Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3119
Peer-review started: March 3, 2023
First decision: April 12, 2023
Revised: April 21, 2023
Accepted: May 4, 2023
Article in press: May 4, 2023
Published online: May 28, 2023
Processing time: 84 Days and 6.2 Hours
Pancreatic mucinous cystic neoplasms (MCNs) represent one of the precursor lesions of pancreatic ductal adenocarcinoma, and their detection has been facilitated by advances in preoperative imaging. Due primarily to the rarity of MCNs, however, there is limited knowledge regarding the prognostic variables and high-risk factors for malignant transformation. A more comprehensive and nuanced approach is necessary to fill this gap and provide a basis for improved treatment decisions and patient outcomes.
To investigate the high-risk factors associated with malignant MCNs and to explore the prognostic factors of MCN with associated invasive carcinoma (MCN-AIC).
All cases of resected MCNs from a single high-volume institution between January 2012 and January 2022 were retrospectively reviewed. Only cases with ovarian-type stroma verified by progesterone receptor staining were included. Preoperative features, histological findings and postoperative course were documented. Multivariate logistic regression was employed to investigate variables related to malignancy. Survival analysis was performed using the Kaplan-Meier curve, and the prognostic factors were assessed to evaluate the postoperative course of patients with MCN-AIC.
Among the 48 patients, 36 had benign MCNs, and 12 had malignant MCNs (1 high-grade atypical hyperplasia and 11 MCN-AIC). Age, tumour size, presence of solid components or mural nodules and pancreatic duct dilatation were identified as independent risk factors associated with malignancy. The follow-up period ranged from 12 mo to 120 mo, with a median overall survival of 58.2 mo. Only three patients with MCN-AIC died, and the 5-year survival rate was 70.1%. All 11 cases of MCN-AIC were stage I, and extracapsular invasion was identified as a prognostic factor for poorer outcomes.
The risk factors independently associated with malignant transformation of MCNs included age, tumour size, presence of solid components or mural nodules, and pancreatic duct dilatation. Our study also revealed that encapsulated invasion was a favourable prognostic factor in MCN-AIC patients.
Core Tip: Pancreatic mucinous cystic neoplasms (MCNs) are a rare tumour with a low incidence and one of the precursor lesions of pancreatic ductal adenocarcinoma. The detection of MCN has been increasing by advances in imaging technology. MCNs associated risk factors, clinicopathological manifestations, and prognosis must be explored to improve our understanding of this rare tumour type and optimize clinical treatment.
- Citation: Xia Q, Li F, Min R, Sun S, Han YX, Feng ZZ, Li N. Malignancy risk factors and prognostic variables of pancreatic mucinous cystic neoplasms in Chinese patients. World J Gastroenterol 2023; 29(20): 3119-3132
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3119
Pancreatic mucinous cystic neoplasm (MCN) is a rare sporadic tumour with a low incidence, accounting for 10%-45% of all resected primary pancreatic cystic neoplasms and 2.5% of exocrine pancreatic tumours[1,2]. With the increasing popularity of imaging evaluation and advances in radiological technology, the rate of MCN identification is on the rise. Pancreatic ductal adenocarcinoma (PDAC) is the leading cause of cancer-related death globally, and its incidence has been consistently increasing[3]. However, the majority of PDAC cases are detected at an advanced stage, highlighting the importance of early diagnosis and treatment of associated precancerous lesions to improve survival rates. MCN is a precancerous lesion of PDAC and evolves from benign adenoma to invasive carcinoma similarly to intraductal papillary mucinous neoplasm (IPMN) and pancreatic intraepithelial neoplasia (Pan IN)[4].
However, MCNs have not been studied in sufficient detail or breadth owing to their infrequency. The risk factors associated with malignancy have not been well characterized, and physicians have not been unanimous in adopting an optimal management profile. Discrepancies in recommendations offered by several medical associations may contribute to further challenges in managing the disease. Moreover, despite PDAC being the most common histopathological type of invasive MCN, notable differences exist in prognosis. However, the precise prognostic determinants of MCNs have yet to be fully elu
Therefore, we conducted a retrospective study of the clinicopathological, radiological and survival data of a sizable cohort of patients. The findings of this study were intended to help identify key risk factors of malignant transformation in MCNs as well as the prognostic determinants of MCN with associated invasive carcinoma (MCN-AIC). The insights gained from this study may be used to inform the development of more effective management strategies for patients with MCNs, ultimately improving their clinical outcomes.
All cases of MCN identified at the Department of Pathology of the First Affiliated Hospital of Bengbu Medical College from January 2012 to January 2022 were retrospectively retrieved. A total of 48 patients who fit the diagnostic criteria were included in the study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. Written informed consent was obtained from all patients for the use of their clinical data.
The inclusion criteria were patients who had been confirmed by surgical pathology to have MCN, underwent imaging examinations, and did not receive preoperative radiotherapy or chemotherapy. The exclusion criteria included poor-quality radiographic images, insufficient pathological diagnostic data, the presence of other pancreatic diseases, and a history of other malignant tumours. These criteria were carefully selected to ensure the accuracy and reliability of the study’s findings.
All patients underwent preoperative imaging examinations, and the imaging data were uploaded to the Picture Archiving and Communication System. After reviewing and interpreting the data, two radiologists evaluated the radiographic tumour size, location, calcification, separation, solid com
From the date of diagnosis to January 31, 2022, 48 patients were followed up by reviewing their medical records, interviewing them via phone, or sending emails to acquire follow-up data, including post
The data were analyzed statistically using IBM SPSS Statistics (version 25.0) and R (version 4.0.0). The χ2 test or Fisher’s exact test was used to compare categorical data. The preoperative risk variables associated with malignant MCNs were evaluated using a multivariate binary logistic regression model. The cumulative survival rate was calculated using the Kaplan-Meier method, and the log rank test was performed to analyse the association between clinicopathological data and survival rate. P < 0.05 was regarded as indicating statistical significance.
A total of 48 patients diagnosed with MCN were included in accordance with strict inclusion and exclusion criteria. MCNs accounted for 2.8% (48/1685) of all pancreatic lesions resected over the same period. The median age of the patients was 47.2 years ± 13.1 years (range, 27-72 years), and 16 patients (33.3%) were more than 50 years old. There were 34 females and 14 males, with a female-to-male ratio of 2.2:1.0. Approximately 41.7% of patients were asymptomatic, including 18 patients whose disease was detected incidentally by imaging evaluation and 2 patients whose disease was identified by elevated preoperative carbohydrate antigen 19-9 (CA19-9) serology. The remaining 28 patients presented with nonspecific clinical symptoms, such as stomach pain, abdominal distension, abdominal discomfort, and jaundice. Serologic CA19-9 Levels were elevated in 16 patients (33.3%).
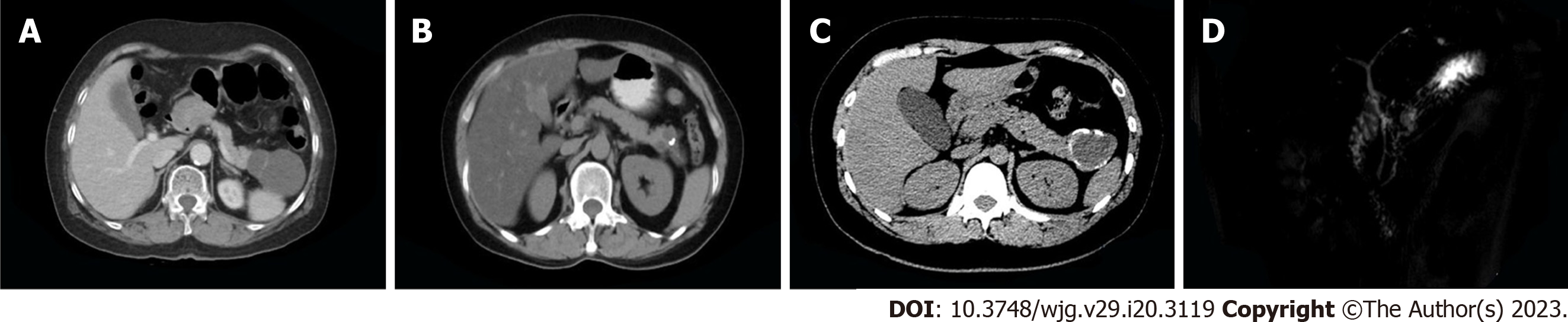
Imaging revealed oval-shaped, lobulated hypodense masses that were well circumscribed, separated linearly, and surrounded by egg-shell-like foci of calcification. The contents of the cyst were also visible. The average radiological diameter of the tumour was 5.8 cm ± 3.7 cm (1.2-14.5 cm), and in 23 patients (47.9%), the tumour measured more than 4 cm. Thirty-five patients (72.9%) had tumours that were located in the distal pancreas. Solid components or mural nodules were identified in 14 patients (29.2%), septations in 21 (43.85%), calcification in 13 (27.1%) and duct dilatation in 18 (37.5%) (Figure 1). Detailed data on the imaging findings and clinicopathological information of MCN patients are shown in Table 1.
| Variable | Benign group (n = 36) | Malignant group (n = 12) | P value |
| Sex | 0.142 | ||
| Male | 8 (22.2) | 6 (50.0) | |
| Female | 28 (77.8) | 6 (50.0) | |
| Age, yr | 0.013 | ||
| ≥ 50 | 8 (22.2) | 8 (66.7) | |
| < 50 | 28 (77.8) | 4 (33.3) | |
| Clinical symptoms | 0.499 | ||
| Yes | 20 (55.6) | 8 (66.7) | |
| No | 16 (44.4) | 4 (33.3) | |
| Serum CA19-9 level | 0.077 | ||
| Elevate | 9 (25) | 7 (58.3) | |
| Normal | 27 (75) | 5 (41.7) | |
| Tumor size, cm | 0.005 | ||
| ≥ 4 | 13 (36.1) | 10 (83.3) | |
| < 4 | 23 (63.9) | 2 (16.7) | |
| Location | 0.348 | ||
| Head and neck | 8 (22.2) | 5 (41.7) | |
| Body and tail | 28 (77.8) | 7 (58.3) | |
| Duct dilatation | 0.039 | ||
| Yes | 10 (27.8) | 8 (66.7) | |
| No | 26 (72.2) | 4 (33.3) | |
| Solid component/mural nodule | 0.039 | ||
| Yes | 10 (27.8) | 8 (66.7) | |
| No | 26 (72.2) | 4 (33.3) | |
| Septations | 0.867 | ||
| Yes | 16 (44.4) | 5 (41.7) | |
| No | 20 (55.6) | 7 (58.3) | |
| Calcification | 0.851 | ||
| Yes | 9 (25) | 4 (33.3) | |
| No | 27 (75) | 8 (66.7) |
MCN was correctly diagnosed using preoperative imaging in 35 patients (72.9%). The most common misdiagnoses involved other subtypes of pancreatic cystic lesions (PCLs) and the use of imprecise terms such as pancreatic cystic or solid masses. The preoperative imaging modalities employed in this study comprised computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS). Of the 48 patients, all underwent CT, 33 underwent MRI, and 21 underwent EUS. The corresponding detection accuracy rates were 64.6%, 87.9%, and 71.4%, respectively. Nevertheless, there was no significant difference in diagnostic accuracy among the three methods (P = 0.64).
Postoperative pathological diagnosis revealed 36 cases of LGD, 1 case of HGD, and 11 cases of MCN-AIC (the histological type of the invasive component was PDAC in all cases). All cases were solitary without multiple foci. The morphology of the cysts generally manifested as unilocular or multilocular with septa, and the fluid aspirated from the cystic cavity was frequently viscous.
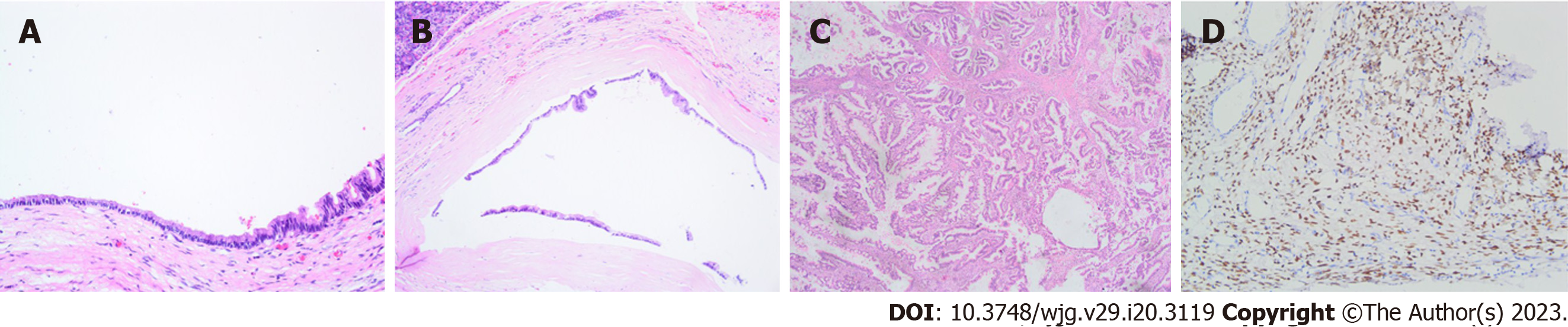
Microscopically, the cyst walls were found to be coated with mucinous columnar epithelium (Figure 2A and B). Subepithelial tissue in the MCN consisted of ovarian-type stroma (OTS) characterized by dense spindle cells expressing PR and possessing round or elongated nuclei with scant cytoplasm. Positive PR staining was observed in all tumours (Figure 2D). The mucin-producing columnar epithelial cells exhibited varying degrees of dysplasia. In cases of LGD, mild to moderate structural and cellular atypia was observed with occasional micropapillae (Figure 2A and B). HGD showed obvious atypia of both structures and cells, including multilayered cell arrangement, nuclear enlargement, loss of alignment polarity, and emergence of aberrant mitotic figures. In cases of MCN-AIC, interstitial infiltration was noted in comparison to HGD, with invasive components growing interspersed in the stroma (Figure 2C). Solid components, mural nodules, or nipple protrusion were also occasionally visible. Notably, 5 cases of MCN-AIC showed atypical hyperplasia adjacent to invasive components.
Based on malignant biological behaviours, LGD was defined as benign MCN in 36 cases (75%), while HGD and MCN-AIC were classified as malignant MCN in 12 cases (25%). Univariate analysis revealed that age ≥ 50 years [8 (66.7%) vs 8 (22.2%), P = 0.013], tumour size ≥ 4 cm [10 (83.3%) vs 13 (36.1%), P = 0.010], pancreatic duct dilatation [8 (66.7%) vs 10 (27.8%), P = 0.039], and presence of a solid component or mural nodule [8 (66.7%) vs 10 (27.8%), P = 0.039] were associated with malignant MCN. However, sex, clinical symptoms, serum CA19-9 Level, tumour location, septations and calcification did not correlate with malignancy (P < 0.05) (Table 1).
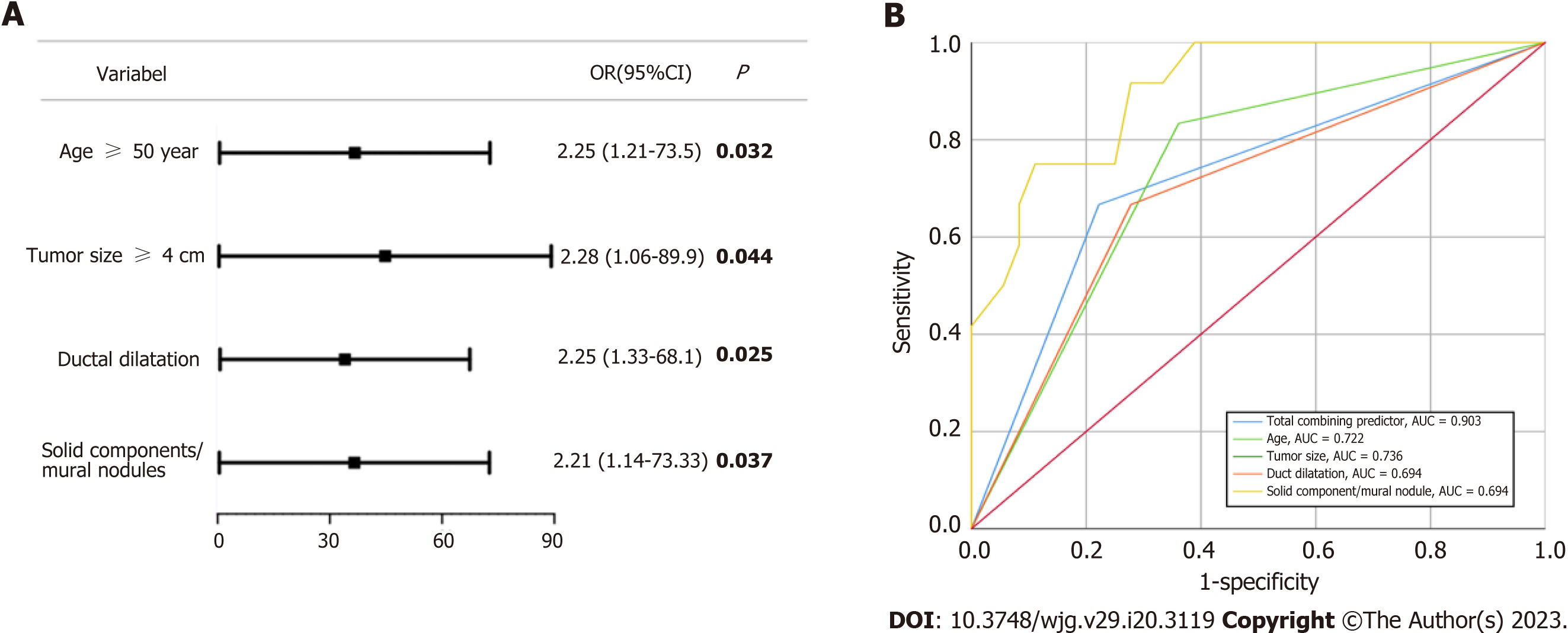
Further multivariate analysis demonstrated that all four statistically significant indicators were independent risk factors for the preoperative diagnosis of malignant MCNs (P < 0.05) (Figure 3). The prediction probability of each indicator for malignancy risk in a cohort of patients was then explored. The results of the analysis showed that age, tumour size, duct dilatation, and solid component/mural nodule all had good performance in predicting malignancy risk, as evidenced by the area under the curve (AUC) values of 0.722 (95%CI: 0.546-0.898, P = 0.220), 0.736 (95%CI: 0.578-0.894, P = 0.015), 0.694 (95%CI: 0.517-0.872, P = 0.046), and 0.694 (95%CI: 0.517-0.872, P = 0.046), respectively. Furthermore, when the four indicators were combined into a total predictor, the AUC value increased to 0.903 (95%CI: 0.814-0.992, P < 0.010), improving the accuracy of the prediction model.
The follow-up period ranged from 12 mo to 120 mo with a mean of 58.2 mo ± 32.7 mo. Of the 36 patients with benign MCNs, 6 were lost to follow-up, and 1 died of an abrupt cerebral infarction. The remaining 29 patients had an excellent prognosis; their 5-year disease-specific survival rate was 100%. Of the 12 patients with malignant MCNs, 3 died at 8, 14, and 46 mo after surgery; the 5-year disease-specific survival rate was 70.1%. All deaths occurred in MCN-AIC patients.
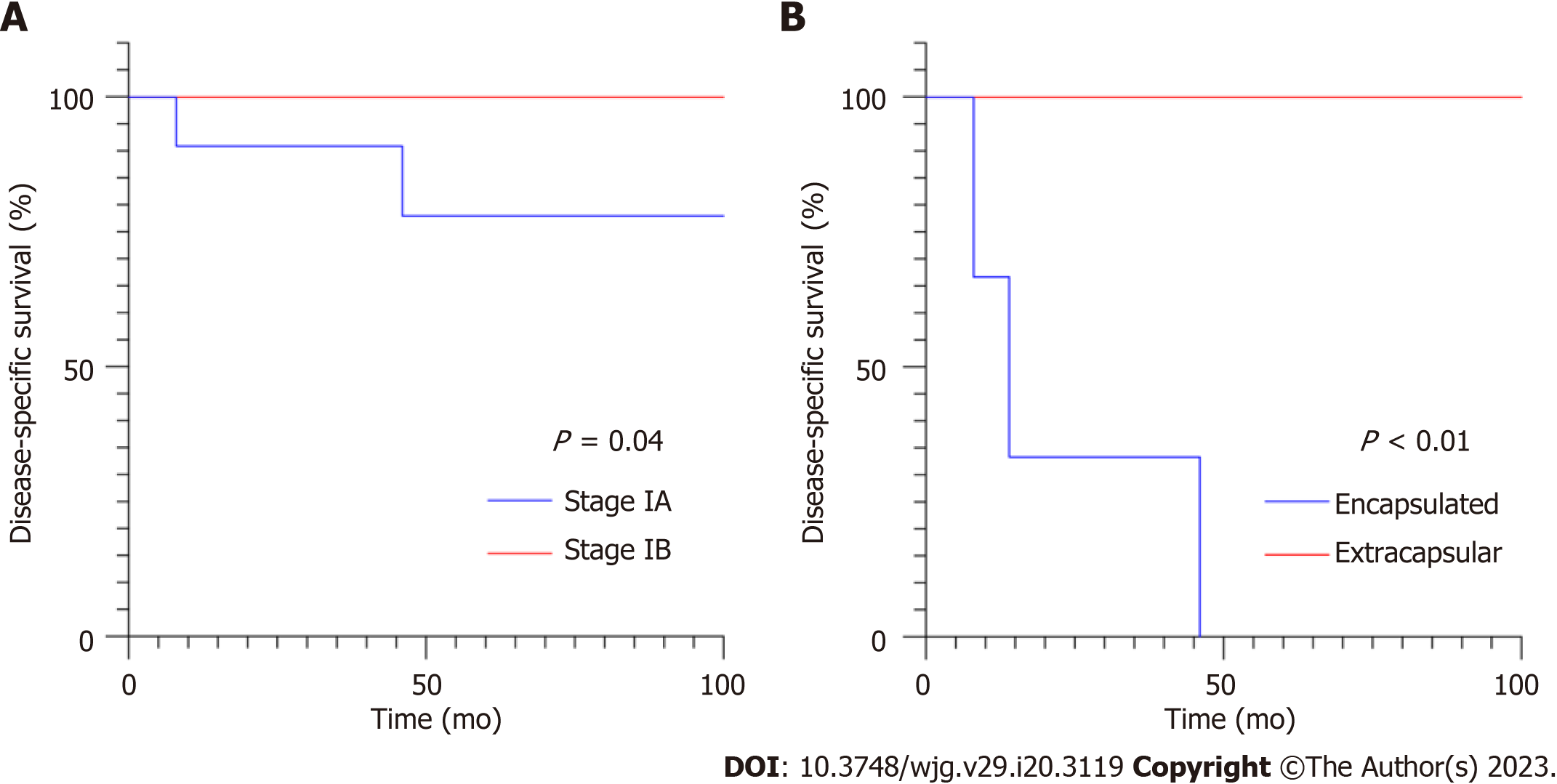
Consequently, the clinicopathological characteristics of the 11 MCN-AIC patients were further explored (Table 2). The tumours examined in this study did not display any signs of lymph node involvement, distant metastasis, or nerve invasion. In addition, all the tumours were successfully resected, and the margins were negative. Based on the AJCC cancer staging system[6], all 11 cases of MAC-AIC were categorized as stage I, with 7 patients having stage IA cancers and 4 having stage IB cancers, depending on the tumour size. The encapsulated invasion of MCN-AIC was defined as infiltrating components not exceeding the outermost layer of the capsule, with or without infiltration into the subepithelial stroma or cystic septa[7]. Using this definition, 9 tumours were encapsulated, while 3 were extracapsular (Cases 1-3). In Case 1 and Case 2, the cancerous tissue extended into the capsule and intersected with the surrounding pancreatic parenchyma. The tumour in Case 3 was located in the head of the pancreas and showed infiltration into the adjacent duodenal muscular layer. There was no significant difference in age (P = 0.301), tumour size (P = 0.109), sex (P = 0.402), postoperative adjuvant therapy (P = 0.952) or TNM stage (P = 0.400). However, the difference in infiltration of the capsule was statistically significant (P < 0.001) (Figure 4).
| Case | Age (yr) | Sex | Tumor size (cm) | Largest dimension (cm) | Invasion pattern | pT stage | pTNM stage | Status | Overall survival (mo) | Adjuvant chemotherapy |
| 1 | 72 | Female | 8.0 | 3.0 | Extracapsular | T2 | IB | Death | 8 | Yes |
| 2 | 60 | Male | 14.0 | 1.2 | Extracapsular | T1 | IA | Death | 14 | Yes |
| 3 | 57 | Male | 7.2 | 4.0 | Extracapsular | T2 | IB | Death | 46 | No |
| 4 | 64 | Female | 5.0 | < 0.1 | Encapsulated | T1 | IA | Alive | 56 | Yes |
| 5 | 56 | Male | 6.4 | 2.0 | Encapsulated | T1 | IA | Alive | 53 | Yes |
| 6 | 43 | Female | 5.0 | < 0.1 | Encapsulated | T1 | IA | Alive | 38 | No |
| 7 | 52 | Female | 4.5 | 3.0 | Encapsulated | T2 | IB | Alive | 47 | No |
| 8 | 28 | Male | 11.0 | 2.0 | Encapsulated | T1 | IA | Alive | 104 | Yes |
| 9 | 34 | Female | 3.0 | < 0.2 | Encapsulated | T1 | IA | Alive | 75 | No |
| 10 | 45 | Male | 4.0 | 1.5 | Encapsulated | T1 | IA | Alive | 24 | No |
| 11 | 63 | Female | 6.0 | < 0.2 | Encapsulated | T1 | IA | Alive | 94 | Yes |
The advancements and widespread use of imaging technology have led to an apparent rise in the prevalence of PCLs[1]. PCLs encompass both neoplastic and nonneoplastic lesions, with pancreatic cystic neoplasms (PCNs) representing a substantial subset. PCNs comprise serous cystic neoplasms (SCNs), solid pseudopapillary neoplasms (SPNs), IPMNs and mucinous cystic neoplasms. SCNs and SPNs are nearly benign and low-grade malignant tumours, respectively. IPMNs and MCNs, also known as pancreatic mucinous neoplasms, produce viscid mucin and have the potential to progress to PDAC. Notably, they are easier to detect by preoperative imaging than Pan IN due to the particular hypodensity of the cystic tumour.
Compagno and Oertel[8] initially distinguished MCN from SCN in 1978, citing its higher potential for malignancy. However, confusion between IPMNs and MCNs persisted until the International Association of Pancreatology designated OTS as the diagnostic criterion for MCN in 2006[9]. Earlier reports on MCNs inevitably included IPMN cases, leading to a high reported incidence of malignant transformation ranging from 6% to 39% over the last few decades[10-13]. In our sample, 25% of MCN cases were malignant, a rate nearly consistent with the findings of Höhn et al[14]. Loss of OTS was reported after the malignant transformation of MCNs[15]. In our study, although some cases showed this particular phenomenon following malignant transformation, with replacement by interstitial fibrous collagen, the remaining OTS was still visible. Once OTS cannot be recognized, detection of positive PR is recommended for a definitive diagnosis[16]. All cases in our study exhibited positive staining, ensuring accurate diagnosis.
Currently, there are no reliable preoperative variables that can be used to accurately predict the malignant potential of PCNs.
As one of the most frequently utilized preoperative assessment modalities, imaging has garnered considerable attention in the field of radiomics research for identifying certain subtypes of PCNs and stratifying the risk of IPMNs[17]. Conversely, MCNs have received far less attention than IPMNs, mainly owing to their less substantial incidence and malignancy rate. The management criteria for MCNs remain ambiguous and differ from those of IPMNs. Due to the inability to accurately predict the malignant potential of MCNs through preoperative inspection, it was formerly recommended that all MCNs amenable to surgery be removed regardless of lesion size[13]. Fortunately, the latest guidelines recommend monitoring for eligible MCNs[4,18], although the inclusion criteria are similar to those for IPMNs. This parallelism with IPMN criteria may warrant further consideration. A substantial proportion of patients diagnosed with pancreatic mucinous cysts prior to surgery are subsequently found to have benign disease and do not need surgery[19], highlighting the need for improved identification of those cases with malignant potential. Therefore, given the limitations of current risk stratification schemes in predicting malignant transformation of MCNs, further investigation regarding potential risk factors is essential. While a variety of clinicopathological features and imaging findings have been proposed as potential risk factors for malignancy, their importance remains controversial across different studies[12,14,20-23]. In addition to the variables included in current guidelines, such as tumour size, wall nodule, and duct dilation, other factors, including sex, location, wall thickening/enhancement, weight, and serum CA19-9 Level, have also been reported to be relevant. Our own research identified age, tumour size, solid component or mural nodule, and duct dilation as inde
To the best of our knowledge, age has not been widely recognized in the literature as a significant risk factor for malignant transformation of MCNs. The mean ages of patients with benign and malignant MCNs were 45.2 years ± 12.7 years and 52.2 years ± 13.4 years, respectively, which were comparable to those previously described in the literature[24]. Age has been shown to be related to malignancy in a cohort of pancreatic mucinous neoplasms[25]. Crippa et al[12] suggested that age was statistically significant in univariate analysis but did not emerge as a significant factor in multivariate analysis for malignant MCNs. Whether age is an independent risk factor for malignant MCNs needs further study. The occurrence of malignant MCNs appears to increase with age and seems to represent a dynamic pathological transition from adenoma to adenocarcinoma over time.
The average diameter of MCN masses in our investigation was found to be 5.8 cm, which is consistent with the previously reported average diameter of 6-11 cm. It has been emphasized that MCNs should be stratified into surveillance and surgery groups based on a diameter threshold of 4.0 cm, as stated in current guidelines[18]. Tumour size ≥ 4 cm was confirmed to be an independent risk for malignant MCNs in our study. In our research, we further identified that the presence of a solid component or mural nodule in MCNs is an independent risk factor for malignancy; it even ranks first in the likelihood of malignancy in certain studies[23]. The combination of solid component or mural nodule and tumour size has been utilized as a tool for identifying malignancy in some studies. In one cohort, it was reported that all malignant MCNs were either larger than 4 cm in diameter or had nodules[12]. Consistent with this, guidelines recommend surgical intervention for MCNs larger than 4 cm in diameter or for those with a mural nodule.
The majority of patients with MCNs are typically asymptomatic. In our analysis, while the percentage of malignant patients with clinical symptoms was higher than that of benign patients, the difference was not statistically significant. The relationship between clinical symptoms and malignancy in MCNs is still debatable[14,25], although surgical excision is advised if symptoms are present[18]. During follow-up, the increase in mass size can often result in nonspecific mass-related clinical complaints. In one case from our cohort, surgical intervention was advised after the tumour became larger than 4 cm and clinical symptoms appeared in the sixth year of imaging surveillance.
Unlike IPMNs, MCNs typically do not communicate with the pancreatic duct, which is one of their distinguishing features. Duct dilatation is a high-risk factor for malignant transformation in IPMNs, with dilatation greater than 10 mm being an unequivocal indication for surgery[18,26]. Similarly, our study found that pancreatic duct dilatation is a concerning imaging finding in MCNs, as verified by other investigations[23].
The management of malignant pancreatic tumours is predominantly centred on postoperative follow-up, whereas the management of MCNs places greater emphasis on the potential risks associated with preoperative monitoring and misdiagnosis. The management plan for IPMN is relatively mature, considering that dedicated management guidelines for IPMN have been published[1]. However, despite MCN being a precursor lesion of pancreatic cancer, similar to IPMN, the management plan for MCN remains unclear, particularly in identifying high-risk factors. Although the risk factors and surgical indications for IPMN and MCN are not differentiated in some guidelines[4], the demographic, cystic, histological and other characteristics of these two tumours differ, so they should be managed differently, particularly with respect to exploring risk factors. Unfortunately, our findings suggest that the risk factors for malignant MCNs are quite similar to those for IPMNs. The size of the lesion and the presence of solid components/wall nodules have been widely recognized as high-risk factors for malignant transformation of MCNs and IPMNs in various guidelines and publications, including our study. While various other malignant risk factors for MCN reported in the literature are statistically significant, they require further confirmation. In the case of IPMNs, the characteristic risk factor is main pancreatic duct dilation, especially when the diameter exceeds 10 mm, given that IPMNs grow within the pancreatic ducts. However, MCNs lack their own characteristic risk factors. Based on their relationship with the pancreatic ducts, IPMNs are classified into the main duct type, mixed duct type, and branch duct type, and direct surgical resection is recommended for the first two types due to a high risk of malignant transformation[18]. However, immediate surgical intervention for MCNs with risk factors remains controversial. Höhn et al[14] conducted a retrospective analysis exploring the risk factors for malignant transformation of MCNs, similar to our own analysis. They recommended radical resection surgery for all eligible patients suspected of having MCNs due to concerns about the potential risk of malignant progression and the level of expertise of pancreatic surgeons in low-volume centres. However, this view seems too radical, and the decision for MCN patients to undergo surgery should be more cautious, as the incidence and malignant transformation rate of MCNs are much lower than those of IPMNs[27]. The main argument for surgical resection in all MCN patients based on eliminating the risk of future malignancy seems invalid. It is essential to increase awareness and continuously conduct research on this rare tumour, especially in terms of preoperative malignant risk factors. Based on the above premise, a multidisciplinary team with expertise in pancreatic cysts and surgery can combine various reported risk factors, patient comorbidities, surgery-related complications, and mortality rates to comprehensively evaluate the potential risks and benefits of surgery and monitoring, thereby making the best treatment decision for the patient.
In addition, preoperative imaging evaluation of various risk indicators for MCNs seems to require the use of different modalities. No single test can accurately diagnose all cases, and in fact, most patients undergo more than one diagnostic procedure[28]. Therefore, the comprehensive use of different detection methods is more conducive to preoperative diagnosis. In our study, this may be attributed to the preference of imaging doctors at our hospital. CT is the preferred initial test for patients undergoing physical examination or with symptoms, and if the CT results are inconclusive or require further confirmation, MRI or EUS will be performed. Unfortunately, there was no significant difference between these three detection methods in our study; nevertheless, combining CT with MRI/EUS increased the preoperative diagnosis rate of our patients from 64.4% to 72.9%. A review of the literature suggests that MRI has slightly higher accuracy in distinguishing between malignant PCNs and benign lesions than CT[29]. MRI was found to be more effective than EUS in distinguishing malignant MCNs[30]. CT combined with MRI was shown to be better than CT alone in the preoperative diagnosis of pancreatic cysts[31]. Moreover, patients with MCNs who have a certain risk of malignant trans
Complete surgical resection of noninvasive MCNs is a curable treatment, resulting in a 5-year disease-specific survival rate of 100%, as previously reported[33], indicating that postoperative surveillance may not be necessary. As in PDAC patients, prognosis in cases of MCN-AIC was formerly believed to be dismal[34]. However, a series of recent studies have shown that the tumour behaviour and biological characteristics of MCN-AIC are entirely distinct from those of PDAC[23,35,36]. MCN-AIC is often diagnosed at an early stage, and surgical resection is the primary treatment. A study based on SEER research data showed that early-stage MCN-AIC (stage I-II) accounted for 82.9% of cases[37]. All 12 MCN-AIC cases in our study were at stage I, and the 5-year disease-specific survival rate was 70.1%, which was higher than the range of 20%-60% reported in previous literature[12,13,38]. Currently, there is a paucity of studies in which the biological behaviour of invasive MCNs is explored. Previous research indicates that patients with early-stage tumours (stages I and II) have better survival rates than those with high-grade tumours (stages III and IV)[37]. The prognostic variables for patients with MCN-AIC, such as pT grade and capsular invasion, have gained considerable attention. In PDAC, stage I is divided into stages IA and IB, which correspond to stages T1 and T2, respectively, without lymph node metastases and distant metastases[6]. A large cohort study has shown that the survival rate of patients with stage IA PDAC is much higher than that of patients with stage IB PDAC[39]. However, survival disparities between stage IA and IB invasive PDAC derived from MCN-AIC are rarely observed. Our findings, consistent with those of another study[7], demonstrate that there is no significant difference between the two stages. The invasion pattern of the capsule may provide a more accurate prediction of prognosis in MCN-AIC patients than stage classification. The notion of encapsulated invasion is not included in the MCN-AIC classification and guidelines, and the terminology varies somewhat across various research efforts. We adopted the term from the most recent publication[7]. The results of multiple studies have demonstrated that the prognosis is favourable when the invasion is confined to the capsule, and patients with encapsulated invasion exhibit a better survival rate than those with extracapsular invasion. Recurrence has been reported in one of sixteen cases of MCN-AIC confined to the OTS, and the probability of widespread invasion cannot be ruled out because of the minimal quantity of tumour sampled in this case[35]. It has been claimed that encapsulated MCN-AIC has a tumour-free survival rate that is comparable to that of noninvasive MCNs[7]. Our findings revealed that all patients with encapsulated invasion survived, while those with extracapsular invasion died regardless of whether MCN-AIC was stage IA or stage IB. It is recommended that MCN-AIC patients undergo standardized surveillance based on pancreatic cancer guidelines for 5 years after surgery[4]. During monitoring for stage I MCN-AIC, it seems that patients with extracapsular infiltration should be prioritized.
For patients with resected pancreatic cancer, the current standard of care mandates the administration of either a modified FOLFIRINOX (mFOLFIRINOX) or a gemcitabine-based regimen, provided that no contraindications are present[40]. However, limited research has been conducted on the benefits and risks of adjuvant treatment for stage I PDAC, which represents a small proportion of all resectable cases[41]. A study based on the NCDB database indicated that none of the patients with stage IA PDAC had to receive adjuvant treatment following resection, as the side effects and economic costs substantially outweighed the benefits[42]. Despite inadequate data to support this approach, current guidelines advocate adjuvant treatment for MCN-AIC patients comparable to that for patients with PDAC[18]. The role of adjuvant treatment in treating MCN-AIC patients after surgery, particularly for stage I tumours, remains unclear. Two of three patients with stage IA MCN-AIC did not receive adjuvant treatment after surgery, and none of them experienced disease recurrence or death[43]. Of the patients with tumours who did not receive adjuvant therapy, seven recovered from stage IA and 1 died from stage IB MCN-AIC[7]. These reported cases could be defined as “encapsulated invasion” based on the definition. Patients with stage IA MCN-AIC (T1a and T1b) seem to have the same favourable prognosis as those with LGD or HGD, particularly in the absence of extracapsular invasion. In our research, three IA patients and one IB patient with tumours confined to the capsule did not receive adjuvant treatment and survived, while those with extracapsular infiltration died regardless of whether adjuvant therapy was offered. Diligent monitoring might be preferred over intensive systemic therapy for stage I MCN-AIC with encapsulated invasion.
MCNs are known to harbour gene mutations associated with PDAC, as they are precursor lesions of cancer[17]. However, the genetic pathways underlying IPMNs and MCNs are distinct from one another. The most prevalent and early genetic event in IPMN and MCN carcinogenesis is the KRAS mutation[44], which is present in 30% of MCNs, whereas the GNAS mutation is almost nonexistent[45,46]. In contrast, the combined detection of KRAS and GNAS mutations exhibits 100% sensitivity for IPMNs[46]. The decreased frequency of genetic mutations in MCNs compared to IPMNs partly explains why MCNs have a lower probability of malignant transformation. Other mutations, including TP53, PIK3CA, PTEN, CDKN2A, and SMAD4, have also been described in MCNs but only in advanced stages[47,48]. KRAS mutation could be detected in all phases of MCNs and increased with the degree of dysplasia. Sawai et al[49] discovered KRAS mutations in 19% of LGD and 100% of HGD cases, whereas TP53 and CDKN2A mutations were exclusively observed only in HGD cases. Gene sequencing may aid in the early identification and tailored treatment of MCNs.
This study was subject to several limitations. First, it was a retrospective study that only included patients who underwent surgical resection and were pathologically confirmed to have MCNs, which may result in selection bias. Second, the rarity of MCNs limited the sample size, leading to a large confidence interval that may have hindered statistical analysis. Third, the MRI/EUS results were based on previous CT information, potentially affecting the interpretation of imaging results. Therefore, further multicentre and large-scale studies are needed to explore the clinical, pathological, imaging, and biological behaviours of MCNs.
In conclusion, this study involved analysing data from 48 patients who underwent complete resection of MCNs. The incidence of malignant MCNs was low, and the specific risk factors for malignancy were age, tumour size, presence of solid components or mural nodules, and duct dilatation. The prognosis of stage I MCN-AIC was closely related to encapsulated invasion, and complete surgical excision alone or in combination with postoperative treatment may be a viable option for invasive masses confined within the capsule.
Mucinous cystic neoplasms (MCNs) are recognized as precursor lesions of pancreatic cancer. Despite their rarity, the detection of MCNs is on the rise due to advancements in preoperative imaging techniques. Thus, there is a pressing need to increase our knowledge of MCNs to ensure that patients receive the most appropriate treatment decisions.
An inadequate understanding of MCNs can hinder the treatment of patients, underscoring the importance of research on MCNs.
To investigate the risk factors for malignancy in MCNs and the prognostic factors associated with MCN-associated invasive carcinoma (MCN-AIC) to advance our comprehension of this uncommon tumour.
This study involved a retrospective analysis of clinical and pathological data, imaging records, and outcomes of patients diagnosed with MCNs at our research centre over a 10-year period. We then investigated the risk factors for malignancy in MCNs and the prognostic factors associated with MCN-AIC.
A total of 48 patients with MCNs, accounting for 2.8% of pancreatic lesions resected during the study period, were included in this study. Among these patients, 36 had benign MCNs, while 12 had malignant MCNs. We conducted a comparative analysis of clinical and imaging features and discovered that age, tumour size, solid components or wall nodules, and pancreatic duct dilation were significantly associated with malignancy. Subsequently, we performed a prognostic analysis of malignant MCNs and observed that all malignant MCNs in our study were at stage I, and extracapsular invasion was identified as a significant prognostic factor for poor outcomes.
Age, tumour size, solid components or wall nodules, and pancreatic duct dilation were independent risk factors associated with malignancy in MCN. In addition, extracapsular invasion was indicative of poor prognosis of MCN-AIC.
The aim of this study was to enhance the management of MCN, a rare disease, by utilizing patient information from our research centre and conducting research from both preoperative and postoperative perspectives. We hope that this study can provide valuable insights into the management of MCNs.
We would like to thank Dr. Xian-Jie Jia for significant contributions to data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tenreiro N, Portugal; Zimmitti G, Italy S-Editor: Chen YL L-Editor: A P-Editor: Zhang YL
| 1. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1157] [Article Influence: 144.6] [Reference Citation Analysis (1)] |
| 2. | Ketwaroo GA, Mortele KJ, Sawhney MS. Pancreatic Cystic Neoplasms: An Update. Gastroenterol Clin North Am. 2016;45:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 694] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 4. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 6. | Shi S, Hua J, Liang C, Meng Q, Liang D, Xu J, Ni Q, Yu X. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2019;269:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 7. | Liang H, Xie W, Lin X, Wang T, Xie J, Wang C, Xiao SY, Guo Y. Pathologic T1 and T2 encapsulated invasive carcinomas arising from mucinous cystic neoplasms of the pancreas have favorable prognosis and might be treated conservatively. J Pathol Clin Res. 2021;7:507-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol. 1978;69:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 311] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S; International Association of Pancreatology. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1442] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 10. | Suzuki Y, Atomi Y, Sugiyama M, Isaji S, Inui K, Kimura W, Sunamura M, Furukawa T, Yanagisawa A, Ariyama J, Takada T, Watanabe H, Suda K; Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of intraductal papillary mucinous tumor and mucinous cystic tumor. Pancreas. 2004;28:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Yamao K, Yanagisawa A, Takahashi K, Kimura W, Doi R, Fukushima N, Ohike N, Shimizu M, Hatori T, Nobukawa B, Hifumi M, Kobayashi Y, Tobita K, Tanno S, Sugiyama M, Miyasaka Y, Nakagohri T, Yamaguchi T, Hanada K, Abe H, Tada M, Fujita N, Tanaka M. Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan pancreas society. Pancreas. 2011;40:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, Capelli P, Pederzoli P, Castillo CF. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 13. | Testini M, Gurrado A, Lissidini G, Venezia P, Greco L, Piccinni G. Management of mucinous cystic neoplasms of the pancreas. World J Gastroenterol. 2010;16:5682-5692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Höhn P, Soydemir MA, Luu AM, Janot-Matuschek M, Tannapfel A, Uhl W, Belyaev O. It’s not all about the size—characteristics and risk factors for malignancy of mucinous cystic neoplasms of the pancreas. Ann Transl Med. 2020;8:1572-1572. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Lam MM, Swanson PE, Upton MP, Yeh MM. Ovarian-type stroma in hepatobiliary cystadenomas and pancreatic mucinous cystic neoplasms: an immunohistochemical study. Am J Clin Pathol. 2008;129:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Izumo A, Yamaguchi K, Eguchi T, Nishiyama K, Yamamoto H, Yonemasu H, Yao T, Tanaka M, Tsuneyoshi M. Mucinous cystic tumor of the pancreas: immunohistochemical assessment of "ovarian-type stroma". Oncol Rep. 2003;10:515-525. [PubMed] |
| 17. | Ardeshna DR, Cao T, Rodgers B, Onongaya C, Jones D, Chen W, Koay EJ, Krishna SG. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J Gastroenterol. 2022;28:624-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (6)] |
| 18. | The European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |
| 19. | Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 20. | Chang YT, Tien YW, Jeng YM, Yang CY, Liang PC, Wong JM, Chang MC. Overweight increases the risk of malignancy in patients with pancreatic mucinous cystic neoplasms. Medicine (Baltimore). 2015;94:e797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Jang KT, Park SM, Basturk O, Bagci P, Bandyopadhyay S, Stelow EB, Walters DM, Choi DW, Choi SH, Heo JS, Sarmiento JM, Reid MD, Adsay V. Clinicopathologic characteristics of 29 invasive carcinomas arising in 178 pancreatic mucinous cystic neoplasms with ovarian-type stroma: implications for management and prognosis. Am J Surg Pathol. 2015;39:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Kim GH, Choi K, Paik N, Lee KT, Lee JK, Lee KH, Han IW, Kang SH, Heo JS, Park JK. Diagnostic Concordance and Preoperative Risk Factors for Malignancy in Pancreatic Mucinous Cystic Neoplasms. Gut Liver. 2022;16:637-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Postlewait LM, Ethun CG, McInnis MR, Merchant N, Parikh A, Idrees K, Isom CA, Hawkins W, Fields RC, Strand M, Weber SM, Cho CS, Salem A, Martin RC, Scoggins C, Bentrem D, Kim HJ, Carr J, Ahmad S, Abbott DE, Wilson GC, Kooby DA, Maithel SK. Association of Preoperative Risk Factors With Malignancy in Pancreatic Mucinous Cystic Neoplasms: A Multicenter Study. JAMA Surg. 2017;152:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Riva G, Pea A, Pilati C, Fiadone G, Lawlor RT, Scarpa A, Luchini C. Histo-molecular oncogenesis of pancreatic cancer: From precancerous lesions to invasive ductal adenocarcinoma. World J Gastrointest Oncol. 2018;10:317-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 25. | Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, Kochman ML, Foley PJ, Drebin J, Oh YS, Ginsberg G, Ahmad N, Merchant NB, Isbell J, Parikh AA, Stokes JB, Bauer T, Adams RB, Simeone DM. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg. 2008;12:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol. 2017;14:911-923. [RCA] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 216] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 27. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 28. | Del Chiaro M, Segersvärd R, Pozzi Mucelli R, Rangelova E, Kartalis N, Ansorge C, Arnelo U, Blomberg J, Löhr M, Verbeke C. Comparison of preoperative conference-based diagnosis with histology of cystic tumors of the pancreas. Ann Surg Oncol. 2014;21:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Mohamed E, Jackson R, Halloran CM, Ghaneh P. Role of Radiological Imaging in the Diagnosis and Characterization of Pancreatic Cystic Lesions: A Systematic Review. Pancreas. 2018;47:1055-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hwang J, Kim YK, Min JH, Jeong WK, Hong SS, Kim HJ. Comparison between MRI with MR cholangiopancreatography and endoscopic ultrasonography for differentiating malignant from benign mucinous neoplasms of the pancreas. Eur Radiol. 2018;28:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Jang DK, Song BJ, Ryu JK, Chung KH, Lee BS, Park JK, Lee SH, Kim YT, Lee JY. Preoperative Diagnosis of Pancreatic Cystic Lesions: The Accuracy of Endoscopic Ultrasound and Cross-Sectional Imaging. Pancreas. 2015;44:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 33. | Xourafas D, Tavakkoli A, Clancy TE, Ashley SW. Noninvasive intraductal papillary mucinous neoplasms and mucinous cystic neoplasms: recurrence rates and postoperative imaging follow-up. Surgery. 2015;157:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, DiMagno EP. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Lewis GH, Wang H, Bellizzi AM, Klein AP, Askin FB, Schwartz LE, Schulick RD, Wolfgang CL, Cameron JL, O'Reilly EM, Yu KH, Hruban RH. Prognosis of minimally invasive carcinoma arising in mucinous cystic neoplasms of the pancreas. Am J Surg Pathol. 2013;37:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Park JW, Jang JY, Kang MJ, Kwon W, Chang YR, Kim SW. Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology. 2014;14:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Doulamis IP, Mylonas KS, Kalfountzos CE, Mou D, Haj-Ibrahim H, Nasioudis D. Pancreatic mucinous cystadenocarcinoma: Epidemiology and outcomes. Int J Surg. 2016;35:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Nilsson LN, Keane MG, Shamali A, Millastre Bocos J, Marijinissen van Zanten M, Antila A, Verdejo Gil C, Del Chiaro M, Laukkarinen J. Nature and management of pancreatic mucinous cystic neoplasm (MCN): A systematic review of the literature. Pancreatology. 2016;16:1028-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Muralidhar V, Nipp RD, Mamon HJ, Punglia RS, Hong TS, Ferrone C, Fernandez-Del Castillo C, Parikh A, Nguyen PL, Wo JY. Association Between Very Small Tumor Size and Decreased Overall Survival in Node-Positive Pancreatic Cancer. Ann Surg Oncol. 2018;25:4027-4034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | NCCN. Pancreatic Adenocarcinoma, Version 2. 2022, NCCN Clinical Practice Guidelines in Oncology.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Gong J, Tuli R, Shinde A, Hendifar AE. Meta-analyses of treatment standards for pancreatic cancer. Mol Clin Oncol. 2016;4:315-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Shaib WL, Narayan AS, Switchenko JM, Kane SR, Wu C, Akce M, Alese OB, Patel PR, Maithel SK, Sarmiento JM, Kooby DA, El-Rayes BF. Role of adjuvant therapy in resected stage IA subcentimeter (T1a/T1b) pancreatic cancer. Cancer. 2019;125:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Hui L, Rashid A, Foo WC, Katz MH, Chatterjee D, Wang H, Fleming JB, Tamm EP. Significance of T1a and T1b Carcinoma Arising in Mucinous Cystic Neoplasm of Pancreas. Am J Surg Pathol. 2018;42:578-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 45. | Reid MD, Saka B, Balci S, Goldblum AS, Adsay NV. Molecular genetics of pancreatic neoplasms and their morphologic correlates: an update on recent advances and potential diagnostic applications. Am J Clin Pathol. 2014;141:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 266] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 47. | Singhi AD, Wood LD. Early detection of pancreatic cancer using DNA-based molecular approaches. Nat Rev Gastroenterol Hepatol. 2021;18:457-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 48. | Theisen BK, Wald AI, Singhi AD. Molecular Diagnostics in the Evaluation of Pancreatic Cysts. Surg Pathol Clin. 2016;9:441-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Sawai H, Kurimoto M, Koide S, Kiriyama Y, Haba S, Matsuo Y, Morimoto M, Koide H, Kamiya A, Yamao K. Invasive Ductal Carcinoma Arising in Mucinous Cystic Neoplasm of Pancreas: A Case Report. Am J Case Rep. 2019;20:242-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |