Published online Jun 14, 2023. doi: 10.3748/wjg.v29.i22.3400
Peer-review started: March 16, 2023
First decision: March 24, 2023
Revised: March 31, 2023
Accepted: May 11, 2023
Article in press: May 11, 2023
Published online: June 14, 2023
Processing time: 82 Days and 9.4 Hours
Small intestinal bacterial overgrowth (SIBO) is defined as an increase in the bacterial content of the small intestine above normal values. The presence of SIBO is detected in 33.8% of patients with gastroenterological complaints who underwent a breath test, and is significantly associated with smoking, bloating, abdominal pain, and anemia. Proton pump inhibitor therapy is a significant risk factor for SIBO. The risk of SIBO increases with age and does not depend on gender or race. SIBO complicates the course of a number of diseases and may be of pathogenetic significance in the development of their symptoms. SIBO is significantly associated with functional dyspepsia, irritable bowel syndrome, functional abdominal bloating, functional constipation, functional diarrhea, short bowel syndrome, chronic intestinal pseudo-obstruction, lactase deficiency, diverticular and celiac diseases, ulcerative colitis, Crohn’s disease, cirrhosis, metabolic-associated fatty liver disease (MAFLD), primary biliary cholangitis, gastroparesis, pancreatitis, cystic fibrosis, gallstone disease, diabetes, hypothy
Core Tip: Small intestinal bacterial overgrowth (SIBO) is common in functional and organic bowel diseases, liver diseases, other diseases of digestive organs, a number of endocrine, nervous, and rheumatic diseases, asthma, heart failure, and certain other diseases. In a number of diseases, including cirrhosis, metabolic-associated fatty liver disease, diabetes mellitus, pancreatitis, and cystic fibrosis, an association was found between disease severity and the presence of SIBO. Further work on the effect of SIBO eradication on the condition and prognosis of patients in various diseases is required.
- Citation: Efremova I, Maslennikov R, Poluektova E, Vasilieva E, Zharikov Y, Suslov A, Letyagina Y, Kozlov E, Levshina A, Ivashkin V. Epidemiology of small intestinal bacterial overgrowth. World J Gastroenterol 2023; 29(22): 3400-3421
- URL: https://www.wjgnet.com/1007-9327/full/v29/i22/3400.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i22.3400
Small intestinal bacterial overgrowth (SIBO) is defined as an increase in the bacterial content of the small intestine above normal values (100000 cells per mL)[1-4]. This leads to excessive gas formation in the small intestine, bloating, and pain in the umbilical region, which may be accompanied by malabsorp
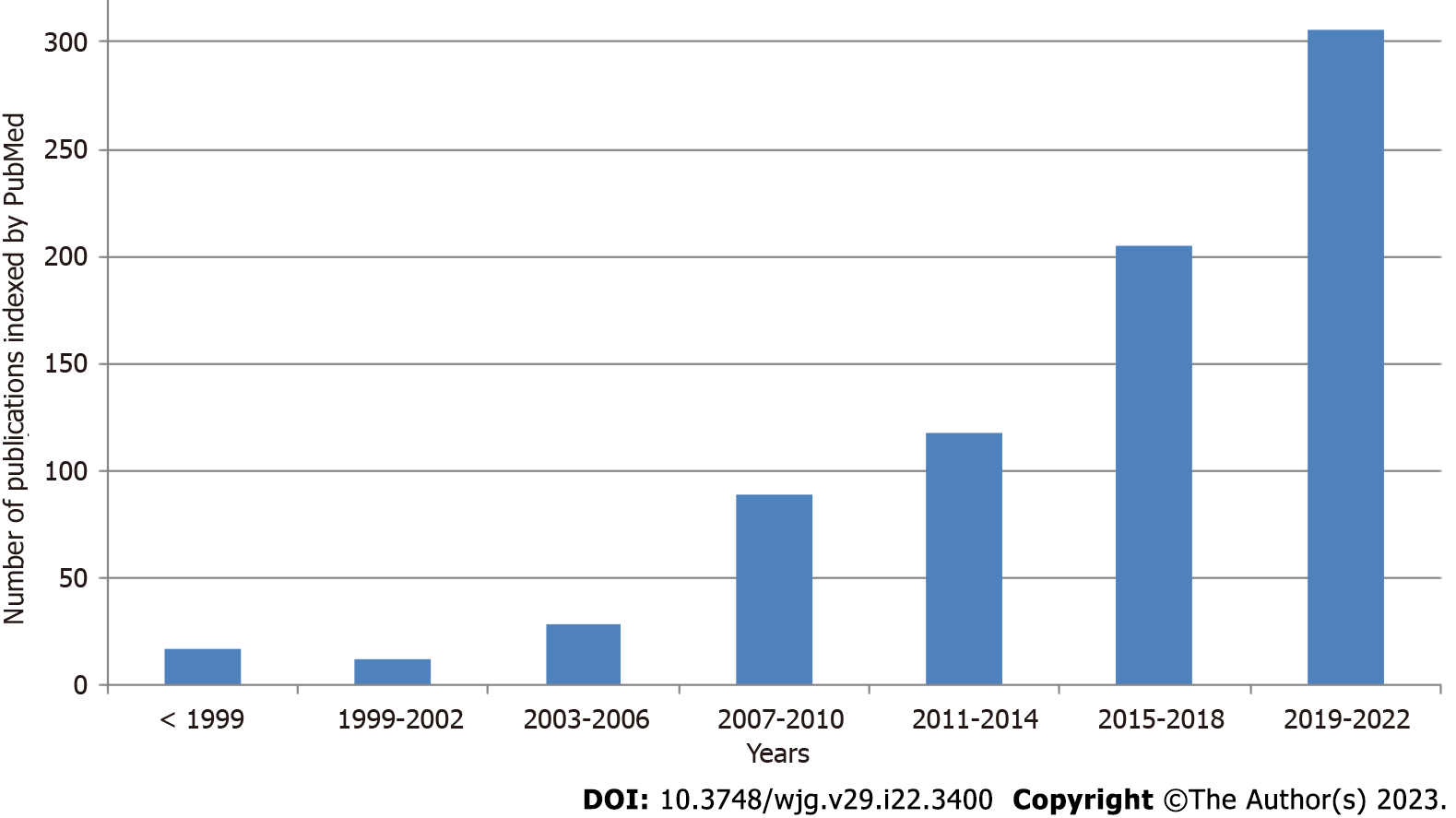
Interest in SIBO is increasing, as evidenced by the continuous growth in publications on this topic indexed in PubMed (Figure 1). An important issue is the standardization of SIBO diagnosis. The main diagnostic methods are lactulose and glucose breath tests (LBT and GBT, respectively), as well as jejunal bacterial culture quantification[5-7]. Unfortunately, the results obtained using these different methods do not always correspond with each other, which may explain the variation in results between different studies that use different tests. In addition, while SIBO was initially associated with hydrogen release, it is now accepted that SIBO can also accompany the formation of methane. Methane SIBO and hydrogen SIBO are not only detected with different frequencies, but can also have different effects. It is important that the test for hydrogen SIBO does not detect methane SIBO and vice versa[8,9].
SIBO complicates the course of a number of diseases and may be of pathogenetic significance in the development of their symptoms[1-4]. The aim of this review is to provide up-to-date information on the association of SIBO with various diseases and their manifestations.
The presence of SIBO was detected in 33.8% of patients with gastroenterological complaints who underwent a breath test, and was significantly associated with smoking [odds ratio (OR) = 6.66], bloating (OR = 5.39), abdominal pain (OR = 4.78), and anemia (OR = 4.08)[10]. The risk of SIBO increased with age [OR = 1.04, 95% confidence interval (CI): 1.01-1.07][11] and did not depend on gender or race[11,12]. Ileocecal junction pressure was lower, small bowel, colonic, and whole gut transit times were longer, and gastric and small bowel pH was higher in patients with SIBO than in those without[13,14]. There were no significant differences in gastric emptying time and other small intestinal motility parameters (small intestinal motility index, contractions per minute, and peak amplitudes)[13,15], or in fecal calprotectin levels[16]. SIBO was more frequent in patients who consumed a moderate amount of alcohol than in abstainers (58% vs 39%)[17]. SIBO was identified in 63% of children with abdominal pain[18].
Patients with SIBO consumed lower amounts of dietary fiber and red meat than SIBO-negative individuals. Those with methane SIBO consumed more fruits, vegetables, and fish compared with those with other variants of SIBO and the control group, while individuals with hydrogen SIBO ate more poultry than those with other SIBO types and controls[19]. SIBO was detected more frequently in preschool children who were on a long-term dairy-free diet than in children on a regular diet (55.0% vs 20.0%)[20].
A meta-analysis of 19 studies revealed that PPI therapy is a significant risk factor for SIBO (OR = 1.71, 95%CI: 1.20-2.43)[21]. The prevalence of SIBO increased after 1 year of continuous PPI use[22]. Interestingly, SIBO following PPI therapy is associated with decreased OCTT, and the concomitant use of prokinetics in patients receiving PPI reduces the risk of SIBO by increasing OCTT[23]. In older patients undergoing long-term PPI therapy, the prevalence of SIBO was higher than that in patients not receiving PPI (77.1% vs 58.3%) and increased significantly when the administration time was longer than 6 mo[24]. The prevalence of SIBO was also higher in older patients undergoing continuous PPI therapy (88.6%) than in those receiving on-demand PPI therapy (65.7%); no significant difference was found in the incidence of SIBO between the on-demand PPI and control groups[24].
Breath tests revealed that 68.1% of patients with functional bowel diseases also had SIBO[25].
A meta-analysis of seven studies found that the prevalence of SIBO in functional dyspepsia is 34.7% (95%CI: 24.8%-45.4%)[26]. Another meta-analysis found SIBO prevalence in functional dyspepsia to be significantly higher using the LBT (53.4%, 95%CI: 33.9%-71.9%) compared with the GBT (17.2%, 95%CI: 8.6%-31.6%). There was no significant difference in SIBO prevalence between patients with different subtypes of functional dyspepsia. The risk of SIBO in patients with functional dyspepsia was significantly higher than that in controls (OR = 4.3, 95%CI: 1.1-17.5)[27].
A meta-analysis of 37 studies revealed that the prevalence of SIBO in irritable bowel syndrome (IBS) was 36.7% (95%CI: 24.2%-44.6%). The lactulose hydrogen breath test (LHBT) for SIBO was positive in patients with IBS no more often than in the control group [risk ratio (RR) = 1.6, 95%CI: 0.9-2.8], while SIBO was detected in patients with IBS significantly more often than in controls using the glucose hydrogen breath test (RR = 4.2, 95%CI: 3.0-5.9) and upper gut aspirate culture (RR = 3.2, 95%CI: 1.4-7.3). Patients with diarrhea-predominant IBS (IBS-D) were more likely to have SIBO than patients with other subtypes (OR = 1.4, 95%CI: 1.02-1.63)[28].
Another meta-analysis of 25 studies showed that SIBO prevalence in patients with IBS was significantly higher than that in controls [31.0% (95%CI: 29.4%-32.6%) vs 20.9% (95%CI: 19.5%-22.2%); OR = 3.7 (95%CI: 2.3-6.0) for all studies, OR = 4.9 (95%CI: 2.8-8.6) for studies using only healthy controls, and OR = 1.3 (95%CI: 0.6-3.3) for studies using non-IBS disease controls]. Using breath testing, SIBO prevalence in patients with IBS was 35.5% (95%CI: 33.6%-37.4%) vs 29.7% (95%CI: 27.6%-31.8%) in controls. Culture-based studies found an SIBO incidence of 13.9% (95%CI: 11.5%-16.4%) in patients with IBS and 5.0% (95%CI: 3.9%-6.2%) in controls. SIBO prevalence as diagnosed by the LBT was much greater in patients with IBS (3.6-fold) and controls (7.6-fold) than that diagnosed by the GBT. Similar differences were observed when LBT was compared with the bacterial culture method of diagnosis. SIBO prevalence was greater in patients with IBS-D (35.5%, 95%CI: 32.7%-40.3%) compared with patients with constipation-predominant IBS [IBS-C; 22.5% (95%CI: 18.1%-26.9%); OR = 1.8 (95%CI: 1.2-2.8) vs IBS-D] and mixed IBS (25.2%, 95%CI: 22.2%-28.4%). PPI use by patients with IBS was not associated with SIBO (OR = 0.8, 95%CI: 0.5-1.5). The prevalence of SIBO both in patients with IBS and controls was greatest in the United States [(54.6%, 95%CI: 51.2%-58.0%) and 37.2% (95%CI: 34.7%-39.7%), respectively], followed by Europe [23.4% (95%CI: 21.1%-26.0%) and 8.1% (95%CI: 6.6%-9.6%), respectively], and lowest in India [14.1% (95%CI: 11.5%-16.7%) and 1.1% (95%CI: 0%-2.2%), respectively][29].
The third meta-analysis revealed that among patients with IBS, female sex (OR = 1.5, 95%CI: 1.0-2.1), older age [standardized mean difference = 3.1 years (95%CI: 0.9-5.4)], and IBS-D (OR = 1.7, 95%CI: 1.3-2.3) compared with other IBS subtypes increased the odds of SIBO[30].
Separately, a meta-analysis of studies investigating methane SIBO in IBS was performed. It showed that the incidence of methane SIBO in patients with IBS was 25.0% (95%CI: 18.8%-32.4%), not significantly different from that in the control group (OR = 1.2, 95%CI: 0.8-1.7). In patients with IBS, the LBT provided positive results for methane SIBO almost three times as often as the GBT [29.0% (95%CI: 20.9%-38.6%) vs 11.5% (95%CI: 5.0%-24.3%)]. In contrast with hydrogen SIBO, the prevalence of methane SIBO in patients with IBS-C was higher than that in patients with IBS-D [37.7% (95%CI: 33.5%-42.1%) vs 12.4% (95%CI: 10.2%-14.9%); OR = 3.1 (95%CI: 1.7-5.6)][31].
Serum interleukin (IL)-1β levels were higher and serum IL-10 levels were lower in SIBO-positive patients with IBS than in the SIBO-negative group[32]. Another study also showed that increased IL-1α and IL-1β levels were associated with SIBO[33]. A third study reported that IL-10 levels were reduced in IBS patients with SIBO compared with those without, but found no significant differences in serum tumor necrosis factor (TNF)-α, IL-6, and IL-8 concentrations[34].
The body mass index (BMI) of patients with IBS-D and SIBO was significantly lower than that of patients without SIBO. IBS-D patients with SIBO had higher scores of abdominal pain than those without SIBO in this study. The proportion of dietary fat was significantly higher in IBS patients with SIBO than in those without. Breath methane peak value was positively correlated with the proportion of fat in the diet[35]. Another study also showed that in non-constipation IBS, patients with SIBO had lower BMI and waist circumference than patients without SIBO. Moreover, multivariate analysis revealed that the OR of SIBO was 0.396 for obesity and 0.482 for abdominal obesity[36].
The presence of SIBO did not affect IBS symptoms in another study[37], although OCTT was significantly increased in SIBO-positive compared with SIBO-negative patients[37]. Intestinal permeability did not differ significantly between IBS patients with and without SIBO[38]. The severity of anxiety, depression, and other psychological disorders was not different between patients with IBS who were positive and negative for hydrogen SIBO[34]. Another study confirmed that the severity of IBS symptoms and associated psychological disorders did not differ between SIBO-positive and -negative patients[39]. However, it has been shown that IBS patients with SIBO who are predominant methane producers have higher urge thresholds for colon contraction and higher baseline levels of colon phasic contractions than SIBO-negative IBS patients, and report more ‘hard or lumpy stools’ compared with patients who are predominant hydrogen producers and those who are SIBO-negative[39]. This is consistent with the findings that hydrogen SIBO is more prevalent in IBS-D, whereas methane SIBO is more prevalent in IBS-C[30,31]. In children with IBS, the prevalence of SIBO was significantly higher than that in control subjects [65% vs 7%; OR = 3.9 (95%CI: 7.3-80.1)]. Among abdominal symptoms, significant differences were observed only in bloating[40].
The LHBT was positive in 59% of patients with and 24% of patients without severe non-steroidal anti-inflammatory drug (NSAID)-induced small intestinal damage. Multiple regression analysis showed that SIBO was significantly associated with severe NSAID-induced small intestinal damage (OR = 6.54, 95%CI: 1.40-30.50). However, there was no significant difference in symptoms between patients with severe small intestinal damage with and without SIBO[43].
SIBO was found in 50% of children with short bowel syndrome and predisposed these patients to ambulatory central line-associated bloodstream infection (RR = 1.87, 95%CI: 1.1-3.17)[44].
Methane SIBO was detected more frequently in patients with chronic intestinal pseudo-obstruction than in patients with other diseases (52.6% vs 11.8%), while no significant difference was found in the prevalence of hydrogen SIBO between these groups (23.7% vs 25.7)[45].
SIBO was detected at a significantly higher rate in patients with lactase deficiency than in healthy controls (27.6% vs 6.7%)[46]. SIBO was found in 90% of asymptomatic well-nourished older people with lactose malabsorption compared with 20% of those without it[47].
SIBO was found in 58.9% of patients with symptomatic uncomplicated diverticular disease (SUDD), more specifically in 93.1% of patients with diarrhea-prevalent disease and 42.6% of patients with constipation-prevalent disease[48]. OCTT was delayed in 92.5% of SUDD patients with SIBO and 48.6% of patients without SIBO (OR = 12.9). Lactose malabsorption was identified in 92.5% of SUDD patients with SIBO and 27.0% of patients without (OR = 33.0)[48].
A meta-analysis of 14 studies showed that SIBO was more prevalent in patients with celiac disease than in controls [18.3% (95%CI: 11.4-28.1)[1]; OR = 5.1, 95%CI: 2.1-12.4]. The prevalence of SIBO as diagnosed by breath tests was 20.8% (95%CI: 11.9%-33.7%), while that of SIBO identified by culture-based methods was 12.6% (95%CI: 5.1%-28.0%). The prevalence of SIBO in patients with celiac disease was lower in the United States [8.7% (95%CI: 6.1%-11.9%)] than in Asia [22.1 (95%CI: 15.4%-30.2%)] and Europe [23.3% (95%CI: 17.5%-29.9%)]. SIBO prevalence in non-responders to a gluten-free diet was similar to that in responders. Antibiotic therapy for SIBO in patients with celiac disease resulted in an improvement in gastrointestinal symptoms in 95.6% (95%CI: 78.0%-99.9%) of cases. There were no significant differences in the degree of intestinal damage as measured by histology or celiac serology between patients with celiac disease with and without SIBO. Patients with celiac disease and SIBO tended to be older and have more severe signs of malabsorption (lower hemoglobin, serum beta-carotene, albumin levels, and higher fecal fat level), compared with those without SIBO[49]. SIBO was present in 67% of celiac patients with persistence of gastrointestinal symptoms who had histological response to a gluten-free diet[50].
Environmental enteric dysfunction is an incompletely defined syndrome of inflammation, reduced absorptive capacity, and barrier dysfunction in the small intestine. It is widespread in low- and middle-income countries[51]. The prevalence of SIBO is 85.3% in children with stunted growth due to this disease[52].
A meta-analysis of 11 studies revealed that the proportion of SIBO in patients with inflammatory bowel disease (IBD) was greater than that in controls [22.3% (95%CI: 19.92%-24.68%); OR = 9.51 (95%CI: 3.39-26.68)]. The severity of abdominal symptoms (bloating, flatus, satiety, and loose stools) was also greater in patients with SIBO than in those without. Patients with SIBO had increased stool frequency, lower body weight, and prolonged OCTT compared with SIBO-negative patients[53]. The prevalence of methane SIBO was assessed in a meta-analysis of six studies, which did not reveal a significant difference between patients with IBD and healthy individuals[31]. The incidence of SIBO was significantly higher in patients with Crohn’s disease (CD) compared with patients with ulcerative colitis (UC)[54].
A meta-analysis showed that the prevalence of SIBO in CD was higher than that in controls [25.4% (95%CI: 22.5%-28.3%); OR = 10.9 (95%CI: 2.8-42.69)]. CD patients who had undergone prior surgery were more likely to test positive for SIBO than those who had not [31.8% (95%CI: 26.7%-36.9%) vs 19.2% (95%CI: 15.4%-23.0%); OR = 2.4 (95%CI: 1.7-3.4)]. The prevalence of SIBO in CD was highest [33% (95%CI: 19.2%-50.6%)] in patients following ileocecal valve surgery. Among different forms of the disease, fibrostenosing CD was associated with an increased risk of SIBO compared to others [OR = 7.5 (95%CI: 2.5-22.2)]. No association was found between the prevalence of SIBO and CD activity index score, or thiopurine or biologic treatment[53].
OCTT was significantly longer in patients with CD with SIBO than in those without[54]. SIBO also occurred more frequently in patients who had CD recurrence within 18 mo than in those who remained in remission (63.0% vs 37.0%; OR = 2.79). Relapses were more frequent in patients with SIBO than in those without (26.5% vs 7.7% at the 6-mo follow-up, and 50.0% vs 25.6% at the 18-mo follow-up)[55].
CD patients with and without SIBO were comparable with regard to demographics, systemic inflammatory biomarkers, and disease characteristics, except for the fibrostenosing phenotype that was more common in patients with SIBO than in those without [43.3% vs 19.3%; OR = 3.8 (95%CI: 1.5-6.8)]. Fecal calprotectin levels were significantly higher in SIBO-positive than in SIBO-negative patients (OR = 9.4, 95%CI: 3.0-11.3)[56].
Another study reported that CD patients with SIBO had increased stool frequency and significant reduction of stool solidity, were older, reported a longer history of CD, and were significantly more likely to have had prior surgery than CD patients without SIBO. An association was identified between the number of surgical procedures and the presence of SIBO (OR = 2.83, 95%CI: 1.15-6.96)[57].
In CD patients in remission, the prevalence of SIBO was 16.8%. No associations were observed between SIBO in these remission patients and the use of thiopurine or biological drugs. Multivariate analysis revealed that the presence of meteorism and a fistulizing phenotype were associated with the presence of SIBO in patients with CD in remission[58].
A meta-analysis showed that the prevalence of SIBO in patients with UC was greater than in controls [14.3% (95%CI: 10.52%-18.1%); OR = 8.0 (95%CI: 1.67-38.4)][31]; OCTT has also been shown to be significantly longer in these patients than in controls[54]. Serum levels of lipopolysaccharide (LPS), and the expression levels of Toll-like receptor (TLR)2 and TLR4 on the surface of peripheral blood mononuclear cells were higher in UC patients with SIBO than in those without. LPS levels were positively correlated with the expression levels of TLR2 and TLR4[59]. Serum levels of pro-inflammatory cytokines (IL-6, IL-8, and TNF-α), anti-inflammatory cytokines (IL-10), and lipid peroxidates (a marker of oxidative stress) were significantly higher, and levels of reduced glutathione (antioxidant) were lower in UC patients with SIBO than in those without[60].
In a mixed cohort of patients with various liver diseases, SIBO prevalence was significantly higher in patients with cirrhosis than in those without (41.2% vs 13.1%). Moreover, both cirrhotic and non-cirrhotic patients had a greater SIBO prevalence than healthy controls[61].
A meta-analysis of 21 studies revealed that the prevalence of SIBO in patients with cirrhosis was greater than that in healthy controls [(40.8%, 95%CI: 34.8%-47.1%) and OR = 6.83 (95%CI: 4.16-11.21) for all studies; 48.0% (95%CI: 27.5%-69.1%) and OR = 5.32 (95%CI: 1.02-27.82) for studies using culture-based methods; 52.7% (95%CI: 43.9%-61.4%) and OR = 3.24 (95%CI: 1.34-7.84) for studies using the LBT; and 35.9% (95%CI: 30.6%-41.6%) and OR = 8.65 (95%CI: 4.30-14.42) for studies using the GBT]. The prevalence of SIBO in patients with decompensated cirrhosis (classes B and C) was higher than that in those with compensated cirrhosis class A [50.5% (95%CI: 40.3%-60.7%) vs 31.2% (95%CI: 19.9%-45.1%); OR = 2.56 (95%CI: 1.60-4.09)]. The prevalence of SIBO was higher in patients with cirrhosis class C than in patients with cirrhosis class B [59.2% (95%CI: 45.0%-71.7%) vs 47.6% (95%CI: 35.9%-59.5%); OR = 1.79 (95%CI: 1.18-2.71)][62].
Patients with cirrhosis and SIBO were more likely to have ascites, minimal hepatic encephalopathy, and spontaneous bacterial peritonitis than those without SIBO. The relationship of SIBO with hypocoagulation, alanine aminotransferase and glutamyl transpeptidase activity, white blood cell and platelet counts, hemoglobin and ascitic fluid albumin levels, or esophageal varices was not found[62]. Recent meta-analyses have shown that SIBO in cirrhosis is associated with hepatic encephalopathy (OR = 4.43, 95%CI: 1.73-11.32)[63]. Short-term treatment with rifaximin effectively reduced blood ammonia levels, improved psychometric test scores, and reduced the rate of SIBO as determined by breath testing[64]. No significant association was found between SIBO and hepatic venous pressure gradient[65]. SIBO in patients with cirrhosis was associated with the presence of bacterial DNA in peripheral blood[66], higher plasma LPS levels[67], a shift to alkaline pH in gastric juices[68], malnutrition[69], prolonged OCTT[70,71], lower systolic blood pressure and systemic vascular resistance, higher cardiac output and serum C-reactive protein levels[72], splanchnic vasodilation, and abdominal hyperdynamic circulation[73].
In patients with cirrhosis, gut microbiota changes with SIBO (a higher abundance of Firmicutes and Fusobacteria, and a lower abundance of Bacteroidetes) did not correspond to cirrhosis-associated gut dysbiosis; therefore, it can be assumed that gut dysbiosis and SIBO are most likely independent disorders of gut microbiota in patients with cirrhosis[74].
The incidence of SIBO in patients with hepatocellular carcinoma (HCC) was higher than that in cirrhosis patients without HCC (71.8% vs 41.1%; OR = 3.7) and healthy controls (71.8% vs 3.0%; OR = 81.5). The expression levels of TLR2 and TLR4 on the surface of peripheral blood mononuclear cells in patients with HCC and SIBO were higher than those in patients without SIBO[75].
Patients with extra-hepatic portal vein obstruction had similar rates of SIBO compared with healthy controls (7.14% vs 1.97%)[76].
A meta-analysis of 18 studies revealed that, among patients with metabolic-associated fatty liver disease (MAFLD), the rate of SIBO was 35.0% (95%CI: 24.4%-47.2%); in patients with metabolic-associated steatohepatitis (MASH), the rate of SIBO was 41.1% (95%CI: 21.9%-63.4%)[77]. The prevalence of SIBO in MAFLD was higher than that in controls (OR = 3.82, 95%CI: 1.93-7.59) in another meta-analysis that included ten studies[78].
Patients with MAFLD and SIBO had increased OCTT; echomarkers of liver steatosis and fibrosis; severity of liver steatosis, fibrosis, lobular and portal inflammation, and ballooning by liver biopsy; levels of hepatic CD14 mRNA, nuclear factor kappa beta mRNA, and TLR4 protein expression; homeostatic model assessment for insulin resistance scores; and serum LPS levels compared with patients without SIBO. However, there was no significant difference in BMI, or in serum levels of TNF-α, adiponectin, glutamyl transpeptidase, triglycerides, or low-density lipoprotein between MAFLD patients with and without SIBO. SIBO was associated with significant fibrosis and MASH in MAFLD[79-81]. However, the presence of abdominal obesity was significantly associated with the absence of SIBO (RR = 0.38; 95%CI: 0.02-0.48)[82]. There was no significant difference in the incidence of SIBO in MASH patients with and without signs of metabolic syndrome[83].
A meta-analysis of three studies showed that children with SIBO were more likely to have MAFLD (OR = 5.27; 95%CI: 1.66-16.68) and children with MAFLD were at an increased risk of developing SIBO (RR = 2.17; 95%CI: 1.54-2.81)[84]. Metabolic syndrome and MAFLD were risk factors for SIBO development in obese children[85].
The prevalence of SIBO was higher in patients with primary biliary cholangitis (PBC) than in controls (32.8% vs 2.5%; OR = 18.9). Patients with PBC and SIBO were significantly more likely to have diarrhea (78.9% vs 35.9%) than those without SIBO. Age, sex, BMI, comorbidities, severity of disease, signs of portal hypertension, and the prevalence of abdominal pain and bloating were similar in PBC patients with and without SIBO[86].
SIBO was detected in 43% of patients who had undergone bariatric Roux-en-Y gastric bypass or one-anastomosis gastric bypass and was associated with a higher frequency of defecation in these patients than in those without SIBO[87]. In a separate study, SIBO was detected in 73.4% of patients who received a Roux-en-Y gastric bypass[88]. A third study reported that SIBO testing was negative in all pre-surgery patients and positive in 37.0% of patients 6 mo after one-anastomosis gastric bypass. SIBO in this study was associated with lower reported dietary intake and folate levels and higher vitamin A deficiency rates. No significant differences in alpha and beta diversities of gut microbiota were observed between patients with and without SIBO at 6 mo post-surgery[89]. Among patients that underwent Roux-en-Y gastric bypass at least 1 year before, the SIBO-positive group had a higher BMI than the SIBO-negative group[90]. SIBO prevalence does not increase after adjustable gastric banding (before, 15%; after, 10%), whereas it increased from 15% before Roux-en-Y gastric bypass to up to 40% afterwards. This study also found an association between SIBO and lower weight loss[91].
SIBO was found in 61.6% of patients who had undergone gastrectomy. No correlation was found with any malnutrition parameter[92]. Another study reported significant differences in SIBO prevalence between postgastrectomy patients and controls (77.6% vs 6.7%)[93]. Abdominal fullness or borborygmus during oral glucose load was more common in patients with SIBO than in those without. The prevalence of dumping syndrome and postprandial hypoglycemia, pulse rate, and hematocrit levels were not different between patients following gastrectomy with and without SIBO[93]. A third study also revealed that SIBO was more frequent in patients after gastrectomy than in controls (71.4% vs 13.3%; OR = 16.3). Specifically, 75% of patients that had undergone a Billroth I or II operation and 50% of patients that had undergone total gastrectomy were positive for SIBO[94].
Patients who had undergone colectomy had a greater incidence of SIBO than healthy controls (62% vs 32%; OR = 3.47). There was a higher prevalence of aerobic organisms and a lower prevalence of anaerobic organisms in the duodenal contents in the colectomy group compared with controls[95]. SIBO was detected in 74% of patients after right-sided hemicolectomy for colorectal cancer; no association between SIBO and bile acid malabsorption, delayed OCTT, or loose stools was found in these patients[96].
Patients that had undergone cholecystectomy were more likely to have SIBO than controls (46.8% vs 13.3%; OR = 5.7)[97]. Another study reported similar results (41.1% vs 13.3%; OR = 4.5)[94]. Cholecy
The presence of asymptomatic chronic pouchitis after ileoanal anastomosis was not associated with SIBO[98].
Patients with erosive esophagitis had SIBO more frequently than controls (65% vs 31%; OR = 4.0)[99].
Among patients with abdominal symptoms, SIBO was detected more frequently in Helicobacter pylori (H. pylori)-infected patients than in uninfected ones (60.4% vs 30.6%; OR = 3.45). The incidence of SIBO after H. pylori eradication decreased to 20.8%. The remission rate of SIBO after eradication was 66.7%[100].
SIBO was present in 60% of patients with gastroparesis, 43.8% of whom had hydrogen SIBO and 22.2% of whom had methane SIBO (6% of patients produced both gases). SIBO was associated with greater disease duration. No significant differences were noted in age, sex, gastric emptying parameters, and etiology of gastroparesis between patients with and without SIBO[101].
A meta-analysis of 13 studies showed that the prevalence of SIBO in patients with chronic pancreatitis (CP) was higher than that in controls in all patients [38.6% (95%CI: 25.5%-53.5%); OR = 5.58 (95%CI: 2.26-13.75)], nonsurgical cases [34.6% (95%CI: 20.7%-51.8%); OR = 4.61 (95%CI: 1.67-12.73)], and cases that needed surgical intervention [54.2% (95%CI: 23.3%-82.2%); OR = 10.86 (95%CI: 0.90-131.72)]. Diabetes mellitus (OR = 2.1; 95%CI: 1.2-3.5) and pancreatic exocrine insufficiency (OR = 2.5; 95%CI: 1.3-4.8), but not narcotic drug or PPI use, were associated with SIBO in patients with CP[102]. SIBO was more prevalent in patients with alcohol-induced CP than in patients with other forms of this disease[103]. A separate study showed no association between the severity of CP symptoms and the presence of SIBO[104].
SIBO was found in 17.8% of patients with acute pancreatitis (AP) and was associated with disease severity, as it was detected in 8.42%, 25.58%, and 25.92% of patients with mild, moderate, and severe AP, respectively. The production of hydrogen by gut microbiota was significantly lower in the late than in the early stage, and SIBO mainly developed within 72 h of AP onset. The incidence of organ failure was significantly higher in patients with SIBO than in those without. However, the incidence of infectious or local complications was not associated with SIBO, and neither was systemic inflammatory response syndrome[105]. Another study reported that SIBO incidence in AP was 12.0%, similar to the results of historic healthy controls, and that glucose tolerance was lower in AP patients with SIBO than in those without[106].
The prevalence of SIBO in cystic fibrosis was 31.6%. SIBO was associated with pancreas insufficiency, and independently associated with lower BMI and serum albumin levels[107]. Another study reported the prevalence of SIBO in cystic fibrosis as 40.0%; fecal calprotectin levels did not differ between patients with and without SIBO[108].
SIBO prevalence in patients with gallstones was higher than that in controls and those who had undergone cholecystectomy (40.5% vs 20.5% and 24.6%; OR = 2.23)[109]. Another study also reported that SIBO was more frequent in patients with gallstones than in controls (14.8% vs 0.7%; OR = 26.9)[110]. OCTT and serum bile acid levels were increased in patients with SIBO compared with patients without SIBO, and there was a positive correlation between OCTT and serum bile acid levels in SIBO-positive patients[110].
Hydrogen SIBO was detected in 42% of children with encopresis and 23% of controls (OR = 2.4). Methane was produced in 56% of children with encopresis and 23.1% of controls[111].
A meta-analysis of five studies reveled that the increased risk of SIBO in obese individuals did not reach statistical significance (OR = 2.08; 95%CI: 0.82-5.31) but was significant when including only studies from Western countries (OR = 3.41; 95%CI: 1.21-9.59)[112]. However, SIBO was not related to BMI. Small intestinal manometry showed a marked increase of clustered contractions in obese individuals with SIBO compared with those without; no other differences were observed in parameters of fasting cyclic activity[113]. Patients with SIBO have higher visceral to subcutaneous fat ratios than the general population. Metabolic syndrome (OR = 2.5; 95%CI: 1.1-5.7) and higher visceral to subcutaneous fat ratio (OR = 3.3; 95%CI: 1.6-7.2) were independently associated with SIBO in the general population[114]. Obese individuals with SIBO ingested more carbohydrates and refined sugars and less total and insoluble fibers than those without SIBO. There were no significant differences in lipid and protein intake between the two groups[115].
The prevalence of SIBO in obese children was greater than that in controls (37.6% vs 3.3%; OR = 17.5). Obese children with SIBO had higher rates of MAFLD, hypertension, and metabolic syndrome, and higher alanine transaminase and aspartate transaminase levels than those without SIBO[116].
A meta-analysis of 14 studies showed that SIBO is detected in patients with diabetes mellitus more frequently than in controls [29% (95%CI: 20%-39%); OR = 4.18 (95%CI: 1.34-13.05)]. The rate of SIBO was 35% (95%CI: 21%-49%) in Western countries and 24% (95%CI: 14%-34%) in Eastern countries. The prevalence of SIBO in type 2 diabetes was similar to that in type 1 diabetes [30% (95%CI: 13%-47%) vs 25% (95%CI: 14%-36%)][117].
Serum levels of biomarkers of oxidative stress (lipid peroxidates, catalase, and superoxide dismutase) and systemic inflammation (TNF-α, IL-6, and IL-10) were higher and serum levels of the antioxidant glutathione were lower in type 2 diabetic patients with SIBO than in those without[118]. SIBO was associated with lower fasting insulin levels, lower insulin release after glucose load, and poorer glucose tolerance and long-term glycemic control (higher glycated hemoglobin) in patients with type 2 diabetes. At the same time, insulin resistance and BMI were lower in patients with SIBO than in those without[119]. In type 2 diabetics, OCTT was more delayed, urinary D-xylose levels were more reduced, and lactose intolerance was more severe in patients with SIBO than in those without[120].
In type 1 diabetes mellitus, OCTT was more delayed and a history of uncontrolled diabetes was more likely in patients with SIBO than in those without. SIBO was significantly higher in patients who had had type 1 diabetes for ≥ 5 years than in those who were diagnosed < 5 years previously[121]. Patients with autonomic neuropathy in type 1 diabetes have a higher prevalence of SIBO, which was associated with higher daily insulin requirements[122].
The rate of SIBO in patients with gestational diabetes mellitus was greater than that in those patients without (54.6% vs 27.5%; OR = 3.16). Patients with SIBO had higher levels of fasting blood glucose, glycated hemoglobin, and C-reactive protein, and lower levels vitamin D than those without; neonates from these patients with SIBO had higher weights and lower levels of blood glucose than those from patients without SIBO[123].
SIBO prevalence in patients with hypothyroidism was higher than that in controls [54% vs 5%; OR = 22.3 (95%CI: 4.8-102.7)]. Abdominal discomfort, flatulence, and bloating were more prevalent in the patients with SIBO[124].
The positive rates of SIBO were 56.7% and 31.6% in pregnant women with and without subclinical hypothyroidism, respectively (OR = 2.83). The incidence of abdominal distension and constipation, presence of thyroid peroxidase antibody, levels of thyroid-stimulating hormone, and BMI were higher, and the levels of thyroxine were lower in women with SIBO compared with those without[125].
Patients with acromegaly showed an increased prevalence of SIBO compared with controls (43.9% vs 3.3%; OR = 22.7)[126].
SIBO was found in 78.9% of patients with hyperlipidemia and 40% of controls. The level of exhaled hydrogen at 120 min was positively correlated with serum triglyceride and low- and very-low-density lipoprotein levels, and negatively correlated with serum high-density lipoprotein levels[127]. Among patients who did not have previous cardiovascular events and presented with gastrointestinal discomfort, asymptomatic atherosclerotic plaques were detected more often in patients with SIBO than in those without. This was true for the abdominal aorta (OR = 4.18; 95%CI: 2.56-6.80), carotid arteries (OR = 1.93; 95%CI: 1.23-3.02), lower extremity arteries (OR = 1.81; 95%CI: 1.14-2.88), and any-territory plaque presence (OR = 5.42; 95%CI: 2.78-10.58)[128]. Arterial stiffness was elevated in patients with SIBO compared with those without, but carotid intima-media thickness and arterial calcifications did not differ between the two groups[129].
SIBO was detected in patients with multiple sclerosis more frequently than in controls [38.1% (95%CI: 29.4%-46.9%); OR = 4.50 (95%CI: 2.38-8.50)]. SIBO was associated with more severe disease according to the Expanded Disability Status Scale and Multiple Sclerosis Severity Score[130].
Patients with restless legs syndrome were more likely to have SIBO than healthy controls (69% vs 10%; OR = 19.8)[131].
SIBO was detected in 49% of patients with Alzheimer’s disease, a higher level than that in controls (OR = 3.35). SIBO was not associated with fecal calprotectin and zonulin levels, degree of cognitive impairment, comorbidities, or drug treatment[132].
Children with autism had an SIBO incidence of 31.0% (95%CI: 25.8%-36.1%), which was higher than that in controls (OR = 4.35). Autistic children with SIBO had higher Autism Treatment Evaluation Checklist scores than those without[133].
SIBO was detected in 21% of patients after spinal cord injury. SIBO was observed more frequently in the subacute than in the chronic phase (37.5% vs 0%), and in tetraplegia more frequently than in paraplegia (55.6% vs 0.5%)[134]. Another study reported that the SIBO rate after spinal cord injury was 38.5% (95%CI: 29.9%-59.0%) and that SIBO was associated with deep vein thrombosis in these patients (OR = 3.72; 95%CI: 1.97-6.62)[135].
A meta-analysis of 11 studies showed that the prevalence of SIBO in patients with Parkinson’s disease was 46% (95%CI: 36%-56%), which was higher than that in controls (OR = 5.22; 95%CI: 3.33-8.19). This incidence was greater in Western countries than in Eastern ones [52% (95%CI: 40%-64%) vs 33% (95%CI: 22%-43%)][136]. Patients with SIBO used smaller doses of dopaminergic drugs and had lower serum triglyceride and total bilirubin levels. SIBO was not associated with motor or abdominal symptoms[137] or weight loss[138]. However, another study reported that disease duration, Hoehn and Yahr stage, unified Parkinson’s disease rating-III and -IV scores, and Non-Motor Symptoms Scale score were associated with SIBO in these patients[139].
A meta-analysis of 14 studies reported that the prevalence of SIBO in patients with systemic sclerosis was 34% (95%CI: 27%-42%), which was higher than that in controls (OR = 12.51; 95%CI: 6.51-24.03). SIBO prevalence was greater in Western countries than in Asian countries [38% (95%CI: 31%-47%) vs 15% (95%CI: 10%-23%)]. Systemic sclerosis patients with SIBO were more likely to have diarrhea (OR = 8.82; 95%CI: 4.09-19) than those without. Sex, diffuse or limited disease, digital ulcer, pulmonary fibrosis, and the presence of anticentromere or Scl-70 antibodies were not associated with SIBO in these patients[140,141]. Fecal calprotectin levels were higher in patients with SIBO than in those without[141]. The average disease duration was longer in patients with SIBO, and these patients had lower levels of hemoglobin, ferritin, total serum protein, phosphorus, calcium, and triglycerides and an elevated erythrocyte sedimentation rate compared with patients without SIBO[142]. The duration of disease (> 5 years) was significantly associated with the presence of SIBO in systemic sclerosis (OR = 9.38; 95%CI: 1.09-80.47)[143].
SIBO was diagnosed in 36% of patients with Behçet’s disease. No significant differences in disease activity, patient age, severity of abdominal symptoms, frequency of drug use, surgery, smoking status, serum total protein, albumin, and C-reactive protein levels, and erythrocyte sedimentation rate were found between patients with and without SIBO[144].
The prevalence of SIBO in patients with spondylarthropathy was higher than that in the control group (63% vs 5%; OR = 32.9) and did not depend on the presence of HLA-B27, or treatment with NSAID, salazopyrin, or PPI[145].
SIBO was diagnosed in 100% of patients with fibromyalgia, compared with 20% of controls. The degree of somatic pain correlated significantly with hydrogen level as measured by the breath test[146].
SIBO was detected in 67% and 43% of patients with allergic and non-allergic asthma, respectively. Patients with SIBO had higher levels of immunoglobulin E in serum and eosinophils in sputum, and more severe impairment of respiratory function[147].
Among patients with heart failure (HF), 45% were positive for SIBO, including 41% of patients with reduced ejection fraction (EF) and 51% of patients with preserved EF. SIBO was associated with an increased risk of rehospitalization in patients with reduced EF and cardiovascular death in patients with preserved EF. Patients with SIBO showed higher rates of New York Heart Association functional class III–IV, atrial fibrillation, peripheral edema, spironolactone and intravenous diuretic use, lower use of beta-blockers, and increased left atrial diameter and pulmonary artery systolic pressure than patients without SIBO. Among patients with reduced EF, those with SIBO had higher serum levels of N-terminal pro-B-type natriuretic peptide (a biomarker of HF) than those without[148]. Another study also reported higher SIBO incidence in HF than in controls (41.7% vs 9.1%; OR = 7.14) but found that SIBO in HF was not associated with the New York Heart Association functional class, echocardiographic data, number of supraventricular and ventricular extrasystoles, or indicators of complete blood count or biochemical blood analysis. However, patients with SIBO had higher C-reactive protein levels and ventricular tachycardia incidence (OR = 6.8; 95%CI: 1.5-30.2) compared with those without[149].
SIBO was found in 63.3% of patients with pancreatic cancer and 46.7% of patients with cholangiocarcinoma, which was greater than that in the healthy controls (OR = 11.2 and 5.7, respectively). TLR4 protein expression in pancreatic carcinoma and cholangiocarcinoma patients was significantly higher in patients with SIBO than in those without[150].
Among patients with obstructive sleep apnea, 30.8% tested positive for SIBO. The incidence of flatulence was significantly greater in patients with SIBO than in those without. Waist-to-hip ratio was indepen
SIBO was detected more frequently in patients with post coronavirus disease 2019 IBS than in those with typical IBS (93.3% vs 60.0%; OR = 9.3)[152].
The prevalence of SIBO in patients with deep vein thrombosis was higher than in controls (69.8% vs 39.9%; OR = 3.5) and SIBO was independently associated with this disease (OR = 3.27; 95%CI: 1.70-6.32)[153].
SIBO was detected in patients with rosacea more frequently than in controls in one study (46.0% vs 5.0 %; OR = 16.2)[154] and was not more common in these patients than in controls in the other (10.0% vs 7.8%)[155]. The prevalence of SIBO in psoriatic patients did not differ from that in the controls (10% vs 5%)[156].
As shown above, SIBO is often detected in functional and organic bowel diseases, liver diseases, other diseases of the digestive organs, a number of endocrine, nervous and rheumatic diseases, asthma, HF, and certain other diseases. We have summarized all the reported information on the incidence and OR for SIBO in various diseases in Table 1. An important issue in comparing data is that the presence of SIBO was determined by different tests in different studies, giving different results. In addition, we have observed pronounced heterogeneity in the frequency of SIBO detection in healthy individuals within the control groups, which ranged from fractions of a percent to several tens of percent. The reason for this is yet to be established. However, the detection of SIBO in such a large number of clinically healthy individuals shows that this disorder can be relatively harmless and often asymptomatic.
| Disease | Prevalence of SIBO | OR |
| Functional digestive diseases | ||
| Functional dyspepsia | 17.2%-53.4% | 4.3 |
| Irritable bowel syndrome | 31.0%-36.7% | 3.7 |
| Functional abdominal bloating/distention | 43%-68% | |
| Functional constipation | 78% | |
| Functional diarrhea | 69% | |
| Organic (non-functional) digestive diseases | ||
| Short bowel syndrome | 50% | |
| Chronic intestinal pseudo-obstruction | 23.7%-52.6% | |
| Lactase deficiency | 27.6% | 5.3 |
| Lactose malabsorption in elderly persons | 90% | 36 |
| Symptomatic uncomplicated diverticular disease | 58.9% | 8.7 |
| Celiac disease | 18.3% | 5.1 |
| Environmental enteric dysfunction | 85.3% | |
| Crohn’s disease | 25.4% | 10.9 |
| Ulcerative colitis | 14.3% | 8.0 |
| Liver diseases | ||
| Cirrhosis | 40.8% | 6.8 |
| Hepatocellular carcinoma | 71.8% | 81.5 |
| Metabolic associated fatty liver disease | 35.0% | 3.8 |
| Primary biliary cholangitis | 32.8% | 18.9 |
| Abdominal surgery | ||
| Bariatric surgery | 37.0%-73.4% | |
| Gastrectomy | 61.6%-77.6% | 16.3 |
| Colectomy | 62%-74% | 3.47 |
| Cholecystectomy | 24.6%-46.8% | 4.5-5.7 |
| Other digestive diseases | ||
| Erosive esophagitis | 65% | 4.0 |
| Helicobacter pylori infection | 60.4% | 3.45 |
| Gastroparesis | 60% | |
| Chronic pancreatitis | 38.6% | 5.58 |
| Acute pancreatitis | 12.0%-17.8% | |
| Cystic fibroris | 31.6%-40.0% | |
| Gallstone disease | 14.8%-40.5% | 2.2-26.9 |
| Encopresis | 42% | 2.4 |
| Endocrine and metabolic diseases | ||
| Diabetes mellitus | 29% | 4.18 |
| Hypothyroidism | 54% | 22.3 |
| Acromegaly | 43.9% | 22.7 |
| Hyperlipidemia | 78.9% | |
| Nervous diseases | ||
| Multiple sclerosis | 38.1% | 4.5 |
| Restless legs syndrome | 69% | 19.8 |
| Azheimer’s disease | 49% | 3.35 |
| Autism | 31.0% | 4.35 |
| Spinal cord injuries | 37.5%-38.5% | |
| Parkinson’s disease | 46% | 5.22 |
| Rheumatic diseases | ||
| Systemic sclerosis | 34% | 12.51 |
| Behçet’s disease | 36% | |
| Spondylarthropathy | 63% | 32.9 |
| Fibromyalgia | 100% | |
| Other diseases | ||
| Allergic asthma | 67% | |
| Non-allergic asthma | 43% | |
| Heart failure | 41.7%-45.0% | 7.14 |
| Pancreatic cancer | 63.3% | 11.2 |
| Cholangiocarcinoma | 46.7% | 5.7 |
| Obstructive sleep apnea | 30.8% | |
| Post-COVID-19 irritable bowel syndrome | 93.3% | 9.3 |
| Deep vein thrombosis | 69.8% | 3.5 |
| Rosacea | 10%-46% | 16.2 |
For many diseases, the development of SIBO is associated with an increased OCTT that decreases the normal clearance of bacteria from the small intestine and is one of the mechanisms underlying the development of SIBO. The slowdown of this transit may be associated with motor dysfunction of the intestine in diseases of the gut, autonomic diabetic polyneuropathy, and portal hypertension, or a decrease in the motor-stimulating influence of thyroid hormones.
Bariatric surgery and gastrectomy lead to a decrease in the barrier function of the stomach which prevents the colonization of the small intestine by oral microbiota. Colectomy leads to a decrease in the barrier function of the ileocecal valve, which prevents the colonization of the small intestine by the colon microbiota. This may explain the increased risk of SIBO following these surgeries.
Interestingly, the frequency of SIBO is increased in a number of nervous and rheumatic diseases, which may support the existence of the gut-brain[157-159] and gut-joint[160-162] axes, suggesting that the products of gut microbiota metabolism can directly or indirectly affect emotional-cognitive and immune function[163], predisposing individuals to the development of these diseases, which is well illustrated by the example of hepatic encephalopathy[164,165] and reactive arthritis[166,167].
The presence of SIBO affects the course of various diseases in different ways. Some diseases, such as IBS, functional diarrhea, and functional constipation, can be mimicked by SIBO with the same symptoms. In these cases, it is not clear what disease is present: SIBO, which manifests itself as IBS and other functional bowel diseases, or these diseases, which are aggravated by the development of SIBO.
In a number of diseases, including cirrhosis, MAFLD, diabetes mellitus, pancreatitis, and cystic fibrosis, an association was found between disease severity and the presence of SIBO. This may be due to the fact that intestinal motility is more severely disturbed in more severe diseases (in cirrhosis or autonomic diabetic polyneuropathy) or that the digestive capabilities of the gut are more affected (in severe pancreatitis and cystic fibrosis) in these cases, which increases the nutrients available for bacteria of the small intestine, leading to their excess growth. However, in SIBO, the overgrown bacteria themselves can, through their metabolic products, affect the metabolism of lipids and carbohydrates, aggravating the course of diabetes[168] and MAFLD[169], and also, through bacterial translocation and systemic inflammation, aggravate the course of portal hypertension in cirrhosis[170-172].
Since SIBO is associated with many diseases, even those not related to the intestines, it seems useful to continue studying the association of SIBO with other diseases and their manifestations. It is also important to clarify the pathogenetic ways in which the underlying disease can contribute to the development of SIBO, and SIBO, in turn, can have a negative effect on the course of the underlying disease. Currently, such studies are being conducted in cirrhosis[72], MAFLD[79-81], diabetes[118,119], and other diseases. An important question is how the course of the underlying disease will respond to SIBO treatment. These results have already been obtained for some diseases[49,154], but their presen
In conclusion, we have summarized almost all published information on the association of SIBO with various diseases and their manifestations. Almost one third of cited studies were published within the last 2 years, highlighting the recent interest in the field and the importance of our review. However, further study on SIBO in various diseases, and particularly on the effect of its eradication on the condition and prognosis of patients, is required.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Duan SL, China; Ji G, China; Wen XL, China; Zhang XL, China S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Bushyhead D, Quigley EMM. Small Intestinal Bacterial Overgrowth-Pathophysiology and Its Implications for Definition and Management. Gastroenterology. 2022;163:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 2. | Ghoshal UC, Sachdeva S, Ghoshal U, Misra A, Puri AS, Pratap N, Shah A, Rahman MM, Gwee KA, Tan VPY, Ahmed T, Lee YY, Ramakrishna BS, Talukdar R, Rana SV, Sinha SK, Chen M, Kim N, Holtmann G. Asian-Pacific consensus on small intestinal bacterial overgrowth in gastrointestinal disorders: An initiative of the Indian Neurogastroenterology and Motility Association. Indian J Gastroenterol. 2022;41:483-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Skrzydło-Radomańska B, Cukrowska B. How to Recognize and Treat Small Intestinal Bacterial Overgrowth? J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 4. | Ivashkin VT, Maev IV, Abdulganieva DI, Alekseeva OP, Alekseenko SA, Zolnikova OY, Korochanskaya NV, Medvedev OS, Poluektova EA, Simanenkov VI, Trukhmanov AS, Khlynov IB, Tsukanov VV, Shifrin OS, Ivashkin KV, Lapina TL, Maslennikov RV, Fadeeva MV, Ulyanin AI. Practical Recommendation of the Scientific Сommunity for Human Microbiome Research (CHMR) and the Russian Gastroenterological Association (RGA) on Small Intestinal Bacterial Overgrowth in Adults. Russ J Gastroenterol Hepatol Coloproctol. 2022;32:68-85. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Broekaert IJ, Borrelli O, Dolinsek J, Martin-de-Carpi J, Mas E, Miele E, Pienar C, Ribes-Koninckx C, Thomassen R, Thomson M, Tzivinikos C, Benninga M. An ESPGHAN Position Paper on the Use of Breath Testing in Paediatric Gastroenterology. J Pediatr Gastroenterol Nutr. 2022;74:123-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Baker JR, Chey WD, Watts L, Armstrong M, Collins K, Lee AA, Dupati A, Menees S, Saad RJ, Harer K, Hasler WL. How the North American Consensus Protocol Affects the Performance of Glucose Breath Testing for Bacterial Overgrowth Versus a Traditional Method. Am J Gastroenterol. 2021;116:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Losurdo G, Leandro G, Ierardi E, Perri F, Barone M, Principi M, Leo AD. Breath Tests for the Non-invasive Diagnosis of Small Intestinal Bacterial Overgrowth: A Systematic Review With Meta-analysis. J Neurogastroenterol Motil. 2020;26:16-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Madigan KE, Bundy R, Weinberg RB. Distinctive Clinical Correlates of Small Intestinal Bacterial Overgrowth with Methanogens. Clin Gastroenterol Hepatol. 2022;20:1598-1605.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | de Lacy Costello BP, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: a review. J Breath Res. 2013;7:024001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Liu Chen Kiow J, Bellila R, Therrien A, Sidani S, Bouin M. Predictors of Small Intestinal Bacterial Overgrowth in Symptomatic Patients Referred for Breath Testing. J Clin Med Res. 2020;12:655-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (4)] |
| 11. | Erdogan A, Rao SS, Gulley D, Jacobs C, Lee YY, Badger C. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil. 2015;27:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Choung RS, Ruff KC, Malhotra A, Herrick L, Locke GR 3rd, Harmsen WS, Zinsmeister AR, Talley NJ, Saito YA. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment Pharmacol Ther. 2011;33:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 13. | Roland BC, Ciarleglio MM, Clarke JO, Semler JR, Tomakin E, Mullin GE, Pasricha PJ. Small Intestinal Transit Time Is Delayed in Small Intestinal Bacterial Overgrowth. J Clin Gastroenterol. 2015;49:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 14. | Chander Roland B, Mullin GE, Passi M, Zheng X, Salem A, Yolken R, Pasricha PJ. A Prospective Evaluation of Ileocecal Valve Dysfunction and Intestinal Motility Derangements in Small Intestinal Bacterial Overgrowth. Dig Dis Sci. 2017;62:3525-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Calderon G, Siwiec RM, Bohm ME, Nowak TV, Wo JM, Gupta A, Xu H, Shin A. Delayed Gastric Emptying Is Not Associated with a Microbiological Diagnosis of Small Intestinal Bacterial Overgrowth. Dig Dis Sci. 2021;66:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Montalto M, Santoro L, Dalvai S, Curigliano V, D'Onofrio F, Scarpellini E, Cammarota G, Panunzi S, Gallo A, Gasbarrini A, Gasbarrini G. Fecal calprotectin concentrations in patients with small intestinal bacterial overgrowth. Dig Dis. 2008;26:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 17. | Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 18. | Siniewicz-Luzeńczyk K, Bik-Gawin A, Zeman K, Bąk-Romaniszyn L. Small intestinal bacterial overgrowth syndrome in children. Prz Gastroenterol. 2015;10:28-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Pilipenko VI, Isakov VA, Morozov SV, Vlasova AV, Naydenova MA. [Association of food patterns with different forms of small intestinal bacterial overgroth syndrome and treatment efficacy]. Ter Arkh. 2019;91:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Nalyotov AV, Svistunova NA. [Assessment of the state of the small intestine microbiota in children on a long-term dairy-free diet]. Vopr Pitan. 2022;91:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (2)] |
| 21. | Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (2)] |
| 22. | Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Revaiah PC, Kochhar R, Rana SV, Berry N, Ashat M, Dhaka N, Rami Reddy Y, Sinha SK. Risk of small intestinal bacterial overgrowth in patients receiving proton pump inhibitors versus proton pump inhibitors plus prokinetics. JGH Open. 2018;2:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 24. | Zhang R, Li Y, Ma JX, Tang S, Li CM, Wan J. [The effects of continuous proton pump inhibitor therapy on small intestinal bacterial overgrowth in elderly]. Zhonghua Nei Ke Za Zhi. 2020;59:706-710. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Plauzolles A, Uras S, Pénaranda G, Bonnet M, Dukan P, Retornaz F, Halfon P. Small Intestinal Bacterial Overgrowths and Intestinal Methanogen Overgrowths Breath Testing in a Real-Life French Cohort. Clin Transl Gastroenterol. 2023;14:e00556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Kucheryavyy YA, Andreev DN, Maev IV. [Prevalence of small bowel bacterial overgrowth in patients with functional dyspepsia: a meta-analysis]. Ter Arkh. 2020;92:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 27. | Gurusamy SR, Shah A, Talley NJ, Koloski N, Jones MP, Walker MM, Morrison M, Holtmann G. Small Intestinal Bacterial Overgrowth in Functional Dyspepsia: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2021;116:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Ghoshal UC, Nehra A, Mathur A, Rai S. A meta-analysis on small intestinal bacterial overgrowth in patients with different subtypes of irritable bowel syndrome. J Gastroenterol Hepatol. 2020;35:922-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Shah A, Talley NJ, Jones M, Kendall BJ, Koloski N, Walker MM, Morrison M, Holtmann GJ. Small Intestinal Bacterial Overgrowth in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. Am J Gastroenterol. 2020;115:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (2)] |
| 30. | Chen B, Kim JJ, Zhang Y, Du L, Dai N. Prevalence and predictors of small intestinal bacterial overgrowth in irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol. 2018;53:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Gandhi A, Shah A, Jones MP, Koloski N, Talley NJ, Morrison M, Holtmann G. Methane positive small intestinal bacterial overgrowth in inflammatory bowel disease and irritable bowel syndrome: A systematic review and meta-analysis. Gut Microbes. 2021;13:1933313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 32. | Yu X, Li Y, Xiang F, Feng J. Correlation between small intestinal bacterial overgrowth and irritable bowel syndrome and the prognosis of treatment. Ann Palliat Med. 2021;10:3364-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 33. | Srivastava D, Ghoshal U, Mittal RD, Ghoshal UC. Associations between IL-1RA polymorphisms and small intestinal bacterial overgrowth among patients with irritable bowel syndrome from India. Neurogastroenterol Motil. 2014;26:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Chu H, Fox M, Zheng X, Deng Y, Long Y, Huang Z, Du L, Xu F, Dai N. Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome: Clinical Characteristics, Psychological Factors, and Peripheral Cytokines. Gastroenterol Res Pract. 2016;2016:3230859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Wei H, Liu ZJ, Wang K, Zheng W, Duan LP. [The dietary features of diarrhea predominant irritable bowel syndrome patients with small intestinal bowel overgrowth]. Zhonghua Nei Ke Za Zhi. 2017;56:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Jung SE, Joo NS, Han KS, Kim KN. Obesity Is Inversely Related to Hydrogen-Producing Small Intestinal Bacterial Overgrowth in Non-Constipation Irritable Bowel Syndrome. J Korean Med Sci. 2017;32:948-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Ding XW, Liu YX, Fang XC, Liu K, Wei YY, Shan MH. The relationship between small intestinal bacterial overgrowth and irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2017;21:5191-5196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Won KH, Park SM. The Relationship between Small-Intestinal Bacterial Overgrowth and Intestinal Permeability in Patients with Irritable Bowel Syndrome. Gut Liver. 2009;3:174-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Grover M, Kanazawa M, Palsson OS, Chitkara DK, Gangarosa LM, Drossman DA, Whitehead WE. Small intestinal bacterial overgrowth in irritable bowel syndrome: association with colon motility, bowel symptoms, and psychological distress. Neurogastroenterol Motil. 2008;20:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 40. | Scarpellini E, Giorgio V, Gabrielli M, Lauritano EC, Pantanella A, Fundarò C, Gasbarrini A. Prevalence of small intestinal bacterial overgrowth in children with irritable bowel syndrome: a case-control study. J Pediatr. 2009;155:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Noh CK, Lee KJ. Fecal Microbiota Alterations and Small Intestinal Bacterial Overgrowth in Functional Abdominal Bloating/Distention. J Neurogastroenterol Motil. 2020;26:539-549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Madrid AM, Defilippi C C, Defilippi G C, Slimming A J, Quera P R. [Small intestinal bacterial overgrowth in patients with functional gastrointestinal diseases]. Rev Med Chil. 2007;135:1245-1252. [PubMed] |
| 43. | Muraki M, Fujiwara Y, Machida H, Okazaki H, Sogawa M, Yamagami H, Tanigawa T, Shiba M, Watanabe K, Tominaga K, Watanabe T, Arakawa T. Role of small intestinal bacterial overgrowth in severe small intestinal damage in chronic non-steroidal anti-inflammatory drug users. Scand J Gastroenterol. 2014;49:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 44. | Seddik TB, Tian L, Nespor C, Kerner J, Maldonado Y, Gans H. Risk Factors of Ambulatory Central Line-Associated Bloodstream Infection in Pediatric Short Bowel Syndrome. JPEN J Parenter Enteral Nutr. 2020;44:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Khan MZ, Lyu R, McMichael J, Gabbard S. Chronic Intestinal Pseudo-Obstruction Is Associated with Intestinal Methanogen Overgrowth. Dig Dis Sci. 2022;67:4834-4840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 46. | Jo IH, Paik CN, Kim YJ, Lee JM, Choi SY, Hong KP. Lactase Deficiency Diagnosed by Endoscopic Biopsy-based Method is Associated With Positivity to Glucose Breath Test. J Neurogastroenterol Motil. 2023;29:85-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 47. | Almeida JA, Kim R, Stoita A, McIver CJ, Kurtovic J, Riordan SM. Lactose malabsorption in the elderly: role of small intestinal bacterial overgrowth. Scand J Gastroenterol. 2008;43:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Transient lactose malabsorption in patients affected by symptomatic uncomplicated diverticular disease of the colon. Dig Dis Sci. 2006;51:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 49. | Shah A, Thite P, Hansen T, Kendall BJ, Sanders DS, Morrison M, Jones MP, Holtmann G. Links between celiac disease and small intestinal bacterial overgrowth: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37:1844-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (2)] |
| 50. | Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol. 2003;98:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Keusch GT, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, Nataro JP, Rosenberg IH, Ryan ET, Tarr PI, Ward H, Bhutta ZA, Coovadia H, Lima A, Ramakrishna B, Zaidi AK, Hay Burgess DC, Brewer T. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59 Suppl 4:S207-S212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 52. | Collard JM, Andrianonimiadana L, Habib A, Rakotondrainipiana M, Andriantsalama P, Randriamparany R, Rabenandrasana MAN, Weill FX, Sauvonnet N, Randremanana RV, Guillemot V, Vonaesch P, Sansonetti PJ; Afribiota Investigators. High prevalence of small intestine bacteria overgrowth and asymptomatic carriage of enteric pathogens in stunted children in Antananarivo, Madagascar. PLoS Negl Trop Dis. 2022;16:e0009849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Shah A, Morrison M, Burger D, Martin N, Rich J, Jones M, Koloski N, Walker MM, Talley NJ, Holtmann GJ. Systematic review with meta-analysis: the prevalence of small intestinal bacterial overgrowth in inflammatory bowel disease. Aliment Pharmacol Ther. 2019;49:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 54. | Rana SV, Sharma S, Malik A, Kaur J, Prasad KK, Sinha SK, Singh K. Small intestinal bacterial overgrowth and orocecal transit time in patients of inflammatory bowel disease. Dig Dis Sci. 2013;58:2594-2598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 55. | Wei J, Feng J, Chen L, Yang Z, Tao H, Li L, Xuan J, Wang F. Small intestinal bacterial overgrowth is associated with clinical relapse in patients with quiescent Crohn's disease: a retrospective cohort study. Ann Transl Med. 2022;10:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 56. | Ricci JER Júnior, Chebli LA, Ribeiro TCDR, Castro ACS, Gaburri PD, Pace FHDL, Barbosa KVBD, Ferreira LEVVDC, Passos MDCF, Malaguti C, Delgado ÁHDA, Campos JD, Coelho AR, Chebli JMF. Small-Intestinal Bacterial Overgrowth is Associated With Concurrent Intestinal Inflammation But Not With Systemic Inflammation in Crohn's Disease Patients. J Clin Gastroenterol. 2018;52:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 57. | Greco A, Caviglia GP, Brignolo P, Ribaldone DG, Reggiani S, Sguazzini C, Smedile A, Pellicano R, Resegotti A, Astegiano M, Bresso F. Glucose breath test and Crohn's disease: Diagnosis of small intestinal bacterial overgrowth and evaluation of therapeutic response. Scand J Gastroenterol. 2015;50:1376-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Sánchez-Montes C, Ortiz V, Bastida G, Rodríguez E, Yago M, Beltrán B, Aguas M, Iborra M, Garrigues V, Ponce J, Nos P. Small intestinal bacterial overgrowth in inactive Crohn's disease: influence of thiopurine and biological treatment. World J Gastroenterol. 2014;20:13999-14003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Yang C, Guo X, Wang J, Fan H, Huo X, Dong L, Duan Z. Relationship between Small Intestinal Bacterial Overgrowth and Peripheral Blood ET, TLR2 and TLR4 in Ulcerative Colitis. J Coll Physicians Surg Pak. 2020;30:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Rana SV, Sharma S, Kaur J, Prasad KK, Sinha SK, Kochhar R, Malik A, Morya RK. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J Crohns Colitis. 2014;8:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Scarpellini E, Abenavoli L, Cassano V, Rinninella E, Sorge M, Capretti F, Rasetti C, Svegliati Baroni G, Luzza F, Santori P, Sciacqua A. The Apparent Asymmetrical Relationship Between Small Bowel Bacterial Overgrowth, Endotoxemia, and Liver Steatosis and Fibrosis in Cirrhotic and Non-Cirrhotic Patients: A Single-Center Pilot Study. Front Med (Lausanne). 2022;9:872428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 62. | Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018;12:567-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Feng X, Li X, Zhang X, Chen W, Tian Y, Yang Q, Yang Y, Pan H, Jiang Z. Hepatic Encephalopathy in Cirrhotic Patients and Risk of Small Intestinal Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Biomed Res Int. 2022;2022:2469513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 64. | Zhang Y, Feng Y, Cao B, Tian Q. Effects of SIBO and rifaximin therapy on MHE caused by hepatic cirrhosis. Int J Clin Exp Med. 2015;8:2954-2957. [PubMed] |
| 65. | Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Jun DW, Kim KT, Lee OY, Chae JD, Son BK, Kim SH, Jo YJ, Park YS. Association between small intestinal bacterial overgrowth and peripheral bacterial DNA in cirrhotic patients. Dig Dis Sci. 2010;55:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Wang J, Chen M, Sun G. Small bowel bacterial overgrowth and endotoxemia in cirrhosis. Zhonghua Nei Ke Za Zhi. 2002;41:459-461. [PubMed] |
| 68. | Shindo K, Machida M, Miyakawa K, Fukumura M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol. 1993;88:2084-2091. [PubMed] |
| 69. | Yao J, Chang L, Yuan L, Duan Z. Nutrition status and small intestinal bacterial overgrowth in patients with virus-related cirrhosis. Asia Pac J Clin Nutr. 2016;25:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 70. | Lunia MK, Sharma BC, Sachdeva S. Small intestinal bacterial overgrowth and delayed orocecal transit time in patients with cirrhosis and low-grade hepatic encephalopathy. Hepatol Int. 2013;7:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 72. | Maslennikov R, Pavlov C, Ivashkin V. Is small intestinal bacterial overgrowth a cause of hyperdynamic circulation in cirrhosis? Turk J Gastroenterol. 2019;30:964-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Maslennikov RV, Tatarkina MA, Mayevskaya MV, Pavlov CS, Zharkova MS, Ivashkin VT. The impact of bacterial overgrowth syndrome and systemic inflammation on abdominal hemodynamics in liver cirrhosis. Russ J Gastroenterol Hepatol Coloproctol. 2017;27:52-61. |
| 74. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Kudryavtseva A, Krasnov G. Gut dysbiosis and small intestinal bacterial overgrowth as independent forms of gut microbiota disorders in cirrhosis. World J Gastroenterol. 2022;28:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 75. | Zhou DX, Ma YJ, Chen GY, Gao X, Yang L. [Relationship of TLR2 and TLR4 expressions on the surface of peripheral blood mononuclear cells to small intestinal bacteria overgrowth in patients with hepatocellular carcinoma]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Lakshmi CP, Ghoshal UC, Kumar S, Goel A, Misra A, Mohindra S, Choudhuri G. Frequency and factors associated with small intestinal bacterial overgrowth in patients with cirrhosis of the liver and extra hepatic portal venous obstruction. Dig Dis Sci. 2010;55:1142-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Gudan A, Jamioł-Milc D, Hawryłkowicz V, Skonieczna-Żydecka K, Stachowska E. The Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Non-Alcoholic Liver Diseases: NAFLD, NASH, Fibrosis, Cirrhosis-A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (1)] |
| 78. | Wijarnpreecha K, Lou S, Watthanasuntorn K, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Keaveny AP, Ungprasert P. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 79. | Shi H, Mao L, Wang L, Quan X, Xu X, Cheng Y, Zhu S, Dai F. Small intestinal bacterial overgrowth and orocecal transit time in patients of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2021;33:e535-e539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 80. | Mikolasevic I, Delija B, Mijic A, Stevanovic T, Skenderevic N, Sosa I, Krznaric-Zrnic I, Abram M, Krznaric Z, Domislovic V, Filipec Kanizaj T, Radic-Kristo D, Cubranic A, Grubesic A, Nakov R, Skrobonja I, Stimac D, Hauser G. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease diagnosed by transient elastography and liver biopsy. Int J Clin Pract. 2021;75:e13947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Kapil S, Duseja A, Sharma BK, Singla B, Chakraborti A, Das A, Ray P, Dhiman RK, Chawla Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016;31:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 82. | Fitriakusumah Y, Lesmana CRA, Bastian WP, Jasirwan COM, Hasan I, Simadibrata M, Kurniawan J, Sulaiman AS, Gani RA. The role of Small Intestinal Bacterial Overgrowth (SIBO) in Non-alcoholic Fatty Liver Disease (NAFLD) patients evaluated using Controlled Attenuation Parameter (CAP) Transient Elastography (TE): a tertiary referral center experience. BMC Gastroenterol. 2019;19:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 83. | De Oliveira JM, Pace FL, Ghetti FF, Barbosa KVBD, Cesar DE, Chebli JMF, Ferreira LEVVC. Non-alcoholic Steatohepatitis: Comparison of Intestinal Microbiota between Different Metabolic Profiles. A Pilot Study. J Gastrointestin Liver Dis. 2020;29:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 84. | Kuang L, Zhou W, Jiang Y. Association of small intestinal bacterial overgrowth with nonalcoholic fatty liver disease in children: A meta-analysis. PLoS One. 2021;16:e0260479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Stepanov YM, Zavhorodnia NY, Yagmur VB, Lukianenko OY, Zygalo EV. Association of nonalcoholic fatty liver disease with small intestine bacterial overgrowth in obese children. Wiad Lek. 2019;72:350-356. [PubMed] |
| 86. | Liu Chen Kiow J, Vincent C, Sidani S, Bouin M. High occurrence of small intestinal bacterial overgrowth in primary biliary cholangitis. Neurogastroenterol Motil. 2019;31:e13691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Novljan U, Pintar T. Small Intestinal Bacterial Overgrowth in Patients with Roux-en-Y Gastric Bypass and One-Anastomosis Gastric Bypass. Obes Surg. 2022;32:4102-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (2)] |
| 88. | Dolan RD, Baker J, Harer K, Lee A, Hasler W, Saad R, Schulman AR. Small Intestinal Bacterial Overgrowth: Clinical Presentation in Patients with Roux-en-Y Gastric Bypass. Obes Surg. 2021;31:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Kaniel O, Sherf-Dagan S, Szold A, Langer P, Khalfin B, Kessler Y, Raziel A, Sakran N, Motro Y, Goitein D, Moran-Gilad J. The Effects of One Anastomosis Gastric Bypass Surgery on the Gastrointestinal Tract. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 90. | Coelho LK, Carvalho NS, Navarro-Rodriguez T, Marson FAL, Carvalho PJPC. Lactulose Breath Testing Can Be a Positive Predictor Before Weight Gain in Participants with Obesity Submitted to Roux-en-Y Gastric Bypass. Obes Surg. 2019;29:3457-3464. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 91. | Sabate JM, Coupaye M, Ledoux S, Castel B, Msika S, Coffin B, Jouet P. Consequences of Small Intestinal Bacterial Overgrowth in Obese Patients Before and After Bariatric Surgery. Obes Surg. 2017;27:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 92. | Pérez Aisa A, García Gavilán MC, Alcaide García J, Méndez Sánchez IM, Rivera Irigoin R, Fernández Cano F, Pereda Salguero T, Rivas Ruiz F. Small intestinal bacterial overgrowth is common after gastrectomy but with little impact on nutritional status. Gastroenterol Hepatol. 2019;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Paik CN, Choi MG, Lim CH, Park JM, Chung WC, Lee KM, Jun KH, Song KY, Jeon HM, Chin HM, Park CH, Chung IS. The role of small intestinal bacterial overgrowth in postgastrectomy patients. Neurogastroenterol Motil. 2011;23:e191-e196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 94. | Kim DB, Paik CN, Kim YJ, Lee JM, Jun KH, Chung WC, Lee KM, Yang JM, Choi MG. Positive Glucose Breath Tests in Patients with Hysterectomy, Gastrectomy, and Cholecystectomy. Gut Liver. 2017;11:237-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Rao SSC, Tan G, Abdulla H, Yu S, Larion S, Leelasinjaroen P. Does colectomy predispose to small intestinal bacterial (SIBO) and fungal overgrowth (SIFO)? Clin Transl Gastroenterol. 2018;9:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 96. | Larsen HM, Krogh K, Borre M, Gregersen T, Mejlby Hansen M, Arveschoug AK, Christensen P, Drewes AM, Emmertsen KJ, Laurberg S, Ladefoged Fassov J. Chronic loose stools following right-sided hemicolectomy for colon cancer and the association with bile acid malabsorption and small intestinal bacterial overgrowth. Colorectal Dis. 2023;25:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 97. | Sung HJ, Paik CN, Chung WC, Lee KM, Yang JM, Choi MG. Small Intestinal Bacterial Overgrowth Diagnosed by Glucose Hydrogen Breath Test in Post-cholecystectomy Patients. J Neurogastroenterol Motil. 2015;21:545-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 98. | Lisowska A, Banasiewicz T, Marciniak R, Drews M, Majewski P, Herzig KH, Walkowiak J. Chronic pouchitis is not related to small intestine bacterial overgrowth. Inflamm Bowel Dis. 2008;14:1102-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Kim KM, Kim BT, Lee DJ, Park SB, Joo NS, Kim YS, Kim KN. Erosive esophagitis may be related to small intestinal bacterial overgrowth. Scand J Gastroenterol. 2012;47:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Zhu D, Wang XL, Dai Y, Li SY, Wang WH. [Influence of Helicobacter pylori infection and its eradication treatment on small intestinal bacterial overgrowth]. Zhonghua Yi Xue Za Zhi. 2022;102:3382-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 101. | Reddymasu SC, McCallum RW. Small intestinal bacterial overgrowth in gastroparesis: are there any predictors? J Clin Gastroenterol. 2010;44:e8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | El Kurdi B, Babar S, El Iskandarani M, Bataineh A, Lerch MM, Young M, Singh VP. Factors That Affect Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Pancreatitis: A Systematic Review, Meta-Analysis, and Meta-Regression. Clin Transl Gastroenterol. 2019;10:e00072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 103. | Ní Chonchubhair HM, Bashir Y, Dobson M, Ryan BM, Duggan SN, Conlon KC. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI). Pancreatology. 2018;18:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 104. | Therrien A, Bouchard S, Sidani S, Bouin M. Prevalence of Small Intestinal Bacterial Overgrowth among Chronic Pancreatitis Patients: A Case-Control Study. Can J Gastroenterol Hepatol. 2016;2016:7424831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 105. | Zhang M, Zhu HM, He F, Li BY, Li XC. Association between acute pancreatitis and small intestinal bacterial overgrowth assessed by hydrogen breath test. World J Gastroenterol. 2017;23:8591-8596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Kim DB, Paik CN, Lee JM, Kim YJ. Association between increased breath hydrogen methane concentration and prevalence of glucose intolerance in acute pancreatitis. J Breath Res. 2020;14:026006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 107. | Furnari M, De Alessandri A, Cresta F, Haupt M, Bassi M, Calvi A, Haupt R, Bodini G, Ahmed I, Bagnasco F, Giannini EG, Casciaro R. The role of small intestinal bacterial overgrowth in cystic fibrosis: a randomized case-controlled clinical trial with rifaximin. J Gastroenterol. 2019;54:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 108. | Lisowska A, Madry E, Pogorzelski A, Szydłowski J, Radzikowski A, Walkowiak J. Small intestine bacterial overgrowth does not correspond to intestinal inflammation in cystic fibrosis. Scand J Clin Lab Invest. 2010;70:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Kim DB, Paik CN, Song DS, Kim YJ, Lee JM. The characteristics of small intestinal bacterial overgrowth in patients with gallstone diseases. J Gastroenterol Hepatol. 2018;33:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Kaur J, Rana SV, Gupta R, Gupta V, Sharma SK, Dhawan DK. Prolonged orocecal transit time enhances serum bile acids through bacterial overgrowth, contributing factor to gallstone disease. J Clin Gastroenterol. 2014;48:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Leiby A, Mehta D, Gopalareddy V, Jackson-Walker S, Horvath K. Bacterial overgrowth and methane production in children with encopresis. J Pediatr. 2010;156:766-770, 770.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Wijarnpreecha K, Werlang ME, Watthanasuntorn K, Panjawatanan P, Cheungpasitporn W, Gomez V, Lukens FJ, Ungprasert P. Obesity and Risk of Small Intestine Bacterial Overgrowth: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2020;65:1414-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 113. | Madrid AM, Poniachik J, Quera R, Defilippi C. Small intestinal clustered contractions and bacterial overgrowth: a frequent finding in obese patients. Dig Dis Sci. 2011;56:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 114. | Fialho A, Fialho A, Thota P, McCullough A, Shen B. Higher visceral to subcutaneous fat ratio is associated with small intestinal bacterial overgrowth. Nutr Metab Cardiovasc Dis. 2016;26:773-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Ierardi E, Losurdo G, Sorrentino C, Giorgio F, Rossi G, Marinaro A, Romagno KR, Di Leo A, Principi M. Macronutrient intakes in obese subjects with or without small intestinal bacterial overgrowth: an alimentary survey. Scand J Gastroenterol. 2016;51:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (3)] |
| 116. | Belei O, Olariu L, Dobrescu A, Marcovici T, Marginean O. The relationship between non-alcoholic fatty liver disease and small intestinal bacterial overgrowth among overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2017;30:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 117. | Feng X, Li XQ. The prevalence of small intestinal bacterial overgrowth in diabetes mellitus: a systematic review and meta-analysis. Aging (Albany NY). 2022;14:975-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 118. | Malik A, Morya RK, Saha S, Singh PK, Bhadada SK, Rana SV. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur J Clin Invest. 2020;50:e13238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 119. | Yan LH, Mu B, Pan D, Shi YN, Yuan JH, Guan Y, Li W, Zhu XY, Guo L. Association between small intestinal bacterial overgrowth and beta-cell function of type 2 diabetes. J Int Med Res. 2020;48:300060520937866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (2)] |
| 120. | Rana SV, Malik A, Bhadada SK, Sachdeva N, Morya RK, Sharma G. Malabsorption, Orocecal Transit Time and Small Intestinal Bacterial Overgrowth in Type 2 Diabetic Patients: A Connection. Indian J Clin Biochem. 2017;32:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 121. | Malik A, Morya RK, Bhadada SK, Rana S. Type 1 diabetes mellitus: Complex interplay of oxidative stress, cytokines, gastrointestinal motility and small intestinal bacterial overgrowth. Eur J Clin Invest. 2018;48:e13021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 122. | Ojetti V, Pitocco D, Scarpellini E, Zaccardi F, Scaldaferri F, Gigante G, Gasbarrini G, Ghirlanda G, Gasbarrini A. Small bowel bacterial overgrowth and type 1 diabetes. Eur Rev Med Pharmacol Sci. 2009;13:419-423. [PubMed] |
| 123. | Zhang M, Xu Y, Zhang J, Sun Z, Ban Y, Wang B, Hou X, Cai Y, Li J, Wang M, Wang W. Application of methane and hydrogen-based breath test in the study of gestational diabetes mellitus and intestinal microbes. Diabetes Res Clin Pract. 2021;176:108818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 124. | Lauritano EC, Bilotta AL, Gabrielli M, Scarpellini E, Lupascu A, Laginestra A, Novi M, Sottili S, Serricchio M, Cammarota G, Gasbarrini G, Pontecorvi A, Gasbarrini A. Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab. 2007;92:4180-4184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 125. | Wang B, Xu Y, Hou X, Li J, Cai Y, Hao Y, Ouyang Q, Wu B, Sun Z, Zhang M, Ban Y. Small Intestinal Bacterial Overgrowth in Subclinical Hypothyroidism of Pregnant Women. Front Endocrinol (Lausanne). 2021;12:604070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 126. | Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, Ferone D. Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92:2119-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Kvit KB, Kharchenko NV, Kharchenko VV, Chornenka OI, Chornovus RI, Dorofeeva US, Draganchuk OB, Slaba OM. The role of small intestinal bacterial overgrowth in the pathogenesis of hyperlipidemia. Wiad Lek. 2019;72:645-649. [PubMed] |
| 128. | Dong C, Wang G, Xian R, Li C, Wang S, Cui L. Association between Small Intestinal Bacterial Overgrowth and Subclinical Atheromatous Plaques. J Clin Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 129. | Ponziani FR, Pompili M, Di Stasio E, Zocco MA, Gasbarrini A, Flore R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J Gastroenterol. 2017;23:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 130. | Zhang Y, Liu G, Duan Y, Han X, Dong H, Geng J. Prevalence of Small Intestinal Bacterial Overgrowth in Multiple Sclerosis: a Case-Control Study from China. J Neuroimmunol. 2016;301:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Weinstock LB, Walters AS. Restless legs syndrome is associated with irritable bowel syndrome and small intestinal bacterial overgrowth. Sleep Med. 2011;12:610-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 132. | Kowalski K, Mulak A. Small intestinal bacterial overgrowth in Alzheimer's disease. J Neural Transm (Vienna). 2022;129:75-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 133. | Wang L, Yu YM, Zhang YQ, Zhang J, Lu N, Liu N. Hydrogen breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in autism. Eur Child Adolesc Psychiatry. 2018;27:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 134. | Vallès M, Antuori A, Mearin F, Serra J. Small intestinal bacterial overgrowth in spinal cord injury patients. Gastroenterol Hepatol. 2021;44:539-545. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 135. | Cheng X, Zhang L, Xie NC, Xu HL, Lian YJ. Association between small-intestinal bacterial overgrowth and deep vein thrombosis in patients with spinal cord injuries. J Thromb Haemost. 2017;15:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Li X, Feng X, Jiang Z. Association of small intestinal bacterial overgrowth with Parkinson's disease: a systematic review and meta-analysis. Gut Pathog. 2021;13:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 137. | Hasuike Y, Endo T, Koroyasu M, Matsui M, Mori C, Yamadera M, Fujimura H, Sakoda S. Bile acid abnormality induced by intestinal dysbiosis might explain lipid metabolism in Parkinson's disease. Med Hypotheses. 2020;134:109436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 138. | DiBaise JK, Crowell MD, Driver-Dunckley E, Mehta SH, Hoffman-Snyder C, Lin T, Adler CH. Weight Loss in Parkinson's Disease: No Evidence for Role of Small Intestinal Bacterial Overgrowth. J Parkinsons Dis. 2018;8:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 139. | Niu XL, Liu L, Song ZX, Li Q, Wang ZH, Zhang JL, Li HH. Prevalence of small intestinal bacterial overgrowth in Chinese patients with Parkinson's disease. J Neural Transm (Vienna). 2016;123:1381-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 140. | Feng X, Li XQ, Jiang Z. Prevalence and predictors of small intestinal bacterial overgrowth in systemic sclerosis: a systematic review and meta-analysis. Clin Rheumatol. 2021;40:3039-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 141. | Polkowska-Pruszyńska B, Gerkowicz A, Rawicz-Pruszyński K, Krasowska D. The Role of Fecal Calprotectin in Patients with Systemic Sclerosis and Small Intestinal Bacterial Overgrowth (SIBO). Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Polkowska-Pruszyńska B, Gerkowicz A, Szczepanik-Kułak P, Krasowska D. Small intestinal bacterial overgrowth in systemic sclerosis: a review of the literature. Arch Dermatol Res. 2019;311:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 143. | Sawadpanich K, Soison P, Chunlertrith K, Mairiang P, Sukeepaisarnjaroen W, Sangchan A, Suttichaimongkol T, Foocharoen C. Prevalence and associated factors of small intestinal bacterial overgrowth among systemic sclerosis patients. Int J Rheum Dis. 2019;22:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 144. | Jo JH, Park SJ, Cheon JH, Kim TI, Kim WH. Rediscover the clinical value of small intestinal bacterial overgrowth in patients with intestinal Behçet's disease. J Gastroenterol Hepatol. 2018;33:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 145. | Laroche JM, Kapel N, Benahmed N, Claudepierre P, Chevalier X, Barbot-Trystram L. High prevalence of small intestinal bacterial overgrowth (SIBO) in spondylarthropathy. Clin Exp Rheumatol. 2021;39:703. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 146. | Pimentel M, Wallace D, Hallegua D, Chow E, Kong Y, Park S, Lin HC. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann Rheum Dis. 2004;63:450-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 147. | Potskhverashvili ND, Zolnikova OY, Kokina NI, Dzhakhaya NL, Sedova AV, Bueverova EL, Trukhmanov AS. Small Bowel Bacterial Overgrowth Syndrome in Patients with Bronchial Asthma. Russ J Gastroenterol Hepatol Coloproctol. 2018;28:47-54. [DOI] [Full Text] |
| 148. | Song Y, Liu Y, Qi B, Cui X, Dong X, Wang Y, Han X, Li F, Shen D, Zhang X, Hu K, Chen S, Zhou J, Ge J. Association of Small Intestinal Bacterial Overgrowth With Heart Failure and Its Prediction for Short-Term Outcomes. J Am Heart Assoc. 2021;10:e015292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (2)] |
| 149. | Fadeeva MV, Skhirtladze MR, Ivashkin VT. Small Intestinal Bacterial Overgrowth Syndrome as a Risk Factor for Ventricular Tachycardia in Chronic Heart Failure with Left Ventricular Systolic Dysfunction. Russ J Gastroenterol Hepatol Coloproctol. 2019;29:38-48. [DOI] [Full Text] |
| 150. | Ma X, Wang H, Zhang P, Xu L, Tian Z. Association between small intestinal bacterial overgrowth and toll-like receptor 4 in patients with pancreatic carcinoma and cholangiocarcinoma. Turk J Gastroenterol. 2019;30:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 151. | Kim DB, Park CS, Paik CN, Kang YJ, Jo IH, Lee JM. Relationship between untreated obstructive sleep apnea and breath hydrogen and methane after glucose load. Saudi J Gastroenterol. 2022;28:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 152. | Nalyotov AV, Guz NP. Prevalence of Small Intestinal Bacterial Overgrowth Syndrome in Patients with Irritable Bowel Syndrome who Have Suffered COVID-19. Russ J Gastroenterol Hepatol Coloproctol. 2022;32:35-39. [DOI] [Full Text] |
| 153. | Fialho A, Fialho A, Schenone A, Thota P, McCullough A, Shen B. Association between small intestinal bacterial overgrowth and deep vein thrombosis. Gastroenterol Rep (Oxf). 2016;4:299-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 154. | Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, Parodi A, Savarino V. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 155. | Gravina A, Federico A, Ruocco E, Lo Schiavo A, Masarone M, Tuccillo C, Peccerillo F, Miranda A, Romano L, de Sio C, de Sio I, Persico M, Ruocco V, Riegler G, Loguercio C, Romano M. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United European Gastroenterol J. 2015;3:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 156. | Drago F, Ciccarese G, Indemini E, Savarino V, Parodi A. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57:112-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 157. | Majumdar A, Siva Venkatesh IP, Basu A. Short-Chain Fatty Acids in the Microbiota-Gut-Brain Axis: Role in Neurodegenerative Disorders and Viral Infections. ACS Chem Neurosci. 2023;14:1045-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (1)] |
| 158. | Anand N, Gorantla VR, Chidambaram SB. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 159. | Lewandowska-Pietruszka Z, Figlerowicz M, Mazur-Melewska K. The History of the Intestinal Microbiota and the Gut-Brain Axis. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 160. | Romero-Figueroa MDS, Ramírez-Durán N, Montiel-Jarquín AJ, Horta-Baas G. Gut-joint axis: Gut dysbiosis can contribute to the onset of rheumatoid arthritis via multiple pathways. Front Cell Infect Microbiol. 2023;13:1092118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 161. | Qaiyum Z, Lim M, Inman RD. The gut-joint axis in spondyloarthritis: immunological, microbial, and clinical insights. Semin Immunopathol. 2021;43:173-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 162. | Gracey E, Vereecke L, McGovern D, Fröhling M, Schett G, Danese S, De Vos M, Van den Bosch F, Elewaut D. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol. 2020;16:415-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 163. | Campbell C, Kandalgaonkar MR, Golonka RM, Yeoh BS, Vijay-Kumar M, Saha P. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 103] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 164. | Luo M, Xin RJ, Hu FR, Yao L, Hu SJ, Bai FH. Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis. World J Gastroenterol. 2023;29:144-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 165. | Won SM, Oh KK, Gupta H, Ganesan R, Sharma SP, Jeong JJ, Yoon SJ, Jeong MK, Min BH, Hyun JY, Park HJ, Eom JA, Lee SB, Cha MG, Kwon GH, Choi MR, Kim DJ, Suk KT. The Link between Gut Microbiota and Hepatic Encephalopathy. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 166. | Berthelot JM, Claudepierre P. Trafficking of antigens from gut to sacroiliac joints and spine in reactive arthritis and spondyloarthropathies: Mainly through lymphatics? Joint Bone Spine. 2016;83:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 167. | García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, Iraheta I. Reactive arthritis: update 2018. Clin Rheumatol. 2018;37:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 168. | Bajinka O, Tan Y, Darboe A, Ighaede-Edwards IG, Abdelhalim KA. The gut microbiota pathway mechanisms of diabetes. AMB Express. 2023;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 169. | Maestri M, Santopaolo F, Pompili M, Gasbarrini A, Ponziani FR. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: Effects of current treatments and future strategies. Front Nutr. 2023;10:1110536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 170. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Gut-liver axis in cirrhosis: Are hemodynamic changes a missing link? World J Clin Cases. 2021;9:9320-9332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (3)] |
| 171. | Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022;34:1700-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 329] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 172. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1249] [Article Influence: 249.8] [Reference Citation Analysis (1)] |