Published online Jun 7, 2023. doi: 10.3748/wjg.v29.i21.3269
Peer-review started: February 2, 2023
First decision: March 8, 2023
Revised: March 13, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 7, 2023
Processing time: 119 Days and 6 Hours
Alcoholism is regarded as a risk factor for vitamin B12 (VB12) deficiency. Because VB12 serves as a coenzyme of methylmalonyl-CoA mutase, a key enzyme in propionate metabolism, the 13C-propionate breath test (PBT) has been studied as a non-invasive diagnostic modality for VB12 deficiency. However, the conventional PBT requires 2 h, which is inconvenient in clinical practice. We hypothesized that a faster PBT can be used to evaluate propionate metabolism and is more easily adaptable for clinical practice.
To evaluate a faster PBT for assessing the effects of long-term ethanol consump
ERs were obtained by replacing standard drinking water (for control rats, CRs) with 16% ethanol solution in descendants of F344/DuCrj rats. Faster PBT was performed by administering 13C-propionate aqueous solution to male and female ERs and CRs by inserting a metal tubule from the mouth to the stomach; exhaled gas was collected in a bag to measure its 13CO2/12CO2 isotope ratio via infrared isotope spectrometry. Serum VB12 and alanine transaminase (ALT) levels were measured via chemiluminescence immunoassay and the lactate dehydrogenase-ultraviolet method, respectively. We evaluated statistical differences in mean body weight, change in 13CO2 (Δ13CO2‰), peak Δ13CO2‰, and serum VB12 and ALT, between males and females and between ERs and CRs using the t-test and Mann-Whitney U test for normally and non-normally distributed variables, respectively.
Males weighed significantly more than females (P < 0.001); CRs weighed significantly more than ERs (P < 0.008). Δ13CO2 reached a peak (Cmax) at 20 min and 30 min in females and males, respectively, decreasing after 20-30 min without rebound in all groups. Males had significantly higher Cmax and Δ13CO2 at 15-45 min than females (P < 0.05; for all pairs). Propionate metabolism was enhanced in male ERs relative to male CRs, whereas metabolism did not differ markedly between ERs and CRs for females. Males had higher serum VB12 levels than females, without prominent differences between the ER and CR groups. Male CRs had notably higher ALT levels than male ERs. Thus, chronic ethanol consumption may trigger fatty acid production via intestinal bacteria and changes in gut microbiome composition.
Faster PBT shows that 16% ethanol consumption promotes propionate metabolism without inducing liver injury. This PBT may be used clinically to evaluate gut flora status.
Core Tip: Alcoholism is a risk factor for vitamin B12 (VB12) deficiency. The 13C-propionate breath test (PBT) is a diagnostic modality for VB12 deficiency, but requires 2 h for completion. We applied a faster PBT to evaluate propionate metabolism using an ethanol-fed rat model. After 13C-propionate administration, the 13CO2/12CO2 isotope ratio of gas collected every 5 min for 60 min was measured using infrared isotope spectrometry. The Δ13CO2 peak occurred within 30 min. Ethanol-fed males showed marked propionate metabolism without associated liver injury. This study demonstrates the potential of the faster PBT to evaluate propionate metabolism under various clinical conditions.
- Citation: Sasaki Y, Kawagoe N, Imai T, Urita Y. Effects of ethanol and sex on propionate metabolism evaluated via a faster 13C-propionate breath test in rats. World J Gastroenterol 2023; 29(21): 3269-3279
- URL: https://www.wjgnet.com/1007-9327/full/v29/i21/3269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i21.3269
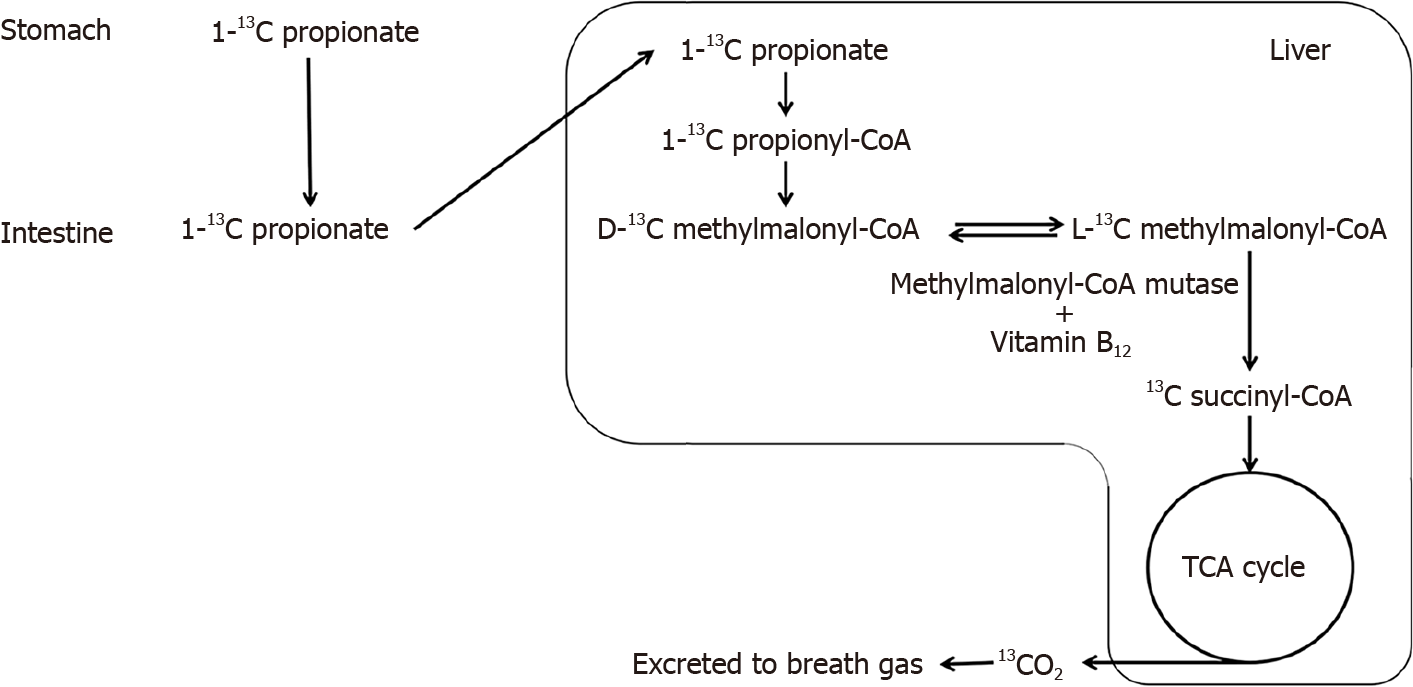
Chronic alcoholism is a risk factor for vitamin B12 (VB12) deficiency[1,2]. Because VB12 works as a coenzyme of methylmalonyl-CoA mutase, a key enzyme in propionate metabolism (Figure 1), the 13C-propionate breath test (PBT) has been studied as a non-invasive diagnostic modality for VB12 deficiency, with favorable results[3,4]. For instance, Wagner et al[3] reported that the conventional PBT could reliably predict VB12 deficiency in humans, with an area under the curve of the receiver operating curve value of 0.88. Propionate is a ubiquitous short chain fatty acid produced by intestinal bacteria, such as Phascolarctobacterium[5]. Emerging evidence suggests that intestinal microbial flora have a healing influence on alcoholic liver damage[6], and propionate produced by intestinal bacteria has protective effects against alcoholic liver damage[7]. Thus, we believe that PBT may provide important information not only regarding VB12deficiency, but also regarding alcohol metabolism and alcoholic liver damage.
However, the conventional PBT requires 2 h to complete, which can be highly inconvenient for patients in clinical settings. Thus, in the present study, we aimed to evaluate the potential of a faster PBT for assessing the effects of long-term ethanol consumption on propionate metabolism as well as VB12 deficiency using ethanol-fed rats (ERs) as an animal model of chronic alcoholism. As the protective effects of estrogen against VB12 deficiency have been reported[8], we also evaluated the effect of sex-related differences on propionate metabolism, as detected by the faster PBT.
All animal experiments were performed with approval of the Toho University School of Medicine, No. 21-51-4960. Descendants of F344/DuCrj rats purchased from CLEA Japan Inc. (Tokyo, Japan) for our previous study[9] were used to establish the ER and control rat (CR) groups for this study. All rats used in the present study were 18th-generation descendants of the originally established ER and CR groups, maintaining the lines within treatments (i.e., parents of ERs were ERs, parents of CRs were CRs).
All rats were housed with their mothers until weaning at 4 wk of age. Subsequently, all rats were individually housed in a controlled environment (temperature, 23 ± 2 °C; humidity, 55% ± 5%) and provided a standard diet (CE-7; CLEA Japan Inc., Tokyo, Japan) and drinking liquid ad libitum. In the ER group, a 16% ethanol solution (Japanese Sake, Ozeki Corporation, Hyogo, Japan) was provided as a substitute for water by replacing the content of water bottles with ethanol solution in all cages of ERs.
A total of 16 ERs (8 males and 8 females) and 16 CRs (8 males and 8 females) aged 27-30 wk were used in the experiments; ERs continuously consumed alcohol for 23-27 wk. We used available descendants of F344/DuCrj rats that we had utilized in previous studies[9]. Therefore, we did not perform sample size calculation, randomization, or blinding.
We purchased 1-13C-sodium propionate from Cambridge Isotope Laboratories (Andover, MA, United States) and prepared a 13C-propionate aqueous solution at 1 g/mL using distilled water immediately before administration. Body weight was measured immediately before administration. We performed gastrointestinal intubation in each rat and used a metal tubule, extending from the mouth to the stomach, to administer 0.1 mL/g of the 13C-propionate solution. Immediately after administration, the rats were individually placed in the chambers of a dedicated exhaled-gas collection machine consisting of sealed chambers, pumps, and collecting bags, designed by Uchida et al[10]. We collected 100-200 mL of exhaled gas in the collecting bag for 90 s every 5 min for a total of 60 min.
Because 13C-propionate is metabolized in the liver and exhaled as 13CO2 (Figure 1), we measured the
After collecting the exhaled gas for 60 min, the rats were immediately anesthetized via sevoflurane inhalation, and 5-10 mL of venous blood was collected from the inferior vena cava and the right atrium under laparotomy. After collecting sufficient blood samples, the animals were euthanized by rapid blood release. The blood was immediately centrifuged (relative centrifugal force: 1700 ×g) for 10 min, and the serum was collected. The serum was promptly frozen and submitted to FUJIFILM VET Systems Co. Ltd. (Tokyo, Japan) for measuring serum VB12 and ALT levels via chemiluminescence immunoassay and the lactate dehydrogenase-ultraviolet method, respectively.
We analyzed Δ13CO2‰ measured every 5 min after 13C-propionate administration for 60 min and serum VB12 levels, comparing the sexes and the ER and CR groups. The normality of the distribution of all variables was evaluated using the Kolmogorov-Smirnov test, and differences between groups were compared using the t-test and Mann-Whitney U test for normally and non-normally distributed variables, respectively. Statistical significance was set at P < 0.05. All statistical analyses were performed using Stata/IC software (version 15.1; Stata Corp., College Station, TX, United States). We used R 4.2.0 for construction of graphics[12]. The statistical methods were reviewed by Yosuke Sasaki from the Toho University School of Medicine (the first author). As Yosuke Sasaki has completed several certified biostatistics courses, we did not obtain additional biostatical review suggestions by external biomedical statisticians.
Body weight was significantly higher (P < 0.0001) in males (335.8 ± 37.0 g) than in females (176.6 ± 21.9 g). In addition, body weight was significantly higher in CRs than in ERs for both males and females (P = 0.0082 and P = 0.005, respectively, Table 1).
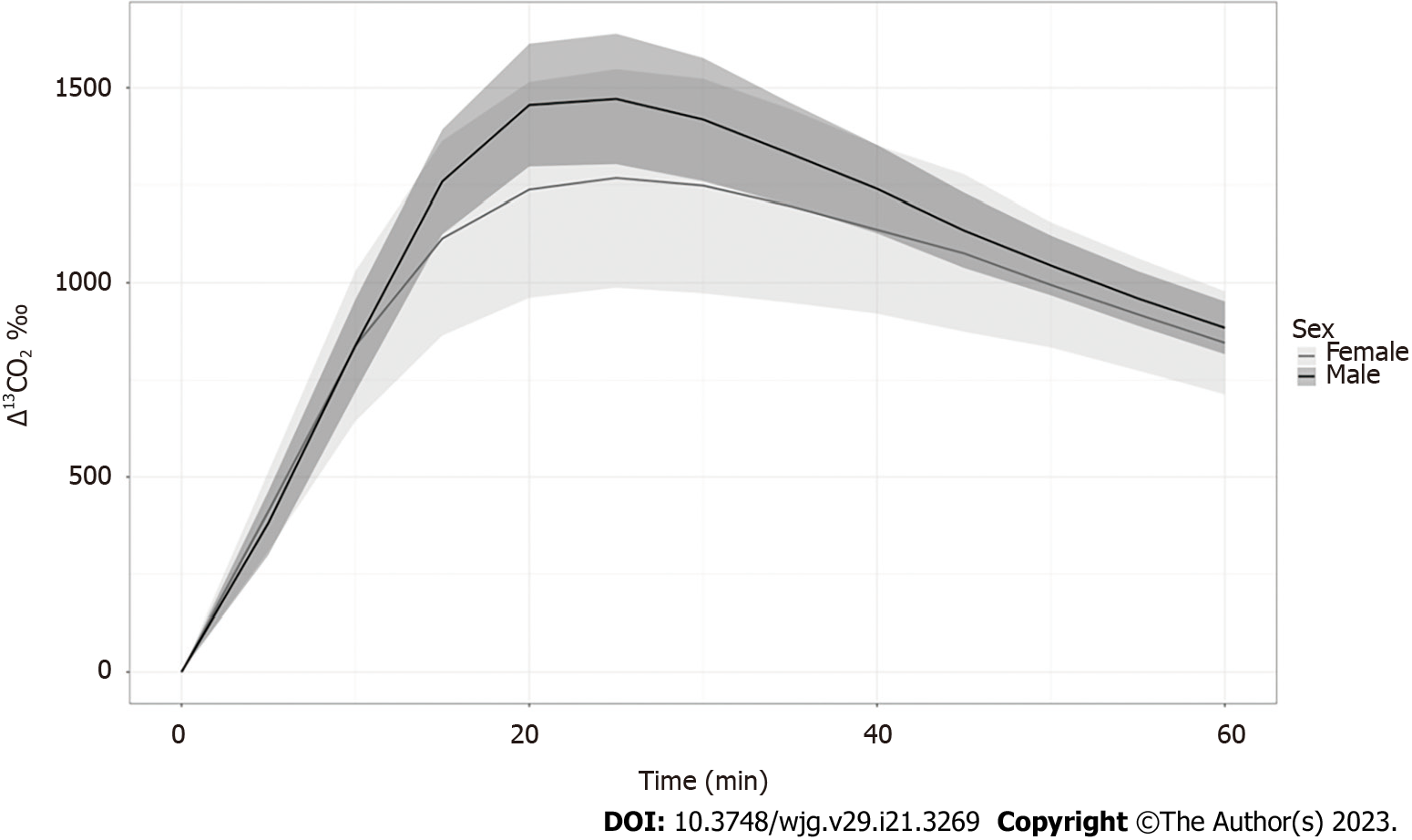
The Δ13CO2 reached its peak (Cmax) at 20 min and 30 min in females and males, respectively. The Δ13CO2 decreased after 20-30 min without rebound in both groups (Table 2 and Figure 2). Therefore, the overall trends in Δ13CO2 over time were similar between males and females, although Cmax was delayed in males and Δ13CO2 was significantly higher in males at 30 min and thereafter (Table 2 and Figure 2). The Cmax and Δ13CO2 values between 15 and 45 min were significantly higher in males than in females (P < 0.05, Table 2). Considering these sex-based differences, we further compared the effects of ethanol in males and females separately.
| Male (n = 16) | Female (n = 16) | P value | |
| Cmax | 1478.0 | 1302.3 | 0.039a |
| 5 min | 381.8 | 412.5 | 0.358 |
| 10 min | 838.6 | 837.4 | 0.983 |
| 15 min | 1259.2 | 1114.1 | 0.049a |
| 20 min | 1455.7 | 1238.2 | 0.011a |
| 25 min | 1471.3 | 1267.9 | 0.007a |
| 30 min | 1418.5 | 1248.2 | 0.008a |
| 35 min | 1330.6 | 1197.1 | 0.010a |
| 40 min | 1240.3 | 1136.5 | 0.013a |
| 45 min | 1134.5 | 1075.9 | 0.035a |
| 50 min | 1044.7 | 994.6 | 0.050 |
| 55 min | 959.9 | 918.8 | 0.083 |
| 60 min | 884.2 | 845.6 | 0.309 |
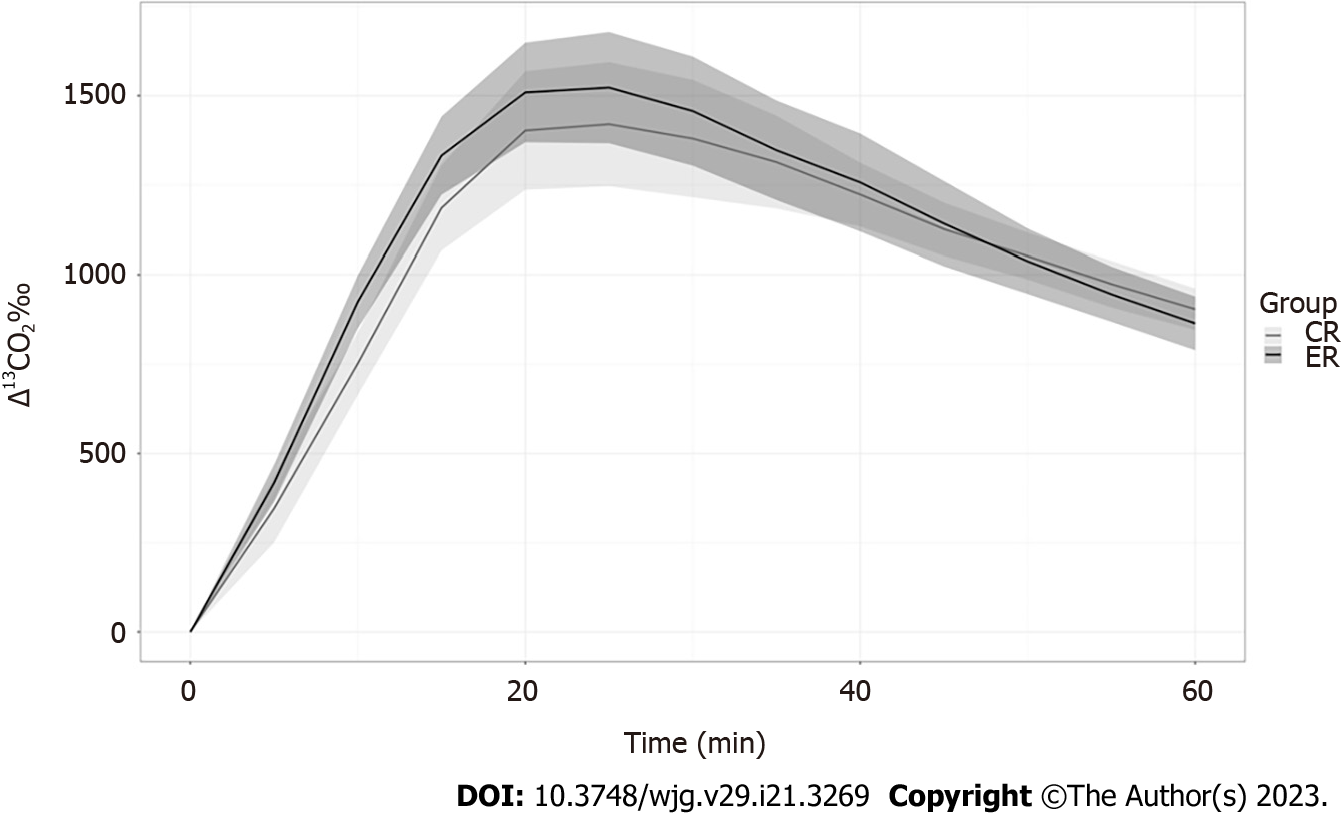
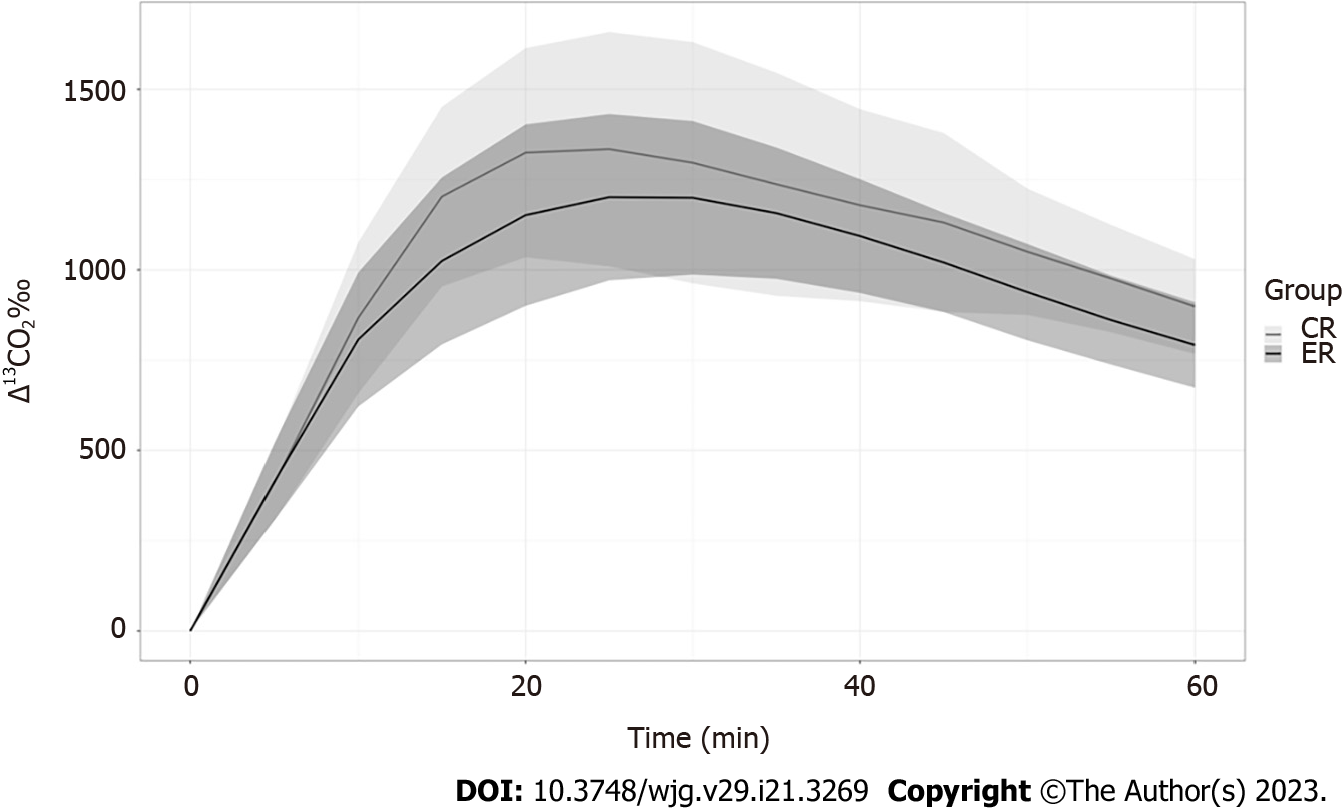
Propionate metabolism was accelerated in the ERs relative to that in the CRs in males (Figure 3), with Δ13CO2 at 10 and 20 min being markedly higher in male ERs. However, Δ13CO2 after 40 min was higher in the CR group (Table 3). The Δ13CO2 reached Cmax earlier in the ERs (at 20 min) than in the CRs (at 30 min). These findings suggest that ethanol promoted propionate metabolism in male rats. However, propionate metabolism was similar between the ER and CR groups among females, without any significant differences (P > 0.110 for all pairs, Table 3, Figure 4).
| Male | Female | |||||
| CR (n = 8) | ER (n = 8) | P value | CR (n = 8) | ER (n = 8) | P value | |
| Cmax | 1422.8 | 1533.1 | 0.192 | 1366.1 | 1238.5 | 0.401 |
| 5 min | 345.8 | 417.9 | 0.080 | 410.8 | 414.2 | 0.950 |
| 10 min | 752.3 | 924.9 | 0.0008a | 867.6 | 807.3 | 0.552 |
| 15 min | 1186.1 | 1332.5 | 0.023a | 1203.2 | 1025 | 0.160 |
| 20 min | 1402.3 | 1509.1 | 0.186 | 1324.7 | 1151.7 | 0.223 |
| 25 min | 1402.1 | 152.6 | 0.235 | 1334.5 | 1201.4 | 0.462 |
| 30 min | 1379.9 | 1457 | 0.348 | 1296.8 | 1199.7 | 0.753 |
| 35 min | 1313.9 | 46 | 0.629 | 1237.2 | 1157.1 | 0.753 |
| 40 min | 1223.2 | 1257.3 | 0.565 | 1179.2 | 1093.8 | 0.529 |
| 45 min | 1126.6 | 1142.3 | 0.756 | 1131.3 | 1020.6 | 0.208 |
| 50 min | 1051.8 | 1037.5 | 0.724 | 1050.4 | 938.7 | 0.172 |
| 55 min | 974.2 | 945.6 | 0.436 | 976.4 | 861.3 | 0.142 |
| 60 min | 904.1 | 864.4 | 0.256 | 898.9 | 792.3 | 0.110 |
The serum VB12 levels were significantly higher in males than in females (P = 0.0013, Table 4); however, no significant differences were observed between the ER and CR groups for either sex (P > 0.05 for all pairs, Table 4). In contrast, serum ALT levels were significantly higher in male CRs than in male ERs (P = 0.0347, Table 5).
In this study, we compared propionate metabolism using a faster PBT in rats and compared serum VB12 and ALT levels between males and females and between ER and CR groups. Overall, our study demonstrates that (1) The faster PBT is useful for evaluating differences in propionate metabolism after administration of a 13C-propionate solution; (2) Males show greater propionate metabolism, with higher serum VB12 levels, than females; (3) Ethanol consumption promotes propionate metabolism in male rats only; and (4) Ethanol consumption reduces body weight and serum ALT levels.
In the faster PBT, Δ13CO2 peaked at 30 min, then decreased over time without rebound in all groups. Accordingly, we consider the faster PBT, which is completed within only 60 min after 13C propionate administration, to be sufficiently sensitive to evaluate propionate metabolism, as a substitute for the conventional PBT that requires collecting exhaled gas for 2 h.
Using the PBT, our study showed a higher Cmax and Δ13CO2 between 15 and 45 min in male rats than in female rats, which suggests that male rats have stronger propionate metabolism. Suppression of carbohydrate metabolism and promotion of lipid metabolism by estrogen in females have been proposed as mechanisms contributing to lower carbohydrate metabolism in females than in males[13]. Furthermore, a protective effect of estrogen against VB12 deficiency in fertile females has been reported, along with higher susceptibility to VB12 deficiency in postmenopausal women[8,14]. Considering that VB12 works as a coenzyme of methylmalonyl-CoA mutase, and that serum VB12 levels were not pathologically low in the rats used in our study, we postulate that the lower propionate metabolism detected by the faster PBT and the lower serum VB12 levels in females than in males may reflect underlying physiological sex-related differences in carbohydrate metabolism associated with estrogen.
As we aimed to use the faster PBT to evaluate impaired propionate metabolism due to VB12 deficiency and liver disease caused by chronic alcohol consumption, we expected to find lower propionate metabolism and higher serum ALT levels in the ER group than in the CR group. However, we obtained contrasting results, with acceleration of propionate metabolism in the ER group and higher serum ALT levels in the CR group. Changes in the gut flora caused by chronic alcohol consumption may explain the promotion of propionate metabolism in the ER group. Using male marmosets, Zhu et al[15] reported that the concentrations of short-chain fatty acids, including propionate, depend on changes in intestinal bacteria, based on an observed reduction in fecal propionate levels along with a reduction in the relative abundance of Phascolarctobacterium in the gut. Moreover, Watanabe et al[5] reported that the substrates of short-chain fatty acids, including propionate, produced by intestinal bacteria depend not only on a single bacterial strain, but also on the specific composition of other bacteria present in the gut. According to these reports, ethanol can serve as both a potential substrate of fatty acid production by intestinal bacteria, such as Phascolarctobacterium, and as a trigger for changes in gut flora. Thus, we hypothesized that chronic alcohol consumption promotes propionate production both as a substrate for various fatty acids and as a trigger for changes in gut flora.
Alternatively, these observations may be due to the well-known difficulties in recapitulating the effects of chronic alcohol consumption in an animal model. We intended to establish a rat model of chronic alcoholism to evaluate the metabolic effect of ethanol consumption by oral administration of a 16% ethanol solution (corresponding to the level of alcohol commonly consumed by Japanese drinkers in the form of sake) for > 20 wk based on a previous study[16]. Therefore, we expected higher serum ALT levels in the ER group. Our contrasting result (lower serum ALT after 16% ethanol consumption) highlights the difficulty in the development of alcoholic animal models. A recent review on the utility of animal models for alcoholic liver disease mentioned that, in contrast to primates, rodent models fail to sufficiently display the full disease spectrum of alcoholic liver disease found in humans, despite many trials under various conditions[17]. The absence of craving in rats, owing to their natural aversion to ethanol[18,19], the faster ethanol catabolism in rodents than in humans[20], and differences in the innate immune systems of the species, have been proposed as the main factors contributing to the difficulty in establishing a useful rat model of human alcoholism[21]. It is therefore possible that our results also reflect failure to generate a chronic alcoholism rat model; thus, studies using primates or other small animals rather than rodents may be more appropriate. Considering the ad libitum diet administration, and the higher body weight in the CR group, fatty liver due to excessive dietary intake may explain the higher serum ALT levels in CRs. Because all of the rats consumed the same diet, it is possible that consumption of 16% ethanol solution had a protective effect against liver damage. Given that propionate itself reportedly has protective effects against steatohepatitis[7], and that improvement of gut flora is an effective way to suppress liver damage[6], enhanced propionate metabolism and favorable changes in the gut flora might suppress liver damage in male ERs. As discussed earlier, the lack of acceleration in propionate metabolism in female ERs can be explained by sex-related differences in carbohydrate metabolism.
In addition to the lack of confirmation of the chronic alcoholism model, our study has other limitations. For instance, the serum methylmalonic acid (MMA) level, rather than the serum VB12 level, is required for the precise diagnosis of VB12 deficiency in humans[3]. However, we were not able to evaluate MMA levels because major domestic commercial laboratories no longer perform MMA testing of human serum or urine, and we could not find or access domestic laboratories measuring serum MMA in animal samples. Similarly, comparing the PBT results with biomarkers, such as aldehyde dehydrogenase and alcohol dehydrogenase, which sensitively and precisely reflect hepatic alcohol metabolism, may provide more information[22]. We believe that comparing levels of serum MMA and the markers evaluated using the faster PBT may provide further insight into the association between VB12 deficiency and alcoholism. Moreover, the present study only focused on the association between propionate metabolism and VB12 deficiency based on a previous study on PBT[3]. However, considering the complexity of intestinal propionate production due to the variety of propionate-producing bacteria, including Clostridium spp., Veillonella spp., Fusobacterium spp., Salmonella ruminantium, and Propionibacterium spp., and the complexity of substrates[23], the findings obtained herein, including the promoted propionate metabolism in male ERs and sex-related difference, may have potential clinical utility and provide a basis for future research into propionate metabolism and intestinal microbiota under various conditions. For instance, comparison of findings between faster PBT and the composition or changes in gut microbiota may provide interesting information on the association between gut microbiota and their products. Despite these limitations and lack of confirmation of VB12 deficiency under our experimental conditions, our study highlights the influence of ethanol and sex-related differences in propionate metabolism.
We evaluated a faster PBT in which Cmax peaked within 30 min. This PBT could serve as a substitute for conventional PBT (which takes at least 2 h) for evaluating propionate metabolism and diagnosing VB12 deficiency. Although we could not evaluate the usefulness of faster PBT as a diagnostic modality for VB12 deficiency as initially intended because we failed to create a rat alcoholism model with VB12 deficiency, our study suggests that chronic consumption of 16% ethanol changed the composition of fatty acids produced by the intestinal flora, likely by changing the intestinal flora composition without causing corresponding liver injury. Considering the accumulating evidence of alteration of the gut flora as one of the mechanisms of alcoholism-related health impacts[24], our study demonstrates the potential utility of the faster PBT as a non-invasive and more convenient modality to evaluate changes in the gut flora associated with ethanol consumption and various other conditions.
The 13C-propionate breath test (PBT) has been studied as a non-invasive diagnostic modality for vitamin B12 (VB12) deficiency by utilizing the role of VB12 as a coenzyme of methylmalonyl-CoA mutase in propionate metabolism. Although alcoholism has been regarded as a risk factor for deficiency, studies on propionate metabolism using the PBT in individuals with alcoholism is limited. Furthermore, conventional PBT requires up to 2 hours of breath collection time, which may undermine its clinical utility.
The scarcity of studies regarding the PBT in alcoholism, and the possibility of improving the clinical utility of the PBT by shortening the breath collection time, motivated us to perform this study.
The aim of this study was to evaluate the change in propionate metabolism due to long-term ethanol consumption in ethanol-fed rats (ERs) as an animal model of chronic alcoholism. We also aimed to evaluate the utility of a faster PBT that requires only 1 hour to collect breath.
The ERs were 18th generation descendants of F344/DuCrj rats that had been bred by replacing standard drinking water with a 16% ethanol solution. A faster PBT was performed by injecting the 13C-propionate aqueous solution from the mouth to the stomach of ERs and control rats (CRs); we collected exhaled gas in bags, and measured the 13CO2/12CO2 isotope ratio using infrared isotope spectrometry. We measured serum VB12 and alanine transaminase (ALT) levels via chemiluminescence immunoassay and the lactate dehydrogenase-ultraviolet method, respectively. We evaluated statistical differences in mean body weight, change in 13CO2 (Δ13CO2‰), peak Δ13CO2‰, and serum VB12 and ALT, between ERs and CRs, and males and females, respectively.
Besides male dominance of body weight (P < 0.001), CRs weighed significantly more than ERs (P < 0.008). The Δ13CO2 reached a peak (Cmax) within 30 min in both sex groups, while males had a significantly higher Cmax and Δ13CO2 at 15-45 min than females (P < 0.05; for all pairs). Enhanced propionate metabolism was observed in male ERs relative to male CRs, and although males had higher serum VB12 levels than females, no prominent differences were observed between the ER and CR groups. Male CRs had notably higher ALT levels than male ERs. These results suggest that chronic ethanol consumption may trigger fatty acid production via intestinal bacteria and changes in gut microbiome composition.
We believe that a faster (1-h) PBT could serve as a substitute for the conventional PBT, as the Δ13CO2 reached a peak (Cmax) within 30 min in both sex groups. We failed to evaluate the usefulness of the faster PBT as a diagnostic modality for VB12 deficiency in the chronic alcoholism rat model; however, our study suggests that instead of inducing alcoholism, chronic consumption of 16% ethanol changed the composition of fatty acids produced by the intestinal flora.
Our study demonstrates the potential utility of the faster PBT as a non-invasive and more convenient modality to evaluate changes in the gut flora associated with ethanol consumption and various other conditions via changes in propionate metabolism.
We thank Ms. Mitsuko Sato for her contributions to this study in a wide variety of areas, including the breeding of rats and the preparation of and participation in experiments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baryshnikova NV, Russia; Wang Z, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Lambert D, Benhayoun S, Adjalla C, Gélot MM, Renkes P, Gérard P, Felden F, Belleville F, Gaucher P, Guéant JL, Nicolas JP. Alcoholic cirrhosis and cobalamin metabolism. Digestion. 1997;58:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Han KM, Chang HS, Choi IK, Ham BJ, Lee MS. CYP2D6 P34S Polymorphism and Outcomes of Escitalopram Treatment in Koreans with Major Depression. Psychiatry Investig. 2013;10:286-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Wagner DA, Schatz R, Coston R, Curington C, Bolt D, Toskes PP. A new 13C breath test to detect vitamin B12 deficiency: a prevalent and poorly diagnosed health problem. J Breath Res. 2011;5:046001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Manoli I, Pass AR, Harrington EA, Sloan JL, Gagné J, McCoy S, Bell SL, Hattenbach JD, Leitner BP, Duckworth CJ, Fletcher LA, Cassimatis TM, Galarreta CI, Thurm A, Snow J, Van Ryzin C, Ferry S, Mew NA, Shchelochkov OA, Chen KY, Venditti CP. 1-(13)C-propionate breath testing as a surrogate endpoint to assess efficacy of liver-directed therapies in methylmalonic acidemia (MMA). Genet Med. 2021;23:1522-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Watanabe Y, Nagai F, Morotomi M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl Environ Microbiol. 2012;78:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Meroni M, Longo M, Dongiovanni P. Alcohol or Gut Microbiota: Who Is the Guilty? Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 7. | Xu Q, Zhang R, Mu Y, Song Y, Hao N, Wei Y, Wang Q, Mackay CR. Propionate Ameliorates Alcohol-Induced Liver Injury in Mice via the Gut-Liver Axis: Focus on the Improvement of Intestinal Permeability. J Agric Food Chem. 2022;70:6084-6096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 8. | Margalit I, Cohen E, Goldberg E, Krause I. Vitamin B12 Deficiency and the Role of Gender: A Cross-Sectional Study of a Large Cohort. Ann Nutr Metab. 2018;72:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Kawagoe N, Kijma S, Tanaka H, Takemoto I, Suzuki K, Saito T, Komatsu F, Yamada A, Kumade E, Sasaki Y, Maeda T, Kido H, Ishii T, Watanabe T, Miyazaki T, Hike N, Zai H, Urita Y, Nakajima H, Arai K, Imai T. Alteration of Breath Hydrogen and Methane in Ethanol-Fed Rats. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2016;51:403-413. [PubMed] |
| 10. | Uchida M, Endo N, Shimizu K. Simple and noninvasive breath test using 13C-acetic acid to evaluate gastric emptying in conscious rats and its validation by metoclopramide. J Pharmacol Sci. 2005;98:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kawagoe N, Kano O, Kijima S, Tanaka H, Takayanagi M, Urita Y. Investigation of Metabolism of Exogenous Glucose at the Early Stage and Onset of Diabetes Mellitus in Otsuka Long-Evans Tokushima Fatty Rats Using [1, 2, 3-13C]Glucose Breath Tests. PLoS One. 2016;11:e0160177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. [cited 14 January 2023]. Available from: https://www.R-project.org/. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Tarnopolsky MA, Ruby BC. Sex differences in carbohydrate metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Miki A, Kinno R, Ochiai H, Kubota S, Mori Y, Futamura A, Sugimoto A, Kuroda T, Kasai H, Yano S, Hieda S, Kokaze A, Ono K. Sex Differences in the Relationship of Serum Vitamin B1 and B12 to Dementia Among Memory Clinic Outpatients in Japan. Front Aging Neurosci. 2021;13:667215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Zhu L, Suhr Van Haute MJ, Hassenstab HR, Smith C, Rose DJ, Mustoe AC, Benson AK, French JA. Fecal Short-Chain Fatty Acid Concentrations Increase in Newly Paired Male Marmosets (Callithrix jacchus). mSphere. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 221] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Lamas-Paz A, Hao F, Nelson LJ, Vázquez MT, Canals S, Gómez Del Moral M, Martínez-Naves E, Nevzorova YA, Cubero FJ. Alcoholic liver disease: Utility of animal models. World J Gastroenterol. 2018;24:5063-5075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (4)] |
| 18. | Brandon-Warner E, Schrum LW, Schmidt CM, McKillop IH. Rodent models of alcoholic liver disease: of mice and men. Alcohol. 2012;46:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 19. | Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc. 2013;8:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 903] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 20. | Holmes RS, Duley JA, Algar EM, Mather PB, Rout UK. Biochemical and genetic studies on enzymes of alcohol metabolism: the mouse as a model organism for human studies. Alcohol Alcohol. 1986;21:41-56. [PubMed] |
| 21. | Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2276] [Cited by in RCA: 2709] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 22. | Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245-254. [PubMed] |
| 23. | Eş I, Khaneghah AM, Hashemi SMB, Koubaa M. Current advances in biological production of propionic acid. Biotechnol Lett. 2017;39:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol Res. 2015;37:223-236. [PubMed] |