Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3168
Peer-review started: March 3, 2023
First decision: March 24, 2023
Revised: April 2, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: May 28, 2023
Processing time: 84 Days and 0.5 Hours
The efficacy of conversion therapy for patients with unresectable hepatocellular carcinoma (HCC) is a common clinical concern.
To analyse the prognostic factors of overall survival (OS) in patients with unresectable HCC who received conversion therapy.
One hundred and fifty patients who met the inclusion criteria were enrolled and divided into a training cohort (n = 120) and a validation cohort (n = 30). Using the independent risk factors in the training cohort, a nomogram model was constructed to predict OS for patients treated with transarterial chemoembolization following hepatic resection. The nomogram was internally validated with the bootstrapping method. The predictive performance of nomogram was assessed by Harrell’s concordance index (C-index), calibration plot and time-dependent receiver operating characteristic curves and compared with six other conventional HCC staging systems.
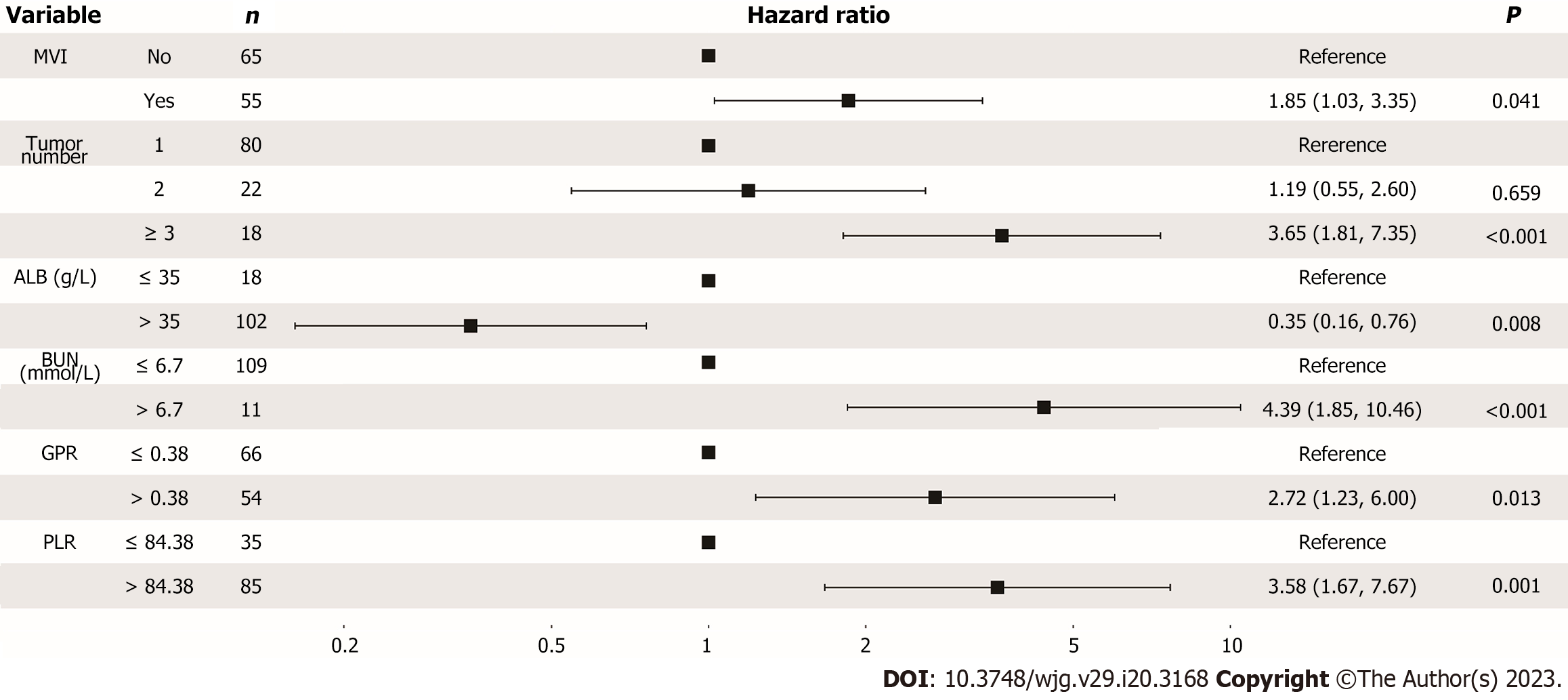
Multivariate Cox analysis identified that albumin, blood urea nitrogen, gamma-glutamyl transpeptidase to platelet ratio, platelet to lymphocyte ratio, macrovascular invasion and tumour number were the six independent prognostic factors correlated with OS in nomogram model. The C-index in the training cohort and validation cohort were 0.752 and 0.807 for predicting OS, which were higher than those of the six conventional HCC staging systems (0.563 to 0.715 for the training cohort and 0.458 to 0.571 for the validation cohort). The calibration plots showed good consistency between the nomogram prediction of OS and the actual observations of OS. Decision curve analyses indicated satisfactory clinical utility. With a total nomogram score of 196, patients were accurately classified into low-risk and high-risk groups. Furthermore, we have deployed the model into online calculators that can be accessed for free at https://ctmodelforunresect
The nomogram achieved optimal individualized prognostication of OS in HCC patients who received conversion therapy, which could be a useful clinical tool to help guide postoperative personalized interventions and prognosis judgement.
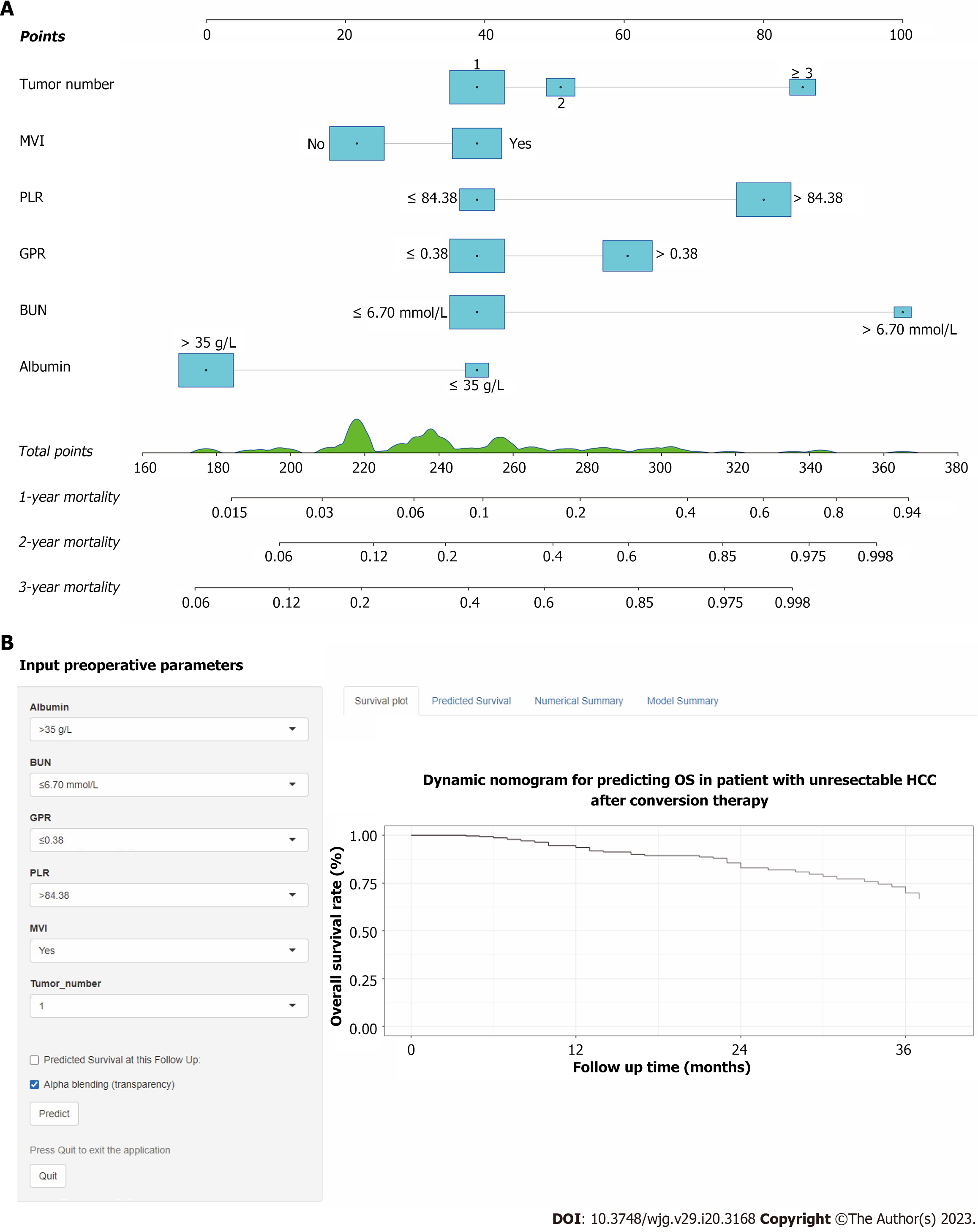
Core Tip: We developed and validated a prognostic nomogram based on inflammation-related biomarkers for patients with unresectable hepatocellular carcinoma after conversion therapy. The proposed conversion therapy model shows increased accuracy, good clinical utility, and better prognostic performance compared with conventional staging systems. Based on the total predictive risk scores, the patients were classified into two groups: low-risk (score < 196) and high-risk group (score ≥ 196), to guide postoperative adjuvant interventions and follow-ups. Furthermore, we have made this prognostic nomogram online for free use (https://ctmodelforunresectablehcc.shinyapps.io/DynNomapp/).
- Citation: Wu JL, Luo JY, Jiang ZB, Huang SB, Chen GR, Ran HY, Liang QY, Huang MS, Lai LS, Chen JW. Inflammation-related nomogram for predicting survival of patients with unresectable hepatocellular carcinoma received conversion therapy. World J Gastroenterol 2023; 29(20): 3168-3184
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3168
Hepatocellular carcinoma (HCC), the most common type of primary liver cancer, ranks as the sixth most prevalent malignancy and third leading cause of cancer-related deaths worldwide[1]. More so than other cancers, hepatitis B virus (HBV) infection is a hallmark of HCC, with 70%-90% of diagnoses occurring with a background of cirrhosis in highly prevalent Asian countries[2]. Hepatic resection (HR) is deemed the most efficacious therapeutic option for patients diagnosed with early to intermediate stages of HCC. Unfortunately, the majority of Chinese patients with HCC are diagnosed at intermediate or advanced stages with massive or multifocal lesions. Nevertheless, HR is possible for a minority of carefully selected patients with the help of a “conversion therapy” strategy, which refers to conversion of an unresectable HCC to achieve adequate tumour shrinkage and downstaging to undergo HR. However, only a limited number of meticulously screened patients with intermediate or advanced stage HCC qualify for HR.
As per the Barcelona Clinic Liver Cancer (BCLC) staging system, transarterial chemoembolization (TACE) is recommended as the first-line treatment for intermediate stage HCC and has been extensively investigated and widely acknowledged as an effective approach for conversion therapy[3]. Previous studies have demonstrated that HR after local treatments, such as TACE, hepatic artery infusion chemotherapy and radiotherapy with or without other systemic therapeutic measures, can improve tumour shrinkage as well as downstaging. Moreover, these treatments have been shown to enhance patient survival rates without causing significant complications[4-7]. While TACE could potentially provide surgical options to previously ineligible HCC patients, its high incidence of short-term recurrence remains a significant challenge for conversion therapy. Meanwhile, active conversion strategies to increase the volume of future liver remnant and planning for patients who cannot achieve R0 resection are worth greater consideration. Thus, the development of a validated clinical risk score based on preoperative indices to assess those at high risk of recurrence and death may help formulate optimal management strategies and prevent disease progression.
Links between inflammation and the development of cancer are well established, and HCC is one of the most classic inflammation-linked tumours. During liver inflammation, inflammatory cells and mediators are present before hepatocarcinogenesis and prompt the malignant activity of cancer cells. Additionally, a persistent cancer-related inflammatory microenvironment leads to immune suppression, which promotes the development of HCC[8]. Recently, numerous previous studies have indicated that preoperative inflammatory biomarkers can be capable of predicting HCC prognosis after HR[9,10]. A previous study by Wang et al[9] assessed the prognostic value of inflammation-related markers prior to surgery and demonstrated their high effectiveness in predicting survival after surgical resection of HCC. In addition, the researchers concluded that two inflammation-related markers, gamma-glutamyl transpeptidase (GGT) to platelet ratio (GPR) and neutrophil to lymphocyte ratio (NLR), were independently associated with prognosis. However, it is unclear whether preoperative inflammatory biomarkers have a high efficacy in predicting the overall survival (OS) of unresectable HCC patients following conversion therapy.
Nomograms have recently been used as an easy-to-operate predictive tool in HCC and have compared favorably to traditional tumour staging systems[10,11]. However, studies focusing on inflammatory biomarkers for the prognosis of HCC treated with TACE following HR are rare. Therefore, the aim of this study was to develop a conversion therapy model (CT model) that combined clinical factors, tumour radiologic features, inflammation biomarkers and TACE strategy for prognosis in patients with unresectable HCC successfully treated with conversion therapy. A browser-based calculator was developed to conveniently help guide individualized follow-up. A precise estimation of OS prognosis can assist clinical surgeons in selecting more appropriate therapeutic measures for recurrent patients through risk-benefit analysis.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Ethical Committee of the Third Affiliated Hospital of Sun Yat-sen University (No. II2023-027). Due to the retrospective design of this study, written informed consent from patients was waived. Consecutive Chinese adult individuals with pathologically confirmed primary unresectable HCC who were treated with TACE following HR in our institution from January 2011 to July 2020 were enrolled. The inclusion criteria were as follows: (1) Age between 18 years and 80 years; (2) pathologically proven HCC; and (3) unresectable HCC according to the Chinese expert consensus and acceptance of TACE as the conventional therapy following HR[3]. The exclusion criteria were as follows: (1) Extrahepatic metastasis; (2) history of other malignancies; (3) previous anticancer therapy; (4) current or recent system infection disease and incomplete clinical data; and (5) death or loss to follow-up within 30 d after resection. One hundred fifty patients from our center were included in this study and randomly assigned to a training cohort (n = 120) and a validation cohort (n = 30). The flow chart of patient selection is shown in Supplementary Figure 1.
For each patient, the following clinical parameters were collected: (1) Preoperative patient characteristics (age, sex, factor of HCC, aetiology of HCC, ascites, and Child-Pugh class); (2) TACE technique [conventional-TACE (cTACE) or drug eluting bead-TACE (DEB-TACE)] and number of TACE treatments; (3) baseline laboratory data, including alpha fetoprotein (AFP) level, white blood cell count, haemoglobin level, platelet count, neutrophil count, lymphocyte ratio, monocyte ratio, neutrophil ratio, aspartate aminotransferase (AST) level, alanine aminotransferase level, GGT level, albumin (ALB) level, total bilirubin level, blood urea nitrogen (BUN) level, serum creatinine level, urea level and prothrombin time (PT); (4) inflammation biomarkers, including GPR, NLR, lymphocyte to monocyte ratio, prognostic nutritional index, platelet to lymphocyte ratio (PLR), systemic immune inflammation index, neutrophil times GGT-to-lymphocyte ratio, AST to platelet ratio index, AST to neutrophil ratio index, and AST (the detailed formulas of inflammation biomarkers are summarized in Supplementary Table 1); (5) baseline tumour radiologic features [tumour number, largest tumour diameter, tumour capsule, macrovascular invasion (MVI)]; and (6) surgical factors (resection margin and blood transfusion).
The cTACE or DEB-TACE procedure was performed according to our previously reported protocol[12]. Repeated TACE was implemented according to a multidisciplinary treatment board (consisting of interventional radiologists, liver surgeons, and medical oncologists) during the follow-up periods and after in-depth discussion with the patient. HR was performed according to the liver surgeons’ assessment that the unresectable HCC had transformed into radically operable HCC. HR was performed by clinicians who possessed more than ten years of experience in hepatectomy. Under general anesthesia, HR was conducted through an L-shaped laparotomy or bilateral subcostal incision with a midline extension. Intraoperative ultrasound (US) was performed as standard practice to assess tumor burden, liver remnant, and resection margin[13]. Curative resection was defined as complete remove of all tumor nodules with no residual tumor margin (R0 resection), which was confirmed through pathological examination.
All patients were monitored in the first month post HR, then at three-month intervals for the first two years, and bi-annual assessments thereafter. During each follow-up, routine blood tests, liver function tests, AFP and imaging examinations (abdominal contrast-enhanced magnetic resonance imaging and computed tomography) were performed. Follow-up ended in July 2022. OS was described as the period from the initial TACE treatment to death attributable to any cause, and patients alive at the end of follow-up were recorded as censored.
The statistical analyses were conducted using R software (Version 4.2.1, http://www.r-project.org). Categorical variables are presented as n (%) and were compared using the chi-square test or Fisher’s exact test. Continuous variables are reported as the mean ± SD, compared by Student’s t test, or median (interquartile range, IQR), compared by the Mann-Whitney U test. We determined the optimal cut-off value for continuous variables and inflammation biomarkers, except AFP and tumour diameter, based on the outcome of OS among all patients, using the “surv_cutpoint” function form the “survminer” R package. OS was calculated using the Kaplan-Meier method, and the log-rank test was used to construct survival curves. Univariate and multivariate Cox proportional hazard regression were conducted to select the independent factors of OS. All factors with P < 0.1 in univariate Cox regression were selected for multivariate Cox regression. The nomograms were created using multivariate analysis in the training cohort. The final model selection for the nomograms was determined by a backwards step-down process with the Akaike information criterion.
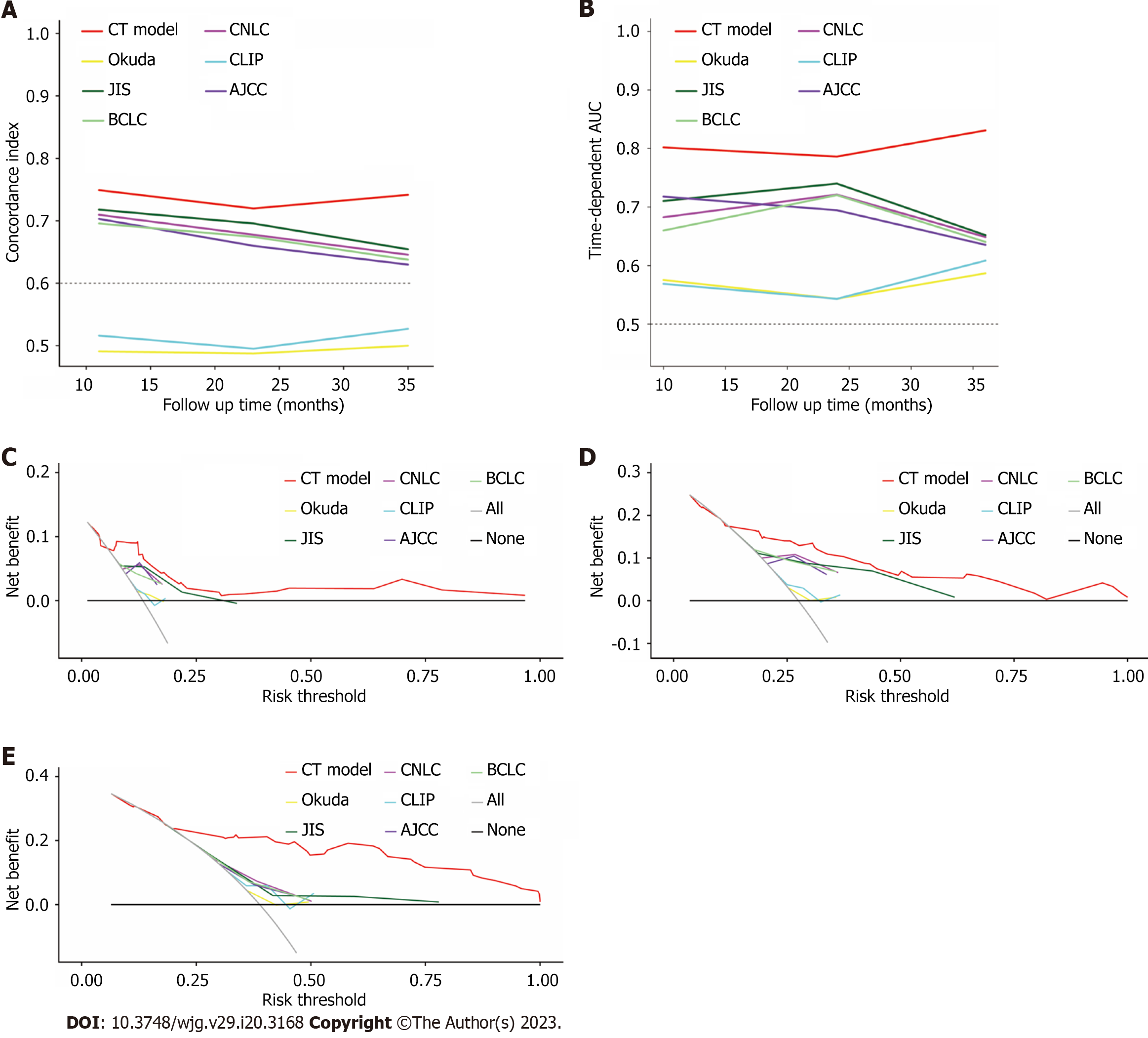
The predictive performance was measured by the concordance index (C-index) and time-dependent area under the curve (AUC). To assess the clinical utility of the nomogram, decision curve analysis (DCA) was performed by quantifying the net benefits relative-e to six other conventional HCC staging systems (including BCLC[14], American Joint Committee on Cancer Tumour-Node-Metastasis eighth edition (AJCC TNM 8th)[15], China liver cancer staging (CNLC)[16], Japan integrated staging (JIS)[17], Cancer of the Liver Italian Program (CLIP)[18] and Okuda staging system[19]). The total score of nomogram was used to categorize patients into low-risk and high-risk groups using X-tile[20]. A 2-sided P value of less than 0.05 in multivariate Cox regression was considered statistically significant.
A total of 150 patients were recruited in this study and randomly divided into training (n = 120) and validation (n = 30) cohorts in an 8:2 ratio. The baseline characteristics of patients in the training and validation cohorts are summarized in Table 1. Among all patients, the mean age was 52.1 years (SD: 11.9), 128 (85.3%) patients were male, and 134 (89.3%) patients were HBsAg positive. Regarding TACE techniques, a total of 100 (66.7%) patients received cTACE therapy. In terms of surgical aspects, a resection margin larger than 1 cm was present in 133 patients (88.7%). Fifty-two patients (34.7%) required a blood transfusion during the perioperative period. The median size of the largest intrahepatic tumours was 62.5 mm (range: 10.0-191.0), and 100 (66.7%) were larger than 5.0 cm. More than half of the patients (n = 96, 64.0%) were with solitary tumour. Tumour radiologic features showed that well-defined tumour capsule, satellite nodules, and MVI were observed in 110 (73.3%), 36 (24.0%) and 68 (45.3%) patients, respectively. There were no statistically significant differences in baseline clinicopathological features between the training and validation cohorts.
| Variables | Training cohort (n = 120) | Validation cohort (n = 30) | Overall cohort (n = 150) | P value | |
| Patient factors | |||||
| Age [yr, mean (SD)] | 51.5 (12.1) | 54.4 (10.9) | 52.1 (11.9) | 0.419 | |
| Sex, male/female | 104/16 (86.7%/13.3%) | 24/6 (80.0%/20.0%) | 128/22 (85.3%/14.7%) | 0.526 | |
| Etiology, HBV/other | 109/11 (90.8%/9.2%) | 25/5 (83.3%/16.7%) | 134/16 (89.3%/10.7%) | 0.39 | |
| Ascites, yes/no | 20/100 (16.7%/83.3%) | 7/23 (23.3%/76.7%) | 27/123 (18.0%/82.0%) | 0.559 | |
| Child-Pugh, A/B | 114/6 (95.0%/5.0%) | 29/1 (96.7%/3.3%) | 143/7 (95.3%/4.7%) | 1 | |
| Laboratory parameters | |||||
| AFP (ng/mL, ≤ 200/> 200) | 68/52 (56.7%/43.3%) | 15/15 (50.0%/50.0%) | 83/67 (55.3%/44.7%) | 0.652 | |
| HGB [g/dL, median (min, max)] | 139 (72, 838) | 139 (100, 162) | 139 (72, 838) | 0.714 | |
| PLT [count, × 109/L, median (min, max)] | 197 (70, 950) | 189 (97, 370) | 192 (70, 950) | 0.873 | |
| AST [U/L, median (min, max)] | 36.0 (2, 471) | 33.0 (16, 251) | 35.0 (2, 471) | 0.925 | |
| ALT [U/L, median (min, max)] | 34.0 (9, 423) | 29.0 (12, 329) | 34.0 (9, 423) | 0.541 | |
| ALB (g/L, ≤ 35/> 35) | 18/102 (15.0%/85.0%) | 2/28 (6.7%/93.3%) | 20/130 (13.3%/86.7%) | 0.368 | |
| TB [μmol/L, median (min, max)] | 12.5 (3.2, 28.2) | 12.9 (4.3, 30.9) | 12.7 (3.2, 30.9) | 0.996 | |
| GGT [U/L, median (min, max)] | 59.5 (15, 435) | 57.0 (22, 375) | 59.0 (15, 435) | 0.987 | |
| BUN (mmol/L, ≤ 6.7/> 6.7( | 109/11 (90.8%/9.2%) | 26/4 (86.7%/13.3%) | 135/15 (90.0%/10.0%) | 0.734 | |
| Creatinine [μmol/L, median (min, max)] | 77.6 (23, 218) | 74.0 (43, 110) | 77.0 (23, 218) | 0.263 | |
| UA [μmol/L, median (min, max)] | 335 (172, 680) | 329 (143, 572) | 334 (143, 680) | 0.899 | |
| PT [s, median (min, max)] | 13.6 (11.1, 16.5) | 13.6 (12.1, 15.6) | 13.6 (11.1, 16.5) | 0.212 | |
| Tumor factors | |||||
| Tumor number (1/2/≥ 3) | 80/22/18 (66.7%/18.3%/15.0%) | 16/7/7 (53.3%//23.3%/23.3%) | 96/29/25 (64.0%/19.3%/16.7%) | ||
| Tumor size (cm, ≤ 5/> 5) | 39/81 (32.5%/67.5%) | 11/19 (36.7%/63.3%) | 50/100 (33.3%/66.7%) | 0.829 | |
| MVI, yes/no | 55/65 (45.8%/54.2%) | 13/17 (43.3%/56.7%) | 68/82 (45.3%/54.7%) | 1 | |
| Tumor capsule, ill-defined/well-defined | 29/91 (24.2%/75.8%) | 11/19 (36.7%/63.3%) | 40/110 (26.7%/73.3%) | 0.249 | |
| Satellite nodules, yes/no | 26/94 (21.7%/78.3%) | 10/20 (33.3%/66.7%) | 36/114 (24.0%/76.0%) | 0.272 | |
| Surgical factors | |||||
| TACE (c-TACE/DEB-TACE) | 79/41 (65.8%/34.2%) | 21/9 (70.0%/30.0%) | 100/50 (66.7%/33.3%) | 0.829 | |
| Resection Margin (cm, < 1/≥ 1) | 13/107 (10.8%/89.2%) | 4/26 (13.3%/86.7%) | 17/133 (11.3%/88.7%) | 0.949 | |
| Blood transfusion, yes/no | 38/82 (31.7%/68.3%) | 14/16 (46.7%/53.3%) | 52/98 (34.7%/65.3%) | 0.184 | |
| Conventional staging system | |||||
| AJCC (I/II/III) | 42/20/58 (35.0%/16.7%/48.3%) | 10/4/16 (33.3%/13.3%/53.3%) | 52/24/74 (34.7%/16.0%/49.3%) | 0.889 | |
| BCLC (A/B/C) | 31/35/54 (25.8%/29.2%/45.0%) | 10/7/13 (33.3%/23.3%/43.3%) | 41/42/67 (27.3%/28.0%/44.7%) | 0.678 | |
| CNLC (I/II/III) | 47/19/54 (39.2%/15.8%/45.0%) | 12/5/13 (40.0%/16.7%/43.3%) | 59/24/67 (39.3%/16.0%/44.7%) | 1 | |
| Okuda (I/II/III) | 65/54/1 (54.2%/45.0%/0.8%) | 15/15/0 (50.0%/50.0%/0%) | 80/69/1 (53.3%/46.0%/0.7%) | 0.749 | |
| JIS (0/1/2/3/4) | 1/42/55/21/1 (0.8%/35.0%/45.8%/17.5%/0.8%) | 0/10/12/8/0 (0%/33.3%/40.0%/26.7%/0%) | 1/52/67/29/1 (0.7%/34.7%/44.7%/19.3%/0.7%) | 0.716 | |
| CLIP (0/1/2/3/4) | 27/28/35/21/9 (22.5%/23.3%/29.2%/17.5%/7.5%) | 7/7/9/4/3 (23.3%/23.3%/30.0%/13.3%/10.0%) | 34/35/44/25/12 (22.7%/23.3%/29.3%/16.7%/8.0%) | 0.962 | |
| Inflammation index | |||||
| GPR (≤ 0.38/> 0.38) | 66/54 (55.0%/45.0%) | 18/12 (60.0%/40.0%) | 84/66 (56.0%/44.0%) | 0.773 | |
| LMR (≤ 2.39/> 2.39) | 26/94 (21.7%/78.3%) | 6/24 (20.0%/80.0%) | 32/118 (21.3%/78.7%) | 1 | |
| NLR (≤ 2.00/> 2.00) | 60/60 (50.0%/50.0%) | 16/14 (53.3%/46.7%) | 76/74 (50.7%/49.3%) | 0.903 | |
| PLR (≤ 84.38/> 84.38) | 35/85 (29.2%/70.8%) | 13/17 (43.3%/56.7%) | 48/102 (32.0%/68.0%) | 0.204 | |
| NrLR (≤ 644.61/> 644.61) | 106/14 (88.3%/11.7%) | 28/2 (93.3%/6.7%) | 134/16 (89.3%/10.7%) | 0.643 | |
| APRI (≤ 0.30/> 0.30) | 91/29 (75.8%/24.2%) | 20/10 (66.7%/33.3%) | 111/39 (74.0%/26.0%) | 0.429 | |
| ANRI (≤ 4.76/> 4.76) | 10/110 (8.3%/91.7%) | 4/26 (13.3%/86.7%) | 14/136 (9.3%/90.7%) | 0.623 | |
| ALRI (≤ 41.96/> 41.96) | 97/23 (80.8%/19.2%) | 24/6 (80.0%/20.0%) | 121/29 (80.7%/19.3%) | 1 | |
| PNI (≤ 52.62/> 52.62) | 93/27 (77.5%/22.5%) | 22/8 (73.3%/26.7%) | 115/35 (76.7%/23.3%) | 0.809 | |
| SII (≤ 336.22/> 336.22) | 48/72 (40.0%/60.0%) | 13/17 (43.3%/56.7%) | 61/89 (40.7%/59.3%) | 0.901 | |
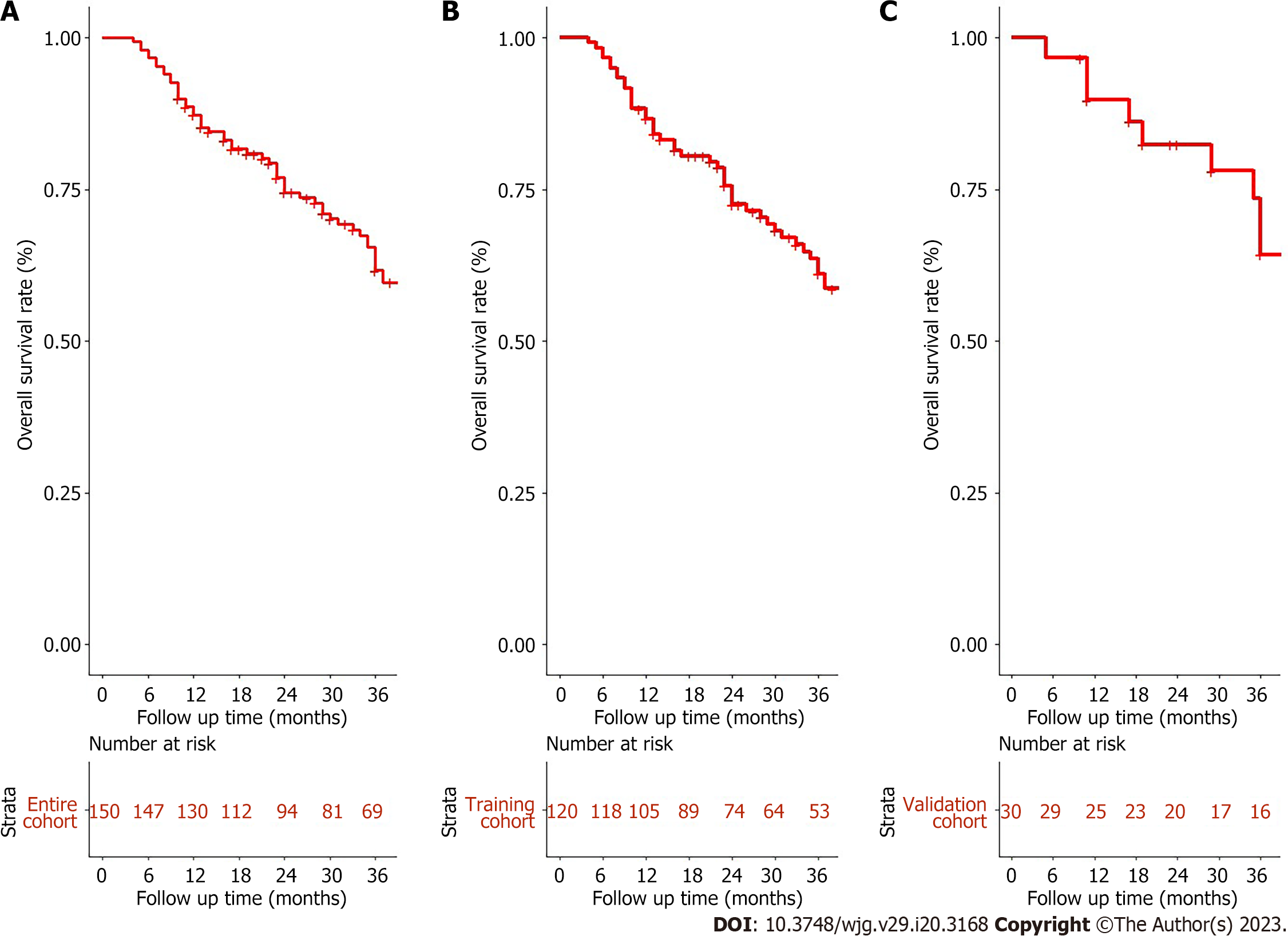
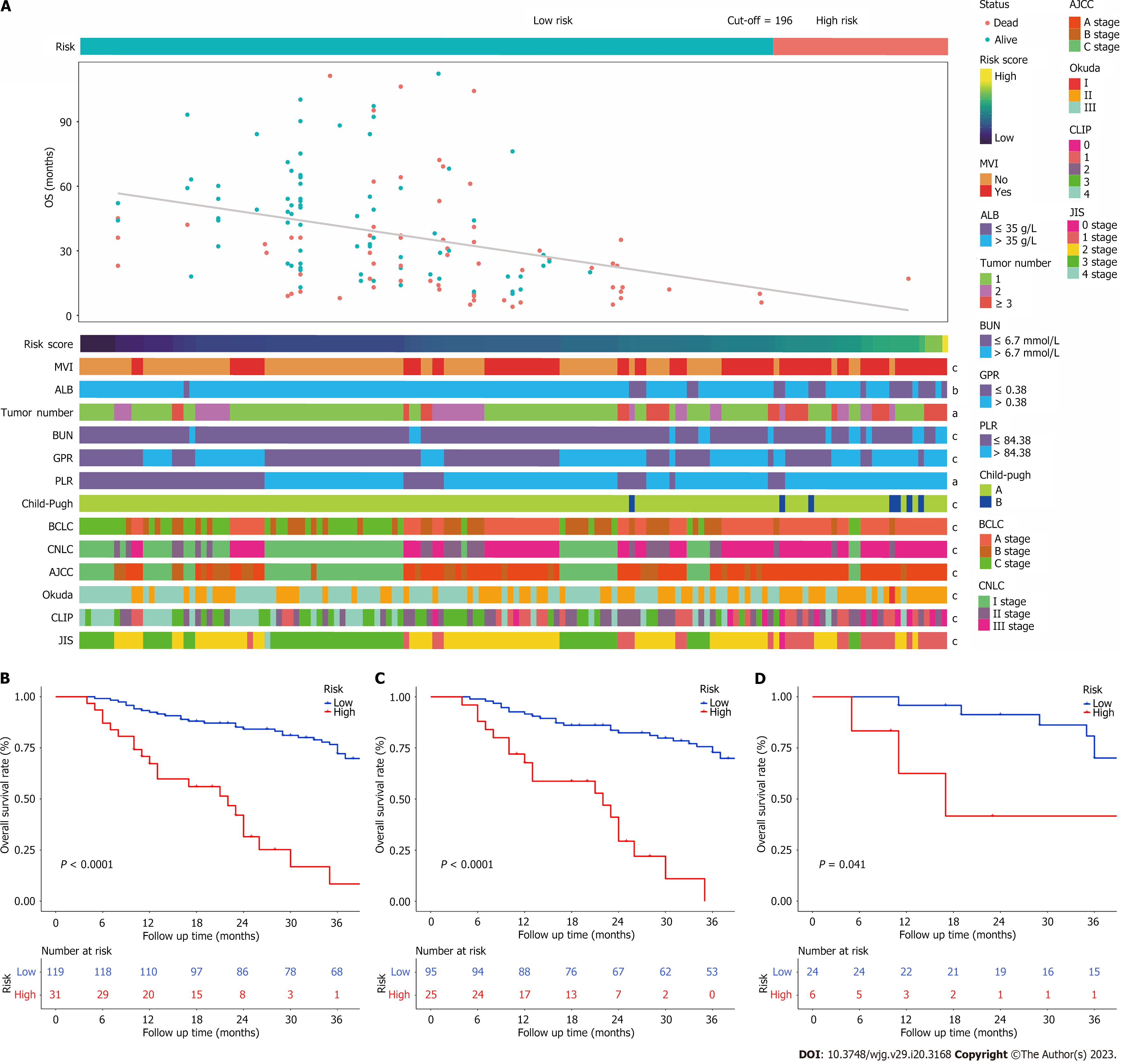
In this study, the median follow-up was 31.5 (range: 4.0-112.0) months. The median survival of the entire cohort was 54 [95% confidence interval (CI): 42-95] mo (Figure 1A). The 1-, 2-, and 3-year OS rates for the training and validation cohorts were 87.5%, 49.3%, 44.2%, and 83.3%, 66.7%, 53.3%, respectively (Figure 1B and C). There was no significant difference observed in OS between the training and validation cohorts [mean OS 37.9 (range, 4-112) mo vs 38.0 (range, 5-93 mo, P = 0.7].
Univariate and multivariate Cox regression analyses were performed in 120 patients from the training cohort to determine the risk factors associated with OS (Supplementary Table 2). As previously described, the optimal cutoff values for inflammation biomarkers were recorded (Supple
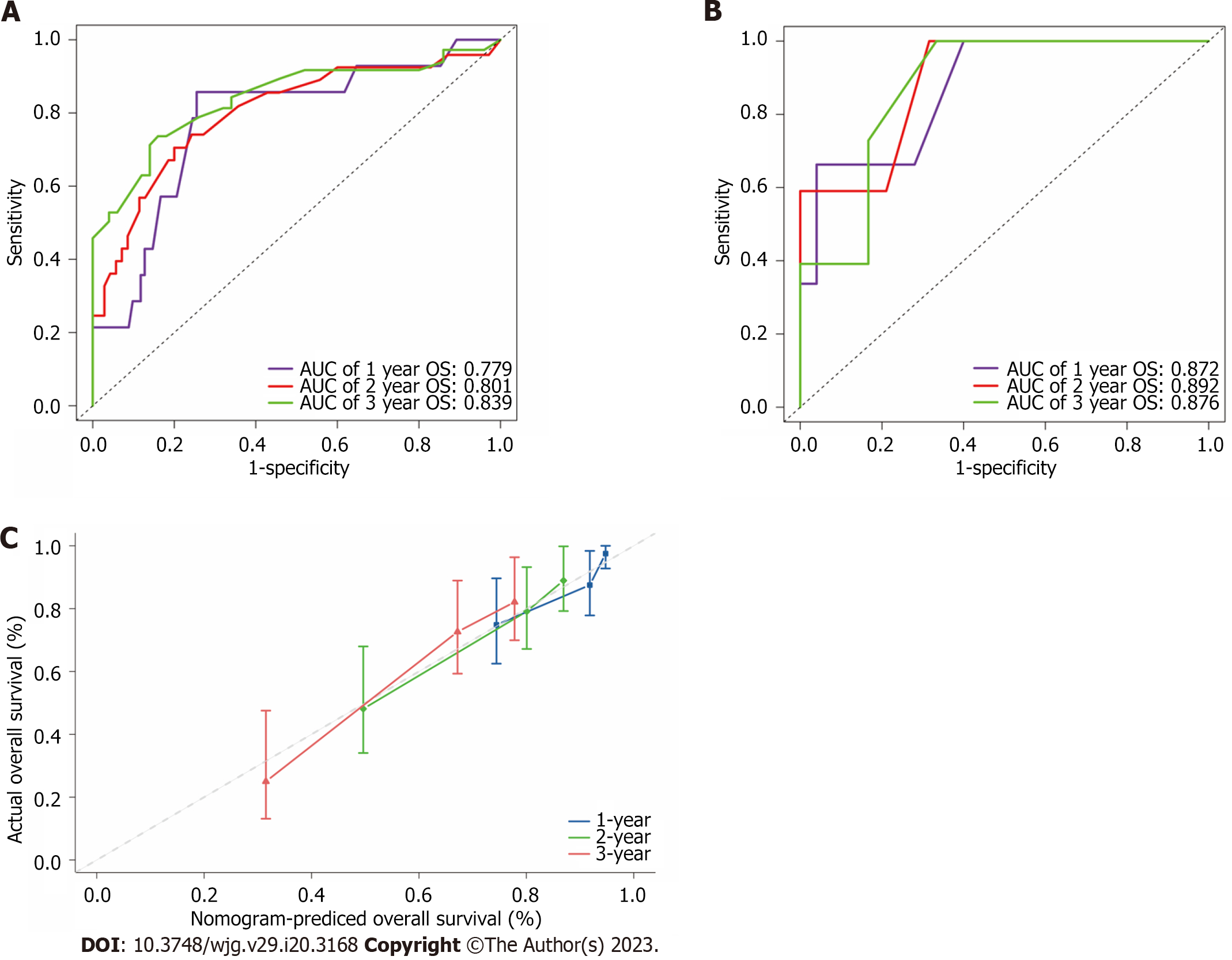
Bootstrapping with 1000 resamples in the primary training cohort revealed good predictive performance for OS, resulting in a C-index of 0.752 (95%CI: 0.688-0.815). The AUC in predicting the
The discrimination power of the nomogram and common staging systems were compared by the C-index and time-dependent AUC (1, 2, and 3 years). In both the training and validation cohorts, the predictive performance of the CT model was superior to that of the other six conventional HCC staging systems (P < 0.01, Figure 5A and B, Supplementary Table 4). DCA indicated a good net benefit compared with six other conventional HCC staging systems at 1, 2 and 3 years after conversion therapy in the training cohort (Figure 5C-E).
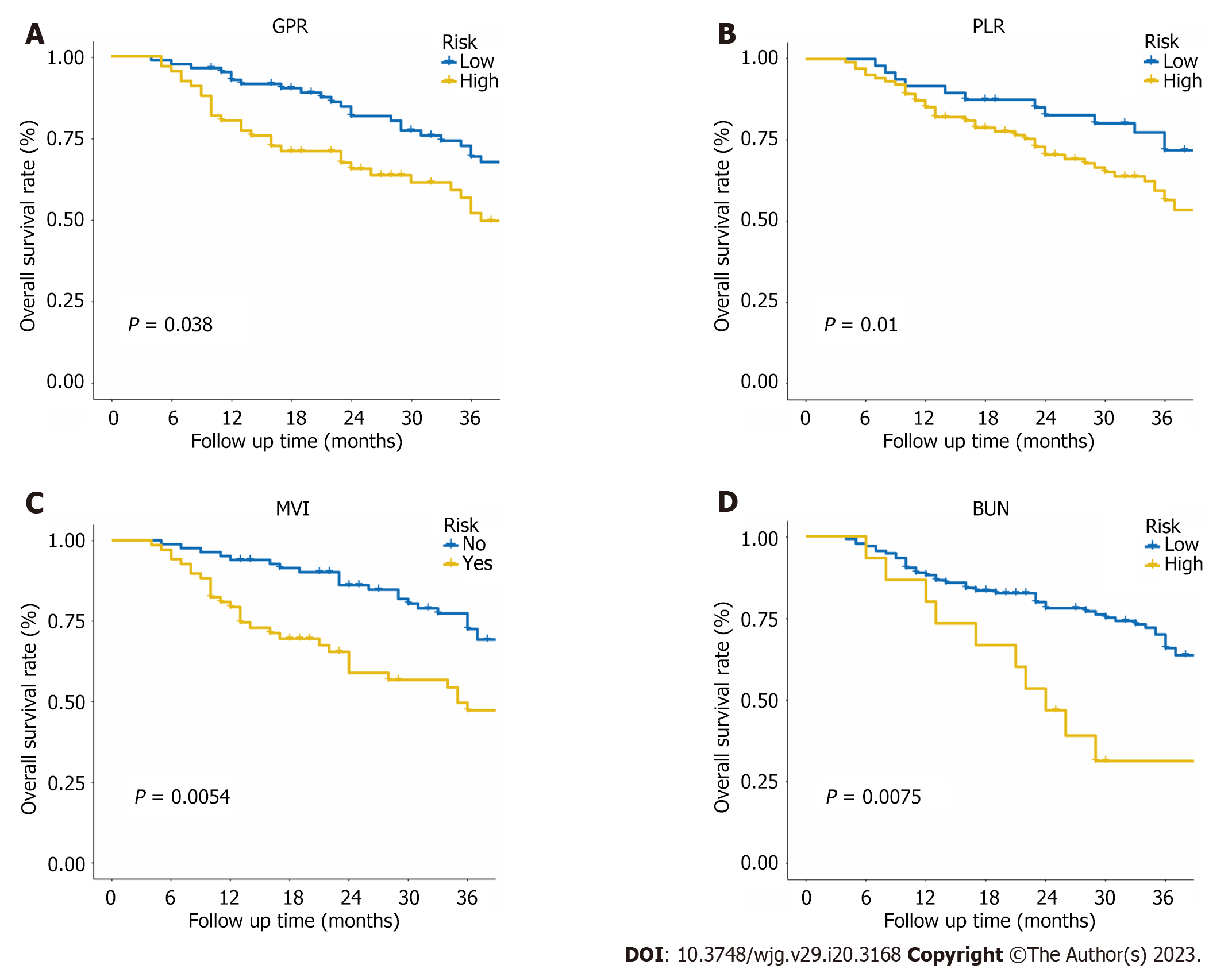
The patients were categorized into two groups based on their overall predictive risk score: Low risk (score < 196) and high risk (score ≥ 196). The distribution of clinical features and corresponding risk scores in each patient are presented in Figure 6A. The OS curves exhibited significant discrimination between the low-risk and high-risk groups among the entire (P < 0.001), training (P < 0.001) and validation (P = 0.041) cohorts (Figure 6B-D). Additionally, given that inflammation biomarkers are associated with postoperative prognosis, we found that high values of both GPR and PLR were significantly correlated with worse prognostic outcomes in patients after conversion therapy. The low-GPR group exhibited higher 1-, 2-, and 3-year OS rates compared to the high-GPR group (0.93, 0.69, and 0.54 vs 0.79, 0.55, and 0.36, P = 0.038). Similarly, the low-PLR group demonstrated greater 1-,
Conversion therapy is a new therapeutic strategy for individuals diagnosed with HCC at an advanced stage and who may not initially be suitable for radical surgery. Several studies have shown that patients with unresectable HCC who underwent conversion following surgical excision have a much longer OS than those who received palliative treatments such as TACE[3,6,21]. Furthermore, 5-year survival rates for patients with unresectable HCC after conversion therapy (25%-57%) are comparable to those for patients with resectable HCC (30%-60%)[22]. Previous studies have developed prognostic models using preoperative factors to predict postoperative outcomes of early-stage HCC patients after curative resection that achieved good results[9-11], but it remains unclear in the CT model. In this study, we developed and validated a CT model that could accurately predicts prognosis for patients with unresectable HCC treated with TACE following HR.
The nomogram demonstrated great accuracy in predicting conversion therapy outcomes of patients with unresectable HCC. The nomogram developed in our study achieved AUC of 0.779, 0.801, and 0.839 in the training cohort and 0.872, 0.892 and 0.876 in the validation cohort, which were superior to those of the other six conventional HCC staging systems. DCA was also performed and revealed that the nomograms have good clinical utility in patients with unresectable HCC who received the CT model.
Using the nomogram score, clinicians can classify unresectable HCC patients into low- and high-risk groups. Effective postoperative therapeutic management and intensive clinical follow-up can be guided for high-risk individuals following this strategy. Additionally, a web-based dynamic nomogram provides a well-calibrated tool for predicting future clinical outcomes.
The present study combined liver and renal function factors, tumour burden and inflammation biomarkers (six significantly independent predictors: ALB, BUN, MVI, tumour number, GPR, and PLR) to establish a more comprehensive model and construct a nomogram CT model that could accurately predict the prognosis for patients with unresectable HCC after conversion therapy. Our findings are consistent with research in previous HCC populations that lower ALB level, MVI presence and multiple tumours were risk factors for long-term survival in patients with unresectable HCC with the CT model[10,23-26].
In terms of clinical utility, the prediction ability of the CT model was significantly improved compared with that of six conventional staging systems (AJCC TNM 8th, CNLC, JIS, CLIP, Okuda, and BCLC staging systems) in both the training and validation cohorts (Figure 5). The conventional staging systems mainly contain factors that may only take certain aspects of the tumour into consideration. Previous studies have noted that inflammatory biomarker combination scores have promising prognostic values in predicting patient survival for different HCC stages in various therapeutic modalities[27,28], indicating that combining these potential indicators would greatly improve the model’s predictive abilities. Meanwhile, the AUC for the prediction of the 1-, 2-, and 3-year OS rates reached 0.755, 0.750, and 0.838 in the training cohort when we only used clinical information (MVI, ALB, tumour number and BUN) to develop a model, and they were unable to perform robustly in the validation cohort (Supplementary Figure 2). Hence, we constructed a nomogram CT model with clinical information (ALB, BUN, MVI, tumour number) and inflammation biomarkers (PLR and GPR).
PLR and GPR have been reported to be risk indicators of prognosis for OS in previous studies[10,29]. Meanwhile, high GPR was also proven to be an independent risk factor for HCC development and recurrence[30-32]. Our findings indicate that the high-PLR (> 84.38) and high-GPR (> 0.38) groups had significantly lower 12-, 24-, and 36-mo survival rates. Since inflammatory factors in the tumor microenvironment are crucial for HCC therapies, this information can assist clinicians in devising follow-up regimens and postoperative therapies for HCC patients at high or low risk of tumor recurrence after hepatectomy.
We also found that BUN was an independent prognostic factor in this study. Renal function impairment represents a clinically significant event in cirrhosis patients[33]. Preoperative renal dysfunction with high levels of BUN may lead to the development of major complications such as intractable ascites and spontaneous bacterial peritonitis (SBP). Moreover, the development of hepatorenal impairment is strongly associated with circulatory dysfunction, which can lead to various complications such as complicated or refractory ascites, SBP, hyponatremia and hepatorenal syndrome, and a high risk of mortality[34]. In clinical practice, serum creatinine concentration is generally used to assess kidney function in patients with liver diseases, which is also a major prognostic factor of cirrhosis included in the Model for End-Stage Liver Disease. However, several reports have shown that BUN levels have greater prognostic accuracy than serum creatinine concentrations[35-37]. In this study, our findings also showed that high BUN levels were related to worse liver function stage and poor OS outcomes (Figure 7D). Further research is needed to better understand this factor in advanced HCC and better classify and manage patients after conversion therapy.
While this nomogram CT model performed well, we do acknowledge a few potential limitations to this study. First, the nomogram was formulated based on a relatively small sample size obtained from our single institution, which unavoidably led to selection bias. Further prospective and multicenter cohorts are required to evaluate its performance. Second, statistical power may be limited because several routine clinical variables were not included in our study. Because our study was retrospective, there were variations in performance score, body mass index, dosage and type of injected drugs during TACE, as well as postoperative treatments, among patients. To further bolster our findings, it is necessary to conduct larger and multicenter prospective cohorts. Hence, we will undertake additional research that incorporates more complete clinicopathological information, treatment details, and postoperative treatment modalities to improve the predictive performance of our model. Third, this study was mainly based on HBV-infected patients. Our results may not be applicable in different geographic regions and aetiologies. Fourth, systematic bioinformatic analyses were not included in this nomogram, which showed excellent predictive ability for early-stage HCC patients[38,39]. We will therefore develop a robust prognostic model that integrates both clinical parameters and multi-omics biomarkers to facilitate an understanding of HCC from a biological perspective and contribute to tailoring therapeutic strategies. Despite these limitations, we believe that our work is meaningful for individualized management in HCC patients following conversion therapy.
We developed and validated a prognostic nomogram combined with inflammation biomarkers for patients with unresectable HCC after conversion therapy. The proposed CT model shows increased accuracy, good clinical utility, and better prognostic performance than conventional staging systems. Furthermore, we have uploaded this prognostic nomogram online for free use (https://ctmodelforunresectablehcc.shinyapps.io/DynNomapp/).
Larger and multicentre prospective cohorts were required to further strengthen our results. We will conduct further investigation that incorporates more complete clinicopathological information, treatment details, and postoperative treatment modalities to improve the predictive performance of our model.
The nomogram achieved optimal individualized prognostication of OS in HCC patients who received conversion therapy. It could be a useful clinical tool to help guide postoperative personalized interventions and prognosis judgement.
Multivariate Cox analysis identified that albumin, blood urea nitrogen, gamma-glutamyl transpeptidase to platelet ratio, platelet to lymphocyte ratio, macrovascular invasion and tumour number were the six independent prognostic factors correlated with OS in nomogram model. The C-indices in the training cohort and validation cohort were 0.752 and 0.807 for predicting OS, which were higher than those of the six conventional HCC staging systems (0.563 to 0.715 for the training cohort and 0.458 to 0.571 for the validation cohort). We have deployed the model into online calculators that are freely available at https://ctmodelforunresectablehcc.shinyapps.io/DynNomapp/.
All patients met the inclusion criteria were enrolled and divided into training and a validation cohort. Using the independent risk factors in the training cohort, nomogram models were constructed to predict OS for patients treated with transarterial chemoembolization following HR. The nomograms were internally validated with the bootstrapping method. The predictive performance of the nomograms was assessed by Harrell’s concordance index, calibration plot and time-dependent receiver operating characteristic curves and compared with six other conventional HCC staging systems.
To develop a nomogram to help guide postoperative personalized interventions and prognosis judgement.
To investigate the prognostic factors of overall survival (OS) in patients with unresectable HCC who received conversion therapy.
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related mortality worldwide. Hepatic resection (HR) is the best therapeutic option for patients with early- and some intermediate-stage HCC. Unfortunately, the majority of Chinese patients with HCC are diagnosed at intermediate or advanced stages with massive or multifocal lesions. HR is possible for a minority of carefully selected patients with the help of a “conversion therapy” strategy, which refers to conversion of an unresectable HCC to achieve adequate tumour shrinkage and downstaging to undergo HR.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li YW, China; Yang M, United States S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3173] [Article Influence: 528.8] [Reference Citation Analysis (37)] |
| 2. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 3. | Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, Zhao M, Bi X, Li G, Bai X, Ji Y, Xu L, Zhu XD, Bai D, Chen Y, Dai C, Guo R, Guo W, Hao C, Huang T, Huang Z, Li D, Li T, Li X, Liang X, Liu J, Liu F, Lu S, Lu Z, Lv W, Mao Y, Shao G, Shi Y, Song T, Tan G, Tang Y, Tao K, Wan C, Wang G, Wang L, Wang S, Wen T, Xing B, Xiang B, Yan S, Yang D, Yin G, Yin T, Yin Z, Yu Z, Zhang B, Zhang J, Zhang S, Zhang T, Zhang Y, Zhang A, Zhao H, Zhou L, Zhang W, Zhu Z, Qin S, Shen F, Cai X, Teng G, Cai J, Chen M, Li Q, Liu L, Wang W, Liang T, Dong J, Chen X, Wang X, Zheng S, Fan J; Alliance of Liver Cancer Conversion Therapy, Committee of Liver Cancer of the Chinese Anti-Cancer Association. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11:227-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 4. | Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, Aikata H, Nagata Y, Chayama K, Ohdan H. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg. 2017;44:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Zhao HT, Cai JQ. Chinese expert consensus on neoadjuvant and conversion therapies for hepatocellular carcinoma. World J Gastroenterol. 2021;27:8069-8080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, Zhou J, Qiu SJ, Lu JZ. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Lau WY, Lai EC. Salvage surgery following downstaging of unresectable hepatocellular carcinoma--a strategy to increase resectability. Ann Surg Oncol. 2007;14:3301-3309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8581] [Cited by in RCA: 8326] [Article Influence: 489.8] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Sun K, Shen J, Li B, Kuang M, Cao Q, Peng S. Novel Prognostic Nomograms Based on Inflammation-Related Markers for Patients with Hepatocellular Carcinoma Underwent Hepatectomy. Cancer Res Treat. 2019;51:1464-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Zeng J, Zeng J, Liu J. Development of pre and post-operative nomograms to predict individual survival for ideal liver resection candidates with hepatocellular carcinoma. Liver Int. 2021;41:2974-2985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Wang JC, Hou JY, Chen JC, Xiang CL, Mao XH, Yang B, Li Q, Liu QB, Chen J, Ye ZW, Peng W, Sun XQ, Chen MS, Zhou QF, Zhang YJ. Development and validation of prognostic nomograms for single large and huge hepatocellular carcinoma after curative resection. Eur J Cancer. 2021;155:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Chen J, Lai L, Luo J, Wang H, Li M, Huang M. DEM-TACE as the initial treatment could improve the clinical efficacy of the hepatocellular carcinoma with portal vein tumor thrombus: a retrospective controlled study. BMC Cancer. 2022;22:1242. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Chen J, Lai L, Lin Q, Huang W, Cai M, Zhu K, Huang M. Hepatic resection after transarterial chemoembolization increases overall survival in large/multifocal hepatocellular carcinoma: a retrospective cohort study. Oncotarget. 2017;8:408-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 15. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4402] [Article Influence: 550.3] [Reference Citation Analysis (4)] |
| 16. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (1)] |
| 17. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 538] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 18. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 19. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 20. | Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252-7259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1947] [Cited by in RCA: 2939] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 21. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization vs radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Zhang T, Merle P, Wang H, Zhao H, Kudo M. Combination therapy for advanced hepatocellular carcinoma: do we see the light at the end of the tunnel? Hepatobiliary Surg Nutr. 2021;10:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Wang YY, Xiang BD, Ma L, Zhong JH, Ye JZ, Wang K, Xing BC, Li LQ. Development and Validation of a Nomogram to Preoperatively Estimate Post-hepatectomy Liver Dysfunction Risk and Long-term Survival in Patients With Hepatocellular Carcinoma. Ann Surg. 2021;274:e1209-e1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Zhang B, Zhang B, Zhang Z, Huang Z, Chen Y, Chen M, Bie P, Peng B, Wu L, Wang Z, Li B, Fan J, Qin L, Chen P, Liu J, Tang Z, Niu J, Yin X, Li D, He S, Jiang B, Mao Y, Zhou W, Chen X. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Zeng J, Zeng J, Lin K, Lin H, Wu Q, Guo P, Zhou W, Liu J. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr. 2022;11:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 26. | Wang X, Mao M, He Z, Zhang L, Li H, Lin J, He Y, Dai S, Hu W, Liu W. Development and Validation of a Prognostic Nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Nakano M, Kuromatsu R, Niizeki T, Okamura S, Iwamoto H, Shimose S, Shirono T, Noda Y, Kamachi N, Koga H, Torimura T; Kurume Liver Cancer Study Group of Japan. Immunological inflammatory biomarkers as prognostic predictors for advanced hepatocellular carcinoma. ESMO Open. 2021;6:100020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Wang C, He W, Yuan Y, Zhang Y, Li K, Zou R, Liao Y, Liu W, Yang Z, Zuo D, Qiu J, Zheng Y, Li B. Comparison of the prognostic value of inflammation-based scores in early recurrent hepatocellular carcinoma after hepatectomy. Liver Int. 2020;40:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 29. | Li J, Liao Y, Suo L, Zhu P, Chen X, Dang W, Liao M, Qin L, Liao W. A novel prognostic index-neutrophil times γ-glutamyl transpeptidase to lymphocyte ratio (NγLR) predicts outcome for patients with hepatocellular carcinoma. Sci Rep. 2017;7:9229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Zhu YF, Tan YF, Xu X, Zheng JL, Zhang BH, Tang HR, Yang JY. Gamma-glutamyl transpeptidase-to-platelet ratio and the fibrosis-4 index in predicting hepatitis B virus-related hepatocellular carcinoma development in elderly chronic hepatitis B patients in China: A single-center retrospective study. Medicine (Baltimore). 2019;98:e18319. [PubMed] |
| 31. | Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, Cooke G, D'Alessandro U, Vray M, Mbaye PS, Njie R, Mallet V, Thursz M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 32. | Wang WL, Zheng XL, Zhang ZY, Zhou Y, Hao J, Tang G, Li O, Xiang JX, Wu Z, Wang B. Preoperative γ-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Medicine (Baltimore). 2016;95:e4087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 34. | Piano S, Tonon M, Angeli P. Management of ascites and hepatorenal syndrome. Hepatol Int. 2018;12:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, Sugimachi K. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Wang Q, Ma L, Li J, Yuan C, Sun J, Li K, Qin L, Zang C, Zhao Y, Zhang Y. A Novel Scoring System for Patients with Recurrence of Hepatocellular Carcinoma After Undergoing Minimal Invasive Therapies. Cancer Manag Res. 2019;11:10641-10649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Yoneyama K, Taniguchi H, Kiuchi Y, Shibata M, Mitamura K. Prognostic index of liver cirrhosis with ascites with and without hepatocellular carcinoma. Scand J Gastroenterol. 2004;39:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Liu F, Liu D, Wang K, Xie X, Su L, Kuang M, Huang G, Peng B, Wang Y, Lin M, Tian J. Deep Learning Radiomics Based on Contrast-Enhanced Ultrasound Might Optimize Curative Treatments for Very-Early or Early-Stage Hepatocellular Carcinoma Patients. Liver Cancer. 2020;9:397-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 39. | Wan S, Lei Y, Li M, Wu B. A prognostic model for hepatocellular carcinoma patients based on signature ferroptosis-related genes. Hepatol Int. 2022;16:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |