Published online Jun 14, 2023. doi: 10.3748/wjg.v29.i22.3534
Peer-review started: February 26, 2023
First decision: March 10, 2023
Revised: March 15, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: June 14, 2023
Processing time: 100 Days and 15.3 Hours
Alterations in plasma and intestinal metabolites contribute to the pathogenesis and progression of alcohol-related liver cirrhosis (ALC).
To explore the common and different metabolites in the plasma and feces of patients with ALC and evaluate their clinical implications.
According to the inclusion and exclusion criteria, 27 patients with ALC and 24 healthy controls (HCs) were selected, and plasma and feces samples were collected. Liver function, blood routine, and other indicators were detected with automatic biochemical and blood routine analyzers. Liquid chromatography-mass spectrometry was used to detect the plasma and feces metabolites of the two groups and the metabolomics of plasma and feces. Also, the correlation between metabolites and clinical features was analyzed.
More than 300 common metabolites were identified in the plasma and feces of patients with ALC. Pathway analysis showed that these metabolites are enriched in bile acid and amino acid metabolic pathways. Compared to HCs, patients with ALC had a higher level of glycocholic acid (GCA) and taurocholic acid (TCA) in plasma and a lower level of deoxycholic acid (DCA) in the feces, while L-threonine, L-phenylalanine, and L-tyrosine increased simultaneously in plasma and feces. GCA, TCA, L-methionine, L-phenylalanine, and L-tyrosine in plasma were positively correlated with total bilirubin (TBil), prothrombin time (PT), and maddrey discriminant function score (MDF) and negatively correlated with cholinesterase (CHE) and albumin (ALB). The DCA in feces was negatively correlated with TBil, MDF, and PT and positively correlated with CHE and ALB. Moreover, we established a P/S BA ratio of plasma primary bile acid (GCA and TCA) to fecal secondary bile acid (DCA), which was relevant to TBil, PT, and MDF score.
The enrichment of GCA, TCA, L-phenylalanine, L-tyrosine, and L-methionine in the plasma of patients with ALC and the reduction of DCA in feces were related to the severity of ALC. These metabolites may be used as indicators to evaluate the progression of alcohol-related liver cirrhosis.
Core Tip: Metabolites in enterohepatic circulation play an important role in cross-talk between liver and gut. More than 300 common metabolites were identified in the plasma and feces of patients with alcohol-related liver cirrhosis (ALC) by liquid chromatography-mass spectrometry. And we found bile acid and amino acid were distributed differently in plasma and feces. More importantly, increased glycocholic acid, taurocholic acid, L-phenylalanine, L-tyrosine, and L-methionine in plasma and decreased deoxycholic acid in feces of patients with ALC were related to the severity of ALC. These metabolites have the potential to evaluate the progression of ALC.
- Citation: Xu YF, Hao YX, Ma L, Zhang MH, Niu XX, Li Y, Zhang YY, Liu TT, Han M, Yuan XX, Wan G, Xing HC. Difference and clinical value of metabolites in plasma and feces of patients with alcohol-related liver cirrhosis. World J Gastroenterol 2023; 29(22): 3534-3547
- URL: https://www.wjgnet.com/1007-9327/full/v29/i22/3534.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i22.3534
Alcohol abuse is one of the major factors causing cirrhosis. According to the research data on global disease burden in 2017, 25% of deaths from liver cirrhosis were caused by alcohol-related liver diseases (ALD)[1]. Several clinical studies have found altered plasma or serum metabolites in patients with alcohol-related liver cirrhosis (ALC)[2]. Previous studies have focused on serum or plasma metabolomics in patients with ALC and demonstrated that alcohol alters the levels of amino acids and bile acids in the plasma or serum[3]. The common changes in plasma or serum include decreased levels of glutamine and increased levels of tyrosine and alanine[4,5]. A few studies on fecal metabolomics showed that fecal metabolomics in patients with alcoholic cirrhosis was changed, while some studies revealed that the fecal amino acids, such as glutamine, isoleucine, phenylalanine, and serine, are reduced[6]. In addition, the production of secondary bile acid, such as deoxycholic acid (DCA), was reduced[6]. Also, intestinal microbiota can metabolize bile acids, aromatic amino acids, carbohydrates, and polysaccharides to produce secondary bile acids, indoles, phenol derivatives, short-chain fatty acids, and polysaccharides[7]. These metabolites can also influence other organs through the gut-liver axis[8]. Therefore, the metabolites in the blood can be either from other organs’ metabolites outside the intestine or from intestinal[9]. The metabolites in the blood or the feces may have homogeneity but may also have specific characteristics related to their respective environments. These products participate in the metabolism of glycogen, amino acid, and exogenous substances and exert anti-inflammatory effects directly in the intestine[10]. However, the correlation between intestinal metabolites and metabolites in plasma for patients with ALC, which might be helpful for the diagnosis and treatment of ALC, has not yet been elucidated. Therefore, the present study aimed to investigate the common and different characteristics of the metabolites in the plasma and feces of patients with ALC, their clinical correlation between plasma metabolomics and fecal metabolomics, and their value in the evaluation of the severity of ALC. This might provide a new direction for understanding the progression of ALC and the diagnosis and treatment of ALD.
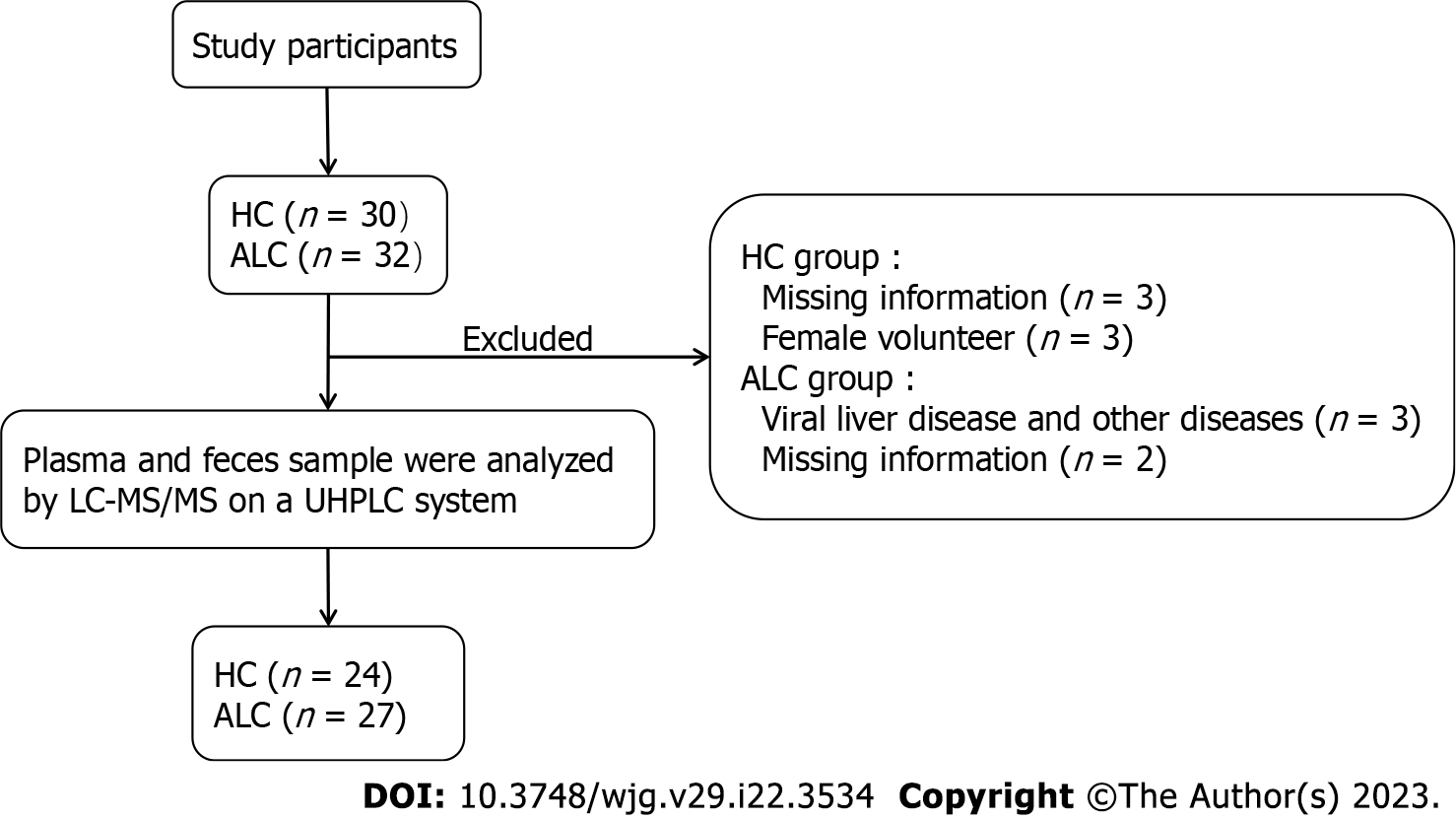
This was a case-controlled observation study. We prospectively enrolled two groups of subjects: individuals with ALC and healthy controls (HCs). ALC subjects were selected from male inpatients admitted to the Liver Disease Center of Ditan Hospital (Beijing, China) from September 30, 2020 to December 31, 2021. The flow-chart demonstrates the selection of study participants (Figure 1). According to the International Clinical Guidelines[11], individuals with ALC were diagnosed if they met the following criteria: (1) Regular drinking and have a prior or ongoing heavy alcohol intake of > 5 years (males >36 g alcohol/day); and (2) ultrasonography, computed tomography, or magnetic resonance imaging should exhibit liver cirrhosis. Additionally, we recruited age-matched healthy males as controls through recruitment advertisements. These controls met the following criteria after clinical assessment and blood and imaging examinations: (1) Normal physical examination (heart rate, blood pressure, and respiratory rate) and body mass index (BMI) < 30 kg/m2; (2) no history of alcohol intake; (3) blood routine, liver function, kidney function, tumor maker, and abdominal ultrasound with normal limits; and (4) no sign of any disease, including liver and heart diseases. The following criteria were excluded for all participants: (1) Liver diseases except for alcohol etiologies, such as hepatitis B virus or hepatitis C virus infection, drug-induced liver diseases, and autoimmune hepatitis; (2) Complications except for ascites, such as hepatic encephalopathy, hepatorenal syndrome, hepatopulmonary syndrome, and esophageal and gastric variceal bleeding; (3) human immunodeficiency virus infection; (4) other digestive diseases (colitis, pancreatitis, and cholecystitis); (5) kidney, heart, lung, neurological, and metabolic diseases (diabetes, thyroid, and adrenal diseases); (6) all types of cancers; and (7) systematically using antibiotics, probiotics, and proton pump inhibitors within 1 mo before enrollment.
Clinical information, including demographic indicators, was collected. Moreover, the plasma and feces of all participants were collected and frozen at -80 °C for subsequent biochemical and hematological indexes tests and untargeted metabolomics detection.
This research scheme was approved by the Ethics Committee of Ditan hospital based on the ethical principles of the Declaration of Helsinki and registered at http://www.chictr.org.cn/ (ChiCTR-2000038216). Written informed consent was obtained from all the participants.
White blood cell, neutrophilic granulocyte percentage, red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), and platelet (PLT) levels were detected on automatic blood routine analyzers (Sysmex, Kobe, Japan). Alanine aminotransferase (ALT), aspartate transaminase (AST), total bilirubin (TBil), albumin (ALB), albumin/globulin (A/G), g-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), cholinesterase (CHE), total bile acid (TBA), total cholesterol, urea nitrogen, and creatinine (Cr) were detected on automatic biochemical analyzers (Hitachi, Tokyo, Japan). Prothrombin time (PT) was detected by automatic coagulation analyzers (Werfan, Barcelona, Spain), and alpha-fetoprotein (AFP) was detected by chemiluminescence immunoassay analyzer (Abbott, Chicago, IL, United States).
Plasma or feces sample was mixed with an extract buffer and homogenized in an ice-water bath. The homogenization and sonication cycle was repeated three times. The supernatant was collected by centrifugation of the homogenates and analyzed by liquid chromatography-mass spectrometry (LC-MS/MS) on a UHPLC system (Thermo Fisher Scientific, Waltham, MA, United States) with a UPLC BEH Amide column coupled to Q Exactive HFX mass spectrometer from Thermo Fisher Scientific. The QE HFX mass spectrometer was used for its ability to acquire MS/MS spectra in information-dependent acquisition (IDA) mode using the acquisition software (Thermo Fisher Scientific, Waltham, MA, United States). The acquisition software continuously evaluates the full scan MS spectrum in this mode. The quality control (QC) sample was prepared by mixing an equal aliquot of the supernatants from all the samples.
The raw data were converted to the mzXML format using ProteoWizard and processed with an in-house program developed using R and based on XCMS for peak detection, extraction, alignment, and integration. Then, an in-house MS2 database (Biotree DB, Shanghai, China) was applied to metabolite annotation. The cutoff for the annotation was set at 0.3. All variables were normalized in MetaboAnalyst 5.0 (https://www.metaboan-alyst.ca) after preprocessing using the Masshunter Profinder software. R script can view details and download them from the website of MetaboAnalyst (https://www.metaboanalyst.ca/MetaboAnalyst/docs/RTutorial.xhtml). The results of the patients’ clinical and biochemical characteristics for continuous variables are expressed as median (upper quartile, lower quartile), and categorical variables are expressed as numbers and percentages. The between-group comparisons were made with Kruskal-Wallis H test. All statistical analyses were performed using SPSS 26.0 software. The significance level for all statistical tests was set at 0.05. Based on the metabolome profile, the phenotype-associated fingerprint metabolites were projected to one eigen metabolite by the first principal component in the unsupervised principal component analysis (PCA). Fold change (FC): the quantitative ratio of the two groups of experimental substances by comparing the ALC group to the HC group. Furthermore, we analyzed significant differences (P < 0.05 and FC > 2 or P < 0.05 and FC < 0.5) by comparing the ALC and HC groups and marked them as ALC/HC. We also detected the metabolic pathways of phenotype-associated fingerprint metabolites. PCA was conducted on the MetaboAnalyst website based on R. Furthermore, the P- and FC values were obtained from the MetaboAnalyst website. Then, the original variables were narrowed down to fingerprint based on P-value and FC.
The clinical characteristics and laboratory tests of participants are shown in Table 1. A total of 51 subjects were included in this study, including 24 HCs and 27 patients with ALC, and all subjects were males. No significant differences were detected in age and BMI between the two groups. The levels of ALT, AST, TBil, GGT, ALP, TBA, and PT were higher in the ALC group than in the HC group, while the number of RBCs and the levels of HGB, HCT, ALB, CHE, PLT, and T-CHO were lower in ALC than in HCs. Compared to HCs, there were no significant differences in UREA, CREA, AFP, and GLU in ALC patients.
| Characteristic | HC (n = 24) | ALC (n = 27) | P value |
| Age (yr) | 53 (48, 57.75) | 51 (43, 60) | 0.527 |
| BMI (kg/m2) | 22.92 (22.04, 24.09) | 24.22 (22.94, 24.94) | 0.101 |
| Clinical lab index | |||
| WBC (109/L) | 5.82 (5.30, 6.76) | 4.57 (3.60, 6.62) | 0.023 |
| NEU (%) | 56.20 (52.1, 60.55) | 56.60 (46.60, 67.90) | 0.678 |
| RBC (1012/L) | 4.83 (4.52, 5.02) | 3.66 (2.90, 4.58) | < 0.01 |
| HGB (g/L) | 148.00 (144.00, 156.00) | 117.00 (100.00, 147.00) | < 0.01 |
| HCT (%) | 43.25(41.85, 46.8) | 33.60 (29.50, 42.50) | < 0.01 |
| MCV (fl) | 91.50 (88.35, 94.68) | 97.00 (92.2, 104.00) | 0.001 |
| PLT (109/L) | 246 (223.25, 268.00) | 86.00 (46.00, 123.00) | < 0.01 |
| ALT (U/L) | 17.35 (12.75, 22.5) | 25.7 (15.1, 36.9) | < 0.01 |
| AST (U/L) | 19.7 (17.78, 21.73) | 36.7 (24.90, 74.20) | < 0.01 |
| TBil (μmol/L) | 13.95 (11.75, 15.20) | 47.3 (28.70, 115.70) | < 0.01 |
| ALB (g/L) | 46.48 (45.68, 47.68) | 34.27 (28.4, 39.5) | < 0.01 |
| A/G | 1.80 (1.63, 1.80) | 1.10 (0.80, 1.60) | < 0.01 |
| GGT (U/L) | 14.7 (10.28, 17.50) | 94.3 (25.00, 243.80) | < 0.01 |
| ALP (U/L) | 58.7 (53.05,70.05) | 97.9 (68.40, 137.50) | < 0.01 |
| CHE (U/L) | 8326.00 (7251.50, 9304.25) | 3547 (1700.00, 5735.00) | < 0.01 |
| TBA (μmol/L) | 1.90 (1.45, 2.50) | 66.1 (29.30, 174.20) | < 0.01 |
| T-CHO (mmol/L) | 4.40(3.72, 4.86) | 3.27 (2.54, 4.78) | < 0.05 |
| UREA (mmol/L) | 5.04 (4.12, 5.87) | 5.05 (4.29, 6.21) | 0.734 |
| CREA (mmol/L) | 75.5 (72.18, 84.93) | 68.8 (60.5, 75.50) | 0.051 |
| GLU (mmol/L) | 5.27 (4.94, 5.76) | 5.24 (4.57, 6.08) | 0.977 |
| PT(s) | 11.45 (11.20, 11.8) | 17.1 (12.10, 22.80) | < 0.01 |
| AFP (ng/mL) | 2.37 (1.97, 3.34) | 4.01 (2.70, 6.97) | 0.002 |
More than 8000 plasma metabolites and more than 10,000 fecal metabolites were detected in this study. HMDB identified > 300 types of co-metabolites in plasma and feces. Amino acids and their derivatives accounted for the largest proportion of co-metabolites in plasma and feces. The remaining metabolites included bile acids, dicarboxylic acids, hydroxy fatty acids, saturated fatty acids, and unsaturated fatty acids (Figure 2A). Principal component analysis on the plasma and feces of patients with ALC showed (Figure 2B and C) that the metabolites in the plasma were clearly distinguished between the two groups based on the first and second principal component scores, while the metabolites in the feces were discretely distributed within the two groups, rendering difficulty in distinguish them between the groups. These findings indicated obvious individual differences in the types and content of fecal metabolites in ALC or HCs.
The common metabolites were analyzed in plasma and feces, respectively, and compared to HCs after t-test and difference analysis. A total of 150 differential metabolites in plasma and 106 differential metabolites in the feces of patients with ALC were screened based on FC > 2 or FC < 0.5. The results showed that 46 metabolites were upregulated and 16 were downregulated in plasma, while 38 metabolites were upregulated and 14 were downregulated in feces (Table 2, SupplementaryTables 1 and 2). Similarly, metabolic pathway analysis showed that the metabolites in plasma and feces of patients with ALC were involved in porphyrin metabolism, phospholipid biosynthesis, inositol metabolism, sugar and starch metabolism, catecholamine biosynthesis, thyroid hormone synthesis, estrone metabolism, betaine metabolism, beta-oxidation of very long chain fatty acids, fatty acid metabolism, caffeine metabolism, and phosphatidylcholine synthesis. The bile acid metabolism pathway was significantly enriched in plasma, while the porphyrin metabolism pathway was significantly enriched in feces (Figures 3A and B). Strikingly, the amino acid metabolism was significantly enriched in both plasma and feces. However, the types of amino acids involved in plasma and feces were different. The metabolic pathways of methionine, phenylalanine, tyrosine, glycine, and serine were significantly enriched in both plasma and feces; however, the metabolic pathways of tryptophan, taurine, hypotaurine, D-arginine, and D-ornithine were enriched in the plasma, while alanine, valine, leucine, isoleucine, arginine, and proline metabolic pathways were significantly enriched in feces. Various substances were involved in the above-mentioned enrichment pathways (Supplemen
| ALC/HC | P < 0.05 | Up (FC > 2) | Down (FC < 0.5) |
| Plasma | 150 | 46 | 16 |
| Feces | 106 | 38 | 14 |
Compared to HCs, metabolites involved in the above metabolic pathways differed in the plasma and stool of ALC (Table 3). In the bile acid metabolism pathway, primary bile acids, including CA, TCA, and glycocholic acid (GCA), increased in the plasma of patients with ALC, while CA, TCA, and GCA did not change significantly in feces. Additionally, secondary bile acid DCA decreased in feces but did not differ in plasma. In the amino acid metabolism pathway, L-threonine, L-phenylalanine, L-tyrosine, and choline increased in both plasma and feces, while betaine decreased concomitantly. GCA, L-methionine, and taurine increased, while sarcosine decreased only in plasma. Glycine elevated only in feces, while L-valine decreased in plasma but increased in feces.
| Metabolites | Metabolic pathway | Plasma (ALC/HC) | Feces (ALC/HC) |
| Cholic acid | Bile acid biosynthesis | ↑ | NS |
| Glycocholic acid | ↑ | NS | |
| Taurocholic acid | ↑ | NS | |
| Deoxycholic acid | ↑ | ↓ | |
| Glycine | Alanine metabolism | NS | ↑ |
| Arginine and proline metabolism | |||
| Glutathione metabolism | |||
| Bile acid biosynthesis | |||
| Taurine | Taurine and hypotaurine metabolism | ↑ | NS |
| Bile acid biosynthesis | |||
| Betaine | Glycine and serine metabolism | ↓ | ↓ |
| Methionine metabolism | |||
| L-Methionine | Glycine and serine metabolism | ↑ | NS |
| Methionine metabolism | |||
| L-Threonine | Glycine and serine metabolism | ↑ | ↑ |
| Choline | Methionine metabolism | ↑ | ↑ |
| Sarcosine | Methionine metabolism | ↓ | NS |
| L-Phenylalanine | Phenylalanine and tyrosine metabolism | ↑ | ↑ |
| L-Tyrosine | Phenylalanine and tyrosine metabolism | ↑ | ↑ |
| Formylanthranilic acid | Tryptophan metabolism | ↓ | NS |
| L-Valine | Valine, leucine and isoleucine degradation | ↓ | ↑ |
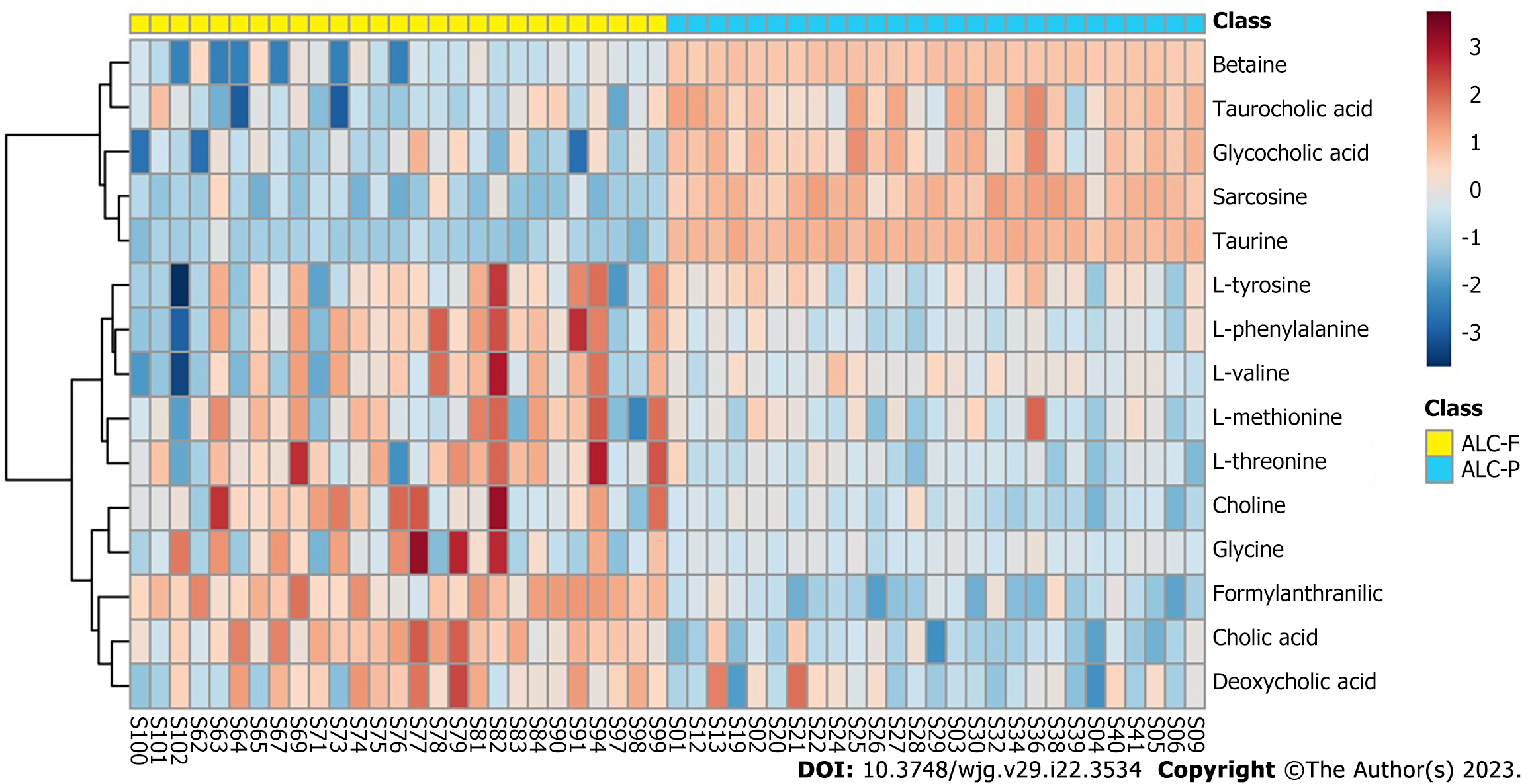
For patients with ALC, the differential enrichment analysis of bile acid and amino acid metabolites in plasma and feces revealed that the distribution of the above-mentioned metabolites differed in the plasma and feces. In the plasma of patients with ALC, betaine, TCA, GCA, sarcosine, and taurine were significantly enriched, while CA, DCA, and formylanthranilic acid, L-threonine, L-phenylalanine, L-tyrosine, L-methionine, and glycine were markedly enriched in feces (Figure 4).
Spearman’s correlation analysis revealed that plasma amino acids L-methionine, L-phenylalanine, and L-tyrosine in patients with ALC were positively correlated to TBA, MDF, TBil, and PT and negatively correlated with CHE and ALB. L-methionine had the highest correlation with TBA, and L-phenylalanine had the highest correlation with MDF, TBil, and PT. Nonetheless, no correlation was established between L-methionine, L-phenylalanine, L-tyrosine and TBA, MDF, TBil, PT in feces (Figure 5).
Primary bile acids GCA and TCA in plasma exhibited a positive correlation with TBil in clinical practice to varying degrees, with GCA having the strongest correlation with a coefficient of 0.86. However, no correlation was established between DCA in feces and clinical TBil. The levels of both GCA and TCA in plasma showed a positive correlation with TBil, MDF, and PT and a negative correlation with CHE and ALB. Conversely, DCA in feces demonstrated a negative correlation with TBil, MDF, and PT but a positive correlation with CHE and ALB. Compared to a single index, the ratio of all primary bile acids in the blood, including GCA and TCA to DCA in the feces (recorded as P/S BA ratio) showed a higher correlation with MDF, TBil, CHE, PT, and ALB (Figure 5).
In patients with ALC, the stability of the intestinal microbiota and the integrity of the intestinal barrier were compromised, altering the intestinal microbiota and their metabolites. Both exogenous molecules, such as amino acids, and endogenously produced molecules from intestinal microorganisms can be transported to the liver via the portal vein. The liver secretes bile acids and other biologically active mediators into the biliary tract and bloodstream, affecting the intestinal microbiota and their metabolites[12]. This raises questions about the similarities and differences between plasma and intestinal metabolites and their implications in the progression of ALD. In order to gain an in-depth understanding of the primary metabolic pathways involved in enterohepatic circulation and their correlation with ALD, we conducted a metabolomics study that analyzed both plasma and feces metabolites. The current comparative analysis was based on the Human Metabolome Database and identified > 300 metabolites, although their distribution varied between plasma and feces. Notably, bile acid and amino acid metabolism pathways were significantly concentrated in both plasma and feces, which might participate in the progression of the disease.
Bile acid is a critical metabolic substance for maintaining the homeostasis of the gut-liver axis[13]. Primary and secondary bile acids (synthesized by the liver and produced by bacterial metabolism, respectively) performed various functions in the small intestine and played critical roles in lipid absorption, cholesterol homeostasis, and hormonal effects via their steroid structure[14]. Firstly, pathway enrichment analysis in patients with ALC showed substantial enrichment of bile acid metabolism in plasma. Next, we found that primary bile acids, including CA, TCA, and GCA of patients with ALC increased in plasma but showed no apparent differences in feces, while secondary bile acid DCA decreased in feces but increased in plasma. Ethanol stimulates bile acid formation in primary human hepatocytes[15]. Moreover, liver cirrhosis affects bile acid secretion, and patients suffer from cholestasis. This phenomenon might be the reason for the increase of CA, TCA, and GCA in the plasma of patients with ALC. Kakiyama et al[16] reported different levels of serum DCA in ALC with varied amounts of alcohol consumption, and the volume of serum binding DCA in ALC was higher than in HCs. These findings indicated that plasma DCA increased in patients with ALC; this phenomenon was associated with alcohol consumption status. However, several studies have demonstrated that fecal DCA and other secondary bile acids in patients with ALD decreased[17,18], which was in agreement with our findings. DCA is the most common bile acid in human feces and cecum samples[19]. Most of the DCA in the intestine is only produced by cholic acid transformed by 7α-dehydroxylation-producing bacteria, and the currently known DCA-producing bacteria include very few Clostridia and closely related bacteria of other genera[20]. Previous studies demonstrated that Clostridiales XIV decreased in the feces of ALC patients[21], which might be related to alcohol-induced dysbiosis and inflammation of the intestine, and Clostridiales XIV was predictive of 90-d hospitalizations. This could be one of the mechanisms for the decrease in fecal DCA, which is presented to the colon[6]. On the other hand, due to the impact of DCA on the stability of intestinal epithelial cell membrane, intestinal permeability increased, which might result in the resorption of DCA in the blood[22]. This further suggested that intestinal microbiota play a significant role in bile acid metabolism and disease progression.
Moreover, our analysis of the correlation between bile acids with clinical indicators showed that GCA and TCA in plasma were positively correlated with TBil, PT, and MDF and negatively correlated with ALB and CHE, while fecal DCA was negatively correlated with TBil, PT, and MDF and positively correlated with ALB and CHE. The ratio of primary bile acids in the plasma to secondary bile acids in the feces was correlated with MDF, TBil, CHE, ALB, and PT. Additionally, a positive correlation was established between fecal DCA and T-CHO in blood, suggesting that secondary bile acids in feces might be closely related to cholesterol homeostasis in the blood. TBil, PT, ALB, and MDF reflect the severity of diseases, while CHE reflects the liver’s composition function. The current results suggested that plasma GCA and TCA and fecal DCA might be related to the severity of diseases. Furthermore, we calculated the ratio of primary bile acids in the plasma to secondary bile acids in feces (P/S BA ratio) and found that the P/S BA ratio is correlated with MDF, TBil, CHE, ALB, and PT, which might be an alternative indicator of the severity of the diseases. In addition, we found a positive correlation between DCA in feces and T-CHO in the blood, which further suggested that secondary bile acids in feces might be closely related to cholesterol homeostasis in the blood. In conclusion, bile acid metabolism plays a significant role in enterohepatic circulation, and a combined analysis of primary bile acids in the plasma and secondary bile acids in feces might provide valuable insights into the progression of ALD.
The current study also revealed that both plasma and feces exhibit a substantial concentration of amino acid metabolism, yet the specific types of amino acids involved in each vary significantly. Branched-chain amino acids and aromatic amino acids partake in the progression of cirrhosis, as shown previously[23,24]. These findings showed a decrease in the plasma concentration of the branched-chain amino acid L-valine and an increase in its concentration in feces. Some studies have shown that the concentration of L-valine in the plasma of ALC patients decreased[25], and supplementing with L-valine could improve the survival rate of cirrhosis patients[26]. The decrease in Faecalibacterium prausnitzii in the feces of cirrhosis patients, which coded for branched-chain amino acid translocators[27], can obstruct the transport of branched-chain amino acids and increase L-valine in feces but decrease it in the plasma. The content of valine in feces is also affected by the dietary intake of branched-chain amino acids. The amino acids with simultaneous changes in plasma and feces are L-phenylalanine, L-tyrosine, and L-threonine, of which L-phenylalanine and L-tyrosine are aromatic amino acids. The elevated levels of aromatic amino acids in cirrhotic patients are linked to the progression of liver disease and the development of hepatic encephalopathy[28]. Previous studies have demonstrated that hydroxylation of L-phenylalanine and L-tyrosine flux is increased in the blood of patients with liver cirrhosis[29]. Phenylalanine is decomposed and hydroxylated to tyrosine, which might explain the elevated levels of L-phenylalanine and L-tyrosine in the plasma. Also, L-phenylalanine and L-tyrosine were raised in feces through enterohepatic circulation, while L-methionine was increased in the plasma but not feces in ALC patients. A previous study by Marchesini et al[30] suggested that the increase of plasma methionine in patients with cirrhosis could be attributed to the damage of liver cells and the decrease in methionine’s metabolic function. In addition, the NMR-based metabolomics analysis by Kumar et al[31] showed increased serum methionine in patients with acute-on-chronic liver failure. Moreover, our study found that plasma L-phenylalanine, L-tyrosine, and L-methionine were positively correlated with TBil and MDF, suggesting that these three amino acids may be related to the severity of diseases, and are designated as markers of disease progression in the blood.
Furthermore, our analysis detected the presence of betaine, a crucial component in the metabolism of amino acids, such as glycine, serine, and methionine, in the enterohepatic circulation. In patients with ALC, betaine decreased in both plasma and feces. It plays a vital role in methylating homocysteine into methionine, a process that is crucial for maintaining liver function stability, cell replication, and detoxification. The reduction in betaine levels in patients with ALC could be due to its depletion or insufficient supply during the disease process. A study by Schofield et al[32] showed that changes in betaine were associated with the development of ALC. Previous studies have shown that supplementing betaine can prevent acute alcoholic liver injury[33], and measuring fecal or plasma betaine levels in patients with ALC can assess its insufficiency and is expected to serve as an indicator for evaluating the severity of disease or a therapeutic target for treatment.
In addition, glycine and taurine play crucial roles in both amino acid metabolism and bile acid metabolism. Alcohol consumption may increase in the proportion of secondary bile acids, the total concentration of bile acids, and the proportion of bile acids bound to glycine rather than taurine[10,13]. Bajaj et al[6] found that the conjugated bile acids in the duodenal fluid and feces turned into toxic glycine-conjugated bile acids from harmless taurine-conjugated bile acids in ALC patients. Our study also observed that taurine was concentrated in the plasma of patients with ALC, while glycine was concentrated in their feces. Taurine/glycine ratio was decreased in the serum and feces of patients with ALC. The imbalance in the taurine/glycine ratio may be due to dysbiosis caused by liver cirrhosis, the subsequent reduction of taurine bioavailability, and an increase in the intestine-liver circulation rate[10,14]. This phenomenon suggested that regulating taurine/glycine ratio might improve the intestinal microecology of ALC patients and alleviate liver diseases.
Taken together, understanding the similarities and differences between plasma and feces metabolites holds significant potential in advancing molecular diagnosis in disease progression. The detection of these metabolites highlights the diversity of substances that can be detected in both plasma and feces. Fecal metabolites are more complex than those in plasma, which might be influenced by several factors, such as race, environment, diet, and medicine. Additionally, changes in intestinal and colon microbiota and their metabolism can alter the fecal metabolites, with secondary metabolites produced by the intestinal microbiota potentially playing a role in disease progression. Thus, further studies with larger samples are required to validate and evaluate bile acid and amino acids as potential markers for disease progression in cirrhosis. Moreover, integrating intestinal microbiome omics and metabonomics analysis is essential to decipher the molecular mechanisms underlying ALC. Since LC-MS analysis used in this study has limitations, further comparisons with other detection methods and complementary studies are required. The analysis of metabolites in plasma and feces provides an in-depth insight into ALC.
Bile acid and amino acid metabolism play a very important role in the progression of ALC. The enrichment of GCA, TCA, L-phenylalanine, L-tyrosine, and L-methionine in the plasma of patients with ALC and the reduction of DCA in feces was related to the severity of ALC. These metabolites may be used as indicators to evaluate the progression of ALC.
Alterations in plasma and intestinal metabolites contribute to the pathogenesis and progression of alcohol-related liver cirrhosis (ALC).
Metabolites in enterohepatic circulation play an important role in cross-talk between liver and gut. The metabolites in the blood or the feces may have homogeneity but may also have specific characteristics related to their respective environments. The correlation between intestinal and plasma metabolites in patients with ALC, which might be helpful for the diagnosis and treatment of ALC, has not yet been elucidated.
To explore the common and different metabolites in the plasma and feces of patients with ALC and evaluate their clinical implications.
This was a case-controlled observation study. We prospectively enrolled two groups of subjects: individuals with ALC and healthy controls (HCs). We recruited age-matched healthy males as controls through recruitment advertisements. According to the inclusion and exclusion criteria, 27 patients with ALC and 24 HCs were selected. The plasma and feces samples were collected. Liver function, blood routine, and other indicators were detected with automatic biochemical and blood routine analyzers. Liquid chromatography-mass spectrometry was used to detect the plasma and feces metabolites. Also, the association of metabolites with clinical features was analyzed.
More than 8000 plasma and more than 10000 fecal metabolites of patients with ALC were detected. Among them, More than 300 metabolites were found both in the plasma and feces. Enrichment analysis showed that these common metabolites are enriched in bile and amino acid metabolic pathways. Moreover, patients with ALC had a higher level of glycocholic acid (GCA) and taurocholic acid (TCA) in plasma and a lower level of deoxycholic acid (DCA) in the feces, while L-threonine, L-phenylalanine, and L-tyrosine increased simultaneously in plasma and feces. These results was consistent with previous studies and have indeed confirmed the disorder of bile acid and amino acid metabolism in patients with ALC. Besides, GCA, TCA, L-methionine, L-phenylalanine, and L-tyrosine in plasma were positively correlated with total bilirubin (TBil), prothrombin time (PT), and maddrey discriminant function score (MDF) and negatively correlated with cholinesterase (CHE) and albumin (ALB). The DCA in feces was negatively correlated with TBil, MDF, and PT and positively correlated with CHE and ALB. AP/S BA ratio of plasma primary bile acid (GCA and TCA) to fecal secondary bile acid (DCA), which was relevant to TBil, PT, and MDF score was established, which may be used as a biomarker of the severity of ALC.
Bile acid and amino acid metabolism play a very important role in the progression of ALC. The enrichment of GCA, TCA, L-phenylalanine, L-tyrosine, and L-methionine in the plasma of patients with ALC and the reduction of DCA in feces was related to the severity of ALC. The P/S BA ratio of plasma primary bile acids (GCA and TCA) to fecal secondary bile acid (DCA), was relevant to TBil, PT, and MDF score.
Integrating intestinal microbiomics and metabolomics analysis is essential to decipher the molecular mechanisms underlying ALC. Since LC-MS analysis used in this study has limitations, further comparisons with other detection methods and complementary studies are required.
The authors would like to thank all patients and healthy volunteers for their participation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Society of Hepatology, Chinese Association of Integrative Medicine; the Microecology Professional Committee of Beijing Association of Preventive Medicine; the Infectious Disease Branch of Chinese Preventive Medicine Association; Chinese Society of Hepatology, Chinese Medical Association; the Microecology Branch of Chinese Preventive Medicine Association; the Hepatology Branch of the Beijing Medical Association.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotlyarov S, Russia; Manautou JE, United States S-Editor: Yan JP L-Editor: A P-Editor: Chen YX
| 1. | GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1158] [Cited by in RCA: 1013] [Article Influence: 202.6] [Reference Citation Analysis (4)] |
| 2. | Voutilainen T, Kärkkäinen O. Changes in the Human Metabolome Associated With Alcohol Use: A Review. Alcohol Alcohol. 2019;54:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (3)] |
| 4. | Lian JS, Liu W, Hao SR, Guo YZ, Huang HJ, Chen DY, Xie Q, Pan XP, Xu W, Yuan WX, Li LJ, Huang JR. A serum metabonomic study on the difference between alcohol- and HBV-induced liver cirrhosis by ultraperformance liquid chromatography coupled to mass spectrometry plus quadrupole time-of-flight mass spectrometry. Chin Med J (Engl). 2011;124:1367-1373. [PubMed] |
| 5. | Harada S, Takebayashi T, Kurihara A, Akiyama M, Suzuki A, Hatakeyama Y, Sugiyama D, Kuwabara K, Takeuchi A, Okamura T, Nishiwaki Y, Tanaka T, Hirayama A, Sugimoto M, Soga T, Tomita M. Metabolomic profiling reveals novel biomarkers of alcohol intake and alcohol-induced liver injury in community-dwelling men. Environ Health Prev Med. 2016;21:18-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Bajaj JS, Kakiyama G, Zhao D, Takei H, Fagan A, Hylemon P, Zhou H, Pandak WM, Nittono H, Fiehn O, Salzman N, Holtz M, Simpson P, Gavis EA, Heuman DM, Liu R, Kang DJ, Sikaroodi M, Gillevet PM. Continued Alcohol Misuse in Human Cirrhosis is Associated with an Impaired Gut-Liver Axis. Alcohol Clin Exp Res. 2017;41:1857-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Sutherland VL, McQueen CA, Mendrick D, Gulezian D, Cerniglia C, Foley S, Forry S, Khare S, Liang X, Manautou JE, Tweedie D, Young H, Alekseyenko AV, Burns F, Dietert R, Wilson A, Chen C. The Gut Microbiome and Xenobiotics: Identifying Knowledge Gaps. Toxicol Sci. 2020;176:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 469] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 9. | Ventura-Cots M, Argemi J, Jones PD, Lackner C, El Hag M, Abraldes JG, Alvarado E, Clemente A, Ravi S, Alves A, Alboraie M, Altamirano J, Barace S, Bosques F, Brown R, Caballeria J, Cabezas J, Carvalhana S, Cortez-Pinto H, Costa A, Degré D, Fernandez-Carrillo C, Ganne-Carrie N, Garcia-Tsao G, Genesca J, Koskinas J, Lanthier N, Louvet A, Lozano JJ, Lucey MR, Masson S, Mathurin P, Mendez-Sanchez N, Miquel R, Moreno C, Mounajjed T, Odena G, Kim W, Sancho-Bru P, Warren Sands R, Szafranska J, Verset L, Schnabl B, Sempoux C, Shah V, Shawcross DL, Stauber RE, Straub BK, Verna E, Tiniakos D, Trépo E, Vargas V, Villanueva C, Woosley JT, Ziol M, Mueller S, Stärkel P, Bataller R. Clinical, histological and molecular profiling of different stages of alcohol-related liver disease. Gut. 2022;71:1856-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Zafari N, Velayati M, Fahim M, Maftouh M, Pourali G, Khazaei M, Nassiri M, Hassanian SM, Ghayour-Mobarhan M, Ferns GA, Kiani MA, Avan A. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022;305:120760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 569] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 12. | Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Liu T, Zhao X, Gao Y. New insights into the bile acid-based regulatory mechanisms and therapeutic perspectives in alcohol-related liver disease. Cell Mol Life Sci. 2022;79:486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Zhong W, Zhou Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol. 2014;5:514-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Nilsson LM, Sjövall J, Strom S, Bodin K, Nowak G, Einarsson C, Ellis E. Ethanol stimulates bile acid formation in primary human hepatocytes. Biochem Biophys Res Commun. 2007;364:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, Nittono H, Ridlon JM, Fuchs M, Gurley EC, Wang Y, Liu R, Sanyal AJ, Gillevet PM, Bajaj JS. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G929-G937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Muthiah MD, Smirnova E, Puri P, Chalasani N, Shah VH, Kiani C, Taylor S, Mirshahi F, Sanyal AJ. Development of Alcohol-Associated Hepatitis Is Associated With Specific Changes in Gut-Modified Bile Acids. Hepatol Commun. 2022;6:1073-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM, White MB, Noble NA, Monteith P, Fuchs M, Thacker LR, Sikaroodi M, Bajaj JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 620] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 19. | Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1644] [Cited by in RCA: 2041] [Article Influence: 102.1] [Reference Citation Analysis (0)] |
| 20. | Streidl T, Karkossa I, Segura Muñoz RR, Eberl C, Zaufel A, Plagge J, Schmaltz R, Schubert K, Basic M, Schneider KM, Afify M, Trautwein C, Tolba R, Stecher B, Doden HL, Ridlon JM, Ecker J, Moustafa T, von Bergen M, Ramer-Tait AE, Clavel T. The gut bacterium Extibacter muris produces secondary bile acids and influences liver physiology in gnotobiotic mice. Gut Microbes. 2021;13:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 21. | Acharya C, Bajaj JS. Altered Microbiome in Patients With Cirrhosis and Complications. Clin Gastroenterol Hepatol. 2019;17:307-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 22. | Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 23. | Fukui A, Kawabe N, Hashimoto S, Kamei H, Yoshioka K. Switching from branched-chain amino acid granules to branched-chain amino acid-enriched nutrient improves the branched-chain amino acid-to-tyrosine ratio in patients with cirrhosis with hypoalbuminemia: a prospective study. Eur J Gastroenterol Hepatol. 2020;32:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Lo EKK, Felicianna, Xu JH, Zhan Q, Zeng Z, El-Nezami H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 25. | Konstantis G, Pourzitaki C, Chourdakis M, Kitsikidou E, Germanidis G. Efficacy of branched chain amino acids supplementation in liver cirrhosis: A systematic review and meta-analysis. Clin Nutr. 2022;41:1171-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 26. | Holeček M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Moran-Ramos S, Macias-Kauffer L, López-Contreras BE, Villamil-Ramírez H, Ocampo-Medina E, León-Mimila P, Del Rio-Navarro BE, Granados-Portillo O, Ibarra-Gonzalez I, Vela-Amieva M, Tovar AR, Torres N, Gomez-Perez FJ, Aguilar-Salinas C, Canizales-Quinteros S. A higher bacterial inward BCAA transport driven by Faecalibacterium prausnitzii is associated with lower serum levels of BCAA in early adolescents. Mol Med. 2021;27:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Parés A, Deulofeu R, Cisneros L, Escorsell A, Salmerón JM, Caballería J, Mas A. Albumin dialysis improves hepatic encephalopathy and decreases circulating phenolic aromatic amino acids in patients with alcoholic hepatitis and severe liver failure. Crit Care. 2009;13:R8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Tessari P, Vettore M, Millioni R, Puricelli L, Orlando R. Effect of liver cirrhosis on phenylalanine and tyrosine metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Marchesini G, Bugianesi E, Bianchi G, Fabbri A, Marchi E, Zoli M, Pisi E. Defective methionine metabolism in cirrhosis: relation to severity of liver disease. Hepatology. 1992;16:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Kumar U, Sharma S, Durgappa M, Gupta N, Raj R, Kumar A, Sharma PN, Krishna VP, Kumar RV, Guleria A, Saraswat VA, Pande G, Kumar D. Serum Metabolic Disturbances Associated with Acute-on-chronic Liver Failure in Patients with Underlying Alcoholic Liver Diseases: An Elaborative NMR-based Metabolomics Study. J Pharm Bioallied Sci. 2021;13:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Schofield Z, Reed MA, Newsome PN, Adams DH, Günther UL, Lalor PF. Changes in human hepatic metabolism in steatosis and cirrhosis. World J Gastroenterol. 2017;23:2685-2695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Arumugam MK, Chava S, Perumal SK, Paal MC, Rasineni K, Ganesan M, Donohue TM Jr, Osna NA, Kharbanda KK. Acute ethanol-induced liver injury is prevented by betaine administration. Front Physiol. 2022;13:940148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |