Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3084
Peer-review started: January 21, 2023
First decision: February 7, 2023
Revised: February 19, 2023
Accepted: April 28, 2023
Article in press: April 28, 2023
Published online: May 28, 2023
Processing time: 124 Days and 14.6 Hours
Capecitabine (CAP) is a classic antimetabolic drug and has shown potential antirejection effects after liver transplantation (LT) in clinical studies. Our previous study showed that metronomic CAP can cause the programmed death of T cells by inducing oxidative stress in healthy mice. Ferroptosis, a newly defined non-apoptotic cell death that occurs in response to iron overload and lethal levels of lipid peroxidation, is an important mechanism by which CAP induces cell death. Therefore, ferroptosis may also play an important role in CAP-induced T cell death and play an immunosuppressive role in acute rejection after trans-plantation.
To investigate the functions and underlying mechanisms of antirejection effects of metronomic CAP.
A rat LT model of acute rejection was established, and the effect of metronomic CAP on splenic hematopoietic function and acute graft rejection was evaluated 7 d after LT. In vitro, primary CD3+ T cells were sorted from rat spleens and human peripheral blood, and co-cultured with or without 5-fluorouracil (5-FU) (active agent of CAP). The levels of ferroptosis-related proteins, ferrous ion concentration, and oxidative stress-related indicators were observed. The changes in mito-chondrial structure were observed using electron microscopy.
With no significant myelotoxicity, metronomic CAP alleviated graft injury (Banff score 9 vs 7.333, P < 0.001), prolonged the survival time of the recipient rats (11.5 d vs 16 d, P < 0.01), and reduced the infiltration rate of CD3+ T cells in peripheral blood (6.859 vs 3.735, P < 0.001), liver graft (7.459 vs 3.432, P < 0.001), and spleen (26.92 vs 12.9, P < 0.001), thereby inhibiting acute rejection after LT. In vitro, 5-FU, an end product of CAP metabolism, induced the degradation of the ferritin heavy chain by upregulating nuclear receptor coactivator 4, which caused the accumulation of ferrous ions. It also inhibited nuclear erythroid 2 p45-related factor 2, heme oxygenase-1, and glutathione peroxidase 4, eventually leading to oxidative damage and ferroptosis of T cells.
Metronomic CAP can suppress acute allograft rejection in rats by triggering CD3+ T cell ferroptosis, which makes it an effective immunosuppressive agent after LT.
Core Tip: Our studies proved that metronomic capecitabine (CAP) alleviated the acute rejection after transplantation in rats without the common side effect of myelosuppression. T cell ferroptosis is the underlying mechanism behind the antirejection effect of CAP, which can induce cell ferrous ions overload and suppress the nuclear erythroid 2 p45-related factor 2, heme oxygenase-1/glutathione peroxidase 4 antioxidant systems, thereby directly increasing the levels of intracellular reactive oxygen species, and leading to severe oxidative damage in T cells. These results revealed a new mechanism of CAP-induced T cell programmed death and suggested the possibility of using CAP as an immunosuppressant after transplantation.
- Citation: Wang H, Wang ZL, Zhang S, Kong DJ, Yang RN, Cao L, Wang JX, Yoshida S, Song ZL, Liu T, Fan SL, Ren JS, Li JH, Shen ZY, Zheng H. Metronomic capecitabine inhibits liver transplant rejection in rats by triggering recipients’ T cell ferroptosis. World J Gastroenterol 2023; 29(20): 3084-3102
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3084
Recurrence of hepatocellular carcinoma (HCC) represents a serious issue after liver transplantation (LT), and is closely related to the usage of immunosuppressant drugs[1-3]. As a classic antimetabolic chemotherapeutic agent, capecitabine (CAP) is widely used in the treatment of cancers and has also shown good effects on HCC[4,5]. Metronomic CAP is suitable for long-term oral administration with low adverse effects and without sacrificing treatment efficacy[6,7]. In patients with recurrent liver cancer after LT, Ravaioli et al[8] showed that metronomic CAP achieved similar efficacy to sorafenib, a protein kinase inhibitor approved for HCC but with a high incidence of adverse effects[9]. Interestingly, no acute rejection was seen, suggesting that CAP may have both immunosuppressive and anticarcinogenic effects. CAP is a fluoropyrimidine prodrug that is metabolized in a three-step process to 5-fluorouracil (5-FU)[10]. The last step requires thymidine phosphorylase (TP), the distribution of which determines CAP distribution. This partially explains its different pharmacological characteristics compared to 5-FU. Because TP is highly expressed in cancer cells and lymphocytes[11,12], the administration of CAP to LT patients with HCC may increase tumor killing while preventing graft rejection. In preliminary work, we found that the differential expression of TP avoided CAP’s side effects of myelosuppression and suppressed normal mouse immune responses by inducing T cell apoptosis[13]. However, it is not clear what effect CAP has during the immune activation that follows transplantation, which is key to its use as an immunosuppressant.
Ferroptosis is a newly defined nonapoptotic cell death that occurs with iron overload and lethal levels of lipid peroxidation[14-16]. Ferroptosis is tightly regulated by iron metabolism, while ferritin is the major intracellular iron storage protein complex. Ferritinophagy activation depends on nuclear receptor coactivator 4 (NCOA4) to transport ferritin to the autophagosome and degrade ferritin to increase intracellular iron levels[17,18]. Subsequently, free ferrous ions overload results in oxidative injury by the Fenton reaction. Although the regulatory mechanisms of ferroptosis are poorly understood, some molecules with antioxidant effects may be implicated, such as nuclear erythroid 2 p45-related factor 2 (Nrf2)[19,20], heme oxygenase-1 (HO-1)[21], and glutathione peroxidase 4 (GPX4)[22]. Overall, free ferrous ions result in reactive oxygen species (ROS) generation and oxidative injury, whereas the Nrf2-HO-1/GPX4 antioxidative system protects cells from oxidative damage induced by ferroptosis[23]. In mouse models and in vitro, 5-FU mediates injuries in intestinal mucosal cells, such as those occurring during chemotherapy, by reducing Nrf2 expression and increasing ferroptosis[24]. 5-FU also leads to ROS and iron homeostasis-dependent ferroptosis in myocardial cells by reducing the expression of GPX4 and ferritin heavy chain (FTH1) but enhancing the expression of transferrin receptor 1[25]. Thus, mechanistically, ferroptosis plays an important role in the cell-killing effect of 5-FU.
Our previous study indicated that metronomic CAP can increase ROS levels and reduce mitochondrial membrane potential (MMP), thus affecting the immune system of mice[13]. Those results prompted us to further explore whether ferroptosis plays a significant role in 5-FU-induced immunosuppression. In this study, we investigated the effect of metronomic CAP on acute rejection in a rat model of LT, and determined how ferroptosis affects 5-FU-induced immunosuppression. Elucidating the possible mechanism of CAP as an immunosuppressant with anticancer effects is of great relevance to optimize drug regimens for HCC patients after LT in the future.
Specific-pathogen-free male Lewis rats (donors) and male Brown Norway rats (recipients) aged 8–10 wk and weighing 250–300 g were obtained from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The rats were housed in a standard room, and fed filtered clean water and standard laboratory food. To acclimatize, the rats were housed 1 wk before transplantation. According to different postoperative medication regimens, the rats were randomized to untreated control group (CON) (0.9% saline, ig) and metronomic capecitabine-treated (MET) groups (CAP 100 mg/kg/d, ig). A total of 12 recipients (6 rats/group) were used for postoperative specimen collection at 7 d after transplantation, and another 12 recipients (6 rats/group) were used to observe survival. All invasive procedures and specimen collection were performed under isoflurane anesthesia to minimize pain or discomfort.
Anesthesia was induced with isoflurane and orthotopic LT was performed based on Kamada’s two-cuff method. The anhepatic phase was controlled within 26 min[26,27]. After transplantation, the rats received 2 mL Ringer’s lactate to replenish blood volume and were rewarmed in an incubator at 37 ℃ for 30 min. On day 7 after LT, an overdose of pentobarbital (150 mg/kg) was injected intraperitoneally for euthanasia. Femur, blood, spleen, and the transplanted liver were collected for further examination.
Whole blood was used for blood routine tests 7 d after LT. These were performed with an automatic hematology analyzer (BC-2800Vet; Mindray, Shenzhen, China).
Peripheral blood lymphocyte CD3+ T cell count was obtained based on peripheral blood lymphocyte count and flow cytometric analysis of the CD3+ T cell ratio.
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), and direct bilirubin (DBIL) were measured using kits (Mindray, Shenzhen, China) and read with an automatic biochemical analyzer (BS-240VET; Mindray, Shenzhen, China).
Rat peripheral blood mononuclear cells were isolated by gradient centrifugation with a rat peripheral blood mononuclear cell separation solution (Solarbio, Beijing, China). Anti-CD3 antibodies (APC anti-rat CD3 Antibody, Cat# 201414; RRID: AB_2563366 BioLegend, San Diego, CA, United States) were used for extracellular staining to identify CD3-positive lymphocytes. Flow cytometry was performed using the Accuri C6 Plus flow cytometry (BD Biosciences, Palo Alto, CA, United States), and FlowJo software (TreeStar, Woodburn, OR, United States) was used for data analysis.
The ferrous levels in T cells were measured using FerroOrange (Cat# MX4559, Maokang Biotechnology, Shanghai, China). Cells were incubated with FerroOrange (37 ℃, 20 min) for fluorescence detection. To measure intracellular ROS, cells were resuspended in phosphate-buffered saline (PBS) containing DCFH-DA (Cat# CA1410, Solarbio, Beijing, China) at 37 ℃ for 30 min and washed twice with PBS for fluorescence detection. To measure the lipid ROS of T cells, C11-BODIPY 581/591 (Cat# MX5211, Maokang Biotechnology, Shanghai, China) was added and incubated at 37 ℃ with 5% CO2 for 30 min. Cells were then washed twice before fluorescence detection. The MMP Assay Kit (Cat# M8650, Solarbio, Beijing, China) was used to measure the MMP in T cells. Cells were incubated with JC-1 working solution at 37 ℃ for 20 min, then washed twice with JC-1 buffer for fluorescence detection. BODIPY emission was recorded on an oxidized C11 signal (FITC channel).
The rat sera were analyzed for cytokine concentrations [interleukin (IL)-10, IL-2, IL-1α, IL-4, interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-5, IL-6, growth-regulated oncogene (GRO)/keratinocyte chemoattractant (KC), IL-12p70] using the Millipore Luminex 200 instrument (Luminex Corporation, Austin, TX, United States), after incubation of the samples with beads according to the manufacturer’s instructions. The quantification was performed with Luminex xPonent version 3.1 software.
Healthy mononuclear cells from the spleen of rats and peripheral blood of humans were collected by gradient centrifugation with respectively, rat tissue mononuclear cell separation solution (Solarbio, Beijing, China) and human peripheral blood mononuclear cell separation solution (Solarbio, Beijing, China). T cells were purified through positive magnetic selection using microbeads (Miltenyi Biotec, Bergisch Gladbac, Germany) and cultivated at 2.0 × 106 cells/well in 6-well plates with RPMI-1640 with 5% fetal bovine serum and 100 IU/mL IL-2. Within the first 72 h, the anti-CD3/CD28 antibody (Cat# 201401, RRID: AB_893302; 2 μg/mL and Cat# 200902, RRID: AB_313891; 1 μg/mL) were used to stimulate the T cells.
The Cell Counting Kit-8 (CCK-8) assay (Boster, Hubei, China) was used to evaluate T cell proliferation in vitro. The CON group (n = 3), 5-FU group (5-FU 15 μmol/L, MCE, Shanghai, China; n = 3); ferrostatin-1 (Fer-1) group (Fer-1 2 μmol/L, MCE, Shanghai, China; n = 3); 5-FU+fer-1 group (5-FU 15 μmol/L + fer-1 2 μmol/L) were included. After 72 h of culture, CCK-8 solution was added to each well of the 96-well plates and allowed to incubate for 2 h at 37 ℃, and the optical density value at 450 nm was recorded.
The femurs and grafts (n = 6 for each group) were removed on postoperative day 7. The femurs were decalcified with EDTA decalcifying solution (Solarbio, Beijing, China). Then both femurs and grafts were fixed in 10% paraformaldehyde, dehydrated, paraffin-embedded, cut into 4 μm slides, and stained with hematoxylin and eosin (H&E; G1120; Solarbio, Beijing, China).
CD3+ cell infiltration was assessed on spleen and liver graft sections as follows: Tissue sections were heated for 15 min in EDTA (pH 8.0) in a microwave for antigen retrieval, incubated in 3% hydrogen peroxide for 30 min to eliminate endogenous peroxidase activity, and blocked in normal goat serum. Then the sections were incubated overnight at 4 ℃ with a rat anti-CD3 antibody (CD3-12, Cat# ab11089, RRID: AB_2889189; Abcam, Cambridge, MA, United States) and incubated with a secondary antibody (Goat Anti-Rat IgG H&L, Cat# ab97057, RRID: AB_10680316; Abcam, Cambridge, MA, United States) or 30 min at room temperature the next day. Finally, the sections were stained with freshly prepared diaminobenzidine solution and counterstained with hematoxylin. ImageJ software was applied to 200 × images to quantify the CD3-positive fields.
Cells were collected after centrifugation and fixed in 2.5% glutaraldehyde. After dehydration through graded ethanol, the cells were embedded in epoxy resins and cut into ultrathin sections. The morphologic changes in mitochondrial ultrastructure were observed using a transmission electron microscope (HT7800; Hitachi, Tokyo, Japan).
Total protein was extracted using RIPA buffer containing protease and phosphatase inhibitors. The mixture was placed on ice for 30 min for cell lysis, and then centrifuged at 12000 g for 15 min at 4 ℃ to discard the cell debris. Total protein concentration was quantified by the BCA Protein Assay Kit (Beyotime Biotechnology, Beijing, China). The proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electrotransferred to polyvinylidene fluoride membranes, and blocked in 5% skimmed milk for 1 h. Then the membranes were incubated overnight at 4 ℃ with primary antibodies against FTH1 (EPR18878, Cat# ab183781; Abcam, Cambridge, MA, United States), NCOA4 (Cat# PA5-96398, RRID: AB_2808200; Thermo Fisher Scientific, Waltham, MA, United States), Nrf2 (E5F1A, Cat# 20733S, Cell Signaling Technology, Danvers, MA, United States), HO-1 (EPR1390Y, Cat# ab68477, RRID: AB_11156457; Abcam, Cambridge, MA, United States), and GPX4 (EPNCIR144, Cat# ab125066, RRID: AB_10973901; Abcam, Cambridge, MA, United States). β-actin (SP124, Cat# ab115777, RRID: AB_10899528; Abcam, Cambridge, MA, United States) antibody served as the internal control. The membranes were washed and then incubated with a secondary antibody (Goat anti-Rabbit IgG H+L, Cat# A16110; Thermo Fisher Scientific, Shanghai, China) for 1 h at RT. Proteins were detected with an imaging system (Bio-Rad, Hercules, CA, United States), and band intensities were analyzed with ImageJ software (National Institutes of Health, Bethesda, MD, United States).
The statistical analyses were performed with SPSS 23.0 (IBM Analytics) and GraphPad 8.0 (GraphPad Software). The statistical methods of this study were reviewed by Yuan Wang from the Department of Biostatistics and Epidemiology, The First Central Clinical School, Tianjin Medical University. Data are presented as the mean ± standard error of the mean. The unpaired t-test was used to assess the differences between the two groups, and a one-way analysis of variance was used to assess the differences among three or more groups. P < 0.05 was considered statistically significant.
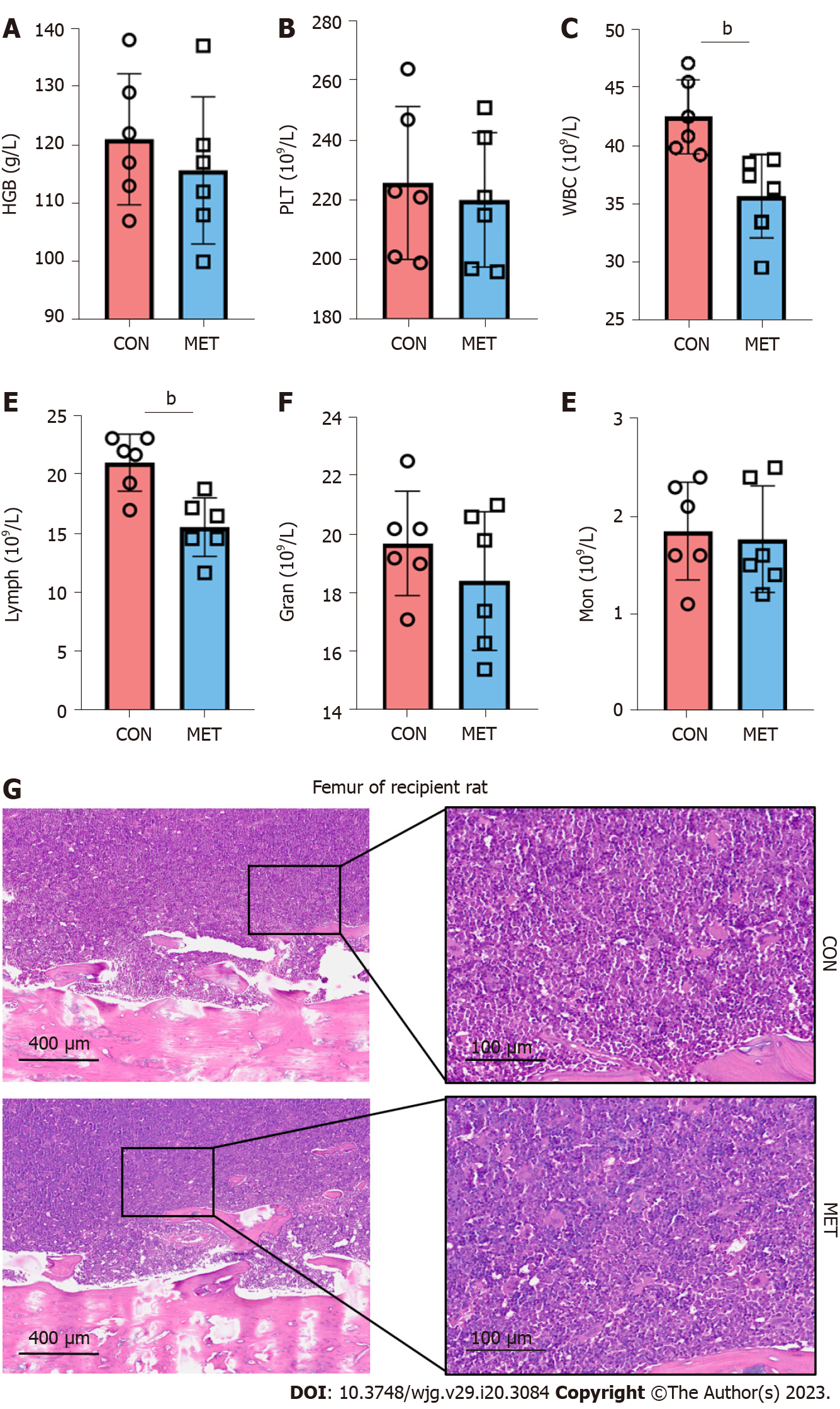
5-FU, the end product of CAP, may induce myelotoxicity. Thus, we first evaluated metronomic CAP’s myelotoxicity on peripheral blood hemoglobin (HGB), platelets (PLTs), and leukocytes in a rat model of orthotopic LT, where recipient Brown-Norway rats received the livers from Lewis rats. Seven days after LT, there was no significant difference in the index of HGB and PLTs between the CON group and the MET group (Figure 1A and B). However, leukopenia with a reduced number of lymphocytes was found in the MET group, while the number of other leukocyte subsets (granulocytes and mononuclear cells) was not significantly different (Figure 1C–F). These results suggest that CAP had a relatively targeted killing effect on lymphocytes, but no obvious effect on PLTs, erythrocytes, monocytes, or granulocytes. In the bone marrow, the rats in the MET group had no pathological manifestation of myelosuppression (Figure 1G), suggesting that the lymphocytopenia caused by CAP did not result from myelosuppression.
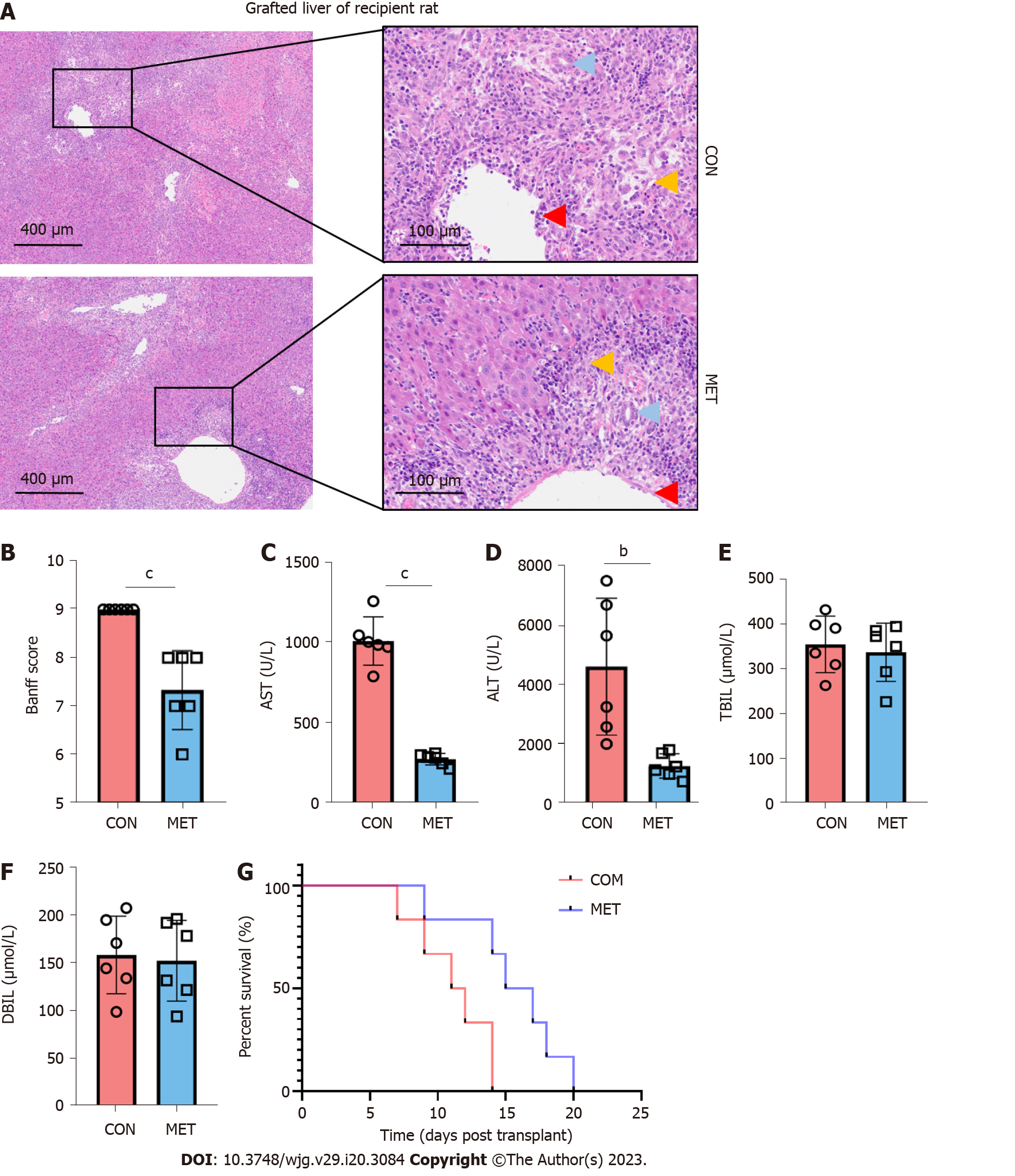
To evaluate the effect of metronomic CAP during acute rejection, the grafted livers were harvested on day 7 after LT for histopathological examination. As shown in Figure 2A, prominent acute rejection with severe bile duct damage, endothelitis, and hepatocyte vacuolation occurred in the grafts of the CON group. Portal areas were infiltrated by inflammatory cells, hepatic sinusoids were greatly expanded, and the sinusoidal endothelial cells were markedly swollen. Compared with the CON group, acute rejection in the MET group was ameliorated, as evidenced by inflammatory cell infiltration being confined to only some of the portal areas and the degeneration of a few bile duct epithelia. The Banff scores[28] decreased in the MET group compared with the CON group (Figure 2B). On day 7 after LT, liver damage was assessed by measuring serum levels of AST and ALT (Figure 2C and D). Compared with the CON group, CAP significantly decreased the serum levels of ALT and AST. However, TBIL and DBIL in both groups showed an increasing trend after the operation, but there was no significant difference between the two groups (Figure 2E and F). In the survival analysis, a log-rank (Mantel Cox) test was conducted. Rats in the MET group had a longer survival time compared with the CON group (Figure 2G, median survival time: 16.5 and 11.5 d; P < 0.01).
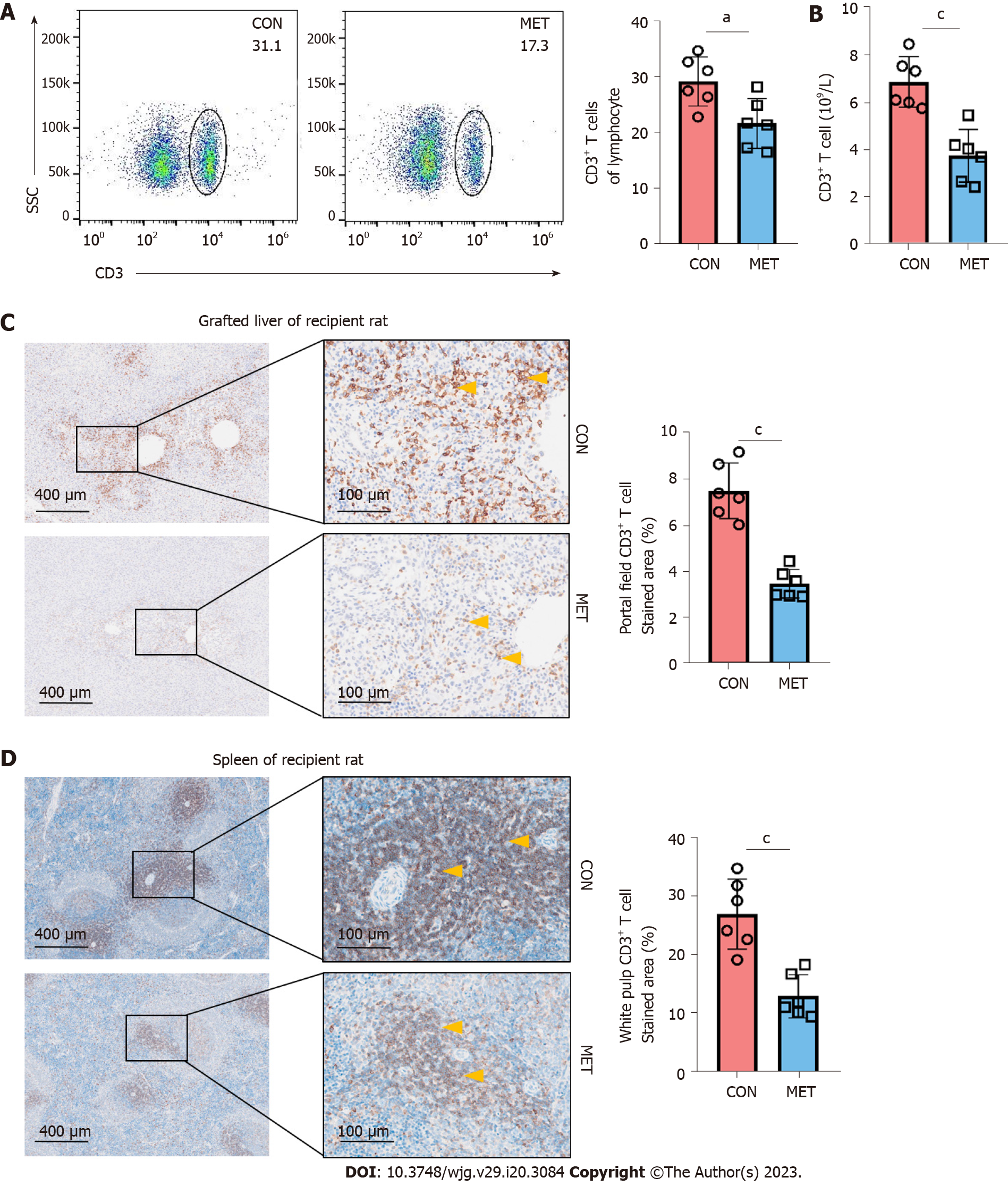
Next, to investigate whether the inhibition of acute rejection by metronomic CAP involved an effect on T cells, CD3+ T cells in the peripheral blood, grafts, and spleens were analyzed. Compared to the CON group, the CD3+ T cells in the peripheral blood were significantly lower in the MET group (Figure 3A and B). In addition, a large number of CD3+ T cells was localized in the portal canal area of the CON group. Infiltrated CD3+ T cells in both the liver and spleen were significantly reduced in the MET group (Figure 3C and D). These results indicate that metronomic CAP can decrease the number of CD3+ T cells in the peripheral blood, liver, and spleen of the recipient rats.
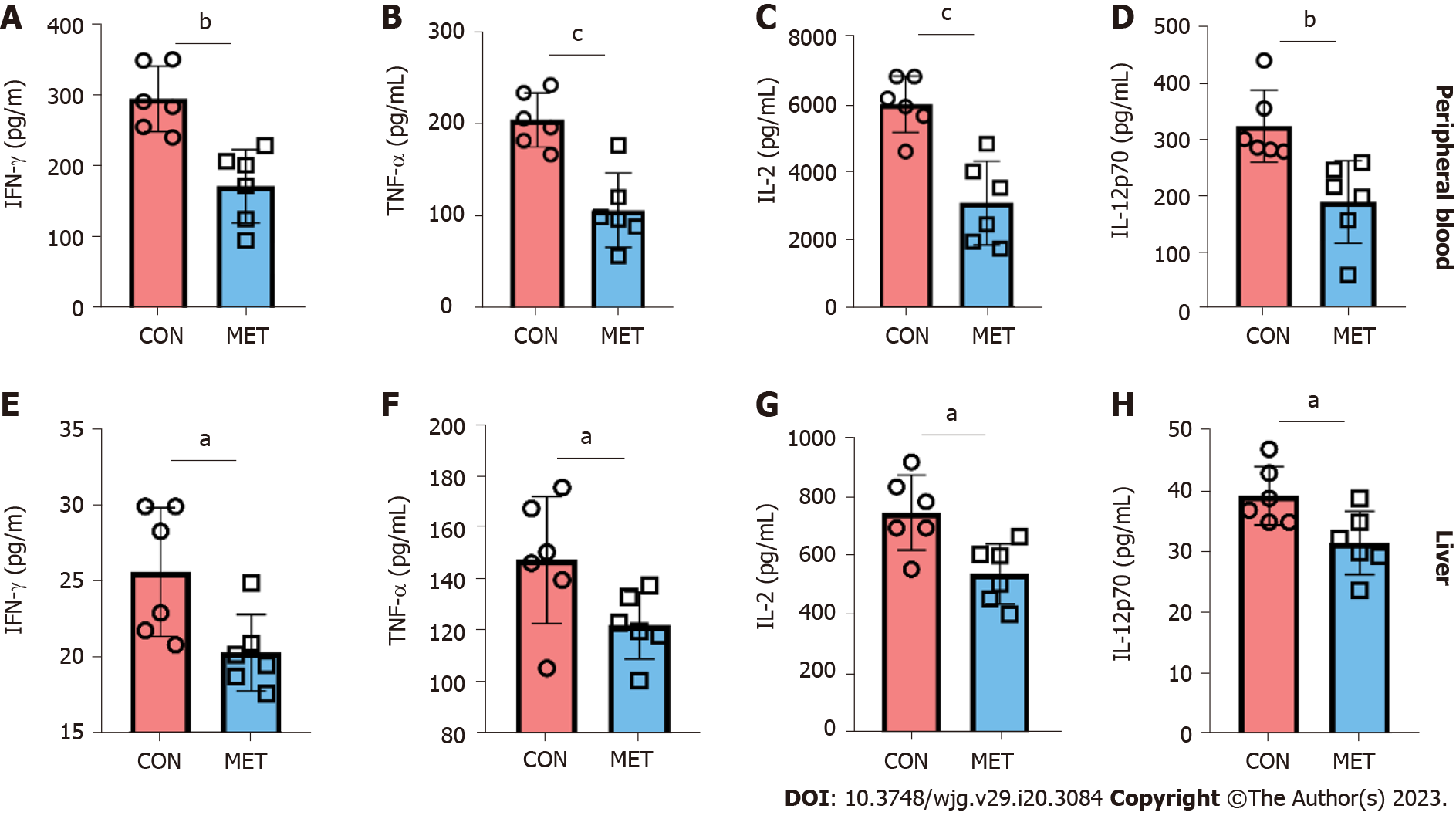
Cytokines are an important reflection of immune cell function and immune status. Thus, the levels of IFN-γ, TNF-α, human GRO/KC, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, and IL-12p70 in peripheral blood and graft were quantified by Luminex assay. As shown in Figure 4, the levels of pro-inflammatory IFN-γ, TNF-α, IL-2, and IL-12p70 in the MET group were significantly lower than those in the CON group. Other cytokines (IL-1β, IL-5, IL-6, IL-4, IL-10, and GRO/KC) did not show significant changes (Supplementary Figure 1). The results showed that metronomic CAP plays an immunosuppressive role by downregulating the concentration of pro-inflammatory cytokines.
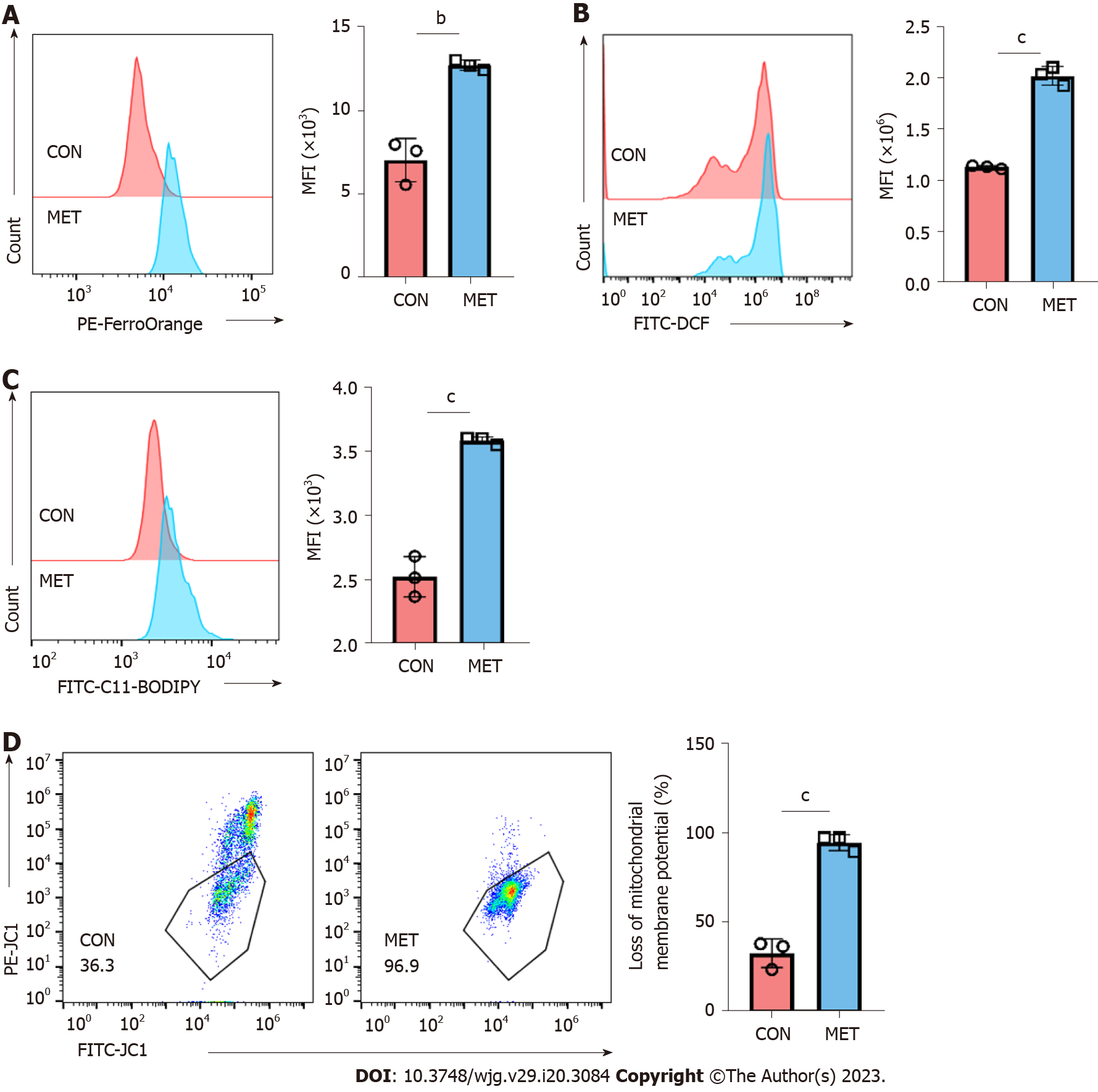
The immune activation of T cells is particularly important in the acute rejection of organ transplants. Similar to many commonly used immunosuppressants, the killing effect of CAP on T cells is also key to its immunosuppressive activity[13]. Therefore, we further explored the mechanism of T cell killing by metronomic CAP. Ferroptosis is a new form of ROS-dependent programmed cell death that may plays an important role in CAP-mediated cytotoxicity. Thus, we measured by flow cytometry the concentration of free ferrous ions, ROS level, and lipid ROS level in peripheral blood CD3+ T cells from transplanted rats of both groups at day 7. Compared with the CON group, free ferrous ion concentration (Figure 5A), total ROS (Figure 5B), and lipid ROS (Figure 5C) levels were increased in the MET group. At the same time, the detection of the MMP showed a decreasing trend in the MET group (Figure 5D). These results indicated that metronomic CAP leads to the generation of ferrous ions and results in lipid peroxidation, which conforms to the typical characteristics of ferroptosis.
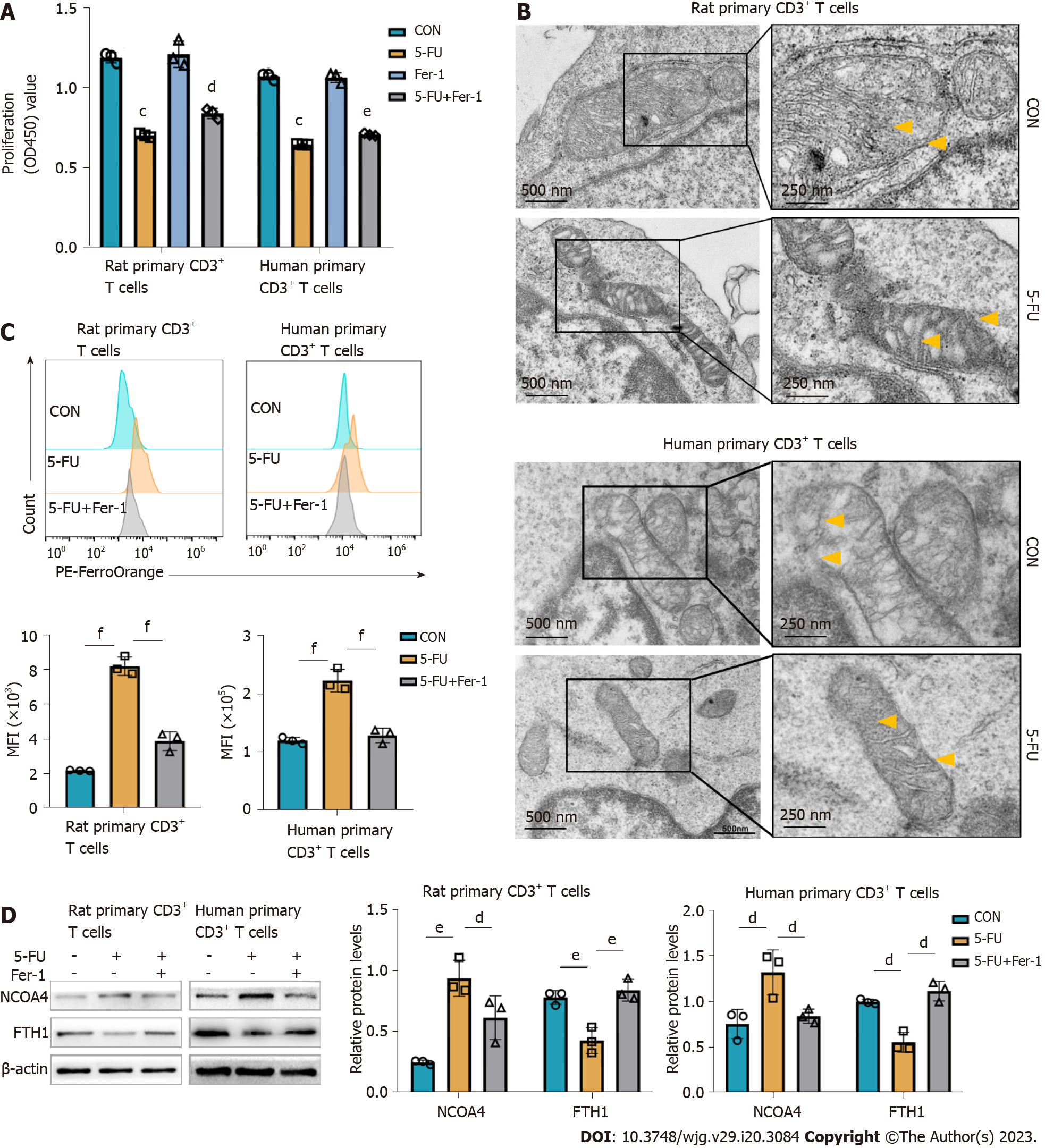
To further explore in vitro the mechanism underlying the CAP-mediated ferroptosis linked to immunosuppression observed in vivo, primary CD3+ T cells were sorted from rat spleens and human peripheral blood (Supplementary Figure 2). Both primary CD3+ T cells were treated with 5-FU in vitro (Supplementary Figure 3). This treatment significantly inhibited cell viability, which was attenuated by the ferroptosis inhibitor Fer-1 (Figure 6A). Transmission electron microscopy revealed typical characteristics of ferroptosis in 5-FU-treated group, namely, smaller mitochondria with increased membrane density, and diminished mitochondrial cristae (Figure 6B). Free ferrous ions concentration was increased in the 5-FU-treated group, whereas Fer-1 partially reversed the increase of ferrous ions induced by 5-FU (Figure 6C). As a selective cargo receptor, NCOA4 mediates the transport of ferritin into lysosomes, inducing ferroptosis. Western blot analysis revealed that 5-FU treatment induced FTH1 decrease, but a tendency toward increasing NCOA4 expression was observed (Figure 6D), consistent with the in vivo studies (Supplementary Figure 4). These results confirmed that the cytotoxicity of 5-FU is at least partly related to the induction of T cell ferroptosis, in which the increase of free ferrous ions caused by NCOA4-mediated ferritinophagy plays an important role.
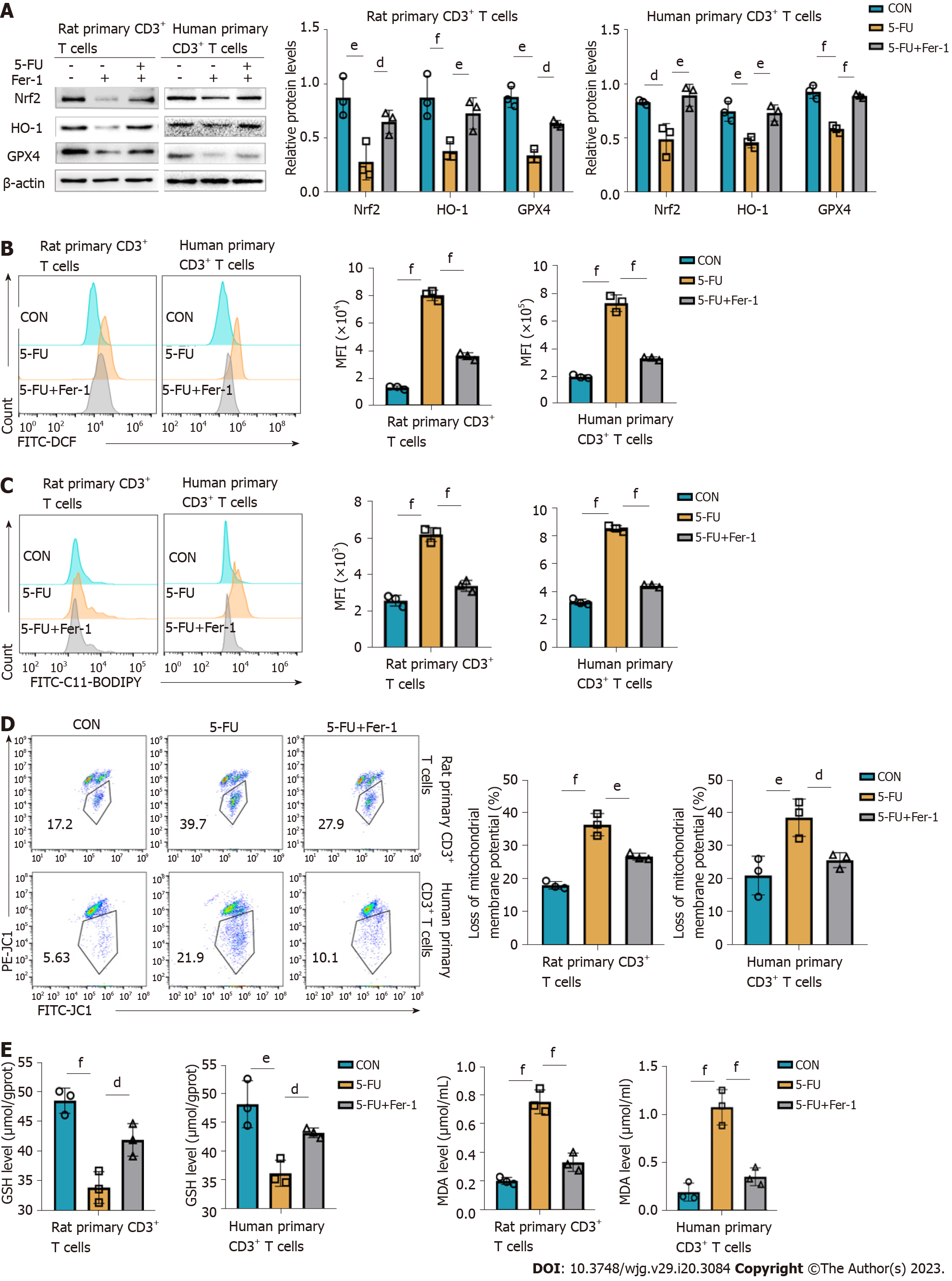
To evaluate the effect of 5-FU on the antioxidant system, antioxidant-related proteins were measured in rat and human primary CD3+ T cells treated with 5-FU. The expression of Nrf-2, HO-1, and GPX4 was evaluated in both groups and compared, and treatment with 5-FU was found to be associated with a marked decrease (Figure 7A), consistent with the in vivo studies (Supplementary Figure 4). The results showed that 5-FU treatment increased total ROS and lipid ROS levels and decreased MMP (Figure 7B–D). It also decreased glutathione (GSH) levels and increased the oxidative stress biomarker malondialdehyde (MDA) (Figure 7E and F). Fer-1 inhibits ferroptosis partly by activating Nrf2[29]. Accordingly, it reversed 5-FU-mediated inhibition of Nrf-2, HO-1, and GPX4, increased GSH level, and decreased MDA content, thereby alleviating cell peroxidation induced by 5-FU (Figure 7A–D). These results suggest that 5-FU inhibits the antioxidant system of T cells, inducing the T cells damaged by lipid peroxidation, which results in ferroptosis.
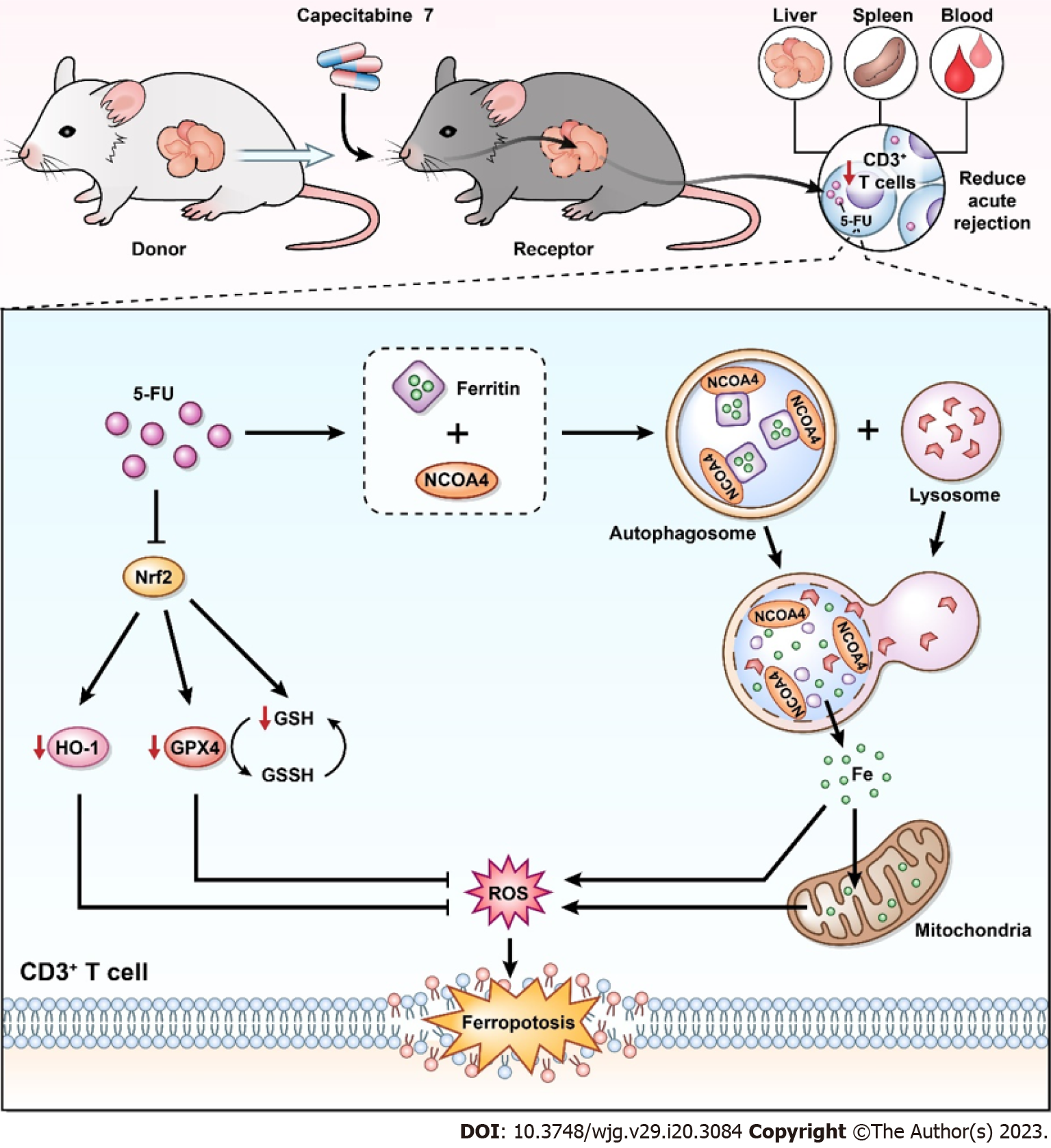
The conflict between the risk of tumor formation and immune rejection after transplantation has always been a concern. Therefore, it is of great clinical value to identify a drug with both immunosuppressive and anticancer effects. CAP is a classic chemotherapy drug for HCC[4,6], which has been shown to have immunosuppressive effects in a previous study[8], suggesting that metronomic CAP can be used as an immunosuppressant after transplantation and bring long-term benefits to LT patients with HCC. In our previous study, we found that metronomic CAP exerts immunosuppressive effects on normal mice by inducing T cell apoptosis[13]; however, the effect of CAP on the immune system in the context of organ transplantation was unclear. Therefore, we explored the safety, availability, and mechanism of metronomic CAP as an immunosuppressant in a model of rat orthotopic LT. Our results suggest that metronomic CAP can inhibit the acute rejection of LT by inhibiting T cells while avoiding the main side effects of 5-FU. Its mechanism is related to the induction of ferritin degradation and the inhibition of antioxidant-related proteins, which ultimately leads to the death of T cells (Figure 8).
The main effects of 5-FU are on rapidly proliferating tissues, specifically bone marrow. 5-FU suppressed bone marrow hematopoiesis including a significant decrease in the number of erythrocytes, platelet, and leukocyte in the peripheral blood[30,31]. As the prodrug of 5-FU, it is of high clinical relevance to show the safety of the medication of CAP. Overall, metronomic CAP is considered safe, with fewer side effects than traditional chemotherapy regimens. Metronomic CAP is well tolerated and safe in clinical practice, including for patients with HCC or patients after LT[8,32-34]. In mice, there was also no obvious myelosuppression in the MET group 7 d after taking CAP, though the related studies showed that 5-FU could cause severe myelosuppression after 7 d of administration[35]. TP is a key enzyme in the metabolism of CAP[36]. The lack of TP in bone marrow may explain the lack of bone marrow suppression of CAP[11,13]. In a past study, we established the safety of CAP in CAP-fed mice, which did not cause significant myelosuppression after 21 d of administration[13]. However, the depletion of lymphocytes is not related to bone marrow suppression, and the high expression of TP in lymphocytes directly leads to the killing effect of CAP[11]. CAP is safe for long-term use because it can circumvent the inhibitory effect of 5-FU on bone marrow hematopoietic function.
When acute rejection occurs, immune cells mainly infiltrate the hilar region of the grafted liver, inducing pericentral inflammation, damaging the vascular and bile duct endothelium, damaging hepatocytes, and destroying the lobular structure[37,38]. CAP can preserve the function and structure of the transplanted liver by inhibiting acute rejection. Compared with the CON group, the Banff score was significantly lower in the MET group at 7 d after transplantation while the elevation of ALT and AST in the MET group was significantly less pronounced than that in the CON group. ALT and AST are primarily expressed in liver cells, and liver allograft destruction caused by rejection is positively associated with ALT and AST expression[39,40]. However, TBIL and DBIL in both groups showed an increasing trend after surgery with no significant difference between the two groups. It may be related to biliary tract injury and bilirubin stasis caused by the lack of hepatic artery reconstruction and the use of cannula reconstruction of the bile duct in the traditional rat “two-cuff method” orthotopic LT[41].
Antigen presentation and T cell activation are key steps in rejection. Donor-derived antigen-presenting cells provide immune stimulation to recipient naive CD4+ T cells, known as the direct antigen presentation pathway, which is the main mode of activation in acute rejection[42,43]. Once rejection is initiated, CD8+ T cells mainly differentiate into cytotoxic T cells, which directly cause graft injury. CD4+ T cells can differentiate into many subtypes, mainly helper T cells (Th1, Th2, and Th17) and regulatory T cells (Treg). In acute rejection, T cells differentiate mainly to Th1, driven by pro-inflammatory cytokines such as IL-12 and IFN-γ. Th1 cells secrete IL-2 and IFN-γ, providing a positive feedback loop to stimulate Th1 cells to proliferate further. IFN-γ is usually associated with proinflammatory and immune activation processes while IL-2 is necessary for survival and effects of activated T cells. T cell activation, proliferation, differentiation, and migration are the immune basis of acute rejection after organ transplantation, and are often used as the targets of clinical immunosuppression[42,44,45]. The results of our study showed that IL-2 and IFN-γ were decreased in CAP treated rats. The changes in cytokines suggest that in addition to decreasing the number of T cells, CAP may also target Th0 cells’ differentiation. Since IFN-γ has a stimulatory effect on the secretion of TNF-α by M1 macrophages[46], the decrease in TNF-α may be related to the inhibition of IL-2 secretion in T cells by CAP. By inducing the programmed death of T cells, CAP mitigates transplant rejection and protects graft function. Moreover, cytokines associated with rejection were also affected by CAP treatment. Metronomic CAP significantly reduced the levels of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-2, and IL-12p70), which are mainly secreted by immune cells and contributing to rejection[47-50].
The data from our study demonstrated that the selective cytotoxic effect of the T cells of metronomic CAP is crucial for the prevention of acute organ transplant rejection. Next, we further explored the mechanism underlying the immunosuppressive effect of metronomic CAP by studying CD3+ T cells in more details. Mitochondria is a main source of ROS and mitochondria ROS is a critical component of T cell activation, proliferation, and effector function of T cells[51-53]. However, excessive ROS production, inducible by a high level of iron, leads to ROS-mediated membrane phospholipid peroxidation and T cell death upon activation[54]. Thus, we investigated the effect of CAP on the level of CD3+ T cell ferrous ions with a ferrous probe and found that CAP treatment induced ferrous ion accumulation, accompanied by increased ROS and lipid ROS and decreased MMP. Mitochondrial ROS production and MMP reduction are two important parameters of mitochondrial damage, and lipid peroxidation following ROS generation plays an important role in ROS-induced cellular damage[55-57]. These results indicate that metronomic CAP increased ferrous ion accumulation and oxidative damage in the CD3+ T cells of rats after transplantation, which may be the mechanism behind CAP-induced T cell reduction. Next, we used rat and human primary CD3+ T cells to further explore the role of ferroptosis in CAP-induced immunosuppression in vitro. Free ferrous iron overload, lipid peroxide accumulation, and specific mitochondrial morphological changes are three key characteristics that distinguish ferroptosis from other programmed cell deaths[14]. In this study, typical mitochondrial changes were observed in T cells of the 5-FU group under transmission electron microscopy. Ferrous ion in rat primary CD3+ T cells of the 5-FU group was increased with the downregulation of FTH1 and upregulation of NCOA4, revealing the occurrence of ferritinophagy. That is, the autophagic degradation of ferritin, contributed to rat T cell ferrous overload, excessive lipid peroxidation, and eventually ferroptosis in the 5-FU group. Nrf2 is a core player in the regulation of antioxidant molecules in cells, which can induce the synthesis of HO-1 and GPX4[20,58]. The Nrf2-HO-1/GPX4 antioxidative system, which participates in the regulation of oxidative damage and inhibits erastin-induced ferroptosis[59], was suppressed by 5-FU in vitro. It also reduced GSH levels, indicative of impairment of antioxidant capacity, and improved the levels of MDA, an end-product of lipid peroxidation.
In this study, we showed that metronomic CAP alleviated rat LT rejection without the common side effect (myelosuppression) of antitumor drugs. T cell ferroptosis plays an important role in the antirejection effect of CAP, which can induce cell iron overload and suppress the Nrf2-HO-1/GPX4 antioxidant systems, directly increasing the levels of intracellular ROS, leading to severe mitochondrial damage in T cells. These results revealed a new mechanism of CAP-induced T cell programmed death and suggested the possibility of using CAP as an immunosuppressant after transplantation. As a traditional antitumor drug, CAP has immunosuppressive effects, that may kill tumor cells and induce T cell death at the same time. CAP may have both antirejection and antitumor effects on patients with HCC after LT, thereby having broad clinical application prospects. In light of our findings, CAP alone as an immunosuppressive agent does not achieve satisfactory therapeutic effects. Therefore, the combination and interaction of CAP with other immunosuppressive agents is an important research direction in the future. In addition, we observed interesting effects of CAP on different cytokines, but further observations were lacking. The effects of CAP on T cell subsets and other immune cells are worth further attention. In the future, we plan to establish tumor-bearing animal models for allotransplantation, to clarify the dual antirejection and antitumor effects of CAP. In conclusion, the immunosuppressive effect of CAP should be further explored to optimize the drug treatment regimen for LT patients with liver cancer.
Metronomic CAP can alleviate liver graft injury and reduce the proliferation and infiltration of CD3+ T cells in peripheral blood, graft, and spleen, thereby inhibiting acute rejection after LT. T cell ferroptosis plays an important role in the antirejection effect of CAP, which can induce cell iron overload and suppress the Nrf2-HO-1/GPX4 antioxidant systems, thereby directly increasing the levels of intracellular ROS and leading to severe oxidative damage in T cells.
As a classical antimetabolite, capecitabine (CAP) has shown potential antirejection effects after liver transplantation (LT) in clinical trials. Our previous study showed that metronomic CAP can cause programmed T cell death in healthy mice by inducing oxidative stress, which is also the key step in ferroptosis. Thus, ferroptosis may play an important role in CAP-induced T cell death and an immunosuppressive role in acute rejection after transplantation.
This study investigated the immunosuppressive effect of metronomic CAP in rat LT and its mechanism, which may be used as an immunosuppressive agent after LT to improve the prognosis of liver transplant patients with hepatocellular carcinoma.
The objective of this study was to investigate the possibility of using CAP as an anti-rejection agent after transplantation. The results showed that metronomic CAP could exert an immunosuppressive effect by inducing T cell ferroptosis, which provided a basis for investigating CAP as part of an immunosuppressive regimen.
A rat LT model of acute rejection was established, and the effect of metronomic CAP on splenic hematopoietic function and acute graft rejection was evaluated 7 d after transplantation. In vitro, primary CD3+ T cells were sorted and co-cultured with or without 5-fluorouracil (5-FU) (active agent of CAP). The levels of ferroptosis-related proteins, ferrous ion concentration, and oxidative stress-related indicators were observed. The changes in mitochondrial structure were observed using electron microscopy.
With no significant myelotoxicity, metronomic CAP alleviated graft injury, prolonged the survival time of the recipient rats, and reduced the infiltration rate of CD3+ T cells in peripheral blood, liver graft, and spleen, thereby inhibiting acute rejection after LT. In vitro, 5-FU, an end product of CAP metabolism, induced the degradation of the ferritin heavy chain by upregulating nuclear receptor coactivator 4, which caused the accumulation of ferrous ions. It also inhibited nuclear erythroid 2 p45-related factor 2, heme oxygenase-1, and glutathione peroxidase 4, eventually leading to oxidative damage and ferroptosis of T cells.
Metronomic CAP can alleviate liver graft injury and reduce the proliferation and infiltration of CD3+ T cells in peripheral blood, graft, and spleen by inducing T cell oxidative damage and ferroptosis, thereby inhibiting acute rejection after LT.
The combination and interaction of CAP with other immunosuppressive agents is an important research direction in the future. In addition, the effects of CAP on T cell subsets and other immune cells are worth further attention. Besides, we plan to establish tumor-bearing animal models for allotransplantation, to clarify the dual antirejection and antitumor effects of CAP.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boteon YL, Brazil; Chun JW, Korea S-Editor: Fan JR L-Editor: A P-Editor: Liu JH
| 1. | Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Gish RG. Hepatocellular carcinoma: overcoming challenges in disease management. Clin Gastroenterol Hepatol. 2006;4:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Kawahara T, Asthana S, Kneteman NM. m-TOR inhibitors: what role in liver transplantation? J Hepatol. 2011;55:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Pelizzaro F, Sammarco A, Dadduzio V, Pastorelli D, Giovanis P, Soldà C, Rizzato MD, Lombardi G, Lonardi S, Peserico G, Imondi A, Sartori A, Maddalo G, Farinati F. Capecitabine in advanced hepatocellular carcinoma: A multicenter experience. Dig Liver Dis. 2019;51:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Changou CA, Shiah HS, Chen LT, Liu S, Luh F, Liu SH, Cheng YC, Yen Y. A Phase II Clinical Trial on the Combination Therapy of PHY906 Plus Capecitabine in Hepatocellular Carcinoma. Oncologist. 2021;26:e367-e373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol. 2016;13:659-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Kerbel RS, Shaked Y. The potential clinical promise of 'multimodality' metronomic chemotherapy revealed by preclinical studies of metastatic disease. Cancer Lett. 2017;400:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Ravaioli M, Cucchetti A, Pinna AD, De Pace V, Neri F, Barbera MA, Maroni L, Frega G, Palloni A, De Lorenzo S, Ripoli MC, Pantaleo MA, Cescon M, Del Gaudio M, Brandi G. The role of metronomic capecitabine for treatment of recurrent hepatocellular carcinoma after liver transplantation. Sci Rep. 2017;7:11305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 379] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 10. | Ishikawa T, Utoh M, Sawada N, Nishida M, Fukase Y, Sekiguchi F, Ishitsuka H. Tumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancer xenografts. Biochem Pharmacol. 1998;55:1091-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Yoshimura A, Kuwazuru Y, Furukawa T, Yoshida H, Yamada K, Akiyama S. Purification and tissue distribution of human thymidine phosphorylase; high expression in lymphocytes, reticulocytes and tumors. Biochim Biophys Acta. 1990;1034:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, Utoh M, Mori K, Weidekamm E, Reigner B. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. 2000;45:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 490] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 13. | Zhang S, Wang Z, Fan S, Liu T, Yoshida S, Yang S, Liu L, Hou W, Cao L, Wang J, Song Z, Li S, Zhang S, Wang H, Li J, Zheng H, Shen Z. Capecitabine Can Induce T Cell Apoptosis: A Potential Immunosuppressive Agent With Anti-Cancer Effect. Front Immunol. 2021;12:737849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4711] [Cited by in RCA: 11753] [Article Influence: 904.1] [Reference Citation Analysis (1)] |
| 15. | Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 2621] [Article Influence: 291.2] [Reference Citation Analysis (0)] |
| 16. | Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 2533] [Article Influence: 506.6] [Reference Citation Analysis (0)] |
| 17. | Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, Ma J, Li W, Kesselman E, Abutbul-Ionita I, Danino D, Gutierrez L, Li H, Li K, Lou H, Regoni M, Poli M, Glaser F, Rouault TA, Meyron-Holtz EG. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood. 2018;131:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 19. | Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1085] [Cited by in RCA: 1531] [Article Influence: 255.2] [Reference Citation Analysis (0)] |
| 20. | Song X, Long D. Nrf2 and Ferroptosis: A New Research Direction for Neurodegenerative Diseases. Front Neurosci. 2020;14:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 21. | Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6:24393-24403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 476] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 22. | Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 1012] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 23. | Deng HF, Yue LX, Wang NN, Zhou YQ, Zhou W, Liu X, Ni YH, Huang CS, Qiu LZ, Liu H, Tan HL, Tang XL, Wang YG, Ma ZC, Gao Y. Mitochondrial Iron Overload-Mediated Inhibition of Nrf2-HO-1/GPX4 Assisted ALI-Induced Nephrotoxicity. Front Pharmacol. 2020;11:624529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 24. | Deng S, Wu D, Li L, Li J, Xu Y. TBHQ attenuates ferroptosis against 5-fluorouracil-induced intestinal epithelial cell injury and intestinal mucositis via activation of Nrf2. Cell Mol Biol Lett. 2021;26:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Li D, Song C, Zhang J, Zhao X. ROS and iron homeostasis dependent ferroptosis play a vital role in 5-Fluorouracil induced cardiotoxicity in vitro and in vivo. Toxicology. 2022;468:153113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Xu HS, Rosenlof LK, Pruett TL, Jones RS. Prostaglandin E1 increases survival with extended anhepatic phase during liver transplantation. Ann Surg. 1994;220:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [PubMed] |
| 28. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1002] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 29. | Li X, Chen J, Yuan S, Zhuang X, Qiao T. Activation of the P62-Keap1-NRF2 Pathway Protects against Ferroptosis in Radiation-Induced Lung Injury. Oxid Med Cell Longev. 2022;2022:8973509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Ishibashi M, Ishii M, Yamamoto S, Mori Y, Shimizu S. Possible involvement of TRPM2 activation in 5-fluorouracil-induced myelosuppression in mice. Eur J Pharmacol. 2021;891:173671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Numazawa S, Sugihara K, Miyake S, Tomiyama H, Hida A, Hatsuno M, Yamamoto M, Yoshida T. Possible involvement of oxidative stress in 5-fluorouracil-mediated myelosuppression in mice. Basic Clin Pharmacol Toxicol. 2011;108:40-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, Serra C, Sama C, Golfieri R, Gramenzi A, Cucchetti A, Pinna AD, Trevisani F, Biasco G; Italian Liver Cancer (ITA. LI.CA) Group. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist. 2013;18:1256-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Granito A, Marinelli S, Terzi E, Piscaglia F, Renzulli M, Venerandi L, Benevento F, Bolondi L. Metronomic capecitabine as second-line treatment in hepatocellular carcinoma after sorafenib failure. Dig Liver Dis. 2015;47:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Casadei Gardini A, Foca F, Scartozzi M, Silvestris N, Tamburini E, Faloppi L, Brunetti O, Rudnas B, Pisconti S, Valgiusti M, Marisi G, Foschi FG, Ercolani G, Tassinari D, Cascinu S, Frassineti GL. Metronomic capecitabine versus best supportive care as second-line treatment in hepatocellular carcinoma: a retrospective study. Sci Rep. 2017;7:42499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Kojima S, Takaba K, Kimoto N, Takeda T, Kakuni M, Mizutani M, Suzuki K, Sato H, Hara T. Protective effects of glutathione on 5-fluorouracil-induced myelosuppression in mice. Arch Toxicol. 2003;77:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Lam SW, Guchelaar HJ, Boven E. The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev. 2016;50:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 37. | Fels Elliott DR, Tavakol M, Lucas CH, Sheahon K, Kakar S, Hameed B, Ferrell LD, Gill RM. Clinicopathological features and outcomes of parenchymal rejection in liver transplant biopsies. Histopathology. 2020;76:822-831. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Demirhan B, Bilezikçi B, Haberal AN, Sevmiş S, Arat Z, Haberal M. Hepatic parenchymal changes and histologic eosinophilia as predictors of subsequent acute liver allograft rejection. Liver Transpl. 2008;14:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | De Lorenzo S, Tovoli F, Barbera MA, Garuti F, Palloni A, Frega G, Garajovà I, Rizzo A, Trevisani F, Brandi G. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: a proof of concept. Sci Rep. 2018;8:9997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 40. | Vionnet J, Miquel R, Abraldes JG, Wall J, Kodela E, Lozano JJ, Ruiz P, Navasa M, Marshall A, Nevens F, Gelson W, Leithead J, Masson S, Jaeckel E, Taubert R, Tachtatzis P, Eurich D, Simpson KJ, Bonaccorsi-Riani E, Feng S, Bucuvalas J, Ferguson J, Quaglia A, Sidorova J, Elstad M, Douiri A, Sánchez-Fueyo A. Non-invasive alloimmune risk stratification of long-term liver transplant recipients. J Hepatol. 2021;75:1409-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 41. | Li GL, Lin HM, Long TZ, Lv LH, Yu JD, Huang YH, Min J, Wan YL. High incidence of biliary complications in rat liver transplantation: can we avoid it? World J Gastroenterol. 2011;17:3140-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Ronca V, Wootton G, Milani C, Cain O. The Immunological Basis of Liver Allograft Rejection. Front Immunol. 2020;11:2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 43. | Safinia N, Vaikunthanathan T, Lechler RI, Sanchez-Fueyo A, Lombardi G. Advances in Liver Transplantation: where are we in the pursuit of transplantation tolerance? Eur J Immunol. 2021;51:2373-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Kumar BV, Connors TJ, Farber DL. Human T Cell Development, Localization, and Function throughout Life. Immunity. 2018;48:202-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 849] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 45. | Wong TC, Lo CM, Fung JY. Emerging drugs for prevention of T-cell mediated rejection in liver and kidney transplantation. Expert Opin Emerg Drugs. 2017;22:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Sun G, Yang S, Cao G, Wang Q, Hao J, Wen Q, Li Z, So KF, Liu Z, Zhou S, Zhao Y, Yang H, Zhou L, Yin Z. γδ T cells provide the early source of IFN-γ to aggravate lesions in spinal cord injury. J Exp Med. 2018;215:521-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Li S, Yu J, Guo C, Jie Y, Pan Z. The Balance of Th1/Th2 and LAP+Tregs/Th17 Cells Is Crucial for Graft Survival in Allogeneic Corneal Transplantation. J Ophthalmol. 2018;2018:5404989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Newstead CG, Lamb WR, Brenchley PE, Short CD. Serum and urine IL-6 and TNF-alpha in renal transplant recipients with graft dysfunction. Transplantation. 1993;56:831-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Ma L, Zhang H, Hu K, Lv G, Fu Y, Ayana DA, Zhao P, Jiang Y. The imbalance between Tregs, Th17 cells and inflammatory cytokines among renal transplant recipients. BMC Immunol. 2015;16:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Assadiasl S, Mooney N, Nicknam MH. Cytokines in Liver Transplantation. Cytokine. 2021;148:155705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1005] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 52. | Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1064] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 53. | Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, Turka LA, Wood KC, Hale LP, Smith PA, Schneider MA, MacIver NJ, Locasale JW, Newgard CB, Shinohara ML, Rathmell JC. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 590] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 54. | Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 504] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 55. | Ogando DG, Choi M, Shyam R, Li S, Bonanno JA. Ammonia sensitive SLC4A11 mitochondrial uncoupling reduces glutamine induced oxidative stress. Redox Biol. 2019;26:101260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev. 2016;2016:4350965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 740] [Cited by in RCA: 1257] [Article Influence: 139.7] [Reference Citation Analysis (0)] |
| 57. | Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 3817] [Article Influence: 347.0] [Reference Citation Analysis (0)] |
| 58. | Kerins MJ, Milligan J, Wohlschlegel JA, Ooi A. Fumarate hydratase inactivation in hereditary leiomyomatosis and renal cell cancer is synthetic lethal with ferroptosis induction. Cancer Sci. 2018;109:2757-2766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 59. | Xu C, Sun S, Johnson T, Qi R, Zhang S, Zhang J, Yang K. The glutathione peroxidase Gpx4 prevents lipid peroxidation and ferroptosis to sustain Treg cell activation and suppression of antitumor immunity. Cell Rep. 2021;35:109235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 304] [Article Influence: 76.0] [Reference Citation Analysis (0)] |