSD-III may exhibit facial abnormalities such as indistinct philtral pillars, bow-shaped lips with a thin vermillion border, a depressed nasal bridge and a broad upturned nasal tip, and deep-set eyes, particularly in younger patients[184]. Some individuals with GSD-III may have an increased risk of developing osteoporosis with reduced bone mineral density which, in part, may be due to suboptimal nutrition, the effects of metabolic abnormalities and muscle weakness[41,185,186]. Bone fractures due to osteopenia and osteoporosis were reported in patients with GSD-III[170]. Polycystic ovary disease has been reported in women with GSD-III with no significant effect on fertility[187]. Type 2 diabetes may occur during the course of the disease in adulthood[188]. Michon et al[189] reported global cognitive impairment in adult GSD-III patients as an underlying cause of psychological and attention deficits seen in this patient group.
Liver histology shows uniform distension of hepatocytes secondary to glycogen accumulation. There is often septal formation, periportal and reticular fibrosis, fine microsteatosis, and less frequently, micronodular cirrhosis without inflammation or interface hepatitis. Skeletal muscle shows subsarcolemmal glycogen accumulation[12]. The diagnosis of GSD-III is made by identification of biallelic AGL pathogenic variants on molecular genetic testing. If the diagnosis cannot be established by genetic analysis, demonstrating enzyme deficiency in peripheral leukocytes or erythrocytes, cultured skin fibroblasts or in the liver or muscle tissue samples is necessary.
A practice guideline was published by the American College of Medical Genetics and Genomics in 2010 providing recommendations on the diagnosis and management of the complications of GSD-III[176]. The mainstay of GSD-III treatment is dietary intervention, which aims to maintain normal blood glucose levels while balancing macronutrient and total caloric intake. This is achieved by the avoidance of fasting, frequent meals enriched in complex carbohydrates and use of UCCS. Continuous enteral feeding may be needed in some cases. Sucrose, fructose, and lactose are not contraindicated unlike GSD-I. UCCS can be used as early as the first year of life to prevent hypoglycemia. As an alternative, Glycosade®, an extended-release cornstarch, can also be used[87]. Caution must be exercised to avoid overtreating with cornstarch or carbohydrates, which may lead to excessive storage of glycogen in the liver and weight gain. In patients with myopathy, along with managing hypoglycemia, a high-protein diet is recommended as it prevents muscle protein breakdown during glucose deprivation, thereby preserving skeletal and cardiac muscle[176]. A ketogenic diet (alone or in combination with high protein and ketone bodies) was also shown to ameliorate cardiomyopathy[190,191]. It has been shown that a high-fat, low-calorie and high-protein diet can reduce cardiomyopathy in individuals with GSD-III[192,193]. The beneficial effects on cardiac or skeletal muscle function of these ketogenic or high-fat diets are possibly related to the increased ketone bodies or fats as fuel sources, or reduced glycogen accumulation through decreased carbohydrate intake. Whether long-term muscular, cardiac, or even liver complications can be prevented by these dietary approaches warrants further studies[194].
Liver transplantation corrects all liver related biochemical abnormalities but does not correct myopathy or cardiomyopathy[107,133,195]. Cirrhosis, liver dysfunction, and/or hepatocellular carcinoma are the main indications for liver transplantation. Detailed information about surveillance recommendations on hepatic, metabolic, musculoskeletal, cardiac, nutritional, and endocrine aspects of the disease can be found elsewhere[176]. Gene therapy and gene-based therapeutic approaches are in development.
The disease was described by Andersen[196] in 1956 as “familial cirrhosis of the liver with storage of abnormal glycogen” and, in 1966, amylo-1,4 to 1,6-transglucosidase [glycogen branching enzyme (GBE)] deficiency was reported[197]. Branching of the chains is essential to pack a very large number of glycosyl units into a relatively soluble spherical molecule. Without GBE, abnormal glycogen with fewer branching points and longer outer chains resembling an amylopectin-like structure (polyglucosan) accumulates in various tissues including hepatocytes and myocytes[198]. The mapping of the GBE1 gene to chromosome 3p12.2 was first accomplished in 1993[199]. Notably, mutations in the same gene are also responsible for adult polyglucosan body disease. GSD-IV accounts for only 0.3% of all GSDs and follows an autosomal recessive inheritance pattern[200]. This rare disorder has a prevalence of 1:600000 to 1:800000[201].
GSD-IV exhibits significant clinical heterogeneity and phenotypic variability, partly due to variations in tissue involvement, which may be influenced by the presence of tissue-specific isozymes[198,200]. The liver is the primary organ affected, with the classical hepatic form appearing normal at birth but progressing rapidly to cirrhosis in early life, leading to liver failure and death between 3 to 5 years of age[196]. Children with GSD-IV experience growth failure, hepatomegaly and/or splenomegaly, and cirrhosis within the first 18 mo of life. Besides the complications of progressive cirrhosis including portal hypertension, ascites and esophageal varices, the development of hepatocellular carcinoma was also reported[202]. In rare cases, the hepatic disease in GSD-IV may not progress or progress slowly[203]. Patients with the non-progressive hepatic form may present with hepatosplenomegaly and mildly elevated liver transaminases, and experience normal growth. Liver size and transaminase levels may return to normal[203]. Patients with the non-progressive hepatic form usually survive into adulthood.
GSD-IV can present with multiple system involvement, with the enzyme deficiency in both liver and muscle[204]. This form of the disease can manifest as peripheral myopathy with or without cardiomyopathy, neuropathy, and liver cirrhosis. Onset of the disease can be from the neonatal period to adulthood[205]. The neuromuscular presentation can be divided into four groups based on age at onset[206]. In the perinatal (fetal) form, which can lead to hydrops fetalis and polyhydramnios, arthrogryposis develops due to akinesia[207]. Detection of cervical cystic hygroma during pregnancy may indicate the disease[200]. Prenatal diagnosis can be performed by determining enzyme activity in cultured amniocytes or chorionic villi samples. Genetic studies can complement uncertain enzyme activity studies, such as equivocal results in prenatal fetal samples and in patients with higher levels of residual enzyme activity that overlap heterozygote levels[208]. Mortality is unavoidable in the neonatal period. Liver cirrhosis or liver failure has not been reported. Severe hypotonia, hyporeflexia, cardiomyopathy, depressed respiration, and neuronal involvement are features of the congenital form of the disease[198,209-211]. Liver disease is not severe, and the child dies in early infancy due to other reasons. The childhood neuromuscular form may start at any age with either myopathy or cardiomyopathy[206,212]. Presenting symptoms mainly include exercise intolerance, exertional dyspnea, and congestive heart failure in advanced stages. The disease can be confined to muscular tissue and serum CK level can be within the normal range. In the adult form, there is isolated myopathy or a multisystemic disease called adult polyglucosan body disease. Onset of symptoms can occur at any age during adulthood, usually after the age of 50, and may exhibit a resemblance to muscular dystrophies. Symptomatology includes progressive gait difficulty and proximal muscle weakness, which is more pronounced in the arms as compared to the legs. Both upper and lower motor neurons are affected in the disorder. The disease may manifest as pyramidal tetraparesis, peripheral neuropathy, early onset of neurogenic bladder, extrapyramidal symptoms, seizures, and cognitive dysfunction leading to dementia[210]. The diagnosis can be established by enzyme activity assay in erythrocytes[213]. Amylopectin-like inclusions are detected through ultrastructural examination of the central nervous system and skeletal muscle. These inclusions are intensely PAS-positive and diastase-resistant, both in neurons and muscular fibers[214]. Magnetic resonance imaging shows white matter abnormalities[215].
Liver biopsy can be diagnostic in patients with hepatic involvement[216]. The histopathological evaluation of the liver reveals abnormal hepatocellular glycogen deposits in the form of PAS-positive, diastase-resistant inclusions. Ultrastructural examination with electron microscopy reveals accumulation of fibrillar aggregations that are typical of amylopectin. Typically, enzyme deficiency can be documented through diagnostic assays performed on hepatocytes, leukocytes, erythrocytes, and fibroblasts. However, patients with cardioskeletal myopathy may exhibit normal leukocyte enzyme activity[198]. The diagnosis of GSD-IV can be confirmed through histopathological examination, detection of enzyme deficiency, and mutation analysis of the GBE1 gene. Genetic confirmation is recommended whenever possible in patients with suspected GSD-IV to provide more data for genotype-phenotype correlations in this extremely rare disease. The genotype-phenotype correlation remains unclear for GSD-IV and the same genetic defect may cause different clinical presentations in unrelated patients[217]. Mutation analysis can also provide crucial diagnostic information in cases with equivocal results of biochemical analyses[218]. Mutations with significant preservation of enzyme activity may be related with milder (e.g., non-progressive hepatic form) and late-onset (e.g., adult polyglucosan body disease) phenotypes of the disease.
Hypoglycemia has traditionally been considered a late manifestation and generally develops due to hepatocellular dysfunction caused by progressive cirrhosis. At this stage of the disease, the biochemical profile of the patients is representative of what is observed in other causes of liver cirrhosis. However, a recent study has reported that fasting intolerance, as indicated by a thorough medical history, along with the presence of hypoglycemia and/or ketosis, can be observed in patients even in the absence of detectable liver injury or dysfunction based on biochemical or radiological assessment[201].
No specific dietary and pharmacological treatments are available for GSD-IV. There is a lack of established guidelines based on either evidence or expert consensus for the dietary management of GSD-IV. Improvement in clinical, anthropometric, and laboratory parameters was reported with a high-protein and low-carbohydrate diet[219,220]. Derks et al[201] recently reported improved clinical and biochemical outcomes after dietary interventions including a late evening meal, continuous nocturnal intragastric drip feeding, restriction of mono- and disaccharides, the addition of UCCS, and protein enrichment in patients with GSD-IV. Individual dietary plans should also aim to avoid hyperglycemia to minimize glycogen accumulation in the liver.
At present, there is no effective therapeutic approach other than liver transplantation for GSD-IV patients who are affected by progressive liver disease. However, anecdotal reports indicate that liver transplantation may not alter the extrahepatic progression of GSD-IV[217]. The presence of extrahepatic involvement, especially amylopectin storage in the myocardium, may lead to fatal complications following liver transplantation[221-223]. Careful assessment of cardiac function even in the absence of clinical decompensation or consideration of combined liver-heart transplantation is warranted for patients with GSD-IV[224]. Liver transplantation may provide beneficial effects not only for patients with liver disease but also for those affected by muscular involvement in GSD-IV[107,225,226]. This may be explained by systemic microchimerism (donor cells presenting in various tissues of the liver recipient) after liver allotransplantation and amelioration of pancellular enzyme deficiencies resulting in a decrease in amylopectin in other organ systems[12]. It has been suggested that the donor cells can transfer enzyme to the native enzyme-deficient cells[226].
In recent years, animal studies have been conducted to prevent glycogen and polyglucosan body accumulation in GSD-IV patients, and GYS inhibitor guaiacol and 144DG11 are promising in this regard[227,228]. The molecular target of 144DG11 is the lysosomal membrane protein lysosome-associated membrane protein 1 (LAMP1), which enhances autolysosomal degradation of glycogen and lysosomal acidification. In the adult polyglucosan body disease mouse model, 144DG11 reduced polyglucosan and glycogen in brain, liver, heart, and peripheral nerve[228].
GSD-VI was first reported by Hers[229] in three patients with hepatomegaly, mild hypoglycemia, an increased glycogen content and deficient activity of glycogen phosphorylase in the liver in 1959. GSD-VI is a rare autosomal recessive genetic disease caused by deficiency of hepatic glycogen phosphorylase. At least three human glycogen phosphorylases exist including muscle, liver, and brain isoforms[230]. In response to hypoglycemia, liver glycogen phosphorylase catalyzes the cleavage of glucosyl units from glycogen which results in the release of glucose-1-phosphate. The glucose-1-phosphate is subsequently converted to glucose-6-phosphate. The PYGL gene is currently the only known genetic locus associated with the development of GSD-VI and was mapped to chromosome 14q21-q22 in 1987[231]. Incidence of the disease is estimated to be 1:100000 and believed to be underestimated due to nonspecific and variable phenotypes, and a paucity of cases confirmed by genetic testing[232]. GSD-VI is more prevalent among the Mennonite community, with a prevalence of 1 in 1000, representing the only known population at higher risk for the disease[232].
GSD-VI is a disorder with broad clinical heterogeneity[232]. Infants with liver phosphorylase deficiency mainly present with hepatomegaly and growth retardation. The condition typically has a benign course, and symptoms tend to improve as the child grows[229]. Hepatomegaly usually normalizes by the second decade of life[233]. The child shows mild to moderate ketotic hypoglycemia related to prolonged fasting, illness, or stressful conditions[232]. As gluconeogenesis is intact in GSD-VI, hypoglycemia is usually mild. Despite gross hepatomegaly, the patient may be largely asymptomatic without hypoglycemia. However, there is a range of clinical severity in GSD-VI, with some patients experiencing severe and potentially life-threatening hypoglycemia. There is generally mild ketosis, growth retardation, abdominal distension due to marked hepatomegaly and mildly elevated levels of serum transaminases, triglycerides, and cholesterol. However, in patients with high residual enzyme activity, biochemical investigations may be normal[234,235]. Hypertriglyceridemia may persist despite treatment[108]. A few patients showing mild muscular hypotonia, muscle weakness or developmental impairment were observed, but otherwise, no neurological symptoms were reported in the literature[232]. Sleep difficulties and overnight irritability are common[236]. In contrast to GSD-I, serum levels of lactic acid and uric acid are generally within the normal range[15]. However, in a recent clinical study including 56 GSD-VI patients, hyperuricemia was reported as a complication in adolescent and adult patients with GSD-VI, which indicates the need for long-term monitoring of uric acid in older GSD-VI patients[237]. CK concentration is usually normal. In some patients, severe and recurrent hypoglycemia, pronounced hepatomegaly, and postprandial lactic acidosis have been reported[238]. Recently, children with GSD-VI have been reported to present with only ketotic hypoglycemia as the sole manifestation of the disease, without the characteristic hepatomegaly[239]. Mild cardiopathy has also been described for GSD-VI[233].
The clinical picture of GSD-VI virtually overlaps with phosphorylase kinase (PHK) deficiency (GSD-IX) and the differential diagnosis includes other forms of GSDs associated with hepatomegaly and hypoglycemia, especially GSD-I and GSD-III[236]. It is not possible to distinguish between GSD-VI and GSD-IX based on clinical or laboratory findings alone[232].
Mutation analysis is the suggested method for the diagnosis of GSD-VI. A liver biopsy is not recommended to establish the diagnosis to avoid an invasive procedure. Excessive glycogen accumulation with structurally normal glycogen in the liver biopsy is consistent with GSD-VI. Fibrosis, mild steatosis, lobular inflammatory activity and periportal copper binding protein staining have also been reported in GSD-VI patients. Although it is possible to document glycogen phosphorylase deficiency in frozen liver biopsy tissue or blood cells including leukocytes and erythrocytes, normal in vitro residual enzyme activity may be seen and prevents establishment of a definitive diagnosis by an enzyme assay alone in some patients[234,235].
In GSD-VI, nutrition therapy aims to improve metabolic control and prevent primary manifestations such as hypoglycemia, ketosis, and hepatomegaly, as well as secondary complications including delayed puberty, short stature, and cirrhosis. Frequent meals, a high-protein diet providing 2-3 g protein/kg body weight/d, limitation but not prohibition of simple sugars such as sucrose, fructose, lactose, a late evening meal and use of UCCS are the main recommendations in GSD-VI patients[236]. The aim of the therapeutic approach is to achieve euglycemia and normoketosis by administration of the appropriate doses of cornstarch. The target level for blood glucose should be within 70-100 mg/dL, while the optimal range for blood ketones is 0.0-0.2 mmol/L[236]. An extended-release corn starch derived from waxy maize, marketed as Glycosade®, has been found to have a positive impact in delaying overnight hypoglycemia in children over 5 years of age and adults[87]. Some individuals with GSD-VI may not require any treatment.
GSD-VI usually has a benign disease course. However, focal nodular hyperplasia, fibrosis, cirrhosis, and a degeneration to hepatocellular carcinoma have been reported in some patients[240-242]. Cirrhosis has been reported in patients as young as preschool age, even within the second year of life[242]. Based on these findings, aggressive treatment of GSD-VI has recently been suggested to maintain optimal metabolic control and prevent long-term complications[243]. Long-term monitoring of hepatic function is also recommended[236].
Glucagon and epinephrine play a critical role in the regulation of glycogenolysis by activation of adenylate cyclase which leads to an increase in the cytosolic concentration of cyclic adenosine monophosphate (cAMP). The increased level of cAMP activates cAMP-dependent protein kinase which activates PHK. In the next step, PHK, a serine/threonine-specific protein kinase, functionally activates glycogen phosphorylase in the liver. PHK is a heterotetramer composed of 4 different subunits (α, β, γ, and δ). Each subunit is encoded by different genes that are located on different chromosomes and differentially expressed in a variety of tissues[244]. α and β subunits have regulatory functions, the γ subunit contains the catalytic site, and δ is a calmodulin protein[245]. PHK has a wide tissue distribution with multiple tissue-specific isoforms.
The α subunit has two isoforms, a muscle isoform, and a liver isoform, which are encoded by two different genes (PHKA1 and PHKA2, respectively) on the X chromosome[244]. The genetic loci of other subunits are mapped to autosomal chromosomes. The γ subunit also has muscle and liver isoforms, each of which is encoded by a distinct gene (PHKG1 and PHKG2, respectively). There is only one gene encoding the β-subunit (PHKB). However, PHKB is expressed in both muscle and liver[246,247].
Liver PHK deficiency (liver GSD-IX) can be classified according to the involved gene, the X-linked form (GSD-IXa, X-linked glycogenosis) and autosomal recessive forms (GSD-IXb and GSD-IXc). GSD-IXa (PHKA2-related GSD-IX) is caused by pathogenic variants in the PHKA2 gene on X chromosome. GSD-IXb (PHKB-related GSD-IX) and GSD-IXc (PHKG2-related GSD-IX) are inherited in an autosomal recessive manner and caused by mutations in PHKB and PHKG2 genes, respectively (Table 1). GSD-IXa is further classified into subtypes XLG-I (formerly GSD-VIII) with no enzyme activity in liver or erythrocytes, and XLG-II with no enzyme activity in liver, but normal activity in erythrocytes[248,249].
GSD-IX is one of the most common forms of GSDs. Approximately 25% of all GSDs can be attributed to PHK deficiency[249]. The frequency of liver PHK deficiency was estimated to be 1:100000[15]. GSD-IXa, the most common subtype of liver PHK deficiency, accounts for 75% of all GSD-IX cases. On the X chromosome, there are two enzyme loci; one for the alpha subunit of muscle PHK, and one for the alpha subunit of liver PHK. In 1992, the liver PHK gene was located to Xp22.2-p22.1[244]. GSD-IXa is more common in males due to the X-linked inheritance pattern. Female carriers may become symptomatic due to X chromosome inactivation[250].
Hepatomegaly, growth retardation, delayed motor development, mild hypotonia, significantly elevated serum transaminase levels, hyperlipidemia, fasting hyperketosis, and hypoglycemia are the main symptoms and findings[251-254]. Rarely described clinical features include splenomegaly, liver cirrhosis, doll-like facies, osteoporosis, neurologic involvement, high serum lactate levels, metabolic acidosis, and renal tubular acidosis[233]. With increasing age, there is a gradual resolution of both clinical symptoms and laboratory abnormalities. Although puberty may be delayed, eventual attainment of normal height and complete sexual development is still possible[253]. Most adult patients are asymptomatic[252]. Unusual presentations including asymptomatic hepatomegaly and isolated ketotic hypoglycemia without hepatomegaly have been reported in affected male children underscoring the importance of screening for GSD-IXa in male patients who are suspected of having GSD with atypical features[239,255]. More severe phenotypes including severe recurrent hypoglycemia and liver cirrhosis have also been reported[243,256,257]. Recent findings suggest that GSD-IXa is not a benign condition as is often reported in the literature and patients may have fibrosis even at the time of diagnosis[258].
GSD-IXc is caused by autosomal recessive mutations in the PHKG2 gene. There are two isoforms, encoded by different genes, for the gamma subunit: The muscle form (PHKG1 gene) and the testis/liver form (PHKG2 gene)[259]. The genetic locus of the liver form was located to 16p12.1-p11.2. The presence of PHKG2 mutations has been linked to more severe clinical and biochemical abnormalities, such as an elevated risk for liver fibrosis and cirrhosis[260-262]. Liver cirrhosis can develop in infancy[263]. Cirrhosis related esophageal varices and splenomegaly, liver adenomas, renal tubulopathy and significant hypocalcemia were other reported clinical findings[236]. Patients with this condition commonly present with severe hypoglycemia requiring overnight feeding, show very low PHK activity in the liver, and exhibit highly elevated serum transaminase levels. A wide range of clinical symptoms can be observed, including hypoglycemia during fasting, hepatomegaly, elevated levels of transaminases, hepatic fibrosis, cirrhosis, muscle weakness, hypotonia, delayed motor development, growth retardation, and fatigue[264].
The genetic cause of GSD-IXb is attributed to mutations in the PHKB gene, which is located on 16q12-q13 and encodes the beta subunit of PHK[265]. The main features of the disease include marked hepatomegaly, increased glycogen content in both liver and muscle, and the development of hypoglycemic symptoms after physical activity or several hours of fasting[265]. Patients with liver fibrosis, adenoma-like mass, mild cardiopathy and interventricular septal hypertrophy were reported[233]. The muscle symptoms are generally mild or absent, affecting virtually only the liver. Distinction between GSD-IXb and individuals with pathogenic variants in PHKA2 or PHKG2 cannot be carried out based on clinical findings alone.
Genetic analysis is the preferred first-line diagnostic test in suspected patients. An approach using next-generation sequencing panels is advised due to the involvement of multiple genes. Liver biopsy can be a valuable diagnostic tool for confirming the diagnosis in cases where there are variants of unknown significance. Histopathological assessment of liver involvement is superior to biochemical parameters[266]. It is important to keep in mind that PHK enzyme activity can be normal in blood cells and even in liver tissue of affected patients. On the other hand, a reduction in PHK enzyme activity can also occur secondary to other metabolic defects such as pathogenic variants in GLUT2 in Fanconi-Bickel syndrome (FBS), PRKAG2 cardiomyopathy syndrome, or mitochondrial complex 1 deficiency[236].
In patients with GSD-IX, close monitoring of long-term liver and cardiac complications is recommended[233]. Aggressive structured dietary treatment with UCCS and relatively high protein intake was associated with considerable improvement in growth velocity, energy, biochemical abnormalities, hepatomegaly, and overall well-being of patients with GSD-IX. Radiographic features of fibrosis were also reported to be improved with early and aggressive dietary management[243]. General nutritional recommendations for GSD-IX are similar to those for GSD-VI and have recently been published[236].
The primary defect in FBS is deficiency of glucose transporter 2 (GLUT2), a monosaccharide carrier that is responsible for the transport of both glucose and galactose across the membranes in hepatocytes, pancreatic β-cells, enterocytes, and renal tubular cells. Utilization of both glucose and galactose is impaired in FBS[267]. Hepatorenal glycogen accumulation and proximal renal tubular dysfunction are the characteristic features of this rare disease[268,269]. FBS follows an autosomal recessive inheritance pattern. The responsible gene, GLUT2 gene (solute carrier family 2 member 2, SLC2A2), was localized to 3q26.1-q26.3 in 1988[270,271]. Since the first patient reported by Fanconi and Bickel in 1949, over 100 cases of FBS with various SLC2A2 mutations including missense, nonsense, frameshift/insertion-deletion, intronic, and compound heterozygous mutations have been reported[272].
Infants with FBS typically present between the ages of 3 to 10 mo. In addition to hepatorenal glycogen accumulation and proximal renal tubular dysfunction, FBS is characterized by fasting hypoglycemia, postprandial hyperglycemia and hypergalactosemia, rickets and marked growth retardation. Patients have entirely normal mental development. In older patients, dwarfism is the most notable finding. Puberty is significantly delayed, with other remarkable observations including a distended abdomen caused by hepatomegaly, deposition of fat on the abdomen and shoulders, and a moon-shaped face[273]. Some patients may not exhibit hepatomegaly during the early stages of the disease[269,274]. Hyperlipidemia and hypercholesterolemia are prominent and may cause acute pancreatitis. The development of generalized osteopenia occurs early and may result in fractures. Hypophosphatemic rickets and osteoporosis are characteristics of the disease that emerge later in life[275]. Tubular nephropathy is characterized by excessive glucosuria, moderate hyperphosphaturia along with persistent hypophosphatemia, hyperuricemia, hyperaminoaciduria, and intermittent albuminuria, collectively referred to as renal Fanconi syndrome[267,268]. Hypercalciuria is also evident. Due to increased renal losses, there is a frequent tendency towards hyponatremia and hypokalemia. Polyuria may develop due to high urinary osmotic load[276]. Progression to renal failure is not the case. Nephrocalcinosis was also reported in one third of the patients in a recent retrospective study[277]. There may be mild metabolic hyperchloremic acidosis with normal anion gap due to renal loss of bicarbonate[267]. Cataracts, a frequently documented consequence of hypergalactosemia, are only present in a small number of patients[278].
Laboratory findings include fasting hypoglycemia and ketonuria, hyperglycemia and hypergala
The management of symptoms involves measures to stabilize glucose homeostasis and compensate for the renal loss of water and various solutes. Patients typically require replacement of water, electrolytes, and vitamin D, while also restricting galactose intake and adhering to a diabetes mellitus-like diet. Frequent small meals with adequate caloric intake and administration of UCCS are important components of symptomatic treatment. In cases of renal tubular acidosis, it may be required to administer alkali to maintain acid-base balance. Catch-up growth was reported to be induced by UCCS[282]. Continuous nocturnal gastric drip feeding may be indicated in some cases with growth failure[283]. With these measures, the prognosis is good. However, a recent retrospective study reported poor outcome despite adequate metabolic management emphasizing the importance of early genetic diagnosis and facilitating prompt nutritional interventions[277].
Pompe disease is a typical example of a lysosomal storage disease. The clinical manifestations of Pompe disease are variable, predominantly due to the varying amounts of residual acid alpha-glucosidase (GAA) activity linked with distinct mutations in the causative gene (GAA). GAA gene is mapped to chromosome 17q25.2-q25.3[284]. Enzyme deficiency results in intra-lysosomal storage of glycogen especially in skeletal and cardiac muscles. There is no genotype-phenotype correlation, but DD genotype in the angiotensin converting enzyme gene and XX genotype in the alpha actinin 3 gene are significantly associated with an earlier age of onset of the disease[285].
There are mainly two types of GSD-II according to age of onset: Infantile-onset and late-onset Pompe disease. Patients with disease onset before the age of 12 mo without cardiomyopathy and all patients with disease onset after 12 mo of age are included in the late-onset form[286]. The combined frequency of infantile onset and late onset GSD-II varies between 1:14000 and 1:100000 depending on ethnicity and geographic region. In the infantile-onset form, cardiomyopathy and muscular hypotonia are the cardinal features and patients die around 1 year of age. The enzyme activity is less than 1% of normal controls, and the enzyme is deficient in all tissues. Patients also have feeding difficulties, macroglossia, failure to thrive, hearing impairment and respiratory distress due to muscle weakness. The liver is rarely enlarged unless there is heart failure. Hypoglycemia and acidosis do not occur[286]. In the late-onset form, involvement of skeletal muscles dominates the clinical picture, and cardiac involvement is generally clinically insignificant depending on the age of onset. Enzyme activity is partially deficient (2% to 40% of normal controls)[286]. Glycogen accumulation in vascular smooth muscle may cause the formation and subsequent rupture of an aneurysm[287]. Both severe infantile and asymptomatic adult forms of the disease were observed in two generations of the same family[288]. Although women with GSD-II do not have an increased risk of pregnancy or delivery complications, pregnancy may worsen muscle weakness and respiratory complications[289]. As a rule, there is an inverse correlation between the age at disease onset and the severity of clinical manifestations with the level of residual enzyme activity[286].
Laboratory testing reveals nonspecific elevations in CK, aldolase, aminotransferases, and lactate dehydrogenase. Elevated urinary tetrasaccharide is highly sensitive but not specific. To establish the final diagnosis, the measurement of enzyme activity in skin fibroblasts or muscle tissue or the demonstration of the responsible mutation is required[286].
Although it is not curative, ERT has changed the course of Pompe disease since its first use in 2001[290]. Alglucosidase alfa, a lysosomal glycogen-specific recombinant enzyme, was approved by the European Medicines Agency (EMA) in 2006 in the European Union and by the Food and Drug Administration (FDA) in 2010 in the United States. The indication criteria were as follows: ≥ 8 years and absence of cardiac hypertrophy (https://www.accessdata.fda.gov/drugsatfda_docs/Label/2010/125291Lbl.pdf; accessed on November 5, 2022). Based on data from later studies, treatment initiation was shifted to the neonatal period. The recommended dosage is 20 mg/kg body weight every two weeks by intravenous administration. A new formulation of GAA enzyme, avalglucosidase alfa, improves the delivery of the enzyme to target cells and has 15 times higher cellular uptake when compared with alglucosidase alfa. The FDA and EMA approved avalglucosidase in 2021 and in 2022, respectively, for the treatment of patients who are one year of age and older with late-onset Pompe disease[291]. Ongoing studies show that avalglucosidase is generally well tolerated in patients with infantile-onset Pompe disease[291]. The recommended dose is 5 to 20 mg/kg every 2 wk[291]. Criteria for starting and stopping ERT in adult patients with GSD-II are similar in different countries. While a confirmed diagnosis and being symptomatic are general criteria for starting ERT, patient wish, severe infusion associated reactions, noncompliance with treatment, and lack of effect are criteria for stopping ERT[292]. Another way to increase the effectiveness of ERT is to use antibodies as an intracellular delivery vehicle. The 3E10 anti-nuclear antibody, that penetrates cells and localizes to the cell nucleus, has been used for this purpose. VAL-1221 is a fusion protein consisting of 3E10 antibody and GAA complex. The presence of 3E10 increases the delivery of GAA to both lysosomal and extra-lysosomal storage of glycogen within cells[293]. The earlier ERT is started, the better its effectiveness. Therefore, it is recommended that ERT is started before irreversible clinical symptoms begin. This concept has led to the development of screening programs for Pompe disease[294]. Recently, it has been shown that in utero alglucosidase alfa treatment, which was started at 24 wk 5 d of gestation and given 6 times at 2-wk intervals through the umbilical vein, was successful[295].
Although antibodies against the enzyme may develop, a recent study showed that the development of antibodies did not affect the clinical course[296]. Whether additional treatments such as oral supplementation of L-alanine is beneficial is being investigated[297]. As an alternative to ERT, studies on gene therapy have also commenced[298].
Although Danon disease was previously classified as a variant of GSD-II with normal alpha-glucosidase activity, it is still controversial whether it is a real GSD. A lysosomal structural protein, LAMP2, is deficient in Danon disease. LAMP2 is involved in autophagosome maturation. Disruption of autophagy leads to accumulation of glycogen granules and autophagic vacuoles[299]. It is an X-linked (Xq24) dominant hereditary disease affecting both skeletal and cardiac muscles, and characterized by skeletal and cardiac myopathy, proximal muscle weakness and intellectual disability. Female patients have a milder disease predominantly involving cardiac muscle[300]. There is currently no treatment for Danon disease. There are ongoing studies evaluating the efficacy and safety of gene therapy[300].
Another glycogen storage cardiomyopathy results from PRKAG2 (the gene encoding gamma-2 non-catalytic subunit of adenosine monophosphate-activated protein kinase) mutations on chromosome 7q36.1. The disease is characterized by left ventricular hypertrophy due to altered glycogen metabolism and glycogen storage in cardiac muscle, similar to Danon disease[301-303]. It is inherited in an autosomal dominant pattern. PRKAG2 gene variants cause a syndrome characterized by cardiomyopathy, conduction disease, and ventricular pre-excitation[302]. It may cause atrial fibrillation/flutter or conduction abnormalities that may cause sudden cardiac death and severe heart failure typically in the third and fourth decade[301,302]. Mutations in the gamma-2 non-catalytic subunit of AMP-activated protein kinase may cause lethal congenital storage disease of the heart, and death in the first year of life[303]. It is important to differentiate the clinical picture related to PRKAG2 mutations from Danon disease, as management and prognosis are different.
GSD-V is caused by mutations in PYGM gene which is the gene encoding the muscle isoform of glycogen phosphorylase. The PYGM gene is located on 11q13.1[304]. In a recently published European registry for patients with muscle glycogenosis, 95% of all patients had GSD-V[305]. The clinical manifestations generally occur during early adulthood with physical activity intolerance and muscle cramps characterized by muscle fatigue and pain, contracture, tachypnea, tachycardia, ptosis, and retinal dystrophy. Most of the patients are symptomatic at pediatric age (< 18 years)[306]. An improvement of exercise induced symptoms, named the “second wind phenomenon”, characterized by the improvement of exercise tolerance after 8 to 10 min of aerobic exercise, is observed[306,307]. Exercise induced rhabdomyolysis can cause transient myoglobinuria, leading to acute renal failure. Hyperuricemia, gout development and thyroid dysfunction are not uncommon[306]. Many patients are diagnosed with an incidental finding of abnormal serum CK levels[307].
Echaniz-Laguna et al[308] studied a family of 13 affected members with adult-onset muscle weakness, and reported a phenotype caused by a dominant myophosphorylase gene mutation (p.Asp639His). The first signs of the disease occurred after 40 years of age with proximal leg weakness, followed by proximal arm weakness. In contrast to McArdle disease, the patients did not have exercise intolerance, second wind phenomenon, markedly increased CK levels, or rhabdomyolysis. The authors concluded that specific PYGM mutations can cause either dominant or recessive GSDs[308].
The responsible gene is located on chromosome 12q13.3[309]. Exercise induced muscle cramps and myoglobinuria are the main characteristics of GSD-VII. Neurological examination does not reveal any abnormalities at rest. Muscle weakness and stiffness invariably occur in muscle groups that are subjected to intense or prolonged exertion. The ischemic exercise test is characterized by the absence of an increase in venous lactate level. Myoglobinuria may develop following exercise. Nausea and vomiting, icterus, elevated CK, hyperuricemia and reticulosis may also be observed[307]. In contrast to GSD-V, glucose intake prior to exercise worsens exercise capacity due to blocked use of both muscle glycogen and blood glucose[307].
The gene is located on chromosome Xq13.1, and the disease is inherited recessively[310]. In most patients, clinical findings appear in adulthood and are characterized by muscle weakness and muscle cramps during exercise. Elevated serum CK level and myopathic findings on electromyography may guide the diagnosis[311].
The last steps of glycogenolysis are abnormal. The disease is inherited in an autosomal recessive manner and characterized by exercise induced muscle cramps, myalgia, rhabdomyolysis and myoglobinuria. Serum CK level is increased between episodes[312].
GSD-XI was first described by Kanno et al[313] in 1980 and characterized by easy fatigue, increase in serum CK, myoglobin, lactate, and pyruvate levels immediately after ischemic work. The gene locus is on chromosome 11p15.1[314].
GSD-XII is a very rare disease resulting from aldolase A deficiency and characterized by muscle glycogen accumulation, crisis of rhabdomyolysis induced by fever and/or exercise and hemolytic anemia with or without myopathy or cognitive dysfunction[315]. It is an autosomal recessive disorder, and the gene is located on chromosome 16p11.2[316].
GSD-XIII was first described by Comi et al[317] in 2001 in a 47-year-old man with severe deficiency of muscle enolase activity. The patient had recurrent exercise induced myalgia without cramps. Serum CK concentration was elevated while serum lactate level was normal following ischemic forearm exercise. The related gene is located on chromosome 17p13.2[317].
Similar to Danon disease and PRKAG2 variants, glycogenin deficiency may cause left ventricular arrhythmogenic cardiomyopathy. Patients present with chest pain, progressive weakness, and vague presyncope spells[318].
There have been significant changes and improvements in the classification, diagnosis, and treatment of GSDs in recent years. We are now more aware that many GSDs, which were previously identified as childhood diseases, may present first in adulthood. Diagnosis can be challenging, especially for GSDs with milder phenotypes and those with only skeletal and/or cardiac muscle involvement. As early diagnosis and aggressive treatment is related to better prognosis, physicians should be aware of these conditions and include GSDs in the differential diagnosis of pediatric and adult patients with not only liver related manifestations but also skeletal and/or cardiac muscle, central nervous system, and multisystemic involvement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt; Rathnaswami A, India; Yao G, China S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Zhao S
| 1. | Ozen H. Glycogen storage diseases: new perspectives. World J Gastroenterol. 2007;13:2541-2553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 213] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (4)] |
| 2. | Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem J. 2012;441:763-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 496] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 3. | Ellingwood SS, Cheng A. Biochemical and clinical aspects of glycogen storage diseases. J Endocrinol. 2018;238:R131-R141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Saltik IN, Ozen H, Ciliv G, Koçak N, Yüce A, Gürakan F, Dinler G. Glycogen storage disease type Ia: frequency and clinical course in Turkish children. Indian J Pediatr. 2000;67:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kanungo S, Wells K, Tribett T, El-Gharbawy A. Glycogen metabolism and glycogen storage disorders. Ann Transl Med. 2018;6:474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 7. | Burda P, Hochuli M. Hepatic glycogen storage disorders: what have we learned in recent years? Curr Opin Clin Nutr Metab Care. 2015;18:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Kollberg G, Tulinius M, Gilljam T, Ostman-Smith I, Forsander G, Jotorp P, Oldfors A, Holme E. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0. N Engl J Med. 2007;357:1507-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Lewis GM, Spencer-Peet J, Stewart KM. Infantile Hypoglycaemia due to Inherited Deficiency of Glycogen Synthetase in Liver. Arch Dis Child. 1963;38:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Orho M, Bosshard NU, Buist NR, Gitzelmann R, Aynsley-Green A, Blümel P, Gannon MC, Nuttall FQ, Groop LC. Mutations in the liver glycogen synthase gene in children with hypoglycemia due to glycogen storage disease type 0. J Clin Invest. 1998;102:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Nuttall FQ, Gannon MC, Kubic VL, Hoyt KJ. The human liver Glycogen synthase isozyme gene is located on the short arm of chromosome 12. Genomics. 1994;19:404-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Hicks J, Wartchow E, Mierau G. Glycogen storage diseases: a brief review and update on clinical features, genetic abnormalities, pathologic features, and treatment. Ultrastruct Pathol. 2011;35:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Kamenets EA, Gusarova EA, Milovanova NV, Itkis YS, Strokova TV, Melikyan MA, Garyaeva IV, Rybkina IG, Nikitina NV, Zakharova EY. Hepatic glycogen synthase (GYS2) deficiency: seven novel patients and seven novel variants. JIMD Rep. 2020;53:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Laberge AM, Mitchell GA, van de Werve G, Lambert M. Long-term follow-up of a new case of liver glycogen synthase deficiency. Am J Med Genet A. 2003;120A:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Wolfsdorf JI, Weinstein DA. Glycogen storage diseases. Rev Endocr Metab Disord. 2003;4:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Weinstein DA, Correia CE, Saunders AC, Wolfsdorf JI. Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia. Mol Genet Metab. 2006;87:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Bachrach BE, Weinstein DA, Orho-Melander M, Burgess A, Wolfsdorf JI. Glycogen synthase deficiency (glycogen storage disease type 0) presenting with hyperglycemia and glucosuria: report of three new mutations. J Pediatr. 2002;140:781-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Kasapkara ÇS, Aycan Z, Açoğlu E, Senel S, Oguz MM, Ceylaner S. The variable clinical phenotype of three patients with hepatic glycogen synthase deficiency. J Pediatr Endocrinol Metab. 2017;30:459-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Tagliaferri F, Massese M, Russo L, Commone A, Gasperini S, Pretese R, Dionisi-Vici C, Maiorana A. Hepatic glycogen storage diseases type 0, VI and IX: description of an italian cohort. Orphanet J Rare Dis. 2022;17:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Browner MF, Nakano K, Bang AG, Fletterick RJ. Human muscle glycogen synthase cDNA sequence: a negatively charged protein with an asymmetric charge distribution. Proc Natl Acad Sci U S A. 1989;86:1443-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Gierke EV. Hepato-nephro-megalia-glycogenica (Glykogenspeicherkrankheit der Leber und Nieren). Beitr Pathol Anat. 1929;82:497-513. |
| 22. | Cori GT, Cori CF. Glucose-6-phosphatase of the liver in glycogen storage disease. J Biol Chem. 1952;199:661-667. [PubMed] |
| 23. | Narisawa K, Igarashi Y, Otomo H, Tada K. A new variant of glycogen storage disease type I probably due to a defect in the glucose-6-phosphate transport system. Biochem Biophys Res Commun. 1978;83:1360-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | van Schaftingen E, Gerin I. The glucose-6-phosphatase system. Biochem J. 2002;362:513-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Chou JY, Jun HS, Mansfield BC. Type I glycogen storage diseases: disorders of the glucose-6-phosphatase/glucose-6-phosphate transporter complexes. J Inherit Metab Dis. 2015;38:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Ekstein J, Rubin BY, Anderson SL, Weinstein DA, Bach G, Abeliovich D, Webb M, Risch N. Mutation frequencies for glycogen storage disease Ia in the Ashkenazi Jewish population. Am J Med Genet A. 2004;129A:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 27. | Scott SA, Edelmann L, Liu L, Luo M, Desnick RJ, Kornreich R. Experience with carrier screening and prenatal diagnosis for 16 Ashkenazi Jewish genetic diseases. Hum Mutat. 2010;31:1240-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Brody LC, Abel KJ, Castilla LH, Couch FJ, McKinley DR, Yin G, Ho PP, Merajver S, Chandrasekharappa SC, Xu J. Construction of a transcription map surrounding the BRCA1 locus of human chromosome 17. Genomics. 1995;25:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Yang Chou J, Mansfield BC. Molecular Genetics of Type 1 Glycogen Storage Diseases. Trends Endocrinol Metab. 1999;10:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr. 2002;161 Suppl 1:S20-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Derks TGJ, Rodriguez-Buritica DF, Ahmad A, de Boer F, Couce ML, Grünert SC, Labrune P, López Maldonado N, Fischinger Moura de Souza C, Riba-Wolman R, Rossi A, Saavedra H, Gupta RN, Valayannopoulos V, Mitchell J. Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Aydemir Y, Gürakan F, Saltık Temizel İN, Demir H, Oğuz KK, Yalnızoğlu D, Topçu M, Özen H, Yüce A. Evaluation of central nervous system in patients with glycogen storage disease type 1a. Turk J Pediatr. 2016;58:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Czapek EE, Deykin D, Salzman EW. Platelet dysfunction in glycogen storage disease type I. Blood. 1973;41:235-247. [PubMed] |
| 34. | Hutton RA, Macnab AJ, Rivers RP. Defect of platelet function associated with chronic hypoglycaemia. Arch Dis Child. 1976;51:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Mühlhausen C, Schneppenheim R, Budde U, Merkel M, Muschol N, Ullrich K, Santer R. Decreased plasma concentration of von Willebrand factor antigen (VWF:Ag) in patients with glycogen storage disease type Ia. J Inherit Metab Dis. 2005;28:945-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Austin SL, El-Gharbawy AH, Kasturi VG, James A, Kishnani PS. Menorrhagia in patients with type I glycogen storage disease. Obstet Gynecol. 2013;122:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Kishnani PS, Austin SL, Abdenur JE, Arn P, Bali DS, Boney A, Chung WK, Dagli AI, Dale D, Koeberl D, Somers MJ, Wechsler SB, Weinstein DA, Wolfsdorf JI, Watson MS; American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014;16:e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 38. | Banugaria SG, Austin SL, Boney A, Weber TJ, Kishnani PS. Hypovitaminosis D in glycogen storage disease type I. Mol Genet Metab. 2010;99:434-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Jacoby JT, Bento Dos Santos B, Nalin T, Colonetti K, Farret Refosco L, F M de Souza C, Spritzer PM, Poloni S, Hack-Mendes R, Schwartz IVD. Bone Mineral Density in Patients with Hepatic Glycogen Storage Diseases. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Minarich LA, Kirpich A, Fiske LM, Weinstein DA. Bone mineral density in glycogen storage disease type Ia and Ib. Genet Med. 2013;14:737-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Cabrera-Abreu J, Crabtree NJ, Elias E, Fraser W, Cramb R, Alger S. Bone mineral density and markers of bone turnover in patients with glycogen storage disease types I, III and IX. J Inherit Metab Dis. 2004;27:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Wang DQ, Carreras CT, Fiske LM, Austin S, Boree D, Kishnani PS, Weinstein DA. Characterization and pathogenesis of anemia in glycogen storage disease type Ia and Ib. Genet Med. 2012;14:795-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Visser G, Rake JP, Kokke FT, Nikkels PG, Sauer PJ, Smit GP. Intestinal function in glycogen storage disease type I. J Inherit Metab Dis. 2002;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Visser G, Rake JP, Fernandes J, Labrune P, Leonard JV, Moses S, Ullrich K, Smit GP. Neutropenia, neutrophil dysfunction, and inflammatory bowel disease in glycogen storage disease type Ib: results of the European Study on Glycogen Storage Disease type I. J Pediatr. 2000;137:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 45. | Lawrence NT, Chengsupanimit T, Brown LM, Derks TG, Smit GP, Weinstein DA. Inflammatory Bowel Disease in Glycogen Storage Disease Type Ia. J Pediatr Gastroenterol Nutr. 2017;64:e52-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Hannah WB, Ong RC, Moreno MN, Pendyal S, Abdelmalak M, Kelsen J, McGreal NM, Kishnani PS. Very early-onset inflammatory bowel disease: Novel description in glycogen storage disease type Ia. Mol Genet Metab Rep. 2022;31:100848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 47. | Smit GP. The long-term outcome of patients with glycogen storage disease type Ia. Eur J Pediatr. 1993;152 Suppl 1:S52-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Talente GM, Coleman RA, Alter C, Baker L, Brown BI, Cannon RA, Chen YT, Crigler JF Jr, Ferreira P, Haworth JC, Herman GE, Issenman RM, Keating JP, Linde R, Roe TF, Senior B, Wolfsdorf JI. Glycogen storage disease in adults. Ann Intern Med. 1994;120:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 140] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Ai J, He W, Huang X, Wu Y, Lei Y, Yu C, Görgülü K, Diakopoulos KN, Lu N, Zhu Y. A case report of acute pancreatitis with glycogen storage disease type IA in an adult patient and review of the literature. Medicine (Baltimore). 2020;99:e22644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Rivers E, Reynolds BC, Bunn S, Leech NJ, Straker J, Lambert HJ. Acute Pancreatitis Secondary to Severe Hypertriglyceridaemia in a Patient with Type 1a Glycogen Storage Disease: Emergent Use of Plasmapheresis. JIMD Rep. 2018;42:1-4. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Chen YT, Coleman RA, Scheinman JI, Kolbeck PC, Sidbury JB. Renal disease in type I glycogen storage disease. N Engl J Med. 1988;318:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 114] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Urushihara M, Kagami S, Ito M, Yasutomo K, Kondo S, Kitamura A, Takahashi A, Kuroda Y. Transforming growth factor-beta in renal disease with glycogen storage disease I. Pediatr Nephrol. 2004;19:676-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Martens DH, Rake JP, Navis G, Fidler V, van Dael CM, Smit GP. Renal function in glycogen storage disease type I, natural course, and renopreservative effects of ACE inhibition. Clin J Am Soc Nephrol. 2009;4:1741-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Yiu WH, Mead PA, Jun HS, Mansfield BC, Chou JY. Oxidative stress mediates nephropathy in type Ia glycogen storage disease. Lab Invest. 2010;90:620-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Reitsma-Bierens WC. Renal complications in glycogen storage disease type I. Eur J Pediatr. 1993;152 Suppl 1:S60-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Restaino I, Kaplan BS, Stanley C, Baker L. Nephrolithiasis, hypocitraturia, and a distal renal tubular acidification defect in type 1 glycogen storage disease. J Pediatr. 1993;122:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Lin CC, Tsai JD, Lin SP, Lee HC. Renal sonographic findings of type I glycogen storage disease in infancy and early childhood. Pediatr Radiol. 2005;35:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Bhowmik E, Ghosh M, Sabui TK, Mondal R. Glycogen Storage Disease Type I Presenting with Hypertension During Infancy. Indian J Pediatr. 2015;82:767. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Gjorgjieva M, Raffin M, Duchampt A, Perry A, Stefanutti A, Brevet M, Tortereau A, Dubourg L, Hubert-Buron A, Mabille M, Pelissou C, Lassalle L, Labrune P, Mithieux G, Rajas F. Progressive development of renal cysts in glycogen storage disease type I. Hum Mol Genet. 2016;25:3784-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur J Pediatr. 1993;152 Suppl 1:S63-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Wang DQ, Fiske LM, Carreras CT, Weinstein DA. Natural history of hepatocellular adenoma formation in glycogen storage disease type I. J Pediatr. 2011;159:442-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Cho JH, Lee YM, Bae SH, Chou JY. Activation of tumor-promoting pathways implicated in hepatocellular adenoma/carcinoma, a long-term complication of glycogen storage disease type Ia. Biochem Biophys Res Commun. 2020;522:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Kishnani PS, Chuang TP, Bali D, Koeberl D, Austin S, Weinstein DA, Murphy E, Chen YT, Boyette K, Liu CH, Li LH. Chromosomal and genetic alterations in human hepatocellular adenomas associated with type Ia glycogen storage disease. Hum Mol Genet. 2009;18:4781-4790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Lee P, Mather S, Owens C, Leonard J, Dicks-Mireaux C. Hepatic ultrasound findings in the glycogen storage diseases. Br J Radiol. 1994;67:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT, Kishnani PS. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Trioche P, Francoual J, Capel L, Odièvre M, Lindenbaum A, Labrune P. Apolipoprotein E polymorphism and serum concentrations in patients with glycogen storage disease type Ia. J Inherit Metab Dis. 2000;23:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Wittenstein B, Klein M, Finckh B, Ullrich K, Kohlschütter A. Radical trapping in glycogen storage disease 1a. Eur J Pediatr. 2002;161 Suppl 1:S70-S74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 68. | Wittenstein B, Klein M, Finckh B, Ullrich K, Kohlschütter A. Plasma antioxidants in pediatric patients with glycogen storage disease, diabetes mellitus, and hypercholesterolemia. Free Radic Biol Med. 2002;33:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Ubels FL, Rake JP, Slaets JP, Smit GP, Smit AJ. Is glycogen storage disease 1a associated with atherosclerosis? Eur J Pediatr. 2002;161 Suppl 1:S62-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Bernier AV, Correia CE, Haller MJ, Theriaque DW, Shuster JJ, Weinstein DA. Vascular dysfunction in glycogen storage disease type I. J Pediatr. 2009;154:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 71. | Melis D, Parenti G, Della Casa R, Sibilio M, Romano A, Di Salle F, Elefante R, Mansi G, Santoro L, Perretti A, Paludetto R, Sequino L, Andria G. Brain damage in glycogen storage disease type I. J Pediatr. 2004;144:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Muzetti JH, do Valle DA, Santos MLSF, Telles BA, Cordeiro ML. Neurological Characteristics of Pediatric Glycogen Storage Disease. Front Endocrinol (Lausanne). 2021;12:685272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 73. | Sechi A, Deroma L, Lapolla A, Paci S, Melis D, Burlina A, Carubbi F, Rigoldi M, Di Rocco M. Fertility and pregnancy in women affected by glycogen storage disease type I, results of a multicenter Italian study. J Inherit Metab Dis. 2013;36:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Ryan IP, Havel RJ, Laros RK Jr. Three consecutive pregnancies in a patient with glycogen storage disease type IA (von Gierke's disease). Am J Obstet Gynecol. 1994;170:1687-90; discussion 1690. [PubMed] |
| 75. | Mairovitz V, Labrune P, Fernandez H, Audibert F, Frydman R. Contraception and pregnancy in women affected by glycogen storage diseases. Eur J Pediatr. 2002;161 Suppl 1:S97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Angaroni CJ, Giner-Ayala AN, Hill LP, Guelbert NB, Paschini-Capra AE, Dodelson de Kremer R. Evaluation of the biotinidase activity in hepatic glycogen storage disease patients. Undescribed genetic finding associated with atypical enzymatic behavior: an outlook. J Inherit Metab Dis. 2010;33:S289-S294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 77. | Paesold-Burda P, Baumgartner MR, Santer R, Bosshard NU, Steinmann B. Elevated serum biotinidase activity in hepatic glycogen storage disorders--a convenient biomarker. J Inherit Metab Dis. 2007;30:896-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Wolf B, Freehauf CL, Thomas JA, Gordon PL, Greene CL, Ward JC. Markedly elevated serum biotinidase activity may indicate glycogen storage disease type Ia. J Inherit Metab Dis. 2003;26:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 79. | Saltik IN, Ozen H, Koçak N, Yüce A, Gürakan F. High biotinidase activity in type Ia glycogen storage disease. Am J Gastroenterol. 2000;95:2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | El-Gharbawy A, Tolun AA, Halaby CA, Austin SL, Kishnani PS, Bali DS. Beyond predicting diagnosis: Is there a role for measuring biotinidase activity in liver glycogen storage diseases? Mol Genet Metab Rep. 2022;31:100856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 81. | Baertling F, Mayatepek E, Gerner P, Baba HA, Franzel J, Schlune A, Meissner T. Liver cirrhosis in glycogen storage disease Ib. Mol Genet Metab. 2013;108:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Göğüş S, Koçak N, Ciliv G, Karabulut E, Akçören Z, Kale G, Cağlar M. Histologic features of the liver in type Ia glycogen storage disease: comparative study between different age groups and consecutive biopsies. Pediatr Dev Pathol. 2002;5:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 83. | Karhan AN, Hizarcioglu-Gulsen H, Gumus E, Akçören Z, Demir H, Saltik-Temizel İN, Orhan D, Özen H. Distinctive Features of Hepatic Steatosis in Children: Is It Primary or Secondary to Inborn Errors of Metabolism? Pediatr Gastroenterol Hepatol Nutr. 2021;24:518-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 84. | Qu Y, Abdenur JE, Eng CM, Desnick RJ. Molecular prenatal diagnosis of glycogen storage disease type Ia. Prenat Diagn. 1996;16:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP; European Study on Glycogen Storage Disease Type I (ESGSD I). Guidelines for management of glycogen storage disease type I - European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr. 2002;161 Suppl 1:S112-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Lee PJ, Dixon MA, Leonard JV. Uncooked cornstarch--efficacy in type I glycogenosis. Arch Dis Child. 1996;74:546-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Ross KM, Brown LM, Corrado MM, Chengsupanimit T, Curry LM, Ferrecchia IA, Porras LY, Mathew JT, Weinstein DA. Safety and Efficacy of Chronic Extended Release Cornstarch Therapy for Glycogen Storage Disease Type I. JIMD Rep. 2016;26:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Bhattacharya K. Dietary dilemmas in the management of glycogen storage disease type I. J Inherit Metab Dis. 2011;34:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Monteillet L, Labrune P, Hochuli M, Do Cao J, Tortereau A, Miliano AC, Ardon-Zitoun C, Duchampt A, Silva M, Verzieux V, Mithieux G, Rajas F. Cellular and metabolic effects of renin-angiotensin system blockade on glycogen storage disease type I nephropathy. Hum Mol Genet. 2022;31:914-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 90. | Melis D, Parenti G, Gatti R, Casa RD, Parini R, Riva E, Burlina AB, Dionisi Vici C, Di Rocco M, Furlan F, Torcoletti M, Papadia F, Donati A, Benigno V, Andria G. Efficacy of ACE-inhibitor therapy on renal disease in glycogen storage disease type 1: a multicentre retrospective study. Clin Endocrinol (Oxf). 2005;63:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Ozen H, Ciliv G, Koçak N, Saltik IN, Yüce A, Gürakan F. Short-term effect of captopril on microalbuminuria in children with glycogen storage disease type Ia. J Inherit Metab Dis. 2000;23:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Nagasaka H, Hirano K, Ohtake A, Miida T, Takatani T, Murayama K, Yorifuji T, Kobayashi K, Kanazawa M, Ogawa A, Takayanagi M. Improvements of hypertriglyceridemia and hyperlacticemia in Japanese children with glycogen storage disease type Ia by medium-chain triglyceride milk. Eur J Pediatr. 2007;166:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Das AM, Lücke T, Meyer U, Hartmann H, Illsinger S. Glycogen storage disease type 1: impact of medium-chain triglycerides on metabolic control and growth. Ann Nutr Metab. 2010;56:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 94. | Mollet-Boudjemline A, Hubert-Buron A, Boyer-Neumann C, Aldea R, Franco D, Trioche-Eberschweiller P, Mas AE, Mabille M, Labrune P, Gajdos V. Perioperative management of hemostasis for surgery of benign hepatic adenomas in patients with glycogen storage disease type ia. JIMD Rep. 2011;1:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 95. | Senior B, Loridan L. Functional differentiation of glycogenoses of the liver with respect to the use of glycerol. N Engl J Med. 1968;279:965-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 96. | Arion WJ, Wallin BK, Lange AJ, Ballas LM. On the involvement of a glucose 6-phosphate transport system in the function of microsomal glucose 6-phosphatase. Mol Cell Biochem. 1975;6:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 97. | Ihara K, Kuromaru R, Hara T. Genomic structure of the human glucose 6-phosphate translocase gene and novel mutations in the gene of a Japanese patient with glycogen storage disease type Ib. Hum Genet. 1998;103:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Annabi B, Hiraiwa H, Mansfield BC, Lei KJ, Ubagai T, Polymeropoulos MH, Moses SW, Parvari R, Hershkovitz E, Mandel H, Fryman M, Chou JY. The gene for glycogen-storage disease type 1b maps to chromosome 11q23. Am J Hum Genet. 1998;62:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 99. | Ihara K, Nomura A, Hikino S, Takada H, Hara T. Quantitative analysis of glucose-6-phosphate translocase gene expression in various human tissues and haematopoietic progenitor cells. J Inherit Metab Dis. 2000;23:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Kim GY, Lee YM, Kwon JH, Jun HS, Chou J. Glycogen storage disease type Ib neutrophils exhibit impaired cell adhesion and migration. Biochem Biophys Res Commun. 2017;482:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Chou JY, Jun HS, Mansfield BC. Neutropenia in type Ib glycogen storage disease. Curr Opin Hematol. 2010;17:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 102. | Leuzzi R, Bánhegyi G, Kardon T, Marcolongo P, Capecchi PL, Burger HJ, Benedetti A, Fulceri R. Inhibition of microsomal glucose-6-phosphate transport in human neutrophils results in apoptosis: a potential explanation for neutrophil dysfunction in glycogen storage disease type 1b. Blood. 2003;101:2381-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Kure S, Hou DC, Suzuki Y, Yamagishi A, Hiratsuka M, Fukuda T, Sugie H, Kondo N, Matsubara Y, Narisawa K. Glycogen storage disease type Ib without neutropenia. J Pediatr. 2000;137:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 104. | Dababneh R, Shawabkeh A, Gharaibeh S, Khouri ZA, Amayreh W, Bissada NF. Periodontal Manifestation of Type Ib Glycogen Storage Disease: A Rare Case Report. Clin Adv Periodontics. 2020;10:150-154. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Melis D, Fulceri R, Parenti G, Marcolongo P, Gatti R, Parini R, Riva E, Della Casa R, Zammarchi E, Andria G, Benedetti A. Genotype/phenotype correlation in glycogen storage disease type 1b: a multicentre study and review of the literature. Eur J Pediatr. 2005;164:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Martin AP, Bartels M, Schreiber S, Buehrdel P, Hauss J, Fangmann J. Successful staged kidney and liver transplantation for glycogen storage disease type Ib: A case report. Transplant Proc. 2006;38:3615-3619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 107. | Matern D, Starzl TE, Arnaout W, Barnard J, Bynon JS, Dhawan A, Emond J, Haagsma EB, Hug G, Lachaux A, Smit GP, Chen YT. Liver transplantation for glycogen storage disease types I, III, and IV. Eur J Pediatr. 1999;158 Suppl 2:S43-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Aeppli TR, Rymen D, Allegri G, Bode PK, Häberle J. Glycogen storage disease type VI: clinical course and molecular background. Eur J Pediatr. 2020;179:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 109. | Saltik-Temizel IN, Koçak N, Ozen H, Yüce A, Gürakan F, Demir H. Inflammatory bowel disease-like colitis in a young Turkish child with glycogen storage disease type 1b and elevated platelet count. Turk J Pediatr. 2005;47:180-182. [PubMed] |
| 110. | Roe TF, Thomas DW, Gilsanz V, Isaacs H Jr, Atkinson JB. Inflammatory bowel disease in glycogen storage disease type Ib. J Pediatr. 1986;109:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Dieckgraefe BK, Korzenik JR, Husain A, Dieruf L. Association of glycogen storage disease 1b and Crohn disease: results of a North American survey. Eur J Pediatr. 2002;161 Suppl 1:S88-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Visser G, Rake JP, Labrune P, Leonard JV, Moses S, Ullrich K, Wendel U, Groenier KH, Smit GP. Granulocyte colony-stimulating factor in glycogen storage disease type 1b. Results of the European Study on Glycogen Storage Disease Type 1. Eur J Pediatr. 2002;161 Suppl 1:S83-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Wicker C, Roda C, Perry A, Arnoux JB, Brassier A, Castelle M, Servais A, Donadieu J, Bouchereau J, Pigneur B, Labrune P, Ruemmele FM, de Lonlay P. Infectious and digestive complications in glycogen storage disease type Ib: Study of a French cohort. Mol Genet Metab Rep. 2020;23:100581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 114. | Gong YZ, Zhong XM, Zou JZ. Infliximab treatment of glycogenosis Ib with Crohn's-like enterocolitis: A case report. World J Clin Cases. 2021;9:5280-5286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 115. | Davis MK, Rufo PA, Polyak SF, Weinstein DA. Adalimumab for the treatment of Crohn-like colitis and enteritis in glycogen storage disease type Ib. J Inherit Metab Dis. 2008;31 Suppl 3:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Melis D, Balivo F, Della Casa R, Romano A, Taurisano R, Capaldo B, Riccardi G, Monsurrò MR, Parenti G, Andria G. Myasthenia gravis in a patient affected by glycogen storage disease type Ib: a further manifestation of an increased risk for autoimmune disorders? J Inherit Metab Dis. 2008;31 Suppl 2:S227-S231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Melis D, Della Casa R, Balivo F, Minopoli G, Rossi A, Salerno M, Andria G, Parenti G. Involvement of endocrine system in a patient affected by glycogen storage disease 1b: speculation on the role of autoimmunity. Ital J Pediatr. 2014;40:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 118. | Melis D, Pivonello R, Parenti G, Della Casa R, Salerno M, Lombardi G, Sebastio G, Colao A, Andria G. Increased prevalence of thyroid autoimmunity and hypothyroidism in patients with glycogen storage disease type I. J Pediatr. 2007;150:300-305, 305.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Melis D, Carbone F, Minopoli G, La Rocca C, Perna F, De Rosa V, Galgani M, Andria G, Parenti G, Matarese G. Cutting Edge: Increased Autoimmunity Risk in Glycogen Storage Disease Type 1b Is Associated with a Reduced Engagement of Glycolysis in T Cells and an Impaired Regulatory T Cell Function. J Immunol. 2017;198:3803-3808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 120. | Schroten H, Roesler J, Breidenbach T, Wendel U, Elsner J, Schweitzer S, Zeidler C, Burdach S, Lohmann-Matthes ML, Wahn V. Granulocyte and granulocyte-macrophage colony-stimulating factors for treatment of neutropenia in glycogen storage disease type Ib. J Pediatr. 1991;119:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Schroten H, Wendel U, Burdach S, Roesler J, Breidenbach T, Schweitzer S, Zeidler C, Welte K. Colony-stimulating factors for neutropenia in glycogen storage disease Ib. Lancet. 1991;337:736-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 122. | Roe TF, Coates TD, Thomas DW, Miller JH, Gilsanz V. Brief report: treatment of chronic inflammatory bowel disease in glycogen storage disease type Ib with colony-stimulating factors. N Engl J Med. 1992;326:1666-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | Alsultan A, Sokol RJ, Lovell MA, Thurman G, Ambruso DR. Long term G-CSF-induced remission of ulcerative colitis-like inflammatory bowel disease in a patient with glycogen storage disease Ib and evaluation of associated neutrophil function. Pediatr Blood Cancer. 2010;55:1410-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 124. | Dale DC, Bolyard AA, Marrero T, Kelley ML, Makaryan V, Tran E, Leung J, Boxer LA, Kishnani PS, Austin S, Wanner C, Ferrecchia IA, Khalaf D, Maze D, Kurtzberg J, Zeidler C, Welte K, Weinstein DA. Neutropenia in glycogen storage disease Ib: outcomes for patients treated with granulocyte colony-stimulating factor. Curr Opin Hematol. 2019;26:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 125. | Li AM, Thyagu S, Maze D, Schreiber R, Sirrs S, Stockler-Ipsiroglu S, Sutherland H, Vercauteren S, Schultz KR. Prolonged granulocyte colony stimulating factor use in glycogen storage disease type 1b associated with acute myeloid leukemia and with shortened telomere length. Pediatr Hematol Oncol. 2018;35:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 126. | Melis D, Della Casa R, Parini R, Rigoldi M, Cacciapuoti C, Marcolongo P, Benedetti A, Gaudieri V, Andria G, Parenti G. Vitamin E supplementation improves neutropenia and reduces the frequency of infections in patients with glycogen storage disease type 1b. Eur J Pediatr. 2009;168:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 127. | Reddy SK, Austin SL, Spencer-Manzon M, Koeberl DD, Clary BM, Desai DM, Smith AD, Kishnani PS. Liver transplantation for glycogen storage disease type Ia. J Hepatol. 2009;51:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 128. | Boers SJ, Visser G, Smit PG, Fuchs SA. Liver transplantation in glycogen storage disease type I. Orphanet J Rare Dis. 2014;9:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 129. | Faivre L, Houssin D, Valayer J, Brouard J, Hadchouel M, Bernard O. Long-term outcome of liver transplantation in patients with glycogen storage disease type Ia. J Inherit Metab Dis. 1999;22:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 130. | Liu PP, de Villa VH, Chen YS, Wang CC, Wang SH, Chiang YC, Jawan B, Cheung HK, Cheng YF, Huang TL, Eng HL, Chuang FR, Chen CL. Outcome of living donor liver transplantation for glycogen storage disease. Transplant Proc. 2003;35:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 131. | Yuen WY, Quak SH, Aw MM, Karthik SV. Long-term outcome after liver transplantation in children with type 1 glycogen storage disease. Pediatr Transplant. 2021;25:e13872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 132. | Emmett M, Narins RG. Renal tranplantation in type 1 glycogenosis. Failure to improve glucose metabolism. JAMA. 1978;239:1642-1644. [PubMed] |
| 133. | Iyer SG, Chen CL, Wang CC, Wang SH, Concejero AM, Liu YW, Yang CH, Yong CC, Jawan B, Cheng YF, Eng HL. Long-term results of living donor liver transplantation for glycogen storage disorders in children. Liver Transpl. 2007;13:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 134. | Shimizu S, Sakamoto S, Horikawa R, Fukuda A, Uchida H, Takeda M, Yanagi Y, Irie R, Yoshioka T, Kasahara M. Longterm Outcomes of Living Donor Liver Transplantation for Glycogen Storage Disease Type 1b. Liver Transpl. 2020;26:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Veiga-da-Cunha M, Chevalier N, Stephenne X, Defour JP, Paczia N, Ferster A, Achouri Y, Dewulf JP, Linster CL, Bommer GT, Van Schaftingen E. Failure to eliminate a phosphorylated glucose analog leads to neutropenia in patients with G6PT and G6PC3 deficiency. Proc Natl Acad Sci U S A. 2019;116:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 136. | Wortmann SB, Van Hove JLK, Derks TGJ, Chevalier N, Knight V, Koller A, Oussoren E, Mayr JA, van Spronsen FJ, Lagler FB, Gaughan S, Van Schaftingen E, Veiga-da-Cunha M. Treating neutropenia and neutrophil dysfunction in glycogen storage disease type Ib with an SGLT2 inhibitor. Blood. 2020;136:1033-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 137. | Hexner-Erlichman Z, Veiga-da-Cunha M, Zehavi Y, Vadasz Z, Sabag AD, Tatour S, Spiegel R. Favorable outcome of empagliflozin treatment in two pediatric glycogen storage disease type 1b patients. Front Pediatr. 2022;10:1071464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 138. | Makrilakis K, Barmpagianni A, Veiga-da-Cunha M. Repurposing of Empagliflozin as a Possible Treatment for Neutropenia and Inflammatory Bowel Disease in Glycogen Storage Disease Type Ib: A Case Report. Cureus. 2022;14:e27264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 139. | Bidiuk J, Gaciong ZA, Sobieraj P. The overall benefits of empagliflozin treatment in adult siblings with glycogen storage disease type Ib: one year experience. Arch Med Sci. 2022;18:1095-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 140. | Grünert SC, Rosenbaum-Fabian S, Schumann A, Selbitz AC, Merz W, Gieselmann A, Spiekerkoetter U. Two successful pregnancies and first use of empagliflozin during pregnancy in glycogen storage disease type Ib. JIMD Rep. 2022;63:303-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 141. | Halligan RK, Dalton RN, Turner C, Lewis KA, Mundy HR. Understanding the role of SGLT2 inhibitors in glycogen storage disease type Ib: the experience of one UK centre. Orphanet J Rare Dis. 2022;17:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 142. | Boulanger C, Stephenne X, Diederich J, Mounkoro P, Chevalier N, Ferster A, Van Schaftingen E, Veiga-da-Cunha M. Successful use of empagliflozin to treat neutropenia in two G6PC3-deficient children: Impact of a mutation in SGLT5. J Inherit Metab Dis. 2022;45:759-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 143. | Mikami M, Arai A, Mizumoto H. Empagliflozin ameliorated neutropenia in a girl with glycogen storage disease Ib. Pediatr Int. 2021;63:1394-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 144. | Grünert SC, Elling R, Maag B, Wortmann SB, Derks TGJ, Hannibal L, Schumann A, Rosenbaum-Fabian S, Spiekerkoetter U. Improved inflammatory bowel disease, wound healing and normal oxidative burst under treatment with empagliflozin in glycogen storage disease type Ib. Orphanet J Rare Dis. 2020;15:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 145. | Grünert SC, Derks TGJ, Adrian K, Al-Thihli K, Ballhausen D, Bidiuk J, Bordugo A, Boyer M, Bratkovic D, Brunner-Krainz M, Burlina A, Chakrapani A, Corpeleijn W, Cozens A, Dawson C, Dhamko H, Milosevic MD, Eiroa H, Finezilber Y, Moura de Souza CF, Garcia-Jiménez MC, Gasperini S, Haas D, Häberle J, Halligan R, Fung LH, Hörbe-Blindt A, Horka LM, Huemer M, Uçar SK, Kecman B, Kilavuz S, Kriván G, Lindner M, Lüsebrink N, Makrilakis K, Mei-Kwun Kwok A, Maier EM, Maiorana A, McCandless SE, Mitchell JJ, Mizumoto H, Mundy H, Ochoa C, Pierce K, Fraile PQ, Regier D, Rossi A, Santer R, Schuman HC, Sobieraj P, Spenger J, Spiegel R, Stepien KM, Tal G, Tanšek MZ, Torkar AD, Tchan M, Thyagu S, Schrier Vergano SA, Vucko E, Weinhold N, Zsidegh P, Wortmann SB. Efficacy and safety of empagliflozin in glycogen storage disease type Ib: Data from an international questionnaire. Genet Med. 2022;24:1781-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 146. | Chou JY, Mansfield BC. Recombinant AAV-directed gene therapy for type I glycogen storage diseases. Expert Opin Biol Ther. 2011;11:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 147. | Kwon JH, Lee YM, Cho JH, Kim GY, Anduaga J, Starost MF, Mansfield BC, Chou JY. Liver-directed gene therapy for murine glycogen storage disease type Ib. Hum Mol Genet. 2017;26:4395-4405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 148. | Chou JY, Cho JH, Kim GY, Mansfield BC. Molecular biology and gene therapy for glycogen storage disease type Ib. J Inherit Metab Dis. 2018;41:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 149. | Lee YM, Conlon TJ, Specht A, Coleman KE, Brown LM, Estrella AM, Dambska M, Dahlberg KR, Weinstein DA. Long-term safety and efficacy of AAV gene therapy in the canine model of glycogen storage disease type Ia. J Inherit Metab Dis. 2018;41:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 150. | Yiu WH, Pan CJ, Allamarvdasht M, Kim SY, Chou JY. Glucose-6-phosphate transporter gene therapy corrects metabolic and myeloid abnormalities in glycogen storage disease type Ib mice. Gene Ther. 2007;14:219-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 151. | Landau DJ, Brooks ED, Perez-Pinera P, Amarasekara H, Mefferd A, Li S, Bird A, Gersbach CA, Koeberl DD. In Vivo Zinc Finger Nuclease-mediated Targeted Integration of a Glucose-6-phosphatase Transgene Promotes Survival in Mice With Glycogen Storage Disease Type IA. Mol Ther. 2016;24:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 152. | Zhang L, Lee C, Arnaoutova I, Anduaga J, Starost MF, Mansfield BC, Chou JY. Gene therapy using a novel G6PC-S298C variant enhances the long-term efficacy for treating glycogen storage disease type Ia. Biochem Biophys Res Commun. 2020;527:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 153. | Shen JJ, Chen YT. Molecular characterization of glycogen storage disease type III. Curr Mol Med. 2002;2:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 154. | Bao Y, Yang BZ, Dawson TL Jr, Chen YT. Isolation and nucleotide sequence of human liver glycogen debranching enzyme mRNA: identification of multiple tissue-specific isoforms. Gene. 1997;197:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 155. | Bao Y, Dawson TL Jr, Chen YT. Human glycogen debranching enzyme gene (AGL): complete structural organization and characterization of the 5' flanking region. Genomics. 1996;38:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | Parvari R, Moses S, Shen J, Hershkovitz E, Lerner A, Chen YT. A single-base deletion in the 3'-coding region of glycogen-debranching enzyme is prevalent in glycogen storage disease type IIIA in a population of North African Jewish patients. Eur J Hum Genet. 1997;5:266-270. [PubMed] |
| 157. | Santer R, Kinner M, Steuerwald U, Kjaergaard S, Skovby F, Simonsen H, Shaiu WL, Chen YT, Schneppenheim R, Schaub J. Molecular genetic basis and prevalence of glycogen storage disease type IIIA in the Faroe Islands. Eur J Hum Genet. 2001;9:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 158. | Rousseau-Nepton I, Okubo M, Grabs R; FORGE Canada Consortium, Mitchell J, Polychronakos C, Rodd C. A founder AGL mutation causing glycogen storage disease type IIIa in Inuit identified through whole-exome sequencing: a case series. CMAJ. 2015;187:E68-E73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 159. | Shen J, Bao Y, Liu HM, Lee P, Leonard JV, Chen YT. Mutations in exon 3 of the glycogen debranching enzyme gene are associated with glycogen storage disease type III that is differentially expressed in liver and muscle. J Clin Invest. 1996;98:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 160. | Goldstein JL, Austin SL, Boyette K, Kanaly A, Veerapandiyan A, Rehder C, Kishnani PS, Bali DS. Molecular analysis of the AGL gene: identification of 25 novel mutations and evidence of genetic heterogeneity in patients with Glycogen Storage Disease Type III. Genet Med. 2010;12:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 161. | Ding JH, de Barsy T, Brown BI, Coleman RA, Chen YT. Immunoblot analyses of glycogen debranching enzyme in different subtypes of glycogen storage disease type III. J Pediatr. 1990;116:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 162. | Van Hoof F, Hers HG. The subgroups of type 3 glycogenosis. Eur J Biochem. 1967;2:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 163. | Derks TG, van Rijn M. Lipids in hepatic glycogen storage diseases: pathophysiology, monitoring of dietary management and future directions. J Inherit Metab Dis. 2015;38:537-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 164. | Bernier AV, Sentner CP, Correia CE, Theriaque DW, Shuster JJ, Smit GP, Weinstein DA. Hyperlipidemia in glycogen storage disease type III: effect of age and metabolic control. J Inherit Metab Dis. 2008;31:729-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 165. | Coleman RA, Winter HS, Wolf B, Chen YT. Glycogen debranching enzyme deficiency: long-term study of serum enzyme activities and clinical features. J Inherit Metab Dis. 1992;15:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 166. | Halaby CA, Young SP, Austin S, Stefanescu E, Bali D, Clinton LK, Smith B, Pendyal S, Upadia J, Schooler GR, Mavis AM, Kishnani PS. Liver fibrosis during clinical ascertainment of glycogen storage disease type III: a need for improved and systematic monitoring. Genet Med. 2019;21:2686-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 167. | Siciliano M, De Candia E, Ballarin S, Vecchio FM, Servidei S, Annese R, Landolfi R, Rossi L. Hepatocellular carcinoma complicating liver cirrhosis in type IIIa glycogen storage disease. J Clin Gastroenterol. 2000;31:80-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 168. | Demo E, Frush D, Gottfried M, Koepke J, Boney A, Bali D, Chen YT, Kishnani PS. Glycogen storage disease type III-hepatocellular carcinoma a long-term complication? J Hepatol. 2007;46:492-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 169. | Labrune P, Trioche P, Duvaltier I, Chevalier P, Odièvre M. Hepatocellular adenomas in glycogen storage disease type I and III: a series of 43 patients and review of the literature. J Pediatr Gastroenterol Nutr. 1997;24:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 180] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 170. | Sentner CP, Hoogeveen IJ, Weinstein DA, Santer R, Murphy E, McKiernan PJ, Steuerwald U, Beauchamp NJ, Taybert J, Laforêt P, Petit FM, Hubert A, Labrune P, Smit GPA, Derks TGJ. Glycogen storage disease type III: diagnosis, genotype, management, clinical course and outcome. J Inherit Metab Dis. 2016;39:697-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 171. | Lucchiari S, Pagliarani S, Salani S, Filocamo M, Di Rocco M, Melis D, Rodolico C, Musumeci O, Toscano A, Bresolin N, Comi GP. Hepatic and neuromuscular forms of glycogenosis type III: nine mutations in AGL. Hum Mutat. 2006;27:600-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 172. | Lee P, Burch M, Leonard JV. Plasma creatine kinase and cardiomyopathy in glycogen storage disease type III. J Inherit Metab Dis. 1995;18:751-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 173. | Coleman RA, Winter HS, Wolf B, Gilchrist JM, Chen YT. Glycogen storage disease type III (glycogen debranching enzyme deficiency): correlation of biochemical defects with myopathy and cardiomyopathy. Ann Intern Med. 1992;116:896-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 174. | Ben Chehida A, Ben Messaoud S, Ben Abdelaziz R, Ben Ali N, Boudabous H, Ben Abdelaziz I, Ben Ameur Z, Sassi Y, Kaabachi N, Abdelhak S, Abdelmoula MS, Fradj M, Azzouz H, Tebib N. Neuromuscular Involvement in Glycogen Storage Disease Type III in Fifty Tunisian Patients: Phenotype and Natural History in Young Patients. Neuropediatrics. 2019;50:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 175. | Mogahed EA, Girgis MY, Sobhy R, Elhabashy H, Abdelaziz OM, El-Karaksy H. Skeletal and cardiac muscle involvement in children with glycogen storage disease type III. Eur J Pediatr. 2015;174:1545-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 176. | Kishnani PS, Austin SL, Arn P, Bali DS, Boney A, Case LE, Chung WK, Desai DM, El-Gharbawy A, Haller R, Smit GP, Smith AD, Hobson-Webb LD, Wechsler SB, Weinstein DA, Watson MS; ACMG. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 177. | Momoi T, Sano H, Yamanaka C, Sasaki H, Mikawa H. Glycogen storage disease type III with muscle involvement: reappraisal of phenotypic variability and prognosis. Am J Med Genet. 1992;42:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 178. | Berling É, Laforêt P, Wahbi K, Labrune P, Petit F, Ronzitti G, O'Brien A. Narrative review of glycogen storage disorder type III with a focus on neuromuscular, cardiac and therapeutic aspects. J Inherit Metab Dis. 2021;44:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Moses SW, Wanderman KL, Myroz A, Frydman M. Cardiac involvement in glycogen storage disease type III. Eur J Pediatr. 1989;148:764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 180. | Vertilus SM, Austin SL, Foster KS, Boyette KE, Bali DS, Li JS, Kishnani PS, Wechsler SB. Echocardiographic manifestations of Glycogen Storage Disease III: increase in wall thickness and left ventricular mass over time. Genet Med. 2010;12:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 181. | Ben Chehida A, Ben Messaoud S, Ben Abdelaziz R, Mansouri H, Boudabous H, Hakim K, Ben Ali N, Ben Ameur Z, Sassi Y, Kaabachi N, Abdelhak S, Abdelmoula MS, Azzouz H, Tebib N. A lower energetic, protein and uncooked cornstarch intake is associated with a more severe outcome in glycogen storage disease type III: an observational study of 50 patients. J Pediatr Endocrinol Metab. 2018;31:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 182. | Austin SL, Proia AD, Spencer-Manzon MJ, Butany J, Wechsler SB, Kishnani PS. Cardiac Pathology in Glycogen Storage Disease Type III. JIMD Rep. 2012;6:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 183. | Focardi M, Bosco A, Bugelli V, Defraia B, Donati MA, Pinchi V. "On air" diagnosis of sudden cardiac death with dynamic Holter ECG in glycogen storage disease type III young female. Minerva Pediatr. 2020;72:142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 184. | Cleary MA, Walter JH, Kerr BA, Wraith JE. Facial appearance in glycogen storage disease type III. Clin Dysmorphol. 2002;11:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 185. | Mundy HR, Williams JE, Lee PJ, Fewtrell MS. Reduction in bone mineral density in glycogenosis type III may be due to a mixed muscle and bone deficit. J Inherit Metab Dis. 2008;31:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 186. | Melis D, Rossi A, Pivonello R, Del Puente A, Pivonello C, Cangemi G, Negri M, Colao A, Andria G, Parenti G. Reduced bone mineral density in glycogen storage disease type III: evidence for a possible connection between metabolic imbalance and bone homeostasis. Bone. 2016;86:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 187. | Lee PJ, Patel A, Hindmarsh PC, Mowat AP, Leonard JV. The prevalence of polycystic ovaries in the hepatic glycogen storage diseases: its association with hyperinsulinism. Clin Endocrinol (Oxf). 1995;42:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 188. | Spengos K, Michelakakis H, Vontzalidis A, Zouvelou V, Manta P. Diabetes mellitus associated with glycogen storage disease type III. Muscle Nerve. 2009;39:876-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 189. | Michon CC, Gargiulo M, Hahn-Barma V, Petit F, Nadaj-Pakleza A, Herson A, Eymard B, Labrune P, Laforet P. Cognitive profile of patients with glycogen storage disease type III: a clinical description of seven cases. J Inherit Metab Dis. 2015;38:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 190. | Valayannopoulos V, Bajolle F, Arnoux JB, Dubois S, Sannier N, Baussan C, Petit F, Labrune P, Rabier D, Ottolenghi C, Vassault A, Broissand C, Bonnet D, de Lonlay P. Successful treatment of severe cardiomyopathy in glycogen storage disease type III With D,L-3-hydroxybutyrate, ketogenic and high-protein diet. Pediatr Res. 2011;70:638-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 191. | Francini-Pesenti F, Tresso S, Vitturi N. Modified Atkins ketogenic diet improves heart and skeletal muscle function in glycogen storage disease type III. Acta Myol. 2019;38:17-20. [PubMed] |
| 192. | Kumru Akin B, Ozturk Hismi B, Daly A. Improvement in hypertrophic cardiomyopathy after using a high-fat, high-protein and low-carbohydrate diet in a non-adherent child with glycogen storage disease type IIIa. Mol Genet Metab Rep. 2022;32:100904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 193. | Sentner CP, Caliskan K, Vletter WB, Smit GP. Heart Failure Due to Severe Hypertrophic Cardiomyopathy Reversed by Low Calorie, High Protein Dietary Adjustments in a Glycogen Storage Disease Type IIIa Patient. JIMD Rep. 2012;5:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 194. | Derks TG, Smit GP. Dietary management in glycogen storage disease type III: what is the evidence? J Inherit Metab Dis. 2015;38:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 195. | Davis MK, Weinstein DA. Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant. 2008;12:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 196. | Andersen DH. Familial cirrhosis of the liver with storage of abnormal glycogen. Lab Invest. 1956;5:11-20. [PubMed] |
| 197. | Brown BI, Brown DH. Lack of an alpha-1,4-glucan: alpha-1,4-glucan 6-glycosyl transferase in a case of type IV glycogenosis. Proc Natl Acad Sci U S A. 1966;56:725-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 198. | Moses SW, Parvari R. The variable presentations of glycogen storage disease type IV: a review of clinical, enzymatic and molecular studies. Curr Mol Med. 2002;2:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 199. | Thon VJ, Khalil M, Cannon JF. Isolation of human glycogen branching enzyme cDNAs by screening complementation in yeast. J Biol Chem. 1993;268:7509-7513. [PubMed] |
| 200. | L'herminé-Coulomb A, Beuzen F, Bouvier R, Rolland MO, Froissart R, Menez F, Audibert F, Labrune P. Fetal type IV glycogen storage disease: clinical, enzymatic, and genetic data of a pure muscular form with variable and early antenatal manifestations in the same family. Am J Med Genet A. 2005;139A:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 201. | Derks TGJ, Peeks F, de Boer F, Fokkert-Wilts M, van der Doef HPJ, van den Heuvel MC, Szymańska E, Rokicki D, Ryan PT, Weinstein DA. The potential of dietary treatment in patients with glycogen storage disease type IV. J Inherit Metab Dis. 2021;44:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 202. | de Moor RA, Schweizer JJ, van Hoek B, Wasser M, Vink R, Maaswinkel-Mooy PD. Hepatocellular carcinoma in glycogen storage disease type IV. Arch Dis Child. 2000;82:479-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 203. | Greene HL, Brown BI, McClenathan DT, Agostini RM Jr, Taylor SR. A new variant of type IV glycogenosis: deficiency of branching enzyme activity without apparent progressive liver disease. Hepatology. 1988;8:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 204. | Schröder JM, May R, Shin YS, Sigmund M, Nase-Hüppmeier S. Juvenile hereditary polyglucosan body disease with complete branching enzyme deficiency (type IV glycogenosis). Acta Neuropathol. 1993;85:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 205. | Servidei S, Riepe RE, Langston C, Tani LY, Bricker JT, Crisp-Lindgren N, Travers H, Armstrong D, DiMauro S. Severe cardiopathy in branching enzyme deficiency. J Pediatr. 1987;111:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 206. | Bruno C, van Diggelen OP, Cassandrini D, Gimpelev M, Giuffrè B, Donati MA, Introvini P, Alegria A, Assereto S, Morandi L, Mora M, Tonoli E, Mascelli S, Traverso M, Pasquini E, Bado M, Vilarinho L, van Noort G, Mosca F, DiMauro S, Zara F, Minetti C. Clinical and genetic heterogeneity of branching enzyme deficiency (glycogenosis type IV). Neurology. 2004;63:1053-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 207. | Alegria A, Martins E, Dias M, Cunha A, Cardoso ML, Maire I. Glycogen storage disease type IV presenting as hydrops fetalis. J Inherit Metab Dis. 1999;22:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 208. | Akman HO, Karadimas C, Gyftodimou Y, Grigoriadou M, Kokotas H, Konstantinidou A, Anninos H, Patsouris E, Thaker HM, Kaplan JB, Besharat I, Hatzikonstantinou K, Fotopoulos S, Dimauro S, Petersen MB. Prenatal diagnosis of glycogen storage disease type IV. Prenat Diagn. 2006;26:951-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 209. | Zellweger H, Mueller S, Ionasescu V, Schochet SS, McCormick WF. Glycogenosis. IV. A new cause of infantile hypotonia. J Pediatr. 1972;80:842-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 210. | McMaster KR, Powers JM, Hennigar GR Jr, Wohltmann HJ, Farr GH Jr. Nervous system involvement in type IV glycogenosis. Arch Pathol Lab Med. 1979;103:105-111. [PubMed] |
| 211. | Giuffrè B, Parini R, Rizzuti T, Morandi L, van Diggelen OP, Bruno C, Giuffrè M, Corsello G, Mosca F. Severe neonatal onset of glycogenosis type IV: clinical and laboratory findings leading to diagnosis in two siblings. J Inherit Metab Dis. 2004;27:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 212. | Reusche E, Aksu F, Goebel HH, Shin YS, Yokota T, Reichmann H. A mild juvenile variant of type IV glycogenosis. Brain Dev. 1992;14:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 213. | Shin YS, Steigüber H, Klemm P, Endres W, Schwab O, Wolff G. Branching enzyme in erythrocytes. Detection of type IV glycogenosis homozygotes and heterozygotes. J Inherit Metab Dis. 1988;11 Suppl 2:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 214. | Ferguson IT, Mahon M, Cumming WJ. An adult case of Andersen's disease--Type IV glycogenosis. A clinical, histochemical, ultrastructural and biochemical study. J Neurol Sci. 1983;60:337-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 215. | Lossos A, Barash V, Soffer D, Argov Z, Gomori M, Ben-Nariah Z, Abramsky O, Steiner I. Hereditary branching enzyme dysfunction in adult polyglucosan body disease: a possible metabolic cause in two patients. Ann Neurol. 1991;30:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 216. | Mizuochi T, Kimura A, Nishiura H, Inomata Y, Okajima H, Sugie H, Mitsubuchi H, Yagi M, Kage M. Liver biopsy is an important procedure in the diagnosis of glycogen storage disease type IV. Pediatr Int. 2011;53:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 217. | Willot S, Marchand V, Rasquin A, Alvarez F, Martin SR. Systemic progression of type IV glycogen storage disease after liver transplantation. J Pediatr Gastroenterol Nutr. 2010;51:661-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 218. | Li SC, Chen CM, Goldstein JL, Wu JY, Lemyre E, Burrow TA, Kang PB, Chen YT, Bali DS. Glycogen storage disease type IV: novel mutations and molecular characterization of a heterogeneous disorder. J Inherit Metab Dis. 2010;33 Suppl 3:S83-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 219. | Goldberg T, Slonim AE. Nutrition therapy for hepatic glycogen storage diseases. J Am Diet Assoc. 1993;93:1423-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 220. | Szymańska E, Szymańska S, Truszkowska G, Ciara E, Pronicki M, Shin YS, Podskarbi T, Kępka A, Śpiewak M, Płoski R, Bilińska ZT, Rokicki D. Variable clinical presentation of glycogen storage disease type IV: from severe hepatosplenomegaly to cardiac insufficiency. Some discrepancies in genetic and biochemical abnormalities. Arch Med Sci. 2018;14:237-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 221. | Rosenthal P, Podesta L, Grier R, Said JW, Sher L, Cocjin J, Watanabe F, Vasiliauskas E, van de Velde R, Makowka L. Failure of liver transplantation to diminish cardiac deposits of amylopectin and leukocyte inclusions in type IV glycogen storage disease. Liver Transpl Surg. 1995;1:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 222. | Sokal EM, Van Hoof F, Alberti D, de Ville de Goyet J, de Barsy T, Otte JB. Progressive cardiac failure following orthotopic liver transplantation for type IV glycogenosis. Eur J Pediatr. 1992;151:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 223. | Choi SY, Kang B, Choe JY, Lee Y, Jang HJ, Park HD, Lee SK, Choe YH. A Case of Glycogen Storage Disease IV with Rare Homozygous Mutations in the Glycogen Branching Enzyme Gene. Pediatr Gastroenterol Hepatol Nutr. 2018;21:365-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 224. | Liu M, Sun LY. Liver Transplantation for Glycogen Storage Disease Type IV. Front Pediatr. 2021;9:633822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 225. | Selby R, Starzl TE, Yunis E, Brown BI, Kendall RS, Tzakis A. Liver transplantation for type IV glycogen storage disease. N Engl J Med. 1991;324:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 226. | Starzl TE, Demetris AJ, Trucco M, Ricordi C, Ildstad S, Terasaki PI, Murase N, Kendall RS, Kocova M, Rudert WA. Chimerism after liver transplantation for type IV glycogen storage disease and type 1 Gaucher's disease. N Engl J Med. 1993;328:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 182] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 227. | Kakhlon O, Ferreira I, Solmesky LJ, Khazanov N, Lossos A, Alvarez R, Yetil D, Pampou S, Weil M, Senderowitz H, Escriba P, Yue WW, Akman HO. Guaiacol as a drug candidate for treating adult polyglucosan body disease. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 228. | Kakhlon O, Vaknin H, Mishra K, D'Souza J, Marisat M, Sprecher U, Wald-Altman S, Dukhovny A, Raviv Y, Da'adoosh B, Engel H, Benhamron S, Nitzan K, Sweetat S, Permyakova A, Mordechai A, Akman HO, Rosenmann H, Lossos A, Tam J, Minassian BA, Weil M. Alleviation of a polyglucosan storage disorder by enhancement of autophagic glycogen catabolism. EMBO Mol Med. 2021;13:e14554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 229. | Hers HG. [Enzymatic studies of hepatic fragments; application to the classification of glycogenoses]. Rev Int Hepatol. 1959;9:35-55. [PubMed] |
| 230. | Newgard CB, Hwang PK, Fletterick RJ. The family of glycogen phosphorylases: structure and function. Crit Rev Biochem Mol Biol. 1989;24:69-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 301] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 231. | Newgard CB, Fletterick RJ, Anderson LA, Lebo RV. The polymorphic locus for glycogen storage disease VI (liver glycogen phosphorylase) maps to chromosome 14. Am J Hum Genet. 1987;40:351-364. [PubMed] |
| 232. | Grünert SC, Hannibal L, Spiekerkoetter U. The Phenotypic and Genetic Spectrum of Glycogen Storage Disease Type VI. Genes (Basel). 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 233. | Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J, Raiman J, Schulze A, Siriwardena K, Mercimek-Mahmutoglu S. The natural history of glycogen storage disease types VI and IX: Long-term outcome from the largest metabolic center in Canada. Mol Genet Metab. 2014;113:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 234. | Tang NL, Hui J, Young E, Worthington V, To KF, Cheung KL, Li CK, Fok TF. A novel mutation (G233D) in the glycogen phosphorylase gene in a patient with hepatic glycogen storage disease and residual enzyme activity. Mol Genet Metab. 2003;79:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 235. | Burwinkel B, Bakker HD, Herschkovitz E, Moses SW, Shin YS, Kilimann MW. Mutations in the liver glycogen phosphorylase gene (PYGL) underlying glycogenosis type VI. Am J Hum Genet. 1998;62:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 236. | Kishnani PS, Goldstein J, Austin SL, Arn P, Bachrach B, Bali DS, Chung WK, El-Gharbawy A, Brown LM, Kahler S, Pendyal S, Ross KM, Tsilianidis L, Weinstein DA, Watson MS; ACMG Work Group on Diagnosis and Management of Glycogen Storage Diseases Type VI and IX. Diagnosis and management of glycogen storage diseases type VI and IX: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2019;21:772-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 237. | Luo X, Duan Y, Fang D, Sun Y, Xiao B, Zhang H, Han L, Liang L, Gong Z, Gu X, Yu Y, Qiu W. Diagnosis and follow-up of glycogen storage disease (GSD) type VI from the largest GSD center in China. Hum Mutat. 2022;43:557-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 238. | Beauchamp NJ, Taybert J, Champion MP, Layet V, Heinz-Erian P, Dalton A, Tanner MS, Pronicka E, Sharrard MJ. High frequency of missense mutations in glycogen storage disease type VI. J Inherit Metab Dis. 2007;30:722-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 239. | Brown LM, Corrado MM, van der Ende RM, Derks TG, Chen MA, Siegel S, Hoyt K, Correia CE, Lumpkin C, Flanagan TB, Carreras CT, Weinstein DA. Evaluation of glycogen storage disease as a cause of ketotic hypoglycemia in children. J Inherit Metab Dis. 2015;38:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 240. | Manzia TM, Angelico R, Toti L, Cillis A, Ciano P, Orlando G, Anselmo A, Angelico M, Tisone G. Glycogen storage disease type Ia and VI associated with hepatocellular carcinoma: two case reports. Transplant Proc. 2011;43:1181-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 241. | Ogawa A, Ogawa E, Yamamoto S, Fukuda T, Sugie H, Kohno Y. Case of glycogen storage disease type VI (phosphorylase deficiency) complicated by focal nodular hyperplasia. Pediatr Int. 2010;52:e150-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 242. | Lu SQ, Feng JY, Liu J, Xie XB, Lu Y, Abuduxikuer K. Glycogen storage disease type VI can progress to cirrhosis: ten Chinese patients with GSD VI and a literature review. J Pediatr Endocrinol Metab. 2020;33:1321-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 243. | Tsilianidis LA, Fiske LM, Siegel S, Lumpkin C, Hoyt K, Wasserstein M, Weinstein DA. Aggressive therapy improves cirrhosis in glycogen storage disease type IX. Mol Genet Metab. 2013;109:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 244. | Davidson JJ, Ozçelik T, Hamacher C, Willems PJ, Francke U, Kilimann MW. cDNA cloning of a liver isoform of the phosphorylase kinase alpha subunit and mapping of the gene to Xp22.2-p22.1, the region of human X-linked liver glycogenosis. Proc Natl Acad Sci U S A. 1992;89:2096-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 245. | Brushia RJ, Walsh DA. Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci. 1999;4:D618-D641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 246. | Burwinkel B, Moses SW, Kilimann MW. Phosphorylase-kinase-deficient liver glycogenosis with an unusual biochemical phenotype in blood cells associated with a missense mutation in the beta subunit gene (PHKB). Hum Genet. 1997;101:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 247. | Wüllrich-Schmoll A, Kilimann MW. Structure of the human gene encoding the phosphorylase kinase beta subunit (PHKB). Eur J Biochem. 1996;238:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 248. | Hendrickx J, Coucke P, Hors-Cayla MC, Smit GP, Shin YS, Deutsch J, Smeitink J, Berger R, Lee P, Fernandes J. Localization of a new type of X-linked liver glycogenosis to the chromosomal region Xp22 containing the liver alpha-subunit of phosphorylase kinase (PHKA2). Genomics. 1994;21:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 249. | Hidaka F, Sawada H, Matsuyama M, Nunoi H. A novel mutation of the PHKA2 gene in a patient with X-linked liver glycogenosis type 1. Pediatr Int. 2005;47:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 250. | Cho SY, Lam CW, Tong SF, Siu WK. X-linked glycogen storage disease IXa manifested in a female carrier due to skewed X chromosome inactivation. Clin Chim Acta. 2013;426:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 251. | Schimke RN, Zakheim RM, Corder RC, Hug G. Glycogen storage disease type IX: benign glycogenosis of liver and hepatic phosphorylase kinase deficiency. J Pediatr. 1973;83:1031-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 252. | Willems PJ, Gerver WJ, Berger R, Fernandes J. The natural history of liver glycogenosis due to phosphorylase kinase deficiency: a longitudinal study of 41 patients. Eur J Pediatr. 1990;149:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 253. | Schippers HM, Smit GP, Rake JP, Visser G. Characteristic growth pattern in male X-linked phosphorylase-b kinase deficiency (GSD IX). J Inherit Metab Dis. 2003;26:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 254. | Burwinkel B, Amat L, Gray RG, Matsuo N, Muroya K, Narisawa K, Sokol RJ, Vilaseca MA, Kilimann MW. Variability of biochemical and clinical phenotype in X-linked liver glycogenosis with mutations in the phosphorylase kinase PHKA2 gene. Hum Genet. 1998;102:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (3)] |
| 255. | Kim JA, Kim JH, Lee BH, Kim GH, Shin YS, Yoo HW, Kim KM. Clinical, Biochemical, and Genetic Characterization of Glycogen Storage Type IX in a Child with Asymptomatic Hepatomegaly. Pediatr Gastroenterol Hepatol Nutr. 2015;18:138-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 256. | Johnson AO, Goldstein JL, Bali D. Glycogen storage disease type IX: novel PHKA2 missense mutation and cirrhosis. J Pediatr Gastroenterol Nutr. 2012;55:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (3)] |
| 257. | Rodríguez-Jiménez C, Santos-Simarro F, Campos-Barros Á, Camarena C, Lledín D, Vallespín E, Del Pozo Á, Mena R, Lapunzina P, Rodríguez-Nóvoa S. A new variant in PHKA2 is associated with glycogen storage disease type IXa. Mol Genet Metab Rep. 2017;10:52-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 258. | Fernandes SA, Cooper GE, Gibson RA, Kishnani PS. Benign or not benign? Deep phenotyping of liver Glycogen Storage Disease IX. Mol Genet Metab. 2020;131:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 259. | Maichele AJ, Burwinkel B, Maire I, Søvik O, Kilimann MW. Mutations in the testis/liver isoform of the phosphorylase kinase gamma subunit (PHKG2) cause autosomal liver glycogenosis in the gsd rat and in humans. Nat Genet. 1996;14:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 260. | Bali DS, Goldstein JL, Fredrickson K, Rehder C, Boney A, Austin S, Weinstein DA, Lutz R, Boneh A, Kishnani PS. Variability of disease spectrum in children with liver phosphorylase kinase deficiency caused by mutations in the PHKG2 gene. Mol Genet Metab. 2014;111:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 261. | Albash B, Imtiaz F, Al-Zaidan H, Al-Manea H, Banemai M, Allam R, Al-Suheel A, Al-Owain M. Novel PHKG2 mutation causing GSD IX with prominent liver disease: report of three cases and review of literature. Eur J Pediatr. 2014;173:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 262. | Burwinkel B, Shiomi S, Al Zaben A, Kilimann MW. Liver glycogenosis due to phosphorylase kinase deficiency: PHKG2 gene structure and mutations associated with cirrhosis. Hum Mol Genet. 1998;7:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 263. | Burwinkel B, Tanner MS, Kilimann MW. Phosphorylase kinase deficient liver glycogenosis: progression to cirrhosis in infancy associated with PHKG2 mutations (H144Y and L225R). J Med Genet. 2000;37:376-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 264. | Waheed N, Saeed A, Ijaz S, Fayyaz Z, Anjum MN, Zahoor Y, Cheema HA. Variability of clinical and biochemical phenotype in liver phosphorylase kinase deficiency with variants in the phosphorylase kinase (PHKG2) gene. J Pediatr Endocrinol Metab. 2020;33:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 265. | Burwinkel B, Maichele AJ, Aagenaes O, Bakker HD, Lerner A, Shin YS, Strachan JA, Kilimann MW. Autosomal glycogenosis of liver and muscle due to phosphorylase kinase deficiency is caused by mutations in the phosphorylase kinase beta subunit (PHKB). Hum Mol Genet. 1997;6:1109-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 266. | Degrassi I, Deheragoda M, Creegen D, Mundy H, Mustafa A, Vara R, Hadzic N. Liver histology in children with glycogen storage disorders type VI and IX. Dig Liver Dis. 2021;53:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 267. | Manz F, Bickel H, Brodehl J, Feist D, Gellissen K, Geschöll-Bauer B, Gilli G, Harms E, Helwig H, Nützenadel W. Fanconi-Bickel syndrome. Pediatr Nephrol. 1987;1:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 62] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 268. | FANCONI G, BICKEL H. [Chronic aminoaciduria (amino acid diabetes or nephrotic-glucosuric dwarfism) in glycogen storage and cystine disease]. Helv Paediatr Acta. 1949;4:359-396. [PubMed] |
| 269. | Saltik-Temizel IN, Coşkun T, Yüce A, Koçak N. Fanconi-Bickel syndrome in three Turkish patients with different homozygous mutations. Turk J Pediatr. 2005;47:167-169. [PubMed] |
| 270. | Fukumoto H, Seino S, Imura H, Seino Y, Eddy RL, Fukushima Y, Byers MG, Shows TB, Bell GI. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988;85:5434-5438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 284] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 271. | Santer R, Schneppenheim R, Dombrowski A, Götze H, Steinmann B, Schaub J. Mutations in GLUT2, the gene for the liver-type glucose transporter, in patients with Fanconi-Bickel syndrome. Nat Genet. 1997;17:324-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 188] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 272. | Sharari S, Kabeer B, Mohammed I, Haris B, Pavlovski I, Hawari I, Bhat AA, Toufiq M, Tomei S, Mathew R, Syed N, Nisar S, Maacha S, Grivel JC, Chaussabel D, Ericsson J, Hussain K. Understanding the Role of GLUT2 in Dysglycemia Associated with Fanconi-Bickel Syndrome. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 273. | Mohandas Nair K, Sakamoto O, Jagadeesh S, Nampoothiri S. Fanconi-Bickel syndrome. Indian J Pediatr. 2012;79:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 274. | Aperia A, Bergqvist G, Linné T, Zetterström R. Familial Fanconi syndrome with malabsorption and galactose intolerance, normal kinase and transferase activity. A report on two siblings. Acta Paediatr Scand. 1981;70:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 275. | Afroze B, Chen M. Fanconi-Bickel Syndrome: Two Pakistani Patients Presenting with Hypophosphatemic Rickets. J Pediatr Genet. 2016;5:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 276. | Massese M, Tagliaferri F, Dionisi-Vici C, Maiorana A. Glycogen storage diseases with liver involvement: a literature review of GSD type 0, IV, VI, IX and XI. Orphanet J Rare Dis. 2022;17:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 277. | Govindarajan S, Khandelwal P, Sharma S, Agarwala A, Sinha A, Hari P, Bagga A. Clinical Features and Genetic Sequencing of Children with Fanconi-Bickel Syndrome. Indian J Pediatr. 2023;90:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 278. | Furlan F, Santer R, Vismara E, Santus F, Sersale G, Menni F, Parini R. Bilateral nuclear cataracts as the first neonatal sign of Fanconi-Bickel syndrome. J Inherit Metab Dis. 2006;29:685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 279. | Sharari S, Abou-Alloul M, Hussain K, Ahmad Khan F. Fanconi-Bickel Syndrome: A Review of the Mechanisms That Lead to Dysglycaemia. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 280. | Demirbilek H, Galcheva S, Vuralli D, Al-Khawaga S, Hussain K. Ion Transporters, Channelopathies, and Glucose Disorders. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 281. | Müller D, Santer R, Krawinkel M, Christiansen B, Schaub J. Fanconi-Bickel syndrome presenting in neonatal screening for galactosaemia. J Inherit Metab Dis. 1997;20:607-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 282. | Lee PJ, Van't Hoff WG, Leonard JV. Catch-up growth in Fanconi-Bickel syndrome with uncooked cornstarch. J Inherit Metab Dis. 1995;18:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 283. | Pennisi A, Maranda B, Benoist JF, Baudouin V, Rigal O, Pichard S, Santer R, Romana Lepri F, Novelli A, Ogier de Baulny H, Dionisi-Vici C, Schiff M. Nocturnal enteral nutrition is therapeutic for growth failure in Fanconi-Bickel syndrome. J Inherit Metab Dis. 2020;43:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 284. | Haines JL, Ozelius LJ, McFarlane H, Menon A, Tzall S, Martiniuk F, Hirschhorn R, Gusella JF. A genetic linkage map of chromosome 17. Genomics. 1990;8:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 285. | De Filippi P, Saeidi K, Ravaglia S, Dardis A, Angelini C, Mongini T, Morandi L, Moggio M, Di Muzio A, Filosto M, Bembi B, Giannini F, Marrosu G, Rigoldi M, Tonin P, Servidei S, Siciliano G, Carlucci A, Scotti C, Comelli M, Toscano A, Danesino C. Genotype-phenotype correlation in Pompe disease, a step forward. Orphanet J Rare Dis. 2014;9:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 286. | Leslie N, Bailey L. Pompe Disease. 2007 Aug 31. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–. [PubMed] |
| 287. | Makos MM, McComb RD, Hart MN, Bennett DR. Alpha-glucosidase deficiency and basilar artery aneurysm: report of a sibship. Ann Neurol. 1987;22:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 288. | Amartino H, Painceira D, Pomponio RJ, Niizawa G, Sabio Paz V, Blanco M, Chamoles N. Two clinical forms of glycogen-storage disease type II in two generations of the same family. Clin Genet. 2006;69:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 289. | Karabul N, Berndt J, Kornblum C, Kley RA, Wenninger S, Tiling N, Mengel E, Plöckinger U, Vorgerd M, Deschauer M, Schoser B, Hanisch F. Pregnancy and delivery in women with Pompe disease. Mol Genet Metab. 2014;112:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 290. | Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, Mackey J, Kishnani P, Smith W, McVie-Wylie A, Sullivan JA, Hoganson GE, Phillips JA 3rd, Schaefer GB, Charrow J, Ware RE, Bossen EH, Chen YT. Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial. Genet Med. 2001;3:132-138. [PubMed] |
| 291. | Dhillon S. Avalglucosidase alfa: First Approval. Drugs. 2021;81:1803-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 292. | van der Ploeg AT, Kruijshaar ME, Toscano A, Laforêt P, Angelini C, Lachmann RH, Pascual Pascual SI, Roberts M, Rösler K, Stulnig T, van Doorn PA, Van den Bergh PYK, Vissing J, Schoser B; European Pompe Consortium. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: a 10-year experience. Eur J Neurol. 2017;24:768-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 293. | Zhou Z, Austin GL, Shaffer R, Armstrong DD, Gentry MS. Antibody-Mediated Enzyme Therapeutics and Applications in Glycogen Storage Diseases. Trends Mol Med. 2019;25:1094-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 294. | Umapathysivam K, Whittle AM, Ranieri E, Bindloss C, Ravenscroft EM, van Diggelen OP, Hopwood JJ, Meikle PJ. Determination of acid alpha-glucosidase protein: evaluation as a screening marker for Pompe disease and other lysosomal storage disorders. Clin Chem. 2000;46:1318-1325. [PubMed] |
| 295. | Cohen JL, Chakraborty P, Fung-Kee-Fung K, Schwab ME, Bali D, Young SP, Gelb MH, Khaledi H, DiBattista A, Smallshaw S, Moretti F, Wong D, Lacroix C, El Demellawy D, Strickland KC, Lougheed J, Moon-Grady A, Lianoglou BR, Harmatz P, Kishnani PS, MacKenzie TC. In Utero Enzyme-Replacement Therapy for Infantile-Onset Pompe's Disease. N Engl J Med. 2022;387:2150-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 296. | van Kooten HA, Ditters IAM, Hoogeveen-Westerveld M, Jacobs EH, van den Hout JMP, van Doorn PA, Pijnappel WWMP, van der Ploeg AT, van der Beek NAME. Antibodies against recombinant human alpha-glucosidase do not seem to affect clinical outcome in childhood onset Pompe disease. Orphanet J Rare Dis. 2022;17:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 297. | Rovelli V, Zuvadelli J, Piotto M, Scopari A, Dionigi AR, Ercoli V, Paci S, Cefalo G, Salvatici E, Banderali G. L-alanine supplementation in Pompe disease (IOPD): a potential therapeutic implementation for patients on ERT? A case report. Ital J Pediatr. 2022;48:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 298. | Ronzitti G, Collaud F, Laforet P, Mingozzi F. Progress and challenges of gene therapy for Pompe disease. Ann Transl Med. 2019;7:287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 299. | Nascimbeni AC, Fanin M, Angelini C, Sandri M. Autophagy dysregulation in Danon disease. Cell Death Dis. 2017;8:e2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 300. | Cenacchi G, Papa V, Pegoraro V, Marozzo R, Fanin M, Angelini C. Review: Danon disease: Review of natural history and recent advances. Neuropathol Appl Neurobiol. 2020;46:303-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 301. | Komurcu-Bayrak E, Kalkan MA, Coban N, Ozsait-Selcuk B, Bayrak F. Identification of the pathogenic effects of missense variants causing PRKAG2 cardiomyopathy. Arch Biochem Biophys. 2022;727:109340. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 302. | Wilkins JL. Challenges and Opportunities Created by the COVID-19 Pandemic. J Nutr Educ Behav. 2020;52:669-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 303. | Akman HO, Sampayo JN, Ross FA, Scott JW, Wilson G, Benson L, Bruno C, Shanske S, Hardie DG, Dimauro S. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2-subunit of AMP-activated protein kinase. Pediatr Res. 2007;62:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 304. | Lebo RV, Anderson LA, DiMauro S, Lynch E, Hwang P, Fletterick R. Rare McArdle disease locus polymorphic site on 11q13 contains CpG sequence. Hum Genet. 1990;86:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 305. | Scalco RS, Lucia A, Santalla A, Martinuzzi A, Vavla M, Reni G, Toscano A, Musumeci O, Voermans NC, Kouwenberg CV, Laforêt P, San-Millán B, Vieitez I, Siciliano G, Kühnle E, Trost R, Sacconi S, Stemmerik MG, Durmus H, Kierdaszuk B, Wakelin A, Andreu AL, Pinós T, Marti R, Quinlivan R, Vissing J; EUROMAC Consortium. Data from the European registry for patients with McArdle disease and other muscle glycogenoses (EUROMAC). Orphanet J Rare Dis. 2020;15:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 306. | Pizzamiglio C, Mahroo OA, Khan KN, Patasin M, Quinlivan R. Phenotype and genotype of 197 British patients with McArdle disease: An observational single-centre study. J Inherit Metab Dis. 2021;44:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 307. | Lucia A, Martinuzzi A, Nogales-Gadea G, Quinlivan R, Reason S; International Association for Muscle Glycogen Storage Disease study group. Clinical practice guidelines for glycogen storage disease V & VII (McArdle disease and Tarui disease) from an international study group. Neuromuscul Disord. 2021;31:1296-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 308. | Echaniz-Laguna A, Lornage X, Laforêt P, Orngreen MC, Edelweiss E, Brochier G, Bui MT, Silva-Rojas R, Birck C, Lannes B, Romero NB, Vissing J, Laporte J, Böhm J. A New Glycogen Storage Disease Caused by a Dominant PYGM Mutation. Ann Neurol. 2020;88:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 309. | Howard TD, Akots G, Bowden DW. Physical and genetic mapping of the muscle phosphofructokinase gene (PFKM): reassignment to human chromosome 12q. Genomics. 1996;34:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 310. | Wehner M, Clemens PR, Engel AG, Kilimann MW. Human muscle glycogenosis due to phosphorylase kinase deficiency associated with a nonsense mutation in the muscle isoform of the alpha subunit. Hum Mol Genet. 1994;3:1983-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 311. | Huang K, Duan HQ, Li QX, Luo YB, Bi FF, Yang H. Expanding the clinicopathological-genetic spectrum of glycogen storage disease type IXd by a Chinese neuromuscular center. Front Neurol. 2022;13:945280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 312. | Nayab A, Alam Q, Alzahrani OR, Khan R, Sarfaraz S, Albaz AA, Rafeeq MM, Sain ZM, Waqas A, Umair M. Targeted exome sequencing identified a novel frameshift variant in the PGAM2 gene causing glycogen storage disease type X. Eur J Med Genet. 2021;64:104283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 313. | Kanno T, Sudo K, Takeuchi I, Kanda S, Honda N, Nishimura Y, Oyama K. Hereditary deficiency of lactate dehydrogenase M-subunit. Clin Chim Acta. 1980;108:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 314. | Maekawa M, Sudo K, Kanno T, Li SS. Molecular characterization of genetic mutation in human lactate dehydrogenase-A (M) deficiency. Biochem Biophys Res Commun. 1990;168:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 315. | Santoro L, Pjetraj D, Velmishi V, Campana C, Catassi C, Dionisi-Vici C, Maiorana A. A new phenotype of aldolase a deficiency in a 14 year-old boy with epilepsy and rhabdomyolysis - case report. Ital J Pediatr. 2022;48:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 316. | Kukita A, Yoshida MC, Fukushige S, Sakakibara M, Joh K, Mukai T, Hori K. Molecular gene mapping of human aldolase A (ALDOA) gene to chromosome 16. Hum Genet. 1987;76:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 317. | Comi GP, Fortunato F, Lucchiari S, Bordoni A, Prelle A, Jann S, Keller A, Ciscato P, Galbiati S, Chiveri L, Torrente Y, Scarlato G, Bresolin N. Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis. Ann Neurol. 2001;50:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 318. | Moslemi AR, Lindberg C, Nilsson J, Tajsharghi H, Andersson B, Oldfors A. Glycogenin-1 deficiency and inactivated priming of glycogen synthesis. N Engl J Med. 2010;362:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (1)] |