Published online Jun 14, 2023. doi: 10.3748/wjg.v29.i22.3519
Peer-review started: January 3, 2023
First decision: March 8, 2023
Revised: March 15, 2023
Accepted: May 17, 2023
Article in press: May 17, 2023
Published online: June 14, 2023
Processing time: 154 Days and 22.7 Hours
It is controversial whether transjugular intrahepatic portosystemic shunt (TIPS) placement can improve long-term survival.
To assess whether TIPS placement improves survival in patients with hepatic-venous-pressure-gradient (HVPG) ≥ 16 mmHg, based on HVPG-related risk stratification.
Consecutive variceal bleeding patients treated with endoscopic therapy + nonselective β-blockers (NSBBs) or covered TIPS placement were retrospectively enrolled between January 2013 and December 2019. HVPG measurements were performed before therapy. The primary outcome was transplant-free survival; secondary endpoints were rebleeding and overt hepatic ence
A total of 184 patients were analyzed (mean age, 55.27 years ± 13.86, 107 males; 102 in the EVL+NSBB group, 82 in the covered TIPS group). Based on the HVPG-guided risk stratification, 70 patients had HVPG < 16 mmHg, and 114 patients had HVPG ≥ 16 mmHg. The median follow-up time of the cohort was 49.5 mo. There was no significant difference in transplant-free survival between the two treatment groups overall (hazard ratio [HR], 0.61; 95% confidence interval [CI]: 0.35-1.05; P = 0.07). In the high-HVPG tier, transplant-free survival was higher in the TIPS group (HR, 0.44; 95%CI: 0.23-0.85; P = 0.004). In the low-HVPG tier, transplant-free survival after the two treatments was similar (HR, 0.86; 95%CI: 0.33-0.23; P = 0.74). Covered TIPS placement decreased the rate of rebleeding independent of the HVPG tier (P < 0.001). The difference in OHE between the two groups was not statistically significant (P = 0.09; P = 0.48).
TIPS placement can effectively improve transplant-free survival when the HVPG is greater than 16 mmHg.
Core Tip: Hepatic venous pressure gradient helps clinicians to assess the prognosis of decompensated cirrhotic patients. The study included 184 patients showed that hepatic-venous-pressure-gradient (HVPG) before therapy as a risk stratification provides prognostic value. Treatment can be given with greater confidence with the management of patients by HVPG.
- Citation: Wang XX, Yin XC, Gu LH, Guo HW, Cheng Y, Liu Y, Xiao JQ, Wang Y, Zhang W, Zou XP, Wang L, Zhang M, Zhu-Ge YZ, Zhang F. Pre-transjugular-intrahepatic-portosystemic-shunt measurement of hepatic venous pressure gradient and its clinical application: A comparison study. World J Gastroenterol 2023; 29(22): 3519-3533
- URL: https://www.wjgnet.com/1007-9327/full/v29/i22/3519.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i22.3519
As a result of portal hypertension related to cirrhosis, variceal bleeding (VB) has been the leading cause of death in cirrhotic patients[1]. More than 50% of cirrhotic patients who undergo the recommended hemostatic treatments are readmitted due to rebleeding within a year. Among them, the mortality rate is nearly 33%[2]. In view of the high rebleeding rate, further therapy to prevent rebleeding, which is defined as secondary prophylaxis, should be performed for patients surviving acute VB. Nonselective beta-blockers (NSBBs) can decrease the portal venous pressure by reducing the cardiac output and splanchnic vasodilation. In addition, to render varices ischemic and necrotic, some endoscopic treatments for VB have been developed. It is advocated that endoscopic variceal ligation (EVL), combined with NSBBs, should be the first line of therapy for the secondary prevention of VB, while transjugular intrahepatic portosystemic shunt (TIPS) placement should be performed in rebleeding patients and preemptive TIPS placement should only be performed for high-risk patients[3,4]. TIPS placement is a fluoroscopy-guided procedure in which a conduit is built between the systemic and portal venous systems with the intent of decreasing the portal pressure[5]. A study with a median follow-up of 16 mo concluded that early TIPS placement could reduce treatment failure and mortality among high-risk patients[6]. Preemptive treatment could also improve the survival of patients with acute-on-chronic liver failure and acute VB, as suggested in a European observational study[7]. However, diversion of the portal flow could also lead to hepatic encephalopathy (HE) resulting from hepatic hypoperfusion and liver failure[6,8]. Holster et al[5] noted that TIPS placement was associated with higher rates of early HE (within one year). Thus, it is not clear whether TIPS placement offers a survival benefit.
The hepatic venous pressure gradient (HVPG) has been shown to have prognostic value in both compensated and decompensated cirrhosis[3,4,9]. In patients with HVPG ≥ 16 mmHg, the mortality rate of acute variceal hemorrhage is increased[10]. Moreover, HVPG ≥ 20 mmHg has been a clinically effective predictor of early rebleeding[11]. In our previous study[12], which had a 1-year follow-up period, we concluded that covered TIPS placement was more effective than standard therapy in preventing rebleeding but that it did not improve survival.
On the basis of our earlier findings, we compared TIPS placement with EVL+NSBBs to assess whether TIPS placement improves survival in cirrhotic patients with VB based on HVPG-related risk stratification over a long-term period to obtain more precise individual treatments and better treatment effects.
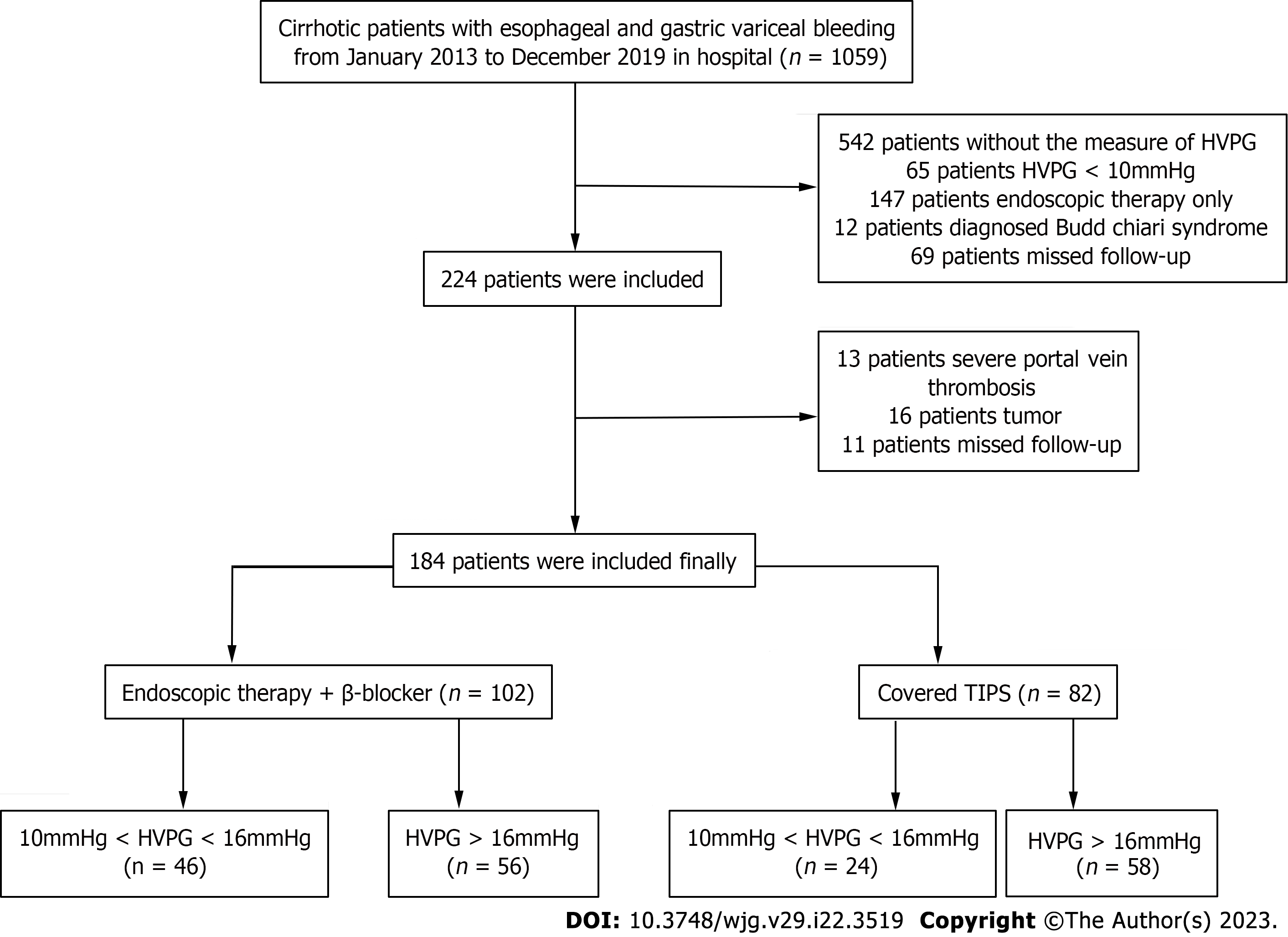
The study protocol was approved by the ethics committees of the Nanjing Drum Tower Hospital (Nanjing, Jiangsu Province, China). We retrospectively screened clinically stable cirrhotic patients with VB who were admitted to the Department of Gastroenterology of Nanjing Drum Tower Hospital from January 2013 to December 2019 in the prospectively maintained database (Figure 1). The included patients were diagnosed with liver cirrhosis by clinical, radiologic, and endoscopic examinations[13], and signed the informed consent form. The HVPG measurement was performed before therapy. Based on the HVPG-guided risk stratification (with the HVPG as a scale variable), patients with HVPG ≥ 16 mmHg were considered high-HVPG patients, and those with 10 mmHg ≤ HVPG < 16 mmHg were considered low-HVPG patients. The exclusion criteria of the study were as follows: (1) Non-standardized sequential endoscopic therapy; (2) previous secondary preventive intervention; (3) concomitant malignant tumors; (4) failure of sequential endoscopic therapy, i.e., rebleeding before the eradication of varices; (5) severe heart failure (New York Heart Association stage IV), respiratory failure (PaO2 < 60 mmHg) or severe kidney dysfunction (stage 5 chronic kidney disease); (6) pregnancy or lactation; and (7) missing follow-up data.
Finally, we included 184 patients in our study. All patients signed the informed consent form. Data for 83 of the 184 patients have been previously reported[12]. The prior article examined the effect of covered TIPS placement vs EVL+β-blockers in patients with HVPG ≥ 16 mmHg after 1 year, whereas in this study, which included all patients with HVPG ≥ 10 mmHg, we report the long-term outcomes after covered TIPS placement.
All included patients underwent preoperative examinations to rule out contraindications followed by HVPG measurements, which were performed under local anesthesia after 6-8 h of fasting (Figure 2). Briefly, the external zero reference point was set at the midaxillary line of the patient. Puncture through the right internal jugular vein was performed. In the TIPS group, a Cobra catheter (RUPS-100, COOK, Bloomington, IN, United States) was introduced into the right or middle hepatic vein, and hepatic venography was performed first to confirm whether stenosis or an obvious lateral shunt was present in the surrounding area. Measurements indicating large shunts were considered meaningless, while measurements indicating small or no shunts were considered meaningful. A 5.5-7 F balloon-tipped catheter (Edwards Lifesciences, Irvine, CA, United States) was guided into the hepatic vein. The free hepatic venous pressure (FHVP) was read after the pressure value stabilized. Air was injected to dilate the balloon to fully block hepatic vein blood flow. The wedged hepatic venous pressure (WHVP) was read after the pressure value stabilized for at least 40 s. The WHVP, FHVP, and inferior vena cava pressure were measured three times. Differences among the WHVP or FHVP measurements should not exceed 1 mmHg. The average of the 3 measurements was taken. After the pressure measurements were completed, a contrast agent was injected to confirm whether the obstruction was complete and whether hepatic vein-to-vein communications were present. The HVPG was calculated according to the following formula: HVPG=WHVP-FHVP.
After the HVPG measurements were acquired, the details of the two therapies were explained to the patients, including the procedures, adverse reactions, cost, and so on. Doctors provided professional medical advice based on the patient’s performance status, past bleeding history, vascular conditions, and HVPG, among other factors. The final treatment was selected by the patients.
Endoscopic therapy and NSBB usage were the same as described in our previous article[12]; specific details are provided in the Supplementary Methods.
Eighty-two patients underwent covered TIPS placement (Figure 2). Briefly, the process of TIPS placement was the same as described in our previous article[12]; specific details are provided in the Supplementary Methods. All operations were completed by a professional gastroenterology interventional therapy team to avoid surgical complications as much as possible.
Etiological treatment was followed throughout the study.
Outpatient follow-up was performed 1, 3, 6 and 12 mo after treatment and once a year thereafter. Follow-up examinations included postoperative history collection, routine blood tests and abdominal ultrasound. Any patients with complications during the follow-up period were admitted to the hospital for active treatment.
The primary endpoint of the study was the transplant-free survival time. The secondary endpoints were: (1) Rebleeding; and (2) the development of overt HE (OHE) diagnosed according to the American Association for the Study of Liver Diseases guidelines[14]. The patients were followed up until November 2021 or until death or liver transplantation. Two physicians regularly reviewed the data to detect errors. Two physicians assessed the accuracy of the data. After verification of the collected clinical variables in our study, statistical analysis was performed.
Our long-term observational study followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines[15]. Data are described as frequencies and percentages, means and standard deviations, or medians and interquartile ranges, as appropriate. Baseline characteristics were compared using Fisher’s exact test for categorical variables, t test for continuous variables, and the Wilcoxon rank-sum test for ordinal and continuous variables. Survival was estimated using the cumulative incidence function. Kaplan–Meier curves were used to illustrate the cumulative rates of survival and variceal rebleeding, and differences were assessed by the log-rank test. Associations of the risk factors with mortality were assessed by univariate and multivariate Cox proportional hazards regression models, and forward regression analysis was used to select the variables included in the multivariate model. All analyses were performed using GraphPad software (version 9.0), Statistical Package for Social Sciences (version 22.0) and R studio (version 4.0.1), and a level of significance was established at the two-sided 5% level.
According to the inclusion and exclusion criteria, 184 patients were analyzed. Based on the HVPG-guided risk stratification, 70 patients were in the low-HVPG tier, and 114 were in the high-HVPG tier. The patient characteristics are shown in Table 1. The median follow-up time was 49.5 mo. Patients who were lost to follow-up were excluded. The average time from registering to the end of research was not significantly different between the two groups. There were some differences in the baseline characteristics, which reflected the more severe conditions of the patients undergoing covered TIPS placement.
| Parameter | 10 mmHg ≤ HVPG < 16 mmHg (n = 70) | HVPG ≥ 16 mmHg (n = 114) | ||||||
| Total | Endoscopic therapy + NSBBs (n = 46) | Covered TIPS (n = 24) | P value | Total | Endoscopic therapy + NSBBs (n = 56) | Covered TIPS (n = 58) | P value | |
| Mean age (yr) | 55.27 ± 13.86 | 55.74 ± 13.44 | 54.38 ± 14.87 | 0.7 | 58.12 ± 11.22 | 57.30 ± 10.89 | 58.91 ± 11.57 | 0.45 |
| Sex | ||||||||
| Male/female | 39/31 | 28/18 | 11/13 | 0.31 | 68/46 | 34/22 | 34/24 | 0.85 |
| Previous bleeding history | ||||||||
| 0/1-2/> 3 | 41/26/3 | 31/15/0 | 10/11/3 | 0.02 | 77/26/11 | 41/10/5 | 36/16/6 | 0.41 |
| Virus/alcoholic/immune/others | 29/6/16/19 | 23/3/10/10 | 6/3/6/9 | 0.21 | 74/8/15/17 | 40/3/4/9 | 34/5/11/8 | 0.23 |
| PLT (× 10^9/L) | 69.80 ± 41.48 | 67.56 ± 34.80 | 74.00 ± 52.37 | 0.54 | 73.3 ± 52.60 | 67.71 ± 38.00 | 78.6 ± 63.32 | 0.27 |
| ALT (U/L) | 28.24 ± 20.56 | 30.10 ± 22.65 | 23.94 ± 14.22 | 0.28 | 29.97 ± 21.50 | 30.5 ± 21.56 | 29.47 ± 21.63 | 0.80 |
| AST (U/L) | 33.09 ± 19.42 | 34.87 ± 21.50 | 29.11 ± 13.27 | 0.27 | 36.26 ± 23.93 | 34.72 ± 17.89 | 37.75 ± 28.67 | 0.51 |
| Tbil (μmol/L) | 21.60 ± 13.80 | 21.76 ± 18.16 | 21.28 ± 12.20 | 0.94 | 20.69 ± 13.19 | 18.61 ± 11.60 | 22.6 9± 14.37 | 0.10 |
| Creatinine (μmol/L) | 64.79 ± 29.51 | 62.23 ± 18.36 | 70.91 ± 46.74 | 0.30 | 62.78 ± 17.31 | 62.89 ± 17.31 | 62.67 ± 17.48 | 0.95 |
| Albumin (g/L) | 35.05 ± 4.31 | 36.27 ± 3.89 | 32.71 ± 4.18 | < 0.01 | 34.06 ± 5.11 | 34.98 ± 4.71 | 33.16 ± 5.37 | 0.06 |
| Prothrombin time (s) | 14.24 ± 2.33 | 14.05 ± 2.16 | 14.67 ± 2.69 | 0.35 | 14.89 ± 2.24 | 14.14 ± 1.84 | 15.59 ± 2.37 | < 0.001 |
| INR | 1.24 ± 0.20 | 1.22 ± 0.19 | 1.27 ± 0.23 | 0.4 | 1.28 ± 0.19 | 1.23 ± 0.16 | 1.34 ± 0.21 | < 0.001 |
| Child–Pugh scores | 6.26 ± 1.15 | 6.02 ± 1.13 | 6.71 ± 1.08 | 0.02 | 6.89 ± 1.37 | 6.46 ± 1.21 | 7.31 ± 1.40 | < 0.001 |
| Child–Pugh stage (A/B/C) | 46/23/1 | 35/10/1 | 11/13/0 | 0.02 | 50/59/5 | 31/24/1 | 19/35/4 | 0.03 |
| MELD score | 16 ± 5.82 | 15 ± 5.83 | 17 ± 5.59 | 0.12 | 16 ± 4.95 | 16 ± 4.37 | 17 ± 5.44 | 0.27 |
| Ascites (yes/no) | 35/35 | 27/19 | 8/16 | 0.12 | 24/90 | 19/36 | 5/54 | < 0.001 |
| Shunt (yes/no) | 10/60 | 6/40 | 4/20 | 0.73 | 9/105 | 6/50 | 3/55 | 0.27 |
| Baseline HVPG (mmHg) | 13.04 ± 1.75 | 12.88 ± 1.78 | 13.35 ± 1.69 | 0.30 | 21.17 ± 4.40 | 18.82 ± 2.45 | 23.44 ± 4.68 | < 0.001 |
| Baseline WHVP (mmHg) | 21.32 ± 3.98 | 21.37 ± 3.96 | 21.23 ± 4.13 | 0.90 | 29.14 ± 5.24 | 26.79 ± 4.01 | 31.41 ± 5.31 | < 0.001 |
| Baseline FHVP (mmHg) | 8.42 ± 3.11 | 8.71 ± 3.05 | 7.88 ± 3.24 | 0.30 | 7.98 ± 3.26 | 7.96 ± 3.07 | 8.01 ± 3.47 | 0.93 |
| Baseline IVCP (mmHg) | 7.58 ± 3.15 | 7.75 ± 3.10 | 7.25 ± 3.30 | 0.53 | 7.12 ± 3.18 | 7.20 ± 3.07 | 7.05 ± 3.30 | 0.81 |
| Baseline RAP (mmHg) | 6.24 ± 3.05 | 6.34 ± 3.05 | 6.06 ± 3.11 | 0.72 | 5.68 ± 3.10 | 5.70 ± 3.10 | 5.66 ± 3.12 | 0.94 |
| Severity of varicosity | ||||||||
| (Mild/Moderate/Severe) | 11/23/36 | 7/13/26 | 4/10/10 | 0.46 | 17/30/67 | 11/13/32 | 6/17/35 | 0.35 |
| EV/GOV1/GOV2 /or IGV (no/yes) | 47/25/16/3 | 31/16/9/1 | 16/9/7/2 | 0.66 | 63/31/29/9 | 31/12/14/4 | 32/19/15/5 | 0.80 |
| Numbers of endoscopic therapy (times) | - | 2.21 ± 1.32 | - | - | - | 2.36 ± 1.19 | - | - |
| Pre-TIPS PPG (mmHg) | - | - | 28.69 ± 5.67 | - | - | - | 32.18 ± 5.90 | - |
| post-TIPS PPG (mmHg) | - | - | 18.79 ± 5.23 | - | - | - | 21.69 ± 5.99 | - |
| Interventions required due to stent dysfunction | - | - | 3 | - | - | - | 9 | - |
| Registration time to research ending (mo) | 65.14 ± 19.83 | 62.47 ± 15.82 | 70.29 ± 25.46 | 0.18 | 55.94 ± 19.90 | 52.91 ± 17.33 | 58.86 ± 21.86 | 0.11 |
| Median follow-up time (mo) | 56 | 56 | 58.5 | - | 49.5 | 45 | 51.5 | - |
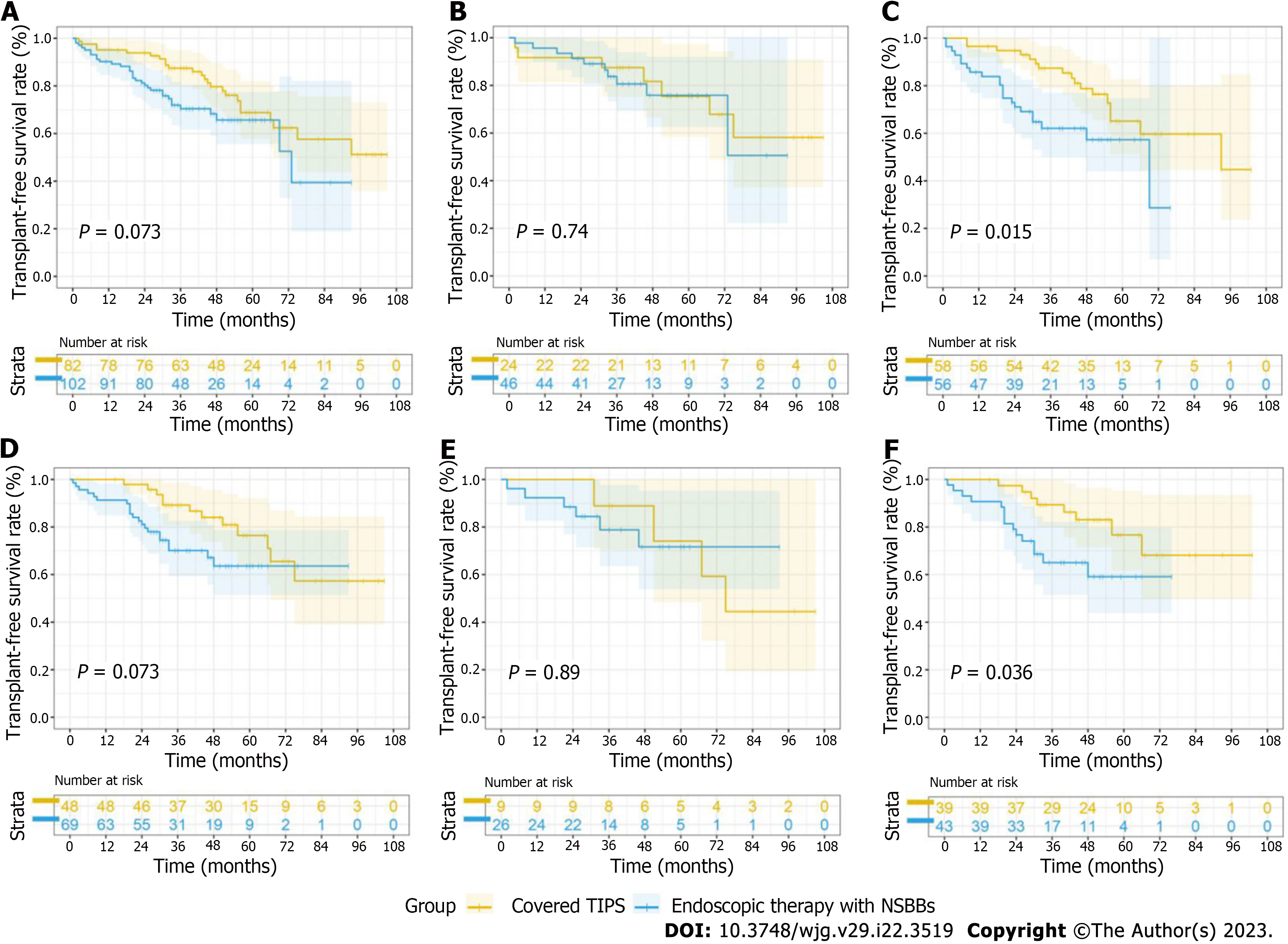
In general, the mortality rate was not significantly different between the two therapies in the whole cohort of patients who were admitted with VB (P = 0.07) (Figure 3A). In the low-HVPG tier, few differences were observed between the two groups (P = 0.74) (Figure 3B). Interestingly, in the high-HVPG tier, covered TIPS placement markedly improved survival. In patients with HVPG ≥ 16 mmHg, 14 (24.1%) patients in the TIPS group and 19 (33.9%) in the EVL+NSBB group died during the follow-up period; 3 (5.4%) patients in the TIPS group and 3 (5.2%) in the EVL+NSBB group underwent liver transplantation. The transplant-free survival rate was higher in the TIPS group (hazard ratio [HR], 0.44; 95% confidence interval [CI]: 0.23-0.85; P = 0.004) (Figure 3C). Similar trends were found among patients without a shunt in the hepatic vein (Supplementary Figure 1).
A subgroup analysis was performed according to etiology. For cirrhotic patients with viral hepatitis and alcoholic hepatitis, the difference in transplant-free survival was negligible (HR, 0.53; 95%CI: 0.26-1.06; P = 0.07) (Figure 3D). Among these patients, TIPS placement did not improve transplant-free survival in the low-HVPG tier (HR, 0.91; 95%CI: 0.23-3.61; P = 0.89) (Figure 3E) but did improve transplant-free survival in the high-HVPG tier (HR, 0.41; 95%CI: 0.18-0.94; P = 0.04) (Figure 3F). For cirrhotic patients who had etiologies unrelated to viral and alcoholic hepatitis, TIPS placement did not confer a significant transplant-free survival benefit, as shown in Supplementary Figure 1.
Subgroup analysis was performed according to the endoscopic appearance of varices, whether there were cardiofundal varices or type 2 isolated gastric varices (IGV)[16]. Among patients suffering only from esophagogastric varices and/or type 1 gastroesophageal varices (GOV1), during the follow-up period, TIPS placement showed a significant transplant-free survival advantage in the high-HVPG tier (HR, 0.29; 95%CI: 0.11-0.71; P = 0.007) (Supplementary Figure 1), while there was no significant difference between the two groups (HR, 0.75; 95%CI: 0.22-2.54; P = 0.64) (Supplementary Figure 1). Among 57 patients with GOV2 and/or IGV, TIPS placement did not improve survival, as shown in Supplementary Figure 1.
In the univariate analysis, baseline age, total bilirubin (Tbil), aspartate aminotransferase, HVPG tier and therapy were included as candidate variables for the multivariate model. The final multivariate Cox regression model showed that age (HR, 1.03 per year of age; 95%CI: 1.02-1.07), Tbil (HR, 1.03 per mol/L of Tbil; 95%CI: 1.00-1.06), HVPG tier (HR, 2.01 compared with low-HVPG tier; 95%CI: 1.04-3.89), and therapy (HR, 0.45 compared with EVL+NSBBs; 95%CI: 0.25-0.81) were independent risk factors for mortality (Table 2).
| Univariate analysis | Multivariate analysis | ||
| Hazard ratio | P value | Hazard ratio | P value |
| 1.03 (1.01, 1.06) | 0.04 | 1.03 (1.01, 1.06) | 0.02 |
| 1.01 (1.01, 1.02) | 0.01 | - | - |
| 1.01 (1.00, 1.02) | 0.03 | 1.03 (1.00, 1.06) | 0.01 |
| 1.63 (0.92, 2.89) | 0.09 | 2.01 (1.04, 3.89) | 0.04 |
| 0.610 (0.35, 1.05) | 0.07 | 0.447 (0.25, 0.81) | 0.01 |
Uncontrolled rebleeding, HE and multiple organ dysfunction syndrome were the most common causes of death (Table 3).
| Uncontrolled rebleeding | OHE | MODS | Liver transplantation | Nonliver related death | ||
| HVPG ≥ 16 mmHg (n = 114) | Endoscopic therapy + NSBBs (n = 56) | 10 | 6 | 3 | 3 | 0 |
| Covered TIPS (n = 58) | 0 | 10 | 2 | 3 | 2 | |
| 10 mmHg ≤ HVPG < 16 mmHg (n = 70) | Endoscopic therapy + NSBBs (n = 46) | 5 | 0 | 1 | 2 | 2 |
| Covered TIPS (n = 24) | 0 | 3 | 0 | 4 | 0 | |
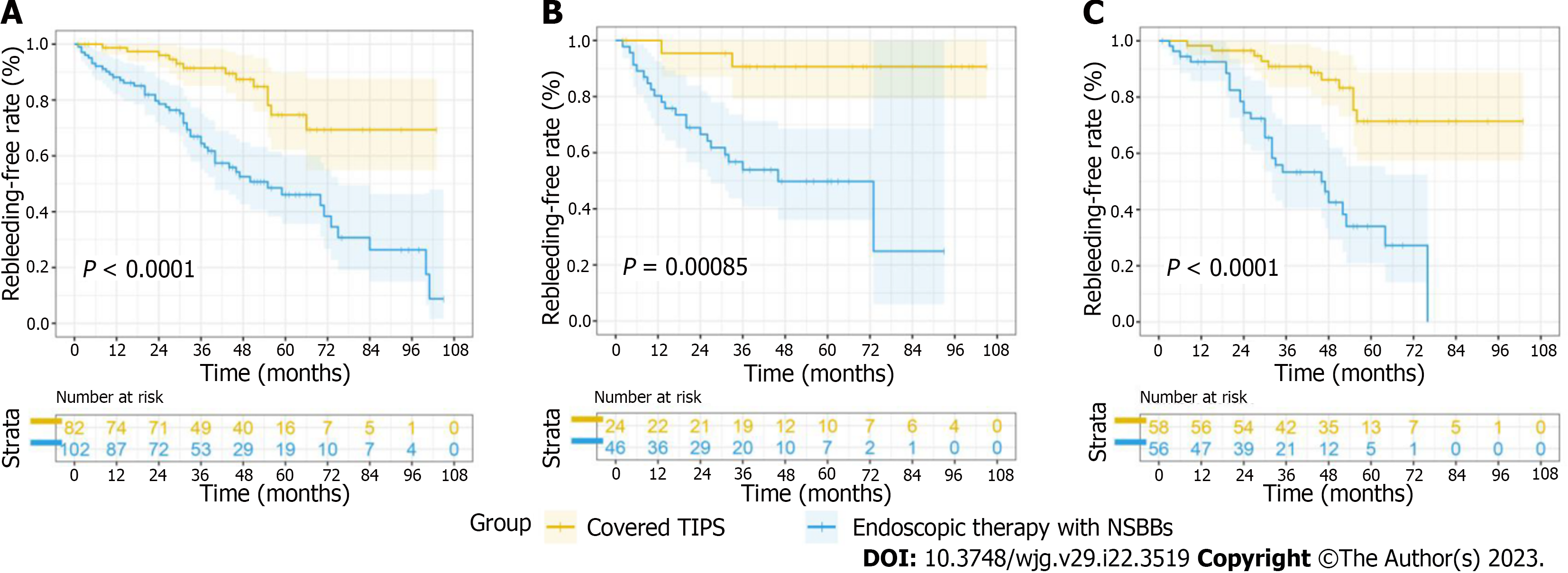
In the whole cohort, covered TIPS placement significantly reduced rebleeding, as previous studies[17] have reported (P < 0.001) in both the low-HVPG and high-HVPG tiers (Figure 4). The same trends were found for the patients without stents and in the subgroup analyses (Supplementary Figure 2). TIPS placement definitely reduced rebleeding, even when ignoring the etiology and endoscopic appearance. The causes of rebleeding are shown in Table 4.
| EGVB | Ectopic variceal bleeding | Ulcer bleeding | Portal hypertensive gastropathy | Venous invasion of HCC | Hematobilia | ||
| HVPG ≥ 16 mmHg (n = 114) | Endoscopic therapy + NSBBs (n = 56) | 24 | 0 | 3 | 1 | 1 | 0 |
| Covered TIPS (n = 58) | 9 | 0 | 1 | 0 | 0 | 1 | |
| 10 mmHg ≤ HVPG < 16 mmHg (n = 70) | Endoscopic therapy + NSBBs (n = 46) | 16 | 1 | 3 | 1 | 0 | 1 |
| Covered TIPS (n = 24) | 3 | 0 | 0 | 0 | 0 | 0 | |
Thirteen patients (16.30%) experienced OHE, including 12 (13.33%) patients in the EVL+NSBB group and 18 (28.12%) in the covered TIPS group (Table 5). The occurrence of OHE was consistently similar between the two groups over the long term, while a significant difference was observed in the occurrence of OHE at 1 year (2.90% vs 10.98%, P = 0.02).
| Complications | 10 mmHg ≤ HVPG < 16 mmHg (n = 70) | HVPG ≥ 16 mmHg (n = 114) | ||||||
| Total | Endoscopic therapy + NSBBs (n = 46) | Covered TIPS (n = 24) | P value | Total | Endoscopic therapy + NSBBs (n = 56) | Covered TIPS (n = 58) | P value | |
| OHE during follow-up (yes/no) | 2/68 | 0/46 | 2/22 | 0.11 | 10/104 | 3/53 | 9/49 | 0.049 |
| Jaundice during follow-up (yes/no) | 22/48 | 14/32 | 8/16 | 0.79 | 46/68 | 17/39 | 29/29 | 0.04 |
| Ascites during follow-up (yes/no) | 29/41 | 18/28 | 11/13 | 0.62 | 75/39 | 38/18 | 37/21 | 0.70 |
| Hypercreatinemia during follow-up(yes/no) | 10/60 | 5/41 | 5/19 | 0.29 | 13/101 | 6/50 | 7/51 | 1.00 |
| Hyponatremia during follow-up (yes/no) | 11/59 | 9/37 | 2/22 | 0.31 | 8/106 | 4/52 | 4/54 | 1.00 |
| OHE in 1 year (yes/no) | 8/62 | 3/43 | 5/19 | 0.09 | 22/92 | 9/47 | 13/45 | 0.48 |
| Jaundice in 1 year(yes/no) | 11/59 | 5/41 | 6/18 | 0.17 | 29/85 | 8/48 | 21/37 | 0.01 |
| Ascites in 1 year (yes/no) | 34/36 | 23/23 | 11/13 | 0.80 | 74/40 | 35/21 | 39/19 | 0.70 |
| Hypercreatinemia in 1 year (yes/no) | 5/65 | 2/44 | 3/21 | 0.33 | 4/110 | 3/53 | 1/57 | 0.36 |
| Hyponatremia in 1 year (yes/no) | 7/63 | 5/41 | 2/22 | 1.00 | 20/94 | 12/44 | 8/50 | 0.33 |
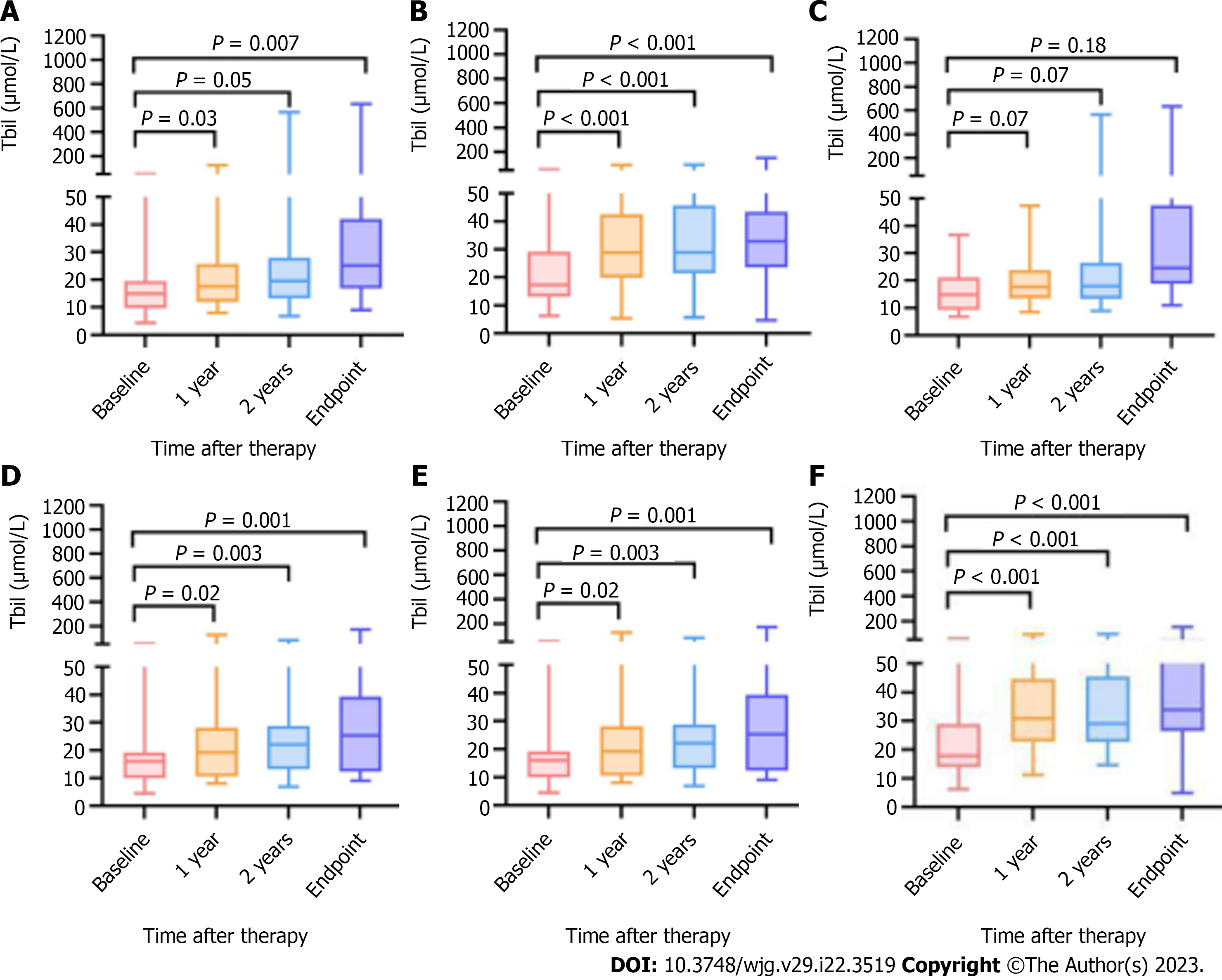
Changes in Tbil after therapy occurred in 184 patients (EVL+NSBB group vs covered TIPS group, 102 vs 82) at baseline, 156 patients at the 1-year follow-up (92 vs 74), and 141 patients at the 2-year follow-up (77 vs 64). The TBIL values for 181 patients were registered at the end of follow-up.
Among the included patients (n = 184), excluding those without complete follow-up examinations (n = 43), the changes in the actual values and in the P values after Bonferroni correction at each point are shown in Figure 5A and B. TIPS placement did not induce hyperbilirubinemia (Tbil > 34 μmol/L[18]) in the low-HVPG tier (Figure 5C and D), while in the high-HVPG tier, more patients who underwent TIPS placement experienced hyperbilirubinemia (Figure 5E and F), as shown in Table 5.
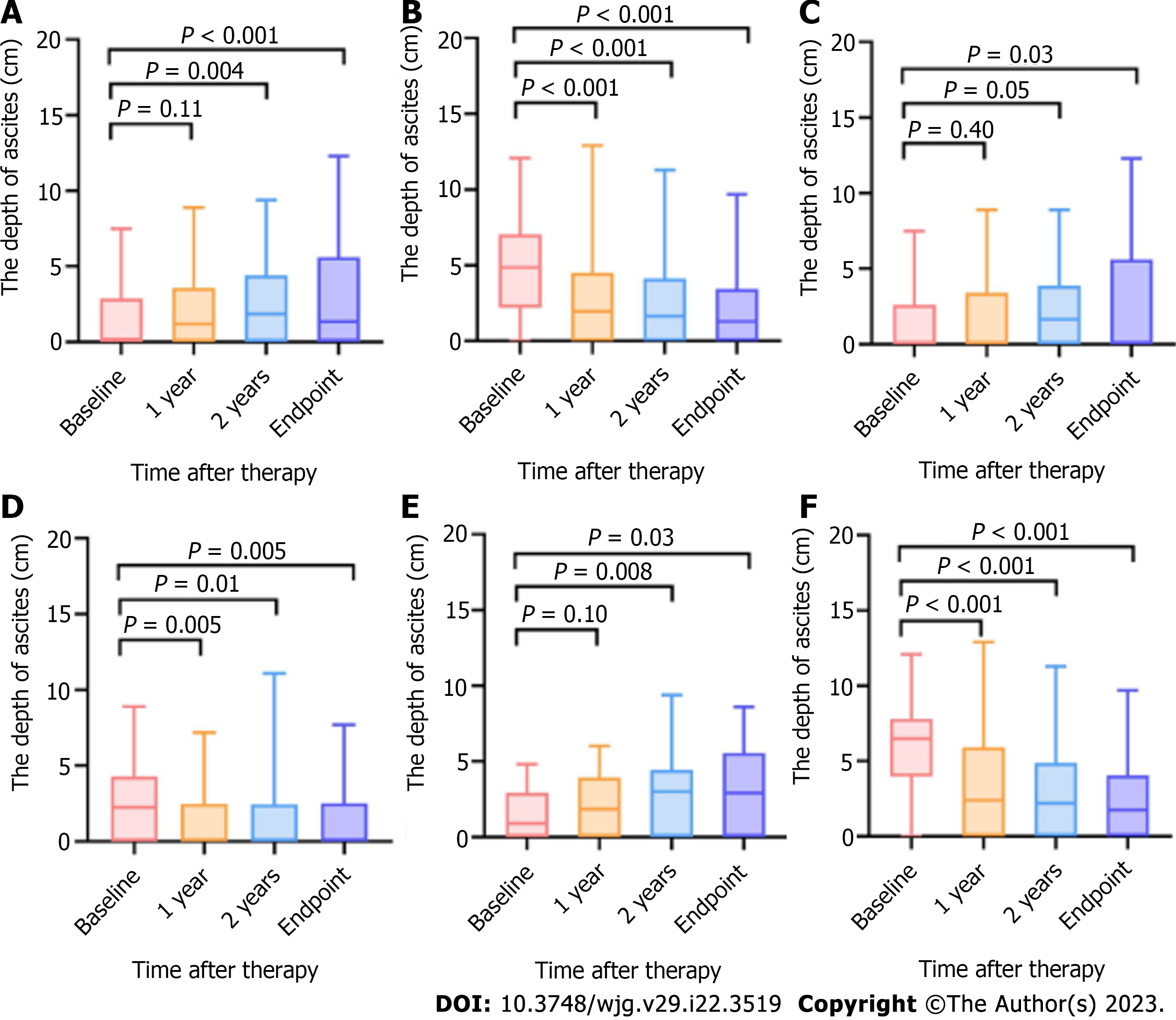
Changes in the depth of ascites after therapy were obtained, as described for Tbil.
The average of the actual values and the P values after Bonferroni correction at each point are shown in Figure 6 and Table 5. In our research, the depth of ascites in the patients after covered TIPS placement decreased, while after endoscopy therapy with NSBBs, it did not, which agreed with the previously reported findings[19].
The changes in serum creatine and Na+ after therapy were the same as the changes in Tbil.
TIPS placement had no effects on hypercreatinemia (creatine > 110 μmol/L for males, > 100 μmol/L for females) or hyponatremia (Na+ < 135 mmol/L) compared with EVL+NSBBs (Supplemental Figure 3), ignoring the HVPG.
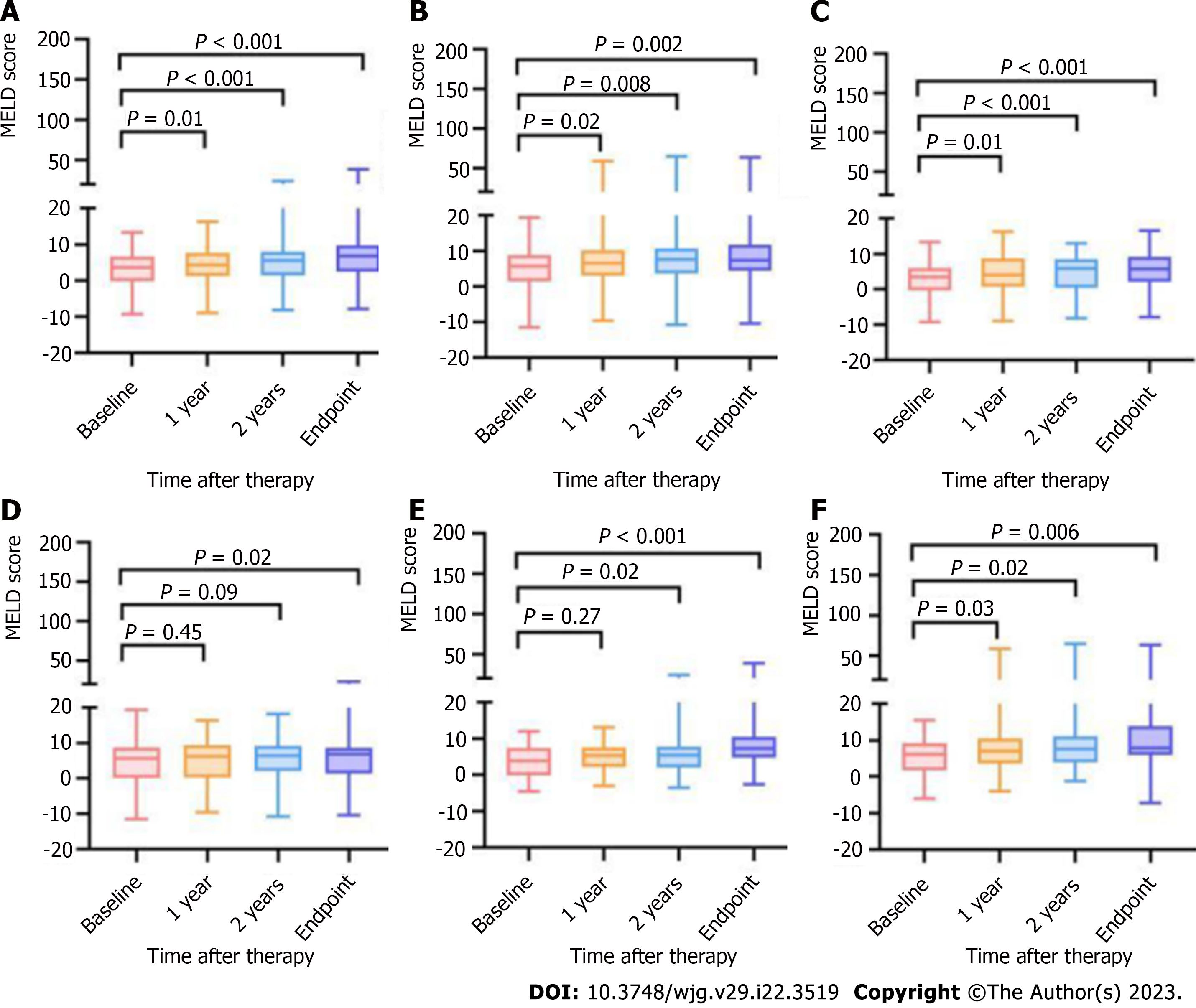
Changes in the model for end-stage liver disease score after therapy were determined the same as described for changes in the Tbil level[20].
In the whole cohort, the scores gradually increased over follow-up after both of the therapies (Figure 7A and B). The patients treated with EVL+NSBBs in the low-HVPG tier showed increased scores after the second year (Figure 7C), while those treated with covered TIPS placement did not. the scores of patients in the high-HVPG tier increased earlier (Figure 7E and F).
In our study, stent loss occurred in 12 of 82 patients, including 3 (12.50%) in the low-HVPG tier and 9 (16.07%) in the high-HVPG tier. Stent dysfunction was discovered in 6 (66.67%) patients in the high-HVPG tier due to rebleeding or ascites, while stent dysfunction was discovered in patients in the low-HVPG tier during regular follow-up.
It would be extremely helpful to be able to distinguish patients with a high risk of uncontrolled rebleeding. Risk stratification is pivotal for secondary prophylaxis in VB. A recent expert consensus has suggested that HVPG-guided treatment is a reasonable clinical strategy[4]. A study in 2015 concluded that HVPG measurement was useful for making decisions in selecting therapies for secondary prophylaxis[21]. However, the HVPG cutoff for risk stratification has not been clearly demonstrated. In 1991, a study demonstrated that the lowest pressure associated with continued bleeding or rebleeding was 16 mmHg[22]. Furthermore, Villanueva et al[23] pointed out that HVPG > 16 mmHg was a predictor of treatment failure. The latest expert consensus is that early TIPS placement reduces treatment failure in patients with HVPG ≥ 20 mmHg[4]. There is established evidence for an HVPG-stratified approach to treatment among compensated cirrhotic patients but very limited data for decompensated patients. On the basis of earlier findings[12], at the beginning of our study and according to the present guidelines, the included patients were divided into 2 groups: the high-HVPG (≥ 16 mmHg) and low-HVPG (10 mmHg ≤ HVPG < 16 mmHg) groups. Consequently, if there were no contraindications, TIPS placement improved survival in patients with HVPG ≥ 16 mmHg (HR, 0.4385; 95%CI: 0.2255-0.8525; P = 0.0037), even though patients who underwent TIPS placement had worse baseline clinical indicators.
A previous study could not conclude whether there were any beneficial effects of TIPS placement on transplant-free survival in all patients. Notably, the median follow-up in all previous studies was shorter than 24 mo, while our median follow-up was 49.5 mo. In our study, the survival benefit can be viewed in the long term. Moreover, the main causes of death were significantly different. Rebleeding in patients treated with covered TIPS placement, which was generally due to stent dysfunction, was controlled in most patients, providing an opportunity for further therapy, while rebleeding in some patients treated with EVL+NSBBs was turbulent.
Our study of HVPG-guided treatment indicated that patients with high HVPG values have a high risk of rebleeding despite treatment with EVL+NSBBs, and in patients with HVPG ≥ 16 mmHg, percutaneous transhepatic variceal embolization or TIPS placement may be a favorable alternative to EVL+NSBBs.
Considering the high rate of OHE in patients treated with covered TIPS placement mentioned in previous research[8,24], we opted for the placement of small-diameter (8 mm) stents, which can provide adequate bleeding prophylaxis and decrease the OHE rate. Regarding the patients in the low-HVPG tier, there was a trend of growth in the rate of OHE among patients treated with covered TIPS placement, and these results are in accordance with earlier findings. Covered TIPS placement did not increase the rate of OHE among patients in the high-HVPG tier. The differences in encephalopathy between the two treatment groups were the largest in the first year, and this result is in accordance with that of a previous study[6]. A Chinese study proposed that the HVPG is a risk factor for liver failure after TIPS placement[24]. Therefore, we assessed hyperbilirubinemia after therapy. Our results were in accordance with those of a previous study[24].
Based on the HVPG-guided risk stratification, patients with a high HVPG were more likely to benefit from TIPS placement in our study. For patients with a low HVPG, the significant reduction in rebleeding did not improve survival outcomes due to the complications of TIPS placement. Furt
Notably, our results cannot be extrapolated to older patients or patients with occlusive portal vein thrombosis or hepatocellular carcinoma, as these populations were not included in our study. Additionally, in our study, the numbers of patients with IGV and/or GOV2 were not sufficient, which contributed to the fact that the differences between the two therapies were not significant; as such, the findings could not confirm previous research[25]. Due to the nature of single-center observational studies, an inherent risk of selection bias is inevitable. Another potential bias may be the lower-than-expected attention to the clinical symptoms in patients. Furthermore, selection bias could not be ignored because the therapy was ultimately chosen by the patient. Even with these limitations, this study produced robust data. Further randomized controlled trials need to be performed to prove the benefit of covered TIPS placement in the secondary prevention of VB.
In conclusion, covered TIPS placement increases long-term transplant-free survival in patients with HVPG ≥ 16 mmHg who are admitted with VB, but this advantage may not be observed in patients with 10 mmHg ≤ HVPG < 16 mmHg. More appropriate management and treatment of cirrhotic patients may be achieved by stratification according to HVPG measurements obtained before therapy.
It is controversial whether transjugular intrahepatic portosystemic shunt (TIPS) placement can improve long-term survival.
To clarify the feasibility of hepatic venous pressure gradient (HVPG) as a risk stratification strategy for patients with decompensated cirrhosis.
To assess whether TIPS placement improves survival in patients with HVPG ≥ 16 mmHg based on HVPG-related risk stratification.
Consecutive variceal bleeding patients treated with endoscopic variceal ligation (EVL) + nonselective β-blockers (NSBBs) or covered TIPS placement were retrospectively enrolled between January 2013 and December 2019. HVPG measurements were performed before therapy. The primary outcome was transplant-free survival; secondary endpoints were rebleeding and overt hepatic encephalopathy (OHE).
A total of 184 patients were analyzed (mean age, 55.27 years ± 13.86, 107 males; 102 in the EVL+NSBB group, 82 in the covered TIPS group). Based on the HVPG-guided risk stratification, 70 patients had HVPG < 16 mmHg, and 114 patients had HVPG ≥ 16 mmHg. The median follow-up time of the cohort was 49.5 mo. There was no significant difference in transplant-free survival between the two treatment groups overall (hazard ratio [HR], 0.61; 95% confidence interval [CI]: 0.35-1.05; P = 0.07). In the high-HVPG tier, transplant-free survival was higher in the TIPS group (HR, 0.44; 95%CI: 0.23, 0.85; P = 0.004). In the low-HVPG tier, transplant-free survival after the two treatments was similar (HR, 0.86; 95%CI: 0.33, 0.23; P = 0.74). Covered TIPS placement decreased the rate of rebleeding independent of the HVPG tier (P < 0.001). The difference in OHE between the two groups was not statistically significant (P = 0.09; P = 0.48).
TIPS placement can effectively improve transplant-free survival when the HVPG is greater.
Further randomized controlled trials need to be performed to prove the benefit of covered TIPS placement in the secondary prevention of decompensated cirrhosis.
The authors would like to thank several researchers from Nanjing Medical University, Nanjing University, Jiangsu University and Southeast University for their help in data collection for the DCDT COHORT STUDY since 2012, Nurse Qin Yin from the Department of Gastroenterology of Nanjing Drum Tower Hospital for help in patient follow-up, Dr. Hao Han and Prof. Jian Yang from the Department of Ultrasound Imaging of Nanjing Drum Tower Hospital for their help in collecting abdominal ultrasound images, Dr. Hao Zhang from the Department of Internal Medicine of Nanjing Drum Tower Hospital for his guidance on visualization, and Dr. Taishun Li from the Department of Biomedicine Statistics of Nanjing Drum Tower Hospital for providing statistical suggestions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papadopoulos VP, Greece; Shalimar, India; Torres US, Brazil S-Editor: Chang KL L-Editor: A P-Editor: Cai YX
| 1. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 526] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 635] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1428] [Article Influence: 178.5] [Reference Citation Analysis (3)] |
| 4. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1435] [Article Influence: 478.3] [Reference Citation Analysis (2)] |
| 5. | Holster IL, Tjwa ET, Moelker A, Wils A, Hansen BE, Vermeijden JR, Scholten P, van Hoek B, Nicolai JJ, Kuipers EJ, Pattynama PM, van Buuren HR. Covered transjugular intrahepatic portosystemic shunt vs endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 837] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 7. | Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, Procopet B, Giráldez Á, Amitrano L, Villanueva C, Thabut D, Silva-Junior G, Martinez J, Genescà J, Bureau C, Llop E, Laleman W, Palazon JM, Castellote J, Rodrigues S, Gluud L, Ferreira CN, Barcelo R, Cañete N, Rodríguez M, Ferlitsch A, Mundi JL, Gronbaek H, Hernández-Guerra M, Sassatelli R, Dell'Era A, Senzolo M, Abraldes JG, Romero-Gómez M, Zipprich A, Casas M, Masnou H, Primignani M, Weiss E, Catalina MV, Erasmus HP, Uschner FE, Schulz M, Brol MJ, Praktiknjo M, Chang J, Krag A, Nevens F, Calleja JL, Robic MA, Conejo I, Albillos A, Rudler M, Alvarado E, Guardascione MA, Tantau M, Bosch J, Torres F, Pavesi M, Garcia-Pagán JC, Jansen C, Bañares R; International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Sauerbruch T, Mengel M, Dollinger M, Zipprich A, Rössle M, Panther E, Wiest R, Caca K, Hoffmeister A, Lutz H, Schoo R, Lorenzen H, Trebicka J, Appenrodt B, Schepke M, Fimmers R; German Study Group for Prophylaxis of Variceal Rebleeding. Prevention of Rebleeding From Esophageal Varices in Patients With Cirrhosis Receiving Small-Diameter Stents Versus Hemodynamically Controlled Medical Therapy. Gastroenterology. 2015;149:660-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 180] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Merkel C, Bolognesi M, Sacerdoti D, Bombonato G, Bellini B, Bighin R, Gatta A. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Lesmana CRA, Raharjo M, Gani RA. Managing liver cirrhotic complications: Overview of esophageal and gastric varices. Clin Mol Hepatol. 2020;26:444-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A, Peñate M, Cruz A, Peña E. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Guo H, Zhang F, Yin X, Zhang M, Xiao J, Wang Y, Zhang B, Zhang W, Zou X, Zhuge Y. Endoscopic therapy + β-blocker vs. covered transjugular intrahepatic portosystemic shunt for prevention of variceal rebleeding in cirrhotic patients with hepatic venous pressure gradient ≥16 mmHg. Eur J Gastroenterol Hepatol. 2021;33:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, Kokudo N, Kokubu S, Sakaida I, Sata M, Tajiri H, Tsukada K, Nonami T, Hashizume M, Hirota S, Murashima N, Moriyasu F, Saigenji K, Makuuchi H, Oho K, Yoshida T, Suzuki H, Hasumi A, Okita K, Futagawa S, Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1405] [Article Influence: 127.7] [Reference Citation Analysis (1)] |
| 15. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 6801] [Article Influence: 618.3] [Reference Citation Analysis (0)] |
| 16. | Sarin SK, Lahoti D. Management of gastric varices. Baillieres Clin Gastroenterol. 1992;6:527-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Jalan R, Forrest EH, Stanley AJ, Redhead DN, Forbes J, Dillon JF, MacGilchrist AJ, Finlayson ND, Hayes PC. A randomized trial comparing transjugular intrahepatic portosystemic stent-shunt with variceal band ligation in the prevention of rebleeding from esophageal varices. Hepatology. 1997;26:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Sullivan JI, Rockey DC. Diagnosis and evaluation of hyperbilirubinemia. Curr Opin Gastroenterol. 2017;33:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Inadomi J, Cello JP, Koch J. Ultrasonographic determination of ascitic volume. Hepatology. 1996;24:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3668] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 21. | Zhang M, Wang G, Zhao L, Wu Z, Zhang W, Zhang C. Second prophylaxis of variceal bleeding in cirrhotic patients with a high HVPG. Scand J Gastroenterol. 2016;51:1502-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Ready JB, Robertson AD, Goff JS, Rector WG Jr. Assessment of the risk of bleeding from esophageal varices by continuous monitoring of portal pressure. Gastroenterology. 1991;100:1403-1410. [PubMed] |
| 23. | Villanueva C, Piqueras M, Aracil C, Gómez C, López-Balaguer JM, Gonzalez B, Gallego A, Torras X, Soriano G, Sáinz S, Benito S, Balanzó J. A randomized controlled trial comparing ligation and sclerotherapy as emergency endoscopic treatment added to somatostatin in acute variceal bleeding. J Hepatol. 2006;45:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Yao Y, Satapathy SK, Fernandes ESM, Ramírez-Fernández O, Vitale A, Chen Z. Hepatic venous pressure gradient (HVPG) predicts liver failure after transjugular intrahepatic portal shunt: a retrospective cohort study. Ann Transl Med. 2022;10:1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Stanley AJ, Jalan R, Ireland HM, Redhead DN, Bouchier IA, Hayes PC. A comparison between gastric and oesophageal variceal haemorrhage treated with transjugular intrahepatic portosystemic stent shunt (TIPSS). Aliment Pharmacol Ther. 1997;11:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |