Published online Jun 21, 2023. doi: 10.3748/wjg.v29.i23.3658
Peer-review started: February 24, 2023
First decision: March 14, 2023
Revised: March 20, 2023
Accepted: May 23, 2023
Article in press: May 23, 2023
Published online: June 21, 2023
Processing time: 112 Days and 7.6 Hours
The expression status of serum and glucocorticoid-induced protein kinase 3 (SGK3) in superficial esophageal squamous cell neoplasia (ESCN) remains unknown.
To evaluate the SGK3 overexpression rate in ESCN and its influence on the prognosis and outcomes of patients with endoscopic resection.
A total of 92 patients who had undergone endoscopic resection for ESCN with more than 8 years of follow-up were enrolled. Immunohistochemistry was used to evaluate SGK3 expression.
SGK3 was overexpressed in 55 (59.8%) patients with ESCN. SGK3 overexpression showed a significant correlation with death (P = 0.031). Overall survival and disease-free survival rates were higher in the normal SGK3 expression group than in the SGK3 overexpression group (P = 0.013 and P = 0.004, respectively). Cox regression analysis models demonstrated that SGK3 overexpression was an independent predictor of poor prognosis in ESCN patients (hazard ratio 4.729; 95% confidence interval: 1.042-21.458).
SGK3 overexpression was detected in the majority of patients with endoscopically resected ESCN and was significantly associated with shortened survival. Thus, it might be a new prognostic factor for ESCN.
Core Tip: This study demonstrated that the expression status of serum and glucocorticoid-induced protein kinase 3 (SGK3) was high in the majority of patients with superficial esophageal squamous cell neoplasia (ESCN) and that high expression of SGK3 predicts a poor prognosis. These findings provide a new prognostic factor for ESCN.
- Citation: Xu N, Li LS, Li H, Zhang LH, Zhang N, Wang PJ, Cheng YX, Xiang JY, Linghu EQ, Chai NL. SGK3 overexpression correlates with a poor prognosis in endoscopically resected superficial esophageal squamous cell neoplasia: A long-term study. World J Gastroenterol 2023; 29(23): 3658-3667
- URL: https://www.wjgnet.com/1007-9327/full/v29/i23/3658.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i23.3658
Esophageal cancer remains a leading cause of cancer-related death, and the incidence rates of esophageal cancer worldwide are increasing[1]. Esophageal squamous cell carcinoma (ESCC) accounts for the majority of esophageal cancer cases[2]. It is usually detected at an advanced stage after the symptoms of dysphagia appear[3]. Thus, its prognosis is the worst among digestive carcinomas[4]. Early detection of the malignant epithelium at an early stage followed by endoscopic resection has become the most effective way to improve clinical outcomes[5]. However, the prognosis of patients with superficial esophageal squamous cell neoplasia (ESCN) remains poor[6,7].
The serum and glucocorticoid-induced protein kinase (SGK) family consists of three isoforms (SGK1, SGK2 and SGK3), and they regulate a range of fundamental cellular processes, such as tumor growth, metastasis, autophagy and survival[8]. SGK is a ubiquitously expressed serine/threonine kinase and plays an active role in a multitude of pathophysiological conditions[9]. It is activated by insulin and growth factors via phosphatidylinositol 3-kinase (PI3K) and is frequently altered in human cancers, including breast cancer and hepatocellular carcinoma[10,11]. Although SGK1, SGK2 and SGK3 share a highly similar domain, the lack of a plasma membrane-targeting domain indicates nonoverlapping functions[12].
Previous studies have shown that SGK3 is expressed in all tissues tested thus far and is especially highly expressed in the heart and spleen[13]. Encoded by chromosome 8q12.2, SGK3 is known as a downstream factor of PI3K signaling and has been reported to play a pivotal role in the development and progression of diverse cancers[14]. Therefore, it has been regarded as a potential target for cancer intervention[15]. However, a minority of studies have evaluated the impact of SGK3 expression on clinical outcomes, and the expression of SGK3 in ESCN has not been investigated[16]. Whether SGK3 is associated with the prognosis of ESCN remains unknown. Herein, we assessed the SGK3 expression levels of patients with ESCN via immunohistochemistry (IHC) to investigate the possible role of SGK3 as a prognostic factor.
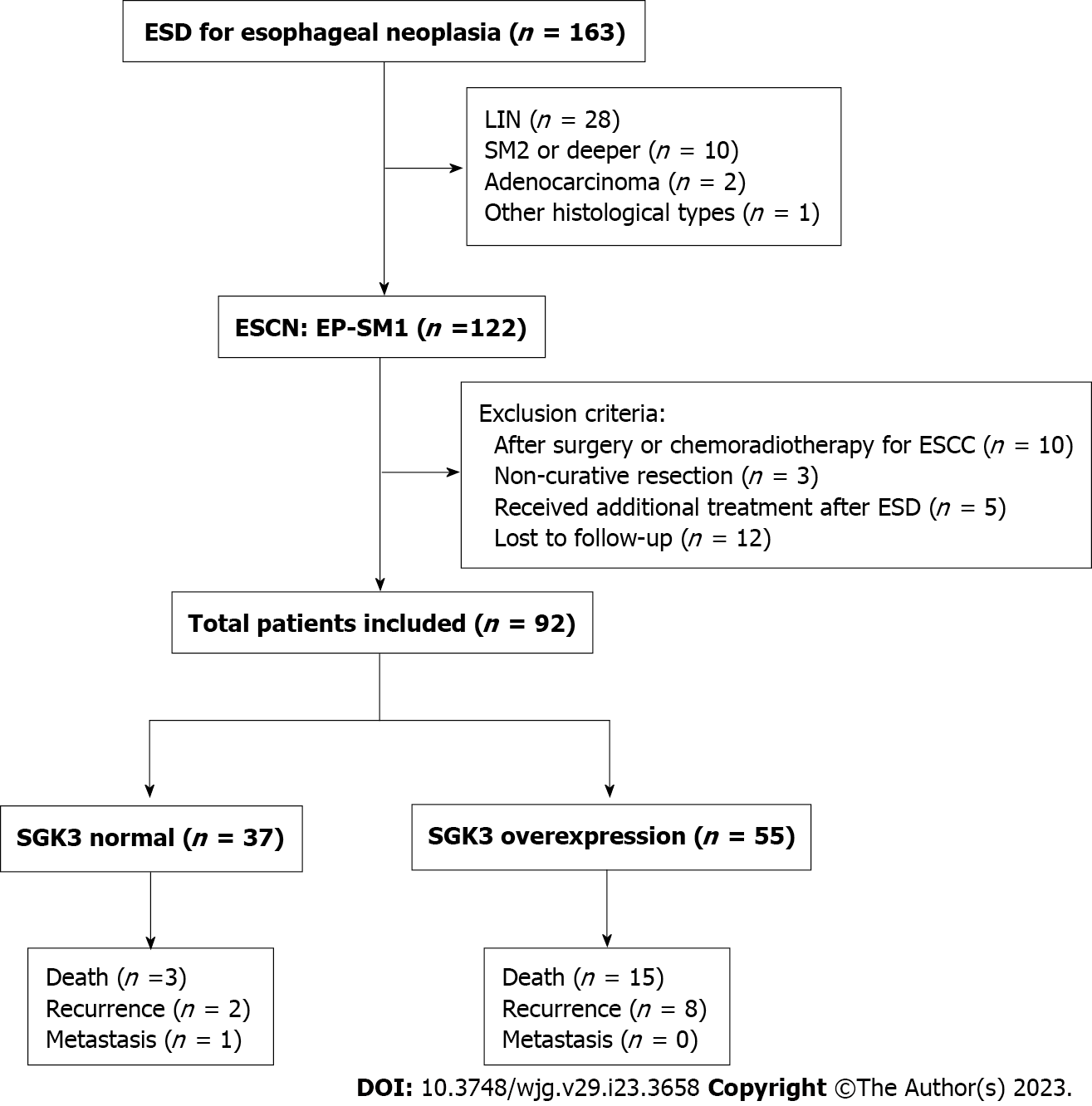
Patients who underwent gastrointestinal endoscopy followed by endoscopic submucosal dissection (ESD) between November 2009 and July 2011 at the First Medical Center of PLA General Hospital were included. The inclusion criteria were as follows: (1) Patients were diagnosed with primary ESCN; (2) the histological type of tumor was ESCN; and (3) the tumor penetrated the submucosal layer < 200 μm or 200 μm from the muscularis mucosa. The exclusion criteria were as follows: (1) ESCN was previously treated with surgery or chemoradiotherapy ESCN; (2) pathological evaluation post-ESD indicated noncurative resection; (3) patients received additional treatment immediately after ESD; and (4) incomplete follow-up. All follow-up data were obtained at least 8 years after the first detection of the tumor. Eventually, 92 patients with complete follow-up data were included in the analysis (Figure 1). This study was approved by the Ethics Board of the First Medical Center of PLA General Hospital (approval No. S2020-251-01).
All procedures were performed under general anesthesia by experienced endoscopists. A single-channel upper gastrointestinal endoscope (GIF-Q230; Olympus, Tokyo, Japan) was used during the procedure. The standard ESD is described as follows: the lesions were detected on endoscopy under white light or narrow band imaging, lesion margins were shown by iodine staining, argon plasma coagulation (ERBE Elektromedizin GmbH) was used to mark 5 mm outside the boundary of the lesion, submucosal circumferential injection was performed to lift the lesions, and submucosal dissection followed by a circumferential mucosal incision was performed to remove the lesion.
Tissue specimens were fixed in formalin. All samples were subjected to histological analysis of differentiation type, depth of invasion, and lymphovascular invasion. Lymphovascular invasion was recognized as any invasion using hematoxylin and eosin staining. Curative resection was defined as complete resection of a mucosal lesion with no lymphovascular invasion, and noncurative resection implied a pathologically positive margin or clinically incomplete resection.
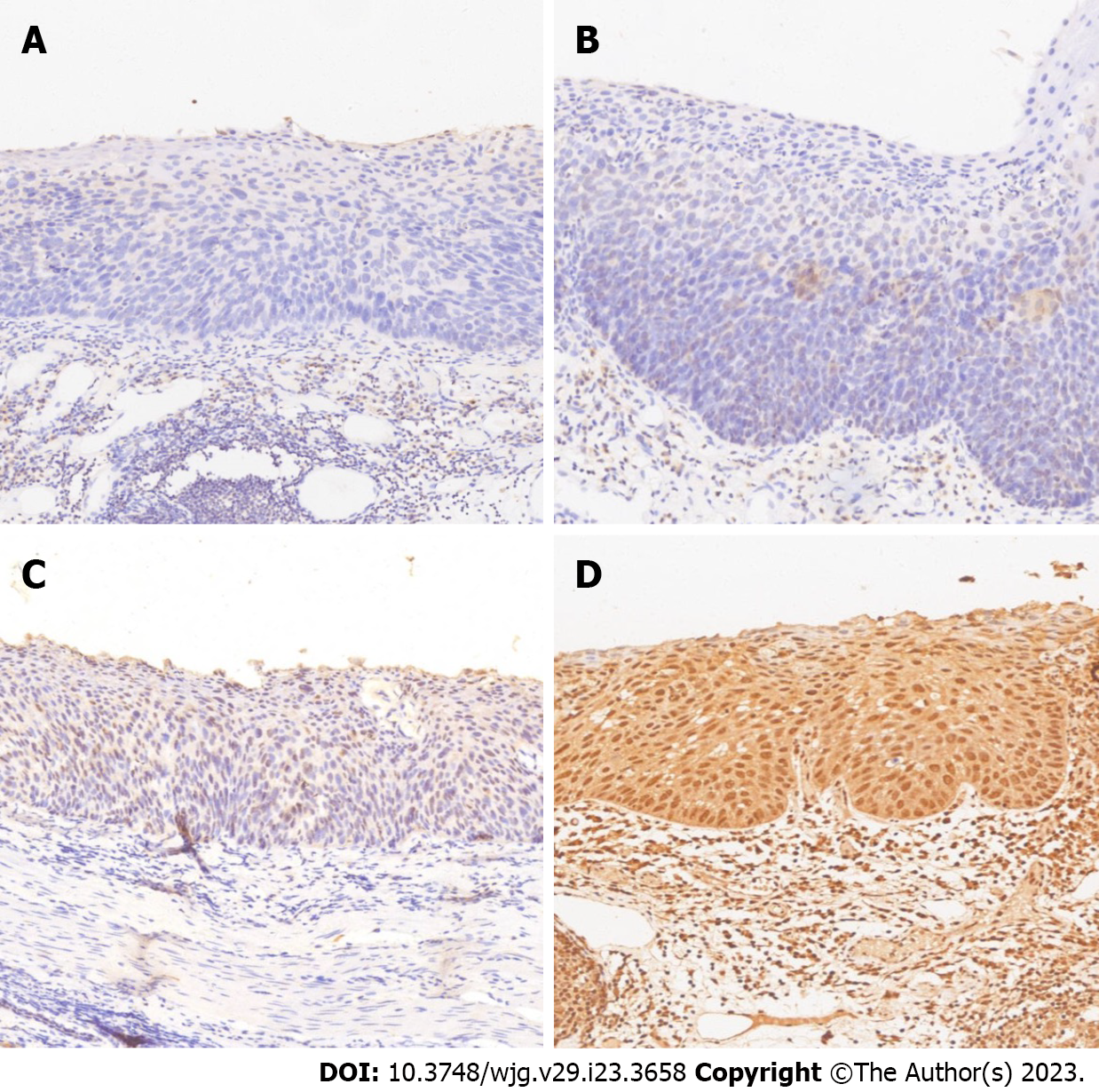
For IHC, we used a rabbit anti-human SGK3 antibody (Roche Diagnostics Japan. Tokyo, Japan) and 4-µm-thick sections. All sections were deparaffinized in xylene 3 times and rehydrated in a graded series of ethanol with decreasing concentrations. Then, we used Tris-buffered saline (pH 7.4) for rinsing. Heat-induced epitope retrieval was conducted by immersing the slides in Coplin jars filled with 10 mmol/L citrate buffer (pH 6.0) and microwaving the buffer for 25 min. The jars were then cooled for 20 min. The reaction products were visualized with diaminobenzidine and counterstained with hematoxylin. Two experienced pathologists independently judged the immunostaining intensity. The staining intensity was graded based on the American Society of Clinical Oncology/College of American Pathology guidelines[17]. The criteria were as follows: 0 = no nuclear staining in tumor cells; 1+ = weak (staining in less than 10% of cells); 2+ = intense complete staining (up to 30% of tumor cells); and 3+ = uniform intense staining (more than 30% of tumor cells). The expression status of SGK3 was classified into 2 groups: normal (0 and 1+) and overexpression (2+ and 3+) (Figure 2).
In each case, clinicopathologic parameters were retrospectively obtained from the pathological results of our hospital. Basic clinical parameters included age, sex, smoking history and family history of cancer. The longest diameter of the lesion in the specimen was defined as the tumor size. Tumor location was categorized as upper thoracic, middle thoracic and lower thoracic. Based on the histological outcome, tumors were characterized into low-grade intraepithelial neoplasia, high-grade intraepithelial neoplasia, well-differentiated squamous cell carcinoma, intermediate differentiated squamous cell carcinoma, and poorly differentiated squamous cell carcinoma. The T stage of the tumor was categorized based on the 11th Japanese classification of esophageal cancer[18]. Tumors limited to the epithelium and tumors that invaded into the submucosa ≤ 200 or > 200 μm from the muscularis mucosae were classified as EP, submucosa 1 (SM1) or submucosa 2 (SM2), respectively. The two recurrence patterns, metachronous recurrence and metastatic recurrence, were both included in this study, and tumor recurrence was identified using endoscopy and histological biopsy. Tumor recurrence was defined as an ESCN or cancerous lesion detected on the esophagus during the follow-up period. Lymph node or distant metastasis was evaluated by contrast-enhanced computed tomography scan or magnetic resonance imaging and verified via histological examination of the biopsy.
The follow-up of patient survival status and cancer recurrence was completed in July 2022. Prognostic outcomes were obtained by calling patients themselves or asking the family members for the recorded death certificate of the patients. Two main variables were assessed: Overall survival (OS) and disease-free survival (DFS). OS was defined as the time that elapsed from the date of endoscopic treatment to the date of death from any cause. DFS was defined as the duration between the endoscopic treatment and any disease recurrence or any cause of death, whichever occurred first.
Continuous variables are expressed as the mean ± SD or median (interquartile range) and were compared using Student’s t test. Categorical variables are expressed as percentages and were compared using the chi-square test or Fisher’s exact test. Multivariate Cox regression was used to further analyze the relationship between SGK3 overexpression and clinical outcomes, and the covariates were screened using stepwise regression. The extended-model method was used to conduct covariate correction: Model 1 was corrected based on SGK3 expression status; Model 2 = model 1 + (age and gender); Model 3 = model 2 + (smoking history + family history of cancer); and Model 4 = model 3 + (tumor location + tumor size + T stage + differentiated types). Multicollinearity was tested using the variance inflation factor (VIF) method, and a VIF ≥ 10 was considered to indicate severe multicollinearity. Kaplan-Meier analysis was used to establish the survival curves based on each prognostic outcome. Survival curves were graphed using GraphPad Prism 8. A P value < 0.05 in the two-tailed tests was considered to indicate statistical significance. All statistical analyses were performed using SPSS 26.0 (SPSS, Inc., Chicago, IL, United States).
A total of 92 patients aged 43 to 80 years were finally included in our study. Table 1 lists the baseline characteristics of the normal SGK3 expression group and the SGK3 overexpression group. The normal SGK3 expression group included 37 patients and consisted of 27 males and 10 females. There were 55 patients (36 males and 19 females) in the SGK3 overexpression group. No significant differences were observed in the baseline indicators (age, sex, tumor location, tumor size, T stage, differentiated types, smoking history, lymphovascular infiltration and family history of cancer) between the normal expression and overexpression groups.
| Characteristics | SGK3 normal, n = 37 | SGK3 overexpression, n = 55 | P value |
| Age (yr, mean ± SD) | 58.73 ± 6.81 | 61.21 ± 8.05 | 0.118 |
| Sex (male), n (%) | 27 (73.0) | 36 (65.5) | 0.447 |
| Tumor location, n (%)1 | 0.560 | ||
| Upper esophagus | 4 (10.8) | 7 (12.7) | |
| Middle esophagus | 14 (37.8) | 26 (47.) | |
| Lower esophagus | 19 (51.4) | 22 (40.0) | |
| Tumor size (cm, mean ± SD) | 3.16 ± 1.94 | 3.30 ± 1.89 | 0.742 |
| Tumor stage, n (%) | 0.720 | ||
| Tis | 25 (67.6) | 38 (69.1) | |
| T1a | 6 (16.2) | 11 (20.0) | |
| T1b | 6 (16.2) | 6 (10.9) | |
| Differentiated types1, n (%) | 0.487 | ||
| HIN | 25 (67.6) | 39 (70.9) | |
| Well-differentiation | 7 (18.9) | 13 (23.6) | |
| Intermediate differentiation | 2 (5.4) | 2 (3.6) | |
| Poor differentiation | 3 (8.1) | 1 (1.8) | |
| Smoking history, n (%) | 0.313 | ||
| Yes | 10 (27.0) | 10 (18.2) | |
| No | 27 (73.0) | 45 (81.8) | |
| Lymphovascular infiltration, n (%)1 | 0.402 | ||
| Present | 1 (2.7) | 0 (0) | |
| Absent | 36 (97.3) | 55 (100) | |
| Family history of cancer1, n (%) | 0.698 | ||
| Yes | 2 (5.4) | 5 (9.1) | |
| No | 35 (94.6) | 50 (90.9) |
Table 2 summarizes the prognostic outcomes in each group. Tumor recurrence occurred in 10 patients. Of these patients, 2 (5.4%) were in the normal SGK3 expression group, and 8 (14.5%) were in the SGK3 overexpression group. Metastasis was detected in only 1 patient in the normal SGK3 expression group during the follow-up period. No significant associations were observed between SGK3 expression and tumor recurrence and metastasis. Death occurred in 3 (8.1%) patients in the normal SGK3 expression group and 15 (27.3%) patients in the SGK3 overexpression group. A significant correlation was observed between SGK3 expression status and death (P = 0.031).
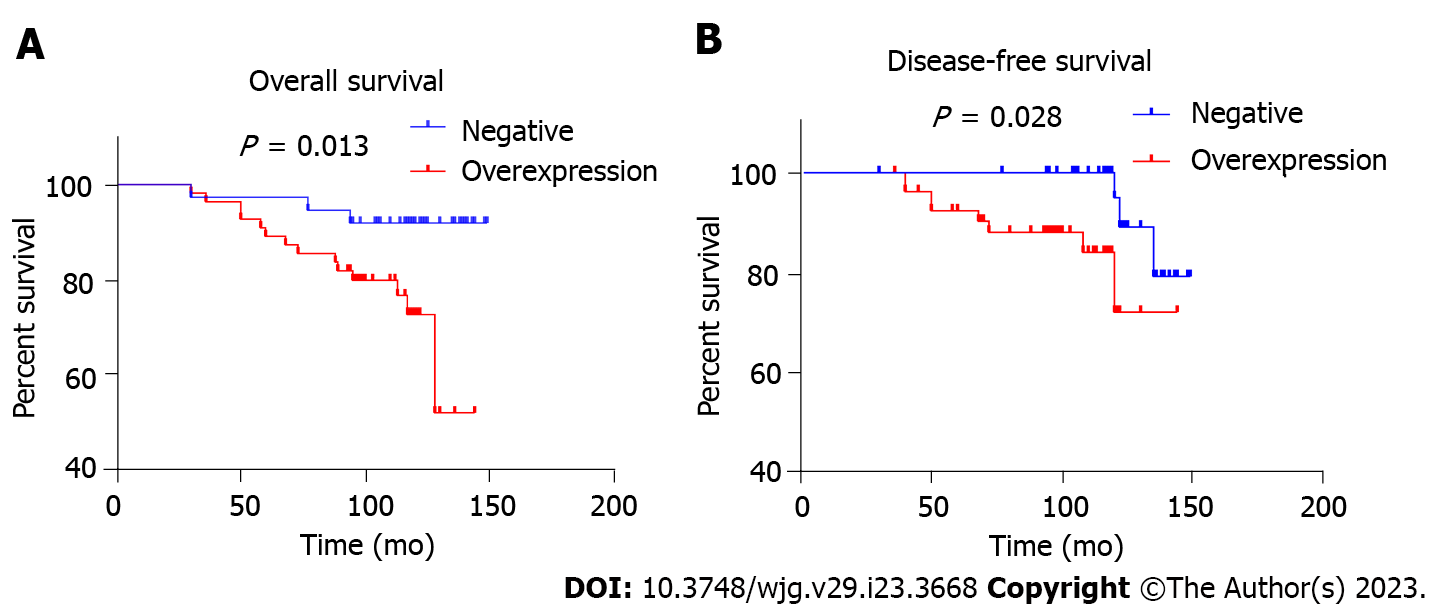
To evaluate the association between SGK3 overexpression and OS, a multivariate Cox regression model was performed (Table 3): Model 1 [hazard ratio (HR) 4.243; 95% confidence interval (CI): 1.219-14.773], Model 2 (HR 3.648; 95%CI: 1.030-12.917), Model 3 (HR 3.637; 95%CI: 1.028-12.866), and Model 4 (HR 4.729; 95%CI: 1.042-21.458). The P value of all four models was less than 0.05. The results of the multicollinearity test showed an average VIF of 1.525 (all VIF < 10). Figure 3 show the Kaplan-Meier curves of the relationship between SGK3 expression status and OS and DFS, respectively. The OS and DFS rates of patients were higher in the normal SGK3 expression group than in the SGK3 overexpression group during the follow-up period (P = 0.013 and P = 0.004, respectively).
| Model | HR (95%CI) | P value |
| Model 1 | 4.243 (1.219-14.773) | 0.023 |
| Model 2 | 3.648 (1.030-12.917) | 0.045 |
| Model 3 | 3.637 (1.028-12.866) | 0.045 |
| Model 4 | 4.729 (1.042-21.458) | 0.044 |
The SGK family is widely dysregulated in numerous human cancers and plays a vital role in tumor development and progression[19]. Among the three members of this family, SGK1 has been well researched, and its correlation with clinical outcomes has been reported in some cancers[20]. It has been suggested that low SGK1 expression in some tumors might induce feedback inhibition by activating the AKT pathway and might result in high SGK3 expression[21]. However, due to the later detection of SGK3, its relationship with tumors or other diseases has been revealed in only a few studies[22,23]. For instance, SGK3, as a novel regulator of renal phosphate transport, is associated with autosomal dominant hypophosphatemic rickets[24,25]. Its relationship with the clinical prognosis of tumors has rarely been reported thus far.
SGK3 exhibits structural and sequence similarity to the AKT family[26]. Its activation is mediated by the PI3K pathway and requires phosphorylation at two regulatory sites, Thr-320 and Ser-486[27]. Deletion of Thr-320 of SGK3 or disruption of the SGK3 protein tertiary structure yields nonfunctional proteins. To our knowledge, the SGK3 expression status of ESCN has not been illustrated. Our analysis of the expression of SGK3 in 92 ESCN tissues identified the expression status of SGK3 in ESCN tissues. Among the 92 tissue samples, the overexpression rate of SGK3 was close to 60%, confirming its high expression in esophageal neoplasia tissues. However, the defined functions of SGK3 in the growth or progression of ESCN have not been clarified.
ESCC is the most common pathological type of esophageal cancer and has a high incidence in some districts of China. It is difficult to detect at the early stage because its symptoms are insidious, and the majority of patients seek medical help when advanced symptoms of significant dysphagia occur. As a consequence, the traditional treatment of ESCN is mainly based on a combination of open surgery and chemotherapy/radiotherapy. Progress in imaging modalities and multidisciplinary comprehensive treatment has improved early diagnosis and clinical outcomes, respectively. However, the 5-year survival rate for ESCC is still lower than 30% because of its invasiveness and metastatic ability[28,29]. Recently, the development of endoscopy techniques has transformed the early diagnosis and treatment of diseases of the entire digestive system. In particular, the introduction of amplification techniques and narrow band imaging enables endoscopists to detect neoplasia at an early stage or in an asymptomatic period. In addition, the minimally invasive removal of lesions by ESD improves the clinical prognosis and even the quality of life. Although the 5-year survival rate of patients who undergo ESD is higher than that of patients who undergo open surgery, death caused by tumor recurrence or other reasons still occurs in some patients during the follow-up period[30]. This research focused on further exploration of ways to improve the survival rates of these patients.
The integration of small datasets in the present study demonstrated the high expression level of SGK3 in ESCN, as well as a significant relationship between its overexpression and OS. Furthermore, the 4 regression models showed that after adjusting for confounding factors, only the expression status of SGK3 was significantly associated with increased mortality. All these results indicate that the overexpression of SGK3 is correlated with the occurrence of death in ESCN patients. However, the influence of SGK3 on the occurrence and development of ESCN or cell cycle pathways has not yet been determined. Based on the results of our study, we speculate that SGK3 can be used as a prognostic indicator to assist in the outcome assessment of ESCN patients.
At present, follow-up of post-ESD patients with ESCN is generally performed via endoscopy as well as biopsy. The application of a standard follow-up method might aid endoscopists in identifying tumor recurrence in time, but this is only effective when endoscopists can detect the recurrence of cancerous lesions followed by accurate biopsy sampling. Otherwise, no further action could be taken to intervene. As the overexpression of SGK3 is an independent factor in prognosis, IHC staining of SGK3 could be used to identify the population with a high mortality risk. Thus, active measures, including shortening the follow-up intervals and using moderate radiotherapy or chemotherapy, can be taken to improve survival.
However, our current study has several limitations. First, the sample size of our study was small, and all the included patients came from one hospital. Thus, these results need to be validated by expanding the sample size and performing a multicenter study. Second, this was a retrospective study. Therefore, we could not collect information without data bias. Thus, a prospective study, similar to this study, is needed in the future to verify the relationship between SGK3 overexpression and mortality.
In conclusion, SGK3 overexpression is significantly correlated with shortened survival in patients with endoscopic resection of superficial ESCN, and SGK3 might be a useful indicator for prognosis evaluation. Further studies are warranted to determine its mechanisms of action in the growth or progression of ESCN.
Serum and glucocorticoid-induced protein kinase 3 (SGK3) regulate a range of fundamental cellular processes, such as tumor growth, metastasis, autophagy and survival, but SGK3 expression levels in patients with esophageal squamous cell neoplasia (ESCN) and their relationship with the prognosis of ESCN remain unknown.
SGK3 expression levels in patients with ESCN were assessed by immunohistochemistry to investigate the possible role of SGK3 as a prognostic factor.
Ninety-two patients who underwent gastrointestinal endoscopy followed by endoscopic submucosal dissection with complete follow-up data between November 2009 and July 2011 were included.
A total of 92 patients who had undergone endoscopic resection for ESCN with more than 8 years of follow-up were enrolled. Immunohistochemistry was used to evaluate SGK3 expression.
Death occurred in 3 (8.1%) patients in the normal SGK3 expression group and 15 (27.3%) patients in the SGK3 overexpression group. A significant correlation was observed between SGK3 status and death (P = 0.031). The overall survival and disease-free survival rates of patients were higher in the normal SGK3 expression group than in the SGK3 overexpression group during the follow-up period.
All these results indicate that the overexpression of SGK3 is correlated with the occurrence of death in ESCN patients and that SGK3 might be a useful indicator for prognosis evaluation.
The sample size of this study was small, and the subjects both came from one hospital. Thus, the results need to be validated by expanding the sample size and performing a multicenter study. A prospective study, similar to this study, is needed in the future to verify the relationship between SGK3 overexpression and mortality.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hwang GS, South Korea; Tsai NM, Taiwan S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Wu Y, Huang F, Zhou X, Yu S, Tang Q, Li S, Wang J, Chen L. Hypoxic Preconditioning Enhances Dental Pulp Stem Cell Therapy for Infection-Caused Bone Destruction. Tissue Eng Part A. 2016;22:1191-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Sánchez-Danés A, Blanpain C. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer. 2018;18:549-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 3. | van Rossum PSN, Mohammad NH, Vleggaar FP, van Hillegersberg R. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol. 2018;15:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Cui Y, Chen H, Xi R, Cui H, Zhao Y, Xu E, Yan T, Lu X, Huang F, Kong P, Li Y, Zhu X, Wang J, Zhu W, Ma Y, Zhou Y, Guo S, Zhang L, Liu Y, Wang B, Xi Y, Sun R, Yu X, Zhai Y, Wang F, Yang J, Yang B, Cheng C, Liu J, Song B, Li H, Wang Y, Zhang Y, Cheng X, Zhan Q, Liu Z. Author Correction: Whole-genome sequencing of 508 patients identifies key molecular features associated with poor prognosis in esophageal squamous cell carcinoma. Cell Res. 2022;32:415-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, Esposito G, Lemmers A, Maselli R, Messmann H, Pech O, Pioche M, Vieth M, Weusten BLAM, van Hooft JE, Deprez PH, Dinis-Ribeiro M. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2022;54:591-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 359] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 6. | Feng Y, Wei W, Guo S, Li BQ. Associated risk factor analysis and the prognostic impact of positive resection margins after endoscopic resection in early esophageal squamous cell carcinoma. Exp Ther Med. 2022;24:457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Yang X, Men Y, Wang J, Kang J, Sun X, Zhao M, Sun S, Yuan M, Bao Y, Ma Z, Wang G, Hui Z. Additional Radiotherapy With or Without Chemotherapy Following Endoscopic Resection for Stage I Esophageal Carcinoma: A Pilot Study. Technol Cancer Res Treat. 2021;20:15330338211048051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kim J, Kim D, Jung H, Lee J, Hong VS. Identification and Kinetic Characterization of Serum- and Glucocorticoid-Regulated Kinase Inhibitors Using a Fluorescence Polarization-Based Assay. SLAS Discov. 2021;26:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 532] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 10. | Cao H, Xu Z, Wang J, Cigliano A, Pilo MG, Ribback S, Zhang S, Qiao Y, Che L, Pascale RM, Calvisi DF, Chen X. Functional role of SGK3 in PI3K/Pten driven liver tumor development. BMC Cancer. 2019;19:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Xu J, Wan M, He Q, Bassett RL Jr, Fu X, Chen AC, Shi F, Creighton CJ, Schiff R, Huo L, Liu D. SGK3 is associated with estrogen receptor expression in breast cancer. Breast Cancer Res Treat. 2012;134:531-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Alonso L, Okada H, Pasolli HA, Wakeham A, You-Ten AI, Mak TW, Fuchs E. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol. 2005;170:559-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344 Pt 1:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Hou M, Lai Y, He S, He W, Shen H, Ke Z. SGK3 (CISK) may induce tumor angiogenesis (Hypothesis). Oncol Lett. 2015;10:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Basnet R, Gong GQ, Li C, Wang MW. Serum and glucocorticoid inducible protein kinases (SGKs): a potential target for cancer intervention. Acta Pharm Sin B. 2018;8:767-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Zhou D, Phung S, Warden C, Rashid R, Chan N, Chen S. SGK3 sustains ERα signaling and drives acquired aromatase inhibitor resistance through maintaining endoplasmic reticulum homeostasis. Proc Natl Acad Sci U S A. 2017;114:E1500-E1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Vergara-Lluri ME, Moatamed NA, Hong E, Apple SK. High concordance between HercepTest immunohistochemistry and ERBB2 fluorescence in situ hybridization before and after implementation of American Society of Clinical Oncology/College of American Pathology 2007 guidelines. Mod Pathol. 2012;25:1326-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus. 2017;14:37-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 19. | Shanmugam I, Cheng G, Terranova PF, Thrasher JB, Thomas CP, Li B. Serum/glucocorticoid-induced protein kinase-1 facilitates androgen receptor-dependent cell survival. Cell Death Differ. 2007;14:2085-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Talarico C, Dattilo V, D'Antona L, Menniti M, Bianco C, Ortuso F, Alcaro S, Schenone S, Perrotti N, Amato R. SGK1, the New Player in the Game of Resistance: Chemo-Radio Molecular Target and Strategy for Inhibition. Cell Physiol Biochem. 2016;39:1863-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Lang F, Perrotti N, Stournaras C. Colorectal carcinoma cells--regulation of survival and growth by SGK1. Int J Biochem Cell Biol. 2010;42:1571-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Gasser JA, Inuzuka H, Lau AW, Wei W, Beroukhim R, Toker A. SGK3 mediates INPP4B-dependent PI3K signaling in breast cancer. Mol Cell. 2014;56:595-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, Hennessy BT, Tseng H, Pochanard P, Kim SY, Dunn IF, Schinzel AC, Sandy P, Hoersch S, Sheng Q, Gupta PB, Boehm JS, Reiling JH, Silver S, Lu Y, Stemke-Hale K, Dutta B, Joy C, Sahin AA, Gonzalez-Angulo AM, Lluch A, Rameh LE, Jacks T, Root DE, Lander ES, Mills GB, Hahn WC, Sellers WR, Garraway LA. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 24. | Cebeci AN, Zou M, BinEssa HA, Alzahrani AS, Al-Rijjal RA, Al-Enezi AF, Al-Mohanna FA, Cavalier E, Meyer BF, Shi Y. Mutation of SGK3, a Novel Regulator of Renal Phosphate Transport, Causes Autosomal Dominant Hypophosphatemic Rickets. J Clin Endocrinol Metab. 2020;105. [PubMed] [DOI] [Full Text] |
| 25. | Trepiccione F, Capasso G. SGK3: a novel regulator of renal phosphate transport? Kidney Int. 2011;80:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Gong GQ, Wang K, Dai XC, Zhou Y, Basnet R, Chen Y, Yang DH, Lee WJ, Buchanan CM, Flanagan JU, Shepherd PR, Wang MW. Identification, structure modification, and characterization of potential small-molecule SGK3 inhibitors with novel scaffolds. Acta Pharmacol Sin. 2018;39:1902-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 949] [Cited by in RCA: 1003] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 28. | Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent Advances From Basic and Clinical Studies of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2015;149:1700-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 29. | Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 249] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 30. | Hirano S, Nagami Y, Yamamura M, Tanoue K, Sakai T, Maruyama H, Ominami M, Nadatani Y, Fukunaga S, Otani K, Hosomi S, Tanaka F, Kamata N, Taira K, Shiba M, Watanabe T, Fujiwara Y. Evaluation of long-term survival in patients with severe comorbidities after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Surg Endosc. 2022;36:5011-5022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |