Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3185
Peer-review started: February 22, 2023
First decision: March 18, 2023
Revised: April 6, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: May 28, 2023
Processing time: 92 Days and 23.7 Hours
Irritable bowel syndrome (IBS) is the most prevalent gastrointestinal disorder in developed countries and reduces patients’ quality of life, hinders their ability to work, and increases health care costs. A growing number of trials have demonstrated an aberrant gut microbiota composition in IBS, also known as ‘gut dysbiosis’. Fecal microbiota transplantation (FMT) has been suggested as a treatment for IBS.
To assess the efficacy and safety of FMT for the treatment of IBS.
We searched Cochrane Central, MEDLINE, EMBASE and Web of Science up to 24 October 2022 for randomised controlled trials (RCTs) investigating the effectiveness of FMT compared to placebo (including autologous FMT) in treating IBS. The primary outcome was the number of patients with improvements of symptoms measured using a validated, global IBS symptoms score. Secondary outcomes were changes in quality-of-life scores, non-serious and serious adverse events. Risk ratios (RR) and corresponding 95%CI were calculated for dichotomous outcomes, as were the mean differences (MD) and 95%CI for continuous outcomes. The Cochrane risk of bias tool was used to assess the quality of the trials. GRADE criteria were used to assess the overall quality of the evidence.
Eight RCTs (484 participants) were included in the review. FMT resulted in no significant benefit in IBS symptoms three months after treatment compared to placebo (RR 1.19, 95%CI: 0.68-2.10). Adverse events were reported in 97 participants in the FMT group and in 45 participants in the placebo group (RR 1.17, 95%CI: 0.63-2.15). One serious adverse event occurred in the FMT group and two in the placebo group (RR 0.42, 95%CI: 0.07-2.60). Endoscopic FMT delivery resulted in a significant improvement in symptoms, while capsules did not. FMT did not improve the quality of life of IBS patients but, instead, appeared to reduce it, albeit non significantly (MD -6.30, 95%CI: -13.39-0.79). The overall quality of the evidence was low due to moderate-high inconsistency, the small number of patients in the studies, and imprecision.
We found insufficient evidence to support or refute the use of FMT for IBS. Larger trials are needed.
Core Tip: We did not find evidence to support the use of fecal microbiota transplantation (FMT) for irritable bowel syndrome (IBS) patients outside of clinical trials in this systematic review and meta-analysis. We report possible beneficial effects when FMT is delivered by endoscopy (colonoscopy or gastroscopy). FMT appears to be safe compared to placebo in patients with IBS, regardless of route of administration. Further randomised clinical trials are necessary to clarify the effect, if any, of FMT in IBS.
- Citation: Halkjær SI, Lo B, Cold F, Højer Christensen A, Holster S, König J, Brummer RJ, Aroniadis OC, Lahtinen P, Holvoet T, Gluud LL, Petersen AM. Fecal microbiota transplantation for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. World J Gastroenterol 2023; 29(20): 3185-3202
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3185.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3185
Irritable bowel syndrome (IBS) is the most prevalent gastrointestinal disorder in developed countries, affecting around 11% of the adult population[1]. The condition reduces patients’ quality of life, hinders their ability to work, and increases health care costs[2,3]. A diagnosis of IBS is based on symptoms, assessed using the Rome criteria, that include abdominal pain and altered bowel habits combined with the absence of organic or structural causes[4]. The criteria have changed over time and the most recent are the Rome IV criteria[5]. IBS can be sub-categorised as diarrhoea-predominant, constipation-predominant, mixed, or unclassified[5]. In most patients, IBS is chronic, with symptoms that fluctuate over time.
The pathogenic mechanisms underlying IBS remain more or less unknown. Genetics[6,7], dietary habits[8], post-infectious conditions[9] and psychological mechanisms[10] are all suspected to be involved. In recent years an increasing number of trials have demonstrated an aberrant gut microbiota composition in IBS[11-14], although not all trials report this aberration and descriptions of it vary between studies[15]. The microbial pathophysiology of IBS remains unknown.
Treating IBS poses a challenge; the syndrome probably represents a heterogeneity of disease mechanisms, which makes it difficult to develop effective therapeutic strategies[16]. Understanding the causes of gut dysbiosis in IBS is crucial[17]. Some trials indicate that probiotics and prebiotics can reduce the symptoms of IBS[18,19]. Fecal microbiota transplantation (FMT) might be an effective therapeutic intervention in IBS[16,20].
FMT is the transfer of stool from a healthy donor to a patient[21]. FMT has been described as far back as the fourth century in China[22]. In modern times, the first published FMT treatment is from 1958, when it was used successfully in four patients with pseudomembranous colitis[23]. Pseudomembranous colitis is now known to be caused by Clostridioides difficile infection (CDI). Based on subsequent placebo-controlled studies, FMT is now accepted in daily clinical practice for the treatment of recurrent CDI[24]. In addition, FMT is being investigated as a treatment option in a range of other diseases, e.g., metabolic syndrome, inflammatory bowel diseases, hepatic encephalopathy and multiple sclerosis[25]. The most promising results with FMT, apart from treating recurrent CDI, are for the treatment of inflammatory bowel disease[26-28].
FMT donors can be healthy relatives or anonymous donors. The advantages of the latter are the possibility of selecting donors with a high microbiota diversity and to store screened donor stool in freezers, to be made use of for multiple patients[29]. A European consensus report recommends that donors are chosen based on detailed information about illnesses with a presumed link to intestinal dysbiosis and rigorous testing of faecal and blood samples to avoid the transfer of infectious diseases[30].
FMT can be delivered in several ways, including through upper or lower endoscopic procedures, or by a gastro-duodenal or a rectal tube[31]. Additionally, capsules can release the stool in the small intestines and have been used successfully for the treatment of CDI[32-34]. In the treatment of recurrent CDI, the highest cure rates have been reported with repeated treatments delivered through lower endoscopy[35]; FMT has proven highly effective and patients are willing to undergo the treatment[36].
The microbial pathophysiology of IBS is not clearly understood, as microbiota alterations in IBS could either be a cause of the disease or a consequence of intestinal secretion and motility altered by IBS[37]. The prevailing hypothesis is that FMT might correct the dysbiosis associated with IBS[38,39], leading to a reversal or improvement of symptoms. Gut dysbiosis in IBS is characterised by a lower diversity of bacteria in the microbiota and abnormal proportions of specific bacteria as compared to the microbiota of healthy individuals[37,40]. In IBS and in other patient groups, FMT has resulted in increased bacterial diversity[41,42] and the coexistence of donor and recipient microbiota strains up to one year after treatment[43-45]. However, this is a new and developing field of study and the long-term effects of FMT on the microbiota remain largely unknown, not least of all because donor stools contain many things other than bacteria.
There is increasing evidence for a connection between gut dysbiosis and IBS[46,47]. The administration of FMT by various methods has been described in published case reports and abstracts, as compiled in an earlier review[48]. A number of smaller trials have examined the effect of FMT on IBS specifically[49-57], and several randomised controlled trials (RCTs), using different methods of administration, have been published with mixed results[43,44,58-63]. The effect of FMT can be difficult to assess due to the absence of reliable outcome measures and high placebo response rates[64]. The short- and long-term safety of FMT in patients with IBS is currently unclear.
The objectives of this systematic review were to examine the benefits and harms of FMT vs placebo (including autologous FMT, i.e., a participant’s own faecal material) for the treatment of patients with IBS.
We conducted a systematic review and meta-analysis following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions[65]. The systematic review was registered a priori as a protocol[66].
We included RCTs comparing FMT to placebo for the treatment of IBS, regardless of publication status and language of publication. For cross-over trials only data from the first intervention were used. For multi-arm trials only the data from intervention groups relevant to the review were used. We excluded trials with quasi-random designs and cluster RCTs. Trials with mixed disease populations were excluded.
Trials were included if their participants were diagnosed with IBS by a physician or according to accepted, symptom-based diagnostic criteria, such as the Rome III or IV criteria[67] (Supple
FMT could be administered in different ways and at different frequencies as there was no standardised procedure. Therefore, we included trials irrespective of FMT procedure, in terms of the quantity of faeces used, the form of faeces (fresh or frozen), the route of administration, the frequency of treatment (i.e., single vs multiple infusions) and donor selection (relatives or not). Only trials that used the whole gut microbiome from the donor were included. Trials that used a placebo, or autologous FMT as a placebo, were included. Trials that used selective microbial communities were excluded.
The primary outcome was the proportion of patients experiencing an improvement of symptoms (patient-reported), as measured by a validated, global IBS symptoms score (e.g., IBS severity scoring system), as defined by each trial’s organisers.
Secondary outcomes were the change in quality of life, as measured by a validated quality of life assessment, e.g., IBS-specific quality-of-life (IBS-QoL), the proportion of patients with non-serious adverse events and serious adverse events according to International Conference on Harmonization-Good Clinical Practice, and dropouts due to adverse events. Outcomes were measured after three and six months.
We searched Cochrane Central, MEDLINE, EMBASE and Web of Science. No language or publication date restrictions were applied to the searches. The detailed search strategy is provided in Supplement
We searched the following sources from the inception of each database up until 24 October 2022 and placed no restrictions on the language of publication (Supplementary Table 2): Cochrane Central (via the Ovid Evidence-Based Medicine Reviews Database, from inception); MEDLINE (via Ovid from 1946); and EMBASE (via Ovid from 1974).
We also searched for ongoing trials on ClinicalTrials.gov (https://clinicaltrials.gov/) and the World Health Organisation International Clinical Trials Registry Platform (https://trialsearch.who.int/).
The reference lists of all trials identified were then scanned for additional relevant trials. We also contacted the first authors of published and ongoing trials to request recent data or additional data, as needed.
Two independent authors performed the study selection (BL, SIH). Disagreements were resolved by consensus using a third author (AMP). The search results were first screened by title and abstract and subsequently excluded if found non-relevant; the remaining results were screened by full text. Data were extracted independently by two investigators (BL, SIH). Any discrepancies were resolved by consensus using a third author (LLG). An attempt to contact the corresponding author by e-mail was made if data were not available.
A data extraction protocol was developed based on the Cochrane Consumers and Communication Review Group’s data and results template and refined accordingly[68]. The following information was extracted from each trial: (1) Author, year of publication, trial design, and study site (country); (2) the mean or median (SD or IQR) change in symptoms, as measured by IBS scoring systems, at the end of the trial; (3) the mean or median (SD or IQR) change in quality of life, as measured by IBS quality of life scoring systems; (4) treatment description (including route of administration, mixed or single donor and fresh or frozen transplant); (5) reported non-serious adverse events and serious adverse events; and (6) dropouts due to adverse events.
The risk of bias was independently assessed by two investigators (BL, FC) using the Cochrane risk of bias tool[69] and the following seven domains were assessed: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias (Supplementary Table 3).
The risk of bias for each domain was rated as either ‘high’, ‘unclear’ or ‘low’. We classified the overall risk of bias in the trials as low if all the bias domains were classified as being at low risk of bias; we classified the overall risk as high if one or more of the bias domains were classified as having an unclear or high risk of bias. Any disagreement was solved by consensus using a third author (LLG).
We compared the fixed-effects and random-effects estimates of the intervention effect. If the estimates were similar, we assumed that any small-study effects had a minimal impact on the intervention effect estimate. If the random-effects estimate showed a larger statistical effect, we re-evaluated whether it was reasonable to conclude that the intervention was more effective in the smaller trials. If the larger trials appeared to be conducted with greater methodological rigour, or were conducted in circumstances more typical of the use of the intervention in practice, we reported the results of meta-analyses only from the larger trials.
Based on predictable clinical heterogeneity, we expected that several analyses would show, at a minimum, moderate heterogeneity (I2 > 30%). For random-effects models precision decreases, and confidence intervals widen, with increasing heterogeneity. We therefore expected the random-effects model would provide the most conservative (and thus a more accurate) estimate of the intervention effect. As such, we planned to report the results of our analyses based on meta-analyses of random-effects models.
We conducted a number of subgroup analyses: fresh vs frozen FMT; quantity of FMT; route of administration (upper gastrointestinal tract (e.g., capsulated, nasogastric, nasoduodenal, gastric tube) vs colonic (e.g., rectal)); type of donor (single vs mixed); frequency of administration (single vs multiple); IBS subtypes (diarrhoea-predominant, constipation-predominant, or mixed type).
We combined data from individual trials for meta-analysis when the interventions, patient groups, and outcomes were sufficiently similar, using the Review Manager version 5.4.1. Risk ratios (RR) were calculated for dichotomous outcomes with 95%CI. For continuous outcomes, we calculated the mean difference (MD) if all studies reported their outcomes using the same scale, and standardised MD with 95%CI if the studies used different scales to report their outcomes. We extracted data for all randomised participants and all participants with missing outcome data. Missing data were described, including dropouts and reasons for dropout, as reported by the authors.
Heterogeneity was assessed through a systematic examination of forest plots and quantified by calculating I2 values. The classification of heterogeneity levels was established using the subsequent thresholds: 0%-40% (insignificant), 40%-60% (moderate), 60%-80% (substantial), and > 80% (considerable). Additionally, the P value for the chi-squared test was included in the evaluation[66].
The outcomes reported in protocols were compared with published trial reports. In addition, for direct meta-analyses with at least 10 randomised clinical trials, we assessed reporting biases through regression analyses and visual inspection of funnel plots from the pairwise meta-analyses.
We used the GRADE approach to evaluate the overall certainty of the evidence and we followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions[65]. We classified the certainty of evidence as ‘high’, ‘moderate’, ‘low’, or ‘very low’.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect.
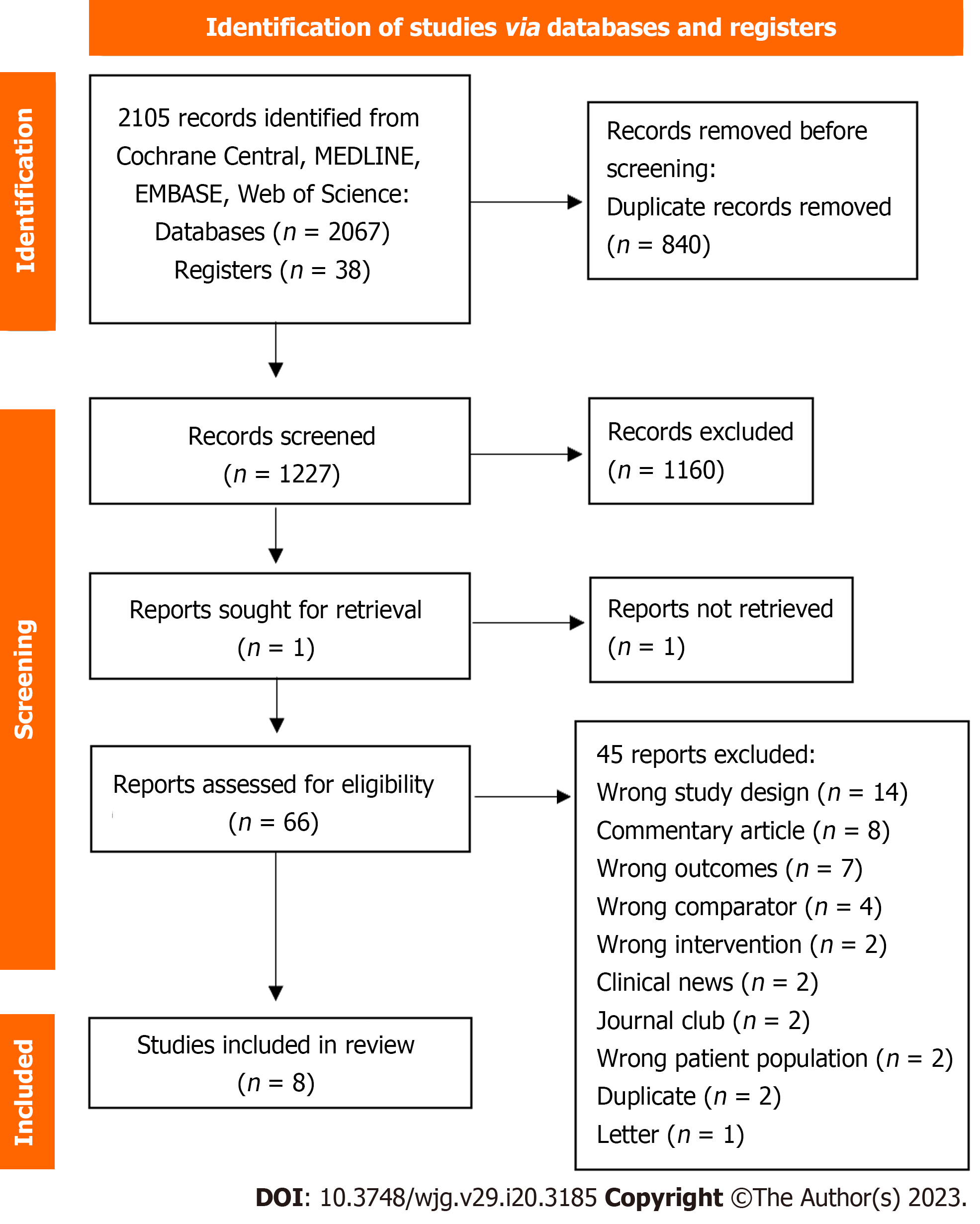
A search conducted on 24 October 2022 identified 2067 records, which were imported for screening into the computer program Covidence (https://www.covidence.org/). Of these records, 840 were removed as duplicates. We screened the titles and abstracts of the remaining 1227. We excluded 1160 reports as non-relevant. In total, 67 records met the criteria for full-text review.
After reading the full texts, we excluded 45 as they did not fulfil our eligibility criteria. The remaining 22 texts, originating from eight different trials, were included in our systematic review (Figure 1)[43,44,58-63].
Supplementary Table 2 contains the complete set of search terms used in each electronic database.
A summary of the trials can be found in Table 1; a full description of them is provided in Supplementary Table 4.
| Ref. | Trial design | Country | Sample size | IBS subtypes | Inclusion criteria | Frequency and route of administration | FMT-content | Placebo content | Pretreatment | Number of donors |
| Aroniadis et al[59], 2019 | RCT, crossover | United States | 48 (25 FMT vs 23 placebo) | IBS-D | Moderate-to-severe IBS symptoms (IBS-SSS > 175) | 3 d of 25 oral capsules | 3 × 25 frozen capsules (0.38 g donor stool/capsule) (Openbiome) | Non-toxic brown pigment | PPI for three days | One donor for one patient (four different donors) |
| El-Salhy et al[60], 2020 | RCT, 3 parallel groups | Norway | 164 (54/30 gram FMT, 55/60 gram FMT, 55 placebo) | All subtypes | Moderate-to-severe IBS symptoms (IBS-SSS > 175) | Single treatment via gastroscope to distal duodenum | Once 30 g or 60 gram of frozen feces in sterile saline solution | Autologous faeces | None | One donor |
| Halkjær et al[43], 2018 | RCT, 2 parallel groups | Denmark | 51 (25 FMT, 26 placebo) | All subtypes | Moderate-to-severe IBS symptoms (IBS-SSS > 175) | 12 d of 25 oral capsules | 25 FMT capsules (one daily dose containing approximately 12 g frozen faecal material) | Saline, glycerol and food colouring E150 | Bowel cleansing | Donor mix from four donors |
| Holster et al[61], 2019 | RCT, 2 parallel groups | Sweden | 16 (8 FMT, 8 placebo) | All subtypes | IBS with small amounts of butyrate-producing bacteria | Single treatment via colonoscopy to the caecum | 30 g frozen stool in sterile saline and glycerol | Autologous feces | Bowel cleansing and 4 mg loperamide | Two donors (three patients received stool from donor 1, the remaining five from donor 2) |
| Holvoet et al[44], 2021 | RCT, 2 parallel groups | Belgium | 62 (43 FMT, 19 placebo) | IBS-D and IBS-M | Refractory IBS with failure of at least three conventional IBS therapies | Single treatment via nasojejunal administration | Fresh feces mixed with saline | Autologous feces | Bowel cleansing | Two donors |
| Johnsen et al[62], 2018 | RCT, 3 parallel groups | Norway | 83 (26 fresh FMT, 29 frozen FMT, 28 placebo) | IBS-D and IBS-M | Moderate-to-severe IBS symptoms (IBS-SSS > 175) | Single treatment administered into the caecum via colonoscopy | 50–80 g fresh or frozen feces mixed with saline and glycerol | Autologous feces | Bowel cleansing and 8 mg loperamid | Donor mix from two donors |
| Lahtinen et al[58], 2020 | RCT, 2 parallel groups | Finland | 51 (25 FMT, 26 placebo) | IBS-D, IBS-M and IBS-U | Patients who remained symptomatic despite receiving conventional treatment | Single treatment administered into the caecum via colonoscopy | 30 g frozen suspension | Autologous feces | Bowel cleansing | One donor |
| Singh et al[63], 2022 | RCT, 4 parallel groups | United States | 23 (11 FMT, 12 placebo) | IBS-D | IBS-SSS > 150 or > 175 | Single treatment with 19 oral capsules | Capsule contain 0.75 frozen fecal filtrate) (Openbiome) | Glycerol with brown coloring agent | Bowel cleansing | Six donors (unknown if donors were mixed) |
We included eight trials that were published between 2018 and 2022[43,44,58-63]. These were either single-centre trials[44,60-63] or multicentre trials[43,58,59] and were conducted in Belgium[44], Denmark[43], Finland[58], Norway[60,62], Sweden[61] and the United States[59,63].
All participants in the trials were diagnosed with IBS by a physician and according to accepted, symptom-based diagnostic criteria (e.g., the Rome criteria)[5]. Participants in the Lahtinen et al[58] trial were diagnosed by a gastroenterologist, Aroniadis et al[59], Halkjær et al[43], Holster et al[61], Holvoet et al[44], Johnsen et al[62] and Singh et al[63] all used the Rome III criteria; El-Salhy et al[60] used the Rome IV criteria.
Four trials included participants with moderate-to-severe IBS symptoms, indicated by a score of 175 or more on the IBS severity scoring system (IBS-SSS)[43,59,60,62]. We are unsure whether Singh et al[63] used a score of 150 or 175 or more on the IBS-SSS, as both are referred to in their article. The remaining three trials used other criteria: Holster et al[61] only included participants with small amounts of butyrate-producing bacteria in faecal samples, Holvoet et al[44] included participants with refractory IBS who had experienced failure of at least three conventional IBS therapies, and Lahtinen et al[58] included participants who remained symptomatic despite receiving conventional treatment.
The trials differed in the IBS subtypes they investigated. All subtypes were included in the trials conducted by El-Salhy et al[60], Halkjær et al[43] and Holster et al[61]. Aroniadis et al[59] and Singh et al[63] included only diarrhoea-predominant participants. Holvoet et al[44] and Johnsen et al[62] included diarrhoea-predominant or mixed participants. Lahtinen et al[58] included diarrhoea-predominant, mixed or un-subtyped participants.
All eight trials used faeces from healthy donors for the FMT. Supplementary Table 5 describes their inclusion and exclusion criteria for donors.
The route of administration varied between the trials. Three trials used colonoscopy[58,61,62], one used gastroscopy[60], one used the nasojejunal route[44] and three used oral capsules[43,59,63].
The frequency of administration varied between trials. El Salhy et al[60], Holster et al[61], Holvoet et al[44], Johnsen et al[62], Lahtinen et al[58] and Singh et al[63] administered FMT just once. Aroniadis et al[59] administered a total of three doses across three consecutive days. Halkjær et al[43] administered a total of 12 doses across 12 consecutive days.
The volume of FMT administered ranged from approximately 100 mL in the El-Salhy et al[60] trial to 300 mL in the Holvoet et al[44] trial. The faecal quantity varied from 30 g[58,61] to 50-80 g[62]. The capsule trials used approximately 28.5 g of minimally processed faecal matter[59], 14.25 frozen faecal filtrate[63] and faecal matter derived from approximately 600 g of faeces[43]. Holvoet et al[44] used fresh FMT transplant, Johnsen et al[62] used both fresh and frozen FMT transplant, while the remaining trials used frozen FMT transplants[43,58-61,63].
Two trials used a single donor for all FMT treatments[58,60]. Holster et al[61], Holvoet et al[44] and Johnsen et al[62] used two donors. Aroniadis et al[59] used four donors, where each participant received a FMT from one donor. Singh et al[63] used six donors, where each participant received a FMT from one donor. Halkjær et al[43] used a FMT donor mix from four donors.
Six trials included bowel cleansing before transplantation[43,44,58,61-63]. Two trials used loperamide before endoscopy to retain the transplant[61,62]. One trial used proton pump inhibitors (PPI) for the three days prior to the transplantation[59].
Five trials used autologous faeces as an alternative to placebo for the comparison group[44,58,60-62]. In the capsule trials, Aroniadis et al[59] and Singh et al[63] used placebo capsules with a non-toxic, brown pigment and Halkjær et al[43] used placebo capsules made from saline, glycerol and food colouring E150.
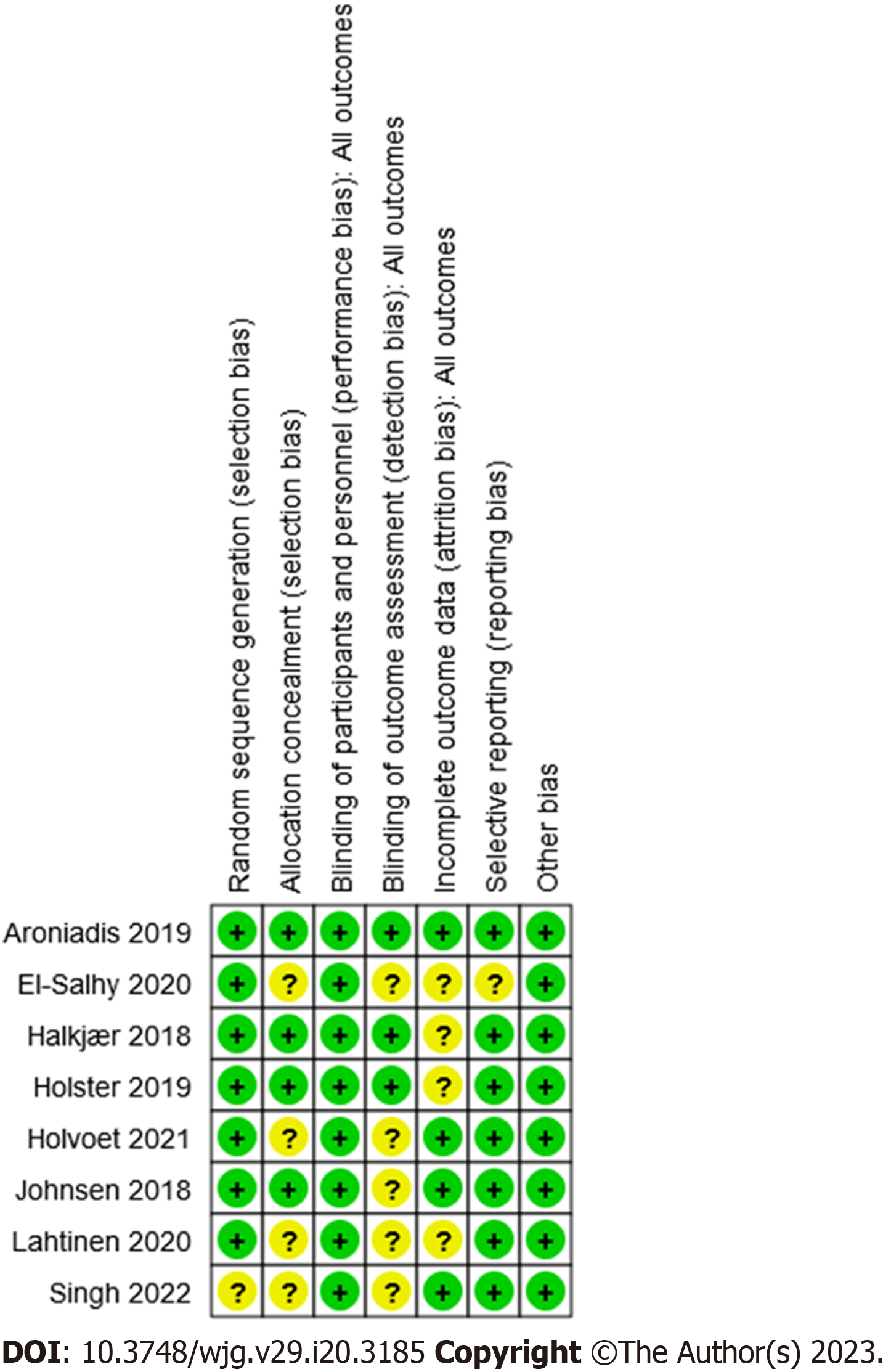
A summary of the risk of bias assessments is reported in Figure 2 and bias assessments for the individual trials are reported in Supplementary Table 4.
Overall, none of the studies had a high risk of bias in any of the seven dimensions considered. However, five of the eight trials[44,58,60,62,63] had an unclear bias for the blinding of outcomes, and four out of eight[43,58,60,61] had a similarly unclear bias in terms of how they reported the handling of incomplete data. In both cases this unclear bias was primarily due to a lack of information.
A summary of the findings is provided in Table 2 for comparing FMT and placebo in treating IBS. We did not assess publication bias as this review only consisted of eight trials. Furthermore, we chose to report the random-effect models’ results despite some of the fixed-effect models being found significant as we did not find any larger trial that was more methodologically rigorous. The significant outcomes of the fixed-effect models were most likely due to the small number of trials available in each analysis and their high heterogeneity.
| Outcomes and timeframe | Anticipated absolute effects | Relative effect (95%CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Effect in placebo | Effect difference with FMT (95%CI) | |||||
| Improvement of symptoms after three months | 42 per 100 | 8 or more per 100 (from 13 or fewer to 46 or more) | RR 1.19 (0.68-2.10) | 484 (8 RCTs) | ++--1 Low | Improvement of symptoms as measured by a validated global IBS symptoms score (e.g., IBS-SSS scale from 0, no symptoms, to 500, maximum symptoms) (as defined by each trial) |
| Improvement of symptoms after six months | 38 per 100 | 5 or fewer per 100 (from 25 or fewer to 52 or more) | RR 0.88 (0.33-2.39) | 99 (3 RCTs) | ++--2 Low | Improvement of symptoms as measured by a validated global IBS symptoms score (e.g., IBS-SSS scale from 0, no symptoms, to 500, maximum symptoms) (as defined by each trial) |
| Adverse events prior to end of trial | 26 per 100 | 4 or more per 100 (from 10 or fewer to 30 or more) | RR 1.17 (0.63-2.15) | 450 (7 RCTs) | ++--3 Low | Common adverse events were mild and self-limiting gastrointestinal symptoms |
| Serious adverse events prior to end of trial | 1 per 100 | 1 or fewer per 100 (from 1 or fewer to 2 or more) | RR 0.42 (0.07-2.60) | 501 (8 RCTs) | ++--4 Low | Serious adverse events included one suicide (placebo), cholecystitis (placebo), and one admission to the hospital due to discomfort after the FMT procedure |
| Dropouts due to adverse events prior to end of trial | 1 per 100 | 1 or fewer per 100 (from 1 or fewer to 1 or more) | RR 0.24 (0.03-2.17) | 502 (8 RCTs) | ++--5 Low | Dropouts due to adverse events include one suicide (placebo) and one for discomfort after the FMT procedure (placebo) |
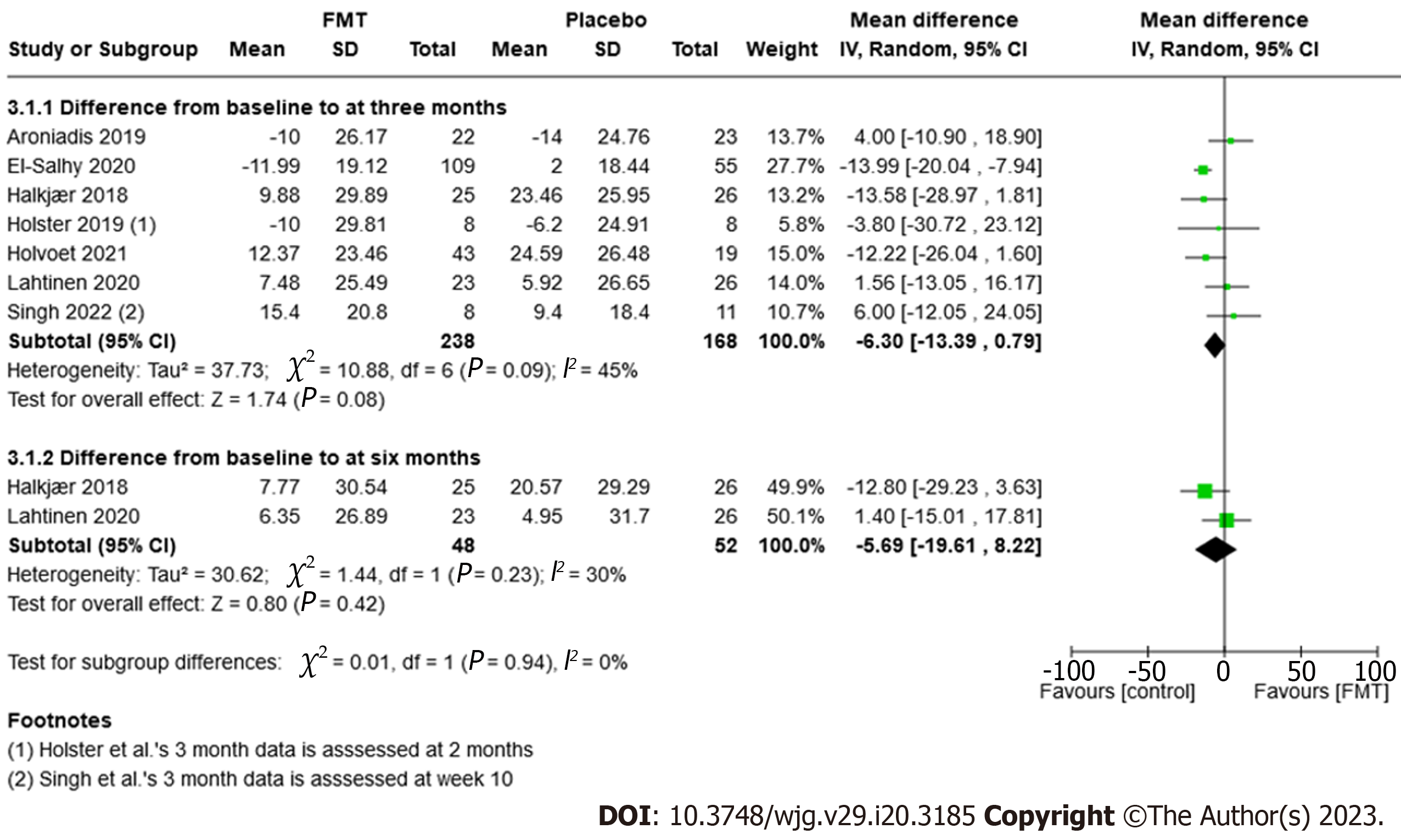
| Improvement in QoL scores after three months | NA | NA | MD -6.30 (-13.39 to 0.79) | 406 (7 RCTs) | ++--6 Low | Improvement of quality of life as measured by a validated scale IBS-QoL, where 34 items are summed and averaged for a total score and then transformed to a 0-100 scale for interpretation (high scores indicate better IBS-QoL) |
The GRADE rating for the certainty of the evidence examined was low due to moderate-high inconsistency, small numbers of patients and imprecision.
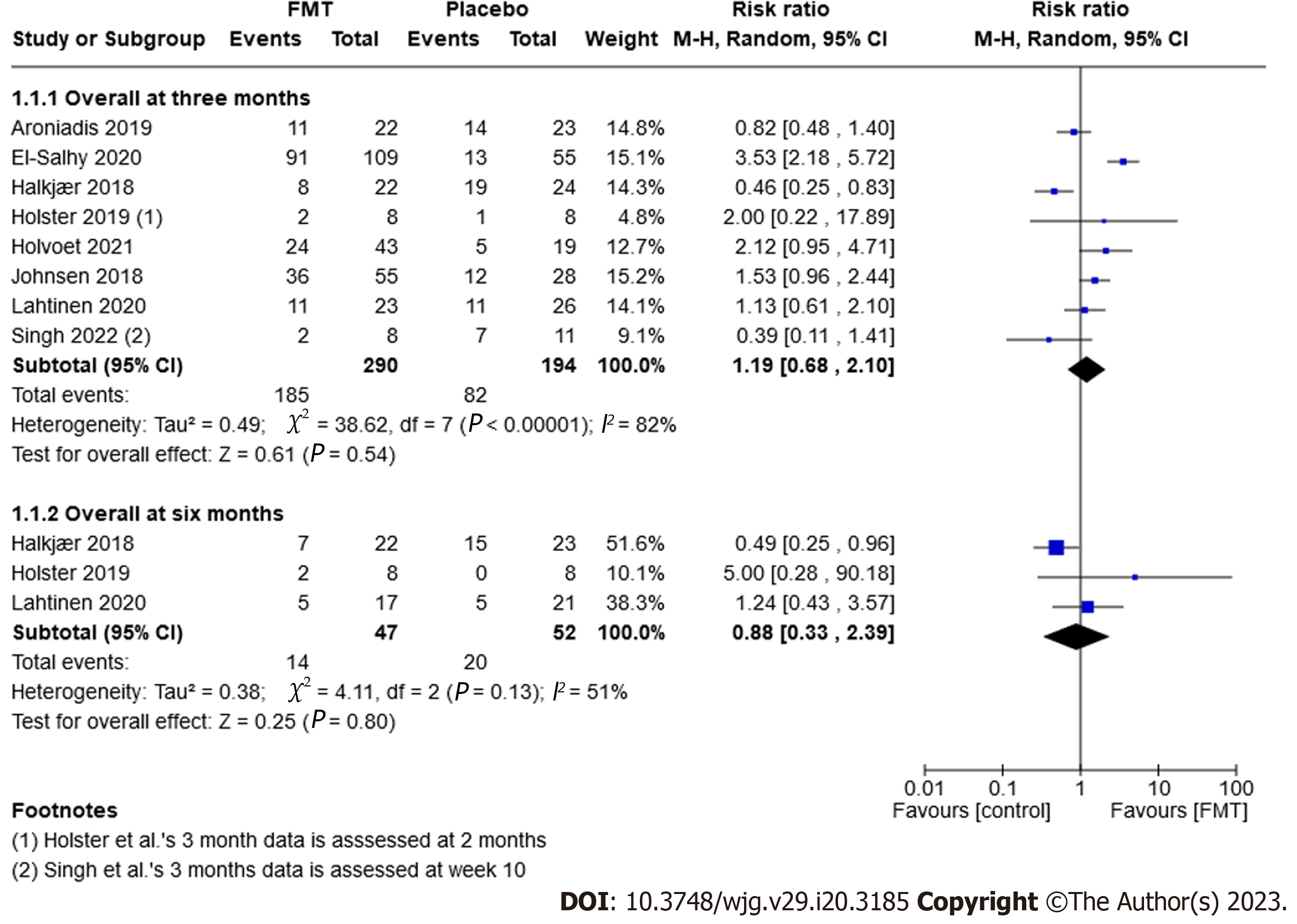
Improvement of symptoms: Eight randomised trials, comprising 484 participants, examined whether IBS symptoms improved after three months. Six trials defined improvement of symptoms as a decrease in IBS-SSS of 50 or more[43,44,59,60,63], while Johnson et al[62] defined it as a decrease of more than 75 points. Holster et al[61] used the gastrointestinal symptom rating scale-IBS and defined improvement as a change of more than 30%. Sixty-four percent (185/290) of FMT participants experienced an improvement of symptoms after three months compared to 42% (82/194) in the placebo group. A meta-analysis showed there was no significant difference between FMT and placebo (RR 1.19, 95%CI: 0.68-2.10, P = 0.54, I2 = 82%; Figure 3).
Three trials (99 participants) reported on the improvement of symptoms after six months. Thirty per cent (14/47) of FMT participants saw an improvement of their symptoms after six months compared to 38% (20/52) of the placebo group (RR 0.88, 95%CI: 0.33-12.39, P = 0.8, I2 = 51%; Figure 3).
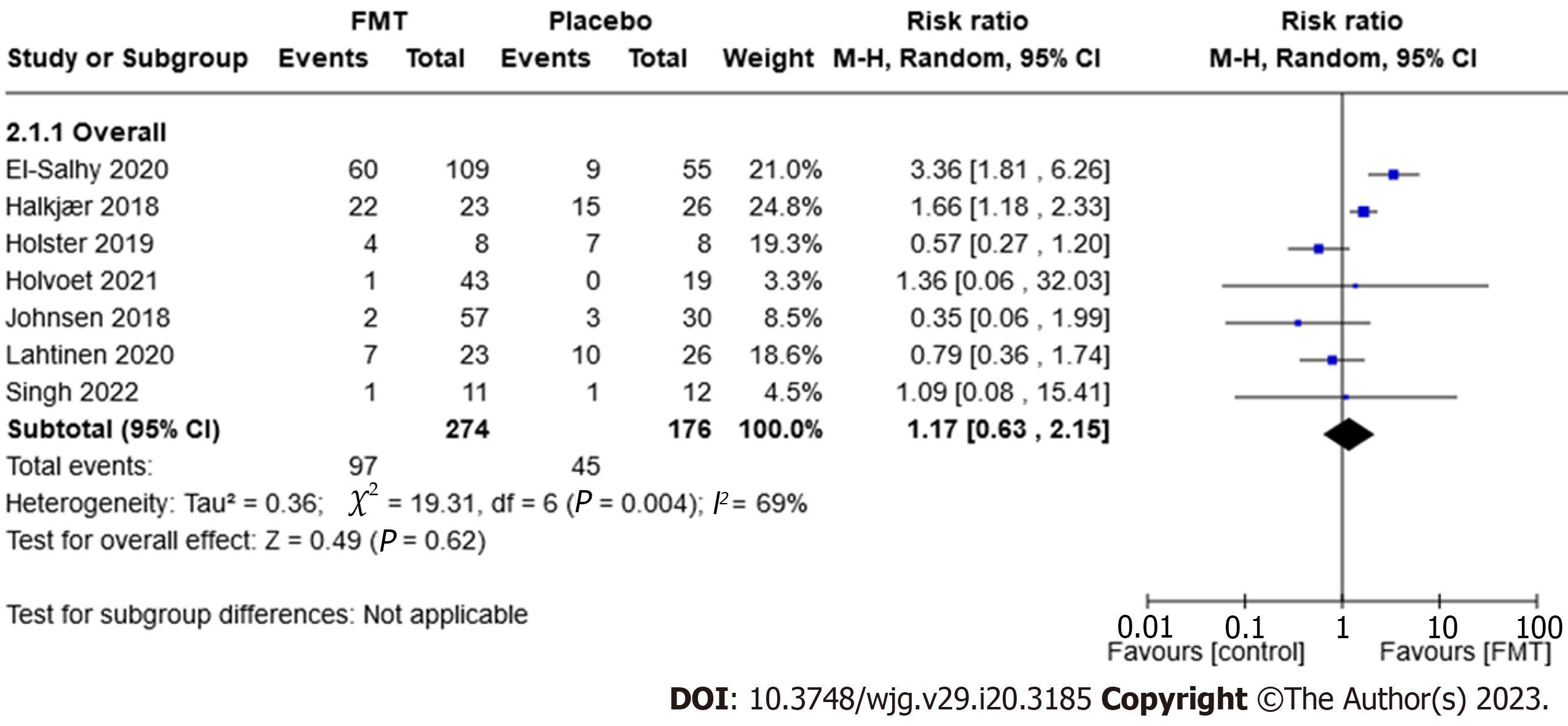
Adverse events: Seven trials, comprising 450 participants, reported on the proportion of participants who experienced adverse events. Thirty-five per cent (97/274) of the FMT group experienced an adverse event compared to 26% (45/176) of the placebo group (RR 1.17, 95%CI: 0.63-2.15, P = 0.62, I2 = 69%; Figure 4).
The most frequent adverse events reported in the trials were mild and transient symptoms of the gastrointestinal system.
Serious adverse events: All eight trials, comprising 501 participants, provided data for serious adverse events. A serious adverse event was reported once in a FMT group and twice in placebo groups. In the FMT group, 0.33 per cent (1/302) reported a serious adverse event, compared to 1% (2/199) in the placebo group (RR 0.42, 95%CI: 0.07-2.60, P = 0.35, I2 = 0%; Supplementary Figure 1).
Holvoet et al[44] reported that one participant from the placebo group committed suicide 10 d after the transplantation procedure. Aroniadis et al[59] reported one participant from the placebo group was admitted to hospital during week 20 of the trial with acute cholecystitis. Johnsen et al[62] reported that one participant from the FMT group was admitted to hospital after the FMT procedure due to transient vertigo and nausea.
Dropouts due to adverse events: Eight trials, comprising 502 participants, reported on dropouts due to adverse events; there were none in the FMT groups, but two instances in the placebo groups. None (0/302) of the FMT groups had dropouts due to adverse events compared to 1% (2/200) in the placebo group (RR 0.24, 95%CI: 0.03-2.17, P = 0.2, I2 = 0%; Supplementary Figure 2).
Holster et al[61] reported that one participant from the placebo group discontinued the trial after the FMT procedure due to discomfort. The dropout due to an adverse event in Holvoet et al[44] was the suicide occurring 10 d after the transplantation procedure in the placebo group.
Seven trials, comprising 406 participants, reported on QoL outcomes. There were no significant differences between the FMT and placebo treatment groups; however, there was a slightly favorable effect seen in the placebo groups (MD -6.30, 95%CI: -13.39 to 0.79, P = 0.08, I2 = 45%; Figure 5).
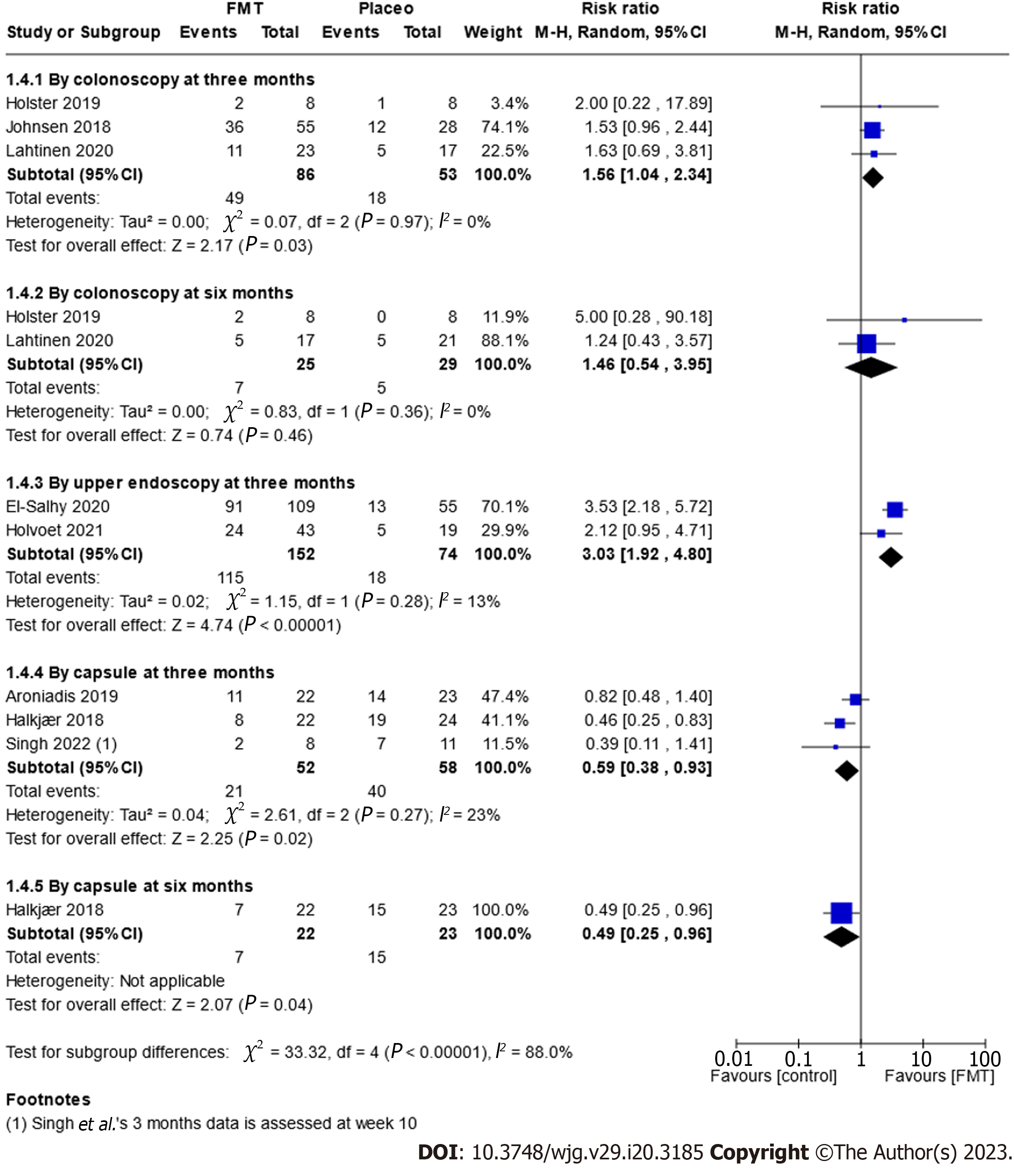
Planned subgroup analyses included fresh vs frozen transplant, quantity of transplant, route of administration, type of donor (single vs mixed donor), frequency of administration and subtype of IBS (Supplementary Figures 3-8, Figure 6).
Overall, we found that endoscopic delivery (colonoscopy and upper endoscopy) of the FMT improved IBS-SSS after three months (RR 1.56, 95%CI: 1.04-2.34, P = 0.03, I2 = 0% and RR 3.03, 95%CI: 1.92-4.80, P ≤ 0.00001, I2 = 13%; Figure 6). Furthermore, administering a single, large dose of FMT resulted in a greater improvement of the IBS-SSS, while increasing the dose across several treatments was comparable to a placebo (Supplementary Figures 4 and 6). None of the other subgroup analyses demonstrated an effect of FMT over placebo.
This review systematically examined the benefits and harms of FMT vs placebo or autologous FMT for the treatment of patients with IBS. Our main objective was to assess the efficacy of FMT for the improvement of symptoms in patients with IBS.
This review combined findings from eight randomised clinical trials that assessed the efficacy of FMT in 465 IBS patients. We found no significant difference in the improvement of symptoms in the FMT groups compared to the placebo groups (P = 0.54). The meta-analysis suggests a favorable, but non-significant, effect on quality of life in patients treated with placebo.
In general, placebo response rates are high in IBS patients. Placebo response estimates in prior meta-analyses range from 16% to 72%[64,70]. Likewise, bowel cleansing might contribute to symptom improvement; however, its effects on the microbiota seem to be transient[71,72].
FMT appears to be safe, with mild and self-limiting gastrointestinal symptoms like nausea, constipation, diarrhoea, and stomach pain - all of which are common IBS symptoms. This conclusion was also reached in a previous review assessing FMT for the treatment of inflammatory bowel disease[73]. FMT was not associated with serious adverse events in the treatment of IBS; three such events were reported in total (two in the placebo group and one in the FMT group) and none were considered to be related to the treatment.
In general, the results from the trials used for this review were highly heterogeneous. Therefore, it is possible that the absence of a positive overall effect is simply the result of how different the trials were from one another. The trials had pronounced differences in their selection processes for participants and donors, the routes of administration, the transplant quantities, and the frequency of administration. These differences make it difficult to draw conclusions about FMT as a treatment for IBS.
There is scientific evidence to support the hypothesis that FMT may be beneficial for patients with IBS. Observational trials have reported that IBS patients have reduced diversity or aberrant microbiota composition when compared to healthy controls[74]. Altered gut microbiota is also referred to as ‘microbiota dysbiosis’ and has been connected with disturbances in the microbiota gut-brain axis signaling[75]. Furthermore, other modulating agents targeting the microbiota, such as specific probiotic strains and antibiotics, have had demonstrable effects in IBS patients[76]. However, the underlying causes and mechanisms of dysbiosis in IBS and other diseases remain largely unknown. It has yet to be determined whether dysbiosis is a cause or a consequence of IBS, and even a ‘healthy’ microbiome has yet to be satisfactorily defined.
All eight trials included in this review reported on changes in gut microbiota after FMT. Aroniadis et al[59], El-Salhy et al[60], Halkjær et al[43], Lahtinen et al[58] and Singh et al[63] reported that participants receiving FMT saw changes in their gut microbiota that made their profiles more like the donors, when compared to placebo participants. Johnsen et al[62] reported these data in a later publication with the same outcome[77]. Holster et al[61] reported that microbiota diversity was not significantly affected by either FMT or placebo (autologous FMT). Holvoet et al[44] reported that responders to FMT had a higher baseline microbial diversity compared to those whose FMT treatment failed.
The possible effects, both positive and negative, of autologous FMT as placebo should be borne in mind.
In the treatment of recurrent CDI, the highest cure rates have been reported with repeated treatments delivered through lower endoscopy, but delivery through capsules is also highly effective[35,78]. In contrast, in IBS, FMT administered via upper or lower endoscopy, rather than capsules, has resulted in significant improvements in IBS-SSS. While much research has focused on FMT capsules[79], it is possible that the engraftment of the donor microbiota is better accomplished through endoscopic methods in IBS patients. Future RCTs in IBS patients that examines the combination of different routes of delivery for strain engraftment could be very interesting. Such studies would also contribute towards a more comprehensive understanding of microbial engraftment dynamics, which is currently lacking. A recent, systematic meta-analysis with shotgun metagenomic results showed that receiving FMT from multiple routes (for example, both via colonoscopy and capsules during the same treatment) resulted in increased engraftment[80]. Likewise, El-Salhy et al[81] present additional data from their trial and argue for using super donors since the efficacy of FMT appears to be donor-dependent. This argument needs further corroboration. Finally, data about patient and donor diets could prove relevant when determining the optimal patient-donor match[82].
The findings of this review have limited applicability and generalisability. More trials are needed to investigate whether FMT is a beneficial treatment strategy for IBS. Several aspects of the methods used in these trials could have influenced the effect of FMT, such as the route of administration, duration and interval between treatments, and the quantity of faecal microbiota transplanted to the patient. Despite the subgroup analyses we conducted as part of this review, firm conclusions cannot be drawn due to the small number of events and participants in the trials. Nonetheless, the results do suggest a possible beneficial effect in delivering FMT by endoscopy (colonoscopy or gastroscopy) over other routes.
Most of the patients in the trials we reviewed had moderate-to-severe IBS and were diagnosed according to the Rome III criteria. The newest, Rome IV criteria are more rigorous and it is not clear whether the greater homogeneity of IBS study populations they encourage will affect the efficacy of FMT. We recommend that future trials use the Rome IV criteria.
Additional investigations of microbiota, both when selecting patients of interest and after interventions, are needed in order to establish the precise mechanism of action of FMT as a potential treatment for IBS.
We did not find evidence to support the use of FMT for IBS patients outside of clinical trials in this systematic review and meta-analysis. We report a possible beneficial effect when delivering FMT by endoscopy (colonoscopy or gastroscopy). FMT appears to be safe, when compared to placebo, in patients with IBS, regardless of route of administration. Further randomised clinical trials are necessary in order to determine the effect of FMT in IBS.
Irritable bowel syndrome (IBS) is a widespread gastrointestinal disorder accompanied by chronic abdominal pain and altered bowel habits. Gut microbiota disturbances have been linked to the pathophysiology of IBS, with fecal microbiota transplantation (FMT) emerging as a potential treatment strategy.
Manipulating gut microbiota composition via FMT could offer a promising avenue for IBS treatment, warranting further investigation into its efficacy and safety.
This review and meta-analysis aimed to evaluate the effectiveness and safety of FMT for treating IBS.
A comprehensive search of Cochrane Central, MEDLINE, EMBASE, and Web of Science to identify randomised controlled trials (RCT) comparing FMT to placebo or autologous FMT in IBS patients. Primary outcome was improvement of symptoms, while secondary outcomes were quality-of-life scores and adverse events.
Our analysis incorporated data from eight RCTs with 484 participants. FMT did not result in significant improvement of symptoms when compared to placebo after three months, and no significant improvement in quality of life was observed. Subgroup analysis indicated that endoscopic FMT delivery led to symptom improvement, whereas FMT capsules did not. FMT was found to be safe.
This systematic review and meta-analysis do not support FMT as a treatment for IBS outside of clinical trials. Nevertheless, FMT was found to be safe.
Large-scale, RCTs are needed to confirm or refute these findings. Investigating the potential significance of combining different FMT delivery routes for strain engraftment could provide a more comprehensive understanding of microbial engraftment dynamics in IBS patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bao CH, China; Rahmati M, Iran; Wu LH, China S-Editor: Zhang H L-Editor: A P-Editor: Cai YX
| 1. | Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712-721.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1415] [Article Influence: 108.8] [Reference Citation Analysis (2)] |
| 2. | Spiegel BM. The burden of IBS: looking at metrics. Curr Gastroenterol Rep. 2009;11:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 330] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 4. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (14)] |
| 5. | Drossman DA. Functional gastrointestinal disorders: what's new for Rome IV? Lancet Gastroenterol Hepatol. 2016;1:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Choi HH, Cho YS. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin Endosc. 2016;49:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 7. | Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, Moore T, Wu G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology. 2015;149:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 423] [Article Influence: 42.3] [Reference Citation Analysis (1)] |
| 8. | Khanna S, Tosh PK. A clinician's primer on the role of the microbiome in human health and disease. Mayo Clin Proc. 2014;89:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 9. | Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 482] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 10. | Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil. 2015;27:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 12. | King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 295] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 499] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 14. | Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, Saarela M. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Hugerth LW, Andreasson A, Talley NJ, Forsberg AM, Kjellström L, Schmidt PT, Agreus L, Engstrand L. No distinct microbiome signature of irritable bowel syndrome found in a Swedish random population. Gut. 2020;69:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos J. A Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther. 2018;35:289-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 17. | Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019;10:1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 18. | Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015;21:3072-3084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 266] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (4)] |
| 19. | Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de Wit N; European Society for Primary Care Gastroenterology. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | El-Salhy M, Mazzawi T. Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2018;12:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 22. | Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755; author reply p.1755-1755; author reply p.1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 409] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 23. | Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, Wu K. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39:1003-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2582] [Cited by in RCA: 2678] [Article Influence: 223.2] [Reference Citation Analysis (0)] |
| 25. | Wortelboer K, Nieuwdorp M, Herrema H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine. 2019;44:716-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 26. | Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, Bourrier A, Le Gall G, Lalande V, De Rougemont A, Kirchgesner J, Daguenel A, Cachanado M, Rousseau A, Drouet É, Rosenzwajg M, Hagege H, Dray X, Klatzman D, Marteau P; Saint-Antoine IBD Network, Beaugerie L, Simon T. Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome. 2020;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (1)] |
| 27. | Stojek M, Jabłońska A, Adrych K. The Role of Fecal Microbiota Transplantation in the Treatment of Inflammatory Bowel Disease. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther. 2017;46:213-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 195] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 29. | Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, Sokol H, Kump P, Satokari R, Kahn SA, Kao D, Arkkila P, Kuijper EJ, Vehreschild MJG, Pintus C, Lopetuso L, Masucci L, Scaldaferri F, Terveer EM, Nieuwdorp M, López-Sanromán A, Kupcinskas J, Hart A, Tilg H, Gasbarrini A. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111-2121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 30. | Keller JJ, Ooijevaar RE, Hvas CL, Terveer EM, Lieberknecht SC, Högenauer C, Arkkila P, Sokol H, Gridnyev O, Mégraud F, Kump PK, Nakov R, Goldenberg SD, Satokari R, Tkatch S, Sanguinetti M, Cammarota G, Dorofeev A, Gubska O, Ianiro G, Mattila E, Arasaradnam RP, Sarin SK, Sood A, Putignani L, Alric L, Baunwall SMD, Kupcinskas J, Link A, Goorhuis AG, Verspaget HW, Ponsioen C, Hold GL, Tilg H, Kassam Z, Kuijper EJ, Gasbarrini A, Mulder CJJ, Williams HRT, Vehreschild MJGT. A standardised model for stool banking for faecal microbiota transplantation: a consensus report from a multidisciplinary UEG working group. United European Gastroenterol J. 2021;9:229-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 31. | König J, Siebenhaar A, Högenauer C, Arkkila P, Nieuwdorp M, Norén T, Ponsioen CY, Rosien U, Rossen NG, Satokari R, Stallmach A, de Vos W, Keller J, Brummer RJ. Consensus report: faecal microbiota transfer - clinical applications and procedures. Aliment Pharmacol Ther. 2017;45:222-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 32. | Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, Weese JS, Collins S, Moayyedi P, Crowther M, Ropeleski MJ, Jayaratne P, Higgins D, Li Y, Rau NV, Kim PT. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2016;315:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 492] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 33. | Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 483] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 34. | Satokari R, Mattila E, Kainulainen V, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection--an observational cohort study. Aliment Pharmacol Ther. 2015;41:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 35. | Baunwall SMD, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, Hvas CL. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;29-30:100642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 36. | Zellmer C, De Wolfe TJ, Van Hoof S, Blakney R, Safdar N. Patient Perspectives on Fecal Microbiota Transplantation for Clostridium Difficile Infection. Infect Dis Ther. 2016;5:155-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Lee KN, Lee OY. Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol. 2014;20:8886-8897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 38. | Fan WT, Ding C, Xu NN, Zong S, Ma P, Gu B. Close association between intestinal microbiota and irritable bowel syndrome. Eur J Clin Microbiol Infect Dis. 2017;36:2303-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Ringel-Kulka T, Benson AK, Carroll IM, Kim J, Legge RM, Ringel Y. Molecular characterization of the intestinal microbiota in patients with and without abdominal bloating. Am J Physiol Gastrointest Liver Physiol. 2016;310:G417-G426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 637] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 41. | Mizuno S, Masaoka T, Naganuma M, Kishimoto T, Kitazawa M, Kurokawa S, Nakashima M, Takeshita K, Suda W, Mimura M, Hattori M, Kanai T. Bifidobacterium-Rich Fecal Donor May Be a Positive Predictor for Successful Fecal Microbiota Transplantation in Patients with Irritable Bowel Syndrome. Digestion. 2017;96:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 42. | Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn's Disease. Inflamm Bowel Dis. 2016;22:2182-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 43. | Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, Petersen AM. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 44. | Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, Verhasselt B, van Vlierberghe H, De Vos M, Raes J, De Looze D. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology. 2021;160:145-157.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 45. | Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojärvi J, Voigt AY, Zeller G, Sunagawa S, de Vos WM, Bork P. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352:586-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 391] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 46. | Pimentel M, Lembo A. Microbiome and Its Role in Irritable Bowel Syndrome. Dig Dis Sci. 2020;65:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 47. | Wang L, Alammar N, Singh R, Nanavati J, Song Y, Chaudhary R, Mullin GE. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. J Acad Nutr Diet. 2020;120:565-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 48. | Halkjær SI, Boolsen AW, Günther S, Christensen AH, Petersen AM. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol. 2017;23:4112-4120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Andrews P, Barnes P, Borody T. Chronic constipation reversed by restoration of bowel flora. A case and a hypothesis. Eur J Gastroenterol Hepatol. 1992;4:245-247. |
| 50. | Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 147] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 52. | Cruz Aguilar R, Buch T, Bajbouj. Fecal microbiota transplantation as a novel therapy for irritable bowel syndrome with predominant diarrhea. Neurogastroenterol Motil. 2015;110. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Holvoet T, Boelens J, Joossens M, Raes J, De Vos M, De Looze D. Fecal Microbiota Transplantation in Irritable Bowel Syndrome With Bloating: Results From a Prospective Pilot Study. Gastroenterology. 2015;148:S963-S964. [DOI] [Full Text] |
| 54. | Hong J, Bang B, Shin Y, Kim H, Kwon K. Treatment of Irritable Bowel Syndrome with Fecal Microbiota Transplantation: A case series of 10 patients. United European Gastroenterol J. 2016;A102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 55. | Mazzawi T, El-Salhy M, Lied GA, Hausken T. The Effects of Fecal Microbiota Transplantation on the Symptoms and the Duodenal Neurogenin 3, Musashi 1, and Enteroendocrine Cells in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front Cell Infect Microbiol. 2021;11:524851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Pinn D, Aroniadis O, Brandt L. Follow-up Study of Fecal Microbiota Transplantation (FMT) for the Treatment of Refractory Irritable Bowel Syndrome (IBS) 1862. Am J Gastroenterol. 2013;108:S563. [DOI] [Full Text] |
| 57. | Syzenko G, Budovska L, Puchkov K. P0397 Efficiency of FMT in cases of ‘Treatment-resistant’ IBS. United European Gastroenterol J. 2016;2:A294. |
| 58. | Lahtinen P, Jalanka J, Hartikainen A, Mattila E, Hillilä M, Punkkinen J, Koskenpato J, Anttila VJ, Tillonen J, Satokari R, Arkkila P. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51:1321-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 59. | Aroniadis OC, Brandt LJ, Oneto C, Feuerstadt P, Sherman A, Wolkoff AW, Kassam Z, Sadovsky RG, Elliott RJ, Budree S, Kim M, Keller MJ. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019;4:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 61. | Holster S, Lindqvist CM, Repsilber D, Salonen A, de Vos WM, König J, Brummer RJ. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin Transl Gastroenterol. 2019;10:e00034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 62. | Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, Goll R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 63. | Singh P, Alm EJ, Kelley JM, Cheng V, Smith M, Kassam Z, Nee J, Iturrino J, Lembo A. Effect of antibiotic pretreatment on bacterial engraftment after Fecal Microbiota Transplant (FMT) in IBS-D. Gut Microbes. 2022;14:2020067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 64. | Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 65. | Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. |
| 66. | Halkjær SI, Lo B, Cold F, Christensen AHH, Gluud LL, Petersen AM. Fecal microbiota transplantation for treatment of irritable bowel syndrome (Protocol). Cochrane Database Syst Rev. 2020;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 67. | Simren M, Palsson OS, Whitehead WE. Update on Rome IV Criteria for Colorectal Disorders: Implications for Clinical Practice. Curr Gastroenterol Rep. 2017;19:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 68. | Cochrane Consumers and Communication La Trobe University, Ryan R, Synnot A, M P, Hill S. Data extraction template. 2018. [DOI] [Full Text] |
| 69. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24848] [Article Influence: 1774.9] [Reference Citation Analysis (3)] |
| 70. | Patel SM, Stason WB, Legedza A, Ock SM, Kaptchuk TJ, Conboy L, Canenguez K, Park JK, Kelly E, Jacobson E, Kerr CE, Lembo AJ. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterol Motil. 2005;17:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 71. | Li M, Qian W, Yu L, Tian F, Zhang H, Chen W, Xue Y, Zhai Q. Multi-Time-Point Fecal Sampling in Human and Mouse Reveals the Formation of New Homeostasis in Gut Microbiota after Bowel Cleansing. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 72. | Jalanka J, Hillamaa A, Satokari R, Mattila E, Anttila VJ, Arkkila P. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2018;47:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 73. | Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, Acra S. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11:CD012774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 74. | Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 75. | Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34:15490-15496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 76. | Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (1)] |
| 77. | Goll R, Johnsen PH, Hjerde E, Diab J, Valle PC, Hilpusch F, Cavanagh JP. Effects of fecal microbiota transplantation in subjects with irritable bowel syndrome are mirrored by changes in gut microbiome. Gut Microbes. 2020;12:1794263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 78. | Cold F, Baunwall SMD, Dahlerup JF, Petersen AM, Hvas CL, Hansen LH. Systematic review with meta-analysis: encapsulated faecal microbiota transplantation - evidence for clinical efficacy. Therap Adv Gastroenterol. 2021;14:17562848211041004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 79. | Franc A, Vetchý D, Fülöpová N. Commercially Available Enteric Empty Hard Capsules, Production Technology and Application. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Ianiro G, Punčochář M, Karcher N, Porcari S, Armanini F, Asnicar F, Beghini F, Blanco-Míguez A, Cumbo F, Manghi P, Pinto F, Masucci L, Quaranta G, De Giorgi S, Sciumè GD, Bibbò S, Del Chierico F, Putignani L, Sanguinetti M, Gasbarrini A, Valles-Colomer M, Cammarota G, Segata N. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. 2022;28:1913-1923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 81. | El-Salhy M, Hausken T, Hatlebakk JG. Current status of fecal microbiota transplantation for irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e14157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Orr MR. The biodiversity dose-response curve translates theory and practice from ecological restoration into research and clinical priorities for fecal microbiota transplantation. Front Med (Lausanne). 2022;9:1059148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |